Introduction
With an aging global population, the morbidity of
Alzheimer's disease (AD), an age-related illness, is increasing
significantly (1). In 2008, the
cost of treatment in Europe reached 177 billion euros, far more
than cost of treating tumors and heart disease which are considered
as diseases with the highest morbidity and death rate (2). Furthermore, the available treatment
options for AD are restricted and therefore optimal outcomes are
not achieved (3). Thus, novel
therapies producing reliable effects and offering ease of
administration and minimal adverse effects, which can be used in
clinical practices, would benefit patients with AD worldwide.
AD is a progressive, neurodegenerative disease
characterized by worsening of cognition and memory, progressive
interference with daily living activities accompanied by
neuropsychiatric symptoms and behavioral disorders (4). The pathogenesis of AD is
characterized by abnormalities in amyloid precursor proteins which
result in the leakage of proteins from the cytomembrane, thereby
causing neurofibrillary tangles and cell death, i.e., selective
neuronal loss. Drugs used to treat AD principally improve the
neurotransmission of choline, cerebral circulation and the
metabolism of brain cells (5).
Furthermore, drugs including calcium antagonists, hormone therapy,
nonsteroidal anti-inflammatory drugs, free radical scavengers,
antioxidants and muscarinic receptor agonists, are also used in the
treatment of AD. without achieving optimal therapeutic outcomes
(6). Thus, the development of
effective treatments for AD is proving to be challenging.
Caffeic acid possesses a number of pharmacologic
functions including anti-inflammatory, antibacterial and antiviral
effects (7–9). Furthermore, it may also increase the
levels of white blood cells and blood platelets (10). Thus, caffeic acid has the
potential to be used to minimize oxidative stress and inflammatory
responses in cardiovascular diseases and brain damage as well as to
prevent and treat viral diseases such as HIV, in addition to
treating leukopenia and thrombocytopenia (11). By exerting antioxidant,
anti-inflammatory, immunoregulatory and antibacterial effects,
caffeic acid may potentially be of value in the treatment of
diseases associated with oxidative stress and inflammatory
responses (7,11,12). To the best of our knowledge, this
is the first study to directly examine the effects of caffeic acid
in AD by evaluating cognitive function and to explore the possible
mechanisms responsible for these effects.
Materials and methods
Animals
Healthy male Sprague-Dawley (SD) rats (7–8 weeks of
age) were purchased from the Experimental Center of Henan
University School of Medicine and were housed in a room maintained
at 23°C under a 12-h light/dark cycle, with ad libitum
access to food and water. The present study was approved by the
Animal Care and Use Committee of Zhengzhou University (Zhengzhou,
China), and all experiments were performed in accordance with the
National Institutes of Health Guidelines for the Care and Use of
Laboratory Animals. Firstly, the amyloid β-peptide Aβ1-40
(Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was dissolved in
deionized water stored at −80°C until use. The rats were subjected
to an intraperitoneal injection of 350 mg/kg chloral hydrate. The
hippocampus was localized at 3.8 mm posterior, 2.9 mm below the top
of the skull and 2.4 mm lateral to the Bregma. Each rat received 5
µl Aβ1-40 in the left side over 10 min in order to establish
a model of AD.
Grouping
The rats were randomly divided into 3 groups
(n=12/group): i) control group, ii) AD model group, and iii)
caffeic acid group. The rats in the caffeic acid group were
injected with 100 mg/kg caffeic acid (Sigma-Aldrich Chemie GmbH)
for 2 weeks; the rats in the control and AD model groups were were
injected with normal saline for 2 weeks.
Morris water maze task
A circular water pool (150×60 cm; containing water
at 24±2°C) was divided into 4 equally spaced quadrants containing
various prominent visual cues. An invisible excape platform (10×10
cm, 1 cm below the water surface) was hidden in the center of
quadrant II during the training period and removed at the time of
the probe task. Five days after the Aβ1–40 injection, memory
training was initiated. The training was recorded twice a day for 5
days. Each rat was allowed to swim onto the platform or until a 120
sec time period had elapsed. A video tracking system (SMART; Panlab
SL, Barcelona, Spain) was used to record the escape latency,
average swim speed, mean path lenth, time spent in the target
quadrant, and the number of times the animal crossed the previous
location of the platform.
Hematoxylin and eosin (H&E)
staining
We examined synaptophysin expression in our model of
AD to analyze the effects of caffeic acid on cerebral damage by
H&E staining. After the rats were sacrificed by decollation
under anestheisia, the hippocampal tissues were quickly removed,
washed with saline and embedded in paraffin. The hippocampal
tissues were then serially sectioned into 30-mm-thick slices. The
slices were stained with H&E and observed under a Mirax Scan
digital microscope slide scanner (Mirax 3D Histech; Carl Zeiss,
Oberkochen, Germany).
Determination of acetylcholinesterase
(AChE) activity in the brain
The hippocampal tissues were quickly removed, washed
with saline and embedded in paraffin. AChE activity was collected
from brain homogenate using the method developed by Ellman et
al (13). A spectrophotometer
(Infinite H200 PRO; Tecan, Männedorf, Switzerland) was used to
measure absorbance at 412 nm and the results are expressed as
µM of acetylthiocholine iodide hydrolysed/min/mg of
protein.
Measurement of nitrite generation in the
brain
A total of 100 µl homogenate was incubated
with an equal volume of Griess reagent at room temperature for 10
min. A spectrophotometer (Infinite H200 PRO; Tecan) was used to
measure nitrite generation in the brain at 550 nm and the results
were calculated from a sodium nitrite standard curve.
Measurement of catalase (CAT),
glutathione (GSH), interleukin-6 (IL-6) and tumor necrosis factor-α
(TNF-α) activities
CAT, GSH, IL-6 and TNF-α ELISA kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) were used to
analyse the supernatant (200 µl, which was removed using a
pipette). A spectrophotometer (Infinite H200 PRO; Tecan) was used
measure the activities of CAT, GSH, IL-6 and TNF-α.
Western blot analysis
The hippocampal tissues were quickly removed and
pre-cooled radio-immunoprecipitation assay (RIPA) buffer was used
to extract the proteins. Miscible liquids were centrifuged at
12,000 × g for 15 min at 4°C and used to determine the protein
content using the bicinchoninic acid method (Joincare
Pharmaceutical Group Industry Co., Ltd., Zhuhai, China). Equal
amounts of protein from each group were subjected to 12% SDS-PAGE
and transferred to polyvinylidene fluoride (PVDF) membranes
(Bio-Rad, Berkeley, CA, USA). The PVDF membranes were blocked with
5% fat milk in Tris-buffered saline for 1 h and incubated
anti-nuclear factor (NF)-κB-p65 (1:2,000), anti-p53 (1:4,000) and
anti-phosphorylated (p)-p38 MAPK (1:4,000) (all from Santa Cruz
Biotechnology, Santa Cruz, CA, USA); and anti-β-actin (1:500; Wuhan
Boster Biological Technology Ltd., Wuhan, China) at 4°C overnight.
The membranes were washed three times with 0.1% Tween-20 TBS and
incubated with horseradish peroxidase-conjugated anti-sheep
secondary antibodies (Wuhan Boster Biological Technology Ltd.). The
proteins were detected with an enhanced chemiluminescence kit
(Millipore Corp., Billerica, MA, USA).
Analysis of caspase-3 activity
The hippocampal tissues were quickly removed and a
pre-cooled RIPA buffer was used to extract proteins. Miscible
liquids were centrifuged at 12,000 × g for 15 min at 4°C and the
protein content was determined using the bicinchoninic acid method
(Joincare Pharmaceutical Group Industry Co., Ltd.). Equal amounts
of protein from each group were incubated with the colorimetric
substrate for caspase-3, Ac-DEVD-pNA, at 37°C for 2 h in the dark,
and detected using a spectrophotometer (Infinite H200 PRO, Tecan)
at a wavelength of 405 nm.
Statistical analysis
All data are expressed as the means ± standard error
of the mean. Statistical analysis was performed using one- or
two-way ANOVA followed by Dunnett's post hoc test. A p-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of caffeic acid on cognitive
function in a model of AD
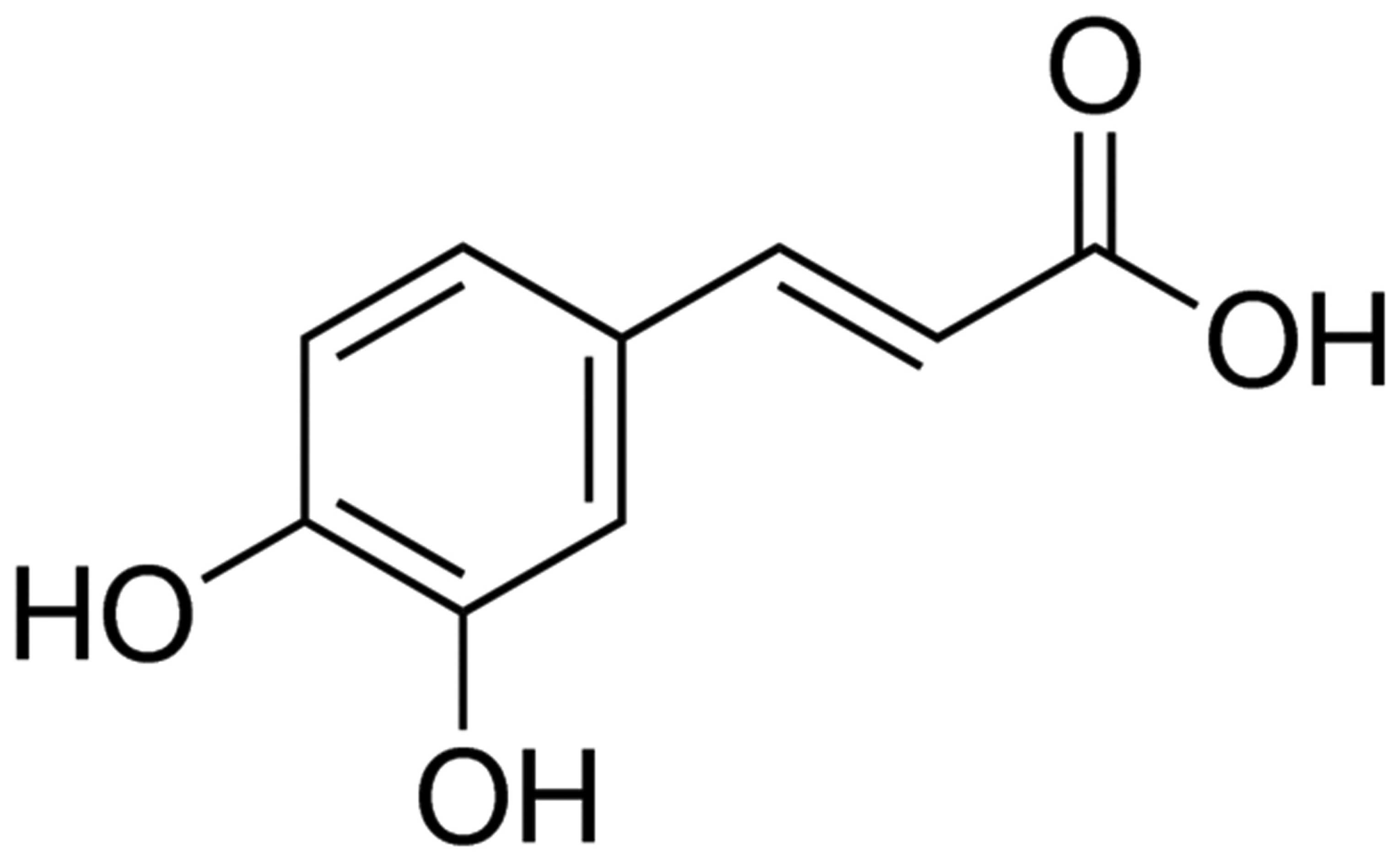
The chemical structure of caffeic acid is presented
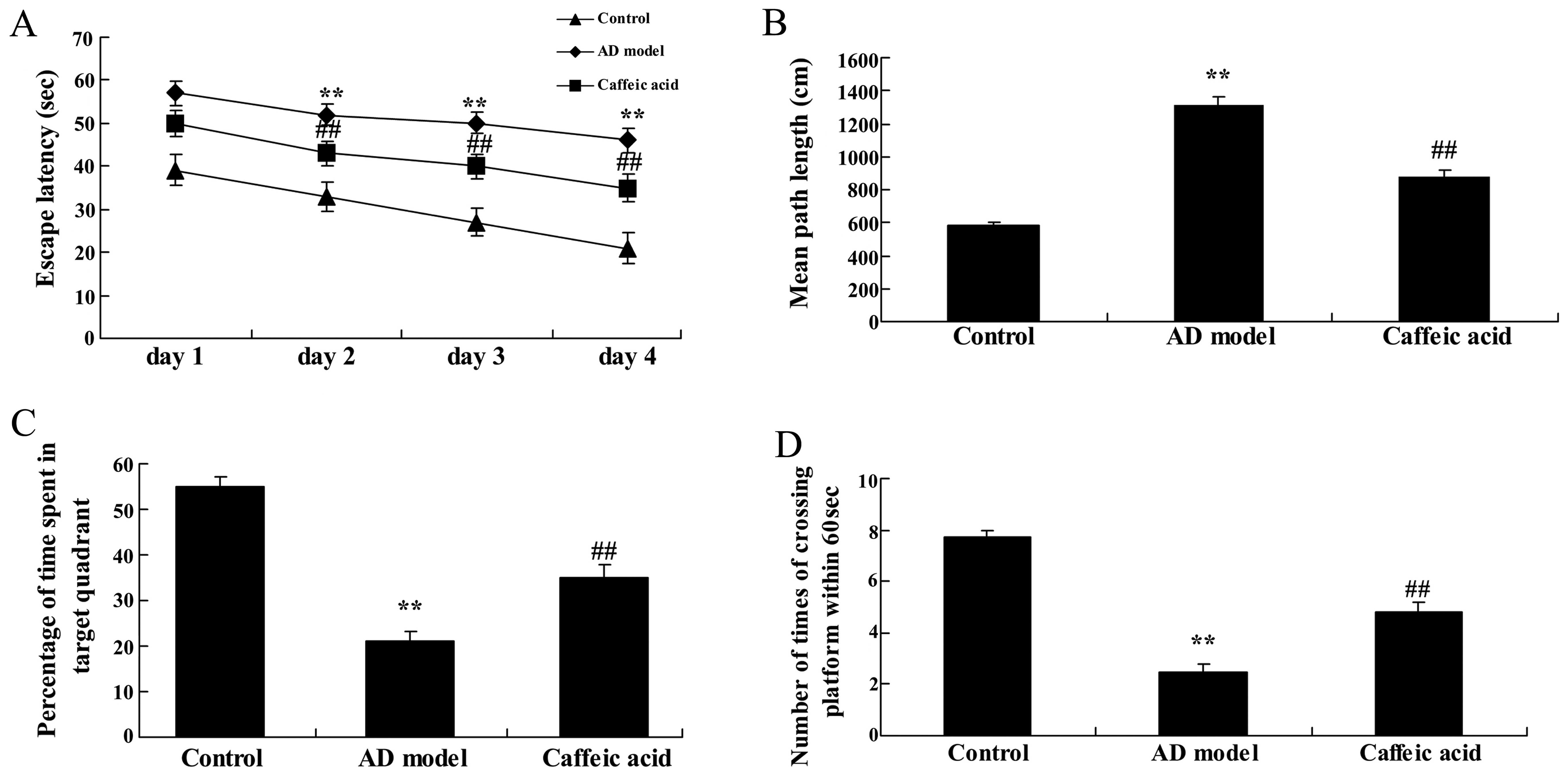
in Fig. 1. The results of the
Morris water maze task are shown Fig.
2. Escape latency in the AD model group significantly increased
compared with that in the control group at different time points
(Fig. 2A). However, there was a
significant decrease in escape latency in the caffeic acid-treated
group compared with that in the AD model group (Fig. 2A). The mean path length taken by
the rats in the AD group was significantly longer than that taken
by the rats in the control group (Fig. 2B). As shown in Fig. 2B, treatment with caffeic acid
significantly decreased the mean path length taken by the rats.
Moreover, the rats in the AD model group spent significantly less
time in the target quadrant and also, crossed the former platform
location within 60 sec fewer times, compared with the rats in the
control group (Fig. 2C and D).
Following caffeic acid treatment, the time spent in the target
quadrant and the number of times the animals crossed the former
platform location were significantly increased compared with the AD
model group (Fig. 2C and D).
Effect of caffeic acid on cerebral damage
in model of AD
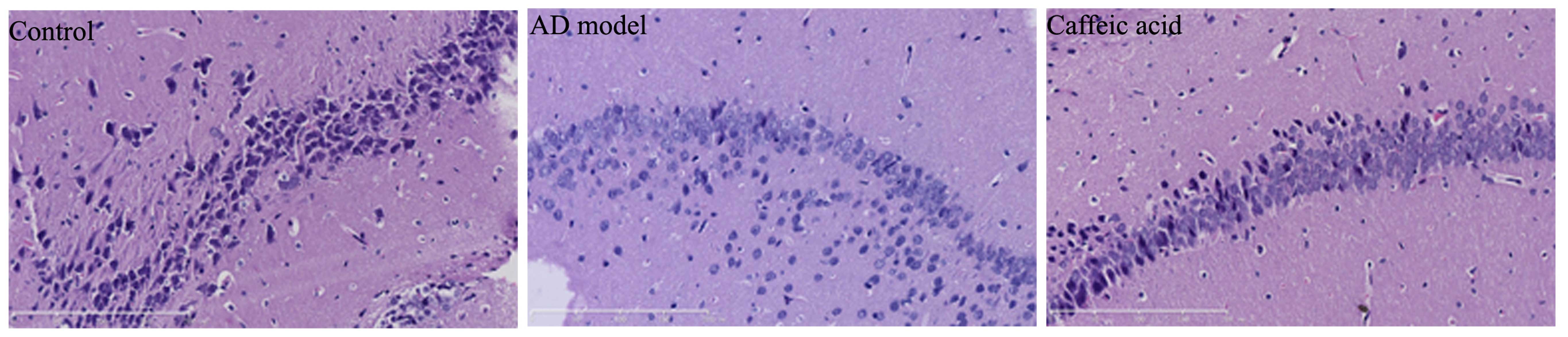
To examine the effect of caffeic acid on cerebral
damage in a model of AD, the hippocampal tissues from each group
were stained with H&E to determine synaptophysin expressoin. As
shown in Fig. 3, synaptophysin
expression was observably weakened and severe cerebral damage had
occurred in the AD model group compared with the control group.
Treatment with caffeic acid markedly increased synaptophysin
expression and weakened the AD-induced cerebral damage in the rats
compared with the AD model group (Fig. 3).
Effect of caffeic acid on AChE activity
in a model of AD
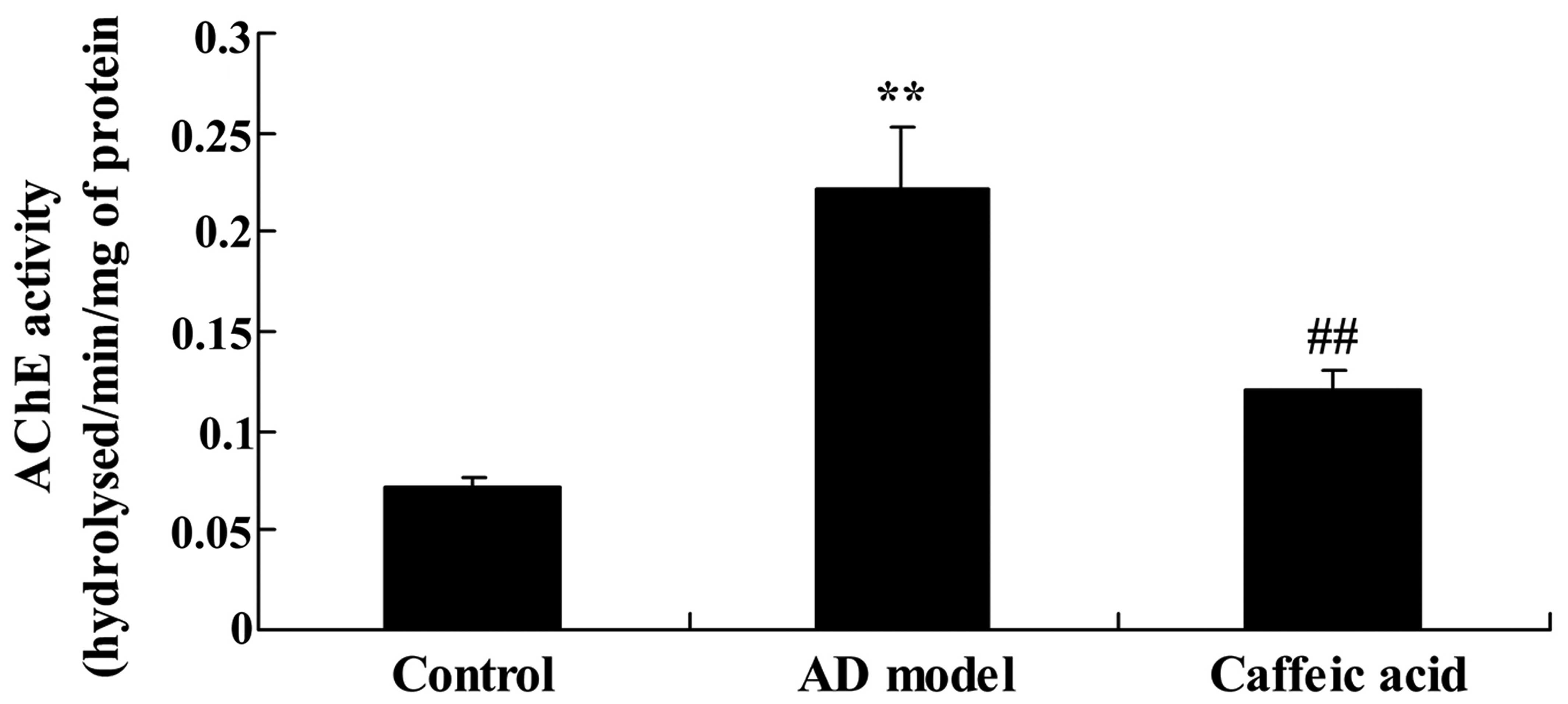
To examine the effect of caffeic acid on AChE
activity in a model of AD, we measured AChE activity in the rat
brain tissue. As shown in Fig. 4,
enhanced AChE activity was observed in the hippocampal tissues from
rats in the AD model group compared with the tissues from the
control group. Furthermore, the enhanced ChE activity was
significantly suppressed by treatment with caffeic acid compared
with that in the AD model group (Fig.
4).
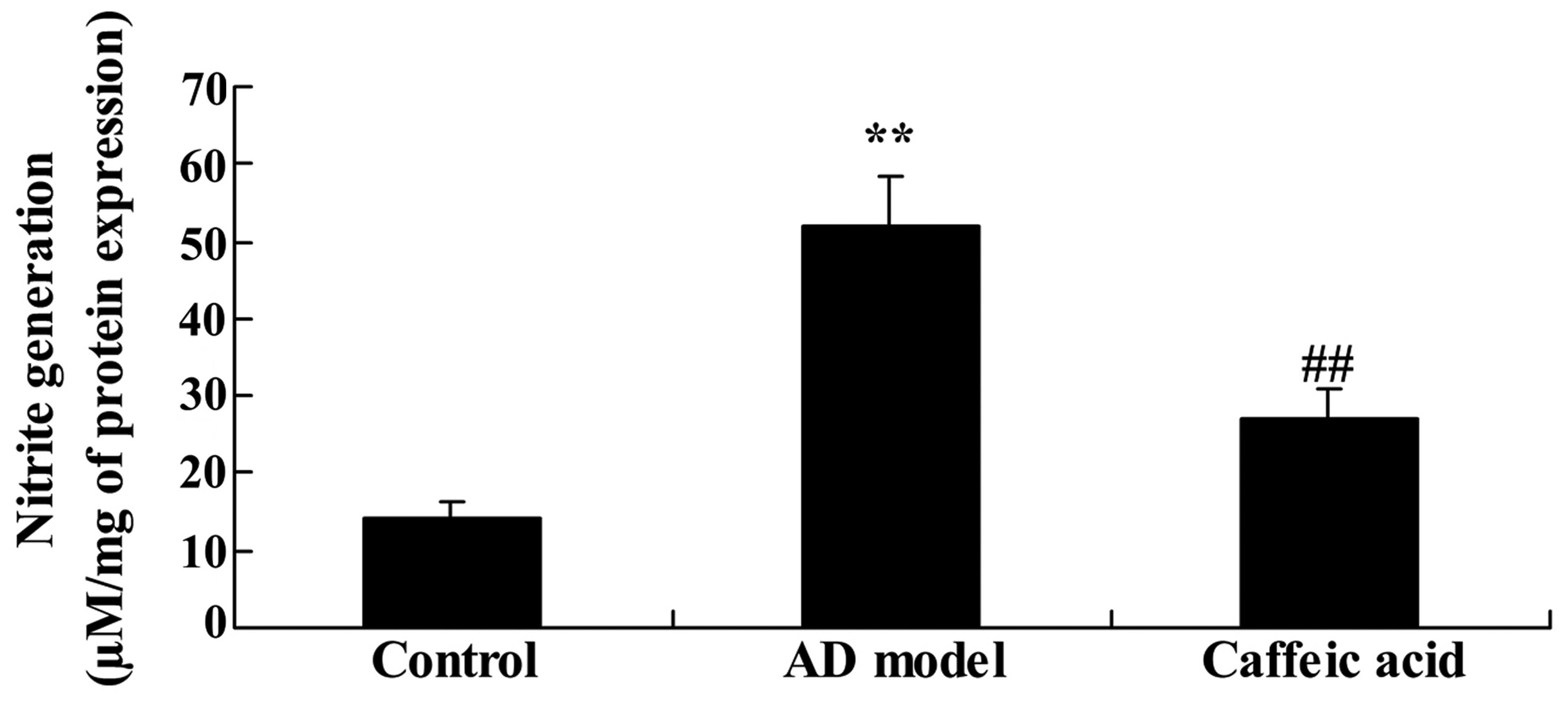
Effect of caffeic acid on nitrite
generation in a model of AD
We next examined the effect of caffeic acid on
nitrite generation in a model of AD. There was a significant
increase in nitrite generation in the hippocampal tissues of the AD
model group compared with that in the control group (Fig. 5). Compared with the AD model
group, caffeic acid significantly reduced the elevated generation
of nitrite (Fig. 5).
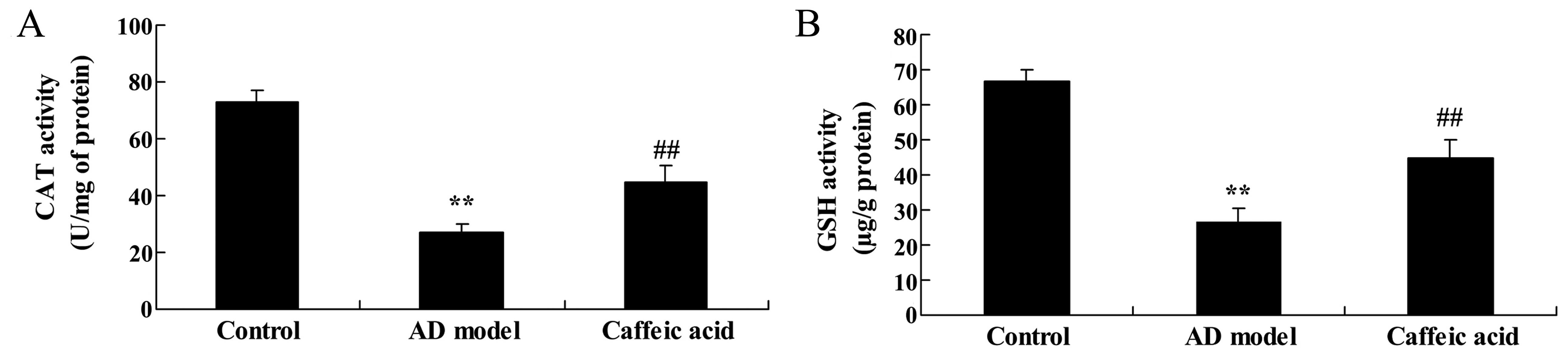
Effect of caffeic acid on oxidative
stress in a model of AD
The activities of CAT and GSH were significantly
reduced in the hippocampal tissues in the AD model group compared
with the control group (Fig. 6).
Treatment with caffeic acid significantly elevated the inhibition
of CAT and GSH activities in the hippocampal tissues compared with
the AD model group (Fig. 6).
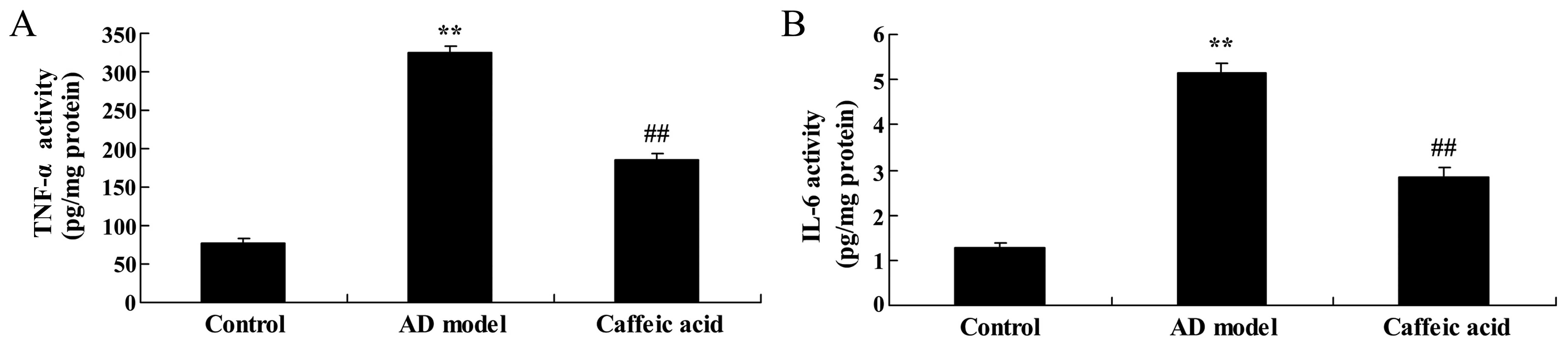
Effect of caffeic acid on inflammation in
a model of AD
Brain IL-6 and TNF-α activities in the AD model
group were increased compared with the control group (Fig. 7). The elevated IL-6 and TNF-α
activities were significantly reduced by caffeic acid treatment
(Fig. 7).
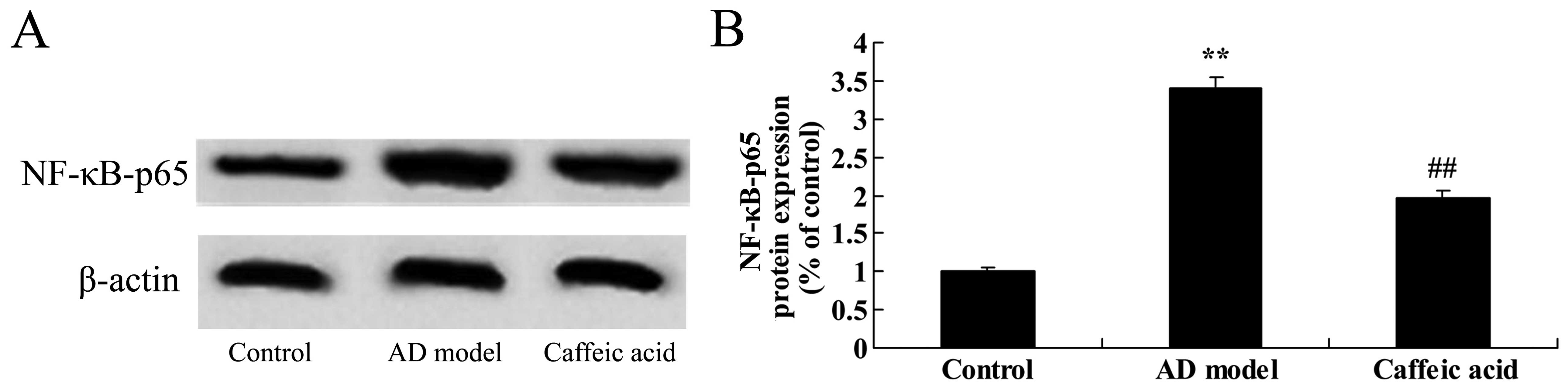
Effect of caffeic acid on NF-κB-p65
expression in a model of AD
The brain tissues from rats in the AD model group
were observed to have significantly increased NF-κB-p65 protein
expression as compared with the control animals (Fig. 8). Caffeic acid treatment
suppressed NF-κB-p65 protein expression significantly compared with
the AD model group (Fig. 8).
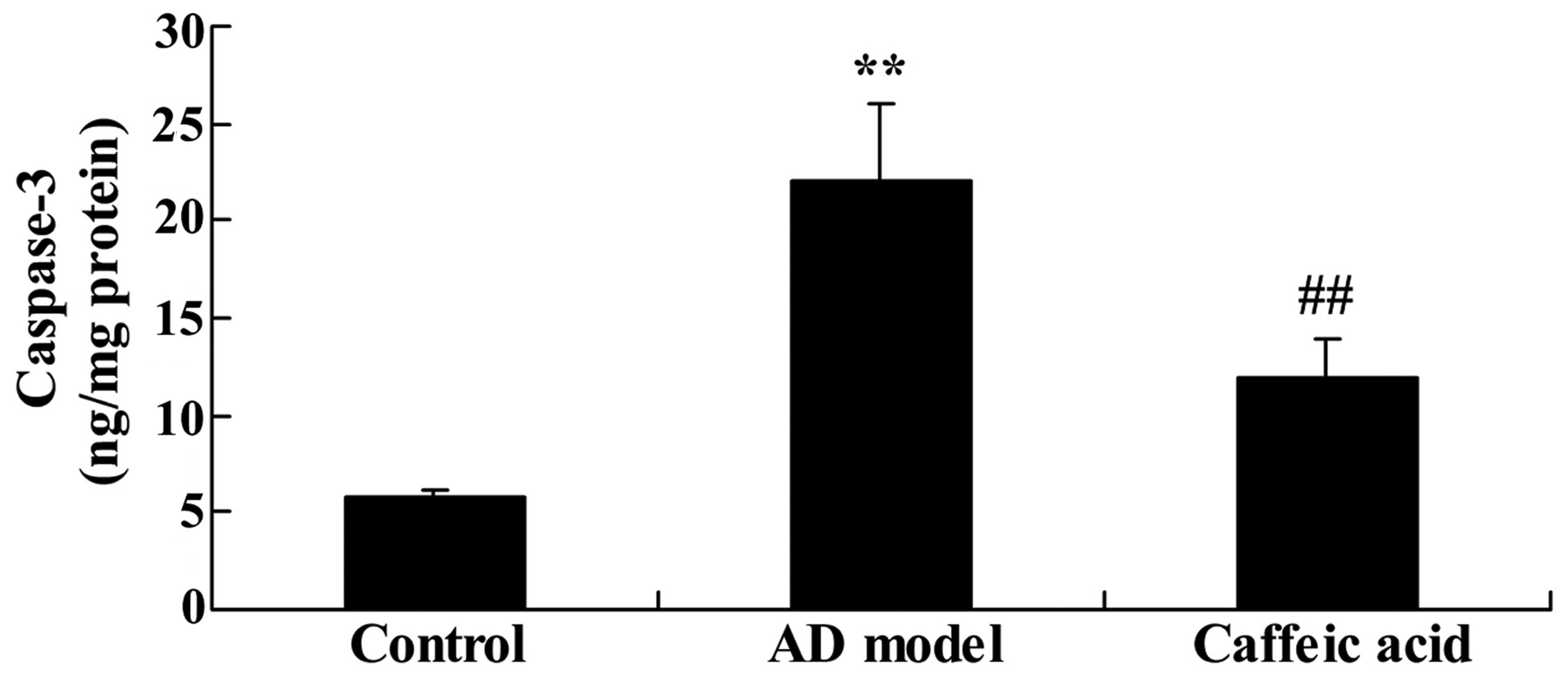
Effect of caffeic acid on caspase-3
activity in a model of AD
The rats in the AD model group showed a significant
increase in caspase-3 activity compared with the control animals
(Fig. 9). However, treatment with
caffeic acid significantly weakened the AD-induced caspase-3
activity compared with that in the AD model group (Fig. 9).
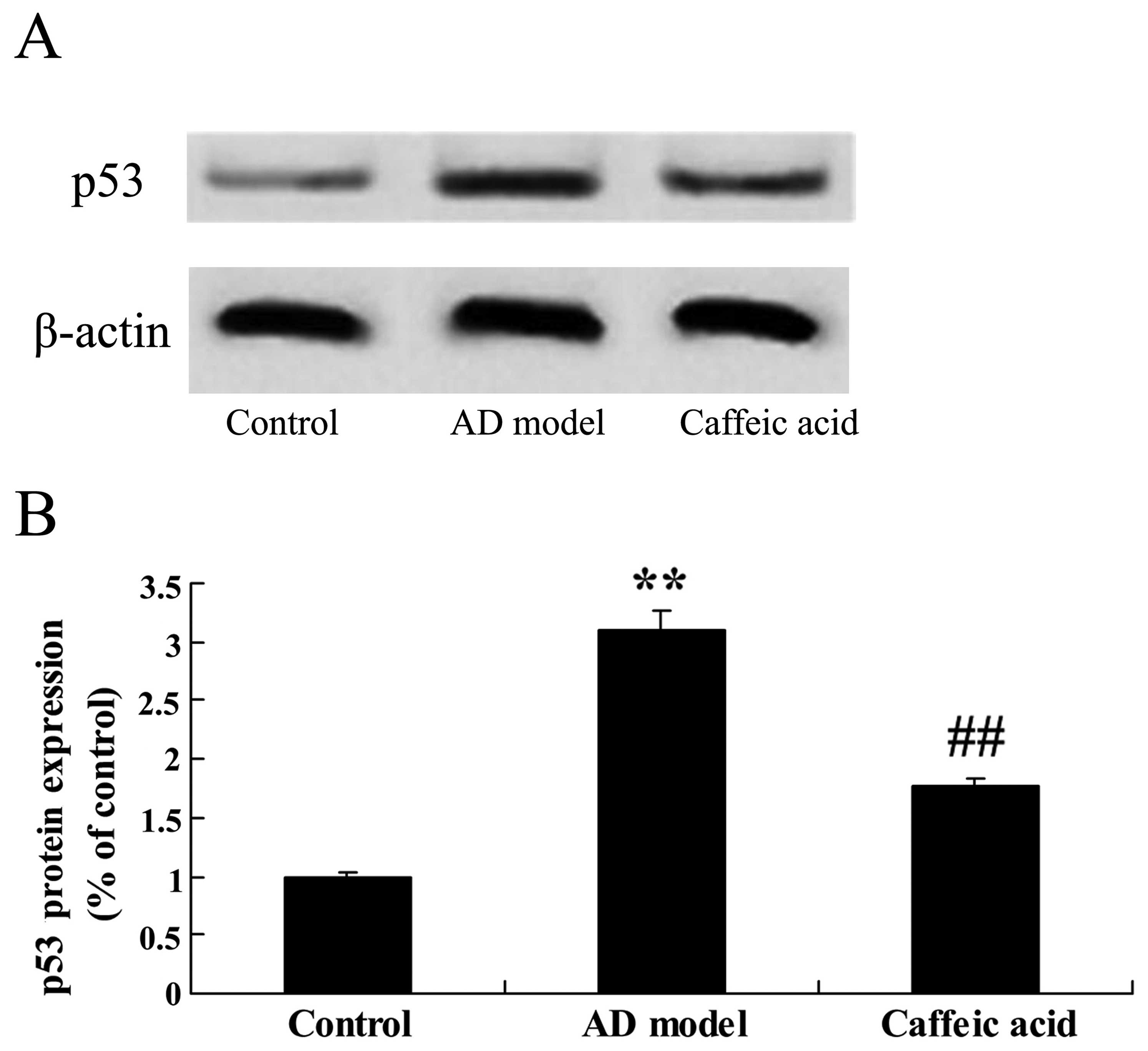
Effect of caffeic acid on p53 expression
in a model of AD
As shown in Fig.
10, a significant increase in p53 protein expression was
observed in the hippocampus of the rats in the AD model group
compared with that in the control group. Moreover, a significant
decrease in p53 protein expression was found in the caffeic
acid-treated group compared with that in the AD model group
(Fig. 10).
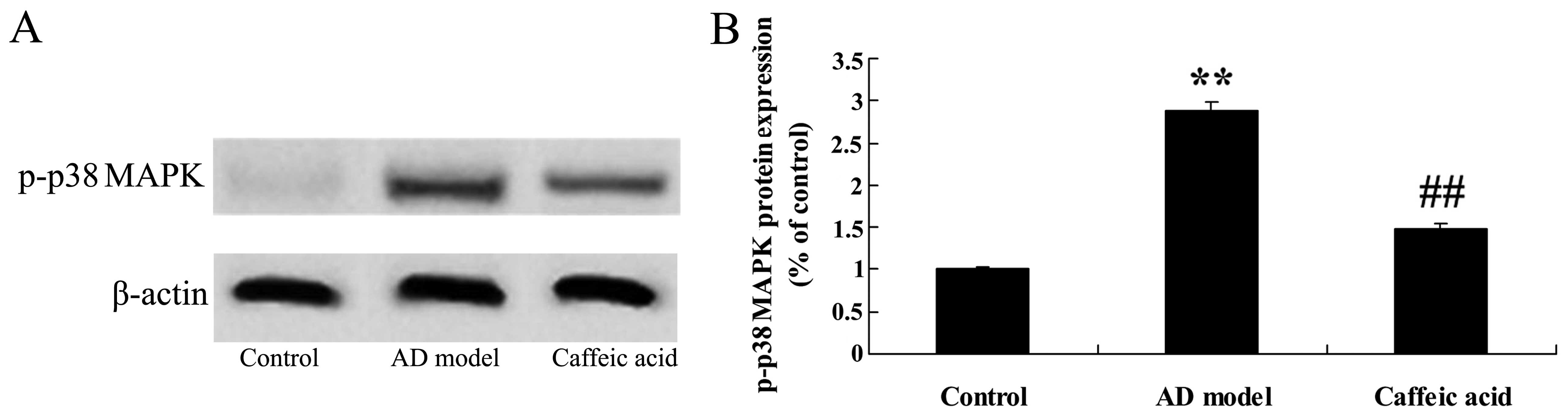
Effect of caffeic acid on p-p38 MAPK
expression in a model of AD
There was a significant increase in the protein
expression of p-p38 MAPK in the AD model group, compared with that
in the control animals (Fig.
11). By contrast, caffeic acid significantly suppressed the
protein expression of p-p38 MAPK compared with that in the AD model
group (Fig. 11).
Discussion
AD is a neurodegenerative disease of the central
nervous system characterized by cognitive decline and the
impairment of memory (14). With
an aging population, the incidence of AD increases which places a
heavy burden on society and families (15). Therefore, medical researchers and
scholars are presented with a great challenge; to discover a
therapeutic drug with high treatment efficacy and low toxicity. The
present study revealed that caffeic acid increased cognitive
function and attenuated cerebral damage compared with the rats in
the AD model group. Khan et al suggested that caffeic acid
prevented AlCl3-induced dementia in rats (16). Pinheiro Fernandes et al
suggested that caffeic acid prevents memory deficits induced by
focal cerebral ischemia (17).
Consistent with these findings, our results indicate that caffeic
acid may be a novel drug for use in the treatment of AD.
In the human brain, there are two types of
cholinesterase: AChE and butyrylcholinesterase (BChE). Research has
shown that BChE also exists in the brain and it may degrade
acetylcholine (ACh). Thus, at times when AChE levels are
insufficient or inhibition has occurred, BChE is capable of
compensating for a lack of AChE (18). In fact, in the brains of patients
with worsening symptoms of AD, the activity of BChE is evidently
increased, which may be due to the development of tolerance to AChE
inhibitors (19). We found that
caffeic acid inhibited the AD-induced AChE activity and nitrite
generation in a rat model of AD. Khan et al suggested that
caffeic acid prevents against AlCl3-induced dementia by
reducing brain AChE activity and nitrite levels in rats (16). Mehrotra et al also reported
that caffeic acid inhibited myeloperoxidase, malondialdehyde and
nitrite generation in mice and rats (20).
In the brains of patients with AD, neurons in the
nucleus basalis of Meynert are seriously denatured or lost and the
activity of choline acetyltransferase is decreased (21). The generation and release of ACh
is lessened and the numbers of presynaptic cholinergic receptors
are diminished. In Aβ42-induced AD, choline acetyltransferase in
synaptosomes experiences oxidative modification and enzymatic
activities are decreased (22).
The pathogenesis of AD involves numerous factors and oxidative
stress may be the trigger for a series of complex events. Oxidative
stress and inflammation leads to the abnormal peroxidation,
glycosylation or nitration of structural and functional materials.
Furthermore, the accumulation of abnormal materials damages
cellular functions (23).
Previous findings have indicated that caffeic acid suppresses the
oxidative state and colonic inflammation in the inflamed colon
(24). In the present study, we
have shown that caffeic acid increased the activities of CAT and
GSH which were inhibited in the AD model group and reduced the
promotion of IL-6 and TNF-α activity induced by AD as well as
NF-κB-p65 protein expression in the rat brains.
Learning and memory formation are closely associated
with the formation and plasticity of synapses in hippocampal
neuron. In the hippocampus, the sites and degree of positive
expression of synaptophysin correspond with the number and
distribution of synapses and neurons (25). The apoptosis of nerve cells
induced by drugs and the central nervous system affects the
expression of synaptophysin. Research has shown that in patients
with AD, neurons in the hippocampal formation are lost and
synaptophysin is significantly decreased, which is mediated by the
p38 MAPK signaling pathway (26).
Moreover, the p38 MAPK signaling pathway as a regulatory center is
associated with the degree of cognitive impairment (27). In the present study, we
demonstrated that caffeic acid suppressed the protein expression of
p53 and p-p38 MAPK in a rat model of AD. Lee et al suggested
that caffeic acid exerts an antidepressant-like effect through the
suppression of p38 MAPK (28).
Yang et al also reported that caffeic acid inhibited the
migratory capability and cancer stem cells-like properties of
malignant human keratinocytes by downregulating the p38 NF-κB/snail
signaling pathway (12).
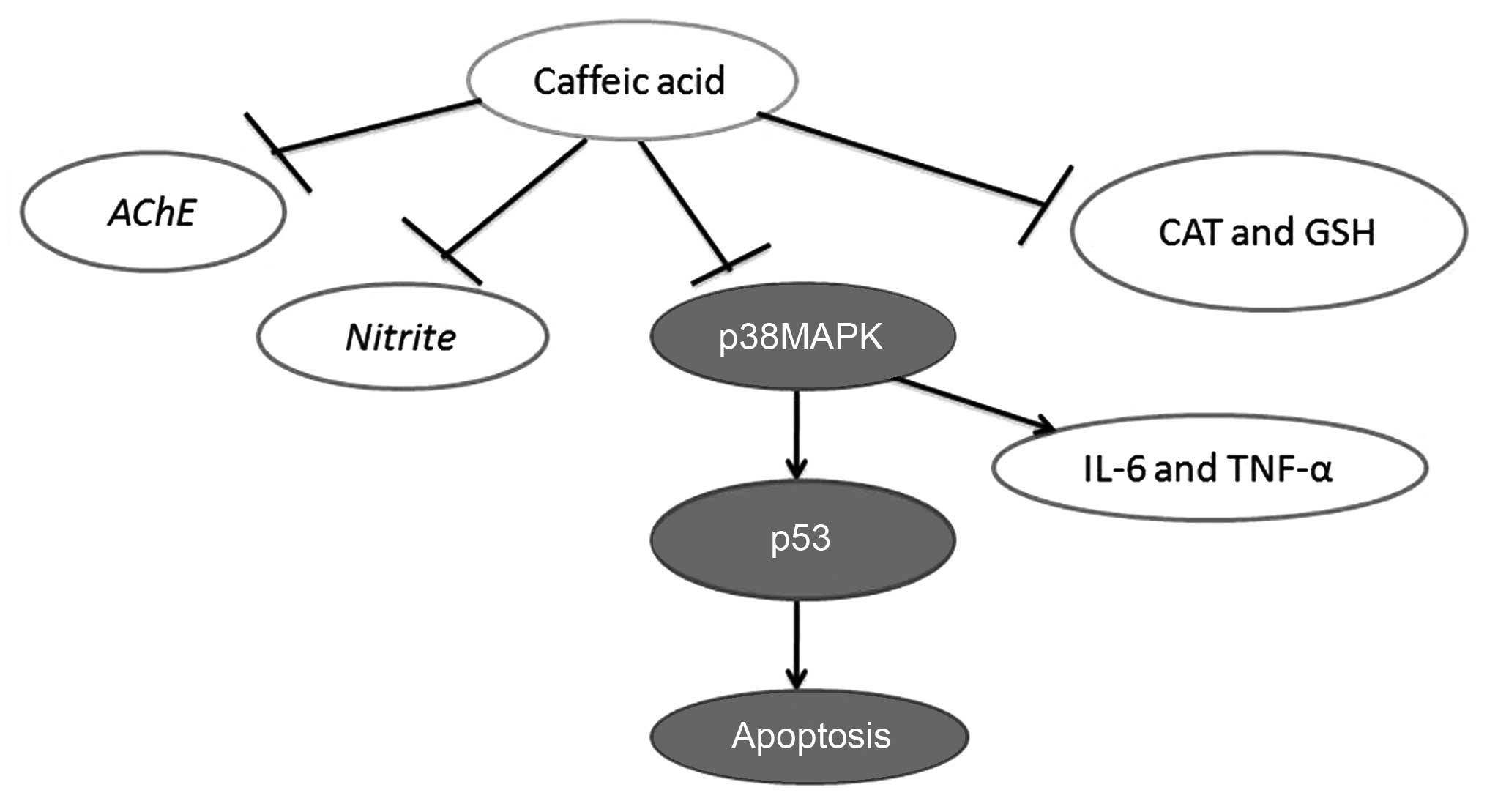
Taken together, the findings of the present study
demonstrated that caffeic acid attenuated the development of AD, by
increasing cognitive function, attenuating cerebral damage, and
inhibiting the AD-induced increase in AChE activity and nitrite
generation in a model of AD. Furthermore, caffeic acid induced the
inhibition of oxidative stress, inflammation and apoptosis through
the p53 and p38 MAPK signaling pathways (Fig. 12). These findings suggest that
the effects of caffeic acid in the treatment of AD occur through
the p53 and p38 MAPK signaling pathways.
References
|
1
|
Muir SW, Speechley M, Wells J, Borrie M,
Gopaul K and Montero-Odasso M: Gait assessment in mild cognitive
impairment and Alzheimer's disease: the effect of dual-task
challenges across the cognitive spectrum. Gait Posture. 35:96–100.
2012. View Article : Google Scholar
|
|
2
|
Pitkala KH, Raivio MM, Laakkonen ML,
Tilvis RS, Kautiainen H and Strandberg TE: Exercise rehabilitation
on home-dwelling patients with Alzheimer's disease - a randomized,
controlled trial. Study protocol. Trials. 11:922010. View Article : Google Scholar :
|
|
3
|
Spires-Jones TL, Mielke ML, Rozkalne A,
Meyer-Luehmann M, de Calignon A, Bacskai BJ, Schenk D and Hyman BT:
Passive immunotherapy rapidly increases structural plasticity in a
mouse model of Alzheimer disease. Neurobiol Dis. 33:213–220. 2009.
View Article : Google Scholar :
|
|
4
|
Olazarán J, Reisberg B, Clare L, Cruz I,
Peña-Casanova J, Del Ser T, Woods B, Beck C, Auer S, Lai C, et al:
Nonpharmacological therapies in Alzheimer's disease: a systematic
review of efficacy. Dement Geriatr Cogn Disord. 30:161–178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang WY, Tan MS, Yu JT and Tan L: Role of
pro-inflammatory cytokines released from microglia in Alzheimer's
disease. Ann Transl Med. 3:1362015.PubMed/NCBI
|
|
6
|
Götz J, Ittner LM, Schonrock N and Cappai
R: An update on the toxicity of Abeta in Alzheimer's disease.
Neuropsychiatr Dis Treat. 4:1033–1042. 2008. View Article : Google Scholar
|
|
7
|
Pittalà V, Salerno L, Romeo G, Siracusa
MA, Modica MN, Romano GL, Salomone S, Drago F and Bucolo C: Effects
of novel hybrids of caffeic acid phenethyl ester and NSAIDs on
experimental ocular inflammation. Eur J Pharmacol. 752:78–83. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinho E, Henriques M and Soares G: Caffeic
acid loading wound dressing: Physicochemical and biological
characterization. Ther Deliv. 5:1063–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanida I, Shirasago Y, Suzuki R, Abe R,
Wakita T, Hanada K and Fukasawa M: Inhibitory effects of caffeic
acid, a coffee-related organic acid, on the propagation of
hepatitis C virus. Jpn J Infect Dis. 68:268–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paracatu LC, Faria CM, Quinello C, Rennó
C, Palmeira P, Zeraik ML, da Fonseca LM and Ximenes VF: Caffeic
Acid phenethyl ester: consequences of its hydrophobicity in the
oxidative functions and cytokine release by leukocytes. Evid Based
Complement Alternat Med. 2014:7936292014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bailly F and Cotelle P: Anti-HIV
activities of natural antioxidant caffeic acid derivatives: toward
an antiviral supplementation diet. Curr Med Chem. 12:1811–1818.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Li Y, Wang K, Wang Y, Yin W and Li
L: P38/NF-κB/snail pathway is involved in caffeic acid-induced
inhibition of cancer stem cells-like properties and migratory
capacity in malignant human keratinocyte. PLoS One. 8:e589152013.
View Article : Google Scholar
|
|
13
|
Ellman GL, Courtney KD, Andres V Jr and
Feather-Stone RM: A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paillard T, Rolland Y and de Souto Barreto
P: Protective effects of physical exercise in Alzheimer's disease
and Parkinson's disease: a narrative review. J Clin Neurol.
11:212–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H, Finkelstein DI and Adlard PA:
Interactions of metals and Apolipoprotein E in Alzheimer's disease.
Front Aging Neurosci. 6:1212014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan KA, Kumar N, Nayak PG, Nampoothiri M,
Shenoy RR, Krishnadas N, Rao CM and Mudgal J: Impact of caffeic
acid on aluminium chloride-induced dementia in rats. J Pharm
Pharmacol. 65:1745–1752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pinheiro Fernandes FD, Fontenele Menezes
AP, de Sousa Neves JC, Fonteles AA, da Silva AT, de Araújo
Rodrigues P, Santos do Carmo MR, de Souza CM and de Andrade GM:
Caffeic acid protects mice from memory deficits induced by focal
cerebral ischemia. Behav Pharmacol. 25:637–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yousof Ali M, Jung HA and Choi JS:
Anti-diabetic and anti-Alzheimer's disease activities of Angelica
decursiva. Arch Pharm Res. 38:2216–2227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann M: Neuronal AChE splice
variants and their non-hydrolytic functions: redefining a target of
AChE inhibitors? Br J Pharmacol. 170:953–967. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mehrotra A, Shanbhag R, Chamallamudi MR,
Singh VP and Mudgal J: Ameliorative effect of caffeic acid against
inflammatory pain in rodents. Eur J Pharmacol. 666:80–86. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sassi C, Ridge PG, Nalls MA, Gibbs R, Ding
J, Lupton MK, Troakes C, Lunnon K, Al-Sarraj S, Brown KS, et al:
Influence of coding variability in APP-Aβ metabolism genes in
sporadic Alzheimer's disease. PLoS One. 11:e01500792016. View Article : Google Scholar
|
|
22
|
Gonzalez P, da Costa VC, Hyde K, Wu Q,
Annunziata O, Rizo J, Akkaraju G and Green KN: Bimodal-hybrid
heterocyclic amine targeting oxidative pathways and copper
mis-regulation in Alzheimer's disease. Metallomics. 6:2072–2082.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hatanaka H, Hanyu H, Hirose D, Fukusawa R,
Namioka N and Iwamoto T: Peripheral oxidative stress markers in
individuals with Alzheimer's disease with or without
cerebrovascular disease. J Am Geriatr Soc. 63:1472–1474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim H, Kim W, Yum S, Hong S, Oh JE, Lee
JW, Kwak MK, Park EJ, Na DH and Jung Y: Caffeic acid phenethyl
ester activation of Nrf2 pathway is enhanced under oxidative state:
structural analysis and potential as a pathologically targeted
therapeutic agent in treatment of colonic inflammation. Free Radic
Biol Med. 65:552–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashabi G, Alamdary SZ, Ramin M and
Khodagholi F: Reduction of hippocampal apoptosis by
intracerebroventricular administration of extracellular
signal-regulated protein kinase and/or p38 inhibitors in amyloid
beta rat model of Alzheimer's disease: involvement of
nuclear-related factor-2 and nuclear factor-κB. Basic Clin
Pharmacol Toxicol. 112:145–155. 2013. View Article : Google Scholar
|
|
26
|
Yang WN, Ma KG, Qian YH, Zhang JS, Feng
GF, Shi LL, Zhang ZC and Liu ZH: Mitogen-activated protein kinase
signaling pathways promote low-density lipoprotein receptor-related
protein 1-mediated internalization of beta-amyloid protein in
primary cortical neurons. Int J Biochem Cell Biol. 64:252–264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim TI, Lee YK, Park SG, Choi IS, Ban JO,
Park HK, Nam SY, Yun YW, Han SB, Oh KW and Hong JT: L-Theanine, an
amino acid in green tea, attenuates beta-amyloid-induced cognitive
dysfunction and neurotoxicity: reduction in oxidative damage and
inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radic
Biol Med. 47:1601–1610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee MS, Kim YH, Lee BR, Kwon SH, Moon WJ,
Hong KS, Song YS, Morita K, Hahm DH, Shim I and Her S: Novel
antidepressant-like activity of caffeic acid phenethyl ester is
mediated by enhanced glucocorticoid receptor function in the
hippocampus. Evid Based Complement Alternat Med. 2014:6460392014.
View Article : Google Scholar : PubMed/NCBI
|