Introduction
Recent statistics from the World Health Organization
indicate that almost 17.5 million people died from cardiovascular
disease (CVD) in 2012, which represents nearly 31% of deaths
worldwide (1). Of these deaths,
an estimated 7.4 million were due to coronary heart disease and 6.7
million were due to stroke (1).
The principal cause of heart disease, including myocardial
infarction and stroke, in Western society is atherosclerosis, a
chronic inflammatory disease characterized by cholesterol and lipid
accumulation within the arterial wall (2). Atherosclerotic cardiovascular
disease (ACD) is affected by several factors including age, gender,
genetic predisposition, smoking, stress and dietary habits
(3–5). However, despite lifestyle
modifications and the development of novel pharmacological
therapies to lower the concentrations of plasma cholesterol, ACD
remains the principal cause of death worldwide (1,6,7).
Atherosclerosis is not only a lipid disorder but
also a chronic inflammatory disease affecting the arterial vessel
wall (4,8,9).
The first step in the pathogenesis of atherosclerosis is triggered
by the deposition of low-density lipoprotein (LDL) cholesterol in
the arterial endothelium. The deposited LDL is modified to oxidized
LDL (oxLDL), which stimulates the endothelial cells to express
adhesion molecules [vascular cell adhesion molecule-1 (VCAM-1) and
intercellular adhesion molecule-1 (ICAM-1)] and chemokines
[monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-8]
(10,11). During this process, the
overexpression of adhesion molecules leads to the recruitment of
monocytes in the arterial endothelium, which eventually migrate
into the intima and differentiate into pro-atherogenic macrophages.
The pro-atherogenic macrophages are activated by residual oxLDL,
endotoxins, and heat-shock proteins, and release inflammatory
cytokines and growth factors, thus resulting in endothelial
activation. This leads to the induction of an inflammatory response
through the increased recruitment of leukocytes and the
proliferation of smooth muscle cells (12), which release various inflammatory
cytokines, such as tumor necrosis factor-α (TNF-α), IL-1β and IL-6,
consequently leading to the accumulation of lipid-laden foam cells
and the development of atherosclerosis (4,13,14).
Humulus japonicus (HJ) of the family
Cannabaceae, is an annual climbing vine originating from East Asia,
including Korea and China (15).
It is wind-pollinated and has a flowering stage that lasts from
August to October. This plant grows rapidly as a vine that forms
dense stands and displaces native vegetation by outcompeting them
for essential resources. It may pose a threat to the environment
when invading riparian habitats. It has been demonstrated that the
pollen of this plant is allergenic, and is considered as one of the
major causes of pollen allergy (16). To date, HJ has been reported to
exert anti-mutagenic (17),
antimicrobial (18), antioxidant
(18,19), anticancer (19), anti-mycobacterial (20), and anti-inflammatory (21) effects. From the results of
previous studies, we speculated that the antioxidant and
anti-inflammatory effects of HJ may also contribute to the
anti-atherosclerotic effect of HJ. Using RAW 264.7 cells, a murine
macrophage cell line, and apolipoprotein E-deficient
(apoE−/−) mice fed an atherogenic diet, a well-known
animal model of atherosclerosis, we performed the present study in
order to examine the anti-inflammatory and anti-atherogenic effects
of HJ.
Materials and methods
Preparation of HJ extract
HJ was purchased from Gangwon Herbs (Wonju, Korea)
in July, 2014. The voucher specimen was identified by Professor
W.-K. Oh, and a voucher specimen (SNU-2014-0004) was deposited at
the College of Pharmacy, Seoul National University, Seoul, Korea.
Then, the HJ extract was prepared and supplied by the Korea
Bioactive Natural Material Bank (Seoul, Korea). Briefly, the dried
aerial parts of HJ were soaked in 100% methanol in an extraction
container for 2 days at room temperature. The methanol-soluble
extract was then filtered through cheesecloth, concentrated
exhaustively, and dried under reduced pressure to afford a methanol
extract. This HJ extract was suspended in 0.5% carboxymethyl
cellulose (CMC) at a concentration of 50 mg/ml as a stock solution,
and the working solution of HJ was adjusted to the intended
concentrations for use in the in vitro and in vivo
experiments in the present study.
Cell culture and HJ treatment
The RAW 264.7 cells, a murine macrophage cell line,
were purchased from the American Type Cell Culture (ATCC; Manassas,
VA, USA), cultured in Dulbecco's modified Eagle's medium (DMEM)
containing 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin antibiotics (Gibco, Carlsbad, CA, USA), and
maintained at 37°C in a humidified 5% CO2 incubator. The
cells were seeded in 12-well plates (1×105 cells/well),
incubated overnight, and thereafter, co-treated with different
concentrations of HJ (200 and 400 µg/ml) and
lipopolysaccharide (LPS; 1 µg/ml; Sigma-Aldrich, St. Louis,
MO, USA) for 24 h. The doses of HJ were determined by preliminary
tests using various concentrations (50–500 µg/ml) without
cell toxicity to examine the anti-inflammatory effect.
Measurement of inflammation-related gene
expression in LPS-stimulated RAW 264.7 cells by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The RAW 264.7 cells were treated with HJ extract for
1 h, and then stimulated with LPS or vehicle for 24 h. The total
RNA from the cells was extracted and the samples were reverse
transcribed using the iScript cDNA synthesis kit (BioRad, Hercules,
CA, USA). The resulting cDNA was amplified using the Exicycler 96
quantitative Real-Time PCR system and SYBR Premix Ex Taq (both from
Bioneer, Daejeon, Korea) according to the manufacturer's
instructions. Oligonucleotide primers [for TNF-α, IL-1β, IL-6,
cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase
(iNOS)] were designed by Primer Express 3.0 software (Applied
BioSystems, Carlsbad, CA, USA); the primer sequences used in the
experiments are listed in Table
I. The cycling conditions were 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec, and 60°C for 1 min. To detect and remove
possible primer-dimer artifacts, a dissociation curve was generated
for the following cycling conditions: 95°C for 15 sec, 60°C for 1
min, and 95°C for 15 sec. The results were normalized to 18s mRNA
levels (reference gene).
 | Table IPrimer sequences for RT-qPCR of
target genes. |
Table I
Primer sequences for RT-qPCR of
target genes.
| Gene | Primer sequence
(5′-3′) |
|---|
| 18s | F:
5′-GACACGGACAGGATTGACAGATTGATAG-3′ |
| R:
5′-GTTAGCATGCCAGAGTCTCGTTCGTT-3′ |
| VCAM-1 | F:
5′-ACTGTTTATTACAGCCCCGC-3′ |
| R:
5′-ACTTCAACGATGGGGACTTG-3′ |
| ICAM-1 | F:
5′-GTCGAAGGTGGTTCTTCTGAGC-3′ |
| R:
5′-TCCGTCTGCAGGTCATCTTAGG-3′ |
| iNOS | F:
5′-GTTCTCAGCCCAACAATACAAGA-3′ |
| R:
5′-GTGGACGGGTCGATGTCAC-3′ |
| COX-2 | F:
5′-GGGTGTCCCTTCACTTCTTTCA-3′ |
| R:
5′-GAGTGGGAGGCACTTGCATT-3′ |
| MCP-1 | F:
5′-CAGCAAGATGATCCCAATGAGTAG-3′ |
| R:
5′-TCTCTTGAGCTTGGTGACAAAAAC-3′ |
| CD68 | F:
5′-TCACAGTTCACACCAGCTCC-3′ |
| R:
5′-TCACAGTTCACACCAGCTCC-3′ |
| TNF-α | F:
5′-TGGCCTCCCTCTCATCAGTT-3′ |
| R: 5′ –
CCTCCACTTGGTGGTTTGCT-3′ |
| IL-1β | F:
5′-CTACAGGCTCCGAGATGAACAAC-3′ |
| R:
5′-TCCATTGAGGTGGAGAGCTTTC-3′ |
| IL-6 | F:
5′-GTTGCCTTCTTGGGACTGATG-3′ |
| R:
5′-GGGAGTGGTATCCTCTGTGAAGTCT-3′ |
| IL-18 | F:
5′-GGCTGCCATGTCAGAAGACT-3′ |
| R:
5′-GTCTGGTCTGGGGTTCACTG-3′ |
Determination of pro-inflammatory
cytokine secretion in LPS-stimulated RAW 264.7 cells
The secretion levels of TNF-α, IL-1-β and IL-6 were
determined using culture supernatants from the LPS-stimulated RAW
264.7 cells by capture ELISA using Nunc-Immuno™ Microwell 96-well
MaxiSorp™ microplates (Nunc A/S, Roskilde, Denmark).
Pro-inflammatory cytokine levels were measured according to the
manufacturer's instructions using the Mouse ELISA kit, BD OptEIA™
(Pharmingen, San Diego, CA, USA). Briefly, the 96-well plates were
coated overnight at 4°C with a capture antibody (anti-mouse TNF-α,
IL-1β and IL-6) in coating buffer (1:250). After the plates were
washed with washing buffer [0.05% Tween-20 in 1X phosphate-buffered
saline (PBS)], the plates were blocked with assay diluent (10% FBS
in 1X PBS) for 1 h at room temperature. After the plates were
washed, standards and samples were added to the wells and incubated
for 2 h at room temperature. After the plates were washed, working
detector (biotinylated anti-mouse cytokine and
streptavidin-horseradish peroxidase conjugate in assay diluent)
were added and incubated for 1 h at room temperature. The wells
were again washed and the substrate solution (tetramethylbenzidine
and hydrogen peroxide) was added and incubated for 30 min at room
temperature in the dark. After 30 min, stop solution (1 N
H2SO4) was added. The absorbance was measured
using a microplate reader (Thermo Multiskan Spectrum, Thermo Fisher
Scientific, Waltham, MA, USA) at 450 nm. A standard curve was
obtained using serial dilutions starting from 0 upto 1,000
pg/ml.
Assessment of nitrite and prostaglandin
E2 (PGE2) production in LPS-stimulated RAW
264.7 cells
The RAW 264.7 cells were plated in a 12-well plate
as mentioned above, and the concentration of nitrite was measured
using Griess reagent. The culture supernatant (100 µl) was
mixed with Griess reagent (100 µl) [equal volumes of 1%
(w/v) sulfanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v)
naphthyl ethylenediamine dihydrochloride], and incubated at room
temperature for 15 min. The light absorbance of the mixture was
read at 540 nm using a microplate reader. The concentration of
nitrite was calculated from a serial dilution standard curve of
sodium nitrite (NaNO2). In addition, the PGE2
concentration was measured by a PGE2 assay kit according
to the manufacturer's instructions (R&D Systems, Inc.,
Minneapolis, MN, USA).
Animal experiments
Eight-week-old male apoE−/− mice were
used in the present study. All animals were obtained from the
Jackson Laboratory (Bar Harbor, ME, USA) and maintained at the
Korea Research Institute of Bioscience and Biotechnology (KRIBB;
Daejeon, Korea) and housed in a controlled temperature (22 ± 1°C)
facility and maintained on a reverse 12 h light/dark cycle. The
mice were randomly divided into three groups: those fed i) an
atherogenic diet (no. 102571; Dyets Inc., Bethlehem, PA, USA) plus
vehicle (0.5% CMC) as the vehicle/control group (n=9); ii) an
atherogenic diet plus 100 mg/kg of HJ as the HJ100 group (n=5); and
iii) an atherogenic diet plus 500 mg/kg of HJ as the HJ500 group
(n=8). The doses of HJ were determined by preliminary tests using
concentrations of 100, 300, and 500 mg/kg for selection of
anti-atherogenic effect. HJ was administered daily by oral gavage
for 12 weeks and changes in body weight were measured each week.
All animal experiments were approved by the Institutional Animal
Care and Use Committee (IACUC) of KRIBB and were performed in
accordance with the institutional guidelines at KRIBB.
Plasma analysis
At the end of the experimental period, blood samples
were collected from the orbital venous sinuses of the mice to
analyze the concentrations of plasma lipid as well as various
biomarkers. Plasma was prepared by centrifuging the blood at 10,000
× g for 5 min at 4°C and subsequently storing it at −70°C until
analysis. Plasma total cholesterol and triglyceride levels were
analyzed using a kit (Asan Pharm, Seoul, Korea), and other
biomarkers including aspartate transaminase (AST), alanine
transaminase (ALT), blood urea nitrogen (BUN), creatinine (Crea),
glucose (Glu), and non-esterified fatty acid (NEFA) were measured
using an automatic blood chemical analyzer (Hitachi, Tokyo,
Japan).
Analysis of atherosclerotic lesion
formation in the aorta of apoE−/− mice
The mice were euthanized with CO2 gas,
and then the whole aortas were isolated from the vehicle group
(n=4), the HJ100 group (n=3), and the HJ500 group (n=4), and the
adventitial tissue was removed. After fixation in 10% neutral
formalin, the whole aortas were longitudinally dissected, and
pinned flat on a rubber plate. Lipid plaques in the whole aorta
were stained with oil-red O (Sigma-Aldrich), and en face images
were captured using a digital camera (Canon, Tokyo, Japan). The
whole aorta surface and stained plaque areas were analyzed by
digital image analysis software (Image Inside; GS Media, Daejeon,
Korea).
Analysis of atherosclerotic lesion
formation in the aortic sinus of apoE−/− mice
The aortic sinuses were isolated from the vehicle
group (n=8), the HJ100 group (n=4), and the HJ500 group (n=7), and
fixed in 10% neutral formalin. After fixation, the aortic sinuses
were embedded in a Tissue-Tek optimal cutting temperature (OCT)
compound and sectioned at a thickness of 8 µm using a
cryotome (both from Sakura Finetek, Tokyo, Japan). Cryostat
sections of the aortic sinus were stained with oil-red O. After
oil-red O staining, photographs were captured under a light
microscope (BX51; Olympus Corp, Tokyo, Japan) and the atheroma
areas of the aortic sinus were quantified using digital image
analysis software (Image Inside).
Analysis of monocyte/macrophage
infiltration in the aortic sinus of apoE−/− mice
The infiltration of monocytes and macrophages was
detected using anti-MOMA-2, a mouse monocyte/macrophage-specific
antibody (Abcam, Cambridge, UK). The aortic sinuses were embedded
in a Tissue-Tek OCT compound, and then sections of the aortic sinus
(8 µm) were incubated with anti-MOMA-2 (1:200), followed by
Alexa Fluor 488 nm goat anti-rat IgG (1:250) (Invitrogen, Carlsbad,
CA, USA) for visualization. Images of the aortic sinus were
captured under a dual fluorescence microscope (Carl Zeiss,
Oberkochen, Germany). The positively stained areas in the aortic
sinus were quantified using digital image analysis software (Image
Inside).
Assessment of the aortic expression of
atherogenic genes in apoE−/− mice
Total RNA from the whole aorta in each group was
extracted using TRIzol reagent (Invitrogen) according to the
manufacturer's instructions, quantified by a NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies, San Diego, CA, USA), and
stored at −70°C until analysis. Relative quantification of the gene
expression was performed using an Exicycler 96 Real-Time PCR System
(Bioneer) with SYBR-Green dye (Takara). Oligonucleotide primers
(VCAM-1, ICAM-1, iNOS, COX-2, MCP-1, CD68, IL-1β, IL-6 and IL-18)
were designed by Primer Express 3.0 software (Applied Biosystems),
and the primer sequences used in the experiments are listed in
Table I. The cycling conditions
were 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec, and
60°C for 1 min. To detect and remove possible primer-dimer
artifacts, a dissociation curve was generated by adding a cycle of
95°C for 15 sec, 60°C for 1 min, and 95°C for 15 sec. The results
were normalized to 18s RNA levels (reference gene).
Statistical analysis
All data are presented as the means ± standard error
of the mean (SEM). For two-group comparisons, a Student's t-test
was used. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
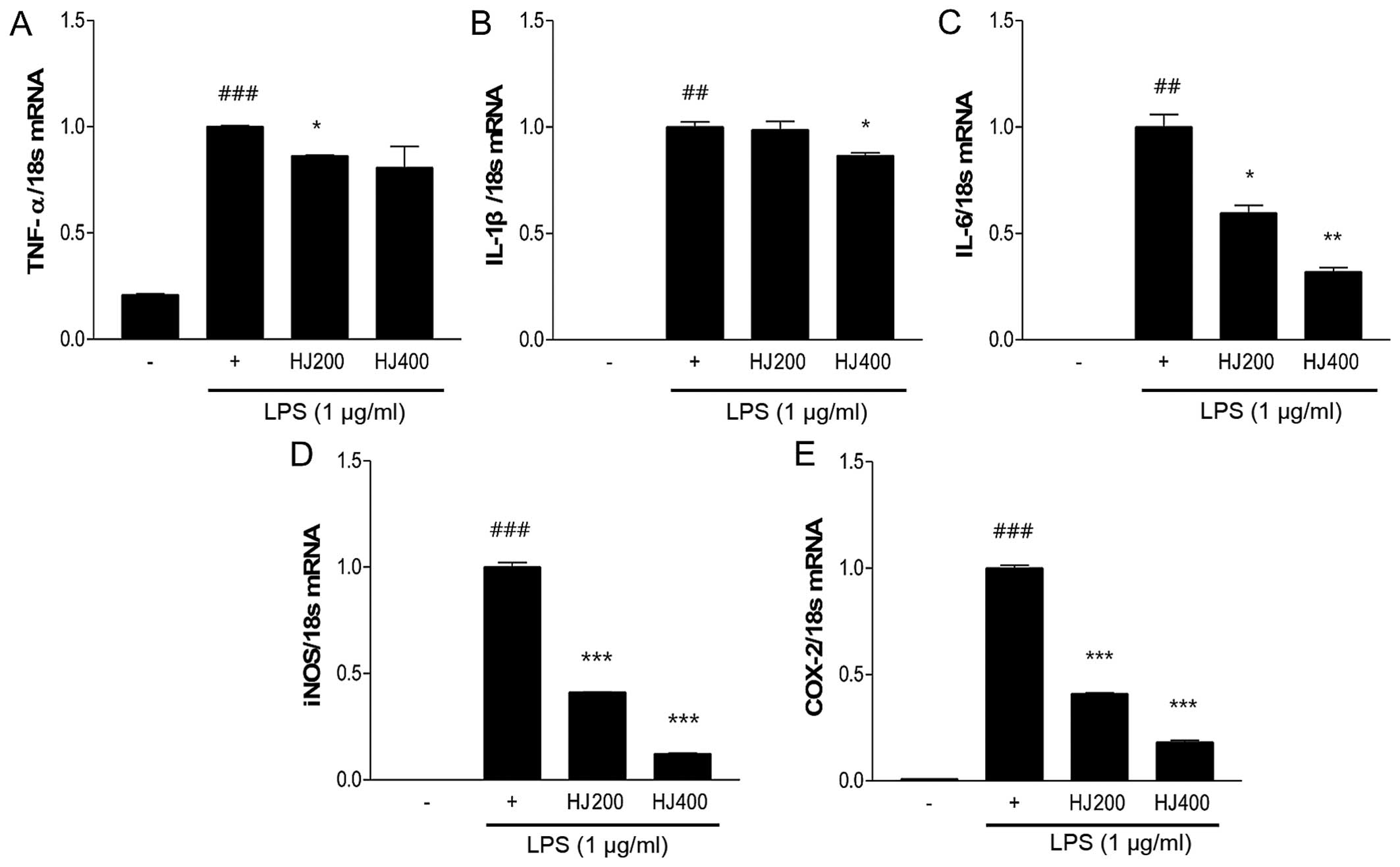
HJ inhibits the mRNA expression of
inflammation-related genes in LPS-stimulated RAW 264.7 cells
To examine the anti-inflammatory effect of HJ, the
mRNA expression of inflammation-related genes (TNF-α, IL-1β, IL-6,
COX-2 and iNOS) was measured in HJ-treated RAW 264.7 cells
stimulated with LPS. LPS stimulation significantly induced the
increased expression of pro-inflammatory genes compared with the
non-treated cells. The mRNA expression of TNF-α and IL-1β was
significantly decreased in the HJ-treated cells (Fig. 1A and B). In addition, IL-6 mRNA
expression was markedly reduced in the HJ-treated groups,
particularly in the HJ400 group (by 68%) compared with IL-6 mRNA
expression in the cells treated with LPS only (Fig. 1C). The expression of other
inflammatory mediators, such as iNOS and COX-2, markedly decreased
after HJ treatment in a dose-dependent manner. Particularly, the
mRNA expression of these two genes decreased by >80% in the
HJ400 group compared with that in the cells treated with LPS only
(Fig. 1D and E).
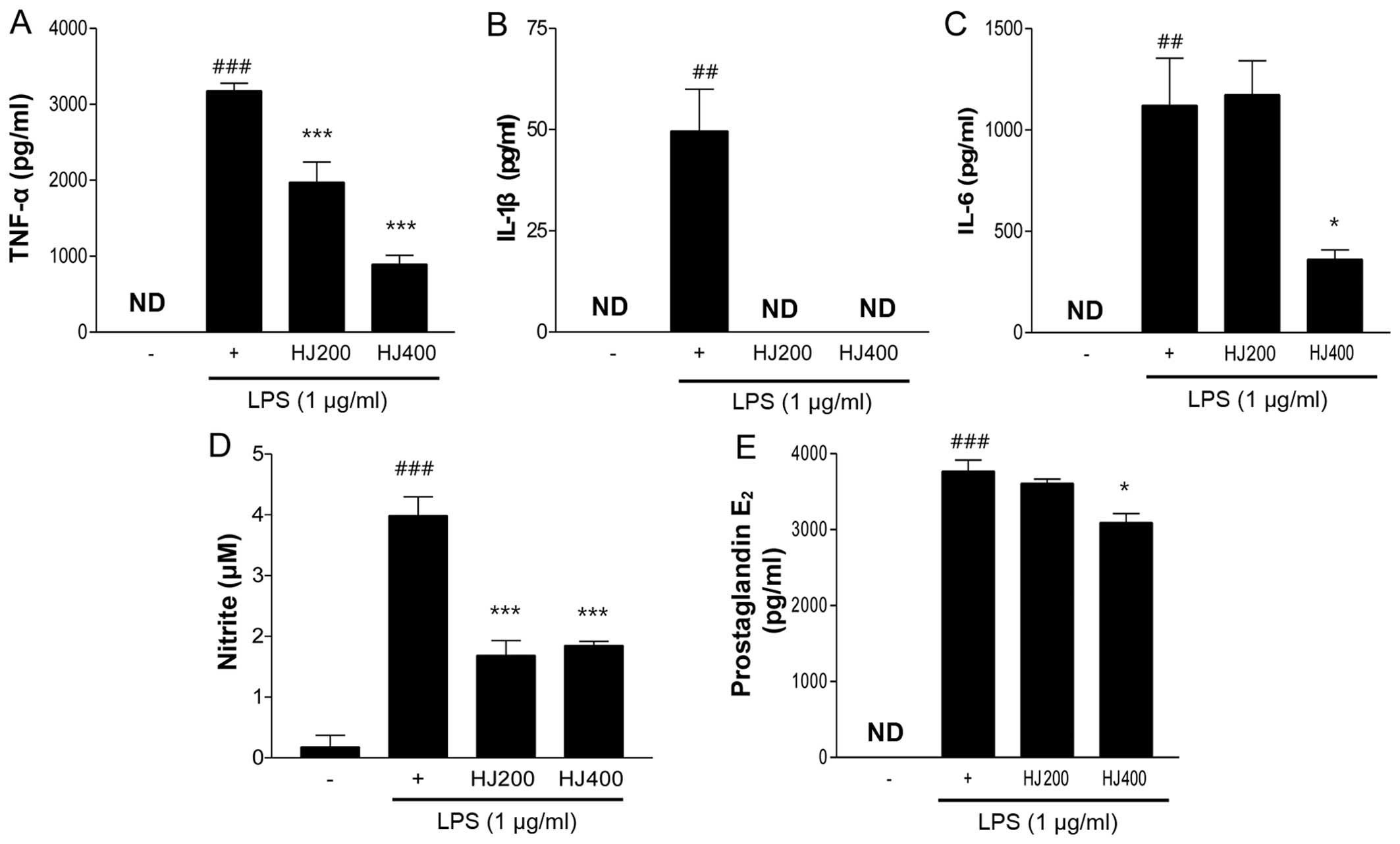
HJ suppresses the secretion of
pro-inflammatory cytokines and production of nitrite and
PGE2 in LPS-stimulated RAW 264.7 cells
In addition to assessing the mRNA expression of
pro-inflammatory cytokines and inflammatory mediators, we measured
the secretion levels of TNF-α, IL-1β, IL-6, nitrite and
PGE2 in the LPS-stimulated RAW 264.7 cells following HJ
treatment. As expected, the TNF-α and IL-6 secretion levels were
significantly decreased by HJ treatment (Fig. 2A and C). Particularly the HJ400
group showed a >50% decrease compared with the cells treated
with LPS only. Notably, IL-1β secretion was completely inhibited by
HJ treatment regardless of the concentration (Fig. 2B). In general, the increased
expression of iNOS leads to the production of high quantities of
nitrite, nitrate and superoxide (O2−)
(22). The release of nitrite in
HJ-treated cells was markedly reduced by >50% compared with the
level of nitrite release in the cells treated with LPS only
(Fig. 2D). The effects of
PGE2, an inflammatory mediator, are mediated through the
induction of COX-2 expression resulting in inflammation (23). The treatment of LPS-stimulated RAW
264.7 cells with 400 µg/ml of HJ significantly reduced
PGE2 levels by 12% compared with the PGE2
levels in the LPS-only treated cells (Fig. 2E). Taken together, these findings
suggest that HJ inhibits the secretion of pro-inflammatory
cytokines and inflammatory mediators in the LPS-stimulated RAW
264.7 cells.
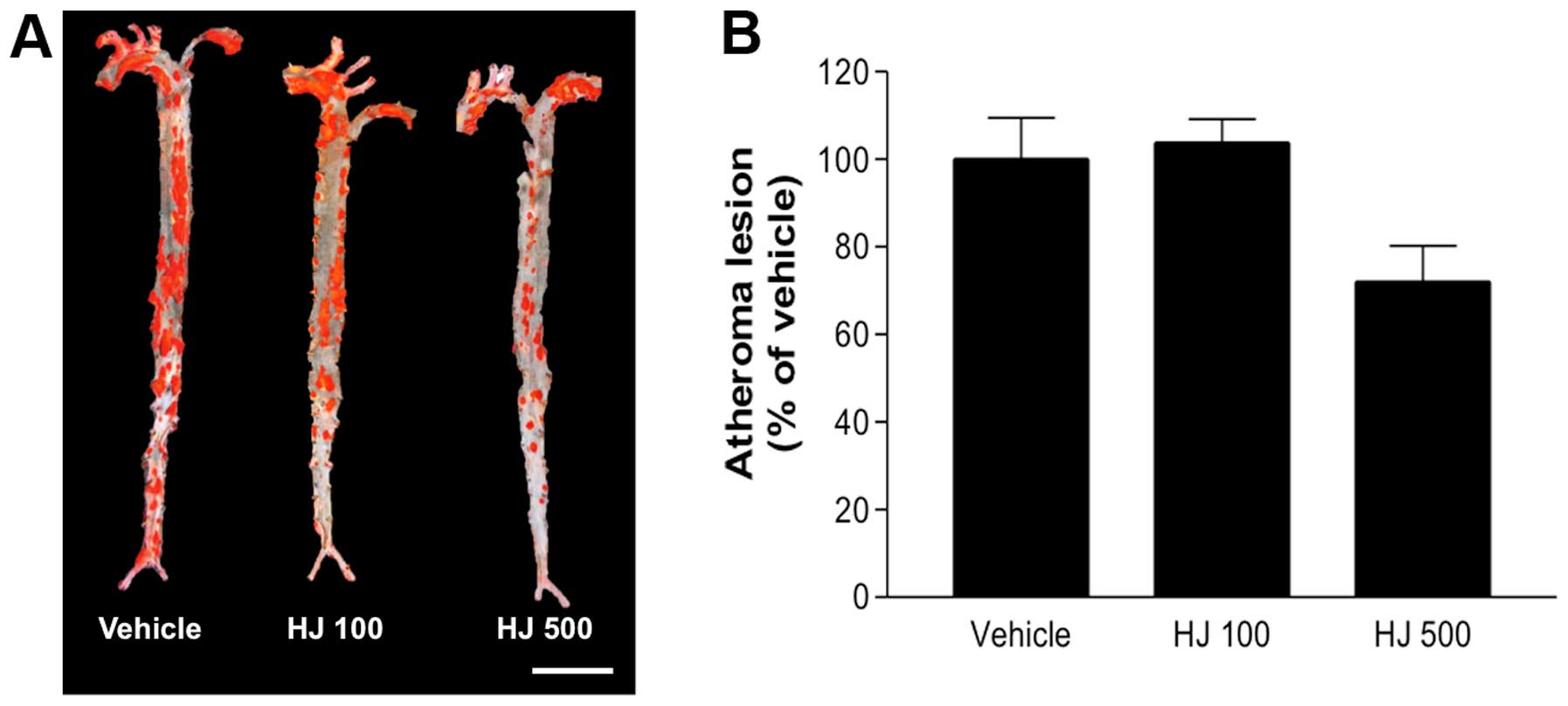
HJ reduces the formation of
atherosclerotic lesions in the aortic sinus and the aorta of
apoE−/− mice
To examine the effect of HJ on the formation of
atherosclerotic lesions in vivo, HJ was administered to
apoE−/− mice for 12 weeks, and the whole aorta was
obtained, stained with oil-red O, and analyzed. No significant
change in the number of atherosclerotic lesions in the aortic en
face images of the HJ100 group was observed, whereas the number of
aortic lesions was markedly reduced in the HJ500 group
(72.09±14.16%), compared with the number of lesions in the vehicle
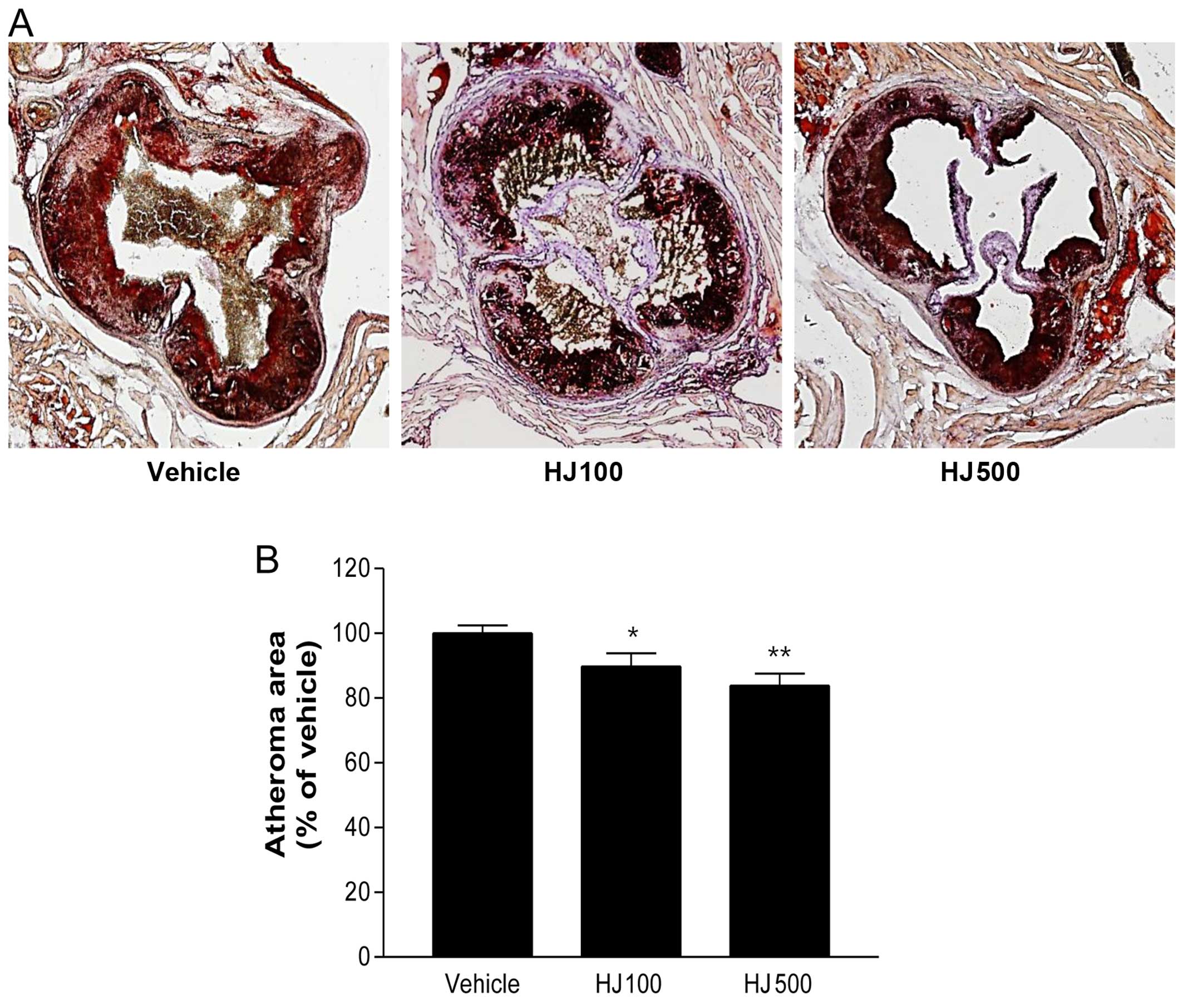
group (Fig. 3). Furthermore, the
number of atherosclerotic lesions in the aortic sinus was compared
following oil-red O staining. The atherosclerotic area was
significantly and dose-dependently decreased in the HJ treatment
groups (HJ100 group, 89.71±4.75%; HJ500 group, 83.87±3.97%),
compared with that in the vehicle group (Fig. 4). These results indicate that
high-dose treatment with HJ clearly reduces the formation of
atherosclerotic lesions.
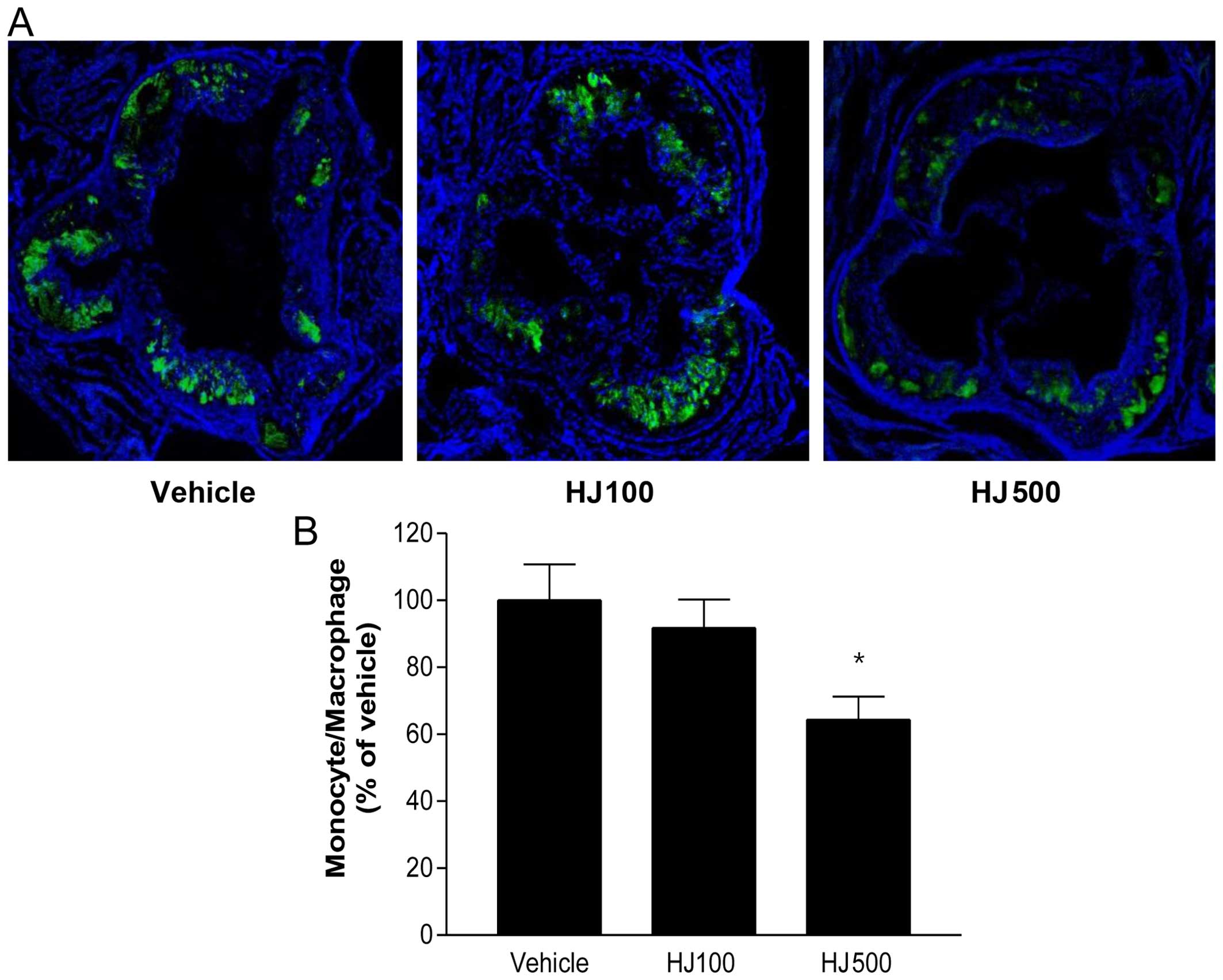
HJ inhibits monocyte/macrophage
infiltration in the aortic sinus of apoE−/− mice
To examine the infiltration of monocytes and
macrophages in the arterial intima, we performed immunofluorescence
staining for MOMA-2 in the aortic sinus (Fig. 5A). HJ decreased the levels of
MOMA-2-positive areas in a dose-dependent manner. Moreover, the
areas of the aortic sinus stained by MOMA-2 were quantified using
image analysis software, and a significantly decreased
monocyte/macrophage infiltration was observed in the HJ-treated
mice, compared with that in the control apoE−/− group
(Fig. 5B). This finding suggests
that HJ treatment strongly inhibits the infiltration of monocytes
and macrophages in the aortic sinus.
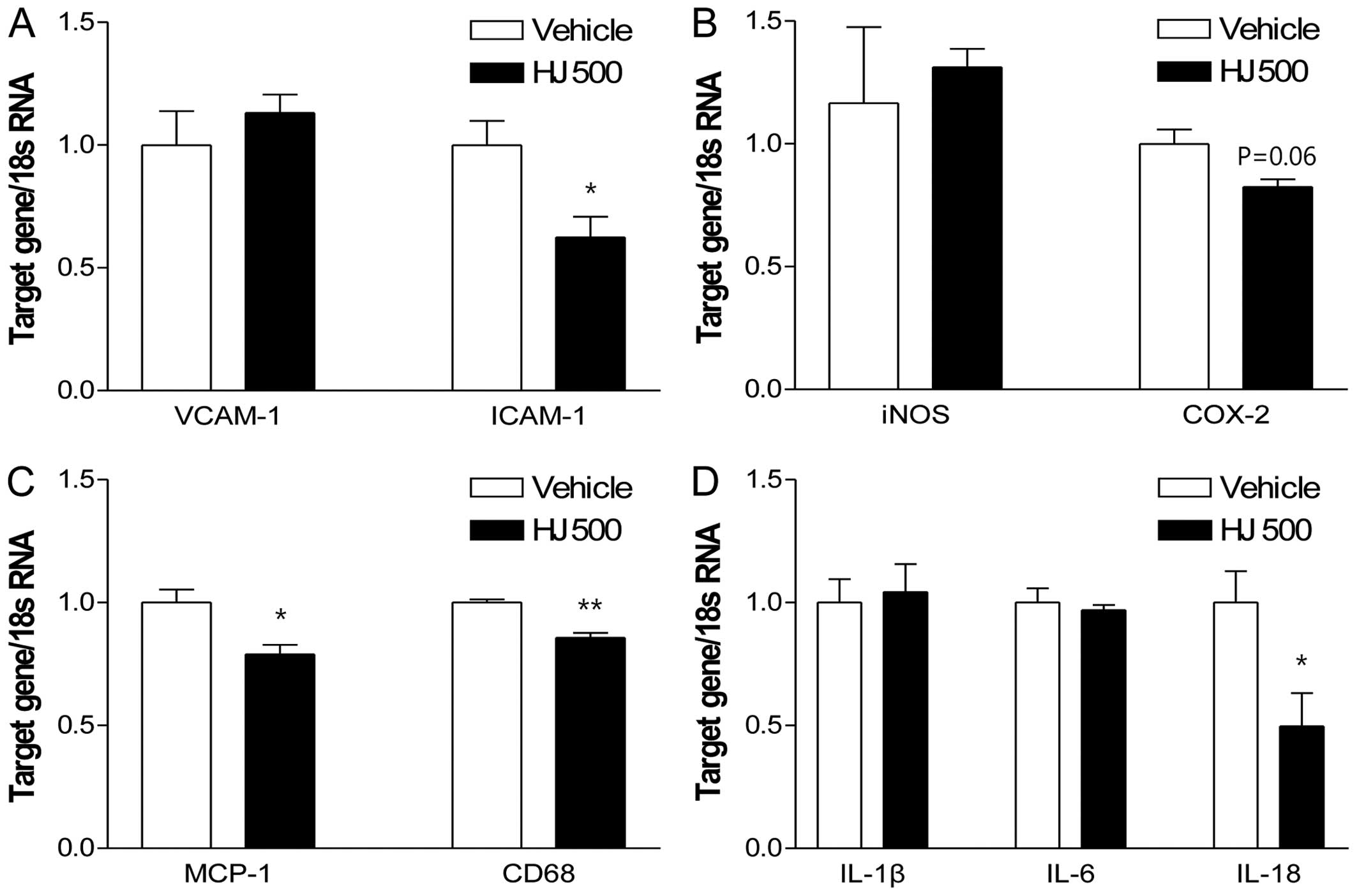
HJ reduces aortic atherogenic gene
expression in apoE−/− mice
To further examine the aortic expression of
atherogenic genes in HJ-treated apoE−/− mice, we
measured the mRNA levels of adhesion molecules (VCAM-1 and ICAM-1),
inflammatory mediators (iNOS and COX-2), the monocyte/macrophage
marker, CD68, and MCP-1, as well as the pro-inflammatory cytokines
(IL-1β, IL-6 and IL-18) in the HJ500 and the control groups. Among
the analyzed parameters, the mRNA expression of ICAM-1, MCP-1, CD68
and IL-18 was significantly decreased in the HJ500 group compared
with that in the control group (Fig.
6). These results indicate that HJ may reduce the expression of
the pro-inflammatory cytokines and atherogenic genes, thereby
regulating the migration and the infiltration of mainly
monocytes/macrophages, in the aorta of HJ-treated
apoE−/− mice.
Plasma analysis
Plasma lipid and other physiological biomarkers were
also determined in the HJ- or vehicle-treated apoE−/−
mice. Apart from a decrease in plasma triglyceride levels in both
the HJ100 and the HJ500 groups compared with the control group, no
significant changes were found in cholesterol, NEFA, AST, ALT, BUN,
Crea and Glu levels (Table II).
Moreover, the changes in body weight were not significantly
different among groups during the whole period of the experiments
(data not shown). Based on these results, it is suggested that
long-term treatment with HJ, at least for 12 weeks in
apoE−/− mice, does not induce significant toxic
effects.
 | Table IIEffects of HJ on plasma biomarkers in
apoE−/− mice. |
Table II
Effects of HJ on plasma biomarkers in
apoE−/− mice.
| Groups | AST (IU/l) | ALT (IU/l) | BUN (mg/dl) | Crea (mg/dl) | Glu (mg/dl) | NEFA (mEq/l) | TG (mg/dl) | TC (mg/dl) |
|---|
| Vehicle | 96.1±4.8 | 35.3±2.6 | 35.0±9.0 | 0.3±0.0 | 112.5±4.9 | 1.6±0.1 | 179.5±12.0 | 1485.8±54.8 |
| HJ100 | 112.2±26.8 | 31.2±3.7 | 28.8±1.9 | 0.3±0.0 | 136.3±14.3 | 1.2±0.1 | 157.7±21.4 | 1504.4±30.2 |
| HJ500 | 96.2±6.4 | 32.8±2.2 | 26.1±1.4 | 0.2±0.0 | 115.9±6.7 | 1.9±0.1 | 139.6±8.7a | 1541.0±23.0 |
Discussion
The present study showed that HJ effectively
inhibits atherogenesis by suppressing inflammation and the
development of atherosclerotic lesions in LPS-stimulated RAW 264.7
cells and apoE−/− mice, respectively. Inflammation
caused by inflammatory cytokines and chemokines is the response of
tissue to injury (24). LPS is
one of the most well-known macrophage-activating factors, and is
usually used for evaluating anti-inflammatory effects (25). In addition, LPS stimulates the
production of various inflammatory cytokines and mediators such as
nitric oxide (NO), TNF-α, and ILs, thereby affecting the immune
response (26,27). To determine whether HJ exerts an
anti-inflammatory effect, we measured the gene expression of
pro-inflammatory cytokines and mediators, and their secretion
levels in LPS-stimulated RAW 264.7 cells. Pro-inflammatory
cytokines (TNF-α, IL-1β and IL-6) play crucial roles in the
initiation of inflammatory processes including immune cell
recruitment, thereby promoting the formation of atherosclerotic
lesions (28). Moreover, iNOS and
COX-2 are crucial mediators of inflammation. iNOS catalyzes the
oxidative deamination of L-arginine to produce NO, a highly
reactive free radical involved in several physiological and
pathological processes (29,30). The modulation of iNOS-mediated NO
release is a major step in the inflammatory process (31). COX-2, an inducible isoform of COX,
plays an important role in inflammation and produces
PGE2, also involved in inflammation (32). In this study, HJ demonstrated a
potent anti-inflammatory effect by suppressing the expression of
pro-inflammatory cytokines and mediators, and their secretion in
LPS-stimulated RAW 264.7 cells. These findings suggest that the
anti-inflammatory activity of HJ may contribute to its
anti-atherosclerotic effect.
In order to observe the effect of HJ on the
development of atherosclerotic lesions, the lesions in the whole
aorta and aortic sinus of apoE−/− mice were analyzed
after staining with oil-red O. Indeed, HJ treatment for 12 weeks
markedly reduced lesion formation in both the aorta (en face) and
the aortic sinus in apoE−/− mice fed an atherogenic
diet, particularly in the HJ500 group. It is well known that
macrophages produce a variety of cytokines and tissue factors in
atherosclerotic lesions. Thus, -the inhibitory effect of HJ on
monocyte and macrophage infiltration was investigated in the aortic
sinus of apoE−/− mice fed an atherogenic diet by
immunofluorescent staining with MOMA-2. Quantitative analysis
showed that the degree of infiltration was significantly reduced in
the HJ500 group compared with the degree of infiltration in the
vehicle group. These histological findings indicate that HJ exerts
an anti-atherogenic effect and may potentially be used as a novel
natural compound for inhibiting the development of
atherosclerosis.
Previously, it was reported that atherosclerotic
lesions form and develop as a consequence of lipid uptake as well
as monocyte recruitment in arterial intima, and subsequent
transformation into macrophage foam cells (4). During this process, activated
arterial endothelial cells express various leukocyte adhesion
molecules and chemokines which affect the progression of
atherogenesis (33,34). The role of adhesion molecules in
atherosclerosis has been reported previously (35,36). These studies suggest that the
expression of adhesion molecules affects the recruitment of immune
cells to the arterial intima. Among them, VCAM-1 and ICAM-1,
well-known endothelial adhesion molecules of the Ig gene
superfamily, may participate in the pathogenesis of atherosclerosis
by promoting monocyte accumulation in the arterial intima.
Particularly ICAM-1 has well-established roles in T cell activation
and in interactions between leukocytes and endothelial cells
(37,38). MCP-1, a member of the
cysteine-cysteine chemokine family, is responsible for both lipid
accumulation and monocyte recruitment into the arterial walls which
affects the progression of atherosclerosis (39). Previously, studies have reported
that the absence of MCP-1 or the inhibition of adhesion molecule
expression ameliorates atherosclerosis in various models of
atherosclerosis, including apoE−/− mice (40), LDL receptor-deficient mice
(41), and double-knockout
(apoE−/−/ICAM-1−/−) mice (42). Moreover, CD68, a pan-macrophage
marker, is involved in the inflammation of carotid plaques. It
contributes to the formation of a fibrous cap and negatively
correlates with plaque cap thickness (43–45). IL-18, a member of the IL-1 family
of cytokines, has been widely studied in inflammatory diseases
including atherosclerosis (46–48). It was highly expressed in
atherosclerotic plaques compared with the normal and control human
carotid atherosclerotic plaques, and was localized in plaque
macrophages (49). Therefore, the
downregulation of pro-atherogenic genes may suppress the
development of atherosclerotic lesions. In the present study, the
expression of pro-atherogenic genes (ICAM-1, MCP-1, CD68 and IL-18)
significantly decreased following HJ treatment in
apoE−/− mice fed an atherogenic diet. These findings
suggest that HJ inhibits atherosclerosis, in part, by
downregulating the expression of atherogenic genes, such as
adhesion molecules (ICAM-1) and cytokines, leading to a reduction
in vascular inflammation, and in monocyte and neutrophil
recruitment.
To the best of our knowledge, this is the first
study to report that HJ suppresses the development of
atherosclerosis by inhibiting the expression of pro-atherogenic
factors, such as pro-inflammatory cytokines, chemokines and
adhesion molecules, which is followed by the prevention of lipid
accumulation in the aortic endothelium in apoE−/− mice.
In conclusion, HJ may have potential applications as an effective,
therapeutically potent natural product for preventing
atherosclerosis.
Abbreviations:
|
VCAM-1
|
vascular cell adhesion molecule-1
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
iNOS
|
inducible nitric oxide synthase
|
|
COX-2
|
cyclooxygenase-2
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-1β
|
interleukin-1β
|
|
IL-6
|
interleukin-6
|
|
IL-18
|
interleukin-18
|
Acknowledgments
The present study was supported by the KRIBB
Research Initiative Program of the Republic of Korea. The authors
wish to thank In-Bok Lee, Jung-Hyun Choi, and Yun-Jeong Seo for
their technical assistance.
References
|
1
|
World Health Organization: Cardiovascular
diseases (CVDs). http://www.who.int/mediacentre/factsheets/fs317/en.
Accessed March 12, 2015.
|
|
2
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosamond WD, Chambless LE, Folsom AR,
Cooper LS, Conwill DE, Clegg L, Wang CH and Heiss G: Trends in the
incidence of myocardial infarction and in mortality due to coronary
heart disease, 1987 to 1994. N Engl J Med. 339:861–867. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zernecke A and Weber C: Chemokines in
atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc
Biol. 34:742–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braunwald E: Shattuck lecture -
cardiovascular medicine at the turn of the millennium: triumphs,
concerns, and opportunities. N Engl J Med. 337:1360–1369. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Breslow JL: Cardiovascular disease burden
increases, NIH funding decreases. Nat Med. 3:600–601. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hansson GK and Libby P: The immune
response in atherosclerosis: a double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wildgruber M, Swirski FK and Zernecke A:
Molecular imaging of inflammation in atherosclerosis. Theranostics.
3:865–884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stocker R and Keaney JF Jr: Role of
oxidative modifications in atherosclerosis. Physiol Rev.
84:1381–1478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witztum JL and Steinberg D: Role of
oxidized low density lipoprotein in atherogenesis. J Clin Invest.
88:1785–1792. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janeway CA Jr and Medzhitov R: Innate
immune recognition. Annu Rev Immunol. 20:197–216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore KJ, Sheedy FJ and Fisher EA:
Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol.
13:709–721. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peiser L, Mukhopadhyay S and Gordon S:
Scavenger receptors in innate immunity. Curr Opin Immunol.
14:123–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JW, Ko SH, Kim CW, Jeoung BJ and Hong
CS: Identification and characterization of the major allergen of
the Humulus japonicus pollen. Clin Exp Allergy. 29:1080–1086. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong KY, Han IS, Choi SY, Lee JH, Lee JS,
Hong CS and Park JW: Allergenicity of recombinant profilins from
Japanese hop, Humulus japonicus. J Investig Allergol Clin Immunol.
23:345–350. 2013.PubMed/NCBI
|
|
17
|
Park SW, Chung SK and Kim SH:
Antimutagenic effects and isolation of flavonoids from Humulus
japonicus extract. Korean Soc Food Sci Technol. 27:879–901.
1995.
|
|
18
|
Park SW, Woo CJ, Chung SK and Chung KT:
Antimicrobial and antioxidative activities of solvent fraction from
Humulus japonicus. Korean Soc Food Sci Technol. 26:464–470.
2000.
|
|
19
|
Lee YR, Kim KY, Lee SH, Kim MY, Park HJ
and Jeong HS: Antioxidant and Antitumor Activities of Methanolic
Extract form Humulus japonicus. Korean Soc Food Sci Technol.
25:357–361. 2012.
|
|
20
|
Hong MS, Son ES, Lee SJ, Lee SK, Lee YJ,
Song SD, Cho SN, Clifton E and Eum SY: Anti-mycobacterial effects
of the extract of Humulus japonicus. Korean Soc Food Sci Technol.
46:94–99. 2014. View Article : Google Scholar
|
|
21
|
Hwang SY, Jung HJ, Jang WS, Jo MJ, Kim SC
and Jee SY: Anti-inflammatory effects of the MeOH extract of
Humulus japonicus in vitro. J Korean Med Ophthalmol Otolaryngol
Dermatol. 22:71–79. 2009.In Korean.
|
|
22
|
Xia Y, Roman LJ, Masters BS and Zweier JL:
Inducible nitric-oxide synthase generates superoxide from the
reductase domain. J Biol Chem. 273:22635–22639. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Surh YJ, Chun KS, Cha HH, Han SS, Keum YS,
Park KK and Lee SS: Molecular mechanisms underlying chemopreventive
activities of anti-inflammatory phytochemicals: down-regulation of
COX-2 and iNOS through suppression of NF-kappa B activation. Mutat
Res. 480–481:243–268. 2001. View Article : Google Scholar
|
|
24
|
Park CM and Song YS: Luteolin and
luteolin-7-O-glucoside inhibit lipopolysaccharide-induced
inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt
signaling cascades in RAW 264.7 cells. Nutr Res Pract. 7:423–429.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chun SC, Jee SY, Lee SG, Park SJ, Lee JR
and Kim SC: Anti-inflammatory activity of the methanol extract of
moutan cortex in LPS-activated Raw264.7 cells. Evid Based
Complement Alternat Med. 4:327–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kubes P and McCafferty DM: Nitric oxide
and intestinal inflammation. Am J Med. 109:150–158. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watson WH, Zhao Y and Chawla RK:
S-adenosylmethionine attenuates the lipopolysaccharide-induced
expression of the gene for tumour necrosis factor alpha. Biochem J.
342:21–25. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adams DO and Hamilton TA: The cell biology
of macrophage activation. Annu Rev Immunol. 2:283–318. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.PubMed/NCBI
|
|
30
|
Wink DA, Vodovotz Y, Laval J, Laval F,
Dewhirst MW and Mitchell JB: The multifaceted roles of nitric oxide
in cancer. Carcinogenesis. 19:711–721. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim ND, Kim EM, Kang KW, Cho MK, Choi SY
and Kim SG: Ginsenoside Rg3 inhibits phenylephrine-induced vascular
contraction through induction of nitric oxide synthase. Br J
Pharmacol. 140:661–670. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Collins RG, Velji R, Guevara NV, Hicks MJ,
Chan L and Beaudet AL: P-Selectin or intercellular adhesion
molecule (ICAM)-1 deficiency substantially protects against
atherosclerosis in apolipoprotein E-deficient mice. J Exp Med.
191:189–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cybulsky MI and Gimbrone MA Jr:
Endothelial expression of a mononuclear leukocyte adhesion molecule
during atherogenesis. Science. 251:788–791. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Braunersreuther V and Mach F: Leukocyte
recruitment in atherosclerosis: potential targets for therapeutic
approaches? Cell Mol Life Sci. 63:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huo Y and Ley K: Adhesion molecules and
atherogenesis. Acta Physiol Scand. 173:35–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Broide DH, Humber D, Sullivan S and
Sriramarao P: Inhibition of eosinophil rolling and recruitment in
P-selectin- and intracellular adhesion molecule-1-deficient mice.
Blood. 91:2847–2856. 1998.PubMed/NCBI
|
|
38
|
Kuhlman P, Moy VT, Lollo BA and Brian AA:
The accessory function of murine intercellular adhesion molecule-1
in T lymphocyte activation. Contributions of adhesion and
co-activation. J Immunol. 146:1773–1782. 1991.PubMed/NCBI
|
|
39
|
Parhami F, Fang ZT, Fogelman AM, Andalibi
A, Territo MC and Berliner JA: Minimally modified low density
lipoprotein-induced inflammatory responses in endothelial cells are
mediated by cyclic adenosine monophosphate. J Clin Invest.
92:471–478. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nagarajan S, Burris RL, Stewart BW,
Wilkerson JE and Badger TM: Dietary soy protein isolate ameliorates
atherosclerotic lesions in apolipoprotein E-deficient mice
potentially by inhibiting monocyte chemoattractant protein-1
expression. J Nutr. 138:332–337. 2008.PubMed/NCBI
|
|
41
|
Gu L, Okada Y, Clinton SK, Gerard C,
Sukhova GK, Libby P and Rollins BJ: Absence of monocyte
chemoattractant protein-1 reduces atherosclerosis in low density
lipoprotein receptor-deficient mice. Mol Cell. 2:275–281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bourdillon MC, Poston RN, Covacho C,
Chignier E, Bricca G and McGregor JL: ICAM-1 deficiency reduces
atherosclerotic lesions in double-knockout mice
(ApoE(−/−)/ICAM-1(−/−)) fed a fat or a chow
diet. Arterioscler Thromb Vasc Biol. 20:2630–2635. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Medbury HJ, James V, Ngo J, Hitos K, Wang
Y, Harris DC and Fletcher JP: Differing association of macrophage
subsets with atherosclerotic plaque stability. Int Angiol.
32:74–84. 2013.PubMed/NCBI
|
|
44
|
Medbury HJ, Tarran SL, Guiffre AK,
Williams MM, Lam TH, Vicaretti M and Fletcher JP: Monocytes
contribute to the atherosclerotic cap by transformation into
fibrocytes. Int Angiol. 27:114–123. 2008.PubMed/NCBI
|
|
45
|
Ren S, Fan X, Peng L, Pan L, Yu C, Tong J,
Zhang W and Liu P: Expression of NF-κB, CD68 and CD105 in carotid
atherosclerotic plaque. J Thorac Dis. 5:771–776. 2013.
|
|
46
|
Ghayur T, Banerjee S, Hugunin M, Butler D,
Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et
al: Caspase-1 processes IFN-gamma-inducing factor and regulates
LPS-induced IFN-gamma production. Nature. 386:619–623. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K,
Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al:
Activation of interferon-gamma inducing factor mediated by
interleukin-1beta converting enzyme. Science. 275:206–209. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Seta Y, Kanda T, Tanaka T, Arai M,
Sekiguchi K, Yokoyama T, Kurimoto M, Tamura J and Kurabayashi M:
Interleukin 18 in acute myocardial infarction. Heart. 84:668–669.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mallat Z, Corbaz A, Scoazec A, Besnard S,
Lesèche G, Chvatchko Y and Tedgui A: Expression of interleukin-18
in human atherosclerotic plaques and relation to plaque
instability. Circulation. 104:1598–1603. 2001. View Article : Google Scholar : PubMed/NCBI
|