Introduction
Alterations in vascular smooth muscle cell (VSMC)
growth, migration, proliferation and plasticity are believed to
contribute to vascular dysfunction associated with cardiovascular
diseases, such as hypertension, atherosclerosis and stenosis
following angioplasty (1,2). Aberrant increases in the plasma
levels of vasoactive peptides are a hallmark of these vascular
diseases. The involvement of the vasoconstrictor endothelin-1
(ET-1) in the activation of signaling events intimately linked to
the migration and proliferation of VSMCs has been documented over
the past years (3,4). In VSMCs, ET-1 exerts its
growth-promoting effects through the activation of its seven
transmembrane domain guanine nucleotide-binding protein (G
protein)-coupled receptor (GPCR) (5), ETA receptor. GPCR stimulation leads
to the activation of several downstream signaling cascades, which
include members of the mitogen-activated protein kinase (MAPK)
family, as well as the phosphatidylinositol 3-kinase
(PI3-K)/protein kinase B (PKB) pathway (6).
MAPKs constitute a family of serine/threonine
protein kinases which are widely conserved among eukaryotes, and
are involved in many cellular responses, such as cell motility,
proliferation, differentiation and survival (7,8).
To date, the most extensively studied members of the MAPK family
include extracellular signal-regulated kinase (ERK)1/2, c-Jun
N-terminal kinase (JNK), and p38 MAPK (7,8),
which are stimulated by mitogens, such as polypeptide growth
factors [insulin-like growth factor (IGF)-1, platelet-derived
growth factor (PDGF) and colony stimulating factor-1 (CSF-1)], as
well as by insulin and phorbol 12-myristate 13-acetate (PMA).
Substantial evidence exists to support a role of MAPK activation in
inducing cell growth and hypertrophy in aortic and mesenteric
artery-derived VSMCs (9–12).
The early growth response factor-1 (Egr-1) is a zinc
finger transcription factor that regulates the transcription of
several genes involved in cardiovascular functions (13,14). Egr-1 has been suggested to
contribute to the progression of vascular disease processes, such
as intimal thickening following vascular injury (15), atherosclerosis and cardiac
hypertrophy (16,17). We recently reported the
upregulation of Egr-1 levels in response to ET-1 in VSMCs (18); however, little is known about the
molecular mechanisms that transduce these signals into an increase
in Egr-1 expression.
We have recently demonstrated that c-Src, also known
as Src or p60c-Src, is a non-receptor tyrosine kinase
(NR-TK) that plays a key role in mediating ET-1-induced PKB
phosphorylation, cell hypertrophy and proliferation (19). However, the involvement of c-Src
in ET-1-induced Egr-1 expression has not yet been investigated and
its role in MAPK signaling remains controversial (38).
Therefore, in the present study, by using a
pharmacological inhibitor of c-Src and cells deficient in c-Src, we
examined the role of c-Src as an upstream regulator of ET-1-induced
ERK1/2, JNK and p38 MAPK phosphorylation, as well as its
involvement in the regulation of Egr-1 expression in VSMCs.
Materials and methods
Materials
Chemicals
Cell culture reagents were purchased from Gibco
(Burlington, ON, USA). ET-1 was purchased from American Peptide
(Sunnyvale, CA, USA).
4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazole(3,4-d)pyrimidine
(PP2; src inhibitor), 4-amino-7-phenylpyrazole(3,4-d) pyrimidine
(PP3; inactive analog of src inhibitor) and PD98059 (MEK
inhibitor), SP600125 (JNK inhibitor), were purchased from
Calbiochem (San Diego, CA, USA). The enhanced chemiluminescence
(ECL) detection system kit was purchased from Amersham Pharmacia
Biotech (Baie d'Urfé, QC, Canada).
Antibodies
Phospho-SAPK/JNK (Thr183/Tyr185) (Cat. no. 4668),
phospho-p38 MAPK (Thr180/Tyr182) (Cat. no. 4511S), total SAPK/JNK
(Cat. no. 9252), β-tubulin (Cat. no. 2146S), total glyceraldehyde
3-phosphate dehydrogenase (GAPDH) (Cat. no. 5174) and anti-rabbit
IgG, horseradish peroxidase-linked secondary antibody (Cat. no.
7074) were procured from Cell Signaling Technology (Danvers, MA,
USA). Phospho-ERK1/2 (Thr202/Tyr204) (Cat. no. sc-16982-R), total
ERK (Cat. no. sc-154), total p38 MAPK (Cat. no. sc-7972), total
Egr-1 (Cat. no. sc-110) and anti-mouse IgG, horseradish
peroxidase-linked secondary antibody (Cat. no. sc-2005) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Methods
Cell culture
Rat aorta A-10 VSMCs (CRL-1476; ATCC, Manassas, VA,
USA) were maintained in 75-cm2 flasks culture with
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum and antibiotics at 37°C in a humidified atmosphere of
5% CO2, as previously described (19). The cells were passed upon reaching
confluence with 0.5% trypsin-containing 0.2% EDTA and plated in 60
mm dishes. The cells were grown to 90% confluence and incubated in
serum-free DMEM 18 h prior to the treatments. Mouse embryonic
fibroblasts (MEFs) deficient for c-Src, Yes and Fyn (SYF)
(CRL-2459) and expressing endogenous wild-type c-Src, but not Yes
and Fyn (Src+/+) (CRL-2497) (both from ATCC) were
cultured and used for the experimentats in the same manner as the
A10 VSMCs.
Cell lysis and immunoblotting
The cells incubated in the absence or presence of
various agents were washed 3 times with ice-cold phosphate-buffered
saline (PBS) and lysed in 200 µl of lysis buffer [25 mM
Tris-HCl, pH 7.5, 25 mM NaCl, 1 mM NaOV, 10 mM Na fluoride, 10 mM
Na-pyrophosphate, 2 mM benzamidine, 2 mM
ethylenebis(oxyethylenenitrilo)-tetraacetic acid, 2 mM
ethylenediamine tetra acetic acid, 1 mM PMSF, 1% Triton X-100, 0.1%
sodium dodecyl sulfate (SDS) and 1% protease inhibitor cocktail
(PIC)] on ice. Cell lysates were centrifuged at 12,000 × g for 10
min at 4°C. Protein concentrations were measured by Bradford assay.
Equal amounts of protein were subjected to 7.5% SDS-polyacrylamide
gel electrophoresis (PAGE), transferred onto PVDF membranes
(Millipore, Billerica, MA, USA) and incubated with the respective
primary antibodies. The antigen-antibody complex was detected by
horseradish peroxidase-conjugated secondary antibody and protein
bands were visualized by ECL. The intensity of specific bands was
quantified using Quantity One Image software (Bio-Rad, Hercules,
CA, USA).
Egr-1 nuclear extraction protocol
The cells incubated in the absence or presence of
pharmacological agents were washed twice in ice-cold PBS and lysed
in 500 µl of buffer solution containing 10 mM HEPES, 10 mM
KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM PMSF, 1 mM protease cocktail
inhibitor and 1 mM NaOV as previously described (20). Briefly, lysates were placed on ice
for 15 min prior to the addition of 10% NP-40 detergent. Lysates
were then vortexed for 10 sec at highest setting before
centrifugation at 18,000 × g for 4 min at 4°C. The pellet was
resuspended in 60 µl of buffer containing 10 mM HEPES, 400
mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM PMSF, 1 mM protease
cocktail inhibitor and 1 mM NaOV. The suspension was sonicated by
performing 6 cycles at 10 sec/cycle with 30 sec intervals and then
centrifuged at 18,000 × g for 5 min at 4°C. The pellet was
discarded and the supernatant, corresponding to the nuclear
fraction, was collected. Protein concentrations were measured by
Bradford assay for subsequent immunoblotting with Egr-1 antibody.
This antibody detects Egr-1 protein as a doublet, since Egr-1 can
also be phosphorylated (21), it
is possible that this doublet represents the phosphorylated and
dephosphorylated forms of the Egr-1.
Preparation of cDNA
Following incubation, total RNA was isolated using
TRIzol reagent (Life Technologies, Burlington, ON, USA). The RNA
concentration was quantified with the Eppendorf BioPhotometer D30
(Eppendorf, Mississauga, ON, Canada). Absorbances were measured at
wavelengths of 260 and 280 nm. The purity of RNA preparation was
confirmed when the ratio A260/A280 was comprised in the range
1.8–2.0. Subsequently, the cDNA was synthesized from 1 µg of
total pure RNA using High Capacity RNA-to-cDNA kit (Applied
Biosystems, Grand Island, NY, USA) as per the manufacturer's
instructions.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed using SYBR-Green (Life
Technologies, Grand Island, NY, USA) with 1 µl of cDNA in a
20 µl reaction. Amplification was performed using 7500 Fast
RT-PCR system (Applied Biosystems, Grand Island, NY, USA).
Sequences used to design Egr-1 primers were as follows: forward,
5′-CTGCTTCATCGTCTTCCTCTG-3′ and reverse,
5′-GTCAGTGTTGGGAGTAGGAAAG-3′. Egr-1 mRNA expression was measured
and normalized to β-actin mRNA levels using primers: forward,
5′-TCTTCCAGCCTTCCTTCCT-3′ and reverse,
5′-CAGCACTGTGTTGGCATAGA-3′.
Statistical analysis
The results presented are the means ± SE of 3 or
more independent experiments. Statistical analyses were performed
by analysis of variance (one-way ANOVA), followed by Dunnett′s
multiple comparison post test, where applicable, using Prism 5
(GraphPad Software Inc., La Jolla, CA, USA). P-values <0.05 were
considered to indicate statistically significant differences.
Results
Inhibition of c-Src attenuates the
ET-1-induced phosphorylation of ERK1/2 in A10 VSMCs
We previously demonstrated that c-Src plays a
critical role in mediating ET-1 and angiotensin-II (Ang-II)-induced
PKB phosphorylation through the Tyr418 phosphorylation of c-Src in
VSMCs (19); however, the
involvement of c-Src in the ET-1-induced MAPK phosphorylation
remains controversial (22).
Therefore, by using PP2, a specific blocker of the Src family of
PTK (19), in this study, we
investigated the role of c-Src in ERK1/2 phosphorylation in A10
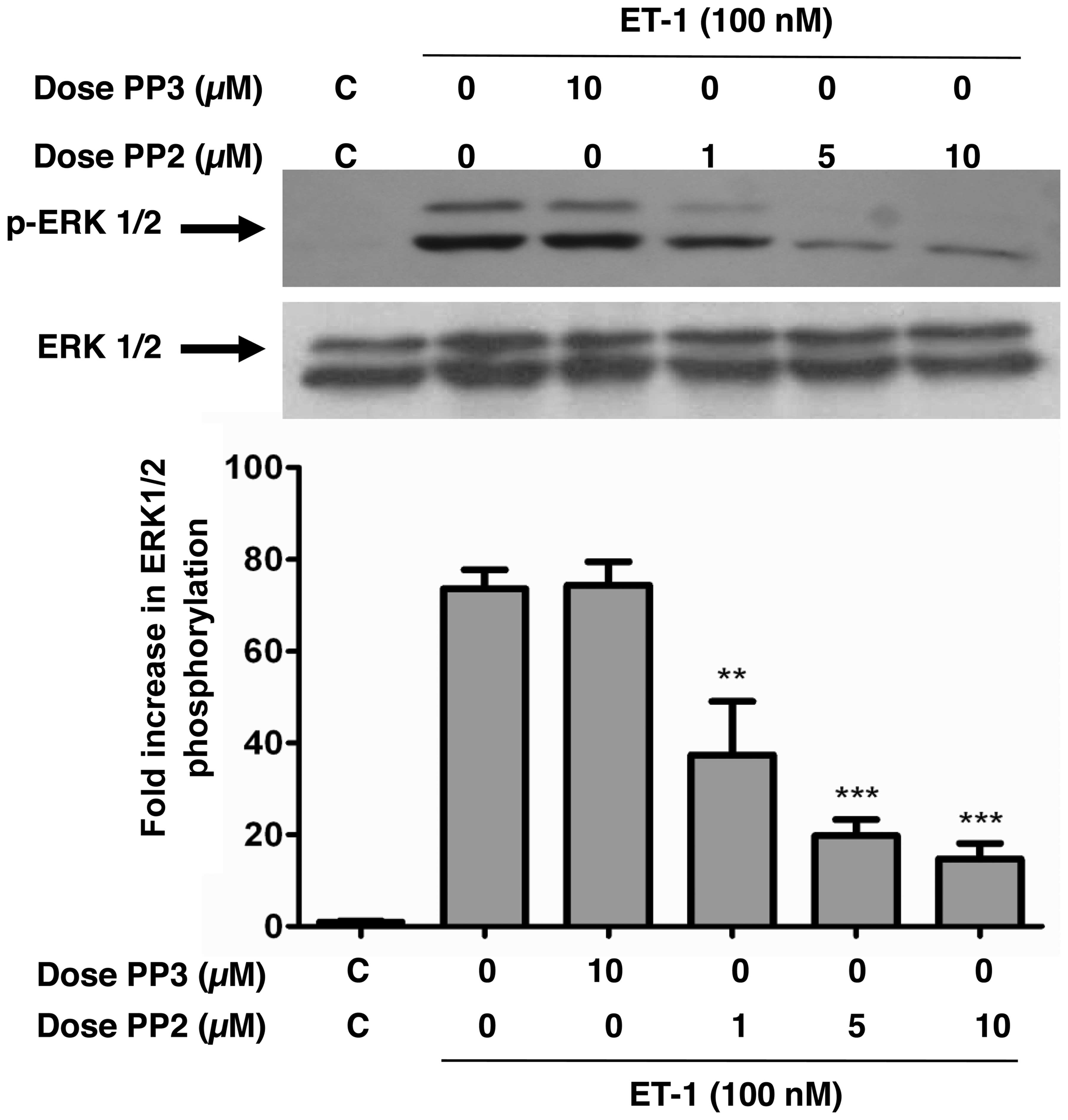
VSMCs. ET-1 potently enhanced the phosphorylation of ERK1/2
(Fig. 1). However, pre-treatment
of the A10 VSMCs with PP2 for 30 min dose-dependently attenuated
the ET-1-induced phosphorylation of ERK1/2, whereas treatment with
PP3, an inactive analog of PP2, had no effect. No alterations in
the total amounts of ERK1/2 were observed under these experimental
conditions.
ET-1-induced phosphorylation of JNK/SAPK
and p38 MAPK is attenuated by c-Src tyrosine kinase inhibition in
A10 VSMCs
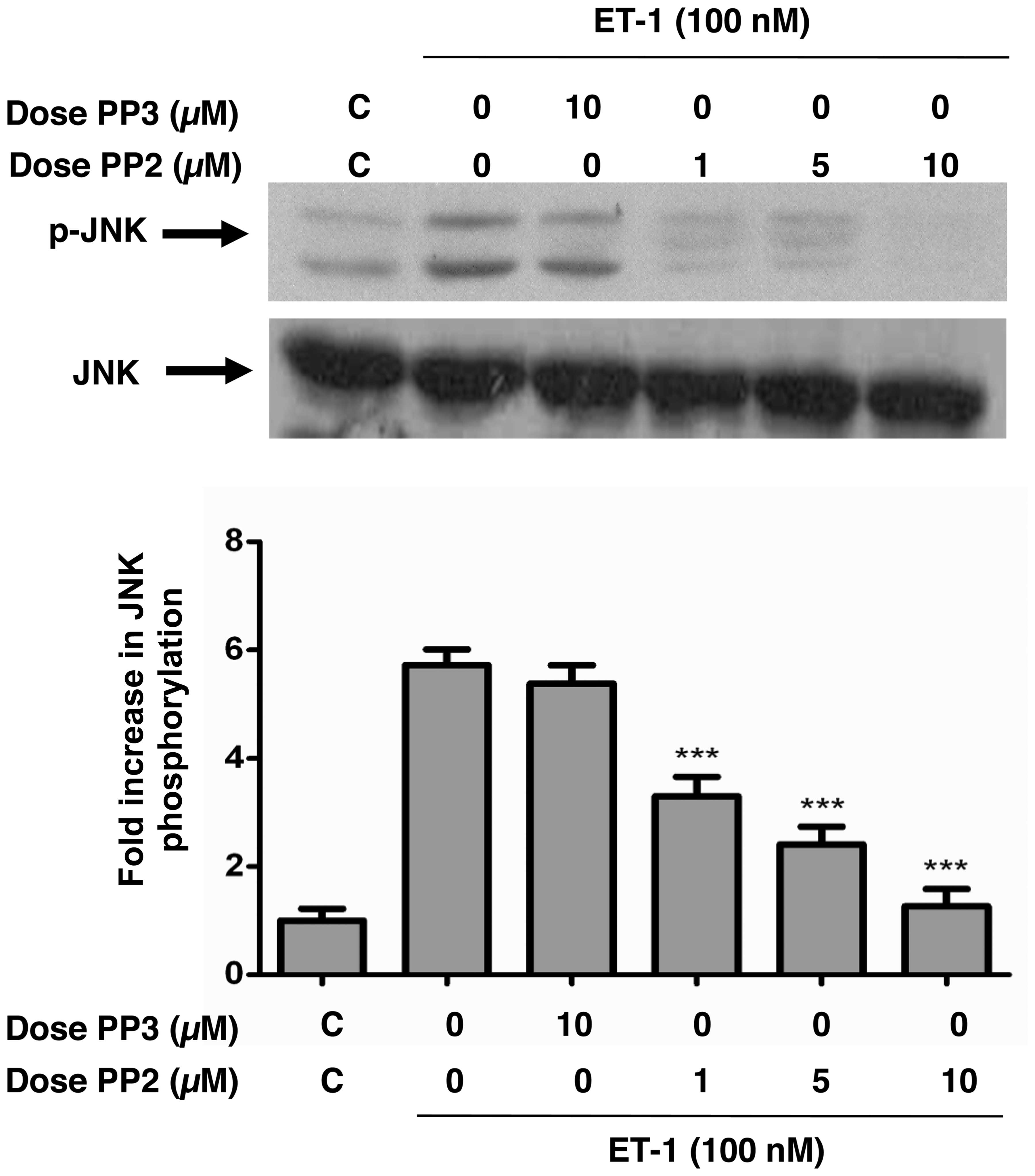
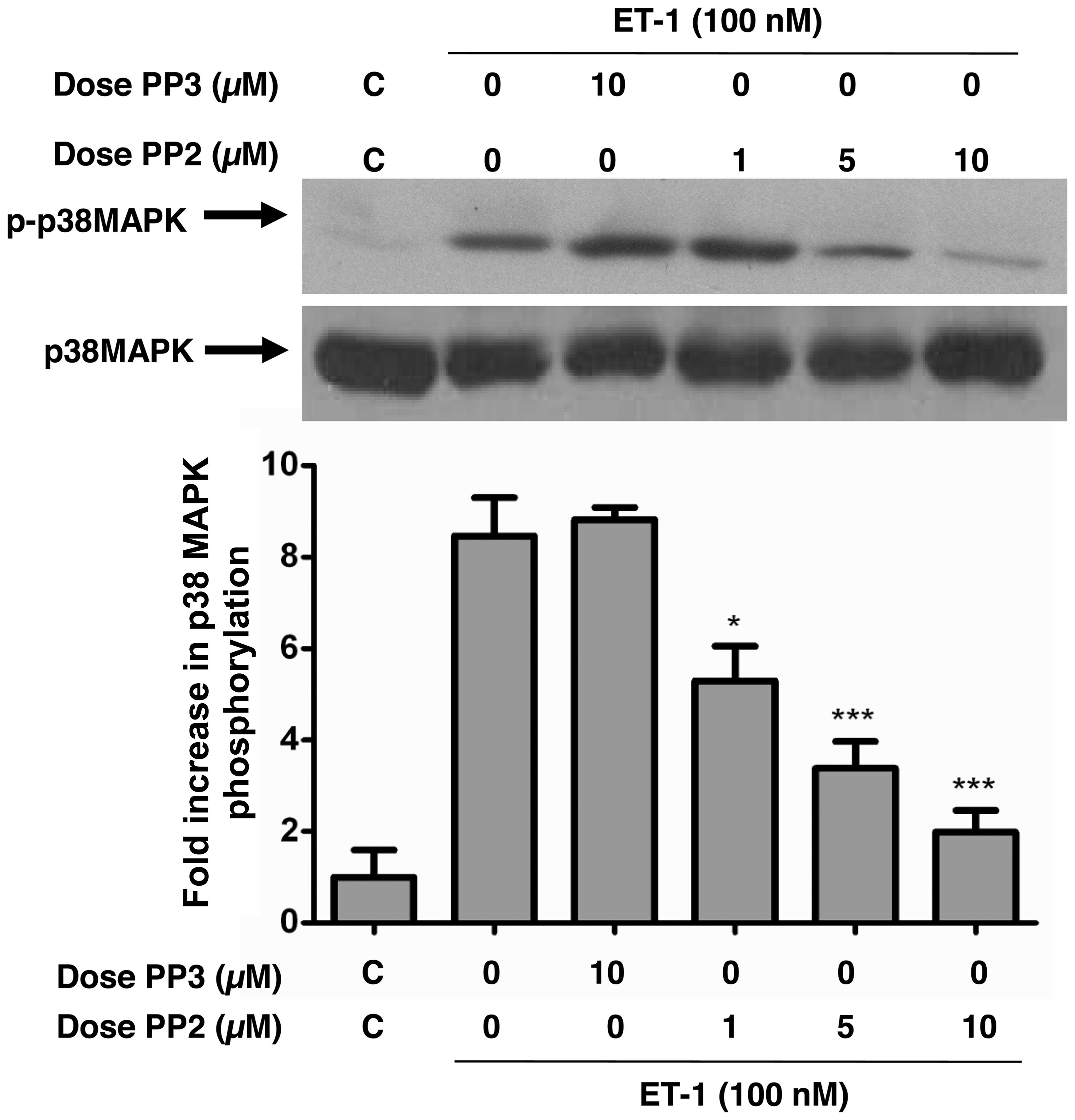
JNK and p38 MAPK are both expressed in VSMCs and are
activated by both Ang-II and ET-1 (22,23); however, the role of c-Src in
mediating this activation remains controversial. Therefore, in this
study, by using PP2, we investigated the role of c-Src in JNK and
p38 MAPK phosphorylation in A10 VSMCs. ET-1 potently enhanced the
phosphorylation of JNK (Fig. 2)
and p38 MAPK (Fig. 3). Treatment
of the A10 VSMCs with PP2 for 30 min prior to ET-1 stimulation
dose-dependently inhibited JNK (Fig.
2) and p38 MAPK (Fig. 3)
phosphorylation induced by ET-1. PP3, on the other hand, was unable
to inhibit JNK or p38 MAPK phosphorylation induced by the peptide.
No alterations in the total amounts of JNK or p38 MAPK were
observed under these experimental conditions.
Inhibition of c-Src tyrosine kinase
attenuates ET-1-induced Egr-1 expression in A10 VSMCs
Previous studies have suggested that the
transcription factor, Egr-1, plays an important role in multiple
processes linked to vascular pathobiology, including the
progression of atherosclerotic lesions and neointimal thickening
after vascular injury (24–26). We have recently demonstrated that
ET-1 upregulates the expression of Egr-1 in a
calcium/calmodulin-dependent manner (18); yet no studies to date have
examined the role of c-Src in this process, at least to the best of
our knowledge. Therefore, we wished to determine the involvement of
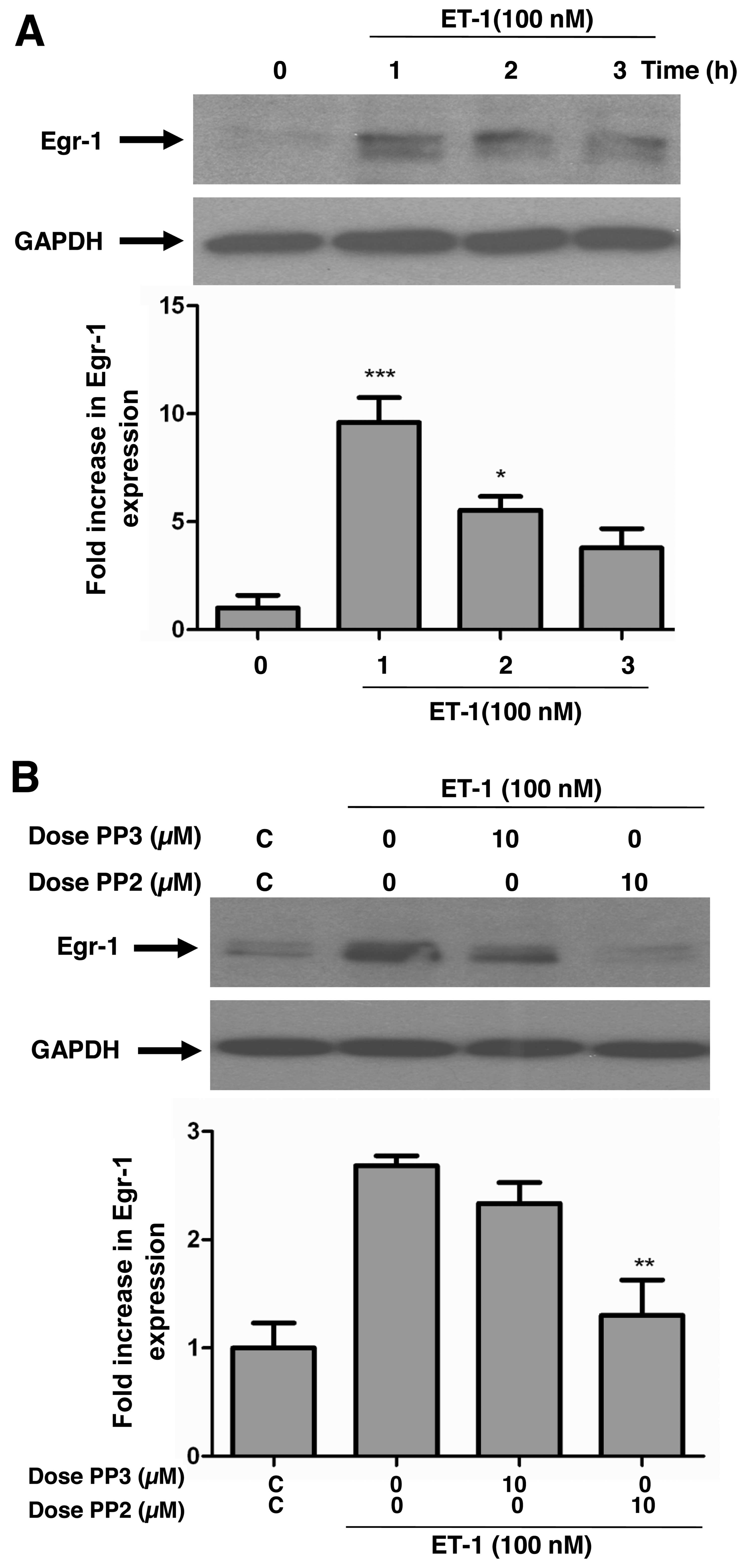
c-Src in the ET-1-induced expression of Egr-1. Stimulation of the
serum-starved VSMCs with 100 nM ET-1 time-dependently increased the
protein expression of Egr-1 (Fig.
4A). Treatment of the cells with 10 µM PP2 prior to
stimulation with ET-1 significantly decreased the ET-1-induced
Egr-1 expression in these cells (Fig.
4B), suggesting a role of c-Src in Egr-1 expression. Treatment
with PP3 did not have any effect.
c-Src knockdown decreases ET-1-induced
ERK1/2, JNK and p38 MAPK phosphorylation, as well as Egr-1
expression
To further confirm the role of c-Src PTK in
ET-1-induced MAPK activation, we utilized MEFs harvested from mouse
embryos which have a functional null mutation in both alleles of
the Src family PTK coding for c-Src (SYF) (27). MEFs expressing endogenous
wild-type c-Src (Src+/+) were used as the control cells
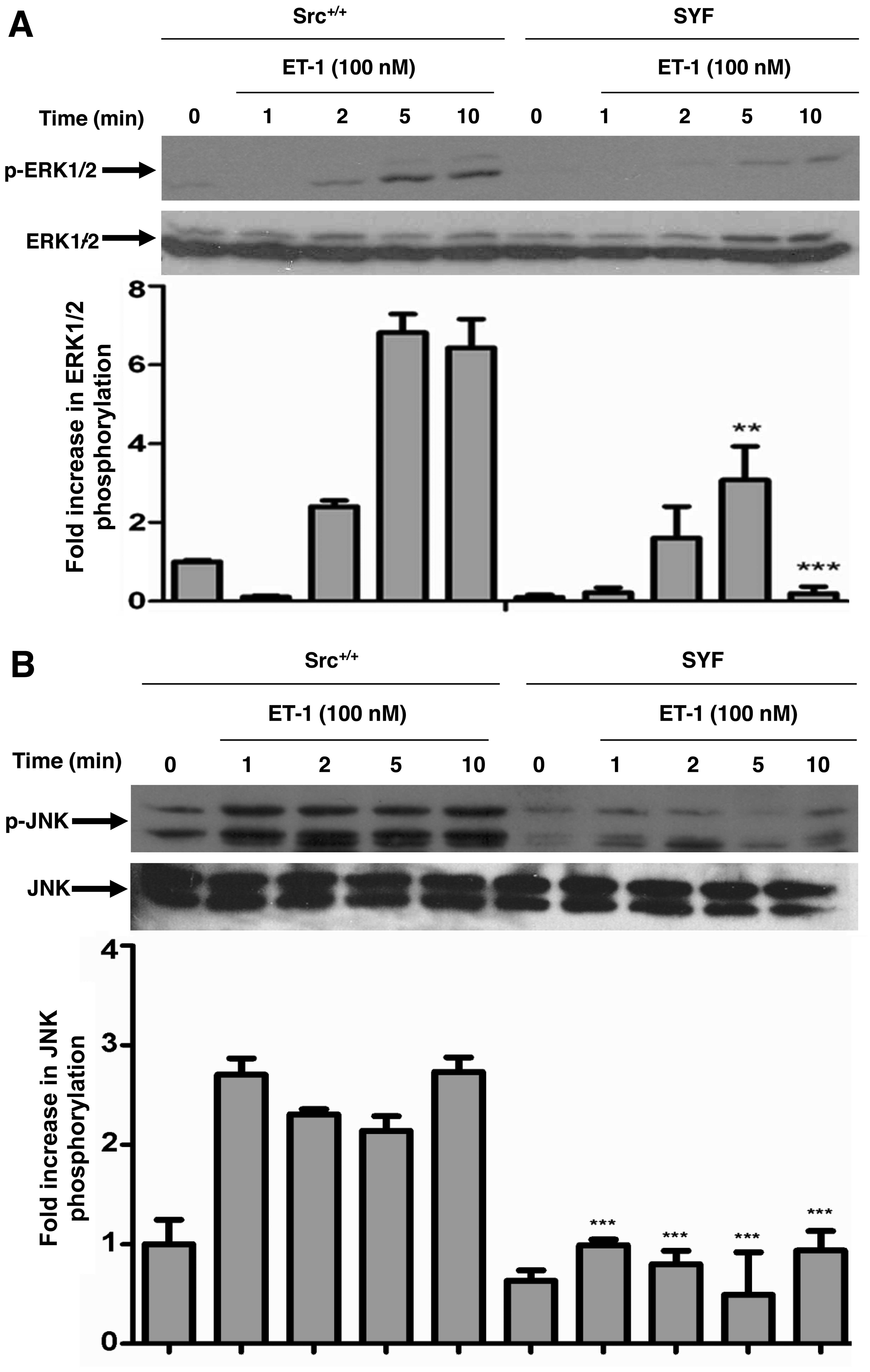
in these experiments. ET-1 treatment resulted in a time-dependent
increase in the phosphorylation of ERK1/2 (Fig. 5A), JNK (Fig. 5B) and p38 MAPK (Fig. 5C) in the c-Src+/+
cells; however, this response was blunted in the SYF cells. No
alterations in the total amounts of ERK1/2, JNK or p38 MAPK were
observed under these experimental conditions. Furthermore, we used
these cells to confirm the role of c-Src in ET-1-induced Egr-1
expression. ET-1-induced Egr-1 expression was blunted in the SYF
cells as compared to the Src+/+ MEFs, demonstrating the
requirement of c-Src in ET-1-induced Egr-1 expression (Fig. 5D).
Pharmacological blockade of ERK1/2 and
JNK activity attenuates ET-1-induced Egr-1 expression in A10
VSMCs
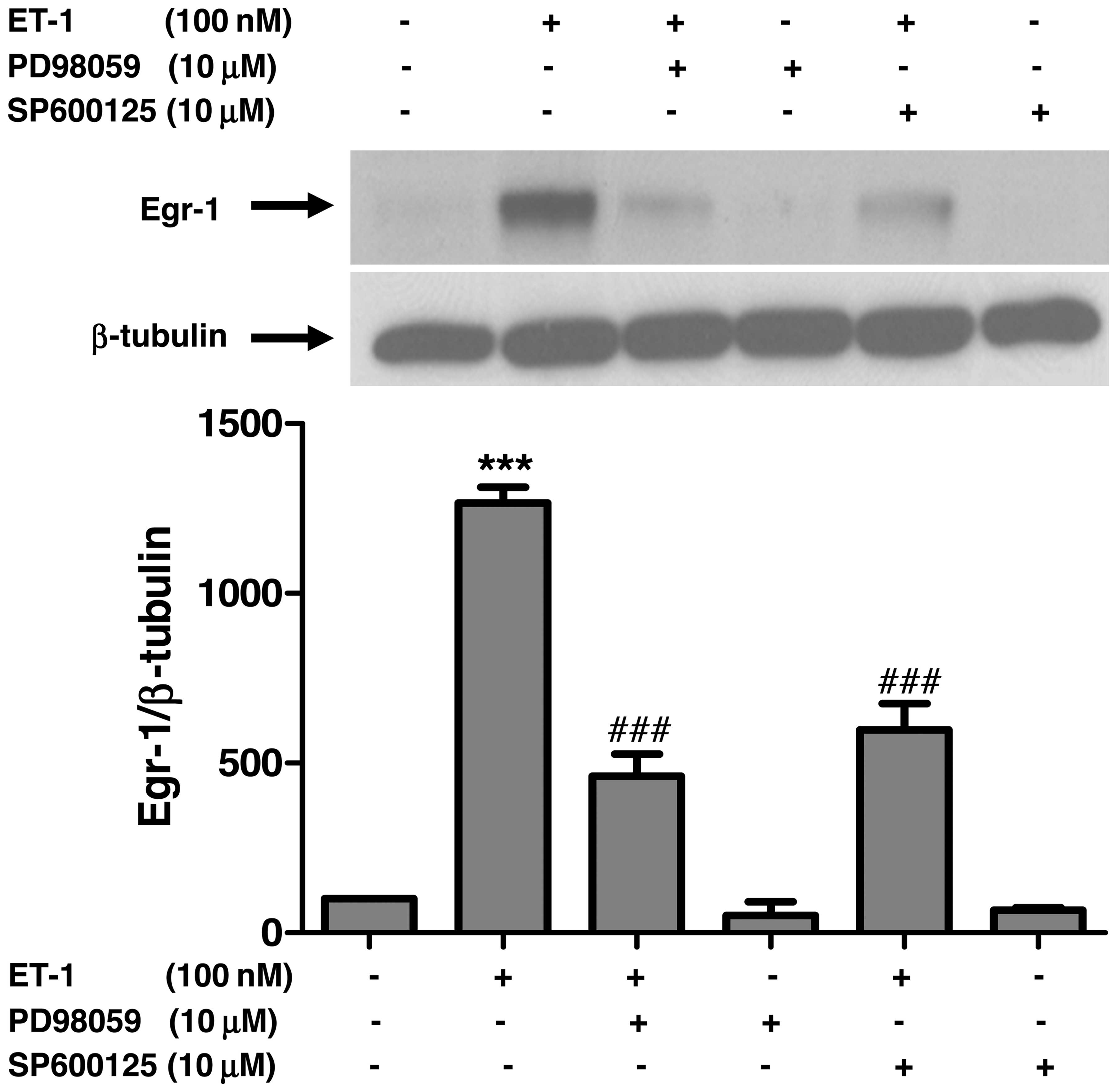
In order to further examine whether the attenuation
of JNK and ERK1/2 activity by the inhibition of c-Src plays a role
in ET-1-induced Egr-1 expression, we examined the expression of
Egr-1 following the pharmacological blockade of ERK1/2 and JNK by
using PD98059 and SP600125, respectively. Treatment of the VSMCs
with PD98059 or SP600125, respectively, prior to stimulation with
ET-1 significantly decreased ET-1-induced Egr-1 expression
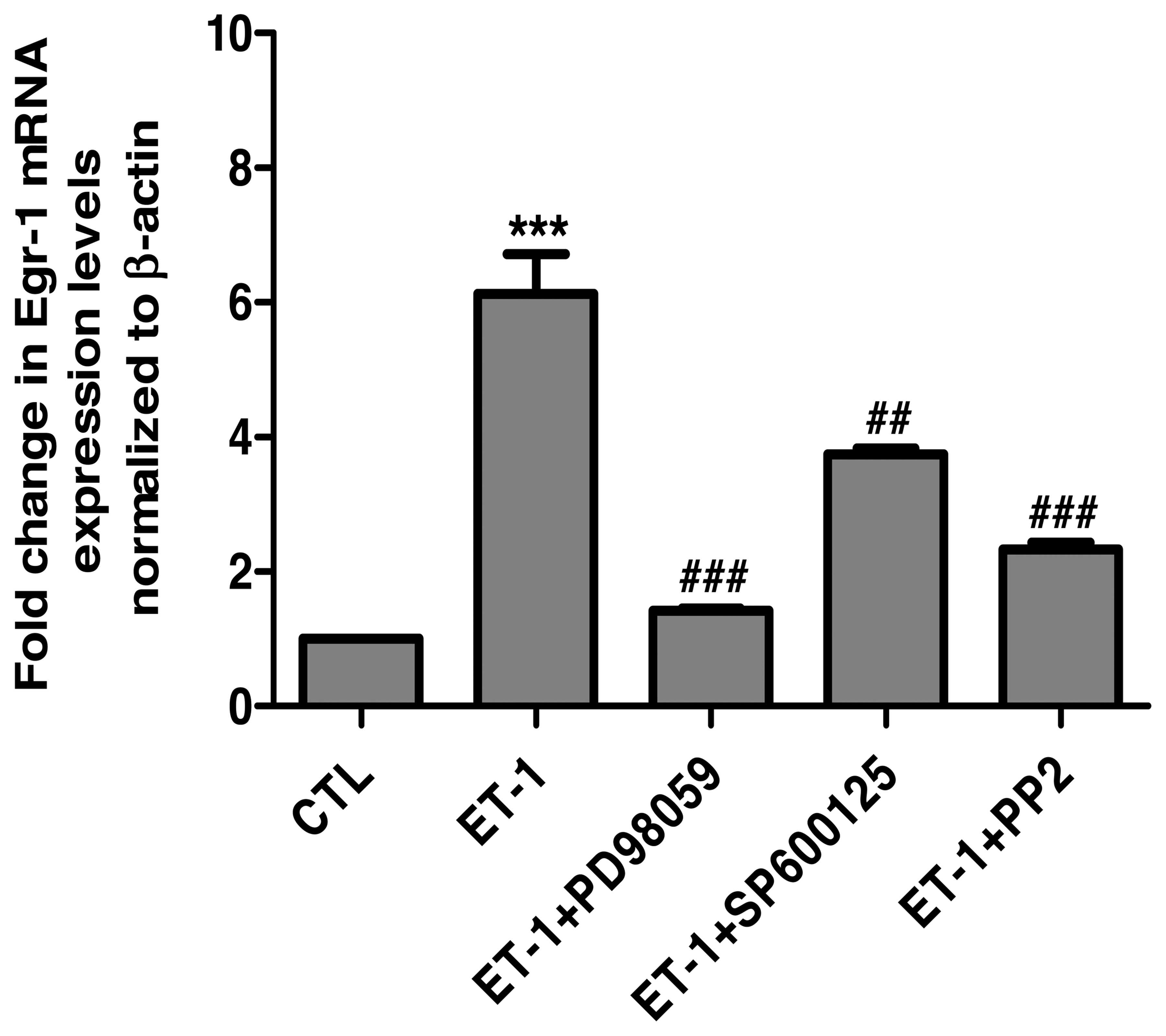
(Fig. 6). In addition, consistent
with the results of the protein expression of Egr-1, the
pharmacological blockade of ERK1/2, JNK and c-Src significantly
reduced the ET-1-induced upregulation of the Egr-1 mRNA levels
(Fig. 7).
Discussion
c-Src is a member of the Src family of NR-TKs that
play a major role in the signaling mechanisms underlying cell
differentiation, proliferation, survival, as well as in cell
adhesion, morphology and motility (reviewed in ref. 28). Our previous study demonstrated
that treatment of VSMCs with ET-1 induced the phosphorylation of
Tyr418 in the activation loop of c-Src and the blockade of c-Src
activity by PP2 resulted in the inhibition of ET-1 and
Ang-II-induced PKB signaling, as well as protein and DNA synthesis
(19). However, whether c-Src is
also involved inthe ET-1-induced activation of MAPK signaling and
subsequent gene expression remains to be established. In the
present study, by using a pharmacological approach to inhibit c-Src
PTK activity, as well as MEFs deficient in c-Src, we demonstrated
that c-Src is essential to propagate ET-1-induced MAPK
phosphorylation. In addition, to the best of our knowledge, we also
report for the first time that the c-Src-dependent activation of
ERK1/2 and JNK is required to induce Egr-1 expression in response
to ET-1 in VSMCs. ERK1/2 and JNK, by their ability to phosphorylate
several transcription factors and co-activators have been
implicated in triggering the transcription of Egr-1 in VSMCs
(29–31). For example, c-Jun is a downstream
target of JNK and a role of ERK1/2- and JNK-induced c-Jun activity
in shear and injury-induced Egr-1 expression in VSMCs has been
demonstrated (32). ERK1/2 also
phosphorylates the transcription factor, Ets-like protein-1 (Elk-1)
(33), and the c-AMP response
element binding protein (CREB) (33,34) both of which have been shown to
transcriptionally regulate the expression of Egr-1 in response to
various stimuli (29,31,33,35).
Although several studies have reported a requirement
of c-Src in MAPK activation in response to vasoactive peptides,
growth factors and oxidative stress (22,36–38), its role in ET-1-induced activation
remains unclear. A lack of c-Src requirement in ET-1-induced MAPK
was reported by Yogi et al in VSMCs isolated from mouse
mesenteric arteries (22),
whereas it was shown to be essential to enhance the MAPK
phosphorylation in rat aortic rings treated with ET-1 (37). Our results showing that both the
pharmacological blockade of c-Src activity by using PP2 and c-Src
deficiency in MEFs resulted in a significant reduction in the
ET-1-induced phosphorylation of ERK1/2, JNK and p38 MAPK, support
the notion that c-Src is essential to propagate the ET-1 signaling
cascade leading to MAPK activation in VSMCs. Consistent with a role
of c-Src as a mediator of ET-1-induced responses in VSMCs, it was
recently reported that the pharmacological blockade of c-Src not
only attenuated the exaggerated levels of ERK1/2 phosphorylation
observed in VSMCs from spontaneously hypertensive rats (SHR), but
also potently inhibited the aberrant proliferation exhibited by
these cells (39). Moreover, an
enhanced phosphorylation of c-Src and ERK1/2 in VSMCs isolated from
the aorta of SHR has been demonstrated (40). These authors also reported that
the pharmacological blockade of c-Src and ET-1 receptors attenuated
the enhanced ERK1/2 phosphorylation, proliferation and growth
observed in VSMC sfrom SHR, suggesting the importance of c-Src in
mediating ET-1-induced ERK signaling in VSMCs from SHR (40,41).
The results presented herein also reveal a role of
c-Src in ET-1-induced Egr-1 expression in VSMCs. Egr-1 belongs to
the family of zinc finger transcription factors and plays an
important role in vascular proliferative responses (16). Egr-1 governs the expression of
several genes that play a deleterious role in vascular biology and
has been implicated in intimal thickening in response to vascular
injury and the development of atherosclerotic lesions (13,42). ET-1, as well as several growth
factors and inflammatory cytokines, has been shown to induce its
expression in several cell types including both endothelial cells
and VSMCs (18,20,43–46). Our results showing that the
pharmacological blockade or genetic knockdown of c-Src inhibits
ET-1-induced Egr-1 expression, to our knowledge, represent the
first to report a role of c-Src in ET-1-induced Egr-1 expression in
VSMCs. Although a role of ERK1/2 and JNK in Egr-1 expression in
VSMCs has been reported earlier (47–49), our data are the first to suggest
that c-Src functions upstream of MAPK in transducing the effect of
ET-1 in the induction of Egr-1 expression in VSMCs.
In conclusion, in this study, we demonstrated that
ET-1 induces the phosphorylation of ERK1/2, JNK and p38 MAPK, as
well as Egr-1 expression through a c-Src PTK-dependent pathway and
tht c-Src-dependent MAPK activation is essential to induce Egr-1
expression in VSMCs. It may be suggested that c-Src is a key
upstream intermediate in ET-1-induced signaling pathways leading to
Egr-1 expression in VSMCs.
Acknowledgments
This study was supported by a grant from the
Canadian Institutes of Health Research (MOP 67037) to A.K.S.
E.R.S.-C. is a recipient of a studentship from the department of
Nutrition, University of Montreal. G.V. was the recipient of Ph.D.
studentships from the Faculties of Medicine and Graduate Studies,
Université de Montréal and the Montreal Diabetes Research Center
(MDRC). The authors would like to thank Ms. Viktoria Youreva for
her technical support.
References
|
1
|
Schwartz SM: Smooth muscle migration in
atherosclerosis and restenosis. J Clin Invest. 100(Suppl 11):
S87–S89. 1997.PubMed/NCBI
|
|
2
|
Cordes KR, Sheehy NT, White MP, Berry EC,
Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN and Srivastava D:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009.PubMed/NCBI
|
|
3
|
Iglarz M and Schiffrin EL: Role of
endothelin-1 in hypertension. Curr Hypertens Rep. 5:144–148. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Touyz RM and Schiffrin EL: Role of
endothelin in human hypertension. Can J Physiol Pharmacol.
81:533–541. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arai H, Hori S, Aramori I, Ohkubo H and
Nakanishi S: Cloning and expression of a cDNA encoding an
endothelin receptor. Nature. 348:730–732. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bouallegue A, Daou GB and Srivastava AK:
Endothelin-1-induced signaling pathways in vascular smooth muscle
cells. Curr Vasc Pharmacol. 5:45–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
8
|
Kyosseva SV: Mitogen-activated protein
kinase signaling. Int Rev Neurobiol. 59:201–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daou GB and Srivastava AK: Reactive oxygen
species mediate endothelin-1-induced activation of ERK1/2, PKB, and
Pyk2 signaling, as well as protein synthesis, in vascular smooth
muscle cells. Free Radic Biol Med. 37:208–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Touyz RM, Yao G, Viel E, Amiri F and
Schiffrin EL: Angiotensin II and endothelin-1 regulate MAP kinases
through different redox-dependent mechanisms in human vascular
smooth muscle cells. J Hypertens. 22:1141–1149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daigle C, Martens FM, Girardot D, Dao HH,
Touyz RM and Moreau P: Signaling of angiotensin II-induced vascular
protein synthesis in conduit and resistance arteries in vivo. BMC
Cardiovasc Disord. 4:62004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bouallegue A, Daou GB and Srivastava AK:
Nitric oxide attenuates endothelin-1-induced activation of ERK1/2,
PKB, and Pyk2 in vascular smooth muscle cells by a cGMP-dependent
pathway. Am J Physiol Heart Circ Physiol. 293:H2072–H2079. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang TR, Yang G and Liu GN: DNA enzyme ED5
depletes egr-1 and inhibits neointimal hyperplasia in rats.
Cardiology. 125:192–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheyou ER, Youreva V and Srivastava AK:
Involvement of the early growth response protein 1 in vascular
pathophysiology: an overview. Indian J Biochem Biophys. 51:457–466.
2014.
|
|
15
|
Lowe HC, Fahmy RG, Kavurma MM, Baker A,
Chesterman CN and Khachigian LM: Catalytic oligodeoxynucleotides
define a key regulatory role for early growth response factor-1 in
the porcine model of coronary in-stent restenosis. Circ Res.
89:670–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blaschke F, Bruemmer D and Law RE: Egr-1
is a major vascular pathogenic transcription factor in
atherosclerosis and restenosis. Rev Endocr Metab Disord. 5:249–254.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khachigian LM: Early growth response-1 in
cardiovascular pathobiology. Circ Res. 98:186–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bouallegue A, Simo Cheyou ER,
Anand-Srivastava MB and Srivastava AK: ET-1-induced growth
promoting responses involving ERK1/2 and PKB signaling and Egr-1
expression are mediated by Ca2+/CaM-dependent protein
kinase-II in vascular smooth muscle cells. Cell Calcium.
54:428–435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouallegue A, Vardatsikos G and Srivastava
AK: Role of insulin-like growth factor 1 receptor and c-Src in
endothelin-1- and angiotensin II-induced PKB phosphorylation, and
hypertrophic and proliferative responses in vascular smooth muscle
cells. Can J Physiol Pharmacol. 87:1009–1018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youreva V and Srivastava AK: Early growth
response protein-1 expression by insulin-like growth factor-1
requires ROS-dependent activation of ERK1/2 and PKB pathways in
vascular smooth muscle cells. J Cell Biochem. 117:152–162. 2016.
View Article : Google Scholar
|
|
21
|
Huang RP and Adamson ED: The
phosphorylated forms of the transcription factor, Egr-1, bind to
DNA more efficiently than non-phosphorylated. Biochem Biophys Res
Commun. 200:1271–1276. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yogi A, Callera GE, Montezano AC, Aranha
AB, Tostes RC, Schiffrin EL and Touyz RM: Endothelin-1, but not Ang
II, activates MAP kinases through c-Src independent Ras-Raf
dependent pathways in vascular smooth muscle cells. Arterioscler
Thromb Vasc Biol. 27:1960–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mugabe BE, Yaghini FA, Song CY,
Buharalioglu CK, Waters CM and Malik KU: Angiotensin II-induced
migration of vascular smooth muscle cells is mediated by p38
mitogen-activated protein kinase-activated c-Src through spleen
tyrosine kinase and epidermal growth factor receptor
transactivation. J Pharmacol Exp Ther. 332:116–124. 2010.
View Article : Google Scholar
|
|
24
|
Lowe HC, Chesterman CN and Khachigian LM:
Catalytic antisense DNA molecules targeting Egr-1 inhibit neointima
formation following permanent ligation of rat common carotid
arteries. Thromb Haemost. 87:134–140. 2002.PubMed/NCBI
|
|
25
|
Harja E, Bucciarelli LG, Lu Y, Stern DM,
Zou YS, Schmidt AM and Yan SF: Early growth response-1 promotes
atherogenesis: mice deficient in early growth response-1 and
apolipoprotein E display decreased atherosclerosis and vascular
inflammation. Circ Res. 94:333–339. 2004. View Article : Google Scholar
|
|
26
|
Khachigian LM, Lindner V, Williams AJ and
Collins T: Egr-1-induced endothelial gene expression: a common
theme in vascular injury. Science. 271:1427–1431. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klinghoffer RA, Sachsenmaier C, Cooper JA
and Soriano P: Src family kinases are required for integrin but not
PDGFR signal transduction. EMBO J. 18:2459–2471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roskoski R Jr: Src protein-tyrosine kinase
structure and regulation. Biochem Biophys Res Commun.
324:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hasan RN and Schafer AI: Hemin upregulates
Egr-1 expression in vascular smooth muscle cells via reactive
oxygen species ERK-1/2-Elk-1 and NF-kappaB. Circ Res. 102:42–50.
2008. View Article : Google Scholar
|
|
30
|
Thiel G, Mayer SI, Müller I, Stefano L and
Rössler OG: Egr-1-A Ca(2+)-regulated transcription factor. Cell
Calcium. 47:397–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui MZ, Laag E, Sun L, Tan M, Zhao G and
Xu X: Lysophosphatidic acid induces early growth response gene 1
expression in vascular smooth muscle cells: CRE and SRE mediate the
transcription. Arterioscler Thromb Vasc Biol. 26:1029–1035. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ni J, Waldman A and Khachigian LM: c-Jun
regulates shear-and injury-inducible Egr-1 expression, vein graft
stenosis after autologous end-to-side transplantation in rabbits,
and intimal hyperplasia in human saphenous veins. J Biol Chem.
285:4038–4048. 2010. View Article : Google Scholar
|
|
33
|
Hodge C, Liao J, Stofega M, Guan K,
Carter-Su C and Schwartz J: Growth hormone stimulates
phosphorylation and activation of elk-1 and expression of c-fos,
egr-1, and junB through activation of extracellular
signal-regulated kinases 1 and 2. J Biol Chem. 273:31327–31336.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Molnar P, Perrault R, Louis S and Zahradka
P: The cyclic AMP response element-binding protein (CREB) mediates
smooth muscle cell proliferation in response to angiotensin II. J
Cell Commun Signal. 8:29–37. 2014. View Article : Google Scholar :
|
|
35
|
Mayer SI and Thiel G: Calcium influx into
MIN6 insulinoma cells induces expression of Egr-1 involving
extracellular signal-regulated protein kinase and the transcription
factors Elk-1 and CREB. Eur J Cell Biol. 88:19–33. 2009. View Article : Google Scholar
|
|
36
|
Lieskovska J, Ling Y, Badley-Clarke J and
Clemmons DR: The role of Src kinase in insulin-like growth
factor-dependent mitogenic signaling in vascular smooth muscle
cells. J Biol Chem. 281:25041–25053. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Romero M, Jiménez R, Sánchez M,
López-Sepúlveda R, Zarzuelo A, Tamargo J, Pérez-Vizcaíno F and
Duarte J: Vascular superoxide production by endothelin-1 requires
Src non-receptor protein tyrosine kinase and MAPK activation.
Atherosclerosis. 212:78–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mehdi MZ, Pandey NR, Pandey SK and
Srivastava AK: H2O2-induced phosphorylation
of ERK1/2 and PKB requires tyrosine kinase activity of insulin
receptor and c-Src. Antioxid Redox Signal. 7:1014–1020. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bou Daou G, Li Y and Anand-Srivastava MB:
Enhanced expression of Giα proteins contributes to the
hyperproliferation of vascular smooth muscle cells from
spontaneously hypertensive rats via MAP kinase- and PI3
kinase-independent pathways. Can J Physiol Pharmacol. 94:49–58.
2016. View Article : Google Scholar
|
|
40
|
Gomez Sandoval YH and Anand-Srivastava MB:
Enhanced levels of endogenous endothelin-1 contribute to the over
expression of Giα protein in vascular smooth muscle cells from SHR:
role of growth factor receptor activation. Cell Signal. 23:354–362.
2011. View Article : Google Scholar
|
|
41
|
Li Y, Lévesque LO and Anand-Srivastava MB:
Epidermal growth factor receptor transactivation by endogenous
vasoactive peptides contributes to hyperproliferation of vascular
smooth muscle cells of SHR. Am J Physiol Heart Circ Physiol.
299:H1959–H1967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu M, Zhu X, Zhang J, Liang J, Lin Y, Zhao
L, Ehrengruber MU and Chen YE: Egr-1 target genes in human
endothelial cells identified by microarray analysis. Gene.
315:33–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaufmann K and Thiel G: Epidermal growth
factor and platelet-derived growth factor induce expression of
Egr-1, a zinc finger transcription factor, in human malignant
glioma cells. J Neurol Sci. 189:83–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Houston P, Dickson MC, Ludbrook V, White
B, Schwachtgen JL, McVey JH, Mackman N, Reese JM, Gorman DG,
Campbell C and Braddock M: Fluid shear stress induction of the
tissue factor promoter in vitro and in vivo is mediated by Egr-1.
Arterioscler Thromb Vasc Biol. 19:281–289. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM,
Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ and
Stern DM: Hypoxia-associated induction of early growth response-1
gene expression. J Biol Chem. 274:15030–15040. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Youreva V, Kapakos G and Srivastava AK:
Insulin-like growth-factor-1-induced PKB signaling and Egr-1
expression is inhibited by curcumin in A-10 vascular smooth muscle
cells. Can J Physiol Pharmacol. 91:241–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Day FL, Rafty LA, Chesterman CN and
Khachigian LM: Angiotensin II (ATII)-inducible platelet-derived
growth factor A-chain gene expression is p42/44 extracellular
signal-regulated kinase-1/2 and Egr-1-dependent and mediated via
the ATII type 1 but not type 2 receptor. Induction by ATII
antagonized by nitric oxide. J Biol Chem. 274:23726–23733. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morimoto M, Kume N, Miyamoto S, Ueno Y,
Kataoka H, Minami M, Hayashida K, Hashimoto N and Kita T:
Lysophosphatidylcholine induces early growth response factor-1
expression and activates the core promoter of PDGF-A chain in
vascular endothelial cells. Arterioscler Thromb Vasc Biol.
21:771–776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iyoda T, Zhang F, Sun L, Hao F,
Schmitz-Peiffer C, Xu X and Cui MZ: Lysophosphatidic acid induces
early growth response-1 (Egr-1) protein expression via protein
kinase Cδ-regulated extracellular signal-regulated kinase (ERK) and
c-Jun N-terminal kinase (JNK) activation in vascular smooth muscle
cells. J Biol Chem. 287:22635–22642. 2012. View Article : Google Scholar : PubMed/NCBI
|