Introduction
Renal cell carcinoma (RCC) represents 2–3% of
malignant tumors in adults. The largest morbidity is noted in the
developed countries, the United States and the European Union
(1,2). Over the past two decades, the
incidence of RCC has steadily increased, prompting researchers to
point at a modern lifestyle and resulting diseases of affluence as
a possible cause. Genetic factors account for only 5% of RCC,
usually as a part of hereditary, such as von Hippel-Lindau
syndrome. The vast majority of renal carcinomas arises from a
sporadic mutation; however, smoking, obesity and hypertension are
linked to an increased risk for this malignancy (3). Histologically, three main types of
RCC have been distinguished: clear cell (80–90%), papillary
(10–15%) and chromophobe (4–5%). Surgical treatment is considered
the only curative approach. Systemic immunotherapy is offered to
patients excluded from radical surgery. Cytokines (interferon-α),
serine-threonine mTOR kinase inhibitors (temsirolimus, everolimus),
monoclonal anti-VEGF (bevacizumab) and tyrosine kinase inhibitors
(TKIs) (sunitinib, sorafenib, pazopanib, axitinib) are commonly
used agents (4). Kinases are
enzymes capable of binding a phosphate particle to other proteins,
which results in the activation or inhibition of the protein. There
are two types of kinases: serine/threonine-specific and
tyrosine-specific, named after the amino acids they interact with.
By inhibiting the kinases, we are able to interfere with a signal
transduction, which blocks cell proliferation. Over 20 TKIs are
currently available; however, all the mechanisms through which they
influence the human body have yet not been identified.
Diabetes mellitus (DM), a group of metabolic
diseases characterized by chronic hyperglycemia, concerns
approximatetly 5–7% of the adult population (5). Type 2 diabetes, associated with
obesity, accounts for approximatetly 90% of cases. The underlying
pathomechanism is insulin resistance caused by an excess of free
fatty acids and the increased production of pro-inflammatory
cytokines. Insulin resistance is initially compensated by the
overproduction of insulin by the pancreas. Gradually, however, a
degeneration of pancreatic β-cells takes place and diabetes
develops.
Over the years, it has become clear that there is a
link between RCC and diabetes, both in the incidence of the disease
and in the prognosis and treatment (6). There is also a growing body of
evidence indicating that immunotherapy used in metastatic RCC has
significant, possibly positive effects on blood glucose levels
(BGLs) in patients with diabetes. Our goal was to deliver a
comprehensive literature review on the subject and to raise
awareness of the phenomenon.
Data collection methods
We researched PubMed for studies concerning our
topic of interest. Retrospective and prospective studies were
included, both on humans and on animals. All articles had at least
an abstract written in English; translation services were used for
further content.
Results of the literature review
Comorbidity: RCC and DM
The first study confirming the bond between diabetes
and RCC was published by Lindblad et al in 1999 (7). Their retrospective study analyzed
153,852 Swedish patients with diabetes. The estimated incidence of
RCC for this group should have been 182.4 cases; however, there
were 267 cases. Clearly, diabetic patients had an increased
incidence of RCC. The statistical terms used to assess this result
are the standardized incidence ratio (SIR) and the standardized
mortality ratio (SMR). SIR describes the ratio of the observed
cases (incidence) to the expected cases (estimated prevalence),
while the SMR describes the ratio of the actual number of deaths to
the number expected. These two indicators prove that there is a
correlation between RCC and DM in both males and females. SIR has
been calculated for incidence in the female population (SIR, 1.7;
95% CI, 1.4–2.0) and the male population (SIR, 1.3; 95% CI,
1.1–1.6), and SMR has been calculated for mortality among women
(SMR, 1.9; 95% CI, 1.7–2.2) and men (SMR, 1.7; 95% CI,
1.4–1.9).
In the current literature, the percentage of
patients with DM and with kidney cancer ranges from 9.1% in the
Italian population (8) to as high
as 25.4% among residents of Texas in the United States (9). In females, this association is more
pronounced (31.4% comorbidity in women and 20.8% in men; p=0.01)
(9). Many studies have proven
that diabetes is an independent risk factor for RCC (7,13–17). In a previous study, the gazard
ratio (HR) or relative risk (RR), which served as an indicator,
reached 1,161 (whereas the confidence indicator was 95% CI,
1.19–2.18) (10). In another
study, this rate was HR, 1.92 (95% CI, 1.06–3.46) (11). Patients with diabetes who
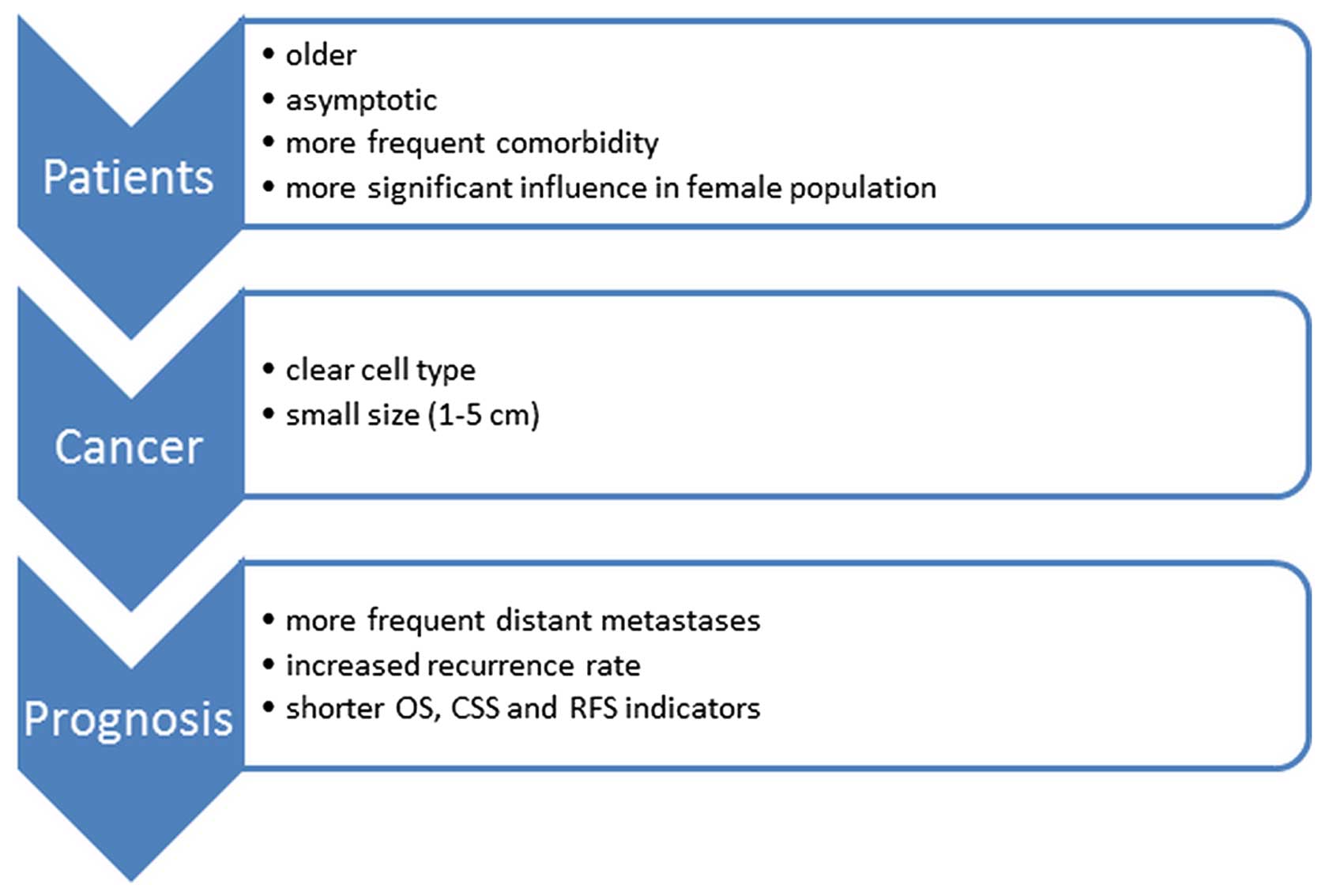
developed kidney cancer were older than patients without DM
(12,13) and more likely to have an
asymptomatic course (13). The
main subtype was clear cell carcinoma (9,12),
with a usually small tumor size (1–5 cm) (9). These patients were also more often
afflicted with multiple diseases (13).
The prognosis for patients who developed both
diseases was also investigated. A previous study on the Turkish
population found, using Kaplan-Meier analysis, that there were
fewer 5-year survivals in the DM group (62.9 vs. 77.7%), p=0.1
(12). Opinions on the impact of
diabetes on mortality in RCC differ. There are sources that suggest
a total lack of effect of DM on mortality (13), whereas other sources report an
increased mortality in these patients (7). In another study, multivariate
analysis on Japanese patients indicated the impact of diabetes on
shorter survival rates, both non-cancer-related survival rates
(survival unrelated to cancer) (HR, 2.22; 95% CI, 1.06–4.64) as
well as overall survival (OS) rates (HR, 1.88; 95% CI, 1.09–13.23)
(14). The study by Vavallo et
al reported a shorter OS, an increased risk of recurrence and a
higher mortality risk associated with kidney cancer [cause-specific
survival (CSS)] (15). The
meta-analysis by Chen et al suggested that DM was
significantly associated with poor OS, CSS and RFS in patients with
RCC (16).
The potential association of pre-existing diabetes
and pre-operative HbA1c with outcomes was tested by Lee et
al. Their multivariate analyses showed that diabetes was an
independent predictor of disease progression (HR, 1.766; p=0.002),
all-cause mortality (OR, 1.825; p=0.001) and cancer-specific
mortality (HR, 2.266; p=0.001). Pre-operative high HbA1c predicted
post-operative disease progression (HR, 2.221; p=0.023) (17). Diabetes also increases the risk of
malignancy recurrence. Fukushima et al proved a significant
difference in the 5-year relapse-free survival between patients
with and without diabetes (75.3 vs. 91.9%, p<0.001) (18). All these data indicate that
diabetes not only affects the incidence of RCC, but also
deteriorates the prognosis of patients (Fig. 1).
Obesity as a factor leading to diabetes
and its role in carcinogenesis
Obesity is the most important risk factor leading to
DM type 2. The greatest risk for a patient is central obesity,
which is characterized by an increased amount of visceral fat. Its
adipocytes secrete substances responsible for insulin resistance,
e.g., free fatty acids.
The question of obesity arises in the incidence of
kidney cancer. Studies have proven the link between excess body
weight and an increased risk of breast cancer in post-menopausal
women, endometrial and colon cancer, adenocarcinoma of the
esophagus and even pancreatic cancer (19,20). The role of excessive body mass in
RCC was confirmed by a study on the population of the United
States, where the RR rate was 1.5 (BMI, 25–30 kg/m2)
compared to 2.5 in obese subjects (BMI>30 kg/m2)
(19). An increased risk
resulting from a greater BMI was more pronounced in women. It is
important to emphasize that obesity is a risk factor independent of
blood pressure, which suggests a different mechanism of action.
Obese subjects are more likely to develop clear cell subtype RCC
than subjects with a normal BMI (OR, 1.48; 95% CI, 1.19–1.84)
(21,22). However, there has also been a
study on the Japanese population which demonstrated that BMI did
not play a statistically significant role (p=0.991) in the
recurrence of RCC (18).
Paraneoplastic syndromes as other
manifestations of diabetes in RCC
Diabetes can be a rare paraneoplastic syndrome. Two
such cases have been reported. The first patient, described in
1999, was a 35-year-old man, suffering from diabetes for 12 years.
He was admitted to the hospital due to a sudden loss of glycemic
control. The insulin demand increased from 80 units s.c. to 600
units i.v. What is more, during a physical examination, he
complained of a pain on the right side, and his blood test revealed
increased levels of C-reactive protein (CRP). Hematuria was
discovered during a urine test. A subsequently ordered CT scan
revealed a bilateral renal tumor. Following a histopathological
examination, he was diagnosed with bilateral papillary carcinoma.
Following a radical left-side nephrectomy and nephron-sparing
surgery (NSS) on the right side, the control of diabetes returned
to normal (22).
The second case was a 64-year-old patient with a
history of nephrectomy due to RCC, clear cell type. Nine years
after surgery, the man was admitted to the hospital with a 2-week
history of polydipsia, polyuria and weight loss. Blood tests
revealed the following levels: glucose, 662 mg%; HbA1c, 11.5%; and
anti-glutamic acid decarboxylase (GAD) 28,680 U/l (normal <9.5
U/l). The abdominal CT scan revealed progression; sunitinib
treatment was ordered. Within 9 months, the dosage of insulin had
been systematically reduced and finally discontinued. This state
lasted at least 7 months, counting from the date of appearance.
After 15 months of follow-up, the levels of anti-GAD were still
elevated (23).
Treatment with TKIs in metastatic RCC and
its influence on diabetic disorders
In a study conducted on mice, Louvet et al
achieved the remission of type 1 diabetes in NOD mice 1 week after
the initiation of therapy (24).
Two years later, Agostino et al studied the average BGLs of
80 patients treated with TKIs, including 30 treated with sunitinib
and 23 with sorafenib. Both groups (DM and non-DM) experienced a
decrease in the average glucose level of 14 and 15 mg/dl for
sunitinib and 12 mg/dl for sorafenib, respectively. Discontinuation
of the treatment resulted in an increase of BGLs to the previous
values associated with DM. In total, 47% of the respondents were
able to stop taking their anti-glycemic agents during treatment
with TKIs (25).
Improvement in glycemic control via oral
anti-diabetic drugs was also described by Billemont et al.
Their report analyzed the impact of sunitinib therapy on 28
patients with metastatic RCC (19 patients with DM). BGL assessment
during the 4-week therapy showed that all DM patients experienced a
significant, yet reversible, reduction in blood glucose (average of
1.77 mmol/l). Two patients discontinued taking the oral
anti-glycemic agents entirely, and five achieved BGL results
defined as normal until the end of the therapy. This result was not
repeated in healthy patients, and blood glucose reduction was not
statistically significant (0.17 mmol/l) (26).
Improvement in glycemic control also occurred during
the administration of pazopanib. A 73-year-old patient stopped
taking glibenclamide due to an average drop in self-measured blood
glucose of 5 mmol/l and HbA1C reduction from 10.9 to 7%. Weight and
kidney functions were unaltered (27). A life-threatening hypoglycemia
during sunitinib administration was described by Lee et al.
In this case, the patient had been diagnosed with diabetes,
although the episode could only be explained as a side-effect of
kinase inhibitors (28).
The latest reports provide further evidence of the
impact of TKIs on the glycemic state. Research carried out by Oh
et al (29) revealed a
significant decrease in the mean BGL in patients with diabetes. The
average score before treatment was 185.2±52.8 and 76.1±29.0 mg/dl 4
weeks after treatment. After such significant changes in blood test
results, 40% of the patients changed their oral dose of
hypoglycemic medication or even discontinued its use. In contrary
to results of the study by Agostino et al (25), patients without diabetes
demonstrated only a slight downward trend in the level of glucose.
Moreover, in the whole group of 48 patients, no significant changes
in BMI were noted (29). In
another case, among 10 patients on sorafenib therapy, four required
a reduction of insulin dose (30).
Multiple case reports showing a reduction of insulin
dose in patients treated with TKI have been published. A case
report from 2014 described a woman with type 1 DM who underwent a
pancreoduodenectomy due to a neuroendocrine tumor of the pancreas.
She was administered sunitinib after metastases were discovered.
Within 3 months of the treatment, the patient's need for insulin
gradually diminished. This state lasted until her death due to
sepsis following pneumonia 3 months later. It is worth noting that
the woman was suffering from type 1 DM for 40 years (31). A similar case was described in
Korea: a 57-year-old man with DM type 2 treated with metformin and
glimepiride was admitted to the hospital due to a decreased level
of consciousness. He was diagnosed with severe hypoglycemia and
metabolic encephalopathy. No changes in physical activity, dietary
habits or present infections were noted; however, he recently began
taking sunitinib (50 mg/day) due to metastatic RCC (23).
Tyrrell and Pwint analyzed a case of a 61-year-old
man with type 2 DM who, prior to sunitinib treatment, had erratic
blood sugars and HbA1c ranging from 55 to 79 mmol/mol in a recent
year. Four months after sunitinib, his HbA1c was down to 49
mmol/mol and his insulin dose had to be reduced (32). After the sunitinib dose was
reduced, his blood sugars rose slightly (from 43–48 to 52
mmol/mol), and this trend continued with every sunitinib dose
reduction until it was stopped entirely, at which point his HbA1c
rose to 68 mmol/mol. A similar phenomenon occurred after axitinib
was administered, although the follow-up period was shorter due to
the patient's death.
Unfortunately, TKIs may also cause hypoglycemia. A
study recently published by Polish researchers investigated the
influence of sunitinib on BGLs in rabbits. In animals with DM,
examined between 6 and 12 h after the sunitinib administration, the
decrease in glucose levels ranged between 14.4 and 69.6%, while
healthy rabbits responded with a 15.4 to 33.6% glucose drop
(33).
Severe hypoglycemia in a patient with hepatocellular
carcinoma due to a possible interaction between glibenclamide and
the first dose of sorafenib has been described by Holstein et
al (34). As no hepatic or
renal dysfunction was present, this effect was attributed to
sorafenib-induced inhibition of the metabolic pathway of
glibenclamide.
Another case of sunitinib inducing severe
hypoglycemia was reported by Demirci et al (35). A 60-year-old man with type 2 DM
treated with glimepiride was started on sunitinib, and 2 weeks
afterwards he had a hypoglycemic episode not linked to drug
overdose or a change in dietary habits. Five days later, the
situation repeated itself, and this time the patient required an
intravenous infusion of dextrose. Over the next 2 weeks,
hypoglycemia appeared less frequently, and he was discharged, but
did not need to continue taking glimepiride during 6 months of
follow-up.
A case of recurring episodes of severe,
life-threatening hypoglycemia 3 months after the initiation of
sunitinib was described by Fountas et al (36). Initially, milder episodes were
corrected by an increased calorie intake; however, soon the patient
required intravenous dextrose administration. Laboratory tests
revealed increased plasma insulin and C-peptide levels. Sunitinib
was discontinued, and there was a gradual improvement in both the
frequency and severity of hypoglycemic episodes until their
disappearance 1 month later.
An important study on humans has recently been
published. Thijs et al (37) recruited 10 patients with
metastatic RCC with an indication to start sunitinib. One patient
had diabetes controlled with metformin. In the week before the
treatment and 1 week after its commencement, a 120-min
hyperinsulinemic euglycemic clamp was performed. The obtained mean
plasma insulin concentrations were significantly higher 1 week
after the sunitinib administration. As several studies (38,39) have shown that the pharmacokinetics
of insulin aspart are not affected by an impaired renal function,
it was decided that a rise in creatinine levels in patients did not
play a significant role in the achieved result, the conclusion
being that sunitinib reduces insulin clearance.
Less obvious results emerge when we analyze the
number of hypo- and hyperglycemic episodes in the full population
of patients administered sunitinib or pazopanib, making no
distinction between those with DM or without it. According to
survey information provided by Pfizer, 19% of respondents reported
hypoglycemic events, whereas hyperglycemia appeared in 15%
(40). Guevremont et al
rated elevated BGLs, which occur in 15% of patients, among the
toxic side-effects of therapy sunitinib (41). In addition, for pazopanib, cases
of both hyper- and hypoglycemia have been reported in patients with
metastatic RCC (42).
Molecular background for diabetes, RCC
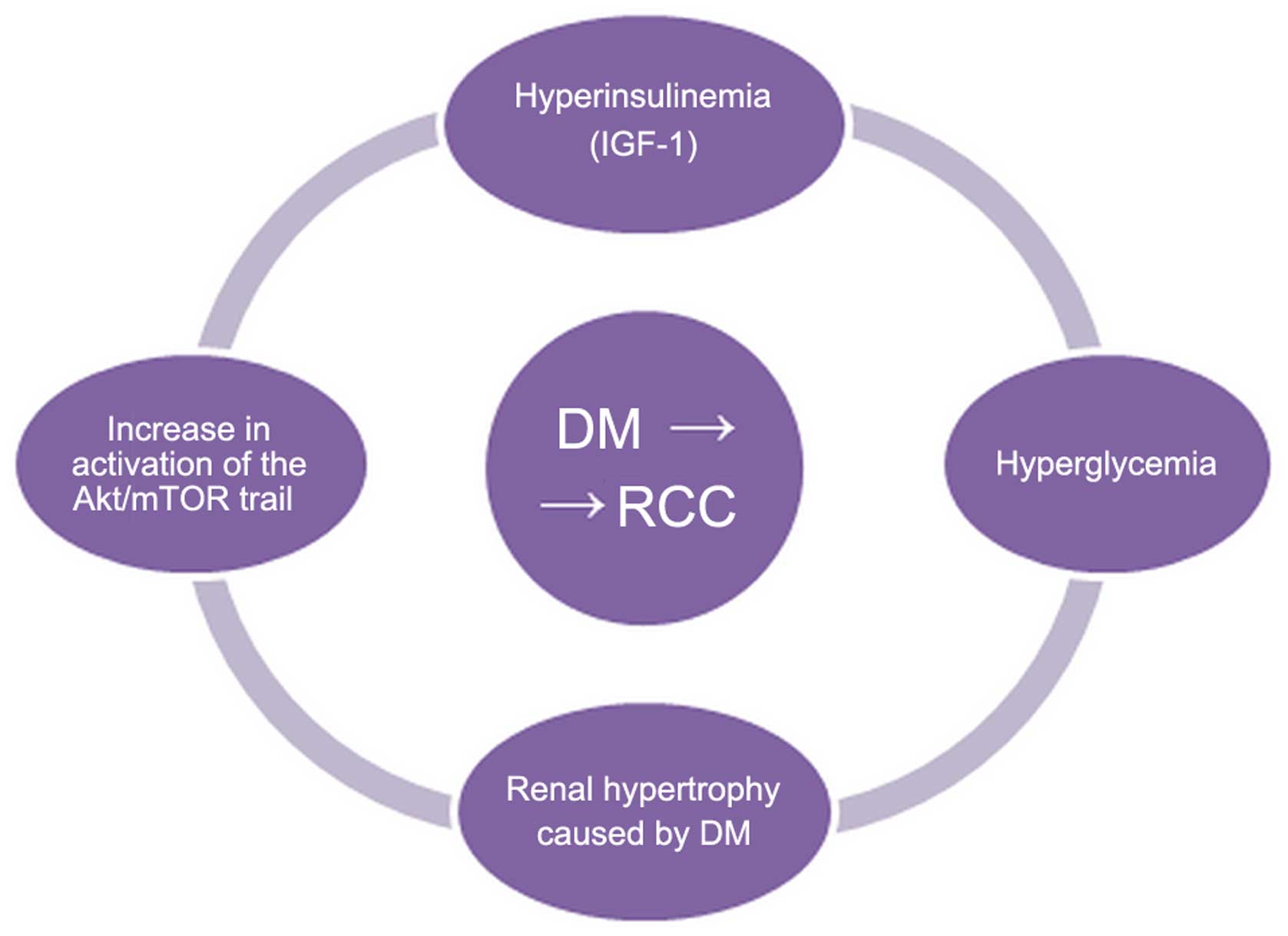
and TKI interactions Risk factors
There are many pathways which, when activated by
diabetes, can contribute to the development of RCC. The most
frequently mentioned carcinogenic factors are the following.
i) Hyperglycemia
According to the Warburg hypothesis, cancer cells
prefer glycolysis over the respiratory chain in the mitochondria.
In order to maintain a proper metabolism, they need an increased
supply of glucose. Hyperglycemia in a diabetic can stimulate the
metabolism of tumor cells. Hyperglycemia also stimulates tumor cell
proliferation by increased levels of protein kinase C (PKC) and
peroxisome proliferator-activated receptors (PPARs), which can
accelerate cellular metabolism and induce proliferation (43).
ii) Hyperactivation of the protein
kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway
Akt regulates the majority of the cellular pathways,
including those responsible for renal carcinogenesis. In a study
examining cancer cells from patients suffering only from RCC and
from those with both RCC and diabetes, a considerably higher
activity in the second group was noted (44). Tuberin is a protein released
during acute renal failure. It inhibits proliferation induced by
reproductive processes. Akt kinase is responsible for the
phosphorylation or the inactivation of tuberin, which activates a
pathway responsible for growth, proliferation and cell traffic
regulation (mTOR). The activation of mTOR kinase causes the
phosphorylation of p70S6K protein. Increased concentrations of
phosphorylated p70S6K were detected in patients with both DM and
RCC and in patients with only DM (44). It can thus be concluded that
patients with diabetes have an increased activity of Akt, which
causes a reduction in the tuberin concentration and the increased
activity of mTOR. All these processes affect the stimulation of the
proliferation of kidney cells.
iii) Hyperinsulinemia and the
insulin-like growth factor (IGF) family
Insulin promotes the synthesis and activity of
IGF-1. Both insulin and IGF-1 stimulate proliferation and inhibit
apoptosis. IGF acts through the IGF-1 receptor. It is a
transmembrane protein of tyrosine kinase activity. Its activity is
required for the transmission of a signal, which occurs through the
activation of phosphatidylinositol 3-kinase (PI3K) and
mitogen-activated protein kinase (MAPK) (45). It is also present in tumor cells,
including RCC (46). In these
cells, particularly those with increased proliferation, IGF-1R is
often located in the nuclear membrane, which enables a direct
transcription regulation (47).
The significance of this phenomenon is disputable. Aleksic et
al suggested that cells with IGF-1R located in the nuclear
membrane have a higher proliferation rate and are associated with a
poorer prognosis (47). However,
in the study by Lkhagvadorj et al, an increased expression
of IGF-1R was demonstrated in RCC of a lower malignancy grade
(Fuhrman grading scale); this result did not apply to cancers with
higher grade tumors (48). The
study by Rasmuson et al showed that increased levels of
IGF-1 were associated with a better prognosis (p=0.017) (49).
The IGF family also includes IGF-binding proteins
(IGFBPs). According to the 'insulin-neoplasia' hypothesis, chronic
hyperinsulinemia reduces the levels of IGFBP-1 and -2, which
results in an increased bioavailability of IGF-1 (20). The most interesting binding
protein is IGFBP-3. IGF-1 has a higher affinity to it than to its
receptor, so that IGFBP-3 is able to reduce bioavailability and,
indirectly, the activity of the growth factor. Reduced levels of
IGFBP-3 are associated with a higher risk of cancer in many organs,
as well as with a poorer prognosis (50) (Fig.
2).
Influence of tyrosine kinase inhibition
on cells and tissues
The mechanisms through which receptor TKIs affect
the metabolism of glucose have not yet been fully elucidated. There
are several mechanisms through which TKIs influence glucose
metabolism:
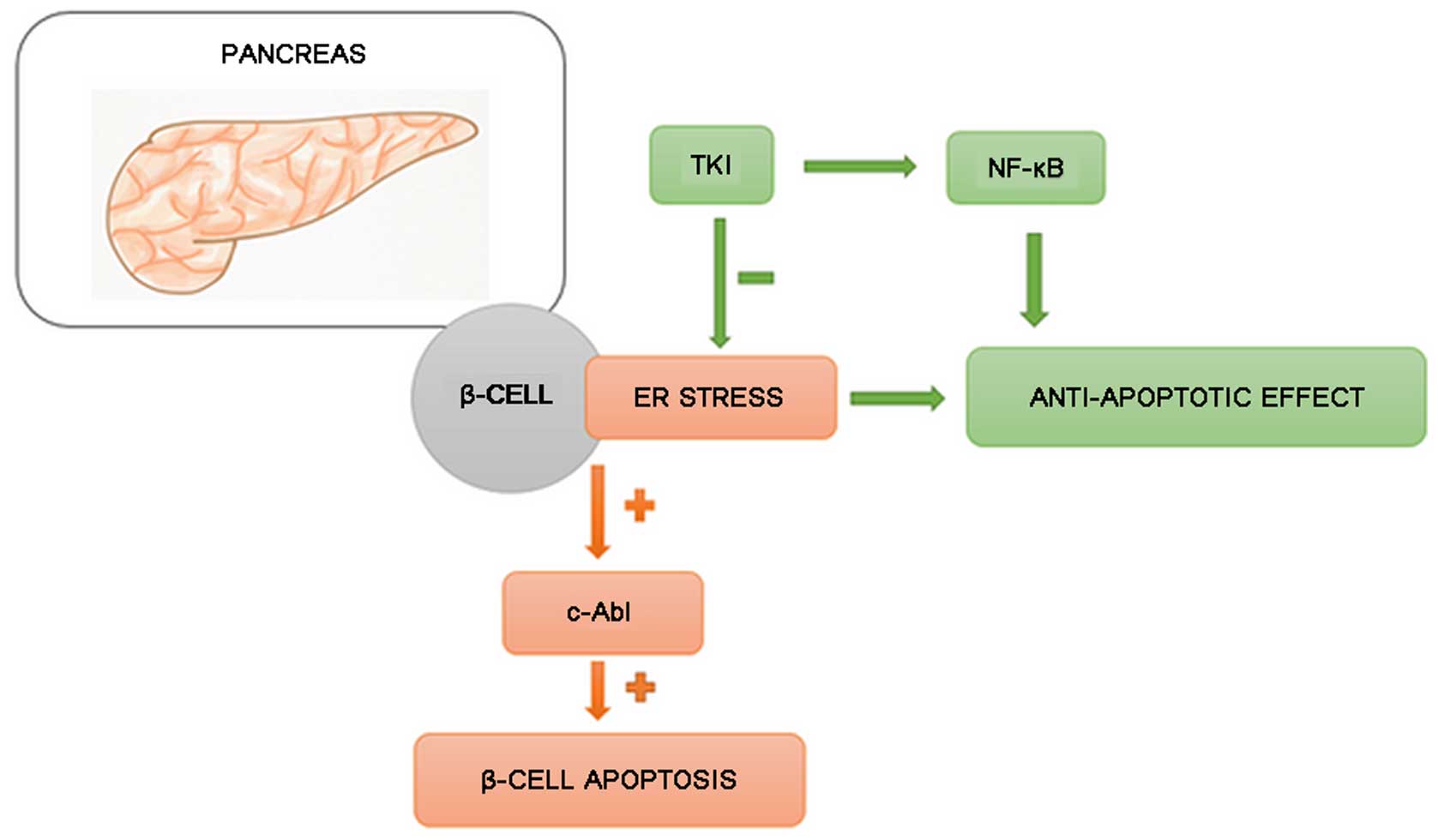
i) Inhibition of β-cell damage and
increased insulin production
Hägerkvist et al hypothesized that imatinib
may help to preserve β-cell mass. Under conditions of stress,
Abelson tyrosine kinase (c-Abl) protein [present in large
quantities in the endoplasmic reticulum (ER)] induces the apoptosis
of β-cells. The protection of β-cells can be achieved by silencing
c-Abl protein with small interfering RNA (siRNA). TKIs inhibit
c-Abl and c-Jun N-terminal kinase (JNK), and create a state similar
to ischemic preconditioning, resulting in increased nuclear factor
(NF)-κB production (51).
Mokhtari et al (52) proved that imatinib activates
NF-κB, which has an anti-apoptotic effect. Additionally, a decrease
in the sensitivity of pancreatic cells to cytokines was noted. This
could reduce the amount of amyloid formation, alleviating its
unfavorable effect on pancreatic islets' performance (52,53). Ono et al (54) suggested that TKIs may increase
insulin production by acting against an SRC-kinase family
responsible for the reduction of insulin secretion.
ii) Influence on adipose tissue
macrophages
It has been proven that excessive adipose tissue is
a location of a mild but chronic inflammation, which causes an
increase in systemic insulin resistance (55). Up to 40% of the cells infiltrating
the adipose tissue in obese mice and humans are macrophages
(56). They can be divided into
two subpopulations: M1 and M2 macrophages. M1 macrophages secrete
cytokines, such as tumor necrosis factor (TNF)α, interleukin (IL)-6
and inducible nitric oxide synthase (iNOS); M2 macrophages reduce
inflammation by producing IL-4 and IL-10 (57). In obese subjects, the balance
between these two groups is shifted toward the M1 phenotype
(58). Prada et al
demonstrated that the administration of TKIs decreased the amount
of circulating IL-6, TNFα and iNOS, which suggested that the drug
had shifted the balance between macrophage subpopulations from M1
to M2 (59) (Fig. 3).
iii) Influence on insulin sensitivity
and ER stress
It has also been suggested that imatinib improves
insulin sensitivity (60). This
could be due to a decrease in ER stress. In the study by Han et
al, imatinib was administered to mice with DM type 2 for 4
weeks. Afterwards, an improvement in fasting glucose levels and
glucose tolerance was noted. The levels of markers of ER stress,
such as phospho-ERK or phospho-eIF2-α decreased. The experiment was
repeated on liver cancer cells, yielding a similar result. Reduced
ER stress was also accompanied by a decrease in JNK activity and
insulin receptor substrate-1 (IRS-1) phosphorylation (61). Hägerkvist et al
demonstrated that the administration of a TKI (PD153035 or
imatinib) reduced the phosphorylation of an insulin receptor
associated with insulin resistance (60). However, in the study conducted by
Thijs et al, this effect was not confirmed (37) (Fig.
4).
Discussion
Multiple studies claim that diabetes is an
independent risk factor for non-hereditary kidney cancer
development and its accelerated progression (7–9).
The co-occurrence of both diseases is more common in elderly
patients and in women. Patients affected often have an asymptotic
course and more comorbidities (10–13). Kidney cancer in diabetic patients
is usually of the clear cell subtype with a small tumor size (1–5
cm) (14). The prognosis is worse
than for patients suffering from kidney cancer alone. This is due
to a higher rate of recurrence and a greater number of distant
metastases. All these factors contribute to a reduction in survival
rates, both OS and CSS. Diabetes can also occur as a paraneoplastic
syndrome, thus contributing to an early diagnosis of RCC (15–24).
Reasons for severe hypoglycemia episodes and
alterations in BGLs in patients treated with TKIs remain
disputable. The first and most obvious explanation that comes to
mind is that body mass and dietary habits change due to the disease
itself. Weight loss could contribute to a decrease in insulin
resistance. However, in the study by Oh et al (29), BMI reduction was very
insignificant, ranging from 0.68 in diabetic to 0.25 in
non-diabetic patients. Other authors, when struggling to explain
numerous hypoglycemic effects, exclude all likely causes, such as
drug overdose, changes in dietary habits, insulin-producing tumors
or effects of corticosteroids. Moderate (grade 2) anorexia related
to sunitinib treatment has been reported in 6% of patients;
however, severe or life-threatening (grades 3 and 4) effects were
not present at all (62).
Sorafenib resulted in a decreased appetite in only 2.9% of patients
in a large international study (63). In a study published in 2009,
Mokhtari and Welsh suggested that trials should be conducted to
verify the following hypotheses: i) TKIs prevent the destruction of
β-cells in patients with recently developed type 1 diabetes; ii)
TKIs inhibit inflammatory and autoimmune processes in pre-diabetic
patients so that the precipitation of type 1 diabetes is
delayed/prevented; and iii) TKIs improve β-cell function and
insulin sensitivity in late decompensated stages of DM type 2 so
that insulin therapy is no longer necessary (64). The authors of this study are aware
that carrying out such trials on real-life patients can be
ethically disturbing; therefore, we emphasize that large-scale
animal studies should precede those on humans. Confirmation of the
inhibitory effects of TKIs on the development of diabetes may
result in the discovery of novel treatment methods for DM, and
disproving this hypothesis would finally confirm the safety of TKIs
for diabetic patients in oncological treatment.
In conclusion, the potential pathomechanisms leading
to the development of kidney cancer in patients with DM remain
unexplained. The most important are probably the following: chronic
hyperglycemia, renal hypertrophy, Akt/mTOR trail hyperactivation
and hyperinsulinemia. The role of insulin and the IGF-1 family is
perhaps the most promising object of future investigation. The
stimulation of cell proliferation by insulin and IGF-1 and the
direct impact on transcription by the presence of IGF-1R in the
cell membrane suggest that this agent is of the greatest importance
in the development of RCC.
In this analysis, we wanted to draw attention to the
cellular pathways leading to carcinogenesis and to potential
targets for novel anticancer therapies, such as blocking the
activity of IGF-1R or increasing the activity of IGFBP-3. As for
the hypoglycemic effects of TKIs, we are aware that retrospective
analysis on small groups of patients and relatively few animal
studies are not sufficient to draw general conclusions. However,
with the growing incidence of both diabetes and various oncological
conditions in developed countries, we believe it is necessary to
expand our knowledge on the pleiotropic impact of TKIs on the human
body.
Acknowledgments
This study was supported by the National Science
Centre (NCN) grant no. UMO-2012/05/D/NZ5/01844 and WIM intramural
grant no. 1/8863 (355).
References
|
1
|
European Network of Cancer Registries.
Eurocim version 4.0: European incidence database V2.3, 730 entity
dictionary. Lyon: 2001
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chow WH, Dong LM and Devesa S:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adeghate E, Schattner P and Dunn E: An
update on the etiology and epidemiology of diabetes mellitus. Ann
NY Acad Sci. 1084:1–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moyad MA: Review of potential risk factors
for kidney (renal cell) cancer. Semin Urol Oncol. 19:280–293.
2001.
|
|
7
|
Lindblad P, Chow WH, Chan J, Bergström A,
Wolk A, Gridley G, McLaughlin JK, Nyrén O and Adami HO: The role of
diabetes mellitus in the aetiology of renal cell cancer.
Diabetologia. 42:107–112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zucchetto A, Dal Maso L, Tavani A,
Montella M, Ramazzotti V, Talamini R, Canzonieri V, Garbeglio A,
Negri E, Franceschi S and La Vecchia C: History of treated
hypertension and diabetes mellitus and risk of renal cell cancer.
Ann Oncol. 18:596–600. 2007. View Article : Google Scholar
|
|
9
|
Habib SL, Prihoda TJ, Luna M and Werner
SA: Diabetes and risk of renal cell carcinoma. J Cancer. 3:42–48.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joh HK, Willett WC and Cho E: Type 2
diabetes and the risk of renal cell cancer in women. Diabetes Care.
34:1552–1556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inoue M, Iwasaki M, Otani T, Sasazuki S,
Noda M and Tsugane S: Diabetes mellitus and the risk of cancer:
Results from a large-scale population-based cohort study in Japan.
Arch Intern Med. 166:1871–1877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Süer E, Oztürk E, Gülpınar O, Kayış A and
Baltacı S: Effect of type 2 diabetes mellitus on prognosis of
nonmetastatic renal cell cancer. Korean J Urol. 54:499–503. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antonelli A, Arrighi N, Corti S, Zanotelli
T, Cozzoli A, Cosciani Cunico S and Simeone C: Pre-existing type-2
diabetes is not an adverse prognostic factor in patients with renal
cell carcinoma: A single-center retrospective study. Urol Oncol.
31:1310–1315. 2013. View Article : Google Scholar
|
|
14
|
Lee S, Hong SK, Kwak C, Kim HH and Lee SE:
Prognostic significance of diabetes mellitus in localized renal
cell carcinoma. Jpn J Clin Oncol. 42:318–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vavallo A, Simone S, Lucarelli G,
Rutigliano M, Galleggiante V, Grandaliano G, Gesualdo L, Campagna
M, Cariello M, Ranieri E, et al: Pre-existing type 2 diabetes
mellitus is an independent risk factor for mortality and
progression in patients with renal cell carcinoma. Medicine
(Baltimore). 93:e1832014. View Article : Google Scholar
|
|
16
|
Chen L, Li H, Gu L, Ma X, Li X, Gao Y,
Zhang Y, Shen D, Fan Y, Wang B, et al: The impact of diabetes
mellitus on renal cell carcinoma prognosis: A meta-analysis of
cohort studies. Medicine (Baltimore). 94:e10552015. View Article : Google Scholar
|
|
17
|
Lee H, Kwak C, Kim HH, Byun SS, Lee SE and
Hong SK: Diabetes mellitus as an independent predictor of survival
of patients surgically treated for renal cell carcinoma: A
propensity score matching study. J Urol. 194:1554–1560. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukushima H, Masuda H, Yokoyama M,
Tatokoro M, Yoshida S, Ishioka J, Matsuoka Y, Numao N, Koga F,
Saito K, et al: Diabetes mellitus with obesity is a predictor of
recurrence in patients with non-metastatic renal cell carcinoma.
Jpn J Clin Oncol. 43:740–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Renehan AG, Roberts DL and Dive C: Obesity
and cancer: Pathophysiological and biological mechanisms. Arch
Physiol Biochem. 114:71–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lowrance WT, Thompson RH, Yee DS, Kaag M,
Donat SM and Russo P: Obesity is associated with a higher risk of
clear-cell renal cell carcinoma than with other histologies. BJU
Int. 105:16–20. 2010. View Article : Google Scholar :
|
|
22
|
Callewaert PR, Van Poppel H,
Vanderschueren D and Baert L: Uncontrollable diabetes mellitus: A
rare paraneoplastic manifestation of renal cell carcinoma. Nephrol
Dial Transplant. 14:2263–2264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho M, Shim H and Park MR: Hypoglycemic
coma in a patient with metastatic renal cell carcinoma treated with
sunitinib. Korean J Med. 87:501–504. 2014. View Article : Google Scholar
|
|
24
|
Louvet C, Szot GL, Lang J, Lee MR,
Martinier N, Bollag G, Zhu S, Weiss A and Bluestone JA: Tyrosine
kinase inhibitors reverse type 1 diabetes in nonobese diabetic
mice. Proc Natl Acad Sci USA. 105:18895–18900. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agostino NM, Chinchilli VM, Lynch CJ,
Koszyk-Szewczyk A, Gingrich R, Sivik J and Drabick JJ: Effect of
the tyrosine kinase inhibitors (sunitinib, sorafenib, dasatinib,
and imatinib) on blood glucose levels in diabetic and nondiabetic
patients in general clinical practice. J Oncol Pharm Pract.
17:197–202. 2011. View Article : Google Scholar
|
|
26
|
Billemont B, Medioni J, Taillade L, Helley
D, Meric JB, Rixe O and Oudard S: Blood glucose levels in patients
with metastatic renal cell carcinoma treated with sunitinib. Br J
Cancer. 99:1380–1382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Böhm S, Hess D, Gillessen S and Brändle M:
Improved glycemic control with the multi-receptor tyrosine kinase
inhibitor pazopanib. Diabetes Care. 33:e822010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee Y, Jung HS, Choi HJ, Kim MJ, Kim TM,
Park KS and Kim SY: Life-threatening hypoglycemia induced by a
tyrosine kinase inhibitor in a patient with neuroendocrine tumor: A
case report. Diabetes Res Clin Pract. 93:e68–e70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oh JJ, Hong SK, Joo YM, Lee BK, Min SH,
Lee S, Byun SS and Lee SE: Impact of sunitinib treatment on blood
glucose levels in patients with metastatic renal cell carcinoma.
Jpn J Clin Oncol. 42:314–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imarisio I, Paglino C, Ganini C, Magnani
L, Caccialanza R and Porta C: The effect of sorafenib treatment on
the diabetic status of patients with renal cell or hepatocellular
carcinoma. Future Oncol. 8:1051–1057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huda MSB, Amiel SA, Ross P and Aylwin SJ:
Tyrosine kinase inhibitor sunitinib allows insulin independence in
long-standing type 1 diabetes. Diabetes Care. 37:e87–e88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tyrrell HE and Pwint T: Sunitinib and
improved diabetes control. BMJ Case Rep. 2014:2014.PubMed/NCBI
|
|
33
|
Szałek E, Karbownik A, Sobańska K,
Grabowski T, Połom W, Lewandowska M, Wolc A, Matuszewski M and
Grześkowiak E: The pharmacokinetics and hypoglycaemic effect of
sunitinib in the diabetic rabbits. Pharmacol Rep. 66:892–896. 2014.
View Article : Google Scholar
|
|
34
|
Holstein A, Kovacs P and Beil W: Severe
hypoglycemia due to possible interaction between glibenclamide and
sorafenib in a patient with hepatocellular carcinoma. Curr Drug
Saf. 8:148–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Demirci A, Bal O, Durnali A, Ekinci AŞ,
Eşbah O, Alkiş N and Oksüzoğlu B: Sunitinib-induced severe
hypoglycemia in a diabetic patient. J Oncol Pharm Pract.
20:469–472. 2014. View Article : Google Scholar
|
|
36
|
Fountas A, Tigas S, Giotaki Z, Petrakis D,
Pentheroudakis G and Tsatsoulis A: Severe resistant hypoglycemia in
a patient with a pancreatic neuroendocrine tumor on sunitinib
treatment. Hormones (Athens). 14:438–441. 2015.
|
|
37
|
Thijs AM, Tack CJ, van der Graaf WT,
Rongen GA and van Herpen CM: The early effect of sunitinib on
insulin clearance in patients with metastatic renal cell carcinoma.
Br J Clin Pharmacol. 81:768–772. 2016. View Article : Google Scholar
|
|
38
|
Holmes G, Galitz L, Hu P and Lyness W:
Pharmacokinetics of insulin aspart in obesity, renal impairment or
hepatic impairment. Br J Clin Pharmacol. 60:469–476. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kulozik F and Hasslacher C: Insulin
requirements in patients with diabetes and declining kidney
function: differences between insulin analogues and human insulin?
Ther Adv Endocrinol Metab. 4:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lodish MB and Stratakis CA: Endocrine side
effects of broad-acting kinase inhibitors. Endocr Relat Cancer.
17:R233–R244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guevremont C, Alasker A and Karakiewicz
PI: Management of sorafenib, sunitinib, and temsirolimus toxicity
in metastatic renal cell carcinoma. Curr Opin Support Palliat Care.
3:170–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ryu TY, Park J and Scherer PE:
Hyperglycemia as a risk factor for cancer progression. Diabetes
Metab J. 38:330–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Habib SL and Liang S: Hyperactivation of
Akt/mTOR and deficiency in tuberin increased the oxidative DNA
damage in kidney cancer patients with diabetes. Oncotarget.
5:2542–2550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chitnis MM, Yuen JS, Protheroe AS, Pollak
M and Macaulay VM: The type 1 insulin-like growth factor receptor
pathway. Clin Cancer Res. 14:6364–6370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ouban A, Muraca P, Yeatman T and Coppola
D: Expression and distribution of insulin-like growth factor-1
receptor in human carcinomas. Hum Pathol. 34:803–808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aleksic T, Chitnis MM, Perestenko OV, Gao
S, Thomas PH, Turner GD, Protheroe AS, Howarth M and Macaulay VM:
Type 1 insulin-like growth factor receptor translocates to the
nucleus of human tumor cells. Cancer Res. 70:6412–6419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lkhagvadorj S, Oh SS, Lee MR, Jung JH,
Chung HC, Cha SK and Eom M: Insulin receptor expression in clear
cell renal cell carcinoma and its relation to prognosis. Yonsei Med
J. 55:861–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rasmuson T, Grankvist K, Jacobsen J,
Olsson T and Ljungberg B: Serum insulin-like growth factor-1 is an
independent predictor of prognosis in patients with renal cell
carcinoma. Acta Oncol. 43:744–748. 2004. View Article : Google Scholar
|
|
50
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
Overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar
|
|
51
|
Hägerkvist R, Makeeva N, Elliman S and
Welsh N: Imatinib mesylate (Gleevec) protects against
streptozotocin-induced diabetes and islet cell death in vitro. Cell
Biol Int. 30:1013–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mokhtari D, Li T, Lu T and Welsh N:
Effects of Imatinib Mesylate (Gleevec) on human islet NF-kappaB
activation and chemokine production in vitro. PLoS One.
6:e248312011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Welsh N: Does the small tyrosine kinase
inhibitor Imatinib mesylate counteract diabetes by affecting
pancreatic islet amyloidosis and fibrosis? Expert Opin Investig
Drugs. 21:1743–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ono K, Suzushima H, Watanabe Y, Kikukawa
Y, Shimomura T, Furukawa N, Kawaguchi T and Araki E: Rapid
Amelioration of hyperglycemia facilitated by dasatinib in a chronic
myeloid leukemia patient with type 2 diabetes mellitus. Intern Med.
51:2763–2766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW Jr: Obesity is associated with
macrophage accumulation in adipose tissue. J Clin Invest.
112:1796–1808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gregor MF and Hotamisligil GS:
Inflammatory mechanisms in obesity. Annu Rev Immunol. 29:415–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Prada PO, Ropelle ER, Mourão RH, de Souza
CT, Pauli JR, Cintra DE, Schenka A, Rocco SA, Rittner R, Franchini
KG, et al: EGFR tyrosine kinase inhibitor (PD153035) improves
glucose tolerance and insulin action in high-fat diet-fed mice.
Diabetes. 58:2910–2919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hägerkvist R, Jansson L and Welsh N:
Imatinib mesylate improves insulin sensitivity and glucose disposal
rates in rats fed a high-fat diet. Clin Sci (Lond). 114:65–71.
2008. View Article : Google Scholar
|
|
61
|
Han MS, Chung KW, Cheon HG, Rhee SD, Yoon
CH, Lee MK, Kim KW and Lee MS: Imatinib mesylate reduces
endoplasmic reticulum stress and induces remission of diabetes in
db/db mice. Diabetes. 58:329–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Motzer RJ, Michaelson MD, Redman BG, Hudes
GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE,
et al: Activity of SU11248, a multitargeted inhibitor of vascular
endothelial growth factor receptor and platelet-derived growth
factor receptor, in patients with metastatic renal cell carcinoma.
J Clin Oncol. 24:16–24. 2006. View Article : Google Scholar
|
|
63
|
Jäger D, Ma JH, Mardiak J, Ye DW,
Korbenfeld E, Zemanova M, Ahn H, Guo J, Leonhartsberger N, Stauch
K, et al: Sorafenib treatment of advanced renal cell carcinoma
patients in daily practice: The large international PREDICT study.
Clin Genitourin Cancer. 13:156–64.e1. 2015. View Article : Google Scholar
|
|
64
|
Mokhtari D and Welsh N: Potential utility
of small tyrosine kinase inhibitors in the treatment of diabetes.
Clin Sci (Lond). 118:241–247. 2009. View Article : Google Scholar
|