Introduction
Glucocorticoids (GCs) have been extensively used in
the treatment of a variety of diseases, due to their potent
anti-inflammatory effects. However, the long-term and excessive use
of GCs is one of the most common causes of atraumatic osteonecrosis
of the femoral head and likely increases the incidence of secondary
osteoporosis (1). These
complications are partially attributed to modifications in the
bioactivity of bone marrow-derived stem cells,
osteoblasts/osteocytes and osteoclasts (2–4).
Previous studies have indicated that GCs antagonize Runt-related
transcription factor 2 (Runx2) during the osteoblast
differentiation of mesenchymal cells and inhibit the osteogenesis
of bone marrow-derived stem cells (3,5).
GCs have also been reported to directly suppress the osteogenic
differentiation of osteoblasts (6), induce osteoblast and osteocyte
apoptosis, and decrease the number of bone-forming cells (7–9).
Another study indicated that GC-induced bone resorption is caused
by a direct effect of the GCs on extending the lifespan of
osteoclasts (10).
Vitamin K (VK), whose active form has been
demonstrated to be a coenzyme for γ-carboxylase, plays an important
role in bone metabolism (11,12). There are two types of VK in
nature, VK1 (phylloquinone) and VK2
(menatetrenone). VK1 is a single compound and is
primarily found in plants, while VK2 is a series of
vitamers with multiple isoprene units at the 3-position of the
naphthoquinone and is named according to the number of these prenyl
units (13,14). Studies have indicated that
VK2 has a more pronounced osteoprotective effect than
VK1 (15,16). In addition to the γ-carboxylation
of osteocalcin (OCN), VK2 has been proven to promote
osteoblast proliferation (13)
and the osteoblast-to-osteocyte transition in vitro
(15,17–19), including OCN accumulation in the
extracellular matrix, the upregulation of Runx2 and alkaline
phosphatase (ALP), and the transcription of osteogenic genes.
Additional studies also revealed the osteoprotective effects of
VK2 in vivo. Akiyama et al and Iwamoto
et al observed that VK2 prevented bone loss in
rats with ovariectomy or sciatic neurectomies (20,21); bone healing was also promoted in
the osteotomy model in the study by Iwamoto et al (22). Based on these findings,
VK2 has been used in the treatment of osteoporosis in
Asian countries for a number of years (23,24).
Several studies have reported the protective effects
of VK2 on prednisolone-treated rats (22,25,26); however, few studies have reported
similar findings in vitro. Thus, the purpose of this study
was to examine the effects of VK2 on GC-treated
osteoblasts.
Materials and methods
Chemicals
The cell culture medium, Dulbecco's modified Eagle's
medium (DMEM; low glucose, 1 g/l), was obtained from HyClone,
Logan, UT, USA. Fetal bovine serum (FBS) and the
penicillin-streptomycin solution (10,000 U/ml penicillin; 10 mg/ml
streptomycin) were purchased from Gibco Laboratories (Grand Island,
NY, USA). VK2, L-ascorbic acid and β-glycerophosphate
disodium salt hydrate were purchased from Sigma Chemical Co. (St.
Louis, MO, USA). Dexamethasone (DEX) was obtained from Sigma and
was used at a concentration of 1 µM in all the experiments
in this study. VK2 was dissolved in anhydrous ethanol
and all other chemicals were dissolved in PBS.
Cell culture
Mouse osteoblastic MC3T3-E1 cells (GNM15) were
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultured in DMEM supplemented with 10% FBS,
penicillin (100 U/ml) and streptomycin (10 µg/ml).
Osteogenic differentiation was induced in DMEM supplemented with
10% FBS, penicillin (100 U/ml), streptomycin (10 µg/ml),
L-ascorbic acid (50 µg/ml) and β-glycerophosphate disodium
salt hydrate (10 mM). All cells were cultured at 37°C in a
humidified atmosphere containing 5% CO2.
Cell proliferation assay
The MC3T3-E1 cells (5,000/well) were plated in
96-well plates and incubated overnight. Ten microliters of Cell
Counting Kit-8 (CCK-8) solution were then added to 100 µl of
culture medium and the wells were incubated for an additional 2 h.
The absorbance values at 450 nm measured using a microplate reader
(Bio-Rad, Hercules, CA, USA) were recorded as the initial values (0
day), and the cells were then treated with both DEX and various
concentrations of VK2 (10−5, 10−6
and 10−7 M), with medium changes every 2 days. CCK-8
detection was performed again at appropriate time points (48, 96
and 144 h) following incubation with the different chemicals, and
the absorbance values were recorded and analyzed.
Cell apoptosis and viability assay
The Annexin V-FITC cell apoptosis detection kit
(Beyotime Biotechnology, Shanghai, China) was used to detect cell
apoptosis. The MC3T3-E1 cells were incubated with or without DEX
and various concentrations of VK2 (10−5,
10−6 and 10−7 M) for 6 days, collected,
resuspended in 200 µl of Annexin V-FITC and 10 µl of
propidium iodide, and incubated for 20 min at room temperature.
Subsequently, flow cytometry was used to evaluate the number of
apoptotic cells. The early apoptotic cells are labeled green and
the dead and late apoptotic cells are labeled red, while the live
cells are not stained.
Trypan blue staining was performed to evaluate cell
viability. The MC3T3-E1 cells were treated with both DEX and
various concentrations of VK2 (10−5,
10−6 and 10−7 M) in FBS-free medium for 6
days and then collected. Ten microliters of trypan blue
(Invitrogen, Carlsbad, CA, USA) were mixed with 10 µl of the
cell suspension, and 10 µl of the mixture were then added to
the cell counting plate. The cell death rates were automatically
calculated with a cell counter (Invitrogen). A
ReadyProbes® Cell Viability Imaging kit (Life
Technologies, Gaithersburg, MD, USA) was also used to detect cell
viability at 6 days after the MC3T3-E1 cells were incubated under
the different conditions. The blue dye stained all living cells,
and the green dye stained the dead cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cells were cultured in osteogenic
differentiation medium and treated with DEX or DEX with various
concentrations of VK2 (10−5, 10−6,
and 10−7 M). Total RNA was extracted using TRIzol
reagent (Invitrogen) at 1, 3 and 7 days following treatment, and
the RNA was then reverse transcribed into cDNA using the EasyScript
one-step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech
Co., Ltd., Beijing, China), according to the manufacturer's
instructions. RT-qPCR for ALP, OCN and Runx2 was performed using
the TransStart Tip Green qPCR SuperMix (TransGen Biotech Co., Ltd.)
with ABI Prism 7900 (Invitrogen). The reaction conditions were 1
cycle of 95°C for 30 sec and 40 cycles of 95°C for 5 sec and 60°C
for 30 sec. Subsequently, a 65–95°C solubility curve was
constructed. The relative amount of each mRNA was normalized to the
β-actin mRNA. The primer sequences of each cDNA are presented in
Table I.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Forward primer | Reverse Primer |
|---|
| Runx2 |
TGGCCGGGAATGATGAGAAC |
TGAAACTCTTGCCTCGTCCG |
| ALP |
CACTCTGTCCCGTTGGTGTC |
TTGACGTTCCGATCCTGCAC |
| OCN |
TCTGACAAAGCCTTCATGTCCA |
AGCCCTCTGCAGGTCATAGA |
| β-actin |
GTCGAGTCGCGTCCACC |
GTCATCCATGGCGAACTGGT |
Determination of ALP activity and
staining
To assay the ALP activity in the cells subjected to
the different treatments, the total protein was harvested at 1, 3
and 7 days after the different treatments, as described above. ALP
activity was evaluated using the ALP assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), according to the
manufacturer's instructions. The values were measured at 520 nm and
normalized to the protein concentration determined using the BCA
protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). In
addition, ALP staining was performed 7 days following incubation
with the conditioning medium using the BCIP/NBT ALP Color
Development kit (Beyotime Biotechnology), according to the
manufacturer's instructions.
Alizarin Red staining
Following incubation with DEX or DEX plus various
concentrations of VK2 (10−5, 10−6,
and 10−7 M), the cell cultures were rinsed 3 times with
PBS, fixed with 4% paraformaldehyde for 30 min, and then stained
with Alizarin Red (Beyotime Biotechnology) for a further 30 min.
The cultures were then evaluated under a light microscope (CKX31;
Olympus, Tokyo, Japan).
Immunofluorescence staining for Runx2 and
OCN
Following 7 days of incubation with the different
conditioning media, the MC3T3-E1 cells were fixed with 4%
paraformaldehyde for 20 min, treated with 0.1% Triton X-100 for 15
min, and blocked with 10% FBS for 30 min at 37°C. The cells were
then incubated with a rabbit anti-Runx2 monoclonal antibody
(1:1,000 dilution; #12556; Cell Signaling Technology, Danvers, MA,
USA) or an anti-OCN antibody (1:200 dilution; AB10911; Millipore,
Billerica, MA, USA), followed by an anti-rabbit Alexa Fluor™ 488
secondary antibody (1:500 dilution; A32731; Invitrogen) for 1 h at
37°C. Finally, the MC3T3-E1 cells were stained with
4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) for a further 30
sec, rinsed with PBS and then examined under a fluorescence
microscope (Leica DM IL LED; Leica, Wetzlar, Germany).
Western blot analysis
To examine the effects of DEX or DEX and
VK2 on the differentiation of the MC3T3-E1 cells, total
protein was harvested from the cells cultured in the osteogenic
medium described above for 1, 3 and 7 days. The protein
concentrations were measured using the BCA protein assay kit
(Thermo Fisher Scientific). The protein samples were then separated
on a 10% SDS-PAGE gel and transferred onto a PVDF membrane. The
membrane was blocked with 5% BSA and incubated with the primary
antibodies overnight at 4°C, followed by incubation with a
horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h.
After rinsing 3 times with PBST, the membrane was scanned in an
Odyssey scanner (Li-COR Biosciences, Lincoln, NE, USA). The
antibodies used for the western blot analysis were as follows:
monoclonal rabbit anti-rat GAPDH antibody (1:1,000 dilution;
#2118), monoclonal rabbit anti-rat Runx2 antibody (1:1,000
dilution; #12556), and an HRP-conjugated rat anti-rabbit antibody
(1:2,000 dilution; #7074) (all from Cell Signaling Technology,
Danvers, MA, USA). The bands were quantified using Quantity One
software and normalized to GAPDH.
Enzyme-linked immunosorbent assay (ELISA)
for OCN in the media
Following incubation in the conditioning medium for
1, 3 and 7 days, the MC3T3-E1 cells were incubated with regular
medium for a further 24 h. The media were then harvested and the
concentrations of OCN in the media were detected using an ELISA kit
(Mlbio, Shanghai, China); the values were normalized to the total
protein concentration, which was determined using a BCA kit
(Invitrogen).
Statistical analysis
SPSS 20.0 software (Microsoft, SPSS, Inc., Chicago,
IL, USA) was used to analyze the values in each group. All the
experiments in this study were performed in triplicate and the data
are expressed as the means and standard deviation (SD). A
statistical comparison of the data between the groups was performed
using one-way analysis of variance (ANOVA) with a
Student-Newman-Keuls (SNK) post hoc test. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
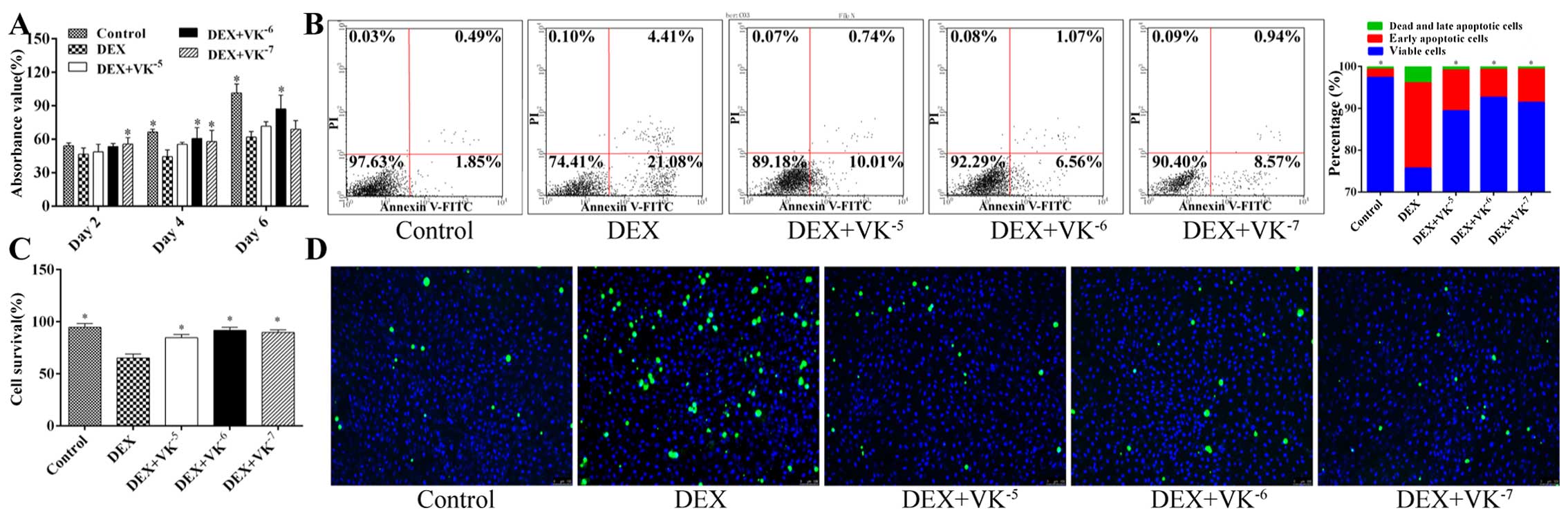
VK2 promotes MC3T3-E1 cell
proliferation and enhances MC3T3-E1 cell survival in the
DEX-treated cultures
A CCK-8 assay was performed at 48, 96 and 144 h
following treatment with DEX alone or with DEX and VK2.
The results revealed that MC3T3-E1 cell proliferation was
significantly suppressed at 96 and 144 h by DEX, although no
significant change was observed at 48 h. However, the addition of
VK2 promoted cell proliferation at these 3 time points,
particularly following treatment with 10−6 and
10−7 M VK2. We did not observe a
dose-dependent effect of VK2 (Fig. 1A).
The results of cell apoptosis assay indicated that
VK2 inhibited apoptosis and enhanced the survival of the
DEX-treated cells, which was also demonstrated by trypan blue
staining. In this experiment, only 65.3% of the MC3T3-E1 cells in
the DEX group survived after being treated with DEX in FBS-free
medium for 6 days, while significantly more cells survived in the
other groups. Cell viability imaging also yielded similar results
(Fig. 1B–D).
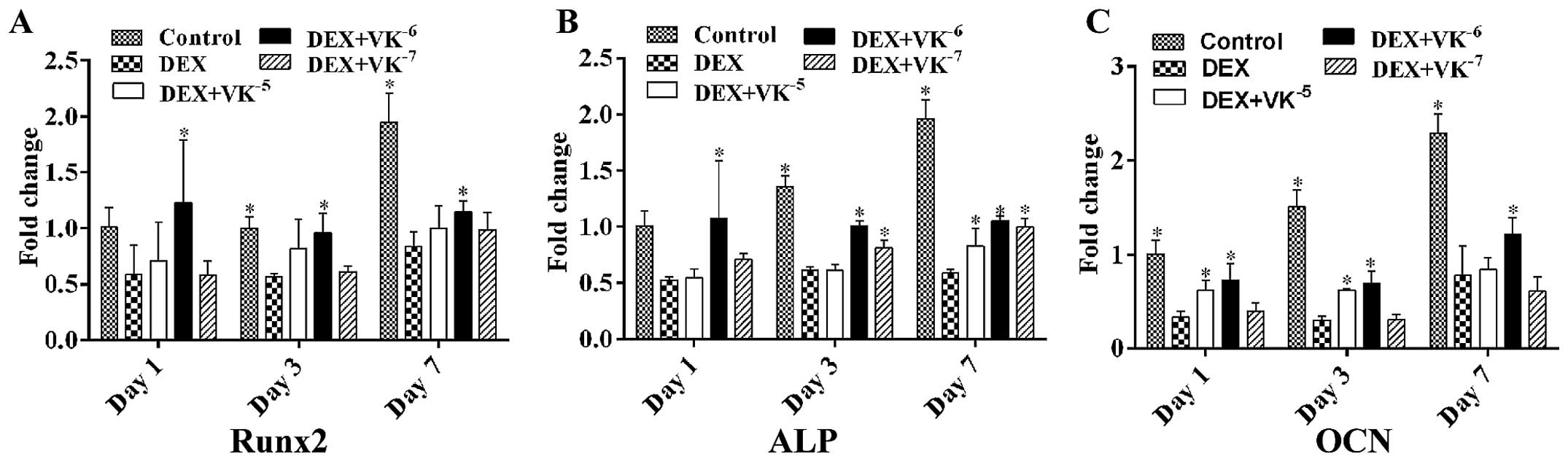
VK2 improves the osteogenic
differentiation potential of DEX-treated MC3T3-E1 cells
To verify whether VK2 enhances the
osteogenic differentiation potential of DEX-treated MC3T3-E1 cells,
we also detected the mRNA expression levels of both early and
mature osteogenic markers in the MC3T3-E1 cells. The results
revealed that, following incubation, the mRNA levels of Runx2, ALP
and OCN were downregulated by DEX and upregulated by
VK2, particularly following treatment with
10−6 M VK2 (Fig. 2).
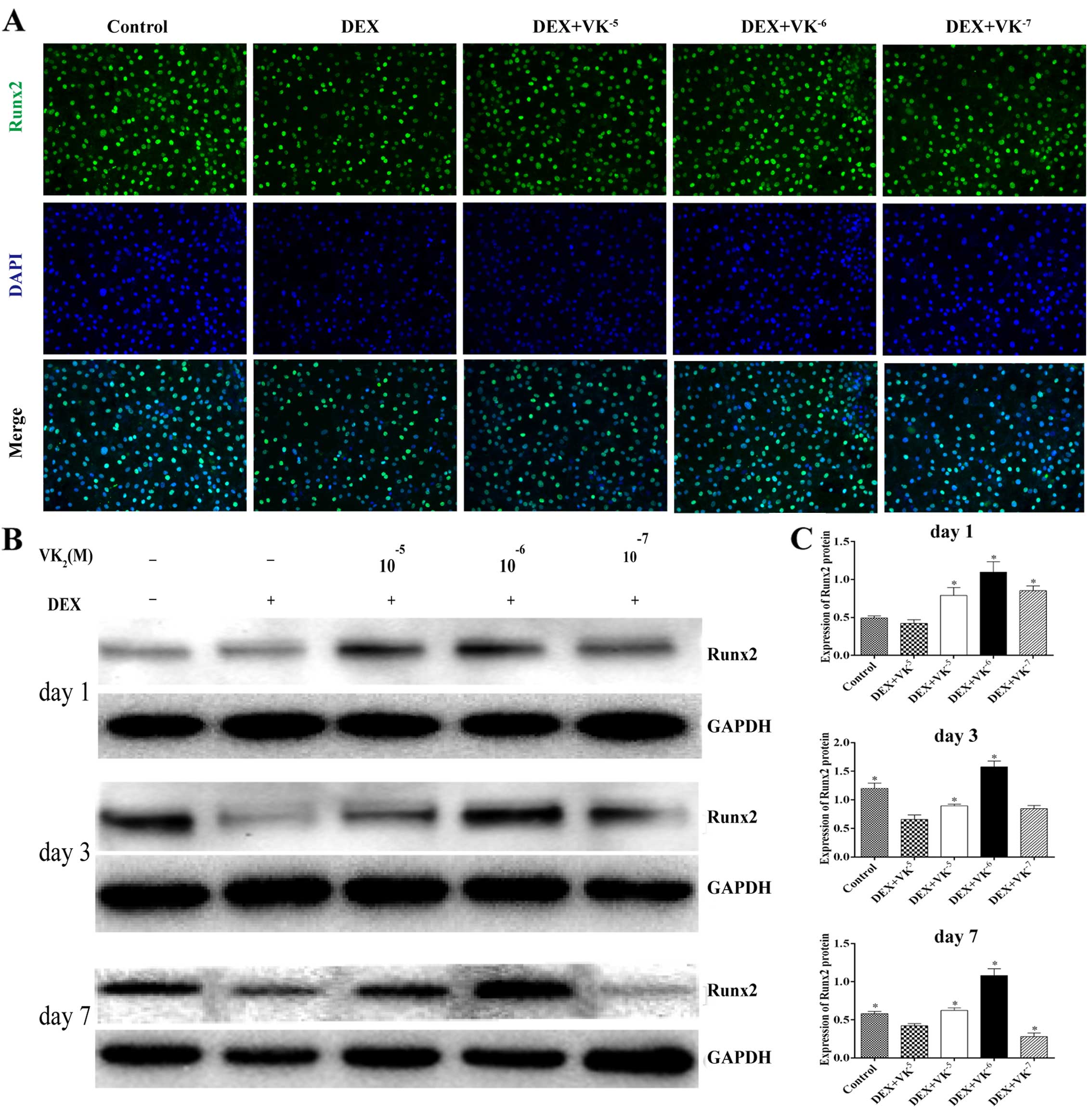
VK2 upregulates Runx2
expression in DEX-treated MC3T3-E1 cells
We performed immunofluorescence staining and western
blot analysis to detect the expression of Runx2, an early
osteogenic marker. We observed that the Runx2 level in the
DEX-treated MC3T3 cells was significantly decreased, while the
Runx2 protein levels were significantly upregulated in the presence
of VK2, particularly 10−6 M VK2
(Fig. 3).
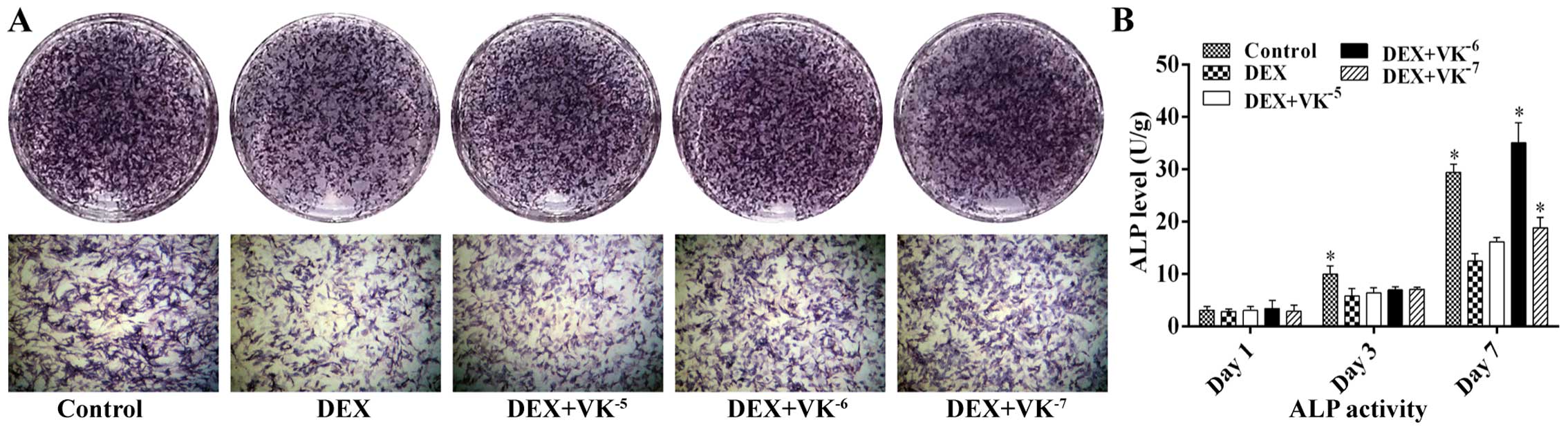
VK2 promotes ALP expression in
the DEX-treated MC3T3-E1 cells
Following 7 days of incubation with DEX or DEX and
various concentrations of VK2, we performed ALP staining
to detect osteogenesis in the MC3T3-E1 cells. As shown in Fig. 4, treatment with 10−5 M
DEX markedly decreased the number of ALP-positive cells, while
VK2 antagonized this effect, showing more bluish
violet-coloured cells. ALP activity was also detected following 1,
3 and 7 days of incubation. The results revealed that ALP activity
increased over time. The MC3T3-E1 cells in the DEX group displayed
less ALP activity than those of the control group, particularly on
days 3 and 7, while the MC3T3-E1 cells in the VK2 groups
exhibited an increased ALP expression, particularly the cells
treated with 10−6 M VK2 on day 7 (Fig. 4).
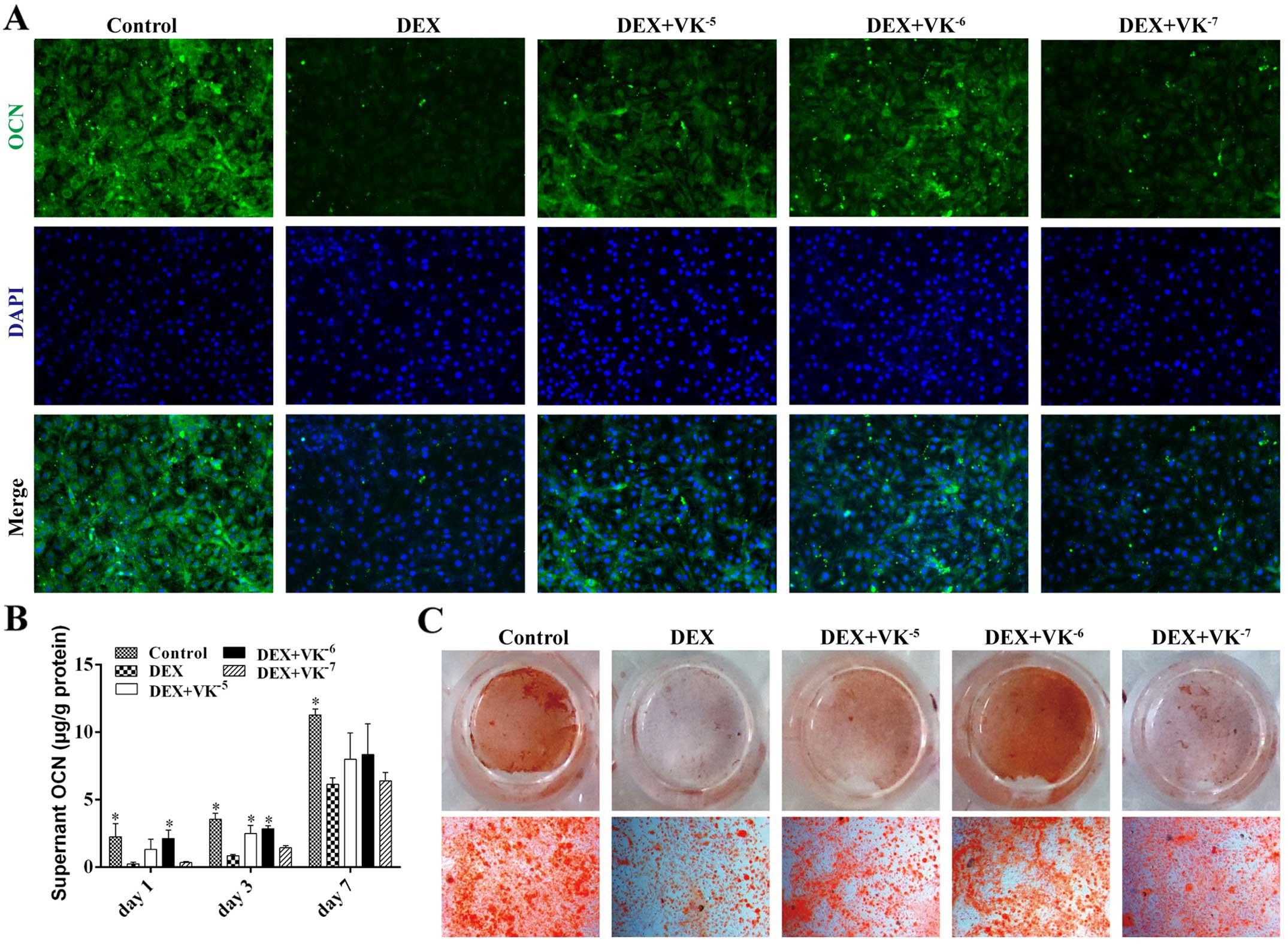
VK2 promotes OCN expression in
the DEX-treated MC3T3-E1 cells
OCN is a mature stage osteogenic marker; therefore,
the OCN levels in the media of the MC3T3-E1 cells treated with DEX
and VK2 were detected on days 1, 3 and 7. The results
revealed that the OCN levels in the media increased over time. The
DEX-treated MC3T3-E1 cells secreted evidently less OCN than the
controls at all time points, while the MC3T3-E1 cells treated with
DEX and VK2 had more OCN in the media, particularly the
cells treated with 10−6 M VK2 (Fig. 5). We also detected OCN expression
in the MC3T3-E1 cells using immunofluorescence staining, and
observed less OCN expression in the DEX-treated MC3T3-E1 cells and
increased OCN expression in the VK2 groups. In addition,
Alizarin Red staining revealed a similar antagonism of
VK2 toward the DEX-treated MC3T3-E1 cells (Fig. 5).
Discussion
GC-induced osteoporosis greatly increases the risk
of fracture, and pharmacological therapy is recommended as soon as
possible and has been studied for many years (27). VK2 has been reported to
be a promising therapy for GC-induced osteoporosis (28). Studies have indicated that
VK2 inhibits bone loss and increases bone formation in
GC-treated rats (25,29). Clinical studies have also reported
increased serum levels of carboxylated OCN and lumbar bone mass
volume in GC-treated patients (26,30). This study further confirmed the
protective effects of VK2 against GC-induced damage in
osteoblasts in vitro.
Several studies have indicated that GCs inhibit
osteoblast proliferation in vivo (31,32), and VK2 has also been
reported to promote osteoblast proliferation (13). In this study, we further confirmed
the proliferative-promoting effect of VK2 on GC-treated
MC3T3-E1 cells. This effect may be related to growth
arrest-specific gene 6 (Gas6), which is a VK-dependent protein that
is involved in cell proliferation by activating Axl (33,34). GC-induced osteoblast apoptosis has
been clearly demonstrated in both in vivo and clinical
studies (7,35). In this study, we observed
significant apoptosis of the DEX-treated MC3T3-E1 cells and an
anti-apoptotic effect of VK2. Studies have demonstrated
that VK2 has some biological effects as a co-factor,
including anti-apoptotic effects in erythroid progenitors (36), maintaining endothelial cell
survival (37) and protecting
neurons from methylmercury-induced cell death (38). The anti-apoptotic effects of
VK2 are believed to be related to its role as an
electron carrier in the regulation of mitochondrial function
(39).
The critical role of Runx2 in osteoblasts has been
well described by previous studies (40,41). Runx2 is a master transcription
factor in osteoblast differentiation and bone formation, and
strongly impacts the expression of osteoblast marker genes and
related proteins, such as ALP. The study by Koromila et al
demonstrated that GC markedly antagonized Runx2 and Runx2-mediated
ALP activity in mesenchymal cells (3). In this study, we also observed that
Runx2 was downregulated by GCs in osteoblastic cells, similar to a
previous study by Kim et al (6). Furthermore, we observed a higher
expression of Runx2 in the MC3T3-E1 cells treated with both GCs and
VK2, which indicated that VK2 promoted the
osteoblast differentiation of GC-treated osteoblastic cells partly
by upregulating Runx2.
OCN is a non-collagenous, VK-dependent protein that
is secreted in the late stage of osteoblast differentiation. One
role of VK2 in bone metabolism is to act as a coenzyme
for the γ-carboxylation of OCN, which combines with hydroxyapatite
to ultimately promote bone mineralization (42). With the exception of its role as a
regulator of bone mineralization, OCN has also been reported to
regulate bone remodeling by modulating osteoblast and osteoclast
activity (43). Runx2 has been
recognized as a key regulator of OCN transcription, and both Runx2
and OCN transcription are inhibited by GCs (3,43).
Studies have also shown that VK2 enhances OCN
accumulation in human osteoblasts (43,19). In this study, we observed an
evident downregulation in the proteins levels of Runx2 and OCN in
the DEX-treated MC3T3-E1 cells and further confirmed the
stimulatory effect of VK2 on osteoblast differentiation.
Furthermore, we observed the upregulated expression of
osteogenesis-related genes in the MC3T3-E1 cells treated with DEX
and VK2; this may be attributed to the transcriptional
effect of VK2 as VK2 can activate steroid and
xenobiotic receptor (SXR) and regulate the transcription of
extracellular matrix-related genes and collagen accumulation in
osteoblastic cells (44–46).
Studies have demonstrated that treatment with
10−5 M VK2 alone promotes mineralization and
increases the Ca2+ concentrations more effectively than
lower concentrations (17,18).
However, in this study, we found that treatment with
10−6 M VK2, which was comparable to the serum
concentrations in patients treated with VK (47), exerted the most effective
protection against DEX. Some other studies have observed similar
results, where 10−6 M VK2 had a stimulatory
effect on colony formation and the proliferation of hematopoietic
progenitors, producing fewer apoptotic cells than those treated
with 10−5 M VK2 (36). Koshihara and Hoshi (19) observed more OCN in the medium of
cells treated with 0.5 µM and 1.5 µM VK2,
and OCN release in the medium was significantly enhanced by 0.5
µM VK2 in the presence of 1,25
(OH)2-D3. Therefore, we speculate that 10−6 M
VK2 may be the most appropriate concentration to
antagonize DEX in vitro.
In conclusion, in this study, we demonstrated that
VK2 promoted osteoblast proliferation and osteogenic
differentiation, inhibited cellular apoptosis and enhanced cellular
survival, supporting the view that VK2 is a promising
option for the prevention and treatment of GC-induced osteoporosis
and osteonecrosis.
Acknowledgments
This study was funded by the National Natural
Science Foundation of China (no. 81301572) and the SMC-Chen Xing
Plan for Splendid Young Teachers of Shanghai Jiao Tong
University.
References
|
1
|
Kim BY, Yoon HY, Yun SI, Woo ER, Song NK,
Kim HG, Jeong SY and Chung YS: In vitro and in vivo inhibition of
glucocorticoid-induced osteoporosis by the hexane extract of
Poncirus trifoliata. Phytother Res. 25:1000–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weinstein RS: Glucocorticoid-induced
osteonecrosis. Endocrine. 41:183–190. 2012. View Article : Google Scholar
|
|
3
|
Koromila T, Baniwal SK, Song YS, Martin A,
Xiong J and Frenkel B: Glucocorticoids antagonize RUNX2 during
osteoblast differentiation in cultures of ST2 pluripotent
mesenchymal cells. J Cell Biochem. 115:27–33. 2014. View Article : Google Scholar
|
|
4
|
Kerachian MA, Séguin C and Harvey EJ:
Glucocorticoids in osteonecrosis of the femoral head: a new
understanding of the mechanisms of action. J Steroid Biochem Mol
Biol. 114:121–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan G, Kang PD and Pei FX: Glucocorticoids
affect the metabolism of bone marrow stromal cells and lead to
osteonecrosis of the femoral head: a review. Chin Med J (Engl).
125:134–139. 2012. View Article : Google Scholar
|
|
6
|
Kim J, Lee H, Kang KS, Chun KH and Hwang
GS: Protective effect of Korean Red Ginseng against
glucocorticoid-induced osteoporosis in vitro and in vivo. J Ginseng
Res. 39:46–53. 2015. View Article : Google Scholar
|
|
7
|
O'Brien CA, Jia D, Plotkin LI, Bellido T,
Powers CC, Stewart SA, Manolagas SC and Weinstein RS:
Glucocorticoids act directly on osteoblasts and osteocytes to
induce their apoptosis and reduce bone formation and strength.
Endocrinology. 145:1835–1841. 2004. View Article : Google Scholar
|
|
8
|
Weinstein RS, Jilka RL, Parfitt AM and
Manolagas SC: Inhibition of osteoblastogenesis and promotion of
apoptosis of osteoblasts and osteocytes by glucocorticoids.
Potential mechanisms of their deleterious effects on bone. J Clin
Invest. 102:274–282. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yun SI, Yoon HY, Jeong SY and Chung YS:
Glucocorticoid induces apoptosis of osteoblast cells through the
activation of glycogen synthase kinase 3beta. J Bone Miner Metab.
27:140–148. 2009. View Article : Google Scholar
|
|
10
|
Weinstein RS, Chen JR, Powers CC, Stewart
SA, Landes RD, Bellido T, Jilka RL, Parfitt AM and Manolagas SC:
Promotion of osteoclast survival and antagonism of
bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J
Clin Invest. 109:1041–1048. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamidi MS, Gajic-Veljanoski O and Cheung
AM: Vitamin K and bone health. J Clin Densitom. 16:409–413. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azuma K, Ouchi Y and Inoue S: Vitamin K:
novel molecular mechanisms of action and its roles in osteoporosis.
Geriatr Gerontol Int. 14:1–7. 2014. View Article : Google Scholar
|
|
13
|
Yamaguchi M, Sugimoto E and Hachiya S:
Stimulatory effect of menaquinone-7 (vitamin K2) on
osteoblastic bone formation in vitro. Mol Cell Biochem.
223:131–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shearer MJ and Newman P: Recent trends in
the metabolism and cell biology of vitamin K with special reference
to vitamin K cycling and MK-4 biosynthesis. J Lipid Res.
55:345–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Igarashi M, Yogiashi Y, Mihara M, Takada
I, Kitagawa H and Kato S: Vitamin K induces osteoblast
differentiation through pregnane X receptor-mediated
transcriptional control of the Msx2 gene. Mol Cell Biol.
27:7947–7954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim M, Na W and Sohn C: Vitamin
K1 (phylloquinone) and K2 (menaquinone-4)
supplementation improves bone formation in a high-fat diet-induced
obese mice. J Clin Biochem Nutr. 53:108–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koshihara Y, Hoshi K, Okawara R, Ishibashi
H and Yamamoto S: Vitamin K stimulates osteoblastogenesis and
inhibits osteoclastogenesis in human bone marrow cell culture. J
Endocrinol. 176:339–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Atkins GJ, Welldon KJ, Wijenayaka AR,
Bonewald LF and Findlay DM: Vitamin K promotes mineralization,
osteoblast-to-osteocyte transition, and an anticatabolic phenotype
by {gamma}-carboxylation-dependent and -independent mechanisms. Am
J Physiol Cell Physiol. 297:C1358–C1367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koshihara Y and Hoshi K: Vitamin
K2 enhances osteocalcin accumulation in the
extracellular matrix of human osteoblasts in vitro. J Bone Miner
Res. 12:431–438. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akiyama Y, Hara K, Ohkawa I and Tajima T:
Effects of menatetrenone on bone loss induced by ovariectomy in
rats. Jpn J Pharmacol. 62:145–153. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwamoto J, Matsumoto H, Takeda T, Sato Y
and Yeh JK: Effects of vitamin K2 on cortical and
cancellous bone mass, cortical osteocyte and lacunar system, and
porosity in sciatic neurectomized rats. Calcif Tissue Int.
87:254–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwamoto J, Seki A, Sato Y, Matsumoto H,
Tadeda T and Yeh JK: Vitamin K2 promotes bone healing in
a rat femoral osteotomy model with or without glucocorticoid
treatment. Calcif Tissue Int. 86:234–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koitaya N, Sekiguchi M, Tousen Y, Nishide
Y, Morita A, Yamauchi J, Gando Y, Miyachi M, Aoki M, Komatsu M, et
al: Low-dose vitamin K2 (MK-4) supplementation for 12
months improves bone metabolism and prevents forearm bone loss in
postmenopausal Japanese women. J Bone Miner Metab. 32:142–150.
2014. View Article : Google Scholar
|
|
24
|
Koitaya N, Ezaki J, Nishimuta M, Yamauchi
J, Hashizume E, Morishita K, Miyachi M, Sasaki S and Ishimi Y:
Effect of low dose vitamin K2 (MK-4) supplementation on
bio-indices in postmenopausal Japanese women. J Nutr Sci Vitaminol
(Tokyo). 55:15–21. 2009. View Article : Google Scholar
|
|
25
|
Hara K, Akiyama Y, Ohkawa I and Tajima T:
Effects of menatetrenone on prednisolone-induced bone loss in rats.
Bone. 14:813–818. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasaki N, Kusano E, Takahashi H, Ando Y,
Yano K, Tsuda E and Asano Y: Vitamin K2 inhibits
glucocorticoid-induced bone loss partly by preventing the reduction
of osteoprotegerin (OPG). J Bone Miner Metab. 23:41–47. 2005.
View Article : Google Scholar
|
|
27
|
van Staa TP, Leufkens HG and Cooper C: The
epidemiology of corticosteroid-induced osteoporosis: a
meta-analysis. Osteoporos Int. 13:777–787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanana I and Oshima H: Vitamin
K2 as a potential therapeutic agent for
glucocorticoid-induced osteoporosis. Clin Calcium. 16:1851–1857.
2006.In Japanese. PubMed/NCBI
|
|
29
|
Iwamoto J, Matsumoto H, Takeda T, Sato Y,
Liu X and Yeh JK: Effects of vitamin K(2) and risedronate on bone
formation and resorption, osteocyte lacunar system, and porosity in
the cortical bone of glucocorticoid-treated rats. Calcif Tissue
Int. 83:121–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inoue T, Sugiyama T, Matsubara T, Kawai S
and Furukawa S: Inverse correlation between the changes of lumbar
bone mineral density and serum undercarboxylated osteocalcin after
vitamin K2 (menatetrenone) treatment in children treated
with glucocorticoid and alfacalcidol. Endocr J. 48:11–18. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanderson M, Sadie-Van Gijsen H, Hough S
and Ferris WF: The role of MKP-1 in the anti-proliferative effects
of glucocorticoids in primary rat pre-osteoblasts. PLoS One.
10:e01353582015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi C, Huang P, Kang H, Hu B, Qi J, Jiang
M, Zhou H, Guo L and Deng L: Glucocorticoid inhibits cell
proliferation in differentiating osteoblasts by microRNA-199a
targeting of WNT signaling. J Mol Endocrinol. 54:325–337. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stenhoff J, Dahlbäck B and Hafizi S:
Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth
of cardiac fibroblasts. Biochem Biophys Res Commun. 319:871–878.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kirane A, Ludwig KF, Sorrelle N, Haaland
G, Sandal T, Ranaweera R, Toombs JE, Wang M, Dineen SP, Micklem D,
et al: Warfarin blocks Gas6-mediated Axl activation required for
pancreatic cancer epithelial plasticity and metastasis. Cancer Res.
75:3699–3705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Calder JD, Buttery L, Revell PA, Pearse M
and Polak JM: Apoptosis - a significant cause of bone cell death in
osteonecrosis of the femoral head. J Bone Joint Surg Br.
86:1209–1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sada E, Abe Y, Ohba R, Tachikawa Y,
Nagasawa E, Shiratsuchi M and Takayanagi R: Vitamin K2
modulates differentiation and apoptosis of both myeloid and
erythroid lineages. Eur J Haematol. 85:538–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hegarty JM, Yang H and Chi NC:
UBIAD1-mediated vitamin K2 synthesis is required for
vascular endothelial cell survival and development. Development.
140:1713–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakaue M, Mori N, Okazaki M, Kadowaki E,
Kaneko T, Hemmi N, Sekiguchi H, Maki T, Ozawa A, Hara S, et al:
Vitamin K has the potential to protect neurons from
methylmercury-induced cell death in vitro. J Neurosci Res.
89:1052–1058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vos M, Esposito G, Edirisinghe JN, Vilain
S, Haddad DM, Slabbaert JR, Van Meensel S, Schaap O, De Strooper B,
Meganathan R, et al: Vitamin K2 is a mitochondrial
electron carrier that rescues pink1 deficiency. Science.
336:1306–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Banerjee C, McCabe LR, Choi JY, Hiebert
SW, Stein JL, Stein GS and Lian JB: Runt homology domain proteins
in osteoblast differentiation: AML3/CBFA1 is a major component of a
bone-specific complex. J Cell Biochem. 66:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shimizu T, Takahata M, Kameda Y, Hamano H,
Ito T, Kimura-Suda H, Todoh M, Tadano S and Iwasaki N: Vitamin
K-dependent carboxylation of osteocalcin affects the efficacy of
teriparatide (PTH(1–34)) for skeletal repair. Bone. 64:95–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neve A, Corrado A and Cantatore FP:
Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol.
228:1149–1153. 2013. View Article : Google Scholar
|
|
44
|
Horie-Inoue K and Inoue S: Steroid and
xenobiotic receptor mediates a novel vitamin K2
signaling pathway in osteoblastic cells. J Bone Miner Metab.
26:9–12. 2008. View Article : Google Scholar
|
|
45
|
Ichikawa T, Horie-Inoue K, Ikeda K,
Blumberg B and Inoue S: Steroid and xenobiotic receptor SXR
mediates vitamin K2-activated transcription of
extracellular matrix-related genes and collagen accumulation in
osteoblastic cells. J Biol Chem. 281:16927–16934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Manolagas SC: Steroids and osteoporosis:
the quest for mechanisms. J Clin Invest. 123:1919–1921. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoshiji H, Kuriyama S, Noguchi R, Yoshii
J, Ikenaka Y, Yanase K, Namisaki T, Kitade M, Yamazaki M, Masaki T
and Fukui H: Combination of vitamin K2 and the
angiotensin-converting enzyme inhibitor, perindopril, attenuates
the liver enzyme-altered preneoplastic lesions in rats via
angiogenesis suppression. J Hepatol. 42:687–693. 2005. View Article : Google Scholar : PubMed/NCBI
|