Introduction
Hydrogen sulfide (H2S), the third member
of the gaso-transmitter family, is gaining acceptance as a
signaling molecule. Increasing evidence has indicated that
H2S has a variety of biological effects that may
participate in the protection of cardiovascular pathophysilology
(1–11). Exogenous H2S has been
shown to protect rat hearts against ischemia-reperfusion (IR)
injury (4–6) and contributes to the
cardioprotective effects of ischemia pre-conditioning in the
isolated, perfused rat heart (6).
In addition, exogenous H2S ameliorates left ventricular
remodeling and dysfunction in the setting of heart failure
(12). Recently, we demonstrated
that exogenous H2S protects H9c2 cardiac cells against
chemical hypoxia (3) or
doxorubicin-induced injury (10,13). In recent years, the roles of
H2S in diabetes-related cardiovascular complications
have attracted considerable attention, since lower circulating
H2S levels have been detected in animal models of
diabetes (14–16) and patients with type 2 diabetes
mellitus (DM) (14,17), and low blood H2S
concentrations may be associated with vascular inflammation
observed in diabetes (14).
Importantly, exogenous H2S protects against
hyperglycemia-induced vascular endothelial injury (15) and provides protection against
myocardial IR-induced damage in db/db mice (18) or diabetic rats (19). Furthermore, we recently revealed
that exogenous H2S exerts protective effects against
high glucose (HG)-induced injury and inflammation in H9c2 cardiac
cells (9,19,20). The mechanisms underlying these
cardioprotective effects of exogenous H2S are associated
with the inhibition of several intracellular signaling pathways,
such as mitogen-activated protein kinase (MAPK) (21), leptin (9) and nuclear factor-κB (NF-κB)
(20). However, the
cardioprotective mechanisms of exogenous H2S are
complex, and other intracellular signaling pathways may also be
involved. Based on the findings introduced in the relative
literatures, we speculated that the heat shock protein
(HSP)90-serine/threonine protein kinase (Akt) pathway may attribute
to the protective effects of exogenous H2S against
HG-induced injury to H9c2 cardiac cells.
HSPs are a family of protective proteins, which
constitute an endogenous cellular defense mechanism against hostile
environmental stress. HSP90, one of the most abundant cytosolic
HSPs, comprises 1–20% of total proteins in cells (22). Accumulating evidence indicates
that HSP90 contributes to cell survival and protection by
regulating the folding and stability of various cellular client
proteins, including survival and apoptotic factor (23). Hypoxia can increase the expression
of HSP90, which efficiently ameliorates myocardial IR-induce
myocardial dysfunction (24). The
inhibition of HSP90 function with HSP90 inhibitor or HSP90 siRNA
markedly diminishes the protective effects of hypoxic
pre-conditioning against prolonged hypoxia/reoxygenation-induced
injury in H9C2 cardiac cells (25). Of note, HSP90-endothelial nitric
oxide synthase (eNOS) interactions are reduced in endothelial cells
cultured in medium containing excess glucose (26). In addition, hyperglycemia impairs
ischemic preconditioning (IPC)-elicited cardioprotection by
disrupting the association of HSP90 with eNOS in rabbits or mice
(27). Of ntoe, in SH-SY5Y cells
(28) and the liver (29), H2S has been shown to
increase HSP90 expression. In our recent studies, we also revealed
that exogenous H2S upregulated the expression levels of
HSP90 in PC12 cells (30) and
H9c2 cells (3). Importantly, we
have demonstrated that HSP90 plays important roles in the
protection of exogenous H2S against chemical
hypoxia-induced neural (30) or
cardiac injury (3). However,
whether HSP90 mediates the cardioprotective effects of exogenous
H2S against HG-induced injury to H9c2 cardiac cells
remains unknown.
Akt, a serine/threonine kinase, is downstream of
phosphatidylinositol 3-kinase (PI3K) to mediate the metabolic
action of insulin (31). Impaired
insulin-stimulated PI3K/Akt has been reported to be involved in a
number of pathological conditions associated with insulin
resistance, such as the cardiovascular complication of diabetes
(32,33). In addition, in
streptozotocin-treated rats, diabetic cardiomyopathy is associated
with the impaired activation of Akt (34,35). Of note, Akt is one of the HSP90
substrates; thus, HSP90 contributes to the functional stabilization
of Akt, the activation of the PI3K/Akt signaling pathways and cell
survival. Additionally, HSP90 modulates Akt activity by suppressing
its dephosphorylation and proteosomal degradation (36). The HSP90/Akt pathway has been
shown to be an important survival and anti-apoptotic pathway in a
variety of cells and settings; this pathway has been shown to play
a role in myocardial calpain-induced caspase-3 activation and
apoptosis during sepsis (37). Of
note, the activation of the Akt pathway also contributes to the
cytoprotective effects of H2S. In 3T3I1 adipocytes, has
been shown to H2S increase glucose utilization by
activating the PI3K/Akt pathway (38). Yao et al reported that
H2S protected cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis via the stimulation of Akt
phosphorylation (39). However,
whether the HSP90/Akt pathway contributes to the protective effects
of exogenous H2S against HG-induced injury to H9c2
cardiac cells remains unclear.
The present study was therefore designed to
determine the effects of HG on the activation of the HSP90/Akt
pathway in H9c2 cardiac cells and to investigate whether exogenous
H2S protects cardiac cells against HG-induced injury by
modulating the activity of the HSP90/Akt pathway.
Materials and methods
Chemicals
NaHS,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1), Hoechst 33258, the superoxide dismutase (SOD) assay kit,
geldanamycin (GA) (an inhibitor of HSP90) and LY294002 [an
inhibitor of Akt] were purchased from Sigma-Aldrich (St. Louis, MO,
USA). The cell counting kit-8 (CCK-8) was supplied by Dojindo
Laboratories (Kumamoto, Japan). Fetal bovine serum (FBS) and
Dulbecco's modified Eagle's medium (DMEM) were obtained from Gibco
BRL (Grand Island, NY, USA). Anti-phosphorylated (p-)Akt antibody
(Cat. no. 12178), anti-Akt antibody (Cat. no. 14702) and anti-HSP90
antibody (Cat. no. 4877) were purchased from Cell Signaling
Technology (Boston, MA, USA); horseradish peroxidase
(HRP)-conjugated secondary antibody (Cat. no. KC5G5) and the BCA
protein assay kit were obtained from KangChen Biotech, Inc.
(Shanghai, China). Enhanced chemiluminescence (ECL) solution was
purchased from KeyGen Biotech (Nanjing, China). β-actin (Cat. no.
KC-5A08), which was used as a control, was supplied by KangChen
Biotech, Inc.
Cell culture and treatment
H9c2 cardiac cells, a rat cardiac myoblast cell
line, were obtained from the Sun Yat-sen University Experimental
Animal Center (Guangdong, China). The cells were grown in DMEM
supplemented with 10% FBS under an atmosphere of 5% CO2
at 37°C and 95% air.
To establish the model of the HG-induced
cardiomyocyte injury, the cells were cultured in DMEM (5.5 mM
glucose) for 12 h prior to the administration of 35 mM glucose
(final concentration) for 24 h. The glucose concentration of the
control group was 5.5 mM. To investigate the protective effects of
exogenous H2S against HG (35 mM glucose)-induced injury,
the cells were treated with 400 µM NaHS (a H2S
donor) for 30 min prior to exposure to HG for 24 h. To determine
whether the HSP90/Akt pathway contributes to the protective effects
of H2S, the H9c2 cardiac cells were treated with 1
µM GA (an inhibitor of HSP90) or 30 µM LY294002 (an
inhibitor of Akt) for 30 min prior to exposure to NaHS and HG for
24 h.
Western blot analysis
As previously described (40), after being subjected to the
indicated treatments, the H9c2 cardiac cells were harvested and
lysed with cell lysis solution at 4°C for 30 min. The total
proteins were quantified using the BCA protein assay kit. Loading
buffer was added to cytosolic extracts, and after boiling for
approximately 5 min, the same amounts of supernatant from each
sample were fractionated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and the
total proteins were then transferred onto polyvinylidene difluoride
(PVDF) membranes (Miniport; Olympus, Hamburg Germany). The
membranes were blocked with 5% fat-free milk for 60 min in fresh
blocking buffer [0.1% Tween-20 in Tris-buffered saline (TBS-T)],
and incubated with either anti-p-Akt antibody (1:1,000 dilution),
anti-Akt antibody (1:1,000 dilution) or anti-HSP90 antibody
(1:1,000 dilution) in freshly prepared TBS-T with 3% fat-free milk
overnight with gentle agitation at 4°C. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as a control for histone
incorporation. GAPDH antibody (Cat. no. KC-5G4) was provided by
KeyGen Biotech. The membranes were washed 3 times with TBS-T,
successively incubated with HRP-conjugated goat anti-rabbit
secondary antibody (1:2,500 dilution) in TBS-T with 3% fat-free
milk for 90 min at room temperature. The membranes were then washed
3 times with TBS-T for 15 min. The immunoreactive signals were
subsequently visualized by ECL detection. In order to quantify
protein expression, the X-ray films were scanned and analyzed using
ImageJ 1.47i software. The experiment was carried out 3 times.
Examination of cell viability
As previously described (40), the H9c2 cardiac cells were
cultured in 96-well plates at a concentration of 1×104
cells/ml, and the CCK-8 assay was employed to measure the viability
of the H9c2 cells. After being subjected to the indicated
treatments, 10 µl of CCK-8 solution at a 1/10 dilution was
added to each well and the plate was then incubated for 3 h in an
incubator. The absorbance at 450 nm was measured using a microplate
reader (Molecular Devices, Sunnyvale, CA, USA), The means of the
optical density (OD) of 4 wells in the indicated groups were used
to calculate the percentage of cell viability according to the
following formula: cell viability (%) = (OD treatment group/OD
control group ×100%. The above experiment was repeated 5 times.
Hoechst 33258 nuclear staining for the
measurement of apoptosis
Apoptotic cell death was measured by Hoechst 33258
staining followed by photofluorography. In brief, the H9c2 cardiac
cells were plated in 35 mm dishes at a density of 1×106
cells/well. After being subjected to the indicated treatments, the
H9c2 cells were cultured with 4% paraformaldehyde in 0.1 mol/l
phosphate-buffered saline (PBS, pH 7.4) for 10 min. The slides were
then washed 3 times with PBS, followed by staining with 5 mg/ml
Hoechst 33258 for 30 min, The H9c2 cells were washed 3 times with
PBS, and the PBS was then discarded and the plates were air dried.
Finally, the cells were visualized under a fluorescence microscope
(Bx50-FLA; Olympus, Tokyo, Japan). Viable H9c2 cells displayed a
uniform blue fluorescence throughout the nucleus and a normal
nuclear size; however apoptotic H9c2 cells exhibited condensed,
fractured or distorted nuclei. The experiment was carried out 5
times.
Measurement of mitochondrial membrance
potential (MMP)
MMP was examined using the fluorescent dye, JC-1, a
cell-permeable carionic dye that preferentially enters the
mitochondria based on the highly negative MMP. The depolarization
of MMP results in the loss of MMP from the mitochondria and a
decrease in the red/green fluorescence ratio. The H9c2 cells were
cultured in a slide with DMEM at a density of 1×106
cells/well. As previously described (40), after being subjected to the
indicated treatments, the slides were washed 3 times with PBS, and
were then incubated with 1 mg/l JC-1 at 37°C for 30 min in an
incubator, washed briefly 3 times with PBS and air dried. The
fluorescence was measured over the hold field of vision using a
fluorescence microscope connected to an imaging system (BX50-FLA;
Olympus). The mean fluorescence intensity (MFI) of JC-1 from 3
random fields was analyzed using ImageJ 1.47i software, and the MFI
was taken as an index of the levels of MMP. The experiment was
carried out 5 times.
Detection of intracellular reactive
oxygen species (ROS) generation
As previously described (40), intracellular ROS generation was
examined by the oxidative conversion of cell-permeable oxidation of
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) to fluorescent
DCF. The H9c2 cells were cultured on a slide with DMEM. After being
subjected to the different treatments, the slides were washed 3
times with PBS. DCFH-DA (10 µM) solution in serum-free
medium was added to the slides, and the H9c2 cells were then
incubated at 37°C for a further 30 min in an incubator. The slides
were washed 5 times with PBS, and DCF fluorescence was measured
over the entire field of vision using a fluorescence microscope
connected to an imaging system (BX50-FLA; Olympus). The MFI of ROS
from 5 random fields was measured using ImageJ 1.47i software and
the MFI was used as an index of the amount of ROS. The experiment
was carried out 5 times.
Measurement of SOD activity
SOD activity was measured by using a SOD assay kit.
As previously described (41),
after being subjected to the indicated treatments, the cells were
washed using PBS and lysed in ice-cold 0.1 M Tris/HCl (pH 7.4)
containing 0.5% Triton-X 100, 5 mM β-mercaptoethanol and 0.1 mg/ml
phenylmethylsulfonyl fluoride. Lysates were clarified by
centrifugation at 14,000 × g at 4°C for 5 min and the cell debris
was discarded. SOD activity was detected using a commercial 'SOD
assay kit' according to the manufacturer's instructions
(Sigma-Aldrich). The absorbance values at 450 nm were measured
using a microplate reader. The experiment was carried out 3
times.
Statistical analysis
All data are presented as the means ± SEM.
Differences between groups were analyzed by one-way analysis of
variance (ANOVA) by using SPSS 13.0 (SPSS, Chicago, IL, USA)
software, followed by the LSD post hoc comparison test. A value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
Exogenous H2S ameliorates the
HG-induced downregulation of the expression level of HSP90 in H9c2
cardiac cells
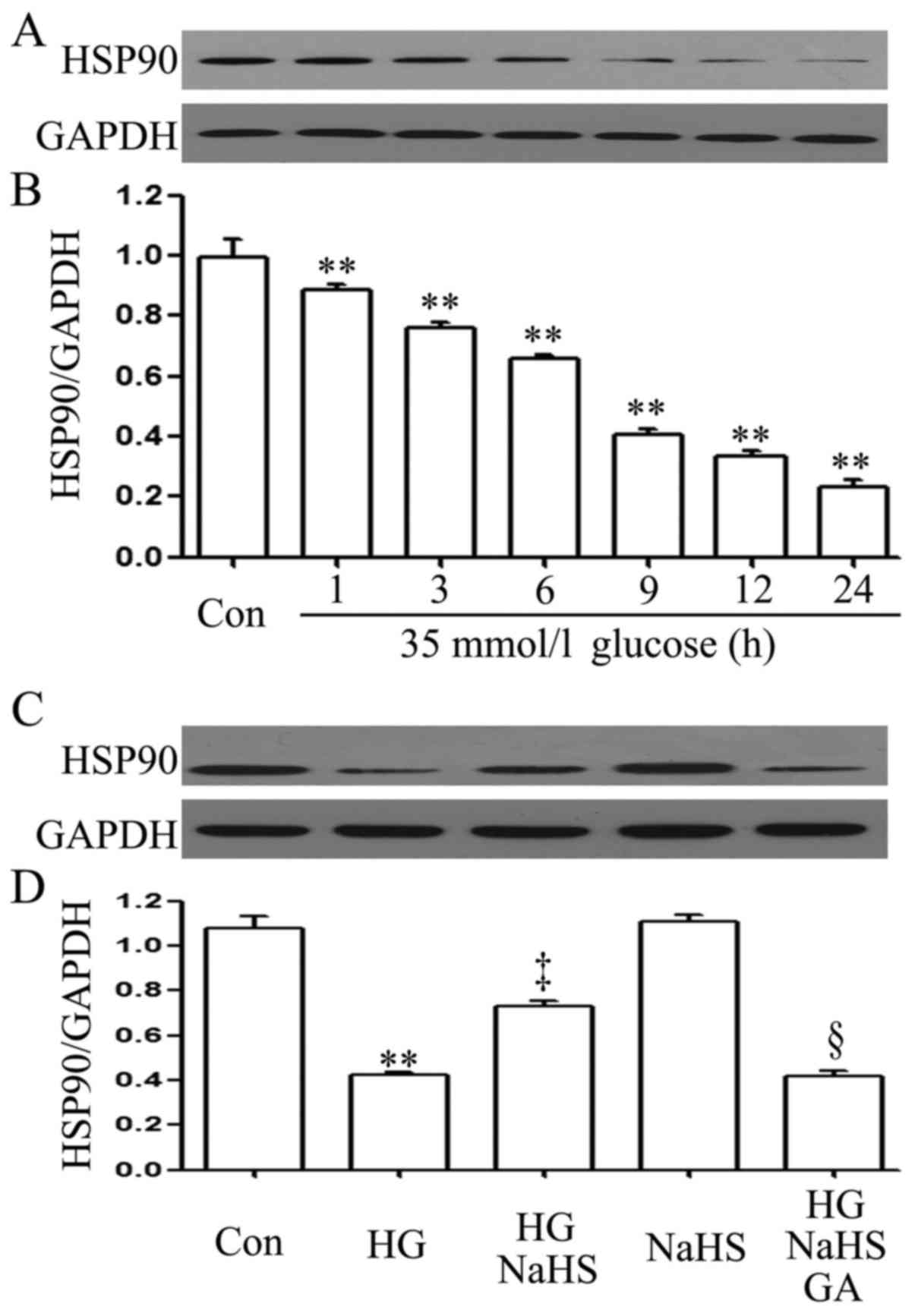
To examine the effect of HG (35 mM glucose) on the
HSP90 expression level in H9c2 cardiac cells, a time-response
experiment on the HSP90 expression level was carried out. After the
cells were exposed to 35 mM glucose for 1, 3, 6, 9, 12 and 24 h,
the expression level of HSP90 was decreased in a time-dependent
manner (Fig. 1A and B).
Importantly, prior to exposure to 35 mM glucose for 9 h, treatment
of the cells with 400 µM NaHS (a donor of H2S)
for 30 min markedly blocked the HG-induced decrease in the HSP90
expression level (Fig. 1C and D).
In addition, treatment of the cells with 1 µM GA (an
inhibitor of HSP90) for 30 min prior to exposure to NaHS and HG
markedly decreased the expression level of HSP90 which was
increased by NaHS (Fig. 1C and
D).
Role of HSP90 in the protective effects
of exogenous H2S against the HG-induced downregulation
of (p)-Akt expression in H9c2 cardiac cells
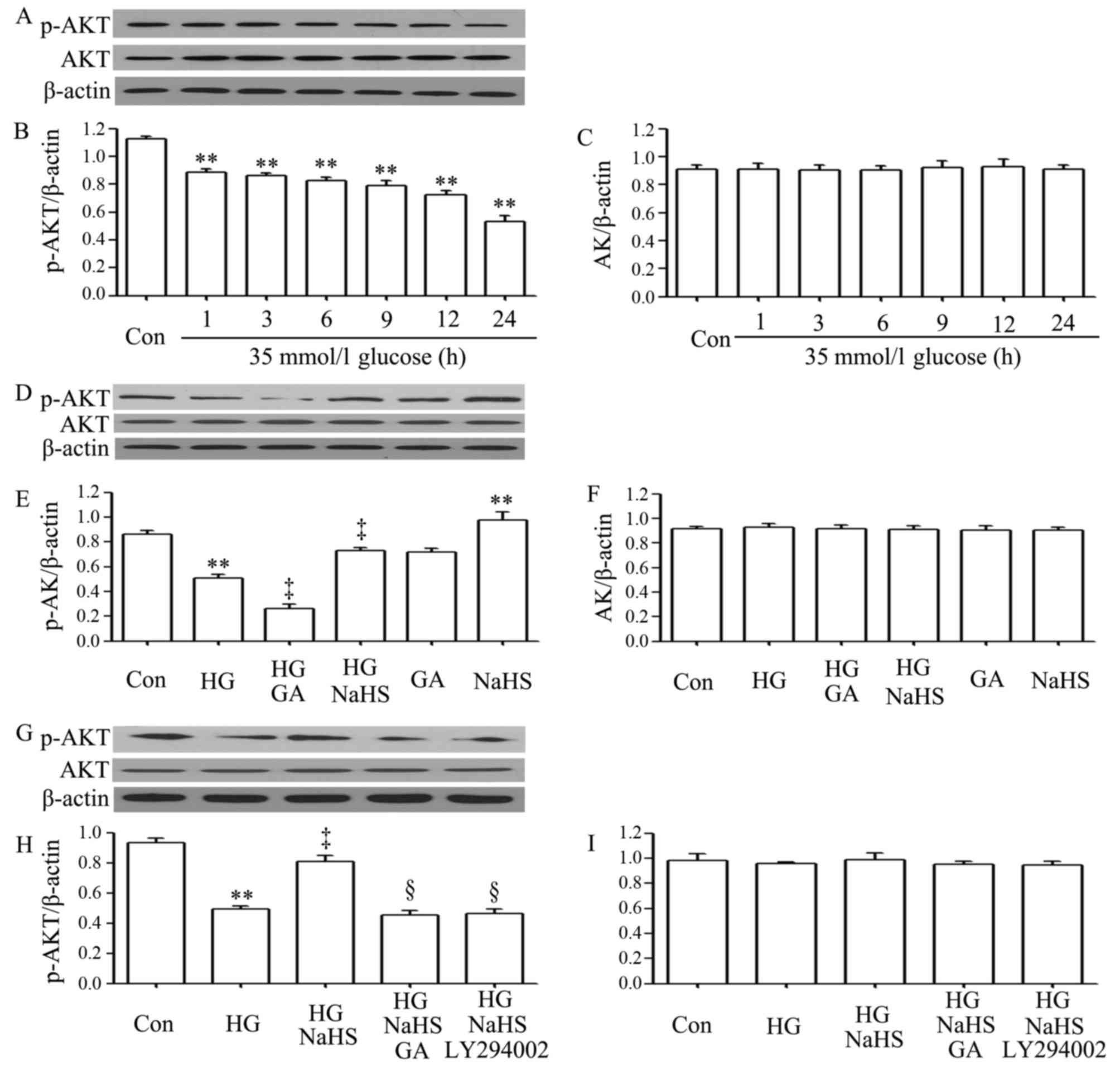
As shown in Fig.
2A–C, exposure of the H9c2 cardiac cells to 35 mM glucose for
1, 3, 6, 9, 12 and 24 h induced a decrease in the expression of
p-Akt, with the maximal decrease observed at 24 h. However,
treatment of the cells with 400 µM NaHS for 30 min prior to
exposure to HG for 12 h significantly inhibited the decrease in the
expression of p-Akt. Furthermore, treatment of the cells with 1
µM GA (an inhibitor of HSP90) for 30 min prior to exposure
to HG for 12 h further reduced the decreased p-Akt expression
level, suggesting the involvement of endogenous HSP90 in the
modulation of Akt activation. Treatment of the cells with 400
µM NaHS alone for 30 min considerably increased the
expression level of p-Akt (Fig.
2D–F). In a separate experiment (Fig. 2G–I), we observed that treatment of
the H9c2 cardiac cells with 1 µM GA for 30 min prior to
exposure to NaHS and HG markedly blocked the increase in p-Akt
expression by NaHS. Additionally, treatment of the cells with 30
µM LY294002 (an inhibitor of Akt) for 30 min prior to
exposure to NaHS and HG also antagonized the promoting effects of
NaHS on p-Akt expression.
Roles of the HSP90/Akt pathway in the
protective effect of exogenous H2S against HG-induced
cytotoxicity in H9c2 cardiac cells
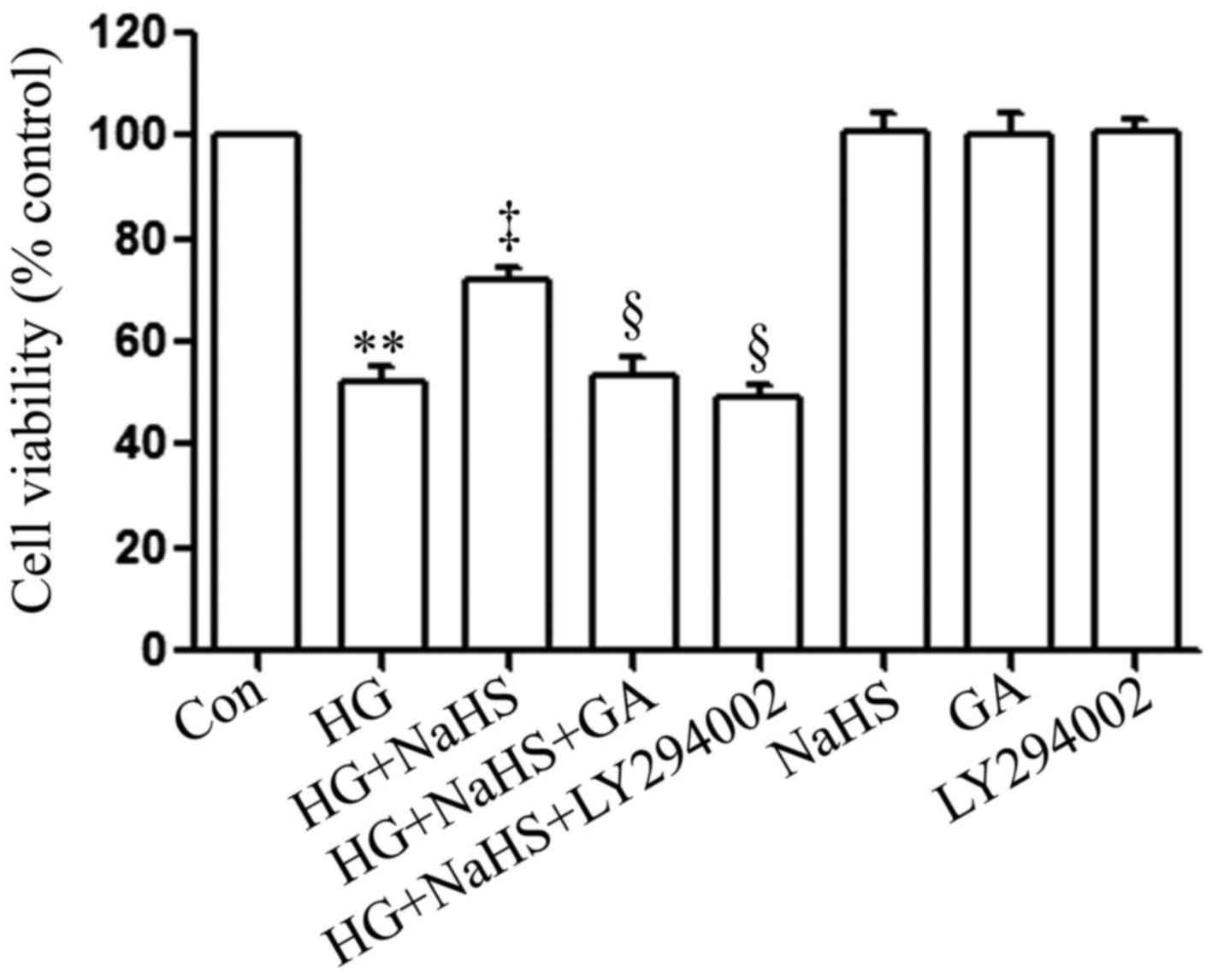
To determine whether the HSP90/Akt pathway is
involved in the protective effects of exogenous H2S
against HG-induced cytotoxicity, the H9c2 cardiac cells were
treated with GA (an inhibitor of HSP90) or LY294002 (an inhibitor
of Akt) prior to exposure to NaHS and HG. As shown in Fig. 3, in agreement with our findings
from our recent studies (9,20),
treatment of the cells with 400 µM NaHS for 30 min prior to
exposure to 35 mM glucose (HG) for 24 h markedly attenuated
HG-induced cytotoxicity, evidenced by an increase in cell
viability. However, treatment of the cells with 1 µM GA or
30 µM LY294002 for 30 min prior to exposure to NaHS and HG
significantly blocked the anti-cytotoxic effects of exogenous
H2S, leading to a decrease in cell viability. When used
alone, GA or LY294002 did not affect the viability of the H9c2
cardiac cells. These results suggest that the HSP90/Akt pathway is
involved in the protective effects of exogenous H2S
against HG-induced cytotoxicity in H9c2 cardiac cells.
Role of the HSP90/Akt pathway in the
protective effects of exogenous H2S against the
HG-induced apoptosis in H9c2 cardiac cells
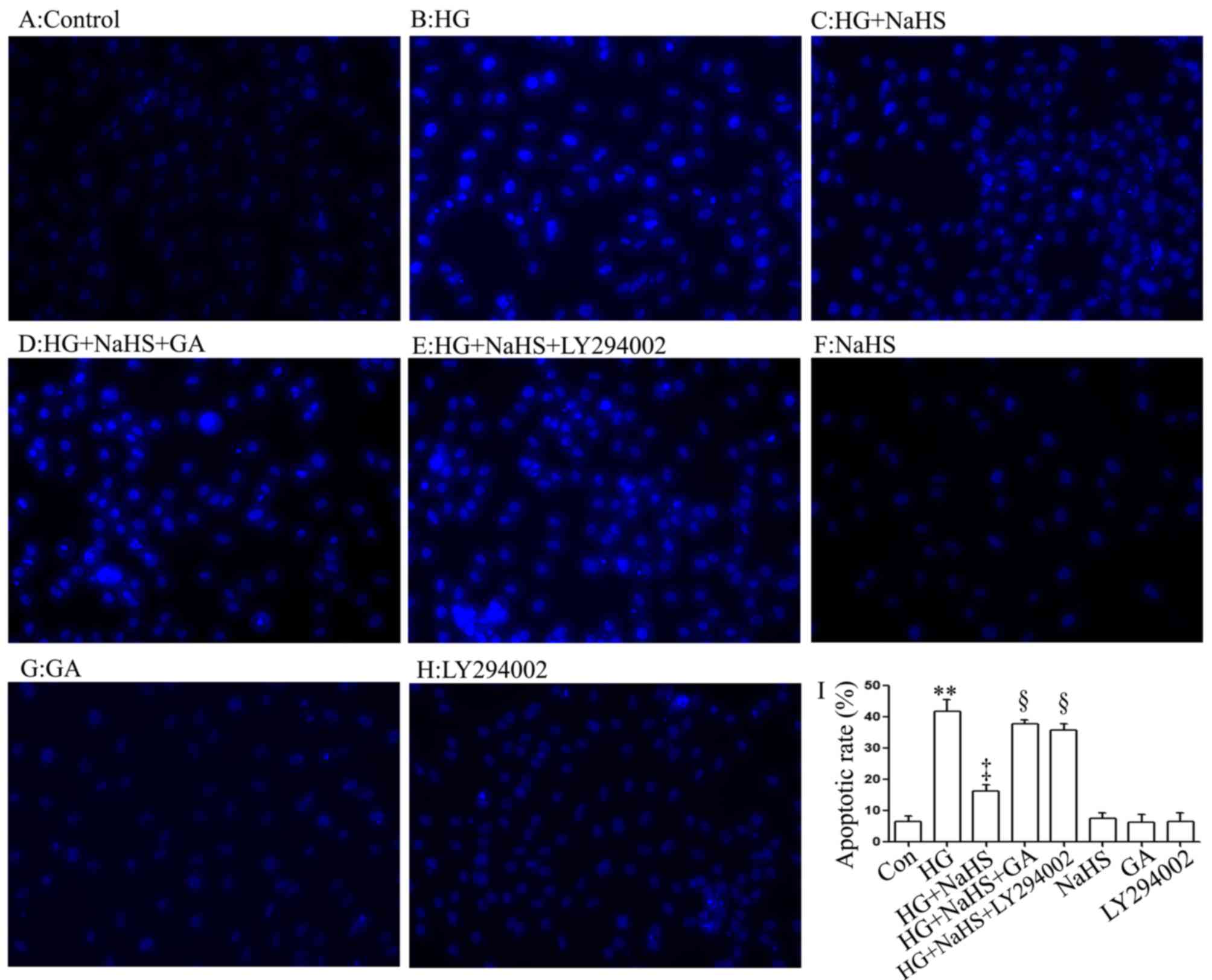
Consistent with the findings of our recent studies
(9,20), treatment of the cells with 400
µM NaHS for 30 min prior to exposure to 35 mM glucose (HG)
for 24 h significantly attenuated the HG-induced increase in the
number of apoptotic cells, which presented nuclear condensation and
fragmentation (Fig. 4C and I).
However, the inhibitory effects of NaHS on the HG-induced increase
in the number of apoptotic cells were markedly attenuated by
treatment of the cells with 1 µM GA (Fig. 4D and I) or 30 µM LY294002
(Fig. 4E and I) for 30 min prior
to exposure to NaHS and HG. The use of GA or LY294002 alone did not
significantly alter the percentage of apoptotic H9c2 cardiac cells
(Fig. 4G–I). These results
indicated that the HSP90/Akt pathway is involved in the protective
effect of exogenous H2S against the HG-induced apoptosis
of H9c2 cardiac cells.
Role of the HSP90/Akt pathway in the
protective effects of exogenous H2S against the
HG-induced loss of MMP in H9c2 cardiac cells
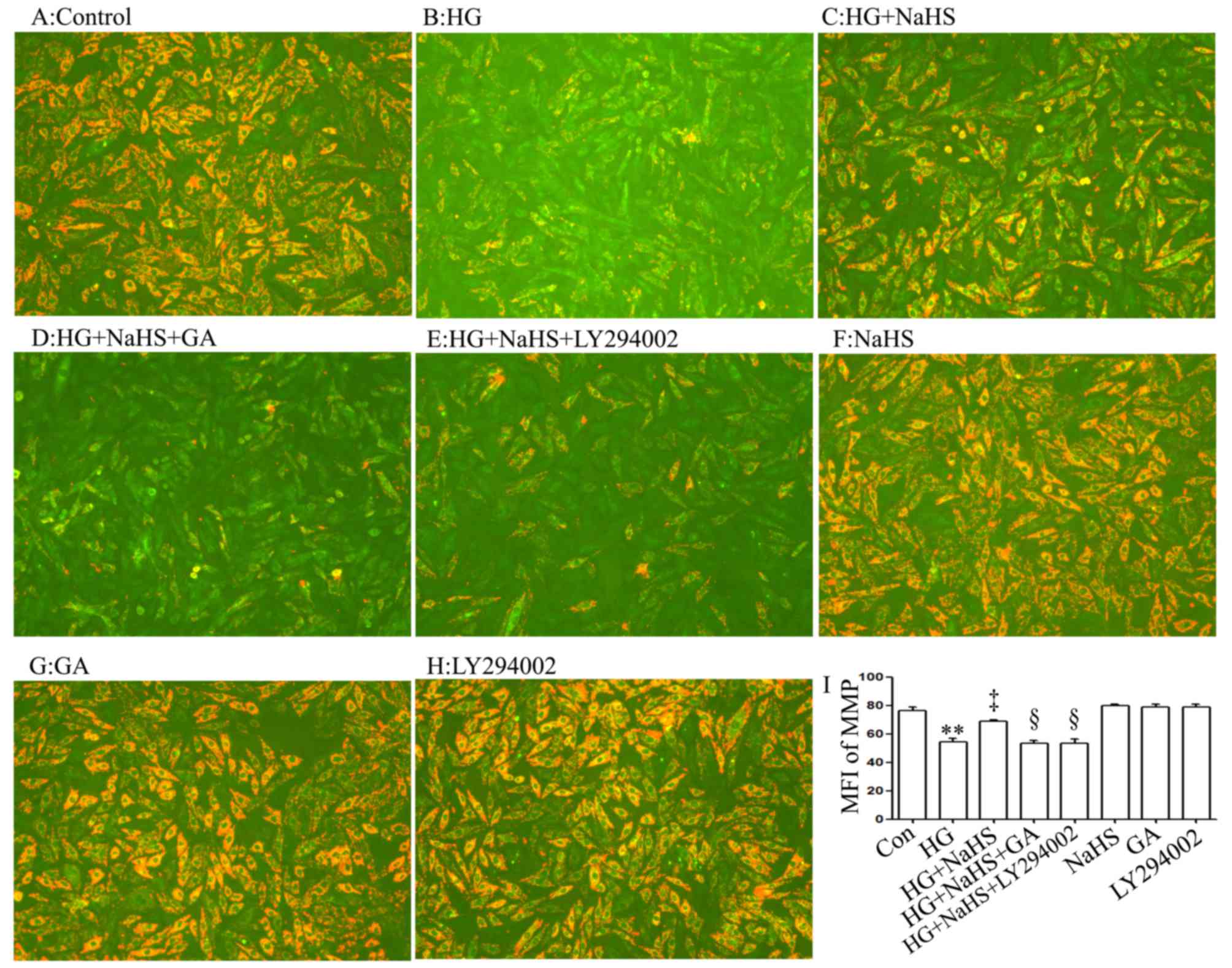
As shown in Fig. 5C
and I, treatment of the cells with 400 µM NaHS for 30
min prior to exposure to HG for 24 h markedly alleviated the
HG-induced mitochondrial insult, as evidenced by a decrease in the
dissipation of MMP. Of note, the decreased dissipation of MMP was
antagonized by treatment of the cells with 1 µM GA (Fig. 5D and I) or 30 µM LY294002
(Fig. 5E and I) for 30 min prior
to exposure to NaHS and HG, suggesting that the HSP90/Akt pathway
is involved in the protective effects of exogenous H2S
against the HG-induced dissipation of MMP in H9c2 cardiac
cells.
Role of the HSP90/Akt pathway in the
protective effects of exogenous H2S against HG-induced
oxidative stress in H9C2 cardiac cells
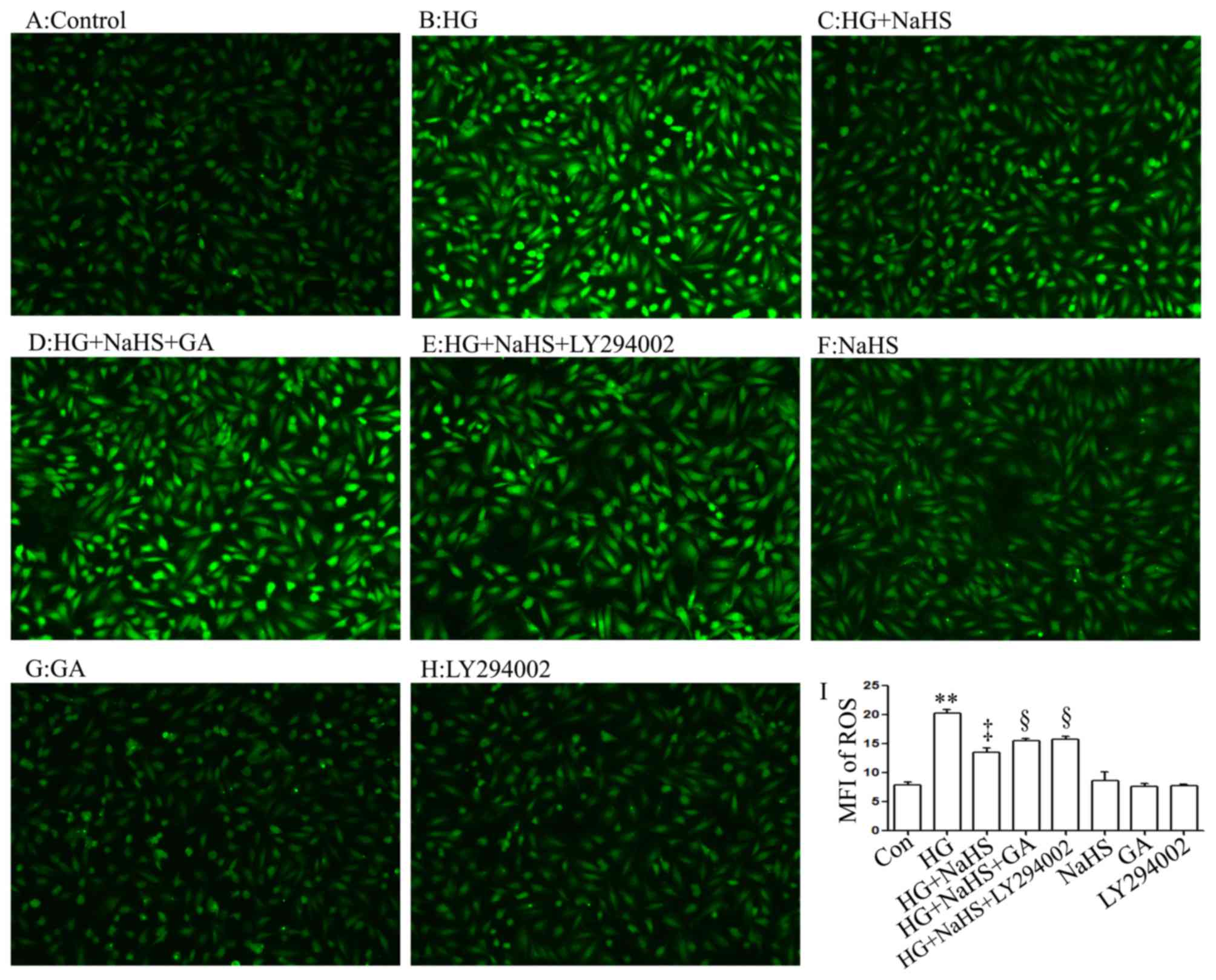
In agreement with the findings of our recent studies
(9,20), treatment of the H9c2 cells with
400 µM NaHS for 30 min prior to exposure to 35 mM glucose
(HG) for 24 h markedly decreased the generation of intracellular
reactive oxygen species (ROS) induced by HG (Fig. 6C and I). Importantly, treatment of
the cells with 1 µM GA (Fig.
6D and I) or 30 µM LY294002 (Fig. 6E and I) for 30 min prior to
exposure to NaHS and HG markedly blocked the inhibitory effects of
NaHS on ROS generation, leading to an increase in ROS generation.
When used alone, NaHS, GA or LY294002 did not alter the basal
levels of ROS generation (Fig. 6F, G
and H).
Role of the HSP90/Akt pathway in the
protective effects of exogenous H2S against the
HG-induced inhibitory effect on the activity of SOD
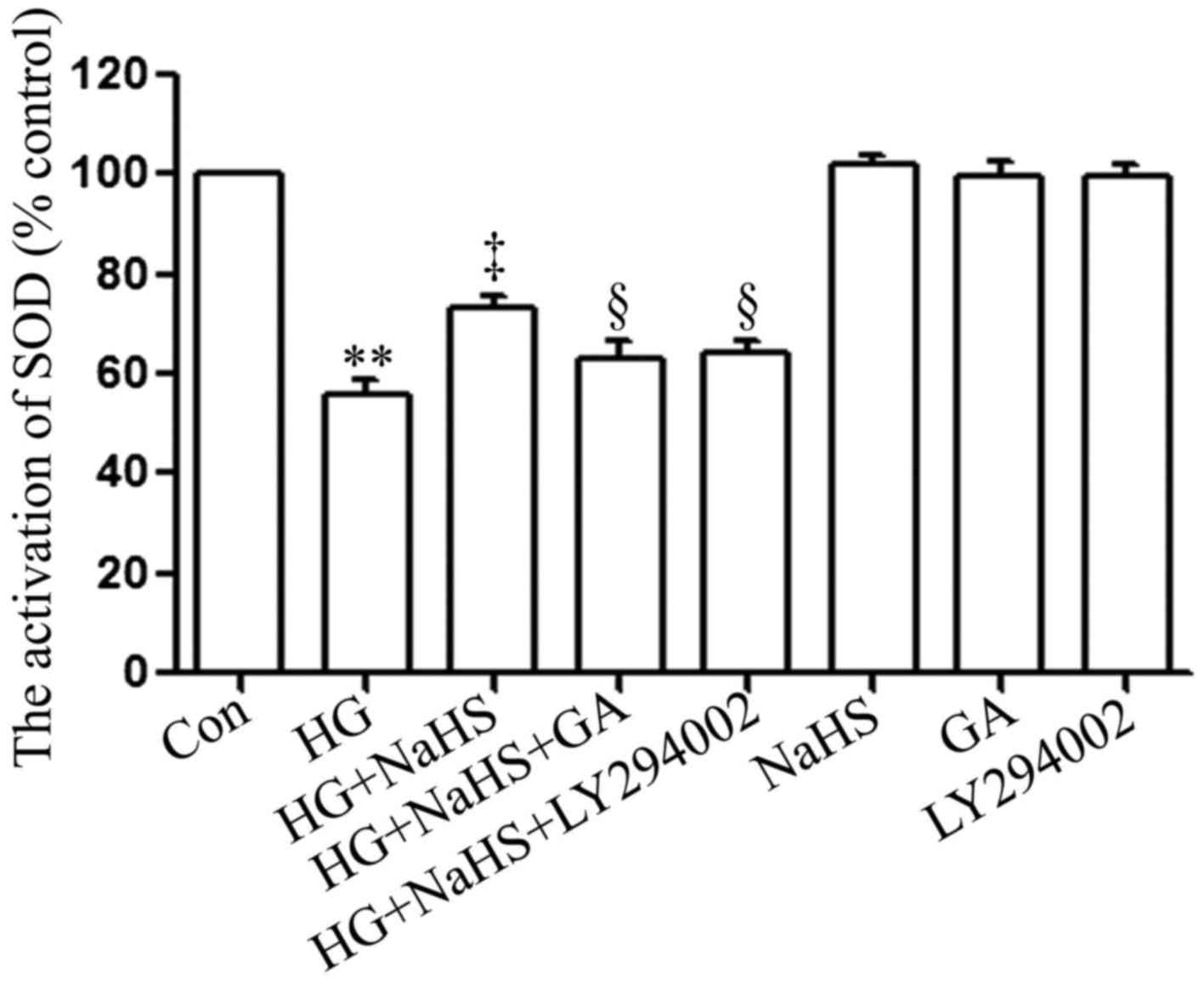
SOD is a significant antioxidant system. As shown in
Fig. 7, following exposure to 35
µM glucose (HG) for 24 h, SOD activity in the H9c2 cardiac
cells was considerably decreased compared with that of the control
group. Notably, the HG-induced decrease in SOD activity was
significantly attenuated by treatment of the cells with 400
µM NaHS for 30 min prior to exposure to HG for 24 h.
However, treatment of the cells with 1 µM GA or 30 µM
LY294002 for 30 min prior to exposure to NaHS and HG markedly
hindered the protective effects of NaHS against the HG-induced
decrease in SOD activity. When used alone, NaHS, GA or LY294002 did
not affect SOD activity in the H9c2 cardiac cells.
Discussion
Previous studies have demonstrated that HSP90
(3,24,25,27) or Akt (34,35,37,42,43) are cardioprotective and that
hyperglycemia impairs HSP90 in endothelial cells (26) and cardiac Akt in
streptozotocin-treated rats (34,35). The present study extends the
findings of previous studies and provides new evidence that
HG-induced H9c2 cardiomyocyte injury is associated with the
impaired HSP90/Akt pathway, as evidenced by the decreased
expression levels of HSP90 and Akt. Importantly, we demonstrate
exogenous H2S protects H9c2 cardiac cells against
HG-induced injury, by preventing cytotoxicity, apoptosis, ROS
overproduction, decreased SOD activity and the dissipation of MMP
through the activation of the HSP90/Akt pathway.
Several lines of evidence have demonstrated the
cardioprotective effects of HSP90. Hypoxia can enhance the
expression of HSP90 (44), which
efficiently ameliorates the myocardial I/R-induced myocardial
dysfunction (24). Recently, we
demonstrated that HSP90 exerts protective effects in H9c2 cardiac
cells exposed to chemically-induced hypoxia (3). However, few studies have explored
the association between HG-induced cardiovascular injury and HSP90.
Mohan et al reported that HG inhibited the HSP90-eNOS
interaction in endothelial cells (26). Hyperglycemia attenuates the
IPC-induced cardio-protective effects by disrupting the association
of HSP90 with eNOS (27). In
agreement with previous findings (26,27), our results revealed that HG
inhibited the expression level of HSP90 in H9c2 cardiac cells in a
time-dependent manner. To further explore the role of endogenous
HSP90 in HG-induced H9c2 cardiac cell injury, the cells were
co-exposed to HG and GA (an inhibitor of HSP90). Our data revealed
that co-treatment of the H9c2 cardiac cells with HG and GA markedly
aggravated HG-induced injury, including cyotoxicity, apoptosis, ROS
overproduction and the loss of MMP (data not shown). These results
suggest that HG-induced cardiomyocyte injury is at least in part
associated with the impaired HSP90 pathway.
Since Akt is downstream of PI3K and one of the HSP90
substrates, its roles in cardiovascular protection have also
attracted considerable attention. Chanoit et al reported
that Akt mediated exogenous zinc-induced cardioprotection against
reperfusion injury (45). The Akt
pathway is also involved in the cardioprotection of substance P
against ischemic/hypoxia-induced myocardial cell death (46). Moreover, the impaired activation
of Akt has been shown to participate in diabetic cardiomyopathy
(34,35). Consistent with previous studies
(34,35), the present study demonstrated that
HG markedly downregulated the expression level of Akt in H9c2
cardiac cells. Additionally, treatment of the cells with GA (an
inhibitor of HSP90) prior to exposure to HG markedly aggravated the
inhibitory effect of HG on the expression level of Akt, suggesting
the modulatory effect of endogenous HSP90 on Akt activation. To
further investigate the role of the impaired Akt pathway in
HG-induced cardiomyocyte injury, the cells were co-exposed to HG
and LY294002 (an inhibitor of Akt). Our findings indicated that
co-treatment of the H9c2 cardiac cells with HG and LY294002
considerably aggravated HG-induced injury, as evidenced by a
decrease in cell viability and SOD activity, and an increase in the
number of apoptotic cells, ROS generation and the dissipation of
MMP (data not shown). Combined with the above-mentioned results
that the impaired HSP90 pathway is implicated in HG-induced
cardiomyocyte injury, these findings indicate that the impaired
HSP90/Akt pathway contributes to HG-induced injury to H9c2 cardiac
cells. A recent study demonstrating that the impaired HSP90/Akt
signaling pathway plays a role in the induction of myocardial
caspase-3 activation and apoptosis in the septic mice (37) supports our results.
Another novel finding of the present study relates
to the role of activation of the HSP90/Akt pathway in the
cardioprotective effects of exogenous H2S against
HG-induced cardiac injury. As a novel gasomolecule with
cardiovascular protective effect, H2S executes the
physiological functions of vasorelaxation, cardioprotection and the
inhibition of vascular remodeling (47). Recently, the protective effects of
H2S against DM-related cardiovascular damage have
received more attention. It has been reported that exogenous
H2S diminishes I/R-induced injury in db/db mice
(18) and diabetic rats (19). Recently, we demonstrated that
exogenous H2S protected H9c2 cardiac cells against
HG-induced injury and inflammation by inhibiting the MAPK (19) and NF-κB (20) pathways. However, the mechanisms
underlying these cardio-protective effects of H2S are
not yet uncompletively clear. Since H2S has been shown
to activate HSP90 (3,28–30) or Akt (38,39) in a variety of cells, including
cardiomyocytes, and this study demonstrated the involvement of the
HSP90/Akt pathway in HG-induced cardiomyocyte insults, this
promotes us to further investigate the roles of the HSP90/Akt
pathway in the cardio-protective effects of exogenous
H2S against HG-induced injury. In agreement with the
findings of our recent studies (19,20) and other studies (9,18,19), our results revealed that exogenous
H2S exerts protective effects against HG-induced
cardiomyocyte injury, leading to an increase in cell viability and
SOD activity, and a decrease in the number of apoptotic cells, ROS
generation and to the decreased dissipation of MMP. Importantly,
exogenous H2S markedly blocked the inhibitory effects of
HG on the expression levels of HSP90 and Akt. In order to explore
the roles of the HSP90/Akt pathway in the cardioprotective effects
of exogenous H2S against HG-induced injuryy, H9c2
cardiac cells were treated with GA (an inhibitor of HSP90) or
LY294002 (an inhibitor of Akt) prior to exposure to NaHS and HG.
Our results revealed that both GA and LY294002 markedly attenuated
the cardioprotective effects of exogenous H2S against
HG-induced injury, resulting in a decrease in cell viability and
SOD activity, and in an increase in the number of apoptotic cells,
ROS generation and the dissipation of MMP. These results suggest
that the activation of the HSP90/Akt pathway plays important roles
in the cardioprotective effects of exogenous H2S against
HG-induced injury.
Taken together, the findings of this study clearly
indicate that the impaired HSP90/Akt pathway may be one of the
important mechanisms responsible for HG-induced cardiomyocyte
injury. This study also provides evidence that the HSP90/Akt
pathway contributes to the cardioprotective effects of exogenous
H2S against HG-induced injury, including cytotoxicity,
apoptosis, oxidative stress and the dissipation of MMP. Further
studies using animal models will likely lead to an improved
understanding of this signaling pathway and its newly discovered
pathophysiological effects in diabites-related cardiovascular
complications.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81270296 and
81370309), Medical Scientific Research Foundation of Guangdong
Province (A2015287), Technology Planning Project (201544-01) of
Huangpu District, Guangdong Natural Science Foundation (no.
S2013010015073) and the Scientific and Technological Projects of
Guangzhou City (201604020129).
References
|
1
|
Wang R: Hydrogen sulfide: The third
gasotransmitter in biology and medicine. Antioxid Redox Signal.
12:1061–1064. 2010. View Article : Google Scholar
|
|
2
|
Li L, Rose P and Moore PK: Hydrogen
sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 51:169–187.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Z, Yang C, Xiao L, Liao X, Lan A,
Wang X, Guo R, Chen P, Hu C and Feng J: Novel insights into the
role of HSP90 in cytoprotection of H2S against chemical
hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol Med.
28:397–403. 2011.PubMed/NCBI
|
|
4
|
Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC,
Huang SH, Tan CS, Whiteman M, Lu J and Moore PK: Hydrogen sulfide
and its possible roles in myocardial ischemia in experimental rats.
J Appl Physiol (1985). 102:261–268. 2007. View Article : Google Scholar
|
|
5
|
Ji Y, Pang QF, Xu G, Wang L, Wang JK and
Zeng YM: Exogenous hydrogen sulfide postconditioning protects
isolated rat hearts against ischemia-reperfusion injury. Eur J
Pharmacol. 587:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bliksøen M, Kaljusto ML, Vaage J and
Stensløkken KO: Effects of hydrogen sulphide on
ischaemia-reperfusion injury and ischaemic preconditioning in the
isolated, perfused rat heart. Eur J Cardiothorac Surg. 34:344–349.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang
Y, Du J and Tang C: Endogenous hydrogen sulfide regulation of
myocardial injury induced by isoproterenol. Biochem Biophys Res
Commun. 318:756–763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen SL, Yang CT, Yang ZL, Guo RX, Meng
JL, Cui Y, Lan AP, Chen PX and Feng JQ: Hydrogen sulphide protects
H9c2 cells against chemical hypoxia-induced injury. Clin Exp
Pharmacol Physiol. 37:316–321. 2010. View Article : Google Scholar
|
|
9
|
Zhuang XD, Hu X, Long M, Dong XB, Liu DH
and Liao XX: Exogenous hydrogen sulfide alleviates high
glucose-induced cardiotoxicity via inhibition of leptin signaling
in H9c2 cells. Mol Cell Biochem. 391:147–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo R, Lin J, Xu W, Shen N, Mo L, Zhang C
and Feng J: Hydrogen sulfide attenuates doxorubicin-induced
cardiotoxicity by inhibition of the p38 MAPK pathway in H9c2 cells.
Int J Mol Med. 31:644–650. 2013.PubMed/NCBI
|
|
11
|
Li H, Wang Y, Wei C, Bai S, Zhao Y, Li H,
Wu B, Wang R, Wu L and Xu C: Mediation of exogenous hydrogen
sulfide in recovery of ischemic post-conditioning-induced
cardioprotection via down-regulating oxidative stress and
up-regulating PI3K/Akt/GSK-3β pathway in isolated aging rat hearts.
Cell Biosci. 5:112015. View Article : Google Scholar
|
|
12
|
Predmore BL, Kondo K, Bhushan S,
Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME and
Lefer DJ: The poly-sulfide diallyl trisulfide protects the ischemic
myocardium by preservation of endogenous hydrogen sulfide and
increasing nitric oxide bioavailability. Am J Physiol Heart Circ
Physiol. 302:H2410–H2418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XY, Yang CT, Zheng DD, Mo LQ, Lan AP,
Yang ZL, Hu F, Chen PX, Liao XX and Feng JQ: Hydrogen sulfide
protects H9c2 cells against doxorubicin-induced cardiotoxicity
through inhibition of endoplasmic reticulum stress. Mol Cell
Biochem. 363:419–426. 2012. View Article : Google Scholar
|
|
14
|
Jain SK, Bull R, Rains JL, Bass PF, Levine
SN, Reddy S, McVie R and Bocchini JA Jr: Low levels of hydrogen
sulfide in the blood of diabetes patients and
streptozotocin-treated rats causes vascular inflammation? Antioxid
Redox Signal. 12:1333–1337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki K, Olah G, Modis K, Coletta C, Kulp
G, Gerö D, Szoleczky P, Chang T, Zhou Z, Wu L, et al: Hydrogen
sulfide replacement therapy protects the vascular endothelium in
hyperglycemia by preserving mitochondrial function. Proc Natl Acad
Sci USA. 108:13829–13834. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmad FU, Sattar MA, Rathore HA, Abdullah
MH, Tan S, Abdullah NA and Johns EJ: Exogenous hydrogen sulfide
(H2S) reduces blood pressure and prevents the
progression of diabetic nephropathy in spontaneously hypertensive
rats. Ren Fail. 34:203–210. 2012. View Article : Google Scholar
|
|
17
|
Whiteman M, Gooding KM, Whatmore JL, Ball
CI, Mawson D, Skinner K, Tooke JE and Shore AC: Adiposity is a
major determinant of plasma levels of the novel vasodilator
hydrogen sulphide. Diabetologia. 53:1722–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peake BF, Nicholson CK, Lambert JP, Hood
RL, Amin H, Amin S and Calvert JW: Hydrogen sulfide preconditions
the db/db diabetic mouse heart against ischemia-reperfusion injury
by activating Nrf2 signaling in an Erk-dependent manner. Am J
Physiol Heart Circ Physiol. 304:H1215–H1224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Y, Yao X, Zhang Y, Li W, Kang K, Sun L
and Sun X: The protective role of hydrogen sulfide in myocardial
ischemia-reperfusion-induced injury in diabetic rats. Int J
Cardiol. 152:177–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu W, Chen J, Lin J, Liu D, Mo L, Pan W,
Feng J, Wu W and Zheng D: Exogenous H2S protects H9c2
cardiac cells against high glucose-induced injury and inflammation
by inhibiting the activation of the NF-κB and IL-1β pathways. Int J
Mol Med. 35:177–186. 2015.
|
|
21
|
Zhou X, An G and Lu X: Hydrogen sulfide
attenuates the development of diabetic cardiomyopathy. Clin Sci
(Lond). 128:325–335. 2015. View Article : Google Scholar
|
|
22
|
Piper PW: The Hsp90 chaperone as a
promising drug target. Curr Opin Investig Drugs. 2:1606–1610.
2001.
|
|
23
|
Terasawa K, Minami M and Minami Y:
Constantly updated knowledge of Hsp90. J Biochem. 137:443–447.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kupatt C, Dessy C, Hinkel R, Raake P,
Daneau G, Bouzin C, Boekstegers P and Feron O: Heat shock protein
90 transfection reduces ischemia-reperfusion-induced myocardial
dysfunction via reciprocal endothelial NO synthase serine 1177
phosphorylation and threonine 495 dephosphorylation. Arterioscler
Thromb Vasc Biol. 24:1435–1441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiao JD, Garg V, Yang B and Hu K: Novel
functional role of heat shock protein 90 in ATP-sensitive
K+ channel-mediated hypoxic preconditioning. Cardiovasc
Res. 77:126–133. 2008. View Article : Google Scholar
|
|
26
|
Mohan S, Konopinski R, Yan B, Centonze VE
and Natarajan M: High glucose-induced IKK-Hsp-90 interaction
contributes to endothelial dysfunction. Am J Physiol Cell Physiol.
296:C182–C192. 2009. View Article : Google Scholar :
|
|
27
|
Vladic N, Ge ZD, Leucker T, Brzezinska AK,
Du JH, Shi Y, Warltier DC, Pratt PF Jr and Kersten JR: Decreased
tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by
hyperglycemia impair myocardial ischemic preconditioning. Am J
Physiol Heart Circ Physiol. 301:H2130–H2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tay AS, Hu LF, Lu M, Wong PT and Bian JS:
Hydrogen sulfide protects neurons against hypoxic injury via
stimulation of ATP-sensitive potassium channel/protein kinase
C/extracellular signal-regulated kinase/heat shock protein 90
pathway. Neuroscience. 167:277–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jha S, Calvert JW, Duranski MR,
Ramachandran A and Lefer DJ: Hydrogen sulfide attenuates hepatic
ischemia-reperfusion injury: Role of antioxidant and antiapoptotic
signaling. Am J Physiol Heart Circ Physiol. 295:H801–H806. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng JL, Mei WY, Dong YF, Wang JH, Zhao
CM, Lan AP, Yang CT, Chen PX, Feng JQ and Hu CH: Heat shock protein
90 mediates cytoprotection by H2S against chemical
hypoxia-induced injury in PC12 cells. Clin Exp Pharmacol Physiol.
38:42–49. 2011. View Article : Google Scholar
|
|
31
|
Dummler B and Hemmings BA: Physiological
roles of PKB/Akt isoforms in development and disease. Biochem Soc
Trans. 35:231–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Semple D, Smith K, Bhandari S and Seymour
AM: Uremic cardiomyopathy and insulin resistance: A critical role
for akt? J Am Soc Nephrol. 22:207–215. 2011. View Article : Google Scholar
|
|
33
|
Yu Q, Gao F and Ma XL: Insulin says NO to
cardiovascular disease. Cardiovasc Res. 89:516–524. 2011.
View Article : Google Scholar
|
|
34
|
Laviola L, Belsanti G, Davalli AM, Napoli
R, Perrini S, Weir GC, Giorgino R and Giorgino F: Effects of
streptozocin diabetes and diabetes treatment by islet
transplantation on in vivo insulin signaling in rat heart.
Diabetes. 50:2709–2720. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gurusamy N, Watanabe K, Ma M, Prakash P,
Hirabayashi K, Zhang S, Muslin AJ, Kodama M and Aizawa Y: Glycogen
synthase kinase 3beta together with 14-3-3 protein regulates
diabetic cardiomyopathy: Effect of losartan and tempol. FEBS Lett.
580:1932–1940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeon YK, Park CH, Kim KY, Li YC, Kim J,
Kim YA, Paik JH, Park BK, Kim CW and Kim YN: The heat-shock protein
90 inhibitor, geldanamycin, induces apoptotic cell death in
Epstein-Barr virus-positive NK/T-cell lymphoma by Akt
down-regulation. J Pathol. 213:170–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X, Luo R, Jiang R, Meng X, Wu X, Zhang
S and Hua W: The role of the Hsp90/Akt pathway in myocardial
calpain-induced caspase-3 activation and apoptosis during sepsis.
BMC Cardiovasc Disord. 13:82013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manna P and Jain SK: Hydrogen sulfide and
L-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3)
and glucose utilization by inhibiting phosphatase and tensin
homolog (PTEN) protein and activating phosphoinositide 3-kinase
(PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ
(PKCζ/λ) in 3T3l1 adipocytes. J Biol Chem. 286:39848–39859. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao LL, Huang XW, Wang YG, Cao YX, Zhang
CC and Zhu YC: Hydrogen sulfide protects cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis by preventing
GSK-3beta-dependent opening of mPTP. Am J Physiol Heart Circ
Physiol. 298:H1310–H1319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, Guo R, Yan H, Tian L, You Q, Li S,
Huang R and Wu K: Naringin inhibits ROS-activated MAPK pathway in
high glucose-induced injuries in H9c2 cardiac cells. Basic Clin
Pharmacol Toxicol. 114:293–304. 2014. View Article : Google Scholar
|
|
41
|
Chen PM, Wu TC, Wang YC, Cheng YW, Sheu
GT, Chen CY and Lee H: Activation of NF-κB by SOD2 promotes the
aggressiveness of lung adenocarcinoma by modulating NKX2-1-mediated
IKKβ expression. Carcinogenesis. 34:2655–2663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Armstrong SC: Protein kinase activation
and myocardial ischemia/reperfusion injury. Cardiovasc Res.
61:427–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bae S and Zhang L: Gender differences in
cardioprotection against ischemia/reperfusion injury in adult rat
hearts: Focus on Akt and protein kinase C signaling. J Pharmacol
Exp Ther. 315:1125–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen JX and Meyrick B: Hypoxia increases
Hsp90 binding to eNOS via PI3K-Akt in porcine coronary artery
endothelium. Lab Invest. 84:182–190. 2004. View Article : Google Scholar
|
|
45
|
Chanoit G, Lee S, Xi J, Zhu M, McIntosh
RA, Mueller RA, Norfleet EA and Xu Z: Exogenous zinc protects
cardiac cells from reperfusion injury by targeting mitochondrial
permeability transition pore through inactivation of glycogen
synthase kinase-3beta. Am J Physiol Heart Circ Physiol.
295:H1227–H1233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jubair S, Li J, Dehlin HM, Manteufel EJ,
Goldspink PH, Levick SP and Janicki JS: Substance P induces
cardioprotection in ischemia-reperfusion via activation of AKT. Am
J Physiol Heart Circ Physiol. 309:H676–H684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhuo Y, Chen PF, Zhang AZ, Zhong H, Chen
CQ and Zhu YZ: Cardioprotective effect of hydrogen sulfide in
ischemic reperfusion experimental rats and its influence on
expression of survivin gene. Biol Pharm Bull. 32:1406–1410. 2009.
View Article : Google Scholar : PubMed/NCBI
|