Introduction
Ischemic cerebral vascular disease is a common
disease in clinical neurology, and is caused by problems with blood
supply to the brain. Ischemic cerebral vascular disease has high
rates of incidence, mortality and recurrence, and is severely
damaging to lifestyle and health (1,2).
Ultra-early thrombolytic therapy is currently being used to reduce
damage to brain tissue in cerebral vascular disease (3); however, the secondary damage caused
to the brain following reperfusion has gained much attention
(4–7). The exact mechanisms of injury in
cerebral ischemia-reperfusion are not yet clear. Findings
demonstrate that the possible mechanisms of ischemia-reperfusion
injury include the oxygen-derived free radical, nitric oxide (NO),
calcium overload in cells, the toxicity of excited amino acids, the
inflammatory reaction in local brain tissues and apoptosis
(8). Neuronal apoptosis plays an
important role in ischemia-reperfusion injury (9,10).
There are three types of apoptosis-related genes, including
pro-apoptotic genes, anti-apoptotic proteins and bidirectional
regulator genes. The Bcl-2 family of proteins consists of both
anti-apoptotic proteins, such as Bcl-2, and pro-apoptotic proteins
such as Bax, which participate in the regulation of apoptosis
(11,12). Caspase-3 has also been identified
as an enzyme that can trigger a cell apoptotic cascade reaction
(13,14).
We previously found that daidzein appeared to have a
local anesthetic action (15). It
has also been shown to exert protective effects against myocardial
ischemia-reperfusion injury in rats following coronary artery
lesion (16). Daidzein has an
antagonizing role towards Ca2+ (17); it impacts on auto-rhythmicity and
contractility of the right atrium in ex vivo rat hearts
(18), and it has also been shown
to induce the contribution of endothelium-derived hyperpolarizing
factor to endothelium relaxation in male rats and in vitro
arteries (19,20).
Daidzein is insoluble in water, which results in low
oral bioavailability and low efficacy; however, 3′-daidzein
sulfonate sodium (DSS) is a newly developed synthetic material with
increased water solubility, resulting from modification of the
structure of daidzein (an active ingredient of kudzu vine root).
There are few studies on the pharmacological effects of DSS
(21), and the protective effects
of DSS against apoptosis in cerebral ischemia have not yet been
reported, to the best of our knowledge. Li et al (22) reported that puerarin downregulated
caspase-3 protein expression and upregulated Bcl-2 protein
expression, which could play a neuroprotective role. However, that
study did not consider whether DSS exerts protective effects on
cerebral ischemia, or whether it is functionally active in reducing
apoptosis.
Thus, in the present study, we aimed to assess the
effects of DSS on blood-brain barrier (BBB) permeability, the
induction of apoptosis and the expression of Bcl-2, Bax and
caspase-3 detected by immunohistochemical methods and western blot
analysis.
Materials and methods
Materials
The Department of Naturally Occurring Drugs and
Chemistry, Shenyang Pharmaceutical University (Shenyang, China)
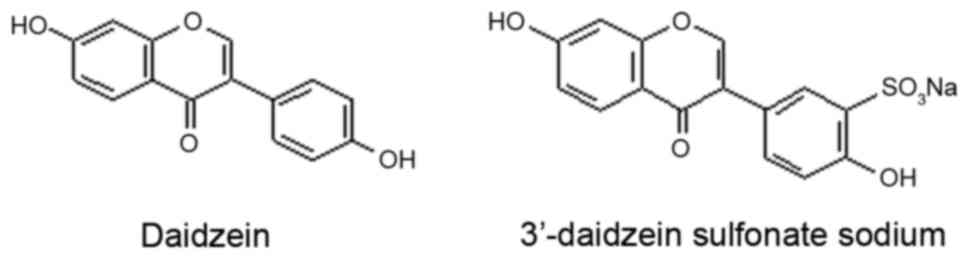
provided DSS (C15H907SNa) as a white crystalline powder, with a
purity of >99%. The chemical structures of daidzein and DSS are
shown in Fig. 1.
Adult male Sprague-Dawley (SD) rats (250–280 g,
n=240) were purchased from Hunan Silaike Jingda Laboratory Animal
Co., Ltd. (Changsha, China). The rats were maintained on a 12-h
light and dark cycle, allowed free access to food and water, and
allowed to adapt to laboratory conditions for 7 days before the
experiment.
All animal experiments were approved by the Animal
Care and Use Committee of Gannan Medical College, and conducted
according to the National Institutes of Health guidelines.
Preparation of the rat model of cerebral
ischemia-reperfusion injury
The rats were anesthetized with 10% chloral hydrate
(350 mg/kg, intraperitoneally), and a rat model of middle cerebral
artery occlusion (MCAO) was established according to the methods of
Longa et al (23). A 4-cm
long, 0.2-mm in diameter nylon thread was slowly inserted
anteriorly towards the direction of the internal carotid artery,
through an incision in the common carotid artery trunk. The common
carotid artery bifurcation was labeled. After inserting the thread
for 18–20 mm, a slight resistance was felt, indicating that the
tiny anterior cerebral artery had been reached. The blood supply of
the middle cerebral artery was blocked for 1 h. The nylon thread
was then pulled out, and the arterial stump was tied. Subcutaneous
tissue and skin were sutured. In the sham surgery group, the common
carotid artery, external carotid artery, and internal carotid
artery were exposed and isolated. The middle cerebral artery was
not occluded. During surgery, room temperature was maintained at
23–25°C.
Experimental grouping
The rats were randomly divided into 5 groups (8 rats
per group) as follows: sham surgery, cerebral ischemia-reperfusion
injury (model group), low-dose daidzein sulfonate sodium (0.5
mg/kg), moderate-dose daidzein sulfonate sodium (1.0 mg/kg) and
high-dose daidzein sulfonate sodium (2.0 mg/kg). Before being
reperfused, the different doses of daidzein sulfonate sodium (0.5,
1.0, 2.0 mg/kg) were administered to the drug treatment groups via
the sublingual vein. The sham surgery and model groups were
administered physiological saline (0.1 ml/100 g body weight) via
the sublingual vein.
Neurological deficit scores
Following 1 h of ischemia and 24 h of reperfusion in
each group, the degree of damage to the nervous system was
evaluated using the Longa grade point standard: a score of 0
indicated no neurologic deficit; a score of 1 (failure to extend
left forepaw fully) a mild focal neurologic deficit; a score of 2
(circling to the contralateral side) a moderate focal neurologic
deficit; a score of 3 (falling to the contralateral side) a severe
neurologic deficit; and a score of 4 was represented by no
spontaneous walk and unconsciousness.
Infarct volume measurement
After the neurological deficit tests, rats were
decapitated and their brains, including the cerebellum, lower brain
stem and olfactory bulb were removed, washed with normal saline
(NS), dried with filter paper and frozen. After freezing, the
forebrain was generally contained within the thickness of the 5
brain slices cut along the coronal plane. These sections were
incubated in 5 ml of 2% triphenyl tetrazolium chloride solution for
30 min at 37°C in the dark. After staining, non-ischemic regions
were colored red, while the infarcted regions were white. The
sections were then fixed with 10% formaldehyde. The infarct volume
was calculated using a DT-200 image analysis system (Nanjing Dongtu
Technology Co., Ltd, Nanjing, China).
Evaluation of the permeability of the
BBB
BBB permeability was measured according to the Evans
blue (EB) content in the brain tissues. After infarct modeling (or
DSS administration for DSS groups), the rats were immediately
injected with 0.25 ml of EB solution (0.5%, dissolved in NS) via
the tail vein. After 24 h, rats were narcotized and then heart
perfused with NS. The fore-brain tissue on the ischemic side was
then removed, and after weighing, tissues were homogenized in 7.5%
trichloroacetic acid (3 ml/g, wet weight). The homogenate was then
centrifuged at 12,000 × g at 4°C for 20 min. The optical density
(OD) value of the supernatant was read at 620 nm. A calibration
curve was set up using a series of EB solution concentrations, with
results being indicated by EB brain wet weight (µg).
Observation of neurovascular unit
ultrastructure by electron microscopy
The rats were narcotized 24 h after MCAO
reperfusion, and then internally fixated with 4% paraformaldehyde.
The parietal cortex brain tissue on the ischemic side was removed
and cut into 1 mm3 cubes, fixated for a further 2 h in a
paraformaldehyde-glutaraldehyde solution, and then 2 h in 1% osmic
acid at 4°C. After dehydration by methanol gradients, and
displacement by epoxypropane, the tissues were embedded and
polymerized in polyphenylene sulfide resin. Semi-thin sections were
then prepared, stained with methylene blue-azure, and observed
under an optical lens. Ultra-thin sections were then stained with
both uranyl acetate and lead citrate, and observed under an
electron microscope (Hitachi H-7650; Hitachi, Tokyo, Japan).
Immunohistochemical detection of Bcl-2,
Bax and caspase-3 expression
Following 24 h of cerebral ischemia-reperfusion, the
rats were anesthetized with 10% chloral hydrate (3.5 ml/kg,
intraperitoneally). A perfusion apparatus filled with 200 ml of NS
was immediately inserted into the left ventricle via the left
ventricular apex, and administration was continued with 300 ml of
4% paraformaldehyde over a period of 30 min. Brain tissues from the
ischemic side were removed and used to prepare paraffin-embedded
sections. These sections were incubated in 4% paraformaldehyde
overnight, washed with running water for 24 h, soaked in 50%
ethanol overnight, then stored in 70% ethanol, followed by 80%
ethanol overnight, followed by soaking in 90% ethanol for 1 h, 95%
ethanol for 45 min (twice), and 100% ethanol for 45 min (twice).
They were then twice-cleared in xylene, for 20 min each time,
before infiltration with wax in a drying machine at a temperature
of 60°C for 1 h and 1.5 h, respectively. They were then embedded in
paraffin, and the paraffin sections were deparaffinized and cut
into 4 µm sections, before dewaxing to water. The expression
of Bcl-2, Bax and caspase-3 were examined using an
immunohistochemistry kit (Booster Bioengineering Institute, Wuhan,
China) used according to the manufacturer's instructions. The
sections were incubated in 3% (v/v) H2O2 and
washed in distilled water at room temperature for 10 min (3 times)
to eliminate endogenous peroxidase activity.
Antigen retrieval protocol
The sections were soaked in 0.01 M citrate buffer
solution (pH 6.0) and boiled using a pressure cooker. The sections
were then rinsed one to two times with phosphate-buffered saline
(PBS) solution (pH 7.2–7.6), incubated in 1% bovine serum albumin
blocking buffer for 20 min at room temperature, and excess liquids
were then shaken off without further washing. The samples were then
incubated with an appropriate diluted primary antibody [Bcl-2
(sc-492; dilution, 1:100; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), Bax (sc-526; dilution, 1:100; Santa Cruz
Biotechnology, Inc.); caspase-3 (9664s; dilution, 1:75; Cell
Signaling Technology, Inc., Danvers, MA, USA] overnight at 4°C,
before washing in PBS solution (pH 7.2–7.6) 3 times, for 2 min each
time. They were then incubated for 20 min at 20–37°C with
biotinylated goat anti-rabbit IgG (SABC, SA2002, dilution, 1:100;
WuHan Boster Biotechnology Limited Company, Wuhan, China), followed
by washing in PBS solution (pH 7.2–7.6) 3 times, for 2 min each
time. The sections were incubated with streptavidin-biotin complex
(SABC; SA2002; WuHan Boster Biotechnology Limited Company) at
20–37°C for 20 min before washing in PBS solution (pH 7.2–7.6) 4
times, for 5 min each time.
DAB color development
The reagents A, B and C in the DAB color development
kit were added to 1 ml distilled water and mixed well. The solution
was then added to sections and the reaction was observed under a
microscope at room temperature for about 6 min. The sections were
then washed in distilled water, stained with hematoxylin,
dehydrated, cleared and mounted. Positive cells were identified by
yellow-brown granules in the cytoplasm under a light microscope.
The percentage of positive neuronal cells was estimated using 10
randomly selected, but representative, high-power visual fields per
section.
Western blot analysis of Bcl-2, Bax and
caspase-3 protein expression
The total proteins in cerebral ischemia-reperfusion
brain were purified, centrifuged, and collected in the supernatant.
Proteins were quantified with a bicinchoninic acid assay, separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), and transferred onto polyvinylidene fluoride membranes.
The membranes were incubated with primary antibodies to Bcl-2
(sc-492; 1:300), Bax (sc-526; 1:300) (both from Santa Cruz
Biotechnology, Inc.) and caspase-3 (9664s; 1:500; Cell Signaling
Technology, Inc.) overnight at 4°C. This was followed by incubation
with horseradish peroxidase secondary antibodies (1:1,000) for 2 h
at 37°C. Images were obtained using a gel image analysis system
(Universal Hood II, 721BR04565; Bio-Rad, Hercules, CA, USA) and OD
was measured using image lab 3.0 software. The ratio of the OD of
the target protein to β-actin was used to represent the relative
expression levels of the target proteins.
Statistical analysis
Data are expressed as the means ± SEM, and were
analyzed using GraphPad Prism version 5.01 software (GraphPad
Software, Inc., La Jolla, CA, USA). The group means were compared
using one-way analysis of variance (ANOVA) and a Q-test.
Comparisons between groups were conducted using paired t-tests. A
probability value of P<0.05 was used to determine a significant
difference.
Results
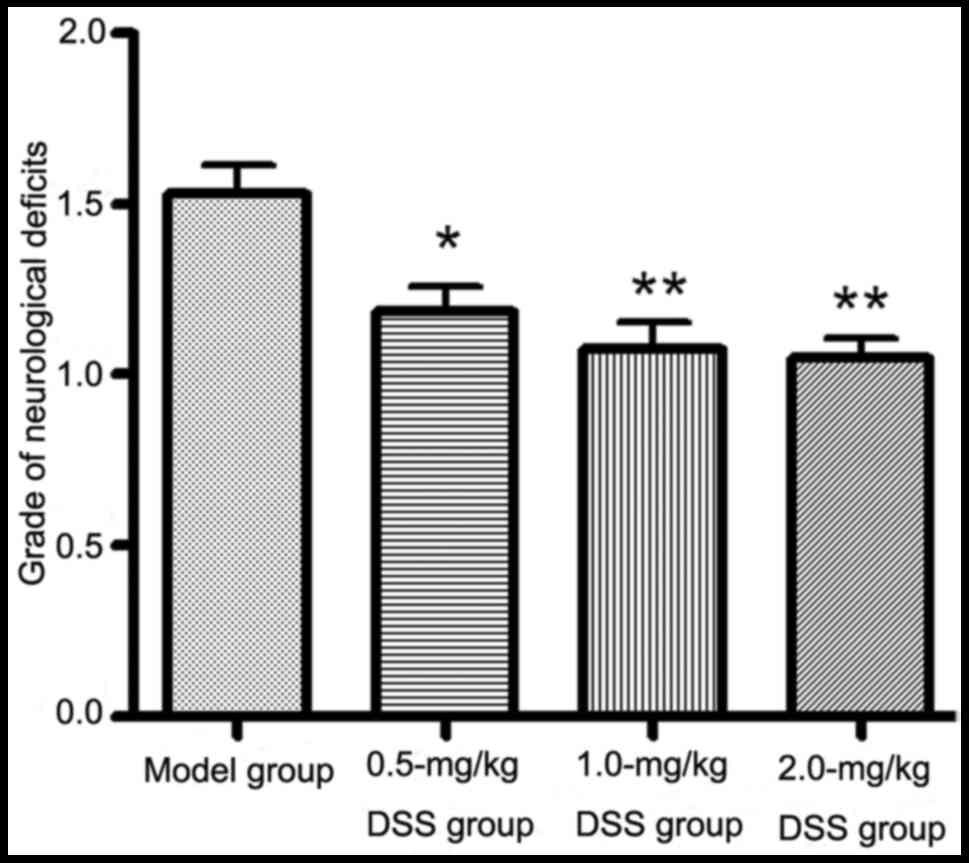
Effects of DSS on neurological
deficits
The neurological deficits in the rats pre-treated
with DSS (0.5, 1.0 and 2.0 mg/kg) were significantly lower than
those in the model group (P<0.05; Fig. 2).
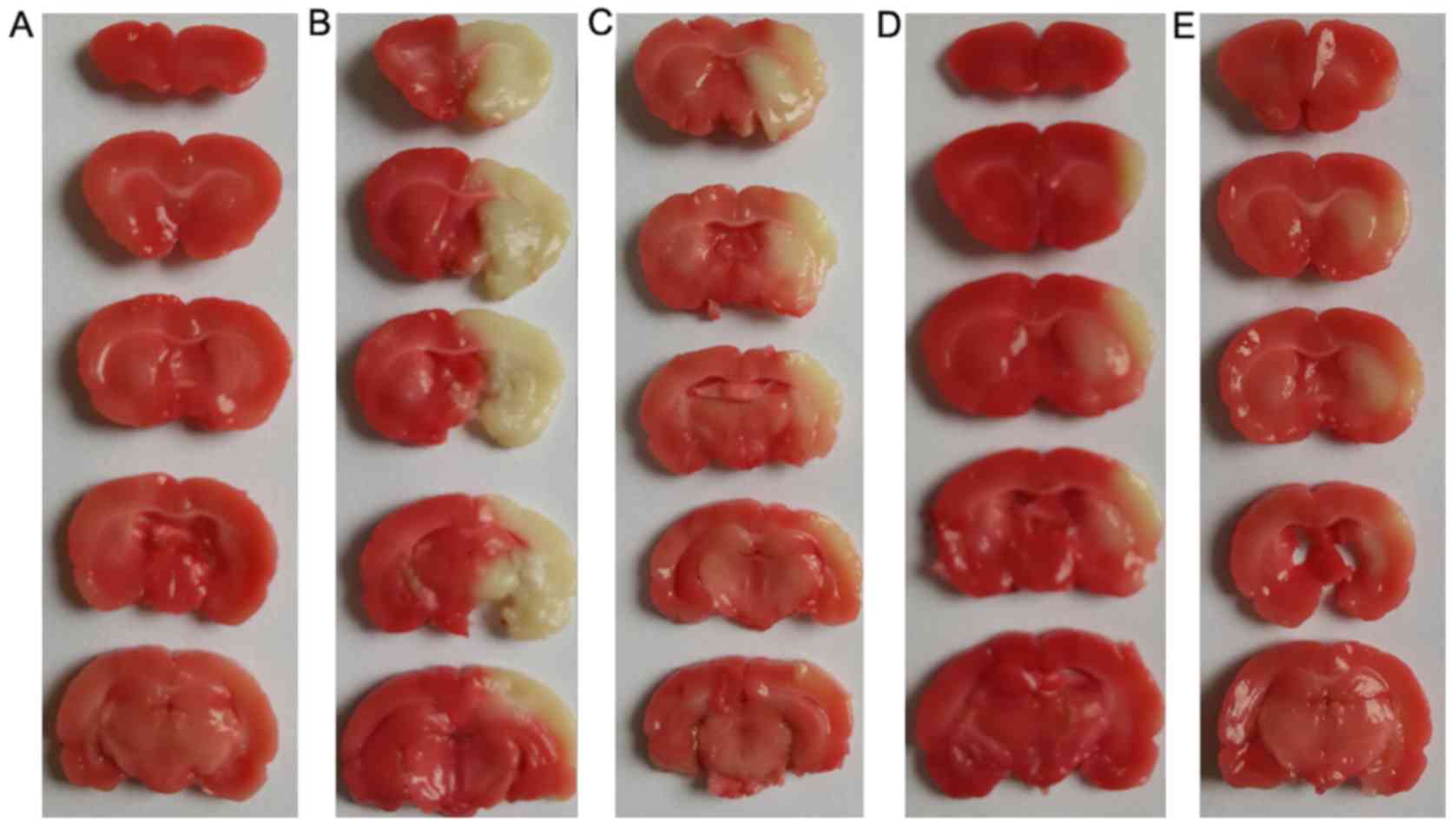
Effects of DSS on cerebral infarction
volume
Triphenyl tetrazolium chloride staining revealed
that after 24 h of cerebral ischemia-reperfusion, the infarcted
areas of the left cerebral hemisphere, which were mainly in the
frontal and parietal cortex, the caudate and the putamen, were
white. Normal tissues were stained red, with part of the brain
tissue in the penumbral area of the cerebral ischemia-reperfusion
injury present as a transition zone of white to red. Compared with
the model group, the cerebral infarction volume ratios exhibited
obvious reductions in all 3 of the DSS-treated groups (Fig. 3).
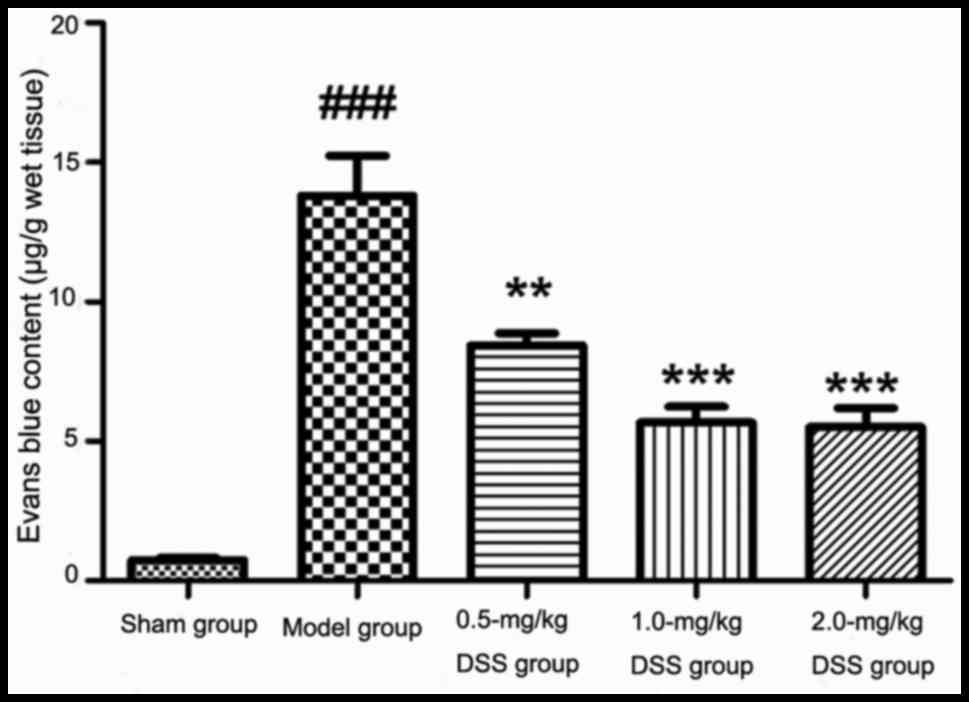
Effects of DSS on BBB permeability
As shown in Fig.
4, in comparison with the sham-operated group, BBB permeability
and the brain EB content were significantly increased in the model
group (P<0.001). The injection of different doses of DSS (0.5,
1.0 and 2.0 mg/kg) resulted in a significantly decreased brain EB
content in comparison with the model group. This indicated that DSS
attenuated the deterioration of BBB permeability in MCAO-induced
cerebral ischemia-reperfusion injury in rats.
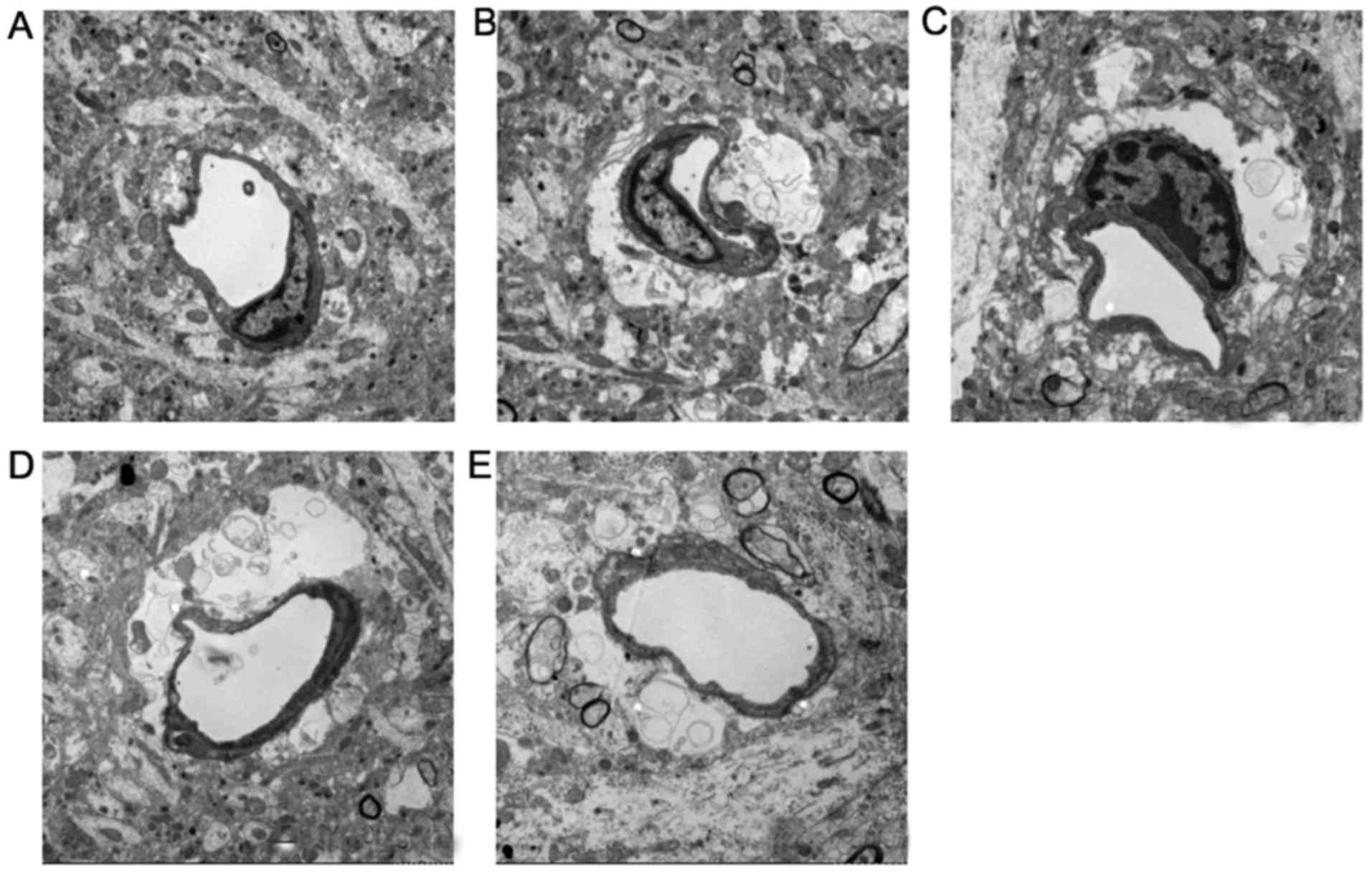
Effects of DSS on the ultrastructure of
the BBB
As shown in Fig.
5, in the sham-operated group, the BBB was intact, with intact
endothelium cells and a vascular wall structure. The perivascular
astrocytic foot processes and pericytes exhibited no swelling, and
the vessel lumen was not affected. In the model group, the
perivascular astrocytes exhibited obvious swelling, including
cytoplasmic vacuolation, edematous fluid, swelling of perivascular
foot processes, separation from basement membranes and narrowing of
the lumen. Compared with the model group, the swelling of the
astrocytes was significantly attenuated in the 0.5-mg/kg DDS group;
the vessel lumen had recovered and blood flow had been restored,
although the swelling of the pericytes could still be observed. In
the 1.0- and 2.0-mg/kg DDS groups, the swelling of the pericytes
and perivascular astrocytes in the BBB had obviously been
inhibited, indicating that DSS attenuated MCAO-induced cerebral
ischemia-reperfusion injury in the rat BBB (Fig. 5).
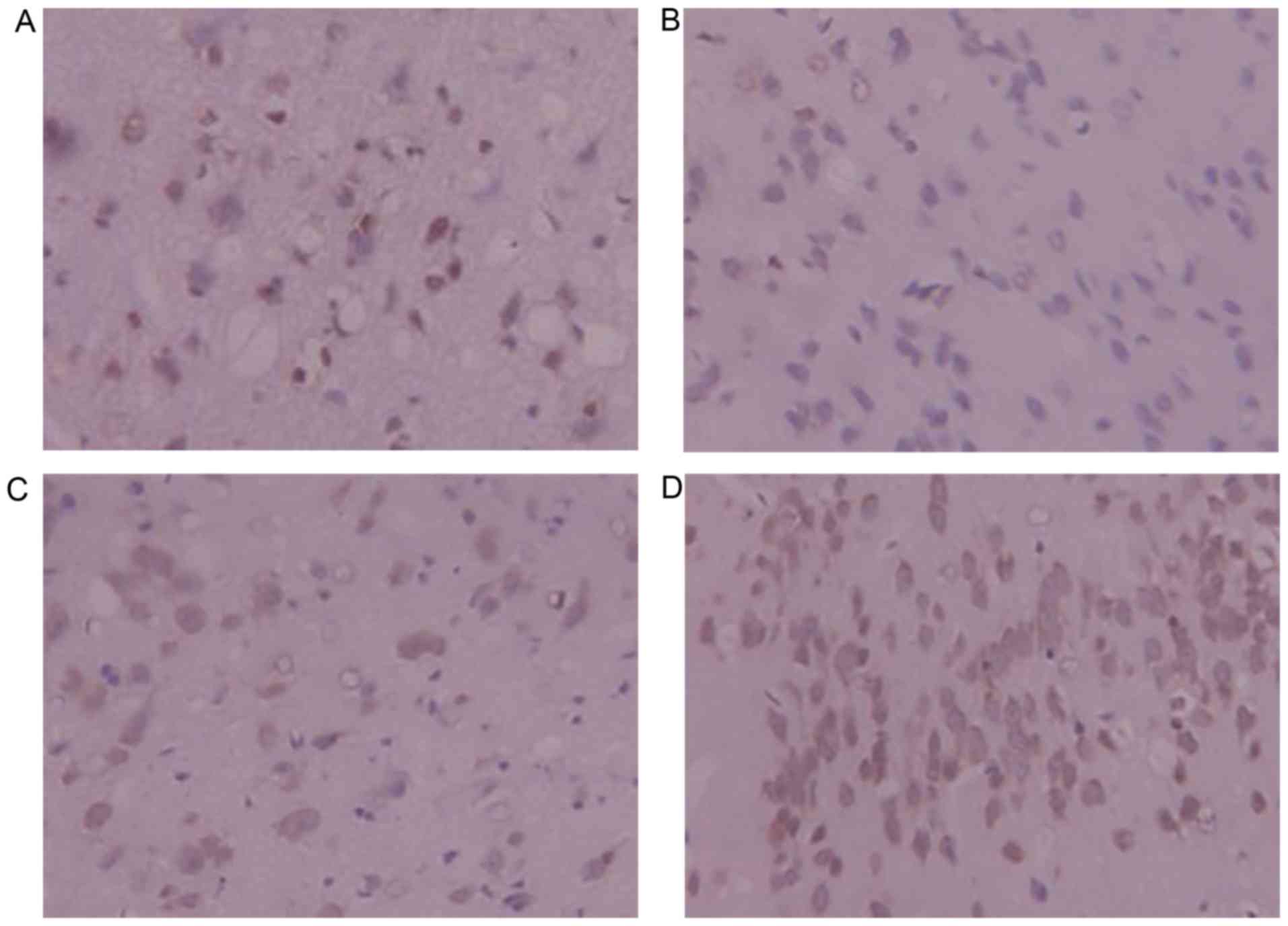
Immunohistochemistry for Bcl-2
Bcl-2-positive yellow-brown granules were observed
in the cytoplasm and some nuclei. Bcl-2-positive cells were
slightly decreased in number in the model group in comparison with
the sham-operated group. Compared with the model group, the number
of Bcl-2-positive cells exhibited a significant increase in the
DSS-treated groups; the positive cells were also stained more
deeply, indicating greater positivity. DSS treatment was shown to
upregulate Bcl-2 expression and inhibit apoptosis following
cerebral ischemia-reperfusion injury in rats (Fig. 6).
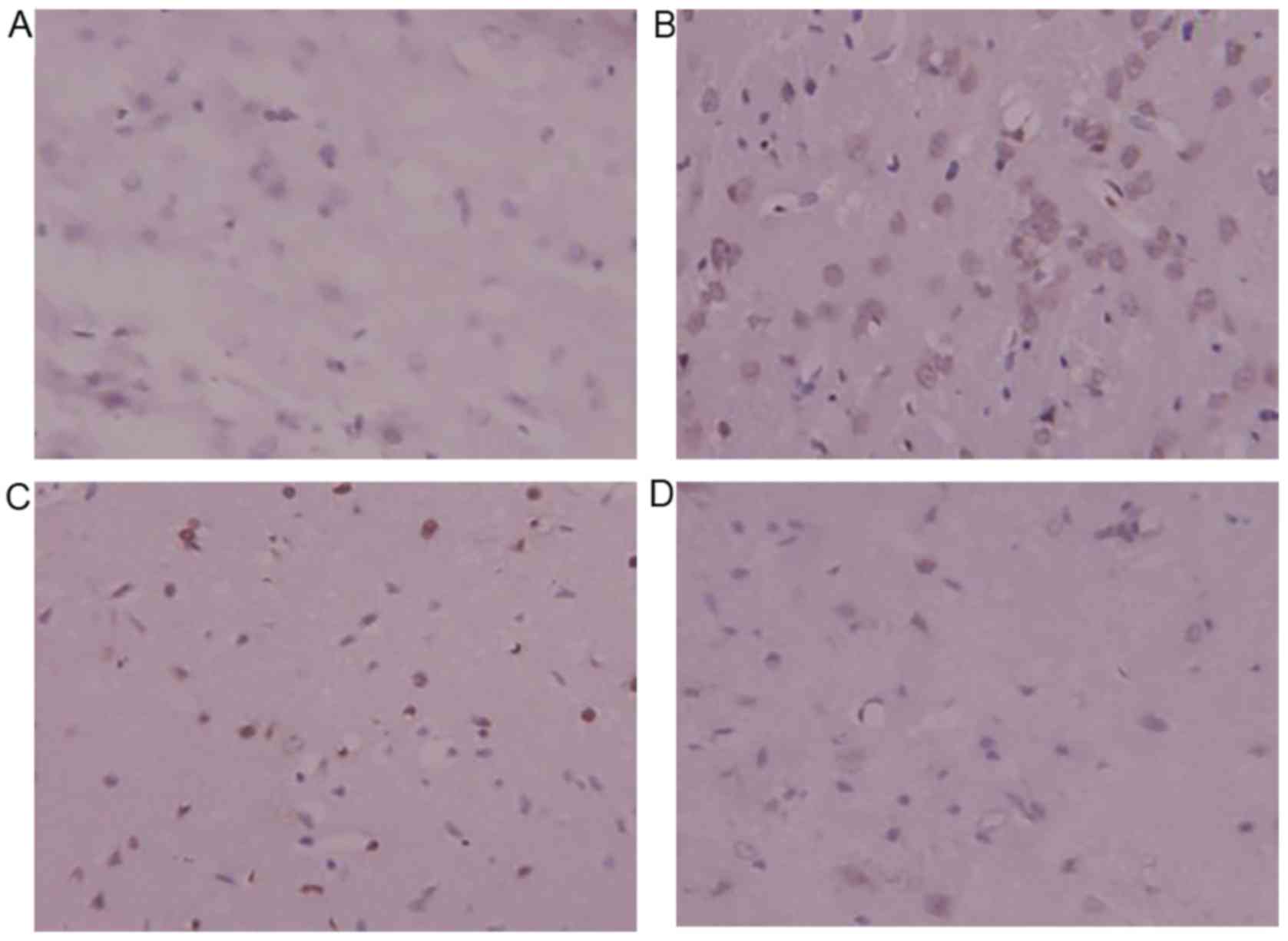
Immunohistochemistry for Bax
Bax-positive yellow-brown granules were located in
the cytoplasm and some nuclei. Compared with the sham surgery
group, the model group exhibited higher numbers of Bax-positive
cells, which indicated that the expression of Bax significantly
increased following ischemia-reperfusion injury. The number of
Bax-positive cells decreased significantly in the DSS-treated
groups compared with the model group. The Bax-positive cells were
also more lightly stained, indicating less positivity in the
DSS-treated groups. DSS treatment decreased the expression of Bax
protein, and inhibited cerebral apoptosis following
ischemia-reperfusion injury in rats (Fig. 7).
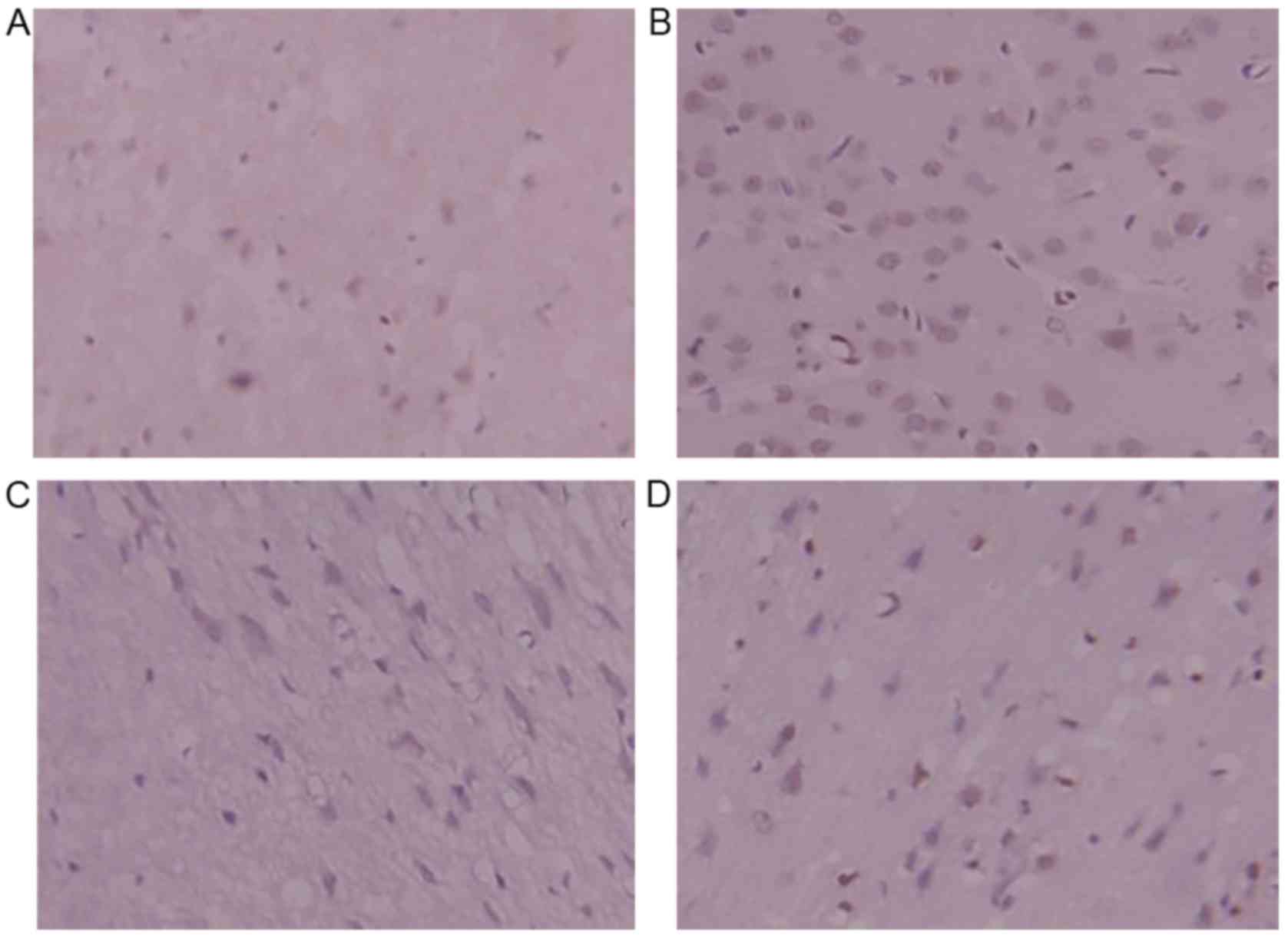
Immunohistochemistry for caspase-3
Caspase-3-positive yellow-brown granules were
located in the cytoplasm and some nuclei. Compared with the sham
surgery group, the model group exhibited a large number of
caspase-3-positive cells, which indicated that the expression of
caspase-3 significantly increased following ischemia-reperfusion
injury. The number of caspase-3-positive cells was significantly
lower in the DSS-treated groups in comparison with the model group.
The positive cells were only lightly stained, indicating that they
were only weakly positive. DSS treatment decreased caspase-3
protein expression, and inhibited cerebral apoptosis following
ischemia-reperfusion injury in rats (Fig. 8).
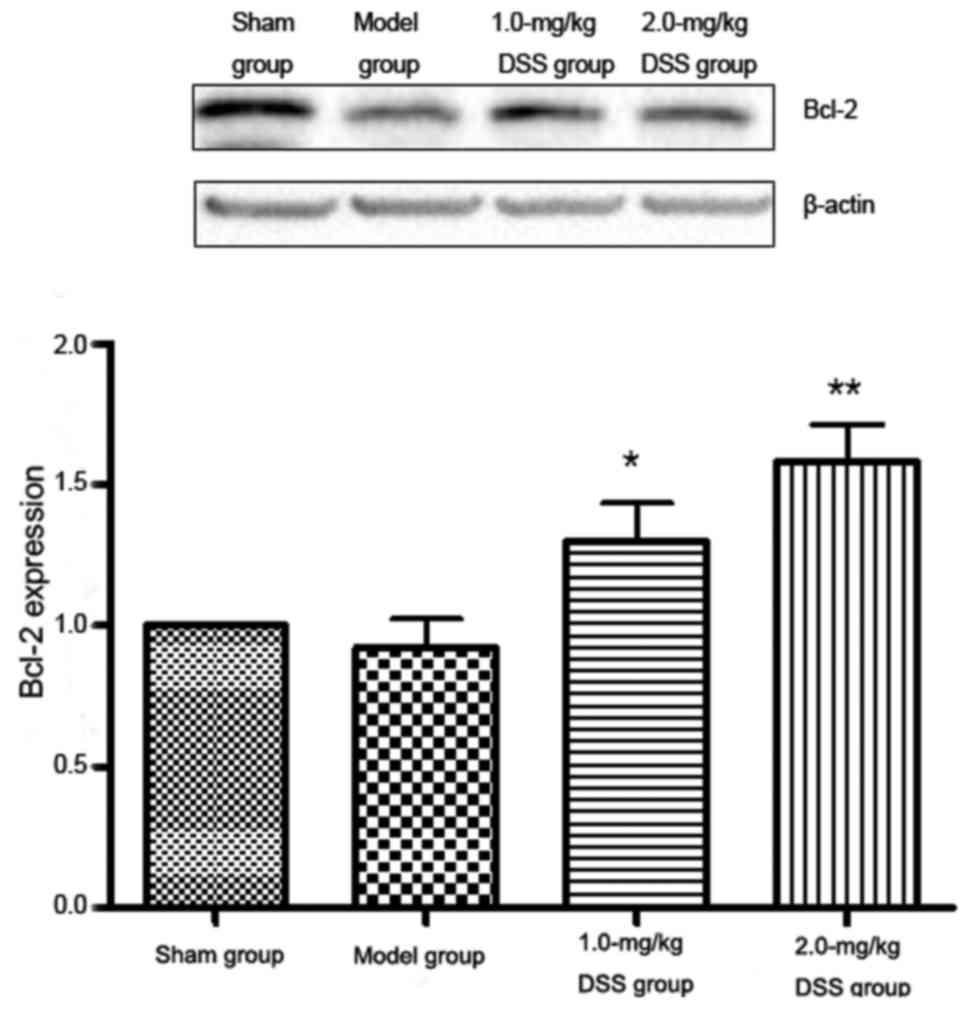
Western blot analysis of Bcl-2, Bax and
caspase-3 Effect of DSS on Bcl-2 expression
The results of western blot analysis revealed that
Bcl-2 expression was significantly reduced in the model group
compared with the sham-operated group. A significant increase in
Bcl-2 expression was detected in the 1.0- and 2.0-mg/kg DSS-treated
groups, compared with the model group (Fig. 9).
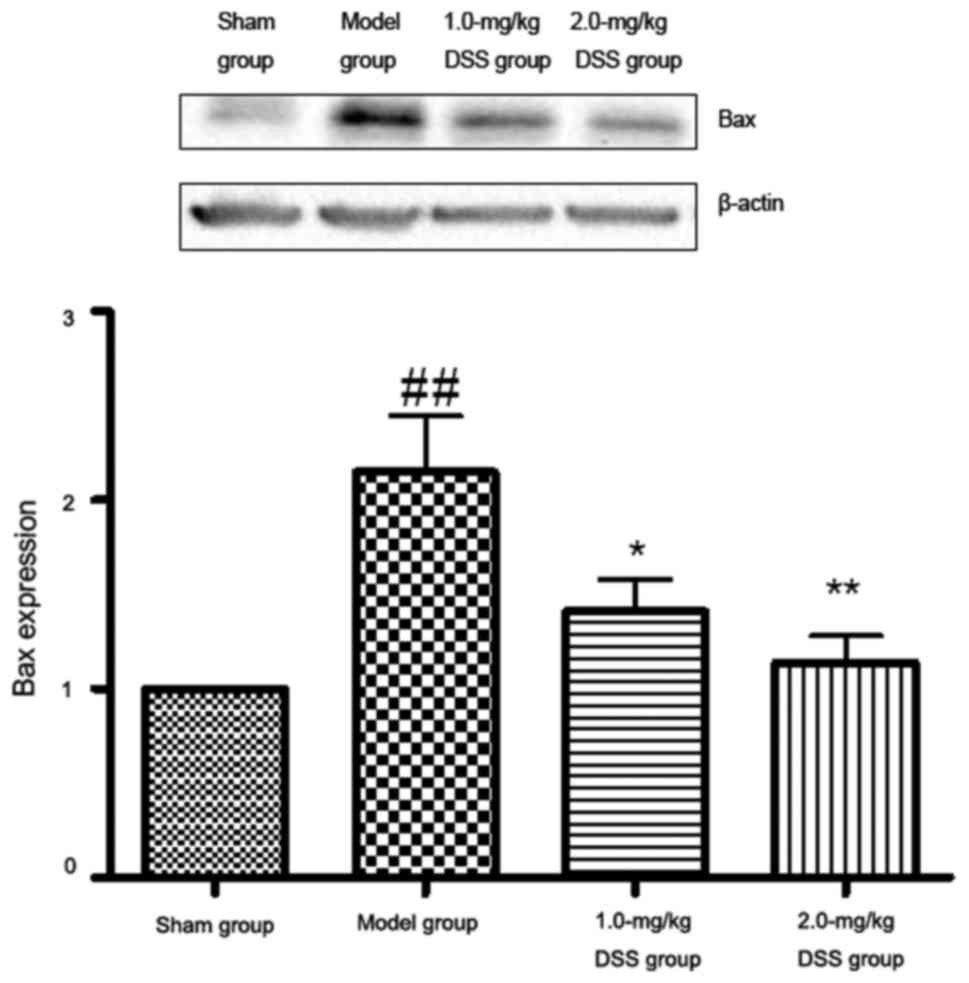
Effect of DSS on Bax expression
The results of western blot analysis revealed that
in comparison with the sham-operated group, Bax expression was
significantly increased in the model group. A significant decrease
in Bax expression was detected in the 1.0- and 2.0-mg/kg
DSS-treated groups in comparison with the model group (Fig. 10).
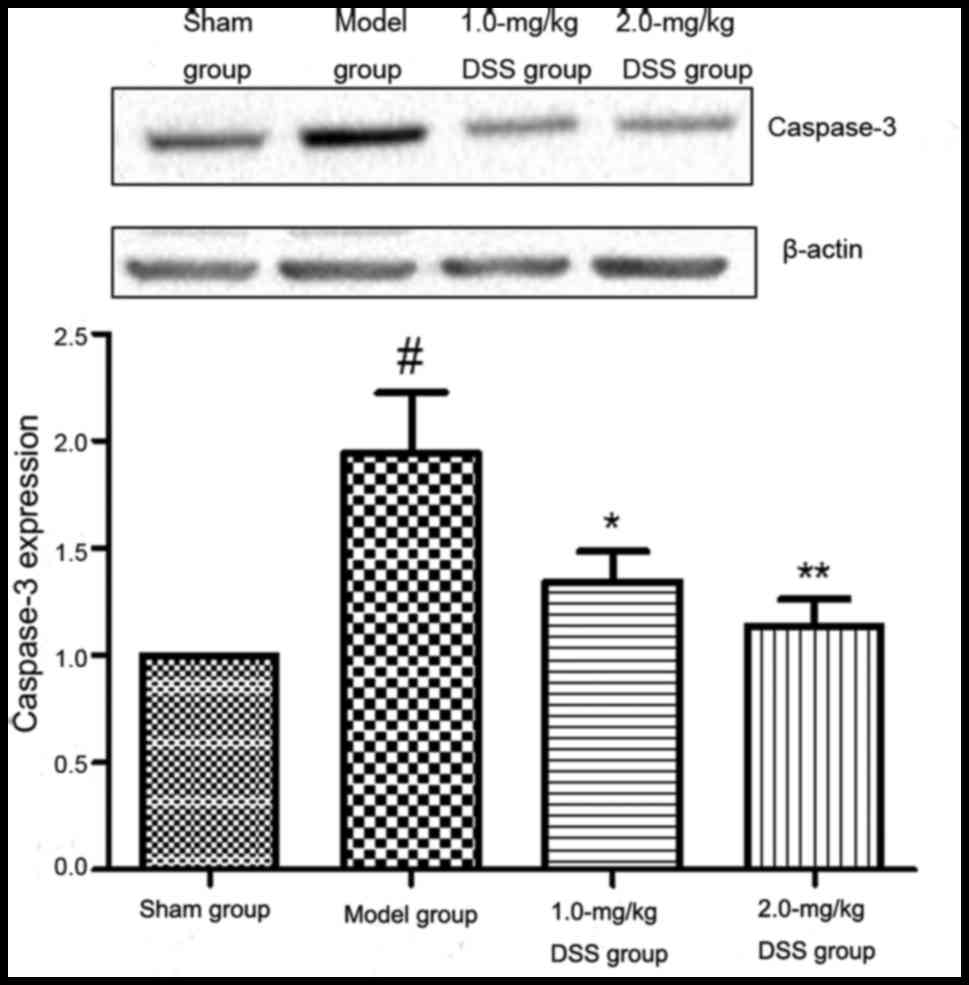
Effect of DSS on caspase-3
expression
The results western blot analysis revealed that in
comparison with the sham-operated group, caspase-3 expression was
significantly increased in the model group. A significant decrease
in caspase-3 expression was detected in the 1.0- and 2.0-mg/kg
DSS-treated groups, compared with the model group (Fig. 11).
Discussion
Ischemia-reperfusion injury is a complex disorder
caused by free radicals, NO, calcium overload, excitatory amino
acids, inflammation and cell apoptosis. Many drugs have been used
in the treatment of cerebral ischemia reperfusion injury, including
calcium antagonists, free radical scavengers (24) and growth factors (25,26). Some traditional Chinese medicines
have been shown to exert neuroprotective effects on cerebral
ischemic injury (27–29). It was recently demonstrated
(30) that DSS has a low acute
toxicity, with the LD50 in rats being 1.75 mg/kg, which
is equivalent to 115-fold the usual daily adult dose. Compared with
other medications, DSS is more effective in the treatment of
cerebral ischemia-reperfusion injury and is also less toxic; DSS
can protect neurons from injury or deterioration.
Cerebral ischemia-reperfusion injury is a complex
process involving many factors, with an increase in neurological
deficit scores, cerebral blood volume and edema. In the present
study, the neurological deficit and the cerebral infarction volume
were significantly reduced in the DSS-treated groups (0.5, 1.0 and
2.0 mg/kg) (P<0.05). We also found that DSS can pass through the
BBB, relieve perivascular edema of the BBB, maintain the integrity
of the vascular wall and reduce ischemia-reperfusion-induced
damage.
Neuronal apoptosis plays an important role in
ischemia-reperfusion injury. Cell apoptosis can be divided into 4
phases: i) apoptotic signal transduction; ii) activation of
apoptotic gene expression; iii) triggering of the execution of cell
apoptosis; and iv) removal of apoptotic cells. Apoptosis-related
genes can be divided into 3 categories: anti-apoptotic genes, such
as Bcl-2; pro-apoptotic genes, such as Bax; and bidirectional
regulator genes (31).
Bcl-2 can regulate the activating factor of caspase,
inhibit cell damage induced by reactive oxygen species, and change
the nuclear-cytoplasmic traffic in cell-cycle regulatory proteins
CDK2, CDC2 and p53 (32). The
co-expression of Bcl-2 with the gene encoding p53 can delay the
apoptosis induced by p53. The synergy between Bcl-2 and Myc prevent
movement of p53 into the nucleus, and block p53-induced apoptosis
(33). Bcl-2 prevents
mitochondrial permeability transition and the release of cytochrome
c from mitochondria into the cytoplasm, it inhibits
apoptosis by altering the calcium current of intracellular
organelles, and promotes the maintenance of calcium homeostasis
(34). Bcl-2 can directly combine
with inactive CED-4 human homolog Apaf-1 and block caspase
(35).
It is known that Bcl-2 and Bax are
apoptosis-regulating proteins that have opposing apoptotic
activities. Bcl-2 can form heterodimers with Bax, and the ratio of
Bcl-2:Bax could reflect the level of apoptosis; lower Bcl-2:Bax
ratios promote apoptosis and higher Bax:Bcl-2 ratios inhibit
apoptosis. Bax can induce release of cytochrome c, Bax is
involved in the regulation pathway of Bcl-xL by combining with it
(36,37). In our study, we observed low
number of Bcl-2-positive cells in the model group in comparison
with the sham-operated group. The number of Bcl-2-positive cells
was higher in the DSS-treated groups than in the model group, and a
high number of Bax-positive cells was observed in the model group
in comparison with the sham-operated group. The number of
Bax-positive cells decreased significantly in the DSS-treated
groups compared with the model group.
Caspase-3 is a key enzyme that is involved in the
execution of apoptosis and leads to disintegration of the cell. It
has been reported that caspase-3 is directly involved in cell
apoptosis following cerebral ischemia, by cleaving DNA repair
proteins, cytoskeletal proteins, and other related caspase
substrate proteins, thereby leading to cerebral ischemia
reperfusion injury (38). In our
study, a large number of caspase-3-positive cells was observed in
the model group compared with the sham-operated group. The number
of caspase-3-positive cells in the DSS-treated groups decreased
significantly compared with that in the model group.
Our findings suggest that DSS at a dose range of
0.5–2.0 mg/kg can significantly promote the expression of Bcl-2,
inhibit the expression of caspase-3 and Bax, and reduce the
Bcl-2:Bax ratio. DSS exerts a neuroprotective effect on cerebral
ischemia-reperfusion by regulating the expression of Bcl-2, Bax and
caspase-3.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (NSFC; nos. 81160399, 81560583)
and from the implement plan of Science and Technology in
Universities of Jiangxi Province (Science Frontier) (no.
KJLD13085).
References
|
1
|
Ministry of Health of the People's
Republic of China: Summary of Chinese health statistics. Beijing:
People's Medical Publishing House; 2012
|
|
2
|
Sauser K, Burke JF, Reeves MJ, Barsan WG
and Levine DA: A systematic review and critical appraisal of
quality measures for the emergency care of acute ischemic stroke.
Ann Emerg Med. 64:235–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang YL, Luo YM and Zhao YM: Protective
effect and the related mechanisms of rhubarb on ischemic
cerebrovascular disease. ShouDu Yikedaxue Xuebao. 36:718–722.
2015.
|
|
4
|
Dong GX and Feng YP: Effects of NBP on
ATPase and antioxidant enzymes activities and lipid peroxidation in
transient focal cerebral ischemic rats. Zhongguo Yi Xue Ke Xue Yuan
Xue Bao. 24:93–97. 2002.
|
|
5
|
Liu R, Gao WJ, Qian T and Wang L:
Astragalus injection inhibits expression of Apaf-1 in rat
hippocampus after cerebral ischemia and reperfusion. Zhongguo
Bingli Shengli Zazhi. 29:872–877. 2013.
|
|
6
|
Qu HY and Yuan J: Effects of Ginsenoside
Rg1 on nNOS and iNOS expressions in rat brain tissue after cerebral
ischemia reperfusion. Tianjin Yiyao. 42:889–892. 2014.
|
|
7
|
Li H, Deng CQ, Chen BY, Chen RF, Zhang SP
and Liang Y: Effects of Panax Notoginseng saponins on expression of
caspase after focal cerebral ischemia-reperfusion in rats. Chin
Pharmacol Bull. 22:189–193. 2006.
|
|
8
|
Li CY and Li WH: Research progress of the
mechanisms of cerebral ischemia reperfusion injury. Zhonghua
Xiandai Zhongxiyi Zazhi. 3:1744–1746. 2005.
|
|
9
|
Kuschinsky W and Gillardon F: Apoptosis
and cerebral ischemia. Cerebrovasc Dis. 10:165–169. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uchino H, Morota S, Hirabayashi G,
Ushijima K, Kakinuma T, Ishii N, Shibasaki F and Kuroda Y:
Molecular mechanism of ischemic brain injuries and perspectives of
drug therapies for neuroprotection. Masui. 56:248–270. 2007.In
Japanese. PubMed/NCBI
|
|
11
|
Zhuo AS, Chen AJ and Hua WJ: Effects of
cerebral ischemia and reperfusion injury on apoptosis of cell and
expression of apoptosis gene in the different tissues of rats.
Zhongguo Linchuang Kangfu. 6:1263–1264. 2002.
|
|
12
|
Xue Q, Zou YA, Zhao AM and He XF: Effects
of Kangnaoye 1 on cerebral ischemia-reperfusion half silent zone
apoptosis and Bcl-2/Bax ratio in rats. Zhongguo Quanke Yixue.
13:498–501. 2010.
|
|
13
|
Khalil H, Peltzer N, Walicki J, Yang JY,
Dubuis G, Gardiol N, Held W, Bigliardi P, Marsland B, Liaudet L and
Widmann C: Caspase-3 protects stressed organs against cell death.
Mol Cell Biol. 32:4523–4533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu L, Miao H, Hou Y, Zhang B and Guo L:
Neuroprotective effect of A20 on TNF-induced postischemic
apoptosis. Neurochem Res. 31:21–32. 2006.PubMed/NCBI
|
|
15
|
Wang QH, Li DL and Huang ZH: The
anti-oxidation effects of daidzin on isolated myocardial
ischemia/reperfusion injury. J Gannan Med Univ. 31:189–191.
2011.
|
|
16
|
Qi JP, Wu AP, Wang DS, Wang LF, Li SX and
Xu FL: Correlation between neuronal injury and Caspase-3 after
focal ischemia in human hippocampus. Chin Med J (Engl).
117:1507–1512. 2004.
|
|
17
|
Ye HY, Chen QY, Qiu F, Huang ZH, Huang YP,
Xiao H and Zeng J: Effect of daidzein on atrial
electrophysiological characteristic in guinea pig. Zhong Yao Cai.
29:312–313. 2006.
|
|
18
|
Ye HY, Hu ZP, Zhou L, Huang ZH and Zeng J:
Protective effect of daidzein on myocardial ischemia injury in
rats. Pharmacol Clin Chin Mater Med. 27:221–223. 2006.
|
|
19
|
Woodman OL and Boujaoude M: Chronic
treatment of male rats with daidzein and 17 beta-oestradiol induces
the contribution of EDHF to endothelium-dependent relaxation. Br J
Pharmacol. 141:322–328. 2004. View Article : Google Scholar
|
|
20
|
Nevala R, Paukku K, Korpela R and
Vapaatalo H: Calcium-sensitive potassium channel inhibitors
antagonize genistein- and daidzein-induced arterial relaxation in
vitro. Life Sci. 69:1407–1417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang YS, Zeng J, Huang YP, Qiu F, Ye HY
and Wang SR: Antagonistic effect of 3′-daidzein sulfonate sodium on
prostatic hyperplasia in mice. Zhonghua Nan Ke Xue. 13:387–390.
2007.In Chinese. PubMed/NCBI
|
|
22
|
Li CT, Wang YL, Wu GT, Cheng XL, She YL,
Huang Y and Chen YF: Effect of Puerarin on Caspase-3 and Bcl-2
expression of hippocampal CA1 neurons in ovariectomized rats.
Zhongguo Zhongyiyao Xinxi Zazhi. 21:40–46. 2014.
|
|
23
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang HF, Guo F, Cao YZ, Shi W and Xia Q:
Neuroprotection by manganese superoxide dismutase (MnSOD) mimics:
antioxidant effect and oxidative stress regulation in acute
experimental stroke. CNS Neurosci Ther. 18:811–818. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Homi HM, Sheng H, Arepally GM, Mackensen
GB and Grocott HP: Aprotinin improves functional outcome but not
cerebral infarct size in an experimental model of stroke during
cardiopulmonary bypass. Anesth Analg. 111:38–45. 2010.PubMed/NCBI
|
|
26
|
Liu J and Wang LN: Gamma aminobutyric acid
(GABA) receptor agonists for acute stroke. Cochrane Database Syst
Rev. CD0096222014.PubMed/NCBI
|
|
27
|
Lin Z, Zhu D, Yan Y and Yu B: Herbal
formula FBD extracts prevented brain injury and inflammation
induced by cerebral ischemia-reperfusion. J Ethnopharmacol.
118:140–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan SJ, Wong WS, Wong PT and Bian JS:
Neuroprotective effects of andrographolide in a rat model of
permanent cerebral ischaemia. Br J Pharmacol. 161:668–679. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun K, Fan J and Han J: Ameliorating
effects of traditional Chinese medicine preparation, Chinese
materia medica and active compounds on ischemia/reperfusion-induced
cerebral microcirculatory disturbances and neuron damage. Acta
Pharm Sin B. 5:8–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng QH: Study on acute toxicity of
3′-daidzein sulfonate sodium in mice. Zhongyiyao Daobao. 19:78–79.
2013.
|
|
31
|
Wang JZ and Yin CH: Pathophysiology.
Beijing: People's Medical Publishing House; 2013
|
|
32
|
He G, Siddik ZH, Huang Z, Wang R, Koomen
J, Kobayashi R, Khokhar AR and Kuang J: Induction of p21 by p53
following DNA damage inhibits both Cdk4 and Cdk2 activities.
Oncogene. 24:2929–2943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goloudina AR, Mazur SJ, Appella E, Garrido
C and Demidov ON: Wip1 sensitizes p53-negative tumors to apoptosis
by regulating the Bax/Bcl-xL ratio. Cell Cycle. 11:1883–1887. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu C, Fujihara H, Yao J, Qi S, Li H,
Shimoji K and Baba H: Different expression patterns of Bcl-2,
Bcl-xl, and Bax proteins after sublethal forebrain ischemia in
C57Black/Crj6 mouse striatum. Stroke. 34:1803–1808. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Inohara N, Gourley TS, Carrio R, Muñiz M,
Merino J, Garcia I, Koseki T, Hu Y, Chen S and Núñez G: Diva, a
Bcl-2 homologue that binds directly to Apaf-1 and induces
BH3-independent cell death. J Biol Chem. 273:32479–32486. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin HM: Pathophysiology. People's Medical
Publishing House; Beijing: pp. 178–180. 2000
|
|
37
|
Behrends M, Martinez-Palli G, Niemann CU,
Cohen S, Ramachandran R and Hirose R: Acute hyperglycemia worsens
hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg.
14:528–535. 2010. View Article : Google Scholar :
|
|
38
|
Harrison DC, Davis RP, Bond BC, Campbell
CA, James MF, Parsons AA and Philpott KL: Caspase mRNA expression
in a rat model of focal cerebral ischemia. Brain Res Mol Brain Res.
89:133–146. 2001. View Article : Google Scholar : PubMed/NCBI
|