Introduction
Medical dressings have been widely applied in the
treatment of external cutaneous wounds, such as abrasions,
lacerations, avulsions, puncture wounds, contusions, blisters,
incisions, burns, split graft donor sites and ulcers (1). Among the many properties of
dressings, the water vapor transmission and water retention
properties are considered key factors for regulating the moisture
balance in the wound area (2).
Specifically, these factors should provide a moderate atmosphere by
transpiring excess exudates promptly and retaining adequate
moisture to promote tissue regeneration (3). To satisfy the above conditions of
dressings, a versatile class of polymers has been extensively
applied as their flexibility during synthesis or modification of
polymers can enable the matching with the physical and mechanical
properties of various tissues or organs in the human body (4). Among these, polymers with good
physical, mechanical and chemical properties have been investigated
as substrates for wound healing (5). Polyurethane (PU) is frequently
applied to wound dressings due to its good barrier properties and
oxygen permeability, while chitin/chitosan have shown positive
effects on wound healing as they are biocompatible, biodegradable,
non-toxic, anti-microbial and hydrating agents (6,7).
Moreover, hydrated hydrogel sheets have shown good coherency,
transparency, flexibility, high oxygen permeability and good skin
adhesion (8), whereas non-woven
fabrics can be readily conditioned to serve as an excellent
dressing material as they contain high porosity and a large surface
area with no dust, are easily processable and have a readily
modifiable surface mechanism (9).
Various types of cellulose have been extensively
applied to the treatment of skin wounds as part of the development
of new materials. Crystalline cellulose membranes (CMs) have been
directly applied to the skin wounds of Wistar rats for 26 days to
evaluate the effects on healing. These membranes contribute to the
maintenance of the humidity of the wound, decrease pain, and ease
the visualization and control of the evolution of the lesion
(10). The hydrocolloid membrane
cellulose containing Styela clava tunic (SCT) powder
(HCM-SCT) has been shown to accelerate the repair process of acute
wounds in Sprague-Dawley (SD) rats through the stimulation of
re-epithelization, collagen deposition and angiogenesis (11). Furthermore, the oxidized
regenerated cellulose (ORC) collagen has been shown to
significantly stimulate diabetic wound closure, resulting in a
measurable improvement in the histological appearance of wound
tissue (12). In our previous
studies, cellulose film was successfully prepared using pure
cellulose powder obtained from SCTs after completing dissolution in
N-methylmorpholine-N-oxide (NMMO)/H2O
(87/13 wt%) (13) or [Amim]Cl
ionic liquid composed of 1-methylimidazol and 3-chloro-1-propene
(14). This film exhibited good
biocompatibility and degradability, as well as a therapeutic effect
on the wounded skin of SD rats (14,15). However, there have been no
attempts to date (to the best of our knowledge) to develop and
invest in novel membranes with enhanced porosity to promote the
repair of surgical wound skin.
In the present study, we compared skin regeneration
and angiogenesis in the surgical wounds of SD rats after the
application of three different SCT-CMs for 14 days. The results of
the present study provide novel evidence that FSCT-CM may be
considered as a wound dressing material which may be used to
accelerate the process of wound healing in the injured skin of SD
rats through the regulation of angiogenesis and connective tissue
formation.
Materials and methods
Preparation of three SCT-CMs
SCT powder was prepared as previously described
(14). Briefly, to remove
sediments and debris, 33 g of SCTs in 10% NaOH aqueous solution
(990 ml) were boiled at 100°C for 2 h after being collected from
the beach of the South Sea in Gosung-gun, Korea. The SCT samples
were subsequently washed with distilled water 3 times, after which
they were boiled in 5% CH3COOH solution at 100°C for 2 h
to neutralize the NaOH solution, then washed with distilled water 3
times. SCT was subsequently bleached by separate boiling and
washing in 10% H2O2 solution. After a final
wash with distilled water, SCT was dried at 100−120°C for 2–3 h,
then ground in a pin mill machine (Daehwa, Yongin, Korea). The
milling for SCT powders was conducted by a proprietary commercial
process consisting of passing through a combination of 30 mesh
sieves for 10 min once, and then 120 mesh sieves for 10 min 2
times.
Three types of SCT-CMs were also prepared using a
modified version of the method described in a previous study
(14). To manufacture SCT-CM (the
first type), 3 g of SCT powder was completely dissolved in 100 ml
of [Amim]Cl ionic liquid composed of 1-methylimidazole and
3-chloro-1-propene (1:1.20 of molar ratio) at 80°C. Additionally,
another cellulose solvent, N-methyl-2-pyrrolidone (NMP) was
added followed by stirring at a high speed for 24 h to accelerate
the dissolution of SCT. These solutions were then cast onto the
glass plate of an automatic film coating apparatus (DAO-CO 02; Dao
Technology, Hwaseong, Korea) to yield a thickness of approximately
3 mm. Following the removal of air bubbles under a vacuum oven, the
glass plates bound with CM were immediately immersed in methanol
and washed with distilled water. Finally, CMs with a thickness of
approximately 3 mm were collected by drying at room temperature for
24 h. FSCT-CM (the second type of CM) was prepared from SCT-CM
through dehydration in a lyophilizer (FDU-540; Tokyo Rikakikai Co.,
Tokyo, Japan) following incubation at −130°C for 24 h. Furthermore,
to prepare ASCT-CM (the third type of CM), SCT powder was
completely dissolved in 100 ml of 8% sodium alginate solution
(Sigma-Aldrich Co., St. Louis, MO, USA) with 0.976 g/ml. Following
immersion in 10% (w/v) CaCl2 solution for 24 h, these
composites were cast onto the glass plate of an automatic film
coating apparatus to give a thickness of approximately 3 mm.
Following the removal of air bubbles under a vacuum oven, the glass
plates bound with CM were immediately immersed in methanol and
washed with distilled water, after which CMs with a thickness of
approximately 3 mm were collected by drying at room temperature for
24 h.
Finally, the three different types of SCT-CMs in our
study were prepared as membranes with 70% moisture content by
dipping in 1X phosphate-buffered saline (PBS) solution immediately
before application onto the skin of SD rats. Gauze (GZ) used as a
negative control was prepared under the same conditions after
sterilization.
Analysis of the physical properties of
the three SCT-CMs
To analyze the morphological features of the three
SCT-CMs, samples were frozen at −70°C for 24 h, after which they
were dehydrated in a lyophilizer for 3 days. These dried SCT-CMs
were then coated with platinum (Pt) using a sputter coater (Jeol
JXA-840A; Jeol, London, UK) for 120 sec under an argon atmosphere,
after which they were observed by scanning electron microscopy
(SEM) (Stereoscan 250 MK III; Cambridge Instruments, London, UK) at
15 kV. After capturing the SEM image, the gap length and the pore
diameter in inside of SCT-CMs was measured using Leica Application
Suite (Leica Microsystems, Wetzlar, Germany).
The mechanical properties, including tensile
strength, elongation, fluid uptake rate and water vapor
transmission rate (WVTR) of the three SCT-CMs were measured by the
methods described in a previous study (14). To perform the tensile strength and
strain analysis, the three SCT-CMs were dried at 105°C for 12 h and
then cut to an appropriate size (1×5 cm). Two properties of these
samples were analyzed (40 kV, 30 mA) at room temperature under a
velocity of 20 mm/min using a United SSTM-1 testing machine (United
Calibration Corp., Huntington Beach, CA, USA) with a 445 N load
cell.
Additionally, the swelling behavior of the three
SCT-CMs (1×5 cm size) was investigated by the immersion in 30 ml of
1X PBS (pH 7.4) at 37°C. At 7 time points (0.167, 0.5, 2, 4, 8, 16
and 24 h) after the immersion, each sample was removed from the
solution, the excess of water at surface withdrawn and their weight
immediately determined. The fluid uptake rate was the calculated
using the following equation, as previously described (16): fluid uptake rate (%) =
[(Wo −
Wi)/Wi) ×100], where,
Wo and Wi represent the wet and dry weight of
the film, respectively. Five samples were used for each
condition.
Furthermore, to determine the moisture permeability
of the three SCT-CMs, the WVTR was measured according to the
American Society for Testing and Materials (ASTM) standard
(17). Each sample from the three
SCT-CMs was cut into a disc with a diameter of 50 mm and mounted on
the mouth of a cylindrical cup with a diameter of 40 mm containing
30 ml of water. The sample was placed into an oven (FO-600M; Jei
Tech, Seoul, Korea) and maintained at 37±2°C and 35±5% relative
humidity. At 7 different time points (1, 2, 4, 8, 16, 24, 48 and 72
h) after the drying of the SCT-CMs began, their weight was
immediately determined and the WVTR was then calculated using the
following equation: WVTR (g/m2/h) =
[(Wi − Wt)/A/24)
×106, where, Wi and Wt represent
the weight of the initial time, the time 't' and the area 'A' at
different periods of time. Five samples were repeatedly measured 3
times for each condition.
Design of animal experiment
The animal protocol used in this study was reviewed
and approved by the Pusan National University-Institutional Animal
Care and Use Committee (PNU-IACUC; approval no. PNU-2014-0520).
Adult male SD rats were purchased from SamTako BioKorea (Osan,
Korea) and handled at the Pusan National University Laboratory
Animal Resources Center accredited by the Korea Food and Drug
Administration (accredited unit no. 00231) and AAALAC International
(accredited unit no. 001525). All rats were provided with a
standard irradiated chow diet (Purina Mills, Seoungnam, Korea)
ad libitum, and were maintained in a specific pathogen-free
state under a strict light cycle (lights on at 06:00 h and lights
off at 18:00 h) at a temperature of 23±2°C and a relative humidity
of 50±10%.
An in vivo wound healing assay was developed
in which 7-week-old SD rats (n=40) were assigned to one of 4 groups
as follows: a GZ-treated group (n=10); a SCT-CM-treated group
(n=10); a FSCT-CM-treated group (n=10); and a ASCT-CM-treated group
(n=10). Each group was then further divided into 2 different
groups: 7 days (n=5) and 14 days (n=5). The animals were
anesthetized by an intramuscular injection of Zoletile (50 mg/kg
body weight) and Rompun (5 mg/kg body weight), after which the back
skins were shaved with an electrical razor and 70% ethanol was
applied. A round wound with a diameter of 8 mm and a depth of 2–4
mm was formed by removing the cutaneous tissue in the back shoulder
region using a biopsy punch (Kasco Com, Sialkot, Pakistan). The
incision wound on each rat was then sterilized with 70% ethanol,
after which it was covered with a 5×4×0.3 mm piece of GZ, SCT-CM,
FSCT-CM or ASCT-CM. The pieces of GZ and the three types of CMs
were replaced with new pieces every 3 days. During replacement, the
condition of the wound skin was observed and photographed using a
Canon® digital camera, while the body weight was
measured using an electronic balance (Mettler Toledo, Greifensee,
Switzerland). On days 7 and 14, all rats were subjected to
euthanasia using carbon dioxide, and samples of damaged skin were
collected for further histological analysis and western blot
analysis. Additionally, blood serum and liver and kidney organs
were collected from the abdominal veins and the abdominal cavity to
analyze the toxicity of the three SCT-CMs.
Macroscopic analyses of surgical
wounds
Photographic data were utilized for the measurement
of the wound size (%), which was calculated as follows:
Wound size(%)=WtW0
where Wt is the wounded area at time 't' and
W0 is the wounded area at the initial time. A multiple
comparisons test was performed for the statistical clarification of
the differences between groups. In addition, the analysis of wound
color (1, red; 2, pink; 3, pale; 4, cyan) and wound edge (1,
without granulation tissue; 2, little granulation tissue; 3, much
granulation tissue) were scored as previously described ().
Serum biochemistry
On days 7 and 14, all SD rats in each group were
fasted for 8 h, after which blood was collected from the abdominal
veins of rats and incubated for 30 min at room temperature. Serum
was then obtained by the centrifugation of blood and serum
biochemical components, including alkaline phosphatase (ALP),
alanine aminotransferase (ALT), aspartate aminotransferase (AST),
blood urea nitrogen (BUN), and creatinine (CRE) were assayed using
an automatic serum analyzer (Hitachi 747; Hitachi, Tokyo, Japan).
All assays were measured using fresh serum and conducted in
duplicate.
Histological analyses
The skin in the region in which GZ and the three
SCT-CMs had been placed was collected and fixed with 10% formalin
for 48 h, embedded in paraffin wax, and then sectioned into
4-µm-thick slices. The skin sections were subsequently
stained with hematoxylin and eosin (H&E; Sigma-Aldrich Co.),
after which they were examined under a light microscope (Leica
Microsystems) for the presence of edema and inflammatory cell
accumulation. Additionally, the thickness levels of the epidermis,
including the stratum germinativum (SG), stratum spinosum (SS) and
stratum granulosum (SGR), as well as the number of neutrophils and
macrophages (cells/0.16 mm2 of field) and blood vessels
(cells/1.4 mm2 of field) were measured using Leica
Application Suite (Leica Microsystems). In addition, the livers and
kidneys collected from all experimental rats were processed using
the same methods applied to treat the skin tissue. After staining
with H&E, pathological changes in the liver and kidney sections
were examined using Leica Application Suite (Leica
Microsystems).
Mast cells in the skin sections were detected by
staining with toluidine blue (Sigma-Aldrich Co.) as previously
described (18). The number of
cells per 0.01 mm2 of observed field in skin tissue
sections was then measured using Leica Application Suite (Leica
Microsystems).
Western blot analysis
Skin tissue isolated from a subset of groups was
homogenized using a PRO-PREP™ Solution kit (Intron Biotechnology,
Sungnam, Korea) supplemented with half of a protein inhibitor
cocktail tablet (Roche, Penzberg, Germany), after which it was
centrifuged at 13,000 rpm for 5 min. The prepared proteins were
then electrophoresed through a 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, after
which they were transferred onto a nitrocellulose membrane
(Amersham Biosciences, Corston, UK) for 2 h at 40 V in transfer
buffer (25 mM Trizma-base, 192 mM glycine, and 20% methanol). The
efficiency of the transfer and equal protein loading were
determined by staining the membrane with Ponceau, while the gel was
stained with Coomassie blue (both from Sigma-Aldrich Co.).
Appropriate dilutions of primary antibodies, rabbit polyclonal
antibodies against anti-vascular endothelial growth factor (VEGF)
(1:3,000, 500-P131; PeproTech, Rocky Hill, NJ, USA), anti-collagen
(1:1,000, ab292; Abcam, Cambridge, UK), anti-JNK (1:1,000, #9252),
anti-phospho-c-Jun N-terminal kinase (JNK; 1:1,000, #9251),
anti-p38 (1:1,000, #9212), anti-phospho-p38 (1:1,000, #9211),
anti-phospho-extracellular signal-regulated kinase (ERK; 1:1,000,
#9101) anti-Smad2/3 (1:1,000, #5678) (all from Cell Signaling
Technology, Danvers, MA, USA), anti-ERK (1:1,000, sc-94; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), rabbit monoclonal
antibodies against anti-phospho-Smad2/3 (1:1,000, #8828; Cell
Signaling Technology) and mouse monoclonal antibodies against
β-actin (1:3,000, A5316; Sigma-Aldrich Co.) were added to the
membranes and allowed to hybridize overnight at 4°C. After the
antibodies were removed, the membrane was washed 3 times in
solution composed of 10 mM Trizma-base (pH 7.6), 150 mM NaCl, and
0.05% Tween-20 for 10 min. The primary antibody-conjugated
membranes were then incubated with horseradish
peroxidase-conjugated anti-secondary antibody for 1 h at room
temperature. The membrane was then washed again as described above
and developed using an enhanced chemiluminescence detection system
(Amersham Bioscience). Finally, the results were quantified using
the Image Analyzer System (Eastman Kodak 2000MM; Eastman Kodak,
Rochester, NY, USA) and expressed as the fold increase over the
control values. All results were confirmed by two independent
researchers conducting the experiments at least twice.
Enzyme-linked immunosorbent assay (ELISA)
for transforming growth factor (TGF)-β1
The concentrations of total TGF-β1 in serum were
measured using the Legend Max Total TGF-β1 ELISA kit (BioLegend,
San Diego, CA, USA) according to the manufacturer's instructions.
Briefly, the capture antibody-coated wells in Nunc C bottom
immunoplates supplied in the kit were washed 3 times with washing
solution. The serum samples and standards were then added to the
wells, after which the plates were incubated at room temperature
for 2 h. After washing 3 times, TGF-β1 detection antibody solution
was added to each well followed by incubation at room temperature
for 1 h with shaking. The wells were then washed with washing
solution, after which HRP-conjugated detection antibodies were
diluted 5,000-fold with conjugate diluent (50 mM Tris, 0.14 M NaCl,
1% BSA, 0.05% Tween-20, pH 8.0) and transferred to each well. The
plates were subsequently incubated at room temperature for 30 min,
then washed 3 times with washing solution. An enzyme reaction was
initiated by adding substrate solution and incubating the plate at
room temperature in the dark for 30 min. Finally, the reaction was
terminated by adding a stop solution, and the absorbance at 450 nm
was measured within 30 min using a microplate reader (Molecular
Device, Sunnyvale, CA, USA).
Statistical analysis
One-way ANOVA (SPSS for Windows, Release 10.10,
Standard Version; SPSS, Inc., Chicago, IL, USA) was used to
identify significant differences between the GZ and the three
SCT-CM-treated groups. In addition, differences in the responses of
the SCT-CM- and other two SCT-CM-treated groups (FSCT-CM and
ASCT-CM) were evaluated using a post-hoc test (SPSS for Windows,
release 10.10, standard version; SPSS, Inc.) of the variance and
significance. All values are reported as the means ± SD, and a
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Physical properties of the three
SCT-CMs
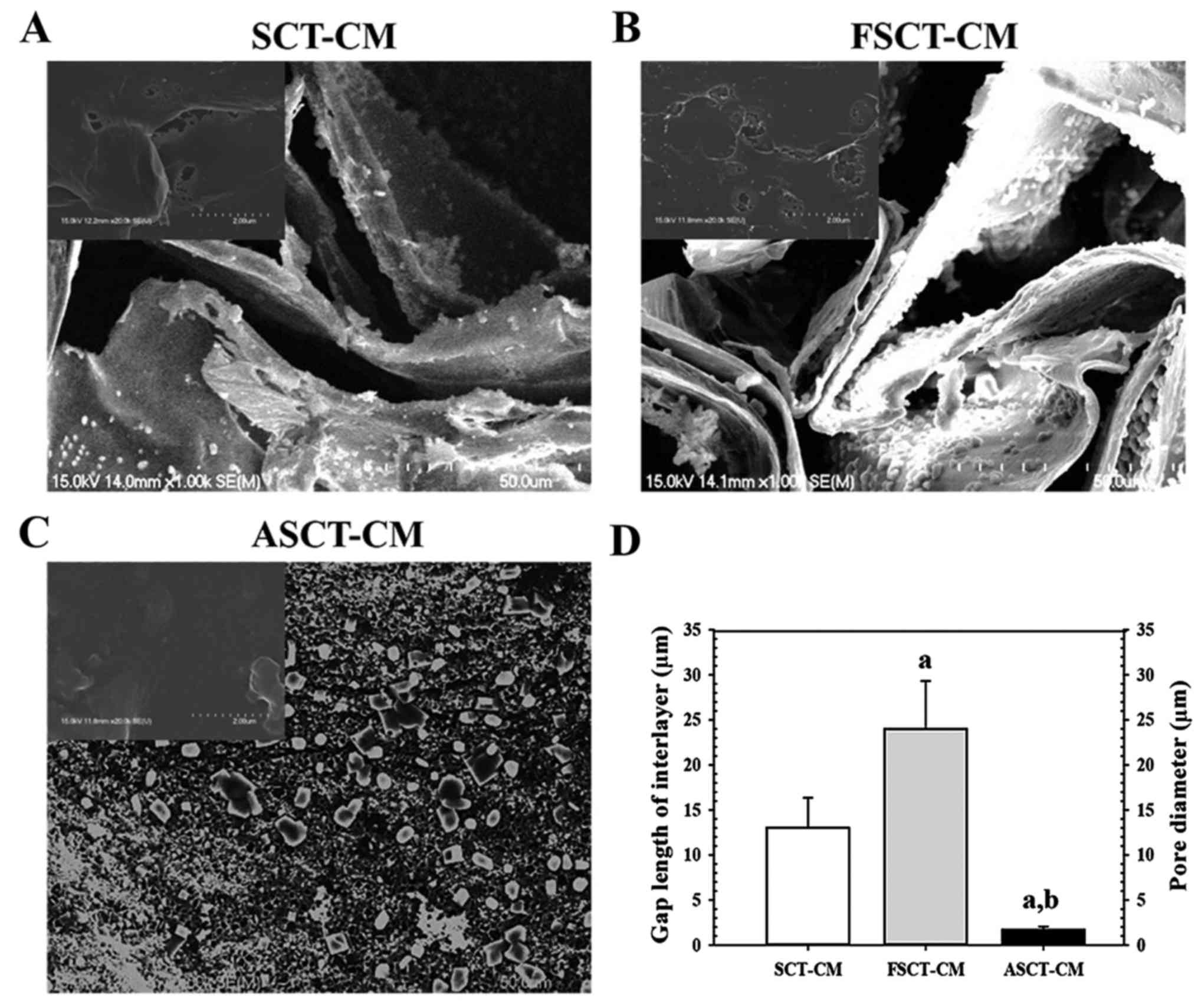
The surface and fracture surface morphology of the
three SCT-CMs was observed by SEM. Two distinct type structures
were detected on the fracture surface the three SCT-CMs. The first
structure consisted of randomly arranged fibrils with a variety of
empty spaces present in the internal matrix of both the SCT-CM and
FSCT-CM, although some differences in the ultrawave structures were
observed between both CMs. The gap length of the interlayer on the
fracture surface was greater (75%) in the FSCT-CM compared with the
SCT-CM (Fig. 1A, B and D). The
second type, which consisted of a large number of small pores
distributed in the same area, was only observed with the ASCT-CM.
The average pore diameter of the ASCT-CM was 1.67 µm
(Fig. 1C and D). However, no
specific differences or unique features were observed upon surface
morphological analysis (Fig.
1A–C, inset on the top left corner of the image). Therefore,
these results suggest that the FSCT-CM had a highly interlayered
structure and a variety of empty spaces that could respond to high
water absorbance and capacity.
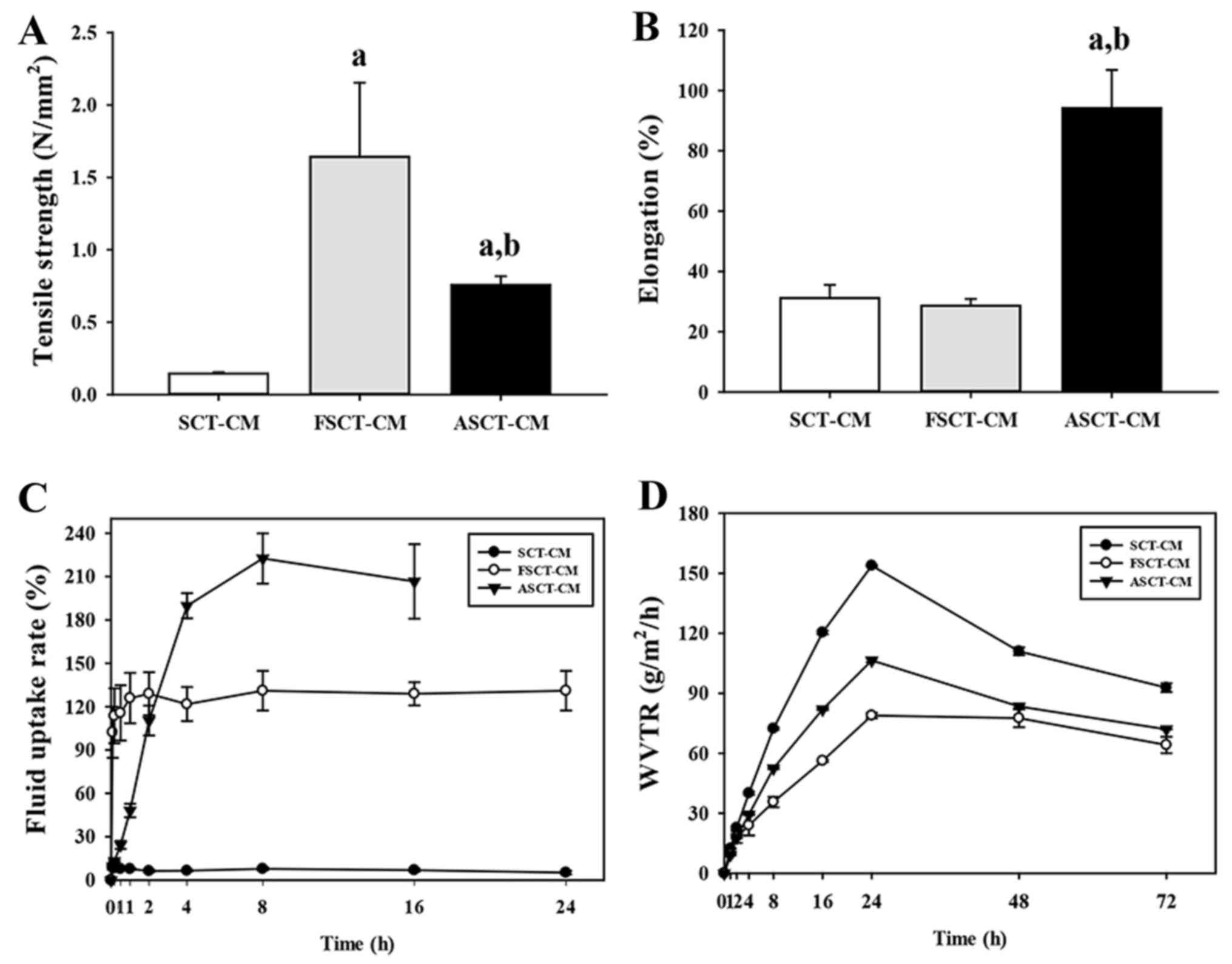
The tensile strength of the FSCT-CM (1.64±0.51
N/mm2) was approximately 993 and 115% greater than that
of the SCT-CM (0.15±0.01 N/mm2) and ASCT-CM (0.76±0.06
N/mm2), although the level of elongation was only higher
in the ASCT-CM (94.17±12.67%) compared with the SCT-CM
(31.14±4.37%) and FSCT-CM (28.59±2.28%) (Fig. 2A and B). Moreover, in the analysis
for moisture regulatory ability, the three SCT-CMs exhibited
different properties as regards the fluid uptake rate and WVTR. The
fluid uptake rate of the FSCT-CM was maintained at a constant level
(approximately 120%), whereas that of the SCT-CM remained at a very
low level. However, the fluid uptake rate of the ASCT-CM rapidly
increased to 210% within 8 h and was maintained at a constant level
(Fig. 2C). In addition, the WVTR
of the three SCT-CMs exhibited a similar pattern during the
experimental period, although that of the FSCT-CM was maintained at
a lower level than the other two CMs. Until the time point of 24 h,
the WVTR of the three CMs was rapidly enhanced; however, these
levels slowly decreased to 102 (SCT-CM), 83 (ASCT-CM) and 69
g/m2/h (FSCT-CM) within 72 h (Fig. 2D). Taken together, the
above-mentioned results provide evidence that the FSCT-CM has good
physical properties and may thus be used as a dressing for surgical
wounds on the skins of mammals.
Effect of the three SCT-CMs on the wound
healing process
To determine how stimulation with the three SCT-CMs
affects the healing process of the wounded skin, the closing rate
of a round area 8 mm in diameter and 2–4 mm in depth was measured
for skin covered with one of the three SCT-CMs for 14 days. We
noted that the most significant changes in the wound area occurred
during days 3–6 and 12–14. In the FSCT-CM sample, the relative area
of the wound decreased to 0.3±0.06 from 1.0±0.08 at 14 days, while
only a 0.77±0.01 change in the same sample was observed during the
first 6 days. Similar results were also observed in the
SCT-CM-treated group, although the decrease rate varied in both
groups. In other words, the wound closing rate was 12.5–37.5%
greater in the FSCT-CM and SCT-CM sample than in the GZ sample
during the first 3–6 days. The closing rate of the FSCT-CM sample
remained the fastest among the samples until the final observation
after 14 days, at which point the wound skin was completely
repaired in all the treatment groups (Fig. 3A and B). However, the
ASCT-CM-treated group did not exhibit any significant alterations
when compared with the GZ-treated group. Additionally, the wound
color score was significantly higher in all the treatment groups at
14 days than at 7 days. Among these groups, the highest level was
detected in the FSCT-CM-treated group (Fig. 3C–a). Furthermore, the score of the
wound edge only decreased in the FSCT-CM-treated group compared
with the GZ-treated group, although the SCT-CM- and ASCT-CM-treated
groups maintained a constant level (Fig. 3C–b). Taken together, these results
suggest that the enhanced ability of the skin to heal the wound and
regenerate tissue could be reasonably attributed to the
FSCT-CM.
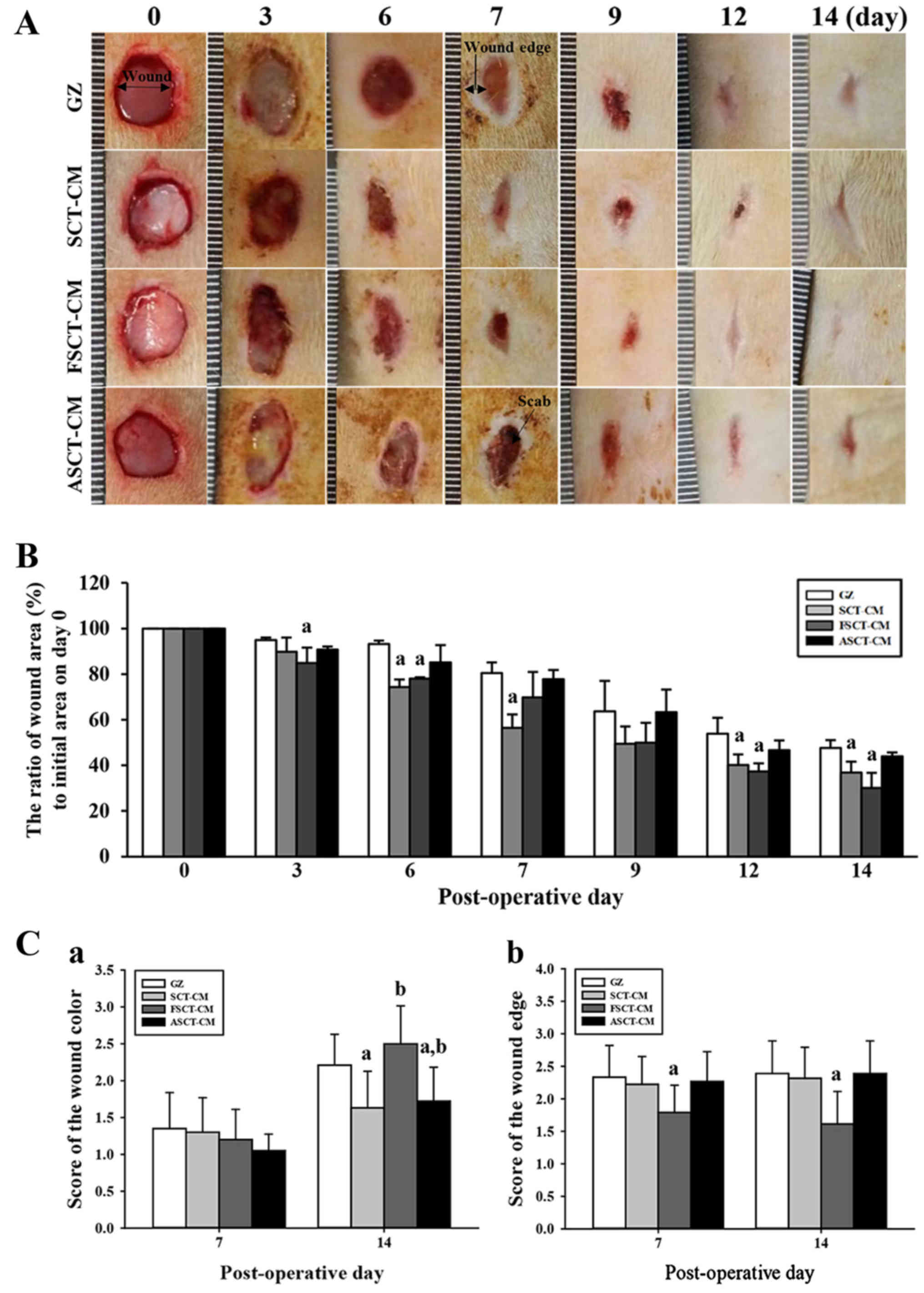 | Figure 3Healing pattern of wound skin with
time. At each time point, images of the surgically wounded skin of
the rats of each group were taken, and the morphological features
were evaluated. (A) Macro-observation of experimental wound healing
at various times post-surgery. (B) The ratio of wound area (%) to
initial area on day 1 was measured with time. The wound size was
measured in triplicate in each test. (C) The total score for wound
color (1, red; 2, pink; 3, pale; 4, cyan) and edge (1, without
granulation tissue; 2, little granulation tissue; 3, very
granulation tissue) were defined as the sum of the individual
scores. Five or six wounds were assayed in triplicate using wound
area analysis. Date are reported as the means ± SD.
aP<0.05 relative to the gauze (GZ)-treated group.
FSCT-CM, freeze-dried SCT-CM; ASCT-CM, sodium alginate-supplemented
SCT-CM. |
Effect of the three SCT-CMs on tissue
regeneration in the wounded skin
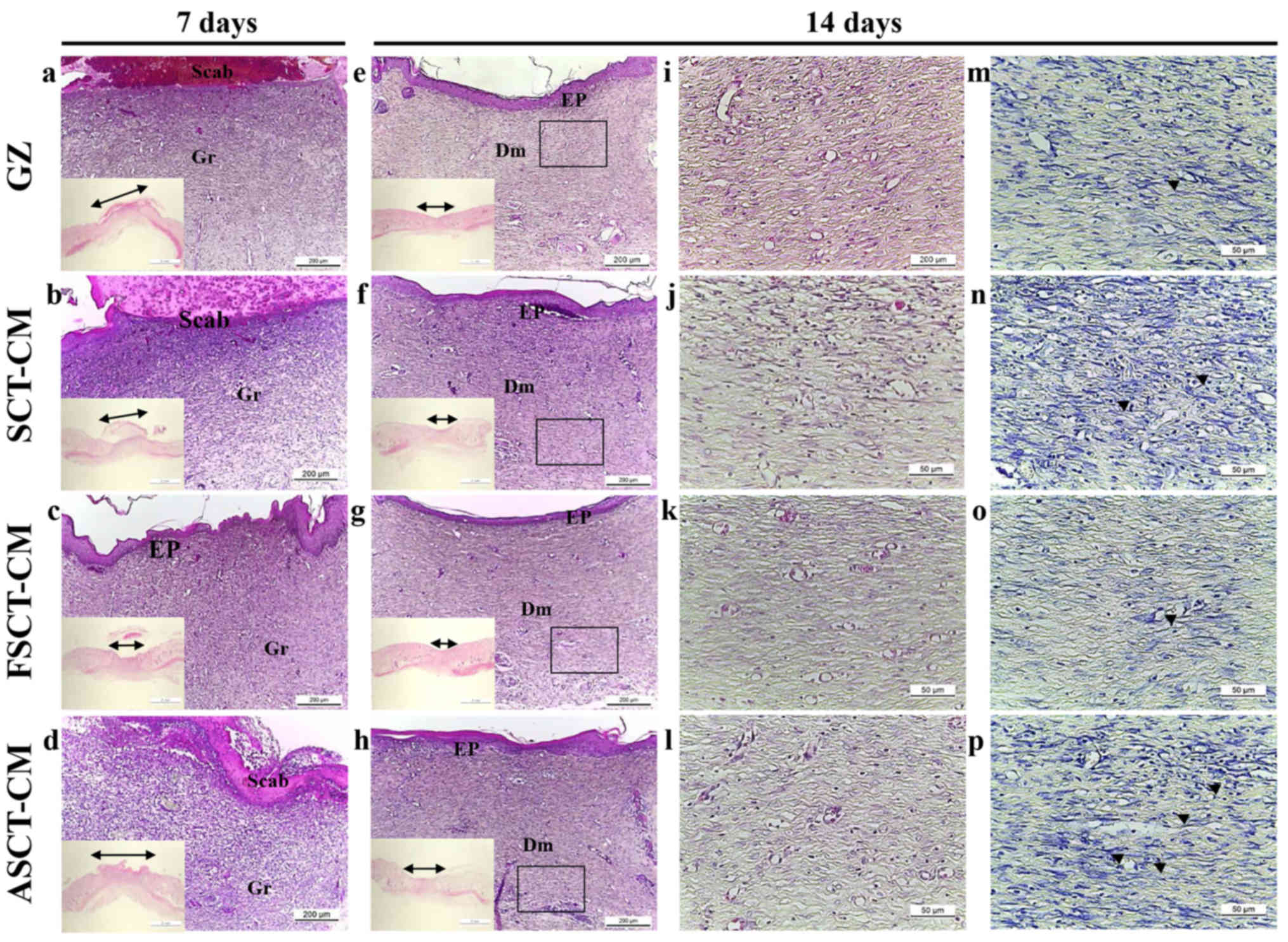
The alteration was determined based on the recovery
of the epidermis, dermis and hyperdermis of the skin tissue of the
rats over the designated period of time (i.e., 7 and 14 days). To
examine the therapeutic effects of the three SCT-CMs on the
histological structure of surgical wounds on the skin, the
thickness of the epidermis and dermis, as well as the number of
blood vessels and mast cells were monitored in the GZ-, SCT-CM-,
FSCT-CM- and ASCT-CM-treated rats. After the first 7 days
post-surgery, the thickness of the total epidermis was
significantly enhanced by approximately 35.9% in the
FSCT-CM-treated group compared with the GZ-treated group, while it
was maintained at a constant level in the SCT-CM- and
ASCT-CM-treated groups. At 14 days post-surgery, both the FSCT-CM-
and SCT-CM-treated groups had a thinner epidermis than the
GZ-treated group, although a larger decrease was detected in the
FSCT-CM-treated group; nevertheless, the rate of change was
reflected in the stratum basal and stratum granulosum (Fig. 4 and Table I). In addition, the inflammatory
reaction was measured by counting the immune cells in the dermis
region. A significant decrease in the number of immune cells was
detected in the FSCT-CM-treated group compared with the GZ-treated
group, while a constant level was maintained in the other groups.
Furthermore, a similar pattern in the number of mast cells was also
observed. Only the FSCT-CM-treated group exhibited a decrease of
approximately 41.0% in the number of mast cells at 7 days
post-surgery, while a decrease of 48.4% was measured at 14 days
post-surgery (Table I). Taken
together, these results suggest that the use of the FSCT-CM
enhanced tissue regeneration, including increasing epidermal
thickness, promoting the formation of blood vessels, and
suppressing the inflammatory response in the skin tissue of SD
rats.
 | Table IAlterations of the histopathological
properties of wounded skin treated with SCT-CMs. |
Table I
Alterations of the histopathological
properties of wounded skin treated with SCT-CMs.
| Items | Histopathological
analysis
|
|---|
| Day | GZ | SCT-CM | FSCT-CM | ASCT-CM |
|---|
| Epidermis |
| Thickness of total
epidermis (µm) | 7 | 23.14±2.44 | 24.85±1.92 | 31.44±3.13a | 21.89±2.63 |
| 14 | 67.52±3.9 | 64.34±4.95 | 32.53±3.34a | 61.92±3.41 |
| Thickness of
stratum basal (SB) (µm) | 7 | NID | NID | NID | NID |
| 14 | 29.49±3.65 | 26.28±3.63 | 13.91±3.74a | 25.07±3.27 |
| Thickness of
stratum spinosum (SS) (µm) | 7 | NID | NID | NID | NID |
| 14 | 30.29±1.91 | 29.07±2.67 | 11.07±2.82a | 23.8±2.98a |
| Thickness of
stratum granulosum (SG) (µm) | 7 | NID | NID | NID | NID |
| 14 | 8.47±1.31 | 4.59±0.35a | 8.68±0.69 | 5.02±0.93a |
| Thickness of dermis
(µm) | 7 | 1656.75±94.97 |
1002.92±13.01a |
1515.08±18.74a |
1813.49±37.48a |
| 14 | 1365±56.69 |
1450.75±23.34a |
1246.75±32.92a |
1544.25±41.25a |
| No. immune cells
(cells/0.16 mm2) | 7 | 41.67±3.79 | 43.67±3.21 | 34.67±4.51 | 41±2 |
| 14 | 25.33±2.52 | 30±5.57 | 15.33±3.5a | 24.67±1.53 |
| No. mast cells
(cells/0.01 mm2) | 7 | 57.67±2.52 | 52.33±9.87 | 34±6.08a | 66.33±7.37 |
| 14 | 22.75±3.77 | 31.75±3.3a | 11.75±2.99a | 29±5.83 |
Effect of the three SCT-CMs on
angiogenesis in the wounded skin
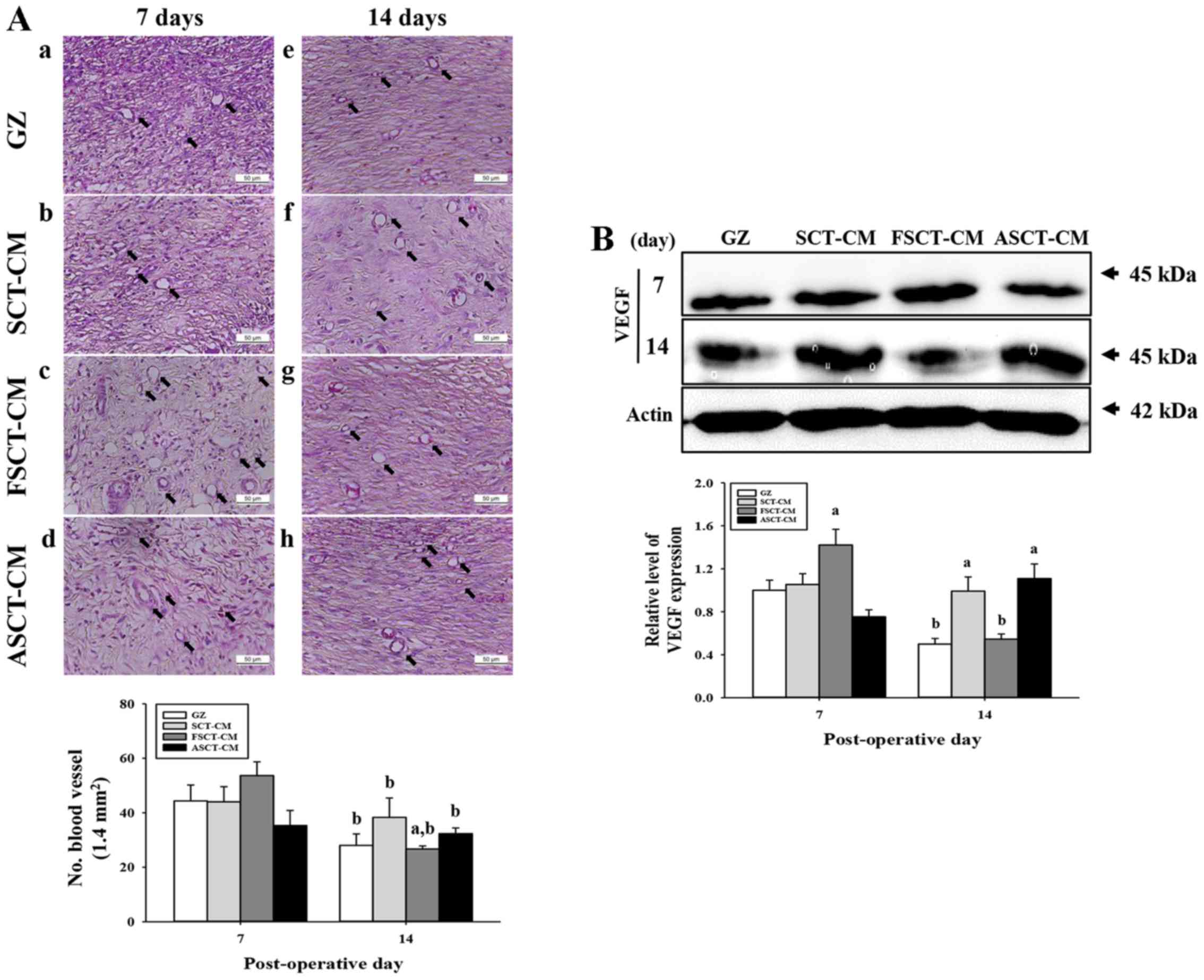
To evaluate the effects of the three SCT-CMs on
angiogenesis in the dermis of wounded skin, we measured the number
of blood vessels and the expression level of VEGF in a subset of
groups for different times. The number of blood vessels in the
dermis was only higher in the FSCT-CM-treated group compared with
the GZ-treated group at 7 days post-surgery, while the levels
remained constant in the SCT-CM- and ASCT-CM-treated groups.
However, the level rapidly decreased in at 14 days post-surgery
(Fig. 5A). In addition, the
expression of VEGF, an important signaling protein involved in both
vasculogenesis and angiogenesis, was similar to the number of blood
vessels at 7 and 14 days post-surgery. A high level of VEGF
expression was measured at day 7 post-surgery in the
FSCT-CM-treated group, although a constant level was maintained in
the other groups. However, this level markedly decreased in the
same group at 14 days post-surgery (Fig. 5B). Therefore, the results of the
present study indicate that the use of the FSCT-CM stimulates
angiogenesis by enhancing the expression of VEGF.
Effect of the three SCT-CMs on the
formation of connective tissue in wounded skin
To evaluate the effects of treatment with the three
SCT-CMs on the formation of connective tissue in the wounded skin,
we measured the expression level of collagen and the concentration
of TGF-β1 in a subset of groups at different times. At 7 days
post-surgery, there was no significant alteration in the expression
of collagen-1 in any of the group subsets. However, these levels
were rapidly increased in the FSCT-CM-treated group at 14 days,
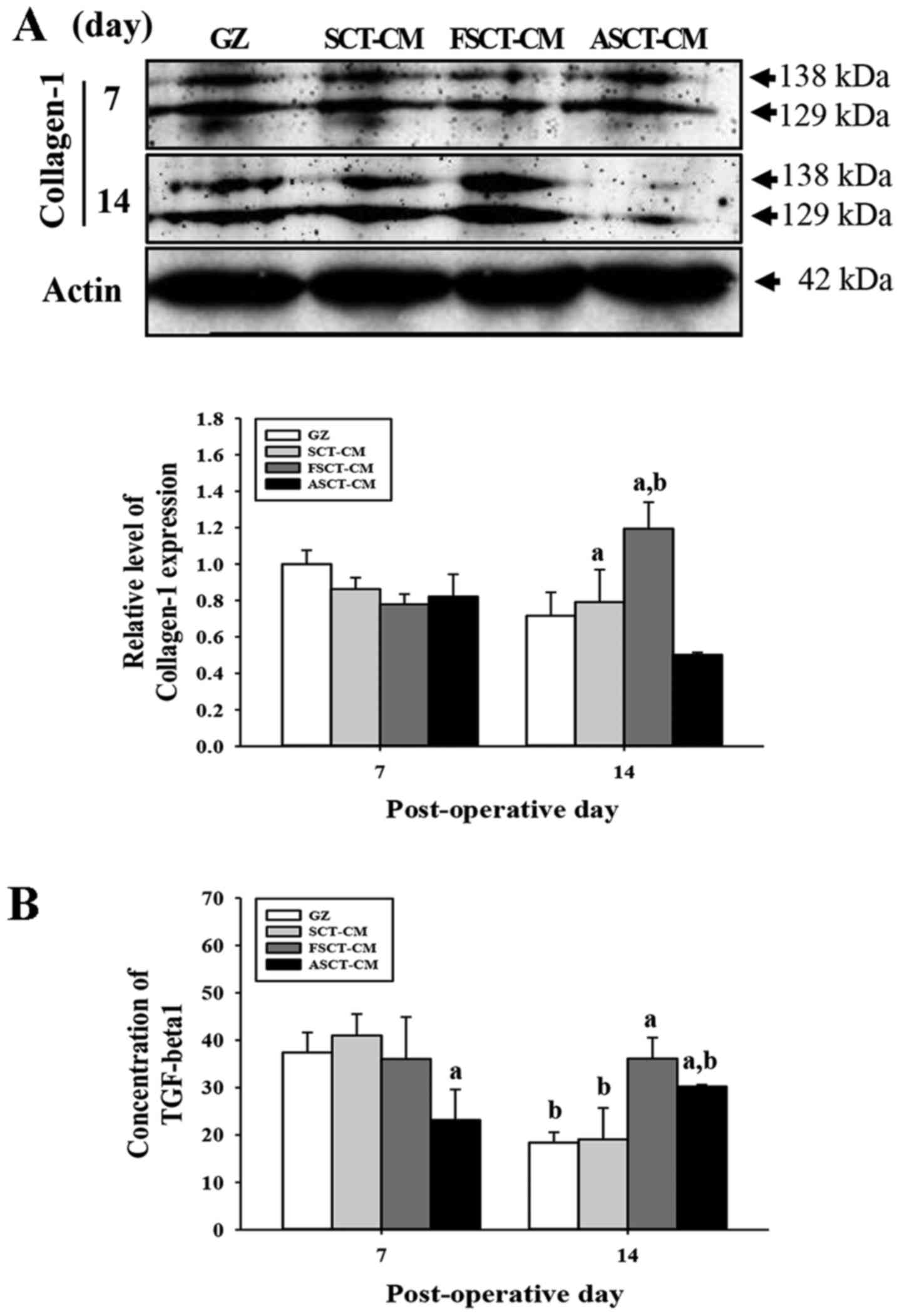
while they decreased in the ASCT-CM-treated group (Fig. 6A). Furthermore, to determine
whether the overexpression of collagen was accompanied by an
increase in the concentration of TGF-β1, the the TGF-β1
concentrations in the serum of the rats in the group subsets were
compared. Overall, the concentration of TGF-β1 was very similar to
the expression of collagen-1, although the level in the
ASCT-CM-treated group differed slightly. At 7 days post-surgery, a
significant decrease in the TGF-β1 concentration was detected in
the ASCT-CM-treated group, whereas the level remained constant in
the other groups. At 14 days post-surgery, the level increased
significantly in the FSCT-CM- and ASCT-CM-treated groups compared
with the GZ-treated group (Fig.
6B). Overall, these findings indicate that the use of the
FSCT-CM induced an increase in collagen levels via the regulation
of TGF-β1 concentration during the later stages of the wound skin
repair process.
Molecular mechanisms underlying skin
re-epithelialization and wound repair with the use of the three
SCT-CMs
Re-epithelialization of wounded skin is accompanied
by the secretion of epidermal growth factors (EGFs), fibroblast
growth factor (FGF) family members, TGF-β, and some chemokines,
which stimulate fibroblasts and increase the expression of smooth
muscle actin in response to wound contraction and collagen
synthesis (19). Among these, the
TGF-β1 signaling pathway includes Smad2/3 in the Smad-dependent
pathway and the MAP kinase protein in the Smad-independent pathway
(20,21). To investigate the molecular
mechanisms of re-epithelialization in wounded skin occurring after
the use of the three SCT-CMs, changes in the regulatory factors of
the TGF-β1 signaling pathway consisting of the Smad-dependent and
-independent pathway were measured by western blot analysis in the
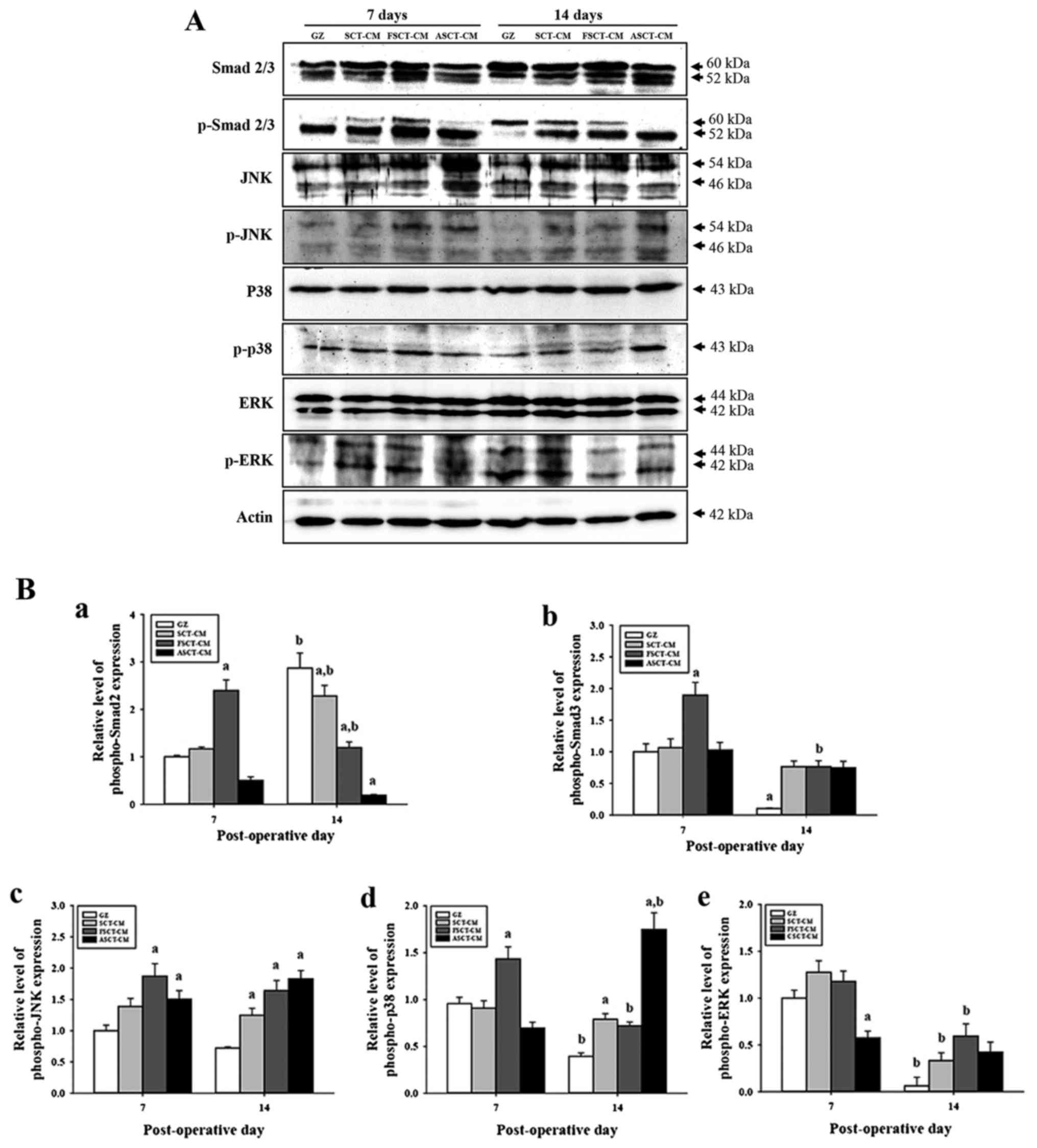
group subsets. In the case of the Smad-dependent pathway, the
expression of p-Smad2/3 at 7 days post-surgery was higher in the
FSCT-CM-treated group than in the GZ-, SCT-CM- and ASCT-CM-treated
groups. However, a different pattern was observed at 14 days
post-surgery. The expression of p-Smad2 was lower in the
SCT-CM-treated groups than in the GZ-treated group, although the
lowest level was observed in the ASCT-CM treated group. However,
the expression of p-Smad3 was higher in the SCT-CM-treated groups
than in the GZ-treated group (Fig.
7). In the case of the Smad-dependent pathway, alterations in
the phosphorylation of downstream regulators in the MAP kinase
pathway were also detected in the three SCT-CM-treated groups. The
expression level of p-JNK and p-p38 reflected the expression level
of p-Smad2 and p-Smad3 well. The highest level of both proteins was
detected in the FSCT-CM-treated group at 7 days post-surgery.
However, at 14 days post-surgery, a high level of p-JNK and p-p38
was detected in the three SCT-CM-treated groups compared with the
GZ-treated group, although the highest level was observed in the
ASCT-CM-treated group. Moreover, the FSCT-CM-treated group
exhibited a higher level of p-ERK than the other groups at 14 days
post-surgery (Fig. 7B–e).
Therefore, these results suggest that the use of the FSCT-CM may
significantly accelerate angiogenesis through the stimulation of
the TGF-β1 signaling pathway during the early stages of wound
healing.
Toxicity of the three SCT-CMs
To investigate the toxicity of the three SCT-CMs
toward the livers and kidneys of the SD rats, alterations in body
weight, metabolic enzymes and histopathology were investigated in
blood serum and liver and kidney tissue using serum biochemical
analysis and histological analysis. No significant alterations in
body weight over 14 days were detected in any of the three
SCT-CM-treated groups when compared with the GZ-treated group
(Table II). Liver toxicity
analysis revealed no increase in the levels of the three liver
toxicity indicators, specifically ALP, AST and ALT, between the
three SCT-CM-treated groups and the GZ-treated group at either time
point, although significantly higher levels of AST and ALT were
detected in the ASCT-CM-treated group on day 7 (Table III). The results of kidney
toxicity analysis were similar to those observed in the liver
toxicity analysis. Specifically, the BUN and CRE levels in serum
did not increase significantly in the three SCT-CM-treated groups,
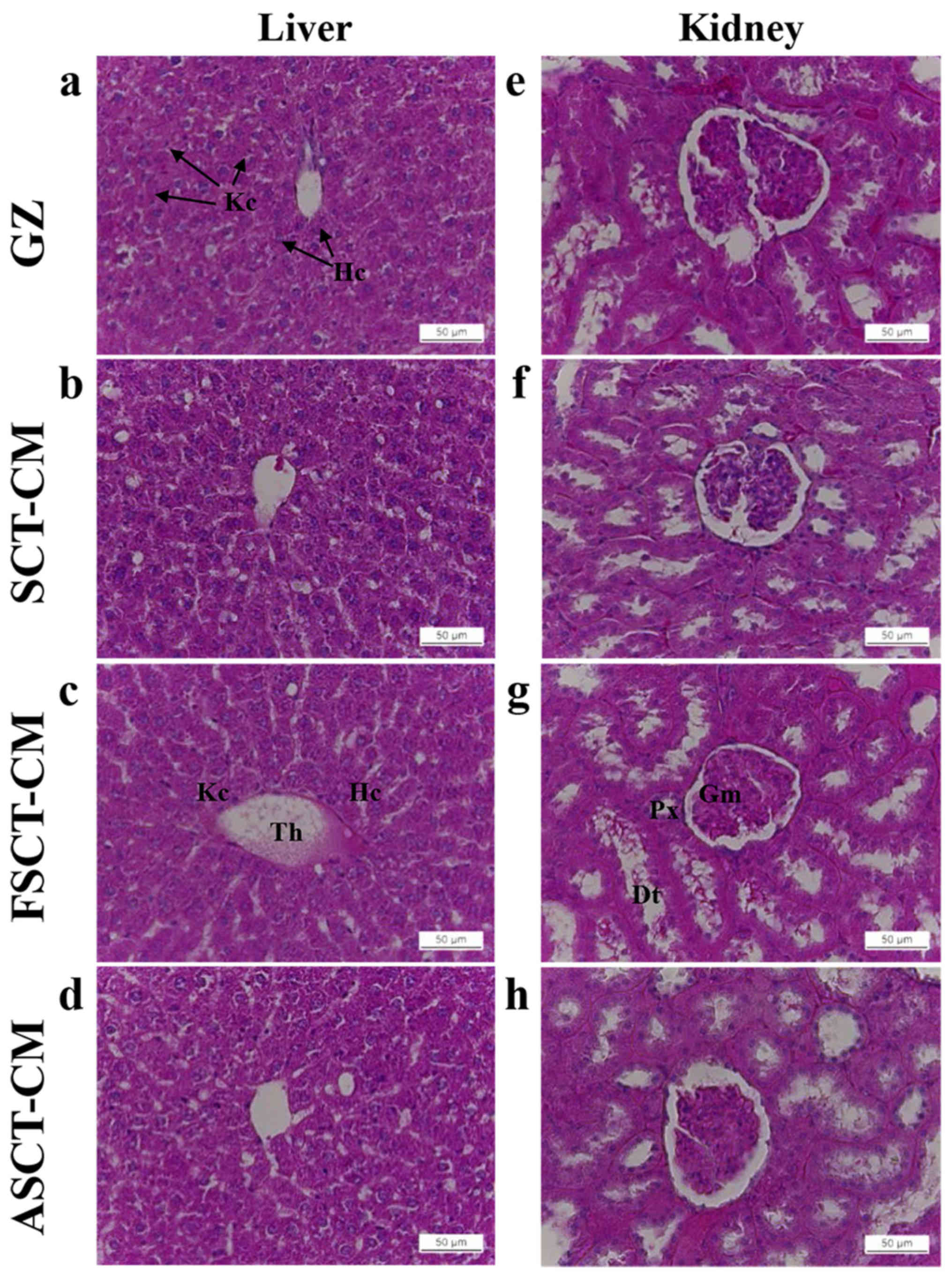
regardless of the exposure time (Table III). To investigate histological
alterations, liver and kidney sections were stained with H&E
and observed microscopically. No significant pathological changes
were detected between the liver and kidney tissue of the rats in
any of the groups (Fig. 8),
suggesting that the use of the three SCT-CMs for a short period of
time did not induce toxicity to the livers and kidneys of the SD
rats.
 | Table IIAlterations in body weight of the SD
rats following the application of the SCT-CMs. |
Table II
Alterations in body weight of the SD
rats following the application of the SCT-CMs.
| Day | GZ | SCT-CM | FSCT-CM | ASCT-CM |
|---|
| 0 | 259.3±12.37 | 262.9±15.07 | 262.6±13.07 | 267.8±10.05 |
| 3 | 267.5±16.32 | 270.2±13.21 | 260.3±13.4 | 270.5±13.89 |
| 6 | 282.3±17.84 | 288.5±11.34 | 267.9±14.11 | 284.6±14.59 |
| 9 | 295±33.85 | 311.6±21.73 | 291±15.62 | 298.4±10.33 |
| 12 | 310.8±29.43 | 324.6±23.29 | 301±18.08 | 308±14.2 |
 | Table IIIAlterations in the serum levels of
the biochemical markers, ALP, AST, ALT, BUN and CRE. |
Table III
Alterations in the serum levels of
the biochemical markers, ALP, AST, ALT, BUN and CRE.
| Day | Items | GZ | SCT-CM | FSCT-CM | ASCT-CM |
|---|
| 7 | ALP | 333±91.92 | 365.33±17.95 | 357±55.58 | 337.33±57.84 |
| AST | 93±2.83 | 98.67±8.5 | 87.67±12.42 | 125.5±37.48a |
| ALT | 49±1.41 | 61.67±5.13 | 55±15.72 | 79±14.14a |
| BUN | 18.73±3.84 | 20.17±2.66 | 17.95±1.75 | 20.48±1.85 |
| CRE | 0.7±0.01 | 0.68±0.06 | 0.68±0.03 | 0.59±0.1 |
| 14 | ALP | 328±43.26 | 361.4±86.71 |
1403.4±132.94a | 380.25±66.09 |
| AST | 86.8±10.21 | 77.4±7.99 | 83.33±15.53 | 98.75±27 |
| ALT | 49.4±5.55 | 51.6±10.14 | 60.2±19.5 | 62.5±13.48 |
| BUN | 20.14±2.12 | 20.64±1.54 | 21.04±2.06 | 20.78±1.81 |
| CRE | 0.66±0.05 | 0.63±0.03 | 0.61±0.04 | 0.6±0.06 |
Discussion
Cellulose and its derivatives have been applied as
drug coating materials, blood coagulants, artificial kidney
membranes, antitumor drugs, blood-compatible materials and supports
for immobilized enzymes in various medical fields (22,23). CM has recently received a great
deal of attention as a novel biomedical material for the treatment
of various wounds as it does not require special treatment for the
elimination of risk factors, such as immune response and viral risk
following chitosan treatment (24,25). Therefore, in this study, we
attempted to obtain additional evidence of the potential for SCT-CM
medical applications and then investigated the therapeutic effects
and toxicity of the three SCT-CMs in surgical wounds of rat back
skin. The results of the present study clearly demonstrated that
the FSCT-CM promoted the improvement of wound skin symptoms,
re-epithelization, granulation tissue formation and angiogenesis
without exerting any toxic effects.
In wound dressing, the porous, sponge and multilayer
structure provide several benefits, including absorbing large
amount of exudate, increasing the efficiency of drug release, good
contacting ability and strong adhesion to the wound surface
(26). In this study, two
different types of structures on the fracture surface were observed
in the SEM image of the three SCT-CMs. The multilayer structure on
the fracture surface of the SCT-CM and FSCT-CM was very similar
with that of the SCT-CF20-F prepared with 3% SCT in [Amim]Cl in a
previous study (26). However,
the porous structures detected in the ASCT-CM (1.67 µm in
diameter) were observed in many regenerated cellulose films and
membranes although their pore size was varied in each one.
Regenerated cellulose film prepared by coagulating with sulfuric
acid displayed a porous structure calculated to be 186 and 80 nm in
diameter on the free surface and fracture surface (27). In addition, regenerated cellulose
hydrogel films presented a fibrous internetwork and porous
structure with diameter of approximatel 20 nm (28).
The enhanced physical properties, including tensile
strength and elongation are required to satisfy the requirements of
a wound dressing. In a previous study, the tensile strength of the
regenerated cellulose film with plasticizers prepared with 5%
cellulose in [Amim]Cl was increased to 70–80 Mpa although
elongation was approximately 6% (29). However, in this study, the three
SCT-CMs prepared in similar conditions with those of the previous
study exhibited an increased level of tensile strength and
elongation. In particular, the FSCT-CM exhibited the highest
strength and lowest elongation level among the three SCT-CMs. These
differences in the value of tensile strength and elongation could
be caused by adding platicizer.
WVTR among various physical properties of wound
dressing tightly correlated with the speed and time required for
wound repair in the skin tissue. The excessively high level of WVTR
value stimulates the scare formation in the wound area as the wound
can dry rapidly. However, the wound repair process can be impeded
by accumulating exudates, resulting in an enhanced risk of
bacterial infection when the WVTR level is too low (30). Generally, the WVTR value was
measured as 8.5 g/m2/h in the normal skin although this
value was approximately 11.6 g/m2/h in the injured skin
(31). In this study, the FSCT-CM
exhibited a stable value on the fluid uptake rate and WVTR compared
with the SCT-CM and ASCT-CM. The WVTR value of the FSCT-CM
gradually increased to approximately 72 g/m2/h within 24
h and then maintained this level from 24 to 72 h. These levels were
also higher, being 8.5-fold those of normal skin (8.5
g/m2/h) (31) and
higher, being 3–3.2-fold those of 23–25 g/m2/h of
regenerated cellulose/collagen composite hydrogel films showing
good equilibrium-swelling ratio, air permeability and water
retention properties (28).
However, this level of FSCT-CMs was significantly lower than wound
dressings available on the market such as Geliperm (375.39
g/m2/h; Geistlich Ltd., Wolhusen, Switzerland) and
Vigilon (390.01 g/m2/h; Bard Ltd., West Sussex, UK)
(32).
The results presented herein demonstrated that the
use of the SCT-CM and FSCT-CM stimulated wound closure in
surgically-injured skin of SD rats. The enhancement of the wound
closure rate, as well as the regeneration of normal tissue,
including a decrease in the wound edge, was strongly detected
within 3–6 and 12–14 days of surgical injury (Fig. 4). These results were consistent
with the results of several previous studies, showing that the
topical application of several cellulose mixtures significantly
increased the rate of wound healing over that of vehicle-treated
groups in animals and humans. The diameter of the wound area was
shown to decrease in hydrocolloid membrane (HCM)-SCT-treated SD
rats when compared with the GZ-treated group (11). Moreover, the topical application
of ORC/collagen was found to accelerate diabetic wound closure in
C57BL/KsJ (db/db) mice and humans with diabetic foot ulcers
(12,33). However, several opposing results
in the investigations of wound closure have been recently reported.
Specifically, the percentage of wound contraction was lower in
crystalline CM-treated Wistar rats than the 0.9% sodium
chloride-treated group (10).
Therefore, we hypothesized that these differences in wound repair
may be caused by differences in the manufacturing process or
additives, even though cellulose was used as a basic building
material.
Another conclusion of this study is that SCT-CM
treatment applied to the back skin of SD rats stimulated the
regeneration of surgical wound tissue. Similar results have been
reported for surgical wounds on SD rats treated with HCM-SCT or
ORC. Application of HCM-SCT into surgical wound skin accelerated
wound reepithelization, development of young granulation tissues,
and decreasing epidermis thickness when compared with that of the
GZ treated group (11). The
thickness of the epidermis at 14 days was rapidly recovered to a
normal level in the FSCT-CM treated group. Therefore, we propose
that FSCT-CM treatment for a longer period of time should be
investigated to determine if the skin regeneration is complete.
Immune cells and mast cells are known to participate
in the three phases of skin wound healing, inflammatory reaction,
angiogenesis and extracellular-matrix reabsorption (34). Therefore, the infiltration of
these cells into the dermis of wounded skin is considered a key
indicator of inflammatory reaction against the wound dressing
materials. Surgical wounds treated with ORC have been shown to have
a significantly lower number of infiltrated inflammatory cells when
compared with the control group at 5 days post-surgery (35). In addition, a decrease in the
infiltration of mononuclear cells was measured in skin wounds
treated with crystalline CM at days 14, 21 and 26, even though a
constant level was maintained until day 7 (10). In the present study, the number of
inflammatory cells and mast cells markedly decreased in the
FSCT-CM-treated group at days 7 and 14, while a decrease in the
number of mast cells was detected in the SCT-CM treated group at
day 14. The results for the later stages agree completely with
those of the two aforementioned studies, even though the results
for the early stage differed.
The wound healing of skin may contribute to
angiogenesis through the production of microvessels that transport
nutrients and oxygen to growing dermal cells (36). Several functional extracts,
including angico extract (Anadenanthera colub- rina var.
cebil) have been shown to induce a significant increase in
the number of blood vessels during the healing of rat skin at 7 and
14 days post-surgery, although such an increase was not measured in
the cellulose-treated animals (37). Alterations in the levels of VEGF,
one of the major regulatory cytokines involved in angiogenesis,
have been detected in surgical wound repair in skin. In a previous
study, the group treated with HCM-SCT exhibited an 18.8% decrease
in VEGF expression when compared with the GZ-treated group at 11
days post-surgery (11).
Moreover, a decrease in the expression of VEGF was observed in the
group treated with microbial cellulose on day 14 after skin
excision, although it was higher in the same group on day 7
(38). In the present study, the
number of blood vessels and the expression level of VEGF were
higher in the FSCT-CM-treated group than the other groups on day 7,
while their levels were lower on day 14. These results are in
complete agreement with those of previous studies, although the
relative ratio of increase or decrease differed.
Collagen is a key component of the extracellular
matrix that plays an important role in all phases of surgical wound
healing (39). The altered
deposition of collagen has been observed in surgically wounded skin
treated with several therapeutic compounds and SCT mixture. In a
previous study, collagen deposition was higher in the dorsal
incision wound treated with total ginseng saponin than in the
control group (40). Moreover,
the expression of collagen was upregulated significantly in the
surgical skin wounds of rats treated with HCM-SCT on day 11
(11). In the present study, the
expression of collagen was only higher in the FSCT-CM-treated group
than the other groups on day 14, although a constant level was
maintained on day 7. These results were similar to those of
previous studies.
TGF-β1 signaling plays a crucial role in
re-epithelialization, inflammation, angiogenesis and granulation
tissue formation during wound healing (41). This signaling mediates their
effects through both the Smad-dependent and Smad-independent
pathway (20,42). The activation of the
Smad-dependent pathway via stimulation with TGF-β1 can induce the
synthesis and secretion of collagen, leading to increased scar
formation (43,44). In addition, the concentration of
TGF-β1 in serum has been shown to change in response to treatment
with cellulose complex. Specifically, an approximately 266%
increase in the TGF-β1 concentration was previously measured in SD
rats treated with HCM-SCT on day 11 when compared to the GZ- or
HCM-treated groups (11). The
results of this study are in agreement with those of previous
studies, although the final analysis point and level of increase
differed (Fig. 6). The
correlation between the Smad-dependent pathway and cellulose wound
dressing on healing has not been reported in any previous studies,
at least to the best of our knowledge. In the present study, the
expression of p-Smad2/3 in the FSCT-treated group was higher on
days 7 and 14 than that of the GZ-treated group. Therefore, the
present study provides novel evidence that FSCT-CM may accelerate
the wound healing of surgically wounded skin through the regulation
of the TGF-β1 signaling pathway.
Finally, several studies have suggested that SCT
complexes and derivatives do not show any toxicity in mammalian
systems. Specifically, SD rats treated with HCM-SCT for 11 days did
not induce any specific toxicity based on body weight or metabolic
enzymes of the liver and kidney (11). In addition, the concentration of
metabolic enzymes representing liver and kidney toxicity in the
serum of SCT-CF-treated SD rats was maintained at a constant level
relative to the vehicle implanted group (14). In this study, most indicators of
liver and kidney toxicity were maintained at a constant level in
the three SCT-CM-treated groups throughout the experimental period.
These results are in agreement with the finding that SCT derived
materials were not correlated with animal toxicity.
Taken together, the results of our study
demonstrated that the topical application of the three SCT-CMs for
2 weeks induced the acceleration of the healing of surgical wounds,
including tissue regeneration, connective formation and
angiogenesis. Among these, FSCT-CM exerted the greatest therapeutic
effect on surgical wound healing of the back skin of SD rats.
Moreover, this study provides insight into the molecular action of
FSCT-CM in a rat model of surgical wound healing using SD rats
(Table IV). Finally, this study
demonstrated that the three SCT-CMs do not induce any significant
toxicity toward the livers and kidneys of SD rats. Overall, our
results provide a rationale for the future development of FSCT-CM
with other functional compounds for topical application to
cutaneous wounds.
 | Table IVSummary of the properties of the
three SCT-CMs applied as wound dressings. |
Table IV
Summary of the properties of the
three SCT-CMs applied as wound dressings.
| Classification | Contents | SCT-CM | FSCT-CM | ASCT-CM |
|---|
| Preparation | Materials | SCT | SCT | SCT with sodium
alginate |
| Drying
conditions | Drying at room
temperature for 24 h | Freeze-drying after
drying at room temperature for 24 h | Drying at room
temperature for 24 h |
| Physical properties
analyses | Morphologic
properties | Randomly arranged
fibrils | Randomly arranged
fibrils | Many small
pores |
| Mechanical
properties | | | |
| Tensile
strength | Low | High | Medium |
| Elongation | Medium | Low | High |
| Fluid uptake
rate | Low | Medium | High |
| WVTR | Low | Medium | High |
| Efficacy
analyses | Wound closing | Medium | High | Low |
|
Re-epithelization | Medium | High | Low |
| Angiogenesis | Low | High | Low |
| Inflammation | Medium | Low | High |
| Connective tissue
regeneration | Medium | High | Low |
| Toxicity
analyses |
Hephatotoxicity | None | None | None |
| Nephrotoxicity | None | None | None |
| Applicability for
wound dressing | | Regular | Excellent | Unsuitable |
Acknowledgments
This study was supported by a grant to Professor Dae
Youn Hwang from the Korea Institute of Marine Science and
Technology Promotion (112088-3). In addition, this manuscript was
proofread and edited by Jeremy Kaman, one of the English editors at
NURISCO (NU-150202).
References
|
1
|
Eaglstein WH: Moist wound healing with
occlusive dressings: A clinical focus. Dermatol Surg. 27:175–181.
2001.PubMed/NCBI
|
|
2
|
Mark GL, Warren RH, John BJ and Ian C:
Treatment of skin disease: comprehensive therapeutic strategies.
Decubitus Ulcers. 4th edition. Joseph AW, Lawrence CP, Caren C and
Jennifer LP: Elsevier Health Sciences; UK: pp. 167–171. 2013
|
|
3
|
Kim JO, Choi JY, Park JK, Kim JH, Jin SG,
Chang SW, Li DX, Hwang MR, Woo JS, Kim JA, et al: Development of
clindamycin-loaded wound dressing with polyvinyl alcohol and sodium
alginate. Biol Pharm Bull. 31:2277–2282. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crépy L, Monchau F, Chai F, Raoul G,
Hivart P, Hildebrand HF, Martin P and Joly N: Evaluation of a
bio-based hydrophobic cellulose laurate film as biomaterial - study
on biodegradation and cytocompatibility. J Biomed Mater Res B Appl
Biomater. 100:1000–1008. 2012. View Article : Google Scholar
|
|
5
|
Thomas S: Wound Management and Dressing.
Pharmaceutical Press; London: 1990
|
|
6
|
Woodley DT, Chen JD, Kim JP, Sarret Y,
Iwasaki T, Kim YH and O'Keefe EJ: Re-epithelialization. Human
keratinocyte locomotion. Dermatol Clin. 11:641–646. 1993.PubMed/NCBI
|
|
7
|
Jayakumar R, Prabaharan M, Sudheesh Kumar
PT, Nair SV and Tamura H: Biomaterials based on chitin and chitosan
in wound dressing applications. Biotechnol Adv. 29:322–337. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Witthayaprapakorn C: Design and
preparation of synthetic hydrogels via photopolymerisation for
biomedical uses as wound dressings. Procedia Eng. 8:286–291. 2011.
View Article : Google Scholar
|
|
9
|
Yang JM and Lin HT: Properties of chitosan
containing PP-g-AA-g-IPAAm bigraft nonwoven fabric for wound
dressing. J Membr Sci. 243:1–7. 2004. View Article : Google Scholar
|
|
10
|
Camargo MC, Nogueira RM, Sanches OC, Saab
MG, Batista A, Vasconcelos D, Luvisotto LY and Lúcio MA:
Applicability of crystalline cellulose membrane in the treatment of
skin wounds induced in Wistar rats. Acta Cir Bras. 29:429–437.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwak MH, Go J, Kim JE, Lee YJ, Lee SH, Lee
HS, Son HJ, Jung YJ and Hwang DY: Property and efficacy analysis of
hydrocolloid membrane containing Styela clava tunic on the wound
repair of skin in SD rats. Biomater Res. 17:91–101. 2013.
|
|
12
|
Hart J, Silcock D, Gunnigle S, Cullen B,
Light ND and Watt PW: The role of oxidised regenerated
cellulose/collagen in wound repair: Effects in vitro on fibroblast
biology and in vivo in a model of compromised healing. Int J
Biochem Cell Biol. 34:1557–1570. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung YJ: Properties of regenerated
cellulose films prepared from the tunicate Styela clava. J Kor Fish
Soc. 41:237–242. 2008.
|
|
14
|
Song SH, Kim JE, Lee YJ, Kwak MH, Sung GY,
Kwon SH, Son HJ, Lee HS, Jung YJ and Hwang DY: Cellulose film
regenerated from Styela clava tunics have biodegradability,
toxicity and biocompatibility in the skin of SD rats. J Mater Sci
Mater Med. 25:1519–1530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung YJ, An BJ, Hwang DY, Kim HD, Park SM,
Cho H and Kim HS: Preparation and properties of regenerated
cellulosic biomaterial made from Styela Clava tunics. Biomater Res.
12:71–76. 2008.
|
|
16
|
Pereira RF, Mendes A and Bártolo PJ: Novel
alginate/Aloe Vera hydrogel blends as wound dressings for the
treatment of several types of wounds. Chem Eng Prog. 32:1009–1014.
2013.
|
|
17
|
ASTM standard E96-00. Standard test
methods for water vapour transmission of materials.
|
|
18
|
Kim HJ, Kim J, Kim SJ, Lee SH, Park YS,
Park BK, Kim BS, Kim SK, Cho SD, Jung JW, et al: Anti-inflammatory
effect of Quercetin on picryl chloride-induced contact dermatitis
in BALB/c mice. Lab Anim Res. 26:7–13. 2010. View Article : Google Scholar
|
|
19
|
Baum CL and Arpey CJ: Normal cutaneous
wound healing: Clinical correlation with cellular and molecular
events. Dermatol Surg. 31:674–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu L, Hébert MC and Zhang YE: TGF-beta
receptor-activated p38 MAP kinase mediates Smad-independent
TGF-beta responses. EMBO J. 21:3749–3759. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matasuzaki K, Yamamoto I, Sato T and
Oshima R: Synthesis of water-soluble branched polysaccharides and
their antitumor activity, 1. Branched polysaccharides from
cellulose acetate. Macromol Chem Phys. 186:449–456. 1985.
View Article : Google Scholar
|
|
23
|
Ito H, Shibata T, Miyamoto T, Inagaki H
and Noishiki Y: Formation of polyelectrolyte complexes between
cellulose derivatives and their blood compatibility. J Appl Polym
Sci. 31:2491–2500. 1986. View Article : Google Scholar
|
|
24
|
Hirano S: Chitin biotechnology
applications. Biotechnol Annu Rev. 2:237–258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Müller FA, Müller L, Hofmann I, Greil P,
Wenzel MM and Staudenmaier R: Cellulose-based scaffold materials
for cartilage tissue engineering. Biomaterials. 27:3955–3963. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seong KY, Koh EK, Lee SH, Kwak MH, Son HJ,
Lee HS, Hwang DY and Jung YJ: Preparation and characterization of
high absorptive cellulose film derived from Styela Clava tunic for
wound dressing. Text Coloration Finish. 27:70–79. 2015. View Article : Google Scholar
|
|
27
|
Zhang L, Ruan D and Gao S: Dissolution and
regeneration of cellulose in NaOH/thiourea aqueous solution. J
Polym Sci Pol Phys. 40:1521–1529. 2002. View Article : Google Scholar
|
|
28
|
Cheng Y, Lu J, Liu S, Zhao P, Lu G and
Chen J: The preparation, characterization and evaluation of
regenerated cellulose/collagen composite hydrogel films. Carbohydr
Polym. 107:57–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pang J, Liu X, Zhang X, Wu Y and Sun R:
Fabrication of cellulose film with enhanced mechanical properties
in ionic liquid 1-Allyl-3-methylimidaxolium chloride (AmimCl).
Materials (Basel). 6:1270–1284. 2013. View Article : Google Scholar
|
|
30
|
Queen D, Evans JH, Gaylor JDS, Courtney JM
and Reid WH: An in vitro assessment of wound dressing
conformability. Biomaterials. 8:372–376. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mi FL, Shyu SS, Wu YB, Lee ST, Shyong JY
and Huang RN: Fabrication and characterization of a sponge-like
asymmetric chitosan membrane as a wound dressing. Biomaterials.
22:165–173. 2001. View Article : Google Scholar
|
|
32
|
Wu P, Fisher AC, Foo PP, Queen D and
Gaylor JD: In vitro assessment of water vapour transmission of
synthetic wound dressings. Biomaterials. 16:171–175. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ulrich D, Smeets R, Unglaub F, Wöltje M
and Pallua N: Effect of oxidized regenerated cellulose/collagen
matrix on proteases in wound exudate of patients with diabetic foot
ulcers. J Wound Ostomy Continence Nurs. 38:522–528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trabucchi E, Radaelli E, Marazzi M, Foschi
D, Musazzi M, Veronesi AM and Montorsi W: The role of mast cells in
wound healing. Int J Tissue React. 10:367–372. 1988.PubMed/NCBI
|
|
35
|
Liu SA, Cheng CC, Chen JS, Hung YW, Chen
FJ and Chiu YT: Effect of oxidized regenerated cellulose on the
healing of pharyngeal wound: An experimental animal study. J Chin
Med Assoc. 75:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andrikopoulou E, Zhang X, Sebastian R,
Marti G, Liu L, Milner SM and Harmon JW: Current Insights into the
role of HIF-1 in cutaneous wound healing. Curr Mol Med. 11:218–235.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pessoa WS, Estevão LR, Simões RS, Barros
ME, Mendonça FS, Baratella-Evêncio L and Evêncio-Neto J: Effects of
angico extract (Anadenanthera colubrina var. cebil) in cutaneous
wound healing in rats. Acta Cir Bras. 27:655–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SU, Lee BK, Kim MS, Park KK, Sung WJ,
Kim HY, Han DG, Shim JS, Lee YJ, Kim SH, et al: The possibility of
microbial cellulose for dressing and scaffold materials. Int Wound
J. 11:35–43. 2014. View Article : Google Scholar
|
|
39
|
Mian M, Beghè F and Mian E: Collagen as a
pharmacological approach in wound healing. Int J Tissue React.
14(Suppl): 1–9. 1992.PubMed/NCBI
|
|
40
|
Kim YS, Cho IH, Jeong MJ, Jeong SJ, Nah
SY, Cho YS, Kim SH, Go A, Kim SE, Kang SS, et al: Therapeutic
effect of total ginseng saponin on skin wound healing. J Ginseng
Res. 35:360–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ramirez H, Patel SB and Pastar I: The role
of TGFβ signaling in wound epithelialization. Adv Wound Care (New
Rochelle). 3:482–491. 2014. View Article : Google Scholar
|
|
42
|
Willis BC, Borok Z and Am J:
TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung
disease. Am J Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ihn H: Autocrine TGF-beta signaling in the
pathogenesis of systemic sclerosis. J Dermatol Sci. 49:103–113.
2008. View Article : Google Scholar
|
|
44
|
Qi SH, Xie JL, Pan S, Xu YB, Li TZ, Tang
JM, Liu XS, Shu B and Liu P: Effects of asiaticoside on the
expression of Smad protein by normal skin fibroblasts and
hypertrophic scar fibroblasts. Clin Exp Dermatol. 33:171–175. 2008.
View Article : Google Scholar : PubMed/NCBI
|