Introduction
Myasthenia gravis (MG) is a typical autoimmune
disease caused by antibodies against the muscle type nicotinic
acetylcholine receptor (nAChR) of the neuromuscular junction.
Anti-nAChR antibodies reduce the number of effective nAChRs at the
endplate, causing the failure of synaptic electrical-chemical
coupling, resulting in muscle weakness and fatigability (1,2).
Anti-nAChR antibodies are detectable in approximately 90% of
myasthenic patient sera (3).
nAChR, from fish electric organs and vertebrate
skeletal muscles, is a transmembrane glycoprotein composed of 5
homologous subunits organized into a cation channel in the order of
α1γα1δβ1 in denervated skeletal muscle, and α1εα1δβ1 in innervated
skeletal muscle (4). Each subunit
in nAChR consists of 2 parts: the extracellular domain (ECD) and
the cytoplasmic loop (CL). Agonists, such as acetylcholine and
competitive antagonists, such as α-bungarotoxin (α-BTX) (5), bind to the two α subunits and
regulate the ion channel. Anti-nAChR antibodies do not bind to the
Ach binding site of nAChR, and antibody-nAChR complexes extracted
from muscle do bind 125Iα-BTX. Therefore, the
concentration of anti-nAChR antibodies in sera can be determined in
terms of the 125Iα-BTX-binding site (6).
In the sera from patietns with MG, most anti-nAChR
antibodies bind to a small region on the extracellular side of the
α1 subunit, the main immunogenic region (MIR) (7–9).
Most of the known anti-nAChR antibodies recognize the extracellular
side of nAChR (10). However, a
growing body of evidence has indicated that anti-nAChR CL
antibodies also exist in the sera of patients with MG (11–13). It has been reported that
approximately 17% of patients with MG are anti-CL antibody-positive
(14). At present, relatively
little is known regarding the association between anti-CL
antibodies and the clinical profile of MG.
Recently, several studies have demonstrated that
anti-CL antibody may play a protective role in animal models of
experimental autoimmune MG (EAMG), in which they discovered a novel
approach to the specific immunosuppression of EAMG with a
therapeutic vaccine consisting of bacterially-expressed human nAChR
CL, which has the potential to specifically suppress MG without the
danger of causing exacerbation (11–13,15). However, to date, information about
the constitutive effects of anti-CL antibody on the process of MG
and its relevance to the degree of MG is lacking. In our
retrospective study, we measured the levels of anti-CL antibody in
the serum samples of 76 patients with MG, and analyzed its
association with the clinical profile and anti-ECD muscle nAChR
titers.
Materials and methods
Human subjects
The sera from 76 patients with MG were collected
from the MG sample bank of the Department of Neurology, Tangdu
Hospital, Xi'an, China. All samples in the MG sample bank were
diagnosed according to the diagnostic criteria. The following
criteria were used to diagnose MG (16,17): i) acquired weakness of skeletal
muscles, including cranial muscles; ii) fluctuation in muscle
strength; iii) 10% decrease in compound muscle action potential
amplitude in response to stimulation at 3–5 Hz and increased jitter
on single-fiber electromyography; and iv) positive response to a
cholinesterase inhibitor. Exclusion criteria were pregnancy. A
total of 40 healthy subjects were recruited as the controls and
were matched with the patients with MG in age, sex and demography.
According to the principles expressed in the Helsinki Declaration
and to the ethics committee-approved protocols, all subjects
provided written and signed informed consent prior to enrollment.
Blood sample collections from human subjects for detailed
immunological assessments were approved by the Ethics Committee of
the Fourth Military Medical University. All patients and healthy
controls provided written informed consent.
Preparation of human muscle native
nAChR
Human skeletal muscle was obtained from the
amputated leg of a diabetic patient treated for gangrene as the
nAChR source. We followed the protocols described in the studies by
Lindstrom et al (6) and
Chang et al (18) and made
necessary changes. The muscle was chopped into small sections of
10–15 g, flash-frozen in liquid nitrogen and stored at −80°C.
Muscle was homogenized with a pre-chilled electrical blender for
1–2 min a 4°C in 300 ml of 0.1 M NaCl, 0.01 M phosphate buffer (pH
7.0). Following centrifugation at 9×103 rpm for 20 min,
the supernatant was discarded. Homogenization and centrifugation
were repeated with the pellet. The pellets were extracted in 160 ml
2% Triton X-100, 0,1 M NaCl, 20 mM EDTA, 0.01 M phosphate buffer
(pH 7.0), 0.01M NaN3 by homogenizing for 20 sec,
followed by gentle shaking for 1 h at 4°C. Following centrifugation
at 105 g/4°C for 30 min, the resulting supernatant was
suctioned using a syringe, pooled, glycerol was added to 10%, mixed
well, and stored as 1 ml aliquot at −80°C.
Preparation of recombinant human muscle
nAChR CL Plasmid and bacterial strain
The plasmid vector pET16b and the prokaryotic
expression host strain E. coli BL21 (DE3) were purchased
from Novagen, Darmstadt, Germany.
Human muscle nAChR CL expression vector
construction
DNA sequences encoding the CL of the human muscle
nAChR subunits, α1 (residues 301–374), β1 (residues 312–412), and δ
(residues 315–416), were codon-optimized to E. Coli
preferred codons and synthesized (Genscript Co., Nanjing, China).
Wild-type CL from human muscle nAChR α1 subunits lacked tyrosine
residue, the key amino acid for iodination labeling. Therefore, 2
additional tyrosine residues were incorporated into the C-terminal
of AChRα1 CL by PCR to generate the coding sequence. For the
wild-type β1 and δ subunits, which already contain the tyrosine
residues, there was no need to change the synthesized coding
sequences. These sequences were inserted into the NdeI and
BamHI sites of pET16b (Novagen), and transformed into
expression host BL21 (DE3) (Novagen). Recombinant clones were
checked for insertion by restriction digestion and sequencing
(Genscript Co.).
Expression and purification of proteins
corresponding to the nAChR CL
Recombinant nAChR CL was generated from the BL21
(DE3) cells harboring the expression plasmids at an OD600 of 4.0
using a homemade fermentor. This was followed by induction with 1
mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 4 h at 37°C.
The cell pellets were collected by centrifugation. For extraction
of recombinant proteins, cell pellets were solubilized in 50 mM
phosphate buffer (pH 8.0), 6 M guanidine hydrochloride, 5 mM
imidazole, sheared with an electric blender to reduce the
stickiness of the solution and centrifuged at 28,000 × g at 4°C for
30 min. The supernatant was then passed through a 5-ml Histrap FF
column (GE Healthcare, Piscataway, NJ, USA). Bound protein was
eluted by 50 mM phosphate buffer (pH 8.0), 6 M guanidine
hydrochloride, 500 mM imidazole. Eluted fractions containing
protein peaks were pooled, dialyzed against 0.1% SDS, 10 mM EDTA,
and then loaded to several preparative SDS-PAGE gels. Protein bands
were visualized by staining with 0.1 M CuSO4 solution
and the band corresponding to the recombinant CL were excised.
Proteins within the bands were electroeluted, dialyzed to remove
residue Cu2+ ion, concentrated by ultrafiltration
(Amicon Ultra 3K filters) and stored at −20°C.
Iodination labeling of α-BTX and
recombinant nAChR CL
The recombinant nAChR CL proteins and α-BTX were
labeled with 125I using the Chloramine T method as
previously described by Lindstrom et al (9) and Greenwood et al (19). Briefly, 2 µCi of
125I (Carrier free, Chengdu Gaotong Isotope Co., Ltd.,
Chengdu, China) was mixed with 12 µl of 0.5 M sodium
phosphate buffer (pH 7.0) and 70 µg (approximately 7 nmol)
of recombinant nAChR CL protein or α-BTX, and then mixed with 2
µl of 1.6 M chloramine T. The mixture was then incubated on
ice for 25 min with occasional shaking and then loaded to a PD-10
Desalting column (GE Healthcare). Protein was eluted using
phosphate sodium buffer containing 0.1% SDS for recombinant nAChR
CL or phosphate sodium buffer for α-BTX. Fractions of 0.3 ml were
collected and monitored for radioactivity. Fractions containing
radioactivity were pooled and the final volume was adjusted with
phosphate buffer saline to yield a protein concentration of
2×10−6 M.
Radioimmunoassay of anti-native nAChR and
anti-CL anti- bodies from the sera of patients with MG
Serum anti-nAChR antibodies were measured using
radioimmunoprecipitation (20) by
125Iα-BTX-labeled native nAChR, 125I-labeled
nAChRα1-CL, 125I-labeled nAChRβ1-CL, and
125I-labeled nAChRδ-CL. For the measurement of
antibodies against nAChR-CL, 500 µl of diluted
125I-labeld nAChR CL protein (final concentration,
approximately 10−9 M) in 0.5% Triton X-100, 0.005% SDS,
were incubated with 10 µl of MG patient serum, followed by
the addition of 10 µl of rabbit anti-human antibody for 2 h.
For the measurement of antibodies against native human nAChR,
125I-labeled-α-BTX was mixed with 1 ml of human skeletal
muscle extract to a final concentration of 10−9 M,
followed by the addition of 10 µl of rabbit anti-human
antibody for 2 h. The immune complex was precipitated by
centrifugation at 13,000 rpm/4°C/5 min, and the pellets washed
twice with PBS (pH7.2) containing 0.5% Triton X-100. The
radioactivity was measured using a γ-counter (CNNC Xi'an Nuclear
Instrument Factory, Xi'an, China). Background radioactivity was
measured without patient serum.
Statistical analysis
The antibody concentrations of anti-native nAChR and
anti-CL are expressed in nM. The data were analyzed using SPSS 17.0
software. Continuous variables are listed as the means ± standard
deviation (SD) and were analyzed using the Student's t-test. The
differences in sex, MG type according to onset age, MG type and
onset MG type were analyzed using Chi-square tests, and the
analysis of antibody of ECD and CL were tested by linear regression
analysis. A two-tailed P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Expression and purification of three CLs
from human muscle nAChR in the α1, β1 and δ subunits
We generated 3 target proteins with a gradient of
molecular masses at approximately 15 kDa (Fig. 1A). We optimized the purification
of CLs from nAChR proteins with recombinant protein fractions
(Fig. 1B).
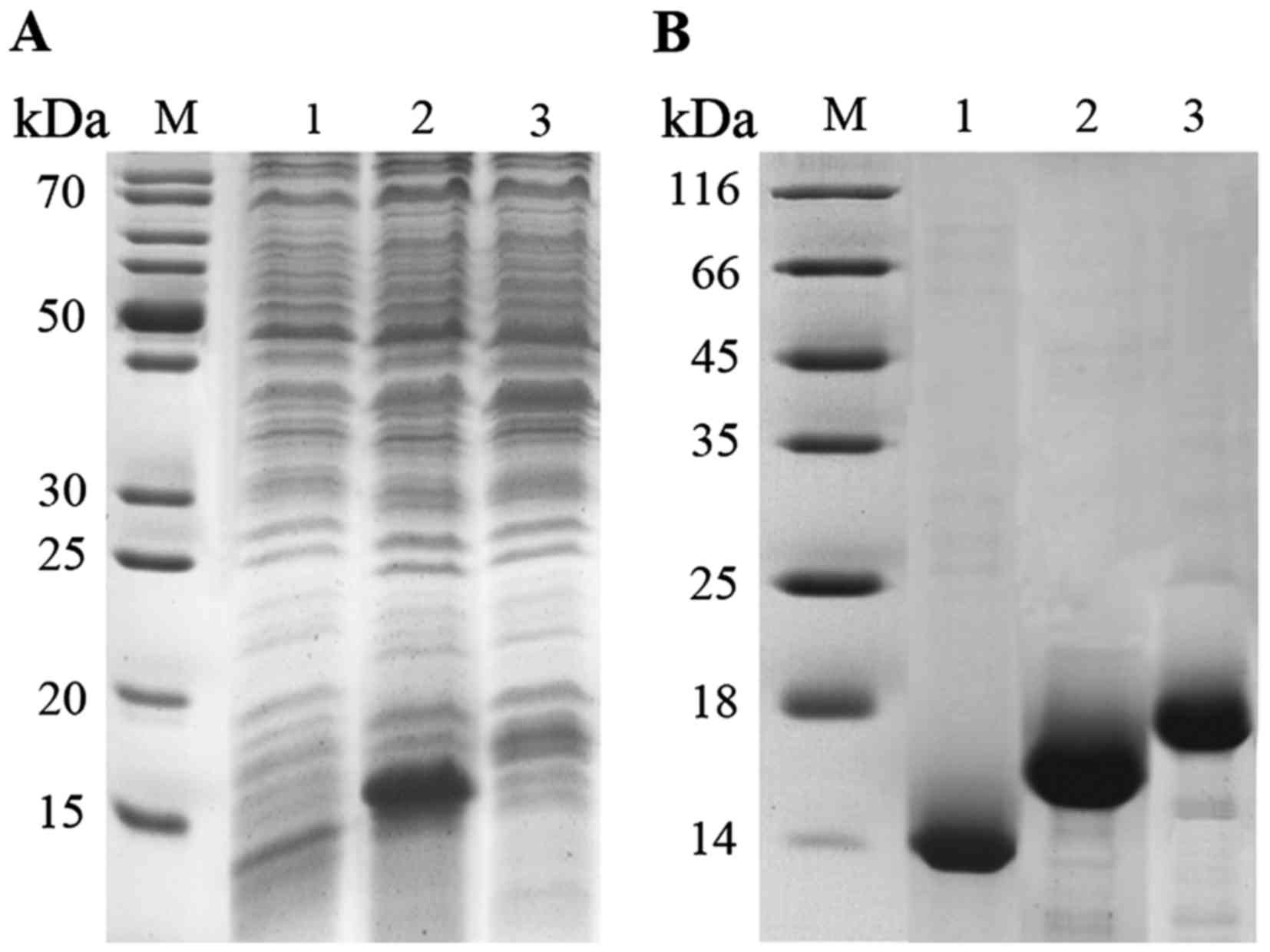 | Figure 1Expression and purification of CLs of
α1, β1 and δ subunits in nAChR. (A) SDS-PAGE of recombinant CLs of
α1, β1 and δ subunits in nAChR before purification. Lane M, marker;
lane 1, CLs of α1 subunit; lane 2, CLs of β1 subunit; lane 3, CLs
of δ subunit. (B) SDS-PAGE of recombinant CLs of α1, β1 and δ
subunit in nAChR after purification. Lane M, marker; lane 1, CLs of
α1 subunit; lane 2, CLs of β1 subunit; lane 3, CLs of δ
subunit. |
Patient data
A total of 76 patients were clinically diagnosed
with MG. The patient characteristics are shown in Table I and summarized in Table II. There were 46 (60.5%) males
and 30 (39.5%) females. The median age at diagnosis was 45 years
(range, 2–79 years). A total of 11 patients (14.5%) had thymoma; 2
patients (2.6%) had hyperthyroidism and 2 patients (2.6%) had
rheumatoid arthritis. In the present study, 29 patients (38.2%)
were clinically classified as ocular MG (OMG) and 47 patients
(61.8%) were classified as generalized MG (gMG). In total, 42
patients (55.3%) were diagnosed with OMG initially and 13 (30.1%)
of these 42 patients then developed gMG. A total of 34 (44.8%)
patients were initially diagnosed with gMG.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| No. | Age (years) | Sex | Disease duration
(months) | Maximum MGFA
class | Thymus change | Other autoimmune
disease | AChR titer
(nmol/l) | All previous
therapies at blood draw |
|---|
| 1 | 46 | M | 9 | II | Thymoma | None | 38.08 | PB, TS |
| 2 | 9 | M | 36 | II | None | None | 6.98 | PB |
| 3 | 46 | M | 180 | II | None |
Hyperthyroidism | 1.03 | PB |
| 4 | 17 | M | 0.3 | I | n.a. | None | 0.19 | None |
| 5 | 13 | M | 120 | I | None | None | 178.04 | PB, Pred |
| 6 | 35 | M | 84 | II | None | None | 0.17 | PB, Pred, MMF |
| 7 | 12 | M | 1 | II | None | None | 0.56 | PB |
| 8 | 38 | F | 4 | II | None | None | 45.48 | PB |
| 9 | 18 | F | 2 | II | Thymoma | None | 166.74 | PB, Pred, TS |
| 10 | 54 | M | 2 | II | None | None | 75.16 | PB |
| 11 | 22 | F | 2 | I | None | None | 135.24 | None |
| 12 | 31 | F | 8 | II | None | None | 60.50 | PB, Pred, CYC |
| 13 | 51 | F | 3 | II | None | Hypothyroidism | 33.78 | PB |
| 14 | 55 | F | 2 | II | Thymoma | None | 31.48 | PB, TS |
| 15 | 50 | M | 84 | II | None | None | 289.84 | PB, Pred |
| 16 | 67 | M | 36 | II | None | None | 29.08 | PB, Pred, CYC |
| 17 | 2.1 | M | 2 | I | None | None | 0.68 | PB |
| 18 | 27 | F | 24 | II | None | None | 26.58 | PB, Pred, AZA |
| 19 | 53 | F | 324 | I | None | None | 1.34 | PB, Pred, MMF |
| 20 | 55 | F | 6 | II | None | RA | 120.24 | PB |
| 21 | 60 | M | 6 | II | None | None | 115.70 | n.a. |
| 22 | 54 | M | 2 | II | Thymoma | None | 8.18 | PB |
| 23 | 23 | F | 12 | II | None | None | 378.14 | PB, Pred |
| 24 | 55 | M | 2 | I | None | None | 1.19 | None |
| 25 | 66 | F | 8 | II | Thymoma | None | 1.44 | PB, TS |
| 26 | 53 | F | 36 | II | None | RA | 125.24 | PB, Pred |
| 27 | 47 | M | 3 | I | None | None | 323.24 | PB |
| 28 | 57 | M | 24 | I | None | None | 324.64 | PB |
| 29 | 51 | M | 3 | II | None | None | 4.35 | n.a. |
| 30 | 30 | M | 11 | I | None | None | 0.90 | n.a. |
| 31 | 51 | M | 0.6 | I | n.a. | None | 87.63 | PB |
| 32 | 39 | F | 360 | II | None | None | 21.18 | PB |
| 33 | 67 | M | 72 | II | None | None | 80.96 | n.a. |
| 34 | 46 | F | 3 | II | None | None | 1.76 | PB |
| 35 | 26 | F | 12 | II | None | None | 506.84 | PB |
| 36 | 23 | M | 36 | I | None | None | 0.47 | PB, Pred, AZA |
| 37 | 16 | M | 2 | I | None | None | 1.70 | None |
| 38 | 50 | F | 0.3 | II | n.a. | None | 23.98 | None |
| 39 | 13 | F | 60 | II | None | None | 91.76 | PB, Pred |
| 40 | 71 | M | 0.6 | II | n.a. | None | 201.64 | None |
| 41 | 39 | M | 3 | II | None | None | 54.10 | None |
| 42 | 13 | M | 132 | I | None | None | 68.02 | PB, Pred, AZA |
| 43 | 17 | M | 36 | II | None | None | 16.28 | PB |
| 44 | 32 | F | 180 | I | None | None | 105.07 | PB, CYC, IVIg |
| 45 | 50 | M | 24 | II | None | None | 51.94 | PB |
| 46 | 61 | M | 480 | III | None | None | 2.01 | PB, Pred, CYC |
| 47 | 61 | F | 84 | II | None | None | 11.38 | n.a. |
| 48 | 47 | M | 60 | II | Thymoma | None | 61.57 | PB, TS |
| 49 | 9 | M | 8 | II | Thymoma | None | 0.44 | PB, TS |
| 50 | 28 | M | 264 | II | None | None | 145.44 | PB, Pred, AZA,
IVIg |
| 51 | 47 | M | 60 | III | Thymoma | None | 483.84 | PB, Pred, AZA,
IVIg, TS |
| 52 | 55 | M | 4 | II | None | None | 0.58 | None |
| 53 | 55 | M | 10 | I | None | None | 397.14 | None |
| 54 | 3 | M | 0.6 | I | n.a. | None | 0.50 | None |
| 55 | 65 | M | 0.3 | II | None | None | 0.52 | None |
| 56 | 64 | M | 2 | II | None | None | 359.54 | None |
| 57 | 34 | F | 120 | II | Thymoma | None | 4.33 | PB, Pred, AZA,
TS |
| 58 | 59 | F | 9 | I | None | None | 0.07 | PB |
| 59 | 50 | M | 4 | II | Thymoma | None | 9.58 | PB, TS |
| 60 | 17 | M | 24 | I | None | None | 458.84 | PB, Pred |
| 61 | 24 | F | 8 | II | None | None | 57.50 | PB |
| 62 | 24 | F | 8 | II | None | None | 156.24 | PB |
| 63 | 79 | F | 24 | II | Thymoma | None | 437.64 | PB, Pred, AZA,
IVIg, TS |
| 64 | 45 | M | 60 | I | Thymoma | None | 226.94 | PB, Pred, TS |
| 65 | 24 | F | 8 | II | None | None | 42.98 | PB |
| 66 | 51 | F | 24 | II | None | None | 18.28 | None |
| 67 | 24 | F | 0.1 | II | None | None | 189.64 | None |
| 68 | 10 | F | 36 | II | None | None | 6.33 | PB, Pred, AZA |
| 69 | 2.6 | M | 1 | I | None | None | 13.48 | PB |
| 70 | 31 | M | 156 | I | None | None | 39.98 | n.a. |
| 71 | 11 | M | 96 | I | None | None | 35.98 | PB |
| 72 | 27 | M | 1 | I | None | None | 214.04 | None |
| 73 | 27 | F | 0.6 | II | n.a. | None | 3.27 | PB, Pred, AZA |
| 74 | 51 | F | 10 | II | None | None | 3.66 | PB |
| 75 | 67 | M | 12 | II | None | None | 7.35 | PB, Pred, CYC |
| 76 | 42 | F | 24 | I | None | None | 48.78 | None |
 | Table IIDemographic and clinical
characteristics of the 76 patients with MG. |
Table II
Demographic and clinical
characteristics of the 76 patients with MG.
| Variable | Value (no. of
patients, %) |
|---|
| Sex |
| Male | 46 (60.5) |
| Female | 30 (39.5) |
| MG type according
to age at onset |
| EOMG | 51 (67.1) |
| LOMG | 25 (32.9) |
| MG type |
| OMG | 29 (38.2) |
| gMG | 47 (61.8) |
| MG type at
onset |
| OMG | 42 (55.3) |
| gMG | 34 (44.8) |
| With thymoma | 11 (14.5) |
| With
hyperthyroidism | 2 (2.6) |
| With RA | 2 (2.6) |
| Native AChR Abs
positivity | 61 (80.3) |
| CL Abs
positivity | 38 (50.0) |
| α1CL Abs
positivity | 29 (38.2) |
| β1CL Abs
positivity | 27 (35.5) |
| δCL Abs
positivity | 25 (32.9) |
| More than one CL
Abs positivity | 23 (30.3) |
| All three CL Abs
positivity | 20 (26.3) |
Anti-CL antibody levels in patients with
different types of MG
In total, 61 (80.3%) were native nAChR
antibody-positive. In addition, 29 (38.2%), 27 (35.5%) and 25
samples (32.9%) were positive for antibodies to α1CL, β1CL and δCL,
respectively. The results revealed that 50% (38 of the patients
with MG) had antibodies to at least one of the three CL domains of
nAChR (Table II). We found that
30.3% (23 of the patients with MG) had antibodies to more than one
of the three CL domains of nAChR. Of these, 20 were positive for
all three CL antibodies. The results also revealed that 38 (38/61,
62.3%) were positive for anti-CL antibody among the 61 anti-ECD
antibody-positive patients. These results suggested that
approximately half of the patients with MG were anti-CL
antibody-positive.
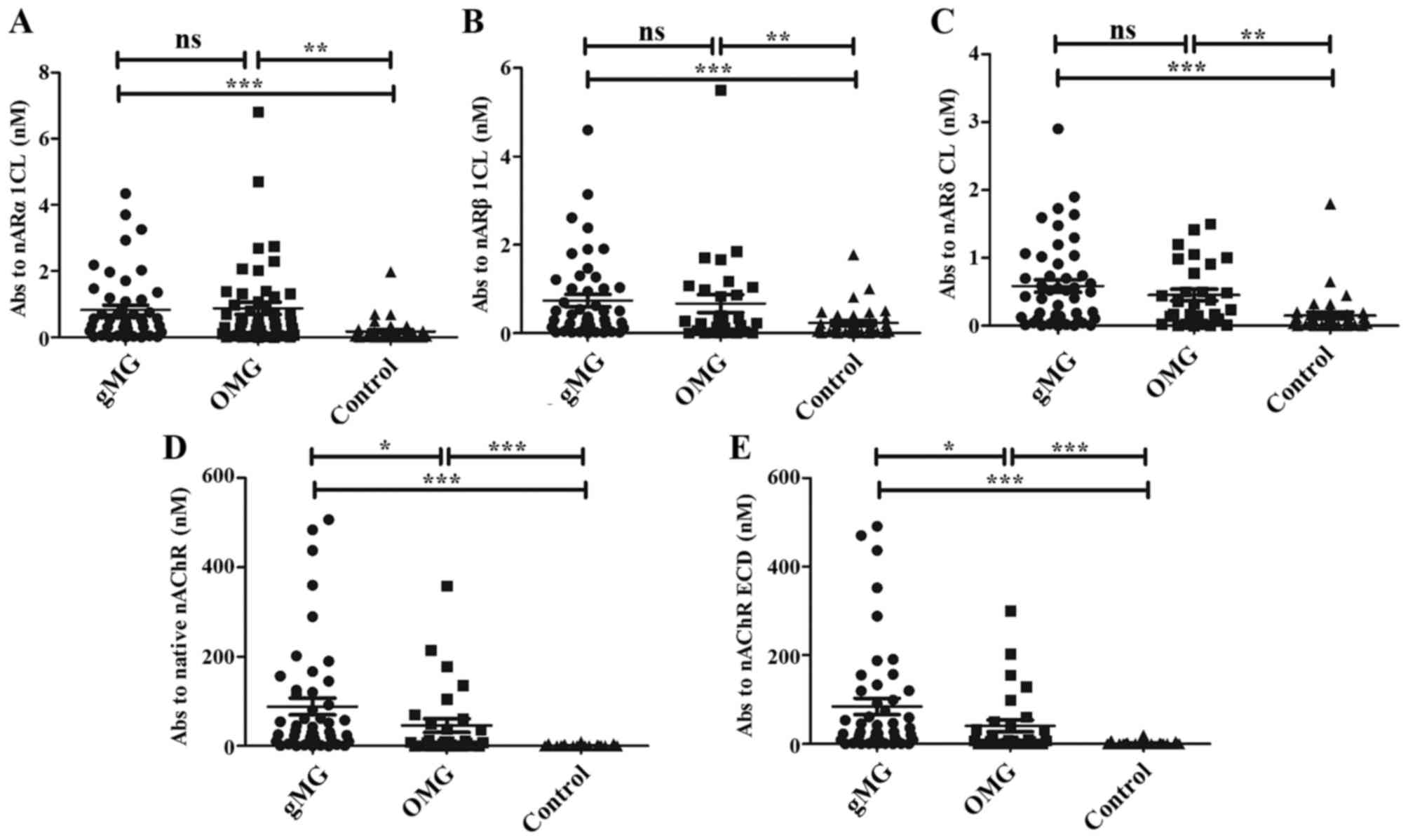
The levels of anti-α1CL antibodies in the patients
with OMG were higher compared with those of the control subjects
(P<0.01). The gMG rates were also significantly higher compared
with the controls (P<0.001); however, there was no difference in
these levels between the patients with OMG and gMG (Fig. 2A). The results of β1 and δ
subunits displayed a similar pattern (Fig. 2B and C). However, the levels of
anti-native nAChR antibodies in gMG were higher than those in OMG
(Fig. 2D). We measured absorption
to the ECD of nAChR as follows: the absorption of
125I-toxin-labeled native nAChR minus the absorption of
125I-labeled CL titers. The levels of anti-ECD
antibodies in gMG were also higher than those in OMG (Fig. 2E). Taken together, our findings
indicated that anti-CL antibodies in the patients with MG were
higher than those in the controls, particularly in the patients
with gMG; however, the levels of anti-CL antibodies did not differ
between the patients with OMG and gMG.
Association between anti-CL antibody and
anti-native nAChR antibody in patients with MG
We tested sera from 76 patients with MG for
antibodies directed against native nAChR and the CLs of nAChR by
immunoprecipitation with 125Iα-BTX-labeled
nAChR and 125I-labeled CLs. Three antibodies against CLs
in the α1, β1 and δ subunits of nAChR were arranged in order of the
increasing concentration of anti-native nAChR antibodies (Fig. 3A). In total, 11 patients (14.5%)
had thymoma (Table II).
Approximately half of these were anti-CL-negative. The other half
of the patients with thymoma, even though they were anti-CL
antibody-positive, the levels of anti-CL antibody were <2 nM
(Fig. 3A).
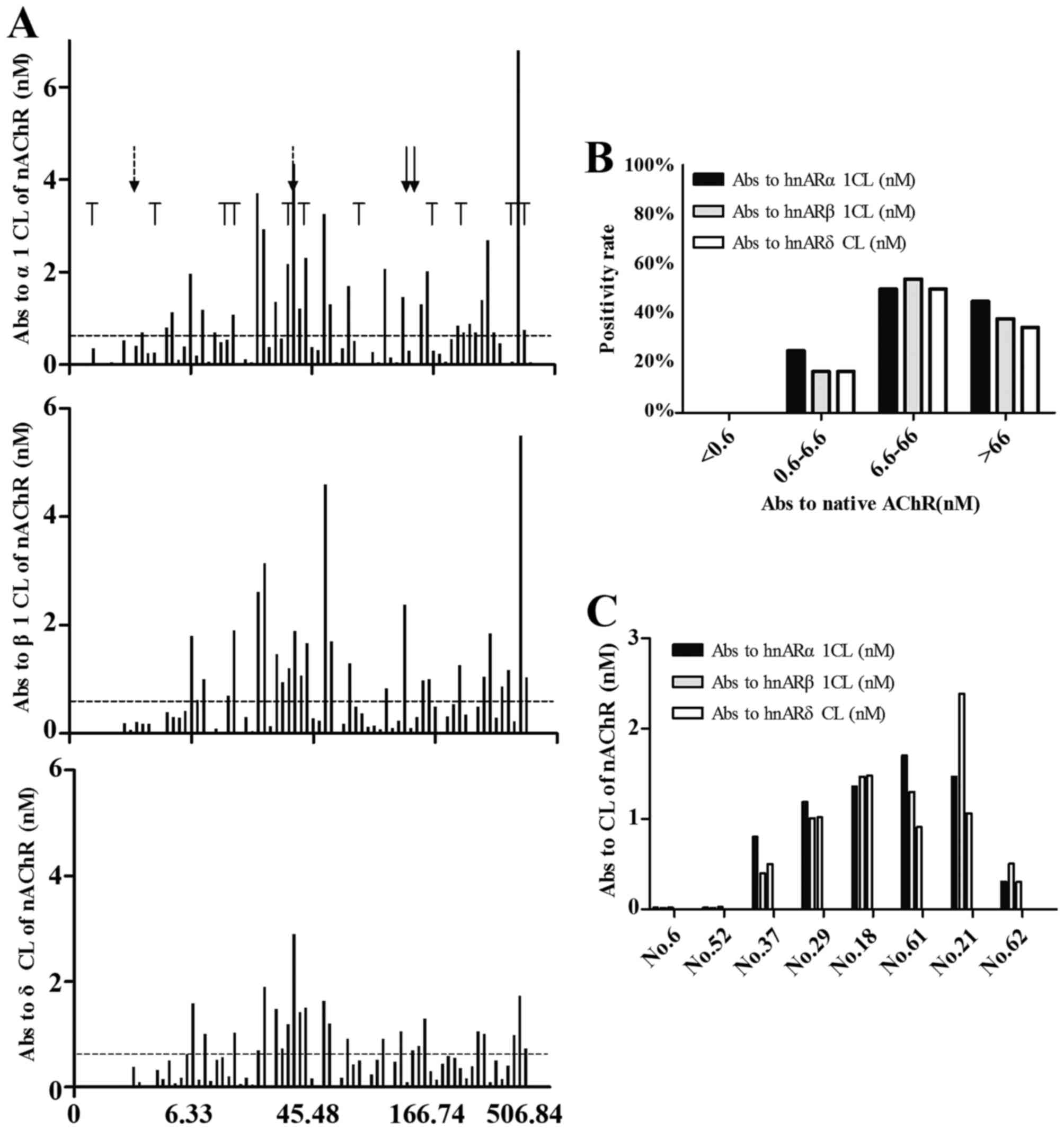 | Figure 3Radioimmunoassay detected antibodies
directed against 3 nAChR subunits (α, β, δ) CL from sera of
patients with MG. 125I-labeled AChRα1CL,
125I-labeled AChRβ1CL and 125I-labeled
AChRδCL were incubated with 10 µl of one of 76 MG patient
sera, the Ab-bound labeled AChRCL was immunoprecipitated using
rabbit anti-human immunoglobulin sera. (A) The sera are arranged in
order of increasing concentration (nM); the 20th, 40th, 60th and
76th sera are indicated. The horizontal dotted lines indicate the
concentration of the antibodies in sera ≥0.6 nM. 'T' marks indicate
sera from 12 patients with MG with thymoma. Arrows with solid and
dashed line indicate patients with MG with hyperthyroidism and RA,
respectively. (B) The positive rate of anti-CL antibodies are
displayed in 4 groups according to the sera titer of anti-native
nAChR. We assume that any type of anti-CL antibody >0.6 nM was
antibody positive. (C) We selected 8 patients randomly from the 76
patients with MG, and compared the coherence of 3 types of anti-CL
antibodies. nAChR, nicotinic acetylcholine receptor; CL,
cytoplasmic loop; MG, myasthenia gravis. |
We classified the patients with MG into 4 groups
according to the concentration of anti-native nAChR antibody as
<0.6, 0.6–6.6, 6.6–66, or >66 nM (Fig. 3B). We compared the positive rates
of anti-CL antibodies among the 4 groups. We found that no patients
were anti-CL antibody-positive in the sera titer <0.6 nM group.
In the group with sera titers between 0.6 and 6.6 nM, anti-CL
positivity was 20%. The anti-CL antibody positive rates in the sera
titer group between 6.6 and 66 nM group was 50%, and 40% in the
>66 nM group. We randomly selected samples from the 4 groups to
further analyze the consistency in the 3 anti-CL antibodies. We
found that the 3 anti-CL antibodies exhibited similar results,
particularly in the low titer of anti-CL antibodies in patient nos.
6 and 52 (Fig. 3C).
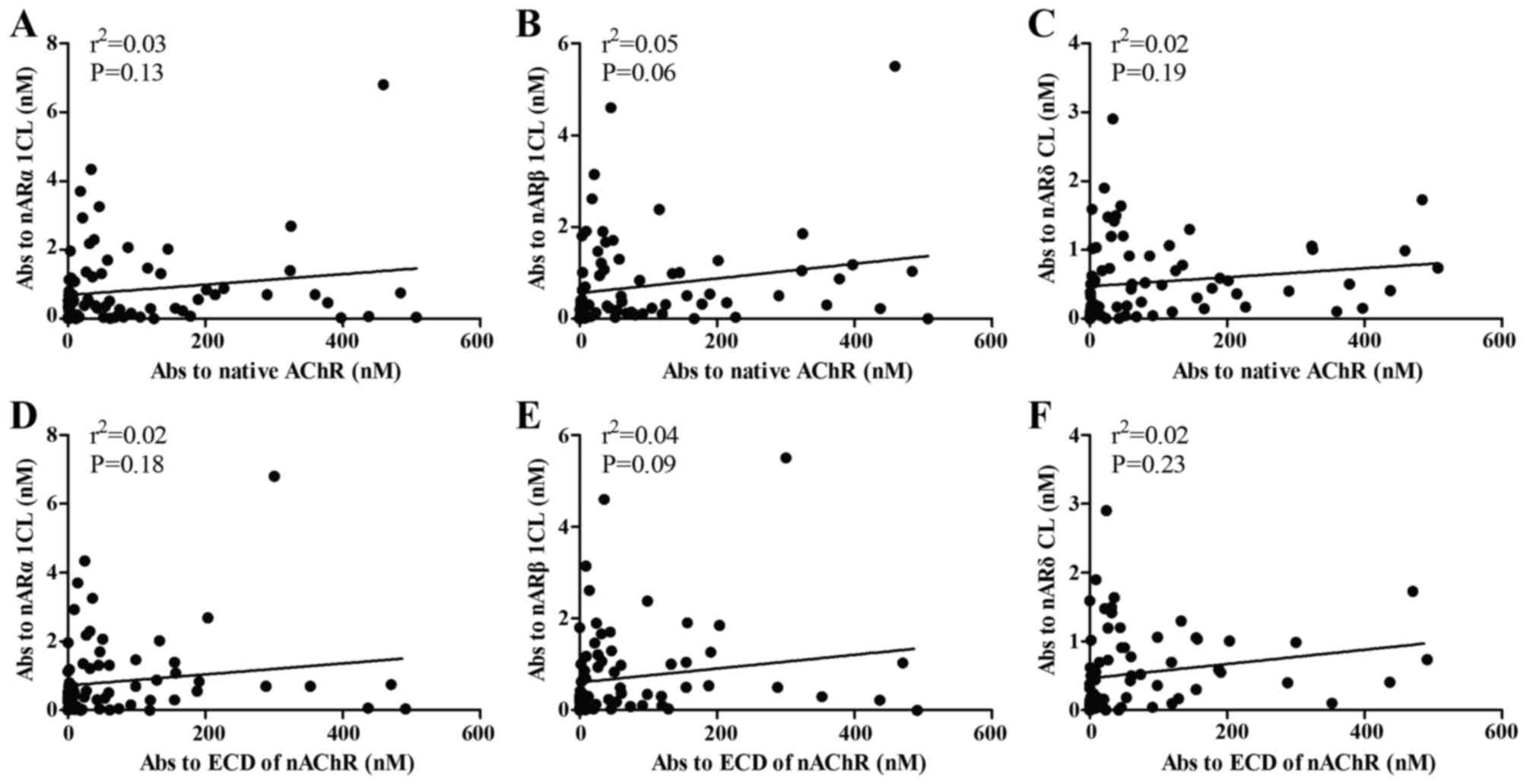
We examined the correlation of antibodies directed
against the CLs of nAChR and native nAChR. The results revealed no
correlation between antibodies to CLs and antibodies to native
nAChR (Fig. 4A–C). Likewise,
antibodies to CLs of nAChR were not associated with antibodies to
ECD of nAChR (Fig. 4D–F).
No relevance of anti-CL antibody with
sex, age and MG type in patients with MG
We assumed the sample was anti-CL antibody-positive
if any of the 3 anti-CL antibody levels (from subunits α1, β1 and
δ) was >0.6 nM (21). Out of
the 29 patients with OMG, 14 (48%) were anti-CL-positive; of the 47
patients with gMG, 24 (51%) were anti-CL-positive. However, there
was no significant difference in anti-CL positivity between the
patients with OMG and gMG. According to the age at onset, MG was
classified into early-onset MG (EOMG) and late-onset MG (LOMG). We
found no statistically significant difference in the anti-CL
antibody positivity rates between the patients with EOMG and LOMG
(Table III). Apart from the
frequency of positive antibody, the levels of anti-CLs, anti-native
nAChR and anti-ECD antibodies were compared between sexes, the type
of MG according to the onset age and the onset MG type. The results
revealed no differences in the level of anti-CL antibodies between
sexes, the type of MG according to the age at onset and the type of
MG at onset. The results were comparable with those of the
anti-native nAChR antibody and anti-ECD antibody (data not
shown).
 | Table IIIClinical differences between patients
who were CL Ab- positive and those that were CL Ab-negative. |
Table III
Clinical differences between patients
who were CL Ab- positive and those that were CL Ab-negative.
| Variable (no. of
patients) | CL Abs (n=38)
| P-value |
|---|
| Positive (%) | Negative (%) |
|---|
| Sex |
| Male (46) | 23 (50) | 23 (50) | 1 |
| Female (30) | 15 (50) | 15 (50) | |
| MG type according
to age at onset |
| EOMG (51) | 27 (53) | 24 (47) | 0.46 |
| LOMG (25) | 11 (44) | 14 (56) | |
| MG type |
| OMG (29) | 14 (48) | 15 (52) | 0.81 |
| gMG (47) | 24 (51) | 23 (49) | |
| MG type at
onset |
| OMG (42) | 19 (45) | 23 (55) | 0.36 |
| gMG (34) | 19 (56) | 15 (44) | |
Discussion
In the present study, we successfully expressed and
purified the α1, β1 and δ subunits of CL in nAChR, which are common
in both fetal and adult nAChR. We detected anti-CL antibodies in 76
patients with MG and found that the levels of any of the 3 anti-CL
antibodies were significantly higher in the patients with MG
compared with the controls.
It is important to address the significance of the
existence of antibodies directed against CL of nAChR. It is
important to determine whether these antibodies directed against CL
of nAChR play a functional role in MG. Several studies have
reported that anti-CL antibody of nAChR plays a protective role in
the development of EAMG (11–13). For example, Luo et al
demonstrated a therapeutic vaccine consisting of
bacterially-expressed human muscle nAChR cytoplasmic domains for
the antigen-specific immunosuppression of MG to be safe, robust and
long-lasting (15). Several
studies have confirmed the existence of anti-CL of nAChR antibodies
in both patients with MG and EAMG (22–24). However, the mechanisms involved
are still unknown. It has been suggested that B cells are
suppressed by the secreting antibody, supporting the inhibitory
receptor FcγRIIB on the surface of B cells (25,26). It is tempting to speculate that
followng the immunization of the CLs of nAChR, the levels of
anti-CL antibodies increase and clinical symptoms are relieved.
This may be due to the combination of the antigen-antibody
complexes and inhibitory receptor FcγRIIB, which strongly inhibits
specific B cells that produce anti-ECD antibodies (27) in patients with MG. Based on these
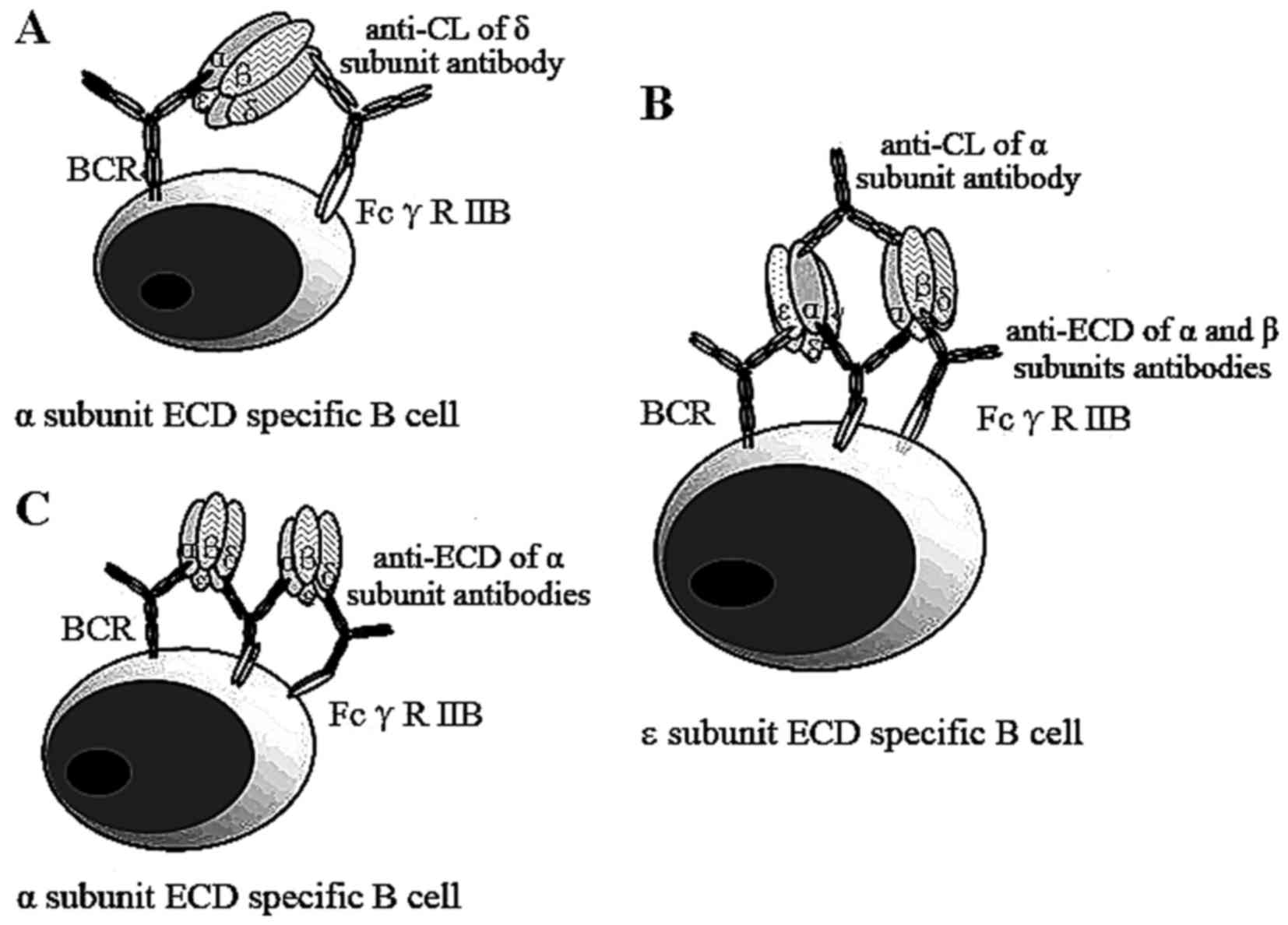
data, we proposed a negative feed-back theoretical model to explain
how the anti-CL loop protects against EAMG in animal models
(Fig. 5). In this proposed
mechanism, B cells specific to the ECD of the α subunit capture the
nAChR, which is bound with an antibody to CL of the δ (Fig. 5A), or α (Fig. 5B) subunits, or the ECD of α
subunit (Fig. 5C). The Fc
portions of these antibodies bind to the FcγRIIB, an inhibitory
receptor on the surface of the same B cell, leading to inactivation
of those B cells.
However, even though the levels of anti-ECD antibody
in patients with gMG were higher than those in patients with OMG,
there were no differences observed in the levels of anti-CL
antibodies between patients with OMG and gMG in our results. There
was also no correlation between anti-CL and anti-ECD antibody.
These findings suggest that anti-CL antibodies may be not
beneficial antibodies in patients with MG.
In fact, EAMG differs from MG in that EAMG is
triggered by immunizing animals with detergent-solubilized native
nAChR, such as Triton X-100. Electron microscopy studies have
indicated that these Triton X-100-solubilized nAChRs are embedded
in tubular and globular structures formed by membrane lipids and
detergent micelles (28,29). The CLs of nAChR were exposed to
the immunological environment rather than protected by the cellular
membranes, which can mediate the immune response at the initiation
of EAMG. Although we can explain the protective effect of anti-CL
antibodies on EMAM using a negative feed-back theory, it does not
confirm that this mechanism exists in patients with MG. This means
that anti-CL antibody may not serve as a negative feed-back bridge
if the CL of nAChR that triggers the autoimmune response in MG
patient is not exposed to the environment.
Since there was no beneficial effect of anti-CL
antibody in patients with MG, it would be benefial to determine how
anti-CL antibody could possibly occur and what the role of anti-CL
antibody is in patients with MG. CLs are not exposed on the cell
surface in healthy humans; thus, no anti-CL antibodies are present.
In patients with MG with anti-nAChR antibody, nAChR is destroyed
through antigen-antibody reaction. The CL is exposed as the nAChR
is destroyed. Therefore, anti-CL antibodies are likely produced as
a result of pathological changes. However, the function of anti-CL
antibody in patients with MG warrants further investigation.
In addition, we found that approximately 50% of MG
sera contained antibodies directed against the CL of nAChR. This is
significantly higher than findings from earlier experiments that
measured the ability of a monoclonal antibody (mAb) to inhibit the
binding of MG sera to the nAChR (30), and also measured the antibody of
MG sera to inhibit the binding of a MoAb detected against the CL of
the nAChR (14). Our results
showed that approximately a quarter of patients with MG with
thymoma were anti-CL antibody-positive. This is higher than the
finding of a previous study that found that no patients with MG
with thymoma were anti-CL antibody-positive (14). This can be explained by the
following reasons: i) the experimental methods were different. Our
radioimmunoassay was direct and more sensitive. Previous methods
used competition experiments that indirectly detected antibodies,
missing minor antibody populations in MG sera. Due to the
specificity of the MoAb used, those experiments only detected the
presence of antibodies against certain epitopes of CL of α and β
subunits. The detection of a narrow portion of anti-CL antibodies
was widened by radioimmunoassay. ii) Patients with MG in different
countries had different conditions. In our study, 14.5% of patients
with MG had thymoma; however, only 5.3% had thymoma in the previous
study (14). iii) Patients with
MG may have been treated with different medicines. Drugs, such as
corticosteroids and immunosuppressive agents affect the immune
system, particularly the expression of antibodies.
No patients in the sera titer <0.6 nM group were
positive for anti-CL antibodies. A higher positivity rate was
concentrated in the sections of sera titer between 6.6 and 66 nM,
and in sera titer >66 nM. These results suggest that the anti-CL
antibodies are prone to be distributed in MG with a higher sera
titer. In 11 patients with thymoma-associated MG, 10 were anti-ECD
positive, but only 4 patients were anti-CL-positive (data not
shown). We thus questioned whether there was an association between
these two antibodies. The number of tested thymoma sera in this
study was too small to draw definitive conclusions.
It should be noted that the present study was
retrospective. The majority of patients coming to our hospital had
been diagnosed and treated in their local primary hospital. Thus,
as a preliminary exploration, we included the ready-made sera from
patients with MG. Currently, we are collecting the sera of patients
with MG before and after treatment for further analysis. Our
preliminary study revealed that the titers of anti-CL antibodies
between patients with MG with and without immunosuppressive therapy
exhibited no statistically significant difference (unpublished
data). Therefore, we assumed that anti-CL antibodies may not be
affected under the immunosuppressive therapy.
The limitation of the present study was that the
sample size was not large enough. We aim to keep collecting the
sera of patients with MG to enlarge the number of samples in future
studies. Despite its preliminary character, our findings still
provide the first clinical evidence that anti-CL antibodies may be
not a beneficial antibody in patients with MG.
In conclusion, we found a phenomenon that there was
no correlation between anti-nAChR CL and the severity of MG.
Anti-CL antibody in patients with MG may not seem to be predictive
of the severity of MG; however, additional studies are warranted to
uncover the clinical implications of anti-CL antibody in patients
with MG.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 31270952 and
81202357). We would like to thank Dr Austin Cape at ASJ Editors for
careful reading the manuscript and providing comments.
Glossary
Abbreviations
Abbreviations:
|
α-BTX
|
α-bungarotoxin
|
|
BCR
|
B-cell antigen receptor
|
|
BME
|
β-mercaptoethanol
|
|
CL
|
cytoplasmic loop
|
|
EAMG
|
experimental autoimmune myasthenia
gravis
|
|
ECD
|
extra cellular domain
|
|
EOMG
|
early-onset myasthenia gravis
|
|
gMG
|
generalized myasthenia gravis
|
|
IPTG
|
isopropyl β-D-1-thiogalacto
pyranoside
|
|
IRB
|
institutional review board
|
|
LOMG
|
late-onset myasthenia gravis
|
|
MG
|
myasthenia gravis
|
|
MoAb
|
monoclonal antibody
|
|
nAChR
|
the nicotinic acetylcholine
receptor
|
|
OMG
|
ocular myasthenia gravis
|
|
SD
|
standard deviation
|
References
|
1
|
Lindstrom JM: Acetylcholine receptors and
myasthenia. Muscle Nerve. 23:453–477. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Palace J and Hilton-Jones D:
Myasthenia gravis. Lancet. 357:2122–2128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huijbers MG, Lipka AF, Plomp JJ, Niks EH,
van der Maarel SM and Verschuuren JJ: Pathogenic immune mechanisms
at the neuromuscular synapse: The role of specific antibody-binding
epitopes in myasthenia gravis. J Intern Med. 275:12–26. 2014.
View Article : Google Scholar
|
|
4
|
Karlin A: Emerging structure of the
nicotinic acetylcholine receptors. Nat Rev Neurosci. 3:102–114.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scarselli M, Spiga O, Ciutti A, Bernini A,
Bracci L, Lelli B, Lozzi L, Calamandrei D, Di Maro D, Klein S and
Niccolai N: NMR structure of alpha-bungarotoxin free and bound to a
mimotope of the nicotinic acetylcholine receptor. Biochemistry.
41:1457–1463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lindstrom JM, Einarson BL, Lennon VA and
Seybold ME: Pathological mechanisms in experimental autoimmune
myasthenia gravis.I.Immunogenicity of syngeneic muscle
acetylcholine receptor and quantitative extraction of receptor and
antibody-receptor complexes from muscles of rats with experimental
automimmune myasthenia gravis. J Exp Med. 144:726–738. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tzartos SJ, Sophianos D and Efthimiadis A:
Role of the main immunogenic region of acetylcholine receptor in
myasthenia gravis. An Fab monoclonal antibody protects against
antigenic modulation by human sera. J Immunol. 134:2343–2349.
1985.PubMed/NCBI
|
|
8
|
Das MK and Lindstrom J: The main
immunogenic region of the nicotinic acetylcholine receptor:
Interaction of monoclonal antibodies with synthetic peptides.
Biochem Biophys Res Commun. 165:865–871. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lindstrom J, Einarson B and Tzartos S:
Production and assay of antibodies to acetylcholine receptors.
Methods Enzymol. 74:432–460. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilhus NE and Verschuuren JJ: Myasthenia
gravis: subgroup classification and therapeutic strategies. Lancet
Neurol. 14:1023–1036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo J and Lindstrom J: Myasthenogenicity
of the main immunogenic region and endogenous muscle nicotinic
acetylcholine receptors. Autoimmunity. 45:245–252. 2012. View Article : Google Scholar :
|
|
12
|
Lindstrom J, Luo J and Kuryatov A:
Myasthenia gravis and the tops and bottoms of AChRs: Antigenic
structure of the MIR and specific immunosuppression of EAMG using
AChR cytoplasmic domains. Ann N Y Acad Sci. 1132:29–41. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo J and Lindstrom J: AChR-specific
immunosuppressive therapy of myasthenia gravis. Biochem Pharmacol.
97:609–619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tzartos SJ and Remoundos M: Detection of
antibodies directed against the cytoplasmic region of the human
acetylcholine receptor in sera from myasthenia gravis patients.
Clin Exp Immunol. 116:146–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo J and Lindstrom J: Antigen-specific
immunotherapeutic vaccine for experimental autoimmune myasthenia
gravis. J Immunol. 193:5044–5055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matney SE and Huff DR: Diagnosis and
treatment of myasthenia gravis. Consult Pharm. 22:239–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaretzki A III, Barohn RJ, Ernstoff RM,
Kaminski HJ, Keesey JC, Penn AS and Sanders DB: Myasthenia gravis:
recommendations for clinical research standards. Task Force of the
Medical Scientific Advisory Board of the Myasthenia Gravis
Foundation of America. Neurology. 55:16–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang HW: Purification and
characterization of acetylcholine receptor-I from Electrophorus
electricus. Proc Natl Acad Sci USA. 71:2113–2117. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greenwood FC, Hunter WM and Glover JS: The
preparation of I-131-labelled human growth hormone of high specific
radioactivity. Biochem J. 89:114–123. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patrick J and Lindstrom J: Autoimmune
response to acetylcholine receptor. Science. 180:871–872. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tindall RS, Kent M and Wells L: A rapid
immunoadsorbent radioimmunoassay for anti-acetylcholine receptor
antibody. J Immunol Methods. 45:1–14. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayashi M, Takaoka T, Manabe K, Yoshinaga
J and Kida K: Comparison of antibody titer to human and rat
acetylcholine receptor in myasthenia gravis. Brain Dev. 17:38–41.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oda K, Goto I, Kuroiwa Y, Onoue K and Ito
Y: Myasthenia gravis: antibodies to acetylcholine receptor with
human and rat antigens. Neurology. 30:543–546. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kordas G, Lagoumintzis G, Sideris S,
Poulas K and Tzartos SJ: Direct proof of the in vivo pathogenic
role of the AChR autoanti-bodies from myasthenia gravis patients.
PLoS One. 9:e1083272014. View Article : Google Scholar
|
|
25
|
Heyman B: Antibody feedback suppression:
Towards a unifying concept? Immunol Lett. 68:41–45. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heyman B: Feedback regulation by IgG
antibodies. Immunol Lett. 88:157–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
PLOS ONE Staff: Correction: Direct proof
of the in vivo pathogenic role of the AChR autoantibodies from
myasthenia gravis patients. PLoS One. 10:e01176732015. View Article : Google Scholar
|
|
28
|
Schürholz T, Kehne J, Gieselmann A and
Neumann E: Functional reconstitution of the nicotinic acetylcholine
receptor by CHAPS dialysis depends on the concentrations of salt,
lipid, and protein. Biochemistry. 31:5067–5077. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang HW and Bock E: Factors influencing
the stability of isolated acetylcholine receptor from Torpedo
californica. Neurochem Int. 2C:269–280. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tzartos SJ, Morel E, Efthimiadis A,
Bustarret AF, D'Anglejan J, Drosos AA and Moutsopoulos HA: Fine
antigenic specificities of antibodies in sera from patients with
D-penicillamine-induced myasthenia gravis. Clin Exp Immunol.
74:80–86. 1988.PubMed/NCBI
|