1. Introduction
Scaffold proteins have evolved for efficiently
interacting with certain signalling pathways and maintaining
cellular structure. This is achieved through the regulation of the
cytoskeleton, cell adhesion, and migration, allowing cells to
survive and grow in variable environments. Dysregulation of certain
scaffold proteins has been implicated in malignancies, including
cancer development and metastasis. In mammals, an example of such
multi-functional transmembrane scaffold proteins, is kinase
D-interacting substrate of 220 kDa/ankyrin repeat-rich membrane
spanning (Kidins220/ARMS). Kidins220 is a highly conversed integral
membrane protein, which was initially identified as a substrate for
protein kinase D (PKD), a serine/threonine kinase responsible for
regulation of several cell processes (1). Despite recent studies demonstrating
the role of Kidins220 in neurotrophin response, it is increasing
apparent that Kidins220 is involved in the regulation of many
cellular functions. Dysregulation of Kidins220 occurs in several
human diseases including neurodegeneration and cancer, and thus
there is increasing effort to pharmacologically target this
protein. Here, we review our current understanding of Kidins220 and
further discuss the possible links of Kidins220 to
malignancies.
2. Kidins220/ARMS
Initially discovered in the nervous systems,
Kidins220 responds to variable environment cues and coordinates
neuronal survival, differentiation and plasticity (2–4).
In the nervous system, Kidins220 is one of the downstream
substrates for tropomyosin-related kinase (Trk) signalling
(5). In mammal neural tissues,
Kidins220 has a high binding affinity for neurotrophins and
localizes to the tips of neurites abundant and enriched in
expression of ephrin receptors and neurotrophins (6). In the nervous system, Kidins220 acts
as a platform for protein-protein interactions, where its multiple
domains recruit downstream receptor substrates resulting in the
activation of signalling pathways which lead to neuronal
differentiation, survival, cytoskeleton remodelling, synaptic
plasticity and dendrite and synapse development (7). Development of the vascular system
involves the interaction of Kidins220 with vascular endothelial
growth factor receptors (VEGFRs). Knockout of Kidins220 resulted in
impairment of the VEGF signalling pathway, while an in vivo
study revealed that mice lacking Kidins220 developed cardiovascular
abnormalities (8).
3. Kidins220 structure, expression and
localization
The Kidins220 gene encodes a protein of 1715 amino
acids. Kidins220 protein is comprised of 11 ankyrin-repeats within
the N-terminal region, a proline-rich stretch, a sterile α motif
(SAM) domain (9), kinase light
chain (KLC)-interacting motif (KIM), and a PSD-95, Dlg,
ZO-1-binding motif (PDZ) at its C-terminal. It contains four
transmembrane segments in the central part of the molecule and N-
and C-terminal tails both exposed to the cytoplasm. Kidins220 is
the target of a molecule called protein kinase D, which first
gained attention due to its broad regulation of neuronal
properties. Biochemical processes led to the purification and
identification of multiple Kidins220 domains which act as
regulators in a variety of cell signalling pathways.
Originating from monocytes, immature dendritic cells
in peripheral blood have been shown to express high levels of
Kidins220. Therefore, currently the cytoskeleton remodelling which
is driven through Kidins220 is best characterised in immature
dendritic cells (10). Upon
migration onto extracellular matrices, highly polarized immature
dendritic cells change stage, from monopolar to symmetrical
bipolar. During this process, Kidins220 is highly expressed at
dendritic cell polarized membrane edges where F-actin localises
(10). Further
immunocytochemistry analysis has revealed that Kidins220 expression
is concentrated around proteins which are associated with the raft
compartment (10). Lipid rafts
are microdomains of the cell plasma membrane and contain
distinctive protein and lipid constituents, which are involved in
the regulation of signalling transduction. Chemically induced
disruption of lipid rafts resulted in the loss of Kidins220 from
the enriched polarized edges, indicating a regulatory role of
Kidins220 in cell morphology changes and motility (10,11). Kidins220 has also been observed at
the neuromuscular junction suggesting that Kidins220 also plays a
role in muscle development (12).
Luo et al proposed a possible model in which Kidins220
bridges a link between α-syntrophin and Eph4, thus contributing to
the regulation of synapse formation and plasticity. Further
evidence has shown that this interaction occurs through
α-syntrophin induction of Kidins220 clustering at the neuromuscular
junction. This Kidins220-mediated localisation subsequently results
in the activation of Eph4 which in turn stimulates postsynaptic
signal cascades (12). By
interacting with a variety of proteins, Kidins220 regulates
neuronal activities. For instance, kinesin light chain 1 (KLC1) is
a binding partner for Kidins220. In PC-12 cells, it was
demonstrated that after nerve growth factor (NGF) stimulation,
intracellular trafficking of Kidins220, from trans-Golgi
network to the plasma membrane, relied on KLC1-based transport
mechanism (13).
4. Interacting partners
Kidins220 binds with a variety of interacting
partners and is involved in various neuronal activities (Fig. 1). Kidins220 acts as a downstream
substrate of protein kinase D (PKD), where enhanced PKD activity
stimulates phosphorylation of Kidins220 at serine 919 in PC12 cells
(1). The most significant role
identified of Kidins220 is to act as a downstream substrate of Trk
receptor tyrosine kinase. Kidins220 is currently the only known
membrane-associated protein which interacts with both Trk and p75
neurotrophin receptors, often forming a ternary complex (14). Kong et al identified that
within hippocampal neurons which were stimulated by neurotrophin,
rapid phosphorylation of Kidins220 was exhibited indicating
Kidins220 as a downstream target of neurotrophin (9). Further research by Arévalo et
al revealed that rapid tyrosine phosphorylation of Kidins220
was induced in primary neurons after treatment with neurotrophin.
They further identified that it was a specific site Tyr1096 which
activated mitogen-activated protein kinase (MAP kinase) activity
through phosphorylated Kidins220 recruits CrkL, an upstream
component of the C3G-Rap1-MAP kinase cascade, in a neutrophin
dependent manner. This sustained MAP kinase activation was affected
in PC-12 cells by a mutation within Tyr1096 affecting cell
differentiation (15). Notably,
it appeared that the mutation or interference of Kidins220 only
reduced neurotrophin-elicited signalling in the ERK pathway,
suggesting an important role played by Kidins220 in mediating
neurotrophin-induced signal transduction via the MAP kinase pathway
(16). Kidins220 was also
phosphorylated in NG108-15 cells following exposure to ephrin B,
suggesting it acts downstream of ephrin receptors (9).
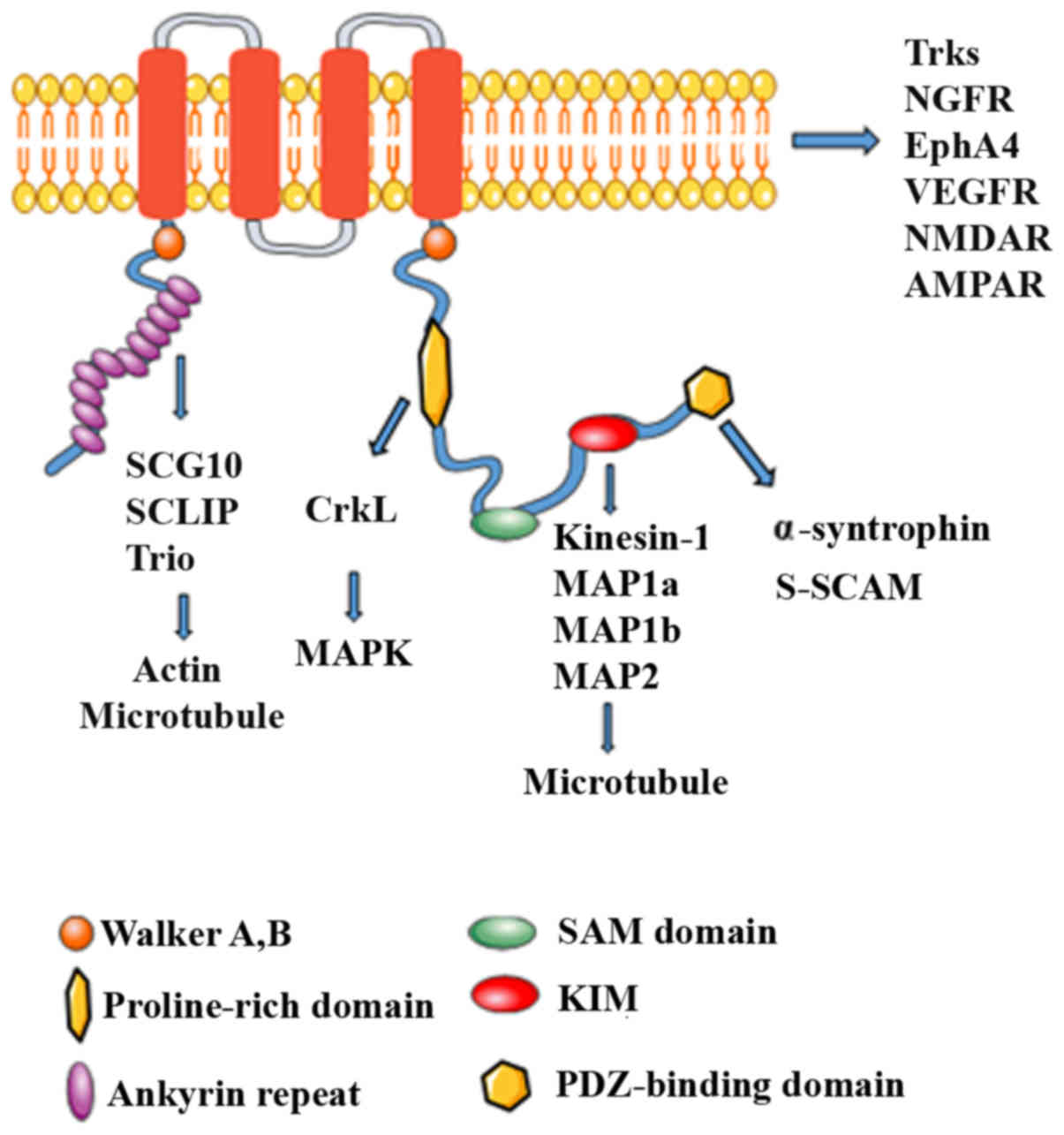 | Figure 1Structure of Kidins220 and its
binding interaction partners. Schematic structure of Kidins220
scaffold protein. An archetypal Kidins220 scaffold protein contains
multiple protein binding domains and post-translational modified
sites, through which Kidins220 coordinates signal transduction of
various pathways, such as Trk and MAPK. The scaffolding role of
Kidins220 appears to be directed based on the different binding
partners within the protein. Kidins2202, kinase D-interacting
substrate of 220 kDa/ankyrin repeat-rich membrane spanning; SAM,
sterile α motif; MAP, microtubule-associated protein; KIM, kinase
light chain (KLC)-interacting motif; PDZ, PSD-95, Dlg, ZO-1-binding
motif; Trks, tropomyosin receptor kinase; NGFR, nerve growth factor
receptor; VEGFR, vascular endothelial growth factor receptor. |
Based on yeast two-hybrid screening, Kidins220
activity in muscle has been linked to the binding of α-syntrophin,
via the PDZ domain. Kidins220 displays clustering in response to
increased expression levels of α-syntrophin which further augments
EphA4 signalling (12). In
nuclear factor-κB (NF-κB) signalling induced by brain-derived
neurotrophic factor (BDNF), a neurotrophin with preference for
targeting tropomyosin receptor kinase B (TrkB), Sniderhan et
al demonstrated that silencing of Kidins220 or targeting the
Kidins220-TrkB interaction abolished NF-κB signalling elicited by
BDNF. Further elucidation of the BDNF-induced interaction between
Kidins220 and TrkB suggested that NF-κB signalling was facilitated
by the activation of MAP kinase and IκB kinase (IKK) leading to the
phosphorylation of RelA (17).
Septin 5, which has been implicated in cytoskeleton
reorganisation, has also been identified as an interacting partner
of Kidins220 and may serve as a regulatory element for
intracellular signalling activities (18). The results of a yeast two-hybrid
screen and co-immunoprecipitation revealed that these two proteins
are co-localized in hippocampal neurons. In PC12 cells, Kidins220
and Septin5 are expressed on the tips of growing neurites induced
by NGF. Andreazzoli et al performed a screening for brain
cDNA products from a phage display library and demonstrated an
interaction between the PDZ-domain of Kidins220 and Pdzrn3, a
protein comprised of a PDZ-domain and RING-finger. The
co-localization of Kidins220 and Pdzrn3 has been observed in PC12
cells in growing neurites induced by NGF (19).
5. Kidins220, neurite outgrowth, survival
and death
Kidins220 binds and activates RhoGEF Trio in
ankyrin-repeats. In NGF-differentiated PC12 cells, Neubrand et
al found that Kidins220 and Trio were colocalized in specific
sites with F-actin and Rac1 (20). Trio is a RhoGEF for Rac1, RhoG and
RhoA and plays an important role in the regulation of neurite
outgrowth. They identified that in PC12 cells, overexpression of
Kidins220 in ankyrin repeats inhibited NGF-dependent neurite
outgrowth regulated by Trio, and a similar mechanism was also
verified in hippocampal neurons (20). Bracale et al conducted a
yeast two-hybrid screen and identified KLC1, the subunit of
kinesin1, as the interacting partner of Kidins220. In NGF-induced
PC12 cells, Kidins220 colocalising with kinesin1 was impacted by
the overexpression of KIM, a KLC-interacting motif in Kidins220,
ultimately interfering with neurite outgrowth (13). Base on the preferential binding of
NGF and TrkA neurotrophin receptor, López-Benito et al
identified a novel signalling pathway, mediated by NGF which
includes TrkA, Kidins220, synembryn-B and Rac1, implicated in
neuronal secretion in PC12 cells (21). Rac1 is the downstream target of
Kidins220 and synembryn-B, which themselves directly interact.
NGF-mediated secretion is blocked by the overexpression of
Kidins220 and synembryn-B; however, basal secretion was unaffected
(20). The secretion defects
caused by high levels of Kidins220 were rescued by the expression
of dominant-negative Rac1 (21).
6. Kidins220 in neuronal polarity, synaptic
plasticity and neurotransmission
The diverse function of neurons depends on the
highly polarised morphology regulated by axon and dendrites.
Kidins220 interacts with tubulin (SCG10 and SCLIP) and
microtubule-associated proteins (MAPs) to regulate neuronal
polarity. As members of MAPs, the phosphorylation of MAP1b and
stathmins were impaired following the downregulation of Kidins220.
Furthermore, downregulation of Kidins220 also led to the aberrant
dendritic arbors and extensions of the longer axon (22). AMPARs produce mature glutamatergic
synapse by targeting the cell surface during neuronal development
(23). As a subunit of AMPAR,
GluA1 can be upregulated when Kidins220 is downregulated. Thus, in
the process of neuronal mutation and synapse formation, the
decreased expression of Kidins220 results in enhanced GLuA1
expression and thus the establishment of a strong synaptic
connection (7). In mature
neuronal cultures, there is also a relevant evidence of interplay
between Kidins220 and NMDA receptors. Upon overstimulation of NMDA
receptors, such as during excitotoxicity, or when neurons are
depolarised, Ca2+ activity-dependent Ca2+
influx, through NMDA receptors leads to a decrease in Kidins220
levels through both transcriptional downregulation and protein
cleavage by calpain (21).
Because Kidins220 knockdown causes a decrease in the amount of
phosphorylated MAPK, a reduction in the expression of Kidins220 may
contribute to neuronal death through a decrease in MAPK signalling
(7). In hippocampal neurons, the
decreased expression of Kidins220 prohibited GABAergic
neurotransmission, whereas the overexpression of Kidins220 reverses
this effect. Furthermore, the GABAergic neurotransmission regulated
by Kidins220 is through a presynaptic mechanism (24). In hippocampal neurons with
overexpression of Kidins220 increased long-term potentiation was
impaired when calpain was prohibited (25). Sutachan et al found that
Kidins220 is implicated in regulating the inhibition of
neurotransmission (24).
7. Kidins220 and vascular development
Cesca et al addressed the potential role of
Kidins220 in vascular development due to its targeting of and
interaction with VEGFRs. Mice with a Kidins220 knockdown phenotype
were found to present with cardiovascular abnormalities. These
abnormalities appeared to be caused by impaired VEGF signalling
pathways induced by lack of Kidins220 (26). Interestingly, Kidins220 interacts
with VEGFR2 and VEGFR3 but not Nrp1, although both VEGFR2 and
VEGFR3 are co-receptors of Nrp1 in endothelial cells. VEGF
signalling is one of the key pathways in the process of
angiogenesis especially mediated by VEGF/VEGFR2, Kidins220 was
demonstrated to interact with VEGFR2 constitutively (27). However, mice with more severe
vascular abnormalities were detected in the model with the
knockdown of VEGF, VEGRFs, Nrp1 than Kidins220−/− itself
(26), suggesting that Kidins220
regulation of angiogenesis may be limited.
8. Kidins220 and immunomodulation
Apart from the role of Kidins220 in modulating
neuronal activities, it also contributes to immunomodulation along
with B cells and T cells. Kidins220 is expressed at the uropod of T
lymphocytes and has been shown to be co-immunoprecipitated with
ICAM-3 and caveolin-1 (28).
Notably, in primary T lymphocytes, the colocalisation of Kidins220
and ICAM-3 is increased with the induction of morphological
polarization. In contrast, Kidins220 displays different
distribution upon change in cell polarity, and colocalisation with
ICAM-3 becomes disrupted. The identification of Kidins220 in the
regulation of T-cell motility was indicated in a
Kidins220-knockdown model of human polarized T-cell lines in which
the basal and stromal cell-derived factor-1α-induced migration was
increased following knockdown of Kidins220 (28). Based on mass spectrometry, Deswal
et al demonstrated the interaction between Kidins220 and
B-Raf in T lymphocytes. In immunoprecipitation and proximity
ligation assays, the sustained ERK signalling relied on Kidins220
induced by T cell receptor (TCR) (29). Furthermore, Fiala et al
reported that Kidins220 interacted with stimulated B cell antigen
receptor (BCR), in an enhanced Src kinase-independent manner via
BCR stimulation. In a B cell-specific Kidins220 knockdown (B-KO)
mouse model, Kidins220 coupled the BCR to PLCγ2, Ca2+,
and ERK signalling and reduced the activation of B cells regulated
by BCR in vitro and in vivo. The role of Kidins220
involved in the PLCγ2 pathway was supported by a 6-fold reduction
in B cells with positive λ light chain (30).
9. Kidins220 in tumours
Several observations support the importance of
Kidins220 in cancer pathogenesis in addition to its function in
neuronal activity. A growing body of evidence points to the
deregulation of Kidins220 at the cell level affecting cell
proliferation, invasion, migration, and apoptosis, playing an
important role in tumour formation and metastasis (Table I). Overexpression of the Kidins220
gene was initially reported in melanoma and was found to be
associated with shorter overall survival. As a cutaneous
malignancy, melanoma ontogenetically originates from the neural
crest and increased expression of Kidins220 was detected in
melanoma cell lines (31,32). Immunohistochemical staining of
different surgical specimens subsequently revealed significantly
increased expression of Kidins220 in primary and metastatic
melanoma tissues with depths >1.0 mm compared with benign tumour
tissues.
 | Table IAlteration of Kidins220 in
tumourigenesis and the related signalling pathway. |
Table I
Alteration of Kidins220 in
tumourigenesis and the related signalling pathway.
| Tumour type | Alteration | Effect | Signalling
pathways | (Refs.) |
|---|
| Cutaneous
melanoma | Increased
expression | Melanoma formation,
migration and invasion | MEK/ERK signalling
pathway | (31,32) |
| Neuroblastoma | Increased
expression | Proliferation | p21/cyclin D1 | (35) |
| | NGF-regulated
signalling | | (33) |
| | Transition from
N-type to S-type | | (34) |
|
Castration-resistant prostate cancer
(CRPC) | Increased
expression | Angiogenesis | VEGF/VEGFR and
PI13K/AKT signalling pathways | (36) |
| Ph-like acute
lymphoblastic leukemia (ALL) | Gene fusion with
PAX5 | Proliferation and
survival | ERK signalling
pathway | (44) |
| Pediatric
high-grade glioma | Intragenic copy
number breakpoint | n.d. | n.d. | (45) |
Kidins220 lies upstream of the BRAF gene that
encodes proteins as part of the RAS/MAPK signalling pathway
controlling several important cell functions. The overexpression of
Kidins220 resulting in sustained activation of MEK/ERK signalling
rather than BRAF mutation, has been identified as leading to acral
lentiginous melanoma tumourigenesis. On the other hand, high levels
of Kidins220 also enhance ultraviolet radiation B (UVB) (290–320
nm)-induced apoptosis in melanoma cells via targeting the activated
ERK signalling pathway (31). The
inhibition of melanoma cell migration and invasion associated with
Kidins220 knockdown indicates that Kidins220 can promote tumour
migration/invasion through MEK/ERK signalling (31,32). Taken together, Kidins220
expression is regarded as a predictor with which to evaluate
melanoma patient outcomes, and its physiologic characteristics also
provide evidence for targeted therapy by inhibiting the
Kidins220/MEK/ERK/MAPK signalling pathways.
The regulatory role of Kidins220 in cancer
development is tumour specific. For example, Kidins220 exerts
different regulatory mechanisms to the MARK signalling pathway in
neuroblastoma tumours. Rogers and Schor first reported that
neuroblastoma tumours overexpress Kidins220, and that its forced
overexpression promotes NGF-stimulated MAPK signalling activity,
but not BDNF driven activation of MAPK signalling. Unlike melanoma,
Kidins220 knockdown did not affect the survival of neuroblastoma
cells under oxidative stress within 24 h. Furthermore, loss of
Kidins220 did not affect migration of neuroblastoma cells (33). During development, neuroblastoma
undergoes a morphologic transition between Schwannian stromal
(S-type) cells and neuroblastic (N-type) cells. S-type cells are
highly adhesive to extracellular matrix (ECM) and are non-invasive,
whereas N-type are less adhesive and highly invasive. DCX and STMN2
markers for neuronal lineage appear to be reduced in
Kidins220-deficient N-type cells, whereas S-type cells containing
low levels of Kidins220 (but not Kidins220 depletion) expressed
considerable levels of both DCX and STMN2. This suggests an
essential role of Kidins220 in regulating morphologic alteration in
neural crest tumour cells (34).
Jung et al generated Kidins220-knockdown neuroblastoma cell
lines to determine whether Kidins220 is involved in cell
proliferation. They found that wild-type cells were 1.8-fold higher
in number than the matching knockdown cells on the fourth day of
culture. Further analysis revealed that the decreased growth rate
of Kidins220-knockdown neuroblastoma cells was due to cell cycle
arrest at the G1 phase, which was accompanied with decreased
expression of both cyclin D1 and CDK4, and also an upregulation of
p21. This suggests that Kidins220 can coordinate the cell cycle
through regulation of p21-cyclinD1/CDK4 (35).
A recent study reported that Kidins220 is a direct
target of miR-4638-5P. miR-4638-5P has been related to the growth
of castration-resistance prostate cancer (CRPC) in vivo and
in vitro. Wang et al discovered a significant
downregulation of miR-4638-5P in CRPC. High expression of Kidins220
was detected in both CRPC cell lines and tissues compared with
androgen-dependent prostate cancer (ADPC). Western blot analysis
showed that only Kidins220 expression was significantly reduced in
PC3 and DU145 cells in the presence of miR-4638-5P. Knockdown of
Kidins220 led to reduced proliferation and growth of CRPC cells
in vitro and in vivo. Furthermore, miR-4638-5P and
Kidins220 regulated prostate cancer (PCa)-associated angiogenesis.
Kidins220 knockdown or overexpression of miR-4638-5P resulted in
similar inhibition of endothelial cell growth and the formation of
vasculogenic mimicry. Their further molecular analysis indicated
that pCDH-miR-4638-5p sponge, a competitive miRNA inhibitor, caused
an increase in expression of VEGF, PI3K and AKT in
androgen-independent PCa cells, whereas all three molecules were
reduced in Kidins220-knockdown PCa cells, suggesting that both
miR-4638-5P and Kidins220 may be involved in androgen-independent
PCa-associated angiogenesis. Interestingly, a reduced level of cell
growth and angiogenesis were also observed in AKT-knockdown cells
which is in accordance with the result from Kidins220-knockdown
cells. Taken together, loss of miR-4638-5 may result in the
activation of Kidins220 and promote neoangiogenesis and
androgen-independent PCa growth through the regulation of
VEGF/VEGFR2 and PI3K/AKT signalling pathways (36).
As previously mentioned, Kidins220 is the substrate
of PKD. At the trans-Golgi network, the family members of
PKD were found to regulate secretory transport (37). PKD1 plays an important role in
stimulating the secretion of neurotensin, a gut peptide, which
modulates gastrointestinal functions such as secretion and growth,
as well as being involved in the proliferation of neurotensin
receptor-positive cancers (38–40). In human carcinoid BON cells, a
novel endocrine cell line that is derived from a human pancreatic
carcinoid tumour, Kidins220 and PKD2 regulated the secretion of
neurotensin (41,42). Further interest is the observation
that in BON cells, Kidins220, PKD1 and PKD2 present the same
localization pattern as neurotensin vesicles, and also co-exist
with neurotensin vesicles. Interestingly the overexpression of PKD1
nullified Kidins220 expression and neurotensin secretion in BON
cells (42). Thus, the
PKD/Kidins220 signalling pathway provides evidence for their
critical role in the regulation of neurotensin hormone secretion.
Since neurotensin is involved in secretion and inflammation in both
normal and tumour cell growth, the PKD/Kidins220 pathway and
neurotensin-containing vesicles are considered as a novel drug
target for clinical application (42,43).
Apart from its role in solid tumours, a recent study
also demonstrated a gene fusion of PAX5 and Kidins220, which leads
to leukemogenesis by inhibiting B lymphocyte differentiation.
Kidins220-mediated activation of the ERK pathway may contribute to
the increased cell proliferation and the survival of leukemic cells
(44).
Controversially, Kidins220 plays a different role in
glioma, which was demonstrated in a profiling study of its function
as a tumour suppressor in pediatric high-grade glioma (45).
10. Conclusion and perspective
Kidins220 is involved in the regulation of diverse
cellular activities, particularly in neural differentiation and
cytoskeleton remodelling (13).
Its different domains mediate the function of Kidins220 as well as
act as a platform for protein-protein interactions, intracellular
signalling and protein transportation. Dysregulation of Kidins220
is evident in several malignancies. Kidins220 binds to Trio,
tubulin, SCG10 and SCLIP to modulate actin and microtubule
cytoskeleton, which is critical in the coordination of cellular
processes, such as cell migration, cell polarity and cell cycle
progression (46). Tumour cell
migration and invasion require the remodelling of the cell
cytoskeleton. Reorganization of the actin cytoskeleton that enables
dynamic cell elongation and directional motility is also found in
epithelial-mesenchymal transition (EMT), a critical process
involved in tumour development (47). Further investigation will shed
light on the role played by Kidins220 in dynamic arrangement of the
cytoskeleton and EMT, and its implication in tumourigenesis and
cancer progression. Kidins220 regulates cell survival and death
through MAPK signalling after the binding of neurotrophins and Trk
receptors (7). Since
dysregulation of MAPK signalling is linked to tumourigenesis in
several types of cancer and inhibition of the MAPK signalling
pathways is critical for the effectiveness of anticancer agents, it
is necessary to gain insight into the corresponding involvement of
Kidins220 (48,49). Targeting its specific multiple
domains such as the proline-rich domain and transmembrane segments
may provide the possibility of more precise targeting. In addition
to its involvement in VEGF/VEGFR-regulated angiogenesis, Kidins220
has been shown as a target gene of miR-4638-5p and its
overexpression has been observed in androgen-independent PCa and
also tumour-associated angiogenesis (36). However, its potential for targeted
therapy in these malignancies and tumour-associated angiogenesis
warrants further investigation.
Acknowledgments
The authors would like to thank Cancer Research
Wales and Ser Cymru Welsh Life Science Research Network for their
support. We also thank Dr Sioned Owen for proofreading the
manuscript. Ms. Shuo Cai is a recipient of the Chinese Medical
Research Scholarship of Cardiff University.
References
|
1
|
Iglesias T, Cabrera-Poch N, Mitchell MP,
Naven TJ, Rozengurt E and Schiavo G: Identification and cloning of
Kidins220, a novel neuronal substrate of protein kinase D. J Biol
Chem. 275:40048–40056. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lipsky RH and Marini AM: Brain-derived
neurotrophic factor in neuronal survival and behavior-related
plasticity. Ann NY Acad Sci. 1122:130–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gómez-Palacio-Schjetnan A and Escobar ML:
Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci.
15:117–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benoit BO, Savarese T, Joly M, Engstrom
CM, Pang L, Reilly J, Recht LD, Ross AH and Quesenberry PJ:
Neurotrophin channeling of neural progenitor cell differentiation.
J Neurobiol. 46:265–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scholz-Starke J and Cesca F: Stepping out
of the shade: Control of neuronal activity by the scaffold protein
Kidins220/ARMS. Front Cell Neurosci. 10:682016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aravind L, Iyer LM, Leipe DD and Koonin
EV: A novel family of P-loop NTPases with an unusual phyletic
distribution and transmembrane segments inserted within the NTPase
domain. Genome Biol. 5:R302004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neubrand VE, Cesca F, Benfenati F and
Schiavo G: Kidins220/ARMS as a functional mediator of multiple
receptor signalling pathways. J Cell Sci. 125:1845–1854. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cesca F, Yabe A, Spencer-Dene B, Arrigoni
A, Al-Qatari M, Henderson D, Phillips H, Koltzenburg M, Benfenati F
and Schiavo G: Kidins220/ARMS is an essential modulator of
cardiovascular and nervous system development. Cell Death Dis.
2:e2262011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong H, Boulter J, Weber JL, Lai C and
Chao MV: An evolutionarily conserved transmembrane protein that is
a novel downstream target of neurotrophin and ephrin receptors. J
Neurosci. 21:176–185. 2001.PubMed/NCBI
|
|
10
|
Riol-Blanco L, Iglesias T, Sánchez-Sánchez
N, de la Rosa G, Sánchez-Ruiloba L, Cabrera-Poch N, Torres A, Longo
I, García-Bordas J, Longo N, et al: The neuronal protein Kidins220
localizes in a raft compartment at the leading edge of motile
immature dendritic cells. Eur J Immunol. 34:108–118. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cabrera-Poch N, Sánchez-Ruiloba L,
Rodríguez-Martínez M and Iglesias T: Lipid raft disruption triggers
protein kinase C and Src-dependent protein kinase D activation and
Kidins220 phosphorylation in neuronal cells. J Biol Chem.
279:28592–28602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo S, Chen Y, Lai KO, Arévalo JC,
Froehner SC, Adams ME, Chao MV and Ip NY: {alpha}-Syntrophin
regulates ARMS localization at the neuromuscular junction and
enhances EphA4 signaling in an ARMS-dependent manner. J Cell Biol.
169:813–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bracale A, Cesca F, Neubrand VE, Newsome
TP, Way M and Schiavo G: Kidins220/ARMS is transported by a
kinesin-1-based mechanism likely to be involved in neuronal
differentiation. Mol Biol Cell. 18:142–152. 2007. View Article : Google Scholar :
|
|
14
|
Chang MS, Arevalo JC and Chao MV: Ternary
complex with Trk, p75, and an ankyrin-rich membrane spanning
protein. J Neurosci Res. 78:186–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arévalo JC, Pereira DB, Yano H, Teng KK
and Chao MV: Identification of a switch in neurotrophin signaling
by selective tyrosine phosphorylation. J Biol Chem. 281:1001–1007.
2006. View Article : Google Scholar
|
|
16
|
Arévalo JC, Yano H, Teng KK and Chao MV: A
unique pathway for sustained neurotrophin signaling through an
ankyrin-rich membrane-spanning protein. EMBO J. 23:2358–2368. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sniderhan LF, Stout A, Lu Y, Chao MV and
Maggirwar SB: Ankyrin-rich membrane spanning protein plays a
critical role in nuclear factor-kappa B signaling. Mol Cell
Neurosci. 38:404–416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HJ, Park HW, Lee SJ, Arevalo JC, Park
YS, Lee SP, Paik KS, Chao MV and Chang MS: Ankyrin repeat-rich
membrane spanning/Kidins220 protein interacts with mammalian Septin
5. Mol Cells. 30:143–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andreazzoli M, Gestri G, Landi E, D'Orsi
B, Barilari M, Iervolino A, Vitiello M, Wilson SW and Dente L:
Kidins220/ARMS interacts with Pdzrn3, a protein containing multiple
binding domains. Biochimie. 94:2054–2057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neubrand VE, Thomas C, Schmidt S, Debant A
and Schiavo G: Kidins220/ARMS regulates Rac1-dependent neurite
outgrowth by direct interaction with the RhoGEF Trio. J Cell Sci.
123:2111–2123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
López-Benito S, Lillo C,
Hernández-Hernández Á, Chao MV and Arévalo JC: ARMS/Kidins220 and
synembryn-B levels regulate NGF-mediated secretion. J Cell Sci.
129:1866–1877. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higuero AM, Sánchez-Ruiloba L, Doglio LE,
Portillo F, Abad-Rodríguez J, Dotti CG and Iglesias T:
Kidins220/ARMS modulates the activity of microtubule-regulating
proteins and controls neuronal polarity and development. J Biol
Chem. 285:1343–1357. 2010. View Article : Google Scholar :
|
|
23
|
Hall BJ and Ghosh A: Regulation of AMPA
receptor recruitment at developing synapses. Trends Neurosci.
31:82–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sutachan JJ, Chao MV and Ninan I:
Regulation of inhibitory neurotransmission by the scaffolding
protein ankyrin repeat-rich membrane spanning/kinase D-interacting
substrate of 220 kDa. J Neurosci Res. 88:3447–3456. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu SH, Arévalo JC, Neubrand VE, Zhang H,
Arancio O and Chao MV: The ankyrin repeat-rich membrane spanning
(ARMS)/Kidins220 scaffold protein is regulated by
activity-dependent calpain proteolysis and modulates synaptic
plasticity. J Biol Chem. 285:40472–40478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cesca F, Yabe A, Spencer-Dene B,
Scholz-Starke J, Medrihan L, Maden CH, Gerhardt H, Orriss IR,
Baldelli P, Al-Qatari M, et al: Kidins220/ARMS mediates the
integration of the neurotrophin and VEGF pathways in the vascular
and nervous systems. Cell Death Differ. 19:194–208. 2012.
View Article : Google Scholar :
|
|
27
|
Guo S, Colbert LS, Fuller M, Zhang Y and
Gonzalez-Perez RR: Vascular endothelial growth factor receptor-2 in
breast cancer. Biochim Biophys Acta. 1806:108–121. 2010.PubMed/NCBI
|
|
28
|
Jean-Mairet RM, López-Menéndez C,
Sánchez-Ruiloba L, Sacristán S, Rodríguez-Martínez M, Riol-Blanco
L, Sánchez-Mateos P, Sánchez-Madrid F, Rodríguez-Fernández JL,
Campanero MR, et al: The neuronal protein Kidins220/ARMS associates
with ICAM-3 and other uropod components and regulates T-cell
motility. Eur J Immunol. 41:1035–1046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deswal S, Meyer A, Fiala GJ, Eisenhardt
AE, Schmitt LC, Salek M, Brummer T, Acuto O and Schamel WW:
Kidins220/ARMS associates with B-Raf and the TCR, promoting
sustained Erk signaling in T cells. J Immunol. 190:1927–1935. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fiala GJ, Janowska I, Prutek F, Hobeika E,
Satapathy A, Sprenger A, Plum T, Seidl M, Dengjel J, Reth M, et al:
Kidins220/ARMS binds to the B cell antigen receptor and regulates B
cell development and activation. J Exp Med. 212:1693–1708. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liao YH, Hsu SM and Huang PH: ARMS
depletion facilitates UV irradiation induced apoptotic cell death
in melanoma. Cancer Res. 67:11547–11556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liao YH, Hsu SM, Yang HL, Tsai MS and
Huang PH: Upregulated ankyrin repeat-rich membrane spanning protein
contributes to tumour progression in cutaneous melanoma. Br J
Cancer. 104:982–988. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rogers DA and Schor NF: Kidins220/ARMS is
expressed in neuroblastoma tumors and stabilizes neurotrophic
signaling in a human neuroblastoma cell line. Pediatr Res.
74:517–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rogers DA and Schor NF: Kidins220/ARMS
depletion is associated with the neural-to Schwann-like transition
in a human neuroblastoma cell line model. Exp Cell Res.
319:660–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung H, Shin JH, Park YS and Chang MS:
Ankyrin repeat-rich membrane spanning (ARMS)/Kidins220 scaffold
protein regulates neuroblastoma cell proliferation through p21. Mol
Cells. 37:881–887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Shao N, Mao X, Zhu M, Fan W, Shen
Z, Xiao R, Wang C, Bao W, Xu X, et al: MiR-4638-5p inhibits
castration resistance of prostate cancer through repressing
Kidins220 expression and PI3K/AKT pathway activity. Oncotarget.
7:47444–47464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yeaman C, Ayala MI, Wright JR, Bard F,
Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, et
al: Protein kinase D regulates basolateral membrane protein exit
from trans-Golgi network. Nat Cell Biol. 6:106–112. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, O'Connor KL, Hellmich MR, Greeley GH
Jr, Townsend CM Jr and Evers BM: The role of protein kinase D in
neurotensin secretion mediated by protein kinase C-alpha/-delta and
Rho/Rho kinase. J Biol Chem. 279:28466–28474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carraway RE and Plona AM: Involvement of
neurotensin in cancer growth: Evidence, mechanisms and development
of diagnostic tools. Peptides. 27:2445–2460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Evers BM: Neurotensin and growth of normal
and neoplastic tissues. Peptides. 27:2424–2433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Evers BM, Townsend CM Jr, Upp JR, Allen E,
Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC and Thompson JC:
Establishment and characterization of a human carcinoid in nude
mice and effect of various agents on tumor growth.
Gastroenterology. 101:303–311. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Chen LA, Townsend CM Jr and Evers
BM: KD1, KD2, and their substrate Kidins220 regulate neurotensin
secretion in the BON human endocrine cell line. J Biol Chem.
283:2614–2621. 2008. View Article : Google Scholar
|
|
43
|
Castagliuolo I, Wang CC, Valenick L, Pasha
A, Nikulasson S, Carraway RE and Pothoulakis C: Neurotensin is a
proinflammatory neuropeptide in colonic inflammation. J Clin
Invest. 103:843–849. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sakamoto K, Imamura T, Kanayama T, Yano M,
Asai D, Deguchi T, Hashii Y, Tanizawa A, Ohshima Y, Kiyokawa N, et
al: Ph-like acute lymphoblastic leukemia with a novel AX5-KIDINS220
fusion transcript. Genes Chromosomes Cancer. 56:278–284. 2017.
View Article : Google Scholar
|
|
45
|
Carvalho D, Mackay A, Bjerke L, Grundy RG,
Lopes C, Reis RM and Jones C: The prognostic role of intragenic
copy number breakpoints and identification of novel fusion genes in
paediatric high grade glioma. Acta Neuropathol Commun. 2:232014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Br J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mirzoeva OK, Das D, Heiser LM,
Bhattacharya S, Siwak D, Gendelman R, Bayani N, Wang NJ, Neve RM,
Guan Y, et al: Basal subtype and MAPK/ERK kinase
(MEK)-phosphoinositide 3-kinase feedback signaling determine
susceptibility of breast cancer cells to MEK inhibition. Cancer
Res. 69:565–572. 2009. View Article : Google Scholar : PubMed/NCBI
|















