Introduction
Retinoblastoma is the most common intraocular
malignancy (1,2). Although early tumor detection and
novel coordinate therapies contribute to significantly improved
survival (80–97%) and eye salvage now, the chemoresistant and
toxicity of chemotherapy and the secondary tumor risk of radiation
all necessitate innovative treatment strategies to combat the
initiation and progression of retinoblastoma.
The revolutionary treatment of acute promyelocytic
leukemia patients through retinoic acid-induced differentiation and
their subsequent improved survival have rendered differentiation
therapy an attractive option in cancer treatment (3,4).
Retinoblastoma was originally thought to be caused by the escape of
cell apoptosis (5). However, a
previous transgenic mice study indicates terminal differentiation,
rather than apoptosis, to be the most important barrier in
retinoblastoma tumorigenesis (6).
Further, clinical evidence revealed that the spontaneous regression
rate of retinoblastoma (1.8–3.2%) is 1,000-fold higher than in
other tumors (7–10), the mechanism of this phenomenon
has not been convinced, but may have some relationship with cell
differentiation (10,11). All render retinoblastoma as a
unique tumor suitable for differentiation therapy.
Retinoblastoma cells have similar characteristics
with retinal progenitor cells (RPCs). Some studies use chemical
agents, such as retinoids, pyruvate and arsenic (12,13), to induce retinoblastoma
differentiate into neuron-like and glia-like cells; however, the
side effects, including headaches, drug resistance, and toxicity
(14–16), have been reported with these
agents. All necessitate optimized agents for retinoblastoma
differentiation.
Cell cycle re-entry and terminal differentiation
disorders transform normal retinal stem cells into malignant
retinoblastoma cells (17). Thus,
specific cell signals related to both cell growth and
differentiation would be critical to regulate retinoblastoma to
differentiate into retinal neurons. Several intrinsic cell signals,
including Wnt, Nodal, bone morphogenetic protein (BMP), Notch and
fibroblast growth factor (FGF) signals, are promising candidates.
Activation of Wnt or Nodal signals can maintain retinal progenitor
cells (RPCs) in an uncommitted state. Inhibition of these signals
induces embryonic stem cells (ESCs) (18–20) or induced pluripotent stem cells
(iPSCs) (21) into
photoreceptors. Basic fibroblast growth factor (bFGF) enhance
photoreceptors and neurons from RPCs (22) and neural stem cells (23). Besides the unique signal, the
signaling cross-talk effect should also be considered. Convincing
evidence has emerged that zebrafish brain resulted from the triple
inhibition of Wnt, Nodal and BMP signaling. The early development
of retinal requires for inhibition of BMP and activation of FGF
signaling. Furthermore, inhibition of Wnt, Nodal, BMP and Notch
signals together with atonal bHLH transcription factor 7
(ATOH7) gene overexpression could generate RGLCs from iPSCs
(21). For retinoblastoma,
activating Wnt signal stimulates tumor stem cell proliferation
(23–26), whereas inhibiting the Notch
(27) and BMP signal (28,29) suppresses tumor growth and
activates apoptosis, respectively. Nevertheless, retinoblastoma
studies mainly focus on cell proliferation and single signal
effects, with none reporting on the differentiation through
multi-signal regulation.
The present study aimed for retinoblastoma
differentiation through the targeted regulation of several critical
cell signaling and key genes. It was identified that the classic
retinoblastoma cell line WERI-Rb-1 cells (W-RBCs) could be directly
induced into retinal neuron-like cells (RNLCs). Importantly, the
differentiated cells show remarkably poorer tumorigenicity in
vivo. Collectively, the present study provides optimized
strategies for regulated WERI-Rb1 cell differentiation and offers
potential targets for the retinoblastoma differentiation
therapy.
Materials and methods
Tissue collection
Two human embryonic neural retinas at the eighth to
ninth week of gestation were donated from legal therapeutic
abortions in the Third Affiliated Hospital, Sun Yat-Sen University
(Guangzhou, China). Informed consent for tissue donation was
obtained before acquisition of the tissue. All the samples were
collected under the auspices of the protocols for the institutional
review boards at Zhongshan Ophthalmic Center and the Third
Affiliated Hospital, Sun Yat-Sen University. The study was approved
by the Ethics Committee of Zhongshan Ophthalmic Center and the
Third Affiliated Hospital, Sun Yat-Sen University.
Cell isolation and culture
The human retinoblastoma cell line WERI-Rb-1 (2011;
American Type Culture Collection, Manassas, VA, USA) were cultured
in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences Chalfont,
UK) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Primary human RPCs were isolated from
human embryonic neural retinas and cultured, as previously
described (30,31).
Retinal neuron-like cell
differentiation
To develop an effective procedure to induce W-RBCs
into RNLCs, two protocols were adopted according to RPC (22), ESC (18,20) and iPSC (21,32) differentiation, with some
modifications: the first protocol involved a two-step process, with
priming and differentiation, whereas the second involved only one
step, with either priming or differentiation. Briefly, single
W-RBCs, with the density of 2×104 cells/ml, were placed
in 24-well plates pre-coated with 0.1 mg/ml poly-D-lysine (PDL) for
24 h and 5 μg/ml laminin (both from Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 1 h. The W-RBCs were primed in 'basic
medium': DMEM/F12, 15% fetal bovine serum (FBS), 0.1 mM
non-essential amino acids, 10 ng/ml bFGF, 0.1 mM 2-mercaptoethanol,
2% B27, and 1% N-2 supplement (all from Gibco; Thermo Fisher
Scientific, Inc.). A total of 10 days later, the medium was
replaced with 'neural medium' (Neurobasal, 2% B27, 1% N-2
supplement, 5% FBS, 0.1 mM non-essential amino acids and 2 mM
L-glutamine; all from Gibco; Thermo Fisher Scientific, Inc.) for a
further 10 days of differentiation. The cytokines Dickkopf-related
protein 1 (Dkk-1, 100 ng/ml) and Lefty-A (500 ng/ml, both from
R&D Systems, Inc., Minneapolis, MN, USA) [Dkk-1+Lefty-A (DL)]
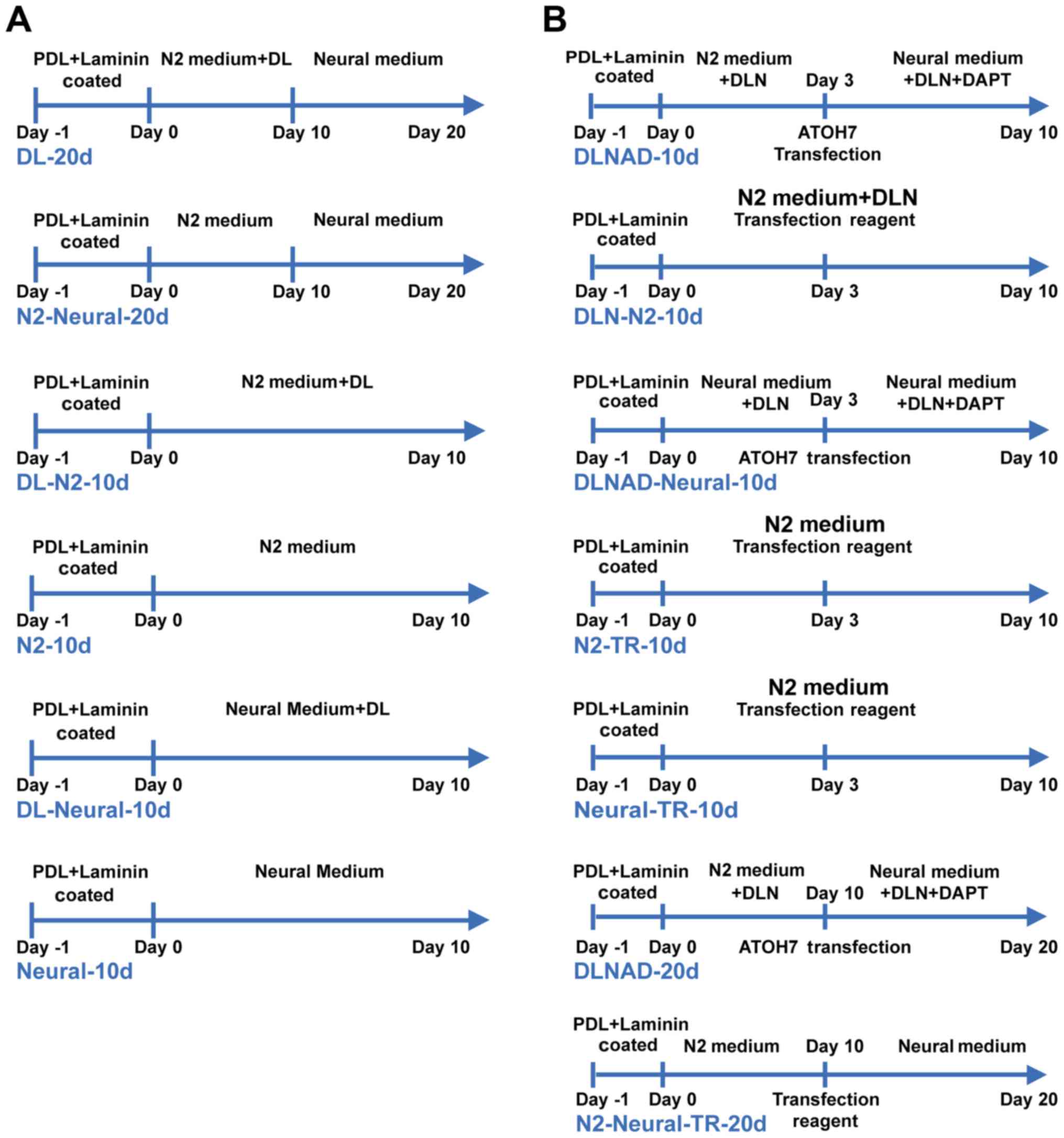
were used in the study. The cells were split into six groups:
groups 1 (DL-20d) and group 2 (N2-Neural-20d) were subjected to a
two-step process with or without DL. Groups 3–6 were subjected to a
one-step process for 10 days: groups 3 (DL-N2-10d) and group 4
(N2-10d) were cultured in basic medium with or without DL. Group 5
(DL-Neural-10d) was cultured in neural medium containing DL and
group 6 (Neural-10d) was cultured in neural medium only (Fig. 1A).
Retinal ganglion-like cell (RGLC)
generation
Single W-RBCs (2×104 cells/ml) were
seeded onto PDL and laminin-coated 24-well plates and then primed
in basic medium, with the addition of Dkk-1 (100 ng/ml), Lefty-A
(500 ng/ml) and Noggin (100 ng/ml; PeproTech, Inc., Rocky Hill, NJ,
USA) [DKK-1+Lefty-A+Noggin (DLN)]. pCMV-ATOH7 expression
plasmids (Vector Gene Technology Co., Ltd., Beijing, China) were
then transfected into the cells at room temperature (RT) using
X-treme GENE HP DNA Transfection reagent (Roche Diagnostics GmbH,
Basel, Switzerland). Following 24 h of transfection, the medium was
changed, and a neural medium containing DLN and 10 μM
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester (DAPT; Merck KGaA, Darmstadt, Germany)
[DKK-1+Lefty−A+Noggin+DAPT (DLND)] was added for further
differentiation. Given the time-dependent distribution of retinal
cell development, the cells were split into short- (10 days) and
long-period (20 days) induction groups. In the 10-day induction
groups, group 1 (DLNAD-10d) was induced by 3 days of priming with
DLN and 7 days of differentiation with DLND. The ATOH7 gene
was transfected into the cells on the third day. Group 2
(DLN-N2-10d) was cultured only in the basic medium with DLN and
without gene transfection. Group 3 (DLNAD-Neural-10d) was similar
to group 1, but the medium was replaced with neural medium). Group
4 (N2-TR-10d) and group 5 (Neural-TR-10d) were cultured in basic
and neural medium, respectively, with a blank transfection reagent
added on the third day at room temperature. In the 20-day
differentiation groups, group 6 (DLNAD-20d) underwent 10 days of
priming with DLN and 10 days of differentiation with DLND, and the
ATOH7 gene was transfected on the tenth day. Treatment of
group 7 (N2-Neural-TR-20d) excluded DLND from the induction system,
yet the blank transfection reagent was added on the tenth day at
room temperature (Fig. 1B)
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative PCR (qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Ambion; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions and then treated with DNase I
(Sigma-Aldrich; Merck KGaA) to remove any genomic DNA
contamination. cDNA synthesis from 3 μg total RNA was
conducted with a second-strand cDNA synthesis kit (PrimeScript™ RT
Master Mix; Takara Bio, Inc., Otsu, Japan). For analysis, 100–200
ng of cDNA was amplified with a master mix (Premix Taq™, Ex Taq™
version 2.0 plus dye; Takara Bio, Inc.) and a PCR system
(TProfessional Thermocycler; Biometra GmbH, Göttingen, Germany).
For quantitative analysis, the cDNA samples were amplified with the
Roche LightCycler® 480 system (Roche Diagnostics GmbH),
and the transcripts were quantified using an RT-PCR kit
(PrimeScript™ RT Master Mix; Takara Bio, Inc.). The quantification
method is the same as the previously described method (33). Human β-actin was used as a
housekeeping gene to calculate the relative mRNA expression. The
primer sequences are listed in Table
I.
 | Table IPrimer sequences used in reverse
transcription-PCR and quantitative PCR. |
Table I
Primer sequences used in reverse
transcription-PCR and quantitative PCR.
| Gene name | Primer sequence
(5′→3′) |
|---|
| NES | F:
GCAGCACTCTTAACTTACGATC |
| R:
GTCACCTCCATTAGCCACA |
| CRX | F:
GAAAGCAACCCGAACC |
| R:
AGAATGGCGTGAACCC |
| PROM1 | F:
AGTGGCATCGTGCAAACCTG |
| R:
CTCCGAATCCATTCGACGATA |
| BMI1 | F:
CTGCAGCTCGCTTCAAGATG |
| R:
TTAGCTCAGTGATCTTGATTCTCGT |
| MKI67 | F:
CCATATGCCTGTGGAGTGGAA |
| R:
CCACCCTTAGCGTGCTCTTGA |
| PRPH2 | F:
CTGCTCAAAGCCCAAACC |
| R:
TCAAATGTGAAACTCAGACCCT |
| OPN1LW | F:
CCCACTCGCTATCATCATGCT |
| R:
CAGTACGCAAAGATCATCACCA |
| RCVRN | F:
AAGCGAGCCGAGAAGAT |
| R:
TCAGTTTGGCAGGGAGC |
| NEUROD1 | F:
GGATGACGATCAAAAGCCCAA |
| R:
GCGTCTTAGAATAGCAAGGCA |
| GFAP | F:
ACGCCAGGCTCTCTGCTTTA |
| R:
GCGGTCCTGAGGGAAGAATC |
| ROM1 | F:
GCTGGCTGTCACCTTCCTAC |
| R:
CCCTGTAGCCATGCTGTTTT |
| POU4F1 | F:
CGCGGACTTTCGGAGTGTTT |
| R:
CAAAGTGAGGCTGCTTGCTG |
| POU4F2 | F:
AAGCCTACTTTGCCATTC |
| R:
GCTCCCTCTTCAGTCCTC |
| MAP2 | F:
CTCGCACAGAGTTATCC |
| R:
GCAGACCTACCACCAA |
| ATOH7 | F:
TTTATTCGCATCATCAGACC |
| R:
CAATCAACCCATTCACAAGA |
| PAX6 | F:
AACGATAACATACCAAGCGTG |
| R:
GGTCTGCCCGTTCAACATC |
| SYP | F:
GGACATGGACGTGGTGAA |
| R:
GGAGTAGAGGAAGGCAAACA |
| TUBB3 | F:
CCGAAGCCAGCAGTGTCTAA |
| R:
AGGCCTGGAGCTGCAATAAG |
| β-actin | F:
CTCTGGCCGTACCACTGGC |
| R:
GTGAAGCTGTAGCCGCGC |
Western blot analysis
W-RBCs, RNLCs and RGLCs were lysed in
radioimmunoprecipitation assay buffer containing a protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Total protein was
extracted by centrifuging (12,000 rpm) at 4°C for 15 min. The
protein was quantified with the BCA kit (Boster, Wuhan, China). A
total of 30 μg protein was electrophoresed on 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel
and then transferred onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The polyvinylidene
difluoride membranes were blocked by 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. Then the
membranes were incubated with retinal neuron-specific primary
antibodies (Table II) overnight
at 4°C. The horseradish peroxidase-conjugated secondary antibodies
were applied to the membranes for 2 h at room temperature. β-actin
served as the normalized protein. The bands were visualized by ECL
chemiluminescence substrates (ECL Plus; PerkinElmer Inc., Waltham,
MA, USA). The bands were quantified by ImageJ software (National
Institutes of Health, Bethesda, MD, USA.)
 | Table IIAntibodies used for
immunofluorescence analysis and western-blot analysis. |
Table II
Antibodies used for
immunofluorescence analysis and western-blot analysis.
| Antigen | Species | Dilutiona | Supplier
details | Catalog no. |
|---|
| PAX6 | Mouse | 1:100 | Merck KGaA | MAB5552 |
| NES | Rabbit | 1:50 | Wuhan Boster
Biological Technology, Ltd. | BA1289 |
| GFAP | Mouse | 1:50/1:200a | Abcam | Ab4648 |
| ISL1 | Rabbit | 1:200 | Abcam | Ab109517 |
| RHO | Mouse | 1:100/2
μg/mla | Abcam | Ab3267 |
| MAP2 | Mouse | 1:100 | Abcam | Ab11267 |
| SYP | Rabbit | 1:250 | Abcam | Ab52636 |
| Recoverin | Mouse | 1:100 | Abcam | Ab31928 |
| SSEA 4 | Mouse | 15
μg/ml | Abcam | Ab16287 |
| MKi 67 | Rabbit | 1:100 | Abcam | Ab16667 |
| OTX2 | Mouse | 1:200/1:500a | Abcam | Ab21990 |
| SOX2 | Rabbit | 1:250 | Abcam | Ab137385 |
| CRX | Mouse | 1:200 | Santa Cruz
Biotechnology, Inc. | Sc377138 |
| Brn3b | Rabbit | 1:100/0.2
μg/mla | Abcam | Ab56026 |
| β-actin | Rabbit | 1:1,000a | Cell Signaling
Technology, Inc. | mAb4970 |
Fluorescence-activated cell sorting
analysis
Single RGLCs and W-RBCs were suspended with a
fluorescence-activated cell sorting analysis buffer (PBS, 0.5% BSA,
2 mM EDTA-2Na-2H2O). The FIX and PERM™ Cell
Permeabilization kit (Invitrogen; Thermo Fisher Scientific, Inc.)
and indirect immunolabeling were applied for flow cytometry
analysis according to the manufacturer's protocols. Briefly, RGLCs
were incubated with POU class 4 homeobox 2 (Brn3b, 1:100)
primary antibody for 15 min at RT, and incubated with rabbit
anti-fluorescein isothiocyanate-conjugated secondary antibody
(1:300) (both from Abcam, Cambridge, UK) for 20 min at room
temperature. The percentage of Brn3b+ cells was detected
by flow cytometry (fluorescence-activated cell sorter Aria; BD
Biosciences, Franklin Lakes, NJ, USA).
Animals
The animal experiments were conducted in accordance
with the ARVO Statement for the Use of Animals in Ophthalmic and
Vision Research and approved by the Animal Experimental Ethical
Inspection to Ethics Committee of Zhongshan Ophthalmic Center (Sun
Yat-sen University). Adult nude mice of either gender (6–8 w, 15–20
g) (Laboratory Animal Center, Sun Yat-sen University) were housed
in cases under a 12:12 h light-dark cycle with water and food
provided ad libitum in the Laboratory Animal Center of
Zhongshan Ophthalmic Center.
Tumorigenicity studies
The nude mice were anesthetized by intraperitoneal
injections of a ketamine (100 mg/kg) and xylazine (10 mg/kg)
cocktail. Then W-RBCs, RNLCs and RGLCs were respectively injected
into one eye of 18 nude mice. Each cell type was also respectively
injected into the anterior chamber (AC, 2 eyes), subretinal space
(SRS, 2 eyes) and vitreous cavity (VC, 2 eyes). Cell suspensions
(105 cells/μl) were injected into the AC (1
μl), SRS (2 μl) or VC (3 μl), as previously
described (34). Changes in the
eyeball were observed with a slit lamp. The mice were sacrificed 27
days following transplantation, and the eyeballs were collected for
hematoxylin and eosin staining.
Immunofluorescence analysis
The suspended W-RBCs were seeded onto PDL-coated
Fisher coverslips (Thermo Fisher Scientific, Inc.) for 1 h at 37°C.
W-RBCs, hRPCs, RNLCs and RGLCs were then subjected to
immunolabeling, as previously reported (20). The images were captured with a
confocal laser scanning microscope (LSM 510) or fluorescence
microscopy (Carl Zeiss AG, Oberkochen, Germany). The primary
antibodies are detailed in Table
II.
Statistical analysis
Each experiment was performed in triplicate. All
data are represented as the mean ± standard deviation. Significance
was assessed with the Student's t-test for the comparison of two
variables or with a two-way analysis of variance for multivariable
comparisons using SPSS software (version 13.0; SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
WERI-Rb-1 cells retained retinal
progenitor cell properties
The data revealed a marked expression of the
hRPC-related genes nestin (NES) and cone-rod homeobox
(CRX), but not paired box 6 (PAX6) in W-RBCs
(Fig. 2A). Interestingly,
expression of the ESC-specific gene prominin 1 (PROM1) and
proliferative genes BMI1 proto-oncogene (BMI-1) and marker
of proliferation Ki-67 (MKI67), especially MKI67, was
remarkably higher in W-RBCs than in hRPCs (Fig. 2A). In addition, W-RBCs and hRPCs
expressed similar markers, with high expression of NES, CRX,
SRY-box 2 (SOX2) and Ki-67, and low expression of glial fibrillary
acidic protein (GFAP, an astrocyte marker). However, the expression
of PAX6 was much lower in W-RBCs than in hRPCs (Fig. 2B). These findings implied the
differentiation potential of retinal neurons and the high
proliferation ability of W-RBCs.
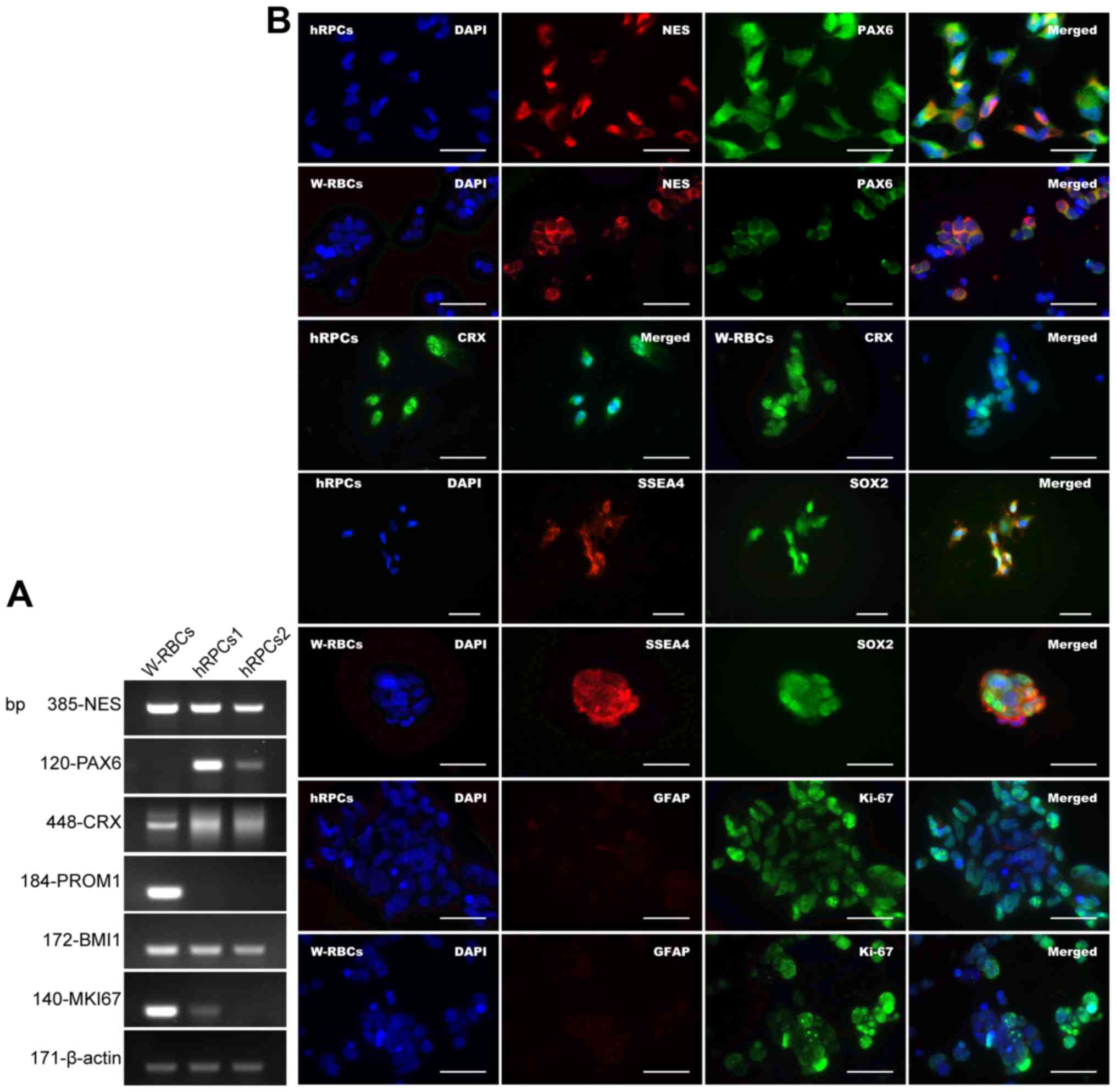 | Figure 2Retinal neuron differentiation
potential of W-RBCs. (A) Reverse transcription-quantitative
polymerase chain reaction revealed that the W-RBCs expressed
RPC-specific genes, NES and CRX, similar to two
controls (hRPCs1 and hRPCs2) obtained from two HENRs at the 8–9th
week of gestation but not PAX6. The expression of
pluripotency- and proliferation-related genes, including
PROM1, BMI1 and MKI67, was also much higher in
the W-RBCs than in the hRPCs. (B) Immunofluorescence confirmed that
both the W-RBCs and hRPCs expressed similar RPC markers (NES and
CRX) but the expression of PAX6 was weaker in the W-RBCs compared
to the hRPCs. In addition, the W-RBCs and hRPCs stained positively
for ESC markers (SSEA4 and SOX2) and the proliferative marker Ki-67
and negatively for the glia cell marker glial fibrillary acidic
protein. Scale bar, 50 μm. W-RBCs, WERI-Rb-1 cells; RPCs,
retinal progenitor cells; hRPCs, human retinal progenitor cells;
NES, nestin; PAX6, paired box 6; CRX, cone-rod homeobox; PROM1,
prominin 1; BMI1, BMI1 proto-oncogene; MKI67, marker of
proliferation Ki-67; ESC, embryonic stem cell. |
Optimization of the regulated W-RBC
differentiation system
With regard to the generation of RNLCs, the results
demonstrated progressively decreased expression of NES, an
intermediate neurofilament of undifferentiated cells, in the
two-step procedure (DL-20 d or N2-Neural-20d). The fold-change of
the NES transcripts decreased to 0.328 after 10-day priming
with DL, and then further declined to 0.118 following the
additional 10 days of differentiation. This trend was also observed
in the group that underwent the two-step process without DL
(N2-Neural-20d), but with a lower reduction in NES (0.352 after
priming and 0.218 after differentiation). NES also declined
significantly in cells cultured in the neural medium without DL for
10 days (Neural-10d). These findings suggested a gradual withdrawal
of the progenitor characteristics of W-RBCs and an engagement into
the differentiation process. In contrast, transcripts corresponding
to retinal neurons, especially photoreceptors, were upregulated in
differentiated W-RBCs. Specifically, the photoreceptor transcripts
peripherin 2 (PRPH2) and recoverin were significantly
increased after the two-step procedure plus DL (DL-20d). Notably,
there was a much higher fold-change of opsin 1, long wave sensitive
(OPN1LW, mature cone) in this group (DL-20d), whereas the
remaining groups showed an obvious reduction. The neural-related
transcript neuronal differentiation 1 (NEUROD1) also
increased notably in the DL-20d group. In contrast, removal of DL
caused a reduction in the retinal neuron-related transcript
expression (Fig. 3A and B).
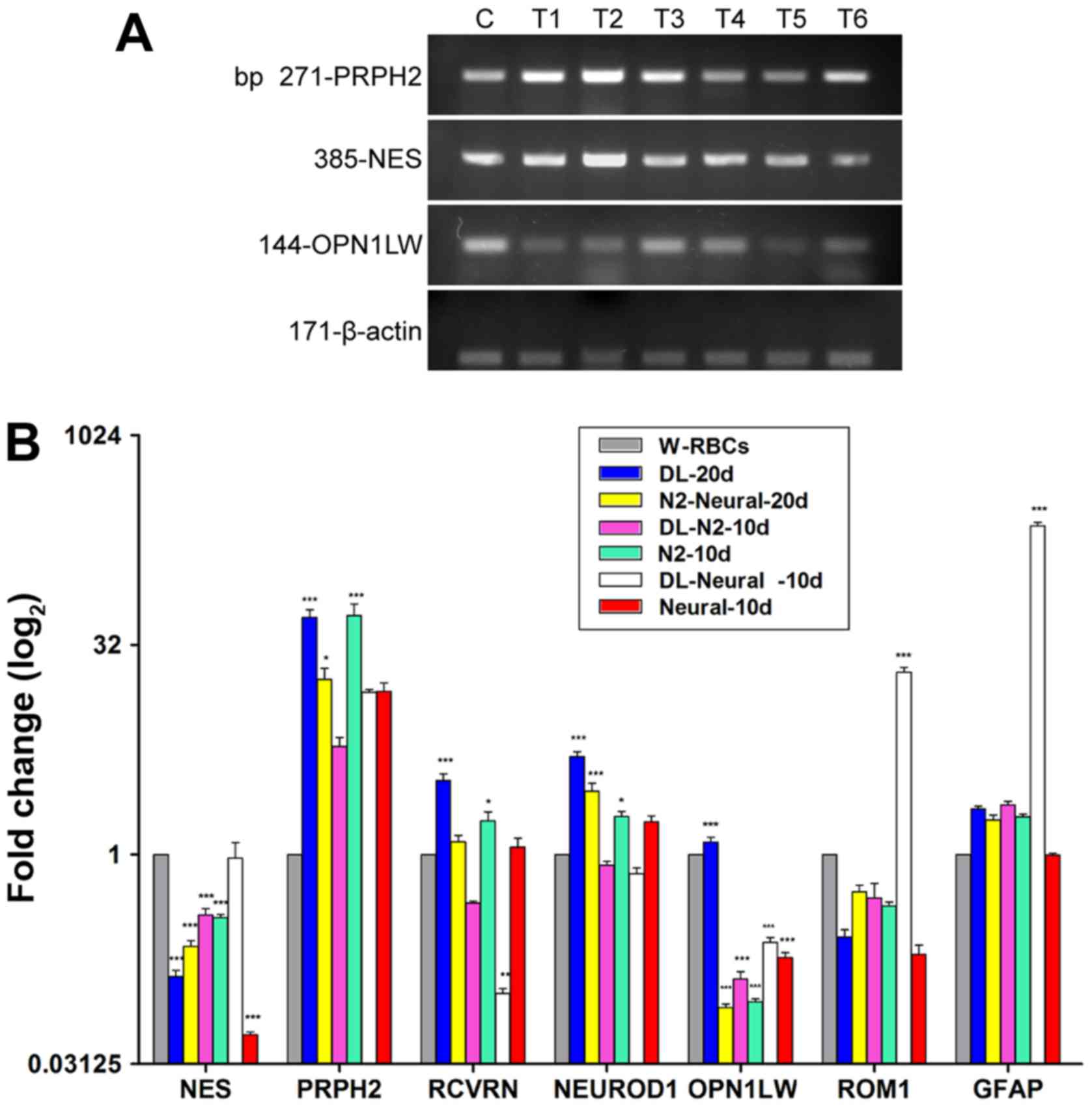 | Figure 3Optimization of retinal neuron-like
cell differentiation system. (A and B) Different expressions of
hRPC-related genes and photoreceptor-related genes among six RNLCs
induction groups and W-RBCs tested by (A) RT-PCR and (B) qPCR. (C)
RT-PCR analysis presented the expression of exogenous ATOH7 and
retinal ganglion cell (RGC)-related genes among retinal
ganglion-like cells induction groups and W-RBCs. (D) qPCR showed
that ATOH7 markedly increased in gene transfected groups. (E)
Real-time PCR revealed the hRPC- and RGC-related gene expressions
among different induction samples and control (W-RBCs). Graphs
present data are mean ± standard error of the mean (n=3).
*P<0.05, **P<0.01 and
***P<0.001 vs. control. (A) C, control (W-RBC); DL,
Dkk-1 + Lefty-A; T1, DL-N2-10d; T2, DL-Neural-10d; T3, DL-20d; T4,
N2-Neural-20d; T5, N2-10d T6, Neural-10d. (C) C, control (W-RBCs);
T1, DLNAD-10d; T2, DLN-N2-10d; T3, DLNAD-Neural-10d; T4, DLNAD-20d;
T5, N2-Neural-TR-20d; T6, N2-TR-10d; T7, Neural-TR-10d. PCR,
polymerase chain reaction; RT, reverse transcription; hRPCs human
retinal progenitor cells; W-RBCs, WERI-Rb-1 cells; qPCR,
quantitative PCR; hRPC, human retinal progenitor cell; RNLCs,
retinal neuron-like cells; RGC, retinal ganglion cell; ATOH7,
atonal bHLH transcription factor 7. |
The direct incubation of W-RBCs in neural medium
with (DL-Neural-10d) or without DL (Neural-10d) had a significantly
weaker effect on RNLC differentiation, with just two transcripts,
retinal outer segment membrane protein 1 (ROM1,
photoreceptor) and GFAP, increasing in the DL group
(DL-Neural-10d) and no effective increase in any related
transcripts in the non-DL group (Neural-10d). Together, these
results demonstrated that bFGF is necessary for the priming of
W-RBCs into a pre-neuronal state and that DL promotes the
differentiation of RNLCs, especially that of mature
photoreceptor-like cells (PLCs) (Fig.
3A and B).
Given that RGCs are specified prior to
photoreceptors during vertebrate retinogenesis, the RGC induction
process was shortened to 10 days, with 20-day procedures used as
controls. The expression of NES decreased significantly in
all the induction samples, providing evidence of cellular
differentiation into mature cells. PAX6 pathway-related genes,
critical genes that directly activate the development of RGCs, and
other RGC-related genes were upregulated in most samples.
Specifically, PAX6, an upstream gene of ATOH7, was
increased in all groups, but only two groups treated without the
neural medium for 10 days presented a significant fold-change
(DLN-N2-10d: 58; N2-TR-10d: 37) (Fig.
3E). The expression of ATOH7, a key gene that regulates
the fate of RGCs, was significantly high in the 10-day
gene-transfected groups (DLNAD-10d: 236; DLNAD-Neural-10d: 694-fold
change) (Fig. 3C and D), and high
in the 20-day transfected and non-transfected groups, albeit with
no statistical significance (Fig. 3C
and D). The upregulation of PAX6 and ATOH7 also
activated the downstream genes POU class 4 homeobox 1 and POU class
4 homeobox 2. Specifically, cells cultured only in basic or neural
media with/without DLN for 10 days all expressed much more Brn3a
(RGC precursor), whereas those cultured in neural medium expressed
less Brn3b (mature RGC) than those in basic medium.
Interestingly, the two-step 10-day protocol (DLNAD-10d)
downregulated Brn3a but significantly upregulated Brn3b and other
mature RGC-related genes, including microtubule associated protein
2 (MAP2) and tubulin β3 class III (TUBB3), which had
a lower expression in the remaining groups (Fig. 3C and E). The 20-day stepwise
strategies (DLNAD-20d or N2-Neural-TR-20d) appeared to have a much
weaker effect on the differentiation of RGLCs, with only
synaptophysin (SYP, neural synapsis specific) showing a
statistically significant increase (Fig. 3E). Collectively, the data
confirmed that the 10-day strategies was superior to the 20-day
strategies with regard to RGLC differentiation and that the
one-step procedure with DLN could effectively induce mature RGLCs,
even without gene transfection (DLN-N2-10d).
W-RBCs differentiated into RNLCs
The cells generated from the 20-day two-step
procedure with the addition of DL (DL-20d) were further
characterized. Spiculate features on cell clusters (Fig. 4A, day 5) were observed 5 days
following W-RBC plating (Fig. 4A,
day 0). By the end of priming on the tenth day, some cells
developed smaller triangular or oval, short neurite features
(Fig. 4A, day 10). Following
completion of differentiation on day 20, PLCs were observed, with
longer and branched neurite-like features (Fig. 4A, day 20). The immunofluorescence
study indicated that RNLCs expressed markers of orthodenticle
homeobox 2 (OTX2), REC, rhodopsin (RHO) and GFAP (Fig. 4B), which were much less expressed
or absent in W-RBCs (Fig. 4C).
The western blot assay confirmed that the OTX2, RHO and GFAP
proteins were remarkably upregulated in RNLCs (Fig. 4D). These results demonstrated that
this procedure could effectively induce W-RBCs into RNLCs, and into
PLCs and gliacytes in particular.
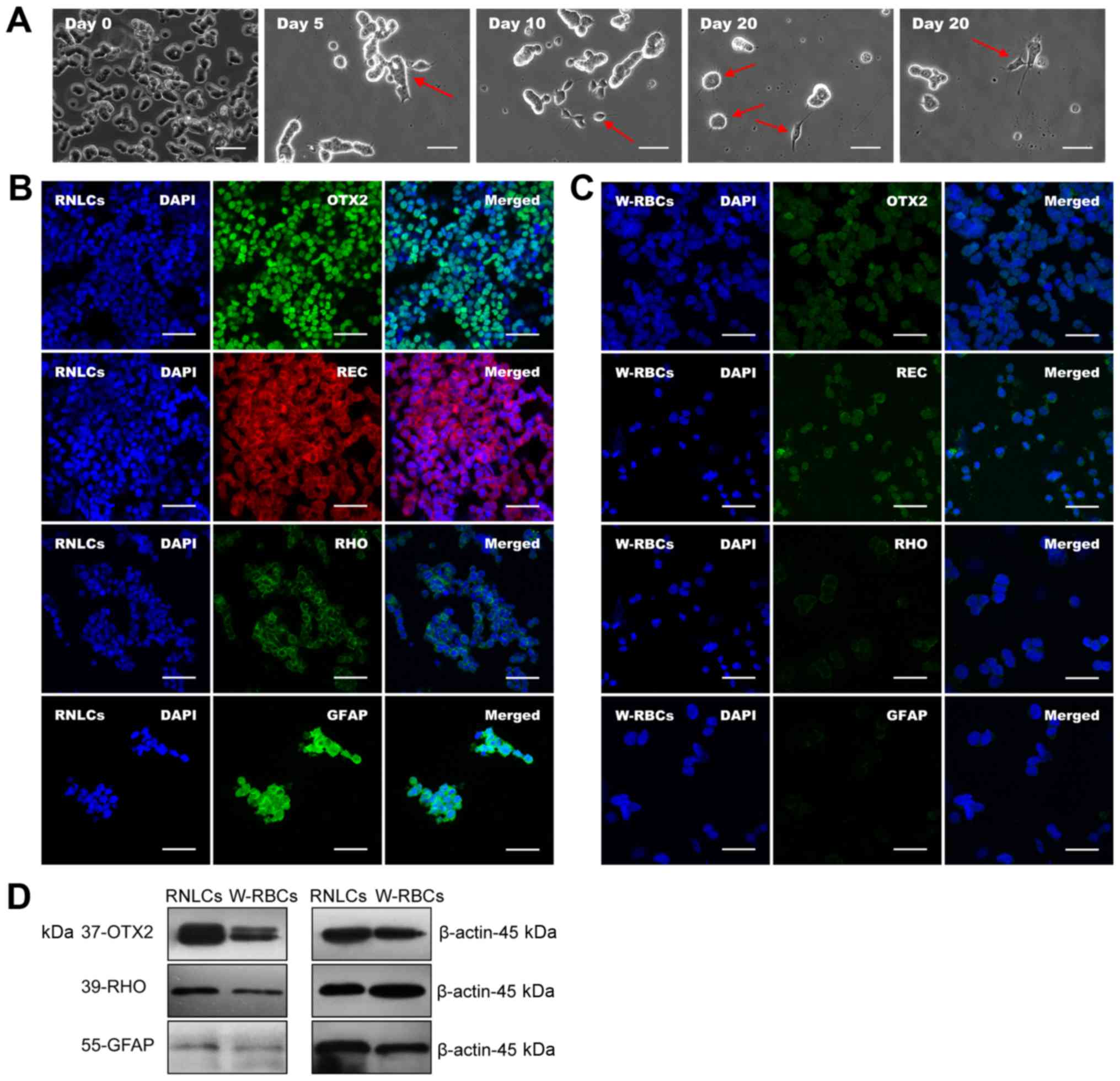 | Figure 4Differentiation of W-RBCs into RNLCs.
(A) Morphological changes in the W-RBCs during differentiation.
When cultured in the conditioned media, the W-RBCs gradually
generated synapse-like and photoreceptor-like morphology from day 0
to 20 (see arrow). (B) Immunofluorescence indicated that the RNLCs
expressed positive photoreceptor-related markers, including OTX2,
REC and RHO, as well as the astrocyte maker GFAP, whereas (C)
W-RBCs showed negative staining. (D) Western blot quantification
revealed that the levels of the OTX2, RHO and GFAP proteins were
much higher in two-step DL-induced sample (DL-20d) compared to
those of the W-RBC control. Scale bar, 50 μm. W-RBCs,
WERI-Rb-1 cells; RNLCs, retinal neuron-like cells; OTX2,
orthodenticle homeobox 2; RHO, rhodopsin; GFAP, glial fibrillary
acidic protein; DL, Dkk-1 + Lefty-A; RNLCs, retinal neuron-like
cells. |
W-RBCs differentiated into RGLCs
The cells generated from the one-step priming with
DLN (DLN-N2-10d) were further characterized through additional
assays. Fig. 5A (day 0) shows
W-RBCs attached to 6-well plates. On day 5, cells displayed a
neuronal morphology, with short-branched neurite features (Fig. 5A, day 5). Following 10-day
differentiation, cells displayed an RGC-like morphology, and a few
showed a bipolar cell-like phenotype (Fig. 5A, day 10). Immunofluorescence
confirmed that the RGLCs expressed Brn3b, ISL LIM homeobox 1 (−1,
RGC), SYP, MAP2 and GFAP (Fig.
5B), whereas these markers were much less expressed or even
absent in W-RBCs (Fig. 5C).
Furthermore, a significant increase in the percentage of
Brn3b+ cells (95.9%) was observed in RGLCs compared to
that in W-RBCs (0.5%) (Fig. 5D).
Western blot analysis indicated that Brn3b and GFAP protein levels
were upregulated following cell differentiation (Fig. 5E). Accordingly, the one-step
priming with DLN (DLN-N2-10d) effectively activated the
differentiation of RGLCs.
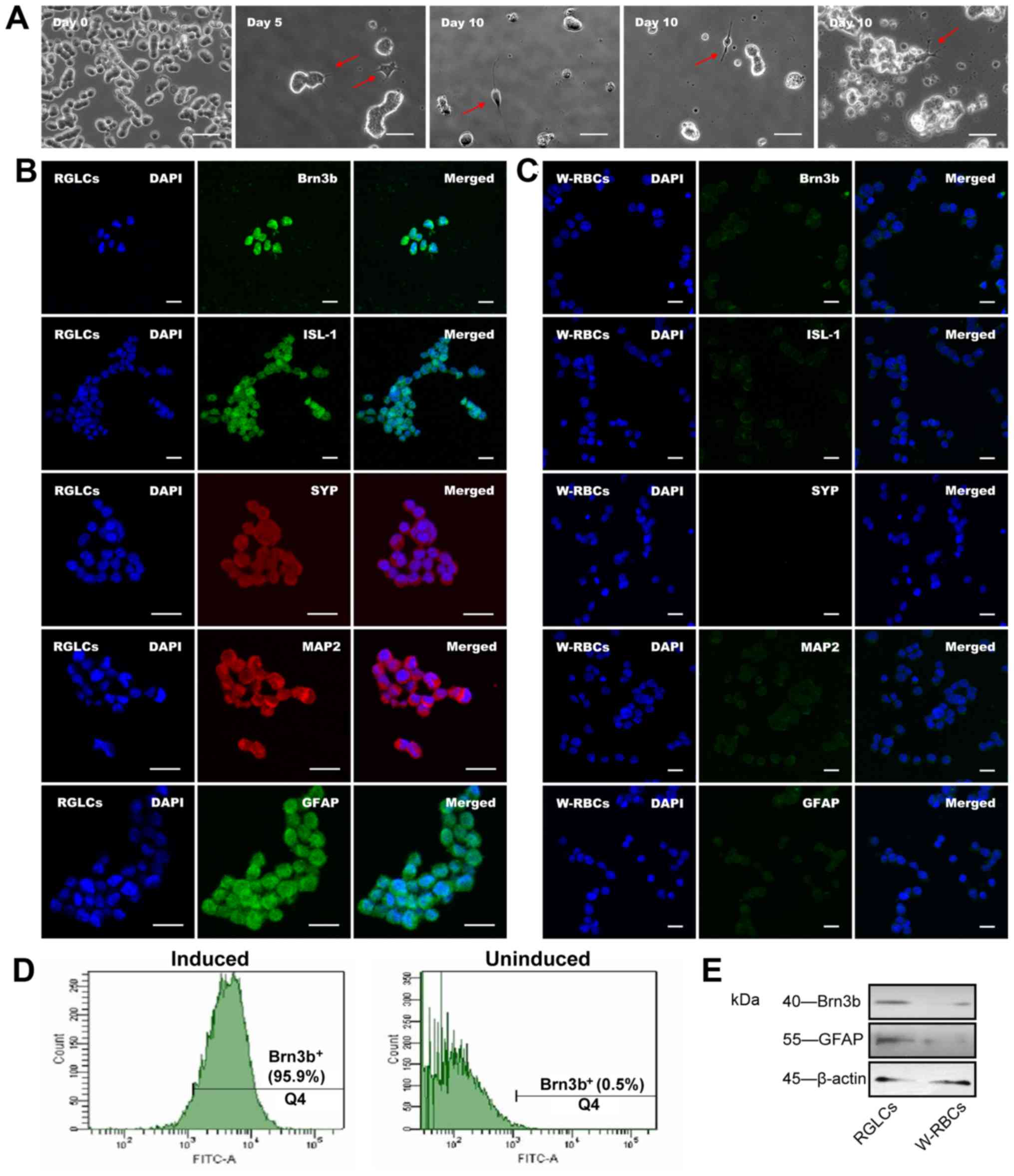 | Figure 5Differentiation of W-RBCs into RGLCs.
(A) Morphological changes in the W-RBCs during differentiation.
When transferred to conditioned media, the W-RBCs gradually
developed retinal neuron-like and ganglion-like morphology from day
0 to 10 (see arrow). (B) The RGLCs presented positive
immunofluorescence staining with Brn3b, ISL1, SYP, MAP2 and GFAP,
whereas (C) W-RBCs showed negative staining. (D) Flow cytometry
revealed that the percentage of Brn3b+ cells climbed to
95.9% after cell differentiation but remained at just 0.5% in the
W-RBCs. (E) Western blot quantification confirmed that the
expression of Brn3b and GFAP were higher in the induced group than
in the non-induced group (W-RBCs). Scale bar, (A) 50 μm and
(B and C) 25 μm. W-RBCs, WERI-Rb-1 cells; RGLCs, retinal
ganglion-like cells; ISL1, ISL LIM homeobox 1; SYP, synaptophysin;
MAP2, microtubule associated protein 2; GFAP, glial fibrillary
acidic protein. |
Differentiated W-RBCs showed poor
tumorigenicity in vivo
Undifferentiated and differentiated W-RBCs were
injected into nude mice eyes to assess tumorigenicity. W-RBCs gave
rise to significant tumors in six receptor eyes (100%) (Fig. 6A1–4). Histopathological
examination indicated that the xenografted tumors were composed of
small, round cells, with scanty cytoplasm and hyperchromatic
nuclei; Homer-Wright rosette structures were also observed
(Fig. 6A2). Importantly, the AC
xenograft tumor not only grew in the AC but also extended into the
VC (Fig. 6A2). In contrast, all
differentiated W-RBCs were located near the injection site and
showed no significant proliferation in any of the eyes (Fig. 6B1-C4). Although the RNLCs/RGLCs
injected in the AC migrated from the AC into the VC, no significant
proliferation was observed (Fig. 6B2
and C2). These results indicated that differentiation
remarkably reduced the tumorigenesis potential of W-RBCs.
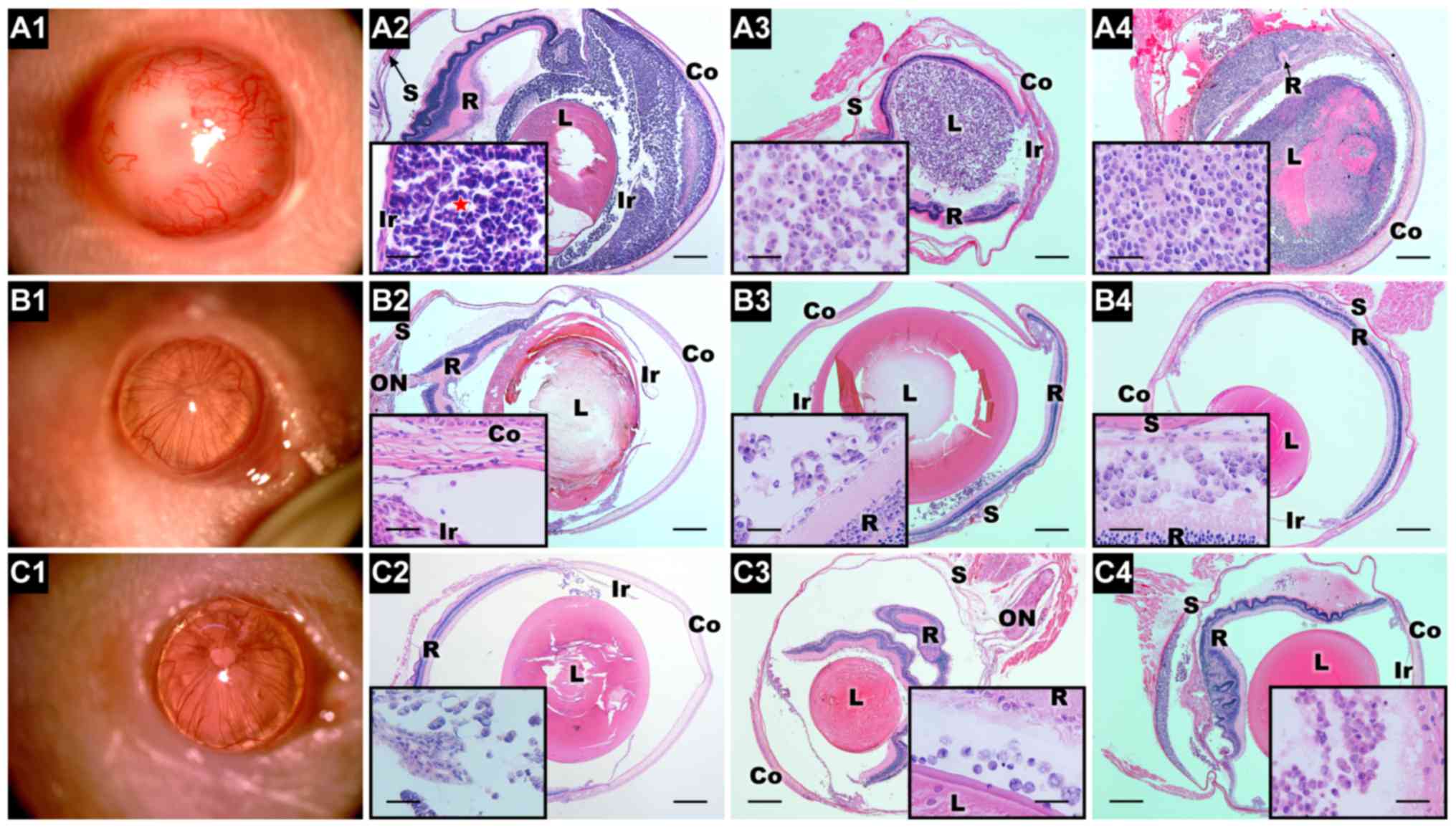 | Figure 6Tumorigenicity of the
undifferentiated and the differentiated W-RBCs in vivo. (A1)
At 25 days after injection into the AC, the W-RBCs developed a
large tumor (slit lamp). (A2–A4) H&E examination showed that
the injected W-RBCs generated a significant tumor in the AC, VC and
SRS. The tumor consisted of highly packed undifferentiated small
rounded cells and exhibited several Homer-Wright rosettes (A2, see
star). Notably, the xenograft tumor extended from the AC to the VC
(A2). (B1 and C1) No tumor (slit lamp) was observed 27 days after
injecting the (B1) RNLCs and (C1) RGLCs into the AC. (B2–B4 and
C2–C4) Hematoxylin and eosin examination showed that grafted
retinal neuron-like cells and retinal ganglion-like cells formed no
significant tumor in the AC, VC or SRS. Scale bar, 500 μm.
Magnification, 50 μm. Abbreviations, Co, cornea; Ir, iris:
L, lens: R, retina; S, sclera; ON, optic nerve;. W-RBCs, WERI-Rb-1
cells; AC, anterior chamber; VC, vitreous cavity; SRS, subretinal
space; H&E, hematoxylin and eosin; RNLCs, retinal neuron-like
cells. |
Discussion
W-RBCs retain retinal neuron
differentiation potency
The homozygous deletion of both RB1 alleles
in W-RBCs could revert cells to a progenitor-like status and
exhibit more advanced neuroblastic differentiation potential
compared to Y79 (12). Thus,
W-RBCs were selected in the present study. The data indicated a
link between W-RBCs and normal hRPCs with regards to gene
expression and molecular markers, thus providing the impetus for
W-RBCs to further differentiate into retinal neurons.
W-RBCs differentiate into RNLCs
Some signals, such as Wnt and Nodal signals, are
involved in both tumorigenesis and retinal development. In
neoplastic stem cells, the canonical Wnt pathway acts as a
mitogenic regulator of its embryonic function (24). The nodal family proteins give rise
to the ectoderm and mesoderm (35), whereas neuroectoderm formation
requires blocking of nodal signaling by DL (36). Moreover, there is cross-talk
between Wnt and Nodal signals, they exert a synergistic effect via
LEF and Smad transcripts. The authors previously adopted the DKK1
and Lefty-A, antagonists of Wnt and Nodal signaling, respectively,
to induce retinal neurons from iPSCs (21) and from retinoblastoma stem cells
(37). However, it has not been
previously reported to use the Dkk-1 and Lefty-A to induce W-RBCs
into retinal neurons.
Herein, six induction protocols were compared to
identify an easy and effective method to harvest sufficient RNLCs,
especially PLCs, from W-RBCs. The decreased rate of NES was
shown to be hindered by bFGF priming but promoted by DL and by
further B27 differentiation. Meanwhile, mature photoreceptor
(PRPH2 and RHO) and neuronal (NEUROD1) genes
were significantly increased in groups treated by both bFGF and
B27, especially through the addition of DL. Furthermore,
OPN1LW, another key gene regulating cone development, was
only upregulated in the two-step procedure with DL (DL-20d). These
results suggested that bFGF priming promotes the neuronal
commitment potency of W-RBCs, which is consistent with previous
studies (22,23) and that DL and B27 further motivate
photoreceptor and neuron differentiation. Nevertheless, some
studies reported that bFGF does not affect RHO expression and even
confines RPCs to glial cells instead of photoreceptors (38,39). Herein, ROM1, expressing in
the retinal outer segment, was significantly increased by direct
treatment with DL and B27, without bFGF (DL-Neural-10d). However,
this procedure also remarkably upregulated GFAP (~229-fold) and
reported a poorer performance in promoting other photoreceptor
genes as compared with bFGF priming. These results suggested that
bFGF priming, together with DL and B27 differentiation, are all
necessary to generate retinal neurons from W-RBCs.
W-RBCs differentiate into RGLCs
The development of RGCs in vertebrate retina occurs
much earlier than photoreceptor development and is regulated by
several critical signals and genes. ATOH7 is a key gene in
RGC generation (40,41). Targeted deletion of ATOH7
blocks terminal RGC differentiation (42). Contrarily, overexpression of
ATOH7 initiates RGCs from iPSCs (32), 293T and ND7 cells. Hes1 maintains
RPC fate and inhibits ATOH7 (32). PAX6, a critical upstream
transcription factor, can motivate ATOH7 by inhibiting Hes1
(43,44). Moreover, suppression of Notch
signaling could directly inhibit Hes1 and increase RGC production.
Notch signaling can also inhibit canonical Wnt signaling (27), while Wnt and BMP signals
antagonize each other (28).
Co-suppression of Nodal, BMP, Wnt, Notch and activation of FGF
signaling could benefit neural and eye-field specification
(21,37).
Thus, seven procedures using various supplements and
induction periods have been designed to assess whether W-RBCs could
initiate RGLCs in an earlier period, which is similar to the human
RGC development timeline. Previous studies have suggested that the
unique inhibition of Wnt and Nodal signaling is not sufficient for
RGC differentiation in both iPSCs and retinoblastoma stem cells
(21,37). However, inhibition of BMP
signaling by Noggin can upregulate PAX6 in ESCs (45,46). Moreover, the synergistic action of
DKK1, Noggin and DAPT (notch signaling inhibitor) together with
ATOH7 gene overexpression induced iPSCs into RGCs. Herein,
the transient transfection of ATOH7 significantly increase ATOH7
expression in short term (10 days); this effect, however, was much
weaker in long term (20 days). Moreover, the 20-day procedures
demonstrated a much weaker effect on upregulating RGC-
(Brn3a/Brn3b) and neuron-related genes (MAP2
and TUBB3) compared to most of the 10-day procedures. This
likely resulted from transient transfection, thus it is possible
that more RGCs would be generated when stable gene transfection was
adopted. Interestingly, however, two 10-day groups (DLN-N2-10d and
N2-TR-10d) without exogenous ATOH7 exhibited remarkable
upregulation of PAX6, Brn3a and Brn3b compared
with transfected samples, implying that ATOH7 is not the
only factor expanding RGLC production. Indeed, other factors, such
as the induction period, possibly serve important roles, meaning
that RGLCs are likely to appear in W-RBCs at an earlier stage
compared to photoreceptors (20 days). Moreover, the onset of ATOH7
expression may be affected by the inactivated RB1 allele because
the cell cycle exit regulator, RB transcriptional corepressor 1
(Rb1), activates the ATOH7 promoter in vivo
(47). Additionally, given the
significantly high expression of PAX6 in these two samples,
it is possible that PAX6 upregulated RGC-related genes
through genes other than ATOH7. The present findings are
consistent with recent studies concluding that ATOH7 is not
the only one essential for RGC specification (48); however, the exact mechanism needs
to be confirmed by further studies.
Furthermore, the bFGF effect of RPC maintenance was
also observed in the present study in relation to NES
expression. The two-step group (DLNAD-10d) treated with both bFGF
and B27 among the short-period induction groups expressed more
MAP2 and TUBB3, but less RGC-specific genes.
Conversely, the one-step processes, involving bFGF treatment only
(DLN-N2-10d and N2-TR-10d), induced more Brn3a and/or
Brn3b expression, indicating that bFGF had a positive effect
on RGC generation other than RPC maintenance, and that B27 was more
likely to motivate general neural cell growth instead of RGLCs.
Additionally, different signaling pathway inhibitors (DKK1, Lefty
A, Noggin and DAPT) were applied for RGC differentiation. DLN, even
without DAPT, was observed to induce a more mature RGC population
(Brn3b+, 95.9%) and a relatively lower increase in
immature RGC gene expression (Brn3a). Thus, to the best of
the authors' knowledge, the present study is the first to confirm
that bFGF plus DLN could effectively promote mature RGC expansion
in W-RBCs.
During the differentiation, however, the authors
found that the RNLCs did not generate long neurite like other stem
cells derived retinal neurons. That may result from the loss of
Rb1 gene in W-RBCs. It was previously determined that the
cell cycle exit regulator Rb1 correlated with the critical
retina development genes such as the ATOH7. The Rb1
gene could promote the ATOH7 expression. The onset of
Rb1 overlaps with the last mitosis in apically located RPCs
at the onset of Ath5 expression (49) and loss of Rb1 has been
reported to cause neuronal differentiation defects (50,51).
Tumorigenicity of differentiated W-RBCs
in vivo
Importantly, the differentiated W-RBCs presented
much lower tumorigenicity, whereas undifferentiated W-RBCs
generated considerable tumorigenesis in nude mice. These results
provided convincing evidence that differentiation of W-RBCs
inhibits their potential tumorigenesis, as previously observed in
Y79 cells (52), thus indicating
that W-RBCs could be transformed into poorly tumorigenetic RNLCs.
This is consistent with the concept that malignant cells could
transform into non-malignant cells after differentiation, as
proposed by Pierce and Wallace (53) and Pierce (54). However, previous studies have
revealed that retinoblastoma-derived horizontal cells could form
metastatic tumors by re-entering the cell cycle (55,56). Therefore, long-term observation
should be considered for further assessment.
The present study comprehensively regulate critical
cell signals to suggest facile and safe strategies to transform
W-RBCs into different types of retinal neurons with low
tumorigenicity. These results imply that differentiation therapy
may be a promising candidate to reverse malignant retinoblastoma to
a lower grade lesion with weaker aggressiveness, and at least to
transform a fatal tumor to one more amenable to conventional
therapies.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81371007, 81430009
and 81170846).
References
|
1
|
Bishop JO and Madson EC: Retinoblastoma.
Review of the current status. Surv Ophthalmol. 19:342–366.
1975.PubMed/NCBI
|
|
2
|
Chintagumpala M, Chevez-Barrios P, Paysse
EA, Plon SE and Hurwitz R: Retinoblastoma: Review of current
management. Oncologist. 12:1237–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Degos L: All trans retinoic acid as a
targeting drug for differentiation therapy in acute promyelocytic
leukemia. Cancer Treat Res. 64:1–13. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peterlin P, Garnier A, Tissot A, Garandeau
C, Houreau-Langlard D, Hourmant M, Vantyghem S, Bonnet A, Guillaume
T, Béné MC, et al: Successful treatment of acute promyelocytic
leukemia with arsenic trioxide and all-trans retinoic acid in a
double lung and kidney transplanted patient. Ann Hematol.
95:1737–1738. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kyritsis AP, Tsokos M, Triche TJ and
Chader GJ: Retinoblastoma: A primitive tumor with multipotential
characteristics. Invest Ophthalmol Vis Sci. 27:1760–1764.
1986.PubMed/NCBI
|
|
6
|
Seigel GM: Differentiation potential of
human retinoblastoma cells. Curr Pharm Biotechnol. 12:213–216.
2011. View Article : Google Scholar
|
|
7
|
Moore LH: Spontaneous regression of
retinoblastoma. Br J Ophthalmol. 50:1101966. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khodadoust AA, Roozitalab HM, Smith RE and
Green WR: Spontaneous regression of retinoblastoma. Surv
Ophthalmol. 21:467–478. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao J, Sun X, Tian Y, Li L, Li B, Ding H
and Xing H: A molecular pathologic study on apoptosis in
retinoblastoma and the mechanism of spontaneous regression in
retinoblastoma. Zhonghua Yan Ke Za Zhi. 32:405–416. 1996.In
Chinese. PubMed/NCBI
|
|
10
|
Xu X, Li B, Wang Y, Gao F and Zhang Z:
Retinoblastoma spontaneous regression: Clinical and histopathologic
analysis. Zhonghua Yan Ke Za Zhi. 50:729–732. 2014.In Chinese.
PubMed/NCBI
|
|
11
|
Challis GB and Stam HJ: The spontaneous
regression of cancer. A review of cases from 1900 to 1987. Acta
Oncol. 29:545–550. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herman MM, Perentes E, Katsetos CD, Darcel
F, Frankfurter A, Collins VP, Donoso LA, Eng LF, Marangos PJ,
Wiechmann AF, et al: Neuroblastic differentiation potential of the
human retinoblastoma cell lines Y-79 and WERI-Rb1 maintained in an
organ culture system. An immunohistochemical, electron microscopic,
and biochemical study. Am J Pathol. 134:115–132. 1989.PubMed/NCBI
|
|
13
|
Khanna H, Akimoto M, Siffroi-Fernandez S,
Friedman JS, Hicks D and Swaroop A: Retinoic acid regulates the
expression of photoreceptor transcription factor NRL. J Biol Chem.
281:27327–27334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saurat JH: Side effects of systemic
retinoids and their clinical management. J Am Acad Dermatol.
27:S23–S28. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fraunfelder FW and Fraunfelder FT: Adverse
ocular drug reactions recently identified by the National Registry
of Drug-Induced Ocular Side Effects. Ophthalmology. 111:1275–1279.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hughes MF: Arsenic toxicity and potential
mechanisms of action. Toxicol Lett. 133:1–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trinh E, Lazzerini Denchi E and Helin K:
Naturally death-resistant precursor cells revealed as the origin of
retinoblastoma. Cancer Cell. 5:513–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikeda H, Osakada F, Watanabe K, Mizuseki
K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N,
et al: Generation of Rx+/Pax6+ neural retinal
precursors from embryonic stem cells. Proc Natl Acad Sci USA.
102:11331–11336. 2005. View Article : Google Scholar
|
|
19
|
Lamba DA, Karl MO, Ware CB and Reh TA:
Efficient generation of retinal progenitor cells from human
embryonic stem cells. Proc Natl Acad Sci USA. 103:12769–12774.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osakada F, Ikeda H, Mandai M, Wataya T,
Watanabe K, Yoshimura N, Akaike A, Sasai Y and Takahashi M: Toward
the generation of rod and cone photoreceptors from mouse, monkey
and human embryonic stem cells. Nat Biotechnol. 26:215–224. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen M, Chen Q, Sun X, Shen W, Liu B,
Zhong X, Leng Y, Li C, Zhang W, Chai F, et al: Generation of
retinal ganglion-like cells from reprogrammed mouse fibroblasts.
Invest Ophthalmol Vis Sci. 51:5970–5978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Merhi-Soussi F, Angénieux B, Canola K,
Kostic C, Tekaya M, Hornfeld D and Arsenijevic Y: High yield of
cells committed to the photoreceptor fate from expanded mouse
retinal stem cells. Stem Cells. 24:2060–2070. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu P, Tarasenko YI, Gu Y, Huang LY,
Coggeshall RE and Yu Y: Region-specific generation of cholinergic
neurons from fetal human neural stem cells grafted in adult rat.
Nat Neurosci. 5:1271–1278. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Silva AK, Yi H, Hayes SH, Seigel GM and
Hackam AS: Lithium chloride regulates the proliferation of
stem-like cells in retinoblastoma cell lines: a potential role for
the canonical Wnt signaling pathway. Mol Vis. 16:36–45.
2010.PubMed/NCBI
|
|
25
|
Denayer T, Locker M, Borday C, Deroo T,
Janssens S, Hecht A, van Roy F, Perron M and Vleminckx K: Canonical
Wnt signaling controls proliferation of retinal stem/progenitor
cells in postembryonic Xenopus eyes. Stem Cells. 26:2063–2074.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuhrmann S: Wnt signaling in eye
organogenesis. Organogenesis. 4:60–67. 2008. View Article : Google Scholar
|
|
27
|
Xiao W, Chen X and He M: Inhibition of the
Jagged/Notch pathway inhibits retinoblastoma cell proliferation via
suppressing the PI3K/Akt, Src, p38MAPK and Wnt/β catenin signaling
pathways. Mol Med Rep. 10:453–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haubold M, Weise A, Stephan H and Dünker
N: Bone morphogenetic protein 4 (BMP4) signaling in retinoblastoma
cells. Int J Biol Sci. 6:700–715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimchi A, Wang XF, Weinberg RA, Cheifetz S
and Massagué J: Absence of TGF-beta receptors and growth inhibitory
responses in retinoblastoma cells. Science. 240:196–199. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klassen H, Ziaeian B, Kirov II, Young MJ
and Schwartz PH: Isolation of retinal progenitor cells from
post-mortem human tissue and comparison with autologous brain
progenitors. J Neurosci Res. 77:334–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmitt S, Aftab U, Jiang C, Redenti S,
Klassen H, Miljan E, Sinden J and Young M: Molecular
characterization of human retinal progenitor cells. Invest
Ophthalmol Vis Sci. 50:5901–5908. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parameswaran S, Balasubramanian S, Babai
N, Qiu F, Eudy JD, Thoreson WB and Ahmad I: Induced pluripotent
stem cells generate both retinal ganglion cells and photoreceptors:
Therapeutic implications in degenerative changes in glaucoma and
age-related macular degeneration. Stem Cells. 28:695–703. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Chen P, Hu H, Chen Z, Cai X, Zhang Z, Yang
Y, Yu N, Zhang J, Xia L, Ge J, et al: BRCA1 silencing is associated
with failure of DNA repairing in retinal neurocytes. PLoS One.
9:e993712014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vallier L, Reynolds D and Pedersen RA:
Nodal inhibits differentiation of human embryonic stem cells along
the neuroectodermal default pathway. Dev Biol. 275:403–421. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith JR, Vallier L, Lupo G, Alexander M,
Harris WA and Pedersen RA: Inhibition of Activin/Nodal signaling
promotes specification of human embryonic stem cells into
neuroectoderm. Dev Biol. 313:107–117. 2008. View Article : Google Scholar
|
|
37
|
Hu H, Deng F, Liu Y, Chen M, Zhang X, Sun
X, Dong Z, Liu X and Ge J: Characterization and retinal neuron
differentiation of WERI-Rb1 cancer stem cells. Mol Vis.
18:2388–2397. 2012.PubMed/NCBI
|
|
38
|
Zhao S and Barnstable CJ: Differential
effects of bFGF on development of the rat retina. Brain Res.
723:169–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Czekaj M, Haas J, Gebhardt M,
Müller-Reichert T, Humphries P, Farrar J, Bartsch U and Ader M: In
vitro expanded stem cells from the developing retina fail to
generate photoreceptors but differentiate into myelinating
oligodendrocytes. PLoS One. 7:e417982012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brown NL, Patel S, Brzezinski J and Glaser
T: Math5 is required for retinal ganglion cell and optic nerve
formation. Development. 128:2497–2508. 2001.PubMed/NCBI
|
|
41
|
Liu W, Mo Z and Xiang M: The Ath5
proneural genes function upstream of Brn3 POU domain transcription
factor genes to promote retinal ganglion cell development. Proc
Natl Acad Sci USA. 98:1649–1654. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang SW, Kim BS, Ding K, Wang H, Sun D,
Johnson RL, Klein WH and Gan L: Requirement for math5 in the
development of retinal ganglion cells. Genes Dev. 15:24–29. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Riesenberg AN, Liu Z, Kopan R and Brown
NL: Rbpj cell autonomous regulation of retinal ganglion cell and
cone photoreceptor fates in the mouse retina. J Neurosci.
29:12865–12877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kayama M, Kurokawa MS, Ueda Y, Ueno H,
Kumagai Y, Chiba S, Takada E, Ueno S, Tadokoro M and Suzuki N:
Transfection with pax6 gene of mouse embryonic stem cells and
subsequent cell cloning induced retinal neuron progenitors,
including retinal ganglion cell-like cells, in vitro. Ophthalmic
Res. 43:79–91. 2010. View Article : Google Scholar
|
|
45
|
Pera MF, Andrade J, Houssami S, Reubinoff
B, Trounson A, Stanley EG, Ward-van Oostwaard D and Mummery C:
Regulation of human embryonic stem cell differentiation by BMP-2
and its antagonist noggin. J Cell Sci. 117:1269–1280. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ying QL, Nichols J, Chambers I and Smith
A: BMP induction of Id proteins suppresses differentiation and
sustains embryonic stem cell self-renewal in collaboration with
STAT3. Cell. 115:281–292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ghiasvand NM, Rudolph DD, Mashayekhi M,
Brzezinski JA IV, Goldman D and Glaser T: Deletion of a remote
enhancer near ATOH7 disrupts retinal neurogenesis, causing NCRNA
disease. Nat Neurosci. 14:578–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang Z, Ding K, Pan L, Deng M and Gan L:
Math5 determines the competence state of retinal ganglion cell
progenitors. Dev Biol. 264:240–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kay JN, Link BA and Baier H: Staggered
cell-intrinsic timing of ath5 expression underlies the wave of
ganglion cell neurogenesis in the zebrafish retina. Development.
132:2573–2585. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen D, Opavsky R, Pacal M, Tanimoto N,
Wenzel P, Seeliger MW, Leone G and Bremner R: Rb-mediated neuronal
differentiation through cell-cycle-independent regulation of E2f3a.
PLoS Biol. 5:e1792007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Souren M, Martinez-Morales JR, Makri P,
Wittbrodt B and Wittbrodt J: A global survey identifies novel
upstream components of the Ath5 neurogenic network. Genome Biol.
10:R922009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
del Cerro M, Notter MF, Seigel G, Lazar E,
Chader G and del Cerro C: Intraretinal xenografts of differentiated
human retinoblastoma cells integrate with the host retina. Brain
Res. 583:12–22. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pierce GB and Wallace C: Differentiation
of malignant to benign cells. Cancer Res. 31:127–134.
1971.PubMed/NCBI
|
|
54
|
Pierce GB: The cancer cell and its control
by the embryo. Rous-Whipple Award lecture. Am J Pathol.
113:117–124. 1983.PubMed/NCBI
|
|
55
|
Ajioka I, Martins RA, Bayazitov IT,
Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS
and Dyer MA: Differentiated horizontal interneurons clonally expand
to form metastatic retinoblastoma in mice. Cell. 131:378–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Seigel GM, Tombran-Tink J, Becerra SP,
Chader GJ, Diloreto DA Jr, del Cerro C, Lazar ES and del Cerro M:
Differentiation of Y79 retinoblastoma cells with pigment
epithelial-derived factor and interphotoreceptor matrix wash:
Effects on tumorigenicity. Growth Factors. 10:289–297. 1994.
View Article : Google Scholar : PubMed/NCBI
|