Introduction
As resident innate immune cells in the central
nervous system (CNS), similar to peripheral macrophages, microglial
cells help to enhance host defence by eliminating pathogen attack
(1). However, the sustained and
uncontrolled activation of microglia leads to the accumulation of
excessive detrimental substances, most notably nitric oxide (NO),
free radicals and pro-inflammatory cytokines [e.g., interleukin
(IL)-1β and tumor necrosis factor-α (TNF-α)] that may finally lead
to neuronal destruction (2).
Indeed, microglial activation is one of the main pathological
characteristics in the processes of many neurological diseases
associated with neuroinflammation, such as Alzheimer's disease,
Parkinson's disease and multiple sclerosis. The suppression of the
activated microglia has been suggested to benefit the prevention of
these neurodegenerative disorders (3,4).
NF-κB, one of the Rel family transcription factors,
is a primary regulator of genes of many pro-inflammatory cytokines
or mediators, such as TNF-α, IL-1β and inducible NO synthase
(iNOS), as well as cyclooxygenase-2 (COX-2) (5,6).
Once activated by phosphorylation, NF-κB is translocated to the
nucleus, and transactivates downstream gene expression. Inhibitors
of NF-κB have been suggested in the treatment of many inflammatory
diseases (7). Lipopolysaccharide
(LPS), a prototypical endotoxin, is able to stimulate the
inflammatory responses in microglia by activating the NF-κB
signaling pathway (8,9). The LPS-induced activation of BV-2
microglial cells has been used widely for the studies of
anti-microglial activation drugs (10–12).
Astragalosides are the main active saponins of
Astragalus membranaceus (A. membranaceus), a
well-known traditional Chinese medicine with antioxidative,
immunoregulatory, anti-inflammatory and neuroprotective properties
(13,14), that have been demonstrated to
benefit both the peripheral nervous system and CNS (15–17). Isoastragaloside I (ISO I) is one
of the saponin molecules found within A. membranaceus
(18), which has been
demonstrated to alleviate insulin resistance and glucose
intolerance in obese mice (19).
However, little is known about whether it can inhibit microglial
activation and whether it is beneficial in neurological diseases.
In this study, the inhibitory effects of ISO I on inflammatory
mediator production in BV-2 microglial cells stimulated with LPS
were explored. Moreover, the possible underlying molecular
mechanisms were investigated. Our findings indicated that ISO I may
be an effective candidate for use in the treatment of various
neurodegenerative disorders characterized with microglial
activation.
Materials and methods
Drugs and reagents
ISO I (HPLC purity ≥98%) was purchased from Shanghai
Lightgoal Industry Co., Ltd. (Shanghai, China). Antibodies to
p-NF-κB (no. 3033), NF-κB (no. 6956), p-IκB (no. 2859S), IκB (no.
4812S), Akt (no. 2920), p-phosphoinositide 3-kinase (PI3K; no.
4228), p-p38 mitogen-activated protein kinase (MAPK; no. 9215), p38
MAPK (no. 9212), p-JNK (no. 9255), JNK (no. 9252), p-extracellular
signal-regulated kinase (ERK)1/2 (no. 4377), ERK1/2 (no. 4348) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; no. 5174) were all
obtained from Cell Signaling Technology Inc. (Danvers, MA, USA).
Lamin B1 (no. 6581-1) and PI3K antibodies (no. 3838-1) were both
obtained from Abcam (Cambridge, UK). The TNF-α ELISA kit was from
eBioscience, Inc. (San Diego, CA, USA).
BV-2 cell culture and treatment
BV-2 microglial cells were supplied by the American
Type Culture Collection (ATCC; Manassas, VA, USA) and were cultured
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 0.1 mg/ml
streptomycin. The cells were seeded in 6-, 12- or 96-well plates at
a density of 2×105 cells/ml according to the requirement
of the different experiments.
For the examination of iNOS and COX-2, and mRNA
expression pattern assay, the cells were pre-treated with ISO I
(100 µM) or dexamethasone (Dex; 10 µM). Two hours
later, they were incu bated with LPS (200 ng/ml) for 20 h, and were
harvested for further western blot analysis and quantitative PCR
(qPCR) assay.
For the analysis of activated PI3K and Akt, the
cells were pre-treated with ISO I (100 µM) for 2 h followed
by LPS (200 ng/ml) stimulation for 5, 15 and 30 min, respectively.
The cells were then lysed in CelLytic™ MT mammalian tissue lysis
reagent with phosphatase and protease inhibitor cocktail (all from
Sigma-Aldrich, St. Louis, MO, USA) on ice for 0.5 h. Following
centrifugation at 12,000 rpm for 15 min at 4°C, the supernatant of
the lysate was subjected to western blot analysis.
For the nuclear translocation assay of p-NF-κB,
nuclear extract of the cells was collected using a respective kit
(NE-PER® Nuclear and Cytoplasmic Extraction Reagents;
Thermo Fisher Scientific, Inc., Rockford, IL, USA), and further
used for western blot analysis.
Cytotoxicity and pro-inflammatory factor
assay
To examine the effects of ISO I on cell viability,
the BV-2 cells were treated with ISO I (0, 25, 50 and 100
µM) for 24 h, the cell viability of which was assessed using
the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto,
Japan). For the subsequent experiments, following pre-treatment
with ISO I (0, 25, 50 and 100 µM) or Dex (10 µM) for
2 h, BV-2 cells were stimulated with LPS (200 ng/ml) for 20 h, and
the culture medium was collected and subjected to further
analysis.
The release of NO in the medium was evaluated by
Griess assay as previously described (20). In brief, following incubation with
nitrate reductase for 1 h at 37°C, the medium was mixed with an
equal volume of Griess reagent (Sigma-Aldrich). The optical density
of the mixture was measured immediately at 540 nm. The
concentration of nitrate in the medium was calculated by a standard
curve of sodium nitrite. The concentration of TNF-α was determined
using a TNF-α ELISA kit according to the manufacturer's
instructions.
Western blot analysis
Proteins were extracted from the cells by sonication
in CelLytic™ MT mammalian tissue lysis reagent with protease and
phosphatase inhibitor cocktails. Following centrifugation at 12,000
× g for 15 min at 4°C, the supernatant of the lysate was collected.
Proteins in the supernatant were separated by 10% SDS-PAGE and
transferred onto PVDF membranes. After blocking in 5% skim milk for
0.5 h, the membranes were incubated with respective antibodies
against p-NF-κB (1:1,000), NF-κB (1:1,000), iNOS (1:1,000), COX-2
(1:1,000), p-IκB (1:1,000), IκB, Lamin B1 (1:1,000), Akt (1:1,000),
p-Akt (1:1,000), p-PI3K (1:1,000), PI3K (1:1,000), p-ERK1/2
(1:1,000), ERK1/2 (1:1,000), p-JNK (1:1,000), JNK (1:1,000), p-p38
MAPK (1:1,000), p38 MAPK (1:1,000) and GAPDH (1:2,000) overnight at
4°C. Thereafter, the membranes were incubated with secondary
antibodies conjugated to horseradish peroxidase (1:5,000). The
blots were developed using the ECL prime kit (GE Healthcare, Little
Chalfont, UK). Protein bands were quantified using ImageJ 1.46r
(National Institutes of Health, Bethesda, MD, USA).
qPCR
Total RNA was extracted using TRIzol reagent (Life
Technologies, Carlsbad, CA, USA) and were reverse transcribed into
cDNA using PrimeScript RT reagent (Takara Biotechnology Co., Ltd.,
Dalian, China). qPCR was performed using the TaqMan SYBR-Green qPCR
kit (Life Technologies). The quantity of target genes was
normalized to that of GAPDH in the same sample. The primer
sequences were listed as follows: iNOS forward,
5′-AACATCAGGTCGGCCATCAC-3′ and reverse,
5′-CCAGAGCCTGAAGTCATGTTTG-3′; TNF-α forward,
5′-AACCTCCTCTCTGCCGTCAAG-3′ and reverse,
5′-CCTCCCAGGTATATGGGCTCAT-3′; IL-1β forward,
5′-TGGGCCTCAAAGGAAAGAATC-3′ and reverse, 5′-GGT
ATTGCTTGGGATCCACACT-3′; GAPDH forward, 5′-ATGTGTCCGTCGTGGATCTGA-3′
and reverse, 5′-ATGCCTGCTTCACCACCTTCT-3′.
Luciferase activity assay
The BV-2 cells were seeded in a 24-well plate at a
density of 2×105 cells/well and cultured overnight. The
cells were then transiently transfected with NF-κB reporter vector
pGL6 (luc2P/NF-κB-RE/Hygro) (Beyotime Institute of Biotechnology,
Shanghai, China) and Renilla luciferase plasmid with
Lipofectamine™ LTX and Plus reagent (Life Technologies). Twelve
hours later, the cells were treated with ISO I (100 µM) for
2 h followed by stimulation with LPS (200 ng/ml) for 20 h.
Afterwards, the cells were harvested and subjected to luciferase
activity assay according to the manufacturer's instructions
(Promega, Madison, WI, USA). The final NF-κB activity was presented
as the ratio of the activity of firefly luciferase to that of
Renilla luciferase.
Immunocytochemistry (ICC)
The BV-2 cells were seeded in 8 chamber slides (BD
Biosciences, San Diego, CA, USA) at a density of 2×105
cells/ml. Tweety-four hours later, the cells were treated with or
without ISO I (100 µM) for 2 h followed by stimulation with
LPS (200 ng/ml) for 20 h. Thereafter, they were washed with
phosphate-buffered saline (PBS), fixed with 4% PFA, permeabilized
and blocked in PBS with 5% normal donkey serum and 0.1% Triton
X-100. Half an hour later, cells were incubated with p-NF-κB p65
antibody overnight at 4°C. After washing in PBS, they were further
incubated with Alexa-594 conjugated secondary antibody at room
temperature for 1 h. At last, cells were sealed in medium with
DAPI. Fluorescence images of the cells were acquired using an
Olympus DX81 fluorescent microscope (Olympus, Tokyo, Japan).
Statistical analysis
All data are presented as the means ± SEM.
Differences among 3 or more groups were analyzed by one-way
analysis of variance (ANOVA) followed by Dunnett's multiple
comparison test using GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA). Moreover, an unpaired Student's t-test
was used to compare the differences between 2 groups. Differences
were considered statistically significant at a value of
P<0.05.
Results
ISO I suppresses the production of
pro-inflammatory mediators induced by LPS
Prior to the assessment of the anti-inflammatory
effects of ISO I, the cytotoxic effects of ISO I on BV-2 cells were
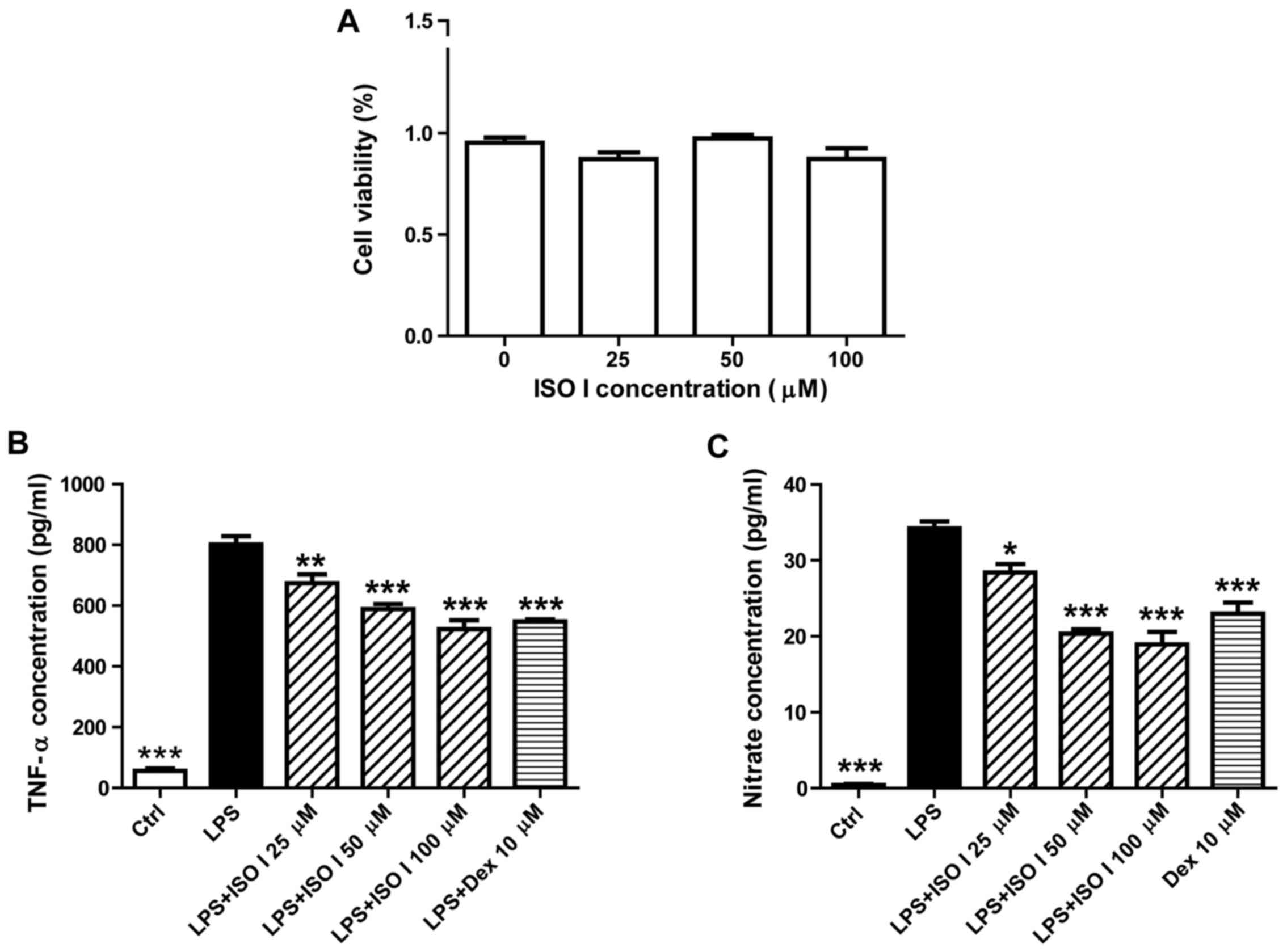
first examined by CCK-8 assay. As shown in Fig. 1A, treatment with ISO I at 25, 50
and 100 µM did not affect the viability of the BV-2 cells.
We then examined the effects of ISO I on LPS-stimulated BV-2 cells.
As shown in Fig. 1B and C, LPS
significantly induced the release of pro-inflammatory factors, such
as TNF-α and NO (P<0.001). Moreover, it also increased the
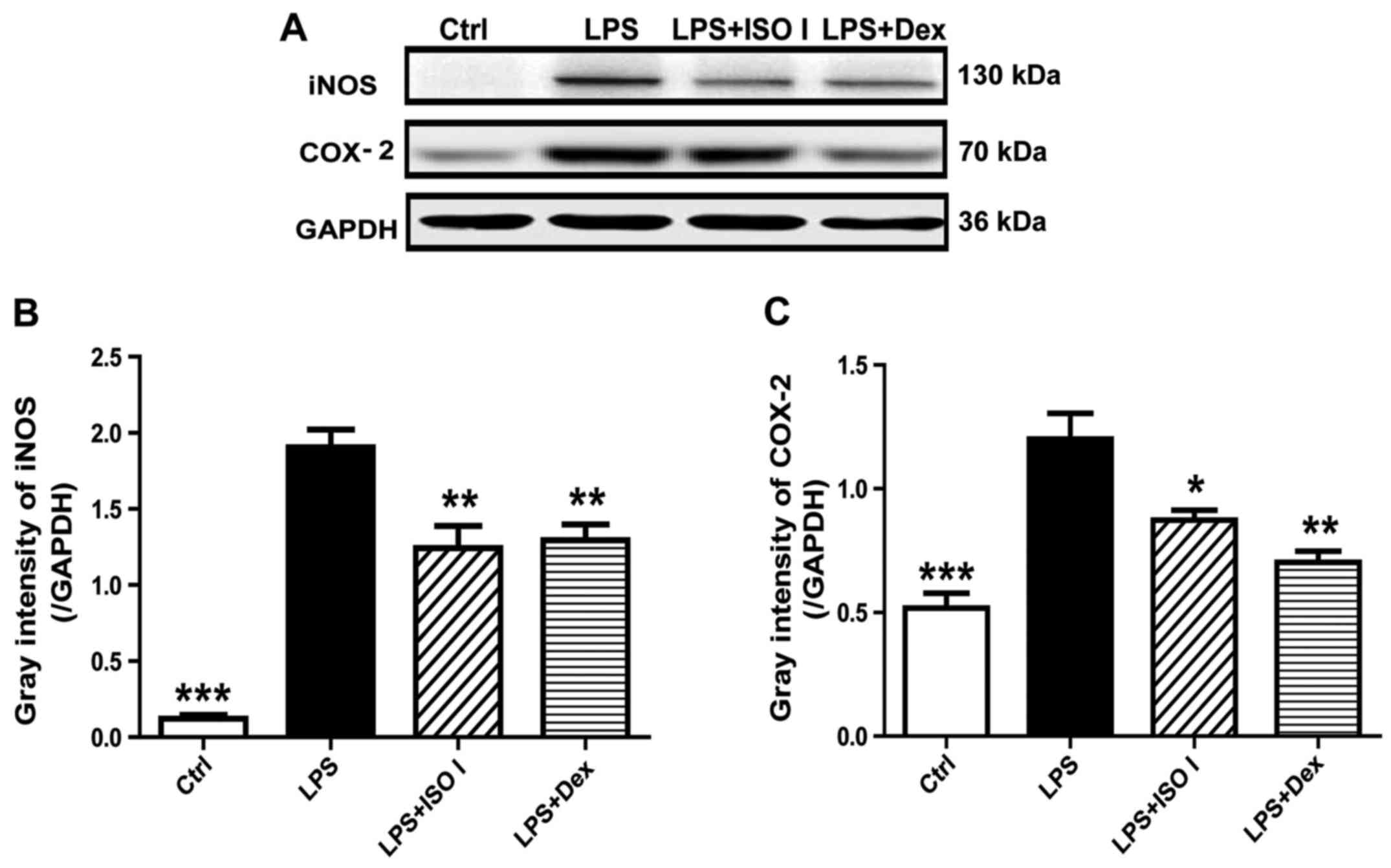
protein expression of iNOS and COX-2 (Fig. 2; P<0.001). Following treatment
with ISO I (25, 50 or 100 µM), the production of all the
pro-inflammatory factors was decreased (P<0.05, P<0.01 or
P<0.001). Similarly, Dex, the positive control drug, also
suppressed the production of all of the pro-inflammatory factors in
the BV-2 cells when used at 10 µM (P<0.01 or P<0.001).
These results indicated that ISO I attenuated the inflammatory
response in microglia upon LPS stimulation. Since ISO I at 100
µM exhibited the optimal inhibitory effect, this
concentration was selected for use in the subsequent
experiments.
ISO I downregulates the mRNA expression
of pro-inflammatory mediators induced by LPS
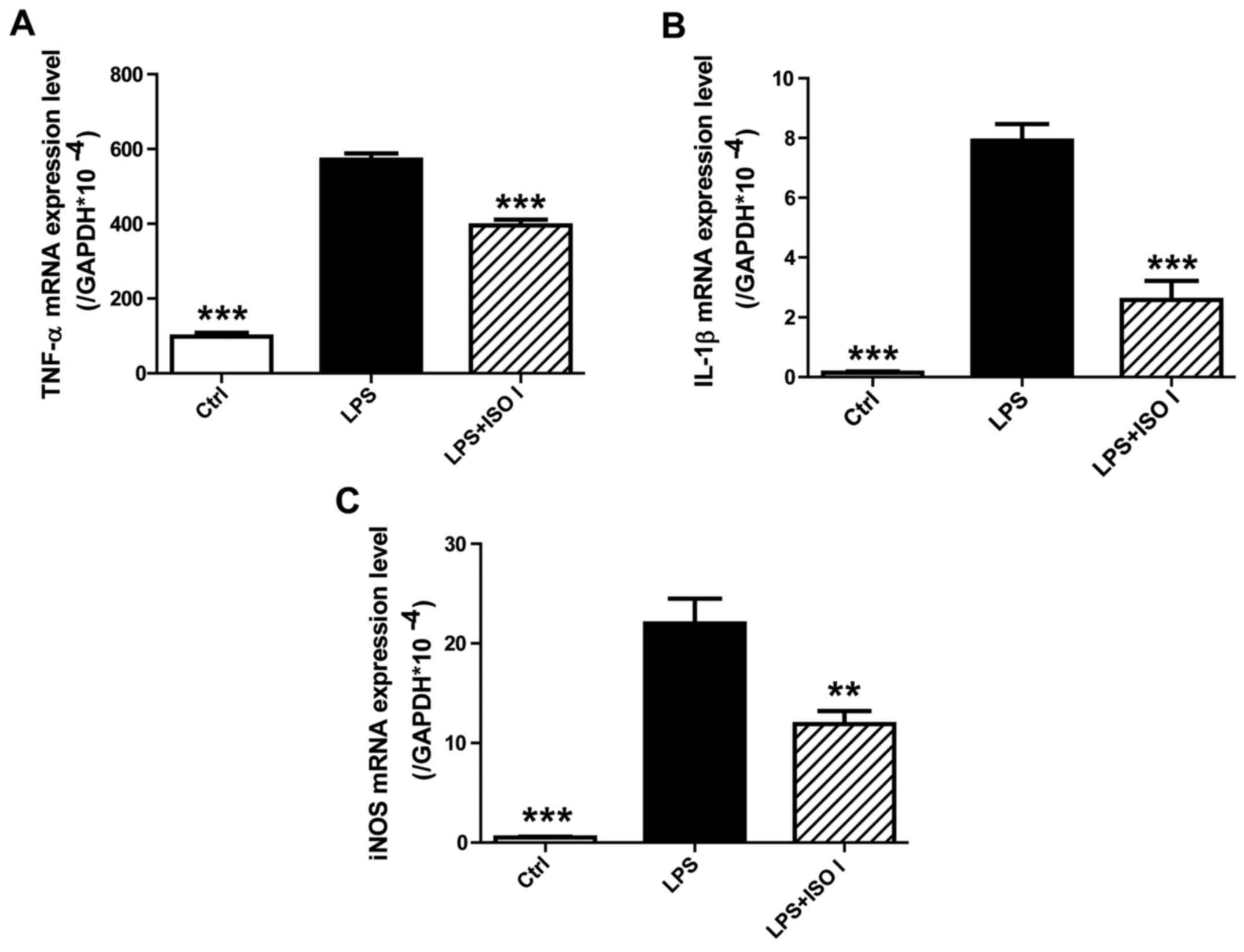
To determine the effects of ISO I on the mRNA
expression of pro-inflammatory mediators in BV-2 cells upon LPS
stimulation, qPCR analysis was performed. As shown in Fig. 3, the mRNA levels of TNF-α, IL-1β
and iNOS were markedly upregulated by LPS (P<0.001). However,
treatment with ISO I (100 µM) significantly inhibited the
overproduction of TNF-α, IL-1β and iNOS mRNA (P<0.01 or
P<0.001), which was consistent with its effects on the protein
levels of these pro-inflammatory mediators.
ISO I prevents the activation of NF-κB
induced by LPS
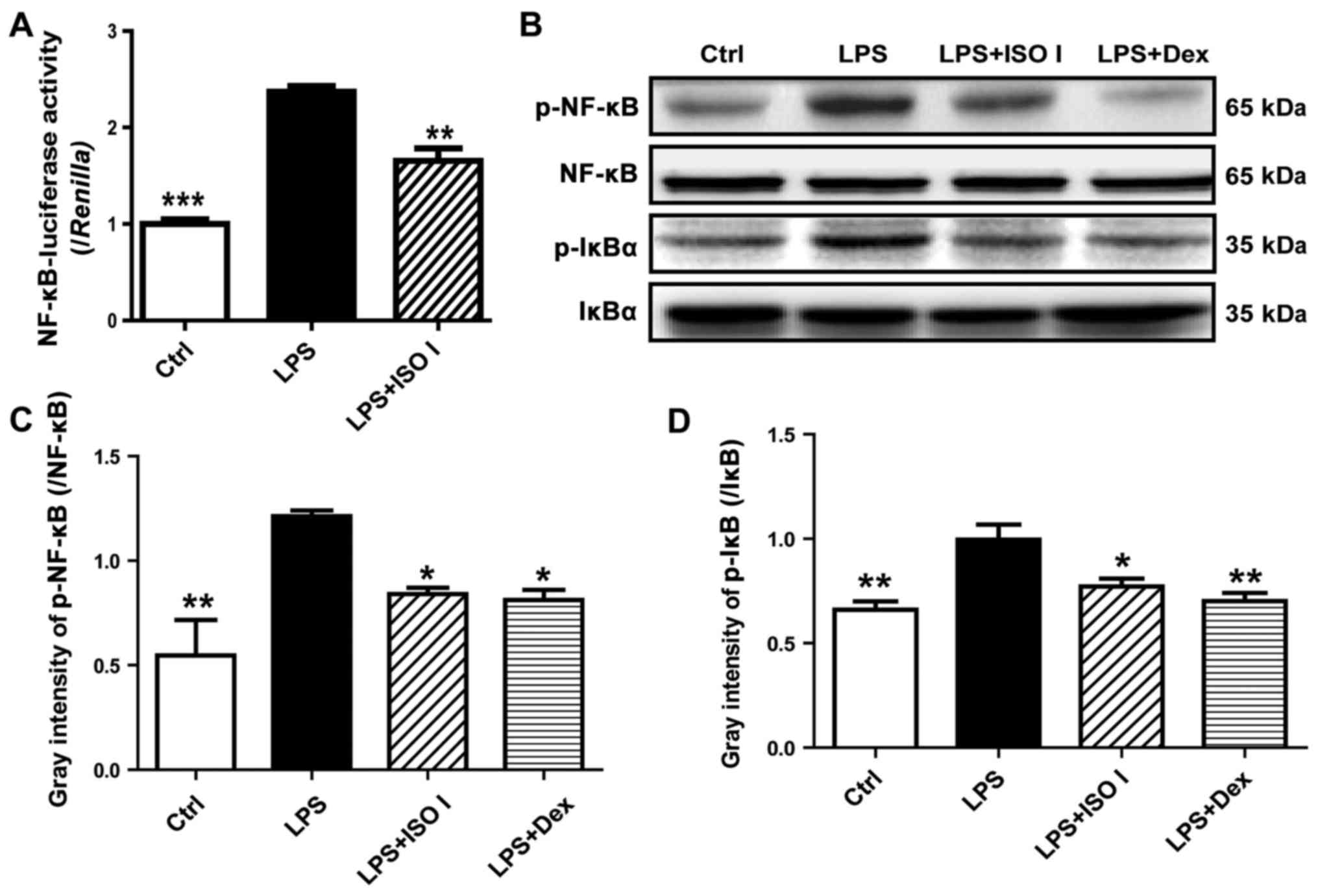
To explore the role of ISO I on NF-κB activation, a
luciferase reporter assay was carried out. As shown in Fig. 4A, LPS stimulation led to a marked
increase in NF-κB-luciferase activity (P<0.001) in the BV-2
cells, which was reversed by ISO I (P<0.01). Moreover, as
revealed by western blot analysis (Fig. 4B and C), ISO I treatment
suppressed the increased phosphorylation of NF-κB induced by LPS
(P<0.05). Accordingly, the elevation of phosphorylated IκB was
lessened (P<0.05).
To further confirm the inhibitory effects of ISO I
on the activation of NF-κB, the nuclear translocation of
phosphorylated NF-κB was analyzed by an immunocytochemistry assay
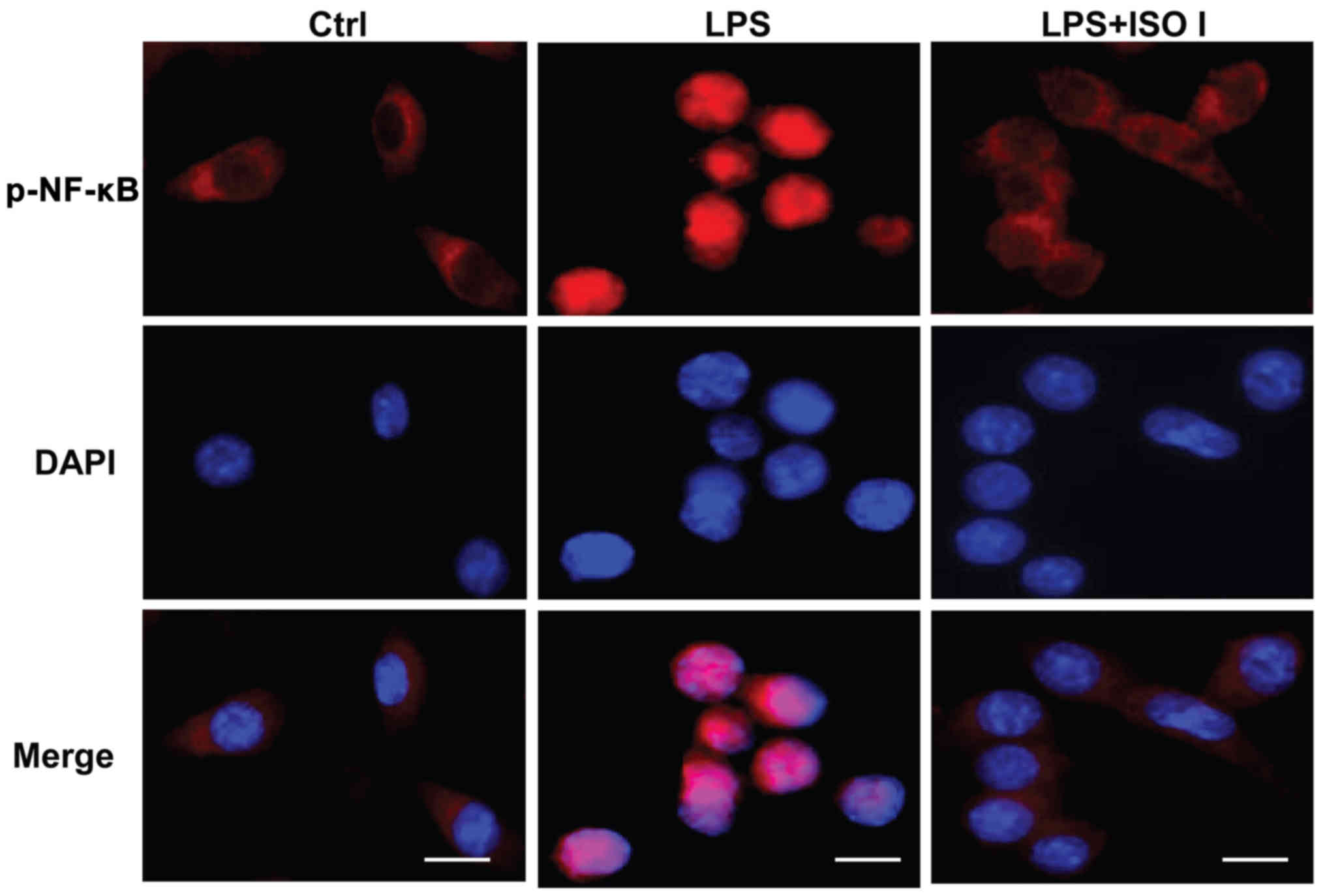
in the BV-2 cells. As shown in Fig.
5, in the non-activated cells, the majority of phosphorylated
NF-κB was localized in the cytoplasm. Upon LPS stimulation, the
fluorescence intensity of phosphorylated NF-κB was enhanced and
NF-κB was mostly translocated to the nucleus. Conversely, ISO I
treatment prevented the nuclear translocation of phosphorylated
NF-κB. All these results suggested that ISO I blocked the
activation of NF-κB induced by LPS.
ISO I reduces the enhanced
phosphorylation of PI3K/Akt and MAPKs induced by LPS
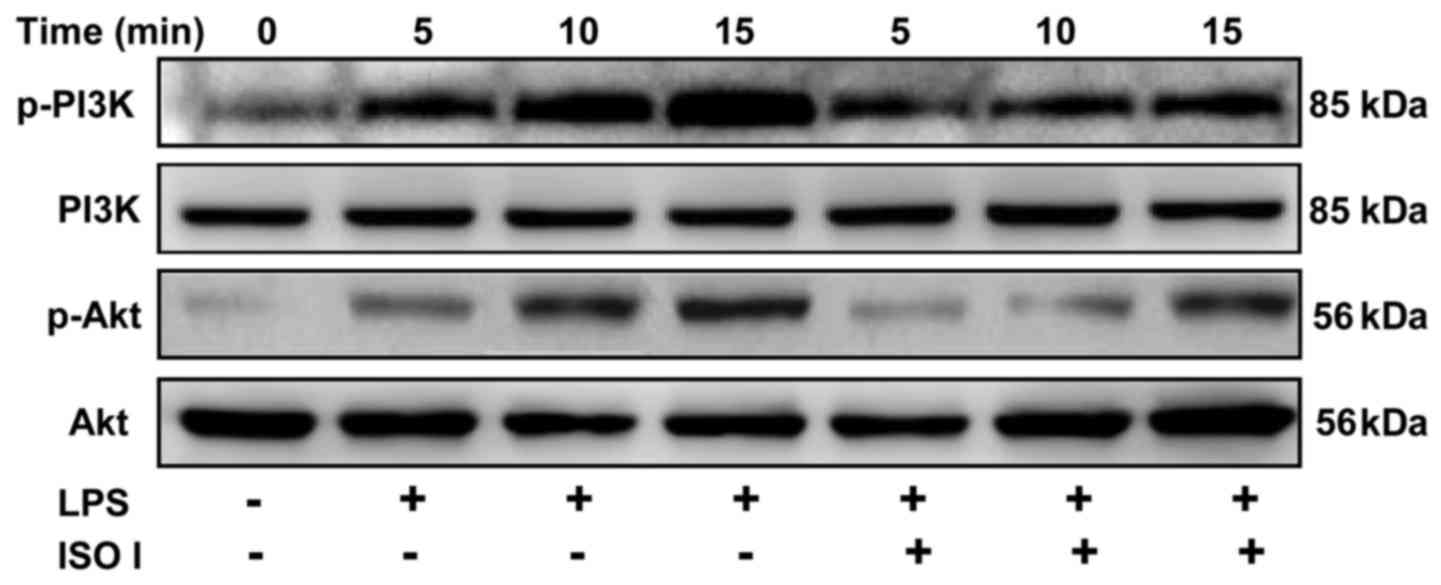
The PI3K/Akt signaling path way has been shown to
control the transactivation of NF-κB (21). Thus, to examine whether ISO I
affects the PI3K/Akt pathway, the phosphorylation of PI3K and Akt
in the BV-2 cells was detected. As shown in Fig. 6, LPS stimulation time-dependently
increased the phosphorylation of both PI3K and Akt, which was
significantly attenuated by ISO I treatment.
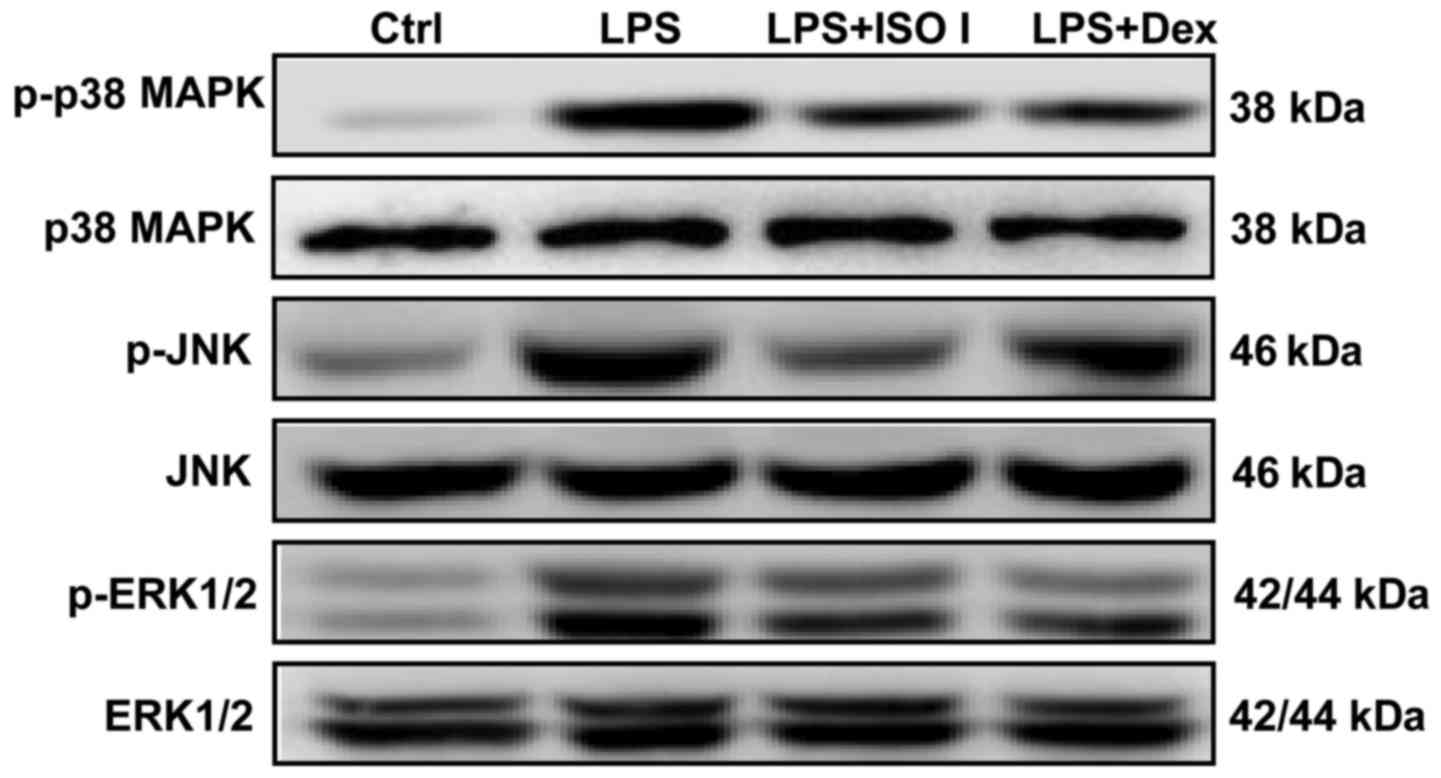
MAPKs, including p38, ERK and JNK, are involved in
NF-κB activation and the synthesis of pro-inflammatory mediators in
activated microglia (22). In our
study, we also detected the expression of MAPKs in LPS-stimulated
BV-2 cells. As shown in Fig. 7,
LPS enhanced the phosphorylation of p38, JNK and ERK1/2. Both ISO I
and Dex significantly alleviated the activation of MAPKs.
Discussion
Neuroinflammation with excessively activated
microglia has been demonstrated to aggravate the pathophysiology of
many neurodegenerative diseases, indicating the beneficial efficacy
of microglia regulatory agents in the therapy of these disorders
(23,24). As disclosed in our previous study,
total astragalosides alleviated neuroinflammation via the
inhibition of iNOS and other inflammatory cytokines in mice with
experimental autoimmune encephalomyelitis (25). Moreover, astragaloside IV, one of
the saponin molecules within total astragalosides, has been shown
to inhibit microglial activation via the glucocorticoid
receptor-mediated signaling pathway (20). In this study, we demonstrated that
another saponin molecule, ISO I, within total astragalosides, also
inhibited the activation of microglia. In the BV-2 cells, it
mitigated the LPS-induced production of pro-inflammatory mediators,
such as NO, TNF-α, iNOS and COX-2. Further experiments revealed
that ISO I attenuated NF-κB activation possibly by regulating the
PI3K/Akt and MAPK signaling pathways.
The persistent and uncontrolled activation of
microglia is one of the characteristics of neurodegenerative
disorders (26,27). Pro-inflammatory cytokines, such as
NO, IL-1β and TNF-α released from overactivated microglia may lead
to progressive neuronal damage and further activate the
inflammatory cascade (28). In
this study, ISO I treatment significantly reduced the release of NO
and TNF-α, decreased the protein levels of COX-2 and iNOS, and
downregulated the mRNA expression levels of iNOS, TNF-α and IL-1β,
indicating that ISO I suppressed the inflammatory responses in
microglia and may thus be beneficial in the treatment of
neurodegenerative diseases.
In the inactive state, the inhibitory protein, IκB,
binds to NF-κB dimmers that blocks the nuclear translocation of the
latter. Upon inflammatory stimulation, IκB is phosphorylated,
ubiquitinated and degraded sequentially. As a result, NF-κB dimers
will be translocated to the nucleus and regulate the transcription
of downstream genes (29). In our
study, ISO I significantly suppressed NF-κB luciferase activity,
reduced the phosphorylation of NF-κB and IκB, and prevented the
nuclear translocation of NF-κB in LPS-stimulated BV-2 cells,
accordingly, decreasing the downstream gene expression of
inflammatory mediators. Therefore, our findings suggested that ISO
I inhibited the inflammatory responses in microglia upon LPS
stimulation via the NF-κB pathway.
Signaling pathways, such as PI3K/Akt and MAPKs are
well known to interact with the NF-κB pathway, and therefore, are
actively involved in the inflammatory cascade in microglia
(30). In agreement with the
reports (31,32), in our study, LPS induced the
prominent activation of PI3K/Akt and MAPKs. The effect of LPS was
abrogated by ISO I, suggesting that the anti-inflammatory effects
of ISO I may be possibly mediated through these signaling
pathways.
In conclusion, the present study demonstrated that
ISO I attenuated LPS-induced inflammatory responses in BV-2
microglia, and these effects were probably mediated via the
inhibition of NF-κB activation through the PI3K/Akt and MAPK
signaling pathways.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (81673626, 81603354), Shanghai Eastern
Scholar Program (2013–59) and Shanghai E-research Institute of
Bioactive Constituent in TCM plan.
References
|
1
|
Glass CK, Saijo K, Winner B, Marchetto MC
and Gage FH: Mechanisms underlying inflammation in
neurodegeneration. Cell. 140:918–934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spencer JP, Vafeiadou K, Williams RJ and
Vauzour D: Neuroinflammation: modulation by flavonoids and
mechanisms of action. Mol Aspects Med. 33:83–97. 2012. View Article : Google Scholar
|
|
3
|
Akiyama H, Barger S, Barnum S, Bradt B,
Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich
BL, et al: Inflammation and Alzheimer's disease. Neurobiol Aging.
21:383–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bi W, Zhu L, Wang C, Liang Y, Liu J, Shi Q
and Tao E: Rifampicin inhibits microglial inflammation and improves
neuron survival against inflammation. Brain Res. 1395:12–20. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright JG and Christman JW: The role of
nuclear factor kappaB in the pathogenesis of pulmonary diseases:
implications for therapy. Am J Respir Med. 2:211–219. 2003.
View Article : Google Scholar
|
|
6
|
Wullaert A: Role of NF-kappaB activation
in intestinal immune homeostasis. Int J Med Microbiol. 300:49–56.
2010. View Article : Google Scholar
|
|
7
|
Calzado MA, Bacher S and Schmitz ML:
NF-kappaB inhibitors for the treatment of inflammatory diseases and
cancer. Curr Med Chem. 14:367–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li N, Liu BW, Ren WZ, Liu JX, Li SN, Fu
SP, Zeng YL, Xu SY, Yan X, Gao YJ, et al: GLP-2 attenuates
LPS-induced inflammation in BV-2 cells by inhibiting ERK1/2, JNK1/2
and NF-κB signaling pathways. Int J Mol Sci. 17:1902016. View Article : Google Scholar
|
|
9
|
Song F, Zeng K, Liao L, Yu Q, Tu P and
Wang X: Schizandrin A inhibits microglia-mediated
neuroninflammation through inhibiting TRAF6-NF-κB and Jak2-Stat3
signaling pathways. PLoS One. 11:e01499912016. View Article : Google Scholar
|
|
10
|
Nan ZD, Zhao MB, Zeng KW, Tian SH, Wang
WN, Jiang Y and Tu PF: Anti-inflammatory iridoids from the stems of
Cistanche deserticola cultured in Tarim Desert. Chin J Nat Med.
14:61–65. 2016.PubMed/NCBI
|
|
11
|
Zhang C, Liu BY, Zeng KW, Guo XY, Jiang Y
and Tu PF: New sesquiterpene and thiophene derivatives from
Artemisia rupestris. J Asian Nat Prod Res. 17:1129–1136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Huang D, Xu J, Tong J, Wang Z,
Huang L, Yang Y, Bai X, Wang P, Suo H, et al: Tiagabine protects
dopaminergic neurons against neurotoxins by inhibiting microglial
activation. Sci Rep. 5:157202015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rios JL and Waterman PG: A review of the
pharacology and toxicology of Astragalus. Phytother Res.
11:411–418. 1997. View Article : Google Scholar
|
|
14
|
Liu G, Song J, Guo Y, Wang T and Zhou Z:
Astragalus injection protects cerebral ischemic injury by
inhibiting neuronal apoptosis and the expression of JNK3 after
cerebral ischemia reperfusion in rats. Behav Brain Funct. 9:362013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng CY, Yao CH, Liu BS, Liu CJ, Chen GW
and Chen YS: The role of astragaloside in regeneration of the
peripheral nerve system. J Biomed Mater Res A. 76:463–469. 2006.
View Article : Google Scholar
|
|
16
|
Lei H, Wang B, Li WP, Yang Y, Zhou AW and
Chen MZ: Anti-aging effect of astragalosides and its mechanism of
action. Acta Pharmacol Sin. 24:230–234. 2003.PubMed/NCBI
|
|
17
|
Li WZ, Wu WY, Huang DK, Yin YY, Kan HW,
Wang X, Yao YY and Li WP: Protective effects of astragalosides on
dexamethasone and Aβ25-35 induced learning and memory impairments
due to decrease amyloid precursor protein expression in 12-month
male rats. Food Chem Toxicol. 50:1883–1890. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirotani M, Zhou Y, Rui H and Furuya T:
Cycloartane triterpene glycosides from the hairy root cultures of
Astragalus membranaceus. Phytochemistry. 37:1403–1407. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte
PM, Wang Y, Wu D, Chu W, Qin G and Lam KS: Selective elevation of
adiponectin production by the natural compounds derived from a
medicinal herb alleviates insulin resistance and glucose
intolerance in obese mice. Endocrinology. 150:625–633. 2009.
View Article : Google Scholar :
|
|
20
|
Liu HS, Shi HL, Huang F, Peterson KE, Wu
H, Lan YY, Zhang BB, He YX, Woods T, Du M, et al: Astragaloside IV
inhibits microglia activation via glucocorticoid receptor mediated
signaling pathway. Sci Rep. 6:191372016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arbibe L, Mira JP, Teusch N, Kline L, Guha
M, Mackman N, Godowski PJ, Ulevitch RJ and Knaus UG: Toll-like
receptor 2-mediated NF-kappa B activation requires a Rac1-dependent
pathway. Nat Immunol. 1:533–540. 2000. View
Article : Google Scholar
|
|
22
|
Leung KW, Yung KK, Mak NK, Chan YS, Fan TP
and Wong RN: Neuroprotective effects of ginsenoside-Rg1 in primary
nigral neurons against rotenone toxicity. Neuropharmacology.
52:827–835. 2007. View Article : Google Scholar
|
|
23
|
Dang Y, Mu Y, Wang K, Xu K, Yang J, Zhu Y
and Luo B: Papaverine inhibits lipopolysaccharide-induced
microglial activation by suppressing NF-κB signaling pathway. Drug
Des Devel Ther. 10:851–859. 2016. View Article : Google Scholar :
|
|
24
|
Luo XL, Liu SY, Wang LJ, Zhang QY, Xu P,
Pan LL and Hu JF: A tetramethoxychalcone from Chloranthus henryi
suppresses lipopolysaccharide-induced inflammatory responses in BV2
microglia. Eur J Pharmacol. 774:135–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He YX, Du M, Shi HL, Huang F, Liu HS, Wu
H, Zhang BB, Dou W, Wu XJ and Wang ZT: Astragalosides from Radix
Astragali benefits experimental autoimmune encephalomyelitis in
C57BL/6 mice at multiple levels. BMC Complement Altern Med.
14:3132014. View Article : Google Scholar
|
|
26
|
Das S and Basu A: Inflammation: a new
candidate in modulating adult neurogenesis. J Neurosci Res.
86:1199–1208. 2008. View Article : Google Scholar
|
|
27
|
Perry VH, Nicoll JA and Holmes C:
Microglia in neurodegenerative disease. Nat Rev Neurol. 6:193–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lull ME and Block ML: Microglial
activation and chronic neurodegeneration. Neurotherapeutics.
7:354–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siddique I and Khan I: Mechanism of
regulation of Na-H exchanger in inflammatory bowel disease: role of
TLR-4 signaling mechanism. Dig Dis Sci. 56:1656–1662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al
Abdulmohsen S, Platanias LC, Al-Kuraya KS and Uddin S: Cross-talk
between NFkB and the PI3-kinase/AKT pathway can be targeted in
primary effusion lymphoma (PEL) cell lines for efficient apoptosis.
PLoS One. 7:e399452012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jung JS, Choi MJ, Lee YY, Moon BI, Park JS
and Kim HS: Suppression of lipopolysaccharide-induced
neuroinflammation by morin via MAPK, PI3K/Akt, and PKA/HO-1
signaling pathway modulation. J Agric Food Chem. 65:373–382. 2017.
View Article : Google Scholar
|
|
32
|
Guo C, Yang L, Wan CX, Xia YZ, Zhang C,
Chen MH, Wang ZD, Li ZR, Li XM, Geng YD and Kong LY:
Anti-neuroinflammatory effect of sophoraflavanone G from Sophora
alopecuroides in LPS-activated BV-2 microglia by MAPK, JAK/STAT and
Nrf2/HO-1 signaling pathways. Phytomedicine. 23:1629–1637. 2016.
View Article : Google Scholar : PubMed/NCBI
|