Introduction
Neuropathic pain is a chronic disorder characterized
by hyperalgesia, allodynia and spontaneous pain affecting the
somatosensory system (1).
Neuropathic pain has emerged as a complicated and refractory
disease affecting an increasing number of people worldwide
(2,3). Unfortunately, there remains a lack
of effective drugs to target and prevent neuropathic pain. Although
conventional drugs, including opioids and non-steroidal
anti-inflammatory drugs, have been used to prevent neuropathic
pain, the therapeutic effect is not fully satisfactory (4). Therefore, it is essential to
investigate the molecular mechanism underlying physiological and
pathological pain status, and to develop novel and effective
therapeutic strategies.
MicroRNA (miRNA), a group of endogenous, small and
non-coding RNA, have emerged as critical regulators of gene
expression (5). miRNA
post-transcriptionally regulate gene expression by binding to the
3′-untranslated region (3′-UTR) of target mRNA, inducing mRNA
instability and degradation (5).
Research has demonstrated that miRNA expression is dysregulated in
various pathological diseases, such as neuropathic pain (6–9).
The targeting of miRNA has indicated promising results in
preventing neuropathic pain development in animal models (10,11). Therefore, an improved
understanding of the role of miRNA in neuropathic pain may help in
developing novel therapeutic approaches for various diseases.
A previous study has demonstrated that the spinal
cord is pivotal in pain perception and modulation (12). Glial activation and neuronal
sensitization in the spinal cord contributes to pain
hypersensitivity (13).
Astrocytes and microglia are activated in the spinal cord dorsal
horn following nerve injury, leading to persistent
neuroinflammation and pain hypersensitivity (14). The release of potent inflammatory
mediators, including tumor necrosis factor-α (TNF-α),
interleukin-1β (IL-1β) and IL-6, contributes to pain
hypersensitivity and persistent pain development (15,16).
High mobility group box 1 (HMGB1) is an ubiquitous
non-histone DNA-binding protein that is a critical regulator of
inflammation (17). Various
studies have indicated that HMGB1 is involved in
inflammation-related diseases, including sepsis (18) and osteoarthritis (19,20). HMGB1 may engage with advanced
glycation end products, Toll-like receptors or integrin to provoke
an inflammatory reaction (21-23). HMGB1 is abundantly expressed in
various cell types, including neurons and glia (24). The injection of HMGB1 into the
sciatic nerve of rats induces neuropathic pain-like behavior
(25). HMGB1 is induced in the
dorsal root ganglion (DRG) following peripheral nerve injury
(26). Inhibition of HMGB1
effectively alleviates neuroinflammation and neuropathic pain
development in animal models (27). Therefore, HMGB-1 has been
suggested as a therapeutic target for the treatment of neuropathic
pain (28,29).
A previous study has suggested that miR-142-3p is a
novel regulator of inflammation that negatively regulates
proinflammatory mediators, including nuclear factor-κB (NF-κB),
TNF-α and IL-6 in macrophages (30). Overexpression of miR-142-3p in
human myeloid inflammatory cells inhibits the production of
inflammatory mediators, including TNF-α and IL-6 (31). A recent study demonstrated that
miR-142-3p inhibits inflammation in chondrocytes of osteoarthritic
conditions (32). These studies
suggest an anti-inflammatory role for miR-142-3p. However, whether
miR-142-3p participates in the neuroinflammation of neuropathic
pain remains unknown.
The present study demonstrated that miR-142-3p was
reduced in the DRG of mice following spinal nerve ligation (SNL)
injury. Overexpression of miR-142-3p inhibited neuropathic pain and
neuroinflammation. HMGB1 was identified as a target gene of
miR-142-3p. Overexpression of miR-142-3p inhibited HMGB1 expression
in vitro and in vivo, while an inverse correlation
was observed between HMGB1 mRNA and miR-142-3p expression in
vivo. Additionally, overexpression of HMGB1 significantly
reversed the protective effects of miR-142-3p. Taken together,
these results suggest that miR-142-3p inhibits neuropathic pain
development through downregulation of HMGB1-mediated
proinflammation, which may serve as a potential therapeutic target
for the treatment of neuropathic pain.
Materials and methods
Animals
A total of 90 adult male 8-week-old ICR mice
weighing 20–25 g were purchased from the Laboratory Animal Center
of Nanjing Medical University (Nanjing, China). The mice were
housed in a 12-h light/dark cycle at a room temperature of 22±1°C
and humidity of 50-60% with ad libitum access to water and
food. All animal experimental procedures were performed in
accordance with the guidelines of the International Association for
the Study of Pain and the National Institute of Health Guide for
the Care and Use of Laboratory Animals (NIH Publications no. 80-23,
revised 1996). The present study was reviewed and approved by the
local Institutional Animal Care and Use Committee of Nanjing
Medical University.
Cell culture
The DRG was cultured as previously described
(33). Briefly, the bilateral L3
and L4 DRG were rapidly dissected from mice (n=3) and digested with
1 mg/ml trypsin and 2 mg/ml collagenase (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). After
mechanical dissociation into single cells using a Pasteur pipette,
the DRG neurons were seeded into 6-well plates at a density of
5×105 cells/well in DMEM for 24 h at 37°C with 5%
CO2. The seeded cells were treated with 5 µg/ml
cytarabine (Sigma-Aldrich; Merck KGaA) to repress the growth of
non-neuronal cells. Following this, the cells were grown in
DMEM/F-12 supplemented with 10% fetal bovine serum (FBS) (both from
Gibco; Thermo Fisher Scientific, Inc.), 10 ng/ml nerve growth
factor (Invitrogen; Thermo Fisher Scientific, Inc.), 0.1 mg/ml
L-glutamine and 1% penicillin/streptomycin mix (Sigma-Aldrich;
Merck KGaA). The medium was refreshed twice a week. 293T cells were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultured in DMEM supplemented with 10% FBS
and 1% penicillin/streptomycin mix. All cells were cultured in a
humidified atmosphere of 5% CO2 at 37°C.
SNL model of neuropathic pain
Mice (n=45) were anesthetized by peritoneal
injection of 50 mg/kg sodium pentobarbital (Merck KGaA). Following
incision of the skin, the paraspinal muscles were bluntly
dissected. The L5 transverse process was removed to expose the L3
and L4 spinal nerves. The exposed L4 spinal nerve was tightly
ligated with a 6-0 silk suture and transected distal to the
ligation. In sham mice (n=15), the L5 transverse process was
exposed, and the L4 spinal nerve was not touched and ligated. SNL
mice were divided into two groups: SNL + lentiviral vector
(LV)-negative control (NC) (n=15) and SNL + LV-miR-142-3p
(n=15).
Intrathecal catheter implantation and
injection
Following anesthesia, the occipital muscles of mice
were separated to expose the cisternal membrane. A polyethylene
catheter (PE-10; Braintree Scientific, Inc., Braintree, MA, USA)
was inserted through an incision in the cisterna magna. The proper
location of the intrathecal implantation was validated by bilateral
hind limb paralysis with injection of 2% lidocaine (1 µl)
(Sigma- Aldrich; Merck KGaA). Animals that failed to display
paralysis by lidocaine were not included in the experiments.
Intrathecal delivery of recombinant LV-miR-142-3p [8×105
transduction units (TU); 1 µl)] or LV-NC (8×105
TU; 1 µl) or HMGB1 small interfering RNA (siRNA) (50
µg, 1 µl) or NC siRNA (50 µg, 1 µl)
(Shanghai GenePharma Co., Ltd., Shanghai, China) was performed by a
microinjection syringe (10 µl) linked with the intrathecal
catheter. The sequence of the HMGB1 siRNA was
5′-CUCGUUAUGAAAGAGAAAUTT-3′ and of the NC siRNA was
5′-UUCUCCGAACGUGUCACGUTT-3′.
Assessment of thermal hyperalgesia
Thermal hyperalgesia was assessed by measuring the
paw withdrawal latencies in response to radiant heat stimulation
using a Plantar Analgesia Meter (IITC Life Science, Inc., Woodland
Hills, CA, USA). The mouse was placed in a plastic chamber with a
glass floor upon a radiant heat source. After acclimatization to
the environment for 1 h, the radiant heat source was launched and
the duration between the start and paw withdrawal was recorded by a
digital timer. A cut-off time was set at 20 sec to avoid tissue
damage and the measurement was repeated five times for each mouse.
A thermal stimulus was delivered at 5-min intervals.
Assessment of mechanical allodynia
Mechanical allodynia was assessed by measuring the
paw withdrawal threshold (PWT) in response to Von Frey hair (IITC
Life Science, Inc.) stimulation. The mouse was placed in a plastic
cage with a plexiglass floor. After acclimatization to the
environment for 1 h, the mouse was subjected to an ascending series
of Von Frey hairs. Brisk withdrawal or paw flinching upon stimulus
was defined as a positive response. The Von Frey hairs were
maintained for 5–6 sec at 5-min intervals and the measurement was
repeated five times for each mouse.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from DRG was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
standard protocols. For detection of miRNA expression, qPCR was
performed using a TaqMan miRNA assay kit (Ambion; Thermo Fisher
Scientific, Inc.) and U6 served as the endogenous control. For
detection of mRNA expression, cDNA was synthesized by M-MLV reverse
transcriptase (BioTeke Corp., Beijing, China) and qPCR
amplification was performed using a SYBR-Green system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
used in the present study were as follows: miR-142-3p, forward,
5′-TGCGGTGTAGTGTTTCCTACTT-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′; HMGB1 forward,
5′-GCTGACAAGGCTCGTTATGAA-3′ and reverse,
5′-CCTTTGATTTTGGGGCGGTA-3′; U6 forward,
5′-TTGGTCTGATCTGGCACATATAC-3′ and reverse,
5′-AAAAATATGGAGCGCTTCACG-3′; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) forward, 5′-AGGTCGGTGTGAACGGATTTG-3′ and
reverse, 5′-GGGGTCGTTGATGGCA ACA-3′. The thermal cycling conditions
were set as 95°C for 3 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 30 sec. GAPDH served as the endogenous control.
Relative gene expression was determined using the 2−ΔΔCq
method (34).
Enzyme-linked immunosorbent assay
(ELISA)
Levels of TNF-α, IL-1β, IL-6 and IL-10 in the L3-L5
DRG were determined using commercial ELISA kits, including TNF-α
Quantikine ELISA kit (cat. no. MTA00B), IL-1β Quantikine ELISA kit
(cat. no. MLB00C), IL-6 Quantikine ELISA kit (cat. no. M6000B) and
IL-10 Quantikine ELISA kit (cat. no. M1000B), purchased from
R&D Systems, Inc. (Minneapolis, MN, USA) according to the
manufacturer's instructions.
Dual-luciferase reporter assay
miRNA targets were predicted using the algorithms of
TargetScan (targetscan.org). A fragment of the HMGB1
3′-UTR containing either the predicted seed-matched or mutant
sequences of miR-142-3p was inserted into pmirGLO Dual-Luciferase
miRNA Target Expression Vector (Promega Corp., Madison, WI, USA).
293T cells were co-transfected with pmirGLO vector with miR-142-5p
mimics (Shanghai GenePharma Co., Ltd.) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated for 48
h. Relative luciferase activity was detected by a dual-lucif-erase
reporter system (Promega Corp.). Relative luciferase activity was
calculated by the formula: Firefly luciferase
activity/Renilla luciferase activity.
Western blot analysis
Proteins were exacted from DRG using
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA).
Protein concentrations were measured using a bicinchoninic acid kit
(Beyotime Institute of Biotechnology, Haimen, China). Samples of 50
µg total protein were separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were then blocked by 5% non-fat
dry milk for 1 h at 37°C, and subsequently incubated overnight at
4°C with primary antibodies, including rabbit monoclonal anti-HMGB1
(cat. no. ab79823; 1:10,000), rabbit monoclonal anti-phosphorylated
(p)-p65 (cat. no. ab86299; 1:2,000) and rabbit polyclonal
anti-GAPDH (cat. no. ab9485; 1:2,500) (Abcam, Cambridge, UK).
Following incubation with horseradish peroxidase-conjugated
secondary antibody (goat anti-rabbit immunoglobulin G; cat. no.
ab6721; 1:2,000; Abcam) for 2 h at room temperature, the protein
bands were detected by enhanced chemiluminescence reagents (Pierce;
Thermo Fisher Scientific, Inc.). The protein band intensities were
quantified with densitometry using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical analyses were performed using the Student's
t-test or one-way analysis of variance followed by the Bonferroni
post hoc test using SPSS 18.0 software (SPSS, Inc., Chicago, IL,
USA). The correlation between miR-142-3p expression and HMGB1 mRNA
expression was determined using Spearman's rank correlation test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
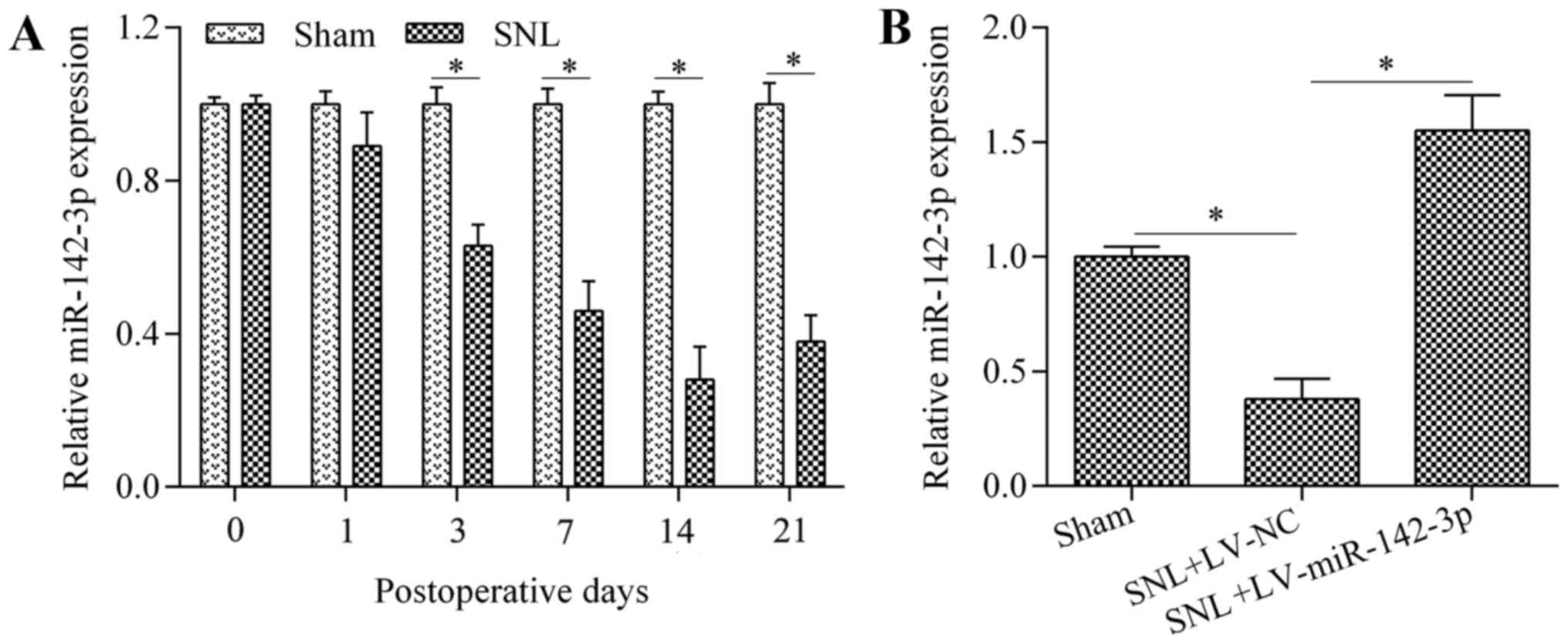
miR-142-3p is downregulated in the DRG of
mice with SNL
To investigate the potential role of miR-142-3p in
neuropathic pain, its expression status was characterized in the
DRG of mice with SNL using RT-qPCR. The results demonstrated that
miR-142-3p was significantly downregulated in mice with SNL
compared with the levels in the sham group by postoperative days 3,
7, 14 and 21 (Fig. 1A). The
results indicate that downregulation of miR-142-3p may be an
important event in the development and maintenance of neuropathic
pain.
Overexpression of miR-142-3p relieves
neuropathic pain in mice with SNL
To investigate the precise function of miR-142-3p in
regulating neuropathic pain, gain-of-function experiments were
performed by intrathecal injection of LV-miR-142-3p. Infection with
LV-miR-142-3p significantly upregulated miR-142-3p expression level
in the DRG of mice with SNL compared with the level in the SNL +
LV-NC group (Fig. 1B). The effect
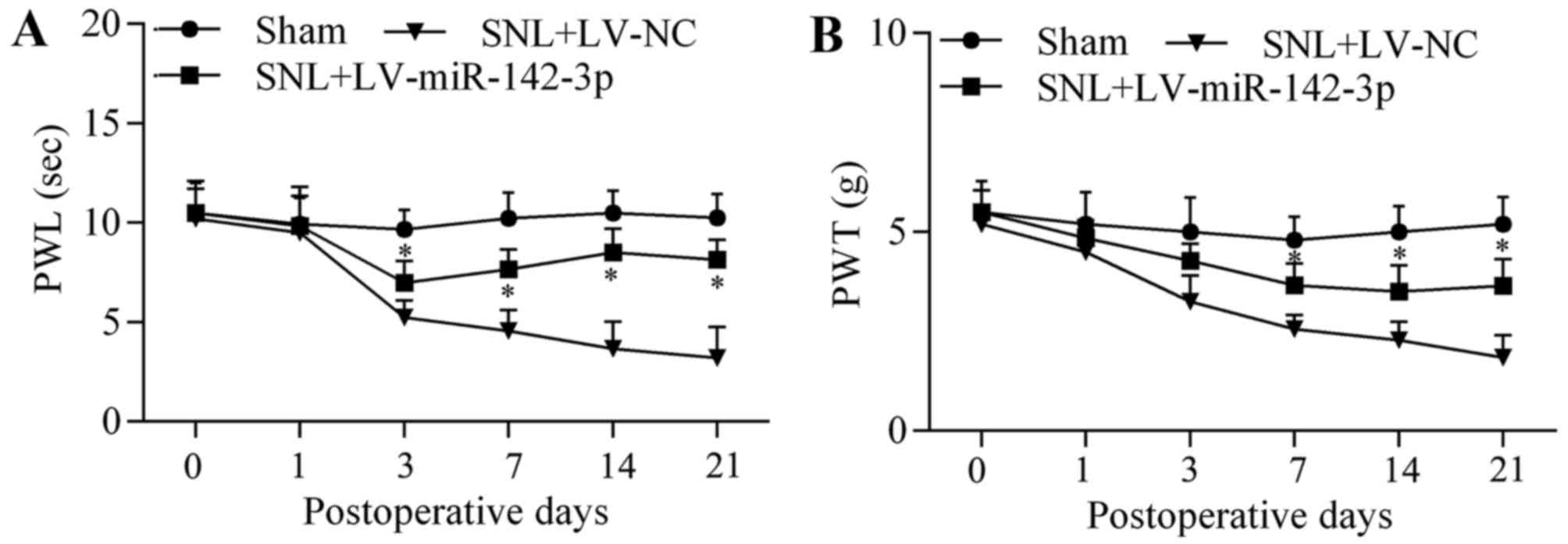
of miR-142-3p overexpression on neuropathic pain development was
investigated by assessment of thermal hyperalgesia and mechanical
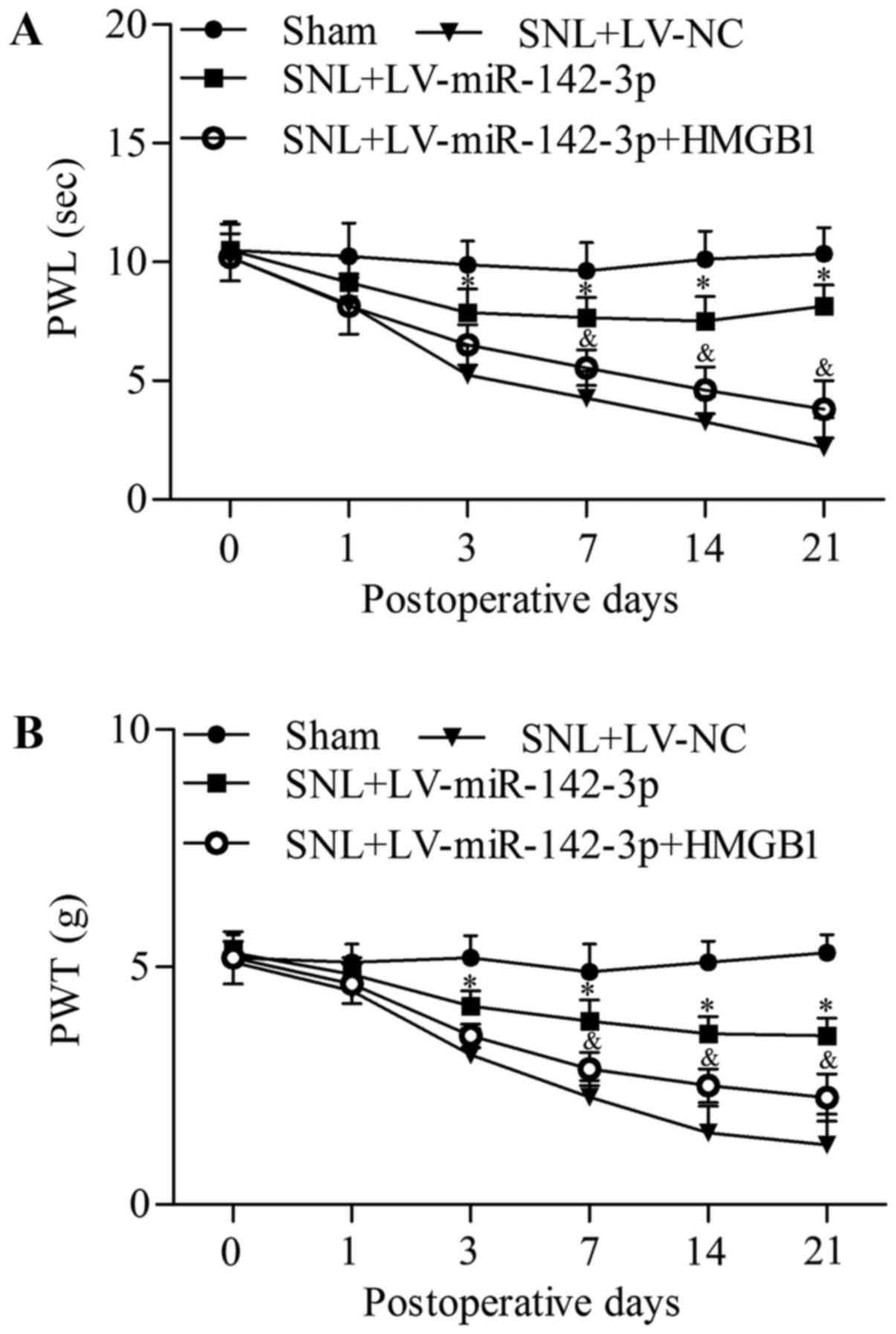
allodynia. The results demonstrated that overexpression of
miR-142-3p significantly increased paw withdrawallatency (PWL)
(Fig. 2A) and PWT (Fig. 2B) in LV-miR-142-3p-infected SNL
mice compared with that in LV-NC-infected SNL mice at postoperative
days 3, 7, 14 and 21, indicating that overexpression of miR-142-3p
relieved thermal hyperalgesia and mechanical allodynia. These
results suggest that miR-142-3p functions as a negative regulator
of neuropathic pain.
Overexpression of miR-142-3p suppresses
neuroinflammation in mice with SNL
To further explore the biological function of
miR-142-3p in regulating neuropathic pain, the effect of miR-142-3p
overexpression on neuroinflammation was investigated by measuring
proinflammatory cytokines (TNF-α, IL-1β and IL-6) and the IL-10
anti-inflammatory cytokine. The results indicated that miR-142-3p
overexpression in mice with SNL was associated with a significant
reduction in the expression levels of TNF-α (Fig. 3A), IL-1β (Fig. 3B) and IL-6 (Fig. 3C), and an increase in the
expression level of IL-10 (Fig.
3D) compared with the levels in the SNL + LV-NC group. These
data suggest an inhibitory role of miR-142-3p on neuroinflammation
in mice with SNL.
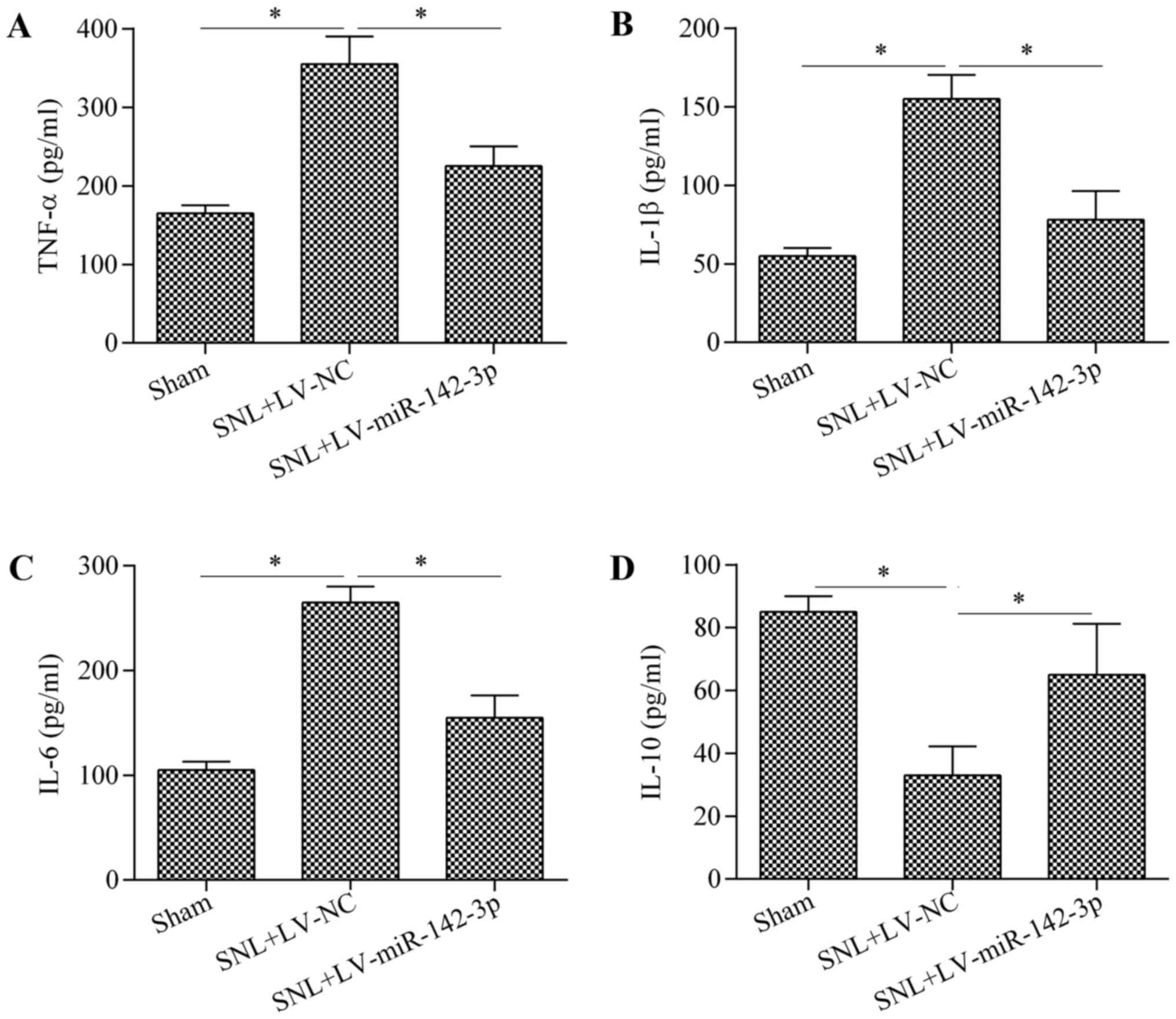 | Figure 3Overexpression of miR-142-3p
suppresses neuroinflammation in mice with SNL. Protein expression
levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-10 in the L3-L5
DRG of mice were determined by ELISA on postoperative day 14. n=3.
*P<0.05 as indicated. miR, microRNA; SNL, spinal
nerve ligation; TNF, tumor necrosis factor; IL, interleukin; DRG,
dorsal root ganglion; NC, negative control; LV, lentiviral vector;
ELISA, enzyme-linked immunosorbent assay. |
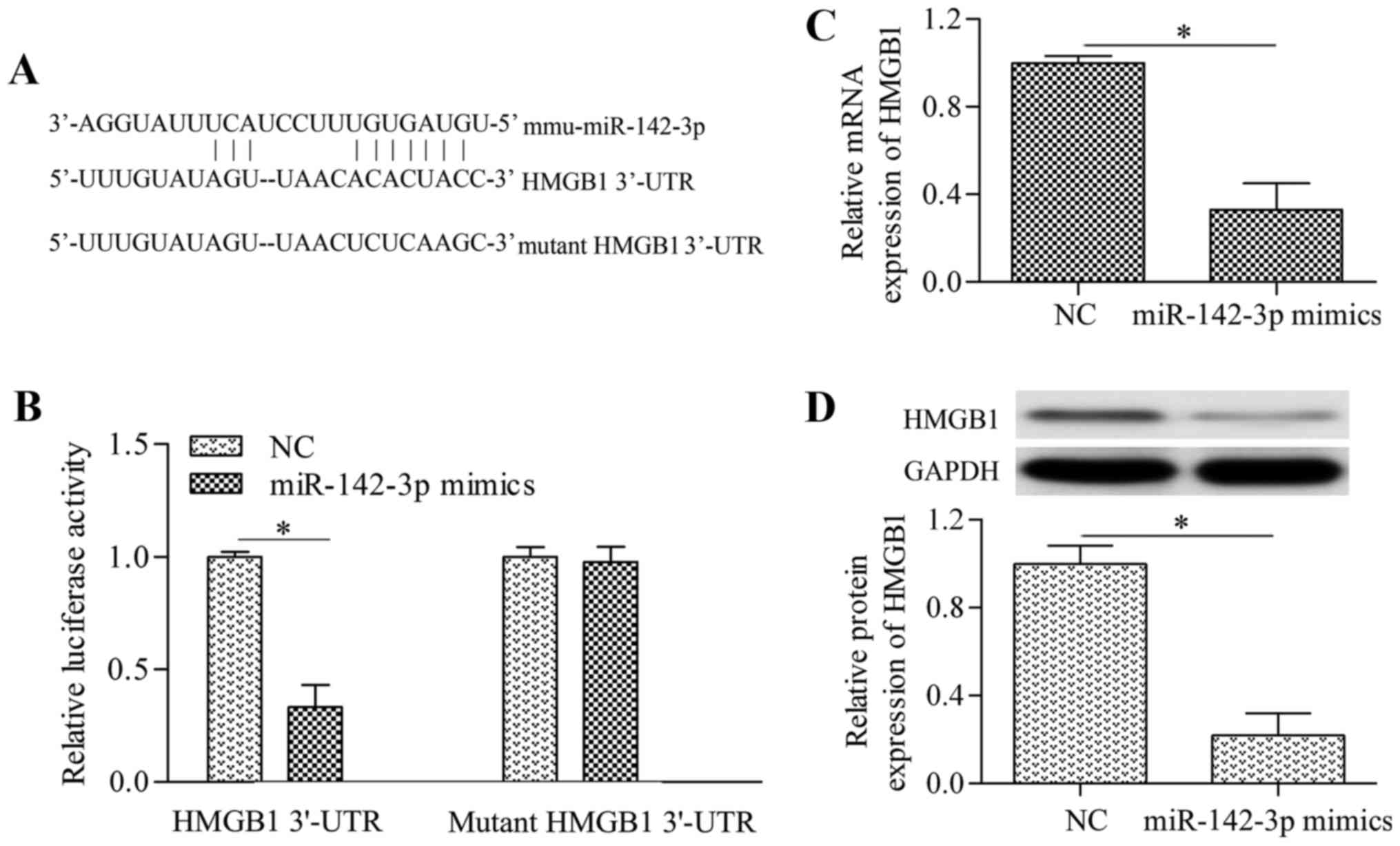
HMGB1 is a direct target gene of
miR-142-3p
To investigate the molecular mechanism underlying
miR-142-3p-induced inhibitory effect on neuropathic pain and
neuroinflammation, predicted targets of miR-142-3p were sought by
established miRNA target prediction programs. Notably, it was
revealed that HMGB1, a critical gene of neuropathic pain and
inflammation (26), was a
predicted target gene of miR-142-3p (Fig. 4A). This prediction was confirmed
by dual-luciferase reporter assays. The putative seed-matched or
mutant sequences of miR-142-3p for the HMGB1 3′-UTR were cloned
into the luciferase reporter vector. Overexpression of miR-142-3p
significantly decreased the luciferase activity of the reporter
vector containing the 3′-UTR of HMGB1 compared with the activity in
the NC group (Fig. 4B). However,
miR-142-3p overexpression demonstrated no significant effect on the
reporter vector containing the mutant HMGB1 3′-UTR compared with
the NC group (Fig. 4B).
Furthermore, the effect of miR-142-3p overexpression on HMGB1
expression was investigated in cultured DRG neurons. The results
indicated that miR-142-3p overexpression significantly decreased
the mRNA (Fig. 4C) and protein
(Fig. 4D) expression levels of
HMGB1 in DRG neurons compared with the levels in the NC group.
Overall, these results suggest that HMGB1 is a direct target gene
of miR-142-3p.
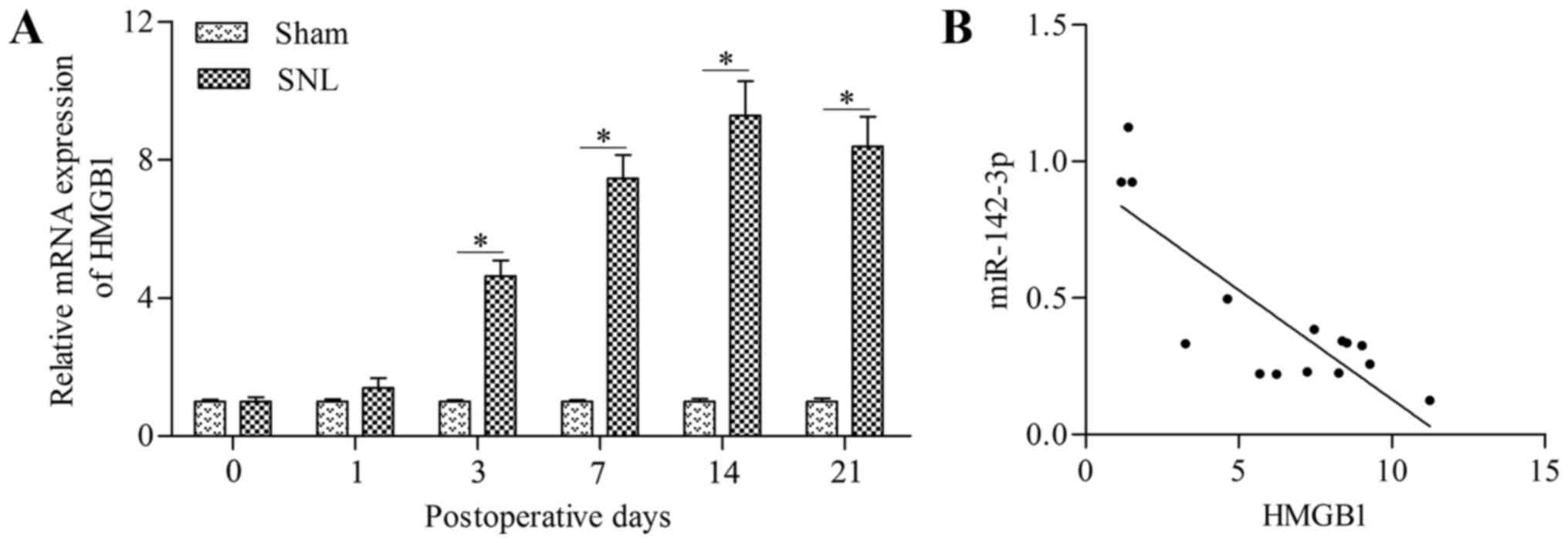
HMGB1 is negatively correlated with
miR-142-3p in mice with SNL
To further confirm the targeted relationship between
miR-142-3p and HMGB1, the correlation between miR-142-3p and HMGB1
in mice with SNL was analyzed. The results demonstrated that the
HMGB1 mRNA expression level was significantly upregulated in the
DRG of mice with SNL at postoperative days 3, 7, 14 and 21 compared
with the levels in the sham group (Fig. 5A). Furthermore, HMGB1 demonstrated
an inverse correlation with miR-142-3p expression (Fig. 5B). These data suggest an inverse
relationship between miR-142-3p and HMGB1 mRNA expression in mice
with SNL.
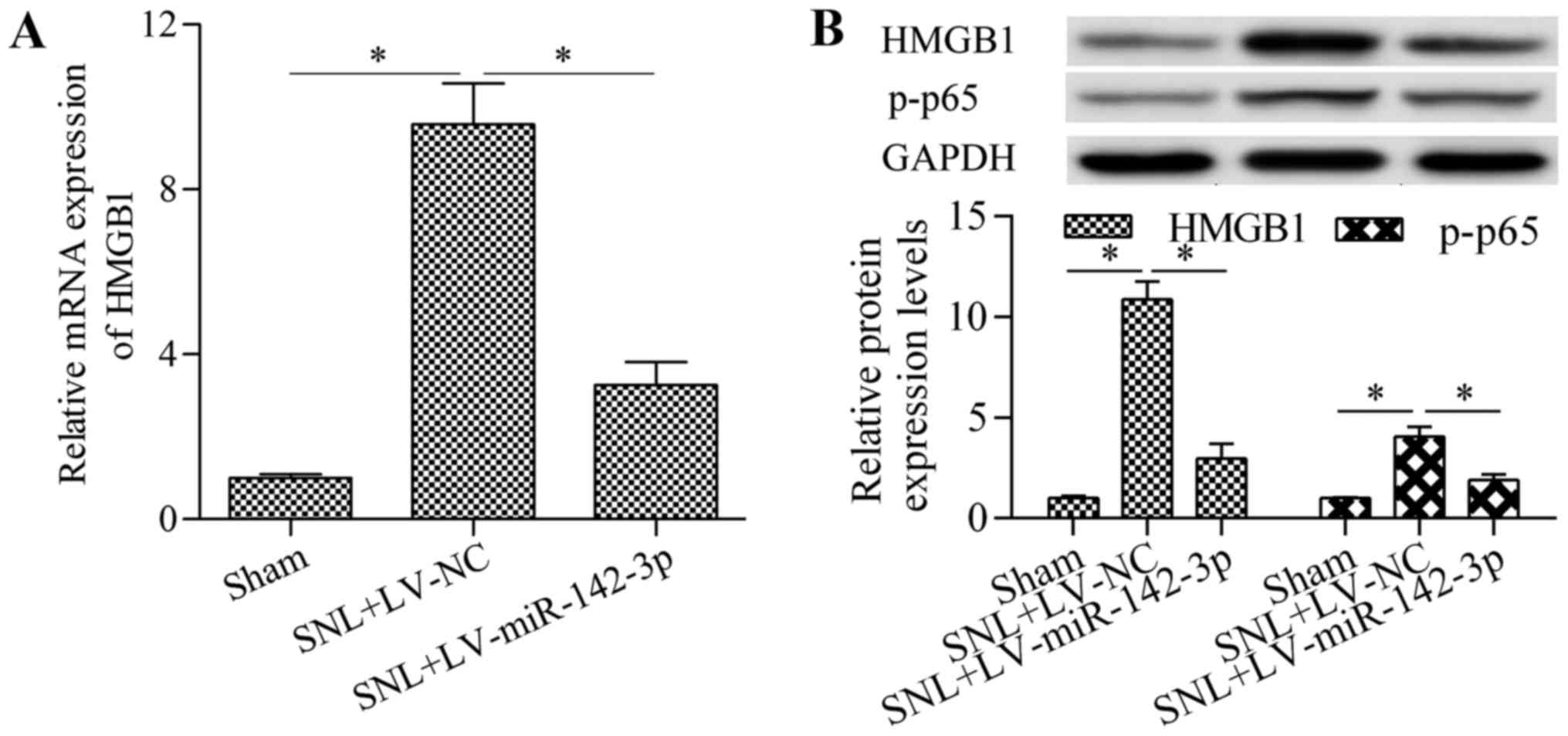
Overexpression of miR-142-3p inhibits
HMGB1 expression in mice with SNL
To investigate whether miR-142-3p regulates HMGB1
expression in vivo, the present study detected the effect of
miR-142-3p overexpression on HMGB1 expression in mice with SNL. The
results demonstrated that overexpression of miR-142-3p
significantly inhibited HMGB1 mRNA (Fig. 6A) and protein (Fig. 6B) expression levels in the DRG of
mice with SNL compared with the levels in the SNL + LV-NC group.
Furthermore, the levels of p-NF-κB p65 protein in mice with SNL
were also significantly reduced by miR-142-3p overexpression
compared with the levels in the SNL + LV-NC group (Fig. 6B). To confirm whether HMGB1 was
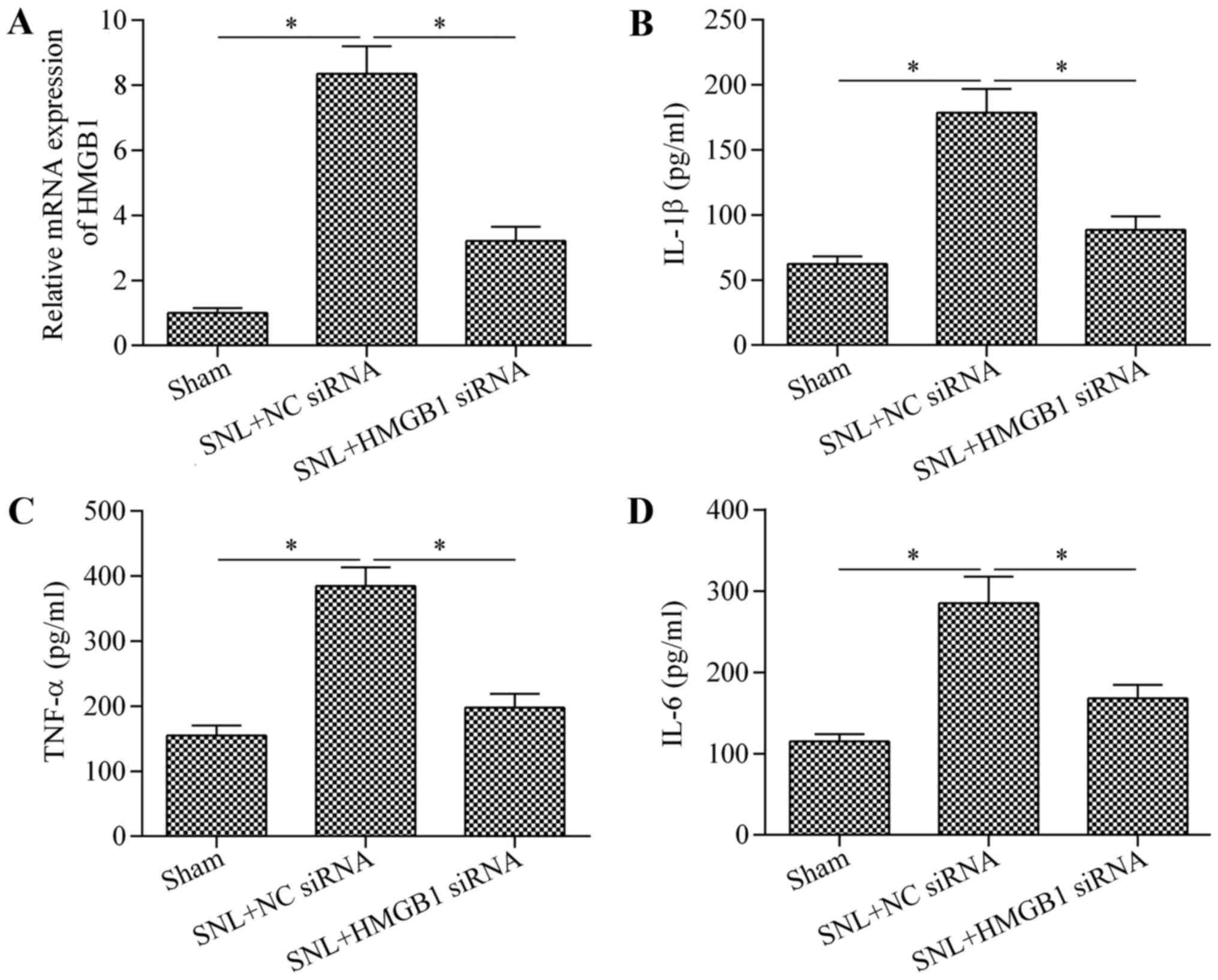
involved in regulating neuroinflammation in mice with SNL, HMGB1
expression was inhibited by transfection of HMGB1 siRNA. The
results demonstrated that treatment with HMGB1 siRNA significantly
reduced the expression of HMGB1 compared with the levels in the SNL
+ NC siRNA group (Fig. 7A).
Additionally, the expression levels of IL-1β (Fig. 7B), TNF-α (Fig. 7C) and IL-6 (Fig. 7D) were significantly downregulated
by HMGB1 siRNA compared with the levels in the SNL + NC siRNA
group. Overall, these data suggest that miR-142-3p regulates
neuroinflammation in mice with SNL through the
HMGB1/NF-κB/inflammatory cytokine production pathway.
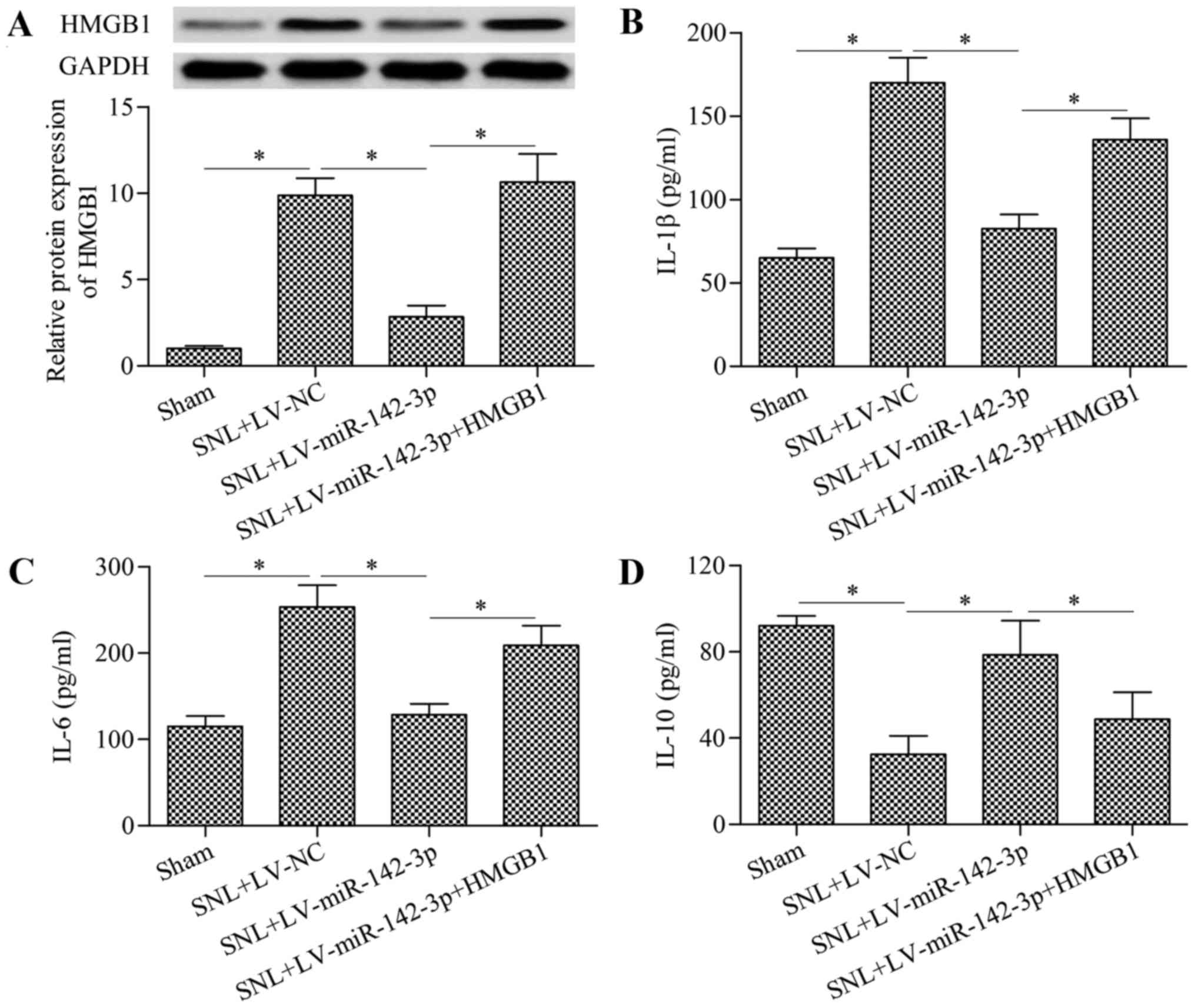
Overexpression of HMGB1 reverses the
inhibitory effect of miR-142-3p on neuropathic pain and
neuroinflammation
To further investigate whether miR-142-3p alleviates
neuropathic pain through targeting HMGB1, rescue experiments were
performed. Mice were infected with LV-miR-142-3p and LV-HMGB1.
Results demonstrated that infection with LV-HMGB1 significantly
restored the decreased HMGB1 expression in LV-miR-142-3p-infected
mice with SNL compared with the level in the SNL + LV-miR-142-3p
group (Fig. 8A). As expected, the
regulatory effects of miR-142-3p overexpression on IL-1β (Fig. 8B), IL-6 (Fig. 8C) and IL-10 (Fig. 8D) were significantly reversed by
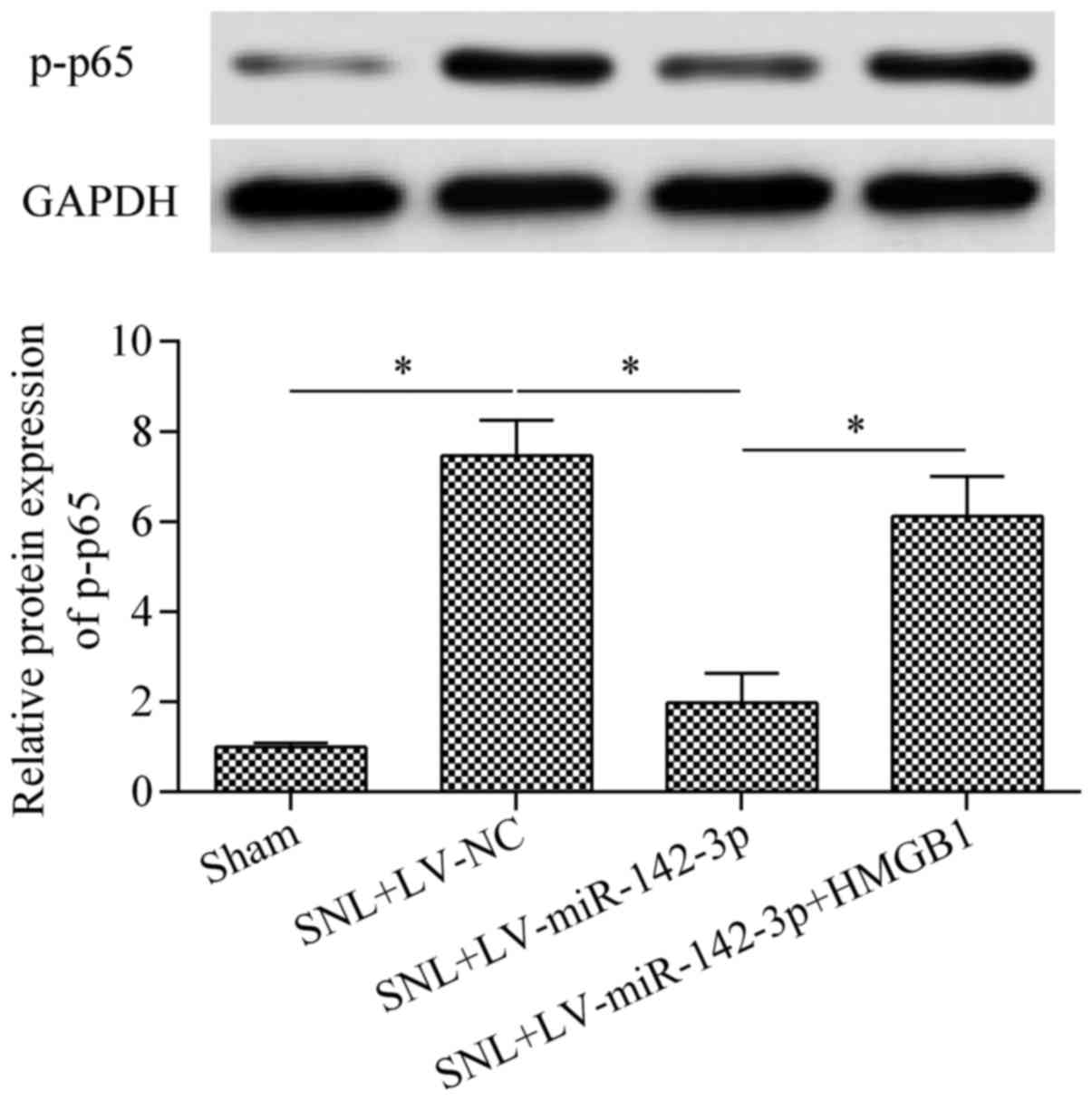
HMGB1 overexpression. The decreased phosphorylation of NF-κB p65
protein in mice with SNL were also significantly restored by HMGB1
overexpression compared with the level in the SNL + LV-miR-142-3p
group (Fig. 9). Furthermore, the
inhibitory effect of miR-142-3p overexpression on neuropathic pain
was also significantly reversed by HMGB1 overexpression at
postoperative days 3, 7,14 and 21 in SNL + LV-miR-142-3p + HMGB1
group as compared with the SNL + LV-miR-142-3p group (Fig. 10). Overall, these results suggest
that miR-142-3p inhibits neuropathic pain and neuroinflammation by
down-regulation of HMGB1.
Discussion
In recent years, miRNA have emerged as critical
regulators of persistent neuropathic pain initiation and
development (35). There is
evidence that miRNA-based therapy may be a promising method for
preventing neuropathic pain (36,37). In the present study, the results
demonstrated that miR-142-3p functions as a novel miRNA involved in
neuropathic pain. The present findings indicated that miR-142-3p
negatively regulated neuropathic pain through targeting
HMGB1-mediated neuroinflammation. These findings suggest that
miR-142-3p may serve as a novel and promising molecular target for
the development of anti-neuropathic pain therapies.
There is growing evidence that miRNA serve an
important role in the pathogenesis of neuropathic pain progression
(6,7,38).
A study by Shi et al (39)
reported that miR-195 induced by SNL aggravated neuropathic pain by
inhibiting Atg14. Suppression of miR-155, miR-19a or miR-221
inhibited neuropathic pain through targeting suppressor of cytokine
signaling 1 (11,40,41). Intrathecal injection of miR-96 and
miR-183 alleviated neuropathic pain by downregulation of sodium
channel Nav1.3 expression (42,43). Inhibiting sodium channel Nav1.7 by
miR-30b inhibited neuropathic pain in nerve injury-induced
neuropathic pain in a rat model (44). Reduced miR-203 expression
contributed to neuropathic pain through targeting Rap1a (10). Overexpression of miR-146a-5p
alleviated neuropathic pain via suppressing TNF receptor-associated
factor-6-mediated neuroinflammation (45). Furthermore, recent studies have
demonstrated that miR-144, miR-132-3p, miR-124a and miR-15b are
also involved in the regulation of neuropathic pain development
(46–49). Together, these results suggest
that miRNA are critical regulators of neuropathic pain and
represent novel and promising targets for the treatment of
neuropathic pain. The present study demonstrated that miR-142-3p is
a novel miRNA involved in neuropathic pain development.
NF-κB is a group of transcription factors that
regulate various genes involved in inflammation, apoptosis,
angiogenesis and cancer metastasis (50,51). Research has indicated that NF-κB
is involved in the pathogenesis of neuropathic pain through
regulating pro-inflammatory cytokine gene expression (52). Inhibition of NF-κB p65 protein
expression significantly attenuates chronic constriction
injury-induced mechanical allodynia and thermal hyperalgesia
(53). Specific participants in
NF-κB signaling transduction have been suggested as molecular
targets for treatment of neuropathic pain (54). In the present study, it was
demonstrated that miR-142-3p inhibited the activation of NF-κB and
expression of pro-inflammatory cytokines, suggesting that
miR-142-3p may be a potential modulator of NF-κB signaling
transduction.
miR-142-3p has been suggested as an
inflammation-related miRNA that is involved in various inflammatory
diseases, including osteoarthritis (32), ulcerative colitis (55), dermatitis (56), psoriasis (57), keratitis (58) and periodontitis (59). miR-142-3p has been shown to
negatively regulate inflammation by suppressing proinflammatory
mediators, including NF-κB, TNF-α and IL-6, in macrophages
(30). Furthermore, miR-142-3p
inhibits the production of inflammatory mediators, including TNF-α
and IL-6, in human myeloid inflammatory cells (31), suggesting an anti-inflammatory
role of miR-142-3p. The present study demonstrated that miR-142-3p
inhibited the production of TNF-α, IL-1β and IL-6, supporting an
anti-inflammatory role for miR-142-3p. Recent studies have reported
that miR-142-3p inhibited inflammation by targeting HMGB1 (32,60). A study by Yuan et al
(60) reported that peroxisome
proliferator-activated receptor γ inhibited inflammation associated
with the upregulation of miR-142-3p, which targeted and inhibited
HMGB1. A study by Wang et al (32) reported that miR-142-3p suppressed
NF-κB, TNF-α and IL-6 in a murine model of osteoarthritis by
targeting HMGB1. Additionally, miR-142-3p targets HMGB1 to inhibit
hypoxia/reoxygenation-induced cardiomyocyte apoptosis and fibrosis
(61). miR-142-3p is also
reported to inhibit tumor growth of lung cancer through targeting
HMGB1 (62). The present results
are consistent with these findings by identifying HMGB1 as a
functional target gene of miR-142-3p.
There is increasing evidence that HMGB1 is an
important regulator of neuropathic pain (25,26,63,64), and HMGB-1 has been suggested as a
promising therapeutic target for the treatment of neuropathic pain
(28,29). Inhibition of HMGB1 by antibody
neutralization effectively improved pain-related behaviors in
animal models of neuropathic pain (27,65). Administration of melatonin,
Tanshinone IIA and IL-10 attenuated neuropathic pain development by
downregulating HMGB1 (66–69).
Furthermore, a recent study indicated that miR-141 inhibits
neuropathic pain development by targeting HMGB1 (46), suggesting that targeting HMGB1
using miRNA may serve as a novel therapeutic strategy for the
treatment of neuropathic pain. The present study demonstrated that
miR-142-3p targeted the 3′-UTR of HMGB1 to regulate HMGB1
expression, providing a novel therapeutic target for the
development of miRNA/HMGB1-based therapy for neuropathic pain.
In conclusion, the results of the present study
demonstrate that miR-142-3p alleviates neuropathic pain through the
downregulation of HMGB1-mediated neuroinflammation. The decreased
expression of miR-142-3p leads to the upregulation of HMGB1, which
contributes to the development and progression of neuropathic pain.
The present study provides novel insight into understanding the
molecular pathogenesis of neuropathic pain. Inhibition of HMGB1 by
miR-142-3p may serve as a novel therapeutic strategy for preventing
neuropathic pain.
Abbreviations:
|
HMGB1
|
high mobility group box 1
|
|
miRNA
|
microRNA
|
|
NF-κB
|
nuclear factor-κB
|
|
SNL
|
spinal nerve ligation
|
|
DRG
|
dorsal root ganglion
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
UTR
|
untranslated region
|
References
|
1
|
Baron R: Peripheral neuropathic pain: From
mechanisms to symptoms. Clin J Pain. 16(Suppl 2): S12–S20. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorge RE, Trang T, Dorfman R, Smith SB,
Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan
M, et al: Genetically determined P2X7 receptor pore formation
regulates variability in chronic pain sensitivity. Nat Med.
18:595–599. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neville A, Peleg R, Singer Y, Sherf M and
Shvartzman P: Chronic pain: A population-based study. Isr Med Assoc
J. 10:676–680. 2008.PubMed/NCBI
|
|
4
|
O'Connor AB and Dworkin RH: Treatment of
neuropathic pain: An overview of recent guidelines. Am J Med.
122(Suppl 10): S22–S32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
von Schack D, Agostino MJ, Murray BS, Li
Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, et al:
Dynamic changes in the microRNA expression profile reveal multiple
regulatory mechanisms in the spinal nerve ligation model of
neuropathic pain. PLoS One. 6:e176702011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aldrich BT, Frakes EP, Kasuya J, Hammond
DL and Kitamoto T: Changes in expression of sensory organ-specific
microRNAs in rat dorsal root ganglia in association with mechanical
hypersensitivity induced by spinal nerve ligation. Neuroscience.
164:711–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakai A and Suzuki H: Emerging roles of
microRNAs in chronic pain. Neurochem Int. 77:58–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang HL, Wang HC, Chunag YT, Chou CW, Lin
IL, Lai CS, Chang LL and Cheng KI: miRNA expression change in
dorsal root ganglia after peripheral nerve injury. J Mol Neurosci.
61:169–177. 2017. View Article : Google Scholar
|
|
10
|
Li H, Huang Y, Ma C, Yu X, Zhang Z and
Shen L: MiR-203 involves in neuropathic pain development and
represses Rap1a expression in nerve growth factor differentiated
neuronal PC12 cells. Clin J Pain. 31:36–43. 2015. View Article : Google Scholar
|
|
11
|
Tan Y, Yang J, Xiang K, Tan Q and Guo Q:
Suppression of microRNA-155 attenuates neuropathic pain by
regulating SOCS1 signalling pathway. Neurochem Res. 40:550–560.
2015. View Article : Google Scholar
|
|
12
|
Moss A, Beggs S, Vega-Avelaira D, Costigan
M, Hathway GJ, Salter MW and Fitzgerald M: Spinal microglia and
neuropathic pain in young rats. Pain. 128:215–224. 2007. View Article : Google Scholar
|
|
13
|
Miljanich G, Rauck R and Saulino M: Spinal
mechanisms of pain and analgesia. Pain Pract. 13:114–130. 2013.
View Article : Google Scholar
|
|
14
|
Scholz J and Woolf CJ: The neuropathic
pain triad: Neurons, immune cells and glia. Nat Neurosci.
10:1361–1368. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moalem G and Tracey DJ: Immune and
inflammatory mechanisms in neuropathic pain. Brain Res Brain Res
Rev. 51:240–264. 2006. View Article : Google Scholar
|
|
16
|
Shen W, Hu XM, Liu YN, Han Y, Chen LP,
Wang CC and Song C: CXCL12 in astrocytes contributes to bone cancer
pain through CXCR4-mediated neuronal sensitization and glial
activation in rat spinal cord. J Neuroinflammation. 11:752014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu B, Wang C, Wang M, Li W, Chen F, Tracey
KJ and Wang H: Molecular mechanism and therapeutic modulation of
high mobility group box 1 release and action: An updated review.
Expert Rev Clin Immunol. 10:713–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andersson U and Tracey KJ: HMGB1 in
sepsis. Scand J Infect Dis. 35:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li ZC, Cheng GQ, Hu KZ, Li MQ, Zang WP,
Dong YQ, Wang WL and Liu ZD: Correlation of synovial fluid HMGB-1
levels with radiographic severity of knee osteoarthritis. Clin
Invest Med. 34:E2982011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mantell LL, Parrish WR and Ulloa L: Hmgb-1
as a therapeutic target for infectious and inflammatory disorders.
Shock. 25:4–11. 2006. View Article : Google Scholar
|
|
21
|
Basta G: Receptor for advanced glycation
endproducts and atherosclerosis: From basic mechanisms to clinical
implications. Atherosclerosis. 196:9–21. 2008. View Article : Google Scholar
|
|
22
|
den Dekker WK, Cheng C, Pasterkamp G and
Duckers HJ: Toll-like receptor 4 in atherosclerosis and plaque
destabilization. Atherosclerosis. 209:314–320. 2010. View Article : Google Scholar
|
|
23
|
Friggeri A, Yang Y, Banerjee S, Park YJ,
Liu G and Abraham E: HMGB1 inhibits macrophage activity in
efferocytosis through binding to the alphavbeta3-integrin. Am J
Physiol Cell Physiol. 299:C1267–C1276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersson U, Erlandsson-Harris H, Yang H
and Tracey KJ: HMGB1 as a DNA-binding cytokine. J Leukoc Biol.
72:1084–1091. 2002.PubMed/NCBI
|
|
25
|
Chacur M, Milligan ED, Gazda LS, Armstrong
C, Wang H, Tracey KJ, Maier SF and Watkins LR: A new model of
sciatic inflammatory neuritis (SIN): Induction of unilateral and
bilateral mechanical allodynia following acute unilateral
peri-sciatic immune activation in rats. Pain. 94:231–244. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibasaki M, Sasaki M, Miura M, Mizukoshi
K, Ueno H, Hashimoto S, Tanaka Y and Amaya F: Induction of high
mobility group box-1 in dorsal root ganglion contributes to pain
hypersensitivity after peripheral nerve injury. Pain. 149:514–521.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Otoshi K, Kikuchi S, Kato K, Sekiguchi M
and Konno S: Anti-HMGB1 neutralization antibody improves
pain-related behavior induced by application of autologous nucleus
pulposus onto nerve roots in rats. Spine. 36:E692–E698. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maeda T, Ozaki M, Kobayashi Y, Kiguchi N
and Kishioka S: HMGB1 as a potential therapeutic target for
neuropathic pain. J Pharmacol Sci. 123:301–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wan W, Cao L, Khanabdali R, Kalionis B,
Tai X and Xia S: The emerging role of HMGB1 in neuropathic pain: A
potential therapeutic target for neuroinflammation. J Immunol Res.
2016:64304232016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu G, Zhang Z, Wei J, Zhang Y, Zhang Y,
Guo L and Liu X: microR-142-3p down-regulates IRAK-1 in response to
Mycobacterium bovis BCG infection in macrophages. Tuberculosis
(Edinb). 93:606–611. 2013. View Article : Google Scholar
|
|
31
|
Naqvi AR, Fordham JB and Nares S: miR-24,
miR-30b, and miR-142-3p regulate phagocytosis in myeloid
inflammatory cells. J Immunol. 194:1916–1927. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Guo Y, Wang C and Yu H, Yu X and
Yu H: MicroRNA-142-3p Inhibits chondrocyte apoptosis and
inflammation in osteoarthritis by targeting HMGB1. Inflammation.
39:1718–1728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang ZJ, Li HC, Cowan AA, Liu S, Zhang YK
and Song XJ: Chronic compression or acute dissociation of dorsal
root ganglion induces cAMP-dependent neuronal hyperexcitability
through activation of PAR2. Pain. 153:1426–1437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
35
|
Norcini M, Sideris A, Martin Hernandez LA,
Zhang J, Blanck TJ and Recio-Pinto E: An approach to identify
microRNAs involved in neuropathic pain following a peripheral nerve
injury. Front Neurosci. 8:2662014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiangpan P, Qingsheng M, Zhiwen Y and Tao
Z: Emerging role of microRNA in neuropathic pain. Curr Drug Metab.
17:336–344. 2016. View Article : Google Scholar
|
|
37
|
Tan PH, Pao YY, Cheng JK, Hung KC and Liu
CC: MicroRNA-based therapy in pain medicine: Current progress and
future prospects. Acta Anaesthesiol Taiwan. 51:171–176. 2013.
View Article : Google Scholar
|
|
38
|
Kusuda R, Cadetti F, Ravanelli MI, Sousa
TA, Zanon S, De Lucca FL and Lucas G: Differential expression of
microRNAs in mouse pain models. Mol Pain. 7:172011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z,
Ding J, Jia L and Yuan W: Increased miR-195 aggravates neuropathic
pain by inhibiting autophagy following peripheral nerve injury.
Glia. 61:504–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Jiang Q, Wang M and Li D: MiR-19a
targets suppressor of cytokine signaling 1 to modulate the
progression of neuropathic pain. Int J Clin Exp Pathol.
8:10901–10907. 2015.PubMed/NCBI
|
|
41
|
Xia L, Zhang Y and Dong T: Inhibition of
microRNA-221 alleviates neuropathic pain through targeting
suppressor of cytokine signaling 1. J Mol Neurosci. 59:411–420.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen HP, Zhou W, Kang LM, Yan H, Zhang L,
Xu BH and Cai WH: Intrathecal miR-96 inhibits Nav1.3 expression and
alleviates neuropathic pain in rat following chronic construction
injury. Neurochem Res. 39:76–83. 2014. View Article : Google Scholar
|
|
43
|
Lin CR, Chen KH, Yang CH, Huang HW and
Sheen-Chen SM: Intrathecal miR-183 delivery suppresses mechanical
allodynia in mononeuropathic rats. Eur J Neurosci. 39:1682–1689.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shao J, Cao J, Wang J, Ren X, Su S, Li M,
Li Z, Zhao Q and Zang W: MicroRNA-30b regulates expression of the
sodium channel Nav1.7 in nerve injury-induced neuropathic pain in
the rat. Mol Pain. Oct 19–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu Y, Cao DL, Jiang BC, Yang T and Gao YJ:
MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6
signaling in the spinal cord. Brain Behav Immun. 49:119–129. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang J, Zhang H and Zi T: Overexpression
of microRNA-141 relieves chronic constriction injury-induced
neuropathic pain via targeting high-mobility group box 1. Int J Mol
Med. 36:1433–1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leinders M, Üçeyler N, Pritchard RA,
Sommer C and Sorkin LS: Increased miR-132-3p expression is
associated with chronic neuropathic pain. Exp Neurol. 283:276–286.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heyn J, Luchting B, Hinske LC, Hübner M,
Azad SC and Kreth S: miR-124a and miR-155 enhance differentiation
of regulatory T cells in patients with neuropathic pain. J
Neuroinflammation. 13:2482016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ito N, Sakai A, Miyake N, Maruyama M,
Iwasaki H, Miyake K, Okada T, Sakamoto A and Suzuki H: miR-15b
mediates oxaliplatin-induced chronic neuropathic pain through BACE1
down-regulation. Br J Pharmacol. 174:386–395. 2017. View Article : Google Scholar
|
|
50
|
Neumann M and Naumann M: Beyond IkappaBs:
Alternative regulation of NF-kappaB activity. FASEB J.
21:2642–2654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Maeda S and Omata M: Inflammation and
cancer: Role of nuclear factor-kappaB activation. Cancer Sci.
99:836–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sakaue G, Shimaoka M, Fukuoka T, Hiroi T,
Inoue T, Hashimoto N, Sakaguchi T, Sawa Y, Morishita R, Kiyono H,
et al: NF-kappa B decoy suppresses cytokine expression and thermal
hyperalgesia in a rat neuropathic pain model. Neuroreport.
12:2079–2084. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM and
Yao SL: Alleviation of neuropathic pain by intrathecal injection of
antisense oligonucleotides to p65 subunit of NF-kappaB. Br J
Anaesth. 97:553–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Niederberger E and Geisslinger G: The
IKK-NF-kappaB pathway: A source for novel molecular drug targets in
pain therapy? FASEB J. 22:3432–3442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schaefer JS, Attumi T, Opekun AR, Abraham
B, Hou J, Shelby H, Graham DY, Streckfus C and Klein JR: MicroRNA
signatures differentiate Crohn's disease from ulcerative colitis.
BMC Immunol. 16:52015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ralfkiaer U, Lindahl LM, Litman T,
Gjerdrum LM, Ahler CB, Gniadecki R, Marstrand T, Fredholm S,
Iversen L, Wasik MA, et al: MicroRNA expression in early mycosis
fungoides is distinctly different from atopic dermatitis and
advanced cutaneous T-cell lymphoma. Anticancer Res. 34:7207–7217.
2014.PubMed/NCBI
|
|
57
|
Pivarcsi A, Meisgen F, Xu N, Ståhle M and
Sonkoly E: Changes in the level of serum microRNAs in patients with
psoriasis after antitumour necrosis factor-α therapy. Br J
Dermatol. 169:563–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Boomiraj H, Mohankumar V, Lalitha P and
Devarajan B: Human corneal microRNA expression profile in fungal
keratitis. Invest Ophthalmol Vis Sci. 56:7939–7946. 2015.
View Article : Google Scholar
|
|
59
|
Perri R, Nares S, Zhang S, Barros SP and
Offenbacher S: MicroRNA modulation in obesity and periodontitis. J
Dent Res. 91:33–38. 2012. View Article : Google Scholar :
|
|
60
|
Yuan Z, Luo G, Li X, Chen J, Wu J and Peng
Y: PPARγ inhibits HMGB1 expression through upregulation of
miR-142-3p in vitro and in vivo. Cell Signal. 28:158–164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang Y, Ouyang M, Wang Q and Jian Z:
MicroRNA-142-3p inhibits hypoxia/reoxygenation induced apoptosis
and fibrosis of cardiomyocytes by targeting high mobility group box
1. Int J Mol Med. 38:1377–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xiao P and Liu WL: MiR-142-3p functions as
a potential tumor suppressor directly targeting HMGB1 in
non-small-cell lung carcinoma. Int J Clin Exp Pathol.
8:10800–10807. 2015.PubMed/NCBI
|
|
63
|
Feldman P, Due MR, Ripsch MS, Khanna R and
White FA: The persistent release of HMGB1 contributes to tactile
hyperalgesia in a rodent model of neuropathic pain. J
Neuroinflammation. 9:1802012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang FF, Morioka N, Harano S, Nakamura Y,
Liu K, Nishibori M, Hisaoka-Nakashima K and Nakata Y: Perineural
expression of high-mobility group box-1 contributes to long-lasting
mechanical hypersensitivity via matrix metalloproteinase-9
upregulation in mice with painful peripheral neuropathy. J
Neurochem. Nov 18–2015.Epub ahead of print. View Article : Google Scholar
|
|
65
|
Nakamura Y, Morioka N, Abe H, Zhang FF,
Hisaoka-Nakashima K, Liu K, Nishibori M and Nakata Y: Neuropathic
pain in rats with a partial sciatic nerve ligation is alleviated by
intravenous injection of monoclonal antibody to high mobility group
box-1. PLoS One. 8:e736402013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin TB, Hsieh MC, Lai CY, Cheng JK, Wang
HH, Chau YP, Chen GD and Peng HY: Melatonin relieves neuropathic
allodynia through spinal MT2-enhanced PP2Ac and downstream HDAC4
shuttling-dependent epigenetic modification of hmgb1 transcription.
J Pineal Res. 60:263–276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang YS, Li YY, Wang LH, Kang Y, Zhang J,
Liu ZQ, Wang K, Kaye AD and Chen L: Tanshinone IIA attenuates
chronic pancreatitis-induced pain in rats via downregulation of
HMGB1 and TRL4 expression in the spinal cord. Pain Physician.
18:E615–E628. 2015.PubMed/NCBI
|
|
68
|
Ma YQ, Chen YR, Leng YF and Wu ZW:
Tanshinone IIA downregulates HMGB1 and TLR4 expression in a spinal
nerve ligation model of neuropathic pain. Evid Based Complement
Alternat Med. 2014:6395632014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
He Z, Guo Q, Xiao M, He C and Zou W:
Intrathecal lentivirus- mediated transfer of interleukin-10
attenuates chronic constriction injury-induced neuropathic pain
through modulation of spinal high-mobility group box 1 in rats.
Pain Physician. 16:E615–E625. 2013.PubMed/NCBI
|