Introduction
Voltage-gated sodium channels (VGSCs) serve a key
role in the initiation and propagation of action potentials in
neurons (1–4). These channels consist of a major
pore-forming α-subunit (Nav1; ~260 kDa) and 1–2 accessory
β-subunits (4). The α-subunit is
the core of the sodium channel and contains the elements essential
for ion conduction and voltage-dependent gating (4–6).
To the best of our knowledge, at present 10 distinct sodium channel
isoforms (Nav1.1-1.9 and NavX) and four small β-subunits (β1-4;
30.4–45 kDa) have been detected and cloned from various mammalian
tissues (5,6). Tetrodotoxin (TTX) is a specific
inhibitor against sodium channels; VGSCs are classified into
TTX-sensitive (Nav1.1-1.4, Nav1.6 and Nav1.7) and TTX-resistant or
insensitive (Nav1.5, Nav1.8 and Nav1.9) types (5).
The intrinsic electrophysiological properties of
human neurons are complex (1).
Therefore, it is important to clarify the distinct isoforms of
sodium channels expressed in the human brain in order to explore
the contribution of specific VGSCs to the excitability of central
neurons. Among the 10 sodium channel isoforms, Nav1.1-1.3 and
Nav1.6 were the first cloned from the brain and functionally
analyzed, as they were originally designated as brain sodium
channel types I, II, III and IV (7-9).
However, previous studies indicated that Nav1.5 mRNA, protein and
its TTX-resistant sodium currents may also be detected in the
mammalian brain and associated cell lines (10–22). In a previous study, we cloned the
full length of Nav1.5 cDNA and demonstrated that two novel variants
of Nav1.5, designated Nav1.5e and Nav1.5f, were expressed in the
human brain cortex (23,24). To the best of our knowledge, at
present, nine Nav1.5 splice variants, including Nav1.5a-f and
truncated variants Nav1.5 E28B-D have been identified, four of
which (Nav1.5a and Nav1.5c-e) are able to generate functional
channels in heterologous expression systems (25). However, the electrophysiological
properties vary among different Nav1.5 splice variants (26,27). As the different expression
patterns of Nav1.5 splice variants may affect the total Nav1.5
sodium current density and kinetics in human brain neurons, the
present study investigated whether additional Nav1.5 splice
variants were expressed in the human brain cortex. Therefore, the
present study systematically investigated the expression of Nav1.5
splice variants, including the functional isoforms in the frontal
lobe of the human brain cortex. In addition, the expression and
distribution of total Nav1.5 protein in the neurons and glial cells
of the gray and white matter within the human brain cortex was
detected.
Materials and methods
Materials
The present study conformed to the principles
outlined in the declaration of Helsinki, and was approved by the
Ethics Committee of China Medical University (Shenyang, China).
Five human brain samples were collected from the First Hospital and
the Department of Anatomy of China Medical University (Shenyang,
China) between January 2013 and December 2015. The samples were
collected from 3 males and 2 females, with an age range of 43–78
years. Three samples (frontal lobe cortexes) were obtained at
autopsy from adults without a history of neurologic diseases at the
Department of Anatomy of China Medical University. All autopsies
were performed within 12 h of mortality. Two human brain samples
were collected blindly from discarded tissues from surgeries to
treat basilar meningiomas at the First Hospital of China Medical
University. All samples were collected with prior understanding and
written informed consent from the patients or their relatives.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from human brain cortexes
using an RNA Extraction kit (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol. First-strand cDNA was
synthesized using an RT-PCR kit (New England BioLabs, Inc.,
Ipswich, MA, USA) with oligo-(dt) and random primers. The RT
reaction conditions consisted of 30°C for 5 min followed by 42°C
for 25 min, 99°C for 5 min and 5°C for 5 min. For the detection and
isolation of Nav1.5 splice variants from the human brain cortex,
primer pairs P1-P6 (Table I) were
used to amplify different fragments of the full-length Nav1.5 cDNA
by the PCR method. The PCR primers were designed from the full
sequences of the SCN5A gene cloned from the human brain cortex
(accession number, EF629346) and the PCR was performed separately,
using a One-Taq One-Step RT-PCR kit (New England Biolabs, Inc.),
according to the specific reannealing temperatures of different
primers. The PCR reaction conditions were as follows: 95°C for 5
min followed by 36 cycles of 95°C for 30 sec, 60–66°C for 30 sec,
72°C for 30 sec and final elongation at 72°C for 5 min.
 | Table IPrimer sequences used for the
isolation of Nav1.5 splice variants. |
Table I
Primer sequences used for the
isolation of Nav1.5 splice variants.
| Primer | Primer sequence
(5′-3′)
| Target | Locationa | Length (bp) |
|---|
| Forward | Reverse |
|---|
| P1 |
ACCAACTGCGTGTTCATGGCCCA |
AGGTCCAGGGATTCCCAGACCA | Exon 6/6A | 425-916 | 492 |
| P2 |
ACCAACTGCGTGTTCATGGCCCA |
GCAGAAGACTGTGAGGACCA | Exon 6/6A | 425-778 | 354 |
| P3 |
GTGCCTCCCACCCGCAAGGAAA |
TGCTGCCCTCGGAGCAACTGT | Nav1.5a,
Nav1.5c | 3054-3420 | 367 |
| P4 |
CTGGGGAACCTGACACTGGTGC |
AGATGATGAATGTCTCGAACC | Nav1.5b | 2514-3636 | 1,123 |
| P5 |
CCAAGAAGAGGATGAGGAGA |
GAGGCAGTCGCTGACACC | Nav1.5c | 3172-3307
132/135 | |
| P6 |
TTCAGTGCAGACAACCTCACA |
TGTTCTCCTCATCCTCTTCTT | Nav1.5d | 2823-3195 | 373 |
DNA sequencing
The PCR products were separated by electrophoresis
on a 2% agarose gel. The different fragments of the expected size
were extracted by using a gel extraction kit (Qiagen, Inc.,
Valencia, CA, USA) and subsequently sequenced directly using a
3730×l DNA Analyzer (Applied BioSystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's
protocol.
Denaturing gel electrophoresis and DNA
argentation
Denaturing gel electrophoresis and the DNA
argentation method was used to detect the relative quantity of
alternatively spliced transcripts of Nav1.5. Denaturing gel
electrophoresis was performed at room temperature, at 5 W for 4 h
on a 12% polyacrylamide (19:1), 7 M urea gel. Subsequently, 2%
AgNO3 was used for the silver staining test of DNA at
room temperature.
Restriction enzyme digestion
Restriction enzyme SacI (cat. no. D1078A;
Takara Bio, Inc., Otsu, Japan) was used to digest the PCR products
to distinguish between different splice variants of Nav1.5. The
total reaction system was 30 µl [4 µl PCR products,
0.5 µl SacI enzyme, 3 µl loading buffer
(Takara Bio, Inc.); 22.5 µl ultrapure water]. Following 1 h
incubation at 38°C, electrophoresis was performed on 2% agarose gel
to detect the digestion results. The expression ratio of Nav1.5
variants vs. total Nav1.5 was detected from the signal
quantification of pre- and post-digestion by autoradiography.
Immunostaining
Human brain cortex specimens were fixed with 4%
paraformaldehyde (4°C for 24 h) immediately after collection. The
tissues were paraffin-embedded after dehydration using a series of
graded ethanol baths (70–100% ethanol), clearing (the transparency
of tissue) using xylene (100%) and wax infiltration. The
streptavidin-peroxidase (SP) immunohistochemical method was
applied. Non-immune goat serum (cat. no. ZLI-9022; without
dilution; OriGene Technologies, Inc., Beijing, China) was added for
blocking followed by incubation at room temperature in a moisture
chamber for 30 min. Immunohistochemistry was performed using a
Histostain-SP kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol. Sections (4 µm) were
incubated overnight with rabbit anti-human Nav1.5 antibodies (cat.
no. ASC-013; 1:100; Alomone Labs, Jerusalem, Israel) at 4°C in a
moisture chamber, followed by three washes with PBS, 5 min each.
Subsequently, sections were incubated with biotinylated goat
anti-rabbit immunoglobulin G secondary antibody (cat. no. 656140;
1:500; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 30 min, followed by three washes with PBS, 5 min
each. PBS replaced the primary antibody to serve as a negative
control and a slide with known positive Nav1.5 expression in the
rat atrial muscle served as a positive control. The
immunohistochemical staining results were observed using a light
microscope (magnification, x40 and x100; Olympus CX31-LV320;
Olympus Corporation, Tokyo, Japan). The expression of brown and
yellow staining on the cells was considered to be positive
immunoreactions when compared with negative and positive
slices.
Results
Expression of adult and neonatal Nav1.5
in the human brain cortex
Primer P1 targeting exon 6 or 6A of SCN5A was used
to detect the expression of adult (wild) and neonatal (Nav1.5e)
Nav1.5 in the frontal lobe cortex of human brains. Nav1.5 protein
encoded by the SCN5A gene including exon 6 is universally
designated as wild or adult Nav1.5, whereas that including exon 6A
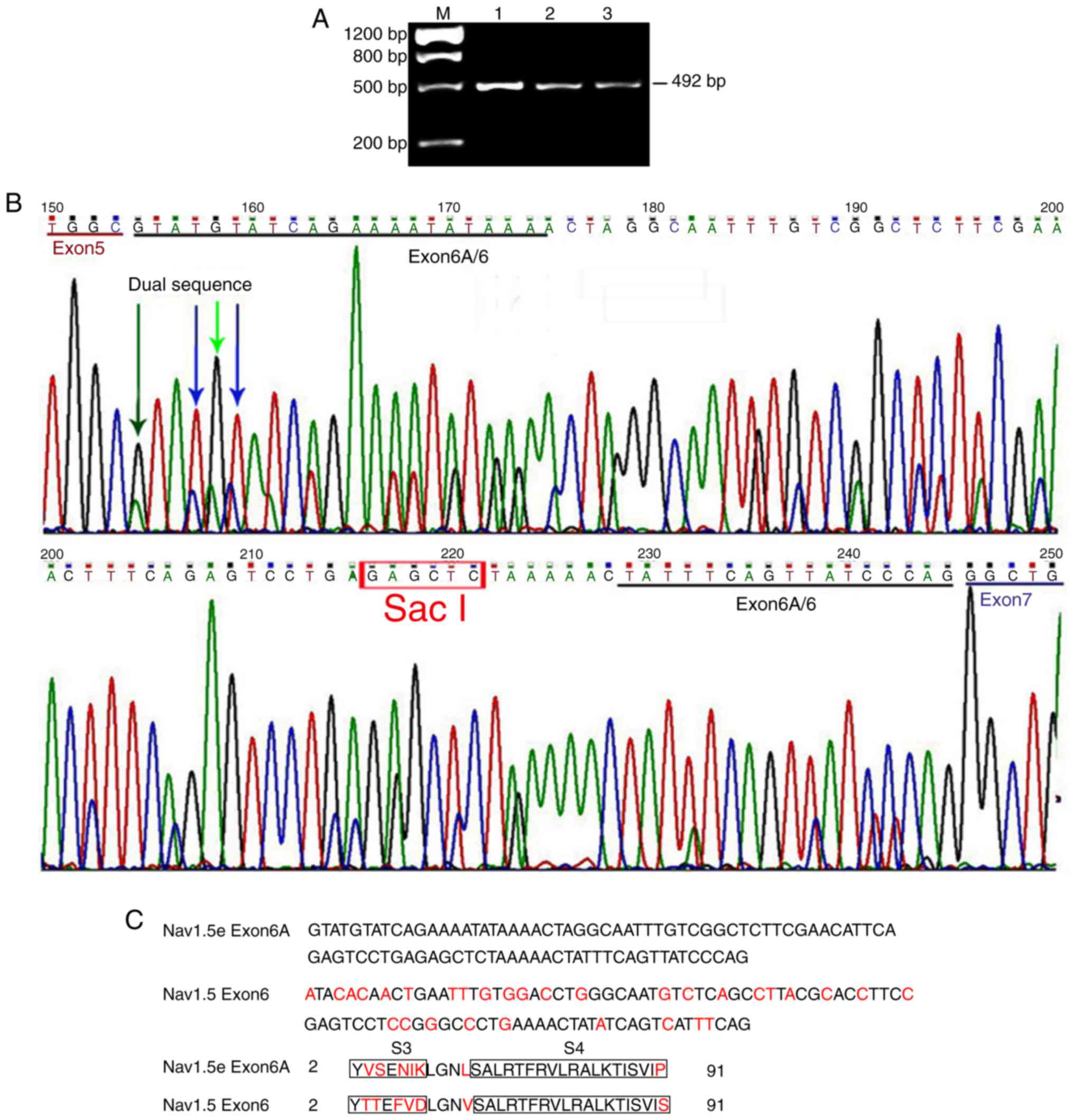
is universally designated neonatal Nav1.5 or Nav1.5e (28). Electrophoresis revealed that a
single clear band with the expected size was visible on the agarose
gel (Fig. 1A). Direct DNA
sequencing of these PCR products revealed that a single sequence
presented in the coding region of exons 5 and 7, whereas a dual
sequence appeared in the coding region of exons 6 and 6A (Fig. 1B). DNA sequence analysis revealed
that exon 6 and 6A were inclusively expressed, indicating the
expression of adult and neonatal Nav1.5 channels in the frontal
lobe of the human brain. Exon 6A and 6 each contained 92
nucleotides; however, 31 of them were different in various
positions (Fig. 1C). The 92
nucleotides encoded 30 amino acid residues. A total of 7 amino acid
residues were different between exon 6 and 6A.
In order to demonstrate the expression
quantification of the two splice variants of Nav1.5, the sequence
differences between exon 6 and 6A were analyzed. Occasionally, a
specific restriction enzyme site of SacI was identified in
exon 6A rather than exon 6. The restriction enzyme SacI was
subsequently used to digest the PCR products to distinguish between
these two variants. The common cDNA from the RT of mRNA and the PCR
products generated by primer P1 (which were demonstrated by DNA
sequencing to include exon 6 and 6A), were used independently as
the templates to perform the usual PCR and the nested PCR. The PCR
products (with primers P1 and P2) containing the total Nav1.5
fragments were digested by the restriction enzyme SacI. As
revealed in Fig. 2, three bands
were observed on the agarose gel following digestion by
SacI, which indicated that fragments containing exon 6A were
digested into two smaller fragments, whereas those containing exon
6 were preserved. Therefore, the relative amounts of digested and
undigested PCR products represented the quantification of neonatal
and adult Nav1.5 cDNA, respectively. Similar results were observed
by using two pairs of primers (P1 and P2) as aforementioned. The
expression ratio of neonatal Nav1.5 vs. adult Nav1.5 was ~5:1, as
calculated from the signal quantification of pre- and
post-digestion by autoradiography (n=5) (data of expression ratio
not shown).
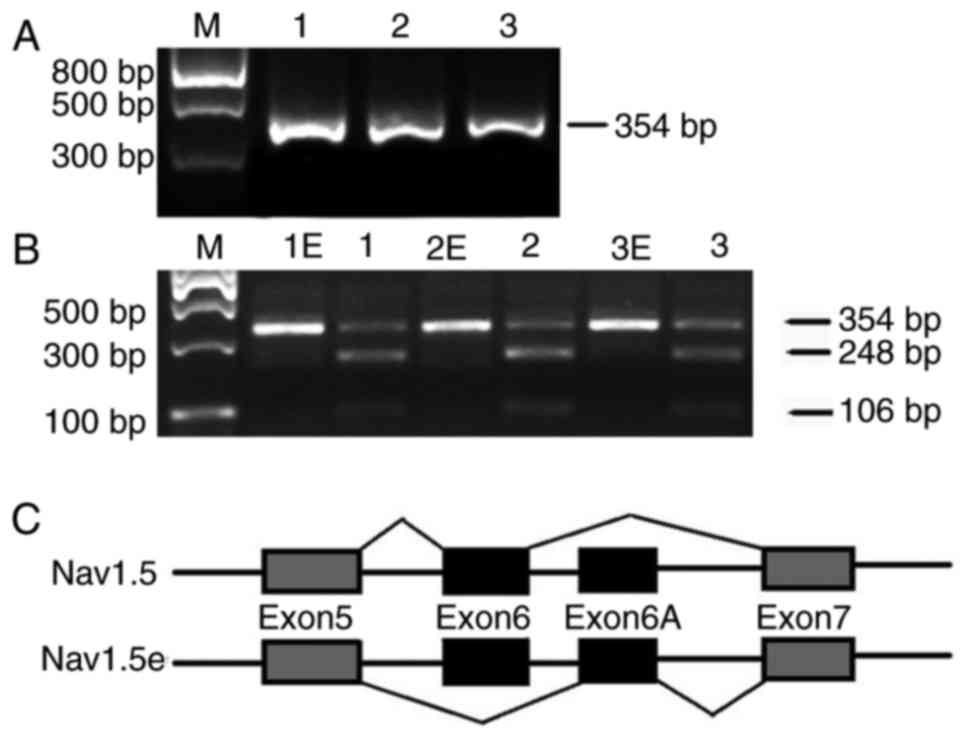 | Figure 2Alternative splicing of exon 6 and 6A
in the SCN5A gene. (A) Agarose gel depicting the electrophoresis
results following polymerase chain reaction with primer P2. A
single, clear band with the expected size of 354 bp was observed.
Lanes 1–3, samples of human brain cortex from the frontal lobe. (B)
Electrophoresis results following digestion by restriction enzyme
SacI. Products with restriction enzyme SacI generated
three bands on the agarose gel, with the expected sizes of 354, 248
and 106 bp, respectively, indicating that fragments including exon
6A were digested into another two fragments, while those containing
exon 6 were preserved. Lanes 1E-3E, PCR products with restriction
enzyme SacI; lanes 1–3, PCR products without restriction
enzyme SacI. (C) Schematic for the alternative splicing of
exons 6 and 6A of the SCN5A gene. Constitutive exons are presented
in gray boxes, alternatively spliced sequences are presented in
solid boxes and introns are presented as solid lines. The two
different pathways are presented as poly lines. M, marker. |
Expression of Nav1.5a in the human brain
cortex
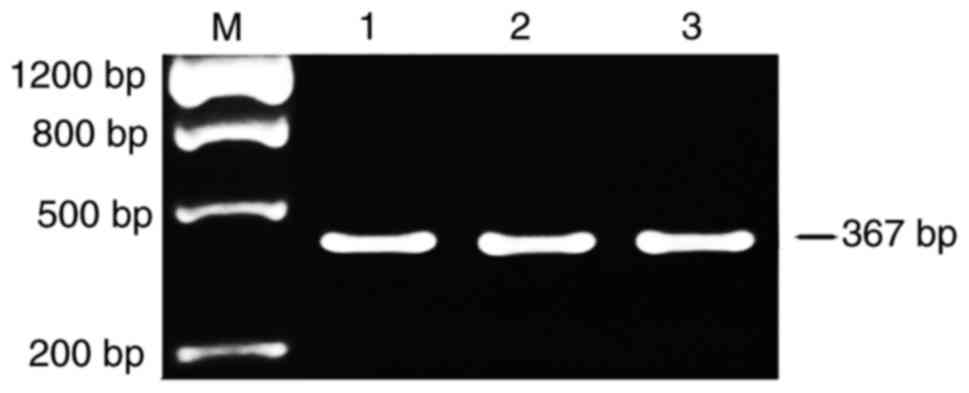
Primer P3 that targeted part of exon 17 and all of
exon 18 of Nav1.5 cDNA was used to detect the expression of Nav1.5a
splice variant (with the alternative splice of exon 18). PCR
products were purified and sequenced directly. The expected
fragment size of 367 bp was observed following electrophoresis
(Fig. 3). The fragment with
alternative splicing of exon 18 (159-bp deletion) was not detected.
DNA sequencing further demonstrated the inclusive expression of
exon 18 in the Nav1.5 cDNA (data of sequencing not shown). These
results indicate that Nav1.5a may not be expressed within the
frontal lobe cortex of the human brain, or it is expressed at a
very low level.
Expression of Nav1.5b in the human brain
cortex
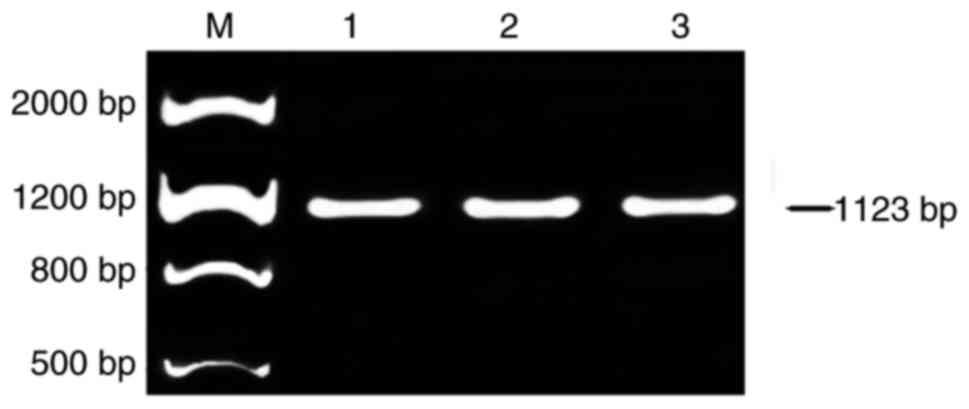
Primer P4 that targeted the full lengths of exons 17
and 18 of Nav1.5 cDNA was used to detect the expression of Nav1.5b.
The expected fragment size of 1,123 bp was observed following
electrophoresis (Fig. 4). The
fragment with alternative splicing of exon 17 and 18 (600-bp
deletion) was not observed on the agarose gel. This result suggests
that Nav1.5b may not be expressed within the frontal lobe cortex of
the human brain, or it is expressed at a very low level.
Expression of alternatively spliced
variant Q1077 (Nav1.5c) and Q1077del (wild Nav1.5) in the human
brain cortex
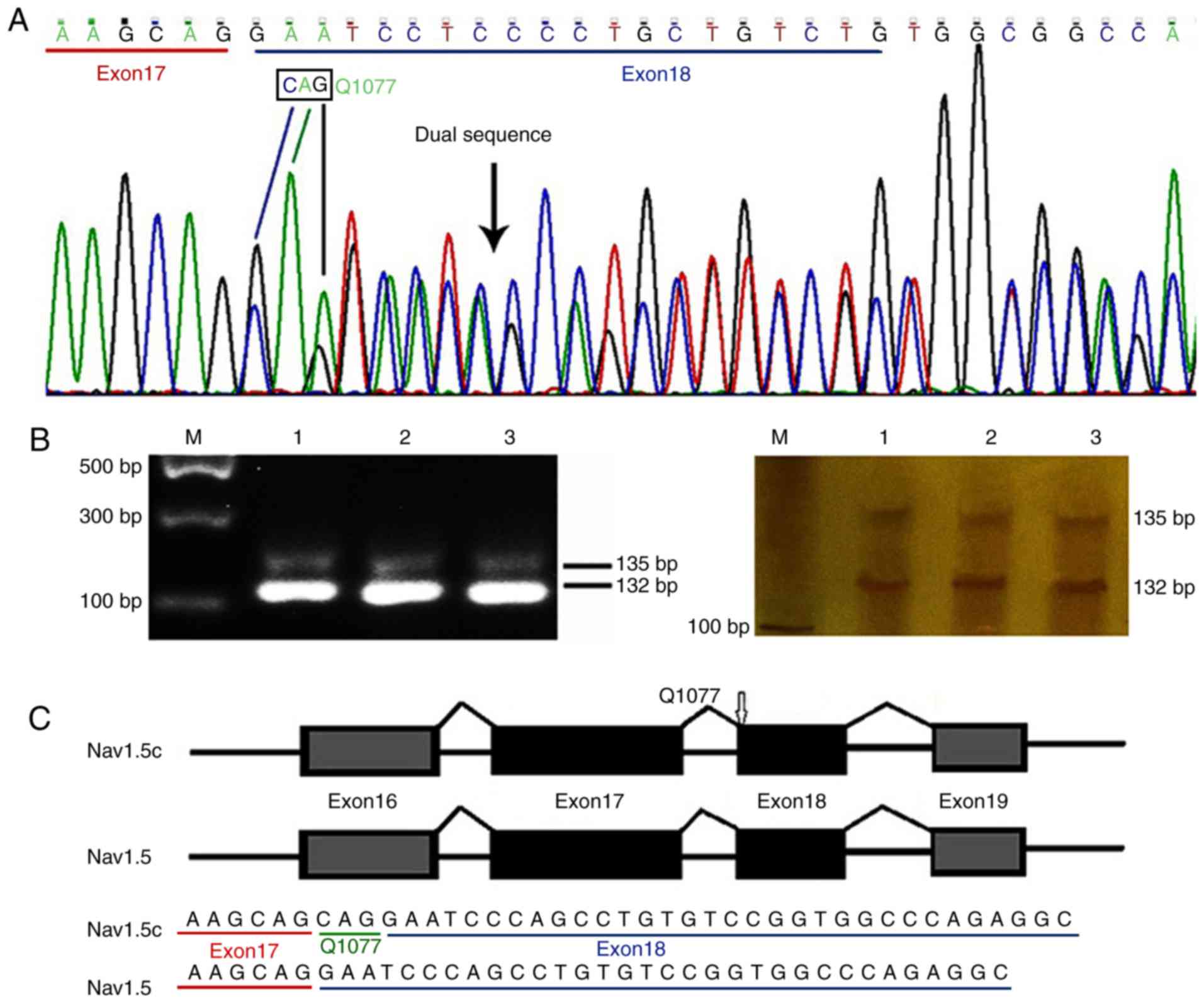
Primers P3, that targeted partial exon 17 and full
exon 18, and P5, that targeted the conjunction region of exons 17
and 18, were used to investigate the expression of Nav1.5c and wild
Nav1.5 (Nav1.5) in the frontal lobe cortex of the human brain,
respectively. Direct DNA sequencing was performed on the PCR
products generated by primer P3. As demonstrated in Fig. 5, the results indicated that a
single sequence presented in the coding region of exons 17 and 19,
whereas a dual sequence presented in the exon 18 coding region
(Fig. 5A). Sequence analysis
demonstrated that two CAG repeats in the conjunction region of
exons 17 and 18 led to the alternative splicing of a glutamine (Q)
at position 1,077, indicating that Nav1.5c and wild Nav1.5 were
expressed in the human brain cortex (Fig. 5C). In order to determine the
expression quantifications of the two Nav1.5 variants, primer P4
was used. It targeted (harboring) the conjunction region of exons
17 and 18; however, it generated shorter products than expected
(Fig. 5B). Electrophoresis
revealed the presence of transcripts with 132 and 135 bp.
Autoradiography was used for signal quantification of the two
alternatively spliced transcripts. The proportion of alternatively
spliced variants containing Q1077 was ~16.6% (n=5) and the Q1077del
transcript was ~83.4% (n=5), the expression ratio of Nav1.5:Nav1.5c
was ~5:1 (data of expression ratio not shown).
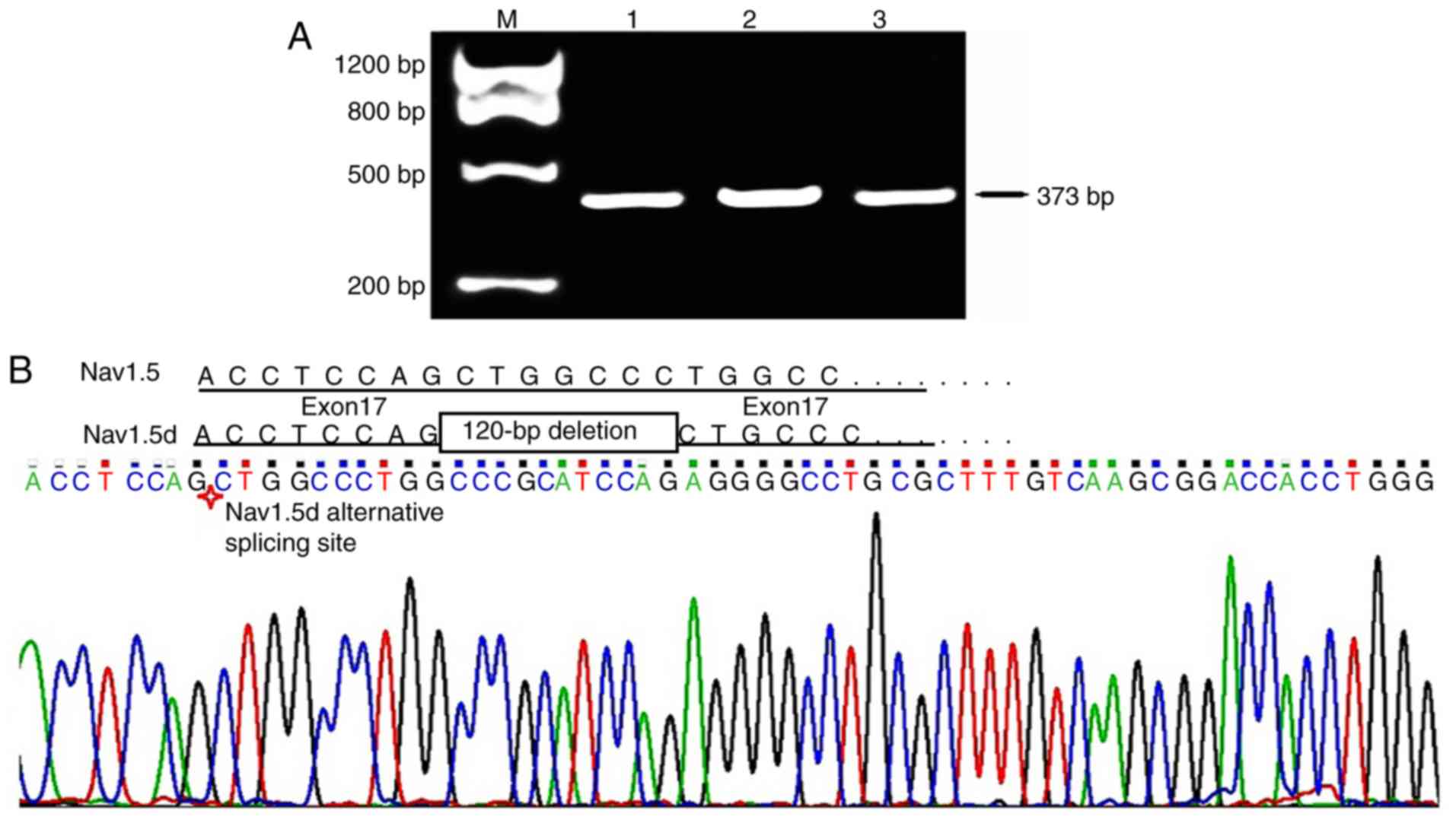
Expression of Nav1.5d in the human brain
cortex
Primer P6 targeting the full length of exon 17 was
used to detect the expression of Nav1.5d, the variant generated by
partial alternative splicing of exon 17 (with an intermediate
120-bp deletion). PCR products with the expected fragment size of
373 bp were observed on 2% agarose gel (Fig. 6A); however, the expected fragments
of 253 bp were not observed. Direct DNA sequencing further
confirmed the inclusive expression of the full-length exon 17
(Fig. 6B). This result was the
same as that obtained using primer P3, indicating no or very low
expression of Nav1.5d in the frontal lobe cortex of the human
brain.
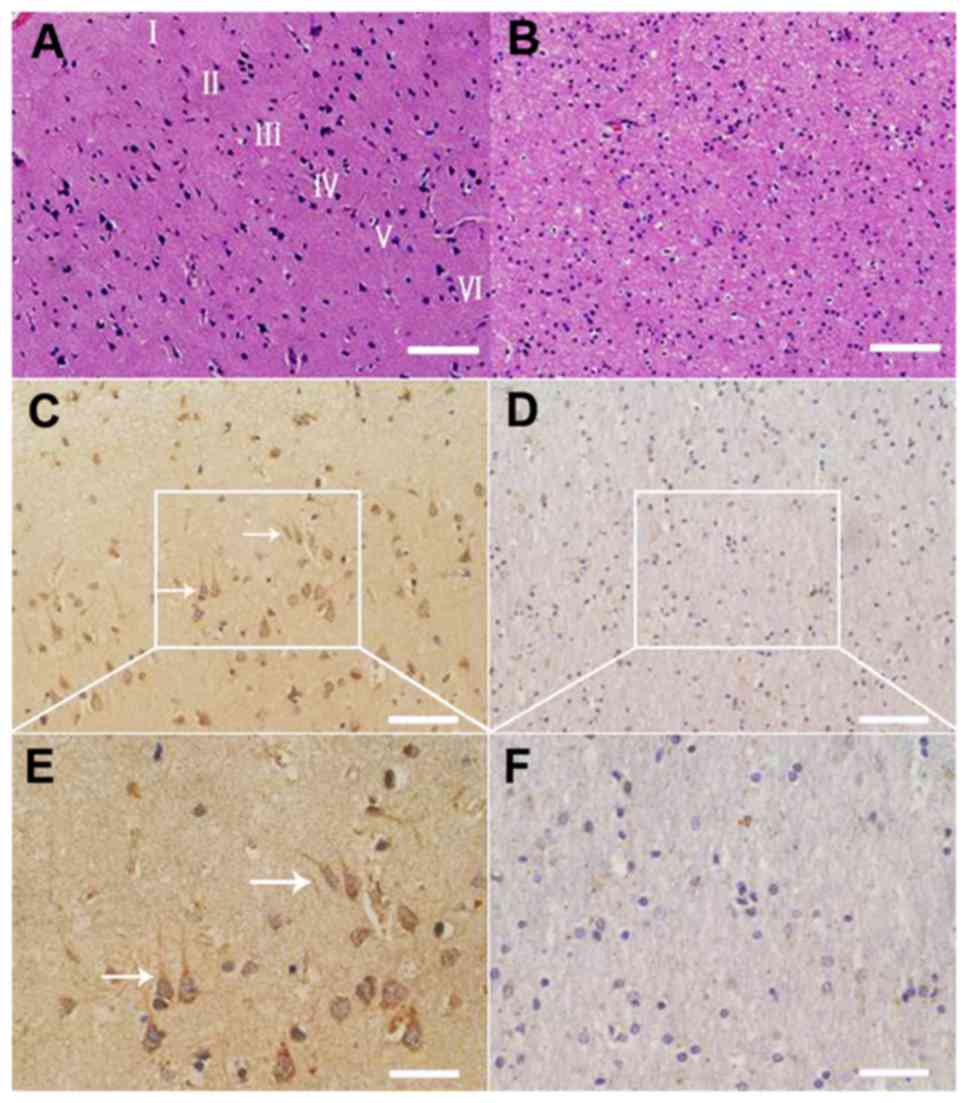
Expression and distribution of total
Nav1.5 protein in the neurons and glial cells of gray and white
matter within the human brain cortex
Tissue staining and immunohistochemistry were used
to investigate the expression and distribution of total Nav1.5
protein in the neurons and glial cells of the frontal lobe cortex
of the human brain (Fig. 7). The
results revealed that Nav1.5 immunoreactivity was predominantly
located within the neuronal cell bodies and processes, including
the axons and dendrites, whereas little or no immunoreactivity was
detected in the glial components. Pyramid cells in layer V of the
frontal lobe cortex of the human brain revealed slightly stronger
immunoreactivity to Nav1.5 antibody compared with neurons in the
other layers (Fig. 7). The
results reveal the expression and distribution of Nav1.5 at the
protein level in the frontal lobe cortex of the human brain.
Discussion
The VGSC isoform Nav1.5, encoded by the SCN5A gene,
is the predominant sodium channel in the heart (28). It was previously referred to as
the cardiac sodium channel as it is essential for action potential
initiation in atrial and ventricular cardiomyocytes (25,28). In 1991, Yarowsky et al
(10) first detected Nav1.5 mRNA
in the brain cortex of neonatal and adult rats. Throughout the
following decade, several studies demonstrated the expression of
Nav1.5 mRNA, protein or its TTX-resistant sodium current within
mammalian brains (11–19,22). The Nav1.5 sodium channel expressed
in the brain was considered to be identical to the one expressed in
the adult heart (20). However,
when full length Nav1.5 cDNA were cloned from the human
neuroblastoma cell line NB-1 and the brain cortex, neonatal Nav1.5
was detected and confirmed as a novel variant of Nav1.5 expressed
in nervous tissue, which was different in structure and function to
the wild Nav1.5 located in adult human hearts (20,23,24). Previous studies have demonstrated
that Nav1.5 may be associated with certain neurological diseases. A
study by Black et al (29)
indicated a central, robust upregulation of the sodium channel
Nav1.5 in reactive astrocytes at the borders of and within active
and chronic multiple sclerosis lesions. Two previous studies
detected a SCN5A mutation in patients with epilepsy (30,31). The function of Nav1.5 within the
normal human brain and its potential association with neurological
diseases is being increasingly studied.
Compared with wild Nav1.5, Nav1.5a is characterized
by a deletion of exon 18 (126 bp) of the SCN5A gene, which encodes
53 amino acid residues in the intracellular loop between domains II
and III of the Nav1.5 channel (32). This splice variant was the first
reported splice variant of the cardiac sodium channel Nav1.5, which
was identified in a rat brain, heart, and hippocampal progenitor
stem cells and mouse cardiac cells (18,33). Subsequently, Nav1.5a expression
was identified in neonatal and adult dorsal root ganglia (DRG)
(32,34), human neuroblastoma cell line NB-1
(20) and rat striatal progenitor
cell line ST14A (35). Although
Nav1.5a has been previously revealed as being expressed in several
tissue types within the nervous system, including the rat brain
(36), it was not detected within
the human brain cortex in the present study. This result was
consistent with the results of our previous study (24). Previous studies have theorized
that the removal of the exon 18 sequence from the Nav1.5 transcript
is specific only to small rodents; higher mammals retained this
exon, suggesting an associated species-specific function (25,37). Therefore, it is important to
specify whether alternative splicing of exon 18 affects the
kinetics of the Nav1.5 channel. Our previous study suggested that
although two alternatively spliced variants, designated hNbR1 and
hNbR1-2 (wild Nav1.5 and Nav1.5a cloned from human neuroblastoma
cell line NB-1), demonstrated similar mRNA expression levels and
sodium current, they were slightly different in the kinetics of
steady-state inactivation and activation (20). Similar results were revealed by
Kerr et al (32) who
investigated Nav1.5a in adult mouse dorsal root ganglia. The above
results of previous studies indicated that alternative splicing of
exon 18 affects the electrophysiological activity of Nav1.5
channel.
Nav1.5b is characterized by alternative splicing of
exons 17 and 18 encoding 200 amino acid residues in S6 of domain
II, and the intracellular loop between domain II and III of the
Nav1.5 channel (28). It was
first detected as being expressed in very low levels in the hearts
of mice (33). However, this
variant was not identified in the hearts of other species,
including rats, pigs and humans (37). Nav1.5b was not detected in the
frontal lobe cortex of the human brain in the present study.
Notably, previous electrophysiological recordings have revealed
that Nav1.5b was a nonfunctional splice variant (25). Alternative splicing of exon 18 may
generate a functional variant of Nav1.5a, therefore the existence
of a partial or full exon 17 was essential to maintain the function
of the Nav1.5 channel (25). It
may be hypothesized that the differential expression of Nav1.5b in
various tissue types may be associated with the tissue-specific
expression patterns of sodium channels.
Nav1.5c is characterized by an additional CAG
trinucleotide (encoding an additional glutamine at position 1077)
at the starting site of exon 18, and it is the most abundant Nav1.5
splice variant detected in the heart (38). A previous study investigated the
expression of Nav1.5c in the heart of humans (38). The results demonstrated that the
Nav1.5:Nav1.5c transcript ratio was ~2:1 and remained constant in
different regions of the human heart (38); however, the expression level of
Nav1.5c was demonstrated to be lower in mouse heart and thigh
muscle, and trigeminal and dorsal root ganglia (32,34). This suggested a distinct
expression pattern of Nav1.5c in different species and tissue
types. Our previous studies also detected the expression of Nav1.5c
in the human brain cortex and neuroblastoma cell line NB-1
(20,24). However, the associated Nav1.5c
transcript level in those tissues was not detected simultaneously,
as an indirect DNA sequencing method was used to analyze the PCR
products (24), which had
limitations. The method used in the present study was improved
compared with previous studies, the PCR products of the present
study were purified and sequenced directly, meaning the dual cDNA
sequences in the products were detected. DNA sequence analysis
revealed that wild Nav1.5 and Nav1.5c were expressed in the frontal
lobe cortex of the human brain. Electrophoresis on agarose gel and
denaturing gel electrophoresis on a polyacrylamide/urea gel were
used independently to determine the expression quantifications of
the two splice variants. The expression ratio of the two variants
in the human brain cortex was ~5:1, which was similar to that
identified in the adult mouse DRG, but different from that
identified in the adult human heart (32,38). These results indicate that the
tissue diversity may not affect the specific expression pattern of
Nav1.5 channels. Previous studies demonstrated that the
electrophysiological properties of wild Nav1.5 and Nav1.5c variants
were indistinguishable when explored in the same wild-type
background Nav1.5 cDNA (25,38–40). However, as demonstrated by
previous studies on SCN5A channelopathies, including SCN5A
mutations in Long QT syndrome or Brugada syndrome, the channel
kinetics of Nav1.5 are altered significantly when expressing the
mutations in wild Nav1.5 compared with that in Nav1.5c background
(25,39–41), indicating a different role of the
respective splicing variants of Nav1.5 in SCN5A
channelopathies.
Nav1.5d is characterized by a deletion of a 120-bp
fragment in the intermediate region of exon 17 of gene SCN5A. A
previous study identified this when exploring the expression of
Nav1.5a and Nav1.5b in the human heart (42). The electrophysiological analysis
revealed that significant changes occurred in channel kinetics,
including the depolarized shift of steady-state activation and
inactivation, and the reduction of whole cell current density when
Nav1.5d was present (42). This
result demonstrated that even partial alternative splicing of exon
17 may alter the function of Nav1.5 channels. To the best of our
knowledge, this splice variant has not been detected in the hearts
of mice, rats, pigs or dogs (37,42). In the present study Nav1.5d was
not detected in the human brain cortex, suggesting that Nav1.5d may
not be expressed in the frontal lobe cortex of the human brain, or
it is only present at very low levels. This result further
suggested a tissue-specific expression pattern of sodium channel
isoforms.
Nav1.5e (neonatal Nav1.5) is encoded by exon 6A of
the SCN5A gene, while wild Nav1.5 (adult Nav1.5) is encoded by exon
6 of the SCN5A gene (20,21,23–25). Gene sequence analysis has revealed
that exon 6 and 6A of the human SCN5A gene has 92 bp, which encodes
30 amino acid residues, 7 of which were different between them
(20,21,23–25). Nav1.5e splice variant has been
previously observed in various cells and tissues, including the
human neuroblastoma cell line NB-1 (20), human brain astrocytoma (43), breast cancer tissue (44,45), human and mouse brain tissue
(21,23,24,36), neonatal mouse heart and several
other types of cancer tissue (21,46). The present study revealed that
adult and neonatal Nav1.5 are expressed within the frontal lobe
cortex of the human brain. As aforementioned, a major reason for
not detecting the expression of adult Nav1.5 in the human brain
cortex in previous studies was the use of an indirect DNA
sequencing method to test the PCR products. Therefore, certain
fragments in the PCR products were missed following subcloning them
into a PGEM-T-easy vector, as it was impossible to test every
colony. In the present study, the PCR products were sequenced
directly following purification and the results suggested that
adult and neonatal Nav1.5 are expressed in the human brain cortex.
Notably, the expression quantification of the two splice variants
of Nav1.5 was different; the expression ratio of neonatal Nav1.5
vs. wild Nav1.5 was ~5:1. This result indicates that Nav1.5e is the
major Nav1.5 channel in the frontal lobe cortex of the human brain.
The total expression level of Nav1.5 in the human brain cortex is
notably lower than that in the heart (23). According to the results of the
present study, adult Nav1.5 accounts for just 1/6 of the total
Nav1.5, explaining why no signal appeared in the agarose gel when
specific PCR primers were used in our previous study (23). To the best of our knowledge, the
present study is the first to demonstrate the expression of
neonatal and adult Nav1.5 isoforms in the human brain cortex,
therefore indicating the abundant expression of VGSC isoforms in
the CNS.
Confirmation of the expression of neonatal and adult
Nav1.5 isoforms in the human brain is critical, as these two splice
variants exhibit distinct electrophysiological properties. Onkal
et al (26) systematically
investigated the channel kinetics of neonatal and adult Nav1.5 and
demonstrated that the neonatal channel exhibited a depolarized
threshold of activation and voltage at which the current peaked,
slower kinetics of activation and inactivation, 50% greater
transient charge influx, a stronger voltage dependence of time to
peak and a slower recovery from inactivation compared to adult
Nav1.5. These results combined with the results of the present
study suggest that the TTX-resistant sodium current in human brain
neurons is generated by neonatal and adult Nav1.5 channels. Further
studies are required to demonstrate the specific contributions of
each Nav1.5 splice variant to the total Nav1.5 sodium channel
function in neurons.
It has been previously hypothesized that Nav1.5 may
have an impact on seizure activity as it has been widely identified
in the limbic regions of the brain, which have a close association
with epilepsy (16,23). Additionally, mutations in the
human heart sodium channel SCN5A gene have been identified to cause
cardiac abnormalities, including congenital Long QT 3 syndrome and
idiopathic ventricular fibrillation (28). Furthermore, myocardial and neural
cells exhibit similarities in electrophysiological activities, such
as the spontaneous generation of action potential (4). This hypothesis has been supported by
several studies, including a study by Aurlien et al
(30) that first reported a novel
missense mutation (R523C) in the SCN5A gene in a patient with
idiopathic epilepsy who suffered mortality. Parisi et al
(31) first reported a
coexistence of epilepsy and Brugada syndrome in a family with an
SCN5A mutation. These previous studies presented a novel view of
Nav1.5 expression, however, further studies are required to
investigate the potential causative association between SCN5A
mutation and epilepsy.
In conclusion, the present study demonstrated the
expression of wild Nav1.5, Nav1.5c, and Nav1.5e in the human brain
cortex, indicating that the TTX-resistant sodium current was a
compound product of different Nav1.5 variants. The present study
also demonstrated a more abundant and complicated expression of
Nav1.5 in the human brain than previously reported, and provided
insights into the functional significance and complexity of Nav1.5
sodium channels in neurons. Further studies are required to explore
the specific contributions of Nav1.5 and its splice variants to the
total sodium current and excitability of neurons.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 31100770) and the
Liaoning Provincial Natural Science Foundation of China (grant no.
2014021097).
References
|
1
|
Eijkelkamp N, Linley JE, Baker MD, Minett
MS, Cregg R, Werdehausen R, Rugiero F and Wood JN: Neurological
perspectives on voltage-gated sodium channels. Brain.
135:2585–2612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu W, Tian C, Li T, Yang M, Hou H and Shu
Y: Distinct contributions of Na(v)1.6 and Na(v)1.2 in action
potential initiation and backpropagation. Nat Neurosci.
12:996–1002. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kole MH, Ilschner SU, Kampa BM, Williams
SR, Ruben PC and Stuart GJ: Action potential generation requires a
high sodium channel density in the axon initial segment. Nat
Neurosci. 11:178–186. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Catterall WA: From ionic currents to
molecular mechanisms: The structure and function of voltage-gated
sodium channels. Neuron. 26:13–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu FH, Yarov-Yarovoy V, Gutman GA and
Catterall WA: Overview of molecular relationships in the
voltage-gated ion channel superfamily. Pharmacol Rev. 57:387–395.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catterall WA: Voltage-gated sodium
channels at 60: Structure, function and pathophysiology. J Physiol.
590:2577–2589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cummins TR, Aglieco F, Renganathan M,
Herzog RI, Dib-Hajj SD and Waxman SG: Nav1.3 sodium channels: Rapid
repriming and slow closed-state inactivation display quantitative
differences after expression in a mammalian cell line and in spinal
sensory neurons. J Neurosci. 21:5952–5961. 2001.PubMed/NCBI
|
|
8
|
Noda M, Ikeda T, Kayano T, Suzuki H,
Takeshima H, Kurasaki M, Takahashi H and Numa S: Existence of
distinct sodium channel messenger RNAs in rat brain. Nature.
320:188–192. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaller KL, Krzemien DM, Yarowsky PJ,
Krueger BK and Caldwell JH: A novel, abundant sodium channel
expressed in neurons and glia. J Neurosci. 15:3231–3242.
1995.PubMed/NCBI
|
|
10
|
Yarowsky PJ, Krueger BK, Olson CE,
Clevinger EC and Koos RD: Brain and heart sodium channel subtype
mRNA expression in rat cerebral cortex. Proc Natl Acad Sci USA.
88:9453–9457. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White JA, Alonso A and Kay AR: A
heart-like Na+ current in the medial entorhinal cortex.
Neuron. 11:1037–1047. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoehn K, Watson TW and MacVicar BA: A
novel tetrodotoxin-insensitive, slow sodium current in striatal and
hippocampal neurons. Neuron. 10:543–552. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng D, Kyle JW, Martin RL, Ambler KS and
Hanck DA: Cardiac sodium channels expressed in a peripheral
neurotumor-derived cell line, RT4-B8. Am J Physiol.
270:C1522–C1531. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deisz RA: A tetrodotoxin-insensitive
[corrected] sodium current initiates burst firing of neocortical
neurons. Neuroscience. 70:341–351. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu XQ, Dib-Hajj S, Rizzo MA and Waxman SG:
TTX-sensitive and -resistant Na+ currents, and mRNA for
the TTX-resistant rH1 channel, are expressed in B104 neuroblastoma
cells. J Neurophysiol. 77:236–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hartmann HA, Colom LV, Sutherland ML and
Noebels JL: Selective localization of cardiac SCN5A sodium channels
in limbic regions of rat brain. Nat Neurosci. 2:593–595. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donahue LM, Coates PW, Lee VH, Ippensen
DC, Arze SE and Poduslo SE: The cardiac sodium channel mRNA is
expressed in the developing and adult rat and human brain. Brain
Res. 887:335–343. 2000. View Article : Google Scholar
|
|
18
|
Gersdorff Korsgaard MP, Christophersen P,
Ahring PK and Olesen SP: Identification of a novel voltage-gated
Na+ channel rNa(v)1.5a in the rat hippocampal progenitor
stem cell line HiB5. Pflugers Arch. 443:18–30. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu L, Nishiyama K, Hollyfield JG and Wang
Q: Localization of Nav1.5 sodium channel protein in the mouse
brain. Neuroreport. 13:2547–2551. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ou SW, Kameyama A, Hao LY, Horiuchi M,
Minobe E, Wang WY, Makita N and Kameyama M: Tetrodotoxin-resistant
Na+ channels in human neuroblastoma cells are encoded by
new variants of Nav1.5/SCN5A. Eur J Neurosci. 22:793–801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chioni AM, Fraser SP, Pani F, Foran P,
Wilkin GP, Diss JK and Djamgoz MB: A novel polyclonal antibody
specific for the Na(v)1.5 voltage-gated Na(+) channel ʻneonatalʼ
splice form. J Neurosci Methods. 147:88–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frenz CT, Hansen A, Dupuis ND, Shultz N,
Levinson SR, Finger TE and Dionne VE: NaV1.5 sodium channel window
currents contribute to spontaneous firing in olfactory sensory
neurons. J Neurophysiol. 112:1091–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Ou SW, Wang YJ, Zong ZH, Lin L,
Kameyama M and Kameyama A: New variants of Nav1.5/SCN5A encode
Na+ channels in the brain. J Neurogenet. 22:57–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Ou SW, Wang YJ, Kameyama M,
Kameyama A and Zong ZH: Analysis of four novel variants of
Nav1.5/SCN5A cloned from the brain. Neurosci Res. 64:339–347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schroeter A, Walzik S, Blechschmidt S,
Haufe V, Benndorf K and Zimmer T: Structure and function of splice
variants of the cardiac voltage-gated sodium channel Na(v)1.5. J
Mol Cell Cardiol. 49:16–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Onkal R, Mattis JH, Fraser SP, Diss JK,
Shao D, Okuse K and Djamgoz MB: Alternative splicing of Nav1.5: An
electrophysiological comparison of ʻneonatalʼ and ʻadultʼ isoforms
and critical involvement of a lysine residue. J Cell Physiol.
216:716–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walzik S, Schroeter A, Benndorf K and
Zimmer T: Alternative splicing of the cardiac sodium channel
creates multiple variants of mutant T1620K channels. PLoS One.
6:e191882011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rook MB, Evers MM, Vos MA and Bierhuizen
MF: Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc
Res. 93:12–23. 2012. View Article : Google Scholar
|
|
29
|
Black JA, Newcombe J and Waxman SG:
Astrocytes within multiple sclerosis lesions upregulate sodium
channel Nav1.5. Brain. 133:835–846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aurlien D, Leren TP, Tauboll E and
Gjerstad L: New SCN5A mutation in a SUDEP victim with idiopathic
epilepsy. Seizure. 18:158–160. 2009. View Article : Google Scholar
|
|
31
|
Parisi P, Oliva A, Coll Vidal M, Partemi
S, Campuzano O, Iglesias A, Pisani D, Pascali VL, Paolino MC, Villa
MP, et al: Coexistence of epilepsy and Brugada syndrome in a family
with SCN5A mutation. Epilepsy Res. 105:415–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kerr NC, Gao Z, Holmes FE, Hobson SA,
Hancox JC, Wynick D and James AF: The sodium channel Nav1.5a is the
predominant isoform expressed in adult mouse dorsal root ganglia
and exhibits distinct inactivation properties from the full-length
Nav1.5 channel. Mol Cell Neurosci. 35:283–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zimmer T, Bollensdorff C, Haufe V,
Birch-Hirschfeld E and Benndorf K: Mouse heart Na+
channels: Primary structure and function of two isoforms and
alternatively spliced variants. Am J Physiol Heart Circ Physiol.
282:H1007–H1017. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kerr NC, Holmes FE and Wynick D: Novel
isoforms of the sodium channels Nav1.8 and Nav1.5 are produced by a
conserved mechanism in mouse and rat. J Biol Chem. 279:24826–24833.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wasner U, Geist B, Battefeld A, Bauer P,
Müller J, Rolfs A and Strauss U: Specific properties of sodium
currents in multipotent striatal progenitor cells. Eur J Neurosci.
28:1068–1079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren CT, Li DM, Ou SW, Wang YJ, Lin Y, Zong
ZH, Kameyama M and Kameyama A: Cloning and expression of the two
new variants of Nav1.5/SCN5A in rat brain. Mol Cell Biochem.
365:139–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Blechschmidt S, Haufe V, Benndorf K and
Zimmer T: Voltage-gated Na+ channel transcript patterns
in the mammalian heart are species-dependent. Prog Biophys Mol
Biol. 98:309–318. 2008. View Article : Google Scholar
|
|
38
|
Makielski JC, Ye B, Valdivia CR, Pagel MD,
Pu J, Tester DJ and Ackerman MJ: A ubiquitous splice variant and a
common polymorphism affect heterologous expression of recombinant
human SCN5A heart sodium channels. Circ Res. 93:821–828. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tan BH, Valdivia CR, Rok BA, Ye B, Ruwaldt
KM, Tester DJ, Ackerman MJ and Makielski JC: Common human SCN5A
polymorphisms have altered electrophysiology when expressed in
Q1077 splice variants. Heart Rhythm. 2:741–747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan BH, Valdivia CR, Song C and Makielski
JC: Partial expression defect for the SCN5A missense mutation
G1406R depends on splice variant background Q1077 and rescue by
mexiletine. Am J Physiol Heart Circ Physiol. 291:H1822–H1828. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang DW, Desai RR, Crotti L, Arnestad M,
Insolia R, Pedrazzini M, Ferrandi C, Vege A, Rognum T, Schwartz PJ
and George AL Jr: Cardiac sodium channel dysfunction in sudden
infant death syndrome. Circulation. 115:368–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Camacho JA, Hensellek S, Rougier JS,
Blechschmidt S, Abriel H, Benndorf K and Zimmer T: Modulation of
Nav1.5 channel function by an alternatively spliced sequence in the
DII/DIII linker region. J Biol Chem. 281:9498–9506. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xing D, Wang J, Ou S, Wang Y, Qiu B, Ding
D, Guo F and Gao Q: Expression of neonatal Nav1.5 in human brain
astrocytoma and its effect on proliferation, invasion and apoptosis
of astrocytoma cells. Oncol Rep. 31:2692–2700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brackenbury WJ, Chioni AM, Diss JK and
Djamgoz MB: The neonatal splice variant of Nav1.5 potentiates in
vitro invasive behaviour of MDA-MB-231 human breast cancer cells.
Breast Cancer Res Treat. 101:149–160. 2007. View Article : Google Scholar
|
|
45
|
Fraser SP, Diss JK, Chioni AM, Mycielska
ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et
al: Voltage-gated sodium channel expression and potentiation of
human breast cancer metastasis. Clin Cancer Res. 11:5381–5389.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
House CD, Vaske CJ, Schwartz AM, Obias V,
Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et
al: Voltage-gated Na+ channel SCN5A is a key regulator
of a gene transcriptional network that controls colon cancer
invasion. Cancer Res. 70:6957–6967. 2010. View Article : Google Scholar : PubMed/NCBI
|