Introduction
Deafness is a serious event that impacts human
health. Maintenance of normal hearing and cochlear function
requires adequate oxygenation and perfusion (1). Hypoxia, especially chronic hypoxia,
e.g. long-term living in special environment, such as the
highlands, has an evident detrimental effect on cochlear function
and hearing sensitivity (2).
Hypoxia in neonates with inadequate blood-inner ear barrier
function causes damage to the inner ear more easily than in adults,
leading to hearing loss and equilibration disorder (3). Intrauterine hypoxia at the prenatal
stage leads to severe damage during fetal development. Intrauterine
hypoxia is a common environmental stressor caused by maternal
(smoking, environmental pollutants), placental (placental
insufficiency) or fetal factors (anemia, cardiac defects) (4). Depending on the severity of the
hypoxic insult, affected fetuses will have varying degrees of
injury, from alterations in metabolic, endocrine and hematological
systems, to overt tissue necrosis in the most severe settings,
including reversible or irreversible injury in the cochlea and
hearing loss (5-7).
Among the factors involved in hearing loss at the
fetal and natal stages, connexin 26 (Cx26), which is coded by gap
junction protein β2 gene, is considered to have an important role
in maintaining normal hearing. Connexins are components of gap
junctions that facilitate the transfer of small molecules between
cells. Cx26, one member of the connexin family, is present in gap
junctions in the sensory epithelia of the inner ear (8). Cx26 mutations are one of the most
common causes of inherited nonsyndromic deafness (8,9),
presbycusis (10), and other
disease types, including skin disorders (11). In various hearing loss models,
Cx26 was frequently observed at low expression levels in inner ear
tissues (12,13) and the recovery of Cx26 was
beneficial to hearing loss (14).
It is less well-established how Cx26 expression is changed and
regulated in the inner ear. A variety of putative
post-translational modifications of Cx26 have been identified,
including acetylation, hydroxylation, γ-carboxyglutamation,
methylation and phosphorylation, some of which occur at sites of
deafness-causing mutations (11).
As methylation of the cytosine residues at CpG dinucleotides of the
promoter region is reported to lead to transcriptional silencing of
many genes (15,16), hypermethylation of the CpG sites
of promoter region of the Cx26 gene was also reported in the
cochlea of inner ear, and was considered to be a cause of Cx26
expression downregulation (12).
In tissues other than ear, the Cx26 expression level is inversely
correlated with the status of methylation. Cx26 expression was
decreased, which was accompanied by the hypermethylation of the 5′
upstream region of this gene in rat lung adenocarcinomas,
colorectal cancer and hepatocellular carcinomas (17-19). Currently, the association between
hearing loss from chronic prenatal hypoxia and Cx26 expression and
regulation is less reported. Thus, the aim of the current study was
to investigate the expression level of Cx26 and the CpG methylation
status of the promoter region in the cochlea of newborn rats that
were exposed to chronic intermittent maternal hypoxia.
Materials and methods
Animals
All experimental procedures were performed in
accordance with National Institutes of Health guidelines, and
approved by the Standing Committee on Ethics and Animal
Experimentation at the Fujian Medical University (Fujian, China).
Virgin female Sprague-Dawley rats used in this study were obtained
from the Shanghai Experimental Animal Center (Shanghai, China).
They were housed under controlled temperature (22±1°C), humidity
(50±10%), fresh air (100%) and light conditions (lights on from
7:00 am to 7:00 pm). Food and water were freely available. For
breeding, 3-month-old females were caged with males of the same
strain (rate 2:1). The vaginal smears were checked each morning for
the presence of spermatozoa. Pregnancy was confirmed by
sperm-positive vaginal smear (day 0). On day 7, the pregnant rats
were randomly assigned to control and maternal hypoxia groups (n=6
each; 12 rats in total; weighing prior to breeding 25–30 g).
Chronic prenatal hypoxia
Chronic prenatal hypoxia was performed by chronic
maternal intermittent hypoxia (20,21), which was initiated on day 7 of
gestation until delivery day (once daily; ~14 days). Pregnant rats
in the hypoxia group were placed in a plexiglass chamber (140 L,
volume), which was continuously infused with nitrogen and
compressed air to maintain an oxygen concentration of 12±1%. Oxygen
concentration in the chamber was monitored using a portable gas
analyzer, which was calibrated daily. The expired CO2
was eliminated by circulating the atmosphere through soda lime, and
the water contained in the expired gas was trapped in a chilled
glass tank. Rats were back to normal room air after 8 h of hypoxia.
Food and water were freely available during hypoxia. Normoxic
control rats were placed into an identical plexiglass chamber, into
which only compressed air, but no nitrogen was continuously
infused. They underwent the same procedures as the animals exposed
to hypoxia. On day 1 of hypoxia administration, arterial blood
samples were with-drawn from the cannulated femoral artery after 1
h of hypoxia for the measurement of blood gas and pH by a blood gas
analyzer (Rapidlab 850; Bayer AG, Leverkusen, Germany).
Deafness screening in offspring rats
Offspring rats (n=120; 60 per group) were tested for
auditory brainstem response (ABR) in the first week following
birth. Acoustic stimulation and recordings were performed with an
Auditory Evoked Potential Workstation (ICS ChartR EP 200;
Otometrics A/S, Copenhagen, Denmark). Briefly, animals were located
in a double shielded booth and were placed inside a
sound-attenuating room. Rats were anesthetized with 10% chloral
hydrate (300 mg/kg) by intraperitoneal injection. Recording
electrodes were placed at the crossing of the sagittal median of
calvaria and the line of bilateral external ears. Electrode needles
were penetrated 0.5 cm into the subderma. Reference electrodes were
placed subcutaneously at bilateral retroauricular areas. Ground
electrodes were placed at the nasal root. Electrodes resistance was
set at <3 KΩ. The parameters of click sound stimuli included
cycle 100 µsec, velocity 21.1, scan time 15 msec, range of
wave filter 100–3,000 Hz, supraposition 1,024 times. Testing
started from auditory stimulus of 100 dB sound pressure level
(SPL), which descended in 5 dB SPL increments until wave III
disappeared. The dB SPL value when wave III disappeared was set as
the ABR threshold. All offspring rats received ABR test. Deafness
was confirmed when the ABR threshold was ≥15 dB on at least one
ear.
Hematoxylin and eosin (H&E)
staining
The cochleae from 56-day-old offspring were
extracted for H&E staining (n=6). The cochleae were dissected
immediately from the auditory vesicles upon sacrifice. Tissues were
fixed with 4% paraformaldehyde overnight at 4°C, decalcified with
10% EDTA 1 h at room temperature, dehydrated with a graded series
of ethanol (100, 90, 80 and 70%, 5 min each step) at room
temperature, cleared in xylene for 10 min at room temperature, and
embedded in paraffin. Sections were cut at a thickness of 3
µm and stained with H&E containing 0.1% eosin B and 0.7%
Hematoxylin solution.
Western blot analysis
The cochleae from 56-day old offspring were used to
examine Cx26 protein expression with western blot analysis. The
cochlear tissues were homogenized in radioimmunoprecipitation assay
lysis buffer (Sigma-Aldrich, St. Louis, MO, USA). Homogenates were
centrifuged at 10,000 × g at 4°C for 5 min, and the soluble protein
content of the supernatant was determined with a bicinchoninic acid
protein assay kit (Xiamen Bioluminor Bio-Technology Co., Ltd.,
Xiamen, China). A total of 30 µg total protein was
electrophoretically separated using 4–12% SDS-PAGE gels and then
transferred to nitrocellulose membranes. Following 30 min of
blocking with 2.5% skimmed milk at room temperature, the membranes
were incubated with rabbit anti-Cx26 polyclonal antibody (1:1,000;
cat. no. sc-130729) or rabbit anti-β-actin polyclonal antibody
(1:5,000; cat. no. sc-130656; both from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) overnight at 4°C, followed by 1 h incubation
with a horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin secondary antibody (1:2,000; cat. no. sc-2004; Santa
Cruz Biotechnology, Inc.). Following treatment with each antibody,
the membranes were washed with phosphate-buffered saline containing
0.5% Tween-20 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Proteins on the membranes were visualized using enhanced
chemiluminescence reagents (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and then exposed to X-ray film. The intensity of
bands was analyzed with Quantity One v4.62 analyzer software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The protein levels
are expressed as the ratio of the band optical intensity to that of
β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to measure the mRNA expression
of Cx26. The cochleae of 56-day old offspring were dissected
immediately from the auditory vesicles upon sacrifice. Total RNA
was extracted using TRIzol reagent (Life Technologies; Thermo
Fisher Scientific, Inc.). The RNA (5 µg) was reverse
transcribed into cDNA using SuperScript II reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) and random
hexonucleotide primers (0.1 µg random hexonucleotide primer
and 10 pmol dNTP) at 65°C for 5 min and incubated for 1 h at 37°C.
qPCR was performed with an iCycler using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd., Dalian, China). Results are presented as
the levels of expression relative to those of the controls
subsequent to normalize to β-actin using the 2−ΔΔCq
method (22). The PCR process was
as follows: first, an initial denaturation was realized at 94°C for
5 min, then 39 cycles were performed with the following cycling
profile: denaturation at 94°C for 30 sec, annealing at 54°C for 30
sec and extension at 68°C for 30 sec each. The primer sequences for
PCR were as follows: Cx26 sense, 5′-ACC ACT ACT TCC CCA TCTCT-3′
and antisense, 5′-TCG TTC TTT ATC TCT CCC TTC-3′; β-actin sense,
5′-GGC TCT CTGC TCC TCCC-3′ and antisense, 5′-CCG TTC ACA CCG
ACCTT-3′.
Bisulfite sequencing
The evaluation of CpG site methylation of the
promoter region of Cx26 gene was performed by bisulfite sequencing
according to previous descriptions (12,23,24). The cochleae of 56-day old
offspring were obtained from the auditory vesicles immediately upon
sacrifice. Genomic DNA was extracted using a genomic DNA
purification kit (Tiangen Biotech Co., Ltd., Beijing, China)
according to the manufacturer's instructions. The purified DNA was
bisulfate treated using EZ DNA Methylation-Gold kit (Zymo Research
Corp., Irvine, CA, USA). Previous publications documented that two
target CpG islands, fragment 1 and 2, are present in the promoter
region of Cx26 (12,23,24). The two regions were then amplified
by PCR using TaKaRa Ex Taq (Takara Biotechnology Co., Ltd.) with
the following primers: Fragment 1 sense, 5′-TTT TTG GTA TTT TGT TTA
AAG TGAT-3′ and antisense, 5′-ATA TAA ACC AAC AAC TTC CAA TATC-3′;
fragment 2 sense, 5′-GGA GTG ATT TAG GTT TTA GGA GAG-3′ and
antisense, 5′-TCC CCA CAA ATC CTA ATA AAA ACTAC-3′. PCR products
were purified and then were inserted into pMD19-T Simple Vector
(Takara Biotechnology Co., Ltd.) and transformed to competent
Escherichia coli DH5α (Beijing Transgen Biotech Co., Ltd.,
Beijing, China). The consequent recombinant plasmids were extracted
with TIANprep mini plasmid extraction kit (Tiangen Biotech Co.,
Ltd.). The extracted plasmids were sequenced to analyze the status
of DNA methylation (Tsingke Biotech Co., Wuhan, China). Methylation
level was expressed as the ratio of methylated CpG sites to the
total CpG site number in fragments 1 and 2.
Statistical analysis
Data re presented as the mean ± standard error. The
unpaired Student's two-tailed t-test was used for comparing two
groups. All data analyses were performed using SPSS 11.5 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Arterial blood gas analysis
At day 1 of hypoxia, arterial blood was withdrawn
from cannulated femoral artery of maternal rats after 1 h of
hypoxia for blood gas analysis. The results showed that arterial
O2 partial pressure and oxygen saturation were
significantly decreased in the hypoxia group compared with the
control group. There were no significant differences in mean
arterial CO2 partial pressure and pH value between the
two groups (Table I). These
results indicate that there was hypoxemia without CO2
retention and acidosis in the hypoxia group, and suggest a reliable
hypoxia animal model.
 | Table IArterial blood gas analysis of
maternal rats. |
Table I
Arterial blood gas analysis of
maternal rats.
| Group | PaO2
(kPa) | SaO2
(%) | PaCO2
(kPa) | pH |
|---|
| Control | 11.17±2.68 | 92.6±3.0 | 6.21±1.15 | 7.4±1.0 |
| Hypoxia | 7.19±2.75a | 75.3±3.4a | 5.98±2.17 | 7.3±2.2 |
Chronic maternal hypoxia results in
intrauterine growth restriction of offspring
Every maternal rat in the two groups gave birth to
12–13 pups. The body weight of offspring was recorded following
birth. The data showed that the body weight at neonatal stage (12 h
after birth) was significantly lower in the hypoxia group than in
the control group (Table II). At
later time-points, 21 and 56 days, there was no significant
difference in body weight between the two groups, although the body
weight of the hypoxia group was a marginally more than in the
control group. Thus, these results suggest that chronic maternal
hypoxia caused a significant intrauterine growth restriction of
offspring.
 | Table IIBody weight of offspring at different
time points after birth. |
Table II
Body weight of offspring at different
time points after birth.
| 12 h | 21 days | 56 days |
|---|
| Control (n=60) | 6.0±0.4 | 52.4±3.0 | 302.3±11.0 |
| Hypoxia (n=60) | 4.8±0.6a | 58.8±9.0 | 306.6±21.0 |
Chronic maternal hypoxia causes hearing
dysfunction in offspring
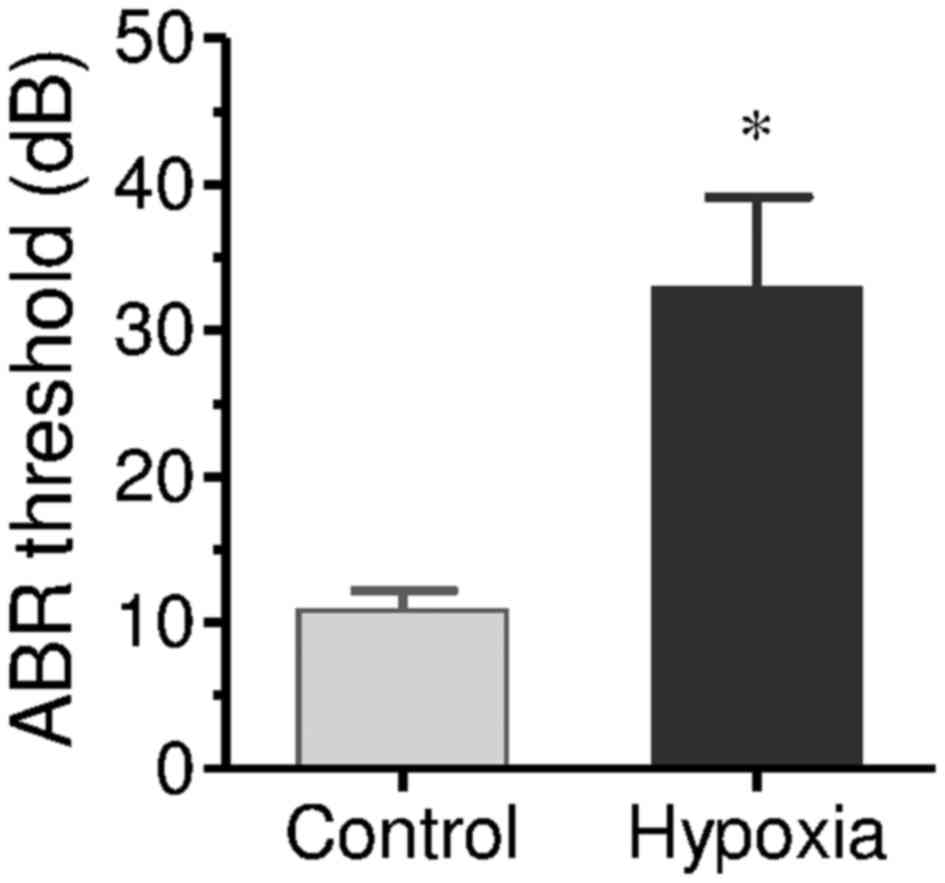
ABR testing was performed on newborn rats 1 week
after delivery. In the offspring rats from the control group, only
one rat exhibited hearing dysfunction. The mean ABR threshold was
10.84 dB. This deaf rat was excluded in further testing. In the
offspring rats from the hypoxia group, 28 rats were confirmed as
deaf. ABR threshold (mean threshold) was 32.45 dB (P<0.01;
Fig. 1). This indicates that
chronic maternal hypoxia effectively caused offspring hearing
dysfunction. The normal hearing rats were excluded, and only the
deaf offspring rats in the hypoxia group were used in further
studies.
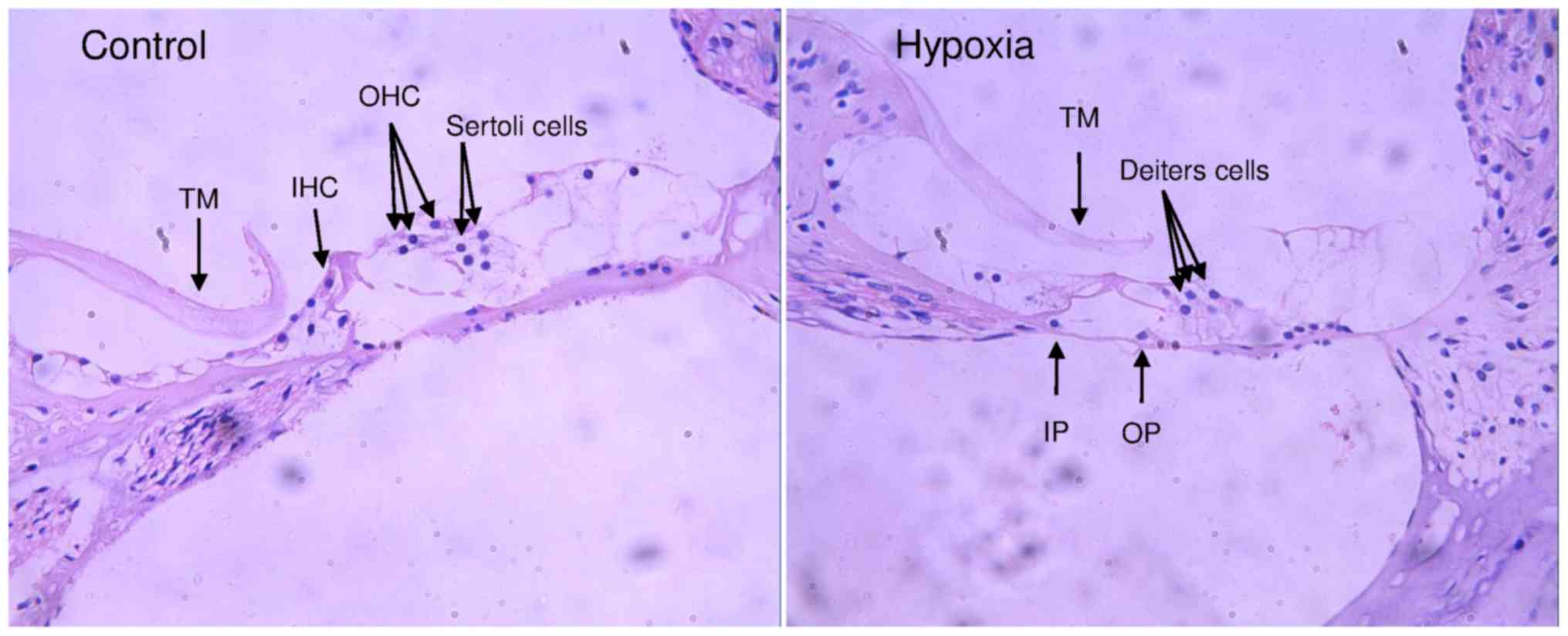
Effect of chronic prenatal hypoxia on
cochlear development
The effect of chronic prenatal hypoxia on cochlear
development was examined with H&E staining (Fig. 2). In offspring from both groups,
no cellular degeneration in cochlear nerve and the spiral ganglion
was observed. There was no inflammatory cell infiltration in the
organ of Corti, and the stria vascularis was clear. In the organ of
Corti of the offspring from control group, sporadic inner hair
cells, outer hair cell and sertoli cells were observed. In the
hypoxia group, only Deiters cells, and some inner pillar cells and
outer pillar cells were observed in organ of Corti, however no
inner and outer hair cells were observed. These results indicate
chronic prenatal hypoxia caused marked abnormal cochlear
development.
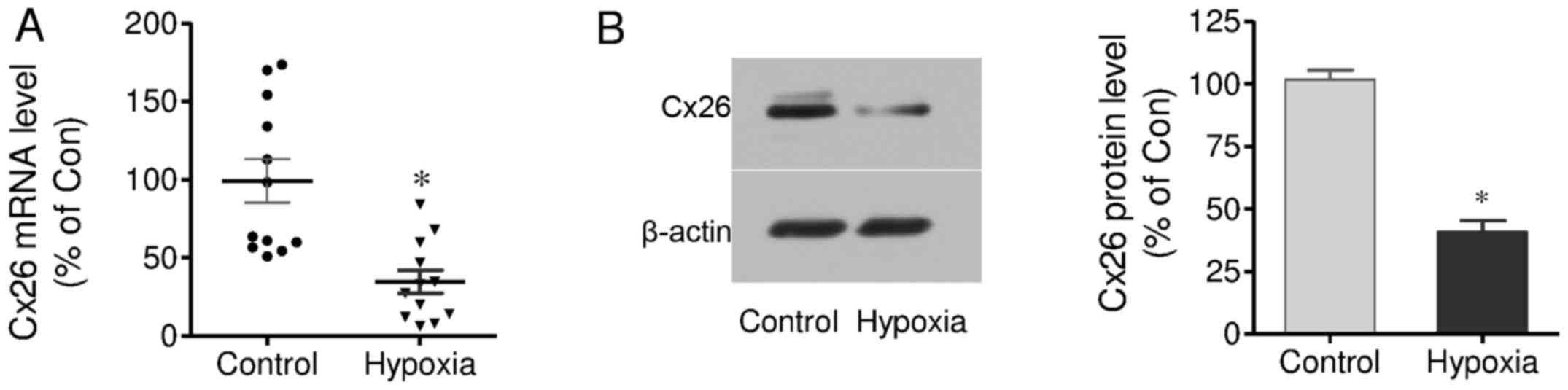
Effect of chronic prenatal hypoxia on
Cx26 mRNA and protein expression in cochlear tissues
The effects of hypoxia on Cx26 mRNA and protein
expression in the cochleae of offspring were measured by RT-qPCR
and western blot analysis, respectively. The results demonstrated
that Cx26 mRNA expression in the hypoxia group was significantly
decreased, compared with control group. The mean value in the
hypoxia group was 34.73% of control group (Fig. 3A). Similarly, Cx26 protein
expression was also significantly decreased in the hypoxia group,
and it was 40.85% of the control group (Fig. 3B). These results demonstrate
chronic prenatal hypoxia negatively regulates Cx26 expression in
cochlear tissues.
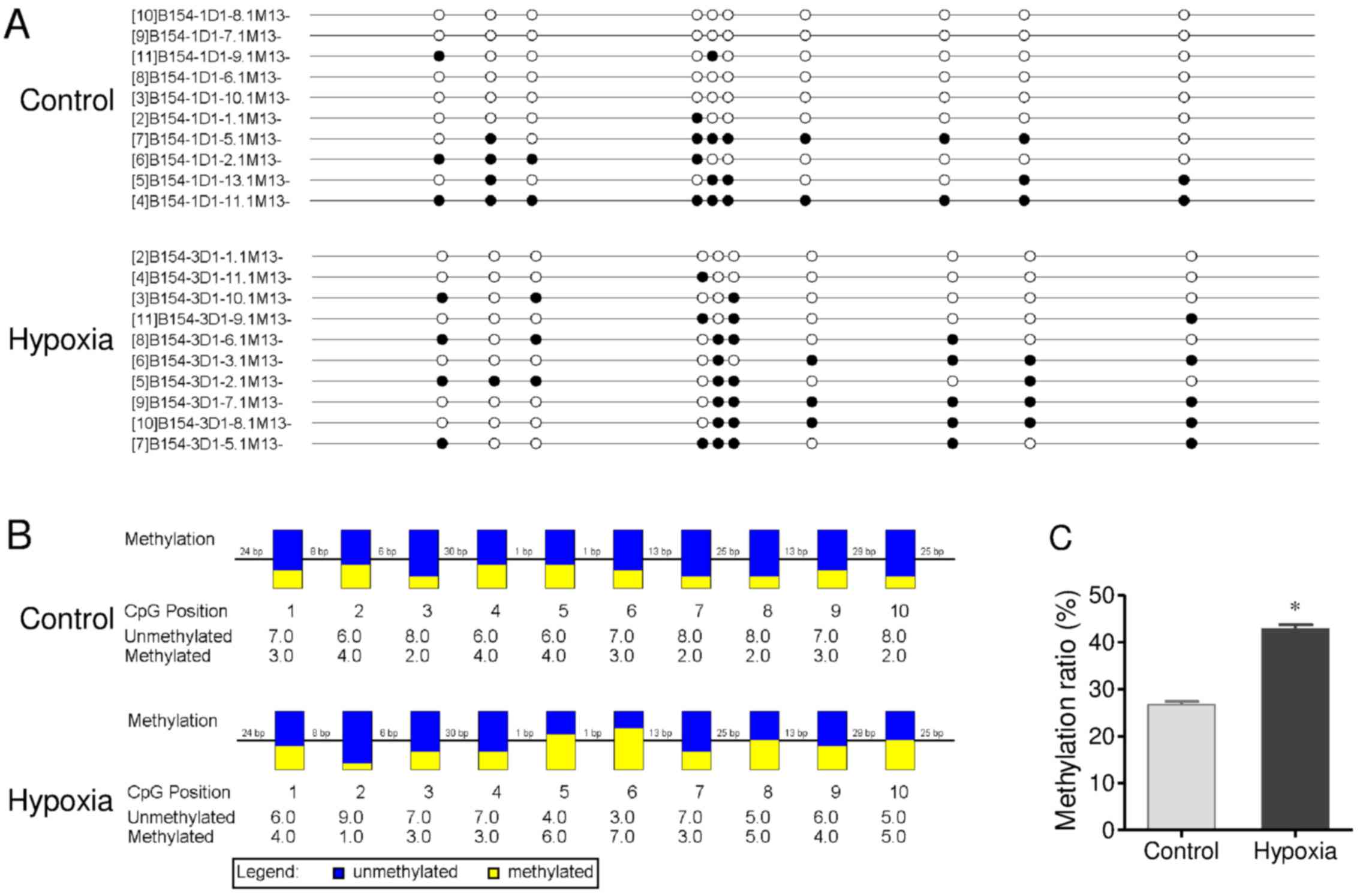
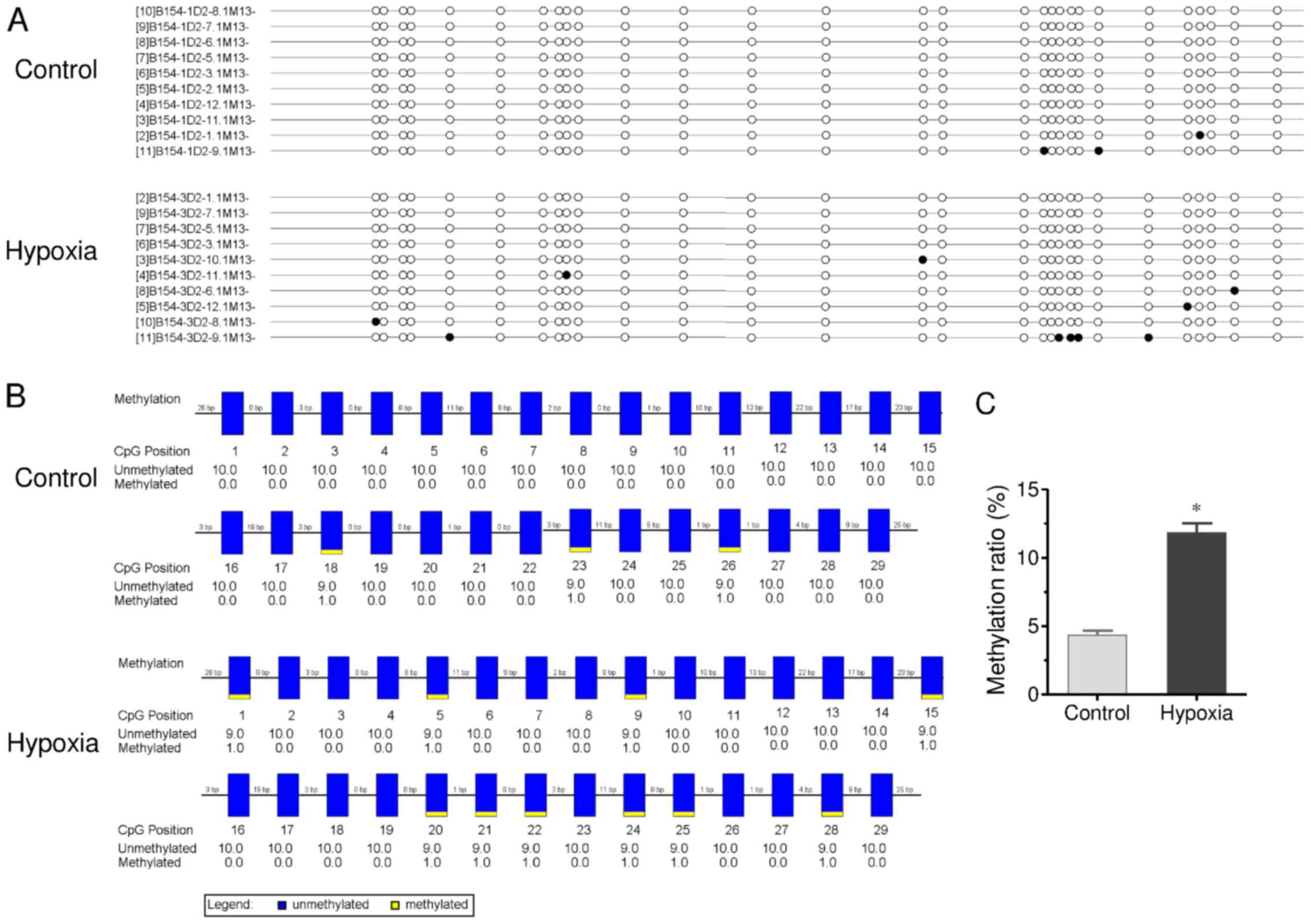
CpG site methylation of the promoter
region of Cx26 gene in cochlear tissues
CpG site methylation of the promoter region of Cx26
(including fragment 1 and 2) in cochlear tissues of offspring
exposed to hypoxia in utero was measured by bisulfite
sequencing PCR (Figs. 4 and
5). As shown in Fig. 4, many methylation sites were
detected in fragment 1 of the promoter region of Cx26. The
methylation ratio was up to 26.7% in the control group. Chronic
maternal hypoxia increased the methylation ratio to 42.8%
(P<0.01 vs. control). In fragment 2 (Fig. 5), although a low methylation ratio
(4.3%) of the promoter region of Cx26 was observed in the control
group, a significantly higher methylation ratio was detected in the
hypoxia group (11.8%; P<0.01). These results demonstrate chronic
maternal hypoxia increases CpG site methylation of the promoter
region of Cx26 gene in cochlear tissues of offspring.
Discussion
The present study aimed to evaluate the effect of
chronic prenatal hypoxia on hearing development and the associated
mechanisms. Long-term prenatal hypoxia, lasting for 2 weeks
preterm, results in hearing dysfunction and deficiency of hair cell
development in the cochlea. These alterations were accompanied by
downregulation of Cx26 expression and hypermethylation of the
promoter region of this gene. These results demonstrate that
chronic prenatal hypoxia is harmful to hearing development in an
experimental rat model. Promoter region hypermethylation and
expression downregulation of Cx26 may underlie the important
functional mechanisms.
Prenatal hypoxia may promote fetal growth
restriction and induce a series of changes in the neural,
cardiovascular, metabolic and endocrine systems of the adult
offspring, which are collectively reflected by decreased body
weight (25,26). Consistent with these studies, the
effect of chronic prenatal hypoxia on fetal hearing was examined in
the current study, and a significant decrease in body weight at the
neonatal stage (day of birth) was observed in the hypoxia group
compared with the control group. However, by adulthood (21- and
56-day), body weights of hypoxia group offspring were restored, and
even greater than normoxic offspring. The increased body weight in
adulthood may be due to increased liver-weight and body fat
deposition (26).
Although hypoxia in adults frequently causes damage
to the brain, but not the inner ear, hypoxia in neonates with
inadequate blood-inner ear barrier function can cause damage to the
inner ear, leading to sensorineural hearing loss and equilibration
disorder (3,27,28). During prenatal stage, hypoxia also
induces sensorineural hearing loss in the fetus (29). The incidence of sensorineural
hearing loss caused by direct damage to the cochlear hair cells of
the inner ear is far more frequent and more serious than disorders
affecting the external ear or the middle ear (30). Damage to the inner ear includes
the degeneration and disappearance of the outer hair cells of the
organ of Corti and edematous changes in the stria vascularis in
neonates exposed to hypoxia (3).
In the present study, inner and outer hair cell defects were
observed in the organ of Corti of hypoxia group rats. These hair
cell defects support that the hearing loss was potentially caused
by chronic prenatal hypoxia.
Cx26 has an essential role in normal neonatal
development, postnatal maturation and homoeostasis of the organ of
Corti prior to the onset of hearing (31-33). Cx26 is one of the major building
blocks of gap junctions in the human cochlea and is widely
expressed in the supporting cells of the sensory epithelial and
fibrocytes in the spiral ligament and spiral limbus (34). Cx26 establishes connectivity in
distinct cochlear compartment and forms hybrid gap junctions with
Cx30 (35). Dysfunction of gap
junctions caused by mutations in Cx26 and Cx30 accounts for nearly
half of all cases of hereditary nonsyndromic deafness cases
(36). Overexpression of cochlear
Cx26 restores gap junction function in the cochlea of conditional
Cx26 knockout mice (37) and
completely rescues hearing in Cx30-null mice deafness model
(38). Dominant negative Cx26
mutation R75W causes severe hearing loss and postnatal programmed
cell death in the organ of Corti (39). Cx26 knockdown causes malformation
of the organ of Corti and distinct cell loss (13). The results of the present study
demonstrated that Cx26 protein and mRNA expression were
significantly decreased by chronic maternal hypoxia. Combined with
the deficiency of hair cell development, the results of the current
suggest a key role of Cx26 downregulation in chronic maternal
hypoxia-induced hearing loss.
Recent studies have reported that silencing of
connexins, including Cx26, may be induced by epigenetic
inactivation through aberrant promoter-region methylation. The
promoter-region hypermethylation caused Cx26 down-regulation at
various tissue types, including colorectal carcinomas (18), lung cancer (17), hepatocellular carcinomas (19), and cochleae (12), although other studies showed Cx26
expression was not mediated by methylation in human esophageal
cancer cells (40). In the
present study, chronic prenatal hypoxia decreased Cx26 expression
in cochlear tissues. When analyzing the two target CpG clusters of
the promoter region of Cx26 (fragment 1 and 2), significantly
increased methylation in fragment 1 and 2 was detected in offspring
from hypoxia maternal rats compared with the control group. This
suggests a negative association between Cx26 expression and
hypermethylation. Notably, the number of methylation sites was far
more in fragment 1 than in fragment 2. In previous studies,
hypermethylation of fragment 2 was considered to be irrelevant to
Cx26 gene repression in human mammary cancer cell lines (41) and in the cochlea of inner ear in
mimetic aging rats (12). In the
present study, the methylation level of fragment 2 was increased in
the hypoxia group compared with the normoxic control group.
In conclusion, the present results demonstrate the
harmful effect of chronic prenatal hypoxia on hearing development.
Promoter region hypermethylation and subsequent reduced expression
of Cx26 may act as important factors that underlie the functional
mechanisms involved.
Acknowledgments
This study was supported by the Educational Research
Project of Fujian Education Department for Young and Middle-aged
Teachers (funding no. JA15718), the Key Project of Science and
Technology of Quanzhou City (funding no. 2015Z46) and the National
Natural Science Foundation (funding no. 81572662).
References
|
1
|
Cristobal R and Oghalai JS: Hearing loss
in children with very low birth weight: Current review of
epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed.
93:F462–F468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan Y, Zhang X, Huang S, Zuo L, Zhang G,
Song Y, Wang G, Wang H, Huang D, Han D, et al: Common molecular
etiologies are rare in nonsyndromic Tibetan Chinese patients with
hearing impairment. PLoS One. 7:e307202012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koyama S, Kaga K, Sakata H, Iino Y and
Kodera K: Pathological findings in the temporal bone of newborn
infants with neonatal asphyxia. Acta Otolaryngol. 125:1028–1032.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hemker SL, Sims-Lucas S and Ho J: Role of
hypoxia during nephrogenesis. Pediatr Nephrol. 31:1571–1577. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eleftheriades M, Creatsas G and Nicolaides
K: Fetal growth restriction and postnatal development. Ann NY Acad
Sci. 1092:319–330. 2006. View Article : Google Scholar
|
|
6
|
Nishioka N, Nishina H, Yoshida K,
Kinoshita K and Ehara Y: Effect of hypoxia on the auditory system
of goat fetuses during extrauterine incubation. J Obstet Gynaecol
Res. 29:109–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Widziszowska A and Namyslowski G:
Assessment of hearing organ activity in a group of neonates with
central nervous system impairment. Int J Pediatr Otorhinolaryngol.
75:1280–1284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee MY, Takada T, Takada Y, Kappy MD,
Beyer LA, Swiderski DL, Godin AL, Brewer S, King WM and Raphael Y:
Mice with conditional deletion of Cx26 exhibit no vestibular
phenotype despite secondary loss of Cx30 in the vestibular end
organs. Hear Res. 328:102–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skvorak Giersch AB and Morton CC: Genetic
causes of nonsyndromic hearing loss. Curr Opin Pediatr. 11:551–557.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lalwani AK and Castelein CM: Cracking the
auditory genetic code: Nonsyndromic hereditary hearing impairment.
Am J Otol. 20:115–132. 1999.PubMed/NCBI
|
|
11
|
Locke D, Bian S, Li H and Harris AL:
Post-translational modifications of connexin26 revealed by mass
spectrometry. Biochem J. 424:385–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X, Wang Y, Sun Y, Chen S, Zhang S, Shen
L, Huang X, Lin X and Kong W: Reduced expression of Connexin26 and
its DNA promoter hypermethylation in the inner ear of mimetic aging
rats induced by D-galactose. Biochem Biophys Res Commun.
452:340–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Sun Y, Lin X and Kong W: Down
regulated connexin26 at different postnatal stage displayed
different types of cellular degeneration and formation of organ of
Corti. Biochem Biophys Res Commun. 445:71–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong M, Zhu Y, Lai H, Fu X, Deng W, Yang
C, He Q and Zheng G: Radix astragali inhibits the down-regulation
of connexin 26 in the stria vascularis of the guinea pig cochlea
after acoustic trauma. Eur Arch Otorhinolaryngol. 272:2153–2160.
2015. View Article : Google Scholar
|
|
15
|
Virmani AK, Muller C, Rathi A,
Zoechbauer-Mueller S, Mathis M and Gazdar AF: Aberrant methylation
during cervical carcinogenesis. Clin Cancer Res. 7:584–589.
2001.PubMed/NCBI
|
|
16
|
Yano T, Ito F, Kobayashi K, Yonezawa Y,
Suzuki K, Asano R, Hagiwara K, Nakazawa H, Toma H and Yamasaki H:
Hypermethylation of the CpG island of connexin 32, a candiate tumor
suppressor gene in renal cell carcinomas from hemodialysis
patients. Cancer Lett. 208:137–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimizu K, Shimoichi Y, Hinotsume D,
Itsuzaki Y, Fujii H, Honoki K and Tsujiuchi T: Reduced expression
of the Connexin26 gene and its aberrant DNA methylation in rat lung
adenocarcinomas induced by N-nitrosobis(2-hydroxypropyl)amine. Mol
Carcinog. 45:710–714. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sirnes S, Lind GE, Bruun J, Fykerud TA,
Mesnil M, Lothe RA, Rivedal E, Kolberg M and Leithe E: Connexins in
colorectal cancer pathogenesis. Int J Cancer. 137:1–11. 2015.
View Article : Google Scholar
|
|
19
|
Tsujiuchi T, Shimizu K, Itsuzaki Y, Onishi
M, Sugata E, Fujii H and Honoki K: CpG site hypermethylation of
E-cadherin and Connexin26 genes in hepatocellular carcinomas
induced by a choline-deficient L-Amino Acid-defined diet in rats.
Mol Carcinog. 46:269–274. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kane AD, Herrera EA, Camm EJ and Giussani
DA: Vitamin C prevents intrauterine programming of in vivo
cardiovascular dysfunction in the rat. Circ J. 77:2604–2611. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen F, Du S, Bian J, You ZB and Wu Y:
Chronic hypoxia exposure during pregnancy is associated with a
decreased active nursing activity in mother and an abnormal birth
weight and postnatal growth in offspring of rats. Horm Behav.
61:504–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Shimizu K, Hanaoka M, Kato A, Fujii H,
Honoki K and Tsujiuchi T: Reduced expression of the E-cadherin gene
and its aberrant DNA methylation in hamster pancreatic tumors.
Biochem Biophys Res Commun. 336:49–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takai D and Jones PA: Comprehensive
analysis of CpG islands in human chromosomes 21–22. Proc Natl Acad
Sci USA. 99:3740–3745. 2002. View Article : Google Scholar
|
|
25
|
Giussani DA, Niu Y, Herrera EA, Richter
HG, Camm EJ, Thakor AS, Kane AD, Hansell JA, Brain KL, Skeffington
KL, et al: Heart disease link to fetal hypoxia and oxidative
stress. Adv Exp Med Biol. 814:77–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iqbal W and Ciriello J: Effect of maternal
chronic intermittent hypoxia during gestation on offspring growth
in the rat. Am J Obstet Gynecol. 209:564.e1–564.e9. 2013.
View Article : Google Scholar
|
|
27
|
Daniel SJ, McIntosh M, Akinpelu OV and
Rohlicek CV: Hearing outcome of early postnatal exposure to hypoxia
in Sprague-Dawley rats. J Laryngol Otol. 15:1–5. 2014.
|
|
28
|
Mencher LS and Mencher GT: Neonatal
asphyxia, definitive markers and hearing loss. Audiology.
38:291–295. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sohmer H and Freeman S: Hypoxia induced
hearing loss in animal models of the fetus in-utero. Hear Res.
55:92–97. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mazurek B, Haupt H, Georgiewa P, Klapp BF
and Reisshauer A: A model of peripherally developing hearing loss
and tinnitus based on the role of hypoxia and ischemia. Med
Hypotheses. 67:892–899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang Q, Tang W, Kim Y and Lin X: Timed
conditional null of connexin26 in mice reveals temporary
requirements of connexin26 in key cochlear developmental events
before the onset of hearing. Neurobiol Dis. 73:418–427. 2015.
View Article : Google Scholar
|
|
32
|
Sun Y, Tang W, Chang Q, Wang Y, Kong W and
Lin X: Connexin30 null and conditional connexin26 null mice display
distinct pattern and time course of cellular degeneration in the
cochlea. J Comp Neurol. 516:569–579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Chang Q, Tang W, Sun Y, Zhou B, Li
H and Lin X: Targeted connexin26 ablation arrests postnatal
development of the organ of Corti. Biochem Biophys Res Commun.
385:33–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu W, Boström M, Kinnefors A and
Rask-Andersen H: Unique expression of connexins in the human
cochlea. Hear Res. 250:55–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahmad S, Chen S, Sun J and Lin X:
Connexins 26 and 30 are co-assembled to form gap junctions in the
cochlea of mice. Biochem Biophys Res Commun. 307:362–368. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P
and Lin X: Gap junction-mediated intercellular biochemical coupling
in cochlear supporting cells is required for normal cochlear
functions. Proc Natl Acad Sci USA. 102:15201–15206. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu Q, Wang Y, Chang Q, Wang J, Gong S, Li
H and Lin X: Virally expressed connexin26 restores gap junction
function in the cochlea of conditional Gjb2 knockout mice. Gene
Ther. 21:71–80. 2014. View Article : Google Scholar
|
|
38
|
Ahmad S, Tang W, Chang Q, Qu Y, Hibshman
J, Li Y, Söhl G, Willecke K, Chen P and Lin X: Restoration of
connexin26 protein level in the cochlea completely rescues hearing
in a mouse model of human connexin30-linked deafness. Proc Natl
Acad Sci USA. 104:1337–1341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Inoshita A, Karasawa K, Funakubo M, Miwa
A, Ikeda K and Kamiya K: Dominant negative connexin26 mutation R75W
causing severe hearing loss influences normal programmed cell death
in postnatal organ of Corti. BMC Genet. 15:12014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Loncarek J, Yamasaki H, Levillain P,
Milinkevitch S and Mesnil M: The expression of the tumor suppressor
gene connexin 26 is not mediated by methylation in human esophageal
cancer cells. Mol Carcinog. 36:74–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singal R, Tu ZJ, Vanwert JM, Ginder GD and
Kiang DT: Modulation of the connexin26 tumor suppressor gene
expression through methylation in human mammary epithelial cell
lines. Anticancer Res. 20:59–64. 2000.PubMed/NCBI
|