Introduction
Previous studies have revealed that oxidative stress
is associated with almost every aspect promoting diabetes and its
complications, thus suggesting the importance of antioxidant
therapy in the development of novel treatments for this disease
(1,2). Particularly, pancreatic β cells are
vulnerable to oxidative stress, as they have a high metabolic
activity and low antioxidant capacity. Once the overproduction of
reactive oxygen species (ROS) exceeds the available antioxidant
defense systems, it may result in cellular membrane, protein, RNA
and DNA damage, dysregulation of ROS signaling pathways and
activation of inflammatory responses, which may eventually lead to
the dysfunction or apoptosis of pancreatic islet β cells (3). Scavenging of ROS by antioxidants and
the re-establishment of redox homeostasis in pancreatic cells
offers a promising strategy to alleviate the suffering of patients
with diabetes. Notably, ROS neutralization by antioxidants has not
yielded the expected outcomes (4). Numerous studies have suggested that
it would be more beneficial to target the pathways involved in ROS
generation (5,6). Therefore, the identification of
antioxidants and the characterization of their associated signaling
pathways are critical for future antidiabetes drug design.
2′,3,4′,5,7-Pentahydroxyflavone (morin) has
exhibited novel activity in reducing ROS production and increasing
the antioxidative capacity of cells; therefore, it has received
increasing attention with regards to its clinical applications.
Morin is a dietary flavonol found in fruits or dietary plants,
including figs, apples, tea and cereal grains (7,8).
Morin treatment has not exhibited any lethal toxicity to
experimental animals, even at high doses (9,10).
In addition, the beneficial effects of morin against oxidative
stress-induced cell damage have been demonstrated in cardiovascular
cells (11), hepatocytes
(12) and neurons (13). Morin is effective in retaining the
normal histological appearance of pancreatic islets, as well as in
preserving insulin-positive β cells in streptozotocin (STZ)-induced
diabetic rats (14). Furthermore,
morin has been reported to increase protein expression and enzyme
activity of catalase in lung fibroblast cells (15). However, in pancreatic cells, the
detailed molecular mechanisms responsible for the protective
effects of morin have yet to be elucidated.
Increasing evidence has indicated that the 5′
adenosine monophosphate-activated protein kinase (AMPK) pathway
exerts protective effects against oxidative stress-induced cell
damage (16–18). The AMPK pathway is a key energy
metabolic signaling pathway involved in ROS regulation (19). It has been reported that AMPK
activation can promote cell survival by inducing autophagy,
mitochondrial biogenesis and the expression of genes, which are
involved in antioxidant defense in response to oxidative stress
(20,21). Forkhead box O (FOXO) transcription
factors are prime candidates that are regulated by AMPK. Previous
studies have revealed the pivotal roles of FOXO3 in cell resistance
to oxidative stress and highlighted the clinical potential to
develop novel therapeutic strategies through approaches that target
FOXO3 (22,23). Inactivation of FOXO3 and its
downstream target genes contributes to oxidative stress in early
diabetic nephropathy, and accelerates renal disease (24). In cardiomyocytes, activated FOXO3
may increase downstream target antioxidant enzymes, including
catalase, effectively manage ROS and alleviate diabetic
cardiomyopathy (25,26). Regulating the transcription of
antioxidant enzymes based on targeting the AMPK-FOXO3 signaling
pathway has been suggested as a promising approach to limit the
progression of oxidative stress-mediated diseases and has merited
intensive investigation (16,27,28).
Although there is some evidence to suggest that the
antidiabetic effects of morin may be due to its capacity to
decrease oxidative stress, few studies have focused on its
cytoprotective effects on pancreatic β cells and the underlying
molecular mechanism. Due to the potential role of the AMPK-FOXO3
pathway in protecting cells against oxidative stress, it is of
great interest to determine whether morin may activate this
pathway, and thus upregulate the antioxidant enzyme catalase.
Therefore, the present study aimed to investigate the protective
effects of morin against STZ-induced β cell damage and to identify
the underlying molecular mechanism. The purpose of the present
study was to characterize a potential antioxidant with precise
targeting for the development of novel therapeutic strategies for
the treatment of diabetes and associated complications.
Materials and methods
Reagents
Morin, 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA), MTT, Hoechst 33342 and STZ were all purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Catalase
(sc-271803) and β-actin (sc-8432) primary antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Phosphorylated-AMPKα (Thr-172; cat. no. 2531), AMPKα (cat. no.
5831) and FOXO3 (cat. no. 2497) antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Anti-TATA binding
protein (TBP; ab818) antibody was obtained from Abcam (Cambridge,
MA, USA). Anti-poly (ADP-ribose) (PAR; cat. no. 4335-MC-100)
antibody was purchased from Trevigen (Gaithersburg, MD, USA). All
other chemicals and reagents were of analytical grade.
Cell culture
RINm5F rat pancreatic β cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). All the cells
used in this study were maintained at 37°C in a humidified
atmosphere containing 5% CO2, and were cultured in
Roswell Park Memorial Institute-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum, streptomycin (100
µg/ml) and penicillin (100 U/ml).
Determination of the toxicity of STZ
Cells were seeded into 96-well plates at a density
of 1×105 cells/ml. After 16 h, cells were incubated for
24 h with various concentrations of STZ dissolved in 0.1 M citrate
buffer (pH 4.5); the final concentrations were as follows: 1, 2, 4,
6, 8 and 10 mM. The toxicity of STZ on RINm5F cells was examined
using the MTT assay, which is based on the cleavage of a
tetrazolium salt by mitochondrial dehydrogenase in viable cells
(29).
Cell viability assay
To evaluate the effects of morin on STZ-induced cell
death, cell viability was examined using the MTT assay. Cells were
seeded into 96-well plates at a density of 1×105
cells/ml. After 16 h, the cells were treated with various
concentrations of morin (10, 25 and 50 µM) for 1 h, followed
by exposure to STZ for 24 h at 37°C. Subsequently, 50 µl MTT
stock solution (2 mg/ml) was added to each well and incubated for a
further 4 h at 37°C. The medium containing MTT was aspirated and
the formazan crystals in the viable cells were then dissolved using
150 µl dimethyl sulfoxide. The absorbance of each well was
determined using a Multiskan GO Spectrophotometer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 490 nm.
Determination of intracellular ROS
Levels of intracellular ROS were determined using
the DCFH-DA method as previously described (15). Briefly, RINm5F cells were seeded
in 6-well plates at 1×105 cells/ml. After 16 h, the
cells were treated with 25 µM morin for 1 h. Following
exposure to STZ for 12 h, cells were collected and incubated with
10 µM DCFH-DA at 37°C for 30 min. Subsequently, the cells
were rinsed twice and resuspended in PBS, and fluorescence
intensity was measured by flow cytometry (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA). The data were analyzed using
CellQuest (version 7.5.3; BD Biosciences). The relative
fluorescence intensity was recorded as the mean value from three
repeated experiments. Image analysis for the generation of
intracellular ROS was also conducted. Briefly, cells were seeded
onto cover slips in 6-well plates at 1×105 cells/ml and
were incubated with 5 µM DCFH-DA for 30 min at 37°C as
previously described. After washing with PBS, the stained cells
were mounted onto a microscope slide in mounting medium (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA). Microscopic
images were captured under a laser confocal microscope (Zeiss GmbH,
Jena, Germany).
Determination of cellular nicotinamide
adenine dinucleotide (NAD+)
Intracellular NAD+ content was measured
using the EnzyChrom™ NAD+/NADH quantification kit
(BioAssay Systems, Hayward, CA, USA), according to the
manufacturer's protocol.
Detection of sub-G1
hypodiploid cell
The proportion of apoptotic sub-G1
hypodiploid cells was determined by flow cytometry (30). Cells were initially treated with
25 µM morin for 1 h. Subsequently, STZ was added and cells
were incubated for a further 24 h. Harvested cells were washed
twice with PBS and fixed in 70% ethanol for 30 min at 4°C. Cells
were then rinsed twice with PBS and were incubated for 30 min in
the dark at 37°C in 1 ml PBS containing 100 µg propidium
iodide (PI) and 100 µg RNase A. Flow cytometric analysis was
performed using a flow cytometer (FACSCalibur; BD Biosciences). The
proportion of sub-G1 hypodiploid cells was assessed
using histograms generated by the computer programs CellQuest
(version 7.5.3; BD Biosciences) and ModFit (version 3.0; Verity
Software House, Inc., Topsham, ME, USA).
Nuclear staining with Hoechst 33342
Cells were plated in 24-well plates at
1×105 cells/ml. After 16 h, cells were treated with 25
µM morin for 1 h and then with STZ at a final concentration
of 6 mM for 24 h. Subsequently, a DNA-specific fluorescent dye,
Hoechst 33342, at a final concentration of 15 µg/ml, was
added to each well and incubated for 10 min at 37°C. The stained
cells were then observed using a fluorescence microscope to examine
the extent of nuclear condensation.
Quantitative polymerase chain reaction
(qPCR) analysis
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
synthesis was performed with 2 µg isolated RNA using
SuperScript III Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.). qPCR was performed in 96-well optical plates
using a CFX-96 Touch™ Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The PCR reaction was
conducted in a total volume of 20 µl: 10 µl 2X SYBR
Premix Ex Taq II kit (Takara Bio, Inc., Otsu, Japan), 1
µl diluted template cDNA (~10 ng) and 10 µM each
forward and reverse primers. The PCR protocol was conducted as
follows: Pre-incubation at 95°C for 30 sec, followed by 40 cycles
of denaturation at 95°C for 5 sec and annealing and extension at
60°C for 34 sec. Primers used in the present study were as follows:
β-actin, forward 5′-GAAGATCCTGACCGAGCGTG-3′, reverse
5′-CGAAGTCTAGGGCAACATAGCA-3′; and catalase, forward
5′-AGGTGCTTTTGGATACTTTGAGG-3′ and reverse,
5′-CGACTGTGGAGAATCGGACGG-3′. The quantification cycle (Cq) method
was employed to evaluate relative alterations in gene expression
and the 2−ΔΔCq value was calculated after β-actin
normalization (31).
Western blot analysis
Cells were rinsed twice with PBS and lysed in 100
µl lysis buffer [40 mM Tris (pH 8.0), 120 mM NaCl, 0.1%
NP-40] on ice for 30 min, after which they were centrifuged at
13,000 × g for 15 min at 4°C. Supernatants were collected and total
protein concentrations were determined using the bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology, Haimen,
China). Aliquots (40 µg total protein) of the collected
supernatants were boiled for 5 min, separated by 10% SDS-PAGE and
transferred to nitrocellulose membranes. The membranes were blocked
with 5% non-fat milk for 1 h, and then incubated with primary
antibodies (targeting catalase (1:1,000), β-actin (1:5,000),
p-AMPKα (1:1,000), AMPKα (1:1,000), FOXO3 (1:1,000), TBP (1:2,000),
and PAR (1:1,000) overnight at 4°C), washed with TTBS, and
incubated with horseradish peroxidase-conjugated
anti-immunoglobulin-G secondary antibodies (cat. nos. 31430 and
31460; 1:5,000 Pierce; Thermo Fisher Scientific, Inc.) for 1 h at
room temperature. The blots were visualized using an enhanced
chemiluminescence western blotting detection kit (GE Healthcare
Life Sciences, Little Chalfont, UK).
Nuclear extract preparation
Cells were seeded in culture dishes at a density of
1.5×105 cells/ml for 16 h. The cultured cells were then
treated with morin for a series of time periods. Cells were
harvested and lysed on ice with 1 ml lysis buffer [10 mM Tris-HCl
(pH 7.9), 10 mM NaCl, 3 mM MgCl2 and 1% NP-40] for 4
min. Cell pellets were collected by centrifugation at 3,000 × g for
10 min at 4°C, suspended in 5 µl extraction buffer [20 mM
HEPES (pH 7.9), 20% glycerol, 300 mM NaCl, 1.5 mM MgCl2,
0.2 mM EDTA, 1 mM DTT and 1 mM PMSF] and incubated on ice for 30
min. The extraction solution was centrifuged at 13,000 × g for 5
min at 4°C, and the supernatants were harvested as nuclear protein
extracts for further blotting analysis and stored at −70°C.
Catalase activity assay
Cells were seeded into Petri dishes at a density of
1.5×105 cells/ml. After 16 h, cells were treated with
morin for a series of time periods. Catalase activity was
determined using a catalase assay kit (S0051; Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. In this
assay, catalase reacts with hydrogen peroxide
(H2O2) to produce water and oxygen;
unconverted H2O2 is then converted to a
chromogenic product, which is measured at 520 nm using a
spectrophotometer. Catalase activity is presented as U/mg, and 1
unit catalase activity is defined as the amount of enzyme
catalyzing the degradation of 1 µmol
H2O2/min under the conditions of 25°C and pH
7.0. In order to detect the effects of morin on STZ-induced
suppression of catalase activity, cells were pretreated with morin
for 1 h, and were then incubated with STZ for 24 h. Catalase
activity was assessed using the aforementioned catalase assay
kit.
Transient transfection of small
interfering RNA (siRNA)
Cells were seeded at 1.5×105 cells/ml in
24-well plates and reached ~50% confluence prior to transfection
with AMPK siRNA (siAMPK), FOXO3 siRNA (siFOXO3) (both from
Invitrogen; Thermo Fisher Scientific, Inc.), catalase siRNA
(siCatalase) or control mismatched siRNA (both from Santa Cruz
Biotechnology, Inc.). Cells were transfected with 10–50 nM siRNA
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A total
of 24 h post-transfection, cells were treated with or without morin
and/or STZ for 24 h and were subjected to western blot analysis and
MTT assay.
Statistical analysis
All experiments were performed in triplicate and all
values are presented as the means ± standard error of the mean. The
results were analyzed by one-way analysis of variance in the
SigmaStat 3.5 and the post hoc Tukey's test was used to assess
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxicity of STZ in RINm5F cells
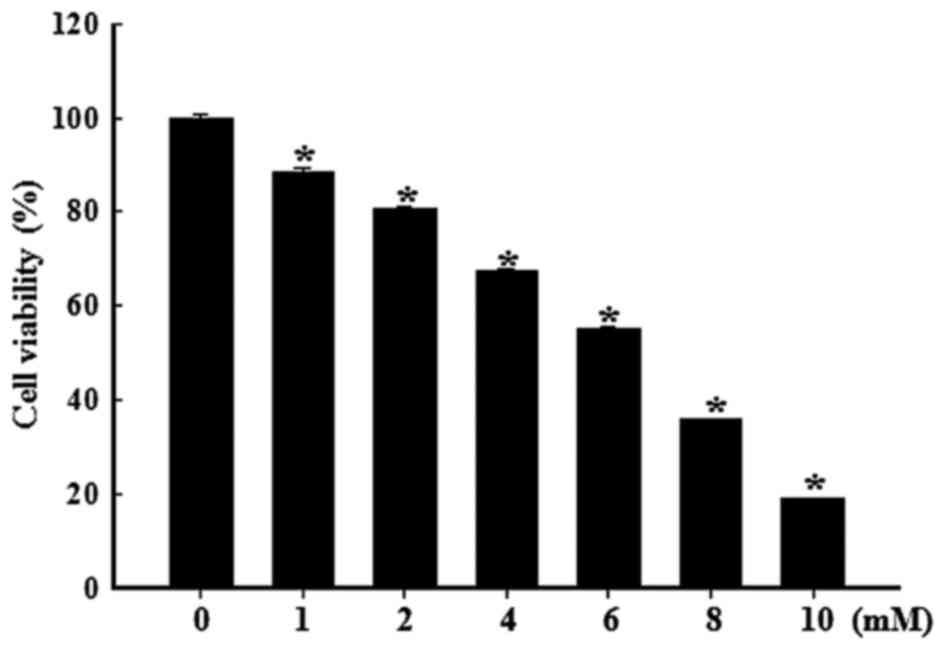
The effects of various concentrations of STZ on
RINm5F cell viability are presented in Fig. 1. A dose-dependent decrease in cell
viability was observed when cells were exposed to various
concentrations of STZ for 24 h. Following treatment with 6 mM STZ,
~50% inhibition of RINm5F cell viability occurred, and at a
concentration of 10 mM, cell viability was markedly reduced to 20%.
Based on these results, 6 mM was selected as the appropriate dose
of STZ for further experiments.
Effects of morin on the viability of
STZ-damaged RINm5F cells
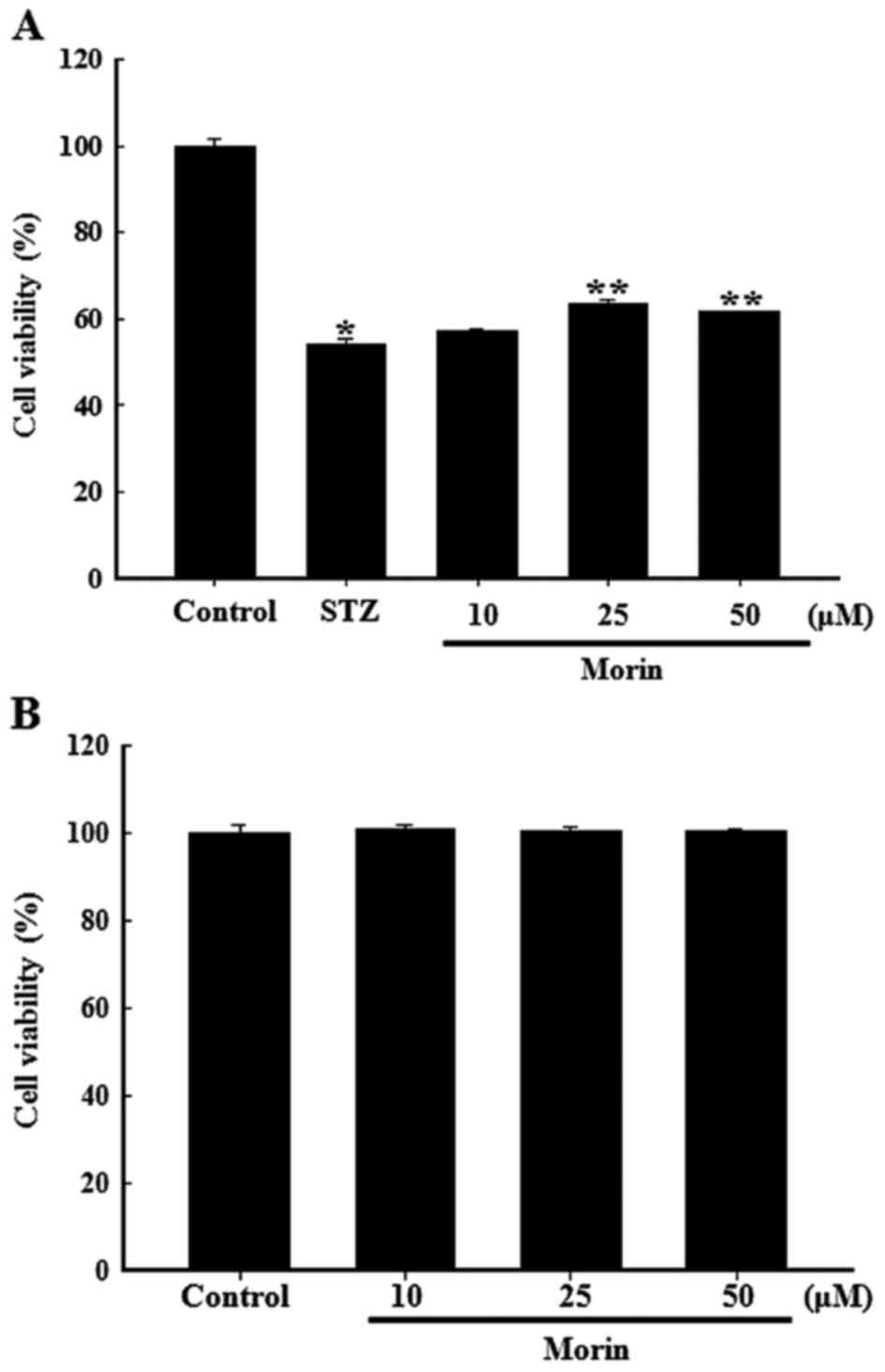
The protective effects of morin on the viability of
STZ-treated cells were measured using the MTT assay. As shown in
Fig. 2A, exposure to 6 mM STZ for
24 h markedly reduced the viability of RINm5F cells, whereas
pretreatment with morin at 10, 25 and 50 µM for 1 h resulted
in amelioration of cell viability. Pretreatment with 25 µM
morin exhibited the highest efficiency with regards to the
protection of RINm5F cells. In addition, treatment with various
concentrations of morin alone did not exhibit any adverse effects
on cell viability (Fig. 2B);
therefore, morin at 25 µM was chosen as the appropriate dose
for subsequent experiments. These results indicated that morin may
be capable of protecting RINm5F cells from STZ-induced cell
damage.
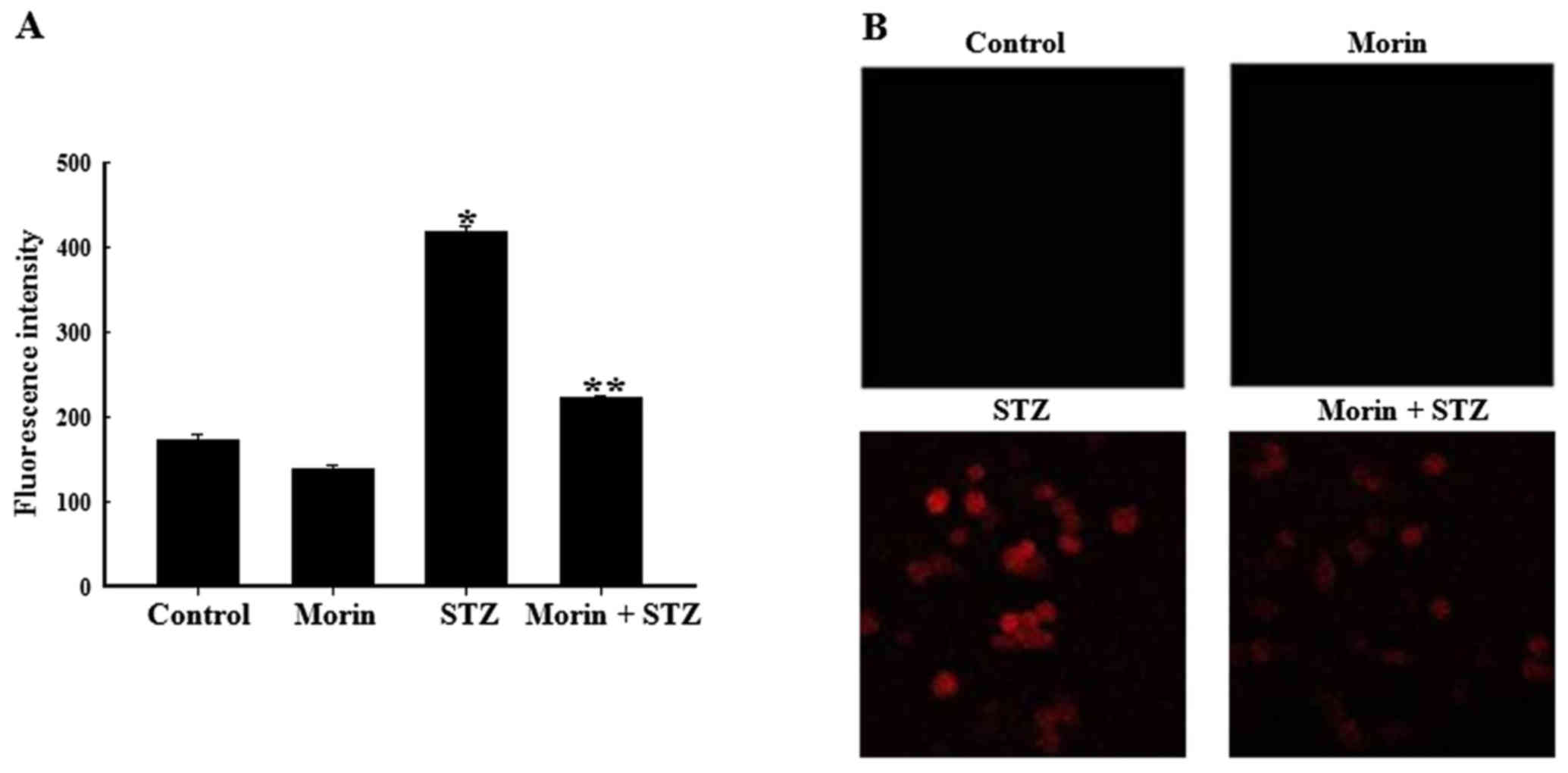
Effects of morin on intracellular
STZ-induced ROS generation in RINm5F cells
To confirm the effects of morin on STZ-induced ROS
production, ROS fluorescence intensity was detected by flow
cytometry. The results of flow cytometric analysis revealed that
STZ increased ROS generation; however, pretreatment with morin
ameliorated the STZ-stimulated increase in ROS content (Fig. 3A). In addition, intracellular ROS
levels were observed under a fluorescence microscope. As presented
in Fig. 3B, exposure to STZ for
12 h markedly increased red fluorescence intensity in RINm5F cells,
whereas pretreatment with morin reduced red fluorescence intensity
upon STZ treatment, thus reflecting a reduction in ROS generation.
These results indicated that morin significantly decreased
STZ-induced elevated ROS levels, thus suggesting that morin
conferred resistance to oxidative stress by suppressing the
increase in intracellular ROS levels.
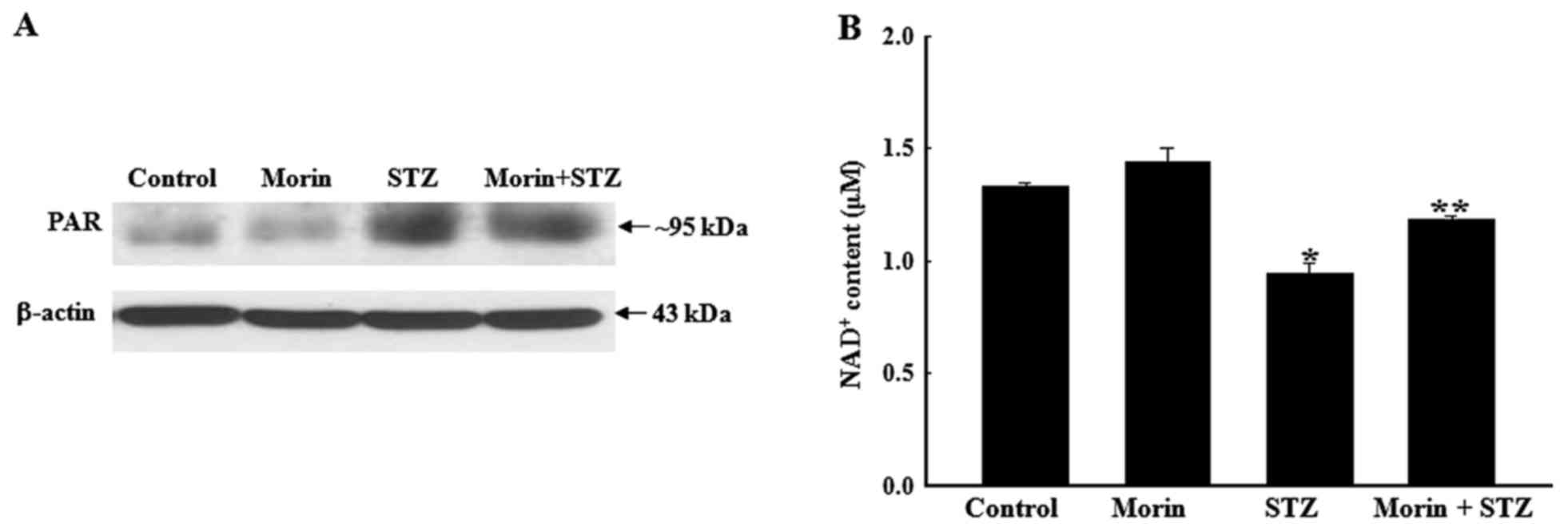
Effects of morin on STZ-induced
alterations in PAR polymerase (PARP) activity and NAD+
levels
PARP is known to be activated under conditions of
oxidative stress and has been well demonstrated in the STZ-induced
model of diabetes (32). PARP
activity may be examined by western blotting of the PAR protein, as
previously described (33). As
shown in Fig. 4A, the expression
levels of PAR were markedly increased in STZ-treated cells compared
with in the control cells, whereas pretreatment with morin
inhibited STZ-induced PAR protein expression. As an important
substrate for PARP, NAD+ is a crucial component in the
modulation of cellular metabolism. Therefore, intracellular
NAD+ levels were measured in the present study. As
expected, pretreatment with morin significantly ameliorated the
loss of NAD+ levels, which was induced by STZ treatment
(Fig. 4B). These results
indicated that PARP may be activated in response to STZ
stimulation, which was accompanied by NAD+ depletion;
however, morin effectively reversed the increase in PARP activity
and the depletion of NAD+ levels in STZ-treated
cells.
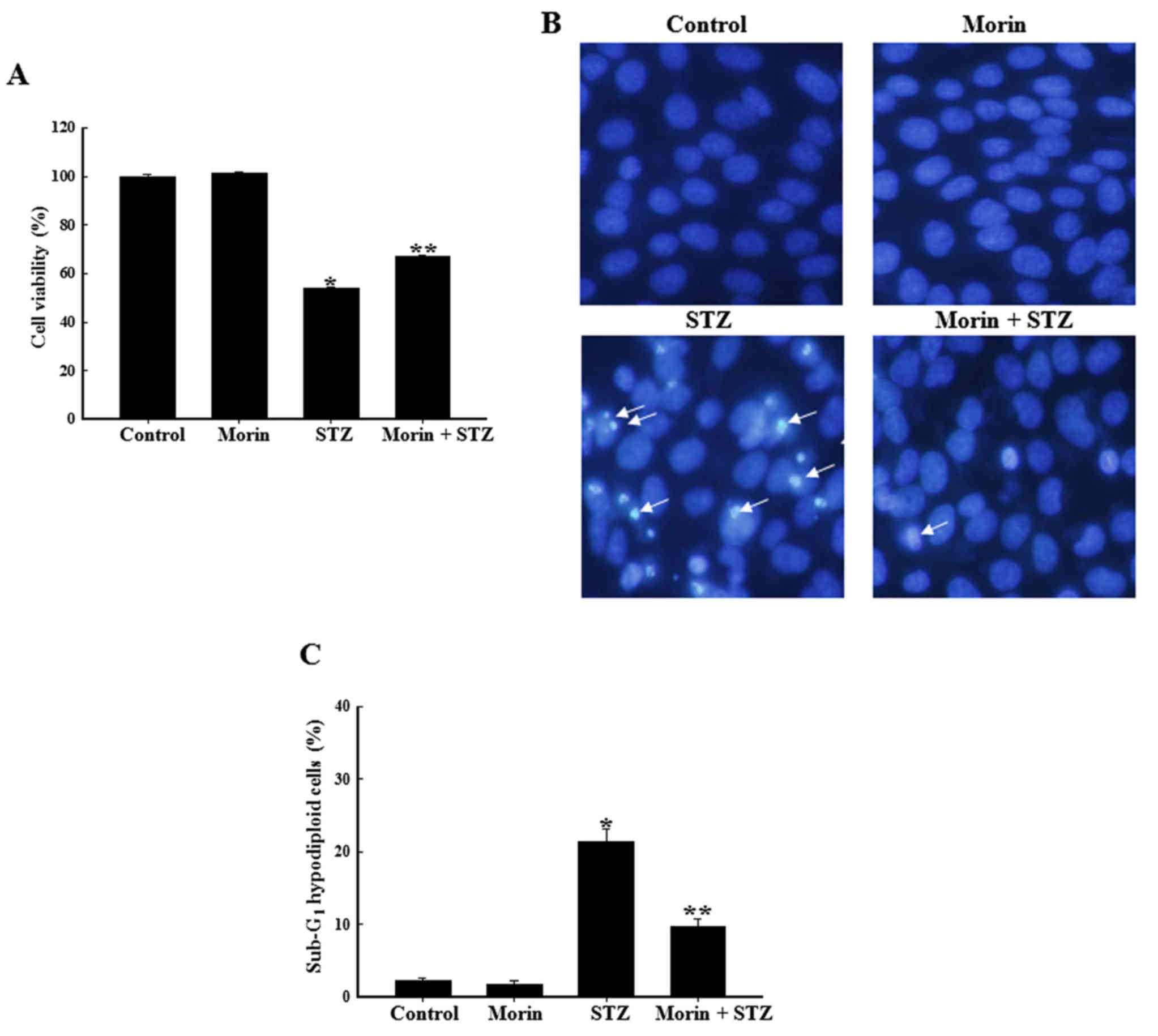
Effects of morin on STZ-induced apoptosis
of RINm5F cells
The protective effects of morin against STZ-induced
apoptotic cell death were assessed. As shown in Fig. 5A, the combination of morin and STZ
markedly increased cell survival rate compared with in STZ-treated
cells. A previous study reported that STZ treatment stimulated
pancreatic β cells to produce large amount of ROS, which in turn
induced cell apoptosis (34). To
determine the cytoprotective effects of morin on STZ-induced
pancreatic cell apoptosis, cell nuclei were stained with Hoechst
33342 for microscopic examination. The microscopic images presented
in Fig. 5B indicated that the
nuclei of the control cells were intact, whereas the STZ-treated
cells exhibited marked nuclear fragmentation, which is a typical
feature of apoptosis. However, when the cells were pretreated with
morin, a marked decrease in nuclear fragmentation was observed. In
addition, the anti-apoptotic effects of morin on STZ-treated cells
were confirmed by flow cytometric analysis with PI staining. As
shown in Fig. 5C, apoptotic
sub-G1 DNA content in STZ-treated cells was increased
compared with in the control cells. However, prior treatment with
morin reduced apoptotic sub-G1 DNA content in
STZ-treated cells. These results indicated that morin reduced
apoptotic cell death, thus suggesting that the cytoprotective
effects of morin on RINm5F cells may be due to the inhibition of
apoptosis.
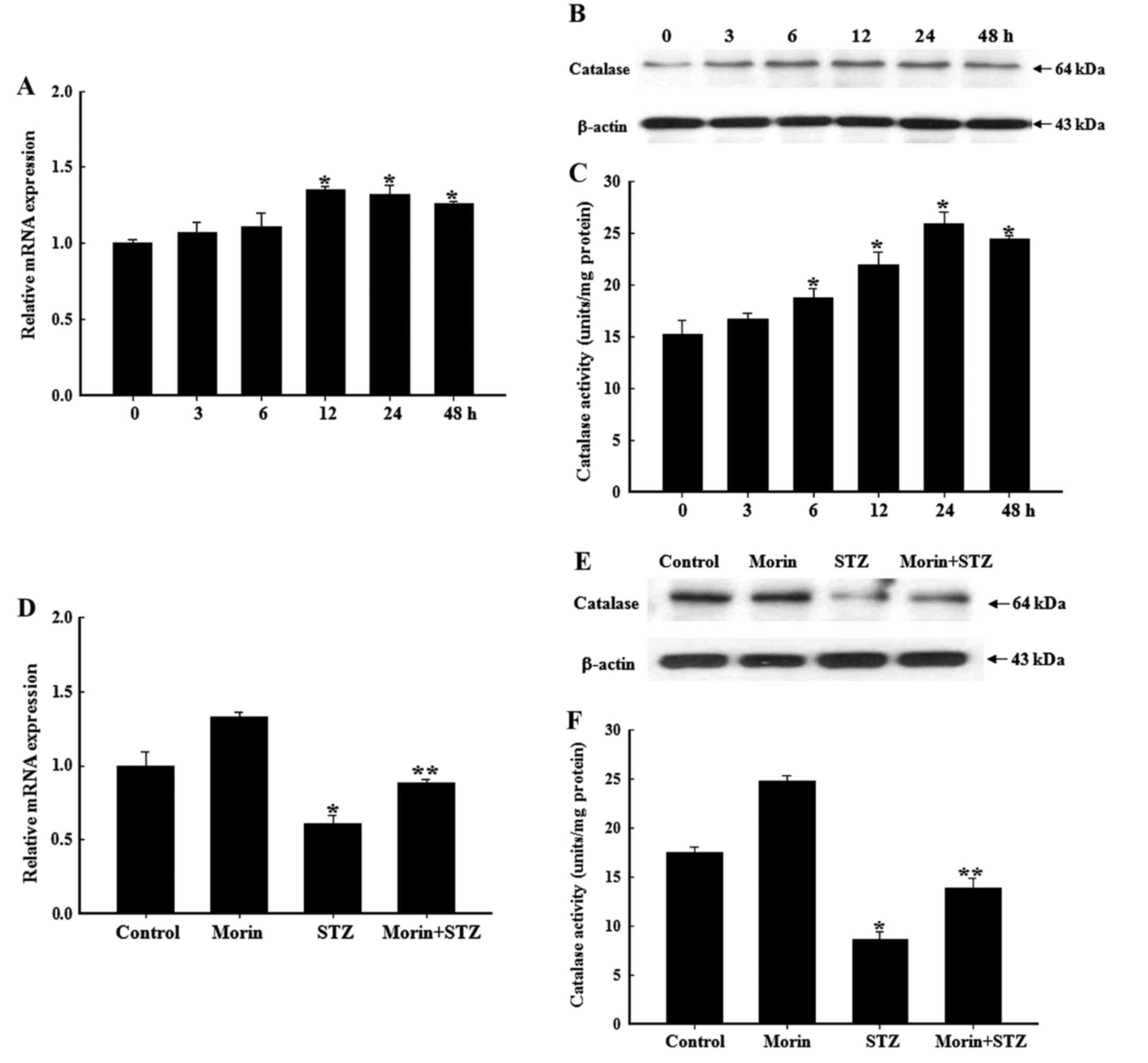
Effects of morin on catalase mRNA
transcription, protein expression and enzyme activity
It is well known that the pancreas is particularly
susceptible to STZ-induced free radical damage, due to the low
levels of endogenous antioxidant enzymes responsible for scavenging
free radicals (35). Since
catalase serves an important role in the cellular defense against
ROS, and morin has been reported to significantly increase protein
expression and enzyme activity of catalase in lung fibroblast cells
(15), the effects of morin on
catalase expression and activity in RINm5F cells were investigated
in the present study. The present results revealed that morin
treatment alone induced catalase mRNA transcription in a
time-dependent manner (Fig. 6A).
In addition, catalase protein expression and enzyme activity were
time-dependently increased following treatment with morin (Fig. 6B and C). Treatment with STZ alone
for 24 h significantly attenuated the mRNA transcription, protein
expression and enzyme activity of catalase in RINm5F cells.
Compared with these findings, pretreatment with morin reduced
STZ-induced attenuation of catalase mRNA transcription and protein
expression (Fig. 6D and E),
leading to a significant increase in catalase activity (Fig. 6F).
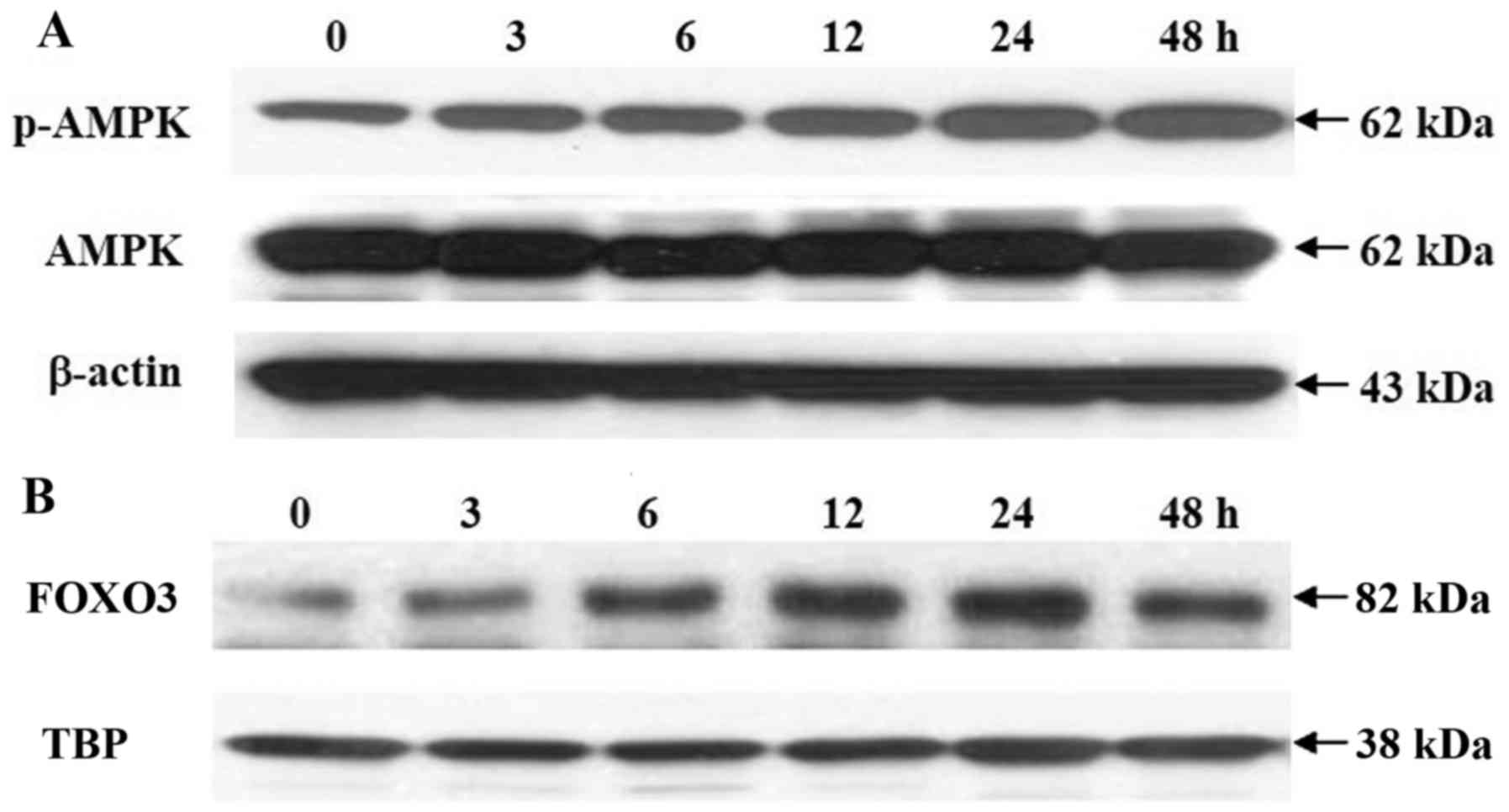
Effects of morin on AMPK activation and
FOXO3a subcellular translocation
Since AMPK is an upstream kinase that regulates
FOXO3 activation, and the AMPK-FOXO3 signaling pathway has been
suggested to be responsible for the induction of antioxidant
enzymes (16,36), the effects of morin on AMPK-FOXO3
activation were examined by western blot analysis. As shown in
Fig. 7A, 25 µM morin
induced phosphorylation of the AMPK catalytic unit AMPKα in a
time-dependent manner, thus promoting the nuclear accumulation of
FOXO3 (Fig. 7B).
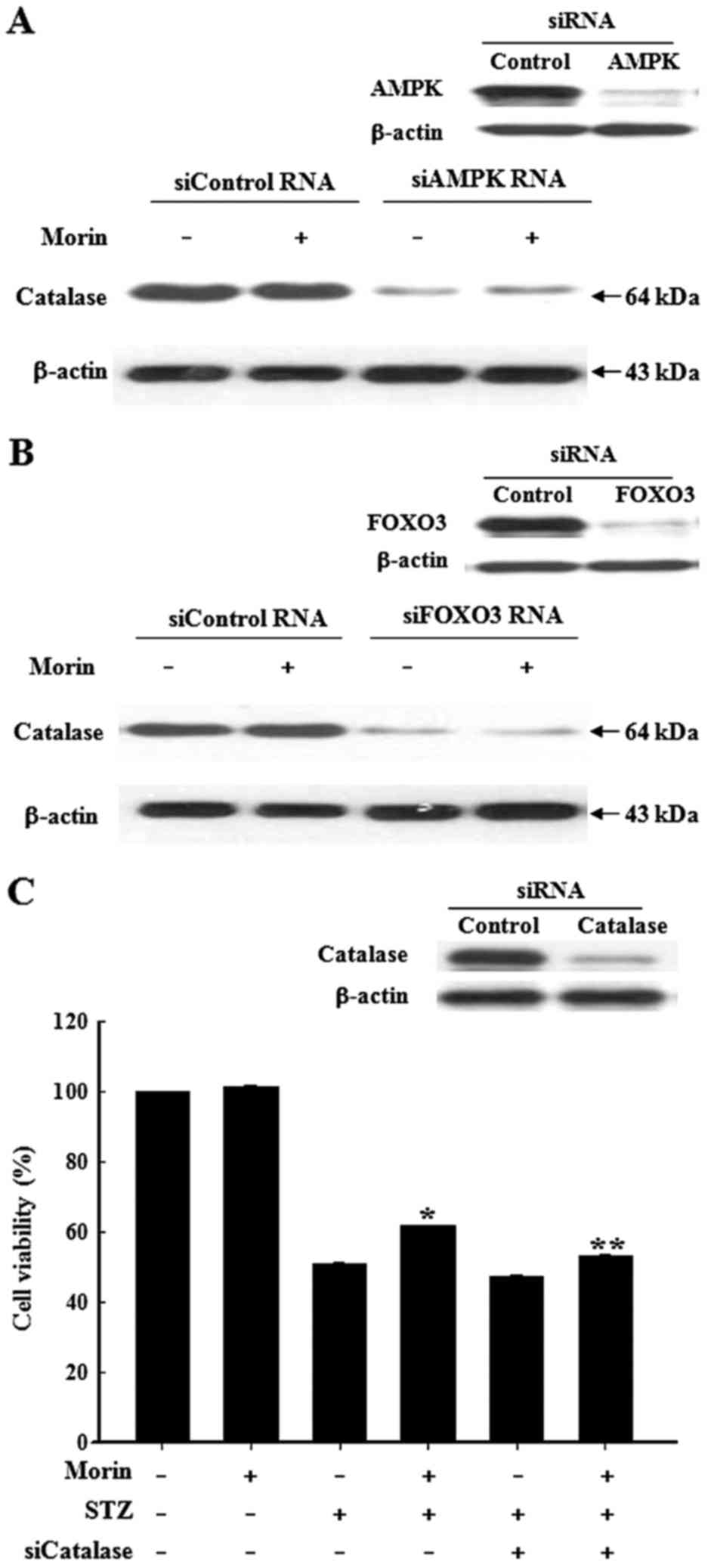
Role of the AMPK-FOXO3 pathway in
regulation of catalase expression
Since catalase is a downstream transcriptional
target of FOXO3, the present study aimed to determine whether the
AMPK-FOXO3 signaling pathway is involved in the induction of
catalase by morin. Following transfection of the pancreatic cells
with siAMPK or siFOXO3, cells were treated with morin for 24 h and
the protein expression levels of catalase were determined.
Silencing of AMPK or FOXO3 expression markedly suppressed the
morin-dependent increase in catalase protein expression (Fig. 8A and B). To determine whether
morin-enhanced catalase activity confers cytoprotection against
oxidative stress, cells were transfected with siCatalase.
siCatalase reduced the protective effects of morin against
STZ-induced cytotoxicity (Fig.
8C). Taken together, these results revealed that AMPK may be
involved in the activation of FOXO3 and the upregulation of
catalase.
Discussion
In the present study, STZ treatment was employed to
mimic the diabetes-associated oxidative stress environment in
pancreatic β cells. STZ is a widely used chemical, which is able to
induce experimental diabetes in animals and can induce cells to
produce several types of ROS, including superoxide anion, hydroxyl
radical and H2O2 (37). Previous studies have demonstrated
that exposure to STZ may result in β cell dysfunction and apoptosis
(38,39). In addition, it has been suggested
that STZ may enter β cells and cause alkylation of DNA; DNA damage
induces activation of PARP. Hyperactivation of PARP is able to
initiate the programmed cell death pathway, resulting in the
depletion of cellular NAD+ (37). In the present study, exposure of
RINm5F cells to STZ significantly increased intracellular ROS
production, which was accompanied by a marked decrease in cell
viability, augmentation of PARP activity, attenuation of
NAD+ levels and an increase in apoptosis. Flavonoids,
including morin, have been reported to possess potent antioxidant
and antidiabetic activity in animal models of experimental diabetes
(40,41). Therefore, the present study
further investigated the protective roles of morin in STZ-treated
RINm5F cells.
The present results suggested that morin could
effectively protect pancreatic cells by augmenting cellular
catalase levels. Pretreatment with morin significantly reduced ROS
levels, restored cell viability, reduced PARP activity, ameliorated
intracellular NAD+ levels and inhibited apoptosis
induced by STZ. Generally, cells can be protected from excessive
ROS via the augmentation of endogenous antioxidant enzymes. A
previous study reported that stable transfection of
insulin-producing RINm5F cells with catalase resulted in defense
against cytokine toxicity (42).
Furthermore, β cell-specific transgenic expression of catalase has
been revealed to shield isolated islets from
H2O2 and reduce the effects of STZ treatment
(43,44). Although a previous study indicated
that morin possesses antioxidant and antidiabetic activities
(45), few studies have
elucidated the molecular mechanisms by which morin may protect
pancreatic β cells and regulate endogenous antioxidant enzymes. The
present study investigated the effects of morin on the regulation
of catalase expression and activity. Notably, morin
time-dependently increased the gene transcription, protein
expression and enzyme activity of catalase, and alleviated the
downregulation of catalase in β cells induced by exposure to STZ.
In addition, knockdown of catalase using specific siCatalase
abolished the protective effects of morin against STZ-induced cell
death, providing evidence verifying the vital role of catalase in
the cytoprotective mechanism of morin in RINm5F cells. These
results suggested that the cytoprotective effects of morin may be
attributed to augmentation of the gene transcription, protein
expression and enzyme activity of catalase.
The present study also demonstrated that
augmentation of catalase was associated with activation of the
AMPK-FOXO3 signaling pathway. Morin treatment time-dependently
increased the phosphorylation of AMPK, thus suggesting that morin
may activate AMPK and function via its downstream network of
signaling pathways. It has previously been reported that AMPK
activation is involved in the antioxidant defense system in
response to oxidative stress (21). Disruption of AMPK activation in
cells under oxidative stress may trigger cell death due to
accumulation of ROS. In addition, knockdown of the catalytic
AMPK-α1 subunit in human umbilical vein endothelial cells
attenuated the expression of key components in the antioxidant
defense system, including catalase (27).
Regarding how the AMPK pathway upregulates catalase,
the present study examined FOXO3 expression. Morin induced FOXO3
expression, and its subsequent translocation into the nucleus. It
has previously been reported that AMPK may directly phosphorylate
and activate FOXO3 (46).
Activated FOXO3 is subsequently translocated into the nucleus and
regulates antioxidant enzymes in response to oxidative stress
(16). In mouse hematopoietic
stem cells, FOXO3 has been identified as a critical mediator of
cell resistance to physiological oxidative stress. Furthermore, the
conditional deletion of FOXO3 can lead to mass ROS generation
(46,47). A previous study indicated that
resveratrol could upregulate FOXO proteins and provide
photoreceptor cells with effective protection against oxidative
stress (48). It has also been
reported that FOXO3 may be involved in the induction of catalase
(49). The AMPK-FOXO3 signaling
pathway has been confirmed to be responsible for the induction of
catalase in vascular endothelial cells (28). In the present study, transfection
of cells with siAMPK or siFOXO3 reduced morin-induced protein
expression of catalase. These findings suggested that activation of
the AMPK-FOXO3 signaling pathway may be critically involved in
morin-induced catalase expression and enzyme activity.
The FOXO3 transcription factor is not the only
transcriptional regulator of catalase. The transcription factor
NF-E2-related factor 2 (Nrf2) has also been reported to be involved
in the regulation of catalase in pancreatic β cells (50). Therefore, future studies may focus
on the association between FOXO3 and Nrf2 in the regulation of
morin-induced catalase. Furthermore, targeting the phosphoinositide
3-kinase/protein kinase B/FOXO3 signaling pathway may lead to the
development of novel approaches for the possible treatment of
diabetic neuropathy (51). In
addition, it will be interesting to determine the signaling network
responsible for the regulation of FOXO3. These findings suggested
that the identification of potential FOXO3 activators may be
promising to limit the progression of oxidative stress-mediated
diseases and merits intensive investigation.
Besides upregulation of catalase, AMPK has been
reported to mediate the expression of manganese superoxide
dismutase, in order to reduce mitochondrial ROS production in human
umbilical vein endothelial cells (52) and Chang liver cells (36). Therefore, it may be hypothesized
that the AMPK-FOXO3 signaling pathway serves an essential role in
the expression of various antioxidant enzymes, including catalase,
in pancreatic β cells. Future studies should aim to investigate the
role of the AMPK pathway in the regulation of other antioxidant
enzymes for the protection of β cells from oxidative stress-induced
cell damage.
In conclusion, the present study demonstrated that
morin may suppress STZ-induced intracellular ROS production and
apoptosis, which may be ascribed, at least partly, to the
upregulation of catalase via the induction of FOXO3 expression and
its subsequent translocation into the nucleus via AMPK activation.
Therefore, morin-induced activation of the AMPK-FOXO3-catalase
pathway may be considered one of the pathways that potentially
mediates the antioxidative functions of morin. Morin may be
considered a promising strategy for the amelioration and/or
prevention of pancreatic β cell dysfunction and diabetes.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81300673), the
Startup Foundation for Advanced Talents of Jiangsu University
(grant no. 13JDG004) and the Cultivation Project for Young Core
Teacher of Jiangsu University.
References
|
1
|
Kaneto H and Matsuoka TA: Involvement of
oxidative stress in suppression of insulin biosynthesis under
diabetic conditions. Int J Mol Sci. 13:13680–13690. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pérez S, Pereda J, Sabater L and Sastre J:
Redox signaling in acute pancreatitis. Redox Biol. 5:1–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keane KN, Cruzat VF, Carlessi R, de
Bittencourt PI Jr and Newsholme P: Molecular events linking
oxidative stress and inflammation to insulin resistance and β-cell
dysfunction. Oxid Med Cell Longev 2015. 181643:2015. View Article : Google Scholar
|
|
4
|
Mishra V: Oxidative stress and role of
antioxidant supplementation in critical illness. Clin Lab.
53:199–209. 2007.PubMed/NCBI
|
|
5
|
Manna P and Jain SK: Obesity, oxidative
stress, adipose tissue dysfunction, and the associated health
risks: causes and therapeutic strategies. Metab Syndr Relat Disord.
13:423–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yadav UC, Rani V, Deep G, Singh RK and
Palle K: Oxidative stress in metabolic disorders: pathogenesis,
prevention, and therapeutics. Oxid Med Cell Longev 2016.
9137629:2016. View Article : Google Scholar :
|
|
7
|
Xie MX, Long M, Liu Y, Qin C and Wang YD:
Characterization of the interaction between human serum albumin and
morin. Biochim Biophys Acta. 1760:1184–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhanasekar C and Rasool M: Morin, a
dietary bioflavonol suppresses monosodium urate crystal-induced
inflammation in an animal model of acute gouty arthritis with
reference to NLRP3 inflammasome, hypo-xanthine phosphoribosyl
transferase, and inflammatory mediators. Eur J Pharmacol.
786:116–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prahalathan P, Kumar S and Raja B: Morin
attenuates blood pressure and oxidative stress in
deoxycorticosterone acetate-salt hypertensive rats: a biochemical
and histopathological evaluation. Metabolism. 61:1087–1099. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abuohashish HM, Al-Rejaie SS, Al-Hosaini
KA, Parmar MY and Ahmed MM: Alleviating effects of morin against
experimentally-induced diabetic osteopenia. Diabetol Metab Syndr.
5:52013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kok LD, Wong YP, Wu TW, Chan HC, Kwok TT
and Fung KP: Morin hydrate: a potential antioxidant in minimizing
the free-radicals-mediated damage to cardiovascular cells by
anti-tumor drugs. Life Sci. 67:91–99. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh MP, Jakhar R and Kang SC: Morin
hydrate attenuates the acrylamide-induced imbalance in antioxidant
enzymes in a murine model. Int J Mol Med. 36:992–1000. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dilshara MG, Jayasooriya RG, Lee S, Choi
YH and Kim GY: Morin downregulates nitric oxide and prostaglandin
E2 production in LPS-stimulated BV2 microglial cells by
suppressing NF-κB activity and activating HO-1 induction. Environ
Toxicol Pharmacol. 44:62–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vanitha P, Uma C, Suganya N,
Bhakkiyalakshmi E, Suriyanarayanan S, Gunasekaran P,
Sivasubramanian S and Ramkumar KM: Modulatory effects of morin on
hyperglycemia by attenuating the hepatic key enzymes of
carbohydrate metabolism and β-cell function in
streptozotocin-induced diabetic rats. Environ Toxicol Pharmacol.
37:326–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang R, Kang KA, Piao MJ, Maeng YH, Lee
KH, Chang WY, You HJ, Kim JS, Kang SS and Hyun JW: Cellular
protection of morin against the oxidative stress induced by
hydrogen peroxide. Chem Biol Interact. 177:21–27. 2009. View Article : Google Scholar
|
|
16
|
Zheng A, Li H, Xu J, Cao K, Li H, Pu W,
Yang Z, Peng Y, Long J, Liu J, et al: Activation of the AMPK-FOXO3
pathway reduces fatty acid-induced increase in intracellular
reactive oxygen species by upregulating thioredoxin. Diabetes.
58:2246–2257. 2009. View Article : Google Scholar
|
|
17
|
Zheng A, Li H, Xu J, Cao K, Li H, Pu W,
Yang Z, Peng Y, Long J, Liu J, et al: Hydroxytyrosol improves
mitochondrial function and reduces oxidative stress in the brain of
db/db mice: role of AMP-activated protein kinase activation. Br J
Nutr. 113:1667–1676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan Y, Cui ZJ, Sun B, Han LP, Li CJ and
Chen LM: Celastrol attenuates oxidative stress in the skeletal
muscle of diabetic rats by regulating the AMPK-PGC1α-SIRT3
signaling pathway. Int J Mol Med. 37:1229–1238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ceolotto G, Gallo A, Papparella I, Franco
L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A,
et al: Rosiglitazone reduces glucose-induced oxidative stress
mediated by NAD(P)H oxidase via AMPK-dependent mechanism.
Arterioscler Thromb Vasc Biol. 27:2627–2633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Essick EE and Sam F: Oxidative stress and
autophagy in cardiac disease, neurological disorders, aging and
cancer. Oxid Med Cell Longev. 3:168–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sid B, Verrax J and Calderon PB: Role of
AMPK activation in oxidative cell damage: implications for
alcohol-induced liver disease. Biochem Pharmacol. 86:200–209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maiese K, Chong ZZ and Shang YC:
OutFOXOing disease and disability: the therapeutic potential of
targeting FoxO proteins. Trends Mol Med. 14:219–227. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nho RS and Hergert P: FoxO3a and disease
progression. World J Biol Chem. 5:346–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kato M, Yuan H, Xu ZG, Lanting L, Li SL,
Wang M, Hu MC, Reddy MA and Natarajan R: Role of the Akt/FoxO3a
pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel
mechanism related to diabetic kidney disease. J Am Soc Nephrol.
17:3325–3335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sundaresan NR, Gupta M, Kim G, Rajamohan
SB, Isbatan A and Gupta MP: Sirt3 blocks the cardiac hypertrophic
response by augmenting Foxo3a-dependent antioxidant defense
mechanisms in mice. J Clin Invest. 119:2758–2771. 2009.PubMed/NCBI
|
|
26
|
Turdi S, Li Q, Lopez FL and Ren J:
Catalase alleviates cardiomyocyte dysfunction in diabetes: role of
Akt, forkhead transcriptional factor and silent information
regulator 2. Life Sci. 81:895–905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Colombo SL and Moncada S: AMPKalpha1
regulates the antioxidant status of vascular endothelial cells.
Biochem J. 421:163–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zrelli H, Matsuoka M, Kitazaki S, Zarrouk
M and Miyazaki H: Hydroxytyrosol reduces intracellular reactive
oxygen species levels in vascular endothelial cells by upregulating
catalase expression through the AMPK-FOXO3a pathway. Eur J
Pharmacol. 660:275–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
30
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Chandak PG, Gaikwad AB and Tikoo K:
Gallotannin ameliorates the development of streptozotocin-induced
diabetic nephropathy by preventing the activation of PARP.
Phytother Res. 23:72–77. 2009. View Article : Google Scholar
|
|
33
|
Guzyk MM, Tykhomyrov AA, Nedzvetsky VS,
Prischepa IV, Grinenko TV, Yanitska LV and Kuchmerovska TM:
Poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors reduce reactive
gliosis and improve angiostatin levels in retina of diabetic rats.
Neurochem Res. 41:2526–2537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen F, Xiong H, Wang J, Ding X, Shu G and
Mei Z: Antidiabetic effect of total flavonoids from Sanguis
draxonis in type 2 diabetic rats. J Ethnopharmacol. 149:729–736.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lenzen S: Oxidative stress: the vulnerable
beta-cell. Biochem Soc Trans. 36:343–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim AD, Kang KA, Piao MJ, Kim KC, Zheng J,
Yao CW, Cha JW, Hyun CL, Kang HK, Lee NH, et al: Cytoprotective
effect of eckol against oxidative stress-induced mitochondrial
dysfunction: involvement of the FoxO3a/AMPK pathway. J Cell
Biochem. 115:1403–1411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szkudelski T: The mechanism of alloxan and
streptozotocin action in B cells of the rat pancreas. Physiol Res.
50:537–546. 2001.
|
|
38
|
Lei H, Han J, Wang Q, Guo S, Sun H and
Zhang X: Effects of sesamin on streptozotocin (STZ)-induced NIT-1
pancreatic β-cell damage. Int J Mol Sci. 13:16961–16970. 2012.
View Article : Google Scholar
|
|
39
|
Zhang R, Kim JS, Kang KA, Piao MJ, Kim KC
and Hyun JW: Protective mechanism of KIOM-4 in
streptozotocin-induced pancreatic β-cells damage is involved in the
inhibition of endoplasmic reticulum stress. Evid Based Complement
Alternat Med. 2011:1–10. 2011. View Article : Google Scholar
|
|
40
|
Bansal P, Paul P, Mudgal J, Nayak PG,
Pannakal ST, Priyadarsini KI and Unnikrishnan MK: Antidiabetic,
antihyperlipidemic and antioxidant effects of the flavonoid rich
fraction of Pilea microphylla (L.) in high fat
diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol.
64:651–658. 2012. View Article : Google Scholar
|
|
41
|
Sendrayaperumal V, Iyyam Pillai S and
Subramanian S: Design, synthesis and characterization of
zinc-morin, a metal flavonol complex and evaluation of its
antidiabetic potential in HFD-STZ induced type 2 diabetes in rats.
Chem Biol Interact. 219:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lortz S, Tiedge M, Nachtwey T, Karlsen AE,
Nerup J and Lenzen S: Protection of insulin-producing RINm5F cells
against cytokine-mediated toxicity through overexpression of
antioxidant enzymes. Diabetes. 49:1123–1130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Benhamou PY, Moriscot C, Richard MJ,
Beatrix O, Badet L, Pattou F, Kerr-Conte J, Chroboczek J,
Lemarchand P and Halimi S: Adenovirus-mediated catalase gene
transfer reduces oxidant stress in human, porcine and rat
pancreatic islets. Diabetologia. 41:1093–1100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu B, Moritz JT and Epstein PN:
Overexpression of catalase provides partial protection to
transgenic mouse beta cells. Free Radic Biol Med. 27:830–837. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ola MS, Aleisa AM, Al-Rejaie SS,
Abuohashish HM, Parmar MY, Alhomida AS and Ahmed MM: Flavonoid,
morin inhibits oxidative stress, inflammation and enhances
neurotrophic support in the brain of streptozotocin-induced
diabetic rats. Neurol Sci. 35:1003–1008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Greer EL, Oskoui PR, Banko MR, Maniar JM,
Gygi MP, Gygi SP and Brunet A: The energy sensor AMP-activated
protein kinase directly regulates the mammalian FOXO3 transcription
factor. J Biol Chem. 282:30107–30119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tothova Z, Kollipara R, Huntly BJ, Lee BH,
Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams
IR, Sears C, et al: FoxOs are critical mediators of hematopoietic
stem cell resistance to physiologic oxidative stress. Cell.
128:325–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang W, Li G, Qiu J, Gonzalez P and
Challa P: Protective effects of resveratrol in experimental retinal
detachment. PLoS One. 8:e757352013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alcendor RR, Gao S, Zhai P, Zablocki D,
Holle E, Yu X, Tian B, Wagner T, Vatner SF and Sadoshima J: Sirt1
regulates aging and resistance to oxidative stress in the heart.
Circ Res. 100:1512–1521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bhakkiyalakshmi E, Shalini D, Sekar TV,
Rajaguru P, Paulmurugan R and Ramkumar KM: Therapeutic potential of
pterostilbene against pancreatic beta-cell apoptosis mediated
through Nrf2. Br J Pharmacol. 171:1747–1757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu MH, Yuan C, He J, Tan TP, Wu SJ, Fu
HY, Liu J, Yu S, Chen YD, Le QF, et al: Resveratrol protects PC12
cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a
pathway. Cell Mol Neurobiol. 35:513–522. 2015. View Article : Google Scholar
|
|
52
|
Kukidome D, Nishikawa T, Sonoda K, Imoto
K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T and
Araki E: Activation of AMP-activated protein kinase reduces
hyperglycemia-induced mitochondrial reactive oxygen species
production and promotes mitochondrial biogenesis in human umbilical
vein endothelial cells. Diabetes. 55:120–127. 2006. View Article : Google Scholar
|