Introduction
Dysregulation of innate immunity and liver
inflammation are triggered by the translocation of gut-derived
endotoxins, such as lipopolysaccharides (LPS), to the liver
(1). LPS-induced experimental
animal models are a common means of studying liver injury, fibrosis
and sepsis (2). Liver injury
triggered by LPS has been identified to be associated with certain
biological processes, including inflammation and apoptosis via
specific signaling pathways. Toll-like receptor 4 (TLR4), one
receptor for LPS, may trigger the myeloid differentiation factor 88
(MyD88)-dependent pathway, which leads to the activation of nuclear
factor (NF)-κB and activates the mitogen-activated protein kinase
(MAPK) pathways (p38, extracellular signal-regulated kinase and
c-Jun N-terminal kinase) (3),
which stimulates the production of proinflammatory cytokines,
chemokines and type I interferon (4). It has been reported that suppression
of TLR4 and MyD88 inhibits the translocation of NF-κB and affects
the levels of interleukin-1β (IL-1β), IL-6 and tumor necrosis
factor α (TNF-α) (5). These
inflammatory mediators contribute to hepatocyte dysfunction,
apoptosis and necrosis, and the generation of extracellular matrix
proteins, leading to characteristic fibrosis (6).
Farnesoid X receptor (FXR) has a key role in
regulating fatty acid and glucose metabolism and also has an
important role in maintaining cholesterol and bile acid levels
(7,8). In addition, FXR has been
demonstrated to have anti-inflammatory functions, and
agonist-activated FXR was reported to inhibit NF-κB target
inflammatory genes in hepatocytes (9). A previous study reported that FXR
knockout (KO) mice displayed prominent liver injury and
inflammation, and developed spontaneous liver tumors as they aged
(10). A similar study indicated
that the expression of inflammatory genes in the liver was elevated
in FXR KO mice (11). GW4064, a
synthetic FXR ligand, has been reported to alleviate LPS-induced
hepatic inflammation by repressing macrophage activation (12) and protects liver cells from
apoptosis induced by serum deprivation in vitro and fasting
in vivo (13). In
addition, activation of FXR was reported to have hepatoprotective
effects against certain toxins, which may be a key function of
FXR(12).
The present study demonstrated that administration
of FXR agonist GW4064 in mice reduced the severity of LPS-induced
liver injury, probably through its anti-inflammatory properties.
The present study revealed novel role for FXR in the control of
liver inflammation by antagonizing the TLR4 signaling pathway.
Based on the results, FXR was suggested to be a potential regulator
of hepatic inflammation and apoptosis and GW4064 may be used to
treat liver inflammatory diseases. This hepatoprotection by GW4064
was totally abolished in FXR KO mice. The unanticipated link
between FXR and the TLR4/p38 MAPK/NF-κB axis highlights a novel
mechanism that contributes to the hepatoprotective effects of FXR
activators.
Materials and methods
Animals and experimental design
A total of 15 male C57BL/6J wild-type (WT) mice
(weight, 24-28 g; age, 13 weeks) were obtained from the National
Laboratory Animal Center (Taiwan) and 15 FXR-knockout (KO) mice
(B6.129X1(FVB)-Nr1h4tm1Gonz/J; stock no. 007214;
weight, 24–28 g; age, 13 weeks) were obtained from the Jackson
Laboratory. Mice were assigned to 6 groups (n=5 per group): i)
C57BL/6J wild-type mice; ii) LPS-treated wild-type mice; iii)
LPS-treated wild-type mice intraperitoneally injected with GW4064;
iv) FXR KO mice; v) LPS-treated FXR KO mice; vi) LPS-treated FXR KO
mice were intraperitoneally injected with GW4064. All animals were
housed under a 12-h light-dark cycle and were provided a standard
laboratory diet ad libitum. The study protocol was reviewed
and approved by the Animal Care and Use Committee of Chang Gung
University (Taoyuan, Taiwan) and was in accordance with the
guidelines of the NIH Guide for the Care and Use of Laboratory
Animals. LPS (cat. no. L2880; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was administered intraperitoneally (i.p.) at 5 mg/kg, and
was applied to induce inflammation-associated disease as previously
described (14,15). WT and FXR KO mice were
intraperitoneally injected with a single dose of LPS (5 mg/kg) and
were intraperitoneally injected with GW4064 twice 24 h later (20
mg/kg i.p.; cat. no. G5172; Sigma-Aldrich, Merck KGaA) (16,17). In a Fisher rat model, GW4064 was
reported to lower serum triglyceride levels in a dose-dependent
manner and to have a median effective dose of 20 mg/kg (18). All mice were euthanized 6 h after
the last GW4064 administration with carbon dioxide. Blood samples
were collected for determining serum alanine transaminase (ALT),
and liver tissues were exsanguinated, immediately frozen in liquid
nitrogen and stored at −80°C for further analysis.
Serum ALT
Serum levels of ALT were determined by
spectrophotometric analysis utilizing the Randox ALT assay kit
(cat. no. AL1205; Randox, Antrim, UK) according to the
manufacturer's instructions.
Histology and immunohistochemistry
For morphological analysis, murine liver specimens
were fixed in 4% paraformaldehyde, embedded in paraffin and cut
into 5-μm sections. Slides were deparaffinized according to
standard procedures and stained with freshly made hematoxylin (cat.
no. H3136) and eosin (H&E; cat. no. 230251) (both from
Sigma-Aldrich; Merck KGaA) followed by microscopic examination. For
H&E staining, tissues were counterstained for 10 min at room
temperature (20–30°C). For protein detection, sections were
rehydrated with PBS prior to being incubated in 3%
H2O2/PBS (cat. no. 31642; Sigma-Aldrich;
Merck KGaA;) to block endogenous peroxidase and blocked in 5%
normal goat serum (cat. no. 566380; Sigma-Aldrich; Merck KGaA) in
PBS, followed by staining with primary antibodies overnight at 4°C.
The following primary antibodies were used: Anti-FXR (cat. no.
SC-13063; 1:90; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
TLR4 (cat. no. ab47093; 1:100; Abcam, Cambridge, UK), MyD88 (cat.
no. AB16529; 1:90; EMD Millipore, Billerica, MA, USA), phospho-p38
MAPK (pT18/pY182; cat. no. 612280; 1:100; BD Biosciences, Santa
Clara, CA, USA), NF-κB (cat. no. SC-8008; 1:90; Santa Cruz
Biotechnology, Inc. Dallas, TX, USA), Bax (cat. no. ab7977; 1:100)
and cytochrome c (cat. no. ab13575; 1:100) (both from
Abcam). Anti-mouse (cat. no. AP124P; 1:100; Sigma-Aldrich; Merck
KGaA) and rabbit (cat. no. ab6721; 1:100; Abcam) horseradish
peroxidase (HRP)-conjugated secondary antibodies were used for 1 h
at room temperature (20–30°C). Finally, immunostaining was
visualized by application of a 3,3′-diaminobenzidine (CN/DAB)
substrate kit (cat. no. 34000; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and viewed under a light microscope (XI71;
Olympus, Tokyo, Japan).
Protein isolation and western blot
analysis
Mouse liver tissues were lysed in lysis buffer [20
mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM
EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM
sodium orthovanadate] containing protease inhibitor cocktail (cat.
no. 78429; Thermo Fisher Scientific, Inc.). The Thermo Scientific
NE-PER Nuclear and Cytoplasmic Extraction kit (cat. no. 78835;
Thermo Fisher Scientific, Inc.) provides for efficient cell lysis
and extraction of separate cytoplasmic and nuclear protein
fractions. Lysates were clarified by centrifugation at 10,000 × g
for 10 min at 4°C and the protein concentration was determined
using a Bio-Rad Protein assay (cat. no. 500-0006; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein was analyzed by
western blot analysis. The boiled samples were separated by 10%
SDS-PAGE and transferred onto nitrocellulose membranes (cat. no.
88018; Thermo Fisher Scientific, Inc.). After blocking with 5%
non-fat skimmed milk or bovine serum albumin (cat. no. A1933;
Sigma-Aldrich; Merck KGaA) in Tris-buffered saline with 0.1%
Tween-20 for 1 h, the membranes were probed with the corresponding
antibodies overnight at 4°C. The following primary antibodies were
used for western blotting: Anti-FXR (cat. no. SC-13063; 1:500;
Santa Cruz Biotechnology, Inc.); TLR4 (cat. no. ab47093; 1:1,000;
Abcam); NF-κB (cat. no. SC-8008; 1:1,000); B-cell lymphoma-2
(Bcl-2; cat. no. SC-7382; 1:750) (both from Santa Cruz
Biotechnology, Inc.) and Bcl-2-associated X protein (Bax; cat. no.
ab7977; 1:1,000; Abcam). β-actin (cat. no. MA5-15739; 1:1,000;
Thermo Fisher Scientific, Inc.) and histone H1 (cat. no. SC-56695;
1:1,000; Santa Cruz Biotechnology, Inc.) antibodies were used to
confirm equal protein loading for all samples. Anti-mouse (cat. no.
AP124P; 1:5,000; EMD Millipore) and rabbit (cat. no. ab6721;
1:5,000; Abcam) horseradish peroxidase (HRP)-conjugated secondary
antibodies were used for 1 h at room temperature. Bound
HRP-conjugated antibodies were visualized by enhanced
chemiluminescence (cat. no. 34080; Thermo Fisher Scientific, Inc.).
Quantification of the blots was performed using ImageQuant 5.2
software (GE Healthcare, Little Chalfont, UK) and calibrated using
β-actin or histone as an internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the liver tissue using
TRIzol reagent (cat. no. 15596018; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions, and the concentration
and integrity of the isolated RNA were determined at the optical
density at 260/280 nm. Complementary (c)DNA was synthesized from
total RNA (4 μg) using the RevertAid™ First Strand cDNA
Synthesis kit (cat. no. 00187457; Thermo Fisher Scientific, Inc.),
followed by qPCR using the LightCycler® 480 SYBR-Green I
Master (cat. no. 04707516001; Roche Applied Science, Penzberg,
Germany) on a LightCycler 1.5 apparatus (cat. no. 03515885001;
Roche Applied Science, Penzberg, Germany). PCR was performed using
the following conditions: 95°C for 10 min, followed by 45 cycles of
95°C for 15 sec, 57°C for 30 sec and 72°C for 30 sec. The qPCR data
were normalized to GAPDH expression as an internal control. The
sequences of primers used for RT-qPCR are listed in Table I.
 | Table ISequences of primers used for reverse
transcription- quantitative polymerase chain reaction. |
Table I
Sequences of primers used for reverse
transcription- quantitative polymerase chain reaction.
| Gene Direction | Primer
sequence |
|---|
| TNF-α | F
5′-TTGACCTCAGCGCTGAGTTG-3′ |
| R
5′-CCTGTAGCCCACGTCGTAGC-3′ |
| IL-1β | F
5′-GCAACTGTTCCTGAACTCA-3′ |
| R
5′-CTCGGAGCCTGTAGTGCAG-3′ |
| IL-6 | F
5′-GTACTCCAGAAGACCAGAGG-3′ |
| R
5′-TGCTGGTGACAACCACGGCC-3′ |
| IFN-γ | F
5′-AGCAACAACATAGCGTCAT-3′ |
| R
5′-CCTCAAACTTGGCAATACTC-3′ |
| FXR | F
5′-GGCCTCTGGGTACCACTACA-3′ |
| R
5′-AAGAAACATGGCCTCCACTG-3′ |
| SHP | F
5′-ACTGGCTGCAGTTCAGTGGC-3′ |
| R
5′-GGTGAAGAGGATCGTGCCC-3′ |
| BAX | F
5′-TTTGCTTCAGGGTTTCATCC-3′ |
| R
5′-CAGTTGAAGTTGCCGTCAGA-3′ |
| BCL-2 | F
5′-TCTTTGAGTTCGGTGGGGTC-3′ |
| R
5′-TGCATATTTGTTTGGGGCAGG-3′ |
| Bcl-xL | F
5′-TTGGACAATGGACTGGTTGA-3′ |
| R
5′-GTAGAGTGGATGGTCAGTG-3′ |
| GAPDH | F
5′-TCACCACCATGGAGAAGGC-3′ |
| R
5′-GCTAAGCAGTTGGTGGTGCA-3′ |
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. The statistical analyses were performed using one-way
analysis of variance followed by the Student Newman-Keuls
multiple-range test. SAS v9.4 software was used for statistical
analysis (SAS Institute, Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of GW4064 on serum ALT,
histopathological changes and inflammatory responses in the
liver
To evaluate whether activation of FXR had a
protective effect on LPS-induced liver injury, WT and FXR KO mice
were used and a highly potent FXR agonist, GW4064, was applied in
the present study. H&E staining revealed that the liver
parenchymal cells were homogeneously swollen and that hypertrophy
and multiple focal hepatic necrosis were present in LPS-treated
mice. FXR KO mice had enlarged livers, whereas those injected with
LPS developed more severe liver injury and hepatocyte ballooning
(Fig. 1A). FXR KO mice exhibited
increased serum ALT levels compared with WT mice (Fig. 1B), while GW4064 administration in
LPS-injected animals resulted in apparently normal hepatocytes in
liver sections (Fig. 1A), as
evidenced by a significant decline in the increase of serum ALT
levels in WT mice, whereas no beneficial effect was seen in FXR KO
mice (Fig. 1B). To further
address whether FXR may modulate inflammatory gene expression in
mice, the present study compared the induction of pro-inflammatory
cytokines by LPS in WT and FXR KO mice. Of note, GW4064 attenuated
liver injury as indicated by inhibition of LPS-induced increases in
pro-inflammatory cytokines in WT mice, but not in FXR KO mice
(Fig. 1C).
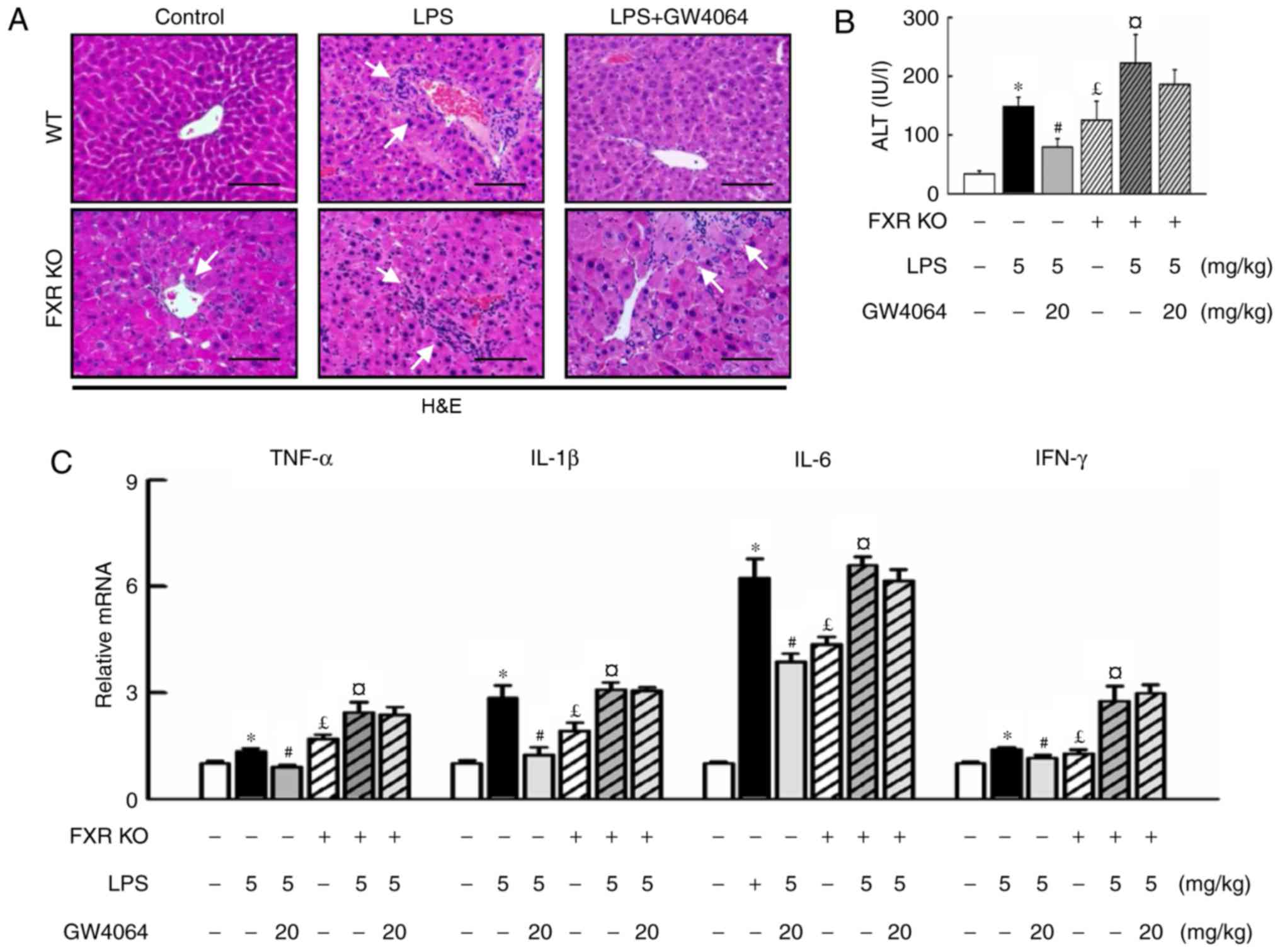 | Figure 1GW4064 ameliorates LPS-induced liver
injury and hepatic inflammation in WT mice. WT and FXR KO mice were
treated with or without GW4064 (20 mg/kg) twice after LPS injection
(5 mg/kg). All mice were sacrificed after the last GW4064
injection. (A) Fresh WT mouse liver tissues were fixed in 4%
paraformaldehyde and 5-μm sections were stained with
H&E. Hepatocyte inflammation and damage was histopathologically
analyzed (indicated by white arrows; magnification, ×200; scale
bar, 100 μm). (B) Serum ALT levels in the livers of WT and
FXR KO mice were determined. (C) Total RNAs from liver tissues were
isolated and hepatic mRNA expression of inflammatory cytokines was
determined by reverse transcription-quantitative polymerase chain
reaction analysis. Values are expressed as the mean ± standard
error of the mean (n=5 mice for each group). *P<0.05,
WT vs. LPS; #P<0.05, LPS vs. LPS+GW4064;
£P<0.05, WT vs. FXR KO; ¤P<0.05, FXR KO
vs. FXR KO+LPS. WT, wild-type; KO, knockout; FXR, farnesoid X
receptor; LPS, lipopolysaccharide; H&E, hematoxylin and eosin;
ALT, alanine transaminase; TNF, tumor necrosis factor; IL,
interleukin; IFN, interferon. |
Effects of GW4064 on FXR and small
heterodimer partner (SHP) levels in liver tissue
Next, the present study determined whether FXR
expression was affected upon LPS injection in mice. LPS injection
reduced FXR protein levels and mRNA expression in WT mice (Fig. 2). Compared with WT mice, FXR KO
mice displayed lowed FXR and SHP mRNA expression in the liver. In
addition, GW4064 significantly inhibited the LPS-mediated reduction
in FXR protein levels and SHP mRNA expression in WT mice, whereas
no effect was observed in FXR KO mice after LPS or GW4064
administration (Fig. 2).
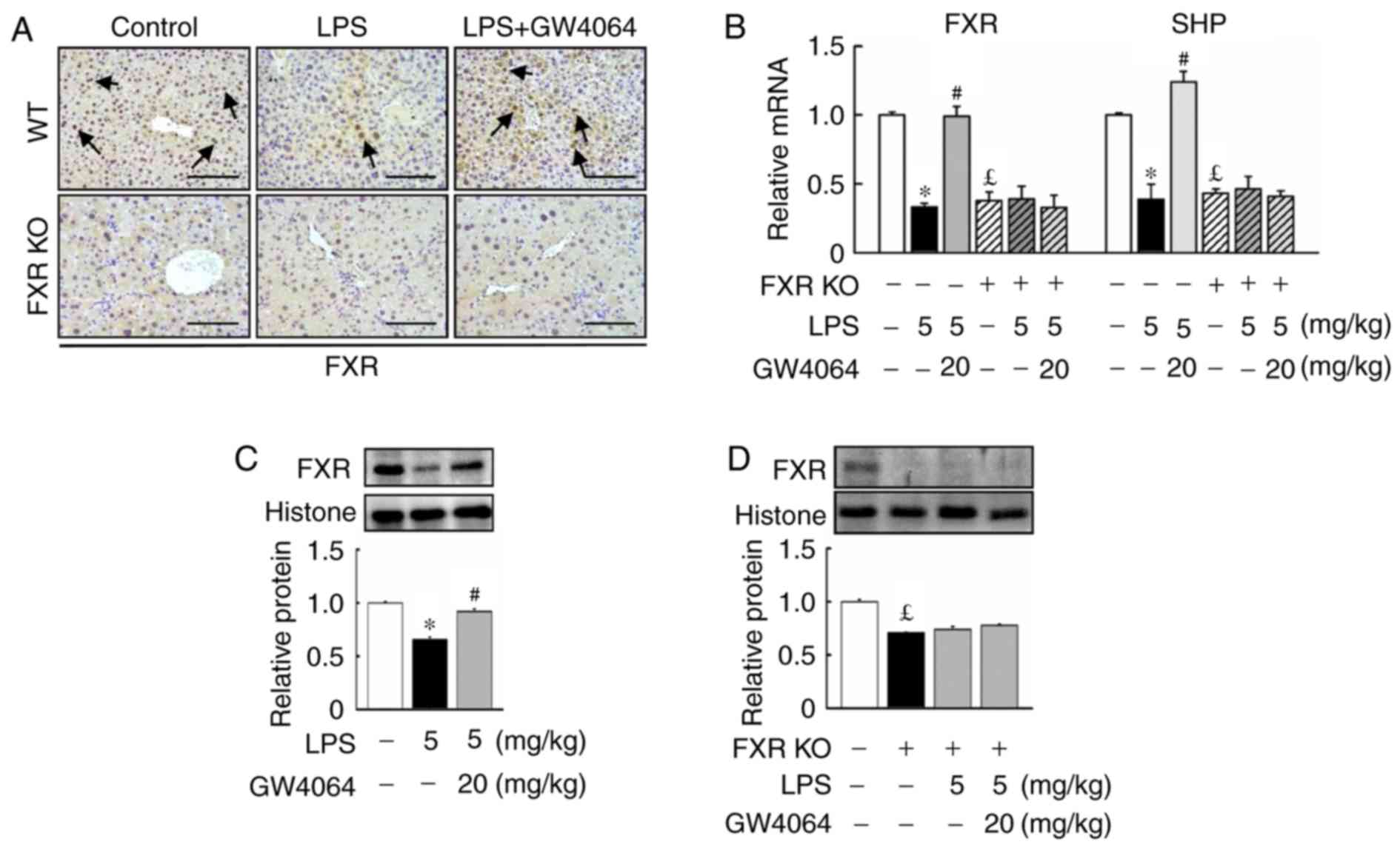 | Figure 2GW4064 administration increases FXR
protein levels and mRNA expression in liver tissues of WT mice
after LPS injection. WT and FXR KO mice were treated with or
without GW4064 (20 mg/kg) twice after LPS injection (5 mg/kg). All
mice were sacrificed after the last GW4064 injection. (A)
Representative immunohistochemical staining images of mouse liver
tissues for FXR are displayed. Staining for FXR is indicated by a
dark-brown color (black arrows; magnification, ×200; scale bar, 100
μm). (B) Hepatic mRNA expression of FXR and SHP were
determined by reverse transcription-quantitative polymerase chain
reaction analysis. (C and D) FXR proteins were determined by
western blot analysis. Histone was used as a control. Expression
levels were densitometrically quantified and presented as relative
intensity units. Values are expressed as the mean ± standard error
of the mean (n=5 mice for each group). *P<0.05, WT
vs. LPS; #P<0.05, LPS vs. LPS + GW4064;
£P<0.05, WT vs. FXR KO. WT, wild-type; KO, knockout;
FXR, farnesoid X receptor; LPS, lipopoly-saccharide; SHP, small
heterodimer partner. |
GW4064 represses LPS-induced hepatic
TLR4/MyD88/p38 MAPK/NF-κB signaling in mice
The present study further determined the role of FXR
in the LPS-induced TLR4/MyD88 pathway in the livers of WT and FXR
KO mice. GW4064 significantly repressed LPS-induced TLR4 (Fig. 3A) and MyD88 (Fig. 3B) protein levels in WT mice. TLR4
(Fig. 3A) and MyD88 (Fig. 3B) protein levels were higher in
FXR KO mouse livers than in WT mice (Fig. 3). Furthermore, GW4064 treatment
did not affect LPS-induced increases in TLR4 and MyD88 protein
levels in FXR KO mice (Fig. 3A and
B).
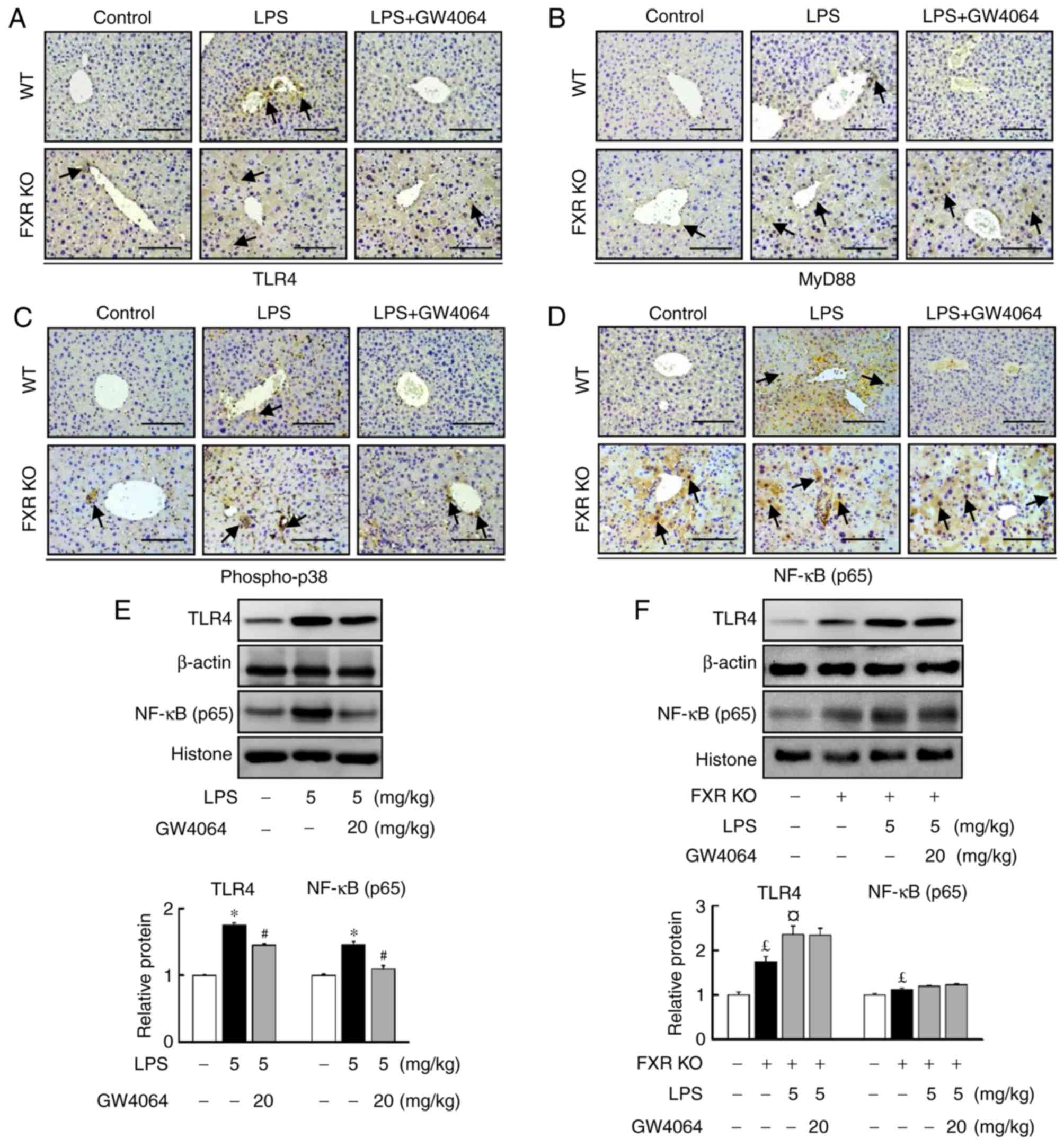 | Figure 3GW4064 attenuates LPS-induced
TLR4/MyD88/p38 MAPK/NF-κB pathway in WT mice. WT and FXR KO mice
were treated with or without GW4064 (20 mg/kg) twice after LPS
injection. At 6 h following the last GW4064 injection, livers were
subjected to IHC staining. Representative images for (A) TLR4, (B)
MyD88, (C) p-p38 and (D) NF-κB from mouse liver sections. IHC
staining for TLR4, MyD88, p-p38 and NF-κB is indicated by a
dark-brown color (indicated by black arrows; magnification, ×200;
scale bar, 100 μm). (E and F) TLR4 and NF-κB protein
expression was assessed by western blot analysis. Quantified levels
were determined by densitometry and data are presented as relative
intensity units. β-actin and histone were used as controls. Values
are expressed as the mean ± standard error of the mean (n=5 mice
for each group). *P<0.05, WT vs. LPS;
#P<0.05, LPS vs. LPS + GW4064; £P<0.05,
WT vs. FXR KO; ¤P<0.05, FXR KO vs. FXR KO + LPS. NF,
nuclear factor; p-p38, phosphorylated p38; WT, wild-type; KO,
knockout; FXR, farnesoid X receptor; LPS, lipopolysaccharide;
MyD88, myeloid differentiation factor 88; TLR, Toll-like receptor;
IHC, immunohistochemical. |
LPS injection induced phopho-p38 (Fig. 3C) and NF-κB (Fig. 3D–F) protein levels in WT mice.
Treatment with GW4064 markedly inhibited the LPS-induced increases
in the protein levels of phopho-p38 and NF-κB in WT mice (Fig. 3C and D). The induction of hepatic
inducible phopho-p38 and NF-κB protein levels by LPS in FXR KO mice
was markedly higher than in WT mice. However, treatment with GW4064
did not affect LPS-induced phopho-p38 (Fig. 3C) and NF-κB (Fig. 3D–F) protein levels in FXR KO mice
(Fig. 3C and D). These results
indicate that GW4064 reduced the LPS-induced upregulation of the
TLR4/p38 MAPK/NF-κB signaling pathway via FXR-dependent signalling
in WT mice.
GW4064 inhibits LPS-induced hepatic
apoptosis in mice
Stimulation of WT and FXR KO mice with LPS induced
similar expression levels of apoptotic proteins and genes. Bax
(Fig. 4A) and cytochrome c
(Fig. 4B) were significantly
higher in FXR KO mice than in WT mice after LPS treatment. In
addition, it was confirmed that the activation of apoptotic
signaling by LPS was higher in FXR KO mice than in WT mice
(Fig. 4A–C). Administration of
LPS and FXR deficiency induced the mRNA expression of the major
apoptotic gene caspase-3 (Fig.
4C) and increased the Bax/Bcl-2 and Bax/Bcl extra-large protein
(Bcl-xL) mRNA ratios (Fig. 4E).
LPS caused a significantly greater increase in the hepatic
Bax/Bcl-2 and Bax/Bcl-xL ratio in FXR KO mice compared with that in
WT mice (Fig. 4C).
GW4064-mediated FXR activation significantly attenuated the
LPS-dependent increase of cytochrome c as well as the
Bax/Bcl-2 and Bax/Bcl-xL ratio, and caspase-3 expression in WT
mice, but not in FXR KO mice (Fig.
4). Thus, the results clearly demonstrated that GW4064 has a
critical role in suppressing apoptosis associated with LPS-induced
liver injury.
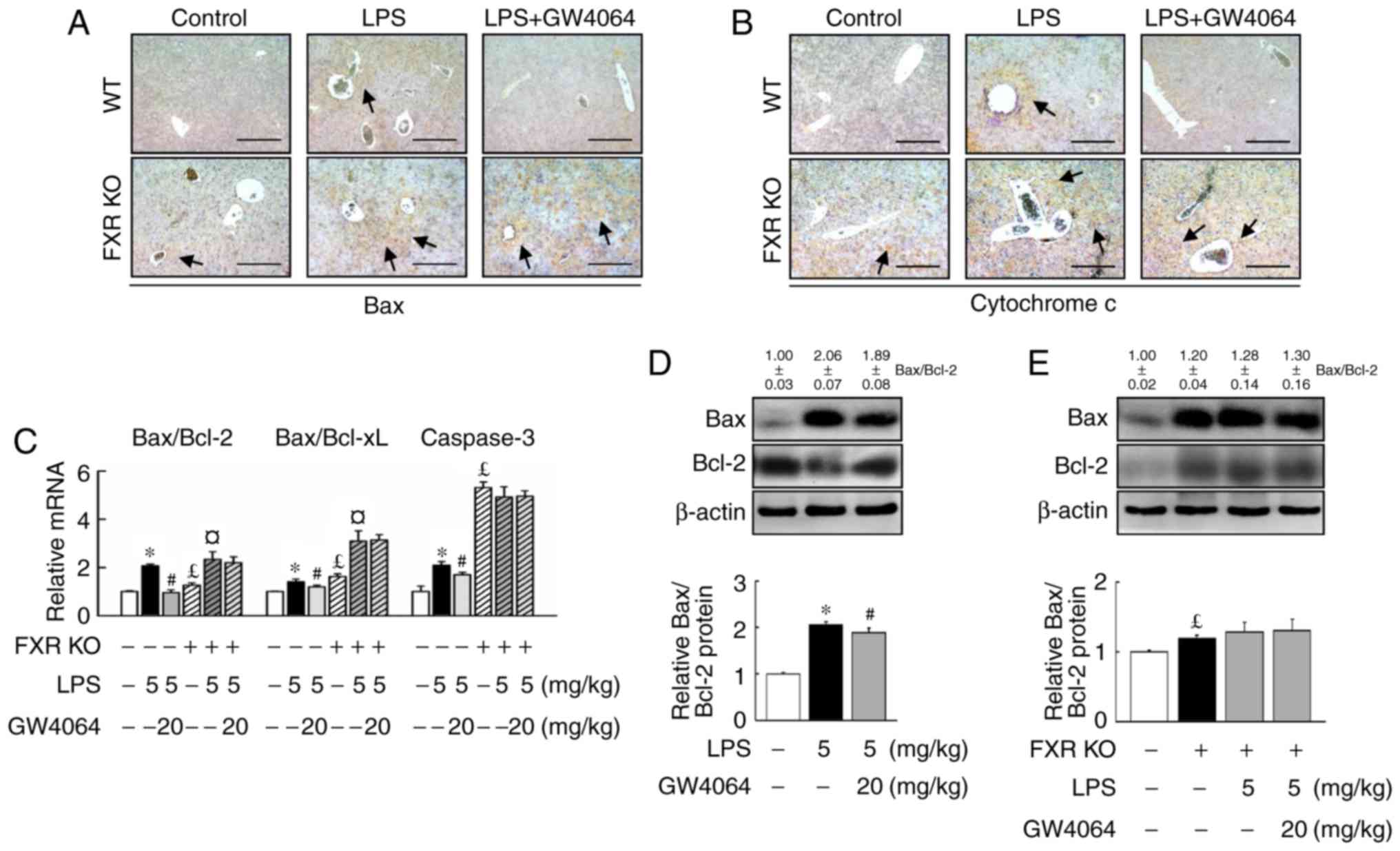 | Figure 4GW4064 attenuates LPS-induced
apoptotic factors in WT mice. WT and FXR KO mice were treated with
or without GW4064 (20 mg/kg) twice after LPS injection. At 6 h
following the last GW4064 injection, livers were removed for
analysis. Representative IHC staining for (A) Bax and (B)
cytochrome c from mouse liver sections. IHC staining for Bax
and cytochrome c are indicated by a dark-brown color (black
arrows; magnification, ×100; scale bar, 200 μm). (C) Total
RNA from liver tissues was isolated and the hepatic Bax/Bcl-2 and
Bax/Bcl-xL ratio as well as caspase-3 were determined by reverse
transcription-quantitative polymerase chain reaction analysis. (D
and E) Bax and Bcl-2 protein was determined by western blot
analysis. Western blot analyses were scanned by densitometry and
data presented as relative intensity units. β-actin was used as a
control. Values are expressed as the mean ± standard error of the
mean (n=5 mice for each group). *P<0.05, WT vs. LPS.
#P<0.05, LPS vs. LPS + GW4064; £P<0.05,
WT vs. FXR KO; ¤P<0.05, FXR KO vs. FXR KO + LPS.
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; Bcl-xL,
Bcl extra-large protein; WT, wild-type; KO, knockout; FXR,
farnesoid X receptor; LPS, lipopolysaccharide; IHC,
immunohistochemical. |
Discussion
Being initially known to regulate liver metabolism,
FXR has recently emerged as a factor participating in hepatic
inflammation, apoptosis and macrophage infiltration (12,19). However, it has remained to be
elucidated whether FXR has a role in LPS-induced hepatic
inflammation and apoptosis mediated via the TLR4/MyD88 signaling
pathway. The present study applied two strategies, namely the use
of the FXR agonist GW4064 and FXR KO mice, and demonstrated that
FXR is indispensable for the effect of GW4064 on the TLR4/MyD88
pathway. The present study revealed that GW4064 has an inhibitory
effect on TLR4/MyD88-mediated inflammatory factors, including TNFα,
IL-6, IL-1β and interferon (IFN)-γ mRNA expression in WT but not in
FXR KO mice. Several lines of evidence suggested that LPS activates
NF-κB mobilization and the p38 MAPK pathway through its direct
binding to TLR4, ultimately causing the production of
pro-inflammatory cytokines such as TNF-α and IL-6 (20,21). NF-κB is activated via
MyD88-dependent and -independent pathways, the former of which
being more robust and immediate, and the latter accounting for a
modest degree in a delayed fashion (22). In addition, the p38 MAPK pathway
is also as a key regulator of the expression of inflammatory
cytokines, including IL-6 and TNF-α, the main determinant of
LPS-stimulated liver damage (23). The results of the present study
may be directly associated with a study by Yao et al
(12), which demonstrated that
FXR agonist GW4064 alleviates endotoxin-induced hepatic
inflammation by repressing macrophage activation in mice. The
results of the present study illustrate that the FXR
counter-regulates the hepatic pro-inflammatory response via the
TLR4 signal pathway. It is therefore suggested that FXR represents
a unique signaling that differentially regulates the inflammatory
response mediated via the TLR4/MyD88 pathway. FXR inhibits the
inflammatory response through several mechanisms, including the
formation of direct complexes with NF-κB family members and/or the
modulation of MAPK activity (9,24).
In addition, GW4064 was reported to alleviate endotoxin-induced
hepatic inflammation by repressing macrophage activation (12) and protect against hepatocellular
inflammation via induction of suppressor of cytokine signaling 3
(25). Therefore, FXR activation
by GW4064 represses the expression of a set of TLR4-regulated
genes, including pro-inflammatory cytokines and apoptotic factors,
and these counter-regulatory effects are lost in FXR KO mice.
Activation of TLR4 signaling in Kupffer cells may
subsequently initiate inflammatory pathways that promote hepatocyte
injury, cholestasis, apoptosis and necrosis, as well as activation
of stellate cells (26,27). An in vitro study indicated
that chenodeoxycholic acid and GW4064, two FXR agonists, rescued
HepG2 cells from serum deprivation-induced apoptosis (9). In addition, an in vivo study
indicated that FXR activation ameliorated hepatocyte apoptosis
during concanavalin A-induced acute liver injury (28). TNF-α and IL-1β induce apoptosis by
inducing caspase-8 and -9 activity, leading to the activation of
caspase-3 in this apoptotic cascade (29). The present study provided a
functional link between these observations and demonstrated that an
increase of apoptosis within the livers of LPS-injected mice is
strongly correlated with an increased TLR4 mRNA expression and
reduced FXR protein levels. These results, together with the
knowledge of the involvement of TLR4 and FXR in hepatic and
microphage injury (9,12,14), motivated us to further elucidate
the role of TLR4 and FXR in liver cell apoptosis. The present
results indicate that LPS treatment led to the upregulation of the
pro-apoptotic Bax and downregulation of the anti-apoptotic Bcl-2 in
WT mice. It was identified that the pro-inflammatory cytokines
TNF-α and IL-1β were elevated alongside LPS-induced liver injury
and apoptosis. In addition, treatment with GW4064 alleviated
LPS-induced hepatocyte apoptosis in the centrilobular area of the
liver of WT mice but not in FXR KO mice.
The present results confirmed that the
hepatoprotective action of GW4064 was mediated through FXR
activation. Induction of hepatic TNF-α and IFN-γ mRNA expression in
response to LPS was significantly greater in FXR KO mice compared
with that in WT mice, suggesting that certain inflammatory genes
are more sensitive to the loss of FXR signaling in mice. Previous
studies indicated that FXR KO livers from 9–12 month-old mice
displayed prominent liver injury and inflammation (10), and that FXR KO mice are more
sensitive to inflammation mediated by NF-κB and LPS than WT mice
(9,30). Of note, according to another
previous study, FXR deficiency completely abolished the ability of
FXR agonist WAY-362450 to decrease the elevations of aspartate
aminotransferase and ALT. In addition, the protective effect of
WAY-362450 on hepatic vascular cell adhesion protein 1, and TIMP
metallopeptidase inhibitor 1 mRNA elevation was also lost in the
FXR KO mice (31). The present
results suggested a potential role of FXR activation in protecting
against liver inflammation by modulation of the TLR4 signaling
pathway and MyD88 activation in the hepatic inflammatory response.
This mutual suppression between FXR and TLR4 signaling may be an
important mechanism for preventing liver apoptosis. Therefore, FXR
activation may inhibit the increase of TLR4/MyD88 activation
through its anti-inflammatory function in the murine liver.
Furthermore, it was observed that activation of FXR repressed
inflammatory cytokines at the mRNA level, which was associated with
changes in the expression of TLR4/MyD88 signaling and NF-κB
activation. Of note, these beneficial effects were seen in WT mice,
while they were not detected in FXR KO mice. Taken together, the
present results suggested that the protective effects of GW4064 are
likely to involve multiple downstream signaling. Regulation of
signaling downstream of FXR by GW4064 may provide a novel approach
for preventing inflammasome overexpression in order to attenuate
hepatic injury. However, to fully dissect the FXR-dependent and
-independent functions of GW4064, it is necessary to further assess
the function of this compound in FXR KO mice.
Acknowledgments
This study was supported by grants from the Ministry
of Science and Technology, Taipei, Taiwan (grant nos.
102-2320-B182-014, 102-2320-B182-015-MY3 and 103-2320-B182-002-MY3)
and the Chang Gung Memorial Hospital (Taoyuan, Taiwan; grant nos.
CMRPD1B0262, CMRPD1D0351 and CMRPD1D0352 to T.-Y.L.).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao B and Bataller R: Alcoholic liver
disease: Pathogenesis and new therapeutic targets.
Gastroenterology. 141:1572–1585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bryant CE, Spring DR, Gangloff M and Gay
NJ: The molecular basis of the host response to lipopolysaccharide.
Nat Rev Microbiol. 8:8–14. 2010. View Article : Google Scholar
|
|
3
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding N, Zhang Y, Loughran PA, Wang Q,
Tsung A and Billiar TR: TIFA upregulation after
hypoxia-reoxygenation is TLR4- and MyD88-dependent and associated
with HMGB1 upregulation and release. Free Radic Biol Med.
63:361–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Wang C, Wang J, Zhao S, Zhang K,
Wang J, Zhang W, Wu C and Yang J: Pseudoginsenoside-F11 (PF11)
exerts anti-neuroinflammatory effects on LPS-activated microglial
cells by inhibiting TLR4-mediated TAK1/IKK/NF-κB, MAPKs and Akt
signaling pathways. Neuropharmacology. 79:642–656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang K: Molecular mechanisms of hepatic
apoptosis. Cell Death Dis. 5:e9962014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Modica S, Gadaleta RM and Moschetta A:
Deciphering the nuclear bile acid receptor FXR paradigm. Nucl
Recept Signal. 8:e0052010.PubMed/NCBI
|
|
8
|
Calkin AC and Tontonoz P: Transcriptional
integration of metabolism by the nuclear sterol-activated receptors
LXR and FXR. Nat Rev Mol Cell Biol. 13:213–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YD, Chen WD, Wang M, Yu D, Forman BM
and Huang W: Farnesoid X receptor antagonizes nuclear factor kappaB
in hepatic inflammatory response. Hepatology. 48:1632–1643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang F, Huang X, Yi T, Yen Y, Moore DD and
Huang W: Spontaneous development of liver tumors in the absence of
the bile acid receptor farnesoid X receptor. Cancer Res.
67:863–867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim I, Ahn SH, Inagaki T, Choi M, Ito S,
Guo GL, Kliewer SA and Gonzalez FJ: Differential regulation of bile
acid homeostasis by the farnesoid X receptor in liver and
intestine. J Lipid Res. 48:2664–2672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao J, Zhou CS, Ma X, Fu BQ1, Tao LS, Chen
M and Xu YP: FXR agonist GW4064 alleviates endotoxin-induced
hepatic inflammation by repressing macrophage activation. World J
Gastroenterol. 20:14430–14441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YD, Yang F, Chen WD, Huang X, Lai L,
Forman BM and Huang W: Farnesoid X receptor protects liver cells
from apoptosis induced by serum deprivation in vitro and fasting in
vivo. Mol Endocrinol. 22:1622–1632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito S, Tanaka Y, Oshino R, Okado S, Hori M
and Isobe KI: GADD34 suppresses lipopolysaccharide-induced sepsis
and tissue injury through the regulation of macrophage activation.
Cell Death Dis. 7:e22192016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao HB, Sun ZL, Zhang HB and Zhang DS:
Berberine inhibits dyslipidemia in C57BL/6 mice with
lipopolysaccharide induced inflammation. Pharmacol Rep. 64:889–895.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fiorucci S, Clerici C, Antonelli E,
Orlandi S, Goodwin B, Sadeghpour BM, Sabatino G, Russo G,
Castellani D, Willson TM, et al: Protective effects of 6-ethyl
chenodeoxycholic acid, a farnesoid X receptor ligand, in
estrogen-induced cholestasis. J pharmacol Exp Ther. 313:604–612.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mencarelli A, Cipriani S, Renga B, D'Amore
C, Palladino G, Distrutti E, Baldelli F and Fiorucci S: FXR
activation improves myocardial fatty acid metabolism in a rodent
model of obesity-driven cardiotoxicity. Nutr Metab Cardiovasc Dis.
23:94–101. 2013. View Article : Google Scholar
|
|
18
|
Maloney PR, Parks DJ, Haffner CD, Fivush
AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis
MC, et al: Identification of a chemical tool for the orphan nuclear
receptor FXR. J Med Chem. 43:2971–2974. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang DG, Zhang C, Wang JX, Wang BW, Wang
H, Zhang ZH, Chen YH, Lu Y, Tao L, Wang JQ, et al: Obeticholic acid
protects against carbon tetrachloride-induced acute liver injury
and inflammation. Toxicol App Pharmacol. 314:39–47. 2017.
View Article : Google Scholar
|
|
20
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McClain CJ, Barve S, Deaciuc I, Kugelmas M
and Hill D: Cytokines in alcoholic liver disease. Semin Liver Dis.
19:205–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagaleekar VK, Sabio G, Aktan I, Chant A,
Howe IW, Thornton TM, Benoit PJ, Davis RJ, Rincon M and Boyson JE:
Translational control of NKT cell cytokine production by p38 MAPK.
J Immunol. 186:4140–4146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen K, Kim ND, Moon JO and Copple BL:
Upregulation of early growth response factor-1 by bile acids
requires mitogen-activated protein kinase signaling. Toxicol Appl
Pharmacol. 243:63–67. 2010. View Article : Google Scholar :
|
|
25
|
Xu Z, Huang G, Gong W, Zhou P, Zhao Y,
Zhang Y, Zeng Y, Gao M, Pan Z and He F: FXR ligands protect against
hepatocellular inflammation via SOCS3 induction. Cell Signal.
24:1658–1664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nolan JP: Intestinal endotoxins as
mediators of hepatic injury-an idea whose time has come again.
Hepatology. 10:887–891. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang P, Zhang M, Wan M, Huang X, Jiang Y,
Xu S and Luo M: Tamoxifen attenuates
lipopolysaccharide/galactosamine-induced acute liver failure by
antagonizing hepatic inflammation and apoptosis. Immunol Invest.
46:284–294. 2017. View Article : Google Scholar
|
|
28
|
Lian F, Wang Y, Xiao Y, Wu X, Xu H, Liang
L and Yang X: Activated farnesoid X receptor attenuates apoptosis
and liver injury in autoimmune hepatitis. Mol Med Rep.
12:5821–5827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zollner G, Wagner M, Fickert P, Geier A,
Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H,
et al: Role of nuclear receptors and hepatocyte-enriched
transcription factors for Ntcp repression in biliary obstruction in
mouse liver. Am J Physiol Gastrointest Liver Physiol.
289:G798–G805. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Wang J, Liu Q and Harnish DC:
Farnesoid X receptor agonist WAY-362450 attenuates liver
inflammation and fibrosis in murine model of non-alcoholic
steatohepatitis. J Hepatol. 51:380–388. 2009. View Article : Google Scholar : PubMed/NCBI
|


















