Introduction
Diabetic nephropathy (DN) is a primary complication
of diabetes mellitus. Oxidative stress has been suggested to have
an important role in DN (1). The
loss of resident renal tubular epithelial cells via apoptosis has
been demonstrated in experimental DN, and renal tubular epithelial
cell apoptosis has been demonstrated to be associated with the
progression of albuminuria (2).
Normal cellular metabolism may produce reactive oxygen species
(ROS), and it has previously been revealed that a number of
environmental factors, including exposure to chemical agents, may
induce oxidative stress (3). The
physiological levels of ROS have important roles in intracellular
signal transduction, follicle development, ovulation and gene
expression (4,5). Furthermore, excessive ROS production
may result in oxidative stress. According to previous studies, it
has been suggested that the suppression of high glucose
(HG)-induced apoptosis in tubular cells is a predominant cause of
renal injury in patients with diabetes (6,7).
The hyperglycemia-induced production of ROS has an important role
in the progression of DN (8). It
has been revealed that HG-induced renal tubular epithelial cellular
apoptosis is significantly decreased following the administration
of N-acetylcysteine and ascorbic acid antioxidants (9,10).
Numerous studies have demonstrated that HG induces the apoptosis of
renal tubular epithelial cells via an oxidant-dependent mechanism.
Therefore, the inhibition of oxidative stress may serve as a
potential therapeutic target for the treatment of DN.
A previous study demonstrated that the intake of
naturally grown fresh vegetables and fruits attenuates the
progression of diabetes and associated complications (11). For example, anthocyanins, the
largest group of water-soluble pigments, belong to a family of
phenolic flavonoid compounds that affect the pigment of numerous
plants. Anthocyanins have been demonstrated to exhibit marked
antioxidative properties in animal models of diabetes mellitus and
to exhibit protective effects against hyperglycemia-induced kidney
injury (12,13). In the present study, in
vivo and in vitro experiments were performed to
investigate the regulation of apoptosis metabolism and the
antioxidative effects exhibited by anthocyanins [grape seed
procyanidin (GSPE) and cyanidin cyanidin-3-O-β-glucoside chloride
(C3G)] in db/db mice. Furthermore, the present study aimed to
investigate the molecular mechanism underlying this process using
HG-stimulated HK-2 cells.
Materials and methods
Animals and treatment
Non-diabetic littermate, 6-8 weeks of age, control
male db/m mice and male BKS db/db C57BL6 mice (body weight 40±2.5
g; total no. of mice, 40) were purchased from the Model Animal
Research Center of Nanjing University (Nanjing, China). According
to the guidelines of the National Institutes of Health (Bethesda,
MD, USA), all experimental animals were housed in pathogen-free
conditions with clean sawdust bedding. All mice were acclimated for
1 week prior to use and housed in polypropylene cages (30×21×10
cm), with free access to food and water, a 12 h light-dark cycle
and a constant temperature of 23±2°C and a humidity of 50–60%. All
procedures and experiments involving mice were reviewed and
approved by the Ethics Committee of Hebei Medical University
(Shijiazhuang, China). At the age of 8 weeks, the mice were divided
randomly into 4 groups: Control group (db/m mice; n=10), db/m +
GSPE group (db/m mice treated with GSPE; n=10), diabetes group
(db/db mice; n=10) and db/db + GSPE group (db/db mice treated with
GSPE; n=10). GSPE was dissolved in normal saline and administered
to mice (once per day) intraperitoneally. GSPE (30 mg/kg body
weight/day; Beijing Solarbio Science and Technology Co., Ltd.,
Beijing, China; purity ≥95%) was dissolved in normal saline and
administered to mice intragastrically for 12 weeks (once per day).
The mice in the control group and the diabetes groups were
administered the same quantity of normal saline (once per day). At
the age of 20 weeks, the mice were placed in individual metabolic
cages in order to collect urine samples over a 24-h time period.
Prior to blood sample collection, mice fasted for 6 h following the
termination of experiments. Plasma and urinary supernatants were
frozen and then stored at −80°C for further analysis. Following
this, animals were sacrificed and kidneys were subsequently stored
for further analysis.
Cell isolation, culture and
treatment
HK-2 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA), and cultured in Dulbecco's
modified Eagle's medium (DMEM)-F12 medium (3:1) supplemented with
5% fetal bovine serum (both Beijing Solarbio Science and Technology
Co., Ltd.) 2 mM l-glutamine, 100 U/ml penicillin and 100
μg/ml streptomycin at 37°C in a 95% air-5% CO2
atmosphere. HK-2 cells were grown to 75–85% confluence, washed once
with serum-free DMEM-F12, and growth-arrested in serum-free
DMEM-F12 at 37°C for 24 h in order to synchronize cell growth.
Following this, cells were treated with normal glucose (NG; 5.6
mM), HG (30 mM), NG plus mannitol (24.4 mM) as an osmotic control,
HG plus C3G [50 μM (14)],
HG plus SB203580 (10 μM), or HG plus PD98059 (50 μM)
for 48 h.
Immunohistochemistry analysis
All samples were incubated with 4% paraformaldehyde
at room temperature for 12 h and then embedded in paraffin for
immunohistochemical analysis used light microscope (magnification,
×400). Paraffin sections were cut into 4-mm sections,
deparaffinized using xylene, and rehydrated using gradient alcohol.
Internal peroxidase was inactivated using 3% hydrogen peroxide in
100% methanol at room temperature for 30 min. Antigen retrieval was
performed via incubation with 10 mM citrate buffer at room
temperature for 15 min. To block nonspecific antibody binding,
sections were incubated with 10% normal goat serum (Beijing
Solarbio Science and Technology Co., Ltd.) in PBS for 30 min at
room temperature. The sections were then incubated overnight at 4°C
with primary antibodies for thioredoxin interacting protein (TXNIP;
1:300; cat. no. ab86983), B-cell lymphoma-2 (Bcl-2; 1:350; cat. no.
ab7973) and Bcl-2-associated X protein (Bax; 1:300; cat. no.
ab7977) (all from Abcam, Cambridge, UK). Following washing with
PBS, sections were incubated with biotinylated secondary antibodies
(SP-9000; 1:4,000; cat. no. 004-2013; ZSGB-BIO; OriGene
Technologies, Inc., Beijing, China) and horseradish
peroxidase-conjugated streptavidin at 37°C for 2 h. Chromogen
detection was performed using 3,3′-diaminobenzidine at room
temperature for 2-3 min to produce a brown color, and sections were
then counterstained using hematoxylin at room temperature for 1-2
min. Negative controls were not incubated with primary
antibodies.
Determination of intracellular and
urinary ROS
The intracellular formation of ROS was determined
using the fluorescence probe 5-(and 6)
chloromethyl-2′,7′-dichlorodihy-drofluorescein diacetate
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Following incubation in 6-well plates under the different
experimental conditions at room temperature for 48 h, the cells
were washed, trypsinized, suspended in PBS, loaded with 10
μM dichlorodihydro-fluorescein diacetate, and incubated at
37°C for 30 min. ROS levels were determined using a flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). To remove particulates,
urine samples were centrifuged at 600 × g for 10 min. The
supernatants were removed, and 8-hydroxy-2-deoxyguanosine levels
were determined using a competitive in vitro ELISA kit (cat.
no. YH8802; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), in accordance with the manufacturer's protocol.
Analysis of mitochondrial membrane
potential (MMP)
MMP was investigated using JC-1 (Shanghai Genmed
Pharmaceutical Technology, Co., Ltd., Shanghai, China) staining.
Cells were seeded in 60 mm culture dishes (2.5×105
cells/well) and incubated with 5 mM JC-1 dye (Shanghai Genmed
Pharmaceutical Technology, Co., Ltd.) at 37°C for 15 min. Cells
were washed three times with PBS and immediately analyzed using a
confocal microscope (magnification, ×400; DM4000B; Leica
Microsystems GmbH, Wetzlar, Germany). The MMP loss was quantified
via the shift of JC-1 emission from red (~590 nm) to green (~525
nm). Red emission revealed membrane potential-dependent JC-1
aggregates in the mitochondria. Green fluorescence revealed the
monomeric form of JC-1 entering the cytoplasm following
mitochondrial membrane depolarization. This assay functions on the
basis of an electrochemical proton gradient at the mitochondrial
inner membrane (15).
Thioredoxin (TRX) activity analysis
TRX activity was determined using the insulin
disulfide reduction assay, as previously described (16). Following incubation of the cells
in 6-well plates under the different experimental conditions for 48
h, total cellular protein was extracted using a lysis buffer (cat.
no. P0013G; Nanjing Jiancheng Bioengineering Institute). A total of
40 μl reaction mixture [200 μl HEPES buffer (1 M, pH
7.6), 40 μl EDTA (0.2 M), 40 μl NADPH (40 mg/ml) and
500 μl insulin (10 mg/ml)] was incubated with cellular
protein extracts at 37°C for 15 min following the administration of
10 μl bovine TRX reductase (Sekisui Diagnostics, LLC,
Lexington, MA, USA). The reaction was terminated by the
administration of 500 μl stop mix (6 M guanidine HCl, 1 mM
DTNB in 0.2 M Tris·HCl pH 8.0), and then the absorption at a
wavelength of 412 nm was determined by Rayto RT-9600 semi-automatic
biochemical analyzer (Rayto Life and Analytical Sciences Co., Ltd.,
Shenzhen, China).
Apoptosis analysis
The apoptosis of proximal tubular epithelial cells
was investigated using a terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay kit
(Promega Corp., Madison, WI, USA). The TUNEL assay was performed in
accordance with the manufacturer's instructions. Fluorescence
images were obtained using a laser-scanning confocal microscope
(magnification, ×400; Leica Microsystems GmbH). A total of 6 fields
of each cover-slip were randomly selected for counting, and 100
cells were randomly counted from each field of vision. The numbers
of apoptotic cells and the total number of cells were counted for
all 6 fields of vision. The apoptosis rate was subsequently
determined.
Following this, slides were immersed in 4%
formaldehyde in PBS at 4°C for 10 min and then incubated in 0.2%
Triton X-100 in PBS for 10 min at room temperature. Equilibration
Buffer (100 μl; Promega Corp.) was added to the cells at
room temperature for 10 min, followed by 50 μl terminal
deoxynucleotidyl transferase reaction mix for 60 min at 37°C.
Subsequently, slides were immersed in 2X saline-sodium citrate and
propidium iodide (PI; 1:10,000), at room temperature for 15 min,
was used to stain all cells. Green fluorescence of apoptotic cells
on a red background was imaged using fluorescence microscopy. In
order to quantify the number of TUNEL-positive (apoptotic) cells,
≥200 cells were counted per group and the percentage of
positively-labeled cells was determined.
The number of apoptotic cells in different groups
was determined using an Annexin V/PI apoptosis detection kit
according to the manufacturer's protocol (MultiSciences Biotech,
Co., Ltd., Hangzhou, China). Briefly, the cell pellet was
resuspended in 1X binding buffer and incubated with 5 ml Annexin V
(conjugated with fluorescein isothiocyanate) and 10 ml PI in the
dark at room temperature for 5 min. Cell fluorescence was analyzed
using a flow cytometer (BD Biosciences). This assay was used to
discriminate between intact cells (Annexin
V−/PI−), early apoptotic cells (Annexin
V+/PI−) and late apoptotic cells (Annexin
V+/PI+).
Western blot analysis
All cells were isolated via scraping in lysis buffer
(cat. no. P0013G; Nanjing Jiancheng Bioengineering Institute). The
protein concentration was determined using Coomassie Protein assay
reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A total of
40 μg proteins was loaded in each lane, separated by
electrophoresis on a 30% gel, and transferred to polyvinylidene
fluoride membranes. Membranes were blocked with 5% dry milk at 37°C
for 2 h and incubated overnight at 4°C with primary antibodies.
Following washing, the membranes, at room temperature for 45 min,
were incubated with goat anti-rabbit or mouse immu-noglobulin G
horseradish peroxidase conjugate (1:5,000; cat. nos. SA00001-2 and
SA00001-1; ProteinTech Group, Inc., Chicago, IL, USA). In order to
perform western blotting analysis, membranes were hybridized with
the following primary antibodies: Rabbit Bax (1:1,000; cat. no.
ab7977), Bcl-2 (1:1,000; cat. no. ab7973), TXNIP (1:1,000; cat. no.
ab86983) (all from Abcam), cleaved caspase-3 (Asp175; 1:1,000; cat.
no. 9661; Cell Signaling Technology, Inc., Danvers, MA, USA),
caspase-3 (1:1,000; cat. no. 19677-1-AP), thioredoxin 2 (TRX2;
1:1,000; cat. no. 13089-1-AP) (both from ProteinTech Group, Inc.),
p-p38 MAPK (Thr180/Tyr182; 1:1,000; cat. no. 4092; Cell Signaling
Technology, Inc.), p-ERK1/2 (Thr202/Tyr204; 1:1,000; cat. no.
1150), p38 MAPK (1:1,000; cat. no. 9212), ERK1/2 (1:1,000; cat. no.
1240) (all from Cell Signaling Technology, Inc.), and mouse
antibody cytochrome c (cyt c; 1:2,000; cat. no.
sc-514435; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Subsequently, the membranes were incubated with goat anti-rabbit or
mouse IgG horseradish peroxidase conjugate, and scanned using the
Odyssey Fc System (LI-COR Biosciences, Lincoln, NE, USA). The
density of the bands was quantified using LabWorks 4.5 software
(UVP, LLC, Phoenix, AZ, USA). Protein expression was quantified via
comparison with the internal control, β-actin (1:2,000; cat. no.
60008-1-Lg; ProteinTech Group, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from granulosa cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The first complementary
DNA strand was synthesized using PrimeScript RT Master Mix (Takara
Biotechnology Co., Ltd., Dalian, China). The primers used for
amplification were as follows: Bax sense, 5′-GCTTGCTTCAGGGTTTCATCC
A-3′ and antisense, 5′-TGTCCACGGCGGCAATCATC-3′; Bcl-2 sense,
5′-GGCGGAGAACAGGGTACGATAAC-3′ and antisense,
5′-CGGGATGCGGCTGGATGGGG-3′; TXNIP sense, 5′-CCGTTAGGATCCTGGCTTGC-3′
and antisense, 5′-GGCGCCTTGTACTCATATTTGTTTC-3′; 18s rRNA sense,
5′-ACACGGACAGGATTGACAGA-3′ and antisense,
5′-GGACATCTAAGGGCATCACAG-3′. The 18s rRNA was used for
normalization. RT-qPCR was performed in a 96-well optical reaction
plate using SYBR Premix Ex TaqII (Takara Biotechnology Co., Ltd.).
The PCR amplification began with a 5-min denaturation at 95°C,
followed by 40 cycles of denaturation at 95°C for 45 sec, annealing
at 55°C for 45 sec, and extension at 72°C for 60 sec. The final
extension was set for 10 min at 72°C. PCR experiments were
performed on an Agilent Mx3000P qPCR System (Agilent Technologies,
Inc., Santa Clara, CA, USA). Relative expression levels were
determined using the 2−ΔΔCq method (17).
Statistical analysis
All data presented in bar graphs are expressed as
mean ± standard deviation. Experiments were performed in
triplicate, and data were analyzed using SPSS 15.0 for Windows
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was
followed by Dunnett's test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Treatment with GSPE reduces the body
weight and the fasting blood glucose (FBG), blood urea nitrogen,
serum creatinine, triglyceride, ROS and urine albumin excretion
(UAE) levels in db/db mice
In order to investigate the role of GSPE in the
progression of DN in db/db mice, 8-week-old db/db mice were
intragastrically administered 30 mg/kg GSPE (dissolved in water)
every second day for 12 weeks. Total body weight and the FBG level
of mice at the termination of the experiments were determined, and
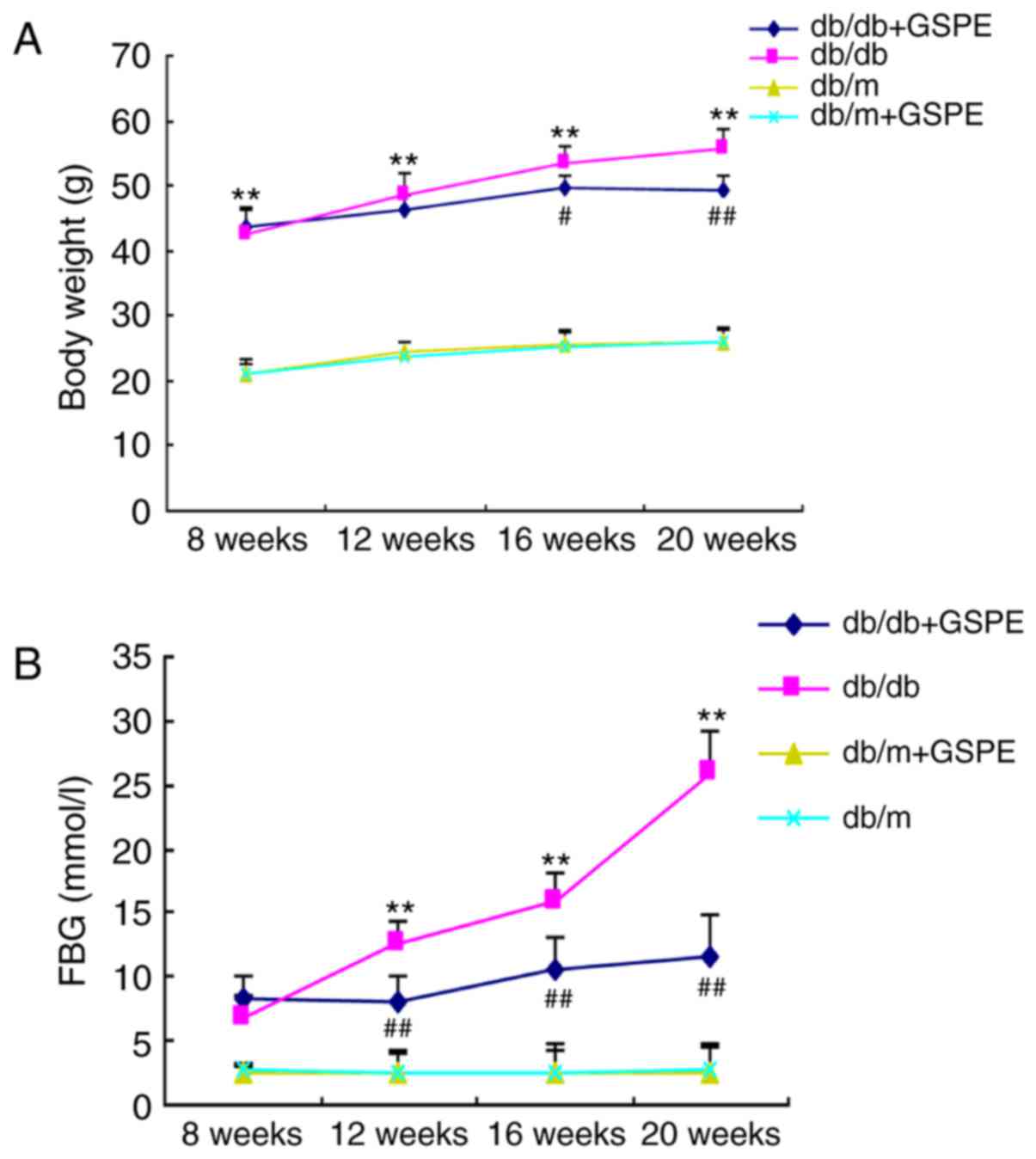
the results are presented in Fig.
1. The body weight and FBG levels were significantly enhanced
in the db/db group compared with the db/m group. Treatment of db/db
mice with GSPE suppressed the increase in body weight. However,
treatment with GSPE significantly increased the body weight of the
db/db + GSPE group compared with the db/db group. All db/db mice
exhibited markedly increased blood glucose levels compared with
db/m mice. Furthermore, db/db mice treated with GSPE demonstrated
significantly decreased blood glucose levels compared with
non-treated db/db mice following 12 weeks of treatment. In
addition, the exhibited levels of blood urea nitrogen, serum
creatinine, were significantly enhanced in the db/db group compared
with the db/m group, however, this was significantly attenuated
following treatment with GSPE (Table
I). These results suggested that GSPE administration
significantly stabilized metabolic parameters and preserved the
renal function of db/db mice. Furthermore, the levels of ROS
[8-Oxo-2′-deoxyguanosine (8-OHdG)] were significantly elevated in
the db/db group compared with the control group (Table I). However, this effect was
attenuated via GSPE administration in the db/db + GSPE group. There
were no significant differences regarding the biochemical and
physical characteristics between the control group and control +
GSPE group.
 | Table IPhysical and metabolic parameters
among different groups. |
Table I
Physical and metabolic parameters
among different groups.
| Parameters | db/m | db/m+GSPE | db/db | db/db+GSPE |
|---|
| Scr
(μmol/l) | 6.92±2.76 | 6.67±3.38 | 11.48±2.06a | 8.52±5.81c |
| Upro (mg/24 h) | 7.16±2.32 | 6.07±1.92 | 29.91±10.01b | 19.96±6.13c |
| BUN (mmol/l) | 4.08±1.31 | 5.91±2.28 | 20.43±10.41b | 11.82±2.19c |
| 8-OHdG (ng/ml) | 27.01±1.67 | 26.08±2.19 | 60.04±3.04b | 31.23±2.28d |
Treatment with GSPE suppresses the
apoptosis of renal tubular cells in db/db mice
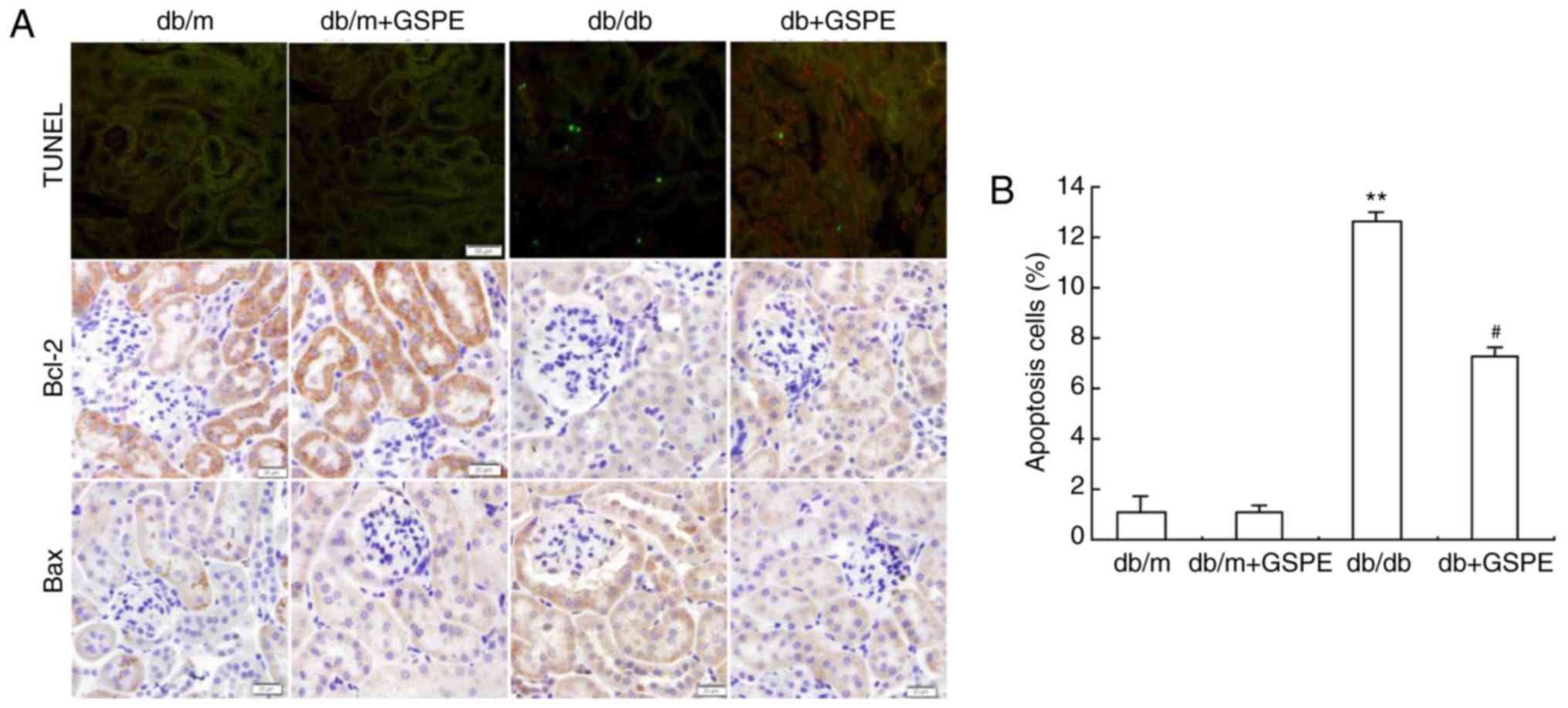
The level of apoptosis of renal tubular cells was
determined using the TUNEL assay. The db/db group exhibited
enhanced levels of renal tubular cell apoptosis compared with the
control group (Fig. 2). This
effect was significantly attenuated following treatment with GSPE
in the db/db + GSPE group (Fig.
2).
Expression of apoptosis-associated
proteins in the kidneys of db/db mice is suppressed following
treatment with GSPE
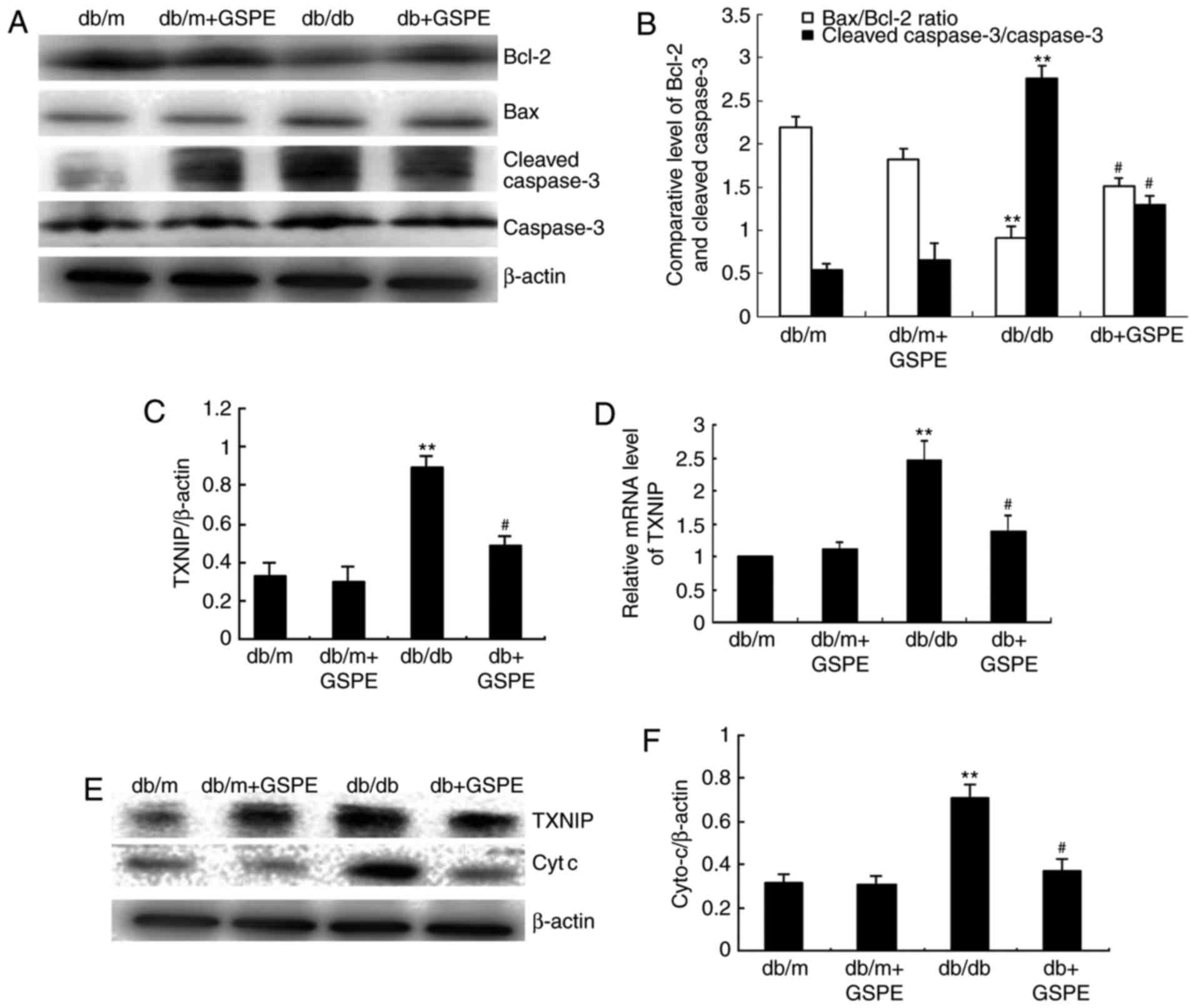
To further investigate the renal protective effects
of GSPE administration in db/db mice, apoptosis-associated protein
expression levels were determined via western blotting. As
demonstrated in Fig. 3A and B,
the Bax/Bcl-2 ratio was suppressed and cleaved caspase-3 was
markedly increased in db/db mice compared with db/m mice. As
revealed in Fig. 3C and D, the
expression level of TXNIP was markedly enhanced in db/db mice, and
treatment with GSPE markedly suppressed TXNIP expression in db/db
mice. This suggests that there is enhanced intrinsic apoptosis in
the kidneys of db/db mice compared with db/m mice; however,
treatment with GSPE significantly attenuated these effects.
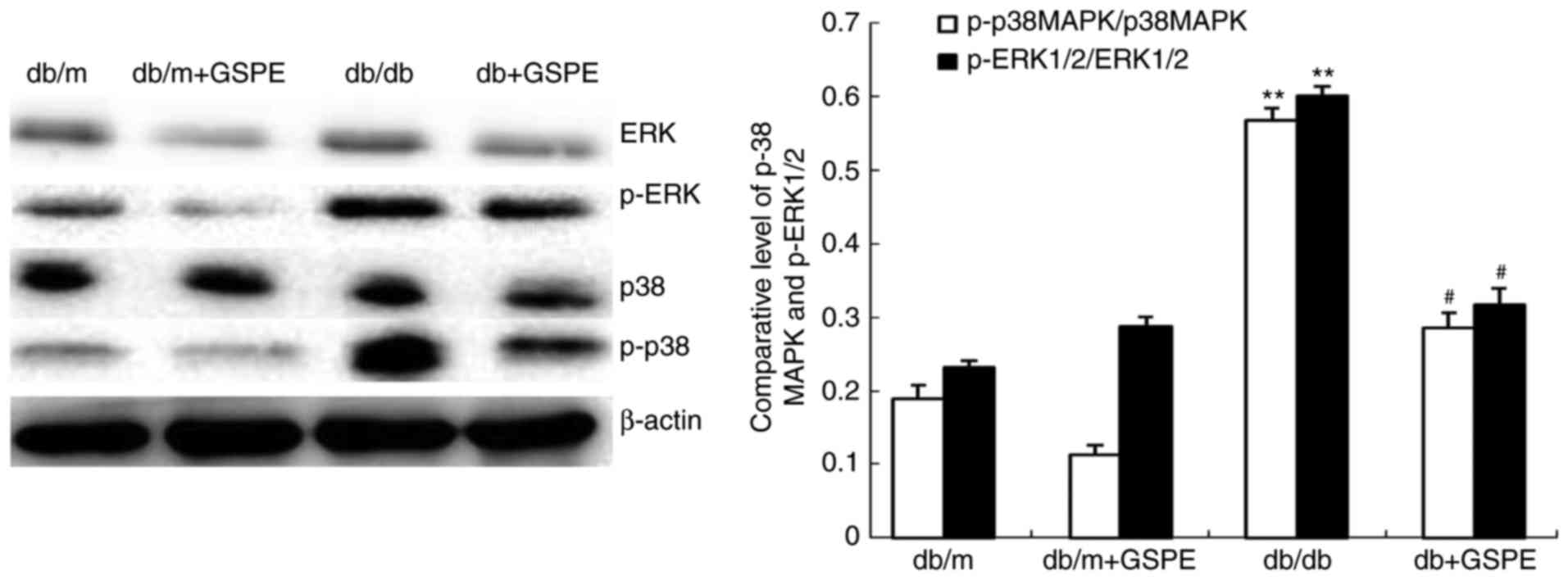
GSPE inhibits the phosphorylation of
ERK1/2, p38 MAPK in db/db mice kidneys
The level of cyt c, in addition to the
p-ERK1/2/ERK1/2 and p-p38/p38 ratios, was investigated in the
kidneys of db/db mice via western blotting. The results revealed
that the level of cyt c (Fig.
3E and F), in addition to the p-ERK1/2/ERK1/2 and p-p38/p38
ratios (Fig. 4), were
significantly enhanced in db/db mice compared with db/m mice.
Furthermore, treatment with GSPE in db/db mice was demonstrated to
significantly attenuate these effects.
C3G protects HK-2 cells from HG-induced
apoptosis
To investigate whether treatment with C3G protects
HK-2 cells from oxidative stress, primary cultured cells were
exposed to HG conditions. Annexin V/PI staining of HK-2 cells
isolated from C3G-pretreated groups was significantly reduced
compared with the HG-treated group (Fig. 5A). In addition, RT-qPCR analysis
was performed in order to determine the mRNA expression levels of
caspase-3, Bax and Bcl-2 (Fig.
5B). The results suggested that the Bcl-2/Bax and cleaved
caspase-3/caspase-3 ratios were significantly suppressed in the HG
+ C3G group compared with the HG-treated group. Furthermore, the
expression of cyt c was demonstrated to be significantly
suppressed in the HG group; however, this effect was significantly
attenuated following treatment with C3G (Fig. 5C).
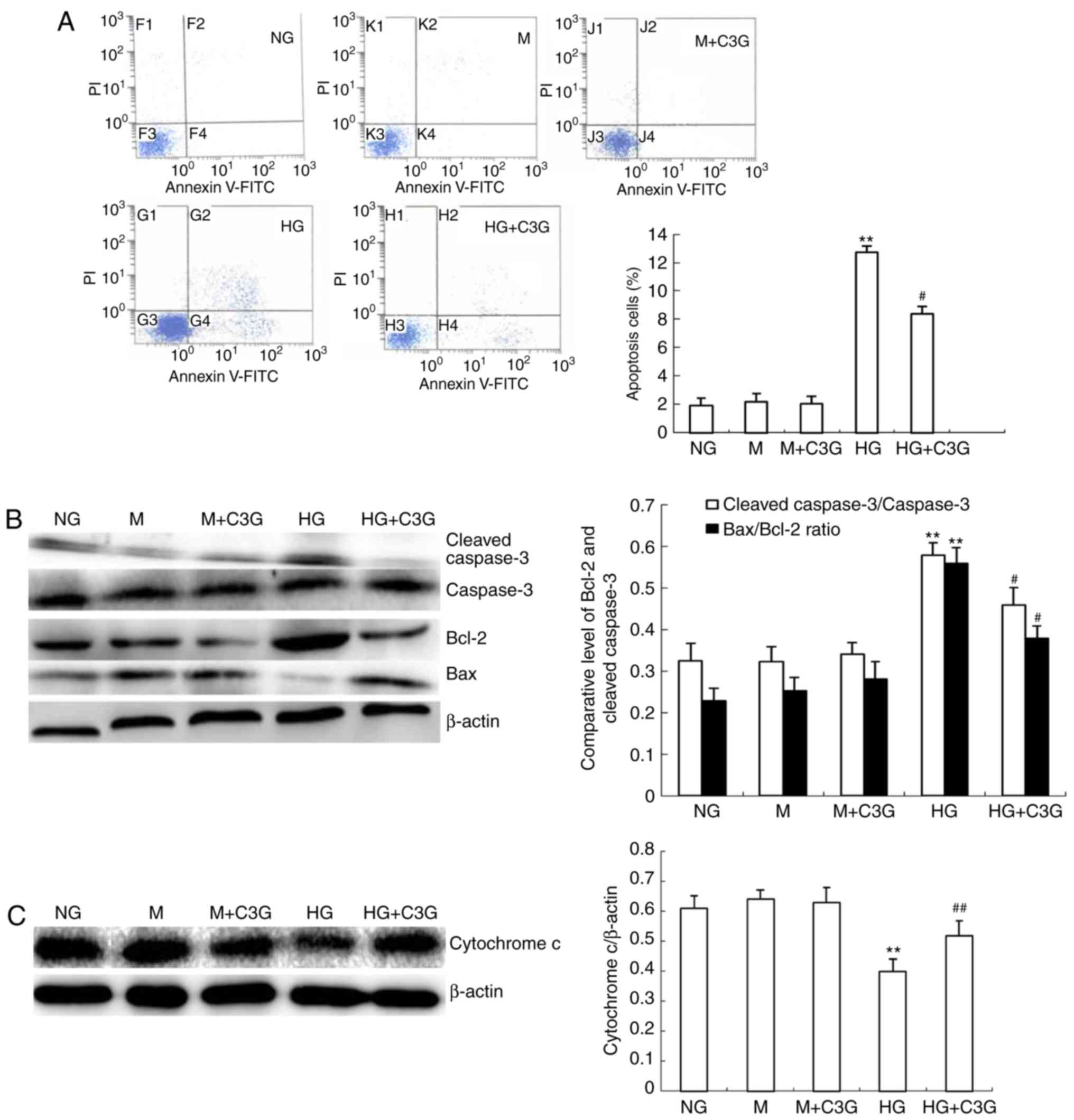 | Figure 5Treatment with C3G suppresses
HG-induced apoptosis in HK-2 cells. HK-2 cells were incubated with
NG (5.6 mM), NG + 24.4 mM M, M + C3G (50 μM, M + C3G), HG
(30 mM) and HG + C3G (50 μM, HG + C3G) for 48 h. (A)
Apoptosis was determined via flow cytometry. C3G was revealed to
significantly suppress HG-induced cell apoptosis. (B) Expression
levels of cleaved caspase-3, caspase-3, Bcl-2 and Bax were
determined using western blotting. The relative intensity of the
cleaved caspase-3 band was normalized to the caspase-3 band.
Normalization of Bax and Bcl-2 against β-actin was performed prior
to the determination of the Bax/Bcl-2 ratio. C3G treatment
significantly suppressed HG-induced expression of cleaved caspase-3
and the Bax/Bcl-2 ratio. (C) Western blot analysis of cyt c
in cytosolic fractions of HK-2 cells. The relative intensity of cyt
c was normalized to β-actin. Cyt c expression in the
cytosol fraction was enhanced following exposure of HK-2 cells to
HG for 48 h; however, this effect was significantly attenuated by
treatment with C3G. Values are expressed as the mean ± standard
deviation (n=6). **P<0.01 vs. NG;
#P<0.05 and ##P<0.01 vs. HG. HG, high
glucose; NG, no glucose; M, mannitol; C3G, cyanidin-3-O-β-glucoside
chloride; cyt c, cytochrome c; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2-associated X protein. |
Treatment with C3G attenuates the
HG-induced enhancement of TXNIP expression, suppression of TRX2
expression, suppression of TRX activity, alteration of MMP, and
enhancement of ROS production in HK-2 cells
The authors' previous study demonstrated that TXNIP
is involved in the HG-induced apoptosis of mouse mesangial cells,
and that knockdown of TXNIP attenuates HG-induced suppression of
TRX activity (18). As revealed
in Fig. 6A–D, HK-2 cells under HG
conditions demonstrated enhanced levels of MMP following treatment
with HG for 48 h. The HG-induced increase in MMP in HK-2 cells was
significantly attenuated following treatment with C3G. In addition,
to investigate whether TXNIP is involved in the protective effect
of C3G in HK-2 cells under HG treatment, RT-qPCR analysis and
western blotting were performed to determine the expression of
TXNIP. Following treatment with HG for 48 h, the expression levels
of TXNIP mRNA were significantly increased in the HG group compared
with the control group, whereas treatment with C3G was revealed to
significantly suppress HG-induced expression of TXNIP in HK-2 cells
(Fig. 6E and F). Following this,
the effect of C3G treatment on TRX2 expression in HK-2 cells
exposed to HG was investigated. As revealed in Fig. 6E, HG exposure did not markedly
affect the expression of TRX2; however, the expression of TRX2 was
significantly enhanced in the HG + C3G group compared with the HG
group. Furthermore, the effect of treatment with C3G on the
activity of TRX in HK-2 cells under HG conditions was investigated.
Furthermore, following exposure to HG for 48 h, the activity of TRX
was significantly suppressed, whereas treatment with C3G under HG
conditions significantly attenuated this effect (Fig. 6G).
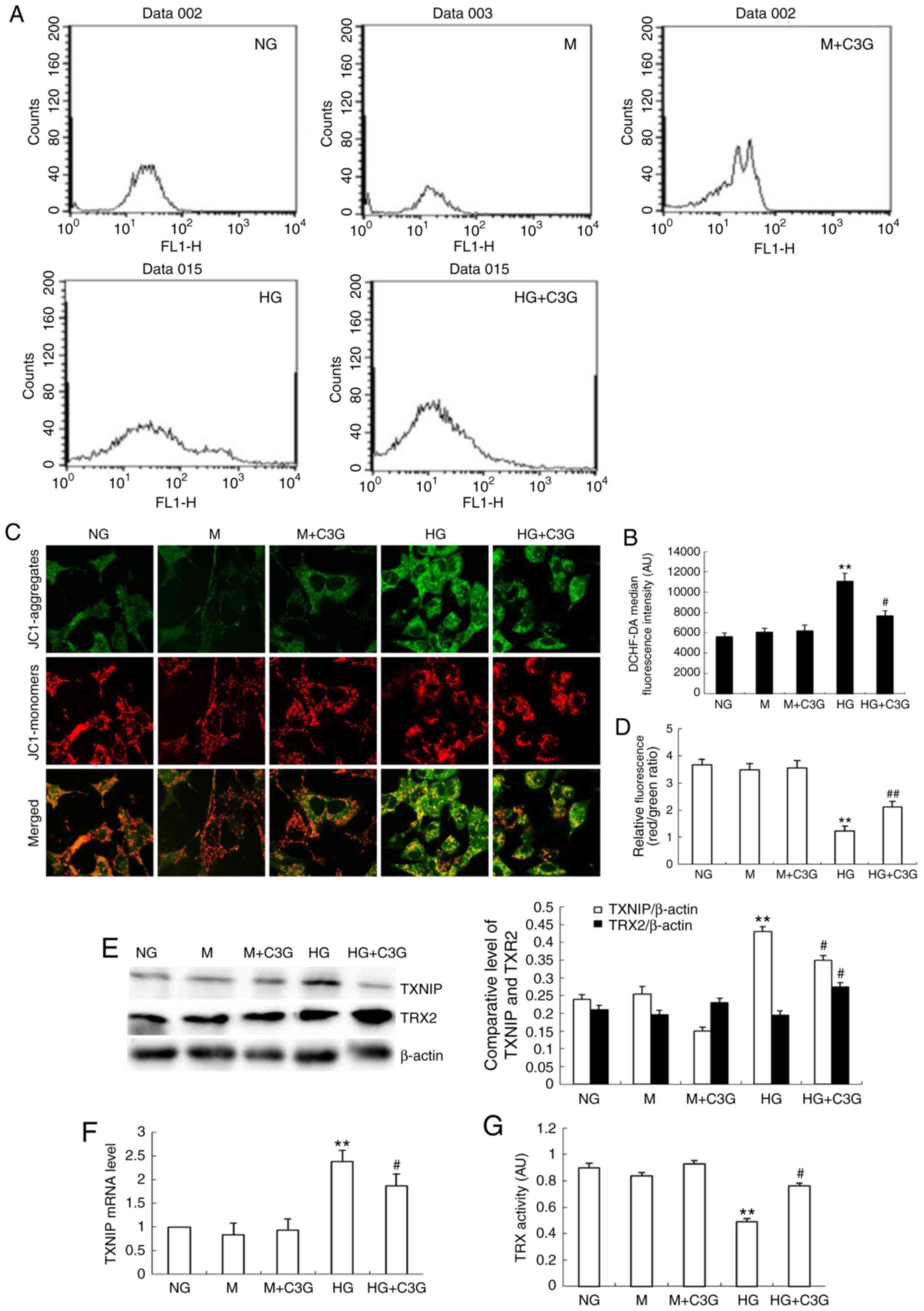 | Figure 6Effects of treatment with C3G on
HG-induced MMP and ROS production, expression of TXNIP and TRX2
expression and TRX activity in HK-2 cells. HK-2 cells were
incubated with NG (5.6 mM), NG + 30 mM M, M + C3G (50 μM, M
+ C3G), HG (30 mM) and HG + C3G (50 μM, HG + C3G) for 48 h.
(A) Intracellular ROS was determined via flow cytometry and (B)
quantified. (C) MMP was determined using a fluorescent probe, JC1,
and (D) quantified. The MMP loss was quantified from the shift of
JC-1 emission from red (~590 nm) to green (~525 nm). The ratio of
red/green fluorescence represented MMP in HK-2 cells. Treatment
with C3G significantly suppressed intracellular ROS production in
HK-2 cells exposed to HG conditions. (E) The expression levels of
TXNIP and TRX2 were determined using western blotting, and the
relative intensities of the TXNIP and TRX2 bands were normalized to
the β-actin band. (F) The mRNA expression of TXNIP was determined
via reverse transcription-quantitative polymerase chain reaction.
(G) TRX activity was determined using an insulin disulfide
reduction assay. Values are expressed as mean ± standard deviation
(n=6). **P<0.01 vs. NG; #P<0.05 vs. HG.
HG, high glucose; NG, no glucose; M, mannitol; C3G,
cyanidin-3-O-β-glucoside chloride; TXNIP, thioredoxin interacting
protein; TRX, thioredoxin; TRX2, thioredoxin 2; MMP, mitochondrial
membrane potential; DCHF-DA, dichlorodihydro-fluorescein
diacetate. |
These results suggest that C3G treatment inhibits
ROS production and suppresses MMP in HK-2 cells otherwise induced
by HG conditions.
C3G suppresses HG-induced apoptosis via
inhibition of p38 MAPK and ERK1/2 phosphorylation
The involvement of p38 MAPK and ERK1/2 in HG-induced
apoptosis in renal tubular epithelial cells has previously been
demonstrated (19). However,
whether C3G is involved in HG-induced apoptosis via p38 MAPK and
ERK1/2 associated pathways remains unclear. As demonstrated in
Fig. 7A–C, the levels of p-p38
MAPK and p-ERK1/2 were significantly increased following HG
exposure, whereas treatment with C3G significantly suppressed the
HG-induced increase of p-p38 MAPK and p-ERK1/2 levels. To
investigate whether p38 MAPK or ERK1/2 signaling affects HG-induced
apoptosis, HK-2 cells were treated with either specific p38 MAPK
SB203580 (10 μM) or ERK1/2 PD98059 (50 μM) inhibitors
for 0.5 h. Following this, cells were incubated under HG
conditions, in the presence of SB203580 or PD98059, for 48 h.
Furthermore, the effects of p38 MAPK and ERK1/2 knockdown regarding
apoptosis were investigated. HG exposure in HK-2 cells was revealed
to significantly enhance cellular apoptosis compared with
non-treated cells (Fig. 7D and
E). In HK-2 cells, the increase in apoptosis exhibited by
HG-treated cells was significantly suppressed following incubation
with either SB203580 or PD98059 (Fig.
7D and E). In addition, administration of C3G to HG-treated
HK-2 cells significantly attenuated the otherwise enhancement of
apoptosis (Fig. 7D and E). In
conclusion, these results suggested that HG-induced apoptosis may
be attenuated by suppression of p38 MAPK and ERK1/2 phosphorylation
in HK-2 cells via treatment with C3G.
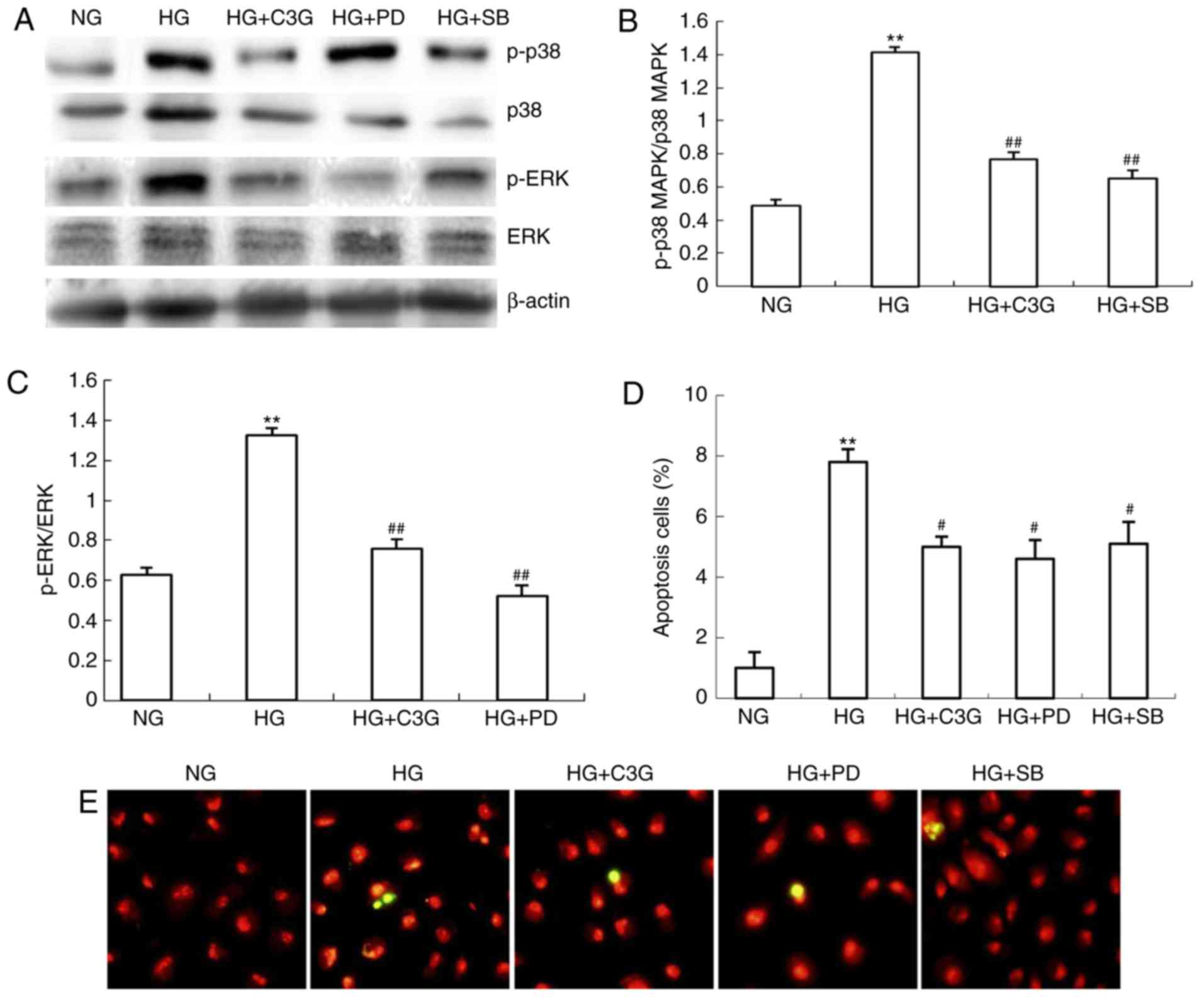 | Figure 7Treatment with C3G suppresses
HG-induced phosphorylation of p38 MAPK and ERK1/2 in HK-2 cells.
HK-2 cells were incubated with NG (5.6 mM), HG (30 mM) and HG + C3G
(50 μM, HG + C3G) and HG + SB203580 (10 μM, HG + SB),
HG + PD94059 (50 μM, HG + PD) for 48 h. (A) Expression
levels of p-p38 MAPK, p38 MAPK, p-ERK1/2 and ERK1/2 were determined
by western blot analysis, and the relative intensities of (B) p-p38
MAPK and (C) p-ERK1/2 were normalized to total p38 MAPK and ERK1/2,
respectively. (D) Apoptosis of HK-2 cells was determined via the
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling assay and (E) images were captured. Values are expressed
as the mean ± standard deviation (n=6; magnification, ×400).
**P<0.01 vs. NG; #P<0.05 vs. HG. HG,
high glucose; NG, no glucose; PD, PD94059; SB, SB203580; C3G,
cyanidin-3-O-β-glucoside chloride; p38 MAPK, p38 mitogen-activated
kinase; p-p38 MAPK, phosphorylated p38 MAPK; ERK1/2, extracellular
signal-regulated kinase 1/2; p-ERK1/2, phosphorylated ERK1/2. |
Discussion
In the present study, it was demonstrated that
treatment with GSPE in db/db mice suppresses kidney cell apoptosis
and superoxide production, thus suggesting that GSPE may have a
therapeutic effect regarding DN. Furthermore, C3G was demonstrated
to suppress HG-induced ROS generation, MMP, cyt c
translocation from the mitochondria to the cytoplasm, TXNIP
expression, and the phosphorylation of p38 MAPK and ERK. In
addition, C3G was demonstrated to upregulate TRX2 expression,
stabilize the biological activity of TRX and suppress HK-2 cellular
apoptosis in response to hyperglycemia.
The enhancement of ROS production is an important
feature of microvascular and macrovascular complications associated
with deformity and death in patients with diabetes mellitus type 2
(20,21). Furthermore, ROS levels have an
important role in the pathogenesis of DN (1,22).
These results suggest that ordinary and catalytic antioxidants may
exhibit protective properties against, or suppress the morbidity
of, DN, and a combination of methods to suppress excessive
production of ROS, and thus increase the elimination of preformed
ROS, may serve as a potential therapeutic agent against the
development and progression of DN. C3G has been suggested to
possess vigorous antioxidant and anti-inflammatory properties
(23,24). Recent studies have revealed that
C3G is able to suppress 1,3-dichloro-2-propanol-induced ROS
generation and suppress the apoptosis of R2C leydig cells (25). In a db/db model of DN, treatment
with grape seed procyanidin B2 (GSPB2) was demonstrated to result
in a suppression of triglyceride concentration in serum, total
cholesterol level, advanced glycation end products and UAE,
compared with vehicle-treated diabetic mice (26). Chronic exposure to Diquat
significantly enhances granulosa cell damage, whereas treatment
with GSPB2 suppresses granulosa cell apoptosis and causes the
autophagy process in ICR mice (27). In the present study, the results
suggested that db/db mice demonstrated markedly enhanced UAE, total
body weight and average glomerular volume at 12 weeks
post-development of diabetes (19). In addition, treatment with GSPE in
db/db mice attenuated albuminuria and the level of urinary 8-OHdG.
Furthermore, the results of the present study demonstrated that
GSPE administration suppressed renal cell apoptosis in db/db mice,
thus suggesting that GSPE exhibits therapeutic effects on the
diabetic kidney. However, Tyagi et al (28) and Li et al (29) suggested that procyanidin B2 leads
to apoptosis in PCa cells of human prostate cancer and in
endothelial cells of diabetes mellitus. The discrepancy between the
results of the present study and previous studies may reflect
differences in the chosen cell lines.
Apoptosis contributes to the development of DN
(30). Previous studies have
suggested that HG-induced cellular apoptosis is an important factor
in the development of kidney injury, and ROS results in the
apoptosis of renal tubular cells (31,32). A predominant factor of apoptosis
is the accumulation of ROS, which enhances intracellular DNA damage
and may induce cell apoptosis. C3G has been demonstrated to
suppress hyperglycemia-induced liver oxidative damage by enhancing
glutathione synthesis via a cyclic adenosine mono-phosphate-protein
kinase A-dependent signaling pathway (33). In the present study, cellular
apoptosis in db/db mouse kidneys was significantly enhanced;
however, this was attenuated by treatment with GSPE. In
vitro, it was revealed that treatment with C3G suppressed
HG-induced ROS generation, apoptosis, the expression of cleaved
caspase-3, the Bax/Bcl-2 ratio, and cyt c translocation from
the mitochondria to the cytoplasm in HK-2 cells. Furthermore, the
results of the present study demonstrated that C3G treatment
reversed the HG-induced alteration in MMP in HK-2 cells. The
results suggest that anthocyanins may attenuate diabetic renal
injury via suppression of ROS, and may thus protect mitochondrial
function. Furthermore, the results suggest that the administration
of anthocyanins enhances antioxidant function and decreases
oxidative stress, thus suppressing apoptosis.
The TRX system, a predominant thiol antioxidant
pathway, regulates the metabolic breakdown of intracellular ROS.
TXNIP, first identified as the product of the 1,25-dihydroxyvitamin
D-3 inducible gene in HL-cells (additionally termed vitamin D3
upregulated protein 1 or thioredoxin binding protein-2), is the
endogenous inhibitor of cellular TRX, inhibiting its antioxidative
role by combining with redox-active cysteine residues (34). TRX/TXNIP, a redox-sensitive
signaling complex, may regulate cellular redox status and has an
important role in the association of redox regulation with the
etiopathogenesis of diseases (35). In a recent study, it was
demonstrated that TXNIP is upregulated under HG conditions in mouse
mesangial cells and renal cells, and that TXNIP knockout may
attenuate the HG-induced suppression of TRX activity, and suppress
HG-induced apoptosis and ROS generation (10,18). In the present study, it was
demonstrated that the administration of anthocyanins significantly
suppressed TXNIP expression in the kidneys of db/db mice and in
HK-2 cells incubated under HG conditions. Furthermore, the results
suggested that C3G treatment enhanced TRX2 expression and
attenuated the HG-induced suppression of TRX activity in HK-2
cells. These results suggest that anthocyanins attenuate oxidative
stress in diabetic kidneys via regulation of TXNIP and TRX
expression.
Increasing studies have suggested that an increase
in the activity of p38 MAPK and ERK1/2 contributes to the
etiopathogenesis of DN (36,37). Numerous studies have suggested
that GSPEs may protect the kidney by suppressing p38 MAPK and
ERK1/2 phosphorylation (38,39). In the present study, it was
demonstrated that the expression levels of p-p38 MAPK and p-ERK1/2
were significantly suppressed in HK-2 cells following treatment
with anthocyanin in vivo and in vitro, thus
suggesting that a reduction in the HG-induced phosphorylation of
p38 MAPK and ERK1/2 may be a downstream effector of the anthocyanin
associated pathway. Furthermore, the present study revealed that
treatment with a p38 MAPK inhibitor (SB203580) or ERK inhibitor
(PD98059) was able to suppress HG-induced apoptosis, thus
suggesting that ROS-MAPK and ROS-ERK is involved in apoptosis.
The present study demonstrated that treatment with
anthocyanins suppressed the TXNIP expression-enhanced p38 MAPK and
ERK1/2 activity, and suppressed renal cellular apoptosis under
diabetic conditions. Therefore, the results suggest that
anthocyanins (GSPE and C3G) may serve as potential therapeutic
agents for the treatment of DN.
Acknowledgments
The present study was supported by the Hebei High
Education Science and Technology Foundation (grant no. NQ2015020),
the Hebei Provincial Natural Science Fund (grant no. H2016206482),
and the Hebei Science and Technology Department Program (grant no.
132777190).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Miranda-Díaz AG, Pazarín-Villaseñor L,
Yanowsky-Escatell FG and Andrade-Sierra J: Oxidative stress in
diabetic nephropathy with early chronic kidney disease. J Diabetes
Res. 2016:70472382016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pesce C, Menini S, Pricci F, Favre A, Leto
G, DiMario U and Pugliese G: Glomerular cell replication and cell
loss through apoptosis in experimental diabetes mellitus. Nephron.
90:484–488. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devine PJ, Perreault SD and Luderer U:
Roles of reactive oxygen species and antioxidants in ovarian
toxicity. Biol Reprod. 86:272012. View Article : Google Scholar :
|
|
4
|
Fujii J, Iuchi Y and Okada F: Fundamental
roles of reactive oxygen species and protective mechanisms in the
female reproductive system. Reprod Biol Endocrinol. 3:432005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sena LA and Chandel NS: Physiological
roles of mitochondrial reactive oxygen species. Mol Cell.
48:158–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu H, Shi Y, Deng X, Su Y, Du C, Wei J,
Ren Y, Wu M, Hou Y and Duan H: Inhibition of c-Src/p38 MAPK pathway
ameliorates renal tubular epithelial cells apoptosis in db/db mice.
Mol Cell Endocrinol. 417:27–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanwar YS, Wada J, Sun L, Xie P, Wallner
EI, Chen S, Chugh S and Danesh FR: Diabetic nephropathy: Mechanisms
of renal disease progression. Exp Biol Med (Maywood). 233:4–11.
2008. View Article : Google Scholar
|
|
8
|
Sun L, Dutta RK, Xie P and Kanwar YS:
Myoinositol oxygenase overexpression accentuates generation of
reactive oxygen species and exacerbates cellular injury following
high glucose ambience: A new mechanism relevant to the pathogenesis
of diabetic nephropathy. J Biol Chem. 291:5688–5707. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mishra R, Emancipator SN, Kern T and
Simonson MS: High glucose evokes an intrinsic proapoptotic
signaling pathway in mesangial cells. Kidney Int. 67:82–93. 2005.
View Article : Google Scholar
|
|
10
|
Wei J, Shi Y, Yan J, Ren Y, Du C, Zhang L,
Li Y and Duan H: Knockdown of thioredoxin-interacting protein
ameliorates high glucose-induced epithelial to mesenchymal
transition in renal tubular epithelial cells. Cell Signal.
25:2788–2796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Punithavathi VR, Stanely Mainzen Prince P,
Kumar MR and Selvakumari CJ: Protective effects of gallic acid on
hepatic lipid peroxide metabolism, glycoprotein components and
lipids in streptozotocin-induced type II diabetic Wistar rats. J
Biochem Mol Toxicol. 25:68–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takikawa M, Inoue S, Horio F and Tsuda T:
Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia
and insulin sensitivity via activation of AMP-activated protein
kinase in diabetic mice. J Nutr. 140:527–533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang MK, Li J, Kim JL, Gong JH, Kwak SN,
Park JH, Lee JY, Lim SS and Kang YH: Purple corn anthocyanins
inhibit diabetes-associated glomerular monocyte activation and
macrophage infiltration. Am J Physiol Renal Physiol.
303:F1060–F1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du C, Shi Y, Ren Y, Wu H, Yao F, Wei J, Wu
M, Hou Y and Duan H: Anthocyanins inhibit high-glucose-induced
cholesterol accumulation and inflammation by activating LXRα
pathway in HK-2 cells. Drug Des Devel Ther. 9:5099–5113. 2015.
|
|
15
|
Rota C, Chignell CF and Mason RP: Evidence
for free radical formation during the oxidation of
2′-7′-dichlorofluorescin to the fluorescent dye
2′-7′-dichlorofluorescein by horseradish peroxidase: Possible
implications for oxidative stress measurements. Free Radic Biol
Med. 27:873–881. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holmgren A and Björnstedt M: Thioredoxin
and thioredoxin reductase. Methods Enzymol. 252:199–208. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Shi Y, Ren Y, Zhao L, Du C, Wang Y, Zhang
Y, Li Y, Zhao S and Duan H: Knockdown of thioredoxin interacting
protein attenuates high glucose-induced apoptosis and activation of
ASK1 in mouse mesangial cells. FEBS Lett. 585:1789–1795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bocanegra V, Gil Lorenzo AF, Cacciamani V,
Benardón ME, Costantino VV and Vallés PG: RhoA and MAPK signal
transduction pathways regulate NHE1-dependent proximal tubule cell
apoptosis after mechanical stretch. Am J Physiol Renal Physiol.
307:F881–F889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hammes HP: Pathophysiological mechanisms
of diabetic angiopathy. J Diabetes Complications. 17(Suppl 2):
S16–S19. 2003. View Article : Google Scholar
|
|
21
|
Suganya N, Bhakkiyalakshmi E, Sarada DV
and Ramkumar KM: Reversibility of endothelial dysfunction in
diabetes: Role of polyphenols. Br J Nutr. 116:223–246. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ha H, Hwang IA, Park JH and Lee HB: Role
of reactive oxygen species in the pathogenesis of diabetic
nephropathy. Diabetes Res Clin Pract. 82(Suppl 1): S42–S45. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Speciale A, Canali R, Chirafisi J, Saija
A, Virgili F and Cimino F: Cyanidin-3-O-glucoside protection
against TNF-α-induced endothelial dysfunction: Involvement of
nuclear factor-κB signaling. J Agric Food Chem. 58:12048–12054.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sivasinprasasn S, Pantan R, Thummayot S,
Tocharus J, Suksamrarn A and Tocharus C: Cyanidin-3-glucoside
attenuates angiotensin II-induced oxidative stress and inflammation
in vascular endothelial cells. Chem Biol Interact:. Oct
28–2016.Epub ahead of print. View Article : Google Scholar
|
|
25
|
Sun J, Xu W, Zhu C, Hu Y, Jiang X, Ou S,
Su Z, Huang Y, Jiao R and Bai W: Cyanidin-3-O-glucoside protects
against 1,3-dichloro-2-propanol-induced reduction of progesterone
by Up-regulation of Steroidogenic Enzymes and cAMP level in leydig
cells. Front Pharmacol. 7:3992016. View Article : Google Scholar :
|
|
26
|
Zhou Y, Li BY, Li XL, Wang YJ, Zhang Z,
Pei F, Wang QZ, Zhang J, Cai YW, Cheng M and Gao HQ: Restoration of
Mimecan expression by grape seed procyanidin B2 through regulation
of nuclear Factor-kappaB in mice with diabetic nephropathy. Iran J
Kidney Dis. 10:325–331. 2016.PubMed/NCBI
|
|
27
|
Zhang JQ, Gao BW, Wang J, Ren QL, Chen JF,
Ma Q, Zhang ZJ and Xing BS: Critical role of FoxO1 in granulosa
cell apoptosis caused by oxidative stress and protective effects of
grape seed procyanidin B2. Oxid Med Cell Longev. 2016:61473452016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tyagi A, Raina K, Shrestha SP, Miller B,
Thompson JA, Wempe MF, Agarwal R and Agarwal C: Procyanidin B2
3,3′-di-O-gallate, a biologically active constituent of grape seed
extract, induces apoptosis in human prostate cancer cells via
targeting NF-κB, Stat3 and AP1 transcription factors. Nutr Cancer.
66:736–746. 2014. View Article : Google Scholar
|
|
29
|
Li BY, Li XL, Cai Q, Cao HQ, Cheng M,
Zhang JH, Wang JF, Yu F and Zhou RH: Induction of lactadherin
mediates the apoptosis of endothelial cells in response to advanced
glycation end products and protective effects of grape seed
procyanidin B2 and resveratrol. Apoptosis. 16:732–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanchez-Niño MD, Sanz AB, Lorz C, Gnirke
A, Rastaldi MP, Nair V, Egido J, Ruiz-Ortega M, Kretzler M and
Ortiz A: BASP1 promotes apoptosis in diabetic nephropathy. J Am Soc
Nephrol. 21:610–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y, Zhang M, Liu R and Zhao C: Oxidative
Stress-activated NHE1 is involved in high Glucose-induced apoptosis
in renal tubular epithelial cells. Yonsei Med J. 57:1252–1259.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Havasi A and Dong Z: Autophagy and tubular
cell death in the kidney. Semin Nephrol. 36:174–188. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu W, Jia Q, Wang Y, Zhang Y and Xia M:
The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases
hepatic glutathione synthesis and protects hepatocytes against
reactive oxygen species during hyperglycemia: Involvement of a
cAMP-PKA-dependent signaling pathway. Free Radic Biol Med.
52:314–327. 2012. View Article : Google Scholar
|
|
34
|
Ren Y, Shi Y, Wang Y, Li Y, Wu S, Li H,
Zhang Y and Duan H: p38 MAPK pathway is involved in high
glucose-induced thioredoxin interacting protein induction in mouse
mesangial cells. FEBS Lett. 584:3480–3485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshihara E, Masaki S, Matsuo Y, Chen Z,
Tian H and Yodoi J: Thioredoxin/Txnip: Redoxisome, as a redoxs
witch for the pathogenesis of diseases. Front Immunol. 4:5142014.
View Article : Google Scholar
|
|
36
|
Sakai N, Wada T, Furuichi K, Iwata Y,
Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Hara A, Yamahana J,
et al: Involvement of extracellular signal-regulated kinase and p38
in human diabetic nephropathy. Am J Kidney Dis. 45:54–65. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou X, Liu R, Duan S, Huang G, Ye Y and
Kong Y: High glucose enhances oxLDL-induced apoptosis in human
renal proximal tubular epithelial cells largely via inducing
Lectin-Like ox-LDL receptor-1. Pharmacology. 98:20–28. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Z, Li BY, Li XL, Cheng M, Yu F, Lu
WD, Cai Q, Wang JF, Zhou RH, Gao HQ and Shen L: Proteomic analysis
of kidney and protective effects of grape seed procyanidin B2 in
db/db mice indicate MFG-E8 as a key molecule in the development of
diabetic nephropathy. Biochim Biophys Acta. 1832:805–816. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takami Y, Uto H, Takeshita M, Kai H,
Akamatsu E, Moriuchi A, Hasegawa S, Oketani M, Ido A, Kataoka H and
Tsubouchi H: Proanthocyanidin derived from the leaves of Vaccinium
virgatum suppresses platelet-derived growth factor-induced
proliferation of the human hepatic stellate cell line LI90. Hepatol
Res. 40:337–345. 2010. View Article : Google Scholar : PubMed/NCBI
|