1. Introduction
Primary liver cancer (PLC) is one of the most common
malignant tumours, and hepatocellular carcinoma (HCC) is the most
frequently occurring primary liver malignancy and a leading cause
of cancer-associated mortality globally (1). Despite advances in prevention,
screening, and novel diagnostic and therapeutic technologies, the
incidence and mortality rates have continued to rise, and the
5-year survival rate of patients with HCC is <20% (2,3).
Therefore, the early diagnosis and treatment of liver cancer is of
particular importance. At present, the serum α-fetoprotein (AFP)
test is a common and important early diagnostic method of liver
cancer, but this biomarker has a low specificity (4). Determining the molecular mechanisms
of the proliferation and metastasis of liver cancer, and
identifying more specific biomarkers to prevent it, is vital to
improve the survival rate for patients.
Exosomes are important as substance transport
carriers for biological information exchange and in the regulation
of the cellular microenvironment via the delivery of a range of
biological molecules, including proteins, lipids and nucleic acids.
Tumour cell-derived exosomes are involved in intercellular
communication, tumour angiogenesis, tumour metastasis, and drug and
radiotherapy resistance (5–7).
Studies have shown that cancer cells release a high level of
exosomes and that the exosome components vary in different
pathological and physiological conditions (8,9).
The contents of exosomes are precisely adjusted in tumour cells,
which suggests that the exosomes may serve an important role in the
process of the tumour formation and development (10). Therefore, the contents of exosomes
appear to be specific diagnostic biomarkers for early-stage tumours
and tumour metastasis (11). In
the present review, recent progress in our understanding of the
biological mechanisms, diagnosis and treatments of exosomes in HCC
are summarized.
2. Source, composition and function of
exosomes
Exosomes are membranous vesicles released into the
extracellular space by cells after fusion of intracellular
multivesicular bodies to the cytomembrane (12). The existence of such vesicles had
been previously observed (13,14), but they were termed exosomes by
Johnstone et al in 1987 (15). These nanoparticles contain a
membrane lipid bilayer and have a cup-like shape with diameters of
between 30 and 150 nm under the electron microscope (16). Exosomes are found in the majority
of, if not all, biological fluids, including urine, plasma, saliva,
bronchial lavage fluid, breast milk, cerebrospinal fluid, amniotic
fluid, abdominal cavity effusion and cell culture supernatant
(17–19). A variety of cells can secrete
exosomes, including B lymphocytes, T cells, mast cells, dendritic
cells, tumour cells, endothelial cells and mesenchymal stem cells
(20).
Exosomes are rich in content, and exosomes from
different sources have been found to contain 9,769 types of
proteins, 3,408 types of mRNAs and 2,838 types of microRNAs
(miRNAs/miRs), according to the latest exosome database (http://www.exocarta.org/). The proteins and RNAs in
exosomes are expressed at different levels in different diseases
and physiological conditions (10). More importantly, the expression of
certain proteins and RNAs in the exosomes is specific to certain
tissues and cell types (20).
Additionally, exosomes contain a variety of lipid molecules that
can not only participate in a variety of biological processes, but
also serve an important role in the morphological stability of
exosomes in the extracellular fluid, protecting their contents from
degradation by extracellular enzymes (21). Accordingly, it can be hypothesized
that the level of exosomes has great clinical potential as a
non-invasive diagnostic method (22).
Exosomes exist in the body fluids and tissues,
suggesting that they may be involved in various physiological or
pathological processes. Exosomes convey information by means of
their vesicle contents and are considered to be the third type of
signalling mechanism between cells, which is as important as cell
contact-dependent signal transduction and signalling transduction
mediated by soluble molecules (23). Under physiological conditions, the
contents of exosomes are precisely regulated by donor cells and
reflect donor cell function. To exchange and transmit biological
information between cells, the donor cell transfers genetic
materials to target cells through the 'transportation' function of
the exosomes (24). Under
pathological conditions, the diseased cells can also transfer their
contents, such as viruses and miRNAs, to normal cells, and cause
the normal cells to be become infected and cytopathic (25), or transfer oncogenes to normal
cells, leading to tumour invasion and metastasis (26).
3. Role of exosomes in tumour initiation and
progression
Exosomes serve a dual role in tumour initiation and
progression. On the one hand, the exosomes of normal cells can
inhibit the proliferation of tumour cells by transferring tumour
suppressor genes into the cancerous cells, allowing the tumour
suppressor genes to block the corresponding signalling pathways
(27,28). The exosomes of tumour cells can
also induce specific antitumour effects. For example, dendritic
cells have been shown to induce potent cluster of differentiation
(CD)8+ T cell-dependent antitumour effects, suggesting
that exosomes are relevant for immuno-interventions (29). On the other hand, exosomes serve
an important role in the tumoural process and have the ability to
promote the occurrence, development and metastasis of tumours. The
exosomes of cancerous cells, which can inhibit natural killer cells
and cytotoxic T cells, promote tumour growth (30); they can transfer the genetic
material of cancerous cells to normal cells, triggering the
uninhibited growth and differentiation of normal cells, which could
be one of the mechanisms of tumour invasion. Exosomes can also be
transported by the blood and body fluids to other tissues and
organs (31). Therefore, exosomes
are an important component of the tumour microenvironment, and are
involved in cell signal transduction and the process of tumour
formation and degradation. Tumour exosomes can assist in
deciphering the process of tumour formation and metastasis, and
provide novel approaches for the clinical diagnosis and treatment
of tumours.
4. Role of exosomes in the occurrence and
development of HCC
Role of exosome miRNAs in HCC
Several studies (32–34) have shown that specific miRNAs are
associated with the occurrence and development of liver cancer. It
has been demonstrated that the expression of miR-21, miR-221 and
miR-222 is increased in liver cancer tissue compared with that in
normal liver tissue (35–37). By contrast, the expression of
miR-122-a, miR-145, miR-199-a and miR-223 is decreased (34,38–40). In addition, miR-338 is closely
associated with the characteristics of liver cancer, including
tumour size, differentiation, vascular invasion and intrahepatic
metastasis (41). miR-221,
miR-103, let-7a, miR-181c, miR-181a and miR-26a (RG-6) are stably
expressed in serum samples of HCC patients, and Fornari et
al (42) also identified that
an exosomal secretion of miR-519d, miR-21, miR-221 and miR-1228 was
present in patients with HCC, together with an association between
tissue and circulating levels of miR-519d, miR-494 and miR-21.
Thus, miRNAs are important in the progression of liver cancer
(Fig. 1) (43). With in-depth research on the
transportation function of these vesicles, exosomal miRNAs have
become a focus of attention. Exosomal miRNAs derived from HCC are
summarized in Table I.
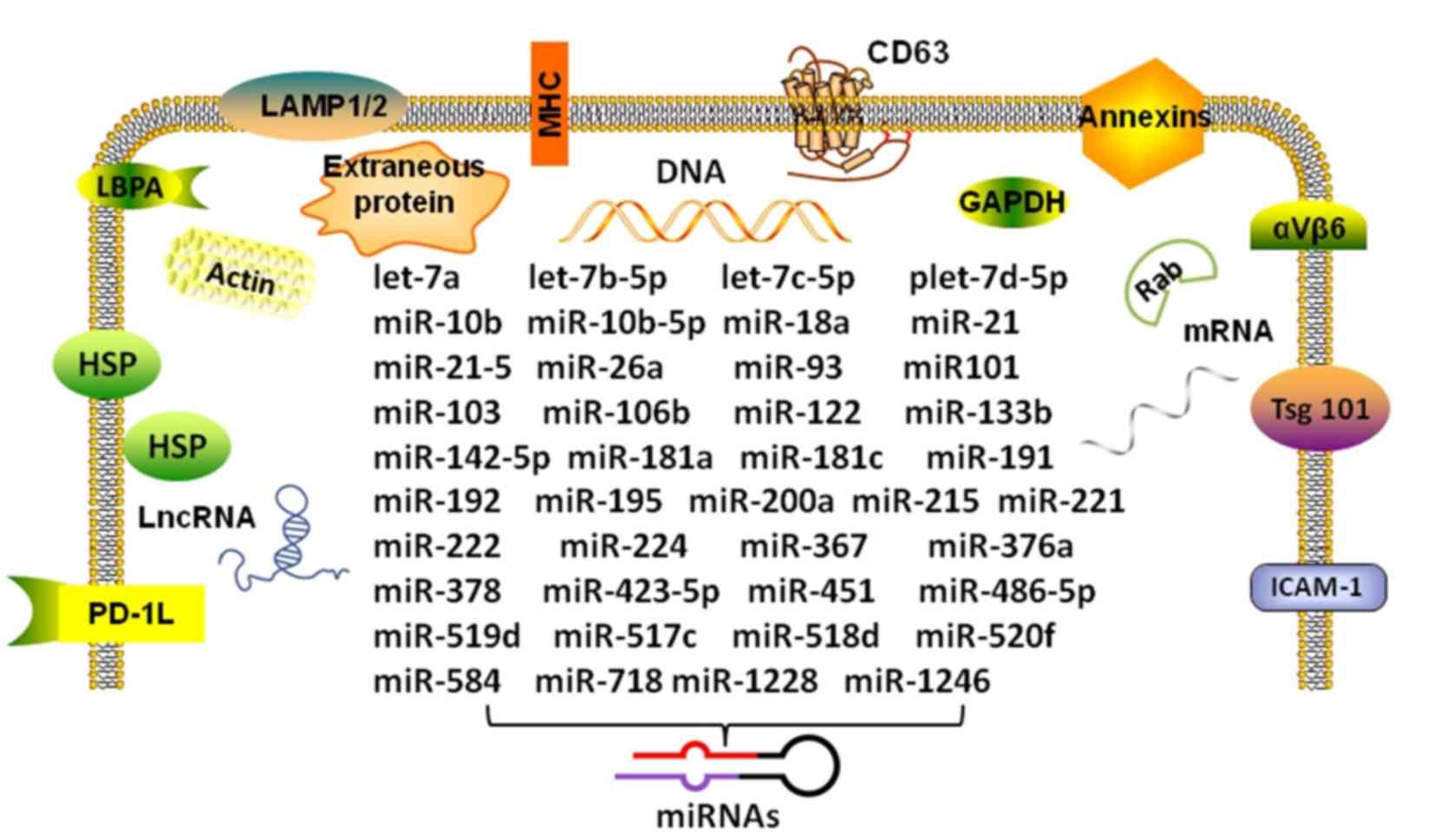 | Figure 1Composition and miRNAs in the
HCC-derived exosome. The figure shows the double membrane structure
of the specific intracellular miRNAs of HCC-derived exosomes. The
transmembrane proteins and cytosolic proteins with membrane binding
capacity of the exosome can be used as markers for isolation and
identification. HCC, hepatocellular carcinoma; miR/miRNA, microRNA;
LncRNA, long non-coding RNAs; HSP, heat shock protein; LBPA,
lactoferrin binding protein A; PD-1L, programmed death-ligand 1;
LAMP, lysosomal-associated membrane protein; MHC, major
histocompatibility complex; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; Tsg101, tumor susceptibility gene 101; ICAM,
intercellular adhesion molecule. |
 | Table ImiRNAs in exosomes of HCC. |
Table I
miRNAs in exosomes of HCC.
| First author/s | Year | Country | Source of
exosomes | miRNAs | (Ref.) |
|---|
| Kogure et
al | 2011 | USA | CCS | miR-584, miR-517c,
miR-378, miR-520f, miR-142-5p, miR-451, miR-518d, miR-215,
miR-376a, miR-133b and miR-367 | (20) |
| Chiba et
al | 2012 | Japan | CCS | miR-21, miR-192 and
miR-221 | (31) |
| Basu and
Bhattacharyya | 2014 | India | CCS | miR-122 | (45) |
| Wang et
al | 2014 | China | Serum | miR-21 | (19) |
| Wei et
al | 2015 | China | CCS | miR-423-5p,
miR-21-5, plet-7d-5p, let-7b-5p, let-7c-5p, miR-486-5p and
miR-10b-5p | (44) |
| Sugimachi et
al | 2015 | Japan | Serum | miR-718 and
miR-1246 | (48) |
| Liu et
al | 2015 | China | Rabbit serum | miR-10b, miR-21,
miR-122 and miR-200a | (58) |
| Li et
al | 2015 | China | Serum | miR-10b, miR-21,
miR-122 and miR-200a | (59) |
| Chen et
al | 2015 | USA | CCS | miR-122 | (53) |
| Sohn et
al | 2015 | Korea | Serum | miR-18a, miR-221,
miR-222, miR-224, miR101, miR-106b, miR-122, miR-195, miR-21 and
miR-93 | (63) |
| Fornari et
al | 2015 | Italy | CCS | miR-519d, miR-21,
miR-221 and miR-1228 | (42) |
| Lou et
al | 2015 | China | CCS | miR-122 | (46) |
| Li et
al | 2015 | China | Serum | miR-221, miR-103,
let-7a, miR-181c, miR-181a and miR-26a | (43) |
A large variation in miRNA expression levels exists
between donor cells and their autocrine or paracrine exosomes. In
Hep3B-derived exosomes, a total of 134 types of miRNAs have been
identified. Of these, 55 were differentially expressed by
>4-fold in exosomes compared with their donor cells; 25 of these
miRNAs were enriched (up to 166-fold), and 30 miRNAs were depleted
(up to 113-fold). It is particularly important to note that 11
types of miRNAs are only expressed in exosomes (20). In another liver cancer cell line,
SMMC-7721, exosomes were found in the cell culture supernatant.
Compared with that in the donor cells, the miR-423-5p and miR-21-5p
levels in the exosomes were not significantly different, whereas
let-7d-5p, let-7b-5p and let-7c-5p exhibited lower levels of
expression, and miR-486-5p and miR-10b-5p exhibited higher levels
of expression (44). Therefore,
donor cells can determine the specific miRNAs loaded into the
exosomes to achieve their functions.
miRNAs can be transferred between cells via exosomes
and can affect target cells. For example, exosomes can transfer
miR-122 between Huh7 and HepG2 human liver cancer cell lines. Huh7
cell-derived exosomes transfer miR-122 to HepG2 cells, which can
inhibit the growth of HepG2 cells and hasten the ageing process of
these cells (45). This
transmission can also occur between different source cells; the
exosomes derived from adipose mesenchymal stem cells can transfer
miR-122 to HepG2 cells (46). In
human and mouse liver cells and primary B cells, exosomes can
shuttle between different cells and transfer endogenous miRNAs
(47). Chiba et al
(31) found that colon cancer
cell-derived exosomes transferred miR-21, miR-192 and miR-221 to
the liver cancer HepG2 cell line and the lung cancer A549 cell
line.
Exosomes can also transport miRNAs to target cells
and then act on the corresponding target genes to alter their
functions. Lou et al (46)
found that miR-122 could be transported to HCC cells via exosomes,
which negatively regulated the target genes of miR-122 and improved
the sensitivity of the HCC cells to chemotherapy drugs.
Additionally, exosomal miR-718 can regulate the target gene
homeobox B8 to inhibit the differentiation of liver HCC cells.
Patients with low levels of serum-derived exosome miR-718 showed
more tumour recurrence after liver transplantation (48). Therefore, exosomes can transfer
miRNAs between cells, and these miRNAs regulate gene expression in
the target cells and serve important roles in tumour invasion,
metastasis and drug resistance.
Exosome-mediated immune escape of
HCC
Viral components can be assembled into exosomes.
Exosomes derived from B cells infected with Epstein-Barr (EB) virus
can be detected by the miRNAs of EB virus (49). With regard to liver cancer,
persistent infection with hepatitis virus is one of the most
important factors in liver tumourigenesis. There is considerable
evidence to suggest that soluble immunoregulatory molecules in the
exosomes secreted by HepG2 cells significantly inhibit lymphocyte
proliferation (50). Recent
evidence indicates that hepatitis A virus (HAV) can be protected
from antibody-mediated neutralization by exosomes (51). In addition, hepatitis C virus
(HCV) released from donor cells by way of exosomes can infect other
hepatocytes. One study reported that HCV-infected Huh7.5.1 liver
cancer cells can transport HCV via exosomes, and that HCV then
escapes immunological surveillance and infects intact Huh7.5.1
cells (52). Another study showed
that exosomes also mediated persistent HCV infection caused by
autophagy (25), and a low Rab27a
level resulted in decreased HCV RNA and protein levels in
virus-infected cells (53). Thus,
exosomes can mediate the escape of hepatitis virus from the human
immune system, resulting in persistent infection.
Exosome-mediated HCC metastasis and
invasion
Exosomes are a unique and important mechanism for
signal transduction between liver cancer cells and target cells.
For example, the paracrine or autocrine exosomes of liver cancer
cells are rich in small RNAs and proteins, and these RNAs and
proteins can be transferred by exosomes to promote HCC cell
metastasis (20). Due to the
natural transportation effect of the exosome, RNAs carried by
exosomes can reach and impact distant cells and produce different
cell phenotypes, and this may be an important mechanism of the
distant metastasis of liver cancer (54). For example, HCC cells pack
selective miRNAs into exosomes by means of ceramide. These exosomes
can regulate the expression and downstream signalling pathways of
transforming growth factor-β-activated kinase 1 of target cells,
and regulate the growth and apoptosis of transformed cells
(20).
In addition, certain RNAs can be transferred to
other cells and tissues by means of exosomes and build a suitable
microenvironment for tumour cell metastasis. A recent study
suggested that in CD90+ liver cancer stem cells
associated with the metastasis and recurrence of liver cancer,
exosomes derived from CD90+ Huh7 cells could induce
effects on tube formation and cell-cell adhesion of human umbilical
vein endothelial cells (HUVECs). CD90+ cells express the
long non-coding RNA (lncRNA) H19 and release it via exosomes that
are able to enter endothelial cells and transfer lncRNA H19 into
the corresponding target cells, which upregulate the synthesis and
release of vascular endothelial growth factor to stimulate
angiogenesis and promote adhesion between CD90 + Huh7 cells and
endothelial cells (26).
In addition to small RNAs, exosomes can also
transfer associated proteins to promote the invasion and metastasis
of liver cancer. For example, the vasorin protein secreted by HCC
HepG2 cells can be transferred to HUVECs by exosomes and enhance
the migration of HUVECs (55).
Accumulating evidence (10,32,41) suggests that exosomes from HCC cell
lines with a high metastatic potential are rich in cancer-promoting
mRNAs and proteins, such as proto-oncogene Met, S100 family members
and the caveolins. One previous study further showed that the
uptake of these shuttled molecules triggered phosphoinositide
3-kinase/AKT and mitogen-activated protein kinase signalling
pathways in MIHA (an immortalized hepatocyte line), with increased
secretion of active matrix metalloproteinase-2 (MMP-2) and MMP-9,
which enhanced the invasion and metastasis of HCC cells (56).
On the other hand, the exosomes from other cancer
cells can also enhance the process of invasion and metastasis. A
recent study demonstrated that exosomes derived from SW480
colorectal cancer cells induced the phosphorylation of
extracellular signal-regulated kinase 1/2 following their uptake
into HepG2 cells and promoted recipient HepG2 cell migration
(57). Therefore, in the
microenvironment of liver cancer, the exosomes can be used to
disseminate cancer genes and proteins, and they serve an important
role in the process of the invasion and metastasis of liver
cancer.
5. Applications of exosomes in HCC
Exosomes as diagnostic markers
Exosomes carry specific biomarkers derived from
tumour cells, and the concentration of their contents is associated
with the invasive ability of the tumour cells and the tumour
microenvironment. Therefore, the basic information on the cancer
cells can be obtained by analysis of the composition of exosomes,
which suggests that the exosomes can serve as a tool for cancer
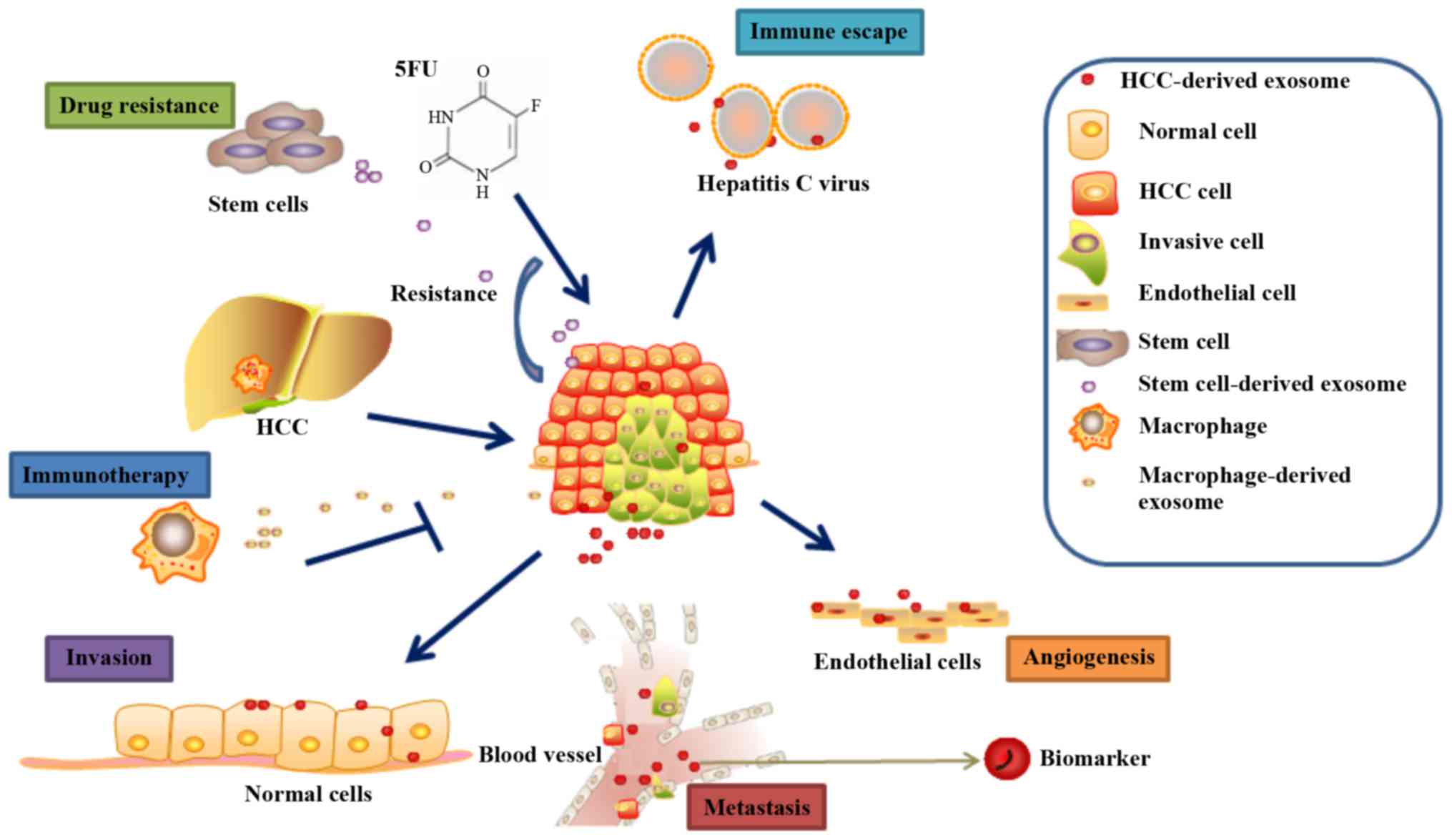
diagnosis (Fig. 2) (58). Owing to the protective lipid
membrane of exosomes, their contents of DNA, RNA and protein cannot
easily be degraded, which renders fresh and preserved samples
available for analysis. More importantly, exosomes can be obtained
from numerous body fluids, which makes detection of exosomes a
promising method for tumour diagnosis and treatment, and possibly
ideal for the method of 'liquid biopsy' (59). Based on the analysis of a large
number of serum samples, previous studies have found that the level
of glypican-1-positive exosomes in the serum of pancreatic cancer
patients is significantly higher than that in healthy individuals.
A further study indicating that patients with early pancreatic
cancer have a higher abundance of glypican-1-positive exosomes in
the serum than healthy subjects provided an important basis for the
application of exosomes to the early diagnosis of pancreatic cancer
(60). In addition, evidence
indicates that miR-21 serves an important role in tumourigenesis
and tumour development. As the miR-21 serum content of tumour
patients is extremely low, previous studies had mainly focused on
the detection of miR-21 expression level in the cancer tissue,
which greatly limited the clinical utility of miR-21 as a
diagnostic tumour marker (61). A
previous study (62) found that
the miR-21 level in serum-derived exosomes increased significantly
in patients with cancer, particularly in those with malignant
glioma and oesophageal squamous carcinoma, compared with that in a
healthy group, which suggests that exosomal miRNAs would likely
become a marker for cancer diagnosis and treatment.
miR-21 can also be detected in the serum of patients
with liver cancer and chronic hepatitis B (CHB). Compared with the
CHB group and healthy individuals, the level of miR-21 expression
in serum-derived exosomes of patients with liver cancer increased
significantly and was associated with liver cirrhosis and liver
cancer staging. More importantly, the abundance of exosomal miR-21
is significantly higher than that in whole serum, which indicates
that exosomal miR-21 would be a more sensitive diagnostic marker
(19). Other exosomal miRNAs may
also be diagnostic markers of liver cancer. For example, in the
serum of patients with HCC, the exosomal contents of miR-18a,
miR-221, miR-222 and miR-224 were found to be significantly higher
than those in hepatitis and liver cirrhosis groups, whereas the
expression levels of miR-101, miR-106b, miR-122 and miR-195 were
significantly reduced (63). For
the identification of specific miRNAs in exosomes from the serum of
patients with recurrent HCC, Sugimachi et al (48) compared the microarray-based
expression profiling of miRs derived from exosomes in the serum of
patients with and without HCC recurrence and found that decreased
expression of miR-718 in the serum exosomes of HCC patients was
associated with HCC tumour aggressiveness. However, the different
expression of these miRNAs in serum exosomes remains controversial.
For example, Wang et al (19) found that miR-21 was more easily
detected in serum-derived exosomes than in complete serum of HCC
patients. Compared with that in a CHB group and a healthy group,
the miR-21 levels in serum-derived exosomes were significantly
increased (30 samples per group). However, Sohn et al
(63) found no significant
difference in the level of miR-21 expression in the serum of HCC
patients or liver cirrhosis patients (30 samples per group). It was
hypothesized that the reported differences may be associated with
the sample selection, the size of the sample and the appraisal
method of the miRNAs.
TUC339 is an lncRNA that is highly enriched within
extracellular vesicles (EVs) released from HCC-derived tumour cells
(64). This lncRNA has been
implicated in modulating tumour cell growth and adhesion. The
emerging data on lncRNAs indicate the presence of several
tumour-associated lncRNAs, certain of which have been functionally
linked to processes involved in tumour growth. The integrity and
the functional role of tumour-specific lncRNAs within EVs have yet
to be established, and their presence or enrichment within tumour
cell-derived EVs suggests their potential as HCC biomarkers
(65).
Role of exosomes in chemotherapy drug
resistance of HCC
The development (46) of drug resistance is the main cause
of failure of cancer chemotherapy. Liver cancer patients easily
develop resistance to conventional chemotherapy drugs (Fig. 2), such as 5-fluorouracil and
doxorubicin. Therefore, it is urgent to improve the efficacy of
liver cancer chemotherapy. miR-122, whose expression is
significantly lower in HCC compared with normal liver tissue,
serves multiple roles in the physiological and pathological
processes of HCC (66). Recent
evidence indicates that miR-122 can alter the chemotherapeutic
sensitivity of HCC cells by downregulating drug resistance-related
genes, including multidrug resistance gene-1, glutathione S
transferase-π and multidrug resistance-associated protein, and by
regulating the expression of apoptosis-related genes, such as
Bcl-2-like 2, and cell cycle-related genes, such as cyclin B1.
The adipose tissue-derived mesenchymal stem cell
(AMSC) can also produce a large number of exosomes. Lou et
al (46) found that AMSCs
transfected with miR-122 can effectively assemble miR-122 into
AMSC-secreted exosomes, which can then be transported into HCC
cells and promote cell apoptosis and cell cycle arrest. miR-122 can
negatively regulate the expression of its target genes, such as
cyclin B1 and insulin-like growth factor 1 receptor, which may
improve the sensitivity of HCC cells to chemotherapeutic drugs.
The exosomes of tumour cells are rich in heat shock
protein (HSP) and common tumour antigen, which can be used as cell
tumour vaccines, but the efficacy of these vaccines has been found
to be limited (67). One study
(68) reported that
exosome-immune mice can induce the response of cytotoxic T
lymphocyte (CTLs) against targeted HCC cells. Following combined
treatment with exosomes and cisplatin, HCC cells showed enhanced
susceptibility to CTLs, which significantly prolonged the survival
time of the mice. To further investigate this mechanism (68), it was hypothesized that following
immunization of mice with exosomes, FasL is expressed on spleen
lymphocytes and binds to the Fas on the surface of target cells,
which induces tumour cell apoptosis. The results indicate that
exosomes can synergize with chemotherapeutic drugs and
significantly enhance the antineoplastic effect of these drugs. On
the other hand, exosomes can also induce drug resistance in HCC.
Isolated exosomes from two invasive HCC cell lines were able to
induce sorafenib resistance in vitro by the activation of
the hepatocyte growth factor/c-Met/Akt signalling pathway and the
inhibition of sorafenib-induced apoptosis. Sorafenib resistance was
also induced in vivo by the inhibition of sorafenib-induced
apoptosis. Furthermore, exosomes derived from highly invasive
tumour cells exhibited greater efficacy compared with exosomes
derived from less invasive cells (69). The aforementioned studies have
shown the important role of exosomes in the drug resistance of
liver cancer cells, thus indicating a novel strategy for improving
the effectiveness of chemotherapy when treating HCC.
Exosomes and immunotherapy for HCC
The shuttle of exosome-bound proteins and genetic
material from one cell type to another suggests the feasibility of
HCC immunotherapy. For example, miR-142 and miR-223 can be
transferred from human macrophages to liver cells by exosomes and
inhibit the proliferation or growth of tumour cells (27). A previous study showed that bone
marrow mesenchymal stem cells developed significant antitumour
activity following sensitization with HCC cell-derived exosomes and
inhibited the proliferation of HCC cells (Fig. 2). These results suggested that
sensitization with HCC cell-derived exosomes may be a novel type of
antitumour therapy (70). In
addition, the immunogenicity of tumour cells can be enhanced by
stimulating exosomes to secrete and express their contents. Drugs
such as 5-Aza-2′-deoxycytidine (5-Aza-CdR) can directly inhibit DNA
methylation and increase the corresponding gene expression. There
is considerable evidence to suggest that 5-Aza-CdR can improve the
antitumour immune response. Subsequent to treatment with 5-Aza-CdR,
the levels of HSP70, human leucocyte antigen-I (HLA-I) and
cancer/testis antigen 1 proteins were increased in exosomes
produced by HCC cells, which provides evidence for
5-Aza-CdR-modified exosome-based anti-HCC immunotherapy (71). MS-275, the histone deacetylase
enzyme inhibitor, had a similar effect by promoting the release of
HSP70, HLA-I and CD80 by HCC HepG2 and Hep3B cells (72). Anticancer drugs can also promote
the production and release by HepG2 cells of more exosomes carrying
HSPs; these exosomes can induce HSP-specific natural killer (NK)
cell activity, which has higher immunogenicity. In addition,
adipose-derived mesenchymal stem cell-derived exosomes promoted NK
and T-cell antitumour responses in rats, thereby facilitating HCC
suppression associated with an early apparent increase of the
diffusion coefficient and low-grade tumour differentiation
(73). However, there has been no
report of the application of exosomes to the immunotherapy of HCC
patients, and further studies are required.
6. Conclusion and future directions
Exosomes are a novel class of intercellular signal
mediators that exhibit involvement in a number of pathological
conditions, including HCC. The present review summarized the roles
and probable mechanisms of exosomes in HCC, and showed the
potential clinical applications of exosomes in the detection and
treatment of the disease. However, the detailed mechanisms of
exosomes in the invasion and metastasis of HCC are not fully
understood, which hinders their application in the diagnosis and
treatment of HCC. Future studies should focus on determining their
mechanisms and identifying potential diagnostic and therapeutic
strategies in HCC.
Multiple histopathological changes occur in HCC,
including angiogenesis and increased vascular permeability, which
are associated with tumourigenesis, progression, invasion and
chemoresistance. Previous studies have shown that tumour
exosome-secreted miR-105 destroys vascular endothelial barriers to
promote metastasis in early-stage breast cancer (74). The high level of expression of
Vasorin in HCC cell exosomes could affect the migration of
endothelial cells (56). Close
connections exist between endothelial cells, pericytes and
exosomes. As HCC-derived exosomes are known to serve important
roles in the tumour microenvironment, greater knowledge of the role
of HCC-derived exosomes in angiogenesis, vascular function and
structure will enhance our understanding of anti-angiogenesis
therapy and possibly improve the efficiency of chemotherapy and
radiation treatment.
Standard technology must be established as soon as
possible for the purification and isolation of exosomes. As the
extracellular milieu is complex, it is not easy to achieve
separation of non-vesicular entities from exosomes (75). A minimal set of biochemical,
biophysical and functional standards is provided by the
International Society for Extracellular Vesicles, which should be
used for the attribution of any specific biological cargo or
functions to extracellular vesicles (76). However, the heterogeneity in size
and the probable subpopulations of exosomes (77) suggest that further improvement of
purification and characterization is required to obtain pure
preparations and easily identify exosomes of interest.
With regard to clinical applications, exosomes have
the potential to be used for the diagnosis and antitumour
treatments of HCC. Clinical trials have been performed to improve
the utilization by antigen-presenting activity and enhance the
antitumour immune response (78,79), but their safety and efficacy
require further confirmation. Therefore, a deeper understanding of
exosomes in HCC should bring breakthroughs and transformative
changes in the diagnosis and treatment of HCC in the future.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81472849), Guangdong
Natural Science Research (no. 2014A030313383) and the Guangdong
High-level University Construction Fund for Jinan University (no.
88016013034).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeon Y, Jang ES, Choi YS, Kim JW and Jeong
SH: Glypican-3 level assessed by the enzyme-linked immunosorbent
assay is inferior to alpha-fetoprotein level for hepatocellular
carcinoma diagnosis. Clin Mol Hepatol. 22:359–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu DD, Wu Y, Shen HY, Lv MM, Chen WX,
Zhang XH, Zhong SL, Tang JH and Zhao JH: Exosomes in development,
metastasis and drug resistance of breast cancer. Cancer Sci.
106:959–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang Y, Cui Y, Li Z, Jiao Z, Zhang Y, He
Y, Chen G, Zhou Q, Wang W, Zhou X, et al: Radiation-induced
miR-208a increases the proliferation and radioresistance by
targeting p21 in human lung cancer cells. J Exp Clin Cancer Res.
35:72016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soung YH, Nguyen T, Cao H, Lee J and Chung
J: Emerging roles of exosomes in cancer invasion and metastasis.
BMB Rep. 49:18–25. 2016. View Article : Google Scholar :
|
|
9
|
Riches A, Campbell E, Borger E and Powis
S: Regulation of exosome release from mammary epithelial and breast
cancer cells - a new regulatory pathway. Eur J Cancer.
50:1025–1034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang N, Li S, Li G, Zhang S, Tang X, Ni S,
Jian X, Xu C, Zhu J and Lu M: The role of extracellular vesicles in
mediating progression, metastasis and potential treatment of
hepatocellular carcinoma. Oncotarget. 8:3683–3695. 2017.
|
|
11
|
Wang Z, Chen JQ, Liu JL and Tian L:
Exosomes in tumor microenvironment: Novel transporters and
biomarkers. J Transl Med. 14:2972016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolf P: The nature and significance of
platelet products in human plasma. Br J Haematol. 13:269–288. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harding C, Heuser J and Stahl P:
Receptor-mediated endocytosis of transferrin and recycling of the
transferrin receptor in rat reticulocytes. J Cell Biol. 97:329–339.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
16
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ,
Street JM, Pound J, Bath LE, Webb DJ, Gregory CD, Bailey MA, et al:
Quantification of human urinary exosomes by nanoparticle tracking
analysis. J Physiol. 591:5833–5842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Street JM, Barran PE, Mackay CL, Weidt S,
Balmforth C, Walsh TS, Chalmers RT, Webb DJ and Dear JW:
Identification and proteomic profiling of exosomes in human
cerebrospinal fluid. J Transl Med. 10:52012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Hou L, Li A, Duan Y, Gao H and
Song X: Expression of serum exosomal microRNA-21 in human
hepatocellular carcinoma. Biomed Res Int.
2014:8648942014.PubMed/NCBI
|
|
20
|
Kogure T, Lin WL, Yan IK, Braconi C and
Patel T: Intercellular nanovesicle-mediated microRNA transfer: A
mechanism of environmental modulation of hepatocellular cancer cell
growth. Hepatology. 54:1237–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuyama K, Sun H, Mitsutake S and Igarashi
Y: Sphingolipid-modulated exosome secretion promotes clearance of
amyloid-β by microglia. J Biol Chem. 287:10977–10989. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herreros-Villanueva M and Bujanda L:
Glypican-1 in exosomes as biomarker for early detection of
pancreatic cancer. Ann Transl Med. 4:642016.PubMed/NCBI
|
|
23
|
Ludwig AK and Giebel B: Exosomes: Small
vesicles participating in intercellular communication. Int J
Biochem Cell Biol. 44:11–15. 2012. View Article : Google Scholar
|
|
24
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shrivastava S, Devhare P, Sujijantarat N,
Steele R, Kwon YC, Ray R and Ray RB: Knockdown of autophagy
inhibits infectious hepatitis C virus release by the exosomal
pathway. J Virol. 90:1387–1396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conigliaro A, Costa V, Lo Dico A, Saieva
L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M,
et al: CD90+ liver cancer cells modulate endothelial
cell phenotype through the release of exosomes containing H19
lncRNA. Mol Cancer. 14:1552015. View Article : Google Scholar
|
|
27
|
Aucher A, Rudnicka D and Davis DM:
MicroRNAs transfer from human macrophages to hepato-carcinoma cells
and inhibit proliferation. J Immunol. 191:6250–6260. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Putz U, Howitt J, Doan A, Goh CP, Low LH,
Silke J and Tan SS: The tumor suppressor PTEN is exported in
exosomes and has phosphatase activity in recipient cells. Sci
Signal. 5:ra702012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Azmi AS, Bao B and Sarkar FH: Exosomes in
cancer development, metastasis, and drug resistance: A
comprehensive review. Cancer Metastasis Rev. 32:623–642. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Whiteside TL: Immune modulation of T-cell
and NK (natural killer) cell activities by TEXs (tumour-derived
exosomes). Biochem Soc Trans. 41:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiba M, Kimura M and Asari S: Exosomes
secreted from human colorectal cancer cell lines contain mRNAs,
microRNAs and natural antisense RNAs, that can transfer into the
human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep.
28:1551–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu Y, Xia F, Ma L, Shan J, Shen J, Yang Z,
Liu J, Cui Y, Bian X, Bie P, et al: MicroRNA-122 sensitizes HCC
cancer cells to adriamycin and vincristine through modulating
expression of MDR and inducing cell cycle arrest. Cancer Lett.
310:160–169. 2011.PubMed/NCBI
|
|
33
|
Wang B, Majumder S, Nuovo G, Kutay H,
Volinia S, Patel T, Schmittgen TD, Croce C, Ghoshal K and Jacob ST:
Role of microRNA-155 at early stages of hepatocarcinogenesis
induced by choline-deficient and amino acid-defined diet in C57BL/6
mice. Hepatology. 50:1152–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li S, Fu H, Wang Y, Tie Y, Xing R, Zhu J,
Sun Z, Wei L and Zheng X: MicroRNA-101 regulates expression of the
v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene
in human hepatocellular carcinoma. Hepatology. 49:1194–1202. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Varnholt H, Drebber U, Schulze F,
Wedemeyer I, Schirmacher P, Dienes HP and Odenthal M: MicroRNA gene
expression profile of hepatitis C virus-associated hepatocellular
carcinoma. Hepatology. 47:1223–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang YF, Wang F, Xiao JJ, Song Y, Zhao YY,
Cao Y, Bei YH and Yang CQ: MiR-222 overexpression promotes
proliferation of human hepatocellular carcinoma HepG2 cells by
downregulating p27. Int J Clin Exp Med. 7:893–902. 2014.PubMed/NCBI
|
|
38
|
Gramantieri L, Ferracin M, Fornari F,
Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E,
Grazi GL, et al: Cyclin G1 is a target of miR-122a, a microRNA
frequently downregulated in human hepatocellular carcinoma. Cancer
Res. 67:6092–6099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang G, Zhu S, Gu Y, Chen Q, Liu X and Fu
H: MicroRNA-145 and microRNA-133a inhibited proliferation,
migration, and invasion, while promoted apoptosis in hepatocellular
carcinoma cells via targeting FSCN1. Dig Dis Sci. 60:3044–3052.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Q, Yu X, Li Q, Qin L, Tan S, Zeng X,
Qiu X, Tang B, Jin J, Liao W, et al: Association between miR-199a
rs74723057 and MET rs1621 polymorphisms and the risk of
hepatocellular carcinoma. Oncotarget. 7:79365–79371.
2016.PubMed/NCBI
|
|
41
|
Huang XH, Wang Q, Chen JS, Fu XH, Chen XL,
Chen LZ, Li W, Bi J, Zhang LJ, Fu Q, et al: Bead-based microarray
analysis of microRNA expression in hepatocellular carcinoma:
miR-338 is downregulated. Hepatol Res. 39:786–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fornari F, Ferracin M, Trerè D, Milazzo M,
Marinelli S, Galassi M, Venerandi L, Pollutri D, Patrizi C, Borghi
A, et al: Circulating microRNAs, miR-939, miR-595, miR-519d and
miR-494, identify cirrhotic patients with HCC. PLoS One.
10:e01414482015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Y, Zhang L, Liu F, Xiang G, Jiang D and
Pu X: Identification of endogenous controls for analyzing serum
exosomal miRNA in patients with hepatitis B or hepatocellular
carcinoma. Dis Markers. 2015:8935942015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin
HM, Zhou R, Shang CZ, Cao J, He H, et al: Vps4A functions as a
tumor suppressor by regulating the secretion and uptake of exosomal
microRNAs in human hepatoma cells. Hepatology. 61:1284–1294. 2015.
View Article : Google Scholar :
|
|
45
|
Basu S and Bhattacharyya SN: Insulin-like
growth factor-1 prevents miR-122 production in neighbouring cells
to curtail its intercellular transfer to ensure proliferation of
human hepatoma cells. Nucleic Acids Res. 42:7170–7185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lou G, Song X, Yang F, Wu S, Wang J, Chen
Z and Liu Y: Exosomes derived from miR-122-modified adipose
tissue-derived MSCs increase chemosensitivity of hepatocellular
carcinoma. J Hematol Oncol. 8:1222015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pan Q, Ramakrishnaiah V, Henry S,
Fouraschen S, de Ruiter PE, Kwekkeboom J, Tilanus HW, Janssen HL
and van der Laan LJ: Hepatic cell-to-cell transmission of small
silencing RNA can extend the therapeutic reach of RNA interference
(RNAi). Gut. 61:1330–1339. 2012. View Article : Google Scholar
|
|
48
|
Sugimachi K, Matsumura T, Hirata H, Uchi
R, Ueda M, Ueo H, Shinden Y, Iguchi T, Eguchi H, Shirabe K, et al:
Identification of a bona fide microRNA biomarker in serum exosomes
that predicts hepatocellular carcinoma recurrence after liver
transplantation. Br J Cancer. 112:532–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pegtel DM, Cosmopoulos K, Thorley-Lawson
DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD,
Würdinger T and Middeldorp JM: Functional delivery of viral miRNAs
via exosomes. Proc Natl Acad Sci USA. 107:6328–6333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiao W, Dong W, Zhang C, Saren G, Geng P,
Zhao H, Li Q, Zhu J, Li G, Zhang S, et al: Effects of the
epigenetic drug MS-275 on the release and function of
exosome-related immune molecules in hepatocellular carcinoma cells.
Eur J Med Res. 18:612013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Feng Z, Hensley L, McKnight KL, Hu F,
Madden V, Ping L, Jeong SH, Walker C, Lanford RE and Lemon SM: A
pathogenic picornavirus acquires an envelope by hijacking cellular
membranes. Nature. 496:367–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ramakrishnaiah V, Thumann C, Fofana I,
Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin
Raj V, Jenster G, et al: Exosome-mediated transmission of hepatitis
C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci
USA. 110:13109–13113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen TC, Hsieh CH and Sarnow P: Supporting
role for GTPase Rab27a in hepatitis C virus RNA replication through
a novel miR-122-mediated effect. PLoS Pathog. 11:e10051162015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tickner JA, Urquhart AJ, Stephenson SA,
Richard DJ and O'Byrne KJ: Functions and therapeutic roles of
exosomes in cancer. Front Oncol. 4:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang A, Dong J, Li S, Wang C, Ding H, Li
H, Su X, Ge X, Sun L, Bai C, et al: Exosomal transfer of vasorin
expressed in hepatocellular carcinoma cells promotes migration of
human umbilical vein endothelial cells. Int J Biol Sci. 11:961–969.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He M, Qin H, Poon TC, Sze SC, Ding X, Co
NN, Ngai SM, Chan TF and Wong N: Hepatocellular carcinoma-derived
exosomes promote motility of immortalized hepatocyte through
transfer of oncogenic proteins and RNAs. Carcinogenesis.
36:1008–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chiba M, Watanabe N, Watanabe M, Sakamoto
M, Sato A, Fujisaki M, Kubota S, Monzen S, Maruyama A, Nanashima N,
et al: Exosomes derived from SW480 colorectal cancer cells promote
cell migration in HepG2 hepatocellular cancer cells via the
mitogen-activated protein kinase pathway. Int J Oncol. 48:305–312.
2016. View Article : Google Scholar
|
|
58
|
Liu WH, Ren LN, Wang X, Wang T, Zhang N,
Gao Y, Luo H, Navarro-Alvarez N and Tang LJ: Combination of
exosomes and circulating microRNAs may serve as a promising tumor
marker complementary to alpha-fetoprotein for early-stage
hepatocellular carcinoma diagnosis in rats. J Cancer Res Clin
Oncol. 141:1767–1778. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li Y, Xiang GM, Liu LL, Liu C, Liu F,
Jiang DN and Pu XY: Assessment of endogenous reference gene
suitability for serum exosomal microRNA expression analysis in
liver carcinoma resection studies. Mol Med Rep. 12:4683–4691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tanaka Y, Kamohara H, Kinoshita K,
Kurashige J, Ishimoto T, Iwatsuki M, Watanabe M and Baba H:
Clinical impact of serum exosomal microRNA-21 as a clinical
biomarker in human esophageal squamous cell carcinoma. Cancer.
119:1159–1167. 2013. View Article : Google Scholar
|
|
63
|
Sohn W, Kim J, Kang SH, Yang SR, Cho JY,
Cho HC, Shim SG and Paik YH: Serum exosomal microRNAs as novel
biomarkers for hepatocellular carcinoma. Exp Mol Med. 47:e1842015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kogure T, Yan IK, Lin WL and Patel T:
Extracellular vesicle-mediated transfer of a novel long noncoding
RNA TUC339: A mechanism of intercellular signaling in human
hepatocellular cancer. Genes Cancer. 4:261–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mohankumar S and Patel T: Extracellular
vesicle long noncoding RNA as potential biomarkers of liver cancer.
Brief Funct Genomics. 15:249–256. 2016. View Article : Google Scholar :
|
|
66
|
Bandiera S, Pfeffer S, Baumert TF and
Zeisel MB: miR-122 - a key factor and therapeutic target in liver
disease. J Hepatol. 62:448–457. 2015. View Article : Google Scholar
|
|
67
|
Mou DL, Jia ZS and Bai XF: Exosome: Trojan
horse in immunotherapy. Sheng Li Ke Xue Jin Zhan. 36:113–118.
2005.In Chinese. PubMed/NCBI
|
|
68
|
Wang SH, Shen Y, Li J, Xiang ZW, Fan WK
and Chen L: Experimental studies on anti-mouse hepatocellular
carcinoma effects of cisplatin combined with exosomes. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 25:49–52. 2009.In Chinese. PubMed/NCBI
|
|
69
|
Qu Z, Wu J, Wu J, Luo D, Jiang C and Ding
Y: Exosomes derived from HCC cells induce sorafenib resistance in
hepatocellular carcinoma both in vivo and in vitro. J Exp Clin
Cancer Res. 35:1592016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ma B, Jiang H, Jia J, Di L, Song G, Yu J,
Zhu Y, Lu Z, Wang X, Zhou X, et al: Murine bone marrow stromal
cells pulsed with homologous tumor-derived exosomes inhibit
proliferation of liver cancer cells. Clin Transl Oncol. 14:764–773.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiao WH, Sanren GW, Zhu JH, Li QW, Kang
HR, Wang RL, Song LP and Ye M: Effect of 5-aza-2′-deoxycytidine on
immune-associated proteins in exosomes from hepatoma. World J
Gastroenterol. 16:2371–2377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li QW, Xiao WH, Sarengaowa G, Dong WW,
Zhao HX, Duan XY, Zhu JH, Kang HR, Fu Y, Hao YX, et al: Histone
deacetylase inhibitor MS-275 treatment alters immune molecule
content and categories in hepatocarcinoma exosomes. Zhonghua Gan
Zang Bing Za Zhi. 20:231–235. 2012.In Chinese. PubMed/NCBI
|
|
73
|
Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC,
Huang CC, Ng SH and Lin JW: Adipose-derived mesenchymal stem cell
exosomes suppress hepatocellular carcinoma growth in a rat model:
Apparent diffusion coefficient, natural killer T-cell responses,
and histopathological features. Stem Cells Int. 2015:8535062015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou W, Fong MY, Min Y, Somlo G, Liu L,
Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al:
Cancer-secreted miR-105 destroys vascular endothelial barriers to
promote metastasis. Cancer Cell. 25:501–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mincheva-Nilsson L, Baranov V, Nagaeva O
and Dehlin E: Isolation and characterization of exosomes from
cultures of tissue explants and cell lines. Curr Protoc Immunol.
115:14.42.1–14.42.21. 2016. View Article : Google Scholar
|
|
76
|
Lötvall J, Hill AF, Hochberg F, Buzás EI,
Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S,
Quesenberry P, et al: Minimal experimental requirements for
definition of extracellular vesicles and their functions: A
position statement from the International Society for Extracellular
Vesicles. J Extracell Vesicles. 3:269132014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bobrie A and Théry C: Exosomes and
communication between tumours and the immune system: Are all
exosomes equal. Biochem Soc Trans. 41:263–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Viaud S, Ploix S, Lapierre V, Théry C,
Commere PH, Tramalloni D, Gorrichon K, Virault-Rocroy P, Tursz T,
Lantz O, et al: Updated technology to produce highly immunogenic
dendritic cell-derived exosomes of clinical grade: a critical role
of interferon-gamma. J Immunother. 34:65–75. 2011. View Article : Google Scholar
|
|
79
|
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H
and Li G: Phase I clinical trial of autologous ascites-derived
exosomes combined with GM-CSF for colorectal cancer. Mol Ther.
16:782–790. 2008. View Article : Google Scholar : PubMed/NCBI
|