Introduction
Glioma is the most common type of malignant tumor of
the central nervous system, accounting for ~46% of brain tumors
(1,2). Glioma is a neuroectodermal tumor
with the highest mortality rate of primary intracranial tumors
(3). Currently, craniotomy is the
most efficient type of therapy to treat gliomas (4). However, due to the invasive growth
of gliomas and its unclear demarcation with normal brain tissues,
it is difficult to completely remove tumor tissues by surgery,
resulting in a high recurrence rate (5,6),
and subsequent treatment with radiotherapy and chemotherapy (which
damage the immune system) are generally not satisfactory (7). Methods to decrease the high
recurrence rates and serious side-effects of glioma treatment are
being investigated to develop specific drugs or therapeutic
strategies with high efficiency and sensitivity, and low toxicity
for the treatment of gliomas (8).
Photodynamic therapy (PDT) is a novel therapeutic
method for tumor treatment (9).
PDT was first discovered by Dougherty et al (10) in 1900, and was first used in
medicine to ablate and destroy unwanted tissue in 1975 (10). PDT utilizes the activation of a
photosensitizer (PS) by light irradiation with a specific
wavelength to generate oxygen radicals and further inhibit growth
of tumor cells (11,12). The advantages of PDT are its
minimally invasive characteristic, repeatability, low toxicity
accumulation when compared with the traditional treatments, in
addition to its low recurrence rate and more definitive outcome
(9). Low toxicity to normal
tissue is a significant advantage of PDT, compared with traditional
treatment. In recent years, PDT has shown a particularly good
therapeutic effect on bladder cancer, early lung cancer, Barrett's
esophagus, head and neck cancer and skin cancer, amongst others
(13–15). For example, when using
2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a as a PS in the
clinical setting, PDT demonstrated a particularly effective outcome
in 16 cases of patients with lung cancer in a treatment cycle, with
a very slight photosensitive side-effect; after 3 days of PDT
treatment, patients with oral cancer recovered (16). Furthermore, PDT treatment for
esophageal cancer and Barrett's esophagus resulted in a good
outcome (17). However,
fundamental difficulties encountered in the treatment of PDT, such
as targeting, light source and light dose have not been addressed.
Advances in chemistry, materials, optics, clinical treatment and
other disciplines contribute to overcoming these difficulties, by
elucidating the underlying mechanism of PDT.
Typically, tumor treatment methods involve enhancing
the antitumor immune response of the body via immunology. In recent
years, PDT was identified as able to inhibit the growth of tumor
cells and regulate the conditional body immune system; thus, has
gradually been adopted as an auxiliary treatment to reduce the
recurrence rate of tumors (18).
In previous studies, PDT was identified as a promising treatment
strategy for glioma (19–22). Using 5-aminolevulinic acid (5-ALA)
as a PS, the expression level of proteasomes was increased in
cultured U251 cells and the growth of glioma cells was inhibited
under light irradiation (19).
Using hematoporphyrin as a PS, PDT induced the transcriptional
level of transporter 1, ATP binding cassette subfamily B member
(TAPI) and the expression level of HLA-1 in U251 cells under light
irradiation, inhibiting the growth of glioma cells in a dose- and
time-dependent manner (20). In
addition, PDT enhanced the immunogenicity of glioma cells,
indicating that a PDT-generated glioma vaccine from dendritic cells
presents as a promising novel method for tumor treatment (21). However, the molecular mechanisms
underlying PDT-inhibited growth of glioma cells remain unclear. In
the current study, based on previous studies, the role of PDT in
the induction of cell apoptosis of glioma cells was investigated
using hematoporphyrin as the PS, as well as microRNAs (miRNAs) and
their corresponding signaling pathways, such as target molecule
networks. The current results provide a potential therapeutic
strategy involving PDT for the treatment of gliomas.
Materials and methods
Cell culture
Human glioma cell lines U87 and U251 cells were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). Cells were cultured in 5% CO2 and 95%
humidified atmosphere in DM 1640 medium (Invitrogen, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (Invitrogen; Thermo Fisher Scientific Inc.) with 100 U/ml
penicillin and 100 μg/ml streptomycin. Cells were passaged
subsequent to reaching 80% confluence.
MTT assay
Cell viability was determined using MTT. Briefly,
5×103 cells per well were seeded in 96-well plates.
Following incubation for 12 h at 37°C, the cells were treated with
phosphate-buffered saline (PBS; control) or various concentrations
of hematoporphyrin (0–120 nM; MedChemExpress, Princeton, NJ, USA)
for 60 min. Following light irradiation using an M8 Spectrum Power
Energy meter (San Diego, Taizhong, Taiwan, China) at 625 nm, 5
mW/cm2 for 60 min (23) and culturing for 22 h at 37°C, 100
μl MTT solution (5.0 mg/ml) was then added to each well and
incubated for 4 h at 37°C, followed by removal of culture medium
and the addition of dimethyl sulfoxide (100 μl/well) to
dissolve the formed formazan crystals. The absorbance value was
determined using a microplate reader (Varioskan Flash, Thermo, USA)
at a wavelength of 520 nm. The relative cell viability was
calculated by normalization to the control. The experiments were
independently performed three times.
Cell apoptosis assays
Cell apoptosis was detected using Hoechst 33342 and
flow cytometry. Briefly, 2×105 cells/well were seeded in
6-well plates. Subsequent to incubation for 12 h at 37°C, the cells
were treated with hematoporphyrin (85 nM, the IC50)
under light irradiation (625 nm, 5 mW/cm2) for 60 min.
The untreated cells served as the control. Cells were subsequently
cultured for 23 h at 37°C, then collected with
trypsin-ethylenediaminetetraacetic acid (EDTA) and resuspended in
PBS. Cell apoptosis was measured by flow cytometry (BD FACSCalibur;
BD Biosciences, Franklin Lakes, NJ, USA) using the Annexin
V-fluorescein isothiocyanate (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's instructions. The
flow cytometry data was analyzed using FlowJo version 10.0 software
(FlowJo LLC, Ashland, OR, USA). For the Hoechst 33342 assay, 10
μl Hoechst 33342 (5 μg/ml) was added to each well and
incubated for 30 min at 37°C in the dark, followed by removal of
the culture medium and two washes with PBS. The apoptotic nuclei
were detected by inverted fluorescence microscopy (SR GSD; Leica
Microsystems, Inc., Buffalo Grove, IL, USA).
TUNEL assay
Cells (2×105/well) were seeded in 12-well
plates. After adherence, cells were treated with hematoporphyrin
(85 nM) under light irradiation (625 nm, 5 mW/cm2) for
60 min. The untreated cells served as the control. Subsequently,
cells were cultured for 23 h at 37°C, and fixed with 4%
paraformaldehyde for 30 min, washed with PBS, incubated with 0.3%
Triton X-100 (Solarbio, Shanghai, China) in PBS for 5 min, and
incubated with 0.3% hydrogen peroxide in PBS for 20 min at 37°C to
inactivate endogenous peroxidase. Cells were then incubated in
TUNEL detection solution (50 μl/well; Beyotime Institute of
Biotechnology) for 60 min at 37°C and 50 μl
streptavidin-horseradish peroxidase solution for 30 min. Cells were
visualized by 3,3′-diaminobenzidine tetrahydro-chloride (DAB)
solution (Solarbio). The nucleus was stained with hematoxylin in 20
min at 37°C.
Singlet oxygen detection
Alterations in singlet oxygen were detected using
2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology) according to the manufacturer's
instruction. Briefly, 2×105 cells per well were seeded
in 6-well plates. After incubation for 12 h at 37°C, the cells were
treated with hematoporphyrin (85 nM, the IC50) under
light irradiation (625 nm, 5 mW/cm2) for 60 min. The
untreated cells served as the control. Subsequently, cells were
cultured for 6 h and incubated with 10 μM DCFH-DA for 30 min
in the dark. The cells were then washed with FBS-free Dulbecco's
modified Eagle's medium (Life, Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), collected with trypsin-EDTA and resuspended in
PBS. The absorbance values were detected using a microplate reader
(using an emission wavelength of 488 nm and an excitation
wavelength of 525 nm. The experiments were independently performed
three times.
Mitochondrial membrane potential
analysis
Alterations in mitochondrial membrane potential were
detected using JC-1 kit (C2005; Beyotime Institute of
Biotechnology, Beijing, China) according to the manufacturer's
instructions. Briefly, 2×105 cells per well were seeded
in 6-well plates. Following incubation for 12 h at 37°C, the cells
were treated with hematoporphyrin (85 nM, the IC50)
under light irradiation (625 nm, 5 mW/cm2) for 60 min.
The untreated cells served as the control. Cells were incubated
with 1 ml work solution of JC-1 for 20 min at 37°C in the dark,
washed three times with JC-1 buffer, collected with trypsin-EDTA,
resuspended in PBS and detected by inverted fluorescence
microscopy. JC-1 dye exhibits potential-dependent accumulation in
mitochondria, which is indicated by a fluorescence emission shift
from green (~529 nm) to red (~590 nm). Consequently, mitochondrial
depolarization is indicated by a decrease in the red/green
fluorescence intensity ratio. Carbonyl cyanide
m-chlorophenylhydrazone (CCCP) served as positive controls (in the
JC-1 kit, Beyotime Institute of Biotechnology). The ratios of the
absorbance values at 525 and 590 nm were determined with a
microplate reader. The experiments were independently performed
three times.
Mature miRNA microarray analysis
The four groups, which included untreated glioma
cells (in the dark; n=3; named Control-dark group), glioma cells
treated with hematoporphyrin and without light irradiation (n=3;
named Hep-dark group), glioma cells that underwent light
irradiation (n=3 named Control-light group), and glioma cells
treated with hematoporphyrin and light irradiation (n=3; named
Hep-light group), were analyzed using Agilent Human miRNA
micro-array chips (8×60K) V21.0 [ShanghaiBio Corp. (SBC), Shanghai,
China]. The differentially expressed miRNAs that exhibited a
two-fold or greater change were screened.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Glioma cells were treated with or without PDT and
their total RNA was separately extracted using an RNA extraction
kit (cat. no. DP431; Tiangen Biotech Co., Ltd., Beijing, China)
according to the manufacturer's instructions. The RNA concentration
was quantified using an ultraviolet spectrophotometer (NanoDrop
2000; Thermo Fisher Scientific, Inc.) and was reverse transcribed
into cDNA using a FastQuant first strand cDNA synthesis kit (cat.
no. KR104; Tiangen Biotech Co., Ltd.) and T100 thermal Cycler for
PCR (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The sequences
of specific primers for each gene (Guangzhou IGE Biotechnology
Ltd., Guangzhou, China) were as follows: hsa-miR-205-5p,
5′-CCTTCATTCCACCGGAGTCTGAA-3′; hsa-miR-21-5p,
5′-GCCGTAGCTTATCAGACTGATGTTGAA-3′; hsa-miR-7641,
5′-TGCCGTTGATCTCGGAAGCTAAG-3′; hsa-miR-9500,
5′-CCAAGGGAAGATGGTGACCACAA-3′; hsa-miR-135b-5p,
5′-TGCTATGGCTTTTCATTCCTATGTGAA-3′; hsa-miR-210-3p,
5′-GCGTGTGACAGCGGCTGAAA-3′; hsa-miR-30b-5p,
5′-TGCCCGTGTAAACATCCTACACTCA-3′; hsa-miR-4459,
5′-GAGGCGGAGGAGGTGGAGAAA-3′; hsa-miR-663a, 5′-GCGCCGCGGGACCGCAA-3′;
U6, 5′-CTCGCTTCGGCAGCACA-3′; hsa-miR-1273e,
5′-TGAACCCAGGAAGTGGAAAAA-3′; hsa-miR-642a-3p,
5′-AGACACATTTGGAGAGGGAACC-3′; hsa-miR-6893-5p,
5′-CAGGCAGGTGTAGGGTGGAG-3′; hsa-miR-7641,
5′-TGTTGATCTCGGAAGCTAAGCA-3′; hsa-miR-135b-5p,
5′-CCTATGGCTTTTCATTCCTATGTG-3′; hsa-miR-210-3p,
5′-AAACTGTGCGTGTGACAGCG-3′; hsa-miR-30b-5p,
5′-TCCTGTAAACATCCTACACTCAGCTA-3′; hsa-miR-31-5p,
5′-TAGGCAAGATGCTGGCATAGAA-3′. qPCR analysis was performed in a
real-time PCR thermocycler (CFX96 real-time PCR; Bio-Rad
Laboratories, Inc.) using the enhanced miRNA SYBR-Green RT-PCK kit
(cat. no. FP411; Biotech Co., Ltd.) and run for 40 cycles at 95°C
for 20 sec and 60°C for 34 sec. The relative miRNA levels were
normalized to that of U6 using the 2−ΔΔCq method
(24). The experiment was
performed three times.
Gene Ontology (GO) and pathway enrichment
analyses
The differentially expressed miRNAs were further
analyzed for predicted gene targets simultaneously using at least
two of the following five databases: TargetMiner, miRDB, microRNA,
TarBase and RNA22 via the SBC analysis system (http://sas.ebioservice.com). GO enrichment analyses
and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analyses were performed by the SBC analysis system,
which uses clusterProfiler data from R and Bioconductor software
(http://www.r-project.org and http://www.bioconductor.org/) with public databases
that include NCBI Entrez Gene (http://www.ncbi.nlm.nih.gov/gene), GO (http://www.geneontology.org), KEGG (http://www.genome.jp/kegg) and BioCarta (http://www.biocarta.com). The hyper-geometric test was
employed with a Benjamini-Hochberg-False Discovery Rate-based
multiple testing correction (correction, P<0.01) (25).
Protein array detection assay
A Human Apoptosis Antibody Array (cat. no.
AAH-APO-1; RayBiotech, Inc., Norcross, GA, USA) was used according
to the manufacturer's instructions. Following development, films
were scanned using ImageQuant LAS 4000 (GE Healthcare, Chicago, IL,
USA) and the images were processed and quantified using the
AAH-APO-1 software.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism Software (version 6.0; GraphPad Software, Inc., La Jolla, CA,
USA) and Student's t-test. Each experiment was performed at least
three times and all figures represent means ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PDT with PS, hematoporphyrin inhibits
cell viability in a dose-dependent manner
The cell viability in U87 and U251 glioma cells
following PDT was detected with PS, hematoporphyrin (Fig. 1). In the U87 and U251 cells
(Fig. 1A), cell viability was
significantly decreased along with the hematoporphyrin
concentration. The level of cell viability was reduced to a greater
extent in the U87 cells than in the U251 cells (IC50: 85
vs. 166 Nm for U87 vs. U251), indicating that the inhibition of PDT
in U87 is better than in U251. The viability of cells that
irradiated with white or red light in the U87 cells was compared
(Fig. 1B) and the results
demonstrated that the inhibition of hematoporphyrin under red light
was better than under white light. Thus, red light-irradiated U87
cells were used in the following experiments.
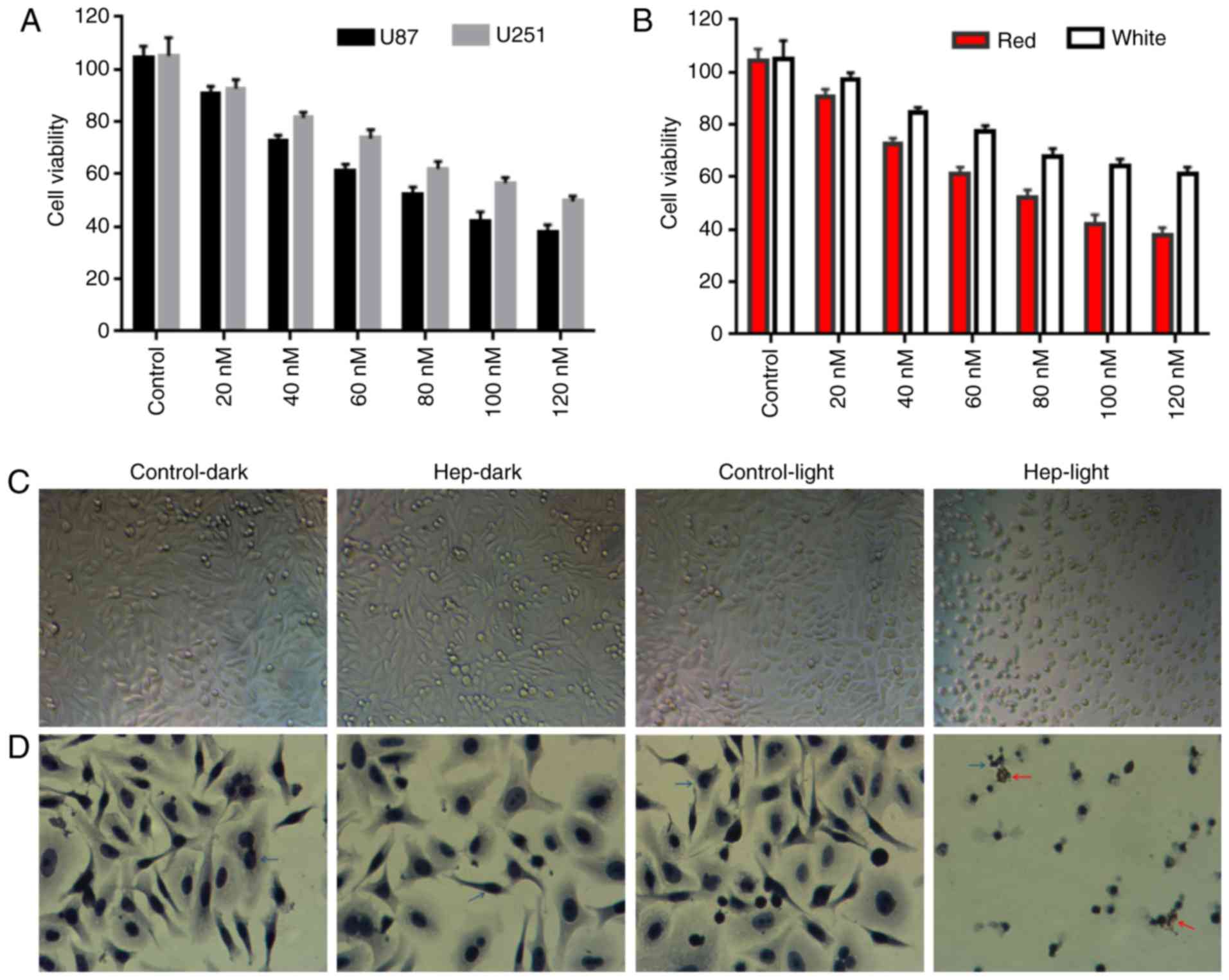 | Figure 1Effect of PDT with photosensitizer,
hematoporphyrin in cell viability of glioma cells. (A) Cell
viability of U87 and U251 cells following PDT treatment with
photosensitizer, hematoporphyrin. (B) The viability of U87 cells
irradiated under white or red light. (C) Effect of photodynamic
activity of hematoporphyrin (85 nM) in U87 cell morphology under
red light. (D) Cell apoptosis was detected by TUNEL assay.
Following hematoxylin staining, the nuclei were stained blue/purple
(blue arrows). Following hematoporphyrin treatment with light
irradiation, the nuclei were stained brown by 3,3′-diaminobenzidine
tetrahydrochloride, indicating apoptotic cells (red arrow).
Hep-light, cells treated with hematoporphyrin and light
irradiation; Control-dark, untreated cells; Hep-dark, cells treated
with hematoporphyrin; Control-light, cells treated by light
irradiation. PDT, photodynamic therapy. |
Photodynamic activity of hematoporphyrin (85 nM)
induced changes in cell morphology in the U87 cells (Fig. 1C and D). The U87 cells
demonstrated morphological changes following PDT-hematoporphyrin
treatment, including shrinking, fragmentation, which was not
observed in cells without hematoporphyrin treatment (in the light
or dark; Fig. 1C and D).
PDT with PS, hematoporphyrin induces cell
apoptosis
Cell apoptosis in U87 cells following PDT with PS,
hematoporphyrin (85 nM) were detected by Hoechst 33342 and flow
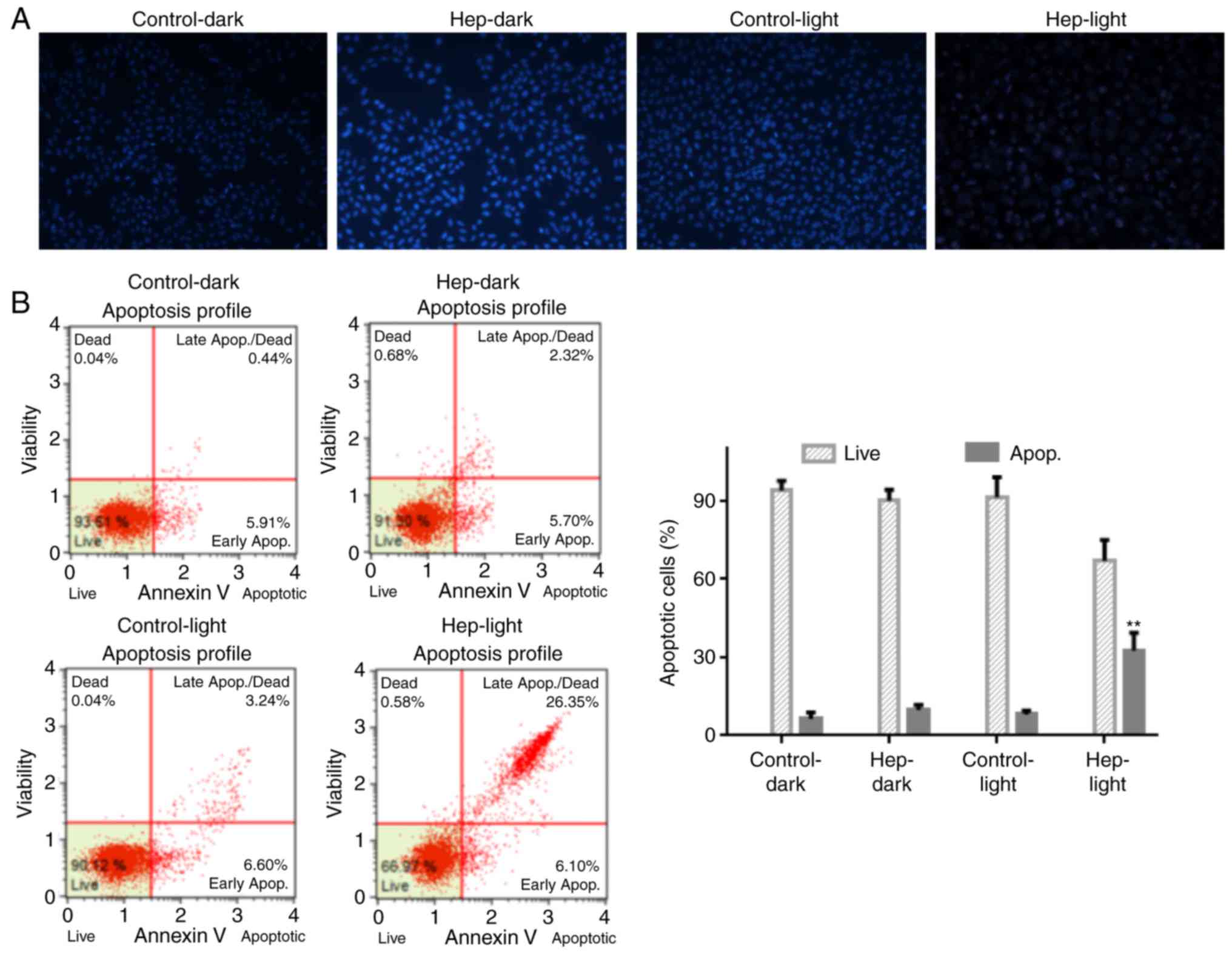
cytometry (Fig. 2). As presented
in Fig. 2A, photodynamic activity
of hematoporphyrin induced apoptotic nuclei in U87 cells with low
cell density. Similarly, the flow cytometric analysis revealed that
photodynamic activity of hematoporphyrin increased cell apoptosis
in early (6.1 vs. 5.91% for PDT vs. control) and later (26.35 vs.
0.44% for PDT vs. control; P<0.05) stages (Fig. 2B). Similar to the Hoechst 33342
and flow cytometry data, the TUNEL assay indicated that the
photodynamic activity of hematoporphyrin increased cell apoptosis
(Fig. 1D). Thus, photodynamic
activity of hematoporphyrin inhibited cell growth via induction of
cell apoptosis.
PDT with PS, hematoporphyrin induces cell
apoptosis via induction of ROS
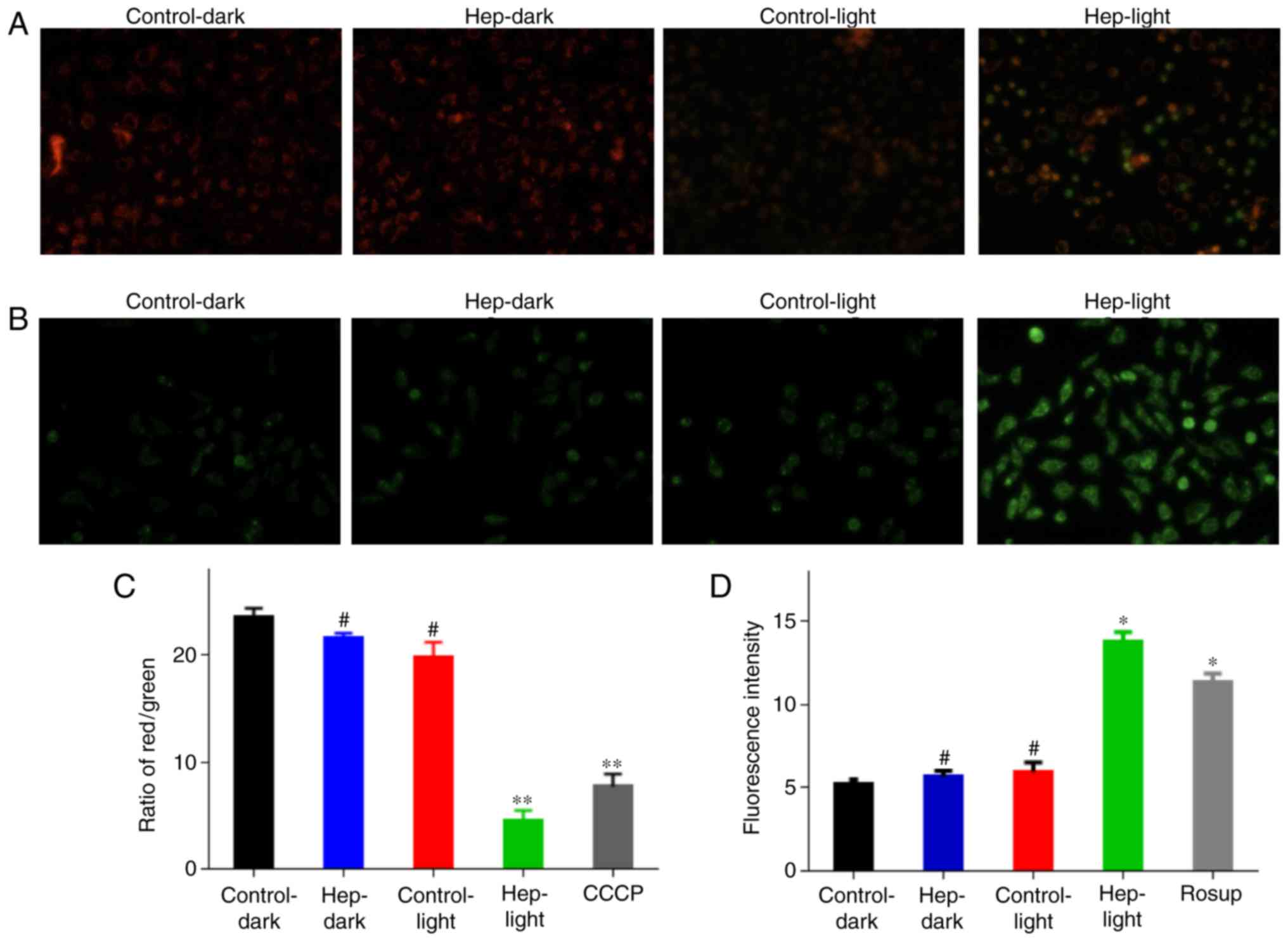
As previous studies demonstrated that, under light
irradiation, hematoporphyrin exerts its action via production of
singlet oxygen and superoxide anions (26,27), the current study investigated the
alterations in singlet oxygen and mitochondrial membrane potential
in cells following PDT with PS, hematoporphyrin treatment (Fig. 3). In healthy cells, the
mitochondrial membrane potential was not markedly changed, while
obvious green fluorescence was observed in the U87 cells following
PDT treatment (Fig. 3A). A
similar result was obtained using a microplate reader, the
mitochondrial membrane potential was significantly decreased
following PDT treatment, which was lower than the CCCP group
(Fig. 3B). The content of
reactive oxygen species (ROS) was significantly increased by PDT,
which was greater than that in the Rosup group (Fig. 3C). Thus, PDT with hematoporphyrin
may result in the induction of ROS and the decrease in
mitochondrial membrane potential, which are important in cell
apoptosis and mitochondrial disorders (28).
Screening and validation of miRNA
involved in PDT with PS, hematoporphyrin-induced cell
apoptosis
The potential target miRNA was analyzed by miRNA
microarray, and the differentially expressed miRNAs between
Hep-light (cells with hematoporphyrin and light irradiation) and
Control-dark (cells without treatment), Hep-dark (cells with
hematoporphyrin) or Hep-light (cells with light irradiation) groups
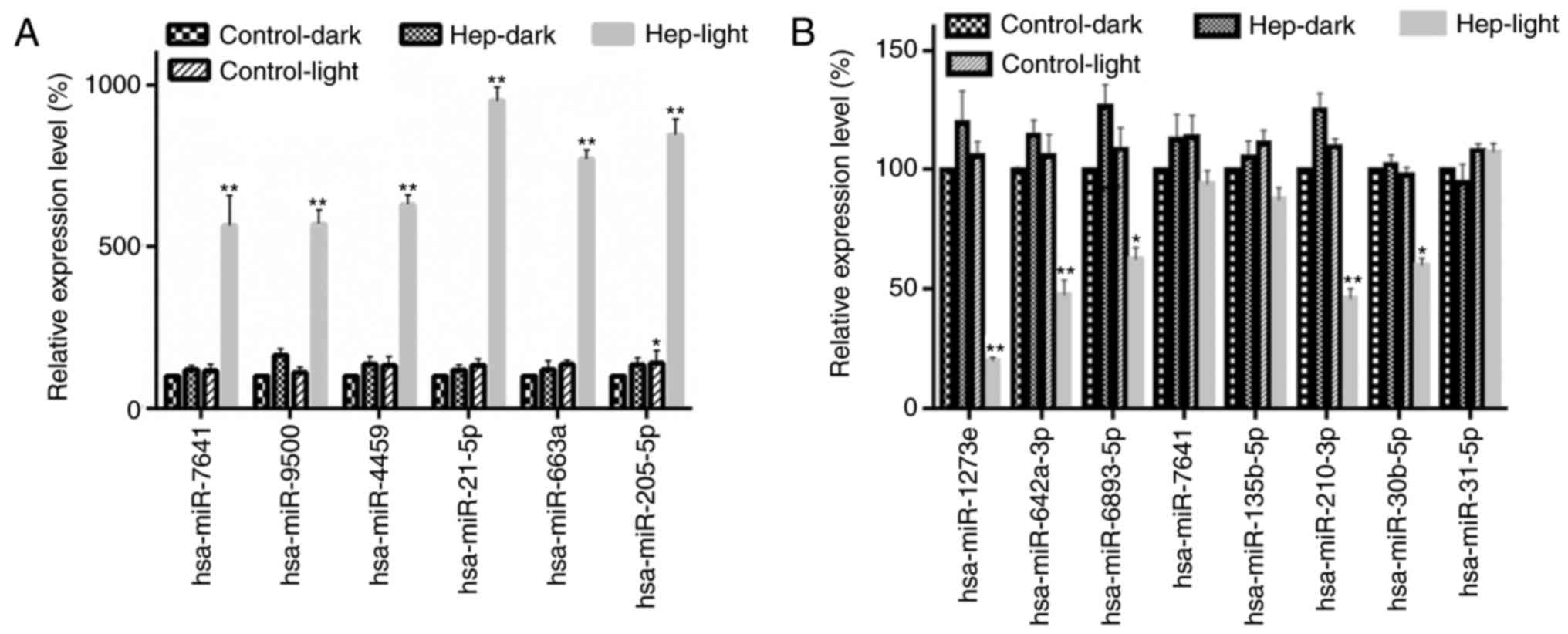
were screened. There were 185 miRNAs differentially expressed in
the PDT treatment groups compared with the other groups. The top
six upregulated miRNAs included hsa-miR-7641, hsa-miR-9500,
hsa-miR-4459, hsa-miR-21-5p, hsa-miR-663a and hsa-miR-205-5p. The
top eight downregulated miRNAs included hsa-miR-1273e,
hsa-miR-642a-3p, hsa-miR-6893-5p, hsa-miR-7641, hsa-miR-135b-5p,
hsa-miR-210-3p, hsa-miR-30b-5p and hsa-miR-31-5p. The expression
levels of the six upregulated miRNAs, including hsa-miR-7641,
hsa-miR-9500, hsa-miR-4459, hsa-miR-21-5p, hsa-miR-663a and
hsa-miR-205-5p were confirmed by RT-qPCR (Fig. 4A). In addition, the expression
levels of the top eight downregulated miRNAs were detected by
RT-qPCR (Fig. 4B). The expression
levels of hsa-miR-1273e, hsa-miR-642a-3p, hsa-miR-6893-5p,
hsa-miR-210-3p and hsa-miR-30b-5p were significantly down-regulated
in the PDT treatment groups, compared with in the other groups.
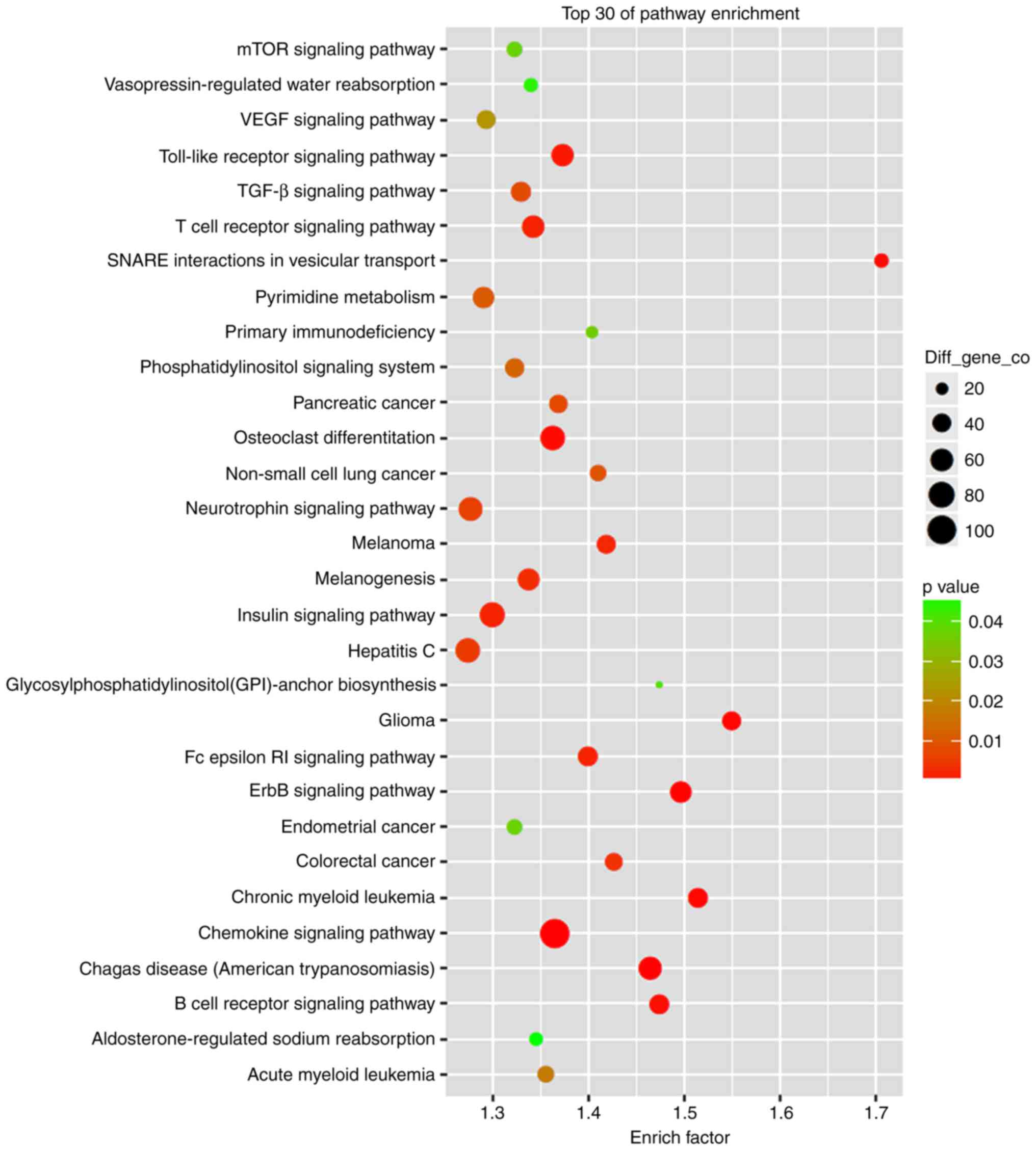
GO and KEGG enrichment analysis demonstrated that
the most significant cellular functions were cell apoptosis, cell
necrosis and ROS-mediated functions, and the most significant
signaling pathways were the chemokine signaling pathway, osteoclast
differentiation and the insulin signaling pathway, indicating that
PDT inhibited glioma cell growth via induction of cell apoptosis
and necrosis (Fig. 5). In the
process, the upregulated miRNAs, including hsa-miR-1273e,
hsa-miR-642a-3p, hsa-miR-6893-5p, hsa-miR-7641, hsa-miR-9500,
hsa-miR-135b-5p, hsa-miR-205-5p, hsa-miR-21-5p, hsa-miR-210-3p,
hsa-miR-30b-5p, hsa-miR-31-5p, hsa-miR-4459 and hsa-miR-663a are
significant and represent important targets for therapy of
PDT-induced cell apoptosis in glioma.
Detection of cell apoptosis-associated
proteins
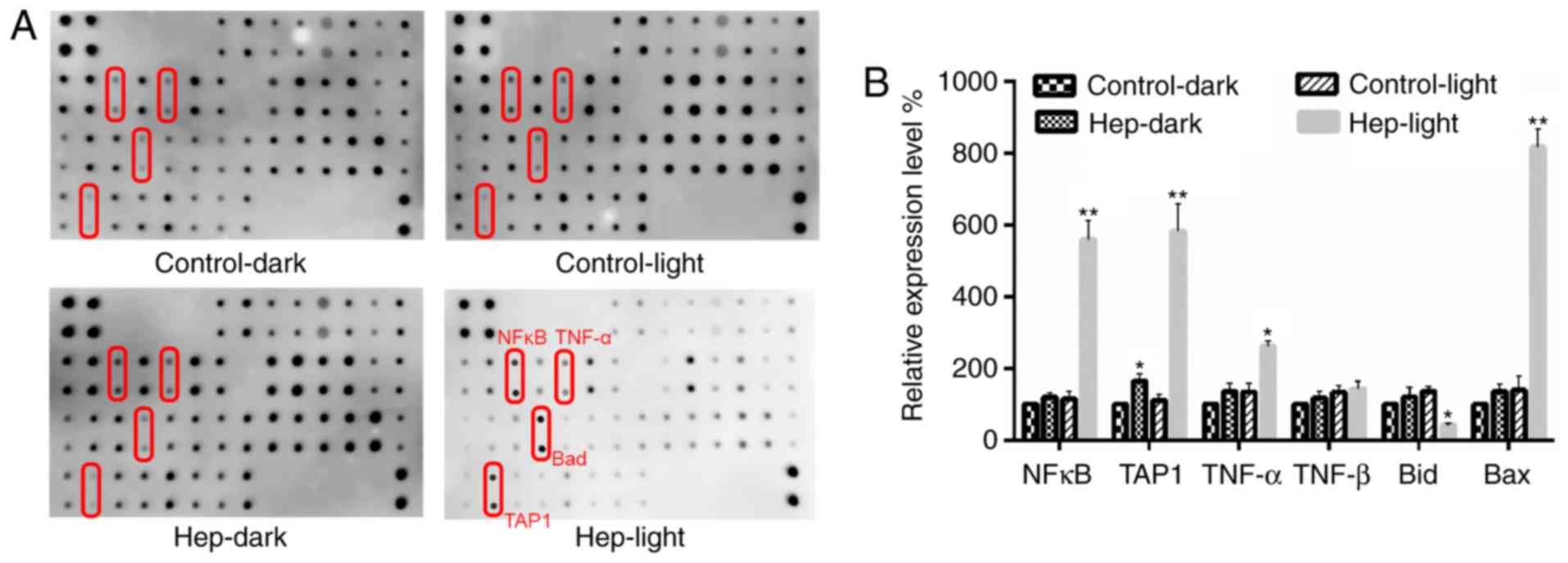
The proteins associated with cell apoptosis were
detected by apoptosis antibody array (Fig. 6). Quantitative analysis revealed
that TAP1- and nuclear factor (NF)-κB-mediated apoptosis pathways
were the most important signaling pathways involved in PDT with
hematoporphyrin-induced apoptosis observed in glioma U87 cells
(Fig. 6A). Following PDT with
hematopor-phyrin treatment in U87 cells, the expression levels of
NF-κB, TAP1, Bcl-2-associated X protein and tumor necrosis factor-α
were significantly upregulated, and the expression level of Bid was
significantly downregulated (P<0.05; Fig. 6B).
Discussion
The current study examined the possibility of using
PDT for the treatment of gliomas, and evaluated the molecular
mechanism of PDT using hematoporphyrin as a PS for the inhibition
of glioma cell growth by screening target miRNAs and
apoptosis-associated proteins. The aim of the study was to provide
a theoretical reference for the design and preparation of novel PSs
for PDT.
Using the first generation of PS for PDT,
hematoporphyrin on U251 and U87 glioma cells, the inhibition of PDT
in U87 cells was identified to be better than that in U251 cells.
Under the same conditions, the IC50 of hematoporphyrin
in the U87 cells was 80 nM, while that in the U251 cells was 120
nM. Physical and chemical factors act on cells together (29), and PS and light source are two key
factors of PDT. Therefore, the effect of single wavelength red
light and non-single wavelength white light on photodynamic
activity of hematoporphyrin were detected by irradiation of U87
cells. The results demonstrated that the inhibition of
hematoporphyrin under red light was better than that under white
light. Thus, red light-irradiated U87 cells were used to
investigate the molecular mechanism.
The inhibition of cell growth by drugs is generally
caused by induction of apoptosis or cell cycle arrest (23). The photodynamic activity of
hematoporphyrin was found to inhibit cell growth via induction of
cell apoptosis at the later stage. This is consistent with the
results reported in previous studies (28). For example, the glucose-modified
porphyrin derivatives, synthesized by Králová et al
(30) under light irradiation
significantly inhibited cell growth of MDA-MB-231 cells via
induction of cell apoptosis, which was also verified in a mouse
model. Thus, the PDT activity of hematoporphyrin on glioma U87
cells appears to be due to the induction of apoptosis.
It was demonstrated that hematoporphyrin under light
irradiation exerts its action via production of singlet oxygen and
superoxide anions (26,27). The current study found that PDT
treatment with hematoporphyrin induced ROS and decreased
mitochondrial membrane potential, which contributed to
mitochondrial disorder.
Previous studies demonstrate that apoptosis is
closely regulated by miRNAs (31,32). The potential target miRNA was
analyzed by miRNA microarray and differentially expressed miRNAs,
including six upregulated and eight downregulated miRNAs between
Hep-light (cells with hematoporphyrin and light irradiation) and
Control-dark (cells without treatment), Hep-dark (cells with
hematoporphyrin), or Hep-light (cells with light irradiation)
groups, were screened. There were 185 miRNAs differentially
expressed in the PDT treatment groups when compared with other
groups. The expression of six upregulated miRNAs, including
hsa-miR-7641, hsa-miR-9500, hsa-miR-4459, hsa-miR-21-5p,
hsa-miR-663a, and hsa-miR-205-5p were confirmed by RT-qPCR. It was
reported that miR-21 regulated proliferation and apoptosis of human
glioma cells by downregulating the phosphatase and tensin homolog
protein (33). In a previous
study, overexpression of miR-663 inhibited proliferation, migration
and invasion in A172 and U87 glioblastoma cells (34). Furthermore, the miR-205 serum
level was identified as an individual diagnostic marker for glioma
(35). However, the roles of
hsa-miR-7641, hsa-miR-9500, hsa-miR-4459, hsa-miR-21-5p,
hsa-miR-663a and hsa-miR-205-5p in PDT-induced cell apoptosis have
not yet bee confirmed using a cell model.
GO and KEGG enrichment analysis identified that the
most significant cellular functions were cell apoptosis, cell
necrosis and ROS-mediated functions, and the most significant
signaling pathways were the chemokine signaling pathway, osteoclast
differentiation and the insulin signaling pathway, indicating that
PDT inhibited glioma cell growth via induction of cell apoptosis
and necrosis. The proteins associated with cell apoptosis were
detected by apoptosis antibody array, and quantitative analysis
revealed that TAP1 and NF-κB-mediated apoptosis signaling pathway
was the most important pathway involved in PDT, with
hematoporphyrin-induced apoptosis observed in glioma U87 cells. A
recent study reported that miR-21 significantly contributes to the
inflammatory response via the sirtuin 1-NF-κB signaling pathway
(36). It implied that the miRNAs
that specifically targeted TAP1 and NF-κB are important therapeutic
targets (Fig. 7). However,
whether changes of AP1- and NF-kB are associated with those miRNAs
remains unclear.
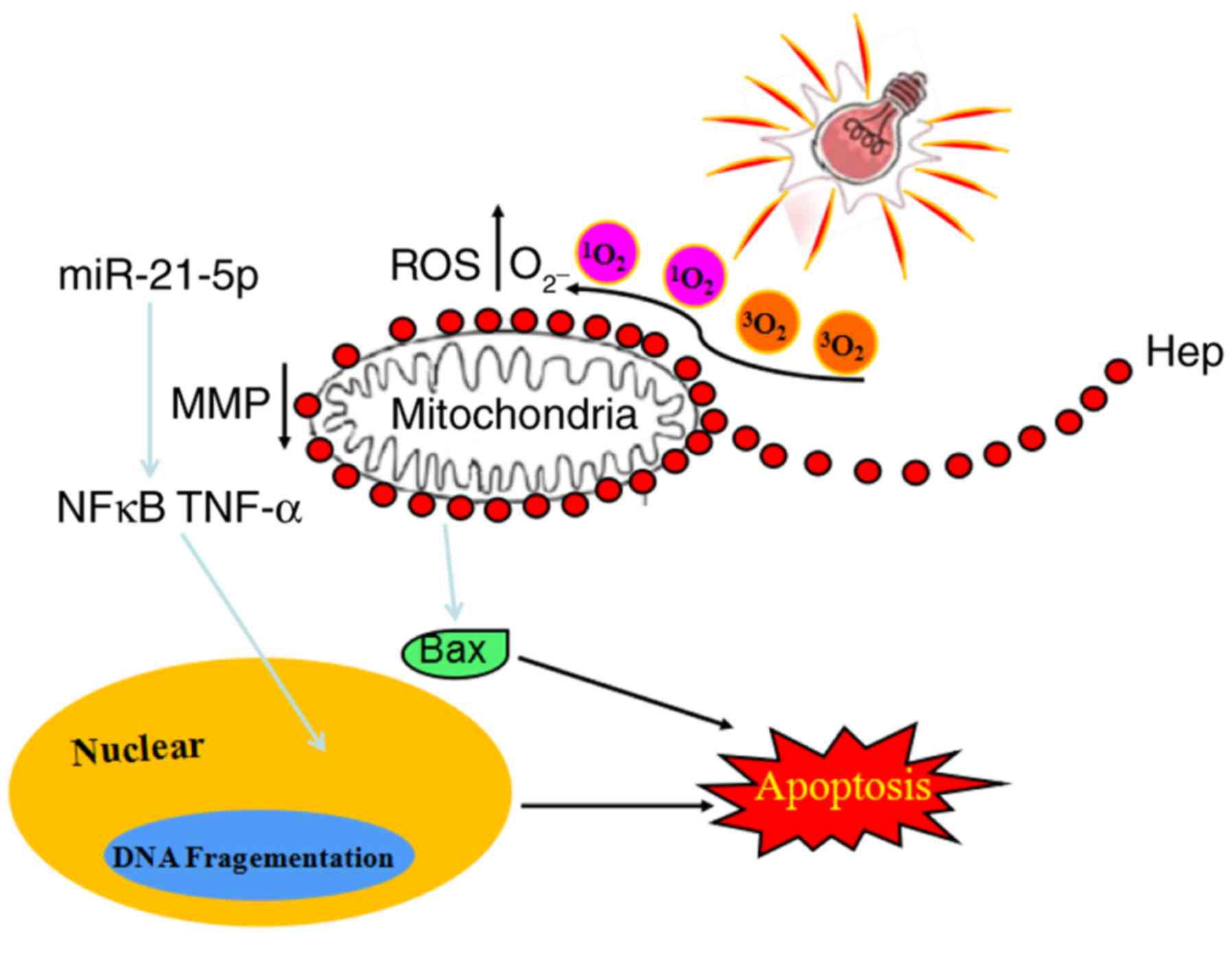 | Figure 7Proposed mechanistic of
hematoporphyrin (Hep)-induced apoptosis in U87 cells under
irradiation. Differentially expressed miRNAs, such as
hsa-miR-21-5p, are involved in PDT-inhibited glioma cell growth via
induction of cell apoptosis and necrosis. Following PDT with
hematoporphyrin treatment in U87 cells, the expression levels of
NF-κB, TAP1, Bax, and TNF-α were significantly upregulated, and the
expression level of Bid was significantly downregulated. The TAP1
and NF-κB-mediated apoptosis pathway was the most important pathway
involved in PDT with hematoporphyrin-induced apoptosis in glioma
U87 cells. It indicates that the miRNAs that specifically targeted
TAP1 and NF-κB are important therapeutic targets. miRNA, microRNA;
PDT, photodynamic therapy; NF-κB, nuclear factor-κB; TAP1,
transporter 1, ATP binding cassette subfamily B member; TNF, tumor
necrosis factor; Bax, Bcl-2-associated X protein; ROS, reactive
oxygen species; MMP, matrix metalloproteinase; Hep,
haematoporphyrin. |
TAPl and NF-KB proteins may be important in the
apoptotic process. Furthermore, 5-ALA may significantly inhibit the
growth of glioma U251 cells under light irradiation, and the
expression levels of TAP1-associated proteasomes has been
demonstrated to be significantly increased (20). In addition, PDT was demonstrated
to induce phosphorylation of proteins in the Bcl-2 family,
enhancing the intracellular oxidative stress response and thereby
accelerating apoptosis of U87 cells (37). According to the results of a
previous study and previous clinical experience (38,39), PDT was hypothesized to be a
potential treatment strategy for glioma via apoptosis induction and
immune enhancement (19–22), which was confirmed in the current
study. PDT represents a potential treatment strategy for glioma,
and the treatment effect appears to be more obvious under a red
light source. PDT inhibited cell growth via induction of cell
apoptosis and immune enhancement. PDT treatment with
hematoporphyrin inhibits cell growth of gliomas, which is regulated
by miRNAs; however, the target genes for miRNAs and the molecular
network remain unclear. Therefore, further studies are required in
future. In the current study, the experiments, such as cell
apoptosis, were only performed at one time point (23 h post-PDT
treatment); thus, further research is required to verify the
mechanism of action at additional time points. Furthermore, in this
study, the human glioma cell lines, U87 and U251, were used; in
future studies, we also aim to use normal control primary glioma
cells from rats or mice to further confirm our findings.
Acknowledgments
The present study was supported by the Natural
Science Foundation of China (grant no. 81272774).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Duan R, Han L, Wang Q, Wei J, Chen L,
Zhang J, Kang C and Wang L: HOXA13 is a potential GBM diagnostic
marker and promotes glioma invasion by activating the Wnt and TGF-β
pathways. Oncotarget. 6:27778–27793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watkins S, Robel S, Kimbrough IF, Robert
SM, Ellisdavies G and Sontheimer H: Disruption of
astrocyte-vascular coupling and the blood-brain barrier by invading
glioma cells. Nature Communications. 5:41962014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li W and Graeber MB: The molecular profile
of microglia under the influence of glioma. Neuro Oncol.
14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito T, Muragaki Y, Maruyama T, Tamura M,
Nitta M and Okada Y: Intraoperative functional mapping and
monitoring during glioma surgery. Neurol Med Chir. 55(Suppl 1):
S1–S13. 2015. View Article : Google Scholar
|
|
5
|
Shi M, Fortin D, Sanche L and Paquette B:
Convection-enhancement delivery of platinum-based drugs and
Lipoplatin™ to optimize the concomitant effect with radiotherapy in
F98 glioma rat model. Invest New Drugs. 33:555–563. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tobias A, Ahmed A, Moon KS and Lesniak MS:
The art of gene therapy for glioma: A review of the challenging
road to the bedside. J Neurol Neurosurg Psychiatry. 84:213–222.
2013. View Article : Google Scholar :
|
|
7
|
Charest G, Sanche L, Fortin D, Mathieu D
and Paquette B: Optimization of the route of platinum drugs
administration to optimize the concomitant treatment with
radiotherapy for glio-blastoma implanted in the Fischer rat brain.
J Neurooncol. 115:365–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rudà R, Bello L, Duffau H and Soffietti R:
Seizures in low-grade gliomas: Natural history, pathogenesis, and
outcome after treatments. Neuro Oncol. 14(Suppl 4): iv55–iv64.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ethirajan M, Chen Y, Joshi P and Pandey
RK: The role of porphyrin chemistry in tumor imaging and
photodynamic therapy. Chem Soc Rev. 40:340–362. 2011. View Article : Google Scholar
|
|
10
|
Dougherty TJ, Gomer CJ, Henderson BW, Jori
G, Kessel D, Korbelik M, Moan J and Peng Q: Photodynamic therapy. J
Natl Cancer Inst. 90:889–905. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krosl G, Korbelik M and Dougherty GJ:
Induction of immune cell infiltration into murine SCCVII tumour by
photofrin-based photodynamic therapy. Br J Cancer. 71:549–555.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kessel D, Luo Y, Deng Y and Chang CK: The
role of subcellular localization in initiation of apoptosis by
photodynamic therapy. Photochem Photobiol. 65:422–426. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dougherty TJ and Levy JG: Biomedical
photonics handbook. CRC Press; New York: 2003
|
|
14
|
Tian YY, Wang LL and Wang W: Progress in
photodynamic therapy on tumors. Laser Physics. 18:1119–1123. 2008.
View Article : Google Scholar
|
|
15
|
Sutedja T, Baas P, Stewart F and van
Zandwijk N: A pilot study of photodynamic therapy in patients with
inoperable non-small cell lung cancer. Eur J Cancer. 28A:1370–1373.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pandey RK, Goswami LN, Chen Y, Gryshuk A,
Missert JR, Oseroff A and Dougherty TJ: Nature: A rich source for
developing multifunctional agents. Tumor-imaging and photodynamic
therapy. Lasers Surg Med. 38:445–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whelpton R, Michael-Titus AT, Basra SS and
Grahn M: Distribution of temoporfin, a new photosensitizer for the
photo-dynamic therapy of cancer, in a murine tumor model. Photochem
Photobiol. 61:397–401. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji J, Fan Z, Zhou F and Wang X, Shi L,
Zhang H, Wang P, Yang D, Zhang L, Chen WR and Wang X: Improvement
of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for
skin squamous cell carcinoma. Oncotarget. 6:17135–17146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen CC, Li JL, Zhang SY, Xu XK, Ouyang LP,
Yu J and Li FC: Photodynamic therapy mediated with 5-aminolevulinic
acid enhances the expression of proteasomes in glioma cell line
U251 cells. Chin J Clin Neurosurg. 17:154–157. 2012.
|
|
20
|
Le-ping O, Shan-yi Z, Jun-liang L, Xin-ke
X, Yin-lun W, Meiguang Z, Sheng-wen W and Fang-cheng L:
Overexpression of TAP1 up-regulates HLA-I in human glioma U251
cells. In: Chin J Pathophysiol. 29. pp. 425–429. 2013
|
|
21
|
Yuan SX, Li FC, Zhou HJ, Sun X and Yin HJ:
Antitumor efficacy of photodynamic therapy-generated glioma vaccine
from dendritic cells. Tumor. 27:962–967. 2007.
|
|
22
|
Zhang SY, Li JL, Xu XK, Zheng MG, Wen CC
and Li FC: Effect of hematoporphyrin monomethyl ether-based
photodynamic treatment on gene expression of transporter associated
with antigen processing 1 in human U87-MG glioma. Chin J Exp Surg.
27:914–916. 2010.In Chinese.
|
|
23
|
Zhang Z, Wen JY, Lv BB, Li X, Ying X, Wang
YJ, Zhang HT, Wang H, Liu HY and Chnag CK: Photocytotoxicity and
G-quadruplex DNA interaction of water-soluble gallium(III)
tris(N-methyl-4-pyridyl)corrole complex. Appl Organometal Chem.
30:132–139. 2016. View
Article : Google Scholar
|
|
24
|
Liu JX, Yan ZP, Zhang YY, Wu J, Liu XH and
Zeng Y: Hemodynamic shear stress regulates the transcriptional
expression of heparan sulfate proteoglycans in human umbilical vein
endothelial cell. Cell Mol Biol. 62:28–34. 2016.PubMed/NCBI
|
|
25
|
Zeng Y, Liu JX, Yan ZP, Yao XH and Liu XH:
Potential microRNA biomarkers for acute ischemic stroke. Int J Mol
Med. 36:1639–1647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moserova I and Kralova J: Role of ER
stress response in photodynamic therapy: ROS generated in different
subcellular compartments trigger diverse cell death pathways. PLoS
One. 7:e329722012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Broekgaarden M, Weijer R, van Gulik TM,
Hamblin MR and Heger M: Tumor cell survival pathways activated by
photody-namic therapy: A molecular basis for pharmacological
inhibition strategies. Cancer Metastasis Rev. 34:643–690. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang ZH, Liu HY, Zhou R, Zhang Z, Ali A,
Han BJ, Liu YJ and Xiao XY: DNA-binding, photocleavage, and
photodynamic anti-cancer activities of pyridyl corroles. J Membr
Biol. 249:419–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng Y: Endothelial glycocalyx as a
critical signalling platform integrating the extracellular
haemodynamic forces and chemical signalling. J Cell Mol Med.
21:1457–1462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Králová J, Bríza T, Moserová I, Dolenský
B, Vasek P, Poucková P, Kejík Z, Kaplánek R, Martásek P, Dvorák M
and Král V: Glycol porphyrin derivatives as potent photodynamic
inducers of apoptosis in tumor cells. J Med Chem. 51:5964–5973.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geng Y, Lin D, Shao L, Yan F and Ju H:
Cellular delivery of quantum dot-bound hybridization probe for
detection of intracellular pre-microRNA using
chitosan/poly(γ-glutamic acid) complex as a carrier. PLoS One.
8:e655402013. View Article : Google Scholar
|
|
32
|
Guo P, Coban O, Snead NM, Trebley J,
Hoeprich S, Guo S and Shu Y: Engineering RNA for targeted siRNA
delivery and medical application. Adv Drug Deliv Rev. 62:650–666.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li SJ, Zhou J, Zhang L, Xiang W, Hu Q, He
YY and Chen LG: The effect of miR-21 on SWOZ2 glioma cells and its
biological mechanism. J BUON. 22:468–473. 2017.PubMed/NCBI
|
|
34
|
Li Q, Cheng Q, Chen Z, Peng R, Chen R, Ma
Z, Wan X, Liu J, Meng M, Peng Z and Jiang B: MicroRNA-663 inhibits
the proliferation, migration and invasion of glioblastoma cells via
targeting TGF-β1. Oncol Rep. 35:1125–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yue X, Lan F, Hu M, Pan Q, Wang Q and Wang
J: Downregulation of serum microRNA-205 as a potential diagnostic
and prognostic biomarker for human glioma. J Neurosurg.
124:122–128. 2016. View Article : Google Scholar
|
|
36
|
Lin Q, Geng Y, Zhao M, Lin S, Zhu Q and
Tian Z: MiR-21 Regulates TNF-α-induced CD40 expression via the
SIRT1-NF-κB pathway in renal inner medullary collecting duct cells.
Cell Physiol Biochem. 41:124–136. 2017. View Article : Google Scholar
|
|
37
|
Misuth M, Horvath D, Miskovsky P and
Huntosova V: Synergism between PKCδ regulators hypericin and
rottlerin enhances apoptosis in U87 MG glioma cells after light
stimulation. Photodiagnosis Photodyn Ther. 18:267–274. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan L, Lin H, Tian S, Bai D, Kong Y and Yu
L: The sensitivity of glioma cells to pyropheophorbide-αmethyl
ester-mediated photodynamic therapy is enhanced by inhibiting
ABCG2. Lasers Surg Med. 49:719–726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Christie C, Pomeroy A, Nair R, Berg K and
Hirschberg H: Photodynamic therapy enhances the efficacy of
gene-directed enzyme prodrug therapy. Photodiagnosis Photodyn Ther.
18:140–148. 2017. View Article : Google Scholar : PubMed/NCBI
|