Introduction
Human induced pluripotent stem cells (hiPSCs) have
broad prospects for application in the field of regenerative
medicine due to their three germ layer differentiation ability and
patient-specific cell source (1,2).
Therefore, through differentiation into neural cells, hiPSCs can
provide a good cell source for the cell therapy of
neurodegenerative disease (3). It
is known that, during the induction period, there is a precise
molecular regulation mechanism, developmental sequence, overlap of
time and overlap of space that should be followed (4,5).
In the present study, the differentiation of mesodermal and
endodermal cells was reduced through inhibition of bone
morphogenetic protein (BMP) and transforming growth factor-β
(TGF-β) signaling using the signaling pathway inhibitors LDN193189
and SB431542. In this manner, a large number of outer germ layer
cells were obtained from which neural progenitors were derived.
Efficient neuron differentiation from derived neural precursor
cells (NPCs) in the cell treatment of neurodegenerative diseases is
critical, but the low differentiation rate and poor survival state
are still to be improved in vitro or in vivo
(6,7). In previous studies, the addition of
antioxidant and anti-inflammatory cytokines to the differentiation
medium, and the combined use of neurotrophic factors during the
differentiation period were able to improve the differentiation
ratio and the survival state (8,9).
However, the mechanism has not yet been clarified.
Leukocyte inhibitory factor (LIF) is a highly
conserved gene of the interleukin (IL)-6 family (10). LIF has multiple functions,
including the maintenance of the undifferentiated state of mouse
embryonic stem cells (mESCs), the proliferation of primordial germ
cells, and functions as a mediator in implantation and
decidualization (11). It has
been found that Janus kinase/signal transducer and activator of
transcription (JAK/STAT3), protein kinase B (AKT), extracellular
signal-regulated protein kinases 1/2 (ERK1/2) and mechanistic
target of rapamycin (mTOR) signaling pathways are involved in the
biological function of LIF (12–15). LIF could improve cell
proliferation in fibroblasts and cardiomyocytes through activating
the PI3K-sensitive AKT kinase (16,17). Phosphatidylinositol 3-kinase
(PI3K)/AKT signaling is involved in numerous events in neuronal
proliferation and differentiation. Downregulating PI3K/AKT
signaling could inhibit neuroendocrine differentiation. By
contrast, upregulating of this signal could increase the neuronal
differentiation from mouse cochlear neural stem cells (18,19). It has been reported that LIF has a
similar function to neurotrophic factor in neuronal differentiation
from the mouse neural crest. Moreover, LIF can improve the
maturation of sensory neurons and maintain their morphological
characteristics (20). In a study
using mice with spinal cord injury, LIF-treated mice manifested
greater recovery of locomotor behavior due to an increase in the
number of neurons and NPCs in the brain (21). However, the mechanism of LIF in
neuron development has not been elaborated.
Anti-inflammation is one of the strategies for the
treatment of neurodegenerative diseases and for neuronal protection
(22,23). Certain studies have demonstrated
that methylprednisolone facilitates the survival of new neurons and
improves the neurological deficit following transient cerebral
ischemia through the suppression of inflammatory reactions
(24). Anti-inflammatory
treatment could protect the dopaminergic neurons in
6-hydroxydopaminelesioned rats through targeting not only the
microglia, but also the other immune cells, including cluster of
differentiation 163-positive macrophages (25). Inflammation of the neurons causes
the release of inflammatory cytokines, including IL-6, IL-5 and LIF
(26). In addition, LIF serves an
important role in the inflammatory responses. It has been reported
that injecting lipopolysaccharide (LPS) into the trachea of rats
could induce the expression and secretion of LIF in bronchoalveolar
cells (27). Moreover,
inflammatory cytokines, including IL-6 and tumor necrosis factor-α
(TNF-α), could increase the mRNA or protein expression of LIF
during cell culture (28,29). However, studies on the stimulation
of an anti-inflammatory effect by LIF in in vitro neuronal
differentiation are rare.
In the present study, we would use LIF to activate
the PI3K/ AKT signal and induce the anti-inflammatory effect during
the neuron differentiation from hiPSCs derived NPCs. This effect
might improve the neuron differentiation ratio and survival
state.
Materials and methods
Culture of undifferentiated hiPSCs
The hiPSC cell line and the H9 embryonic stem cell
line (gifts from Professor Lan Feng, Beijing Anzhen Hospital
Beijing Institute of Heart Lung and Blood Vessel Disease, Capital
Medical University, Beijing, China) were maintained in essential 8
medium (Table I) (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in Matrigel (B&D
Technologies, Macon, GA, USA) pre-coated dishes, 37˚C, 5%
CO2. The hiPSCs were passaged every 4–7 days when the
cells reached 80–90% confluence.
 | Table ICulture media and reagents. |
Table I
Culture media and reagents.
| Medium | Reagents |
|---|
| hiPSC | E8 and its
supplements |
| NPC induction | DMEM/F12a |
| Neurobasal
mediuma |
| 1% N2a |
| 2% B27a |
| 10 μM
SB431542b |
| 100 nM
LDN193189b |
| Neuron
differentiation | DMEM/F12a |
| 2% B27a |
| 1% N2a |
| 20 ng/ml
BDNFc |
| 20 ng/ml
GDNFc |
| 0.2 mM AAb |
| 0.5 mM dibutyryl
cAMPb |
| 5 ng/ml of
LIFc |
| LY294002 (20
μΜ)b |
Neural induction
For neural induction, the hiPSCs were split using
accutase (Thermo Fisher Scientific, Inc.) for 5 min and seeded in a
Matrigel pre-coated 6-well plate at a density of
1.6–2×104 cells/cm2 in the presence of 5
μM ρ-associated, coiled-coil-containing protein kinase
(ROCK) inhibitor (Selleck Chemicals, Houston, TX, USA). Following 1
day of incubation, medium containing ROCK inhibitor was aspirated,
and N2/B27 basic medium (Table I)
containing a mixture of 1:1 Dulbecco's modified Eagle's medium
(DMEM)/F12 and neurobasal medium, supplemented with 1% N2 and 2%
B27 (all Gibco; Thermo Fisher Scientific, Inc.) was added. SB431542
(10 μM) and 100 nM LDN193189 (both Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) were added to the basal medium to trigger
the neural induction. The neural induction lasted 11 days. On day 5
of the induction, the SB431542 was removed. During the induction
period, the culture medium was changed every day.
Neural differentiation
On day 11 of induction, the induction medium was
changed to differentiation medium (Table I) consisting of DMEM/F12,
supplemented with 2% B27, 1% N2, 20 ng/ml brain-derived
neurotrophic factor (Peprotech, Rocky Hill, NJ, USA), 0.2 mM
ascorbic acid (Sigma-Aldrich; Merck KGaA), 20 ng/ml glial cell
line-derived neurotrophic factor (Peprotech) and 0.5 mM dibutyryl
cAMP (Sigma-Aldrich; Merck KGaA). To prepare the LIF-positive
differentiation group, 5 ng/ml LIF (Peprotech) was added to the
differentiation medium. To prepare the PI3K/AKT inhibition
differentiation group, 20 μM PI3K/AKT inhibitor LY294002, or
50 nM wortmannin (both Sigma-Aldrich; Merck KGaA) combined with 5
ng/ml LIF was added to the differentiation medium. To prepare the
mTOR inhibition differentiation group, 100 nM rapamycin
(Sigma-Aldrich; Merck KGaA) combined with 5 ng/ml of LIF was added
to the differentiation medium. Derived NPCs were differentiated for
9 days. On culture day 20 (counting the first culture day as the
day of the initial derived NPC induction from hiPSCs), the cells
were harvested and prepared for further experimentation.
Immunofluorescence
The immunofluorescence method was performed as
previously described (30), with
minor modifications. Briefly, the cells were washed with
phosphate-buffered saline (PBS), fixed at room temperature for 20
min with 4% paraformaldehyde, washed again in PBS 3 times, for 5
min each time, and then permeabilized with 0.3 % Triton X-100/PBS
for 10 min. Next, the cells were blocked with 5% bovine serum
albumin (BSA)/PBS for 40 min. The cells were then exposed to
primary antibodies diluted in PBS plus 1% BSA at 4̊C overnight,
followed by incubation with corresponding secondary antibodies at
room temperature for 2 h. Cell nuclei were counterstained with
4′,6-diamidino-2-phenylindole for 15 min. Samples were analyzed
using an inverted fluorescence microscope (IX71; Olympus, Tokyo,
Japan) or a confocal Leica TCS SP8 (Leica Microsystems, Inc.,
Buffalo Grove, IL, USA). All images were acquired under identical
settings using IPP7.0 Image Browser software (Olympus) or LAS AF
Lite (Leica Microsystems, Inc.). The relative fluorescence
intensity was qualified using ImageJ software. Primary antibodies
used in this study were: POU domain class 5 transcription factor 1
(OCT4; sc-5279; 1:100 dilution; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), Nestin (A0484; 1:50 dilution; ABclonal
Biotechnology Co., Ltd., Cambridge, MA, USA), paired box protein
Pax-6 (PAX6; ab5790; 1:750 dilution; Abcam, Woburn, MA, USA),
microtubule-associated protein 2 (MAP2; 4542; 1:200 dilution; Cell
Signaling Technology, Inc., Danvers, MA, USA), neuron-specific
class III β-tubulin (TUJ1; T2200; 1:200 dilution; Sigma-Aldrich;
Merck KGaA), AKT (9272; 1:200 dilution) and phosphorylated (p)-AKT
(4060; 1:200 dilution) (both Cell Signaling Technology, Inc.).
Secondary antibodies used in this study were fluorescein
isothiocyanate-conjugated goat anti-rabbit and Cy3-conjugated goat
anti-rabbit antibodies (F-2765 and T-2767; 1:200 dilution; both
Thermo Fisher Scientific, Inc.)
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA (hiPSCs, H9, hiPSCs-derived NPCs and
neurons differentiated from hiPSCs-derived NPCs) used in the
present study was isolated using TRIzol reagent (Takara Bio, Inc.,
Otsu, Japan). Isolated RNA (1 μg) was converted into cDNA
using the SuperScript III First-Strand cDNA Synthesis kit (Takara
Bio, Inc.). The RT-qPCR was performed as previously described
(31) on an Applied Biosystems
7500/7500 Fast Real-Time PCR system (Thermo Fisher Scientific,
Inc.) using SYBR Premix Ex Taq™ (Takara Bio, Inc.) in a
20-μl reaction mixture containing 1 μl of each cDNA
and 0.2 μM of each pair of primers. Samples were preheated
at 95˚C for 30 sec. RT-PCR conditions consisted of 40 cycles of 5
sec at 95˚C and 34 sec at 60˚C. The dissociation stage consisted of
15 sec at 95˚C, 1 min at 60˚C and 15 sec at 95˚C. The pairs of
primers are shown in Table II.
GAPDH was used as an endogenous control. The thermocycling
conditions and the method of quantification were performed as
previously described (31).
 | Table IIPrimers used in this study for
reverse transcription-quantitative polymerase chain reaction. |
Table II
Primers used in this study for
reverse transcription-quantitative polymerase chain reaction.
| Name | Sense primers | Antisense
primers |
|---|
| GAPDH
CCCAT |
GTTCGTCATGGGTGT |
GATGGCATGGACTGTGGTCA |
| OCT4 |
TGAGGCCCTGGAGAAAGAGT |
TTGCTGGCCTGTCTTCTCTG |
| NANOG |
GCAGGGATGCCTGGTGAAC |
GGACTGTTCCAGGCCTGATT |
| SOX2 |
GGATAAGTACACGCTGCCC |
ATGTGCGCGTAACTGTCCAT |
| LIN28 |
GGAAAGAGCATGCAGAAGCG |
TGATGCTCTGGCAGAAGTGG |
| PAX6 |
TGAGGCCCTGGAGAAAGAGT |
TTGCTGGCCTGTCTTCTCTG |
| NES |
AGTGATGCCCCTTCACCTTG |
GCTCGCTCTCTACTTTCCCC |
| IL-10 |
CGAGATGCCTTCAGCAGAGT |
CGCCTTGATGTCTGGGTCTT |
| TGF-β |
TTGACTTCCGCAAGGACCTC |
CTCCAAATGTAGGGGCAGGG |
| IL-1α |
CCTGAGCTCGCCAGTGAAAT |
GGTGGTCGGAGATTCGTAGC |
| TNF-α
TCCTCTCT | GCCATCAAGAGC |
AGTAGACCTGCCCAGACTCG |
| MAP2 |
TCTGCACACTCACATCCACC |
CTGAGGTCAGCTCTCCGTTG |
| TUJ1 |
GCTGGTGGAAAACACGGATG |
GCCGATACCAGGTGGTTGAG |
Western blotting
Total proteins were extracted from different cell
stages (day 11 or day 20 after induction) using
radioimmunoprecipitation assay buffer and a cocktail mixture
(Thermo Fisher Scientific, Inc.). The concentration of protein was
measured using the Pierce Bicinchoninic Acid Protein assay kit
(Thermo Fisher Scientific, Inc.). Protein (30 μg) was
subjected to a 10% resolving gel for electrophoresis. The
SDS-polyacrylamide gel electrophoresis was performed at 80 V first
for 20 min, and then at 100 V for 80 min. Next, the protein was
transferred to polyvinylidene difluoride membranes at 276 mA for
150 min using a semidry transfer apparatus (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The blot membranes were washed in
Tris-buffered saline with 0.1% Tween-20 (TBST) and blocked with 5%
BSA in TBST for 1 h at room temperature. The membranes were then
incubated with primary antibodies at 4̊C with agitation overnight.
Following this, the membranes were washed in TBST three times for 5
min each time, incubated with their corresponding secondary
antibodies for 2 h, and rinsed in TBST three times again. Enhanced
chemiluminescence substrate was then applied (Thermo Fisher
Scientific, Inc.). The value of the chemiluminescence was recorded
and the band density was quantified using ImageJ software. Primary
antibodies used in this study were MAP2 (4542; 1:1,000 dilution;
Cell Signaling Technology, Inc.), TUJ1 (T2200; 1:1,000 dilution;
Sigma-Aldrich; Merck KGaA), AKT (9272; 1:1,000 dilution) and p-AKT
(4060; 1:1,000 dilution) (both Cell Signaling Technology, Inc.).
Secondary antibodies used in this study were horseradish
peroxidase-conjugated goat anti-rabbit antibody (7074; 1:5,000
dilution; Cell Signaling Technology, Inc.).
Cell Counting kit-8 (CCK-8) assay
The CCK-8 method was used to detect the viability of
neurons differentiated from hiPSC-derived NPCs on culture day 20 in
each differentiation group. Briefly, 20-day differentiated cells
were seeded on 96-well plates at a density of 5,000/well, and then
incubated with 10 μl CCK-8 (KeyGen Biotech Co., Ltd.,
Nanjing, China) in a total volume of 100 μl culture medium
for 2 to 4 h at 37̊C. Next, the 96-well plate was placed on an
enzyme standard instrument (ELx800; BioTek Instruments, Inc.,
Winooski, VT, USA). The plate was read at a wavelength of 450 nm.
The neuron control differentiation medium was used as the
control.
Apoptosis and flow cytometry
analyses
Neurons differentiated from hiPSC-derived NPCs on
culture day 20 in the control differentiation group and the
LIF-positive differentiation group were subjected to standard
procedure of cell apoptosis analysis. Cells (1×105) were
fixed and labeled with Annexin V-FITC, 45 min at room temperature
and propidium iodide using the Annexin V-FITC and propidium iodide
using the Annexin-V-FLUOS staining kit (BD Biosciences, San Jose,
CA, USA) according to the manufacturer's protocols, followed by
flow cytometry analysis. The Annexin V-positive cells were counted
as apoptotic cells.
Statistical analysis
All the experiments were independently repeated at
least three times, and statistical analysis was performed using the
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). The data are
expressed as the mean ± standard deviation. Pairwise comparisons
between groups were performed using the independent sample t-test
and one-way analysis of variance followed by pairwise t tests. The
correction test we used here is Bonferroni's correction. P<0.05
was considered to indicate a statistically significant
difference.
Results
hiPSCs exhibit similar characteristics to
the H9 embryonic stem cell line
hiPSCs exhibited the similar morphological
characteristics as the H9 embryonic stem cell line (Fig. 1A). Mycoplasma contamination
detection showed that the hiPSCs were not contaminated by
mycoplasma (Fig. 1B). RT-qPCR
analysis showed that the relative mRNA expression of the
pluripotent markers in hiPSCs, including OCT4, Nanog
homeobox, SRY-box 2 and lin-28 homolog A, showed no significant
differences with the control H9 cell line (Fig. 1C). As with the H9 cells, the
hiPSCs cells were also OCT4-positive, as shown in the
immunofluorescence images (Fig.
1D).
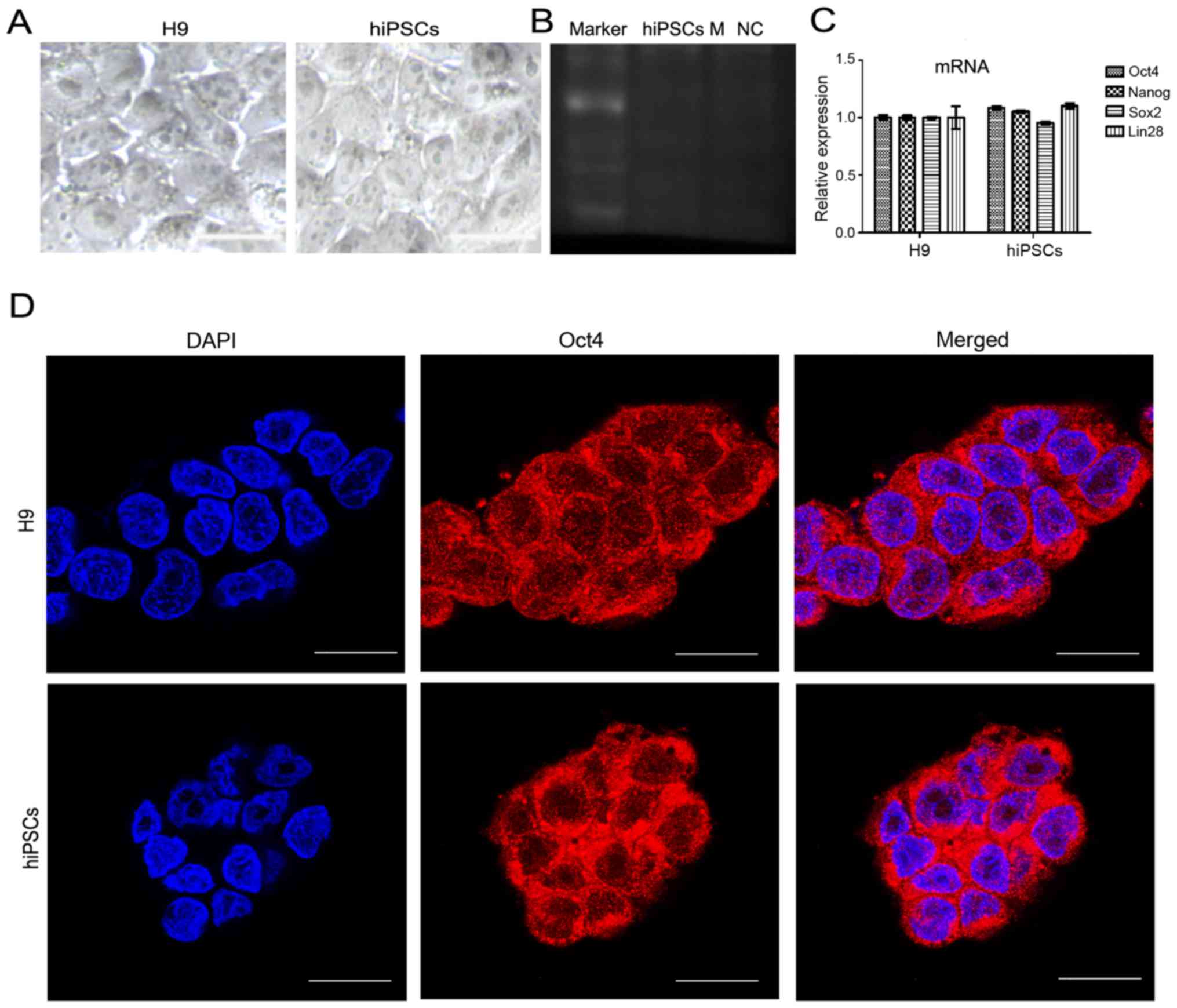 | Figure 1Pluripotency detection of hiPSCs. (A)
The structure of hiPSCs and human H9 embryonic stem cell line under
bright field microscopy Scale bar, 20 μm. (B) RT-PCR showing
no mycoplasma contamination. (C) The mRNA expression of pluripotent
genes OCT4, NANOG, SOX2 and LIN28 in
hiPSCs were detected by RT-qPCR, with H9 used as a control. Results
are presented as the mean ± standard deviation. (D) The protein
expression of OCT4 in hiPSCs and the H9 cell line, observed using
immunofluorescence. Scale bar, 20 μm. hiPSCs, human induced
pluripotent stem cells; RT-PCR, reverse transcription-quantitative
polymerase chain reaction; NANOG, Nanog homeobox;
OCT4, POU class 5 homeobox 1; LIN28, lin-28 homolog
A; SOX2, SRY-box 2; DAPI, 4′,6-diamidino-2-phenylindole. |
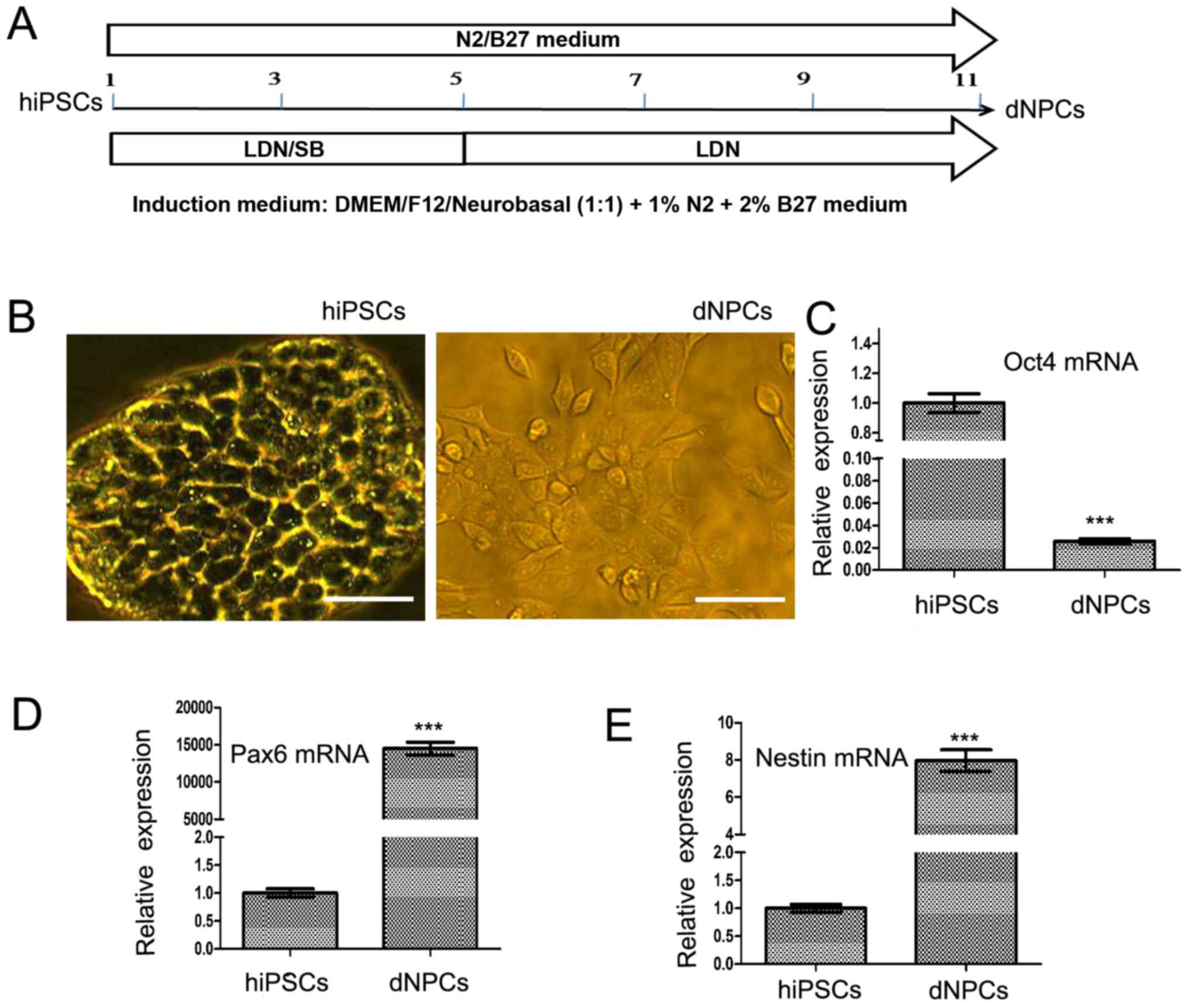
A large proportion of hiPSC-derived NPCs
can be harvested after neural induction
In the present study, hiPSCs were transformed into
NPCs by adding BMP signaling inhibitor LDN193189 and TGF-β
signaling inhibitor SB431542 into the N2/B27 basal culture medium.
The transformation of NPCs was finished after 11 days of induction.
The induction time course is shown in Fig. 2A. The derived cells have different
morphological characteristics compared with the hiPSCs (Fig. 2B). The relative mRNA expression of
pluripotent gene OCT4 was significantly downregulated in the
derived NPCs compared with that in the hiPSCs (Fig. 2C; P<0.001). By contrast, the
mRNA expression of NPC markers, including nestin (NES) and
PAX6, were significantly upregulated in the derived NPCs
compared with that in the hiPSCs (Fig. 2D and E; both P<0.001).
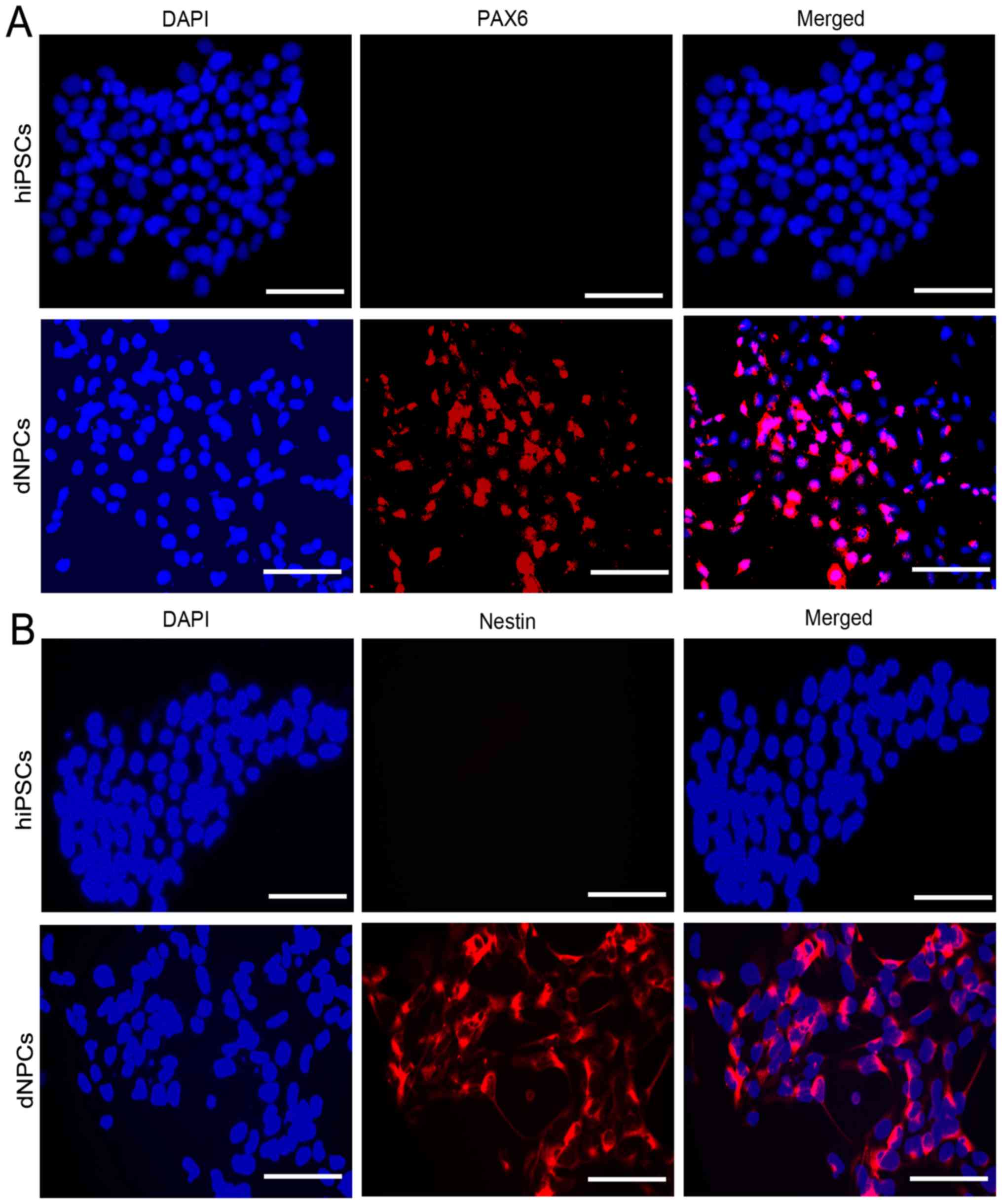
Furthermore, immunofluorescence data clearly showed that the Nestin
and PAX6 proteins were highly expressed in the derived NPCs, but
rarely expressed in the hiPSCs (Fig.
3A and B).
LIF upregulates the expression of
neuronal markers TUJ1 and MAP2 during the differentiation
period
Following the derivation of NPCs from hiPSCs, the
derived NPCs were differentiated into neurons. The normal
differentiation medium differentiation group was used as the
control differentiation group. The differentiation time course is
shown in Fig. 4A The effect of
LIF on neuronal differentiation was tested on culture day 20. The
neuronal markers TUJ1 and MAP2 were detected by immunofluorescence
methods (Figs. 4B and 5B). The relative fluorescence intensity
analysis by ImageJ revealed that the expression of TUJ1 and MAP2 in
the LIF (5 ng/ml) positive differentiation group was significantly
higher than that of the control differentiation group (Figs. 4C and 5C; both P<0.01). The relative mRNA
expression of TUJ1 and MAP2 in the LIF-positive
differentiation group was significantly higher than that in the
control differentiation group (Figs.
4D and 5D both P<0.01).
The protein expression of TUJ1 and MAP2 in the LIF-positive
differentiation group was significantly higher than that in the
control differentiation group too (Figs. 4E and F and 5E and F; both P<0.01). LIF
concentration test experiments showed that adding 5 ng/ml LIF to
the control differentiation medium resulted in the strongest mRNA
expression of TUJ1 and MAP2, as detected by RT-qPCR
on culture day 20 (Fig. 5A).
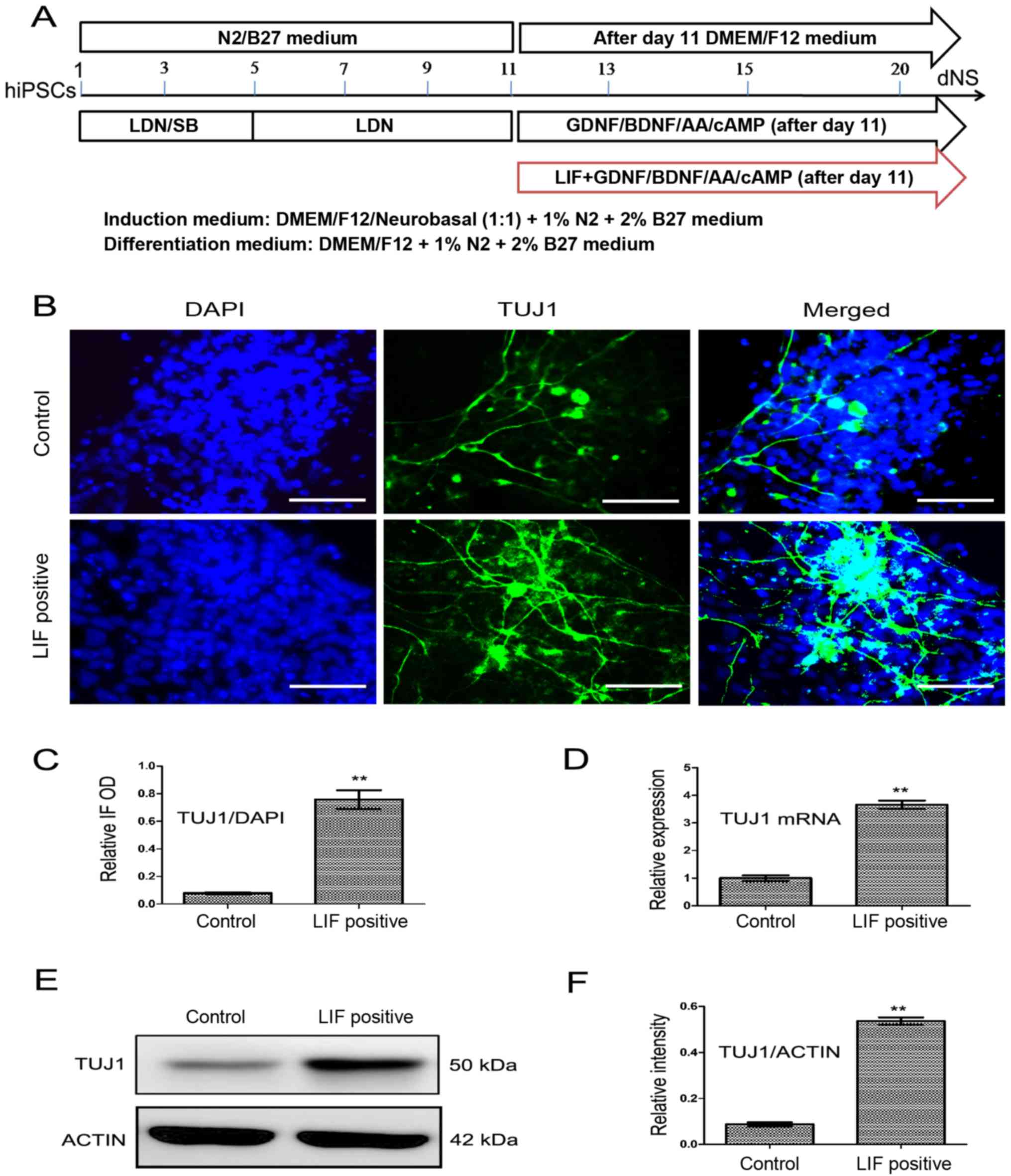 | Figure 4LIF improves the expression of TUJ1.
(A) The time course of neuronal differentiation in the presence or
absence of LIF. (B) The expression of TUJ1 (green) and DAPI (blue)
was observed by immunofluorescence on culture day 20 in the LIF
differentiation group and the control group. (C) The relative
fluorescence intensity value of TUJ1 was qualified by ImageJ
software and the data is expressed as the relative OD value
(TUJ1/DAPI). (D) The relative mRNA expression of TUJ1 was detected
by reverse transcription-quantitative polymerase chain reaction in
the LIF differentiation and control groups. (E) The TUJ1 protein
expression was detected by western blotting in the LIF
differentiation and control groups. (F) The relative band intensity
of TUJ1 was qualified by ImageJ software. Results are presented as
the mean ± standard deviation. **P<0.01. Scale bar,
20 μm. GDNF, glial cell line-derived neurotrophic factor;
BDNF, brain-derived neurotrophic factor; DMEM, Dulbecco's modifed
Eagle's medium; AA, ascorbic acid; cAMP, cyclic adenosine
monophosphate; LIF, leukocyte inhibitory factor; DAPI,
4′,6-diamidino-2-phenylindole; TUJ1, neuron-specific class III
β-tubulin; IF, immunofluorescence; OD, optical density. |
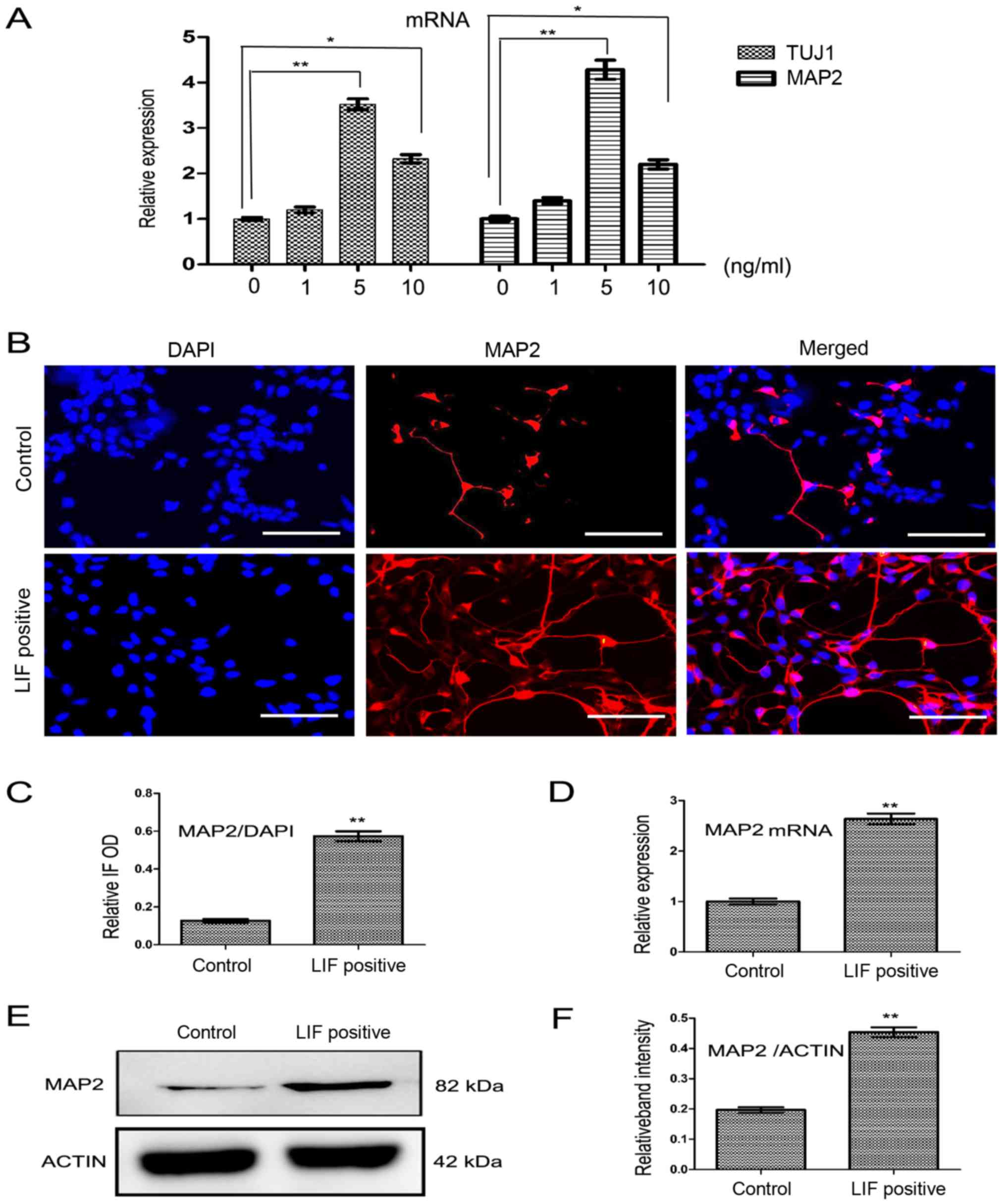 | Figure 5LIF enhances the expression of MAP2.
(A) The relative mRNA expression of TUJ1 and MAP2 on
culture day 20 in the different LIF concentration treated groups.
Results showed that, in the 5 ng/ml LIF differentiation group, the
mRNA expression of TUJ1 and MAP2 was strongest. (B)
The protein expression of MAP2 (red) and DAPI (blue) on culture day
20 was observed by immunofluorescence. (C) The relative
fluorescence intensity of MAP2 was qualified using ImageJ and the
data are expressed as the relative OD value (MAP2/DAPI). (D) The
relative mRNA expression of MAP2 was detected by reverse
transcription-quantitative polymerase chain reaction in the LIF
differentiation group and the control group. (E) The MAP2 protein
expression in the LIF differentiation group and the control group
was detected by western blotting. (F) The relative band intensity
of MAP2 protein was qualified by ImageJ software. Results are
presented as the mean ± standard deviation. *P<0.05
and **P<0.01. Scale bar, 20 μm. MAP2,
microtubule-associated protein 2; TUJ1, neuron-specific class III
β-tubulin; DAPI, 4′,6-diamidino-2-phenylindole; IF,
immunofluorescence; OD, optical density; LIF, leukocyte inhibitory
factor. |
LIF improves cell activity during
differentiation
On culture day 20, the cells were observed under
light microscope and the cell state (structure under the optical
microscope) was found to be better in the LIF-positive
differentiation group than that in the control group (Fig. 6A). The number of differentiated
neurons in the LIF-positive differentiation group was greater than
that of the control group counted on culture day 20 (Fig. 6B; P<0.05). The viability of the
cells was detected by CCK-8 method and it was found that the
viability of neurons in the LIF-positive differentiation group was
greater than that in the control group on culture day 20 (Fig. 6C; P<0.01). Apoptosis and flow
cytometry analyses showed that there were significantly less
apoptotic cells in the LIF differentiation group than in the
control differentiation group (Fig.
6D; P<0.05).
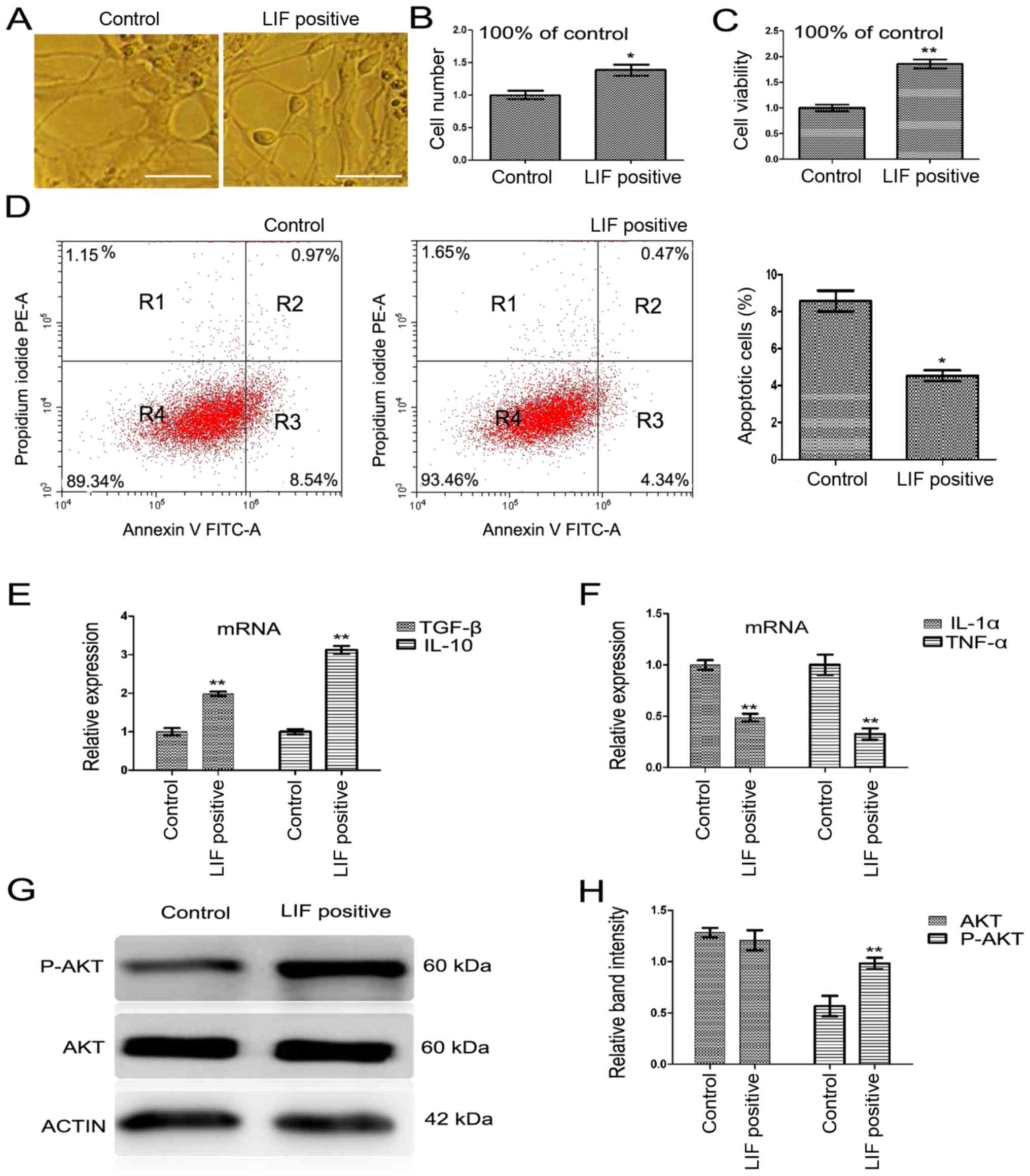 | Figure 6LIF affects the cell viability and
the mRNA expression of inflammatory cytokines. (A) The neurons
differentiated in LIF differentiation and control groups under
bright field microscopy on culture day 20. (B) The cell number was
counted on culture day 20. (C) The cell viability was measured by
cell counting kit-8 method on culture day 20. (D) Cells on culture
day 20 were labeled with Annexin V-FITC and PI. Cells in the R3
area were counted as apoptotic cells. (E) The relative mRNA
expression of the anti-inflammatory cytokines TGF-β and IL-10 in
LIF differentiation and control groups, and (F) the
pro-inflammatory cytokines IL-1α and TNF-α on culture day 20 was
detected by reverse transcription-quantitative polymerase chain
reaction. (G) The protein expression of AKT and p-AKT was analyzed
by western blotting. (H) The relative band intensity of AKT and
p-AKT was qualified by ImageJ software. Results are presented as
the mean ± standard deviation. *P<0.05 and
**P<0.01. Scale bar, 20 μm. LIF, leukocyte
inhibitory factor; FITC, fluorescein isothiocyanate; PI, propidium
iodide; PE, phycoerythrin; TGF-β, transforming growth factor-β; IL,
interleukin; TNF-α, tumor necrosis factor-α; p-AKT, phosphorylated
protein kinase B. |
LIF influences the mRNA expression of
inflammatory cytokines
Inflammation is a primary pathological driving force
of a number of neurodegenerative disorders (32); in the process of nervous system
injury, pro-inflammatory cytokines (IL-1β and TNF-α) are robustly
released, which may affect normal NPC differentiation, and lead to
a vast number of astrocytes and a diminished neural population. In
the present study, the expression of inflammation-related factors
was checked by RT-qPCR on culture day 20 when neurons were
differentiated from derived NPCs. The results showed that the
expression of the pro-inflammatory cytokines, including IL-1α and
TNF-α, was decreased, whilst the expression of anti-inflammatory
cytokines, including IL-10 and TGF-β, was increased in the
LIF-positive differentiation group compared with that in the
control differentiation group (Fig.
6E and F; all P<0.05).
LIF upregulates the expression of
p-AKT
A previous study showed that the inflammatory
reaction could affect the PI3K/AKT signaling pathway (33), and the activation of the PI3K/AKT
signaling pathway could be involved in the inflammatory reaction.
The present study found that in the LIF-positive differentiation
group, p-AKT, the key composition factor of PI3K/AKT, was
significantly increased (Fig. 6G and
H; P<0.01). This finding revealed that the improved neuronal
differentiation may be mediated by the PI3K/AKT signaling
pathway.
The effect of LIF could be prevented by
PI3K/AKT inhibitor LY294002
In order to confirm the effect of LIF on neuron
differentiation was mediated by the activation of the PI3K/AKT
signaling pathway. PI3K/AKT inhibitor LY294002 was used. Following
the addition of LY294002 during the differentiation period, the
protein expression of TUJ1 and MAP2 was downregulated compared with
that in the LIF differentiation group (Fig. 7A and B; both P<0.05).
Additionally, the expression of p-AKT was downregulated compared
with that in the LIF-positive group (Fig. 7C and D; P<0.05). Furthermore,
the mRNA expression of inflammatory cytokines IL-10, TGF-β, IL-1
and TNF-α was returned to a more normal level compared with that in
the LIF-positive differentiation group (Fig. 7E and F; all P<0.01). The number
and the viability of differentiated neurons decreased after the
addition of LY294002 compared with that in the LIF-positive
differentiation group (Fig. 7G and
H; both P<0.01).
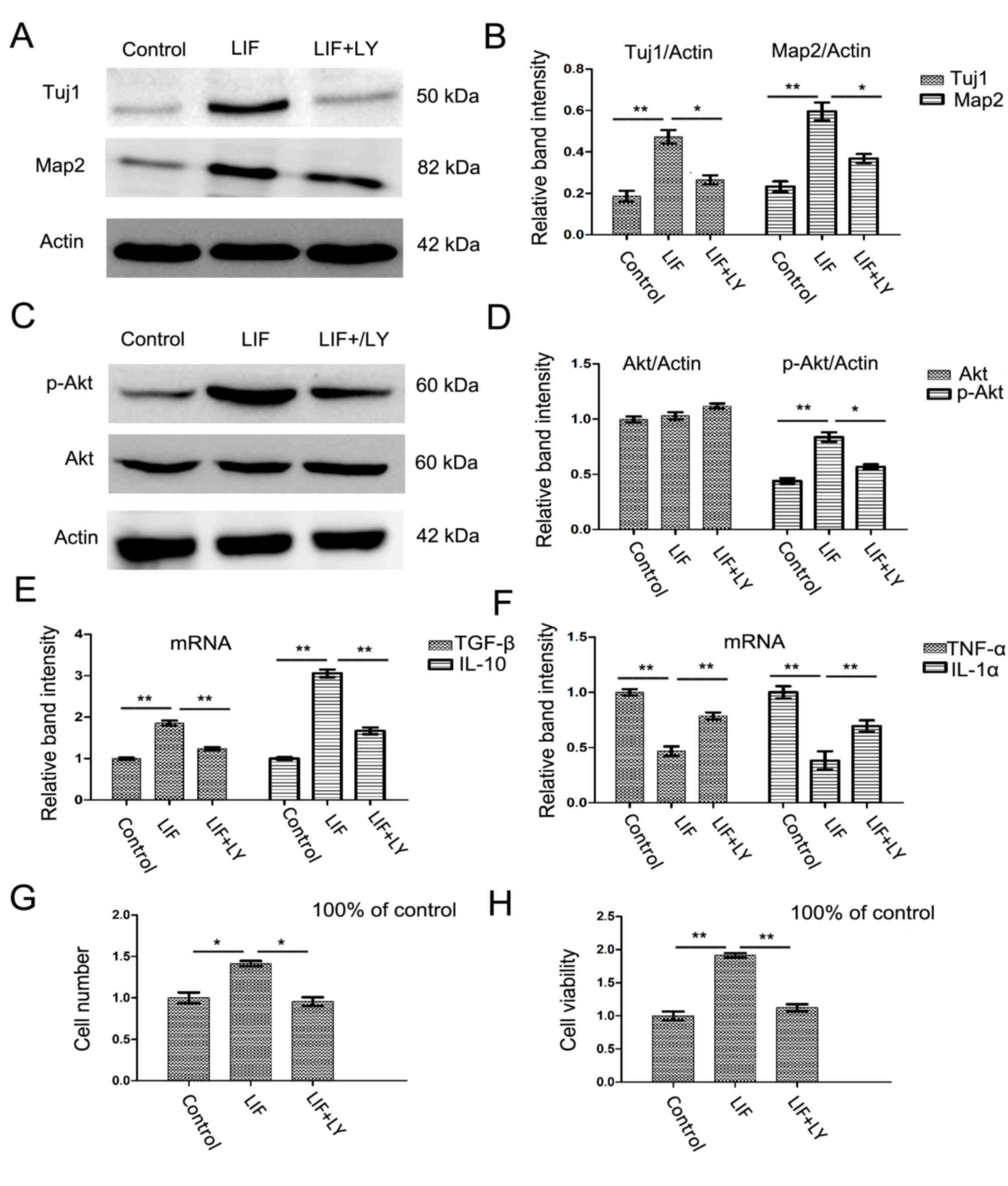 | Figure 7Inhibition of phosphatidylinositol
3-kinase/AKT by LY294002 prevents the LIF-induced changes in the
expression of neuronal markers and converts the mRNA expression of
inflammatory cytokines. (A) The protein expression of TUJ1 and MAP2
was examined on culture day 20 in control, LIF and LIF with
LY294002 differentiation group cells by western blotting. (B) The
relative band intensity of (A) was qualified by ImageJ software.
(C) The protein expression of AKT and p-AKT was detected on culture
day 20 in control, LIF and LIF with LY294002 differentiation group
cells by WB. (D) The relative band intensity of (C) was qualified
by ImageJ software. (E) The relative mRNA expression of
anti-inflammatory factors TGF-β and IL-10 on culture day 20 in
control, LIF and LIF with LY294002 differentiation group cells by
RT-qPCR. (F) The relative mRNA expression of pro-inflammatory
factors TNF-α and IL-1α on culture day 20 in control, LIF and LIF
with LY294002 differentiation group cells were detected by RT-qPCR.
(G) The number of neurons counted on culture day 20 in control, LIF
and LIF with LY294002 differentiation group cells. (H) The cell
viability was detected on culture day 20 in control, LIF and LIF
with LY294002 differentiation group cells by cell counting kit-8.
Results are presented as the mean ± standard deviation.
*P<0.05 and **P<0.01. p-AKT,
phosphorylated protein kinase B; LY, LY294002; LIF, leukocyte
inhibitory factor; MAP2, microtubule-associated protein 2; TUJ1,
neuron-specific class III β-tubulin; TGF-β, transforming growth
factor-β; IL, interleukin; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; TNF-α, tumor
necrosis factor-α. |
The effect of LIF could be prevented by
PI3K/AKT inhibitor wortmannin but not rapamycin
In order to confirm the effect of LIF on neuron
differentiation was mediated by the PI3K/AKT signaling
pathway. Another PI3K/AKT signaling pathway inhibitor,
wortmannin, and an mTOR signaling inhibitor, rapamycin, were used
here. Following the addition of wortmannin to the LIF-positive
differentiation group, it was found that the changes in the protein
expression of TUJ1 and MAP2 was similar to that found following the
addition of LY294002 (Fig. 8A and
B; P<0.05). However, this phenomenon was not found in the
rapamycin-treated group (Fig. 8C and
D; P<0.05). The changes in mRNA expression of inflammatory
cytokines IL-10, TGF-B, IL-1 and TNF-α in the wortmannin-treated
group were similar to those found in the LY294002-treated group,
compared with the LIF-positive differentiation group (Fig. 8G and H; P<0.05). However, this
phenomenon was not found in the rapamycin-treated differentiation
group compared with the LIF-positive differentiation group
(Fig. 8I and J).
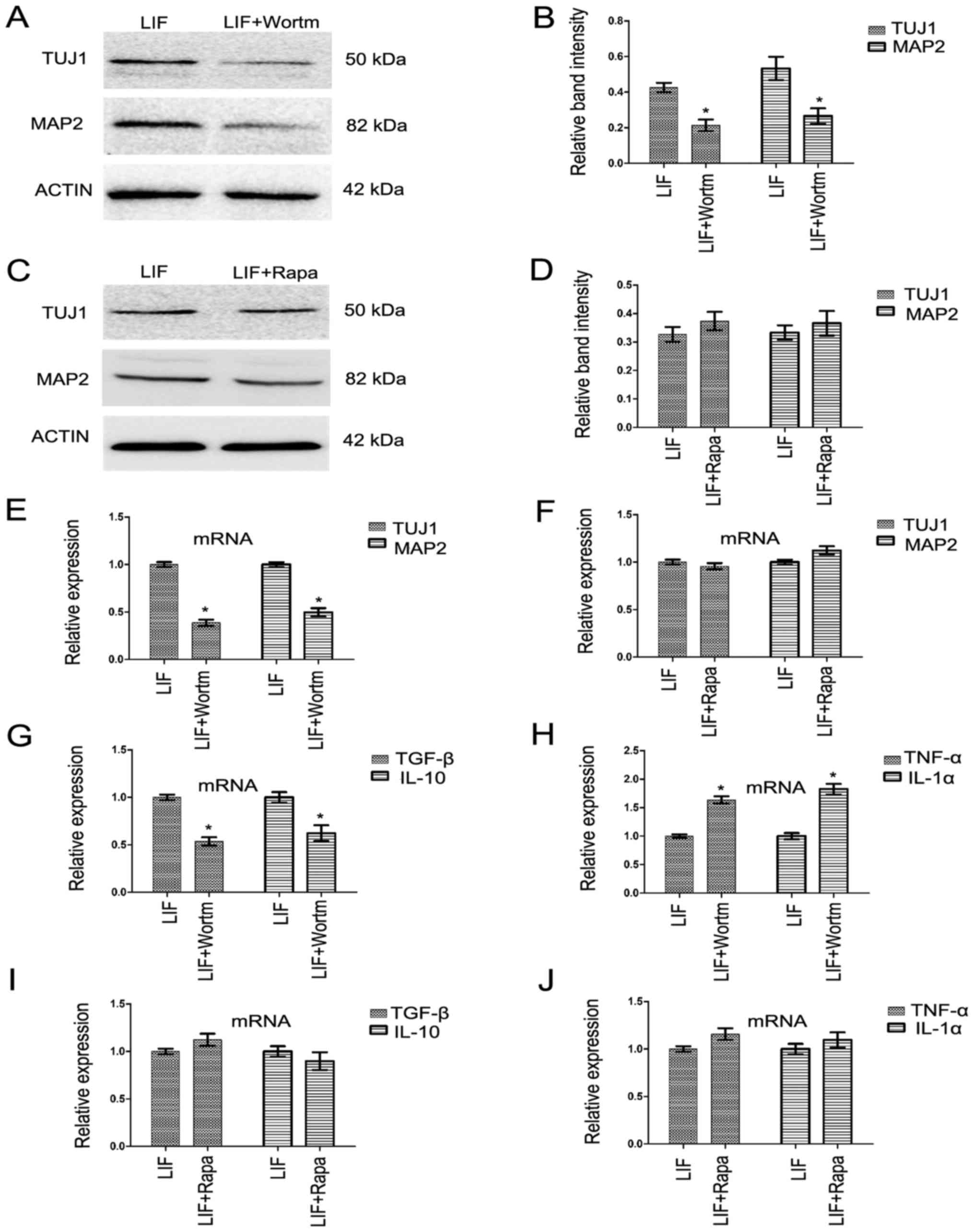 | Figure 8Inhibition of phosphatidylinositol
3-kinase/AKT by wortmannin could also prevent the LIF-induced
changes in the expression of neuronal markers and the mRNA
expression of inflammatory cytokines, but this effect was not found
in the rapamycin differentiation group. (A and B) The protein
expression of TUJ1 and MAP2 was examined by western blotting in LIF
and LIF combined with wortmannin differentiation groups. The
relative band intensity of TUJ1 and MAP2 was qualified by ΙmageJ
software. (C and D) The protein expression of TUJ1 and MAP2 was
detected by western blotting in LIF and LIF combined with rapamycin
differentiation groups. The relative band intensity of TUJ1 and
MAP2 was qualified by ImageJ software. (E) The relative mRNA
expression of TUJ1 and MAP2 on culture day 20 was detected by
RT-qPCR in LIF and LIF combined with wortmannin differentiation
groups. (F) The relative mRNA expression of TUJ1 and MAP2 on
culture day 20 was detected by RT-qPCR in LIF and LIF combined with
rapamycin differentiation groups. (G and H) The relative mRNA
expression of anti-inflammatory factors TGF-β and IL-10, and
pro-inflammatory factors TNF-α and IL-1α on culture day 20 was
detected by RT-qPCR in LIF and LIF combined with wortmannin
differentiation groups. (I and J) The relative mRNA expression of
anti-inflammatory factors TGF-β and IL-10, and pro-inflammatory
factors TNF-α and IL-1α on culture day 20 was detected by RT-qPCR
in LIF and LIF combined with rapamycin differentiation groups.
Results are presented as the mean ± standard deviation.
*P<0.05. AKT, protein kinase B; LY, LY294002; LIF,
leukocyte inhibitory factor; MAP2, microtubule-associated protein
2; TUJ1, neuron-specific class III β-tubulin; TGF-β, transforming
growth factor-β; IL, interleukin; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; TNF-α, tumor
necrosis factor-α; wortm, wortmannin; Rapa, rapamycin. |
Discussion
The transformation of hiPSCs into neural cells
provides one source of stem cells for the cell therapy of
neurodegenerative diseases, including Parkinson's disease and
Alzheimer's disease (34). A high
derivation quality and proportion of NPCs from hiPSCs is a crucial
step in the whole cell therapy procedure. There are several
induction methods to convert the pluripotent stem cells into NPCs,
relying on co-culture with stromal cells or initiating by the
formation of embryoid bodies (35,36). However, the low conversion
efficiency, the contamination of miscellaneous cells and the
complexity of experimental operation has made these methods
impractical. In the present study, the dual inhibition method of
neural induction used by Chambers et al (37) was meliorated using monolayer
culture and N2/B27 medium to induce neuron differentiation. During
the induction period, the BMP inhibitor LDN193189 and the TGF-β
inhibitor SB431542 were used as the induction factor. Under these
conditions, a high proportion of cells were converted into nerve
cells, which were defined as NPCs, expressing Nestin and PAX6. This
may be attributed to the induction inhibition of endodermal and
mesodermal cells by LDN193189 and SB431542. Neuronal
differentiation following successful induction of NPCs is another
key step for the cell treatment of diseases of the nervous system.
However, the low differentiation ratio and poor survival state
require improvement.
LIF, a member of the IL-6 superfamily, acts through
binding to its specific receptor, LIFR. It conscribes gp130 and
then an affinity receptor complex is formed, which can activate the
downstream pathways, including the PI3K/AKT, ERK1/2, JAK/STAT3 and
mTOR signaling pathways (12–15). LIF can regulate cell
proliferation, differentiation and phenotype; its main function is
inhibiting the differentiation of mESCs and promoting the
proliferation of muscle cells. Under certain conditions, LIF can
promote a neonatal rat dorsal root ganglion neuronal phenotype to
change from the adrenergic type to the cholinergic type. Therefore,
LIF is also known as cholinergic neuronal differentiation factor
(20). In addition, LIF can
promote the survival of cells differentiated from the transplanted
neural crest (38). Laterza et
al (39) revealed that
transplanted mouse iPSC-derived NPCs exert neuroprotection through
the secretion of LIF, which promotes the survival and
differentiation capacity of oligodendrocytes. However, the in
vitro effect of LIF on neuron differentiation is rare. In the
present study, a concentration of 5 ng/ml LIF was added to the
differentiation medium, and it was found to strongly improve the
expression of the neuronal markers TUJ1 and MAP2, and the
expression of p-AKT. p-AKT is a key composition of the PI3K/AKT
signaling pathway; it serves an important role in neuronal
proliferation and differentiation (18). Upregulating p-AKT could increase
the neuronal differentiation from mouse cochlear neural stem cells
(19). In the present study, the
PI3K/AKT signal was inhibited by its inhibitor LY294002 and
wortmannin in the LIF-positive differentiation group, and it was
found that the protein expression of neuron markers TUJ1 and MAP2
was returned to a similar level in the two inhibitor groups as the
control group. However, the same phenomenon was not found in the in
the rapamycin-treated group, This may suggest that the greater
neuronal differentiation effect in the LIF-positive differentiation
group compared with that in the control differentiation group may
occur through the activation of the PI3K/AKT signal, and that the
mTOR signaling inhibition caused by LY294002 inhibitor or rapamycin
may not participate in the greater neuronal differentiation and
anti-inflammatory effect during the neuron differentiation from
hiPSC-derived NPCs.
Anti-inflammation is one of the strategies to
promote neuron protection and cell survival; it can reduce
apoptosis and improve the survival of new derived neurons (25). The reaction of inflammation could
regulate the expression of LIF. Injecting LPS into the trachea of a
rat could induce the expression and secretion of LIF in
bronchoalveolar cells (27).
Meanwhile, inflammation-related cytokines such as IL-6 and TNF-α
could increase the mRNA or protein expression of LIF during the
cell culture (28,29). LIF upregulated the expression of
anti-inflammatory cytokines, including IL-10 and TGF-β, and
downregulated the expression of pro-inflammatory cytokines,
including IL-1α and TNF-α, during the neuronal differentiation in
the present study. Furthermore, LIF promoted the viability of the
neurons during the differentiation. These data suggest the
anti-inflammatory effect and cell survival effect of LIF on neuron
differentiation. However, the expression of inflammatory cytokines
and the cell viability were all reversed following the addition of
PI3K/AKT inhibitor LY294002 and wortmannin to the LIF-positive
differentiation group. Several studies revealed the involvement of
PI3K/AKT signaling in metabolic dysfunction and inflammation. For
example, furotrilliumoside prevented the LPS-induced upregulation
of PI3K/AKT, as a result of which the expression of inflammatory
cytokines TNF-α, IL-6 and IL-1β decreased (40). However, salvianolic acid A, a
chemical type of caffeic acid trimer, blocks inflammatory responses
through the activation of PI3K/AKT signaling (41). The present results suggested that
the effect of LIF on inflammation and cell viability may be through
the activation of PI3K/AKT signaling.
In conclusion, by use of a monolayer culture method,
and N2/B27 basic medium combined with BMP inhibitor LDN193189 and
TGF-β inhibitor SB431542 during neural induction, a large
proportion of derived NPCs was harvested. During the neuronal
differentiation period from the derived NPCs, LIF can improve the
differentiation by upregulating the protein expression of MAP2 and
TUJ1, and increasing the number of TUJ1 and MAP2-positive neurons,
which may be through the activation of PI3K/AKT signaling. In
addition to this, LIF could exert the anti-inflammatory effect and
improve the cell viability through activation of PI3K/AKT during
neuronal differentiation. These data suggest that LIF serves an
important role in the differentiation of neurons in vitro
and may have a prospective application in the stem cell treatment
of central nervous system diseases.
Acknowledgments
The authors appreciate the support of the Natural
Science Foundation of Guangdong Province, China (grant no.
2016A030313584), the Characteristic Innovation Foundation of
Innovative and Strong University Project of Guangdong Province,
China (Education and Science Missive of Guangdong Province; grant
no. 2014-65) and the Medical Research foundation of Guangdong
Province, China (grant no. B1432023). The authors would like to
thank Professor Lan Feng for providing the hiPSCs and H9 cell
lines.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests
References
|
1
|
Shiba Y, Gomibuchi T, Seto T, Wada Y,
Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, et
al: Allogeneic transplantation of iPS cell-derived cardiomyocytes
regenerates primate hearts. Nature. 538:388–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanna JH, Saha K and Jaenisch R:
Pluripotency and cellular reprogramming: facts, hypotheses,
unresolved issues. Cell. 143:508–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X,
Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, et al: Synaptic
dysregulation in a human iPS cell model of mental disorders.
Nature. 515:414–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bakken TE, Miller JA, Ding SL, Sunkin SM,
Smith KA, Ng L, Szafer A, Dalley RA, Royall JJ, Lemon T, et al: A
comprehensive transcriptional map of primate brain development.
Nature. 535:367–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charrier JB, Lapointe F, Le Douarin NM and
Teillet MA: Dual origin of the floor plate in the avian embryo.
Development. 129:4785–4796. 2002.PubMed/NCBI
|
|
6
|
Yan Y, Bejoy J, Xia J, Guan J, Zhou Y and
Li Y: Neural patterning of human induced pluripotent stem cells in
3-D cultures for studying biomolecule-directed differential
cellular responses. Acta Biomater. 42:114–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brennand K, Savas JN, Kim Y, Tran N,
Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran
I, et al: Phenotypic differences in hiPSC NPCs derived from
patients with schizophrenia. Mol Psychiatry. 20:361–368. 2015.
View Article : Google Scholar
|
|
8
|
Davila J, Chanda S, Ang CE, Südhof TC and
Wernig M: Acute reduction in oxygen tension enhances the induction
of neurons from human fibroblasts. J Neurosci Methods. 216:104–109.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu F, Xuan A, Chen Y, Zhang J, Xu L, Yan
Q and Long D: Combined effect of nerve growth factor and brain
derived neurotrophic factor on neuronal differentiation of neural
stem cells and the potential molecular mechanisms. Mol Med Rep.
10:1739–1745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Metcalfe SM: LIF in the regulation of
T-cell fate and as a potential therapeutic. Genes Immun.
12:157–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue X, Wu L and Hu W: The regulation of
leukemia inhibitory factor. Cancer Cell Microenviron.
2:e8772015.
|
|
12
|
Burdon T, Smith A and Savatier P:
Signalling, cell cycle and pluripotency in embryonic stem cells.
Trends Cell Biol. 12:432–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pera MF and Tam PP: Extrinsic regulation
of pluripotent stem cells. Nature. 465:713–720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Yang Q, Yu H, Wu L, Zhao Y, Zhang C,
Yue X, Liu Z, Wu H, Haffty BG, et al: LIF promotes tumorigenesis
and metastasis of breast cancer through the AKT-mTOR pathway.
Oncotarget. 5:788–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu SC, Tsang NM, Chiang WC, Chang KP,
Hsueh C, Liang Y, Juang JL, Chow KP and Chang YS: Leukemia
inhibitory factor promotes nasopharyngeal carcinoma progression and
radioresistance. J Clin Invest. 123:5269–5283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanda M, Nagai T, Takahashi T, Liu ML,
Kondou N, Naito AT, Akazawa H, Sashida G, Iwama A, Komuro I, et al:
Leukemia inhibitory factor enhances endogenous cardiomyocyte
regeneration after myocardial infarction. PLoS One.
11:e01565622016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laszlo GS and Nathanson NM: Src family
kinase-independent signal transduction and gene induction by
leukemia inhibitory factor. J Biol Chem. 278:27750–27757. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morell C, Bort A, Vara D, Ramos-Torres A,
Rodríguez-Henche N and Díaz-Laviada I: The cannabinoid WIN 55,212-2
prevents neuroendocrine differentiation of LNCaP prostate cancer
cells. Prostate Cancer Prostatic Dis. 19:248–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, He Q, Dong J, Jia Z, Hao F and
Shan C: Effects of epigallocatechin-3-gallate on proliferation and
differentiation of mouse cochlear neural stem cells: involvement of
PI3K/Akt signaling pathway. Eur J Pharm Sci. 88:267–273. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murphy M, Reid K, Hilton DJ and Bartlett
PF: Generation of sensory neurons is stimulated by leukemia
inhibitory factor. Proc Natl Acad Sci USA. 88:3498–3501. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y and Zang D: The neuron regrowth is
associated with the proliferation of neural precursor cells after
leukemia inhibitory factor administration following spinal cord
injury in mice. PLoS One. 9:e1160312014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin C, Lin HY, Chen JH, Tseng WP, Ko PY,
Liu YS, Yeh WL and Lu DY: Effects of paeonol on
anti-neuroinflammatory responses in microglial cells. Int J Mol
Sci. 16:8844–8860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR,
Zeng YL, Li SN, Huang BX, Lv QK, Wang W, et al: Anti-inflammatory
effects of BHBA in both in vivo and in vitro Parkinson's disease
models are mediated by GPR109A-dependent mechanisms. J
Neuroinflammation. 12:2015. View Article : Google Scholar
|
|
24
|
Jing Yh, Hou Yp, Song Yf and Yin J:
Methylprednisolone improves the survival of new neurons following
transient cerebral ischemia in rats. Acta Neurobiol Exp (Wars).
72:240–252. 2012.
|
|
25
|
Tentillier N, Etzerodt A, Olesen MN,
Rizalar FS, Jacobsen J, Bender D, Moestrup SK and Romero-Ramos M:
Anti-inflammatory modulation of microglia via CD163-targeted
glucocorticoids protects dopaminergic neurons in the 6-OHDA
Parkinson's disease model. J Neurosci. 36:9375–9390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pal R, Tiwari PC, Nath R and Pant KK: Role
of neuroinflammation and latent transcription factors in
pathogenesis of Parkinson's disease. Neurol Res. 38:1111–1122.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ulich TR, Fann MJ, Patterson PH, Williams
JH, Samal B, Del Castillo J, Yin S, Guo K and Remick DG:
Intratracheal injection of LPS and cytokines. V. LPS induces
expression of LIF and LIF inhibits acute inflammation. Am J
Physiol. 267:L442–L446. 1994.PubMed/NCBI
|
|
28
|
Knight DA, Lydell CP, Zhou D, Weir TD,
Robert Schellenberg R and Bai TR: Leukemia inhibitory factor (LIF)
and LIF receptor in human lung. Distribution and regulation of LIF
release. Am J Respir Cell Mol Biol. 20:834–841. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palmqvist P, Lundberg P, Lundgren I,
Hänström L and Lerner UH: IL-1beta and TNF-alpha regulate IL-6-type
cytokines in gingival fibroblasts. J Dent Res. 87:558–563. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong Q, Zhang H, Zhao T, Zhang W, Yan M,
Dong X and Li P: Tangshen formula attenuates hepatic steatosis by
inhibiting hepatic lipogenesis and augmenting fatty acid oxidation
in db/db mice. Int J Mol Med. 38:1715–1726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
32
|
Chen E, Xu D, Lan X, Jia B, Sun L, Zheng
JC and Peng H: A novel role of the STAT3 pathway in brain
inflammation-induced human neural progenitor cell differentiation.
Curr Mol Med. 13:1474–1484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YH, Fu HL, Tian ML, Wang YQ, Chen W,
Cai LL, Zhou XH and Yuan HB: Neuron-derived FGF10 ameliorates
cerebral ischemia injury via inhibiting NF-κB-dependent
neuroinflammation and activating PI3K/Akt survival signaling
pathway in mice. Sci Rep. 6:198692016. View Article : Google Scholar
|
|
34
|
Chau MJ, Deveau TC, Song M, Gu X, Chen D
and Wei L: iPSC transplantation increases regeneration and
functional recovery after ischemic stroke in neonatal rats. Stem
Cells. 32:3075–3087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen C, Wang Y, Goh SS, Yang J, Lam DH,
Choudhury Y, Tay FC, Du S, Tan WK, Purwanti YI, et al: Inhibition
of neuronal nitric oxide synthase activity promotes migration of
human-induced pluripotent stem cell-derived neural stem cells
toward cancer cells. J Neurochem. 126:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meneghini V, Frati G, Sala D, De Cicco S,
Luciani M, Cavazzin C, Paulis M, Mentzen W, Morena F, Giannelli S,
et al: Generation of human induced pluripotent stem cell-derived
bona fide neural stem cells for ex vivo gene therapy of
metachromatic leukodystrophy. Stem Cells Transl Med. 6:352–368.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chambers SM, Qi Y, Mica Y, Lee G, Zhang
XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, et al: Combined
small-molecule inhibition accelerates developmental timing and
converts human pluripotent stem cells into nociceptors. Nat
Biotechnol. 30:715–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kirby ML, Kumiski DH, Myers T, Cerjan C
and Mishima N: Backtransplantation of chick cardiac neural crest
cells cultured in LIF rescues heart development. Dev Dyn.
198:296–311. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Laterza C, Merlini A, De Feo D, Ruffini F,
Menon R, Onorati M, Fredrickx E, Muzio L, Lombardo A, Comi G, et
al: iPSC-derived neural precursors exert a neuroprotective role in
immune-mediated demyelination via the secretion of LIF. Nat Commun.
4:25972013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan T, Yu X, Sun X, Meng D and Jia JM: A
new steroidal saponin, furotrilliumoside from Trillium tschonoskii
inhibits lipopolysaccharide-induced inflammation in Raw264.7 cells
by targeting PI3K/Akt, MARK and Nrf2/HO-1 pathways. Fitoterapia.
115:37–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chien MY, Chuang CH, Chern CM, Liou KT,
Liu DZ, Hou YC and Shen YC: Salvianolic acid A alleviates ischemic
brain injury through the inhibition of inflammation and apoptosis
and the promotion of neurogenesis in mice. Free Radic Biol Med.
99:508–519. 2016. View Article : Google Scholar : PubMed/NCBI
|