Introduction
Essential hypertension is considered to be a highly
prevalent pathological condition contributing to the morbidity of
crucial risk factors such as cardiovascular disease,
cerebrovascular disease, chronic renal failure and increased
mortality (1). According to
statistics, approximately 90–95% of the cases of hypertension
affecting more than 2 billion adults worldwide are categorized as
the essential subtype (2). To
date, a large amount of evidence has shown that vasoactive
substances such as urotensin II (3,4),
endothelin (5,6), and adrenomedullin (7,8)
play a major role in the pathophysiology of essential hypertension
(9). Therefore, the study of the
pathogenesis of hypertension is substantial. Essential hypertension
is a multifactorial disease caused by the combined action of
several genetic, environmental, and behavioral factors (10). Some studies have attempted to
identify the genetic abnormalities (11). However, these results are only
'the tip of the iceberg' in the research of a new field concerning
the pathogenesis of hypertension.
MicroRNAs (miRNAs or miRs) are 22-nucleotide
non-coding RNA molecules that regulate the expression of other
genes by inhibiting translation or cleaving of complementary target
messenger RNAs (12), thereby
providing a mechanism for protein dose regulation. Five years ago,
the discovery of the first miRNA gene, lin-4, in the nematode
Caenorhabditis elegans (13), rapidly unraveled the field of
miRNA biology. Since then many more of these short regulatory RNA
genes have been identified in flowering plants, worms, flies, fish,
frogs, and mammals (12).
Currently, about 2% of the known human genes encode miRNAs
(14).
miRNAs are transcribed as long RNA precursors
(pri-miRNAs) that contain a stem-loop structure of approximately 80
bases (15). Pri-miRNAs are
processed in the nucleus by the RNase III enzyme Drosha and
DGCR8/Pasha (16), which excise
the stem-loop to form the pre-miRNA. These RNA molecules are then
exported from the nucleus by Exportin-5 (17) to the cytoplasm, wherein another
RNase III enzyme, Dicer, cuts the pre-miRNA to generate the mature
miRNA as a part of a short RNA duplex. Subsequently, the RNA is
unwound by helicase activity and incorporated into an RNA-induced
silencing complex (RISC) (18).
The RISC directs the gene silencing, where it is enabled to target
specific mRNAs through complementary sequences in the
3′-untranslated regions (3′-UTRs) (19,20). The perfect base pairing triggers
mRNA degradation through a mechanism similar to that operating
during RNA interference (RNAi) induced by small interfering RNAs
(siRNAs). On the other hand, miRNAs regulate gene expression by
imperfect base pairing to the 3′-UTR of target mRNAs and causing
mRNA degradation or inhibiting protein synthesis (21).
The miRNAs expressing in specific tissues consitute
a predominant population, which suggests that the miRNAs are highly
tissue-specific (22). miR-126 is
an endothelial cell-specific miRNA (20). Recent studies have demonstrated
the vital roles for miRNAs as a response to injury and stress to
the cardiovascular system. This characteristic illustrates that a
single miRNA can regulate vascular integrity and angiogenesis
(20,23). miR-126 was found to reduce
atherosclerosis (24), and can
regulate adhesion molecule expression, thereby further controlling
vascular inflammation (25). Ding
et al demonstrated that the levels of inflammation are
significantly enhanced in the early stage of hypertension (26).
Some researches have previously investigated the
gene functions associated with hypertension and high blood pressure
leading to organ damage (27,28). Previous studies have shown that
miR-126 is involved in cardiovascular disease by modulating the
transition of endothelial progenitor cells, which is indispensable
for the occurrence of hypertension (29–31). Thus, further research by us
focused on the relationship between miR-126 and the pathogenesis of
hypertension in animal experiments. In the present study, the
research process and results of animal experiments are
reported.
miR-126 is closely related to endothelial function,
and endothelial dysfunction is indispensable for the occurrence of
hypertension. Therefore, we hypothesized that dysregulated miR-126
expression impairs endothelial function, resulting in hypertension.
However, relevant data have not yet been reported.
RNA interference (RNAi) occurs in normal individuals
as a phenomenon of specific inhibition of a target gene. When
double-stranded RNAs (dsRNAs) homologous to the endogenous mRNA
coding region are introduced into cells, the mRNA degrades
resulting in specific gene silencing, thus, playing an important
role in cell culture and in vivo studies. Based on the above
principles, the present study utilized the lentiviral vector system
to integrate the antisense oligonucleotides of miR-126, and thereby
knock down the miR-126 expression to treat hypertension through
gene therapy strategy.
Taken together, miR-126 is crucial for endothelial
function. Since hypertension is closely associated with endothelial
dysfunction, we speculated that dysfunctional endothelium caused by
abnormal miR-126 expression may play a pivotal role in primary
hypertension. Our preliminary investigation used serum samples from
4 healthy individuals and 6 patients with essential hypertension
who were comparable in age, sex, and weight. We also found that
miR-126 expression was upregulated in the patients with
hypertension compared with the healthy controls. Moreover, although
miR-126 down-regulation through RNAi is theoretically feasible, no
evidence has yet been shown for miR-126 application in hypertension
therapy. To the best of our knowledge, the present study for the
first time elucideated the relationship between miR-126 and human
hypertension and is also the first in vivo assessment of the
role of miR-126 in hypertension.
Materials and methods
A total of 4 healthy individuals and 6 patients with
essential hypertension from Beijing Chao-Yang Hospital (Beijing,
China) were selected for assessment of miRNA expression in our
preliminary experiment who were comparable in age, sex, and weight.
The present study was approved by the Ethics Committee of Beijing
Chaoyang Hospital (reference no. 2009-S-1). All the patients
provided written informed consent for the use of their samples for
scientific research prior to enrollment. A total of 23
spontaneously hypertensive rats (SHR), 8 weeks of age, were
obtained from Capital Medical University Laboratory Animals
Research Center (Beijing, China) and acclimatized in a controlled
room at 21±2°C and 60±5% humidity in which a 12:12 h light:dark
cycle was maintained. All the animals were maintained under
specific pathogen-free conditions. The study was approved by the
Animal Ethics Committee (reference no. 2012/A-99) and was conducted
in accordance with the 'Animal Welfare Act and the Guide for the
Care and Use of Laboratory Animals (NIH publication no. 5377-3,
1996)'.
Instruments
The instruments used included rat tail-artery
non-invasive blood pressure tail-cuff apparatus (BP-98A; Softron,
Tokyo, Japan), inverted fluorescence microscope BX51, polarization
microscope BH2 (both from Olympus, Tokyo, Japan) and GenePix 4000B
Microarray Scanner (Axon Instruments, Union City, CA, USA).
Reagents
The reagents were purchased as follows: TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA), RNeasy Mini kit
(Qiagen, Inc., Valencia, CA, USA), microRNA-126 gene knockdown
lentiviral vector, microRNA-126 interference control lentiviral
vector (both from Shanghai Shengbo Biotech Co., Ltd., Shanghai,
China), nitric oxide (NO) kit (R&D Systems, Minneapolis, MN,
USA), Sirius red F2B stain in carbazotic acid (Sigma, St. Louis,
MO, USA), and ELISA kit (R&D Systems).
miRCURY LNA™ microRNA arrays
Our preliminary investigation reserved the blood
samples from healthy individuals and patients with essential
hypertension. Total RNA was harvested using TRIzol and RNeasy Mini
kit according to the manufacturer's instructions. After having
undergone RNA measurement on the NanoDrop instrument, the samples
were labeled using the miRCURY™ Hy3™/Hy5™ Power Labeling kit and
hybridized on the miRCURY™ LNA Array (v.11.0). Scanning was
performed with the Axon GenePix 4000B Microarray scanner. GenePix
Pro v6.0 (Molecular Devices LLC, USA) was used to read the raw
intensity of the image.
Construction of the miR-126 gene
knockdown lentiviral vector (lenti-miR-126-KD)
Cloning and vector construction was carried out as
previously described (19). The
rno-mir-126 mature sequence was >rno-miR-126 MIMAT0000832;
UCGUACCGU GAGUAAUAAUGCG. For gene knockdown, the target gene
expression was driven by the U6 promoter. A lentiviral vector for
miR-126 gene knockdown was constructed by U6-promoter-driven target
gene expression. The target sequence was anti-sense and
complementary to the mature miR-126. Primers included: MS2117_1,
CCGGCGCATTATTACTCACGGTACGATTTTTTG; MS2117_2,
AATTCAAAAAATCGTACCGTGAGTAATAATGCG. For scramble-miR design, a
non-specific DNA sequence was inserted after the U6 promoter. For
annealing of the primer pairs, pMagic 4.0 vector was constructed,
and MS2117_1 and MS2117_2 primers were used to evaluate the gene
knockdown. The scramble-miR was not identified. Vectors were sent
for sequencing, and titers of the lentiviral vector were
estimated.
miR-126 gene knockdown for hypertension
therapy
For the experimental groups, 23 SHRs were randomly
divided into 4 groups. The lentiviral vectors were diluted in PBS
to a final volume of 350 μl and injected through the tail
vein into the rats. The high dose group (HD) received
lenti-miR-126-KD (1×108) injection, n=5. The low dose
group (LD) received lenti-miR-126-KD (1×107) injection,
n=6. The scramble miR group received lenti-scramble-miR
(5×107) injection, n=6. As a control, PBS was injected
into each mouse, n=6.
In regards to the observation index of the
experimental animals, the rat tail artery non-invasive blood
pressure (BP-98A; Softron) was used to concurrently measure the
tail artery systolic pressure, mean blood pressure, diastolic blood
pressure, and heart rate in a quiet environment when the rats were
awake. The blood pressure of each rat was tested at least 3 times
consecutively to obtain an average. Eight weeks of continuous
observation was performed, and body weights before and after the
dosage were recorded.
In regards to the observation index of organs and
blood, heart and body ratio (Hw/Bw), 3% of pentobarbital sodium was
administered at a dose of 0.2 g/100 g body weight as anesthesia to
the rats. The heart weight was measured on electronic scales and
Hw/Bw was calculated. For tissue sections, the rats were
anesthetized using a compound anesthetic (3% pentobarbital sodium
at a dose of 30 mg pentobarbital/kg animal body weight). After the
removal of the tissues, the rats were sacrificed. For perfusion,
the syringe needle penetrated the left ventricle of the heart and
opened up the right auricle. A total of 0.9% saline was used for
perfusion until a clear effluent was recovered. For fixation, the
perfusion was rapidly carried out through the left ventricle of the
heart followed by slow perfusion for ~30 min. The heart, liver,
kidneys, lungs and brain were excised from each animal. A total of
36 h of sedimentation was followed by 4% paraformaldehyde fixation
in 30% sucrose solution. The brain tissue was directly embedded
into n-hexane for 25–26 sec for quick freezing. Regarding the
tissue sections, brain tissue was sectioned at the 20 μm
thickness, whereas all the other tissues were sectioned at a
thickness of 14 μm. Green fluorescence was monitored under a
fluorescence microscope for the detection of successful
transfection. H&E staining and occasionally Sirius red F2B in
carbazotic acid staining were also performed for heart and kidney
histomorphology. For collagen specificity by polarization
microscopy, type I collagen fibers were arranged intimately,
showing strong birefringence, with yellow or red fibers; type II
collagen fiber showed weak birefringence, loose mesh distribution
with multiple colors; type III collagen fiber also showed weak
birefringence, with green fine fibers; type IV collagen fiber
exhibited yellow basilemma with weak birefringence. The scale of
fibrosis of each group could thus be observed directly and compared
to the degree of injury in the target organs of the SHRs.
Roles of miR-126 in hypertension
pathogenesis and progression
Serum sample preparation
A total of 3% of pentobarbital sodium was used at a
dose of 0.2 g/100 g body weight for rat anesthesia. A total of 6–7
ml whole blood sample was withdrawn from the cardiac apex before
the removal of the tissues by thoracotomy and transferred into
yellow capped tubes. The serum samples were collected by
centrifugation for 15–20 min at 3,000 rpm.
NO level test
ELISA kit was used, following the manufacture's
protocol, to estimate the NO levels.
Biochemical measurements
ELISA was performed to estimate ALB, AST, ALT, CHOL,
HDL-C, LDL-C, TG, BUN and Cr (38 parameters in total). The
differences in the biochemical indices among groups were
compared.
Statistical analysis
Data were analyzed by SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA) and GraphPad Prism 4.0 statistical package.
Measurement data are represented as mean ± standard deviation (mean
± SD). Normality and homogeneity of variance were tested before
comparison: data that fulfilled the normal distribution and
homogeneity of variance were analyzed by one-way analysis of
variance; for data with the heterogeneity of variance, the
logarithm of normality and homogeneity was used for the variance
test. Data that did not fulfill the homogeneity criteria were
analyzed by Kruskal-Wallis non-parametric test. P<0.05 and
P<0.01 were considered to indicate a statistically significant
difference.
Results
miRNA-126 expression level in
hypertension
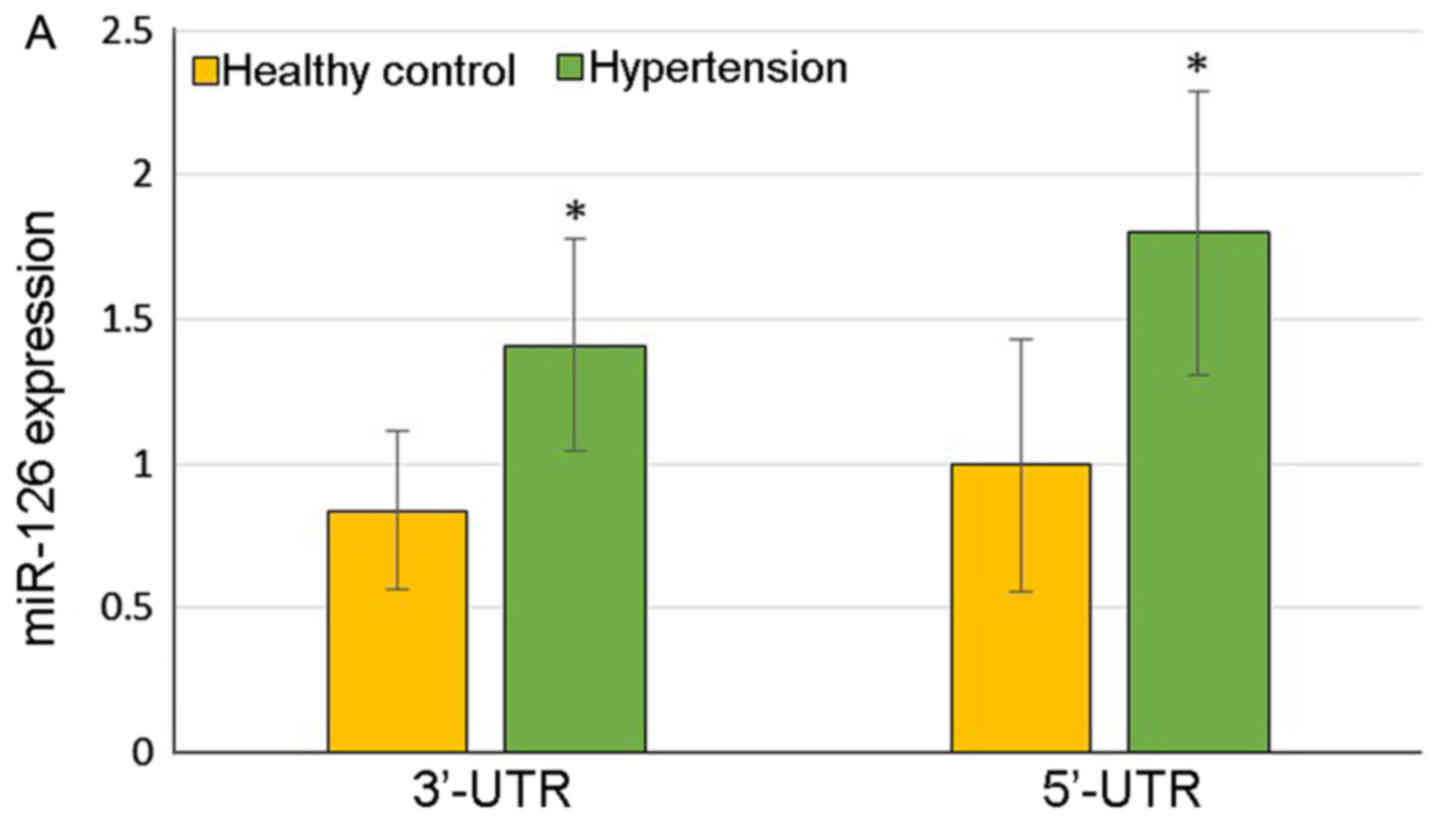
The hypertensive patients demonstrated significantly
higher expression of 3′-UTR of miR-126 (1.410±0.369 vs.
0.838±0.274, P=0.03) and 5′-UTR of miR-126 (1.799±0.490 vs.
0.997±0.437, P=0.03) compared with the healthy controls (Fig. 1A). At the same time, the color of
the heat map diagram scale shown at the top illustrates the
relative expression level of an miRNA in a certain slide: red color
represents a higher expression level and green color represents a
lower expression level, indicating that miR-126 was upregulated in
the patients with hypertension (Fig.
1B).
Construction of the lentiviral vector for
miR-126 gene knockdown
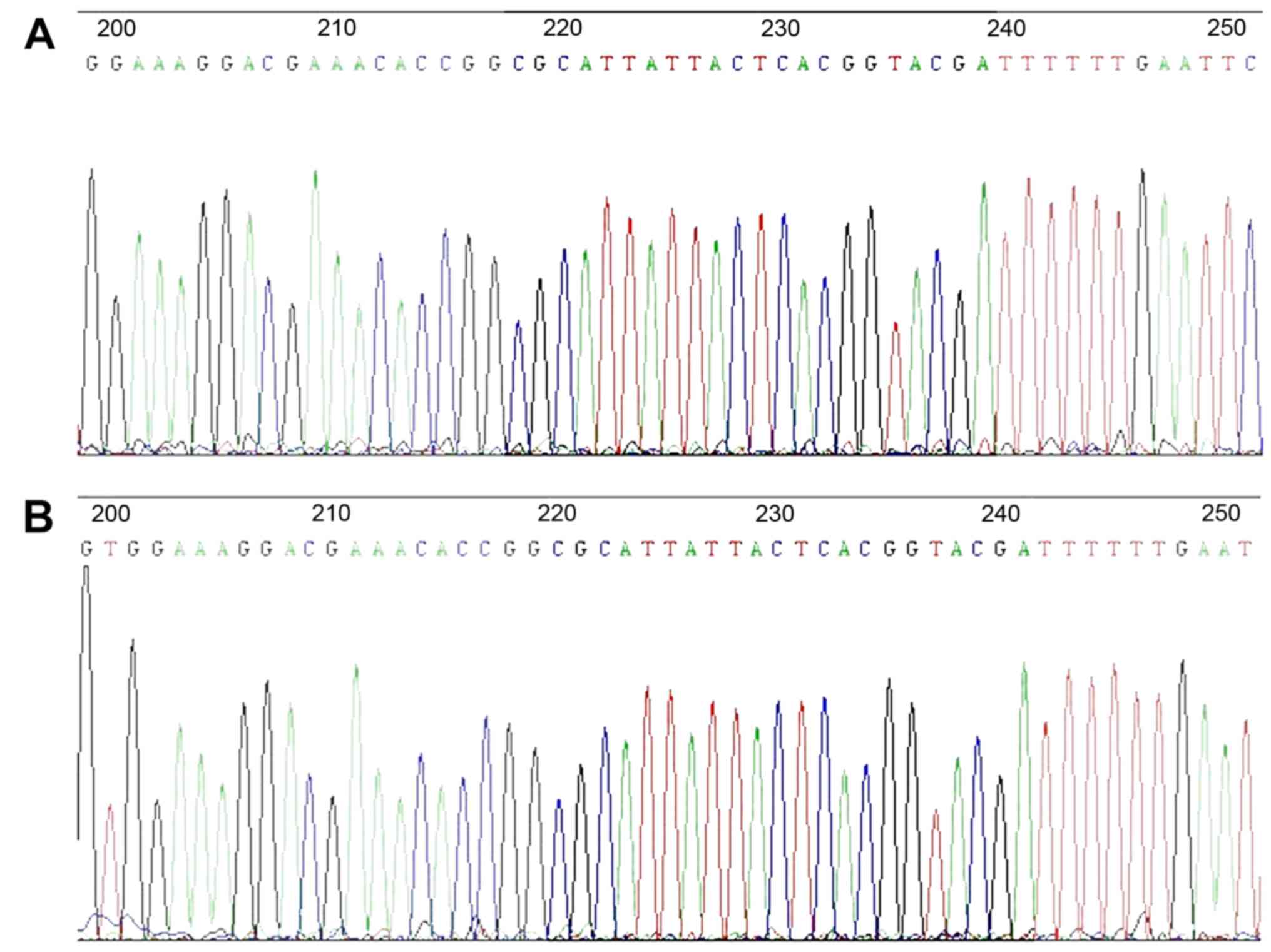
The DNA sequencing result for the lentiviral vector
for miRNA-126 gene knockdown is demonstrated in Fig. 2. The highlighted areas indicate
the antisense sequence complementary to the mature miR-126:
5′-CGCATTATTACTCACGGTACGA-3′, indicating successful construction of
the lentiviral vector.
miR-126 gene knockdown for hypertension
treatment
Blood pressure and heart rate
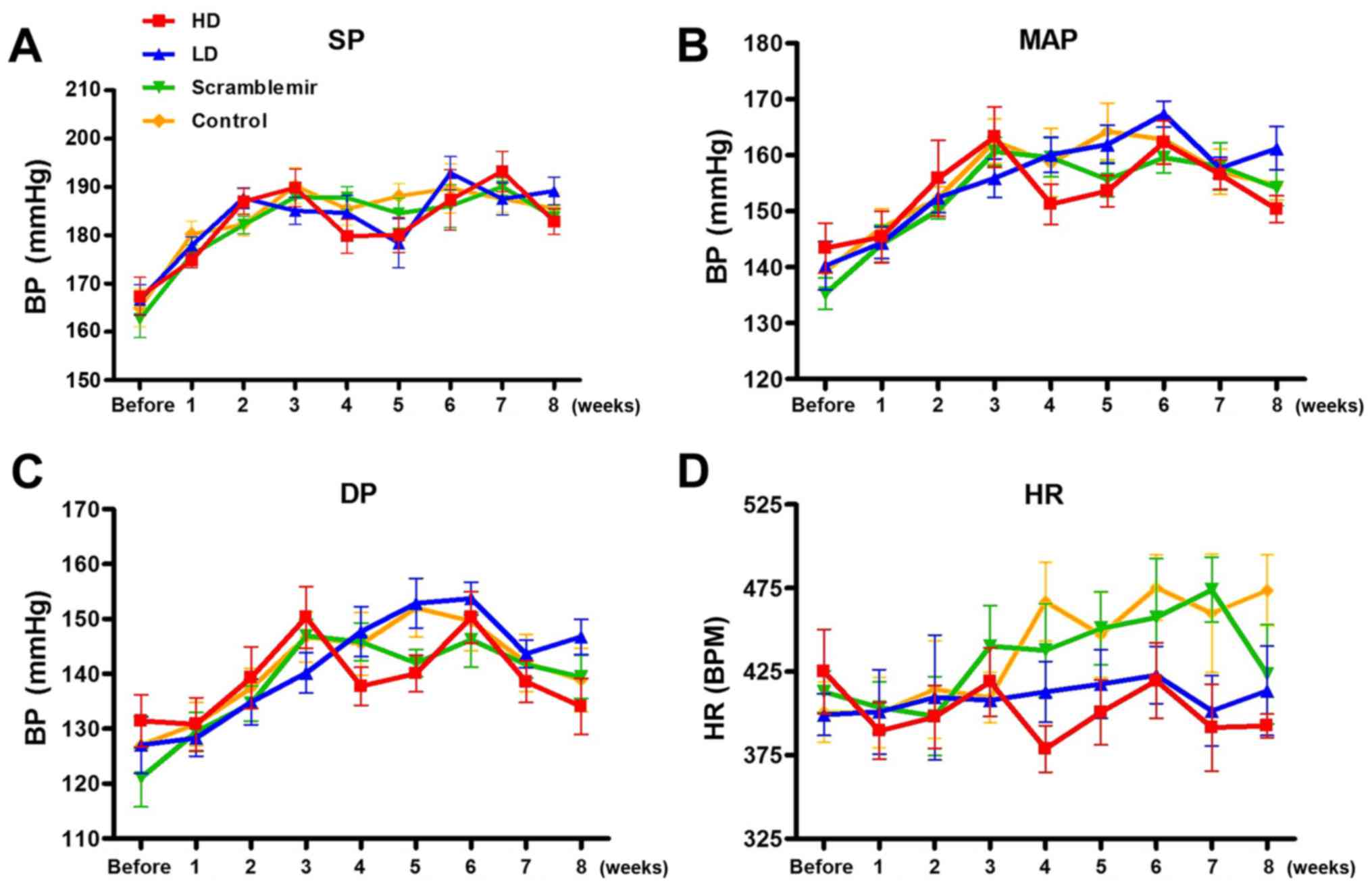
After tail vein injection, changes in the systolic
pressure, mean blood pressure, diastolic blood pressure, and heart
rate with time were continuously monitored for 8 weeks. The results
are summarized in Tables
I–IV, and plots are
represented in Fig. 3. A
statistical significant difference was neither observed in the
blood pressure among each SHR group before dose (P>0.05), nor at
each time point after the drug administration (P>0.05),
suggesting that the blood pressure was not affected by dose.
According to Fig. 3, the heart
rates of the HD and LD groups started declining from 4 weeks after
drug administration; however, this was not statistically
significant (P>0.05).
 | Table IComparison of the systolic pressure
among groups. |
Table I
Comparison of the systolic pressure
among groups.
| Time | HD (n=5) | LD (n=6) | Scramble-miR
(n=6) | Control (n=6) | P-value |
|---|
| Before dose | 167.32±8.83 | 166.67±7.54 | 162.62±9.31 | 164.95±9.48 | 0.809 |
| 1 week after
dose | 174.80±3.12 | 177.86±4.31 | 175.97±4.32 | 180.22±6.77 | 0.364 |
| 2 weeks after
dose | 186.98±5.34 | 187.71±5.30 | 182.16±4.42 | 182.22±5.45 | 0.163 |
| 3 weeks after
dose | 189.86±7.73 | 185.07±6.96 | 187.94±7.21 | 190.2±8.99 | 0.667 |
| 4 weeks after
dose | 179.83±7.06 | 184.62±8.93 | 187.75±5.65 | 185.44±8.20 | 0.469 |
| 5 weeks after
dose | 180.08±7.18 | 178.39±12.39 | 184.60±7.69 | 188.14±6.24 | 0.268 |
| 6 weeks after
dose | 187.35±12.50 | 192.85±8.44 | 186.06±10.89 | 189.71±12.53 | 0.740 |
| 7 weeks after
dose | 193.25±8.39 | 187.54±8.11 | 190.06±4.51 | 187.58±7.01 | 0.570 |
| 8 weeks after
dose | 182.88±5.33 | 189.17±7.12 | 183.65±4.29 | 185.50±8.03 | 0.405 |
 | Table IVComparison of heart rates among
groups. |
Table IV
Comparison of heart rates among
groups.
| Time | HD (n=5) | LD (n=6) | Scramble-miR
(n=6) | Control (n=6) | P-value |
|---|
| Before dose | 425.07±56.25 | 399.28±30.59 | 412.91±30.54 | 425.07±56.25 | 0.705 |
| 1 week after
dose | 389.52±33.68 | 400.97±62.05 | 403.62±37.63 | 389.52±33.68 | 0.974 |
| 2 weeks after
dose | 397.88±36.72 | 409.52±91.75 | 398.48±58.01 | 397.88±36.72 | 0.973 |
| 3 weeks after
dose | 419.91±40.97 | 407.85±23.48 | 440.32±59.09 | 419.91±40.97 | 0.538 |
| 4 weeks after
dose | 378.84±27.79 | 412.23±44.57 | 437.79±68.45 | 378.84±27.79 | 0.105 |
| 5 weeks after
dose | 400.67±38.67 | 417.57±50.22 | 450.89±53.38 | 400.67±38.67 | 0.405 |
| 6 weeks after
dose | 419.62±45.17 | 422.95±41.81 | 457.44±86.22 | 419.62±45.17 | 0.365 |
| 7 weeks after
dose | 391.58±52.02 | 401.56±51.51 | 473.94±47.49 | 391.58±52.02 | 0.111 |
| 8 weeks after
dose | 392.62±13.94 | 413.52±65.57 | 423.39±72.69 | 392.62±13.94 | 0.178 |
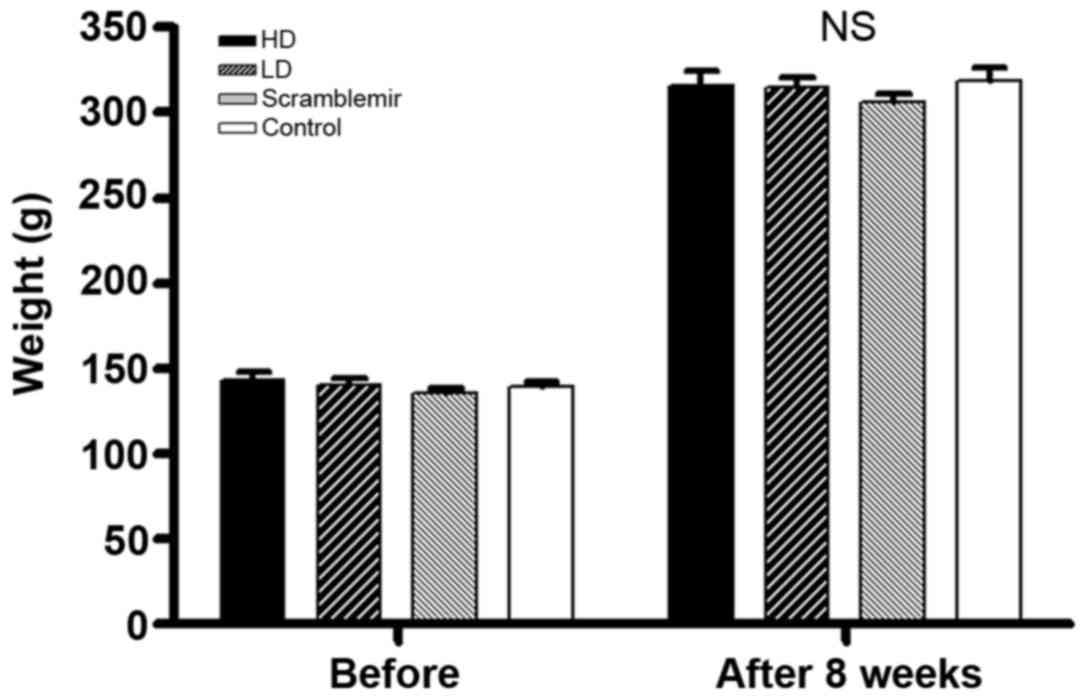
Body weight and heart body ratio
As shown in Table
V and Fig. 4, no statistical
significance was noted (one-way analysis of variance
P>0.05).
 | Table VComparison of body weights before and
after experiments. |
Table V
Comparison of body weights before and
after experiments.
| Group | Body weight before
dose (g) | Body weight after
dose (g) | Heart body ratio
(mg/g) |
|---|
| HD (n=5) | 143.4±9.9 | 316.1±16.2 | 4.41±0.52 |
| LD (n=6) | 140.3±10.5 | 314.6±15.9 | 4.06±0.29 |
| Scramble-miR
(n=6) | 135.3±6.8 | 305.9±12.0 | 3.97±0.20 |
| Control (n=6) | 139.3±8.5 | 318.3±18.5 | 4.25±0.40 |
| P-value | 0.527 | 0.561 | 0.230 |
Green fluorescence of frozen
sections
Since the lentiviral vector encodes an eGFP gene,
successful transfection of the lentiviral vector can be observed as
green fluorescence in the tissue. Although miR-126 has tissue
specificity, with high expression in the heart and lung, it is also
expressed in brain, liver, and kidney at a low level (15). The lentiviral vector is not topic
for organs. It is not only highly expressed in the lung but
expressed in all other organs. The GFP is demonstrated in Fig. 5 in all the organs; less in the
brain, maximum in the lung. The expression data are consistent with
the previous studies, indicating successful transfection of the
lentiviral vectors, and detectable levels in the SHRs even after
experiments.
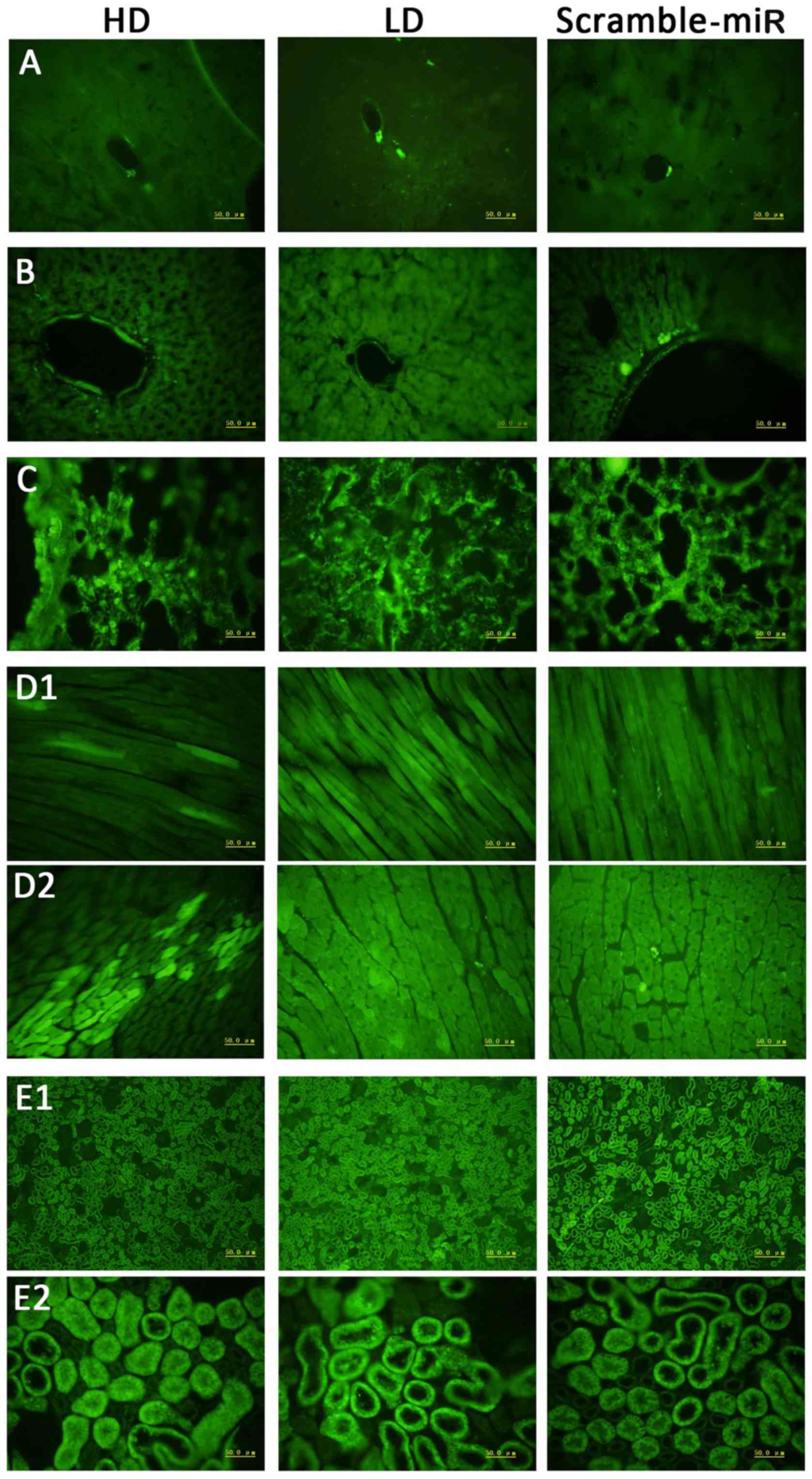 | Figure 5Green fluorescence expression in each
organ under fluorescence microscope. 1st column, HD; 2nd column,
LD; 3rd column, scramble-miR. (A) Brain, (B) liver, (C) lung, (D1)
longitudinal section of cardiac muscle, (D2) cross section of
cardiac muscle, (E1) kidney under low power, (E2) kidney under high
power. HD, high dose; LD, low dose. |
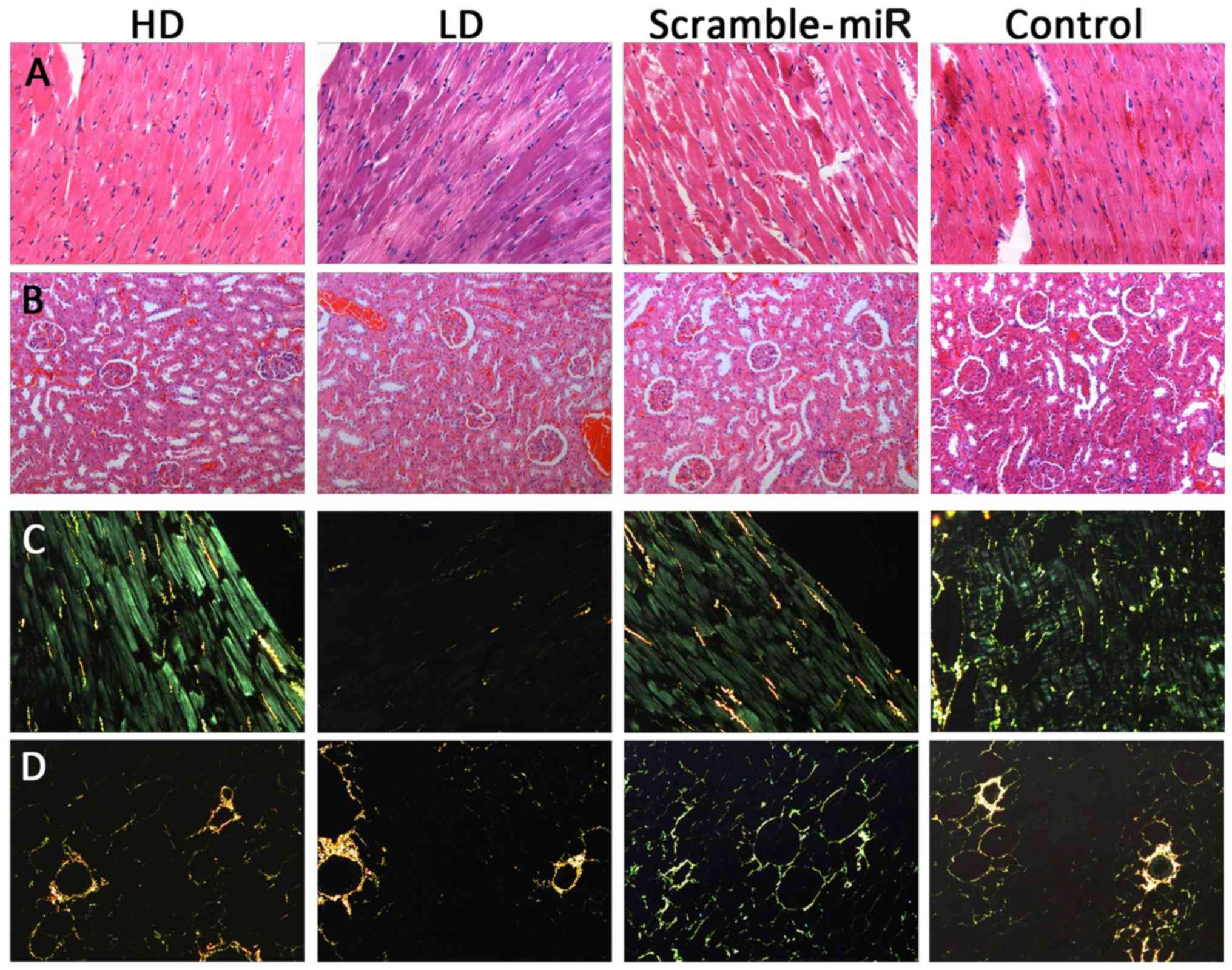
H&E and Sirius red F2B in
carbazotic acid staining
H&E and Sirius red F2B in carbazotic acid
staining of the heart and kidney are shown in Fig. 6. Although the quantitative
analysis was not performed, a difference in the histomorphology was
not observed among the groups.
Effects of miR-126 on hypertension
pathogenesis and progression
NO and major biochemical criterion of SHR groups are
shown in Table VI; no
statistical significant difference was noted among the groups
(P>0.05).
 | Table VIComparison of NO and major
biochemical criterion of the SHR groups. |
Table VI
Comparison of NO and major
biochemical criterion of the SHR groups.
| Test index | HD (n=4) | LD (n=5) | Scramble-miR
(n=5) | Control (n=5) | P-value |
|---|
| NO
(μmol/l) | 8.299±5.001 | 8.081±6.353 | 14.00±10.01 | 4.492±2.055 | 0.233 |
| ALB (g/l) | 16.0±1.7 | 16.6±1.4 | 17.1±1.7 | 18.6±0.5 | 0.131 |
| CHOL(mmol/l) | 1.74±0.32 | 1.59±0.06 | 1.69±0.12 | 1.64±0.05 | 0.552 |
| LDL-C (mmol/l) | 0.22±0.14 | 0.14±0.12 | 0.15±0.10 | 0.06±0.04 | 0.280 |
| TG (mmol/l) | 0.27±0.07 | 0.22±0.09 | 0.28±0.32 | 0.29±0.10 | 0.601 |
| AST (U/l) | 311.0±56.7 | 213.8±63.4 | 244.8±119.8 | 205.0±165.6 | 0.610 |
| ALT (U/l) | 59.3±8.6 | 57.6±8.9 | 61.4±13.6 | 65.3±9.1 | 0.764 |
| BUN (mmol/l) | 8.44±1.20 | 7.67±1.30 | 7.09±1.27 | 6.75±1.57 | 0.398 |
| CREA
(μmol/l) | 41.1±4.7 | 36.7±11.9 | 29.9±3.9 | 34.4±3.3 | 0.244 |
| Na+
(mmol/l) | 146.2±2.2 | 147.9±1.3 | 147.3±1.4 | 146.3±1.8 | 0.422 |
| K+
(mmol/l) | 6.8±1.0 | 6.0±0.6 | 6.7±0.5 | 6.6±0.7 | 0.250 |
| Ca2+
(mmol/l) | 2.42±0.04 | 2.40±0.12 | 2.42±0.11 | 2.40±0.06 | 0.976 |
Discussion
The pathogenesis of primary hypertension is complex
(1). Genetics, activation of the
renin-angiotensin-aldosterone system, and vascular endothelial
dysfunction may all be associated with the pathogenesis and
progression of hypertension. However, the pathogenesis of primary
hypertension remains unknown in nearly 90% of patients.
RNAi is a biological process of gene silencing
induced by dsRNA (including siRNA and miRNA). RNAi interferes with
gene translation or transcription to inhibit specific gene
expression. Once dsRNA homologous to the endogenous mRNA is induced
into a cell, the mRNA degrades resulting in gene silencing
(20). Compared to other gene
therapies, RNAi is highly specific, with high efficiency and
stability (21,22). However, it is primarily used in
tumors (23), virus infectious
diseases (24,25), and genetic diseases with single
gene defects (26). In 2003,
Rubinson et al (27)
reported successful RNAi in mammalian primary stem cells and
transgenic mice by the virus system. It was confirmed that RNAi
lowers target gene expression efficiently in vitro (28). Chen et al (32) injected adenovirus-mediated ATIR
shRNA into mouse brains for hypertension therapy.
miRNA is an endogenous non-coding RNA, approximately
20–22 nucleotides in length (2).
It has been proved that miRNA-126 is endothelium-specific, and has
been studied extensively in tumors (33–38) and hematological system diseases
(39,40). Our preliminary studies indicated
that miR-126 expression was increased in hypertensive patients by
miRCURY LNA™ microRNA arrays. Thus, we hypothesized that there may
be a close correlation between miR-126 and the pathogenesis and
progression of hypertension. Accordingly, in this study, we focused
on the relationship between miRNA-126 and the pathogenesis of
hypertension in animal experiments. We inhibited the miR-126
expression by RNAi to explore the possibility of its usage in gene
therapy for hypertension and the results of animal experiments were
reported.
Lentiviral vector is a retroviral vector with
defective replication. The principle of lentiviral vector
construction is to separate the cis-acting elements of human
immunodeficiency virus 1 (HIV-1) genome and the sequence encoding
the trans-acting proteins. This eliminates the virulence
gene from the wild-type virus gradually through reconstruction, and
completely removes the U3 sequence at the 3′ LTR, weakening its
transcriptional activation capacity. Thus, a self-inactivating
vector system is established. It retains the cis-acting
element LTR of the wild-type virus and packaging signals as well as
the responding element of the Rev gene, but removes the virulence
genes including vif, vpr, vpu, and nef, ensuring the
biosafety of the vector (41). It
stably integrates into the genome of target cells for effective
exogenous gene expression without any alterations in the cloned
gene. Therefore, the lentiviral vector is widely used to transfer
genes due to its high efficiency and stability (42–46). Other retroviral vectors
accommodate exogenous genes <8 kb, which were unfavorable for
the present study. The adenovirus is also commonly used as a vector
for gene therapy. However, in this case, the target genes are free
in the nucleus and do not integrate into the chromosomes, thereby
impeding long-term expression.
Based on the above features, a lentivirus was
adopted as a vector for miR-126 gene knockdown in the present
study. Hence, pMagic 4.0 vector with two promoters was used: CMV
promoter driving the expression of GFP, resulting in cells with
green fluorescence, which is a robust marker for successful gene
transfection. The other is the U6 promoter, downstream of which the
target gene segment is inserted. For gene knockdown, the target
gene segment is complementary to the mature miRNA-126 gene. The
sequencing result of the vector construct is shown in Fig. 2, and indicates a successful vector
construct. Eight weeks after the in vivo experiment, the
heart, kidney, liver, brain, and lung tissue sections were prepared
from the SHRs. Green fluorescence was observed in all the cells
(Fig. 5), suggesting successful
construction of the lentiviral vector and SHR transfection with a
detectable expression even after the experiments.
It is considered that inflammation and immune
responses are both critical mechanisms for primary hypertension
pathogenesis and progression (47,48). Existing data have shown marked
upregulated inflammatory factors in the serum of hypertension
patients (16). Notably,
upregulation of IL-6 and TNF-α was found to lead to vascular
endothelial dysfunction and excessive proliferation and migration
of vascular smooth muscle cells (49,50). Blocking of the IL-6 or TNF-α
inflammatory signaling pathway inhibited Ang II-induced
hypertension (51,52). Reduced endothelial nitric oxide
synthase (eNOS) activity and NO hyposecretion are crucial for
vasomotor dysfunction induced by endothelial malfunction, thereby
resulting in hypertension (53).
NO is a vital humoral factor which regulates endothelial functions.
In vitro studies have confirmed that NO interacts with
vascular endothelial growth factor (VEGF), upregulating its mRNA
expression. On the other hand, there is a positive feedback from
VEGF through upregulated eNOS mRNA to promote NO secretion
(54,55). Furthermore, increased VEGF
synthesis favors the repair of damaged endothelial cells,
inhibiting the thickening of impaired intima, thereby improving
vascular remodeling. Some studies have shown that in addition to
promoting the proliferation of endothelial cells, vascular
proliferation, and vascular remodeling, VEGF is closely related to
endothelial inflammatory reaction (56–60).
Liu et al found that miR-126 downregulated
the VEGF expression in cells and tissues (61). Based on the previous results that
miR-126 is highly expressed in hypertensive patients, we speculated
that it reduces the expression of endogenous VEGF, weakening the
repair of vascular endothelial cells, and therefore, accelerates
the thickening of the endangium, resulting in aggravation of
vascular remodeling. On the other hand, due to the positive
feedback correlation between VEGF and NO, NO production is reduced,
limiting vasodilation and ultimately resulting in hypertension.
In the present study, a lentiviral vector with
miR-126 gene knockdown sequence was introduced into the rats
through tail vein injection. After 8 weeks of continuous
observation, measurements of blood pressure, heart rate, and
comparison to the control groups, there was no decrease in the
blood pressure. In addition, the heart body ratio, histomorphology
of heart and kidney, serum NO, and other biochemical criterion did
not differ significantly (P>0.05).
Knockdown of miR-126 neither reduced the blood
pressure nor prevented target organ damage. Therefore, miR-126 may
potentially serve as a compensatory mechanism in hypertensive
patients. Wang et al (12)
found that target knockout of miR-126 resulted in angiorrhexis,
bleeding, and embryonic mortality. This was due to impaired
vascular integrity, defects in endothelial cell division and
proliferation or migration, which might be caused by the reduction
of angiogenic factor signaling (e.g., VEGF and FGF). However, the
regulatory mechanism of angiogenesis signaling cascade
amplification remains unclear. Nevertheless, Fish et al
(13) demonstrated that miR-126
directly restricts the negative regulatory factors of the VEGF
pathway, including Spred-1 protein and phosphoinositol-3 kinase
regulatory subunit 2 (PIK3R2/p85-β). For overexpression of Spred-1
or blocking of VEGF signaling in zebrafish, both these pathological
phenomena were mimicked by miR-126 gene knockout. These were
inconsistent with data from Liu et al (61), which proved that miR-126 enhanced
the VEGF pathway and promoted angiogenesis, protecting vascular
integrity. Therefore, it may be justified that while under high
blood pressure, endothelial function is impaired, activating
inflammatory responses, which might promote the compensatory
increase in miR-126. Strikingly, this activates the VEGF pathway,
promoting endothelial cell proliferation and repair of injured
cells, inhibiting thickening of vascular walls, and improving
vascular remodeling. On the other hand, since there is a positive
feedback relationship between VEGF and NO, activated VEGF may
facilitate eNOS to produce NO, reversing the disorder of the
renin-angiotensin-aldosterone system.
Although there was no anticipated therapeutic
outcome, the present study was the first to elucidate the
correlation between miRNA-126 and primary hypertension in human. To
the best of our knowledge, this is the first attempt to target
miR-126 for hypertension therapeutics. In addition, the miR-126
gene knockdown lentiviral vector was constructed successfully. We
speculate that hyper-expression of miR-126 in hypertensive patients
is a compensatory mechanism. Taken together, we demonstrated the
possible mechanisms of miR-126 during the pathogenesis and
progression of hypertension, providing substantial evidence for
miR-126 target gene therapy for hypertension.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu LS; Writing Group of 2010 Chinese
Guidelines for the Management of Hypertension: 2010 Chinese
guidelines for the management of hypertension. Zhonghua Xin Xue
Guan Bing Za Zhi. 39:579–615. 2011.In Chinese. PubMed/NCBI
|
|
2
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao Y, Samal E and Srivastava D: Serum
response factor regulates a muscle-specific microRNA that targets
Hand2 during cardiogenesis. Nature. 436:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of primary microRNAs by the
Microprocessor complex. Nature. 432:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gregory RI, Yan KP, Amuthan G, Chendrimada
T, Doratotaj B, Cooch N and Shiekhattar R: The microprocessor
complex mediates the genesis of microRNAs. Nature. 432:235–240.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pillai RS, Bhattacharyya SN and Filipowicz
W: Repression of protein synthesis by miRNAs: How many mechanisms.
Trends Cell Biol. 17:118–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zernecke A, Bidzhekov K, Noels H,
Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh
MN, Lutgens E, et al: Delivery of microRNA-126 by apoptotic bodies
induces CXCL12-dependent vascular protection. Sci Signal.
2:ra812009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harris TA, Yamakuchi M, Ferlito M, Mendell
JT and Lowenstein CJ: MicroRNA-126 regulates endothelial expression
of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA.
105:1516–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stumpf C, John S, Jukic J, Yilmaz A, Raaz
D, Schmieder RE, Daniel WG and Garlichs CD: Enhanced levels of
platelet P-selectin and circulating cytokines in young patients
with mild arterial hypertension. J Hypertens. 23:995–1000. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai J, Yi FF, Yang L, Shen DF, Yang Q, Li
A, Ghosh AK, Bian ZY, Yan L, Tang QZ, et al: Targeted expression of
receptor-associated late transducer inhibits maladaptive
hypertrophy via blocking epidermal growth factor receptor
signaling. Hypertension. 53:539–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai J, Yi FF, Bian ZY, Shen DF, Yang L,
Yan L, Tang QZ, Yang XC and Li H: Crocetin protects against cardiac
hypertrophy by blocking MEK-ERK1/2 signalling pathway. J Cell Mol
Med. 13:909–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar
|
|
20
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caplen NJ: Gene therapy progress and
prospects. Downregulating gene expression: The impact of RNA
interference. Gene Ther. 11:1241–1248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wall NR and Shi Y: Small RNA: Can RNA
interference be exploited for therapy. Lancet. 362:1401–1403. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uchida H, Tanaka T, Sasaki K, Kato K,
Dehari H, Ito Y, Kobune M, Miyagishi M, Taira K, Tahara H and
Hamada H: Adenovirus-mediated transfer of siRNA against survivin
induced apoptosis and attenuated tumor cell growth in vitro and in
vivo. Mol Ther. 10:162–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCaffrey AP, Nakai H, Pandey K, Huang Z,
Salazar FH, Xu H, Wieland SF, Marion PL and Kay MA: Inhibition of
hepatitis B virus in mice by RNA interference. Nat Biotechnol.
21:639–644. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Randall G, Grakoui A and Rice CM:
Clearance of replicating hepatitis C virus replicon RNAs in cell
culture by small interfering RNAs. Proc Natl Acad Sci USA.
100:235–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding H, Schwarz DS, Keene A, Affar B,
Fenton L, Xia X, Shi Y, Zamore PD and Xu Z: Selective silencing by
RNAi of a dominant allele that causes amyotrophic lateral
sclerosis. Aging Cell. 2:209–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubinson DA, Dillon CP, Kwiatkowski AV,
Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus
MT, et al: A lentivirus-based system to functionally silence genes
in primary mammalian cells, stem cells and transgenic mice by RNA
interference. Nat Genet. 33:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vázquez J, Correa de Adjounian MF, Sumners
C, González A, Diez-Freire C and Raizada MK: Selective silencing of
angiotensin receptor subtype 1a (AT1aR) by RNA interference.
Hypertension. 45:115–119. 2005. View Article : Google Scholar
|
|
29
|
Schober A, Nazari-Jahantigh M, Wei Y,
Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H,
Hristov M, et al: MicroRNA-126-5p promotes endothelial
proliferation and limits atherosclerosis by suppressing Dlk1. Nat
Med. 20:368–376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Zhang Z, Zhang DY, Zhu J, Zhang T
and Wang C: MicroRNA 126 inhibits the transition of endothelial
progenitor cells to mesenchymal cells via the PIK3R2-PI3K/Akt
signalling pathway. PLoS One. 8:e832942013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goerke SM, Kiefer LS, Stark GB, Simunovic
F and Finkenzeller G: miR-126 modulates angiogenic growth
parameters of peripheral blood endothelial progenitor cells. Biol
Chem. 396:245–252. 2015. View Article : Google Scholar
|
|
32
|
Chen Y, Chen H, Hoffmann A, Cool DR, Diz
DI, Chappell MC, Chen AF and Morris M: Adenovirus-mediated
small-interference RNA for in vivo silencing of angiotensin AT1a
receptors in mouse brain. Hypertension. 47:230–237. 2006.
View Article : Google Scholar
|
|
33
|
Fridman E, Dotan Z, Barshack I, David MB,
Dov A, Tabak S, Zion O, Benjamin S, Benjamin H, Kuker H, et al:
Accurate molecular classification of renal tumors using microRNA
expression. J Mol Diagn. 12:687–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Du YY, Lin YF, Chen YT, Yang L,
Wang HJ and Ma D: The cell growth suppressor, mir-126, targets
IRS-1. Biochem Biophys Res Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang F, Zheng Z, Guo J and Ding X:
Correlation and quantitation of microRNA aberrant expression in
tissues and sera from patients with breast tumor. Gynecol Oncol.
119:586–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miko E, Czimmerer Z, Csánky E, Boros G,
Buslig J, Dezso B and Scholtz B: Differentially expressed microRNAs
in small cell lung cancer. Exp Lung Res. 35:646–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li XM, Wang AM, Zhang J and Yi H:
Downregulation of miR-126 expression in colorectal cancer and its
clinical significance. Med Oncol. 28:1054–1057. 2011. View Article : Google Scholar
|
|
38
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fulci V, Colombo T, Chiaretti S, Messina
M, Citarella F, Tavolaro S, Guarini A, Foà R and Macino G:
Characterization of B- and T-lineage acute lymphoblastic leukemia
by integrated analysis of MicroRNA and mRNA expression profiles.
Genes Chromosomes Cancer. 48:1069–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cammarata G, Augugliaro L, Salemi D,
Agueli C, La Rosa M, Dagnino L, Civiletto G, Messana F, Marfia A,
Bica MG, et al: Differential expression of specific microRNA and
their targets in acute myeloid leukemia. Am J Hematol. 85:331–339.
2010.PubMed/NCBI
|
|
41
|
Lever AM, Strappe PM and Zhao J:
Lentiviral vectors. J Biomed Sci. 11:439–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pfeifer A, Ikawa M, Dayn Y and Verma IM:
Transgenesis by lentiviral vectors: Lack of gene silencing in
mammalian embryonic stem cells and preimplantation embryos. Proc
Natl Acad Sci USA. 99:2140–2145. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lai Z and Brady RO: Gene transfer into the
central nervous system in vivo using a recombinanat lentivirus
vector. J Neurosci Res. 67:363–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zufferey R, Dull T, Mandel RJ, Bukovsky A,
Quiroz D, Naldini L and Trono D: Self-inactivating lentivirus
vector for safe and efficient in vivo gene delivery. J Virol.
72:9873–9880. 1998.PubMed/NCBI
|
|
46
|
Yu X, Zhan X, D'Costa J, Tanavde VM, Ye Z,
Peng T, Malehorn MT, Yang X, Civin CI and Cheng L: Lentiviral
vectors with two independent internal promoters transfer high-level
expression of multiple transgenes to human hematopoietic
stem-progenitor cells. Mol Ther. 7:827–838. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lifton RP, Gharavi AG and Geller DS:
Molecular mechanisms of human hypertension. Cell. 104:545–556.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wilson EM, Diwan A, Spinale FG and Mann
DL: Duality of innate stress responses in cardiac injury, repair,
and remodeling. J Mol Cell Cardiol. 37:801–811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fernandez-Real JM, Vayreda M, Richart C,
Gutierrez C, Broch M, Vendrell J and Ricart W: Circulating
interleukin 6 levels, blood pressure, and insulin sensitivity in
apparently healthy men and women. J Clin Endocrinol Metab.
86:1154–1159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moon SK, Cha BY and Kim CH: ERK1/2
mediates TNF-alpha-induced matrix metalloproteinase-9 expression in
human vascular smooth muscle cells via the regulation of NF-kappaB
and AP-1: Involvement of the ras dependent pathway. J Cell Physiol.
198:417–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coles B, Fielding CA, Rose-John S,
Scheller J, Jones SA and O'Donnell VB: Classic interleukin-6
receptor signaling and interleukin-6 trans-signaling differentially
control angiotensin II-dependent hypertension, cardiac signal
transducer and activator of transcription-3 activation, and
vascular hypertrophy in vivo. Am J Pathol. 171:315–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Davis JR, Giardina JB, Green GM, Alexander
BT, Granger JP and Khalil RA: Reduced endothelial NO-cGMP vascular
relaxation pathway during TNF-alpha-induced hypertension in
pregnant rats. Am J Physiol Regul Integr Comp Physiol.
282:R390–R399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shesely EG, Maeda N, Kim HS, Desai KM,
Krege JH, Laubach VE, Sherman PA, Sessa WC and Smithies O: Elevated
blood pressures in mice lacking endothelial nitric oxide synthase.
Proc Natl Acad Sci USA. 93:13176–13181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chin K, Kurashima Y, Ogura T, Tajiri H,
Yoshida S and Esumi H: Induction of vascular endothelial growth
factor by nitric oxide in human glioblastoma and hepatocellular
carcinoma cells. Oncogene. 15:437–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gélinas DS, Bernatchez PN, Rollin S, Bazan
NG and Sirois MG: Immediate and delayed VEGF-mediated NO synthesis
in endothelial cells: Role of PI3K, PKC and PLC pathways. Br J
Pharmacol. 137:1021–1030. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miska EA, Alvarez-Saavedra E, Townsend M,
Yoshii A, Sestan N, Rakic P, Constantine-Paton M and Horvitz HR:
Microarray analysis of microRNA expression in the developing
mammalian brain. Genome Biol. 5:R682004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chivukula RR and Mendell JT: Circular
reasoning: microRNAs and cell-cycle control. Trends Biochem Sci.
33:474–481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pan Q and Chegini N: MicroRNA signature
and regulatory functions in the endometrium during normal and
disease states. Semin Reprod Med. 26:479–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fontana L, Sorrentino A, Condorelli G and
Peschle C: Role of microRNAs in haemopoiesis, heart hypertrophy and
cancer. Biochem Soc Trans. 36:1206–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Finnegan EJ and Matzke MA: The small RNA
world. J Cell Sci. 116:4689–4693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|