Introduction
Osteosarcoma (OS) is the most frequent primary
malignant bone tumor, which is commonly diagnosed in children and
young adolescents, with a male predominance (1). OS is highly aggressive and primarily
metastasizes to the lung (2).
Surgical tumor resection and multi-agent chemotherapy are the main
current therapeutic strategies used to treat OS. It has previously
been reported that chemotherapy may increase the 5-year survival
rate for localized disease by >50% compared with surgery alone.
Conversely, patients diagnosed with metastases exhibit a poor
prognosis, with a 5-year survival rate of 20–30% following surgical
resection and/or radiotherapy (3,4).
Furthermore, currently approved agents exhibit severe side effects
(3,4); therefore, the development of a novel
agent with increased efficiency and reduced toxicity in OS
treatment is required.
Honokiol (HNK) is a biphenolic compound extracted
from the magnolia tree, which has been used to treat anxiety,
thrombotic stroke and gastrointestinal symptoms in traditional
Chinese and Japanese medicine (5). HNK has long been known to exert
antimicrobial (6),
anti-inflammatory (7) and
antiangiogenic (8,9) effects. Increasing evidence has
revealed that HNK exerts antineoplastic functions in various types
of cancer, including angiosarcoma (8), colorectal carcinoma (10), breast cancer (11) and gastric cancer (12). Furthermore, HNK may trigger
apoptotic pathways that result in mitochondrial dysfunction
(13), influence retinoblastoma
function and E2F transcription factor 1 transcriptional activity
(14), and suppress the
phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin
(mTOR) pathway (15). However,
the molecular mechanism underlying the anticancer effects of HNK on
OS cells remains to be elucidated.
MicroRNAs (miRNAs/miRs) are a class of small (19–24
nucleotide) noncoding RNAs that mediate post-transcriptional
regulation of target genes by suppressing translation or promoting
RNA degradation. miRNAs have crucial functions in various
biological and pathological processes, including cellular
proliferation, differentiation, apoptosis and carcinogenesis
(16). In recent years, it has
been demonstrated that some natural products are able to control
tumor-suppressive and oncogenic miRNAs, including curcumin
(diferuloylmethane), which inhibits hepatocellular cancer cell
proliferation via modulating miRNA expression (17). Furthermore, previous studies have
reported that Chinese medicinal herbs exert antitumor effects by
modulating miRNA expression (18,19). Zhang et al demonstrated
that HNK suppresses bladder tumor growth by inhibiting the enhancer
of zeste homolog 2/miR-143 axis (20). Avtanski et al also revealed
that HNK rescued leptin-induced tumor progression by suppressing
the Wnt1-metastasis associated 1-β-catenin signaling pathway in a
miR-34a-dependent manner (11).
Therefore, it may be hypothesized that HNK inhibits proliferation
and induces apoptosis, via the modulation of miRNA expression, in
human OS cells.
The present study investigated the effects of HNK on
OS tumor growth inhibition and explored the underlying molecular
mechanisms. The results indicated that HNK may inhibit growth and
promote apoptosis of human OS cells in a dose-dependent manner.
Furthermore, the results verified that HNK induces aberrant
expression of miRNAs in human OS cells, and miR-21 suppresses
phosphatase and tensin homolog (PTEN) by directly targeting its
3′-untranslated region (3′-UTR). Notably, the results indicated
that HNK blocks the PI3K/protein kinase B (AKT) signaling pathway
by inhibiting miR-21 expression in human OS cells. Collectively,
these results suggested that the molecular mechanism by which HNK
induces apoptosis was modulated by the miR-21/PTEN/PI3K/AKT axis in
human OS cells.
Materials and methods
Reagents and cell culture
HNK was obtained from the National Institute for the
Control of Pharmaceutical and Biological Products (Beijing, China).
HNK was dissolved in 10 µM dimethyl sulfoxide (DMSO) and was
maintained at 4°C. The human OS cell lines Saos-2 and MG-63 were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and were grown in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 50 U/ml penicillin and 50 µg/ml
gentamicin (both Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
2.5 µg/ml amphotericin B, 1% glutamine and 2% HEPES at 37°C
in a humidified incubator containing 5% CO2.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The MTT assay was used to investigate the
anti-proliferative effects of HNK on OS cells. Briefly,
1×104 cells were seeded into 96-well plates overnight.
After treatment with 1–100 µM HNK for 24 h at 37°C, the
cells were washed with PBS and incubated for 48 h at 37°C in fresh
medium. The cells in the control group were only treated with 100
µl DMSO for 24 h at 37°C. Subsequently, 20 µl 5 mg/ml
MTT (Sigma-Aldrich; Merck KGa) solution was added to each well and
incubated at 37°C for an additional 4 h. The supernatant was then
discarded and 150 µl DMSO was added to each well. Finally,
absorbance of the samples was measured at 490 nm using a microplate
reader (Sunrise™; Tecan Group Ltd., Männedorf, Switzerland).
Apoptosis analysis
Flow cytometric analysis was used to detect cell
apoptosis. Briefly, the cells were treated with 1–100 µM HNK
for 24 h at 37°C, after which 5×105 cells were obtained
from the culture and were washed with cold PBS. The cells in the
control group were only treated with 100 µl DMSO for 24 h at
37°C. Cell apoptosis was evaluated using the Annexin V/propidium
iodide (PI) staining kit (BioVision, Inc., Milpitas, CA, USA)
according to the manufacturer's protocol. Flow cytometry was
conducted at the Flow Cytometry Core Facility at Cedars-Sinai
Medical Center (Los Angeles, CA, USA) using FACScan (BD
Biosciences, San Jose, CA, USA). Data were analyzed using the Cell
Quest program version 6.0 (FACScan; BD Biosciences).
Western blot analysis
Cells were lysed as described previously (21). Subsequently, a bicinchoninic acid
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used
to measure protein concentration. Total proteins (60 µg)
were separated by 10% SDS-PAGE (Sigma-Aldrich; Merck KGaA) and were
then transferred onto polyvinylidene difluoride (PVDF) membranes
(BD Biosciences). After blocking with 5% non-fat milk at room
temperature for 1 h, PVDF membranes were incubated with primary
antibodies (dilution 1:500) for 2 h at room temperature and
followed with a horseradish peroxidase conjugated secondary
antibody (dilution 1:1,000) for 1 h at room temperature. Mouse
anti-PTEN (sc-7974), mouse anti-AKT (sc-6546) and mouse anti-p-AKT
(Ser473; sc-33437) primary antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit anti-mTOR
(39408), rabbit anti-p-mTOR (32199), rabbit anti-p70S6K (32505),
rabbit anti-p-p70S6K (66134) and β-actin (79467) antibodies were
purchased from Abcam (Cambridge, MA, USA). Antibodies of
apoptosis-associated proteins were as follows: cleaved PARP
(ab32064), Bax (ab25901), cleaved caspase-3 (ab13847) and Bcl-2
(ab32503), purchased from Abcam. The corresponding rabbit
anti-mouse (315-065-003) and goat anti-rabbit (305-065-003)
secondary antibodies were purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA). Subsequently, protein
bands were scanned on X-ray film using the enhanced
chemiluminescence detection system (PerkinElmer, Inc., Waltham, MA,
USA). AlphaImager software version 2000 (ProteinSimple, San Jose,
CA, USA) was used to measure relative intensity of each band on the
blots. Measurements were conducted independently at least three
times with similar results.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted using ZR RNA
MicroPrep™ kit (Zymo Research Corp., Irvine, CA, USA) according to
the manufacturer's protocol. RNA concentration was measured using a
spectrophotometer (Eppendorf, Hamburg, Germany). The high capacity
cDNA synthesis kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used to synthesize cDNA using miRNA-specific primers
according to the manufacturer's protocol. The primers for
miR-188-5p, miR-202, miR-623, miR-21, miR-532-5p, miR-628-3p and
the internal control RNU44 gene were obtained from Ambion (Thermo
Fisher Scientific, Inc.). The primers were as follows: miR-188-5p
forward, 5′-TGTGGCTATCTTGCTGCCC-3′ and reverse,
5′-GAGTCATTCTCCTTCCCACC-3′; miR-202 forward,
5′-TTAGGCCAGATCCTCAAAGAAG-3′ and reverse,
5′-ATAGGAAAAAGGAACGGCGG-3′; miR-623 forward,
5′-ATCCCTTGCAGGGGCTGTTGGGT-3′ and reverse,
5′-GCCAGCACAGAATTAATACGAC-3′; miR-21 forward,
5′-TAGCTTATCAGACTGATGTTGA-3′ and reverse,
5′-GCCAGCACAGAATTAATACGAC-3′; miR-532-5p forward,
5′-GCCCATGCCTTGAGTGTAG-3′ and reverse, 5′-GTGCGTGTCGTGGAGTCG-3′;
miR-628-3p forward, 5′-GGGGGATGCTGACATATTTAC-3′ and reverse,
5′-CAGTGCGTGTCGTGGAGT-3′; RNU44 forward,
5′-CCTGGATGATGATAGCAAATGC-3′ and reverse,
5′-GAGCTAATTAAGACCTTCATGTT-3′. qPCR was conducted using TaqMan Gene
Expression assay (Applied Biosystems; Thermo Fisher Scientific,
Inc.) on an Applied Biosystems 7500 real-time PCR machine (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR reaction was
performed at 95°C for 5 min followed by 40 cycles of 95°C for 30
sec, 60°C for 30 sec, and 72°C for 30 sec. The 2−ΔΔCq
method was used to analyze relative miRNA expression (22). All reactions were performed in
triplicate.
Transfection assay
Saos-2 and MG-63 (5×103) cells were
seeded into each well of 6-well plates, maintained in DMEM
containing 10% FBS, and treated with either control vehicle (DMSO)
or HNK. Subsequently, the cells were transfected with miR-21 mimics
or miR-21 inhibitor (Guangzhou RiboBio Co., Ltd., Guangzhou, China)
at a final concentration of 50 µM using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Cells were collected 48 h
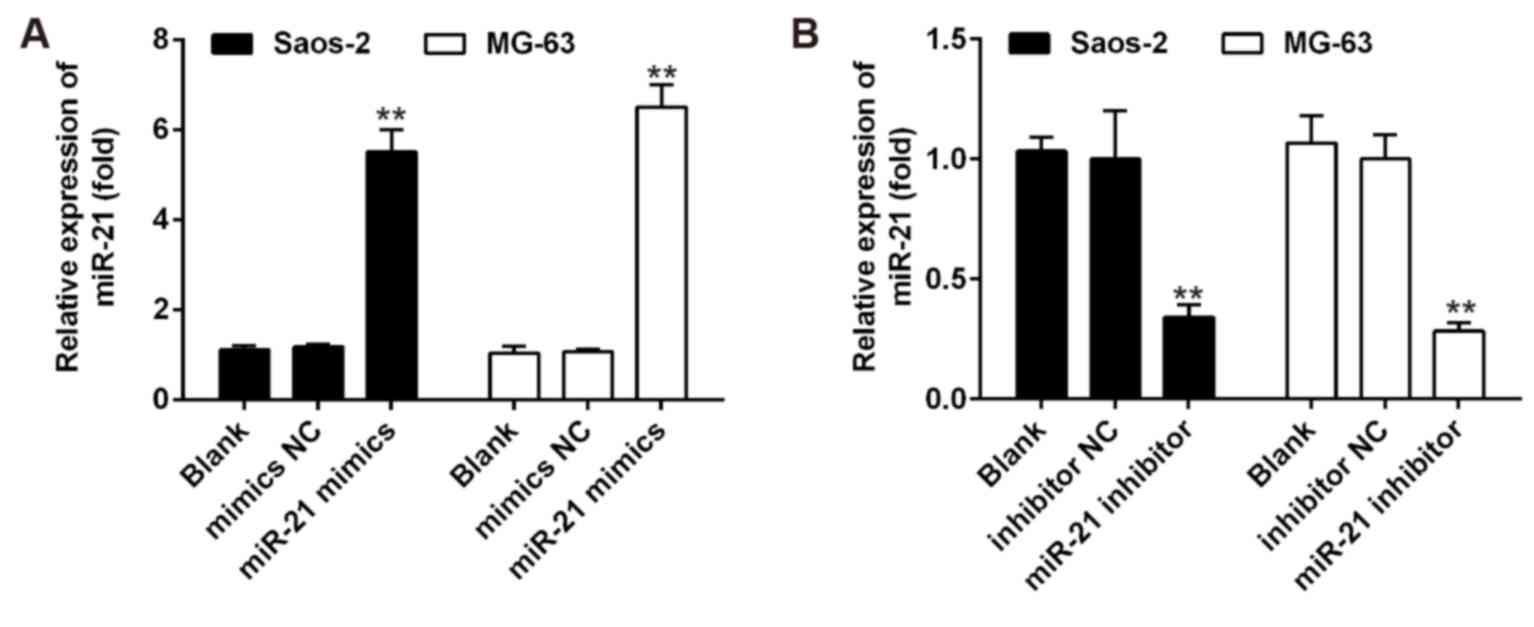
post-transfection. RT-qPCR was used to confirm that miR-21
expression was specifically upregulated/knocked down following
transfection with mimics/inhibitor (Fig. 1). Subsequently, OS cell
proliferation and apoptosis were assessed by MTT assay and flow
cytometric analysis, respectively.
Luciferase assay
The potential binding site between PTEN and miR-21
was identified using TargetScan (http:/www.targetscan.org). The miR-21 mimics/inhibitor
and corresponding negative control (NC) were synthesized by
Guangzhou RiboBio Co., Ltd. Wild-type (wt) PTEN-3′-UTR and mutant
(mut) PTEN-3′-UTR containing the putative binding site of miR-21
were established and cloned into the firefly luciferase-expressing
vector pMIR-REPORT (Ambion; Thermo Fisher Scientific, Inc.). For
the luciferase assay, Saos-2 cells at a density of
2×105/well were seeded into 24-well plates and were
co-transfected with 0.8 µg pMIR-PTEN-3′-UTR or
pMIR-PTEN-mut-3′-UTR and 50 nM miR-21 mimic/inhibitor or
corresponding NC using Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). A total of 48 h post-transfection,
luciferase activity was measured using the dual-light luminescent
reporter gene assay (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Each experiment was repeated at least three times in
independent experiments. The ratio of Renilla luciferase to
firefly luciferase was calculated for each well.
Choice of differentially expressed miRNAs
list using heat map analysis
We obtained the microarray date from Gene Expression
Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), and the GEO
accession no. is GSE85871. Observations with adjusted P-values
≥0.05 were removed, and thus excluded from further analysis. The
heat map of the miRNAs most obvious differences was created using a
method of hierarchical clustering by GeneSpring GX, version 7.3
(Agilent Technologies, Santa Clara, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS
14.0 software (SPSS, Inc., Chicago, IL, USA). Each experiment was
repeated at least three times. Numerical data are presented as the
mean ± SD. For numerical variables, the results were evaluated by
the Student's t-test (comparison between 2 groups) or one way ANOVA
to make multiple-group comparisons followed by the post hoc Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
HNK inhibits growth of human OS
cells
To investigate the antiproliferative effects of HNK
on OS cells, Saos-2 and MG-63 cells were treated with various
concentrations of HNK for 24 h, and the MTT assay was used to
evaluate cell viability. The results indicated that treatment with
1–100 µM HNK reduced cell viability of Saos-2 and MG-63
cells in a dose-dependent manner (Fig. 2A and B). The half maximal
inhibitory concentration (IC50) values of HNK were 37.85
µM in Saos-2 and 38.24 µM in MG-63 cells. Similar
IC50 values of HNK were detected in human Saos-2 and
MG-63 OS cells.
 | Figure 2HNK inhibits proliferation and
induces apoptosis of human OS cells. (A and B) Human Saos-2 and
MG-63 OS cells were treated with or without 1–100 µM HNK for
24 h, and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide assay was conducted to assess viability. (C) Saos-2 and
MG-63 cells were treated with or without 10 or 40 µM HNK for
24 h, and apoptosis was measured using flow cytometry. (D)
Proportion of apoptotic cells was quantified in three independent
experiments. (E) Saos-2 and MG-63 cells were treated with or
without 10 or 40 µM of HNK for 24 h, and the protein
expression levels of cleaved-caspase-3, cleaved-PARP, Bax and Bcl-2
were analyzed by western blot analysis. Representative data from
one of three individual experiments with similar results are
presented. Data are presented as the mean ± standard deviation of
three independent experiments. *P<0.05 and
**P<0.01 vs. 0 µM group. Bax, Bcl-2-associated
X protein; Bcl-2, B-cell lymphoma 2; HNK, honokiol; OS,
osteosarcoma; PARP, poly (ADP-ribose) polymerase. |
HNK induces apoptosis of human OS
cells
It has been widely reported that HNK may induce
apoptosis of various malignant cell types (9,23).
To examine HNK-induced apoptosis of OS cells, the cells were
analyzed by Annexin V-PI staining following treatment with HNK. The
results demonstrated that the proportion of apoptotic cells was
markedly increased following HNK treatment compared with in the
control group (P<0.01). Furthermore, following treatment with 10
or 40 µM HNK, the number of apoptotic cells increased in a
dose-dependent manner (Fig. 2C and
D). With regards to apoptotic induction, Saos-2 and MG-63 had
similar results (Fig. 2D). To
further explore the apoptotic mechanism, the intracellular
apoptotic signaling pathway was investigated in OS cells following
treatment with various concentrations of HNK. The results revealed
that the protein expression levels of cleaved-caspase-3,
cleaved-PARP and Bax were significantly upregulated, and Bcl-2 was
significantly downregulated following HNK treatment. Furthermore,
HNK regulated these protein expression levels in a dose-dependent
manner in human OS cells (Fig.
2E). These data suggested that HNK may induce apoptosis of
human OS cells by activating the intracellular apoptotic signaling
pathway.
HNK induces miRNA aberrant expression in
human OS cells
A recent study revealed that some miRNAs were
upregulated in the T24 human bladder cancer cell line following
treatment with 9.6 µg/ml HNK (20). Microarray data obtained from the
Gene Expression Omnibus (GEO) database (accession no. GSE85871,
http:/www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE85871)
indicated that HNK resulted in aberrant expression of miRNAs in a
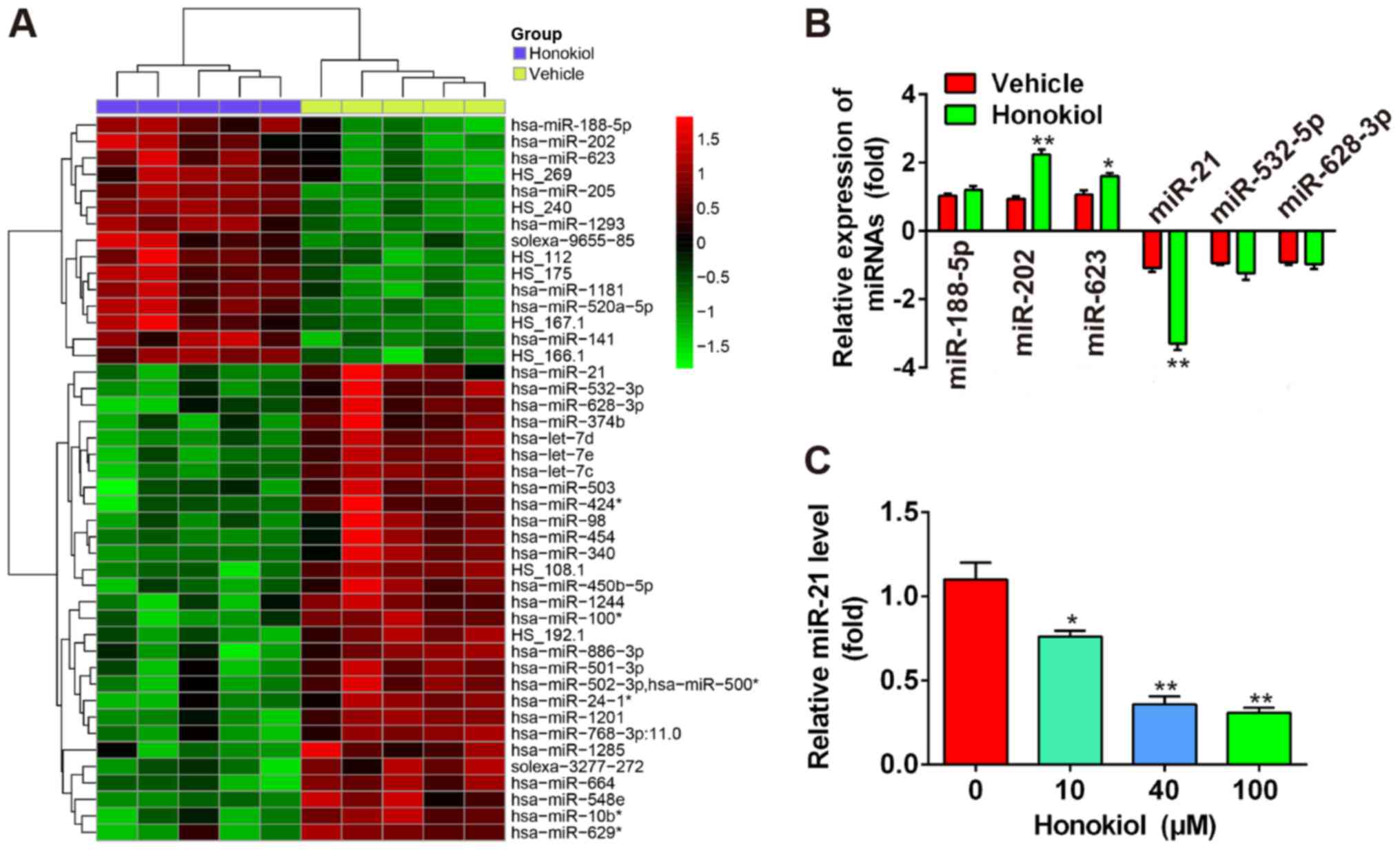
breast cancer cell line (Fig.
3A). To determine whether HNK also induces aberrant expression
of miRNAs in human OS cells, six miRNAs (miR-188-5p, miR-202 and
miR-623 were the most significantly upregulated; miR-21, miR-532-5p
and miR-628-3p were the most significantly downregulated) were
selected based on the microarray data, and were verified by
RT-qPCR. The results indicated that miR-202 and miR-623 were
markedly upregulated, and miR-21 was significantly downregulated in
human Saos-2 OS cells following HNK treatment (P<0.01), whereas
miR-188-5p, miR-532-5p and miR-628-3p were not significantly
different compared with in the vehicle group (Fig. 3B). miR-21 has previously been
reported to be associated with cell apoptosis and proliferation
(24). Therefore, the present
study further investigated the function of miR-21 in human OS
cells. Saos-2 cells were treated with 10–100 µM HNK for 24 h
and the expression levels of miR-21 were determined by RT-qPCR. The
results demonstrated that HNK reduced miR-21 levels in a
dose-dependent manner in human OS cells (Fig. 3C). These results indicated that
HNK may exert antitumor effects via modulating miR-21 expression in
human OS cells.
Overexpression of miR-21 rescues the
suppressive effects of HNK on OS cells
The present study revealed that miR-21 was
downregulated in Saos-2 cells following HNK treatment. Furthermore,
mounting evidence has confirmed that miR-21 may serve a crucial
role in regulating the expression of gene products involved in
phenotypic characteristics of cancer cells, including cell
proliferation, apoptosis and cell cycle (24,25). Therefore, it may be hypothesized
that HNK suppresses cell growth and induces apoptosis of OS cells
by modulating miR-21 expression. Saos-2 and MG-63 cells were
transfected with miR-21 mimics following HNK treatment for 24, 48
and 72 h (HNK + miR-21 group); treatment with vehicle (vehicle
group) or HNK alone (HNK group) served as negative and positive
control groups, respectively. Subsequently, cell proliferation in
each group was investigated by MTT assay, the results indicated
that HNK significantly suppressed cell proliferation; however, the
suppressive effects of HNK on OS cells were significantly rescued
post-transfection with miR-21 mimics (P<0.01; Fig. 4A and B). To further validate these
results, cell apoptosis was measured by flow cytometry. As
expected, the proportion of apoptotic cells was significantly
decreased in the HNK + miR-21 group compared with in the HNK group
in Saos-2 and MG-63 cells (P<0.01; Fig. 4C and D). These data indicated that
HNK may exert suppressive effects on human OS cells via
downregulating miR-21.
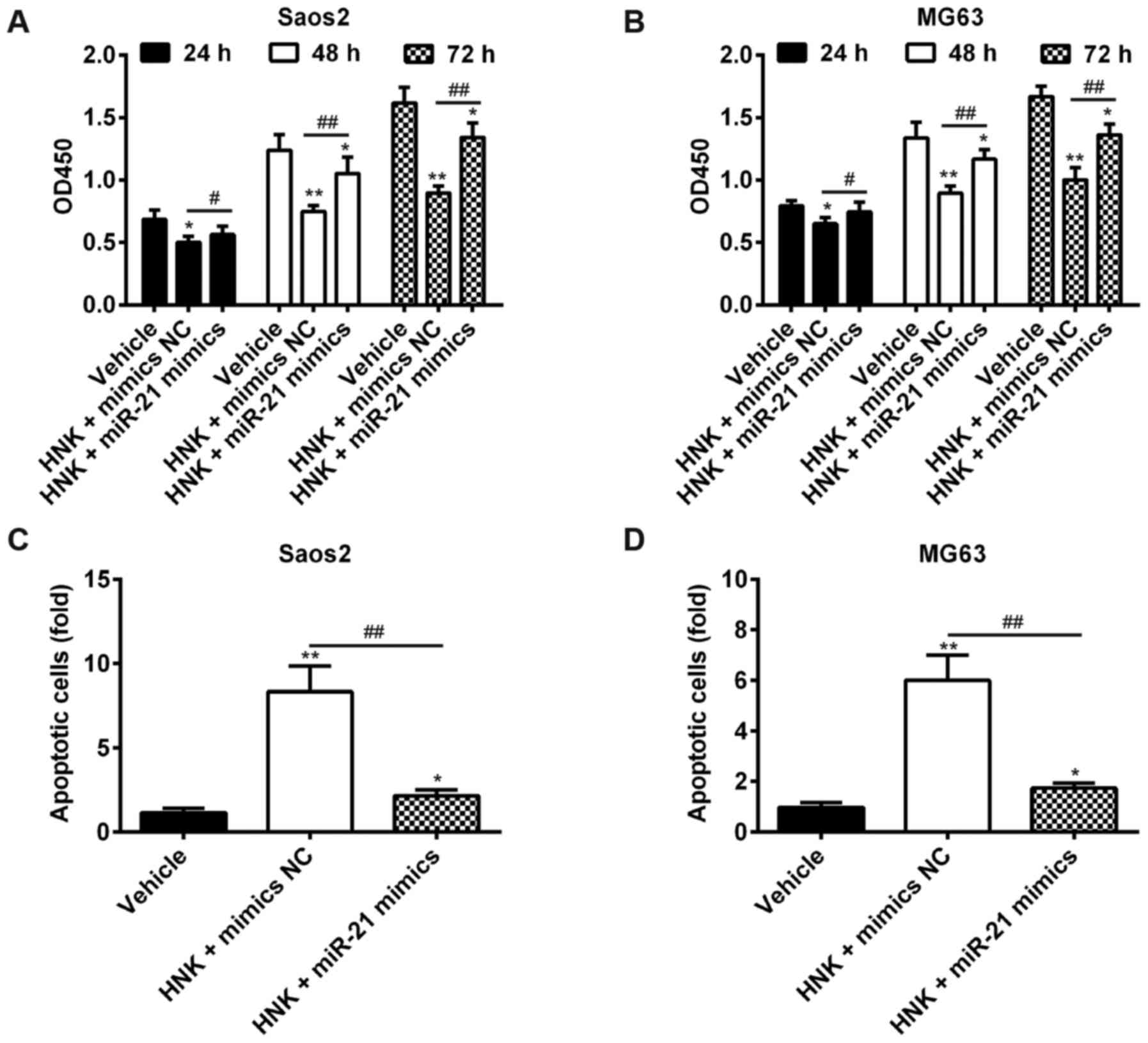 | Figure 4Overexpression of miR-21 rescues the
suppressive effects of HNK on OS cells. (A and B) Saos-2 and MG-63
cells were transfected with miR-21 mimics following HNK treatment
for 24, 48 and 72 h (HNK + miR-21 mimics group); treatment with
vehicle (vehicle group) or HNK alone (HNK + mimics NC group) served
as negative and positive control groups, respectively. Cell
proliferation in each group was determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
(C and D) Saos-2 and MG-63 cells were transfected with miR-21
mimics following HNK treatment for 48 h, and cell apoptosis was
determined using flow cytometric analysis. Data are presented as
the mean ± standard deviation of three independent experiments.
*P<0.05, **P<0.01 vs. the vehicle
group. #P<0.05, ##P<0.01 vs. HNK +
mimics NC group. HNK, honokiol; miR-21, microRNA-21; OD, optical
density; OS, osteosarcoma. |
miR-21 suppresses PTEN expression by
directly targeting its 3′-UTR
Previous studies have reported that miR-21 may
post-transcriptionally suppress PTEN expression in numerous human
cancer cells, including lung cancer and esophageal cancer cells
(25,26); however, whether PTEN is a direct
target of miR-21 in human OS cells remains to be further
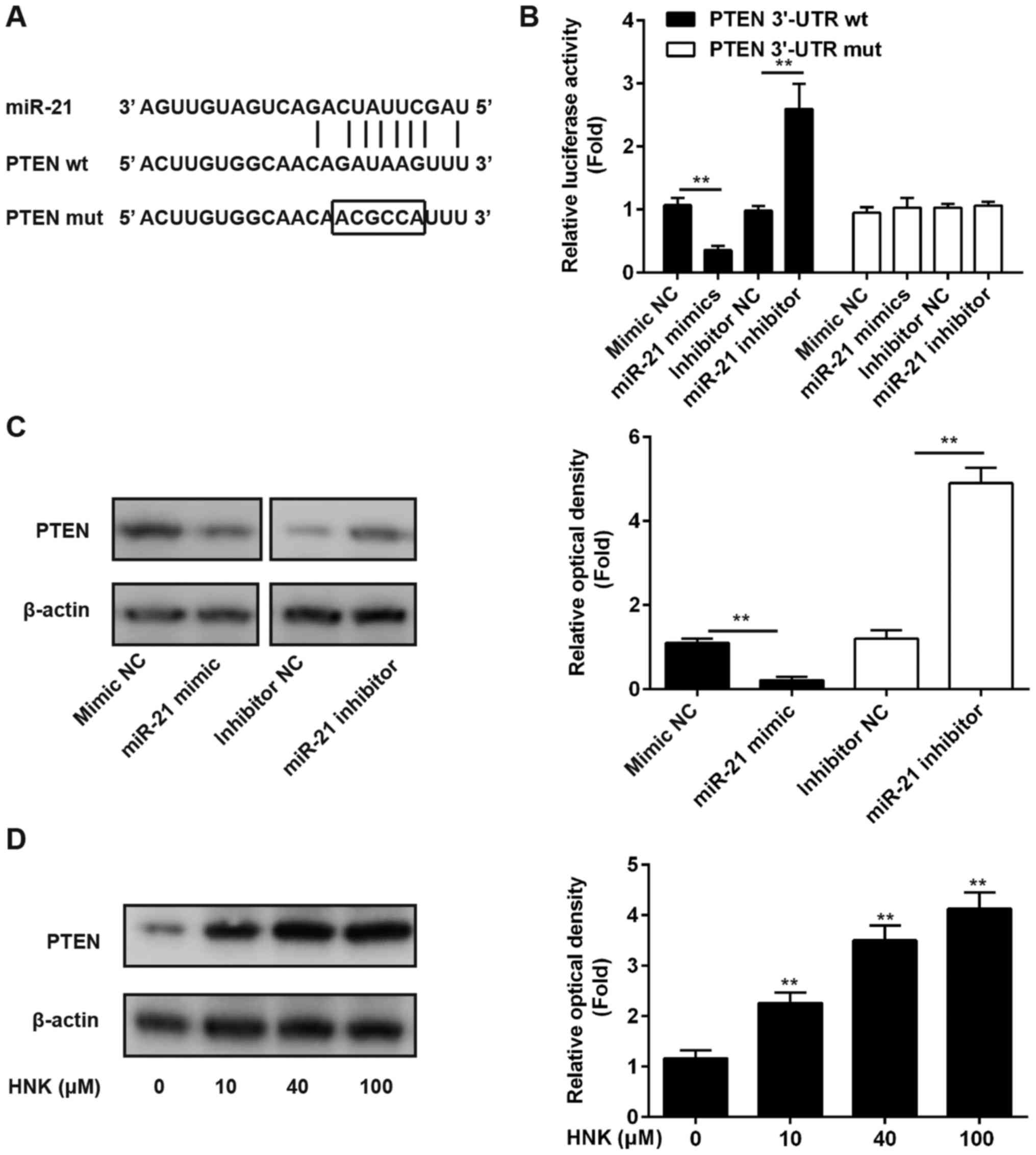
elucidated. In the present study, luciferase-reporter plasmids
containing wt or mut type 3′-UTR segments of PTEN were constructed
(Fig. 5A). The reporters were
cotransfected alongside miR-21 mimics/inhibitor or NC into Saos-2
cells, after which luciferase activity was measured. The results
demonstrated that miR-21 mimic significantly suppressed luciferase
activity compared with mimic NC; however, the miR-21 inhibitor
markedly enhanced luciferase activity compared with inhibitor NC in
the presence of wt 3′-UTR (P<0.01; Fig. 5B). In addition, miR-21 did not
affect luciferase activity of the reporter vector containing mut
PTEN-3′-UTR (Fig. 5B). These
findings indicated that miR-21 inhibits PTEN by directly targeting
PTEN-3′-UTR. To further confirm that PTEN levels are modulated by
miR-21, the Saos-2 human OS cell line was transfected with miR-21
mimic/inhibitor or NC, and the protein expression levels of PTEN
were determined using western blot analysis. The results indicated
that miR-21 inhibited PTEN expression in OS cells compared with the
NC (Fig. 5C). To further verify
whether PTEN expression was modulated by HNK, Saos-2 cells were
treated with 10–100 µM HNK for 24 h and PTEN expression was
measured by western blotting. The results demonstrated that HNK
enhanced PTEN expression in a dose-dependent manner in human OS
cells (Fig. 5D).
HNK suppresses the PI3K/AKT signaling
pathway via modulating miR-21 expression in human OS cells
It has previously been reported that the PI3K/AKT
signaling pathway serves a critical role in cell survival, and
exerts protective effect against tumorigenesis-associated apoptosis
in cancer cells (27).
Furthermore, PI3K and AKT are negatively modulated by PTEN, which
is a key molecule in various diseases that modulates cell
proliferation, survival, apoptosis and metabolism. A recent study
revealed that miR-21 may mediate proliferation, apoptosis,
migration, invasion and cell cycle progression in human esophageal
cancer cells by targeting key proteins of the PTEN/PI3K/AKT
signaling pathway (25).
Therefore, the present study aimed to determine whether
HNK-mediated miR-21 modulation regulates the PI3K/AKT signaling
pathway in OS cells. Saos-2 and MG-63 cells were transfected with
or without miR-21 mimics following treatment with or without HNK,
and western blot analysis was used to determine the expression
levels of p-AKT, p-mTOR and p-p70S6K, which are major components of
the PI3K/AKT signaling pathway (28). The results indicated that the
expression levels of AKT, mTOR and p70S6K were significantly
downregulated following HNK treatment compared with mock
vehicle-treated cells or miR-21 transfection in Saos-2 and MG-63
cells; however, the expression levels of these proteins were
significantly upregulated in HNK-treated OS cells post-transfection
with miR-21 mimics compared with transfection without miR-21 mimics
(P<0.01; Fig. 6). These
results demonstrated that HNK may suppress the PI3K/AKT signaling
pathway in human OS cells; however, it could be activated by miR-21
overexpression. Taken together, these data suggested that HNK
suppresses the PI3K/AKT signaling pathway by inhibiting miR-21
expression in human OS cells.
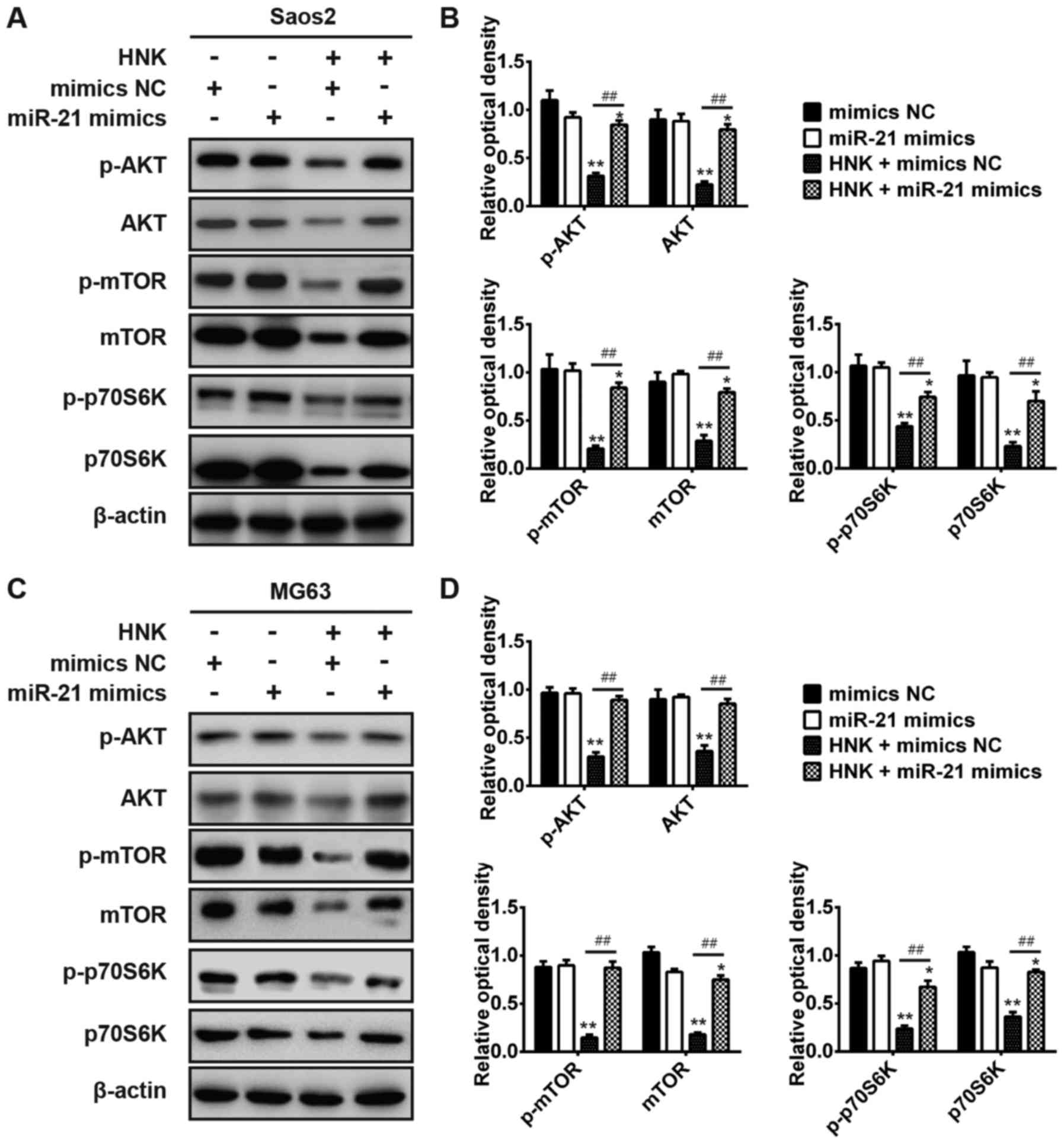 | Figure 6HNK suppresses the phosphoinositide
3-kinase/AKT signaling pathway via modulating miR-21 expression in
human OS cells. Saos-2 and MG-63 cells were transfected with or
without miR-21 mimics following treatment with or without HNK, and
the expression levels of p-AKT, p-mTOR, p-p70S6K, AKT, mTOR and
p70S6K were detected by western blot analysis in (A and B) Saos-2
and (C and D) MG-63 cells. Data are presented as the mean ±
standard deviation of three independent experiments.
*P<0.05, **P<0.01 vs. mimics NC group;
##P<0.01 vs. HNK + mimics NC group. AKT, protein
kinase B; HNK, honokiol; miR-21, microRNA-21; mTOR, mammalian
target of rapamycin; OD, optical density; OS, osteosarcoma; p70S6K,
p70S6 kinase; p-, phosphorylated. |
Discussion
HNK has been widely used to treat various diseases
in traditional Chinese medicine, and it has been reported to exert
anticancer functions (9,23). However, it remains unclear whether
HNK may be used as a stand-alone natural compound to exert strong
anticancer effects against human OS. In the present study, the
potential of HNK-induced apoptosis of human OS cells was examined
and the underlying molecular mechanisms were investigated. The
results demonstrated that HNK inhibited cell growth in a
dose-dependent manner in Saos-2 and MG-63 cells. Furthermore, the
results indicated that HNK-induced apoptosis was dependent upon
caspase activation, and that HNK increased expression of the
proapoptotic protein Bax, and reduced expression of the
anti-apoptotic protein Bcl-2. Notably, the results verified that
HNK may induce aberrant miRNA expression in human OS cells, and
miR-21 inhibits PTEN by directly targeting its 3′-UTR. Furthermore,
HNK was revealed to induce apoptosis through modulating the
miR-21/PTEN/PI3K/AKT signaling pathway in human OS cells.
Natural products that are able to prevent and treat
cancers are the source of numerous medically beneficial drugs,
including camptothecin, curcumin, isoflavone, luteolin, matrine,
HNK, phenolic leaf extract of Heimia myrtifolia (Lythraceae)
and xanthoangelol (29,30). A recent study demonstrated that
xanthoangelol, which is isolated from Angelica keiskei
roots, may inhibit tumor growth, metastasis to the lung and liver,
and tumor-associated macrophage expression in tumors (30). In addition, it is well known that
some natural compounds possess anticancer effects in human OS
(31–33). Steinmann et al revealed
that HNK exhibits prominent antimetastatic activity in OS and is
able to induce rapid cell death in vitro (34). In the present study, the MTT assay
and flow cytometry were used to determine cell viability and
apoptosis in HNK-treated human OS cells; the results indicated that
HNK significantly reduced cell viability and induced apoptosis of
OS cells. Furthermore, it has been reported that HNK may trigger
apoptosis via activation of the intrinsic or extrinsic apoptotic
pathways (9,35). HNK-induced apoptosis may also
enhance caspase expression and PARP cleavage (23). In the present study, the results
further confirmed that HNK significantly upregulated the expression
levels of proapoptotic proteins (caspase-3, cleaved-PARP and Bax)
and downregulated the levels of the anti-apoptotic protein Bcl-2.
These data suggested that HNK may serve a crucial role in the
apoptosis of OS cells.
Mounting evidence has demonstrated that miRNAs have
important roles in numerous types of cancer, whereas some Chinese
medicinal herbs harbor anticancer effects by targeting miRNAs. Liu
et al revealed that berberine may regulate cisplatin
sensitivity via mediating the miR-21/programmed cell death 4 axis
in the ovarian cancer cells (18). Zeng et al demonstrated that
camptothecin promotes cancer cell apoptosis via miRNA-mediated
mitochondrial pathways (36).
Furthermore, a recent study identified that HNK triggers the liver
kinase B1-miR-34a axis and resists the oncogenic effects of leptin
in breast cancer (11). Previous
studies have also indicated that miR-34a modulates tumor cell
growth and cell cycle progression in OS (37,38). The present study revealed that HNK
induced aberrant miRNA expression in a breast cancer cell line,
according to microarray data obtained from the GEO database
(accession no. GSE85871; http:/www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE85871).
Based on these data, six miRNAs, which were most significantly
upregulated or downregulated in breast cancer cells following HNK
treatment, were selected and RT-qPCR was conducted to validate
these results in human OS cells. The results demonstrated that
miR-202 and miR-623 were significantly upregulated, whereas miR-21
was significantly downregulated in the Saos-2 human OS cell line,
whereas miR-188-5p, miR-532-5p and miR-628-3p were not
significantly different compared with in the vehicle group. In
addition, the present study indicated that HNK treatment reduced
miR-21 expression in a dose-dependent manner in human OS cells.
Conversely, restoration of miR-21 expression abrogated the
suppressive effects of HNK on OS cells. These data indicated that
HNK may reduce cell viability and induce apoptosis via inhibiting
miR-21 expression in human OS cells. Previous studies also reported
that HNK induces apoptosis and G1 cell cycle arrest in
various types of cancer cell (39–42). Furthermore, miR-21 has been
identified as an important regulator of diverse cellular functions
via the regulation of cancer cell growth (43–45). However, the possible molecular
mechanism underlying HNK-induced apoptosis of human OS cells
requires further research.
Increasing evidence has confirmed that miR-21
modulates PTEN by directly targeting its 3′-UTR in various types of
cancer, including hepatocellular cancer (24) and lung cancer (46). PTEN is a tumor suppressor gene
that regulates the cell cycle and apoptosis in numerous solid
tumors (47). Consistent with
these results, the present study confirmed that miR-21 inhibits
PTEN by directly targeting its 3′-UTR in human OS cells. Datta
et al revealed that the PI3K/AKT signaling pathway acts as a
key oncogenic pathway to induce cell growth and survival (48), which is negatively regulated by
PTEN (49). Furthermore, Bai
et al reported that the PI3K/AKT signaling pathway may be
modulated by miR-21 in hepatocytes (50). The present study revealed that HNK
suppresses the PI3K/AKT signaling pathway; however, it could be
reactivated by miR-21 overexpression. Taken together, these data
indicated that HNK induced apoptosis of human OS cells via
modulating the miR-21/PTEN/PI3K/AKT signaling pathway.
In conclusion, the present study provided a novel
insight into the molecular mechanism underlying HNK-induced
apoptosis of human OS cells. Notably, the present results confirmed
that HNK induces aberrant miRNA expression and HNK-induced
apoptosis was modulated by the miR-21/PTEN/PI3K/AKT signaling
pathway in human OS cells. These results suggested that HNK may
exert anticancer effects to prevent OS progression.
Notes
[1] Competing
interests
The authors declare there is no competing
interest.
References
|
1
|
Hung GY, Horng JL, Yen HJ, Yen CC, Chen
WM, Chen PC, Wu HT and Chiou HJ: Incidence patterns of primary bone
cancer in taiwan (2003–2010) A population-based study. Ann Surg
Oncol. 21:2490–2498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steinmann P, Walters DK, Arlt MJ, Banke
IJ, Ziegler U, Langsam B, Arbiser J, Muff R, Born W and Fuchs B:
Antimetastatic activity of honokiol in osteosarcoma. Cancer.
118:2117–2127. 2012. View Article : Google Scholar
|
|
6
|
Ho KY, Tsai CC, Chen CP, Huang JS and Lin
CC: Antimicrobial activity of honokiol and magnolol isolated fro
Magnolia officinalis. Phytother Res. 15:139–141. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ou HC, Chou FP, Lin TM, Yang CH and Sheu
WH: Protective effects of honokiol against oxidized LDL-induced
cytotoxicity and adhesion molecule expression in endothelial cells.
Chem Biol Interact. 161:1–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai X, Cerimele F, Ushio-Fukai M, Waqas M,
Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, et
al: Honokiol, a small molecular weight natural product, inhibits
angiogenesis in vitro and tumor growth in vivo. J Biol Chem.
278:35501–35507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishitsuka K, Hideshima T, Hamasaki M, Raje
N, Kumar S, Hideshima H, Shiraishi N, Yasui H, Roccaro AM,
Richardson P, et al: Honokiol overcomes conventional drug
resistance in human multiple myeloma by induction of
caspase-dependent and -independent apoptosis. Blood. 106:1794–1800.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen F, Wang T, Wu Y-F, Gu Y, Xu X-L,
Zheng S and Hu X: Honokiol: A potent chemotherapy candidate for
human colorectal carcinoma. World J Gastroenterol. 10:3459–3463.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Avtanski DB, Nagalingam A, Kuppusamy P,
Bonner MY, Arbiser JL, Saxena NK and Sharma D: Honokiol abrogates
leptin-induced tumor progression by inhibiting Wnt1-MTA1-β-catenin
signaling axis in a microRNA-34a dependent manner. Oncotarget.
6:16396–16410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheu ML, Liu SH and Lan KH: Honokiol
induces calpain-mediated glucose-regulated protein-94 cleavage and
apoptosis in human gastric cancer cells and reduces tumor growth.
PLoS One. 2:e10962007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YJ, Wu CL, Liu JF, Fong YC, Hsu SF,
Li TM, Su YC, Liu SH and Tang CH: Honokiol induces cell apoptosis
in human chondrosarcoma cells through mitochondrial dysfunction and
endoplasmic reticulum stress. Cancer Lett. 291:20–30. 2010.
View Article : Google Scholar
|
|
14
|
Hahm ER and Singh SV: Honokiol causes
G0-G1 phase cell cycle arrest in human prostate cancer cells in
association with suppression of retinoblastoma protein
level/phosphorylation and inhibition of E2F1 transcriptional
activity. Mol Cancer Ther. 6:2686–2695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crane C, Panner A, Pieper RO, Arbiser J
and Parsa AT: Honokiol mediated inhibition of PI3K/mTOR pathway: A
potential strategy to overcome immunoresistance in glioma, breast
and prostate carcinoma without impacting T cell function. J
Immunother. 32:585–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamani M, Sadeghizadeh M, Behmanesh M and
Najafi F: Dendrosomal curcumin increases expression of the long
non-coding RNA gene MEG3 via up-regulation of epi-miRs in
hepatocellular cancer. Phytomedicine. 22:961–967. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Fang Y, Shen H, Xu W and Li H:
Berberine sensitizes ovarian cancer cells to cisplatin through
miR-21/PDCD4 axis. Acta Biochim Biophys Sin (Shanghai). 45:756–762.
2013. View Article : Google Scholar
|
|
19
|
Hong M, Wang N, Tan HY, Tsao SW and Feng
Y: MicroRNAs and Chinese Medicinal Herbs: New possibilities in
cancer therapy. Cancers (Basel). 7:1643–1657. 2015. View Article : Google Scholar
|
|
20
|
Zhang Q, Zhao W, Ye C, Zhuang J, Chang C,
Li Y, Huang X, Shen L, Li Y, Cui Y, et al: Honokiol inhibits
bladder tumor growth by suppressing EZH2/miR-143 axis. Oncotarget.
6:37335–37348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai YJ, Lin CI, Wang CL and Chao JI:
Expression of survivin and p53 modulates honokiol-induced apoptosis
in colorectal cancer cells. J Cell Biochem. 115:1888–1899.
2014.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Battle TE, Arbiser J and Frank DA: The
natural product honokiol induces caspase-dependent apoptosis in
B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood.
106:690–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu YR, Qi HJ, Deng DF, Luo YY and Yang SL:
MicroRNA-21 promotes cell proliferation, migration, and resistance
to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal
cancer. Tumour Biol. 37:12061–12070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar
|
|
27
|
Guo H, German P, Bai S, Barnes S, Guo W,
Qi X, Lou H, Liang J, Jonasch E, Mills GB, et al: The PI3K/AKT
pathway and renal cell carcinoma. J Genet Genomics. 42:343–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ayoub N, Singab AN, El-Naggar M and
Lindequist U: Investigation of phenolic leaf extract of Heimia
myrtifolia (Lythraceae): Pharmacological properties (stimulation of
mineralization of SaOS-2 osteosarcoma cells) and identification of
polyphenols. Drug Discov Ther. 4:341–348. 2010.PubMed/NCBI
|
|
30
|
Sumiyoshi M, Taniguchi M, Baba K and
Kimura Y: Antitumor and antimetastatic actions of xanthoangelol and
4-hydroxyderricin isolated from Angelica keiskei roots through the
inhibited activation and differentiation of M2 macrophages.
Phytomedicine. 22:759–767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia YZ, Ni K, Guo C, Zhang C, Geng YD,
Wang ZD, Yang L and Kong LY: Alopecurone B reverses
doxorubicin-resistant human osteosarcoma cell line by inhibiting
P-glycoprotein and NF-kappa B signaling. Phytomedicine. 22:344–351.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang T, Gong X, Jiang R, Li H, Du W and
Kuang G: Ferulic acid inhibits proliferation and promotes apoptosis
via blockage of PI3K/Akt pathway in osteosarcoma cell. Am J Transl
Res. 8:968–980. 2016.PubMed/NCBI
|
|
33
|
Er S and Dikmen M: Camellia sinensis
increased apoptosis on U2OS osteosarcoma cells and wound
healing potential on NIH3T3 fibroblast cells.
Cytotechnology. May 16–2017.Epub ahead of print. View Article : Google Scholar
|
|
34
|
Steinmann P, Walters DK, Arlt MJ, Banke
IJ, Ziegler U, Langsam B, Arbiser J, Muff R, Born W and Fuchs B:
Antimetastatic activity of honokiol in osteosarcoma. Cancer.
118:2117–2127. PubMed/NCBI
|
|
35
|
Raja SM, Chen S, Yue P, Acker TM, Lefkove
B, Arbiser JL, Khuri FR and Sun SY: The natural product honokiol
preferentially inhibits cellular FLICE-inhibitory protein and
augments death receptor-induced apoptosis. Mol Cancer Ther.
7:2212–2223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeng CW, Zhang XJ, Lin KY, Ye H, Feng SY,
Zhang H and Chen YQ: Camptothecin induces apoptosis in cancer cells
via microRNA-125b-mediated mitochondrial pathways. Mol Pharmacol.
81:578–586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Novello C, Pazzaglia L, Conti A, Quattrini
I, Pollino S, Perego P, Picci P and Benassi MS: p53-dependent
activation of microRNA-34a in response to etoposide-induced DNA
damage in osteosarcoma cell lines not impaired by dominant negative
p53 expression. PLoS One. 9:e1147572014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Tu MJ, Yu YF, Wang WP, Chen QX,
Qiu JX, Yu AX and Yu AM: Combination therapy with bioengineered
miR-34a prodrug and doxorubicin synergistically suppresses
osteosarcoma growth. Biochem Pharmacol. 98:602–613. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin CJ, Chang YA, Lin YL, Liu SH, Chang CK
and Chen RM: Preclinical effects of honokiol on treating
glioblastoma multiforme via G1 phase arrest and cell apoptosis.
Phytomedicine. 23:517–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo LX, Li Y, Liu ZQ, Fan XX, Duan FG, Li
RZ, Yao XJ, Leung EL and Liu L: Honokiol induces apoptosis, G1
arrest, and autophagy in KRAS mutant lung cancer cells. Front
Pharmacol. 8:1992017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen HC, Hsu HT, Weng JW, Chang YF, Hsia
CY, Lee HC and Chi CW: Combined effect of honokiol and
rosiglitazone on cell growth inhibition through enhanced G0/G1
phase arrest in hepatoma cells. J Chin Med Assoc. 79:415–421. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Benhamouche-Trouillet S and Postic C:
Emerging role of miR-21 in non-alcoholic fatty liver disease. Gut.
65:1781–1783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu B, Xia H, Cao J, Wang Z, Yang Y and Lin
Y: MicroRNA-21 inhibits the apoptosis of osteosarcoma cell line
SAOS-2 via targeting caspase-8. Oncol Res. Jan 20–2017.Epub ahead
of print. View Article : Google Scholar
|
|
44
|
Wu YR, Qi HJ, Deng DF, Luo YY and Yang SL:
MicroRNA-21 promotes cell proliferation, migration, and resistance
to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal
cancer. Tumour Biol. 37:12061–12070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lv C, Hao Y and Tu G: MicroRNA-21 promotes
proliferation, invasion and suppresses apoptosis in human
osteosarcoma line MG63 through PTEN/Akt pathway. Tumour Biol.
37:9333–9342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Z, Fang S, Di Y, Ying W, Tan Y and Gu
W: Modulation of NF-κB/miR-21/PTEN pathway sensitizes non-small
cell lung cancer to cisplatin. PLoS One. 10:e01215472015.
View Article : Google Scholar
|
|
47
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One.
9:e1036982014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bai YN, Yu ZY, Luo LX, Yi J, Xia QJ and
Zeng Y: MicroRNA-21 accelerates hepatocyte proliferation in vitro
via PI3K/Akt signaling by targeting PTEN. Biochem Biophys Res
Commun. 443:802–807. 2014. View Article : Google Scholar
|