Introduction
Cataracts are a cause major cause of blindness
worldwide. It is reported that a number of factor lead to the
development and progression of cataracts, including
H2O2, glucose and prolonged exposure to
sunlight (1,2). Ultraviolet B (UVB) is associated
with the formation of cataracts leading to cortical lens opacity.
The lens epithelium is a single layer of cells that regulates lens
homeostasis; however, the lens epithelium is exposed to UVB
irradiation, as the top layer of cells in the eye (3). Even relatively low UVB irradiation
damages the lens epithelium and causes a loss of ion homeostasis,
which contributes to the development of cataracts in the aging
population (4). The precise
mechanism of the association of UVB irradiation with cataract
development in humans unknown.
Apoptosis is a regulated cell death mechanism that
maintains homeostasis, and has a role in cataract formation in
humans (5). Loss of mitochondrial
transmembrane potential and the production of reactive oxygen
species (ROS) cause damage to cells and induce apoptosis. UVB
irradiation induces apoptosis through a number of molecular
processes, such as targeting Bcl-2 family proteins, and activating
caspase and cell apoptosis signaling pathways (6). Bcl-2 has a negative role in the
cellular apoptotic pathway, whereas Bax, a Bcl-2 homologue, can
reverse the inhibitory effect of Bcl-2 in apoptosis. A previous
study demonstrated that Bax expression and caspase-3 activity were
decreased in cataracts, suggesting a pivotal role in cataract
formation (7). The
mitogen-activated protein kinases (MAPKs), including extracellular
signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and
p38 MAPK, are involved in the regulation of cell apoptosis and are
activated during UVB-induced apoptosis of human retinal pigment
epithelial cells (8,9).
Oxidative stress caused by ROS has been reported in
numerous diseases, particularly aged-associated disorders, such as
cataracts (10). Previous studies
demonstrated that the lens has an antioxidant system to counter the
toxic damage of ROS, including the activity of antioxidantive
enzymes and antioxidants, such as superoxide dismutase (SOD),
catalase (CAT), glutathione peroxidase (GSH-PX) and
malondialde-hyde (MDA) (11-13). Chang et al (14) reported that the serum SOD, CAT and
GSH-PX activities were decreased and the level of MDA was increased
in patients with cataracts.
Cellular calcium (Ca2+) homeostasis in
the lens epithelial cells is essential for maintaining lens
clarity. Inappropriate Ca2+ signaling and abnormal
Ca2+ levels are associated with various clinical
disorders, including stroke, cardiac dysfunction, obesity, aging
and Alzheimer's diseases (15-18). Ca2+ is also involved in
cell growth, differentiation and motility during the transformation
and progression of cancer. Calmodulin like 3 (CALML3) is an
epithelial-specific calcium sensing protein that highly expressed
in oral mucosa, skin, breast and prostate (19,20). A previous study reported that
CALML3 is regulated by short-term blockade of the MaxiK channel
associated with an increase in basal Ca2+ concentration
(21). However, the function of
CALML3 in cataracts has not been fully elucidated and requires
further investigation.
The current study investigated the effect of UVB
irradiation on apoptosis, and whether oxidative stress,
Ca2+ homeostasis and apoptosis-associated signaling
pathways have a role in UVB-induced apoptosis in human lens
epithelium cells.
Materials and methods
Cell culture
Human lens epithelial cells (HLEC; an immortalized
cell line) used in this study obtained from Cell Bank of Academia
Sinica (Shanghai, China), were cultured in Dulbecco's modified
Eagle's medium (DMEM)-high glucose (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS) and 1% penicillin-streptomycin solution in an incubator
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C with 5%
CO2. Cells were treated with 0.04 mM nimodipine for 24
h
UVB treatment
The UVB treatment was performed by using a UVB
radiometer with a total output of 4 W/m2 (wavelengths of
297 nm). Prior to irradiation, the HLECs were washed twice with
phosphate-buffered saline (PBS). The HLECs were then irradiated at
the desired intensity and time: 60 min at 0.5, 1, 2 and 4
W/m2; 2 W/m2 for 5, 15, 30, 60, 90, 120 and
180 min. Following UVB irradiation the HLECs were incubated in
DMEM-high glucose (HyClone; GE Healthcare Life Sciences)
supplemented with 10% FBS and 1% penicillin-streptomycin solution
in an incubator (Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2.
Lentivirus and small interfering RNA
transduction
The CALML3 coding sequence (GENEWIZ lnc., Suzhou,
China) was cloned into the pLVX-AcGFP-C1 lenti-virus vector
(Addgene, Inc., Cambridge, MA, USA). Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect
small interfering RNA (siRNA; 40 nM; 5′-AUCUCUCUGUUGCUACUCA-3′)
targeting human CALML3 mRNA (position 1288-1310
5′-ACCUGUUCCUAUUCAUCUG-3′) and constructs according to the
manufacture's instruction. Empty lentivirus vector and nonspecific
siRNA were used as the negative control (NC). Cells were analyzed
at 48 h after transduction and transfection.
Flow cytometry analysis of apoptosis, ROS
production and calcium concentration
Prior to analysis of analysis of apoptosis, ROS
production and calcium concentration, the HLECs were trypsinized,
centrifuged and resuspended twice with PBS. The HLECs
(1×105 cell/ml) were treated with UVB with 2
W/m2 for 60 min, and subsequently collected and
incubated with 5 μl Annexin V buffer and 5 μl
propidium iodide (PI) for 10 min for apoptosis detection, incubated
with medium containing 10 μM dichlorodihydrofluorescein
diacetate (DCFH-DA) for 30 min for ROS detection and incubated with
Fluo-3 (10 μM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 30 min for intracellular calcium concentration
detection.
Determination of SOD, MDA, CAT and GSH-PX
activities
The HLECs (1×105 cell/ml) were treated
with UVB with 2 W/m2 for 60 min. The SOD (WST-1 method),
MDA (TBA method), CAT (visible light) and GSH-PHX assay kits
(obtained from Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) were used to determine the antioxidant activities. SOD, MDA,
CAT and GSH-PHX activities were measured at the absorbance of 450,
532, 405 and 412 nm, respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was isolated with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. Complementary DNA was synthesized using a
cDNA synthesis kit (Thermo Fisher Scientific, Inc.). The cDNA
synthesis conditions were as follows: 37°C for 60 min, followed by
85°C for 5 min and 4°C for 5 min. qPCR was performed to detect the
mRNA levels of the indicated genes. qPCR was performed on ABI 7300
(Applied Biosystems; Thermo Fisher Scientific, Inc.) thermal cycler
using a standard SYBR-Green PCR kit (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. The qPCR
cycling conditions were as follows: 95°C for 10 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 45 sec, and a final
extension step of 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec
and 60°C for 15 sec. The relative mRNA expression of target gene
compared with GAPDH was calculated using the 2−ΔΔCq
(22) method. The primers used in
this study listed in Table I.
 | Table IPrimers sequences used in this
study. |
Table I
Primers sequences used in this
study.
| Gene | Forward | Reverse |
|---|
| CALML3 |
5′-GGCCAGGTCAATTATGAAG-3′ |
5′-TCAGGGAAGAAGGAGAAAG-3′ |
| Caspase-3 |
5′-AACTGGACTGTGGCATTGAG-3′ |
5′-ACAAAGCGACTGGATGAACC-3′ |
| Bax |
5′-AGCTGAGCGAGTGTCTCAAG-3′ |
5′-TGTCCAGCCCATGATGGTTC-3′ |
| Bcl-2 |
5′-AGACCGAAGTCCGCAGAACC-3′ |
5′-GAGACCACACTGCCCTGTTG-3′ |
| GAPDH |
5′-CACCCACTCCTCCACCTTTG-3′ |
5′-CCACCACCCTGTTGCTGTAG-3′ |
Western blot assay
HLECs were seeded at a density of 5×105
cells per well in 6-well plates, cultured overnight and then
treated with UVB 2 W/m2 for 60 and 180 min. Total
proteins were isolated from the HLECs and were subjected to 12%
SDS-PAGE, electroblotted onto to a polyvinylidene fluoride membrane
(Roche Diagnostics, GmbH, Mannheim, Germany). Following blocking
with 5% skimmed milk at 4°C overnight, membranes were first
incubated with mouse polyclonal antibody anti-CALML3 (cat. no.
ab88761; 1:1,000); rabbit monoclonal antibodies anti-caspase-3
(cat. no. ab32351; 1:5,000), anti-Bax (cat. no. ab32503; 1:1,000;
all from Abcam, Cambridge, MA, USA), anti-ERK1/2 (cat. no. 4695;
1:1,000), anti-phospho-ERK1/2 (cat. no. 4376; 1:800) and anti-GAPDH
(cat. no. 5174; 1:1500; all from Cell Signaling Technology, Inc.,
Danvers, MA, USA); mouse monoclonal antibodies anti-Bcl-2 (cat. no.
ab117115; 1:1,000; Abcam) and antibodies anti-phospho-JNK1/2 (cat.
no. 9255; 1:1,000); rabbit poly-clonal antibodies anti-JNK1/2 (cat.
no. 9252; 1:1,000; both from Cell Signaling Technology, Inc.).
Blots were then incubated with goat anti-mouse or anti-rabbit
secondary antibody (cat. nos. A0208 and A0216; 1:1,000; Beyotime
Institute of Biotechnology, Haimen, China) and visualized using
enhanced chemiluminescence (Thermo Fisher Scientific, Inc.). GAPDH
antibody was used as an internal control. The blotting bands were
quantified with ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
The data was presented as the mean value + standard
deviation. Data were analysed by one-way analysis of variance
followed by Tukey's post hoc test. Statistical analyses were
performed using GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
UVB induces apoptosis of HLECs
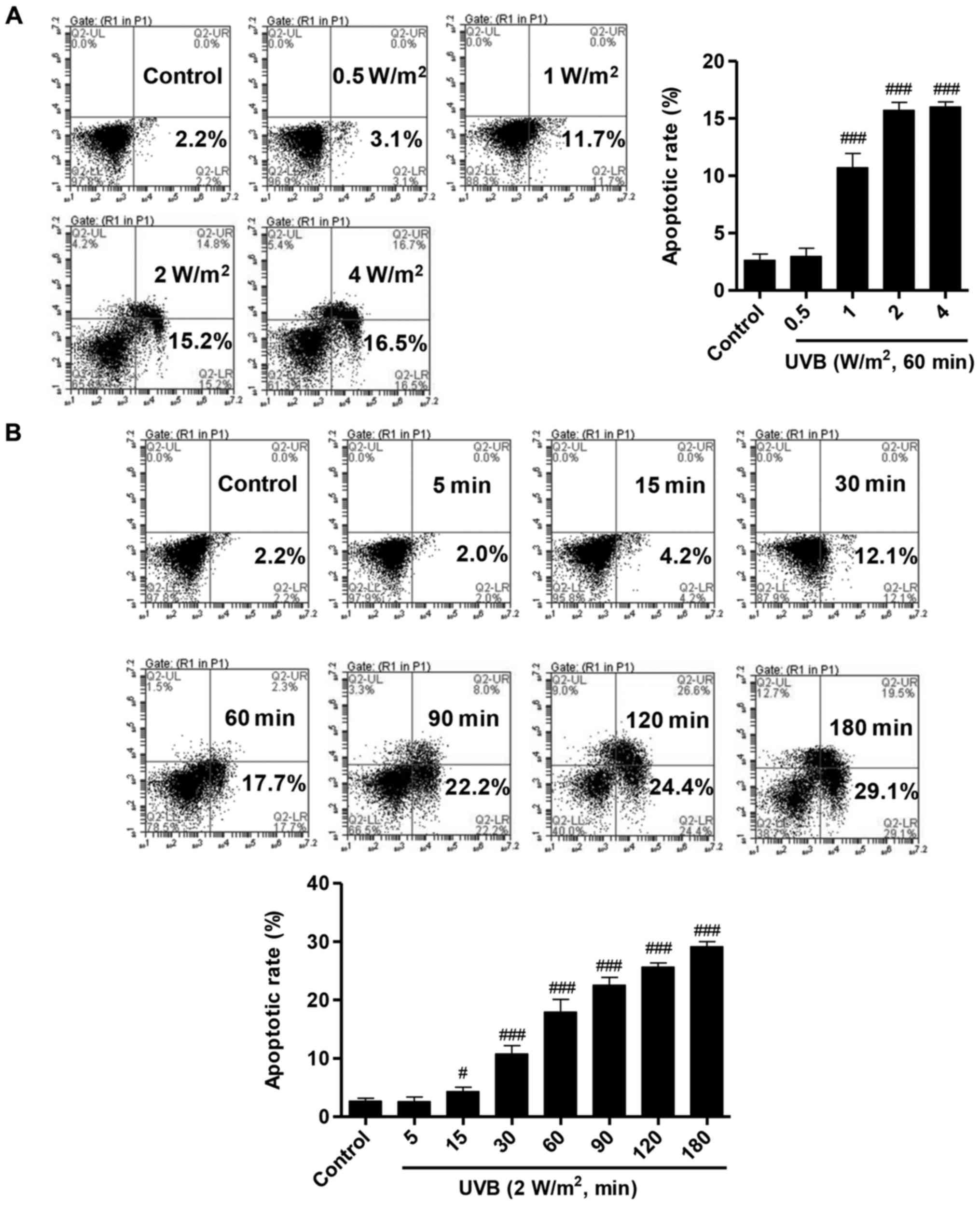
To investigate the effects of UVB irradiation on
HLECs, the HLECs were treated with several of doses of UVB (0.5, 1,
2 and 4 W/m2) for 60 min. Apoptosis analysis was
performed following UVB irradiation using Annexin V/PI labeling
followed by flow cytometry. The percentage of apoptotic cells in
HLECs with different doses of UVB irradiation was significantly
increased compared with that of the control group without UVB
irradiation (Fig. 1A). There was
no significant difference in the number of apoptotic between the
groups treated with 2 W/m2 and 4 W/m2 UVB
irradiation, thus 2 W/m2 UVB irradiation was used in
subsequent experiments. To further determine the effects of UVB
irradiation on apoptosis of HLECs, the cells were also treated with
2 W/m2 UVB for various durations (5, 15, 30, 60, 90, 120
and 180 min). After 15 min irradiation, the number of apoptotic
cells ~4.2%, and the percent of apoptotic cells was increased with
the increasing duration of UVB irradiation (Fig. 1B). These results observed that UVB
irradiation induces apoptosis in the HLECs in dose- and
time-dependent manners.
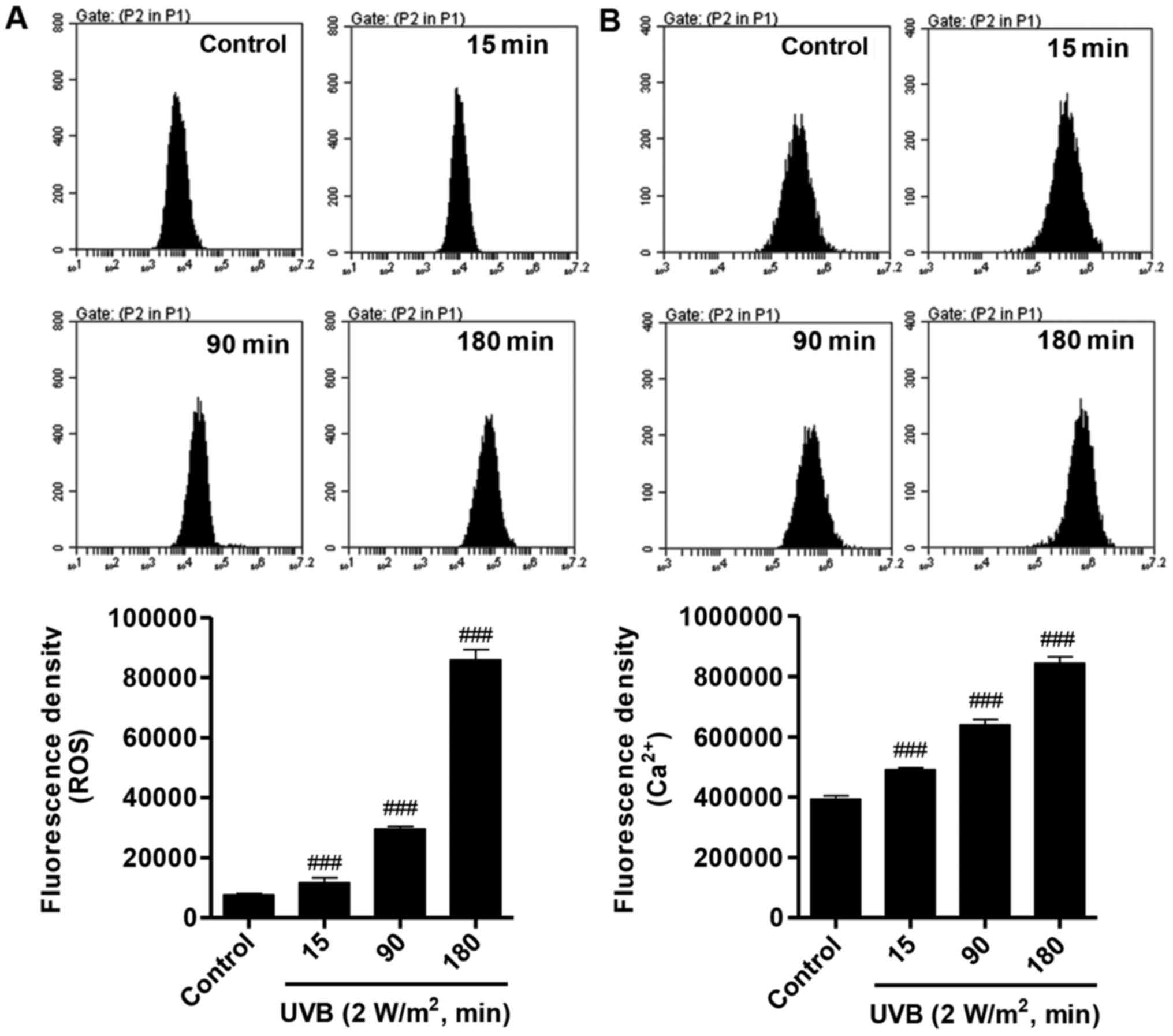
UVB increases ROS production and
intracellular Ca2+ concentration
A number of studies have reported that oxidative
stress is associated with the cell damage that occurs during
exposure to UV irradiation. Whether UVB irradiation can affect the
ROS production and intracellular Ca2+ concentration was
investigated. DCFH-DA is a dye that reacts with ROS used for the
detection of ROS level. The results demonstrated that ROS was
notably increased following 2 W/m2 UVB irradiation in a
time-dependent manner, compared with the control group (Fig. 2A). Similarly, following 2
W/m2 UVB irradiation, the intracellular Ca2+
concentration was increased in a time-dependent manner (Fig. 2B). These data indicated that UVB
irradiation increases oxidative stress in HLECs.
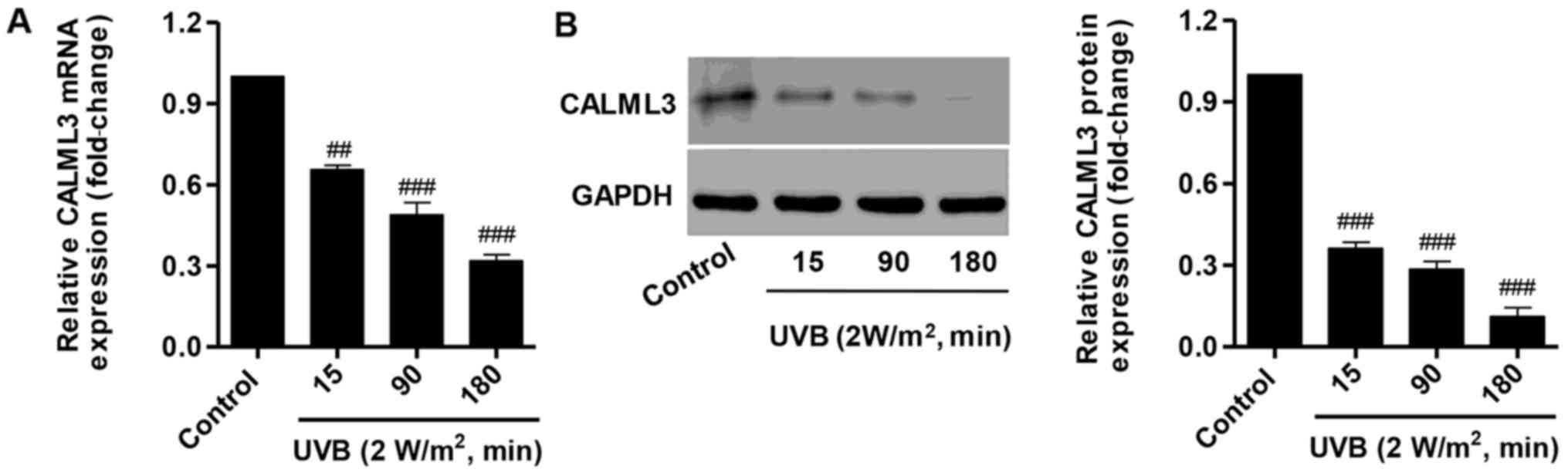
Effect of UVB on CALML3 expression
To further examine the effect of UVB irradiation on
calcium metabolism, CALML3 expression was determined using RT-qPCR
and western blot analysis. Following treatment with 2
W/m2 UVB for 15, 90 and 180 min, CALML3 was
significantly decreased at the mRNA and protein levels (Fig. 3). These results suggest that the
pro-apoptotic activity of UVB may be associated with downregulating
CALML3 expression.
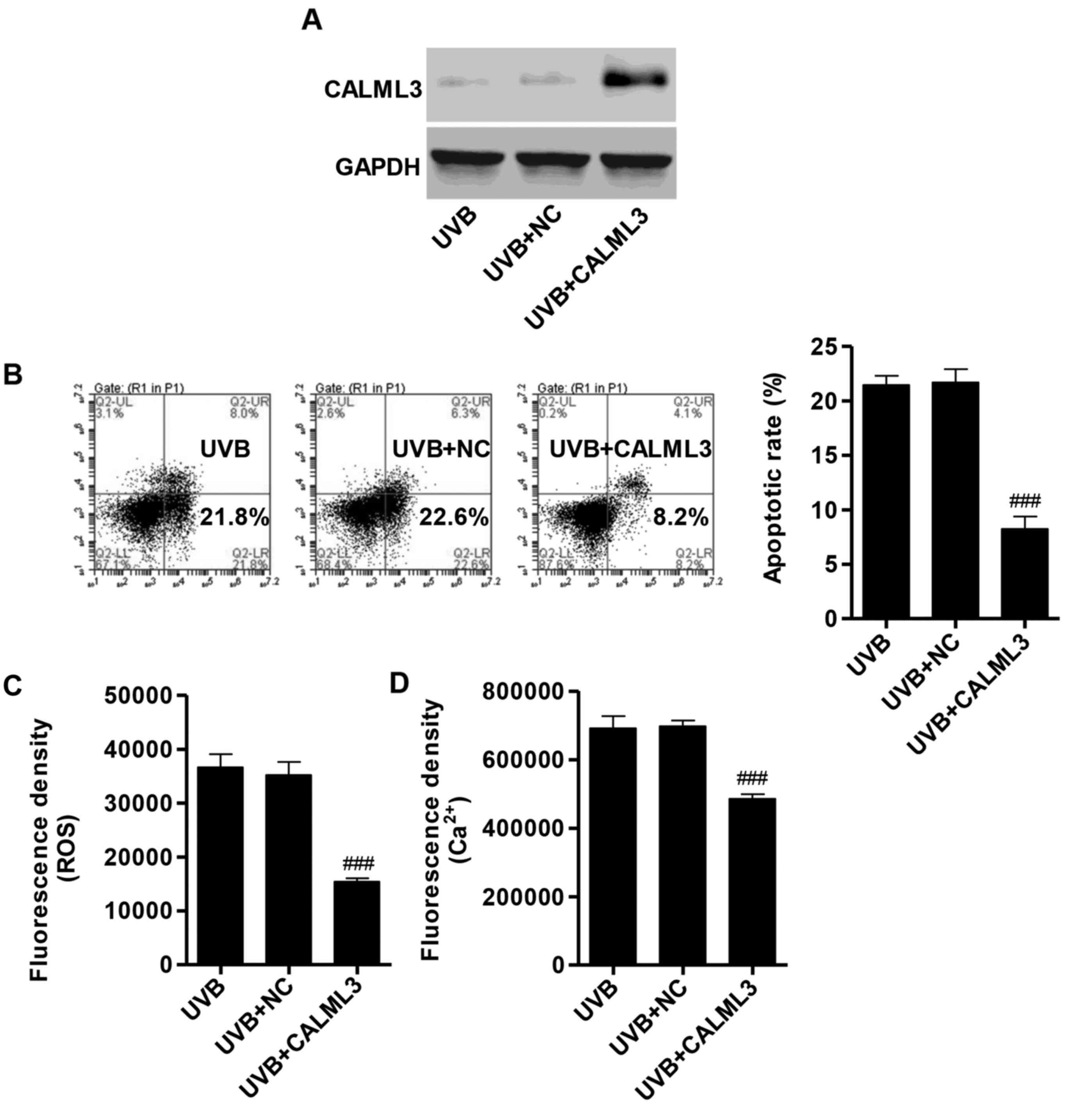
Overexpression of CALML3 reduces
apoptosis, ROS production and intracellular Ca2+
concentration
To elucidate whether the pro-apoptotic activity of
UVB irradiation was associated with the downregulation of CALML3
expression in HLECs, a CALML3 overexpression vector was introduced
into HLECs. The expression of CALML3 in the overexpression group
was increased compared with the NC group, measured by western blot
analysis (Fig. 4A). In HLECs
treated with 2 W/m2 UVB irradiation for 90 min, the
percentage of apoptotic cells in CALML3 overexpression group was
significantly reduced compared with the NC group (Fig. 4B). Furthermore, flow cytometry
revealed that the fluorescence densities of ROS and Ca2+
were significantly reduced in CALML3-overexpressed HLECs following
UVB irradiation (Fig. 4C and D).
These results demonstrated that the apoptotic effect of UVB may
mainly through downregulation of CALML3.
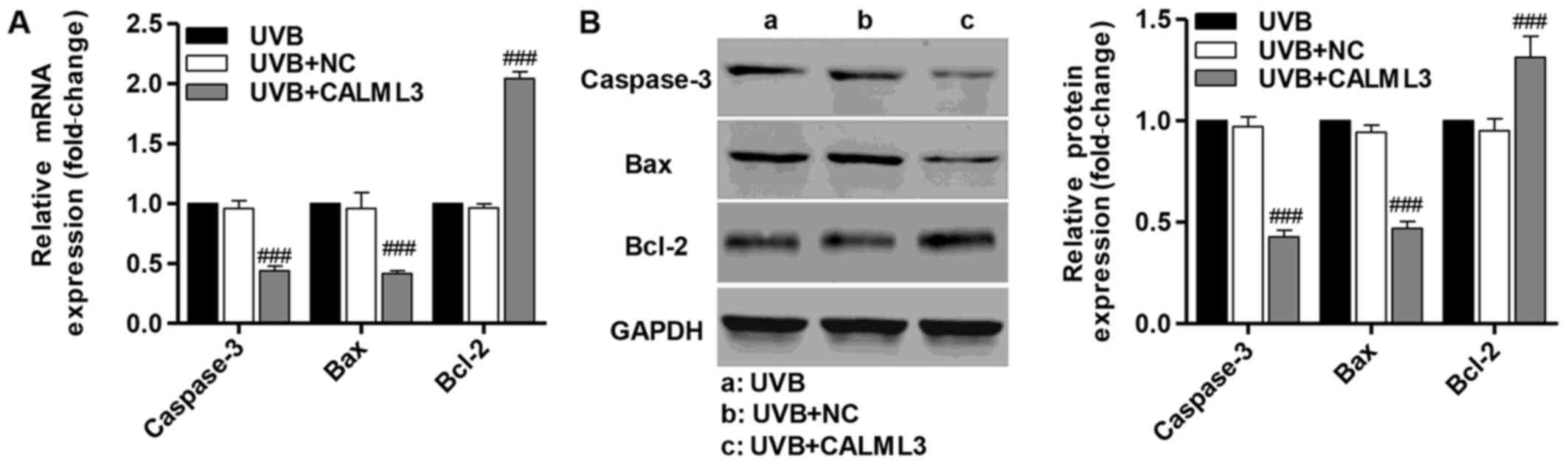
Overexpression of CALML3 alters the
expression of apoptosis-associated proteins
RT-qPCR and western blot analysis were performed
following UVB treatment for 180 min to detect caspase-3, Bax and
Bcl-2 in UVB-induced HLECs in the presence of CALML3
overexpression. CALML3 overexpression markedly increased the
expression of the anti-apoptotic protein Bcl-2, and reduced the
expression of the pro-apoptotic proteins caspase-3 and Bax compared
with the negative control UVB-induced HLECs without CALML3
overexpression (Fig. 5). These
findings demonstrate that CALML4 regulates the expression of
apoptosis-associated proteins and may be involved in UVB-induced
HLEC apop-tosis.
Measurement of SOD, MDA, CAT and GSH-PX
levels
To determine potential changes of SOD, MDA, CAT and
GSH-PX in HLECs upon UVB irradiation, biochemistry assay kits were
used to measure the oxidative stress-induced alteration in HLECs.
Compared with the NC group with 2 W/m2 UVB irradiation
for 90 min, the SOD, CAT and GSH-PX levels of HLECs were
significantly increased by CALML3 overexpression (Fig. 6A–C). Contrary to the increased
SOD, CAT and GSH-PX levels, the MDA level of HLECs in the presence
of UVB irradiation was decreased by CALML3 overexpression (Fig. 6D). These results indicated that
CALML3 overexpression can reverse the UVB-induced oxidative stress
in HLECs.
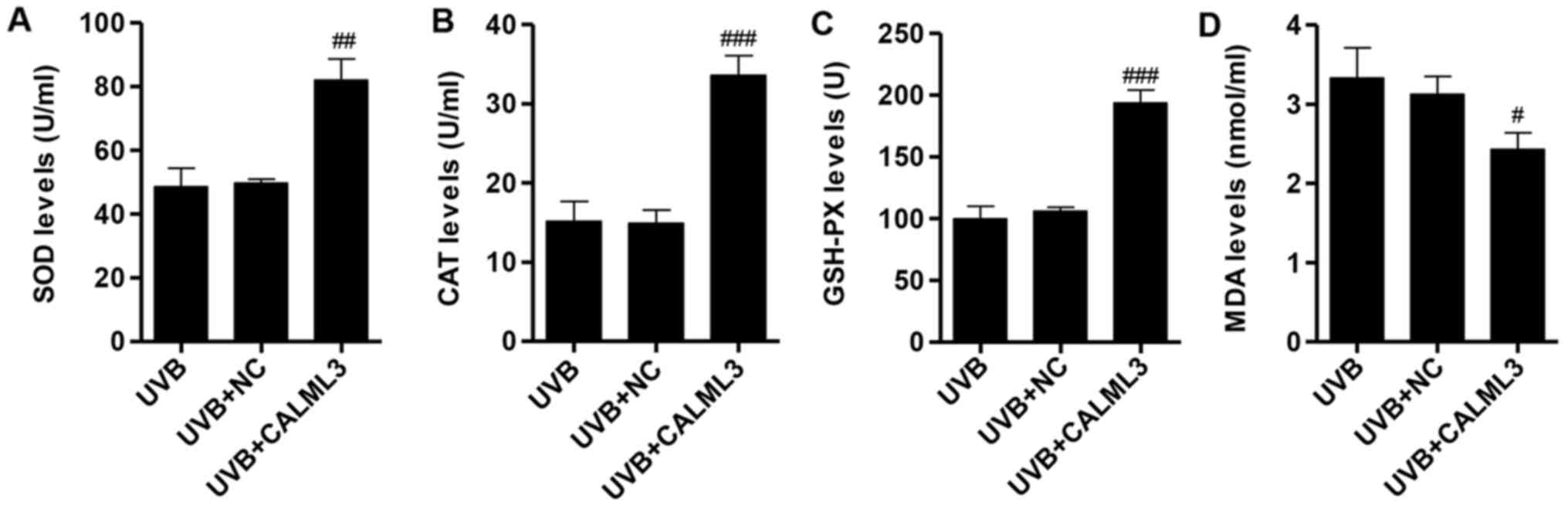 | Figure 6Evaluation of oxidative stress after
exposure with UVB irradiation in CALML3-overexpressed HLECs. The
levels of (A) SOD, (B) CAT and (C) GSH-PX were significantly
increased in UVB-induced HLECs with CALML3 overexpression. (D) MDA
was significantly decreased in UVB-induced HLECs with CALML3
overexpression. #P<0.05, ##P<0.01 and
###P<0.001 vs. UVB. SOD, superoxide dismutase; CAT,
catalase; GSH-PX, glutathione peroxidase; MDA, malondialdehyde;
CALML3, calmodulin like 3; UVB, ultraviolet B; NC, negative
control; HLECs, human lens epithelial cells. |
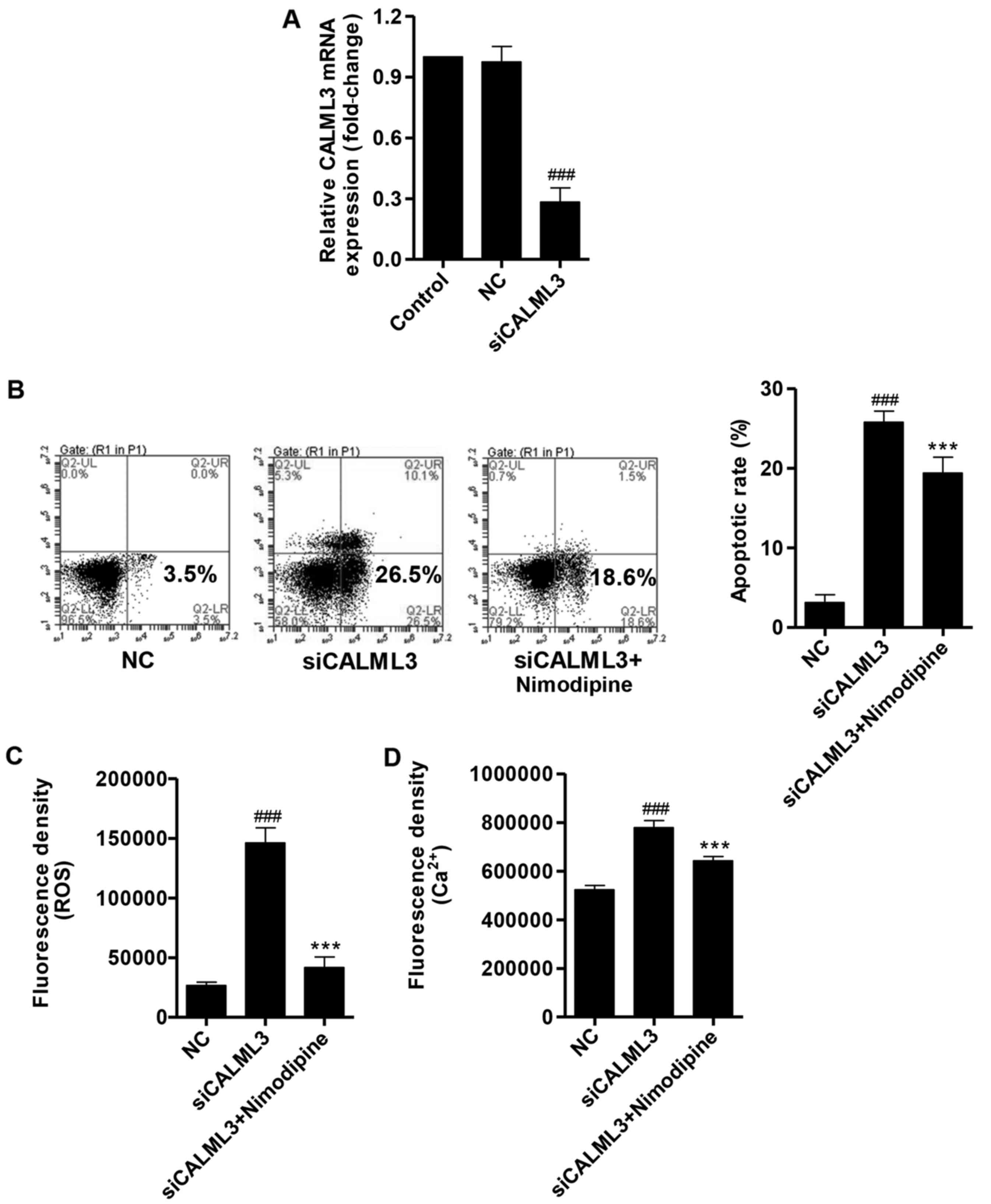
Silencing of CALML3 increases apoptosis,
ROS production and intracellular Ca2+ concentration
To elucidate whether CALML3 downregulation has
similar effects to UVB irradiation, CALML3 siRNA (siCALML3) was
transfected into HLECs and the expression of CALML3 was significant
decreased by the siRNA compared with NC group, as measured by
RT-qPCR (Fig. 7A). The number of
apoptotic HLECs was significantly increased by CALML3
downregulation compared with NC, however, when combined with
nimodipine, a Ca2+-channel antagonist, apoptosis was
significantly decreased compared with siCALML3 (Fig. 7B). The fluorescence densities of
ROS and Ca2+ in HLECs were significantly increased by
siCALML3 compared with NC, and nimodipine decreased the levels of
ROS and Ca2+ compared with the siCALML3 group (Fig. 7C and D). Nimodipine treatment
significantly reduced the modulations in apoptosis, ROS production,
and intracellular Ca2+ concentration induced by siCALML4
treatment alone, (Fig. 7B–D).
These results suggest that the calcium signaling pathway is
associated with the CALML3-induced apoptosis in HLECs.
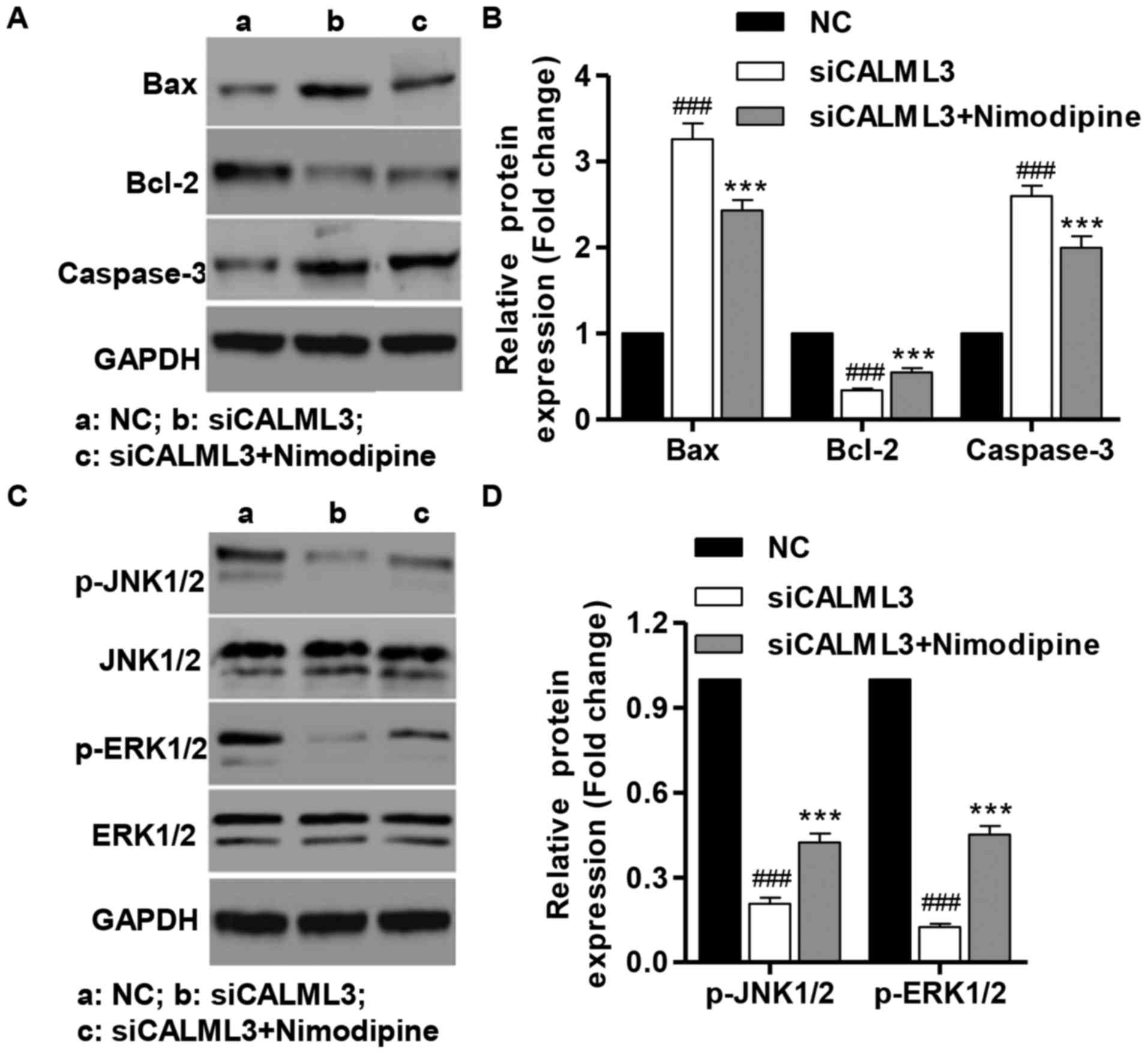
Effect of CALML3 silencing in
apoptosis-associated proteins, and activation of JNK1/2 and
ERK1/2
Western blot analysis was performed after UVB
treatment for 180 min to detect Bax, Bcl-2 and caspase-3 in HLECs.
CALML3 downregulation markedly decreased the expression of the
anti-apoptotic protein Bcl-2, and increased the expression of the
pro-apoptotic proteins caspase-3 and Bax compared with the NC group
(Fig. 8A and B). Furthermore,
CALML3 down-regulation markedly suppressed the activation of JNK1/2
and ERK1/2 compared with the NC group (Fig. 8C and D) after UVB treatment for 60
min of HLECs. However, nimodipine treatment significantly inhibited
the effects of siCALML3 in HLECs. These findings indicate that
JNK1/2 and ERK1/2 are involved in apoptosis induced by CALML3
downregulation in HLECs.
Discussion
Cataracts are a major cause of blindness and the
only cure is surgical removal of the lens and replacement with a
clear one. HLECs are the center of metabolic activities in lens,
and to prevent the onset of cataract is important determine the
mechanisms of cataract development. UVB is a part of the sunlight
spectrum and can cause retina damaged even <1% of the UVB
penetrates it. In the present study, the effects of UVB irradiation
on apoptosis, ROS production and Ca2+ concentration of
HLECs were examined using different intensities and duration of UVB
exposure. Following different intensities and times of exposure to
UVB irradiation, the number of apoptotic cells, ROS production and
Ca2+ concentration was significantly increased in HLECs
in both dose- and time-dependent manner. In agreement with our
findings that UVB irradiation could induce HLECs apoptosis, produce
ROS and cause Ca2+ elevation (3,23).
Whether the mechanism of cataract induced apoptosis
is involved in Ca2+ elevation has to be investigated.
The current study examined the association of UVB irradiation with
CALML3. After 2 W/m2 UVB irradiation for 15, 90 and 180
min, the mRNA and protein levels of CALML3 were decreased in a
time-dependent manner. The interesting pattern of expression
indicated that may be a CALML3 a potentially attractive marker of
cataract progression. Notably, overexpression of CALML3 in HLECs
significantly reduced apoptosis, ROS production and intracellular
Ca2+ concentration following exposure of HLECs with 2
W/m2 UVB irradiation for 90 min. Similar to the
ubiquitous Ca2+ sensor protein calmodulin, CALML3 acts
in pre-apoptotic cells by interacting with a nuclear protein
involved in chromosome fragmentation (20). The findings of the current study
suggest that UVB irradiation induces apoptosis by downregulating
expression of CALML3, and upregulation of CALML3 can reverse the
UVB-induced damage of HLECs.
Numerous genes are associated with apoptosis, such
as the Bcl-2 family, Bax and caspase-3. Among the Bcl-2 family,
Bcl-2 is an anti-apoptotic protein; however the Bax and caspase-3
are pro-apoptotic (24,25). The current study measured the mRNA
and protein expression levels of these proteins. The results
demonstrated that overexpression of CALML3 significantly decreased
the expression of Bax and caspase-3, and increased the expression
of Bcl-2, suggesting that upregulation of CALML3 reverses
UVB-induced apoptosis by decreasing caspase-3 and the Bax/Bcl-2
ratio. In agreement with these findings, Bax/Bcl-2 ratio and
caspase-3 activity were highly positively correlated to the
proportion of apoptotic cells in HLECs following UVB irradiation
(7).
Numerous scientific investigations have confirmed
the presence of oxidative stress in ocular disease. Oxidative
damage caused by ROS has an important role in the patho-physiology
of many forms of cataract (1,26).
In order to protect against toxic effects of ROS, the cells have
antioxidant defense systems, which consist of antioxidative enzymes
including SOD, CAT and GSH-PX, and antioxidants such as MDA. Under
normal conditions, HLECs maintain ROS at low levels via activation
of SOD, CAT and GSH-PX, and MDA inhibition (27). However, some reported decreased
SOD, CAT and GSH-PX, but increased MDA levels in cataracts
(28), although other studies
have reported conflicting results (29). In the present study, UVB-induced
HLECs with CALML3 overexpression decreased the activities of SOD,
CAT and GSH-PX, and increased the activity of MDA, compared with
UVB-induced HLECs without CALML3 upregulation.
To further examine the association of CALML3 with
cataracts, siRNA was used to knockdown CALML3 in HLECs. Silencing
of CALML3 significantly increased the number of apoptotic cells,
ROS production and intracellular Ca2+ concentration,
which was similar to the effects of UVB in HLECs. However,
nimodipine, a Ca2+-channel antagonist, inhibited ROS
production and intracellular Ca2+ concentration, and had
no effect on apoptosis. By contrast, other studies reported that
nimodipine inhibited of apoptosis and ROS production (30) and is considered to be a potent
antioxidant in vivo and in vitro (31,32). In addition, nimodipine treatment
partially rescued changes in expression of Bax, caspase-3 and Bcl-2
caused by CALML3 downregulation. These findings suggest that CALML3
downregulation induced apoptosis through activating the calcium
signaling pathway.
It has been reported that the activation of the MAPK
signaling cascade is essential for oxidative stress-induced
apoptosis in HLECs (33), and the
UVB-activated signal transduction pathways are predominantly
mediated through signaling cascades involving MAPKs, including JNK,
ERK and p38 kinases (23). Among
these MAPKs, ERK is considered to be essential for cell survival
(34), and JNK and p38 activation
are considered to have a role in apoptotic signaling (8,35).
Herein, the association of CALML3 downregulation with apoptosis in
HLECs was also investigated. The results demonstrated that
downregulation of CALML3 significantly inhibited the activation of
JNK1/2 and ERK1/2, whereas nimodipine treatment reversed the
inhibition of JNK1/2 and ERK1/2 caused by CALML3A downregulation.
Similar to these findings, the protein levels of p-JNK and p-ERK
were negatively regulated by treatment with nimodipine in oxygen
and glucose deprivation/reperfusion-induced cell injury in primary
culture of rat cortical neurons (36). Thus, results of the present study
provided evidence to suggest that JNK1/2 and ERK1/2 are involved in
CALML3 downregulation and UVB-induced apoptosis.
Investigating the mechanism of apoptosis in HLECs
may provide important insight into understanding the processes
involved in cataract development. In the current study, UVB
irradiation induced apoptosis, ROS production and intracellular
Ca2+ concentration elevation in HLECs. Overexpression of
CALML3 inhibited the damage caused by UVB irradiation and decreased
the levels of caspase-3, Bax/Bcl-2 ratio and ROS production. In
addition, downregulation of CALML3 induced HLEC apoptosis
potentially through inhibition of JNK1/2 and ERK1/2 activation.
These results suggest that the UVB induces apoptosis in HLECs via
oxidative stress, Ca2+, and JNK1/2 and ERK1/2 signaling
pathways.
Acknowledgments
Not applicable
Notes
[1]
Funding
This study was supported by grants from the National
973 Foundation (no. 2013CB531606), the National Science Foundation
of China (nos. 81300748, 81471605, 81273282, 81202353 and
81170834), the Shanghai Shenkang Grant (no. CHDC22014014), the
Grant of the Army Scientific Research (no. BWS14J023).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
YJ and QQ conceived and designed the experiments.
CPF, WS and TTS performed the experiments. YLH analyzed the data.
WJL and AMD contributed as regards the reagents/materials/analysis
tools. YJ, WJL and AMD wrote the paper. All authors read and
approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable
[5] Consent for
publication
Not applicable
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Shibata S, Singh DP, Shibata N, Sasaki H
and Kubo E: Hyperglycemia-and oxidative stress-induced
hyperoxidation of Prdx 6 during lens epithelial cell damage and rat
lens opacity in vitro and in vivo. Invest Ophthalmol Vis Sci.
55:5723. 2014.
|
|
2
|
Giblin FJ, Lin L-R, Leverenz VR and Dang
L: A class I (Senofilcon A) soft contact lens prevents UVB-induced
ocular effects, including cataract, in the rabbit in vivo. Invest
Ophthalmol Vis Sci. 52:3667–3675. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Q, Guo D, Bi H, Wang D and Du Y: UVB
irradiation-induced dysregulation of plasma membrane calcium
ATPase1 and intracellular calcium homeostasis in human lens
epithelial cells. Mol Cell Biochem. 382:263–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Yan H, Löfgren S, Tian X and Lou
MF: Ultraviolet radiation-induced cataract in mice: The effect of
age and the potential biochemical mechanism. Invest Ophthalmol Vis
Sci. 53:7276–7285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim J, Kim OS, Kim C-S, Sohn E, Jo K and
Kim JS: Accumulation of argpyrimidine, a methylglyoxal-derived
advanced glycation end product, increases apoptosis of lens
epithelial cells both in vitro and in vivo. Exp Mol Med.
44:167–175. 2012. View Article : Google Scholar :
|
|
6
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: The mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji Y, Cai L, Zheng T, Ye H, Rong X, Rao J
and Lu Y: The mechanism of UVB irradiation induced-apoptosis in
cataract. Mol Cell Biochem. 401:87–95. 2015. View Article : Google Scholar
|
|
8
|
Zwang Y and Yarden Y: p38 MAP kinase
mediates stress-induced internalization of EGFR: Implications for
cancer chemotherapy. EMBO J. 25:4195–4206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roduit R and Schorderet DF: MAP kinase
pathways in UV-induced apoptosis of retinal pigment epithelium
ARPE19 cells. Apoptosis. 13:343–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang D, Zhang X, Rong S, Sha Q, Liu P,
Han T and Pan H: Serum antioxidative enzymes levels and oxidative
stress products in age-related cataract patients. Oxid Med Cell
Longev. 2013:5878262013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wojcik M, Burzynska-Pedziwiatr I and
Wozniak LA: A review of natural and synthetic antioxidants
important for health and longevity. Curr Med Chem. 17:3262–3288.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yonar ME: The effect of lycopene on
oxytetracycline-induced oxidative stress and immunosuppression in
rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol.
32:994–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dechandt CR, De Souza DL, Siqueira JT,
Pereira MP, De Assis RP, Da Silva VC, De Sousa PT Jr, Brunetti IL,
Andrade CM, Kawashita NH, et al: Changes in the oxidative stress
biomarkers in liver of streptozotocin-diabetic rats treated with
combretum lanceolatum flowers extract. Br J Pharm Res. 4:2340–2356.
2014. View Article : Google Scholar
|
|
14
|
Chang D, Zhang X, Rong S, Sha Q, Liu P,
Han T and Pan H: Serum antioxidative enzymes levels and oxidative
stress products in age-related cataract patients. Oxid Med Cell
Longev. 2013:5878262013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strehler EE: Emanuel Strehler's work on
calcium pumps and calcium signaling. World J Biol Chem. 2:67–72.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang KC, Lee AS, Chen WY, Lin YN, Hsu JF,
Chan HC, Chang CM, Chang SS, Pan CC, Sawamura T, et al: Increased
LDL electronegativity in chronic kidney disease disrupts calcium
homeostasis resulting in cardiac dysfunction. J Mol Cell Cardiol.
84:36–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu S, Yang L, Li P, Hofmann O, Dicker L,
Hide W, Lin X, Watkins SM, Ivanov AR and Hotamisligil GS: Aberrant
lipid metabolism disrupts calcium homeostasis causing liver
endoplasmic reticulum stress in obesity. Nature. 473:528–531. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walton JR: Aluminum disruption of calcium
homeostasis and signal transduction resembles change that occurs in
aging and Alzheimer's disease. J Alzheimers Dis. 29:255–273.
2012.PubMed/NCBI
|
|
19
|
Brooks MD, Bennett RD, Weaver AL, Sebo TJ,
Eckert SE, Strehler EE and Carr AB: Human calmodulin-like protein
CALML3: a novel marker for normal oral squamous mucosa that is
downregulated in malignant transformation. Int J Dent.
2013:5928432013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bennett RD, Pittelkow MR and Strehler EE:
Immunolocalization of the tumor-sensitive calmodulin-like protein
CALML3 in normal human skin and hyperproliferative skin disorders.
PLoS One. 8:e623472013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calenda G, Suadicani SO, Iglesias R, Spray
DC, Melman A and Davies KP: Silencing MaxiK activity in corporal
smooth muscle cells initiates compensatory mechanisms to maintain
calcium homeostasis. J Sex Med. 8:2191–2204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
23
|
Cao G, Chen M, Song Q, Liu Y, Xie L, Han
Y, Liu Z, Ji Y and Jiang Q: EGCG protects against UVB-induced
apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19
cells. Mol Med Rep. 5:54–59. 2012.
|
|
24
|
Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe,
Ahmed S, Kunugita N, Arashidani K and Fujimaki H: Inhalation of
low-level formaldehyde increases the Bcl-2/Bax expression ratio in
the hippocampus of immunologically sensitized mice.
Neuroimmunomodulation. 13:63–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu GX and Wang TT: Apoptosis of lens
epithelial cells induced by cinobufagin in vitro. Int J Ophthalmol.
3:128–131. 2010.PubMed/NCBI
|
|
26
|
Klein B, Myers C, Cruickshanks K, Lee K
and Klein R: Inflammatory and oxidative stress markers and the
20-year cumulative incidence of age-related cataract: The beaver
dam eye study. Invest Ophthalmol Vis Sci. 54:897. 2013.
|
|
27
|
Berthoud VM and Beyer EC: Oxidative
stress, lens gap junctions, and cataracts. Antioxid Redox Signal.
11:339–353. 2009. View Article : Google Scholar :
|
|
28
|
Brennan LA and Kantorow M: Mitochondrial
function and redox control in the aging eye: Role of MsrA and other
repair systems in cataract and macular degenerations. Exp Eye Res.
88:195–203. 2009. View Article : Google Scholar :
|
|
29
|
Scharf J and Dovrat A: Superoxide
dismutase molecules in human cataractous lenses. Ophthalmic Res.
18:332–337. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang D, Wang Z, Li Y, Chen X and Sun GY:
Nimodipine inhibits N-methyl-N-nitrosourea-induced retinal
photoreceptor apoptosis in vivo. Indian J Pharmacol. 45:149–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ambhore N, Prasanna M, Antony A, Satish
Kumar M and Elango K: Pharmacological and anti-oxidant evaluation
of Aspirin, nimodipine and its combination for anti-Parkinson's
activity in MPTP induced rat model. Int J Health Allied Sci.
3:142014.
|
|
32
|
Yu L, Wang N, Zhang Y, Wang Y, Li J, Wu Q
and Liu Y: Neuroprotective effect of muscone on glutamate-induced
apoptosis in PC12 cells via antioxidant and Ca(2+) antagonism.
Neurochem Int. 70:10–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amin KK and Shah A: Prevention of
oxidative stress and cell death in human lens epithelial cells by
guggulsterone. Invest Ophthalmol Vis Sci. 53:1070. 2012.
|
|
34
|
Dent P, Yacoub A, Fisher PB, Hagan MP and
Grant S: MAPK pathways in radiation responses. Oncogene.
22:5885–5896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li T, Dai W and Lu L: Ultraviolet-induced
junD activation and apoptosis in myeloblastic leukemia ML-1 cells.
J Biol Chem. 277:32668–32676. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang CP, Li GC, Shi YW, Zhang XC, Li JL,
Wang ZW, Ding F and Liang XM: Neuroprotective effect of schizandrin
A on oxygen and glucose deprivation/reperfusion-induced cell injury
in primary culture of rat cortical neurons. J Physiol Biochem.
70:735–747. 2014. View Article : Google Scholar : PubMed/NCBI
|