Introduction
Acute lung injury (ALI), as an early and basic
pathophysiologic change in acute respiratory distress syndrome
(ARDS), is a significant contributor to morbidity and mortality
rates in critically ill patients, and is most commonly caused by
aspiration, infection, trauma and chemicals (1–3).
The majority of current treatments for ARDS predominantly focus on
restrictive intravenous fluid management and lung-protective
ventilation, and there are currently few specific pharmacological
therapies able to attenuate ALI and promote lung repair (4–6).
ALI is a systemic inflammatory reaction syndrome, which is involved
in a variety of inflammatory cells and inflammatory cytokines.
Therefore, modulating immune responses and re-establishing the
balance of the immune system is critical for the treatment of ALI
(7).
Mesenchymal stem cells (MSCs) are one of the most
promising adult stem cells, which have the ability of multiple
differentiation potential for regenerative medicine, and can
interact with the immune system in a complex and coordinate
dmannerto induce a selective and balanced response (8). Current available evidence shows that
MSCs are involved in the repair of the injured lung and effectively
attenuateALI caused by various factors (9–11).
Due to the uncertainty of their biological behavior, including
distribution and chemo-taxis in vivo following MSC
transplantation, whether the use of MSCs in treating ALI is likely
to be limited in their further application in clinical practice
remains to be elucidated. Therefore, it is important to develop a
suitable and noninvasive imaging probe to monitor the transplanted
MSCs.
In optical molecular imaging, fluorescence imaging
utilizes a fluorescence molecular probe to mark specific molecules
or cells, and its spatial resolution can reach the mm level
(12). The probe has numerous
advantages, including high sensitivity, high temporal resolution,
low cost, and relatively high flux. In addition, it does not
require any reaction substrates or other cofactors, and sensitive
optical detection apparatus can directly track the space and time
distribution of cells to understand its associated biological
processes (13–15). Therefore, noninvasive fluorescence
imaging techniques for the investigation of biological processes in
small animal models are rapidly becoming an important tool in
biomedical research. It has been successfully and diffusely applied
in all fields, including life science, molecular biology and drug
development (16,17).
In the present study, monoclonal antibody
anti-ganglioside (GD2) was used, which binds to the specific
antigen GD2 expressed on hUC-MSCs as a carrier (18). It has been shown that cells
selected by GD2 are a subpopulation of MSCs with the features of
primitive precursor cells, and Xu et al (19) provided evidence that GD2 is a cell
surface marker suitable for the isolation and purification of
hUC-MSCs. A novel fluorescent molecule probe was first synthesized
through the covalent coupling of anti-GD2 and fluorophore CyDye
mono-reactive NHS esters (Cy7) (20). In the present study, according to
the specific binding of antigen and antibody, synthetic
anti-GD2-Cy7 wasincubated with hUC-MSCs in a standard culture
medium (21). The labeled
hUC-MSCs were transplanted into a lipopolysaccharide (LPS)-induced
ALI mouse model to preliminarilyinvestigate the distribution
processes of hUC-MSCs in treating ALI, which provides a theoretical
basis for the establishment of an hUC-MSC treatment strategy for
ALI in clinical work.
Materials and methods
Animal care
Healthy specific pathogen free male Balb/c mice (4–6
weeks old), purchased from Southern Medical University Experimental
Animal Center (Guangzhou, China), were bred in the animal facility
at the north campus of Sun Yat-sen University Laboratory Animal
Center (Guangzhou, China) in a clean, temperature-controlled and
independently ventilated environment, at 21°C under a 12/12 h
dark/light cycle with ad libitum access to sterile water and
standard pellet food under standardized environmental conditions.
All experimental protocols were approved by the Institutional
Animal Care and Use Committee of Sun Yat-sen University. Prior to
commencing the experiments, all animals were subjected to a 2-week
period of adaptation to the environment.
Materials
The Cy7 esters were purchased from Beijing Rich
Encyclopedia Biological Technology Company (Beijing, China), The
low-glucose Dulbecco's modified eagle medium (DMEM), fetal bovine
serum and Dulbecco's phosphate-buffered saline (PBS) were purchased
from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The
mouse anti-human GD2 monoclonal antibody (14.G2a; cat no. 554272)
was purchased from BD Biosciences (San Jose, CA, USA)The Cell
Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc.(Kumamoto, Japan). The LPS (Escherichia
coli; 0111:B4), Evans blue dye (EBD) and trypsin were purchased
from the Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
Hematoxylin and eosin (HE) was obtained from the Pathology
Department of The Third Affiliated Hospital of Sun Yat-sen
University. hUC-USC isolation for experiments was performed by The
Biological Treatment Center The Third Affiliated Hospital of Sun
Yat-Sen University according to a protocol previously described
(19). The obtained cryo
preserved hUC-USCs were suitable for culture and were routinely
passaged following thawing; apreliminary experiment confirmed that
the hUC-USCs were capable of differentiating into osteogenic and
adipogenic lineages (22).
Conjugation of antibody to
fluorophore
The GD2-specific monoclonal antibody (anti-GD2) was
labeled with the fluorophore Cy7 esters based on a previously
described protocol (23,24). Briefly, the monoclonal antibody
was reconstituted at 1 mg/ml in 0.1 M sodium bicarbonate; and 100
µl was added to the reactive dye for conjugation. The
antibody-dye mixtures were allowed to incubate for 1 h at room
temperature, and then overnight at 4°C. The conjugated antibody was
then separated from the remaining un conjugated dye on a
purification column by centrifugation for 5 min at 1,100 × g and
4°C.
Absorption spectrum and determining the
degree of labeling
A small quantity of anti-GD2, Cy7-NHS and Cy7-GD2
was separately diluted with PBS (1:100) and transferred into a
cuvette with a 1-cm path-length. The absorbance of the final
samples was measured in the ultraviolet (UV)-visible
spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA) and UV
spectra (wavelength from 200 to 900 nm). According to the Cy7-GD2
absorbance at 280 nm (A280) and 750 nm (A750), the concentration of
protein was calculated in the sample: Protein concentration
(M)=[A280-(A750×0.04)] × dilution factor/203,000, where 203,000 is
the molar extinction coefficient (ε) in cm−1
M−1 of a typical IgG at 280 nm. The degree of labeling
was also determined: Moles dye per mole protein=A750 × dilution
factor/εdye × protein concentration (M), where 250,000 is the
approximate molar extinction coefficient of Cy7.
Cell labeling
The hUC-MSCs were seeded at a density of
5×105 cells/well on 24-well plates in 1 mL of complete
DMEM and cultured for 24 h at 37°C in a fully humidified atmosphere
of 5% CO2. Cy7-GD2 was then directly added to the
culture medium to label cells for 2 h in a standard culture medium
at 37°C and 5% CO2 by the specific binding of antigen
and antibody. Following labeling, the medium was discarded, and the
cells were washed three times with PBS to eliminate residual
fluorescent-conjugated antibodies and resuspended in PBS. The
labeled hUC-MSCs [1×106 cells in 200 µl of normal
saline (NS)], unlabeled hUC-MSCs (1×106 cells in 200
µl of NS), 10 µl Cy7-GD2, and 20 µg/ml Cy7
added to 200 µl of NS were respectively collected in tubes
and were detected on a small animal fluorescent imager.
In vitro cytotoxicity assay
A total of 5×103 hUC-MSCs or labeled
hUC-MSCs were seeded per well with 100 µl culture medium in
96-well plates. The culture medium was carefully replaced following
1, 3, 12, 24 and 48 h of incubation at 37°C and 5% CO2
with Cy7-GD2. CCK-8 (10 µl) solution was added to each well
and incubated for an additional 2 h. The absorbance at 450 nm was
measured on an Infinite F200 Multimode plate reader (Tecan
Deutschland GmbH, Crailsheim, Germany). Cell viability was
calculated by the labeled hUC-MSCs/unlabeled hUC-MSCs absorbance
ratio.
Experimental design and LPS-induced lung
injury
Briefly, the Balb/c mice were randomly assigned into
four groups: NS+NS group, NS+MSC group, LPS+NS group and LPS+MSC
group (n=16 per group). Following anesthetization of the mice with
chloral hydrate (10 mg/kg), ALI was induced by the instillation of
LPS (in the LPS+NS group and LPS+MSC group) from E. coli
O111:B4 at 5 mg/kg intratracheally, whereas the mice in the NS+NS
group and NS+MSC group were treated with sterile NS as controls
(25). At 4 h post-LPS challenge,
the mice in the NS+MSC group and LPS+MSC group were administered
with hUC-MSCs (1×106 cells in 200 µl NS) and
those in the NS+NS group and LPS+NS group were administered with
200 µl of NSvia injection into the tail vein. The animals
were anesthetized and sacrificed by incision of the abdominal aorta
at 24 and 48 h post-transplantation to harvest lung tissue
samples.
Lung histopathology
The inferior lobes of the right lungs were cut into
5-µm thick sections at each time point and subsequently
stained with HE for histological analysis. Lung pathology was
evaluated in a blinded-manner by a pathologist using a light
microscope, according to four criteria: i) alveolar congestion, ii)
hemorrhage, iii) infiltration oraggregation of neutrophils in the
airspace or vessel wall, and iv) thickness of alveolar wall/hyaline
membrane formation. Each category was graded on a 0–4 point scale:
0, no injury; 1, injury up to 25% of the field; 2, injury up to 50%
of the field; 3, injury up to 75% of the field; and 4, diffuse
injury. The total lung injury score was calculated as the sum of
the four criteria (26).
Lung wet-to-dry (W/D) weight ratio
The superior and middle lobes of the right lungs
were collected from the mice at each time point for assessment of
the lung W/D ratio. The samples were weighed immediately following
collection and then placed in a drying oven at 60°C for 72 h, and
the dry mass was subsequently determined. The wet lung mass was
divided by the dry lung mass to give the W/D ratio.
Protein leakage from capillaries
Pulmonary micro vascular permeability was determined
using the EBD extravasation method as described in a previous
experiment (27). The EBD (20
mg/kg in 80 µl PBS) was injected into the tail veinof the
mice in every group at each time point. After 30 min, the right
ventricle of the heart was perfused with 10 ml PBS to flush the
lungs of the intravascular dye. Complete perfusion was confirmed
when all blood had been cleared from the lung. The right lung
tissue was then incubated in formamide for 48 h at 60°C and
centrifuged for 30 min at 5,000 × g at 4°C. The concentration of
EBD in lung tissues was determined via spectrophotometric means at
an optical density of 620 nm.
Fluorescence imaging analyses
The NS+MSC group of mice (n=12) and LPS+MSC group of
mice (n=12) were subjected to optical imaging. ALI was induced by
instillation of LPS (5 mg/kg) intratracheally, as above. At 4 h
post-LPS administration, the labeled hUC-MSCs were transplanted
into mice in the NS+MSC group and LPS+MSC group by tail vein
injection. The mice were sacrificed 30 min, 1 day, 3 days and 7
days following the labeled hUC-MSC injection, and the lungs,
livers, hearts and kidneys were removed. The excised lungs, livers,
hearts and kidneys at each time point were imaged on a Maestro
In-Vivo Optical Imaging system (excitation, 747 nm;
emission, 774 nm; exposition time, 5,000 ms; Caliper Life Sciences,
Woburn, MA, USA) (28). Images
were captured and analyzed using Maestro 2.10.0 software (Caliper
Life Sciences). The fluorescence intensity of each organ was
measured by placing regions of interest (ROIs) on the organ and
determined by an individual blinded to the experimental groups. The
average signals were normalized to the exposure time and the area
of the ROI (scaled counts/sec) (29).
Statistical analysis
Statistical analysis of the data was performed using
one-way analysis of variance followed by Dunnett's post-hoc testor
anun paired Student's t-test with SPSS software (version 17.0,
SPSS, Inc., Chicago, IL, USA). All data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference. All statistical tests were
two-sided.
Results
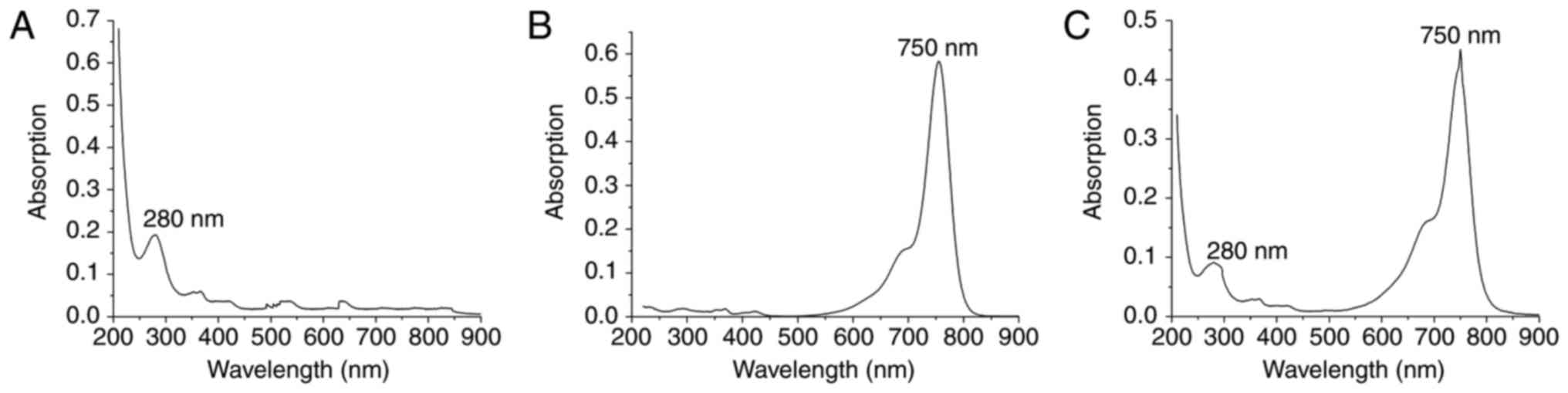
Absorption spectrum of Cy7-GD2
The absorption spectrum is the characteristic
absorption curve of a material and is strictly consistent with
molecular structure (30). The
molecular structures of different materials have a unique spectrum.
UV-Vis the absorption spectrum commonly used to examine the
structure of a substance. There is a maximum absorption wavelength
foranti-GD2 and Cy7-NHS, namely 280 nm (Fig. 1A) and 750 nm (Fig. 1B), respectively. The absorption
spectrum of Cy7-GD2 has two peaks at 280 and 750 nm (Fig. 1C). The product Cy7-GD2 is able to
maintain the properties of the monoclonal antibody and Cy7 dyes,
which does not alter the structures or biological characteristics
of these chemicals due to a conjugation reaction. According to
A280=0.09143; A750=0.45129: Protein concentration
(M)=[A280-(A750×0.04)] × dilution factor/20
3,000=(A280-0.04×A750)/203,000=(0.09669×0.04×0.41529)/203,000=3.61×10−7
M Degree of label: Moles dye per mole protein=A750 × dilution
factor/εdye × M=F/P=A750/250,000 × M=5.014.
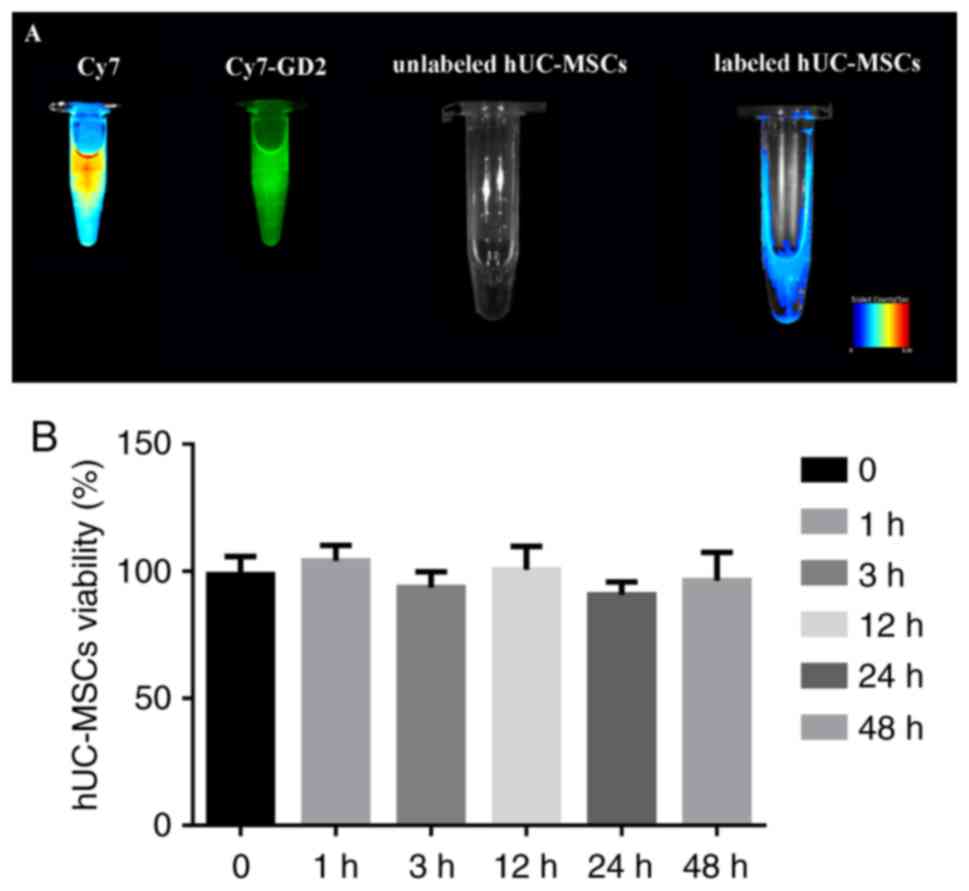
In vitro fluorescence imaging and CCK-8
assay
Based on the fluorescence imaging, the Cy7 reactive
dye solution exhibited intense fluorescence; Cy7-GD2 emitted
secondary fluorescence, the labeled hUC-MSC suspension exhibited
moderate fluorescence, and the fluorescence signal of the unmarked
hUC-MSC suspension was not fully detected (Fig. 2A). The CCK-8 assay confirmed that
there was no significant difference in the cell viability of the
experimental group and control group following extra incubation for
1, 3, 12, 24 and 48 h (Fig. 2B);
it revealed that Cy7-GD2 had no cytotoxic effect on the
hUC-MSCs.
ALI mouse model and assessing the
validity of hUC-MSCs in treating ALI
The histological analysis indicated that LPS caused
capillary expansion and congestion, in addition to neutrophil
infiltration into the lung tissue. In addition, lung septa were
noticeably thickened. The administration of hUC-MSCs in the LPS+MSC
group improved the lung injury at 24 and 48 h (Fig. 3A). Compared with the LPS+NS group,
the lung tissue pathological score was relatively lower in the
LPS+MSC group at 24 and 48 h. However, there were no significant
differences in the lung tissue pathological scores of the NS+MSC
group and NS+NS group at the two time-points (Fig. 3B). In addition, the LPS+MSC group
showed a trend toward a lower lung W/D ratio, compared with that in
the LPS+NS group at 24 and 48 h (Fig.
3C). Compared with the LPS+NS group, the LPS+MSC group
demonstrated improvement in pulmonary micro vascular permeability
at 24 and 48 h (Fig. 3D).
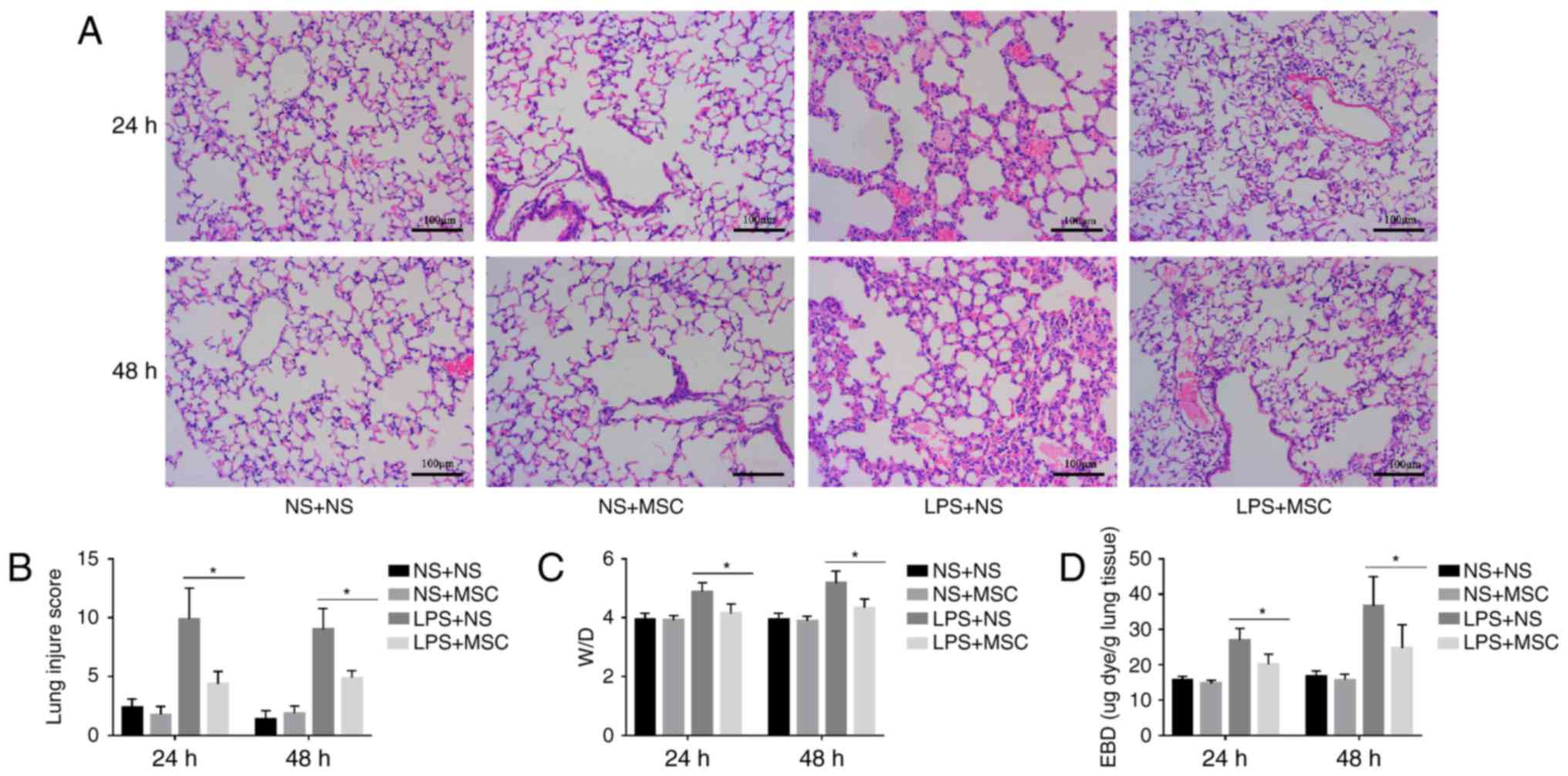 | Figure 3Effects of hUC-MSCs on LPS-induced
acute lung injury. (A) HE staining (magnification, ×200; scale
bar=100 µm) of lung sections from LPS-challenged mice at 24
and 48 h showed marked interalveolar septal thickening, extensive
inflammatory infiltration, diffuse interstitial and alveolar edema,
and severe interstitial hemorrhage. The administration of hUC-MSCs
in the LPS+MSC group improved the lung histopathology to differing
degrees. (B) At 24 and 48 h, HE staining of the lung sections from
hUC-MSC-treated mice in the LPS+MSC group had significantly less
injury, compared with mice in the LPS+NS group. (C) Mice
administered with hUC-MSCs in the LPS+MSC group showed a trend
towards a lower lung W/D weight ratio at 24 and 48 h. (D) Dye
accumulation in the lungs increased markedly at 24 and 48 h in the
LPS+NS group, and was reduced by hUC-MSC treatment at 24 and 48 h.
The results are expressed as the mean ± standard deviation (n=8 per
group). *P<0.05. hUC-MSCs, human umbilical
cord-derived mesenchymal stem cells; LPS, lipopolysaccharide; NS,
normal saline; W/D, wet/dry ratio; EBD, Evans blue dye. |
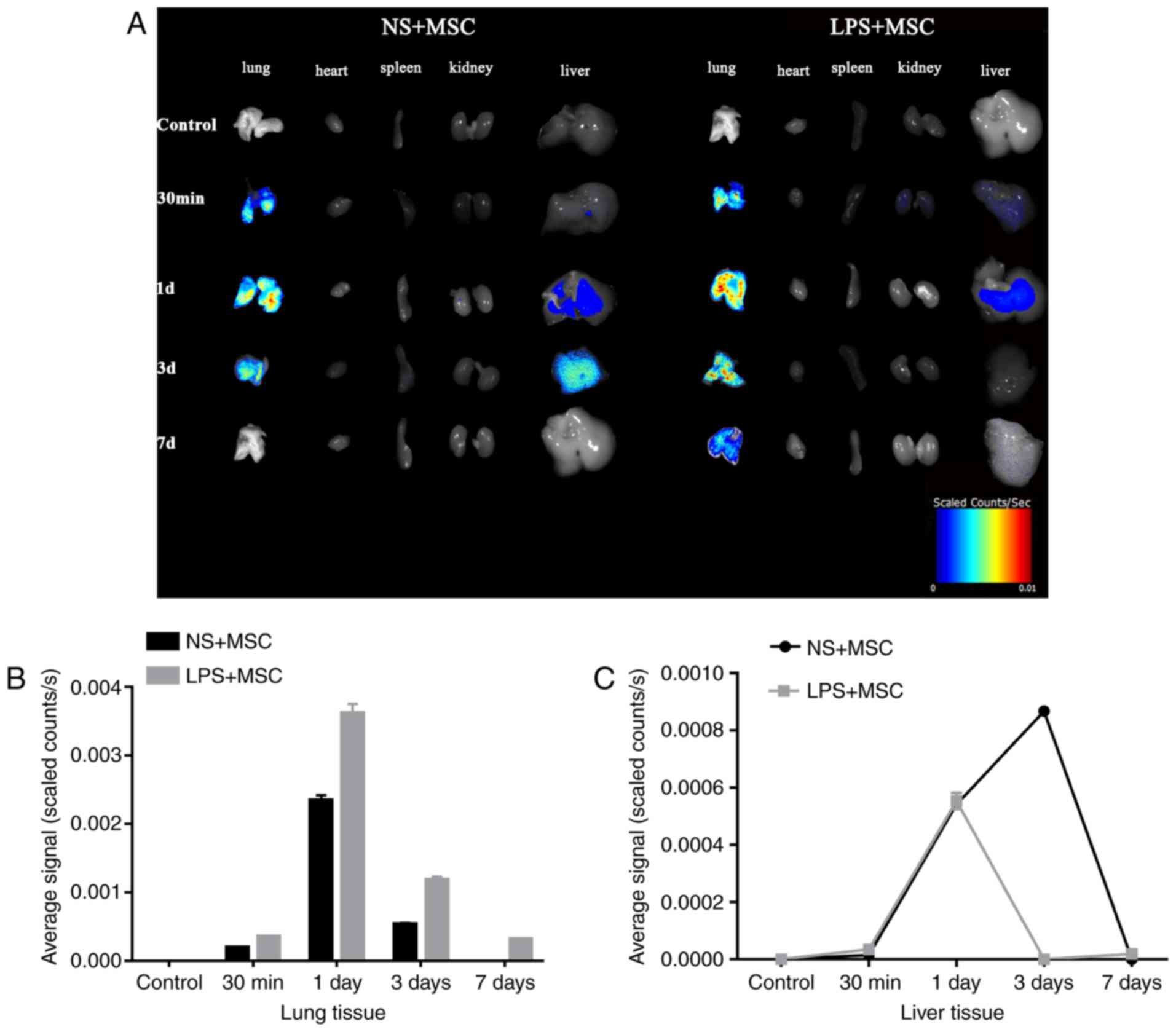
Fluorescence imaging
The fluorescent tracing showed enhanced fluorescence
signal in the lung 30 min following injection in the LPS+MSC group
and NS+MSC group. The signal in the lungs of the LPS+MSC group and
NS+MSC group peaked at day, with that of the LPS+MSC group
decreasing gradually over time and detected 7 days following
transplantation. However, the signal in the lungs of the NS+MSC
group declined sharply and had disappeared completely at day 7. The
fluorescence signal in the livers of the LPS+MSC group and NS+MSC
group appeared at day 1, and that of the LPS+MSC group had almost
disappeared at day 3. However, the fluorescence signal in the
livers of the NS+MSC group enhanced gradually, peaking at day 3 and
fading away at day 7 post-injection. The fluorescence signals of
the heart, spleen and kidneys were minimal in the LPS+MSC group and
NS+MSC group (Fig. 4A). The
average fluorescence signal intensity of each organ was
semi-quantitatively measured at each time point. The results showed
that the signal intensity in the lungs of the LPS+MSC group was
significantly higher, compared with that of the NS+MSC group at
each time point: 30 min (3.53±0.06×10−4, vs.
1.95±0.05×10−4 scaled counts/sec); 1 day
(36.20±0.77×10−4, vs. 23.45±0.43×10−4 scaled
counts/sec); 3 days (11.83±0.26×10−4, vs.
5.39±0.10×10−4 scaled counts/sec); 7 days
(3.14±0.04×10−4, vs. 0.00±0.00×10−4 scaled
counts/sec), as shown in Fig. 4B
and Table I (all P<0.05). The
fluorescence intensity in the liver of the LPS+MSC group, vs.
NS+MSC group was as follows: 30 min (0.00±0.00×10−4, vs.
0.00±0.00×10−4 scaled counts/sec); 1 day
(5.53±0.08×10−4, vs. 5.44±0.16×10−4 scaled
counts/sec); 3 days (0.00±0.00×10−4, vs.
8.67±0.05×10−4 scaled counts/sec); 7 days
(0.00±0.00×10−4, vs. 0.00±0.00×10−4 scaled
counts/sec). Detection of the signal intensity of the heart, spleen
and kidney was minimal (Fig.
4C).
 | Table IFluorescence intensity of the
lungs. |
Table I
Fluorescence intensity of the
lungs.
| Group | 30 min | 1 day | 3 days | 7 days |
|---|
| LPS+MSC | 3.53±0.06a | 36.20±0.77a | 11.83±0.26a | 3.14±0.04a |
| NS+MSC | 1.95±0.04 | 23.45±0.43 | 5.39±0.10 | 0.00±0.00 |
| T-value | 19.90 | 14.45 | 22.79 | 77.69 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 |
Discussion
The present study aimed to develop a suitable and
noninvasive imaging tool to track the bio distribution processes of
hUC-MSCs in treating ALI. A novel class of fluorescence molecular
probe was synthesized, which targeted and effectively labeled
hUC-MSCs. It was hypothesized that, using this method, transplanted
hUC-MSCs may be successfully monitored using this fluorescence
molecular probe.
Stem cell transplantation is considered as a
potential therapy to prevent or reverse the deleterious effects of
various types of tissue injury and immunological disorders
(31,32). MSCs derived from human umbilical
cord have the capacity to differentiate into various components of
the hematopoietic niche, including bone and adipose under
appropriate conditions (33).
hUC-MSCs also possess high cell vitality, low immunogenicity, high
paracrine potential and potent immune modulation to accelerate the
repair process of injured tissue (34,35). Animal models demonstrate that MSCs
can induce the repair of injured organs and ameliorate inflammatory
processes, including ALI/ARDS (36). The present study found that the
infusion of hUC-MSCs had protective effects on attenuating lung
injury and inhibiting lung inflammation in ALI mice induced by
intratracheal LPS challenge. Lung histopathology and pathological
scores, lung W/D ratio and pulmonary microvascular permeability
were assessed to evaluate the effectiveness of hUC-MSCs in treating
ALI, the results of which indicated that the lung histopathology
and scores, pulmonary edema and permeability of lung
microvasculature in the MSC+ALI group showed improvement, compared
with those in the NS+ALI group, which were in accordance with a
previous study that hUC-MSC administration attenuated lung
inflammation in ALI, regardless of whether the cells were delivered
at 24 or 48 h (37).
MSCs modified by different labeling strategies can
be visualized using imaging systems, including magnetic resonance
imaging (MRI), radionuclide imaging, including single photon
emission computed tomography and positron emission tomography, and
optical imaging, including fluorescence imaging and bioluminescence
imaging (38). MRI appears to be
the preferred option for imaging the distribution of
magnetically-labeled stem cells due to its high spatial/temporal
resolution and free selection of imaging plane (39). However, considering the principle
of MRI and low H1 proton density of lung tissue
(40), the efficacy of common MRI
in monitoring the processes of hUC-MSCs in treating ALI is far from
satisfactory. There exists radiation to viable cells in
radionuclide imaging, which can lead to considerable toxicity to
labeled cells and their normal organization, and can increase the
risk of cancer (41). Another
popular in vivo image tracing method is transfecting
reported genes, including GFP, YFP or RFP, to express visible
fluorescent proteins. The main advantage of this technique is that
the fluorescence signal is stably expressed in offspring cells.
However, gene editing increases the risk of viral infection and
potentially carcinogenesis (42).
Fluorescence imaging offers the benefits of high
contrast and sensitivity, low cost, ease of use, and safety, and is
a suitable tracer method for the visualization of cells and tissues
(43). Fluorescence imaging
techniques have become an indispensable tool in biomedical
investigations, as advances in photonic technology and noninvasive
strategies have led to the widespread application of biological
processes (44). In the present
study, near-infrared (650–900 nm) fluorescent Cy7 dye was used for
labeling several molecules for optical imaging due to its
characteristics of small size, ideal aqueous solubility and a free
amino structure (45). The
Cy7-GD2 conjugate was synthesized by the coupling of Cy7-NHS with
anti-GD2 consisting of N-terminal amines. The UV-vis spectrum
showed that the complex had two distinctive peaks at 280 and 750
nm: The first peak was derived from anti-GD2 and the second peak
was the characteristic peak originating from Cy7-NHS. No other
molecule peaks were found, which not only contained the optical
properties of Cy7, but also maintained the immune activity of
monoclonal anti-GD2 antibody. This feature also provided the basis
to calculate the F/Pratio of the conjugate, namely, the number of
Cy7 units in the Cy7-GD2 conjugate. An F/P ratio of 5.014 is the
ideal label status, with a critical 'optimum' at 4–9
labels/protein. Based on cell fluorescence imaging, the labeled
hUC-MSCs suspension showed visible and moderate fluorescence. The
results confirmed that the novel class of fluorescence molecular
probe labeling hUC-MSCs was feasible. In addition, the complexes
did not significantly affect the viability of the hUC-MSCs,
demonstrating a low toxicity towards hUC-MSCs, which is essential
for cell transfection.
To investigate the distribution of MSCs in a
relevant ALI model, the hUC-MSCs labeled with fluorescent probe
were administered into the ALI mice via tail vein injection.
Multiple studies have demonstrated that the management of ALI/ARDS
with MSCs involves two mechanisms (46). One of the mechanisms is termed the
cell engraftment mechanism, during which transplanted MSCs can
differentiate into type I and type II epithelial cells, fibroblasts
and endothelial cells, subsequently leading to regeneration to
repair the damaged tissue (27,47). Another is the paracrine/endocrine
mechanism, in which implanted MSCs secrete numerous and varied
soluble mediators, including anti-inflammatory factors and growth
factors, through paracrine/endocrine modes of action (48). However, the exact functions they
exert in the repair of ALI remain to be fully elucidated, although
the migration of MSCs to the diseased organs or tissues is required
(49). The present study found
that a host of hUC-MSCs migrated into the normal and injured lungs
30 min following cell engraftment, however, there was notable
recruitment and increasing retention in the injured lungs at 1 day,
extending to 7 days. These results indicated that hUC-MSCs possess
the ability to gather in injured tissues, which is in accordance
with previous reports that bone marrow-derived progenitor cells
migrated to inflammatory areas of the lungs (50,51). However, other studies have
reported that there was only a low level of MSC retention in the
injured lung (52,53). The result of the present study
revealed that the retention of hUC-MSCs in the LPS+MSC group was
more marked, compared with that in the NS+MSC group at the same
time between 30 min and 7 days post-cell transplantation. In
particular, the localization of hUC-MSCs in the LPS+MSC group
exhibited marked retention, whereas those in the NS+MSC group had
completely disappeared on day 7. Notably, it was also found that
hUC-MSC recruitment to the liver appeared higher in the NS+MSC
group, which suggested that a smaller proportion of hUC-MSCs
accumulated in the injured lungs of the LPS+MSC mice, compared with
that of the NS+MSC mice with the same MSC treatment strategy.
Therefore, this discrepancy in benefits of hUC-MSCs between the two
groups indicated that the persistence of transplanted hUC-MSCs in
the lung was vital in promoting lung repair.
However, the mechanism underlying hUC-MSC migration
and homing to the injured lung remains to be fully elucidated.
Whether the injured lungs secrete several chemokines or whether the
receptors and adhesion molecules for hUC-MSCs increase in the lungs
to recruit hUC-MSCs to injured sites remains to be elucidated. To
determine what types of homing cytokines are involved and how they
function to recruit hUC-MSCs further investigation is
warranted.
Taken together, the present study successfully
constructed a novel targeted fluorescence molecular probe, Cy7-GD2,
which not only contained the optical properties of Cy7, but also
maintained the immune activity of anti-GD2 monoclonal antibodies.
The hUC-MSCs also effectively alleviated the LPS-induced ALI in the
mouse model. The hUC-MSCs labeled with fluorescent probe were also
engrafted into the ALI mice to investigate the distribution of MSCs
in the ALI mouse model. The novel fluorescent molecular probe,
Cy7-GD2, was confirmed as a candidate for use to track the
distribution of hUC-MSCs in treating ALI.
Abbreviations:
|
hUC-MSCs
|
human umbilical cord-derived
mesenchymal stem cells
|
|
ALI
|
acute lung injury
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
GD2
|
ganglioside
|
|
Cy7
|
CyDye mono-reactive NHS esters
|
|
LPS
|
lipopolysaccharide
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
PBS
|
phosphate-buffered saline
|
|
CCK-8
|
Cell Counting Kit-8
|
|
EBD
|
Evans blue dye
|
|
HE
|
hematoxylin and eosin
|
|
NS
|
normal saline
|
|
W/D
|
wet-to-dry ratio
|
Acknowledgments
The authors would like to thank the Fundamental
Research Funds of Science and Technology Planning Project of
Guangdong Province, China (grant no. 411265834060) and the Key
Scientific and Technological Program of Guangzhou City (grant no.
201508020262) for their support and the Biological Treatment Center
The Third Affiliated Hospital of Sun Yat-Sen University for
providing the hUC-MSCs.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
ARDS Definition Task Force; Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin Definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
2
|
Bellani G, Laffey JG, Pham T, Fan E,
Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley
DF, et al: Epidemiology, patterns of care, and mortality for
patients with acute respiratory distress syndrome in intensive care
units in 50 countries. JAMA. 315:788–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zafar K: Incidence of acute respiratory
distress syndrome. JAMA. 316:3472016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papazian L, Forel JM, Gacouin A,
Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D,
Seghboyan JM, et al: Neuromuscular blockers in early acute
respiratory distress syndrome. N Engl J Med. 363:1107–1116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sueblinvong V and Weiss DJ: Cell therapy
approaches for lung diseases: Current status. Curr Opin Pharmacol.
9:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Briel M, Meade M, Mercat A, Brower RG,
Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, et
al: Higher vs. lower positive end-expiratory pressure in patients
with acute lung injury and acute respiratory distress syndrome:
Systematic review and meta-analysis. JAMA. 303:865–873. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prockop DJ and Oh JY: Mesenchymal
stem/stromal cells (MSCs): Role as guardians of inflammation. Mol
Ther. 20:14–20. 2012. View Article : Google Scholar :
|
|
8
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akram KM, Samad S, Spiteri M and Forsyth
NR: Mesenchymal stem cell therapy and lung diseases. Adv Biochem
Eng Biotechnol. 130:105–129. 2013.
|
|
10
|
Ho MS, Mei SH and Stewart DJ: The
Immunomodulatory and therapeutic effects of mesenchymal stromal
cells for acute lung injury and sepsis. J Cell Physiol.
230:2606–2617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shalaby SM, El-Shal AS, Abd-Allah SH,
Selim AO, Selim SA, Gouda ZA, Abd El Motteleb DM, Zanfaly HE,
El-Assar HM and Abdelazim S: Mesenchymal stromal cell injection
protects against oxidative stress in Escherichia coli-induced acute
lung injury in mice. Cytotherapy. 16:764–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shim SH, Xia C, Zhong G, Babcock HP,
Vaughan JC, Huang B, Wang X, Xu C, Bi GQ and Zhuang X:
Super-resolution fluorescence imaging of organelles in live cells
with photoswitchable membrane probes. Proc Natl Acad Sci USA.
109:13978–13983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chi C, Du Y, Ye J, Kou D, Qiu J, Wang J,
Tian J and Chen X: Intraoperative imaging-guided cancer surgery:
From current fluorescence molecular imaging methods to future
multi-modality imaging technology. Theranostics. 4:1072–1084. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hussain T and Nguyen QT: Molecular imaging
for cancer diagnosis and surgery. Adv Drug Deliv Rev. 66:90–100.
2014. View Article : Google Scholar
|
|
15
|
Kim JC, Lee JL, Yoon YS, Alotaibi AM and
Kim J: Utility of indocyanine-green fluorescent imaging during
robot-assisted sphincter-saving surgery on rectal cancer patients.
Int J Med Robot. 12:710–717. 2016. View
Article : Google Scholar
|
|
16
|
Chi C, Zhang Q, Mao Y, Kou D, Qiu J, Ye J,
Wang J, Wang Z, Du Y and Tian J: Increased precision of orthotopic
and metastatic breast cancer surgery guided by matrix
metalloproteinase-activatable near-infrared fluorescence probes.
Sci Rep. 5:141972015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sonn GA, Behesnilian AS, Jiang ZK,
Zettlitz KA, Lepin EJ, Bentolila LA, Knowles SM, Lawrence D, Wu AM
and Reiter RE: Fluorescent image-guided surgery with an
anti-prostate stem cell antigen (PSCA) diabody enables targeted
resection of mouse prostate cancer xenografts in real time. Clin
Cancer Res. 22:1403–1412. 2016. View Article : Google Scholar
|
|
18
|
Martinez C, Hofmann TJ, Marino R, Dominici
M and Horwitz EM: Human bone marrow mesenchymal stromal cells
express the neural ganglioside GD2: A novel surface marker for the
identification of MSCs. Blood. 109:4245–4248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Liao W, Gu D, Liang L, Liu M, Du W,
Liu P, Zhang L, Lu S, Dong C, Zhou B and Han Z: Neural ganglioside
GD2 identifies a subpopulation of mesenchymal stem cells in
umbilical cord. Cell Physiol Biochem. 23:415–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jasmin, Torres AL, Nunes HM, Passipieri
JA, Jelicks LA, Gasparetto EL, Spray DC, Campos de Carvalho AC and
Mendez-Otero R: Optimized labeling of bone marrow mesenchymal cells
with superparamagnetic iron oxide nanoparticles and in vivo
visualization by magnetic resonance imaging. J Nanobiotechnology.
9:42011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camacho X, Machado CL, García MF, Gambini
JP, Banchero A, Fernández M, Oddone N, Bertolini Zanatta D, Rosal
C, Buchpiguel CA, et al: Technetium-99m- or Cy7-Labeled Rituximab
as an Imaging Agent for Non-Hodgkin Lymphoma. Oncology. 92:229–242.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan GZ, Yang Y, Zhang J, Liu W, Wang GY,
Zhang YC, Yang Q, Zhai FX, Tai Y, Liu JR, et al: Bone marrow
mesenchymal stem cells ameliorate hepatic ischemia/reperfusion
injuries via inactivation of the MEK/ERK signaling pathway in rats.
J Surg Res. 178:935–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Metildi CA, Kaushal S, Luiken GA, Talamini
MA, Hoffman RM and Bouvet M: Fluorescently labeled chimeric
anti-CEA antibody improves detection and resection of human colon
cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse
model. J Surg Oncol. 109:451–458. 2014. View Article : Google Scholar
|
|
24
|
Park JY, Hiroshima Y, Lee JY, Maawy AA,
Hoffman RM and Bouvet M: MUC1 selectively targets human pancreatic
cancer in orthotopic nude mouse models. PLoS One. 10:e01221002015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tai WL, Dong ZX, Zhang DD and Wang DH:
Therapeutic effect of intravenous bone marrow-derived mesenchymal
stem cell transplantation on early-stage LPS-induced acute lung
injury in mice. Nan Fang Yi Ke Da Xue Xue Bao. 32:283–290.
2012.PubMed/NCBI
|
|
26
|
Li J, Li D, Liu X, Tang S and Wei F: Human
umbilical cord mesenchymal stem cells reduce systemic inflammation
and attenuate LPS-induced acute lung injury in rats. J Inflamm
(Lond). 9:332012. View Article : Google Scholar
|
|
27
|
He H, Liu L, Chen Q, Liu A, Cai S, Yang Y,
Lu X and Qiu H: Mesenchymal stem cells overexpressing
angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced
lung injury. Cell Transplant. 24:1699–1715. 2015. View Article : Google Scholar
|
|
28
|
Zhang Y, Fan S, Yao Y, Ding J, Wang Y,
Zhao Z, Liao L, Li P, Zang F and Teng GJ: In vivo, near-infrared
imaging of fibrin deposition in thromboembolic stroke in mice. PLoS
One. 7:e302622012. View Article : Google Scholar
|
|
29
|
Wang XY, Ju S, Li C, Peng XG, Chen AF, Mao
H and Teng GJ: Non-invasive imaging of endothelial progenitor cells
in tumor neovascularization using a novel dual-modality
paramagnetic/near-infrared fluorescence probe. PLoS One.
7:e505752012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin J, Swartz TE, Zhang J, Patapoff TW,
Chen B, Marhoul J, Shih N, Kabakoff B and Rahimi K: Validation of a
spectral method for quantitative measurement of color in protein
drug solutions. PDA J Pharm Sci Technol. 70:382–391. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garbern JC and Lee RT: Cardiac stem cell
therapy and the promise of heart regeneration. Cell Stem Cell.
12:689–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu DX, Marchetto MC and Gage FH:
Therapeutic translation of iPSCs for treating neurological disease.
Cell Stem Cell. 12:678–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Soleimani M and Nadri S: A protocol for
isolation and culture of mesenchymal stem cells from mouse bone
marrow. Nat Protoc. 4:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon
SJ, Chang JW, Choi SJ, Oh W, Yang YS, et al: Low immunogenicity of
allogeneic human umbilical cord blood-derived mesenchymal stem
cells in vitro and in vivo. Biochem Biophys Res Commun.
446:983–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El Omar R, Beroud J, Stoltz JF, Menu P,
Velot E and Decot V: Umbilical cord mesenchymal stem cells: The new
gold standard for mesenchymal stem cell-based therapies. Tissue Eng
Part B Rev. 20:523–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maron-Gutierrez T, Silva JD, Asensi KD,
Bakker-Abreu I, Shan Y, Diaz BL, Goldenberg RC, Mei SH, Stewart DJ,
Morales MM, et al: Effects of mesenchymal stem cell therapy on the
time course of pulmonary remodeling depend on the etiology of lung
injury in mice. Crit Care Med. 41:e319–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tong L, Zhou J, Rong L, Seeley EJ, Pan J,
Zhu X, Liu J, Wang Q, Tang X, Qu J, et al: Fibroblast Growth
Factor-10 (FGF-10) mobilizes lung-resident mesenchymal stem cells
and protects against acute lung injury. Sci Rep. 6:216422016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nguyen PK, Riegler J and Wu JC: Stem cell
imaging: From bench to bedside. Cell Stem Cell. 14:431–444. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu C, Li J, Pang P, Liu J, Zhu K, Li D,
Cheng D, Chen J, Shuai X and Shan H: Polymeric vector-mediated gene
transfection of MSCs for dual bioluminescent and MRI tracking in
vivo. Biomaterials. 35:8249–8260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Triphan SM, Breuer FA, Gensler D, Kauczor
HU and Jakob PM: Oxygen enhanced lung MRI by simultaneous
measurement of T1 and T2 * during free breathing using ultrashort
TE. J Magn Reson Imaging. 41:1708–1714. 2015. View Article : Google Scholar
|
|
41
|
Gu E, Chen WY, Gu J, Burridge P and Wu JC:
Molecular imaging of stem cells: Tracking survival,
biodistribution, tumorigenicity, and immunogenicity. Theranostics.
2:335–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Herberts CA, Kwa MS and Hermsen HP: Risk
factors in the development of stem cell therapy. J Transl Med.
9:292011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feng T, Ai X, Ong H and Zhao Y:
Dual-responsive carbon dots for tumor extracellular
microenvironment triggered targeting and enhanced anticancer drug
delivery. ACS Appl Mater Interfaces. 8:18732–18740. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Landau MJ, Gould DJ and Patel KM: Advances
in fluorescent-image guided surgery. Ann Transl Med. 4:3922016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin X, Zhu H, Luo Z, Hong Y, Zhang H, Liu
X, Ding H, Tian H and Yang Z: Near-infrared fluorescence imaging of
non-Hodgkin's lymphoma CD20 expression using Cy7-conjugated
obinutuzumab. Mol Imaging Biol. 16:877–887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang YY, Li XZ and Wang LB: Therapeutic
implications of mesenchymal stem cells in acute lung injury/acute
respiratory distress syndrome. Stem Cell Res Ther. 4:452013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao YD, Ohkawara H, Vogel SM, Malik AB
and Zhao YY: Bone marrow-derived progenitor cells prevent
thrombin-induced increase in lung vascular permeability. Am J
Physiol Lung Cell Mol Physiol. 298:L36–L44. 2010. View Article : Google Scholar :
|
|
48
|
Qin ZH, Xu JF, Qu JM, Zhang J, Sai Y, Chen
CM, Wu L and Yu L: Intrapleural delivery of MSCs attenuates acute
lung injury by paracrine/endocrine mechanism. J Cell Mol Med.
16:2745–2753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sohni A and Verfaillie CM: Mesenchymal
stem cells migration homing and tracking. Stem Cells Int.
2013:1307632013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yamada M, Kubo H, Kobayashi S, Ishizawa K,
Numasaki M, Ueda S, Suzuki T and Sasaki H: Bone marrow-derived
progenitor cells are important for lung repair after
lipopolysaccharide-induced lung injury. J Immunol. 172:1266–1272.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu L, He H, Liu A, Xu J, Han J, Chen Q,
Hu S, Xu X, Huang Y, Guo F, et al: Therapeutic effects of bone
marrow-derived mesenchymal stem cells in models of pulmonary and
extrapulmonary acute lung injury. Cell Transplant. 24:2629–2642.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Beckett T, Loi R, Prenovitz R, Poynter M,
Goncz KK, Suratt BT and Weiss DJ: Acute lung injury with endotoxin
or NO2 does not enhance development of airway epithelium from bone
marrow. Mol Ther. 12:680–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1. PLoS Med. 4:e2692007. View Article : Google Scholar : PubMed/NCBI
|