Introduction
Diabetes is a severe disorder since it strongly
increases the risk of cardiovascular complications, such as
coronary artery disease, myocardial infarction, hypertension and
dyslipidemia (1,2). The cardiovascular injury mainly
targets two important organs, eyes and kidneys (3). This has made diabetic nephropathy
(DN) one of the most common complications for diabetic patients
(4,5). The main typical renal histological
changes of DN are caused by the changes in the extracellular matrix
(ECM). The ECM accumulation in DN results in mesangial expansion,
tubulointerstitial fibrosis, and irreversible deterioration of
renal function (6,7).
It is reported that transforming growth factor-β1
(TGF-β1) plays an important role in the development of DN (8–10).
After binding to its receptor, TGF-β1 activates two critical
downstream mediators, Smad2 and Smad3, to exert its biological
activities such as ECM production, which is negatively regulated by
Smad7, an inhibitor Smad. The effect of TGF-β1/Smad3 signaling
pathway on mediating renal fibrosis has been well recognized
(11,12), suggesting that the block of
TGF-β1/Smad3 can provide an effective approach for preventing the
progression of DN.
However, no proper effective treatment has been
detected so far for retarding the progression of renal failure.
Therefore, new therapeutic strategies in the management of DN are
required (13).
Sitagliptin (SIT), a dipeptidyl peptidase-4 (DPP-4)
inhibitor, exhibited a modest beneficial effect on glycated
hemoglobin levels when used in combination with metformin for
treatment of diabetes mellitus type 2 (14). Recent studies have shown that SIT
alone can offer cardiovascular and neuropathic protections which
are probably mediated through antioxidant, anti-inflammatory, and
anti-apoptotic mechanisms (15–21). It is reported that SIT can also
ameliorate renal ischemia reperfusion injury in rats (22). However, whether SIT has beneficial
effect on prevention of DN remains unknown.
In this study the therapeutic effect of SIT for
prevention of DN was systematically verified. Moreover, the
possible mechanism through TGF-β1/Smad signaling was investigated
for the first time.
Materials and methods
Ethics statements
This study was carried out in strict accordance with
the recommendations in the Guiding Principles for Care and Use of
Laboratory Animals of Xuzhou Medical University. The protocol was
approved by the Committee on the Ethics of Laboratory Animals of
Xuzhou Medical University (Xuzhou, China). All surgery was
performed under sodium pentobarbital anesthesia, and all efforts
were made to minimize suffering.
Animal experiments
Male Sprague-Dawley rats (certificate no.
SYXK2010-0011), weighing 180–200 g were obtained from the
Laboratory Animal Center of Xuzhou Medical University (Xuzhou,
China). The animals were housed in standard metabolic cages and
maintained under standard condition at constant room temperature of
20–25°C and humidity of 40–70% with a 12:12 h light and dark cycle.
All rats had free access to commercial regular chow and water for
one week before the dietary manipulation. After one week's
adaptation, fasting blood glucose (FBG) values were measured by
OneTouch II blood glucose meter with overnight fasting. Thirty rats
were randomly divided into two groups: control group and study
group. The control group was fed with regular chow and the study
group was given a high fat diet (HFD) and high calorie diet (36.3%
fat, 45% carbohydrate and 18.7% protein) for four weeks. Thereafter
the values of FBG were calculated after 12 h fasting. The study
group rats were initially injected intraperitoneally (i.p.) with
streptozotocin (Sigma, St. Louis, MO, USA) at a low dose of 30
mg/kg diluted in citrate buffer (pH 4.4) (23–25), while the negative control ones (NC
group) were injected with citrate buffer. One week later, the study
group rats with an FBG <7.8 mmol/l were injected with repeated
low dose of STZ (30 mg/kg). After four weeks of STZ injection, the
study group rats with an FBG of ≥7.8 mmol/l twice or with random of
≥11.1 mmol/l were considered as type 2 diabetic rats (26). The type 2 diabetic rats were
randomly divided into 2 groups: DN group (n=9) was treated daily
with normal saline solution and SIT group (n=8) was administrated
with SIT (Meck Sharp and Dohme Pty Ltd., Macquarie Park, NSW,
Australia) at dose of 10 mg/kg (27). The same volume of normal saline
solution was administered to the negative control group (NC group,
n=9). The study period lasted 12 weeks.
Sample collection
After 12 weeks of treatment, the urine and blood
samples were collected from the rats. After the animals were
sacrificed, their kidneys were removed and weighed. The fresh
kidney cortices were stored in formaldehyde solution for
measurements. The kidney was fixed in 4% paraformaldehyde and the
rest kidney tissues were snap frozen in liquid nitrogen and then
stored at −80°C for later analysis.
Biochemical analysis
The values of urine protein were determined by using
urinary protein kits from NanJing JianCheng Bioengineering
Institute (Nanjing, China). The values of creatinine and blood urea
nitrogen (BUN) were determined by enzyme-linked immunosorbent assay
(ELISA) kits from Wuhan ColorfulGene Biological Technology Co.,
Ltd. (Wuhan, China). The kidney index was calculated by ratio of
kidney weight (g)/body weight (kg).
Renal histological examination
After being fixed in 4% paraformaldehyde and
embedded in paraffin, the kidney tissues were cut into 4-µm
sections and stained with hematoxylin and eosin (H&E), periodic
acid schiff (PAS), and Masson staining as previously described
(28). Briefly, for Masson
staining, the 4-µm section samples were firstly
deparaffinized in water and then stained with Masson composite
staining solution for 5 min, washed successively with 0.2% acetic
acid solution, 5% phosphotungstic acid, 0.2% acetic acid solution,
and finally stained with bright green staining solution for 5 min,
washed twice with 0.2% acetic acid solution, dehydrated in absolute
alcohol, put in xylene for transparency, and mounted with neutral
gum. After this staining procedure, the collagen fibers were bluish
green in color.
Immunohistochemistry examination
The immunohisto-chemistry analysis was performed
according to the procedure reported previously (29). After the deparaffinization and
hydration procedure, the 4-µm section samples were then
washed with Tris-buffered saline (TBS; 10 mmol/l Tris HCl, 0.85%
NaCl, pH 7.2). Endogenous peroxidase activity was quenched by
incubating the section samples in 3% H2O2
deionized water. After overnight incubation with rabbit polyclonal
anti-collagen IV antibody and rabbit monoclonal anti-fibronectin
antibody (both from Abcam, Cambridge, MA, USA) at 4°C, the section
samples were washed with TBS. Polymer helper was then added into
the samples, and the section samples were incubated at room
temperature for another 10 min. The slides were thereafter washed
with TBS, incubated with poly-HRP anti-rabbit IgG at room
temperature for final 10 min. A negative control without primary
antibody was included in the experiment to verify antibody
specificity. Finally, the sections were counterstained with
3,3′-diamino-benzidine (DAB) for 2 min. Brownish yellow granular or
linear deposits were interpreted as positive areas. Accumulation of
collagen IV and fibronectin was determined using the quantitative
Image Analysis system. After ten random fields in both glomeruli
and tubulointerstitium under light microscopy (×400) were outlined
and positive staining patterns were identified, the percentage of
positive area in the examined field was then determined. The
arterial lumen space was excluded from the study. Data were
expressed as percentage of positive area examined.
Reverse transcription-polymerase chain
reaction (RT-PCR)
A RT-PCR procedure was performed to determine the
relative mRNA quantities of TGF-β1 and Smad7. Total RNA was
extracted from rat kidney cortices by using TRIzol reagent
(Tiangen, Beijing, China) according to the manufacturer's
instructions. The obtained total RNA was converted into cDNA using
the TIANScript RT kit (Tiangen). The upsteam and downstrean primers
(Shanghai Sangon Co., Shanghai, China) of these genes are shown in
Table I. Levels of mRNA
expression were subjected to housekeeping gene β-actin.
 | Table IPrimer sequences for RT-PCR. |
Table I
Primer sequences for RT-PCR.
| Gene names | Forward
sequences | Reverse
sequences |
|---|
| TGF-β1 |
5′-ATGTGCAGGATAATTGCTGCC-3′ |
5′-TGGTGTTGTACAGGCTGAGG-3′ |
| Smad7 |
5′-TGGTGCGTGGTGGCATACTGG-3′ |
5′-GACTCTTGTTGTCCGAATTGAGCT-3′ |
| β-actin |
5′-TCAGGTCATCACTATCGGCAAT-3′ |
5′-AAAGAAAGGGTGTAAAACGCA-3′ |
Western blotting
As described in previous studies (30), renal cortical tissues were rapidly
dissected and homogenized in lysis buffer containing protease
inhibitor cocktail (MedChem Express, Princeton, NJ, USA). After
centrifugation at 12,000 × g for 10 min, total protein was
collected. The protein concentration was determined by using the
BCA protein assay kit (Beyotime Institute of Biotechnology,
Shanghai, China) following the manufacturer's protocol. The protein
samples were then mixed with sodium dodecyl sulfate-poly-acrylamide
gel electrophoresis (SDS-PAGE) loading buffer (Beyotime Institute
of Biotechnology) and boiled for 10 min. The protein samples were
separated by 10% SDS polyacrylamide gel, and then transferred to
nitrocellulose membranes. The transferred membranes were then
blocked with blocking buffer (Beyotime Institute of Biotechnology)
for 1 h at room temperature. Transferred membranes were incubated
at 4°C overnight with rabbit polyclonal anti-TGF-β1 antibody,
rabbit monoclonal anti-Smad3 antibody, rabbit monoclonal
anti-p-Smad3 antibody, rabbit monoclonal anti-Smad7 antibody,
rabbit monoclonal anti-GAPDH antibody (all from Abcam). The
antigen-antibody complexes were washed three times for 5 min each
and incubated with IRDye® 800CW goat anti-rabbit for 1 h
at room temperature. After further three washes, the bands were
added to infrared imaging system (Odyssey Sa; Li-COR, Lincoln, NE,
USA). The intensity of TGF-β1 and Smad7 were subjected to GAPDH
protein analysis while Smad3 activation was assessed by the ratio
of phosphorylated form among its corresponding total protein
levels.
Cell experiments
Rat mesangial cells (MCs), cultured in Dulbecco's
modified Eagle's medium, and were incubated at 37°C in a humidified
atmosphere of 5% CO2. During the experiments, the cells
were first exposed to a normal concentration of glucose (NC, 5.56
mmol/l) for 24 h, and then retreated with high glucose (HG, 30
mmol/l glucose), high glucose with 0.1 µmol/ml sitagliptin
(SITL), high glucose with 1 µmol/ml sitagliptin (SITM), and
high glucose with 10 µmol/ml sitagliptin (SITH). Sitagliptin
was dissolved in 1‰ (v/v) dimethyl sulfoxide (DMSO) and DMSO group
was adopted as a control to rule out the effect of vehicle.
Mannitol (MA) was adopted as a control to rule out the effect of
osmotic pressure. The cells were harvested for analysis after 24
h.
Immunofluorescence
MCs were seeded into 12 well-plates with glass
bottom inserts. Then the normal glucose medium of the 12
well-plates was replaced with various concentrations of sitagliptin
or other controlling medium. After 24 h, cells were washed with
phosphate-buffered saline (PBS) and fixed with cold polysorbate for
15 min at room temperature. After being washed intensively three
times with PBS, the cells were added with 0.1% Triton X-100 in PBS
for 15 min and blocked with 5% BSA in PBS buffer for 30 min at room
temperature, and incubated with TGF-β1 and p-Smad3 antibodies at
4°C overnight. The cells were then incubated with DyLight 594
AffiniPure Donkey anti-rabbit IgG (H + L) 1 h at room temperature.
The nuclei were counter-stained by 4,6-diamidino-2 phenylindole
(DAPI) for 2 min, then washed again with PBS two times before
mounting with fluorescence mounting medium. Images were captured by
Olympus BX43F fluorescence microscope (Olympus, Tokyo, Japan).
Enzyme-linked immunosorbent assay
The levels of Smad7 protein in the MCs were
determined by ELISA (R.B. Scientific, Waltham, MA, USA). The levels
of Smad7 was determined according to the manufacturer's
instructions. The colorimetric reaction was measured at 450 nm.
Statistical analysis
Data were expressed as means ± SE. Statistical
analyses were performed using the paired t-test for two data
comparison and one-way analysis of variance (ANOVA) for multiple
data comparison. A value of P<0.05 was considered statistically
significant.
Results
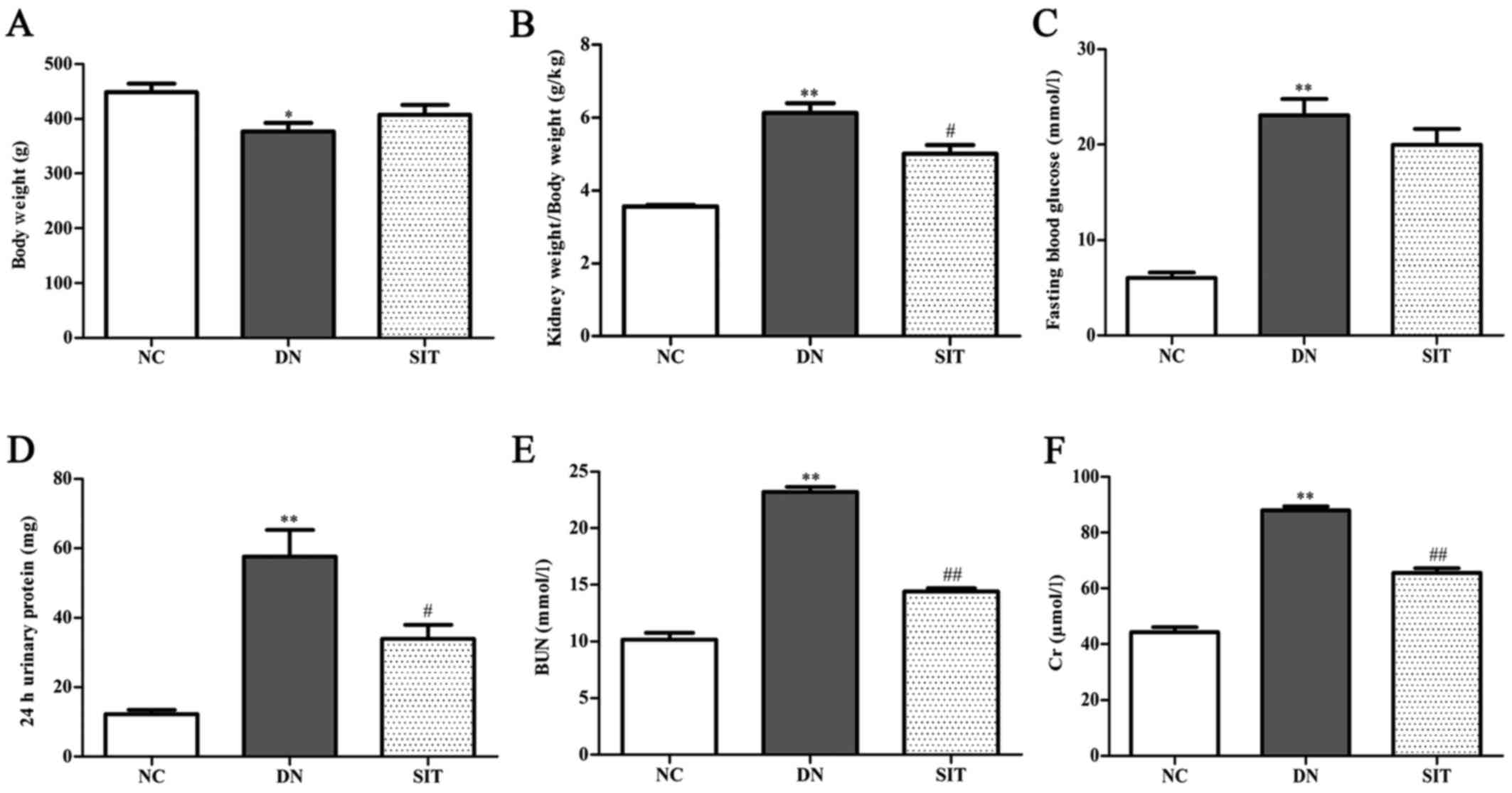
Biochemical analysis
As shown in Fig.
1, the kidney index and levels of FBG, 24 h urinary protein,
BUN and Cr for the DN group were significantly higher than those of
the NC group (P<0.01), suggesting that our early DN model was
successful. The SIT group showed remarkably reduced kidney index
and levels of 24 h urinary protein (P<0.05), BUN (P<0.01),
and Cr (P<0.01), suggesting that SIT can improve the function of
DN.
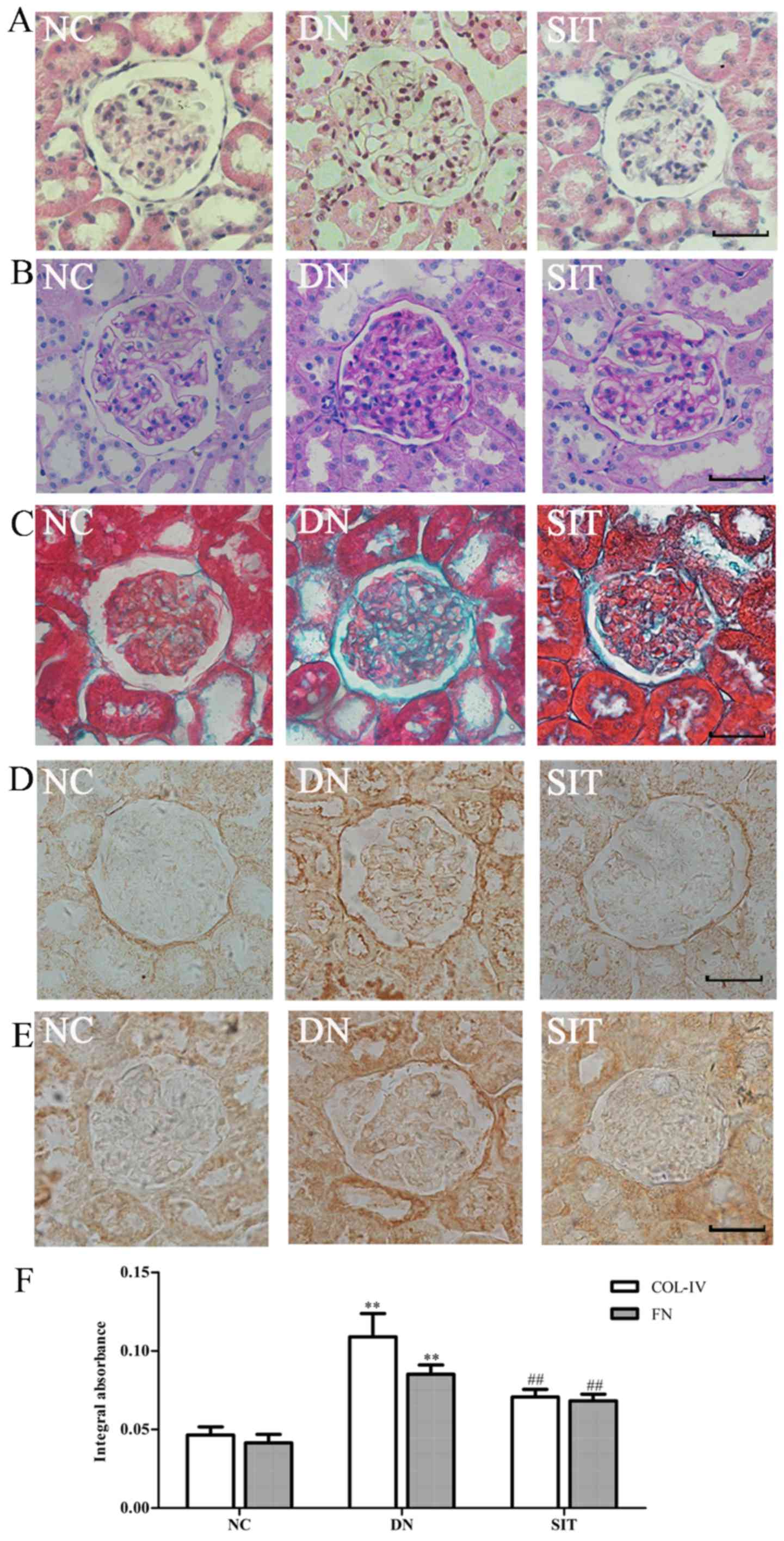
Histological and immunohistochemistry
analysis
The H&E staining (Fig. 2A) and PAS staining (Fig. 2B) of the kidney sections from the
control group revealed a normal glomerulus surrounded by Bowman's
capsule and proximal and distal convoluted tubules without any
inflammatory changes. The DN group showed renal tubule atrophy,
thickening of the basement membrane. The SIT group exhibited a
significantly-attenuated basement membrane. Compared with control
group, the DN group exhibited a maximum accumulation of collagen IV
(Col IV) and fibronectin (FN) in the renal cortex revealed by
Masson staining (Fig. 2C) and
immunohistochemistry (Fig. 2D–E).
Compared with the DN group, significantly-reduced renal fibrosis
was observed in the SIT-treated group. All of these fibrotic
changes in the diabetic kidney were largely attenuated by treatment
with SIT as indicated in Fig. 2F
(P<0.01).
Effects of sitagliptin on TGF-β1
expression in DN rats and high glucose-induced MCs
As illustrated in Fig.
3A–D, the DN group exhibited remarkably higher level of TGF-β1
mRNA and TGF-β1 when compared to the NC group (P<0.01).
Interestingly, SIT-treated group showed significantly lower level
of TGF-β1 mRNA and TGF-β1 than DN group (P<0.01). The above
indicates that the increased TGF-β1 expression was observed in DN
rats through both western blotting and PCR as well as in high
glucose-induced MCs by immunofluorescence. SIT-treated groups
reduced the increased TGF-β1 expression (Fig. 3E). Taken together, these results
suppport that treatment with SIT reverses the overexpression of
TGF-β1.
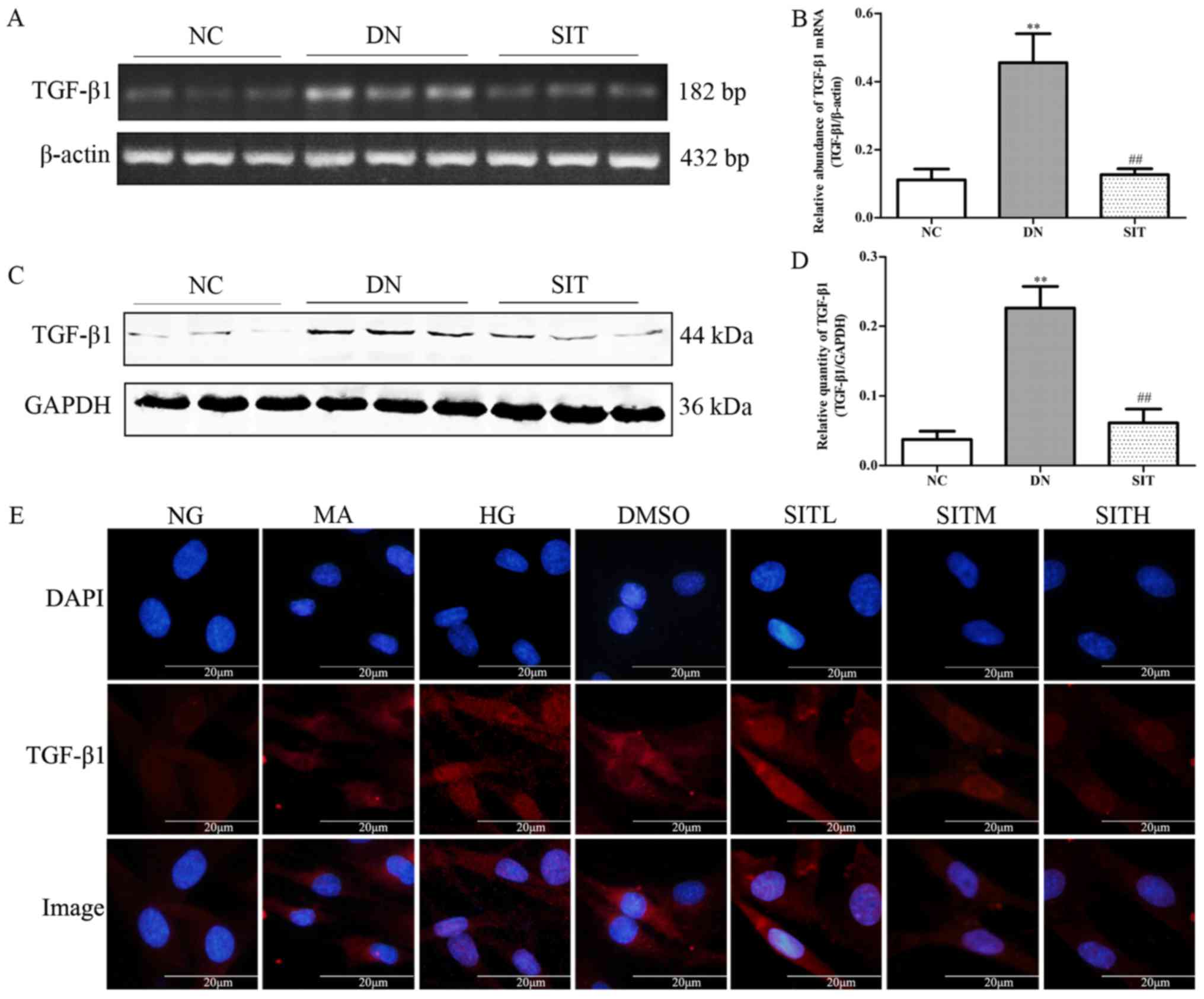 | Figure 3Effects of sitagliptin (SIT) on
transforming growth factor-β1 (TGF-β1) expression in vivo
and in vitro. (A) RT-PCR bands of TGF-β1 mRNA in DN rats.
(B) Relative abundance of TGF-β1 mRNA in DN rats. (C) Western blot
bands of TGF-β1 in DN rats. (D) Relative abundance of TGF-β1 in DN
rats. (E) Expression of TGF-β1 in mesangial cells induced by high
glucose through immunofluorescence. NC, negative control group; DN,
diabetic nephropathy group; SIT, administration of SIT at dose of
10 mg/kg; NG, cells treated with normal glucose 5.56 mmol/l; MA,
cells treated with normal glucose 5.56 mmol/l + mannitol 24.44
mmol/l; HG, cells treated with high glucose 30 mmol/l; dimethyl
sulfoxide (DMSO), cells treated with high glucose + 1‰ DMSO; SITL,
SITM, SITH, cells treated with high glucose + 0.1, 1 and 10
µmol/ml sitagliptin, respectively. Data are expressed as
mean ± SE. n=3. **P<0.01 vs. NC group;
##P<0.01 vs. DN group. |
Effects of sitagliptin on p-Smad3
expression in DN rats and high glucose-induced MCs
In Fig. 4A and B,
relative quantity of p-Smad3 was upregulated in DN group when
compared to the NC group (P<0.01). However, administration of
SIT significantly decreased the expression of p-Smad3 compared with
DN group (P<0.01). In correlation with the finding from western
blotting in DN rats, the p-Smad3 expression in MCs induced by high
glucose was increased through immunofluorescence. However,
SIT-treated groups could downregulate the increased p-Smad3
expression (Fig. 4C). Thus, the
results indicated that administration of SIT significantly
decreased the level of p-Smad3 in DN rats and MCs induced by high
glucose.
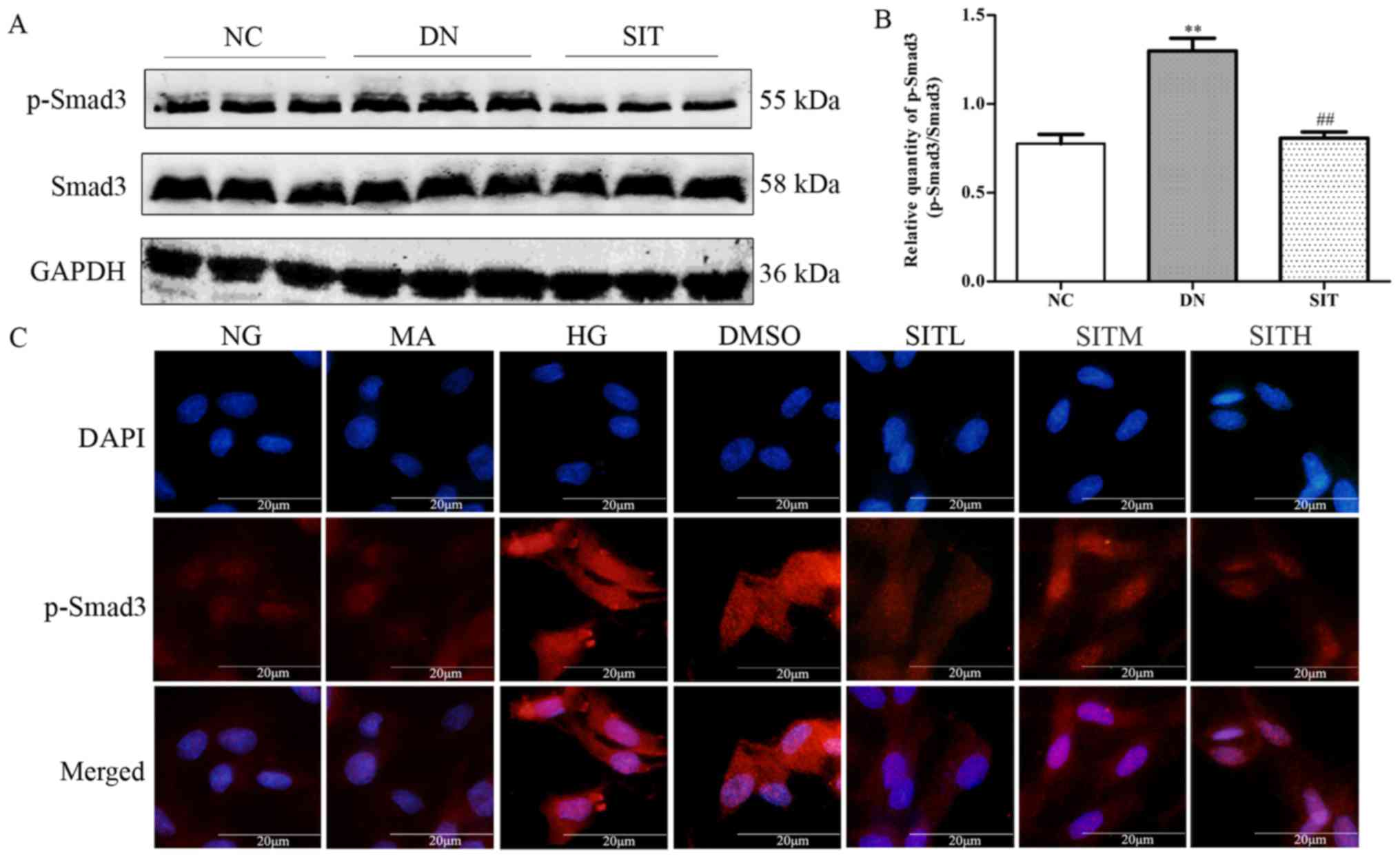 | Figure 4Effects of sitagliptin (SIT) on
p-Smad3 expression in vivo and in vitro. (A) Western
blot bands of p-Smad3 in DN rats. (B) Relative abundance of p-Smad3
in DN rats. (C) Expression of p-Smad3 in mesangial cells induced by
high glucose through immunofluorescence. NC, negative control
group; DN, diabetic nephropathy group; SIT, administration of SIT
at dose of 10 mg/kg; NG, cells treated with normal glucose 5.56
mmol/l; MA, cells treated with normal glucose 5.56 mmol/l +
mannitol 24.44 mmol/l; HG, cells treated with high glucose 30
mmol/l; dimethyl sulfoxide (DMSO), cells treated with high glucose
+ 1‰ DMSO; SITL, SITM, SITH, cells treated with high glucose + 0.1,
1 and 10 µmol/ml sitagliptin, respectively. Data are
expressed as mean ± SE. n=3. **P<0.01 vs. NC group;
##P<0.01 vs. DN group. |
Effects of sitagliptin on Smad7
expression in DN rats and high glucose-induced MCs
The DN group exhibited lower amount of inhibitory
Smad7 than NC group (P<0.01). On the contrary, the SIT-treated
group showed higher amount of inhibitory Smad7 than DN group
(P<0.05) (Fig. 5A–D). In MCs
induced by high glucose the levels of Smad7 were remarkably
down-regulated compared to those of the NG group at 24 h through
ELISA (P<0.01). The levels of Smad7 in SITL, SITM and SITH
groups were significantly higher than those of the HG group
(P<0.01). No significant difference was found between the MA
group and the NG group in terms of the level of Smad7. The Smad7
level in DMSO group was almost identical to that of the HG group,
indicating that no obvious effects were generated by the osmotic
pressure and the vehicle. The above results indicated that
administration of SIT significantly upgraded the levels of Smad7 in
DN rats and MCs induced by high glucose.
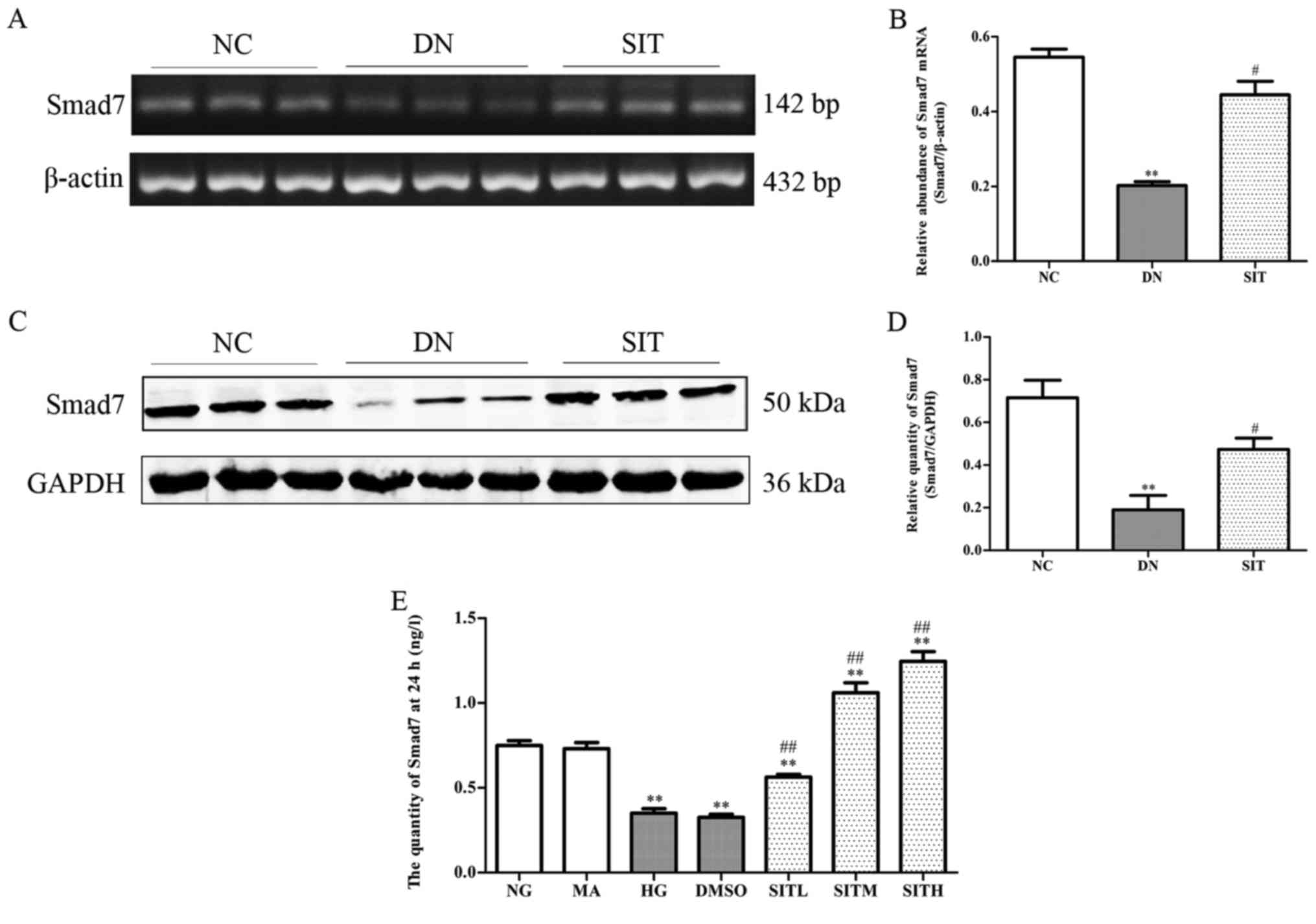 | Figure 5Effects of sitagliptin (SIT) on Smad7
expression in vivo and in vitro. (A) RT-PCR bands of
Smad7 mRNA in DN rats. (B) Relative abundance of Smad7 mRNA in DN
rats. (C) Western blot bands of Smad7 in DN rats. (D) Relative
abundance of Smad7 in DN rats. (E) Expression of Smad7 in mesangial
cells induced by high glucose through enzyme-linked immunosorbent
assay (ELISA). NC, negative control group; DN, diabetic nephropathy
group; SIT, administration of SIT at dose of 10 mg/kg; NG, cells
treated with normal glucose 5.56 mmol/l; MA, cells treated with
normal glucose 5.56 mmol/l + mannitol 24.44 mmol/l; HG, cells
treated with high glucose 30 mmol/l; dimethyl sulfoxide (DMSO),
cells treated with high glucose + 1‰ DMSO; SITL, SITM, SITH, cells
treated with high glucose + 0.1, 1 and 10 µmol/ml
sitagliptin, respectively. Data are expressed as mean ± SE. n=3.
**P<0.01 vs. NC group; #P<0.05 vs. DN
group; ##P<0.01 vs. DN group. |
Discussion
DN is one of the most important causes leading to
endstage renal disease, which affects 15–25% of T1DM patients and
30–40% of type 2 diabetes mellitus (T2DM) patients (31,32). DN, also known as nodular diabetic
glomerulosclerosis, is characterised by mesangial cell
proliferation and excessive accumulation of ECM, which may
ultimately lead to chronic renal failure (33). The major ECM proteins such as
collagen IV and fibronectin are often used as markers of
fibrogenesis in various kidney fibrotic diseases including DN
(34–36).
It is now well-known that TGF-β1/Smad3 mediates
fibrosis (11,12). In this study, we examined the
therapeutic effect of SIT on DN using a rat model of T2DM and high
glucose-induced rat MCs. T2DM was induced in rats using a HFD plus
repeated low dose STZ injections. Our data showed that the kidney
index, the level of 24 h urinary protein, levels of BUN and Cr for
the DN rats were significantly increased, indicating a successful
renal dysfunction in T2DM rats. The increased intensity of PAS and
Masson staining, ECM accumulation also suggested renal lesions in
the T2DM rats. These results demonstrated that the T2DM rat model
was well established and DN rats were successfully induced renal
dysfunction (37). By treating
the DN rats with SIT in our study, we found that the SIT
significantly inhibited kidney fibrosis and remarkably decreased
the levels of proteinuria, BUN and Cr. This indicated that the SIT
effectively improved the renal function.
An interesting finding of this study was that SIT
works through inhibiting TGF-β1/Smad3 signaling and activating
Smad7 expression to alleviate renal fibrosis of the diabetic
kidney. It is well-known that both inhibition of TGF-β1/Smad3
pathway and the increasing Smad7 expression results in reducing ECM
accumulation (12). Moreover, we
also found that the induced DN from T2DM by HFD plus repeated low
dose injections of STZ and high glucose-induced rat MCs were all
associated with a marked activation of Smad3 but a loss of Smad7,
suggesting imbalance between Smad3 and Smad7 signaling in the
pathogenesis of DN model. In contrast, treatment with SIT weakened
diabetic renal injury by rebalancing the TGF-β1/Smad signaling
pathway.
Thus, SIT exhibited potent therapeutic potential in
preventing the progression of DN through blocking
TGF-β1/Smad3-mediated renal fibrosis and upregulating inhibitory
Smad7, which may provide the basis of treatment for DN patients in
the future.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was funded by the National Innovative
Practice Training Program for Students of Higher Education
Institutions (no. 201410313024), the Innovative Practice Training
Program for Students of Jiangsu Higher Education Institutions (no.
201410313024Z), the School of Pharmacy, Xuzhou Medical College
Innovative Practice Training Program for Graduates (no.
2014YKYCX013), Young Medical Talents of Wuxi (no. QNRC020), and the
Young Project of Wuxi Health and Family Planning Research (no.
Q201706). This study was also funded by School of Pharmacy, Xuzhou
Medical College Innovative Practice Training Program for Graduates
(no. 2014YKYCX013).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
QW, YG, and DW conceived and designed the study. DW,
GZ, XC, TW, CL and CC performed the experiments. DW and TW wrote
the paper. CL and CC reviewed and edited the manuscript. All
authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Roberts KT: The potential of fenugreek
(Trigonella foenum-graecum) as a functional food and nutraceutical
and its effects on glycemia and lipidemia. J Med Food.
14:1485–1489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dixit P, Ghaskadbi S, Mohan H and
Devasagayam TP: Antioxidant properties of germinated fenugreek
seeds. Phytother Res. 19:977–983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kashihara N, Haruna Y, Kondeti VK and
Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem.
17:4256–4269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reutens AT and Atkins RC: Epidemiology of
diabetic nephropathy. Contrib Nephrol. 170:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Collins AJ, Foley RN, Herzog C, Chavers B,
Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, et al:
US Renal Data System 2010 Annual Data Report. Am J Kidney Dis.
57(Suppl 1): A8e1–e526. 2011. View Article : Google Scholar
|
|
6
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al Renal Pathology Society: Pathologic classification of
diabetic nephropathy. J Am Soc Nephrol. 21:556–563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Huang XR, Li AG, Liu F, Li JH,
Truong LD, Wang XJ and Lan HY: Signaling mechanism of TGF-beta1 in
prevention of renal inflammation: Role of Smad7. J Am Soc Nephrol.
16:1371–1383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belghith M, Bluestone JA, Barriot S,
Mégret J, Bach JF and Chatenoud L: TGF-beta-dependent mechanisms
mediate restoration of self-tolerance induced by antibodies to CD3
in overt autoimmune diabetes. Nat Med. 9:1202–1208. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang C, Kim Y, Caramori ML, Fish AJ, Rich
SS, Miller ME, Russell GB and Mauer M: Cellular basis of diabetic
nephropathy: II. The transforming growth factor-beta system and
diabetic nephropathy lesions in type 1 diabetes. Diabetes.
51:3577–3581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oujo B, Muñoz-Félix JM, Arévalo M,
Núñez-Gómez E, Pérez-Roque L, Pericacho M, González-Núñez M, Langa
C, Martínez-Salgado C, Perez-Barriocanal F, et al: L-Endoglin
overexpression increases renal fibrosis after unilateral ureteral
obstruction. PLoS One. 9:e1103652014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao M, Zheng S, Yang J, Wu Y, Ren Y, Kong
X, Li W and Xuan J: Suppression of TGF-β1/Smad signaling pathway by
sesamin contributes to the attenuation of myocardial fibrosis in
spontaneously hypertensive rats. PLoS One. 10:e01213122015.
View Article : Google Scholar
|
|
13
|
Sato S, Kawamura H, Takemoto M, Maezawa Y,
Fujimoto M, Shimoyama T, Koshizaka M, Tsurutani Y, Watanabe A, Ueda
S, et al: Halofuginone prevents extracellular matrix deposition in
diabetic nephropathy. Biochem Biophys Res Commun. 379:411–416.
2009. View Article : Google Scholar
|
|
14
|
Ballav C and Gough SC: Safety and efficacy
of sitagliptin-metformin in fixed combination for the treatment of
type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes.
6:25–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nade VS, Kawale LA and Patel KM:
Protective effect of sitagliptin and rosuvastatin combination on
vascular endothelial dysfunction in type-2 diabetes. Indian J Pharm
Sci. 77:96–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brenner C, Kränkel N, Kühlenthal S, Israel
L, Remm F, Fischer C, Herbach N, Speer T, Grabmaier U, Laskowski A,
et al: Short-term inhibition of DPP-4 enhances endothelial
regeneration after acute arterial injury via enhanced recruitment
of circulating progenitor cells. Int J Cardiol. 177:266–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai TH, Sun CK, Su CH, Sung PH, Chua S,
Zhen YY, Leu S, Chang HW, Yang JL and Yip HK: Sitagliptin
attenuated brain damage and cognitive impairment in mice with
chronic cerebral hypo-perfusion through suppressing oxidative
stress and inflammatory reaction. J Hypertens. 33:1001–1013. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Sahar AE, Safar MM, Zaki HF, Attia AS
and Ain-Shoka AA: Sitagliptin attenuates transient cerebral
ischemia/reperfusion injury in diabetic rats: Implication of the
oxidative-inflammatory-apoptotic pathway. Life Sci. 126:81–86.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bachor TP, Marquioni-Ramella MD and Suburo
AM: Sitagliptin protects proliferation of neural progenitor cells
in diabetic mice. Metab Brain Dis. 30:885–893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Picatoste B, Ramírez E, Caro-Vadillo A,
Iborra C, Ares-Carrasco S, Egido J, Tuñón J and Lorenzo O:
Sitagliptin reduces cardiac apoptosis, hypertrophy and fibrosis
primarily by insulin-dependent mechanisms in experimental type-II
diabetes. Potential roles of GLP-1 isoforms. PLoS One.
8:e783302013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gault VA, Lennox R and Flatt PR:
Sitagliptin, a dipeptidyl peptidase-4 inhibitor, improves
recognition memory, oxidative stress and hippocampal neurogenesis
and upregulates key genes involved in cognitive decline. Diabetes
Obes Metab. 17:403–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang MW, Chen CH, Chen YC, Wu YC, Zhen
YY, Leu S, Tsai TH, Ko SF, Sung PH, Yang CC, et al: Sitagliptin
protects rat kidneys from acute ischemia-reperfusion injury via
upregulation of GLP-1 and GLP-1 receptors. Acta Pharmacol Sin.
36:119–130. 2015. View Article : Google Scholar :
|
|
23
|
Sheela N, Jose MA, Sathyamurthy D and
Kumar BN: Effect of silymarin on
streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in
rats. Iran J Kidney Dis. 7:117–123. 2013.PubMed/NCBI
|
|
24
|
Hou J, Zheng D, Zhong G and Hu Y:
Mangiferin mitigates diabetic cardiomyopathy in
streptozotocin-diabetic rats. Can J Physiol Pharmacol. 91:759–763.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Epp RA, Susser SE, Morissette MP, Kehler
DS, Jassal DS and Duhamel TA: Exercise training prevents the
development of cardiac dysfunction in the low-dose streptozotocin
diabetic rats fed a high-fat diet. Can J Physiol Pharmacol.
91:80–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang M, Lv XY, Li J, Xu ZG and Chen L:
The characterization of high-fat diet and multiple low-dose
streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res.
2008:7040452008. View Article : Google Scholar
|
|
27
|
Mega C, de Lemos ET, Vala H, Fernades R,
Oliveira J, Mascarenhas-Melo F, Teixeira F and Reis F: Diabetic
nephropathy amelioration by a low-dose sitagliptin in an animal
model of type 2 diabetes (Zucker diabetic fatty rat). Exp Diabetes
Res. 2011:1620922011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li WG, Chen YL, Chen JX, Qu L, Xue BD,
Peng ZH and Huang ZQ: Portal venous arterialization resulting in
increased portal inflow and portal vein wall thickness in rats.
World J Gastroenterol. 14:6681–6688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arumugam S, Thandavarayan RA, Veeraveedu
PT, Nakamura T, Arozal W, Sari FR, Giridharan VV, Soetikno V,
Palaniyandi SS, Harima M, et al: Beneficial effects of edaravone, a
novel antioxidant, in rats with dilated cardiomyopathy. J Cell Mol
Med. 16:2176–2185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang M, Zhou W, Zhou X, Zhuang F, Chen Q,
Li M, Ma T and Gu S: Antidepressant-like effects of alarin produced
by activation of TrkB receptor signaling pathways in chronic stress
mice. Behav Brain Res. 280:128–140. 2015. View Article : Google Scholar
|
|
31
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar
|
|
33
|
Kanwar YS, Wada J, Sun L, Xie P, Wallner
EI, Chen S, Chugh S and Danesh FR: Diabetic nephropathy: Mechanisms
of renal disease progression. Exp Biol Med (Maywood). 233:4–11.
2008. View Article : Google Scholar
|
|
34
|
Wang JY, Yin XX, Wu YM, Tang DQ, Gao YY,
Wan MR, Hou XY and Zhang B: Ginkgo biloba extract suppresses
hypertrophy and extracellular matrix accumulation in rat mesangial
cells. Acta Pharmacol Sin. 27:1222–1230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gonzalez J, Klein J, Chauhan SD, Neau E,
Calise D, Nevoit C, Chaaya R, Miravete M, Delage C, Bascands JL, et
al: Delayed treatment with plasminogen activator inhibitor-1 decoys
reduces tubulointerstitial fibrosis. Exp Biol Med (Maywood).
234:1511–1518. 2009. View Article : Google Scholar
|
|
36
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Yin Q, Tang X, Bai L, Zhang J, Gou
S, Zhu H, Cheng J, Fu P and Liu F: C3a receptor antagonist
ameliorates inflammatory and fibrotic signals in type 2 diabetic
nephropathy by suppressing the activation of TGF-β/smad3 and IKBα
pathway. PLoS One. 9:e1136392014. View Article : Google Scholar
|