Introduction
Hepatocellular carcinoma (HCC) is a malignant tumor
that has one of the highest morbidity and mortality rates, and its
tumorigenesis is closely related to HBV and HCV infection (1). There were estimated 782,500 hepatic
carcinoma cases and 745,500 related deaths globally in 2012,
according to Torre et al (2). Moreover, estimated 70–90% of primary
liver cancers are HCC. Despite advances in chemotherapy,
radiotherapy and surgery, the low 5-year survival and quality of
life observed in HCC patients remain intractable issues. Many
etiological factors, such as alcohol consumption and aflatoxin B1
exposure, are widely accepted to facilitate HCC occurrence
(3,4); however, the precise molecular
mechanisms remain unknown. Thus, there is an urgent need for a
reliable biomarker that can be used to predict HCC progression and
prognosis.
MicroRNAs (miRNAs), which are limited to a length of
19–22 nucleotides, are small non-coding RNAs that
post-transcriptionally influence gene expression (5). A vast array of oncogenes and
anti-oncogenes are potentially regulated by miRNAs, which act as
gene regulators by conjugating the 3′-untranslated region (3′-UTR)
of their mRNAs. In regulating genes, miRNAs play a crucial
biological role in tumor development, especially during initiation,
proliferation, differentiation, invasion and metastasis (6). Thus, miRNAs may serve as promising
diagnostic and prognostic indexes.
Validating a potential target of miRNA is arduous
and time-consuming due to the tremendous amounts of target sites in
miRNA. However, predicting the targets of miRNA, which is a vital
step in performing miRNA-target interactions, contributes to
narrowing down prospective target sites and promoting experimental
verification. Following the principle of sequence complementarity,
many algorithms were manipulated to figure out the predicted miRNA
targets.
miR-23b-3p was identified as a tumor suppressor that
showed a tendency toward downregulated expression in different
classes of human malignant tumors such as prostate cancer, renal
cell carcinoma, acute myeloid leukemia and osteosarcoma (7–10).
Nevertheless, increasing evidence has indicated that the
upregulation of miR-23b-3p promoted cell proliferation and invasion
in glioma, gastric cancer and breast cancer (11–13). miR-23b-3p was also found to be
involved in liver stem cell differentiation, and its reduced
expression may contribute to liver regeneration after partial
hepatectomy (14,15). However, to our knowledge, only one
study (16) has explored the
relationship between miR-23b-3p expression and HCC but did not
comment on the clinicopathological significance and prognosis.
Therefore, further inquiry is urgently needed.
Our objectives were as follows: to study the
pathophysiologic expression quantity of miR-23b-3p in HCC tissue
through comparison with matching adjacent tissues as well as to
elucidate the correlation between miR-23b-3p expression and HCC
clinicopathological parameters. Additionally, we aimed to identify
the probable target genes of miR-23b-3p and determine the potential
role of miR-23b-3p in HCC development and progression.
Materials and methods
miR-23b-3p expression in HCC based on GEO
datasets
Data acquisition and exclusion
criteria
Twenty-one micro-array datasets were obtained from
the GEO database (http://www.ncbi.nlm.nih.gov/geo/), and 11 were
eliminated after screening. The search strategy was formulated as
follows: (malignan* OR cancer OR tumor OR tumour OR
neoplas* OR carcinoma) AND (hepatocellular OR liver OR
hepatic OR HCC). The last dataset search was on April 2016. The
exclusion criteria were as follows: i) datasets without information
on miR-23b-3p; ii) datasets without complete data for analysis;
iii) samples based on cell lines; iv) not all subjects of the
included studies were human; or v) miR-23b-3p was determined in the
HCC patients without a comparison. To identify the clinical
relevance of miR-23b-3p in HCC, we collected data on miR-23b-3p
expression from the 6 datasets and analyzed their association with
the clinicopathological characteristics of HCC. The clinical
features, which could be obtained from more than two microarray
datasets, were used for the meta-analysis.
Statistical analysis
The meta-analysis was carried out with Stata 12.0
(StataCorp LP, College Station, TX, USA). A standard mean
difference (SMD) and a 95% confidence interval (CI) were utilized
to measure continuous outcomes, including age, sex, HBV infection,
invasion and metastasis. Fixed or random effects models were
applied to pool the effect sizes. Cochrane's Q test (Chi-square
test; Chi2) (17) and
inconsistency (I2) (18) were conducted to assess
heterogeneity. A P<0.05 or I2 >50% indicates
significant heterogeneity (19),
and a random effects model (20)
was applied. Otherwise, the fixed effects model would be adopted.
Begg's funnel plot for asymmetry and Egger's funnel plot for
quantitation were generated to evaluate publication bias. In
addition, sensitivity analysis was performed to evaluate the
reliability of results by elimination of a study each time.
miR-23b-3p expression in HCC based on
TCGA dataset
Retrieval of public data
Altogether, 377 anonymized HCC tissues and 50 normal
tissues were retrieved from the TCGA database (http://cancergenome.nih.gov/publications/publica-tionguidelines).
The non-HCC samples or samples with data deficiency were excluded;
361 HCC patients were finally included in this study. Additionally,
a total of 50 normal liver tissues were retrieved for comparison.
The clinicopathological features of HCC patients in TCGA are
available in Table II.
 | Table IICharacteristics of the HCC patients
included in TCGA. |
Table II
Characteristics of the HCC patients
included in TCGA.
| Clinicopathological
features | N | miR-23b-3p relevant
expression (2−ΔCq)
| Correlation
|
|---|
| Mean ± SD | t-value | P-value | r-value | P-value |
|---|
| Tissue | | | | | | |
| Normal | 50 |
13.4039±0.51072 | −8.286 | <0.001 | 0.246 | <0.001 |
| HCC | 371 |
12.6722±0.97815 | | | | |
| Age (years) | | | | | | |
| <60 | 166 |
12.7336±0.97231 | 0.85 | 0.396 | −0.045 | 0.396 |
| ≥60 | 194 | 12.6464±0.9000 | | | | |
| Sex | | | | | | |
| Male | 246 |
12.6581±0.93294 | −0.613 | 0.54 | 0.032 | 0.54 |
| Female | 115 | 12.7259±1.0132 | | | | |
| Neoadjuvant
treatment | | | | | | |
| No | 359 |
12.6798±0.97924 | 0.036 | 0.972 | −0.002 | 0.972 |
| Yes | 2 |
12.6550±1.07244 | | | | |
| Radiation
therapy | | | | | | |
| No | 231 |
12.6570±0.95382 | 0.998 | 0.319 | −0.065 | 0.319 |
| Yes | 4 |
12.1762±1.03021 | | | | |
| Pharmaceutical
treatment | | | | | | |
| No | 218 |
12.6475±0.96107 | 0.041 | 0.967 | −0.003 | 0.967 |
| Yes | 12 |
12.6358±0.90350 | | | | |
| Alcohol
consumption | | | | | | |
| − | 226 |
12.7394±0.99963 | 1.374 | 0.17 | −0.074 | 0.17 |
| + | 117 |
12.5870±0.92032 | | | | |
| Hepatitis B | | | | | | |
| − | 237 |
12.5999±0.97009 | −2.506 | 0.013 | 0.134 | 0.013 |
| + | 106 |
12.8831±0.96070 | | | | |
| Hepatitis C | | | | | | |
| − | 289 |
12.6963±0.97544 | 0.388 | 0.698 | −0.021 | 0.698 |
| + | 54 |
12.6401±0.97811 | | | | |
| Non-alcoholic fatty
liver | | | | | | |
| − | 324 |
12.6763±0.98327 | −0.874 | 0.383 | 0.047 | 0.383 |
| + | 19 |
12.8774±0.81188 | | | | |
| Smoking | | | | | | |
| − | 326 |
12.7127±0.96181 | 2.115 | 0.035 | −0.114 | 0.035 |
| + | 17 |
12.2025±1.11847 | | | | |
| Cirrhosis | | | | | | |
| − | 337 |
12.6956±0.95014 | 0.564 | 0.597 | −0.063 | 0.248 |
| + | 6 |
12.2312±2.01359 | | | | |
| Grade | | | | | | |
| GI–II | 23 |
12.6505±0.76988 | 1.493 | 0.147 | −0.214 | 0.256 |
| GIII–IV | 7 |
12.0712±1.26499 | | | | |
| Vascular
invasion | | | | | | |
| No | 199 |
12.8350±0.94514 | 2.149 | 0.032 | −0.122 | 0.032 |
| Yes | 107 |
12.5905±0.95709 | | | | |
| Recurrence after
treatment | | | | | | |
| No | 166 |
12.5805±0.98050 | −0.589 | 0.556 | 0.037 | 0.556 |
| Yes | 94 |
12.6537±0.92925 | | | | |
| Survival
status | | | | | | |
| Dead | 86 |
12.6050±1.01283 | −0.811 | 0.418 | 0.043 | 0.418 |
| Alive | 275 |
12.7031±0.96776 | | | | |
| T status | | | | | | |
| T1 | 177 |
12.7653±0.93707 | F=1.605a | 0.202a | −0.083 | 0.117 |
| T2–T4 | 181 |
12.5926±1.01626 | | | | |
| TX | 1 |
12.0451±0.00000 | | | | |
| N status | | | | | | |
| N0 | 247 |
12.7780±0.96867 | F=5.116a | 0.006a | −0.158 | 0.003 |
| N1 | 3 |
11.7684±0.75393 | | | | |
| NX | 110 |
12.4733±0.96540 | | | | |
| M status | | | | | | |
| M0 | 262 |
12.7389±0.96998 | F=1.778a | 0.170a | −0.101 | 0.055 |
| M1 | 4 |
12.6180±0.12946 | | | | |
| MX | 95 |
12.5190±1.00762 | | | | |
Statistical analysis
The statistics were analyzed with SPSS 22.0 software
(IBM Corp., Armonk, NY, USA). The final data after calculation are
shown as the mean ± standard deviation (SD). Student's t-test was
conducted for a comparative analysis of two independent groups. A
one-way analysis of variance (ANOVA) test was utilized to study the
data, which were divided into three or four groups, such as T
status, N status or M status. Spearman's method was performed to
evaluate the association between miR-23b-3p expression and other
clinicopathological parameters. The relationship between miR-23b-3p
and recurrence was obtained with the Kaplan-Meier survival approach
along with a log-rank test. To differentiate between controls and
HCC tissues, a diagnostic value was identified using a receiver
operator characteristic (ROC) curve. A P-value <0.05 denoted a
statistically significant difference. At least 5 individual tests
were applied without ambiguity.
miR-23b-3p expression in HCC hospital
tissues
Patients and data collected from
medical records
Regarding the cases recruited, 101 FFPE HCC tissues
and their counterpart adjacent non-cancerous HCC were obtained from
the First Affiliated Hospital of the Guangxi Medical University
March, 2010 and December, 2011. Before curative hepatectomy, none
had received radiotherapy or chemotherapy. Formalin-fixed and
paraffin embedded (FFPE) tissues were assessed in both the HCC
tissues and adjacent non-cancerous HCC; we retrospectively acquired
the following clinicopathological parameters: age, sex, cirrhosis,
tumor size, tumor nodes, differentiation, clinical TNM stages,
metastasis, existence or inexistence of portal vein tumor embolus,
capsular infiltration, vaso-invasion, as well as other biomarkers,
such as serum AFP level, microvessel density (MVD). Two independent
pathologists diagnosed all the cases. The project had already
gained ethics approval from the Ethics Committee of First
Affiliated Hospital of Guangxi Medical University and the patients
signed informed consents and agreed their samples to be used in
scientific research. The clinicopathological characteristics of the
population are shown in Table
III.
 | Table IIIThe clinicopathological
characteristics related to miR-23b-3p expression in patients. |
Table III
The clinicopathological
characteristics related to miR-23b-3p expression in patients.
| Clinicopathological
features | N | miR-23b-3p relevant
expression (2−ΔCq)
| Correlation
|
|---|
| Mean ± SD | t-value | P-value | r-value | P-value |
|---|
| Tissue | | | | | | |
| Adjacent
non-cancerous | 101 | 4.4694±1.99328 | −6.847 | <0.001 | 0.46 | <0.001 |
| HCC | 101 | 2.7376±1.57739 | | | | |
| Age (years) | | | | | | |
| <50 | 50 | 2.7160±1.53973 | 0.136 | 0.892 | −0.019 | 0.85 |
| ≥50 | 51 | 2.7588±1.62852 | | | | |
| Sex | | | | | | |
| Male | 80 | 2.7162±1.55445 | −0.265 | 0.792 | 0.057 | 0.568 |
| Female | 21 | 2.8190±1.69930 | | | | |
| Cirrhosis | | | | | | |
| − | 54 | 2.8426±1.53274 | −0.715 | 0.476 | 0.112 | 0.256 |
| + | 47 | 2.6170±1.63539 | | | | |
| Tumor size | | | | | | |
| <5 cm | 21 | 2.5905±1.59371 | −0.478 | 0.633 | 0.067 | 0.505 |
| ≥5 cm | 80 | 2.7762±1.58092 | | | | |
| Tumor nodes | | | | | | |
| Single | 57 | 3.0158±1.45979 | 2.049 | 0.043 | −0.255 | 0.01 |
| Multiple | 44 | 2.3773±1.66606 | | | | |
|
Differentiation | | | | | | |
| High | 7 | 2.9857±1.65673 | F=0.394a | 0.675a | −0.044 | 0.66 |
| Moderate | 64 | 2.8062±1.71722 | | | | |
| Low | 30 | 2.5333±1.23995 | | | | |
| Clinical TNM
stage | | | | | | |
| I–II | 25 | 3.0520±1.9796 | 1.151 | 0.253 | −0.192 | 0.055 |
| III–IV | 76 | 2.6342±1.67766 | | | | |
| Metastasis | | | | | | |
| − | 49 | 3.3959±1.57506 | 4.435 | <0.001 | −0.447 | <0.001 |
| + | 52 | 2.1173±1.31762 | | | | |
| Portal vein tumor
embolus | | | | | | |
| − | 69 | 3.1029±1.60797 | 3.618 | <0.001 | −0.34 | 0.001 |
| + | 32 | 1.9500±1.19055 | | | | |
| Tumor capsular
infiltration | | | | | | |
| With complete
capsule | 49 | 2.7449±1.60663 | 0.045 | 0.964 | 0.001 | 0.989 |
| Infiltration or no
capsule | 52 | 2.7308±1.56500 | | | | |
| Vaso-invasion | | | | | | |
| − | 63 | 2.9635±1.63917 | 1.876 | 0.064 | −0.169 | 0.092 |
| + | 38 | 2.3632±1.41123 | | | | |
RT-qPCR
The total RNAs were isolated from clinical tissue
samples with a miRNeasy FFPE kit (Qiagen, Limburg, The Netherlands)
according to the manufacturer's instructions. To assess the miRNA
purity and concentration, a quantitative real-time PCR method was
utilized in 101 HCC tissues and their counterpart adjacent
non-cancerous HCC.
Statistical analysis
The statistical software and methods that were used
to analyze the data from clinical patient files downloaded from
TCGA were also used to identify the relationship between miR-23b-3p
expression and HCC based on qPCR data.
Meta-analysis for GEO datasets, TCGA
datasets and PCR verification in-house
Data acquisition
We next added the data from TCGA datasets and PCR
verification in-house into meta-analysis to expand the sample size
and enhance the credibility. The expression of hsa-miR-23b-3p in
HCC tissues and adjacent non-cancerous tissues were extracted in
meta-analysis.
Statistical analysis
As for statistical analysis of meta-analysis of GEO
datasets, the same statistical methods and software were used to
perform the meta-analysis of GEO datasets, TCGA datasets and qPCR
data.
Biological information analysis
Prediction targets of miR-23b-3p
collection
Ten algorithms, including those from TargetScan
(http://www.targetscan.org), microRNA.org (http://www.microrna.org), RNA22 (https://cm.jefferson.edu/rna22/Precomputed),
PicTar-vert (http://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi),
miRDB (http://mirdb.org/miRDB), PolymiRTS
Database (http://compbio.uthsc.edu/miRSNP/), PITA (http://genie.weiz-mann.ac.il/pubs/mir07/mir07_dyn_data.html#),
TargetMiner (http://www.isical.ac.in/~bioinfo_miu/targetminer20.htm),
TarBase (http://diana.imis.athenainnovation.gr/DianaTools/index.php?r=tarbase),
and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw), were applied. Only
target genes appearing more than or equal to five times amongst all
10 algorithms were finally applied for investigation of the next
step.
Validation targets of miR-23b-3p
collection
We searched the PubMed database using the following
search strategy: (MIRN23 OR microRNA23 OR microRNA-23 OR miR-23 OR
hsa-mir-23 OR miR-23b OR microRNA-23b OR miRNA-23b OR 'miR23b' OR
'miRNA23b' OR 'microRNA23b' OR miR-23b-3p OR miRNA-23b-3p OR
microRNA-23b-3p) and target*. The validation targets of
hsa-miR-23b-3p were recorded from the identified articles by two
authors (P-R Wu and X-L Xiang), and the third author resolved
controversies. These targets were integrated with prediction
targets after combination.
Bioinformatics analysis of
miR-23b-3p
Gene Ontology (GO) analysis. GO analysis was
conducted using the Database for Annotation, Visualization and
Integrated Discovery (DAVID) (http://David.abcc.ncifcrf.gov/). The targets of
miR-23b-3p were divided into three primary classes: biological
processes, cellular components and molecular functions.
Pathway analysis
For pathway analysis, we downloaded pathway data
from the Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.ad.jp/KEGG) to investigate gene
interactions. miR-23b-3p targets were mapped to the KEGG pathway
database using KOBAS 2.0 (http://www.genome.jp/kegg/pathway.html), and P-values
[and a corresponding false discovery rate (FDR)] were applied to
estimate each enriched pathway.
Network analysis
The Search Tool for the Retrieval of Interacting
Genes (STRING), which provides information for experimental and
predicted interactions, is an online database. STRING was applied
to search and to determine an interaction network of targets of
miR-23b-3p via confidence score calculation.
Results
miR-23b-3p expression in HCC based on GEO
datasets
Characteristics of the included
datasets
The characteristics of the GEO datasets included are
presented in Table I. A total of
10 datasets, including GSE6857 (USA, 2013) (21), GSE10694 (China, 2008) (22), GSE21362 (Japan, 2010) (23), GSE12717 (China, 2008) (24), GSE54751 (USA, 2014) (25), GSE57555 (Japan, 2015) (26), GSE41874 (Japan, 2013) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE41874),
GSE67138 (USA, 2015) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE67138),
GSE69580 (China 2015) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE69580+),
and GSE67139 (USA, 2015) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE67139),
were identified to meet the standards. All of the datasets were
derived from tissues.
 | Table ICharacteristics of hsa-miR-23b-3p
gene expression profiling datasets included in meta-analysis. |
Table I
Characteristics of hsa-miR-23b-3p
gene expression profiling datasets included in meta-analysis.
| Citation | Country | Data source | HCC patients | Healthy
controls | Platform |
|---|
| Budhu et al
2013 (21) | USA | GEO: GSE6857 | 240 | 241 | GPL4700 |
| Li et al
2008 (22) | China | GEO: GSE10694 | 75 | 87 | GPL6542 |
| Sato et al
2011 (23) | Japan | GEO: GSE21362 | 73 | 73a | GPL10312 |
| Su et al
2009 (24) | USA | GEO: GSE12717 | 5 | 3 | GPL7274 |
| Shen et al
2015 (25) | USA | GEO: GSE54751 | 10 | 10a | GPL18262 |
| Murakami et
al 2015 (26) | Japan | GEO: GSE57555 | 5 | 5a+11 | GPL16699 |
| | | | | GPL18044 |
| Morita 2013b | Japan | GEO: GSE41874 | 6 | 4 | GPL7722 |
| Hung 2015b | China | GEO: GSE69580 | 5 | 5a | GPL10850 |
| Barry 2015b | USA | GEO: GSE67138 | 23 | 34 | GPL8786 |
| Barry 2015b | USA | GEO: GSE67139 | 60 | 60 | GPL8786 |
Value of miR-23b-3p as a biomarker for
HCC
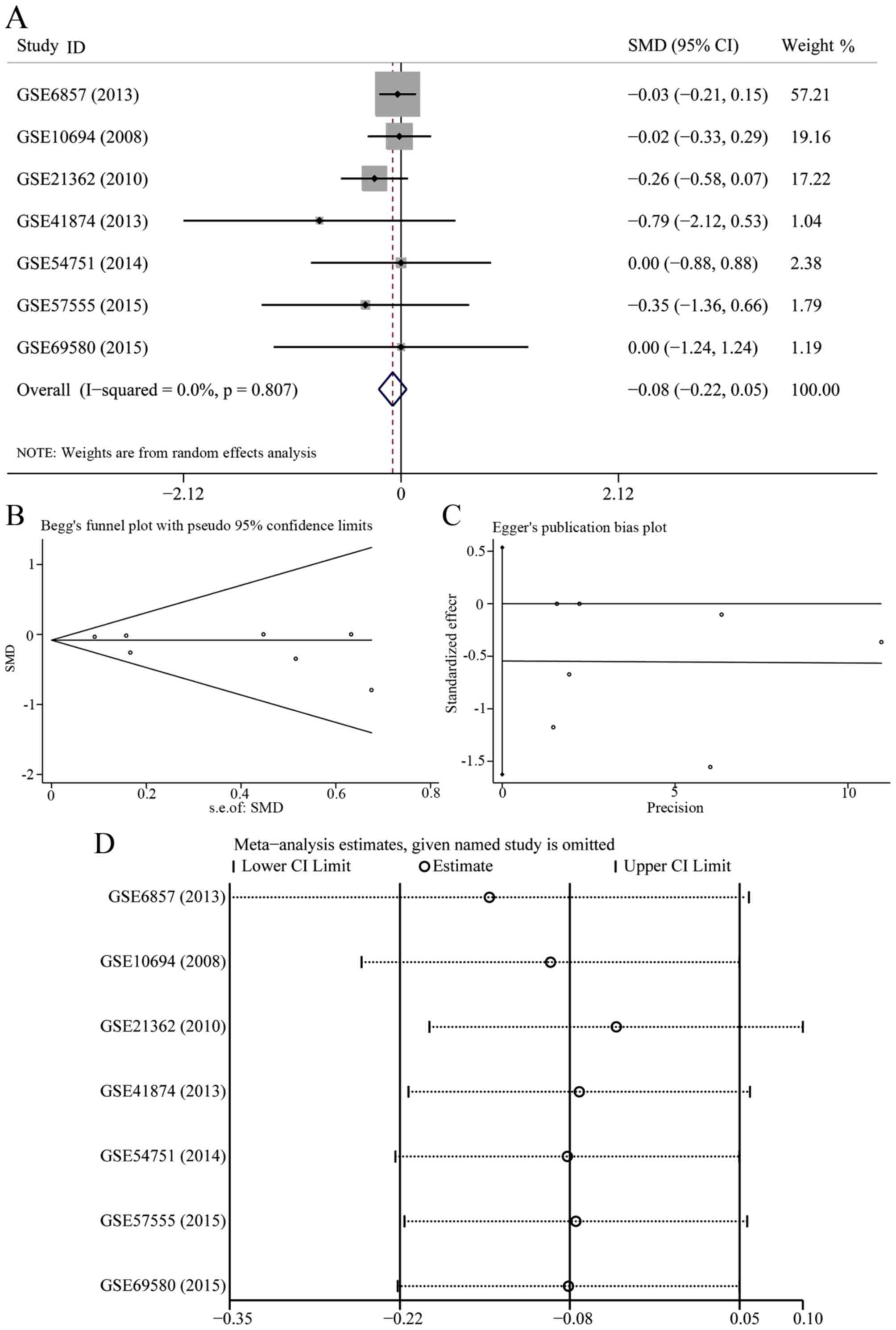
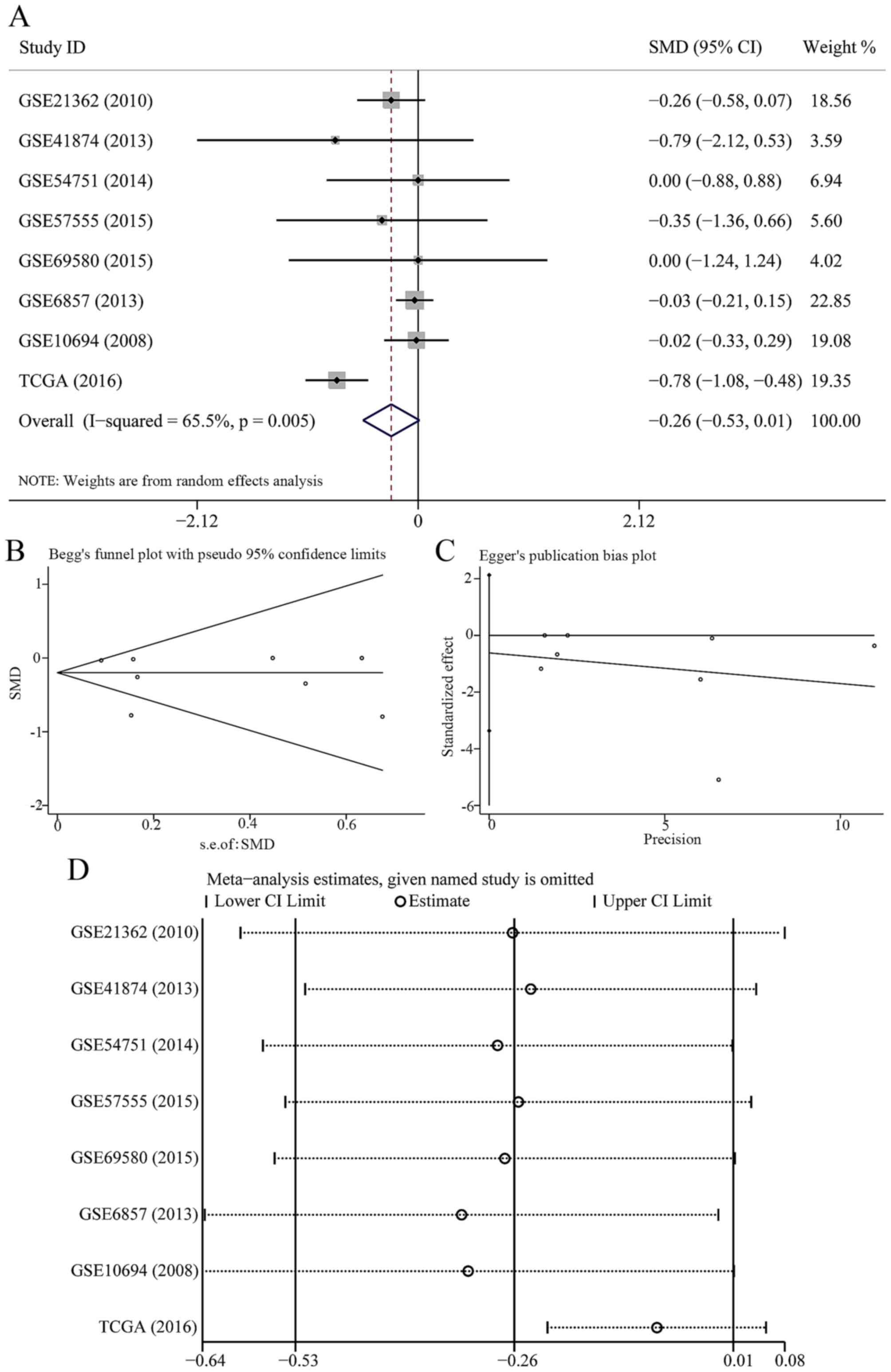
In 7 data-sets presenting miR-23b-3p expression with
comparable groups, 414 patients with HCC and 343 healthy subjects
were recruited. The expression of miR-23b-3p was lower in HCC
patients than healthy controls (Fig.
1A); however, no statistical significance was found
(SMD=−0.081; 95% CI, −0.216 to 0.054; P=0.239). A fixed effects
model was selected to pool the effect variables that did not show
heterogeneity (P=0.807, I2=0%). The results of Begg's
and Egger's test shown in Fig. 1B and
C was 0.133 and 0.251, respectively. As a consequence, no
publication bias was discovered. According to sensitivity analysis
shown in Fig. 1D, the result was
altered when we eliminated the study of GSE6857, suggesting that
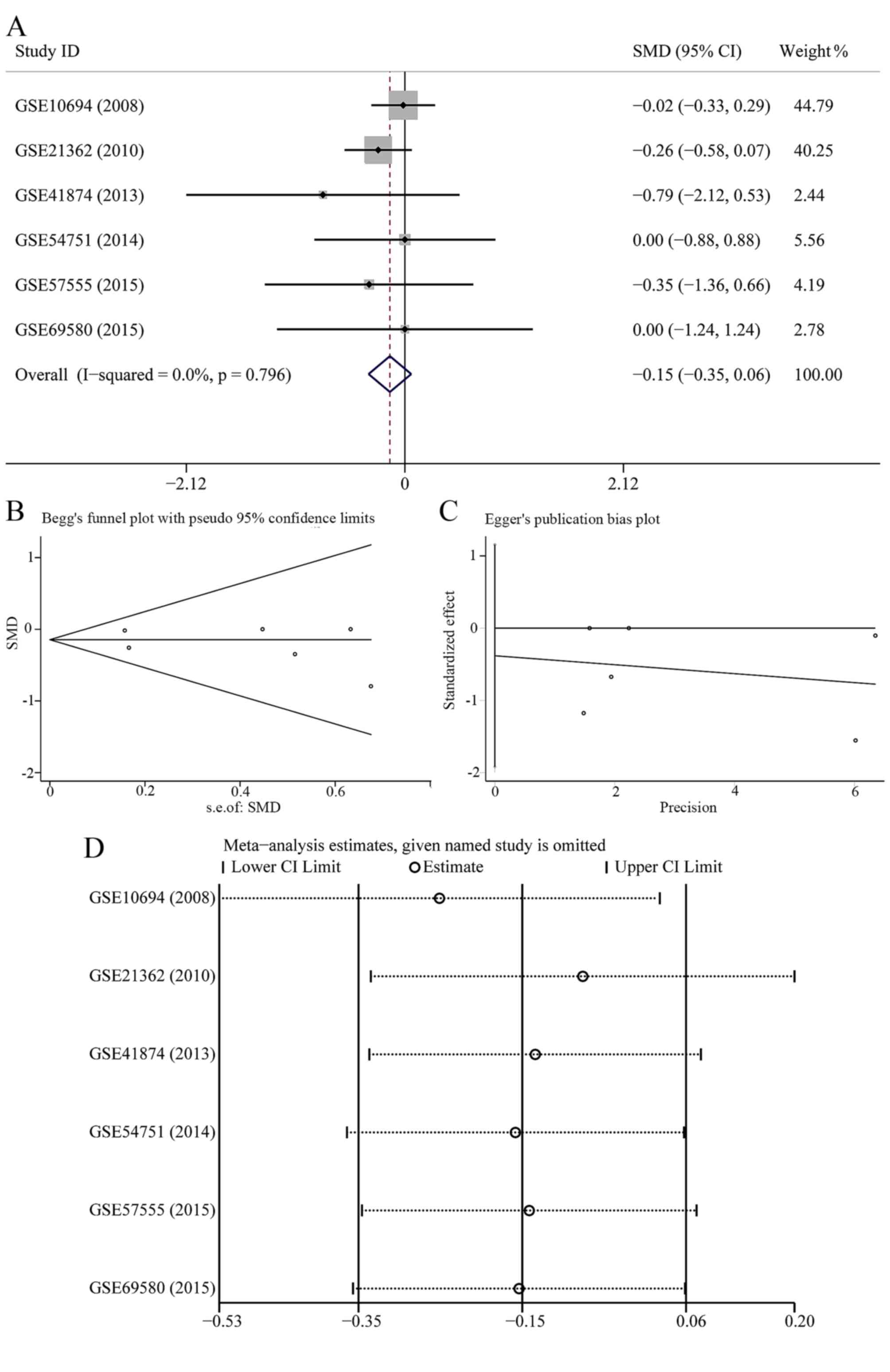
this study may influence the pooled SMD and 95% CI. The forest plot
after the removing of GSE6857 is shown in Fig. 2A; however, no statistical
significance was found (SMD=−0.145; 95% CI, −0.352 to 0.061;
P=0.168). According to Begg's and Egger's test (Fig. 2B and C), no publication bias was
observed (P>0.05). The sensitivity analysis did not show any
alteration when we removed a single study (Fig. 2D).
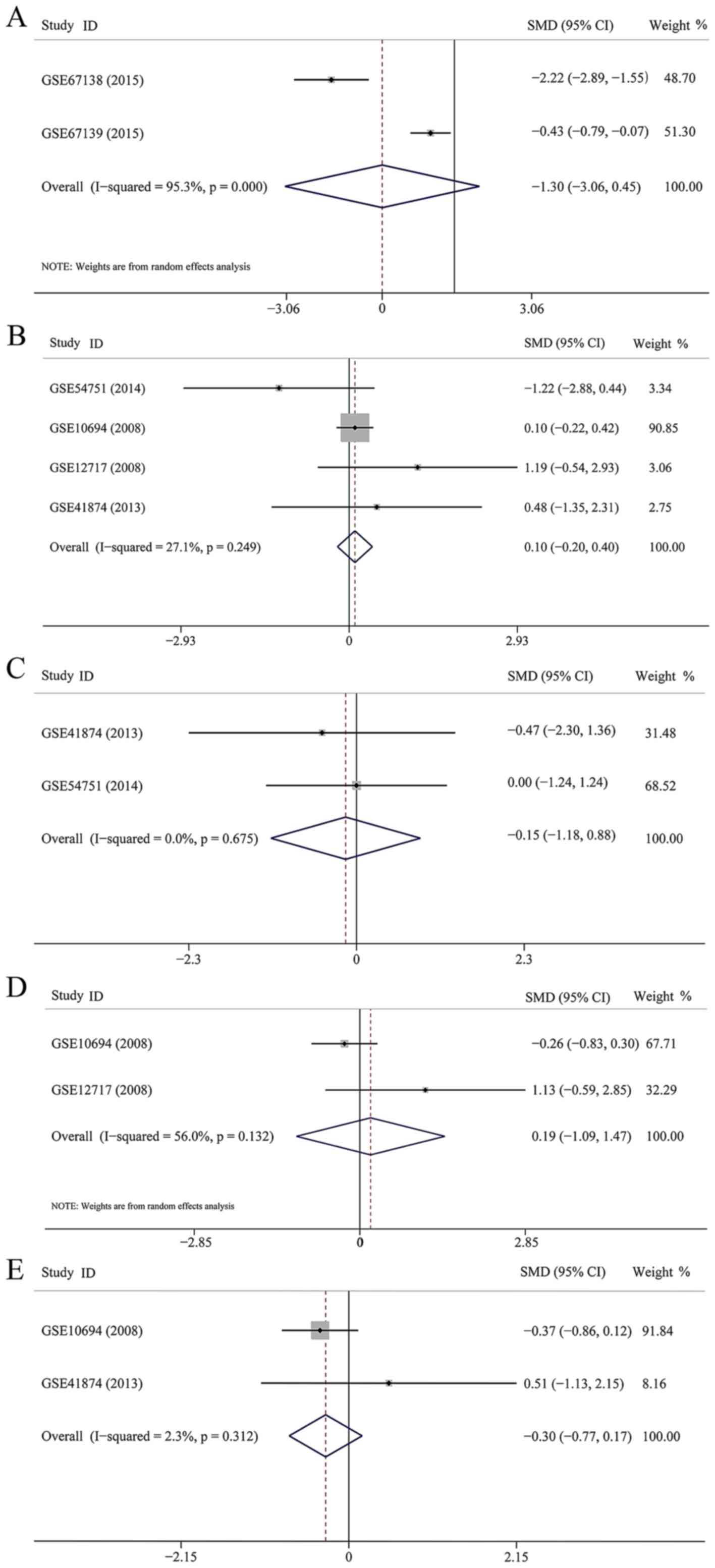
Six datasets were enrolled to assess the association
between miR-23b-3p expression and the clinical aspects of HCC.
However, five parameters, including invasion (83 patients), age (90
patients), sex (8 patients), HBV infection (66 patients) and
metastasis (27 patients) were estimated in this study. The pooled
SMD were −1.302 (95% CI, −3.055 to 0.451, P=0.146) (Fig. 3A), 0.101 (95% CI, −0.203 to 0.405,
P=0.515) (Fig. 3B), −0.266 (95%
CI, −1.293 to 0.762, P=0.613) (Fig.
3C), −0.186 (95% CI, −1.093 to 1.465, P=0.775) (Fig. 3D), and −0.288 (95% CI, −0.787 to
0.210, P=0.257) (Fig. 3E),
respectively. None of the five parameters were associated with the
level of miR-23b-3p expression.
miR-23b-3p expression in HCC based on the
TCGA dataset
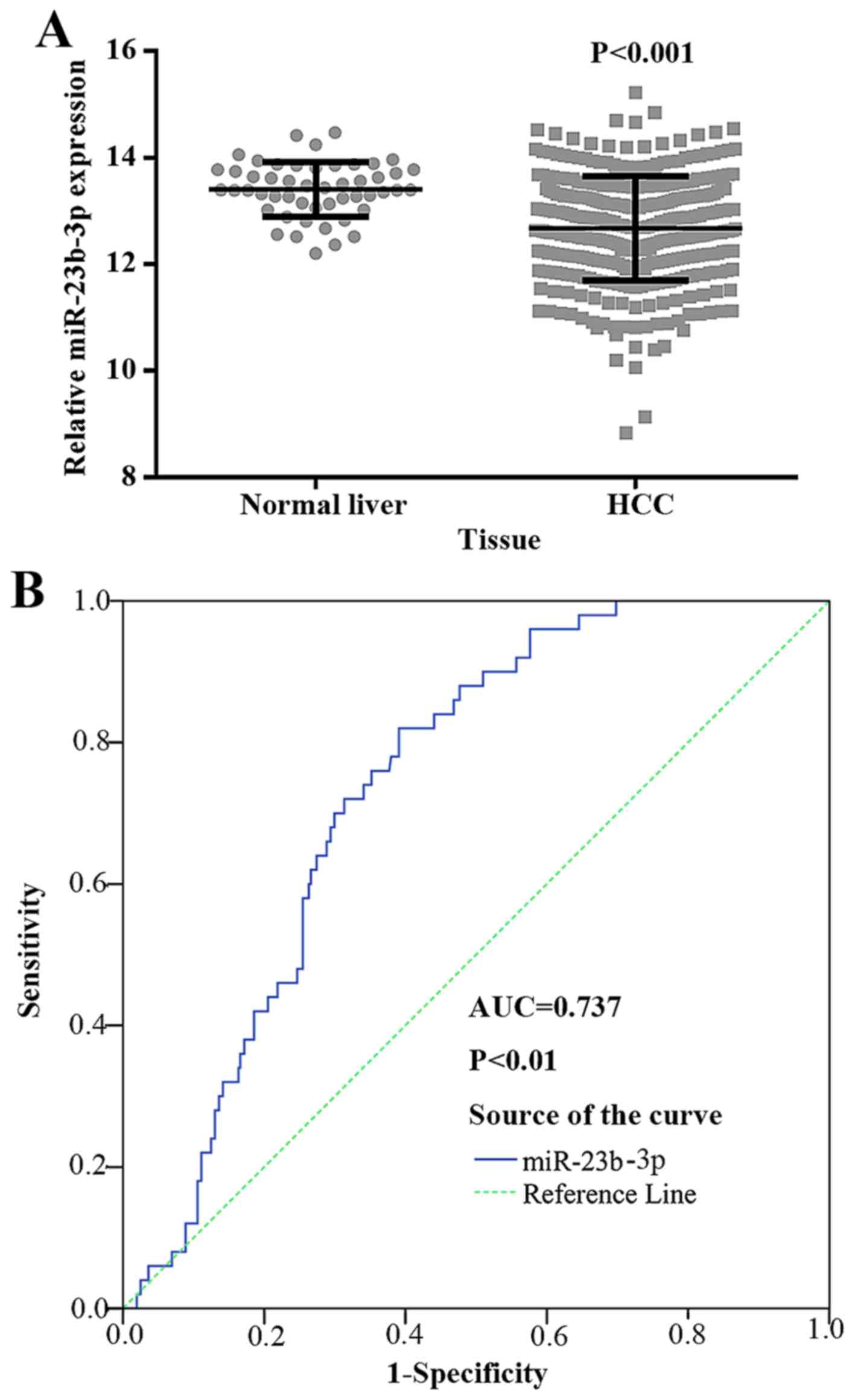
The expression level of miR-23b-3p was
downregulated in HCC samples
To explore the relationship between miR-23b-3p and
HCC, we further obtained data for 361 HCC tissues and 50 normal
liver tissues from TCGA. The clinicopathological features are
presented in Table II. According
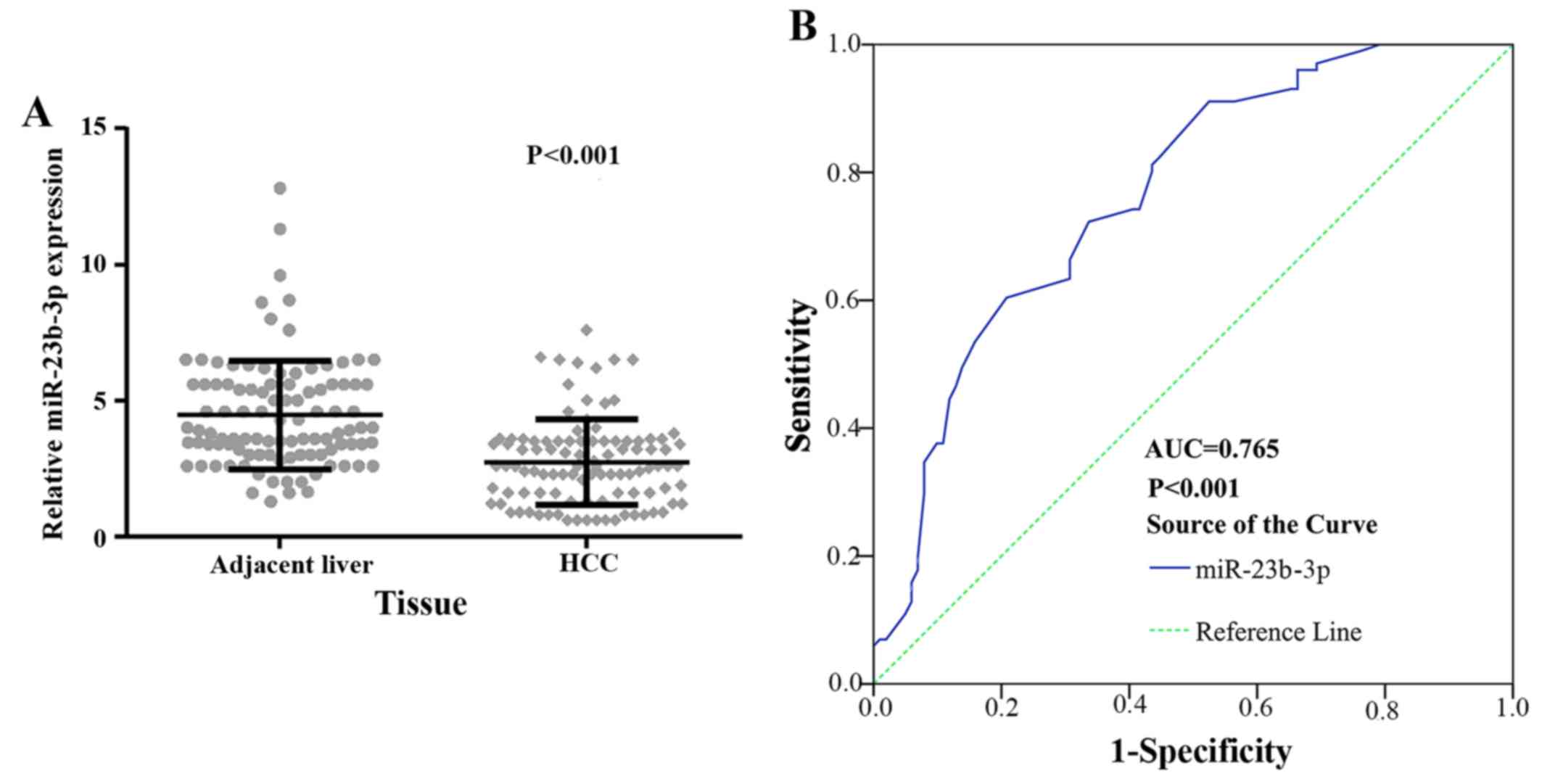
to the TCGA data, the expression level of miR-23b-3p was
substantially downregulated in HCC (12.6722±0.978150) being
compared to normal liver tissues (13.4039±0.51072, P<0.001)
(Fig. 4A).
Relationships between miR-23b-3p and
clinicopathological features
Due to the downregulated miR-23b-3p expression
observed in HCC, the association between clinicopathological
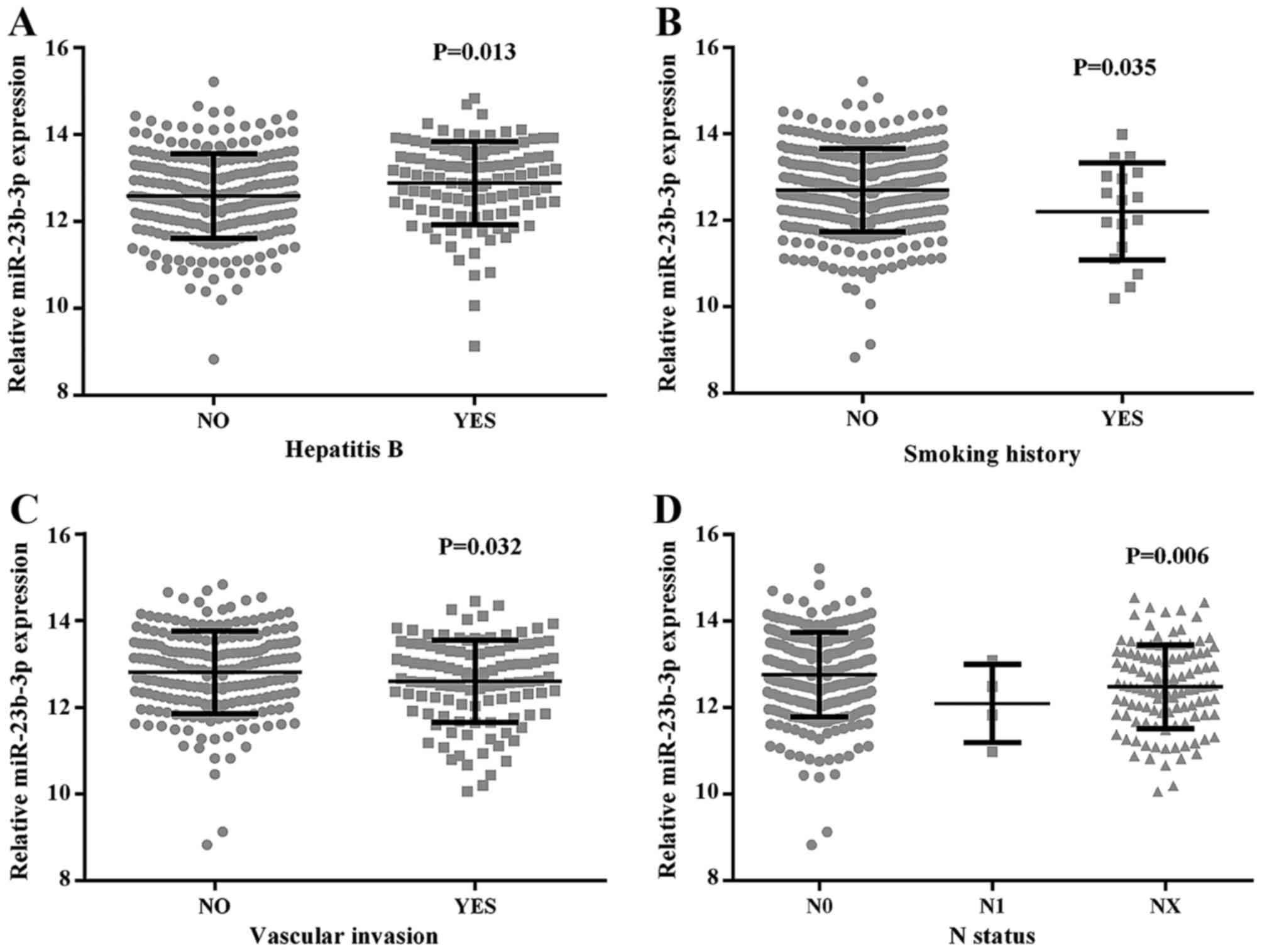
features and miR-23b-3p was studied (Table II and Fig. 5). The data indicated that a higher
level of miR-23b-3p was closely related with hepatitis B
(12.8831±0.96070), in contrast to that without hepatitis B
infection (12.5999±0.97009, P=0.013). miR-23b-3p was also
downregulated in smoking patients (12.2025±1.11847) compared to
those without smoking history (12.7127±0.96181, P=0.035). The
results also gave a demonstration that the downregulated expression
of miR-23b-3p was closely associated with vascular invasion
(12.5905±0.95709) compared with non-vascular invasion
(12.8530±0.94514, P=0.032). According to the universal standard
pathologic neoplasm staging methods of AJCC, there was an obvious
negative relationship between tumor node metastasis and miR-23b-3p.
The expression level of miR-23b-3p was screened in regional
lymphatic metastasis patients (11.7684±0.75393). The expression
level was 12.7780±0.96867 in the patients who never had lymphatic
metastasis; those with samples that remained in an undefined stage
had a level of 12.4733±0.96540 (P=0.006). No other
clinicopathological parameters had a significant relationship with
miR-23b expression, such as HCV infection or alcohol consumption,
nor did neoadjuvant therapy, radiation therapy or pharmaceutical
treatment. In accordance with Table
II, Spearman's method was applied when correlating miR-23b-3p
with clinicopathological parameters, including hepatitis B
(r=0.134, P=0.013), smoking (r=−0.014, P=0.035), vascular invasion
(r=−0.122, P=0.032), and N status (r=−0.158, P=0.003). There were
no other positive correlations with the remaining clinical
features.
The diagnostic accuracy of miR-23b-3p
in HCC tissues
To further determine miR-23b-3p as a biomarker in
diagnosing HCC, we performed ROC for verification. As shown in
Fig. 4B, the AUC of miR-23b-3p in
HCC and its counterpart normal tissues was 0.737 (95% CI, 0.680 to
0.794; P<0.001). The cut-off value reached 0.429; the
sensitivity and specificity were 82.0 and 60.9%, respectively. The
result suggested that miR-23b-3p may be treated as a reliable
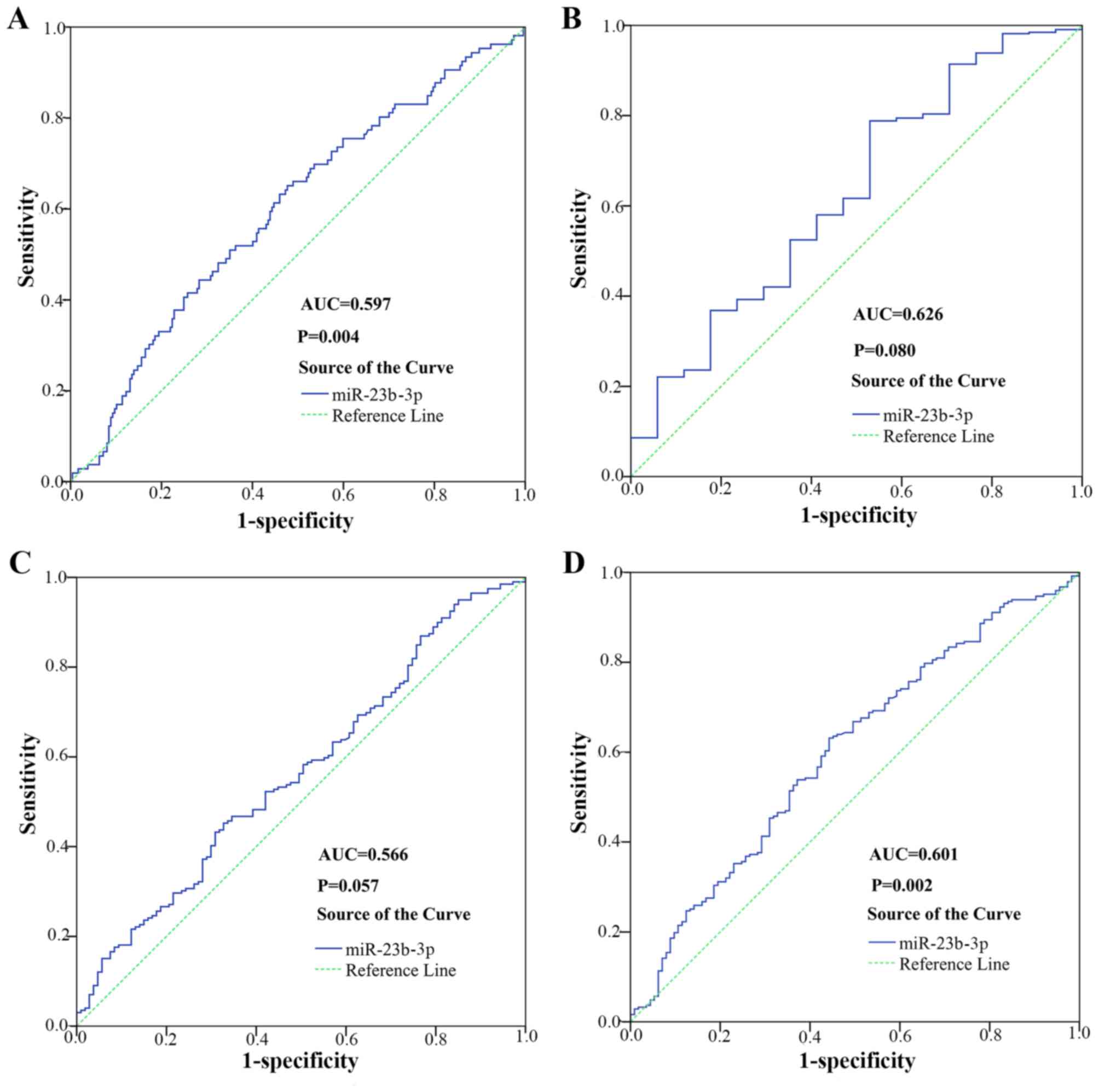
biomarker in diagnosing HCC. As shown in Fig. 6, the AUC to judge hepatitis B was
0.597 (95% CI, 0.532 to 0.662; P=0.004). The cut-off value for
miR-23b-3p was 0.174; the sensitivity and specificity were 65.1 and
52.3% respectively. The AUC to judge smoking was 0.626 (95% CI,
0.485 to 0.767; P=0.080). The cut-off value for miR-23b-3p was
0.259; the sensitivity and specificity were 78.8 and 47.1%,
respectively. The AUC to judge vascular invasion was 0.566 (95% CI,
0.499 to 0.633, P=0.057). The cut-off value for miR-23b-3p was
0.125; the sensitivity and specificity were 45.2 and 67.3%,
respectively. The AUC to judge N status was 0.601 (95% CI, 0.538 to
0.664, P=0.002). The cut-off value for miR-23b-3p was 0.125; the
sensitivity and specificity were 63.2 and 55.8%, respectively.
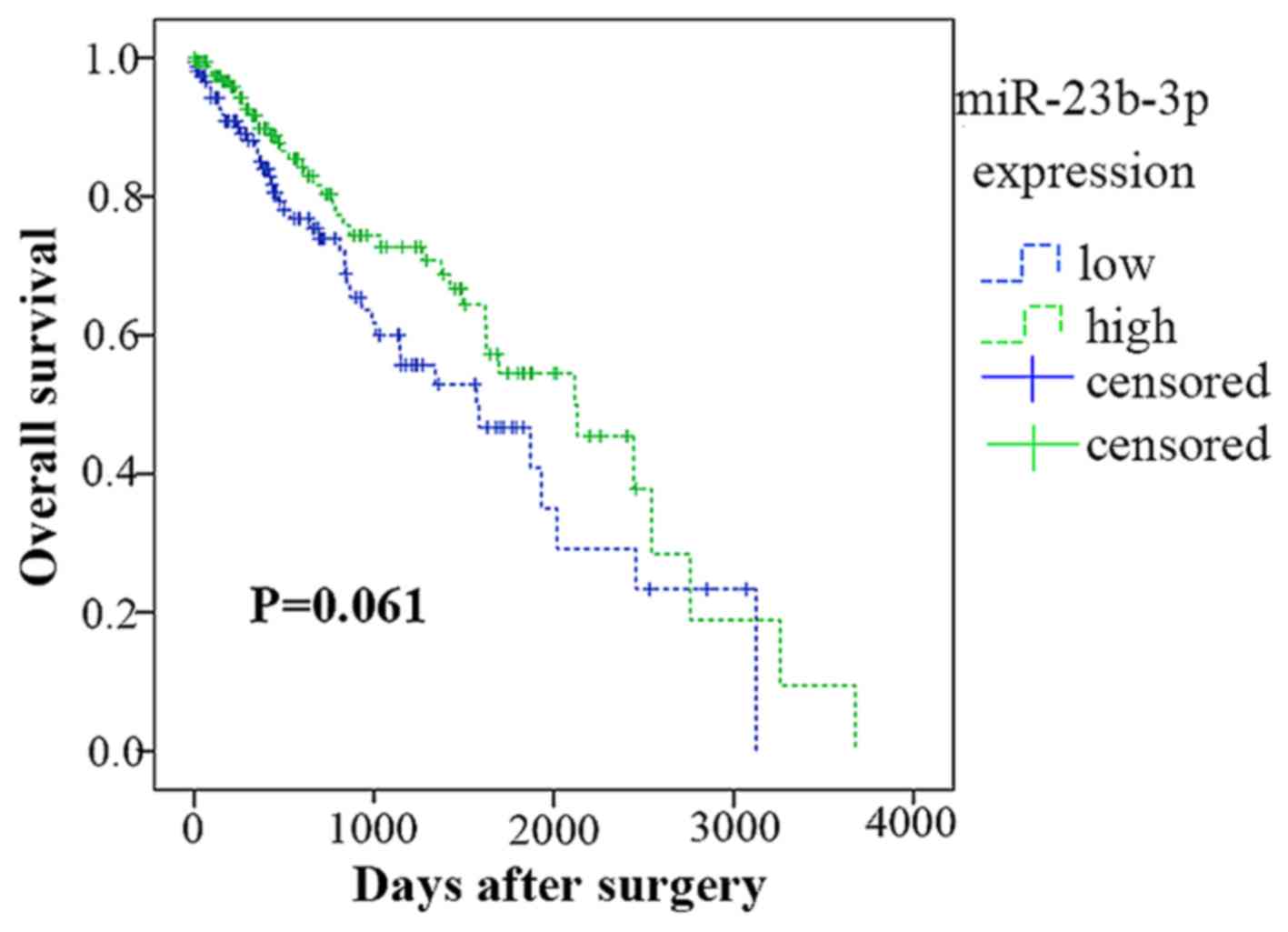
miR-23b-3p expression in surviving
HCC
According to the data from TCGA, 359 of the 361
patients were followed up. The Kaplan-Meier analysis in Fig. 7 showed that 176 patients had a
lower miR-23b-3p expression (lower than the mean expression level
of 12.6797), whereas the remaining 183 patients had a higher level
(higher than the mean expression level of 12.6797). In contrast,
the lower group had an average survival time of 1,617.668±151.722
days, whereas the higher group had a survival time of
1,944.626±160.587 days. However, the survival times of the high and
low miR-23b-3p expression groups were not significantly different
(Chi-square =3.351, P=0.061).
miR-23b-3p expression in HCC tissues from
PCR verification in-house
The expression of miR-23b-3p was
downregulated in HCC
To study the expression level of miR-23b-3p, a
quantitative real-time PCR method was performed in 101 HCC tissues
and counterpart adjacent non-cancerous HCC. The clinicopathological
features of the patients, obtained from medical records, are
available in Table III. The
expression of miR-23b-3p was obviously downregulated in HCC cases
(2.7376±1.99328) in contrast to their counterpart adjacent
non-cancerous HCC (2.7376±1.57739, P<0.001) (Fig. 8A). The TCGA and PCR data provided
similar results.
The relationships between miR-23b-3p
and clinicopathological features
As noted above, the expression of miR-23b-3p was
remarkably lower in the HCC tissues than their counterpart adjacent
non-cancerous HCC, which indicated that miR-23b-3p may perform the
function of a feasible tumor suppressive miRNA in HCC. We
investigated the relationships between miR-23b-3p expression and
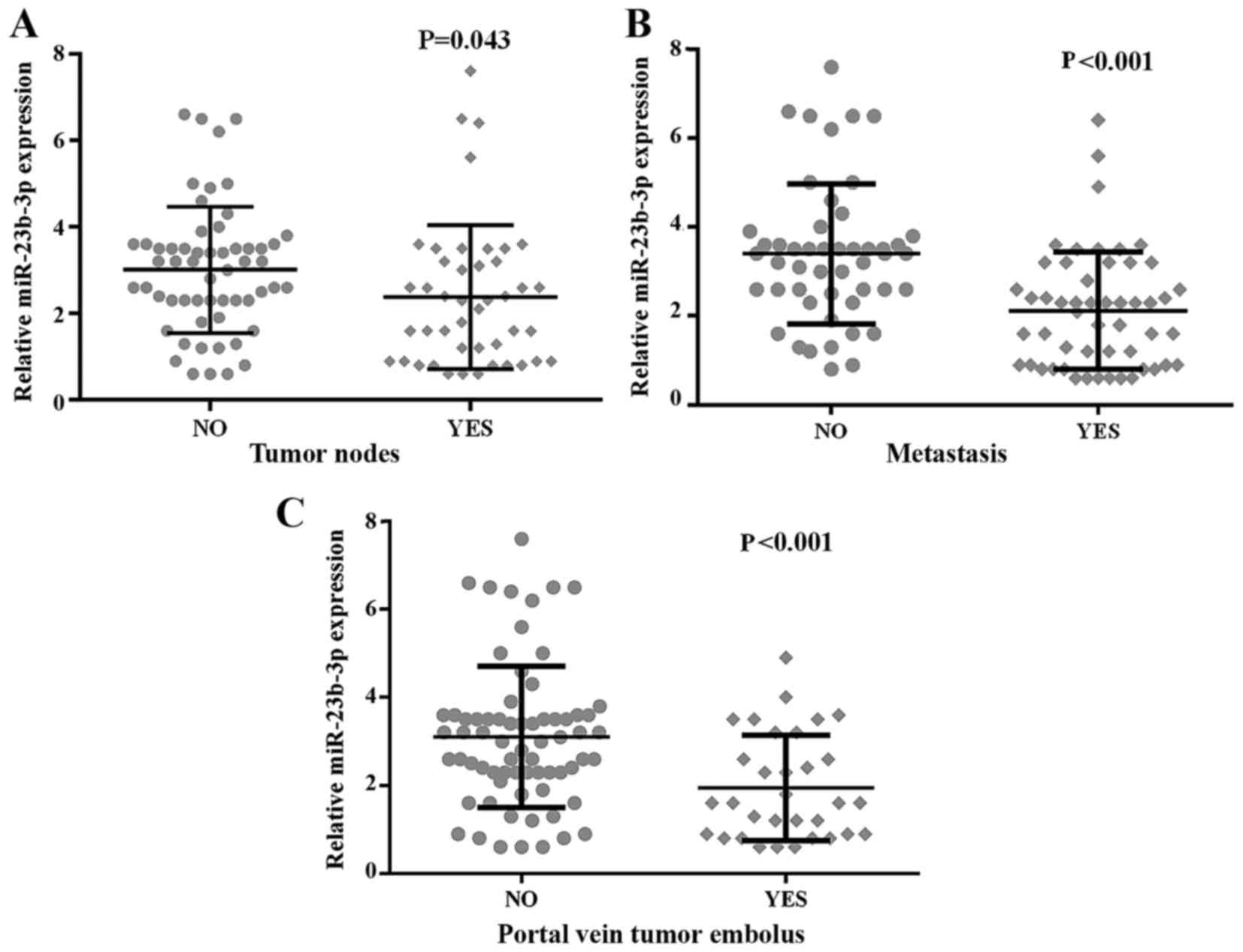
clinicopathological features. The results shown in Table III and Fig. 9 validated that the down-regulated
level of miR-23b-3p was positively related to patients without
metastasis (3.3959±1.57506), in contrast with those with metastasis
(2.1173±1.31762, P<0.001). Subsequently, the miR-23b-3p level
was significantly positively associated with multiple tumor nodes
(3.0158±1.45979) compared with single nodes (2.3773±1.66606,
P=0.043). The presence of a portal vein tumor embolus showed a
lower expression of miR-23b-3p (1.9500±1.19055) than did its
absence (3.1029±1.60797, P<0.001). Furthermore, we used
Spearman's method to record the correlations between the expression
of miR-23b-3p and clinicopathological parameters, including tumor
nodes (r=−0.255, P=0.010), metastasis (r=−0.447, P<0.001), and
portal vein embolus (r=−0.340, P=0.001). Nonetheless, we failed to
gain any positive correlations between miR-23b-3p expression and
other clinical features.
The diagnostic value of the miR-23b-3p
level in HCC tissues
To verify the diagnostic value of the miR-23b-3p
level in HCC tissues, a ROC curve was utilized. As shown in
Fig. 8B, the area under the curve
(AUC) of miR-23b-3p in HCC and adjacent non-cancerous HCC was 0.765
(95% CI, 0.700 to 0.830, P<0.001). At 0.396 (the cut-off value
of miR-23b-3p), the sensitivity and specificity was 88.2 and 55.4%,
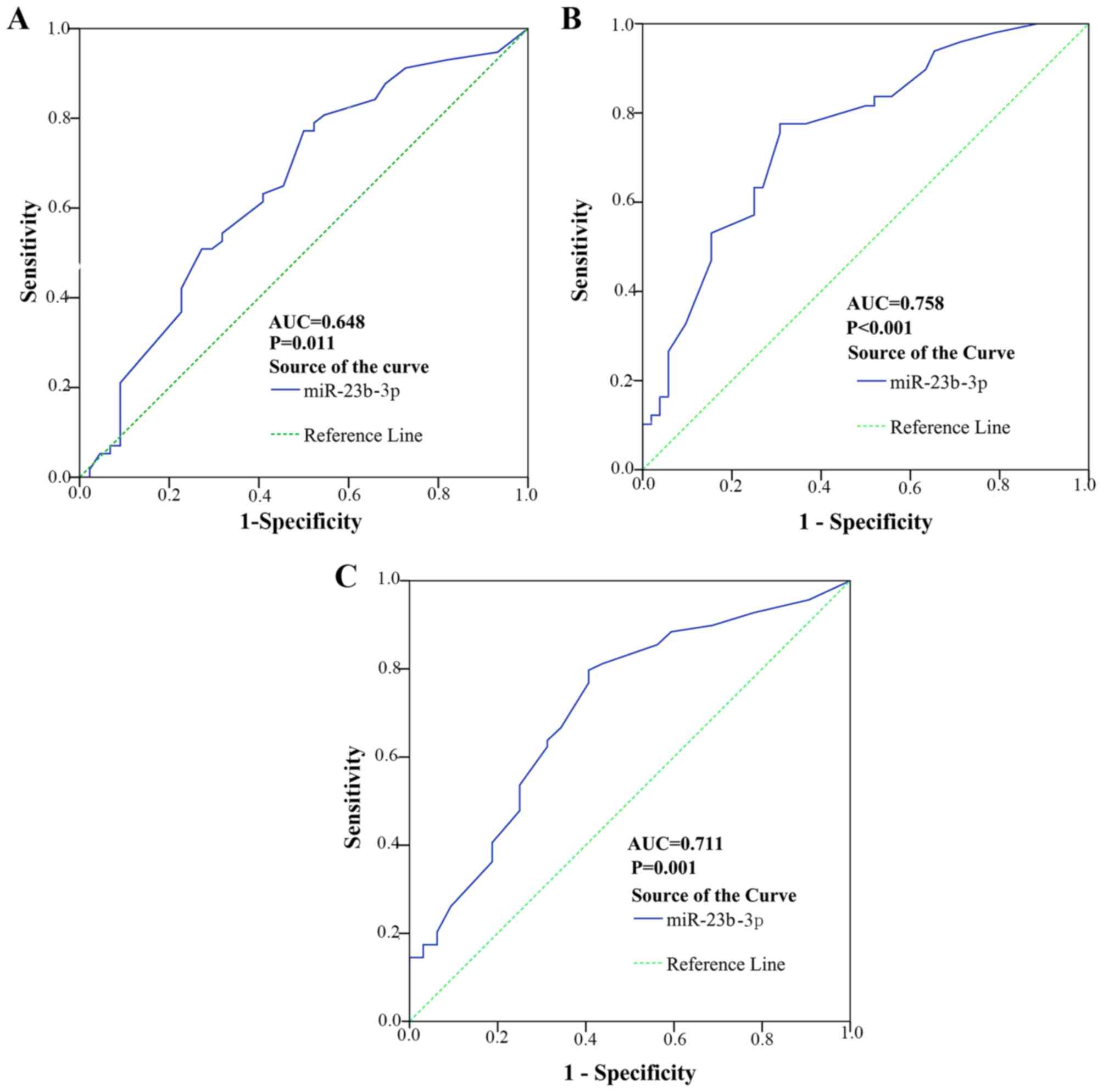
respectively. In Fig. 10, the
AUC to judge tumor nodes was 0.648 (95% CI, 0.538 to 0.758,
P=0.011). The cut-off value for miR-23b-3p was 0.272; the
sensitivity and specificity was 52.6 and 68.2%, respectively. The
AUC to judge metastasis was 0.758 (95% CI, 0.664 to 0.851,
P<0.001). The cut-off value for miR-23b-3p was 0.468; the
sensitivity and specificity was 77.6 and 69.2%, respectively. The
AUC to judge portal vein tumor embolus was 0.711 (95% CI, 0.601 to
0.820, P=0.001). The cut-off value for miR-23b-3p was 0.29; the
sensitivity and specificity was 88.4 and 40.6%, respectively.
miR-23b-3p expression in HCC
recurrence
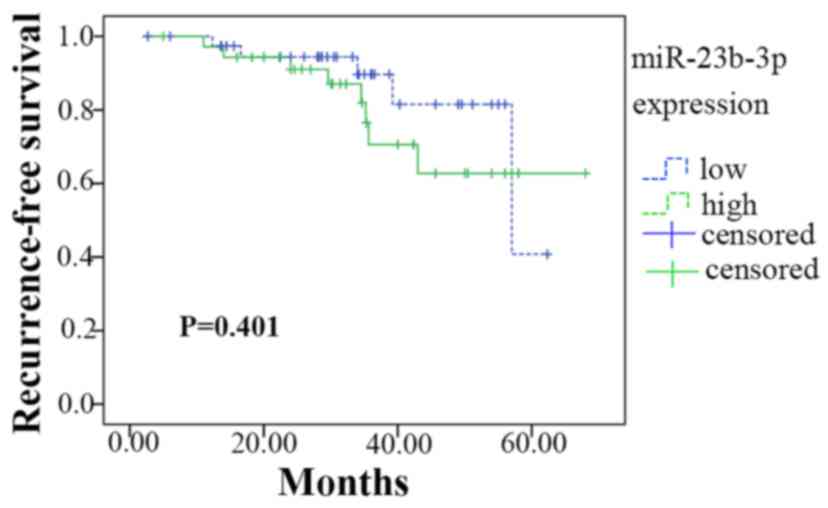
Of the 101 patients recruited for the present study,
76 completed the follow-up. A Kaplan-Meier analysis is presented in
Fig. 11. Among the 76 patients,
45 had a lower expression of miR-23b-3p (lower than the median
level of 2.600), whereas 31 had higher miR-23b-3p expression. The
lower expression group had 54.257±2.943 months of survival time
without recurrence; the higher expression group had 54.509±3.962
months. Thus, there were no significant differences in survival
time between high and low miR-23b-3p expression (Chi-Square =0.706,
P=0.401).
Meta-analysis for GEO datasets, TCGA
datasets and PCR verification in-house
Characteristics of included
studies
Ten GEO datasets, TCGA dataset and the data derived
from medical records, as mentioned above with 886 HCC patients and
587 normal persons in total, were included in the new
meta-analysis. The characteristics of the studies were previously
described (which were shown in sections 'Characteristics of the
included data-sets', 'The expression level of miR-23b-3p was
downregulated in HCC samples' and 'The expression of miR-23b-3p was
downregulated in HCC') in detail.
Value of miR-23b-3p as a biomarker for
HCC
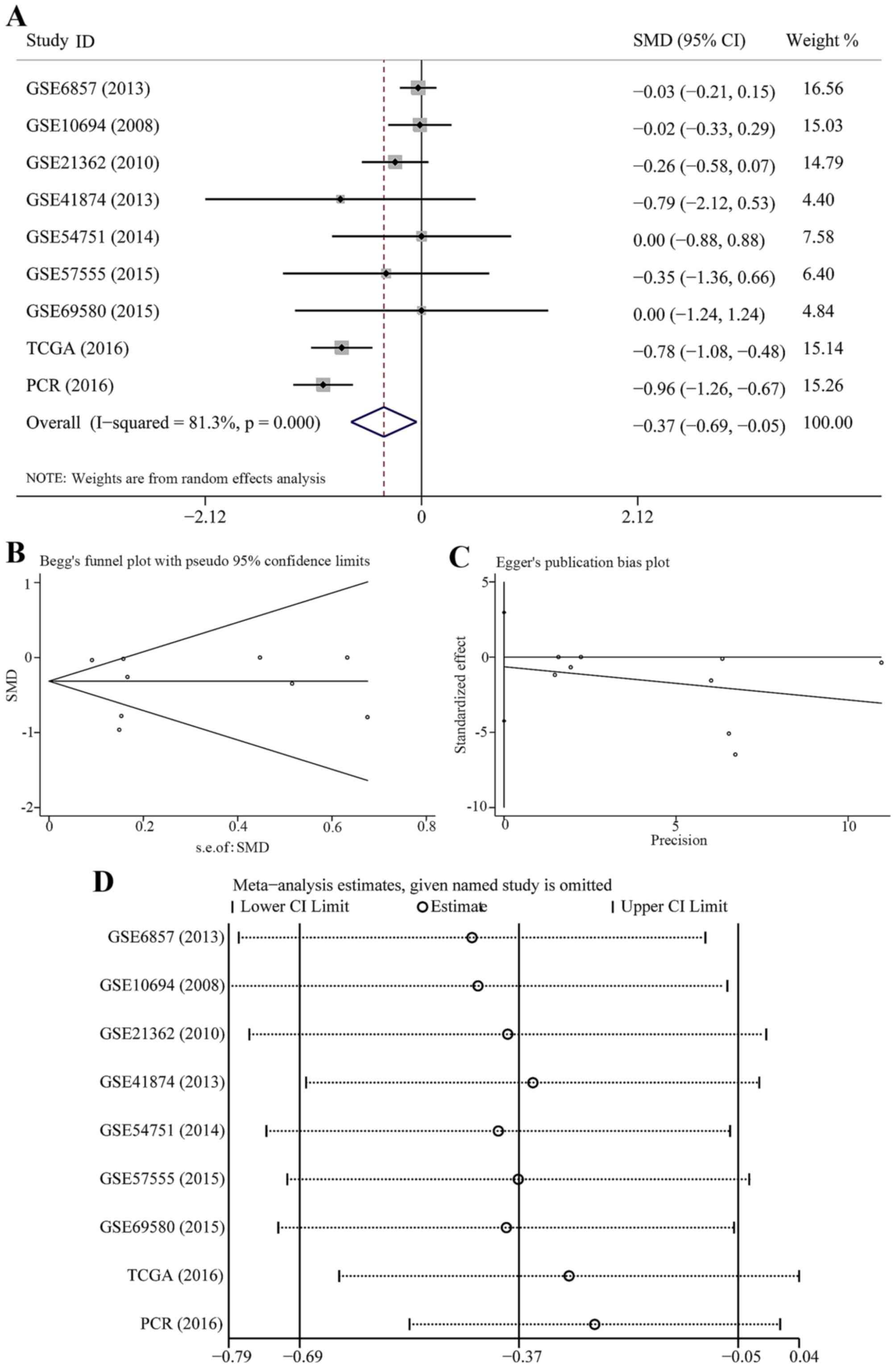
The pooled SMD and its 95% CI (Fig. 12A) indicated that people with
lower expression of miR-23b-3p had a significant risk for HCC
(SMD=−0.368; 95% CI, −0.689 to −0.048; P=0.024). A random effects
model was selected to pool the effect variables with heterogeneity
(P=0.000, I2=81.3%). The results of Begg's and Egger's
test were 0.754 and 0.687, respectively (Fig. 12B and C); however, no publication
bias was discovered. The sensitivity analysis indicated that our
own PCR data exercised a certain influence on that result (Fig. 12D). Therefore, we removed PCR
data and constructed the forest plot again (Fig. 13A). The SMD was −0.258 (95% CI,
−0.529 to 0.014) and no statistical significance was found
(P=0.063). The results of Begg's and Egger's test were 0.386 and
0.602, respectively (Fig. 13B and
C); therefore, no publication bias was observed. The
sensitivity analysis showed that the results were altered when we
removed the study of TCGA dataset (Fig. 13D).
Bioinformatics analysis
Target genes of miR-23b-3p collection
and integration
Using the 10 algorithms, 14,810 target genes were
predicted to be modulated potentially by miR-23b-3p. The number of
miR-23b-3p prediction targets of the 10 algorithms varied, ranging
from 74 to 6,192 with an average of 2,375. Only targets that
appeared more than or equal to five times among all 10 algorithms
were finally applied for further analysis. In total, 357 targets
from the algorithms were included in the next step.
After screening 203 articles from PubMed, we
ultimately gathered 69 validation targets and found that 21 targets
(ATG12, CA2, CFL2, CHUK, GLS, HMGB2, IL6R, KIAA1467, MET, NOTCH2,
PLAU, PNRC2, PRDM1, PTEN, PTK2B, RRAS2, SEMA6D, SPRY2, TAB3, VHL
and WBP2) were duplicated with prediction targets. By combining
targets from algorithms and articles, we finally used 405 targets
to conduct the bioinformatics analysis.
Bioinformatics analysis of miR-23b-3p
targets
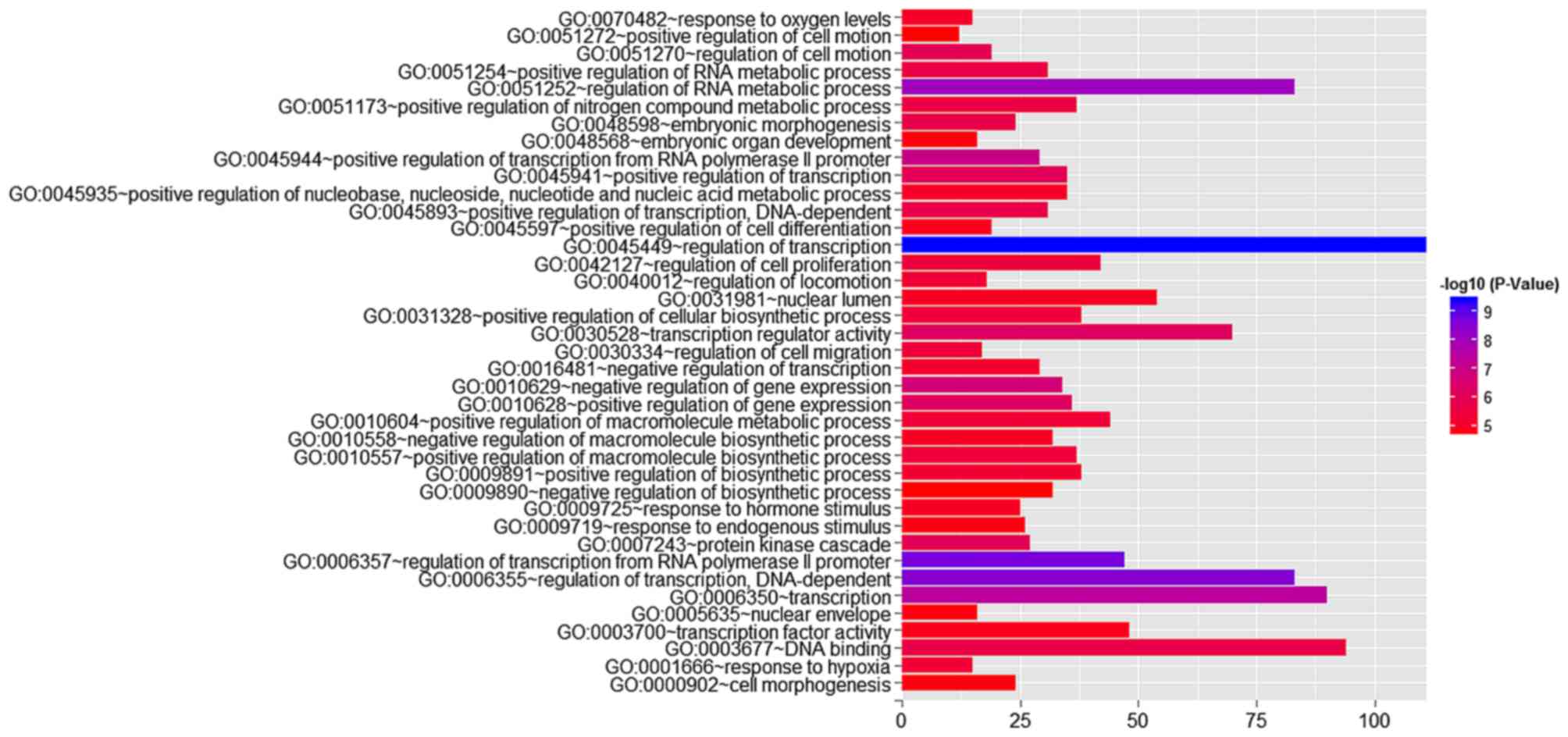
Using GO analysis, 405 targets regulated by
miR-23b-3p were classified as biological processes, cellular
components, and molecular functions (Table IV and Fig. 14). In the pathway analysis,
targets of miR-23b-3p were primarily enriched in the signaling
pathways of renal cell carcinoma, hepatitis B (shown in http://www.genome.jp/kegg-bin/show_pathway?hsa05161/hsa:6777%09red/hsa:4318%09red/hsa:8503%09red/hsa:353376%09red/hsa:596%09red/hsa:317%09red/hsa:1147%09red/hsa:5728%09red/hsa:6714%09red/hsa:4214%09red/hsa:355%09red/hsa:4088%09red/hsa:4089%09red/hsa:2185%09red/hsa:1869%09red/hsa:207%09red)
and pancreatic cancer (corrected P<0.05). In the protein-protein
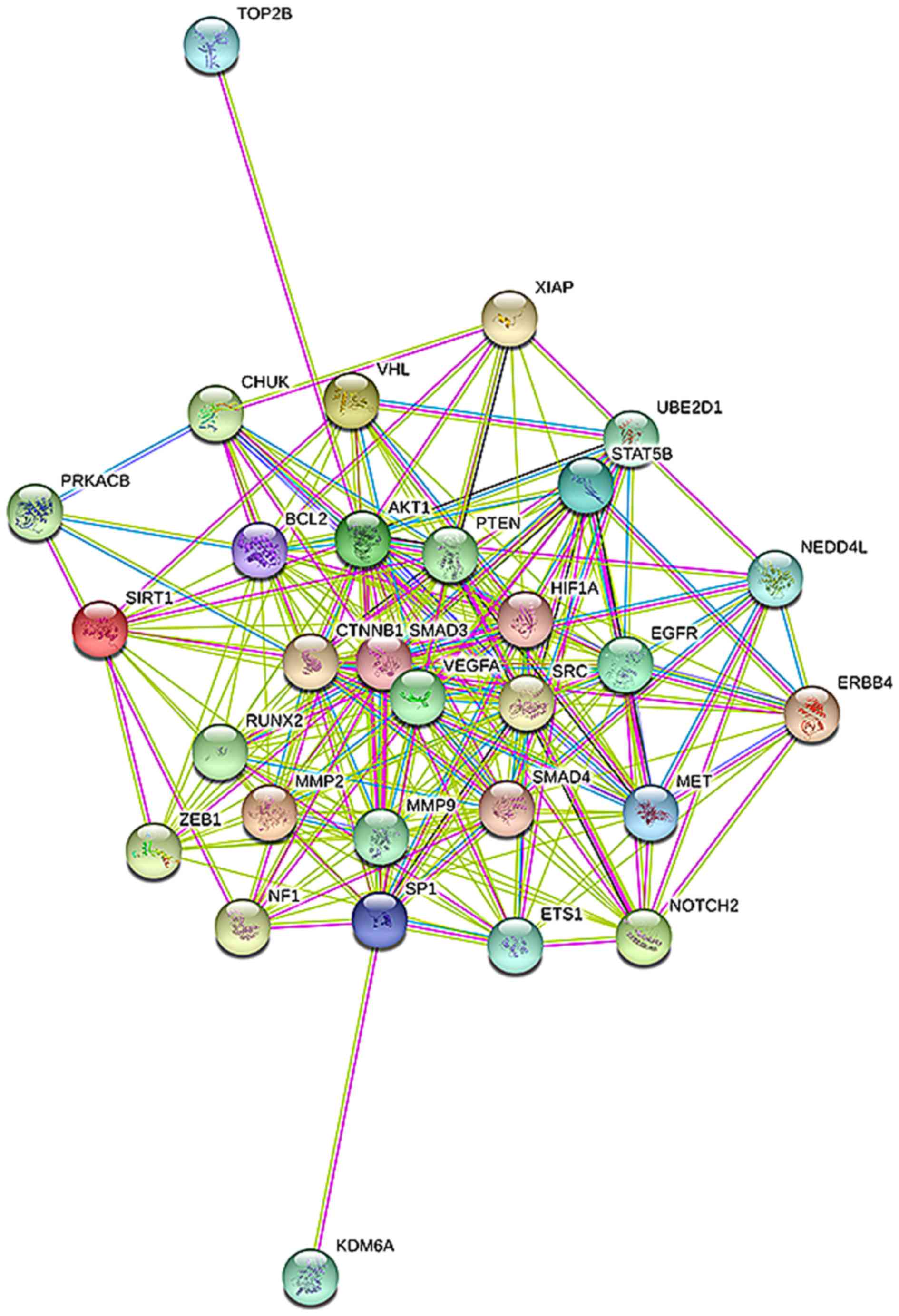
interaction (PPI) network for miR-23b-3p (Fig. 15), 338 target genes were
identified to be involved in 1,074 nodes. A total of 8 targets,
including SRC, AKT1, EGFR, CTNNB1, BCL2, SMAD3, PTEN and KDM6A,
were located in the key nodes with high degree (>35).
 | Table IVFour hundred five genes were
categorized in GO as biological processes, cellular components and
molecular functions. |
Table IV
Four hundred five genes were
categorized in GO as biological processes, cellular components and
molecular functions.
| Term | Count | P-value | FDR |
| Biological
process | | | |
| Regulation of
transcription | 111 | 3.87E-10 | 6.73E-07 |
| Regulation of
transcription from RNA polymerase II promoter | 47 | 1.98E-09 | 3.44E-06 |
| Regulation of
transcription, DNA-dependent | 83 | 2.93E-09 | 5.10E-06 |
| Regulation of RNA
metabolic process | 83 | 8.33E-09 | 1.45E-05 |
| Transcription | 90 | 3.70E-08 | 6.44E-05 |
| Positive
regulation of transcription from RNA polymerase II promoter | 29 | 1.03E-07 | 1.80E-04 |
| Negative
regulation of gene expression | 34 | 2.12E-07 | 3.68E-04 |
| Positive
regulation of gene expression | 36 | 6.48E-07 | 0.001128 |
| Positive
regulation of transcription | 35 | 9.33E-07 | 0.001624 |
| Regulation of cell
motion | 19 | 1.07E-06 | 0.001868 |
| Protein kinase
cascade | 27 | 1.19E-06 | 0.002065 |
| Positive
regulation of transcription, DNA-dependent | 31 | 1.79E-06 | 0.003114 |
| Positive
regulation of RNA metabolic process | 31 | 2.12E-06 | 0.003697 |
| Positive
regulation of nitrogen compound metabolic process | 37 | 2.57E-06 | 0.004477 |
| Regulation of cell
proliferation | 42 | 2.93E-06 | 0.005101 |
| Regulation of cell
migration | 17 | 3.52E-06 | 0.006119 |
| Positive
regulation of macromolecule biosynthetic process | 37 | 3.65E-06 | 0.006356 |
| Positive
regulation of cellular biosynthetic process | 38 | 4.10E-06 | 0.007132 |
| Positive
regulation of macromolecule metabolic process | 44 | 4.31E-06 | 0.007507 |
| Regulation of
locomotion | 18 | 4.36E-06 | 0.007589 |
| Response to
hypoxia | 15 | 4.57E-06 | 0.007949 |
| Positive
regulation of biosynthetic process | 38 | 5.68E-06 | 0.009878 |
| Negative
regulation of transcription | 29 | 7.16E-06 | 0.012463 |
| Response to oxygen
levels | 15 | 8.29E-06 | 0.014432 |
| Positive
regulation of nucleobase, nucleoside, nucleotide and nucleic acid
metabolic process | 35 | 8.58E-06 | 0.014931 |
| Negative
regulation of macromolecule biosynthetic process | 32 | 1.01E-05 | 0.01756 |
| Response to
hormone stimulus | 25 | 1.05E-05 | 0.018246 |
| Positive
regulation of cell differentiation | 19 | 1.21E-05 | 0.021016 |
| Response to
endogenous stimulus | 26 | 1.84E-05 | 0.032008 |
| Embryonic organ
development | 16 | 1.91E-05 | 0.033319 |
| Cell
morphogenesis | 24 | 1.96E-05 | 0.034027 |
| Positive
regulation of cell motion | 12 | 2.46E-05 | 0.042821 |
| Negative
regulation of biosynthetic process | 32 | 2.51E-05 | 0.04366 |
| Cellular
component | | | |
| Nuclear lumen | 54 | 1.14E-05 | 0.015418 |
| Nuclear
envelope | 16 | 1.76E-05 | 0.023821 |
| Molecular
function | | | |
| Transcription
regulator activity | 70 | 6.80E-07 | 9.94E-04 |
| DNA binding | 94 | 2.12E-06 | 0.0031 |
| Transcription
factor activity | 48 | 1.39E-05 | 0.020262 |
Discussion
HCC is one of the most prevalent forms of liver
cancer, and nearly one million cases of HCC occur annually. Thus,
it is urgent to explore the mechanism of HCC tumorigenesis and
progression and to determine treatment and prevention strategies.
Exploiting a new self-assembled cell microarray, Zhang et al
carried out high-throughput screening for miRNAs that are involved
in cell transplantation. They discovered miR-23b-3p, which
functioned as a tumor suppressor in human colon cancer (27). Since then, substantial research
has studied the functions of miR-23b-3p in many physiological and
pathological processes, especially tumor progression. As noted
above, several studies showed that miR-23b-3p played a dual role in
various cancers; these studies also investigated the mechanisms of
miR-23b-3p. This study focused on determining the role of
miR-23b-3p in HCC and its effect on patient prognosis.
In the meta-analysis of data downloaded from GEO
data-sets, the pooled SMD indicated that miR-23b-3p expression
showed no remarkable difference between HCC patients and normal
subjects. No heterogeneity was noted. Additionally, based on GEO
datasets, we investigated the association between miR-23b-3p and
the clinicopathological aspects of HCC and observed that miR-23b-3p
was significantly correlated with invasion. We failed to prove the
relevance of miR-23b-3p and other clinical parameters. Therefore,
diagnosis and targeted therapy based on the miR-23b-3p expression
level required further research.
We identified miR-23b-3p as a HCC biomarker by
analyzing TCGA data. miR-23b-3p expression was obviously
down-regulated in HCC tissues compared to normal liver tissues.
Additionally, miR-23b-3p expression was lower (P<0.05) in
patients with hepatitis B, smoking history, vascular invasion and
lymphatic metastasis. However, only hepatitis B history and
lymphatic metastasis of HCC revealed diagnostic values. HCC
patients with high miR-23b-3p expression presented longer survival;
however, this finding was non-significant.
According to the results above, we propose that
miR-23b-3p may play a crucial part in HCC tumorigenesis and
development. Therefore, we recruited 101 fresh HCC tissues and
counterpart adjacent non-cancerous HCC to inspect the expression of
miR-23b-3p in HCC tissues and its association with the
clinicopathological aspects and prognosis of HCC patients.
However, in contrast with the counterpart adjacent
non-cancerous HCC, we observed that miR-23b-3p was aberrantly
downregulated in HCC tissue (P<0.001). This consequence was
supported by a statistical analysis of TCGA data but in contrast to
the microarray meta-analysis. Additionally, HCC patients with
multiple tumor nodes showed a lower level of miR-23b-3p expression
than those with single tumor nodes (P<0.05). In addition to
other parameters that indicated HCC progression, the deterioration
of metastasis and the portal vein tumor embolus were strongly
associated with a decrease in miR-23b-3p (P<0.001). In line with
the aforementioned findings, miR-23b-3p was closely linked to HCC
aggressiveness and metastasis. Additionally, Salvi et al
transfected miR-23b-3p into HCC cells (SKHeplC3), which resulted in
a reduction in motility and proliferative potential; they also
found that miR-23b-3p was deregulated in HCC (28). To some extent, our study also
revealed that increased miR-23b-3p was associated with an increased
time-to-recurrence (months) in HCC patients. However, there was no
obvious alteration between the two groups concerning the levels of
miR-23b-3p. The lack of a study population may explain this result.
Thus, a larger cohort of patients is required to study this in the
near future. Additionally, only tumor nodes, metastasis portal vein
tumor embolus and EGFR expression of HCC were found to correlate
with miR-23b-3p expression and presented diagnostic values.
Altogether, the data derived from GEO datasets, TCGA
datasets and qPCR were included to perform a comprehensive
meta-analysis. The pooled SMD indicated that pronounced
downregulation of miR-23b-3p expression was noted in HCC patients
as compared to healthy subjects, which indicated that low level of
miR-23b-3p may play an important role in HCC tumorigenesis and
diagnosis. Nevertheless, high heterogeneity indicated that this
conclusion was less reliable. According to sensitivity analysis,
the data from TCGA and qPCR contributed to the high heterogeneity.
The difference of source of specimen and the detection methods of
miR-23b-3p expression may be the major factors.
Ten prediction algorithms (utilizing different
matching criteria and computational algorithms) were employed in
miR-23b-3p target gene prediction. In contrast to the algorithms
that provided validated targets and literature extraction, the
prediction algorithms could not predict with accuracy. Some
factors, such as varying approaches and rules for miRNA targeting,
can affect the target prediction results. To reduce the defects
associated with target prediction, we combined the results of these
algorithms and stipulated that only the genes that appeared no
fewer than five times would be involved in the next analysis.
In addition to targets based on experimental
validation, prediction targets combined with algorithms were
enrolled for GO analysis, pathways analysis and network
interaction. A bioinformatics analysis predicted that miR-23b-3p
was involved in extensive target regulation. Because these targets
took part in multiple phases of tumorigenesis and tumor
progression, we speculated that miR-23b-3p was dysregulated in
various tumors and led to changes in biological characteristics by
regulating a series of target genes in different phases. In a GO
analysis, the targets of miR-23b-3p were involved in biological
processes (like the regulation of transcription and transcription),
cellular components (such as the nuclear lumen and nuclear
envelope) and molecular functions (such as transcription regulator
activity and DNA binding). These targets play a vital role in major
cell processes, and a number of them participate in the
carcinogenesis of various cancers, including HCC. According to KEGG
analysis, miR-23b-3p participated in the hepatitis B pathway, which
was recognized as a key factor of hepatic cirrhosis. Moreover,
hepatic cirrhosis could result in hepatic carcinoma. We also found
that miR-23b-3p was correlated with hepatitis B in the HCC patients
who were included in TCGA. Thus, we speculated that miR-23b-3p may
play an important role in HCC tumorigenesis.
A network analysis was ultimately conducted to
predict the interaction networks of potential targets. Highly
connected genes were regarded to play critical roles in
stabilization, interaction, and gene network regulation. A
connectivity analysis showed the highest connectivity between SRC
and AKT1. SRC is a proto-oncogene encoding a tyrosine-protein
kinase that belongs to Src family kinases, which are considered
vital to cell proliferation, differentiation, apoptosis and
invasion (29,30). Src family kinases have been shown
to play a vital role in several malignancies, including breast
cancer (30), colon cancer
(31), ovarian cancer (32) and HCC (33), as well as melanoma, glioma, and
various types of sarcoma (34).
Akt1 or protein kinase B (PKB)α, which is regularly activated in
human cancers, can phosphorylate downstream molecules and cope with
a wide range of cell processes (35). Moreover, Akt1 was directly or
indirectly controlled by Src kinase activity (35,36). We conjectured that Src kinase and
Akt1 may function in the same signal transduction pathways that
result in carcinogenesis and development. This suggests that
researchers should consider factors related to the hub genes for
improving diagnosis and treatment.
Inquiries in various cancers indicated that
miR-23b-3p, functioning as an oncogenic miRNA, can accelerate tumor
processes such as proliferation and invasion. Jin et al
reported that miR-23b/27b expression was elevated in breast cancer
and that it indicated a poor prognosis (12). Nischarin partially participated in
reducing the level of miR-23b/27b expression by inhibiting NF-κB
phosphorylation and disturbing cancer aggressiveness in vivo
(2). Additionally, the AKT/NF-κB
pathway, which responds to TNFα and EGF, is the pivotal signaling
cascade for Her2/neu-dependent miR-23b/27b in vitro
(2). Li et al demonstrated
that Fas mRNA, which enhances the cell multiplication and apoptosis
rates, was directly silenced by miR-23b-3p upregulation in thymic
lymphoma cells (37).
Many studies also noted that the role of miR-23b-3p
as a tumor suppressor was downregulated in several cancers. Majid
et al discovered that miR-23b-3p was downregulated in
prostate cancer, and its decreasing function of directly inhibiting
proto-oncogene Src kinase may lead to the acceleration of neoplasm
growth (38). The same author in
another study stated that miR-23b-3p played a key role in migration
of bladder cancer cells via posttranscriptionally directly
regulating at Zebl, which could modulate the
epithelial-to-mesenchymal transition (EMT) (39). All of the mechanisms by which
miR-23b-3p acts as a tumor suppressor may contribute to HCC
aggressiveness; further studies are required.
Furthermore, extensive study suggested that
miR-23b-3p was associated with other clinicopathological features
in HCC. Bisio et al confirmed the cis-mediated regulation of
miR-23 by p53 in the MCF7 cell line (40). However, we detected the reverse
(upregulated miR-23b-3p) in p53-positive tissue (P>0.05). There
were two possible reasons for this. First, miR-23b-3p served as
oncogenic miRNA in the MCF7 cell line but acted as a tumor
suppressor in HCC. Notably, the specific mechanism remained
unclear. Secondly, our data was less trustworthy due to its lack of
significance. Chen et al reported that the downregulated
expression of miR-23b-3p inhibited HIF-1α/VEGF and β-catenin/Tcf-4
signaling by enhancing the level of VHL (41). The quantity of VEGF in our study
was also identical with miR-23b-3p expression; however, this was
without significance (P>0.05). Patients with occult hepatitis
virus B infection (OBI), who are generally negative for HBsAg, were
discovered to have a differential expression of miR-23b-3p
(42). Notably, as Salvi et
al reported, miR-23b-3p overexpression contributed to a
decrease in urokinase-type plasminogen (uPA) and c-met. This was
determined by identifying the 3′-UTR of uPA and c-met in two and
four sites, respectively, as well as by observing a reduction in
cell proliferation and metastasis in HCC (28). The present study partially
supports the above study. To our knowledge, the present study is
also the first one to explore the clinicopathological significance
of miR-23b-3p and contributed to a more comprehensive understanding
of the correlation between miR-23b-3p and HCC progression.
Expression of miR-23b-3p was studied in HCC and
their relationship explored. A preliminary analysis of the
biological characteristics and function of miR-23b-3p was performed
via bioinformatical methods. Despite existing limitations,
miR-23b-3p was thought to be a tumor suppressor affecting the
carcinogenesis and aggressiveness of HCC and may represent a
predictive biomarker and therapeutic target for HCC. These results
may contribute to a theoretical basis for HCC diagnosis and
therapy.
Acknowledgments
The authors thank GEO and TCGA for the public
available data.
Notes
[1]
Funding
This study was supported in part by the Natural
Science Foundation of Guangxi, China (2015GXNSFBA139157 and
2017GXNSFAA198026) and Youth Science Foundation of Guangxi Medical
University (WLXSZX18001).
[2] Availability
of data and material
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
RQH and PRW designed the study, did the experiments,
analyzed and interpreted the data, and wrote the manuscript. They
contributed equally to this work. XLX, XY and HWL performed the
statistical analysis, designed and filled-out the figures and
tables. XHQ, LHY, ZGP recruited the specimens, carried out the
miRNA isolation and real-time RT-qPCR. LHY, ZGP and GC participated
in designing the study, supervised all experiments and corrected
the manuscript. All the authors read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
All experiments referred to patient tissues were
authorized by the Ethics Committee of the First Affiliated Hospital
of Guangxi Medical University (no. 2016-KY-NSFC-094). This study
was a sub-study of the project mentioned in ethics approval. Method
and participants are utilized in the same way.
[5] Consent for
publication
The consents for publication were obtained from all
the patients.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
de Martel C, Maucort-Boulch D, Plummer M
and Franceschi S: World-wide relative contribution of hepatitis B
and C viruses in hepatocellular carcinoma. Hepatology.
62:1190–1200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yun EH, Lim MK, Oh JK, Park JH, Shin A,
Sung J and Park EC: Combined effect of socioeconomic status, viral
hepatitis, and lifestyles on hepatocelluar carcinoma risk in Korea.
Br J Cancer. 103:741–746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
M'Bengue AK, Doumbia M, Denoman SR,
Ouattara DN, Adoubi I and Pineau P: A major shift of viral and
nutritional risk factors affects the hepatocellular carcinoma risk
among Ivorian patients: A preliminary report. Infect Agent Cancer.
10:182015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Hara SP, Mott JL, Splinter PL, Gores GJ
and LaRusso NF: MicroRNAs: Key modulators of posttranscriptional
gene expression. Gastroenterology. 136:17–25. 2009. View Article : Google Scholar
|
|
6
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Francis P, Moon SY, Bilke S, Zhu YJ and
Meltzer PS: Role of the microRNA-23 27 24 clusters in osteosarcoma.
Cancer Res. 72(Suppl 8): 11132012. View Article : Google Scholar
|
|
8
|
Goto Y, Nishikawa R, Kojima S, Sakamoto S,
Kawamura K, Imamoto T, Chiyomaru T, Enokida H, Kinoshita T, Naya Y,
et al: The functional significance and its regulated molecular
targets of microrna-23b/27b/24-1 cluster in prostate cancer. J
Urol. 191:e456–e457. 2014. View Article : Google Scholar
|
|
9
|
Ishihara T, Chiyomaru T, Inoguchi S,
Enokida H, Seki N and Nakagawa M: The clustered microRNA-23b/27b
function as tumor suppressors and useful prognostic markers in
renal cell carcinoma. J Urol. 191:e2432014. View Article : Google Scholar
|
|
10
|
Jiang W, Min J, Sui X, Qian Y, Liu Y, Liu
Z, Zhou H, Li X and Gong Y: MicroRNA-26a-5p and microRNA-23b-3p
up-regulate peroxiredoxin III in acute myeloid leukemia. Leuk
Lymphoma. 56:460–471. 2015. View Article : Google Scholar :
|
|
11
|
Han L, Chen L, Zhang K, Shi Z, Zhang J,
Zhang A, Wang Y, Song Y, Zheng Y, Jiang T, et al: MicroRNA-23b
expression is regulated by VHL and effects on glioma cell survival
and invasion. Cancer Res. 72:82012. View Article : Google Scholar
|
|
12
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Prooncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer
Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma G, Dai W, Sang A, Yang X and Gao C:
Upregulation of microRNA-23a/b promotes tumor progression and
confers poor prognosis in patients with gastric cancer. Int J Clin
Exp Pathol. 7:8833–8840. 2014.
|
|
14
|
Rogler CE, Levoci L, Ader T, Massimi A,
Tchaikovskaya T and Norel R: MicroRNA-23b cluster microRNAs
regulate transforming growth factor-beta/bone morphogenetic protein
signaling and liver stem cell differentiation by targeting Smads.
Hepatology. 50:575–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan B, Dong R, Shi D, Zhou Y, Zhao Y,
Miao M and Jiao B: Down-regulation of miR-23b may contribute to
activation of the TGF-β1/Smad3 signalling pathway during the
termination stage of liver regeneration. FEBS Lett. 585:927–934.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salvi A, Sabelli C, Moncini S, Venturin M,
Arici B, Riva P, Portolani N, Giulini SM, De Petro G and Barlati S:
MicroRNA-23b mediates urokinase and c-met downmodulation and a
decreased migration of human hepatocellular carcinoma cells. FEBS
J. 276:2966–2982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lau J, Ioannidis JP and Schmid CH:
Quantitative synthesis in systematic reviews. Ann Intern Med.
127:820–826. 1997. View Article : Google Scholar
|
|
18
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zamora J, Abraira V, Muriel A, Khan K and
Coomarasamy A: Meta-DiSc: A software for meta-analysis of test
accuracy data. BMC Med Res Methodol. 6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan N: Meta-analysis: A quantitative
approach of data pooling. Pak Oral Dental J. 20:214–221. 2000.
|
|
21
|
Budhu A, Roessler S, Zhao X, Yu Z, Forgues
M, Ji J, Karoly E, Qin LX, Ye QH, Jia HL, et al: Integrated
metabolite and gene expression profiles identify lipid biomarkers
associated with progression of hepatocellular carcinoma and patient
outcomes. Gastroenterology. 144:1066–1075.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Xie L, He X, Li J, Tu K, Wei L, Wu
J, Guo Y, Ma X, Zhang P, et al: Diagnostic and prognostic
implications of microRNAs in human hepatocellular carcinoma. Int J
Cancer. 123:1616–1622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato F, Hatano E, Kitamura K, Myomoto A,
Fujiwara T, Takizawa S, Tsuchiya S, Tsujimoto G, Uemoto S and
Shimizu K: MicroRNA profile predicts recurrence after resection in
patients with hepatocellular carcinoma within the Milan Criteria.
PLoS One. 6:e164352011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y
and Zhuang SM: MicroRNA-101, down-regulated in hepatocellular
carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer
Res. 69:1135–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen J, LeFave C, Sirosh I, Siegel AB,
Tycko B and Santella RM: Integrative epigenomic and genomic
filtering for methylation markers in hepatocellular carcinomas. BMC
Med Genomics. 8:282015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murakami Y, Kubo S, Tamori A, Itami S,
Kawamura E, Iwaisako K, Ikeda K, Kawada N, Ochiya T and Taguchi YH:
Comprehensive analysis of transcriptome and metabolome analysis in
intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Sci
Rep. 5:162942015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salvi A, Sabelli C, Moncini S, Venturin M,
Arici B, Riva P, Portolani N, Giulini SM, De Petro G and Barlati S:
MicroRNA-23b mediates urokinase and c-met downmodulation and a
decreased migration of human hepatocellular carcinoma cells. FEBS
J. 276:2966–2982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dehm SM and Bonham K: SRC gene expression
in human cancer: The role of transcriptional activation. Biochem
Cell Biol. 82:263–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Finn RS: Targeting Src in breast cancer.
Ann Oncol. 19:1379–1386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Scott A, Zhang P, Hao Y, Feng X,
Somasundaram S, Khalil AM, Willis J and Wang Z: Regulation of
paxillin-p130-PI3K-AKT signaling axis by Src and PTPRT impacts
colon tumorigenesis. Oncotarget. 8:48782–48793. 2017.
|
|
32
|
Sun L, Wang D, Li X, Zhang L, Zhang H and
Zhang Y: Extracellular matrix protein ITGBL1 promotes ovarian
cancer cell migration and adhesion through Wnt/PCP signaling and
FAK/SRC pathway. Biomed Pharmacother. 81:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong J, Wu JS, Mao SS, Yu XN and Huang
XX: Effect of saracatinib on pulmonary metastases from
hepatocellular carcinoma. Oncol Rep. 36:1483–1490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shor AC, Keschman EA, Lee FY, Muro-Cacho
C, Letson GD, Trent JC, Pledger WJ and Jove R: Dasatinib inhibits
migration and invasion in diverse human sarcoma cell lines and
induces apoptosis in bone sarcoma cells dependent on SRC kinase for
survival. Cancer Res. 67:2800–2808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vojtechová M, Turecková J, Kucerová D,
Sloncová E, Vachtenheim J and Tuhácková Z: Regulation of mTORC1
signaling by Src kinase activity is Akt1-independent in
RSV-transformed cells. Neoplasia. 10:99–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park S, Kim D, Kaneko S, Szewczyk KM,
Nicosia SV, Yu H, Jove R and Cheng JQ: Molecular cloning and
characterization of the human AKT1 promoter uncovers its
up-regulation by the Src/Stat3 pathway. J Biol Chem.
280:38932–38941. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li B, Sun M, Gao F, Liu W, Yang Y, Liu H,
Cheng Y, Liu C and Cai J: Up-regulated expression of miR-23a/b
targeted the pro-apoptotic Fas in radiation-induced thymic
lymphoma. Cell Physiol Biochem. 32:1729–1740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Majid S, Dar AA, Saini S, Arora S,
Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G, et
al: miR-23b represses proto-oncogene Src kinase and functions as
methylation-silenced tumor suppressor with diagnostic and
prognostic significance in prostate cancer. Cancer Res.
72:6435–6446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Majid S, Dar AA, Saini S, Deng G, Chang I,
Greene K, Tanaka Y, Dahiya R and Yamamura S: MicroRNA-23b functions
as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS
One. 8:e676862013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bisio A, De Sanctis V, Del Vescovo V,
Denti MA, Jegga AG, Inga A and Ciribilli Y: Identification of new
p53 target microRNAs by bioinformatics and functional analysis. BMC
Cancer. 13:5522013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen L, Han L, Zhang K, Shi Z, Zhang J,
Zhang A, Wang Y, Song Y, Li Y, Jiang T, et al: VHL regulates the
effects of miR-23b on glioma survival and invasion via suppression
of HIF-1α/VEGF and β-catenin/Tcf-4 signaling. Neuro Oncol.
14:1026–1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Y, Li L, Zhou Z, Wang N, Zhang CY and
Zen K: A pilot study of serum microRNA signatures as a novel
biomarker for occult hepatitis B virus infection. Med Microbiol
Immunol. 201:389–395. 2012. View Article : Google Scholar : PubMed/NCBI
|