Introduction
Acute kidney injury (AKI) is a common clinical
disease with high morbidity and mortality rates (1-3).
Despite fundamental advances in the understanding of AKI
pathophysiology, definitive therapies remain limited. The
utilization of bone marrow-derived mesenchymal stem cells (BMSCs)
to treat AKI has increased in recent years. For example, studies
have demonstrated that the infusion of BMSCs is able to protect
against and accelerate recovery from AKI induced by cisplat-inum
(4), glycerol (5) and ischemia/reperfusion (I/R)
(6-8).
However, the therapeutic efficacy of BMSCs is
greatly limited by the poor survival of donor BMSCs in the ischemic
kidney (9-12). Following transplantation into the
ischemic kidney, BMSCs face a complex environment with numerous
factors that can cause cell death, among which oxidative stress is
the most adverse. Under normal conditions, the naturally-occurring
antioxidant enzymes in the kidney counteract the adverse cellular
effects of oxygen-containing free radicals. However, under AKI
conditions, the protective ability of these scavengers is
overwhelmed by the rapid generation of reactive oxygen species
(ROS) (13). As a result, the
increased levels of ROS induce the death of the transplanted BMSCs
and reduce their differentiation potential (14-16).
Heme oxygenase-1 (HO-1), a stress-inducible
rate-limiting enzyme, is sensitive to upregulation by a variety of
stress mediators, including ischemia, hypoxia, oxidative stress and
inflammatory cytokines (17).
Studies have shown that HO-1 is an antioxidant enzyme, possessing
cytoprotective activity in the ischemic environment (18), which enhances cell survival and
activity (19,20). Thus, it is also possible that HO-1
may prevent BMSCs from being injured by the AKI microenvironment.
However, BMSCs themselves only secrete trace amounts of HO-1
(21), and only a little HO-1 is
expressed in the renal medulla under the stress state because
normal renal tissues express the HO-2 isoform (22). In addition, the stress-induced
expression of HO-1 in the renal medulla is rate-limited and the
generated HO-1 metabolizes quickly (23). Thus, the cytoprotective effect of
the induced HO-1 is not able to balance the stress-induced injury
to BMSCs or exert a positive effect. Therefore, obtaining the
stable and efficient expression of HO-1 in BMSCs becomes a key
issue that may be beneficial for BMSC transplantation in AKI.
In the present study, the expression of HO-1 in
BMSCs was increased using a gene transfection technique. It was
hypothesized that the HO-1 gene augmentation would improve the
survival and differentiation of BMSCs under AKI conditions, and
thereby potentially improve the effect of BMSC therapy on AKI.
Materials and methods
Construction of HO-1-BMSCs and enhanced
green fluorescent protein (eGFP)-BMSCs
Sprague-Dawley (SD) passage 3 BMSCs purchased from
American Type Culture Collection (Manassas, VA, USA), were used for
the preparation of infected cells, according to our previous study
(24). Briefly, the target
plasmids pLV.ExBi.P/Puro-EF1α-HO-1-IReS-eGFP and
pLV.Ex2d.P/puro-EF1A>eGFP were successfully prepared using
Gateway technology (25). The
plasmids were co-transfected into 293FT cells (Shanghai Institute
of Biochemistry and Cell Biology, Chinese Academy of Sciences,
Shanghai, China) together with envelope helper plasmids
(pLV/helper-SL3, pLV/helper-SL4 and pLV/helper-SL5; Guangzhou Saiye
Biotechnology Co., Ltd., Guangzhou, China) to harvest
lenti-HO-1-eGFP/puro (lenti-HO-1) or lenti-eGFP/puro (lenti-eGFP).
The titers of lenti-HO-1 and lenti-eGFP were 1.7×108 and
6.5×108 TU/ml, respectively.
BMSCs were plated in 6-well plates at a density of
2×105 cells/well. Following cell attachment, the BMSCs
were infected with lenti-HO-1 or lenti-eGFP (25 µl/well) and
selected by 2.5 µl puromycin (1 µg/ml). The infection
efficacies of the BMSCs were evaluated using fluorescence
microscopy. SD HO-1-BMSCs and SD eGFP-BMSCs were successfully
prepared for further use.
Cell grouping
BMSCs, eGFP-BMSCs and HO-1-BMSCs resuspended in the
medium were plated in 6-well plates with low-glucose Dulbecco's
modified eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Shanghai, China) at the density of 4×105 cells/well. A
Transwell chamber with a 0.4-µm pore-sized polycarbonate
filter (Corning Incorporated, Corning, NY, USA) was introduced in
order to add the interventions. The Transwell chambers were plugged
into the plates and AKI-kidney homogenate supernatant AKI-(KHS) or
normal-KHS (N-KHS) was added to the upper chambers. The protocols
for the preparation of AKI-KHS and N-KHS were as previously
described (24). Briefly, models
of I/R-AKI were created using 6-8-week-old SD rats weighing 150-200
g; (Experimental Animal Center of the Second Military Medical
University, Shanghai, China; animal production license, no. SCXK
2007-0003) by clamping the two renal pedicles for 45 min followed
by clamp-release to allow reperfusion. At 45 min following the
initiation of reperfusion, bilateral kidneys were excised and the
corticomedullary junction of the kidneys was obtained and used to
prepare a 20 g/l homogenate. The homogenate was then centrifuged
and the supernatant was filtered with a 30-µm-mesh
disposable sterile filter to obtain the AKI-KHS. This AKI-KHS was
used to generate AKI in vitro. The N-KHS from control
healthy SD rats was used as the control. Ethical approval for the
animal experiments conducted in the present study was provided by
the Animal Experimentation Institution of the Second Military
University (Shanghai, China).
Five groups were established: Blank group (BMSCs
group; 1.5 ml low-glucose DMEM was added to the upper chamber),
control group (BMSCs/N-KHS group; 1.5 ml N-KHS was added to the
upper chamber), BMSCs/AKI-KHS group, eGFP-BMSCs/AKI-KHS group and
HO-1-BMSCs/AKI-KHS group. For the last three groups, 1.5 ml AKI-KHS
was added to the upper chamber. All the groups were incubated at
37°C for 3 days in humidified atmosphere with 5%
CO2.
Cell cycle profiles
Cells were trysinized and harvested by
centrifugation at 167.7 × g at 4°C for 10 min. The cell pellets
were incubated in 10 µl propidium iodide (50 µg/ml)
at room temperature for 30 min. The proportions of cells in the
G0/G1, S and G2 phases were analyzed by flow cytometry using BD
CellQuest software (version 341753 rev.A; BD Biosciences, San Jose,
CA, USA) with an excitation at 488 nm according to the
manufacturer's instructions.
Immunohistochemical staining of
proliferating cell nuclear antigen (PCNA)
PCNA, a marker of mitogenesis (26), was detected using
immunohistochemical staining. The cells were fixed in 4%
paraformaldehyde for 30 min at room temperature. Sections were
incubated with rabbit anti-rat PCNA polyclonal antibody (A0264;
dilution 1:2,000; ABclonal, Woburn, MA, USA) at 4°C for 16 h, and
then incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (PAB29760; dilution 1:2,000; Abnova,
Walnut, CA, USA) at 37°C for 1 h. Non-overlapping view fields
(n=15; ×200 magnification) were selected and the mean value of the
proportion of PCNA+ cells for each section was used for
statistical analysis.
Cell ultrastructure
Cells were trysinized and harvested by
centrifugation at 168 × g at 4°C for 5 min. The cell pellets were
rinsed with 0.1 M phosphate buffer and then fixed with 1% osmic
acid for 1 h at 4°C. Following a further rinse, the pellets were
dehydrated using a graded acetone series and embedded in Araldite
resin. Ultrathin sections (50-70 nm) were double-stained with
uranyl acetate at room temperature for 15 min and lead citrate and
subjected to ultrastructural evaluation using the Hitachi H-7500
transmission electron microscope (TEM; Hitachi, Ltd., Tokyo,
Japan).
Renal epithelial-differentiation of the
BMSCs
Immunohistochemical staining was performed to detect
whether BMSCs expressed cytokeratin 18 (CK18). The protocol was as
described above for PCNA, with the exception that goat anti-rat
CK18 polyclonal antibody (ABP50186; dilution 1:2,000; ABclonal) was
used as the primary antibody.
Quantification of signaling protein
phosphorylation in the BMSCs
The phosphorylation of the signaling proteins
protein kinase B (Akt) and extracellular signal-regulated kinase
(ERK) were assayed by western blot analysis. BCA was used to
determine the protein concentration. Briefly, a 0.1-ml pipette of
each standard and unknown protein sample was replicated into an
appropriately labeled test tube. Two blank tubes were set in
duplicate. For a standard curve, 0.1 ml H2O was added
instead of BSA solution. For the protein samples, 0.1 ml protein
preparation buffer was added. Subsequently, 2.0 ml WR were added to
each tube and mixed well. The tubes were then covered and incubated
at 37°C for 30 min. All tubes were kept at room temperature for 10
min before measurement. Finally, absorbance readings were taken at
562 nm. The BMSCs, eGFP-BMSCs and HO-1-BMSCs were scraped in
radioimmunoprecipitation assay buffer (Gibco; Thermo Fisher
Scientific) including protease inhibitors. Following separation by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5%
concentrated and 10% separation), the proteins (10 µl per
lane) were transferred to a polyvinylidene difluoride (PVDF)
membrane and blocked with Tris-buffered saline and Tween 20
containing 1% bovine serum albumin (Gibco; Thermo Fisher
Scientific) at room temperature for 4 h. The PVDF membrane was
incubated with rabbit anti-rat phospho-Akt (pAkt) monoclonal
antibody (4060s; dilution 1:2,000) and rabbit anti-rat phospho-ERK
(pERK; 5683s; dilution 1:2,000) monoclonal antibody (both from Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight.
HRP-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) was added and the membrane was incubated for 1 h
at room temperature. Following 1-2 min incubation with ECL reagent
(Shanghai Ruisai Biotechnology Co., Ltd., Shanghai, China), the
PVDF membrane was placed into a FluorChem HD2 gel image analysis
system (ProteinSimple, San Jose, CA, USA) for observation of the
protein blots. β-actin was used as the internal reference and
protein expression was calculated as the ratio of the band
intensity of the protein of interest to that of β-actin.
Phosphatidylinositol 3-kinase (PI3K)/Akt
and mitogen-activated protein kinase (MEK)/ERK inhibition
studies
In these experiments, the PI3K/Akt inhibitor
LY294002 or the MEK inhibitor PD98059 (both from Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) were applied at a concentration of
5 µM. The HO-1-BMSCs were pre-incubated with LY294002 or
PD98059 for 1 h, and then cultured under the aforementioned AKI-KHS
treatment conditions for 3 days. The cell cycle profile, proportion
of PCNA+ HO-1-BMSCs and proportion of CK18+
HO-1-BMSCs were detected as already described.
Induction of I/R-AKI and BMSC
implantation in vivo
Rat models of I/R-AKI were prepared using the
protocols described in Cell grouping. At the time of reperfusion,
BMSCs (2×106 cells suspended in 0.2 ml low-glucose DMEM)
were injected into the renal artery. Prior to implantation, the
BMSCs, eGFP-BMSCs and HO-1-BMSCs were labeled with
4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich; Merck KGaA).
The labeling efficiencies were all >98% without significant
differences.
The rats were randomly assigned to five experimental
groups (n=10 for each group) as follows: Control group (the kidneys
of the SD rats were exposed for 45 min, and then 0.2 ml low-glucose
DMEM was injected into the renal artery), model group (the I/R-AKI
group; 0.2 ml low-glucose DMEM was injected into the renal artery
at the time of reperfusion), and BMSCs, eGFP-BMSCs and HO-1-BMSCs
groups. All rats were kept at a temperature of 24°C and 50%
humidity with an unlimited supply of water and food and were housed
under a normal 12 h light-12 h dark cycle. At 3 days following the
injection of BMSCs, the rats were humanely euthanized and
transcardially-flushed with phosphate-buffered saline (PBS). The
excised kidneys were flushed with PBS again, and were fixed in 4%
paraformaldehyde for further use. All procedures were performed in
accordance with the principles of the Guidelines for Animal
experimentation of The Second Military Medical University.
Acute tubular necrosis (ATN) score
Kidneys were fixed in 10% phosphate-buffered
formalin, sectioned, and stained with periodic acid-Schiff (PAS) by
standard methods (27). A renal
pathologist conducted a histological examination in a blinded
manner. The ATN score was quantified by counting the percentage of
tubules that displayed cell necrosis, brush border loss, naked
basement membrane and vacuolar degeneration as follows: 0, none; 1,
≤10%; 2, 11-25%; 3, 26-45%; 4, 46-75% and 5, ≥76%.
Proliferation and differentiation of
BMSCs in the injured kidney in vivo
PCNA+ BMSCs in the kidney were detected
using immunofluorescent staining. Briefly, following
deparaffination, the kidney sections (1.5-µm thick) were
dehydrated with graded ethanol and incubated with a
peroxidase-blocking reagent (5% BSA; Thermo Fisher Scientific) for
30 min at room temperature. The sections were incubated with rabbit
anti-rat PCNA polyclonal antibody (A0264; dilution 1:2,000;
ABclonal) at 4°C overnight. After being washed with PBS, the
sections were incubated with TRITC-conjugated anti-rabbit antibody
(6909-250; dilution 1:1,000; BioVision, Milpitas, CA, USA) at 37°C
for 1.5 h. The treatment with the TRITC-conjugated antibody was
followed by DAPI counterstaining at room temperature for 5 min.
PCNA+ nuclei appeared red, DAPI+ nuclei
appeared blue and the nuclei of PCNA+DAPI+
cells (PCNA+ BMSCs) appeared purple. The proportion of
PCNA+ BMSCs was calculated as the following ratio:
Number of PCNA+DAPI+ cells / number of
DAPI+ cells.
Immunofluorescent staining was also performed to
detect whether the BMSCs trapped in the kidney expressed CK18. The
protocol was conducted as described for PCNA, using goat anti-rat
CK18 polyclonal antibody (ABP50186; dilution 1:2,000; ABclonal) as
the primary antibody. CK18+DAPI+ cells
(CK18+ BMSCs) were those having blue nuclei with red
cytoplasm. The results were expressed as the following ratio:
Number of CK18+DAPI+ cells / number of
DAPI+ cells.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Student's t-tests were performed to analyze the
differences between two groups. Multiple-group comparisons were
performed using one-way analysis of variance followed by
Student-Newman-Keuls tests. SPSS 19.0 statistical software (IBM
Corp., Armonk, NY, USA) was used for the analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
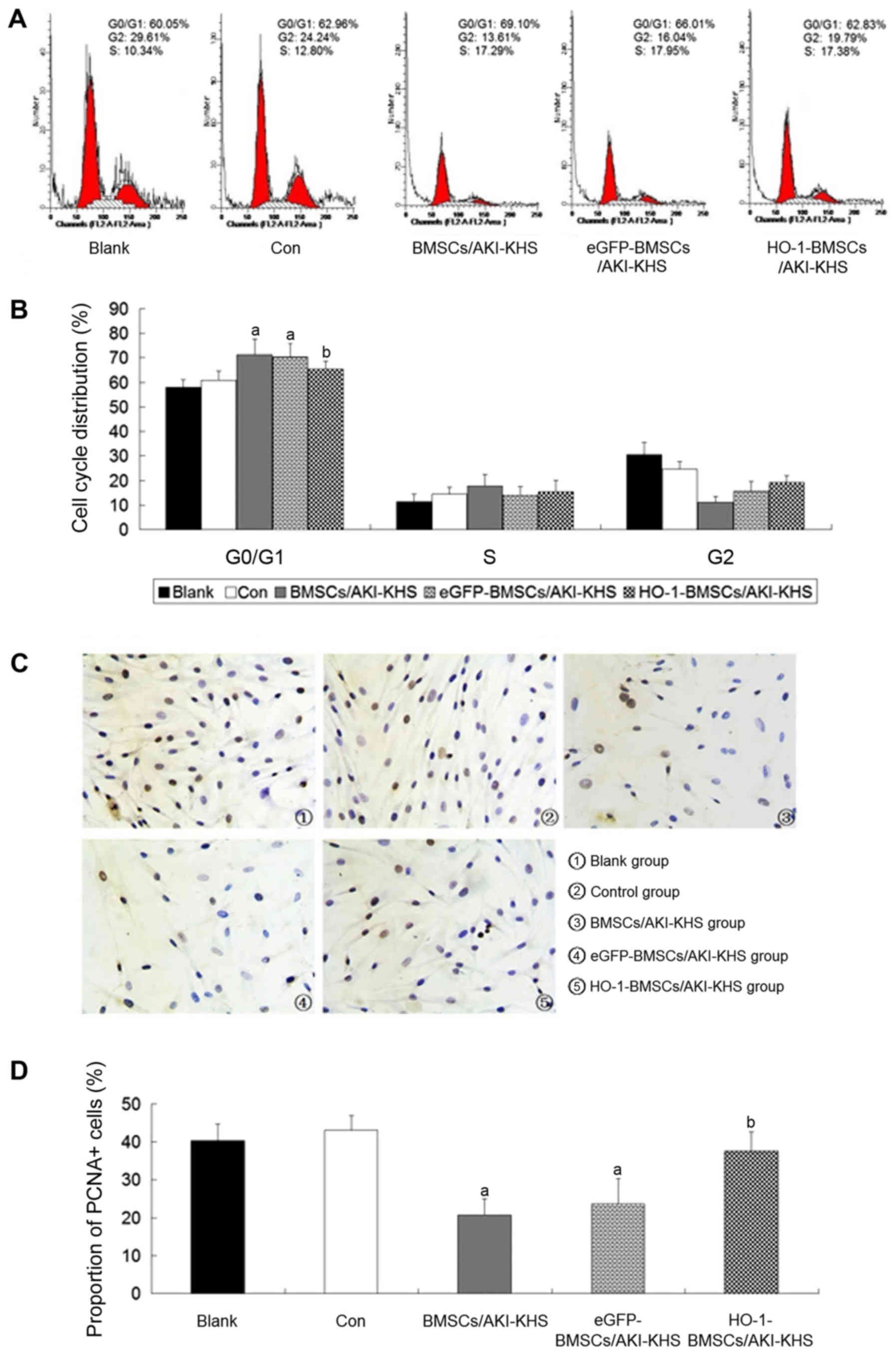
HO-1 overexpression improves the survival
of BMSCs in the AKI microenvironment
BMSCs were indirectly cultured with AKI-KHS to
simulate implanted BMSCs trapped in the AKI kidney in vivo.
Flow cytometric analysis demonstrated that the N-KHS intervention
had no effect on the cell cycle profile of the cultured BMSCs when
compared with the blank group. However, compared with the control
group, the AKI-KHS intervention inhibited the normal mitosis of the
cultured BMSCs and induced cell-cycle arrest at the G0/G1 phase.
The proportion of cells in the G0/G1 phase was increased
significantly in the BMSCs/AKI-KHS group (P<0.05 vs. control
group; Fig. 1A and B). As a
result, there appeared to be fewer BMSCs in the G2 phase, and PCNA
expression was significantly decreased (P<0.05 vs. control
group; Fig. 1C and D). No
significant differences were detected between the BMSCs/AKI-KHS
group and the eGFP-BMSCs/AKI-KHS group. However, HO-1 gene
modification significantly attenuated the cell-cycle arrest,
decreased the proportion of cells in the G0/G1 phase, and increased
the proportion of PCNA+ cells (P<0.05 vs.
BMSCs/AKI-KHS group). Notably, the proportion of PCNA+
cells in the HO-1-BMSCs/AKI-KHS group was similar to those of the
blank and control groups (Fig. 1C and
D).
Cell ultrastructure
Under the TEM, the BMSCs of the blank and control
groups were characterized by large nuclei and few organelles.
AKI-KHS induced epithelial differentiation of the BMSCs. Increased
quantities of granular endoplasmic reticulum, lysosomes and
mitochondria were visible in the cytoplasm of the BMSCs in the
BMSCs/AKI-KHS group (Fig. 2A). In
addition, microvilli and intercellular junctions, which are
specific to epithelial cells, were observed on the surface of the
BMSCs (Fig. 2A). These changes
are indicative of the differentiation of the cultured BMSCs. These
ultrastructural changes were also observed in the cells of the
eGFP-BMSCs/AKI-KHS group and the HO-1-BMSCs/AKI-KHS group when
observed using TEM (data not shown).
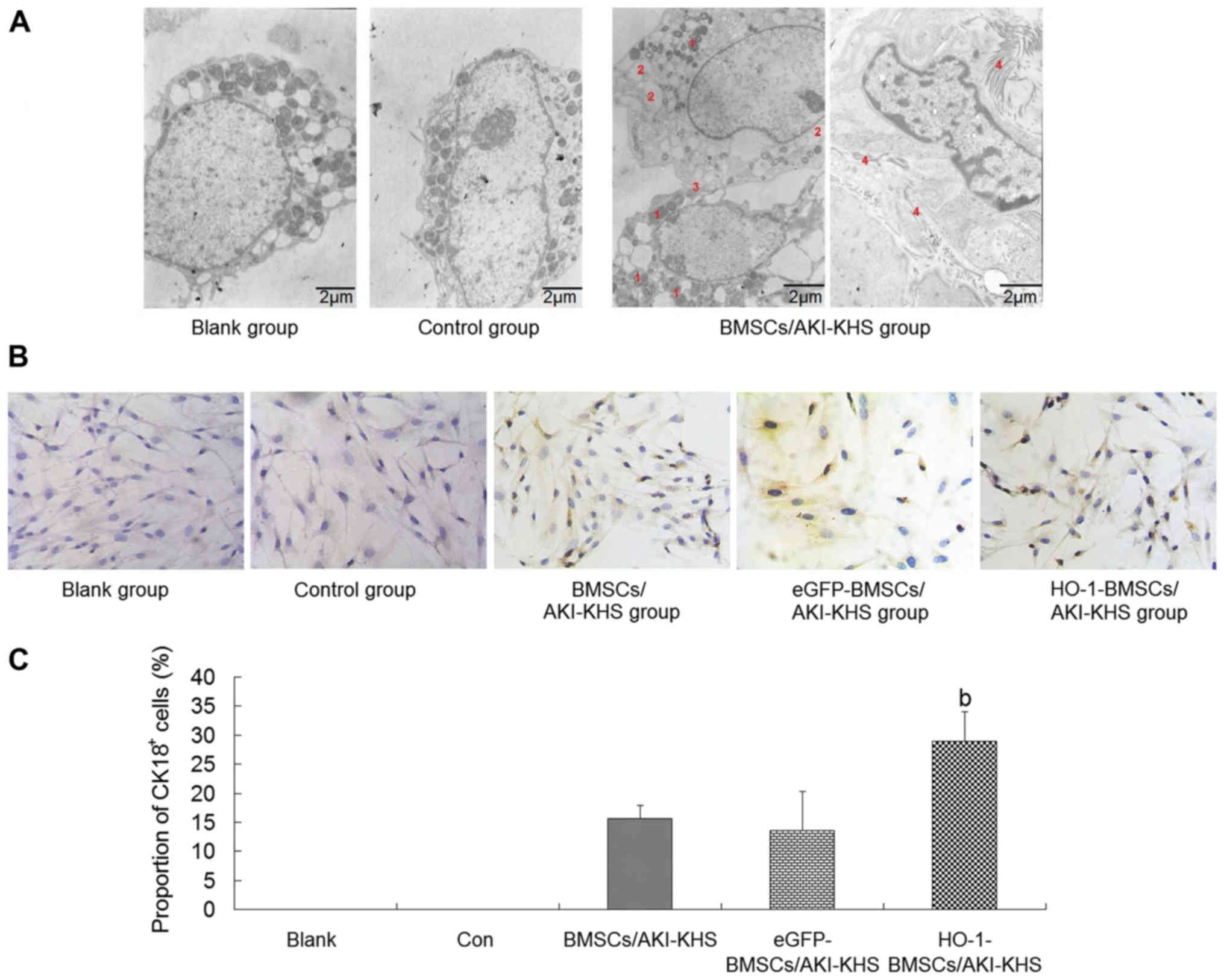 | Figure 2Renal-epithelial differentiation of
BMSCs in vitro. (A) Transmission electron microscopy images
of BMSCs treated with different KHSs. BMSCs of the blank and
control groups were characterized by large nuclei with few
organelles in the cytoplasm. AKI-KHS induced the renal-epithelial
differentiation of BMSCs. Greater numbers of cytoplasm organelles
and some epithelial-specific structures (the intercellular junction
and the microvilli) emerged in the BMSCs. Scale bar, 2 µm.
The red numbers indicate the following features: 1, lysosomes; 2,
granular endoplasmic reticulum; 3, intercellular junction; and 4,
microvilli. (B) Immunohistochemical staining of CK18 in BMSCs
(magnification, ×200). Cells with brown cytoplasm represented
CK18+ cells. (C) Quantitative analysis of the proportion
of CK18+ cells. The HO-1-BMSCs/AKI-KHS group exhibited a
significant increase in the proportion of CK18+ cells.
Results are presented as the mean ± standard deviation (n=6).
bP<0.05 vs. BMSCs/AKI-KHS group. BMSCs, bone
marrow-derived mesenchymal stem cells; KHS, kidney homogenate
supernatant; AKI, acute kidney injury; CK18, cytokeratin 18; HO-1,
heme oxygenase-1; blank, blank group (BMSCs with medium); con,
control group (BMSCs with normal-KHS); eGFP, eGFP, enhanced green
fluorescent protein. |
Renal-epithelial differentiation of BMSCs
in vitro
To test whether HO-1 overexpression had a positive
effect on the renal-epithelial differentiation of BMSCs, the
expression of CK18, a specific marker of renal tubular epithelial
cells (RTeCs) (28,29), was examined. Cells with brown
cytoplasm represented the CK18+ BMSCs (Fig. 2B). The immunohistochemical
staining results demonstrated that the AKI microenvironment induced
the expression of CK18 in BMSCs, and HO-1 overexpression enhanced
this capacity. The proportion of CK18+ cells in the
HO-1-BMSCs/AKI-KHS group was significantly higher compared with
that in the BMSCs/AKI-KHS group (P<0.05; Fig. 2C).
PI3K/Akt and MEK/ERK signal pathways are
involved in the enhanced survival and differentiation of
HO-1-BMSCs
Previous studies demonstrated that the PI3K/Akt and
MEK/ERK pathways are two important signaling pathways involved in
BMSC survival (30). The
phosphorylation of Akt and ERK is also reported to be involved in
the osteogenic differentiation of mesenchymal stem cells (31,32). In the present study, to explore
the signaling pathways of the cytoprotective effect of HO-1
overexpression on BMSCs, the intracellular phosphorylation levels
of Akt and ERK were examined. The levels of pAkt and pERK were
significantly decreased in the BMSCs and eGFP-BMSCs treated with
AKI-KHS compared with the control group (P<0.05); however, the
levels of these proteins were increased significantly in the
HO-1-BMSCs compared with the BMSCs/AKI-KHS (P<0.05; Fig. 3). These results suggest that the
cytoprotective effect of HO-1 overexpression may involve the
PI3K/Akt and MEK/ERK signaling pathways.
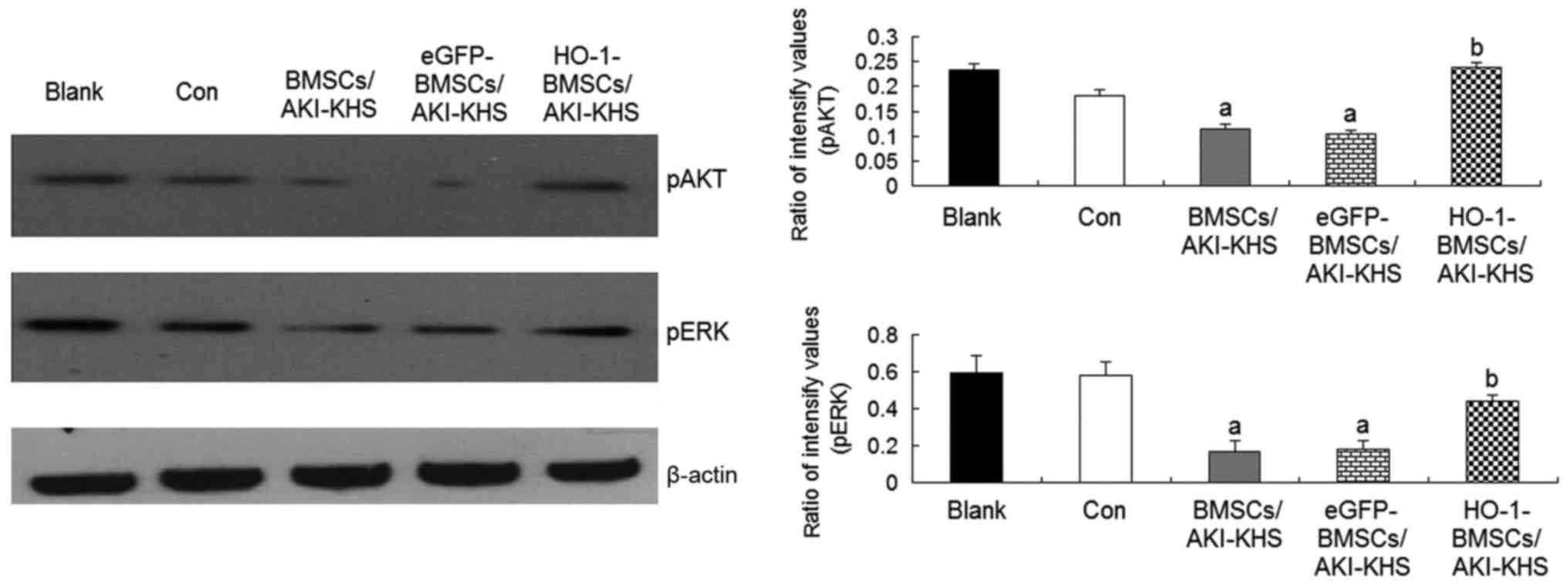 | Figure 3Phosphorylation of Akt and eRK in
BMSCs. HO-1 gene modification increased the phosphorylation of Akt
and eRK in BMSCs in the AKI microen-vironment. Results are
presented as the mean ± standard deviation (n=6).
aP<0.05 vs. control group; bP<0.05 vs.
BMSCs/AKI-KHS group. ERK, extracellular signal-regulated kinase;
pERK, phosphorylated ERK; Akt, protein kinase B; pAkt,
phosphorylated Akt; BMSCs, bone marrow-derived mesenchymal stem
cells; HO-1, heme oxygenase-1; AKI, acute kidney injury; KHS,
kidney homogenate supernatant; blank, blank group (BMSCs with
medium); con, control group (BMSCs with normal-KHS); eGFP, enhanced
green fluorescent protein. |
Effects of PI3K/Akt and MEK/ERK
inhibition of HO-1-BMSCs
To further explore whether the PI3K/Akt and MEK/ERK
signaling pathways are involved in the improved survival and
differentiation of HO-1-BMSCs in the AKI microenvironment, LY294002
and PD98059 were used to block PI3K/Akt and MEK/ERK activities
respectively. The cell cycle profile of the HO-1-BMSCs in the
presence of LY294002 or PD98059 exhibited no significant difference
from that of the HO-1-BMSCs/AKI-KHS group (Fig. 4A). However, as shown in Fig. 4B and C, significantly decreased
proportions of PCNA+ and CK18+ cells were
detected for the HO-1-BMSCs in the presence of LY294002 or PD98059
(P<0.05 vs. the HO-1-BMSCs/AKI-KHS group).
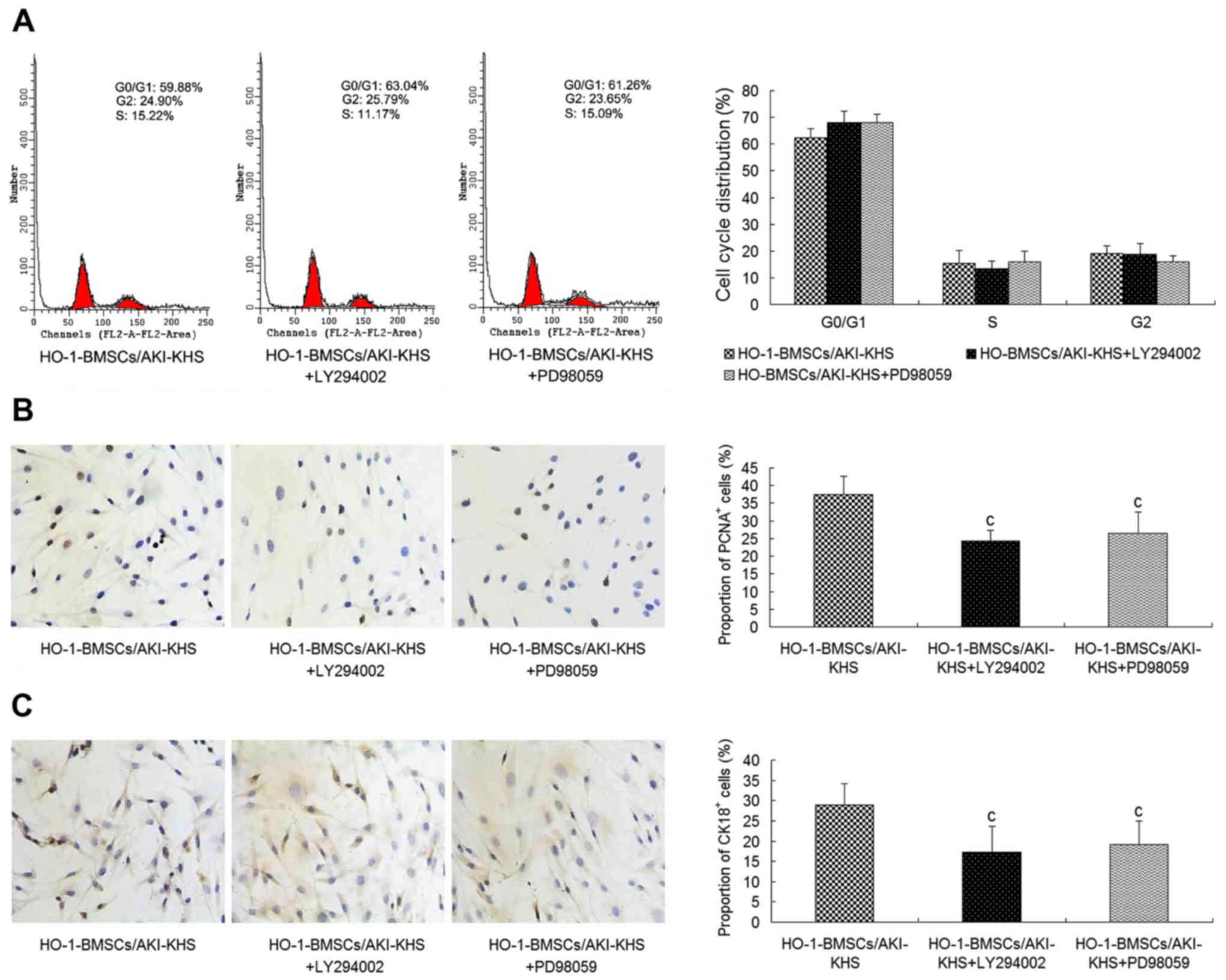 | Figure 4Inhibition of the PI3K/Akt and
MeK/eRK pathways by the PI3K inhibitor LY294002 and MeK inhibitor
PD98059. Pre-incubation with LY294002 or PD98059 attenuated the
cytoprotective effect of HO-1 overexpression on BMSCs. (A) No
significant differences in cell cycle profiles was observed among
the three groups. LY294002 and PD98059 significantly decreased the
expression of (B) PCNA and (C) CK18 in the HO-1-BMSCs (×200
magnification). Results are presented as mean ± standard deviation
(n=6). cP<0.05 vs. HO-1-BMSCs/AKI-KHS group. PI3K,
phosphatidylinositol 3-kinase; Akt, protein kinase B; MeK,
mitogen-activated protein kinase; ERK, extracellular
signal-regulated kinase; HO-1, heme oxygenase-1; BMSCs, bone
marrow-derived mesenchymal stem cells; PCNA, proliferating cell
nuclear antigen; CK18, cytokeratin 18; AKI, acute kidney injury;
KHS, kidney homogenate supernatant; blank, blank group (BMSCs with
medium); con, control group (BMSCs with normal-KHS); eGFP, enhanced
green fluorescent protein. |
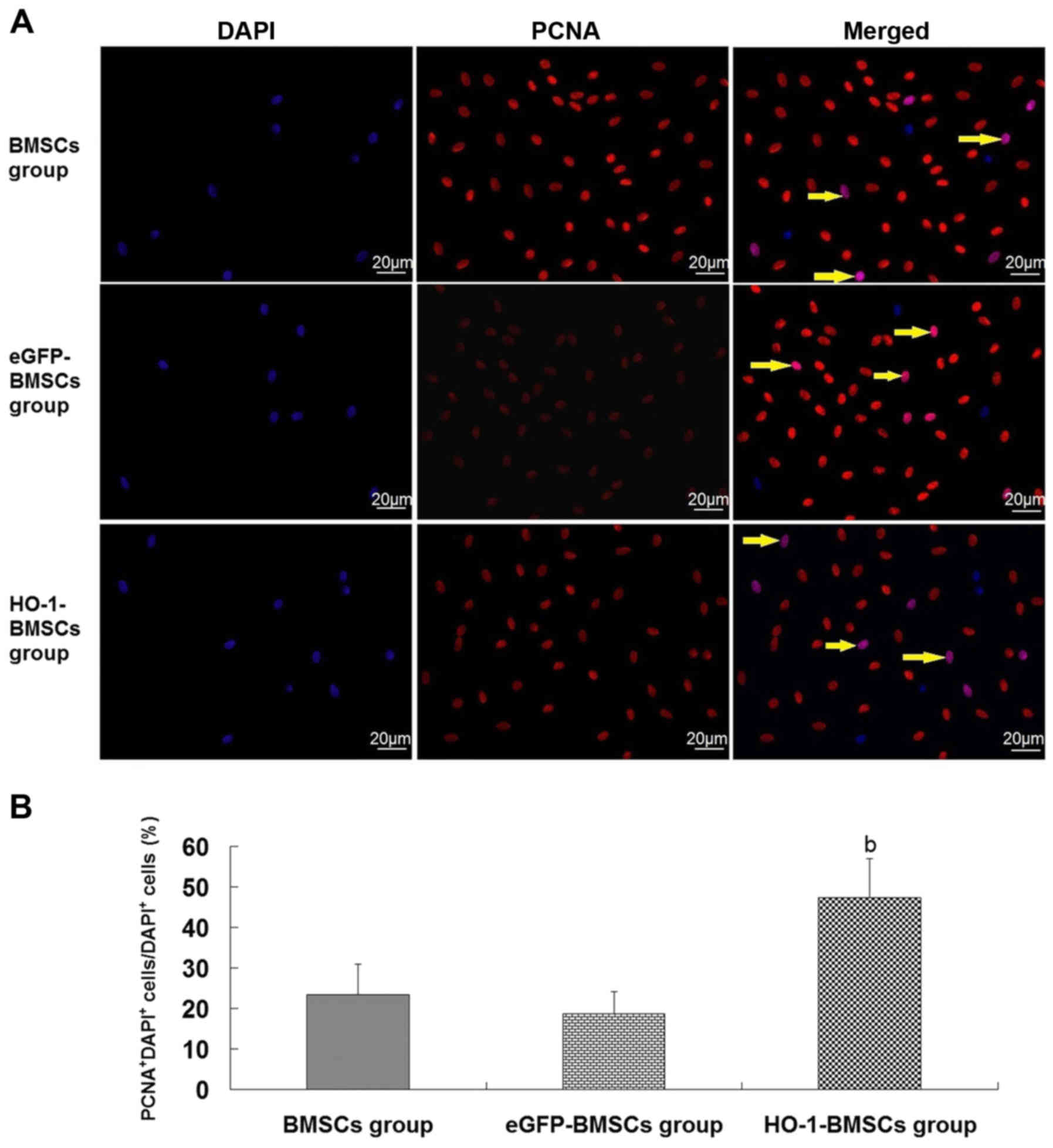
Proliferation and renal-epithelial
differentiation of implanting BMSCs in the injured kidney in
vivo
BMSCs, eGFP-BMSCs and HO-1-BMSCs were labeled with
DAPI. Three days following their injection into the renal artery,
the trapped DAPI+ BMSCs in the injured kidney were
assessed by PCNA assay. Cells with red nuclei were PCNA+
cells and cells with purple nuclei were
PCNA+DAPI+ cells (PCNA+ BMSCs). No
difference was observed in the proportions of PCNA+
BMSCs between the BMSCs group and the eGFP-BMSCs group (Fig. 5). However, in the HO-1-BMSCs
group, the proportion of HO-1-BMSCs that were positive for PCNA
expression was significantly increased (P<0.05 vs. BMSCs group;
Fig. 5).
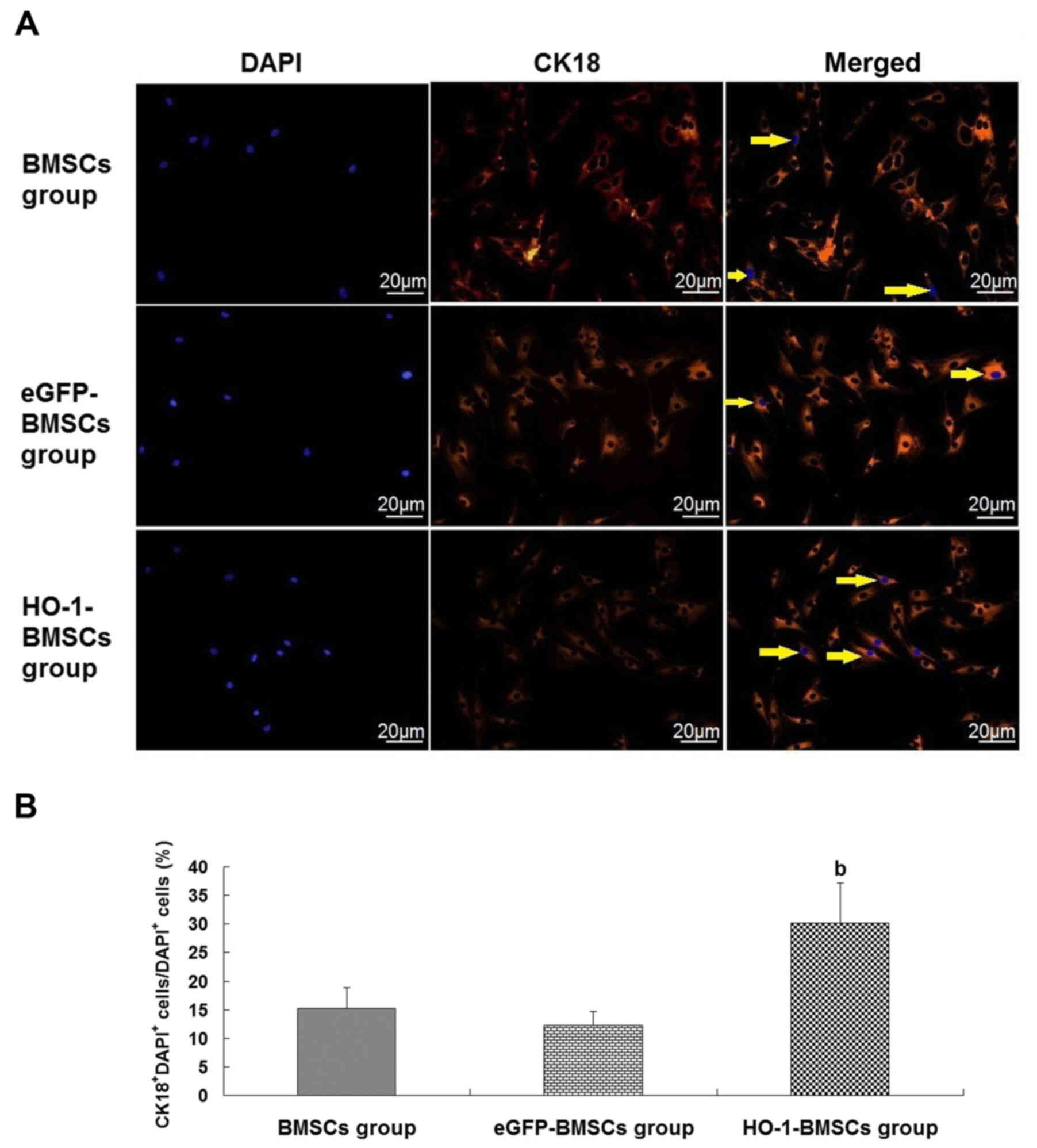
In the renal-epithelial differentiation assay, cells
with red fluorescence in the cytoplasm were CK18+ cells.
The immunofluorescence staining results demonstrated that the
cytoplasm of some of the injected BMSCs was red, indicating that
the AKI conditions induced the renal-epithelial differentiation of
the trapped BMSCs. HO-1 overexpression increased the
renal-epithelial differentiation capacity of the BMSCs in
vivo. The ratio of CK18+DAPI+
cells/DAPI+ cells was higher in the HO-1-BMSCs group
compared with the BMSCs and the eGFP-BMSCs groups (P<0.05;
Fig. 6).
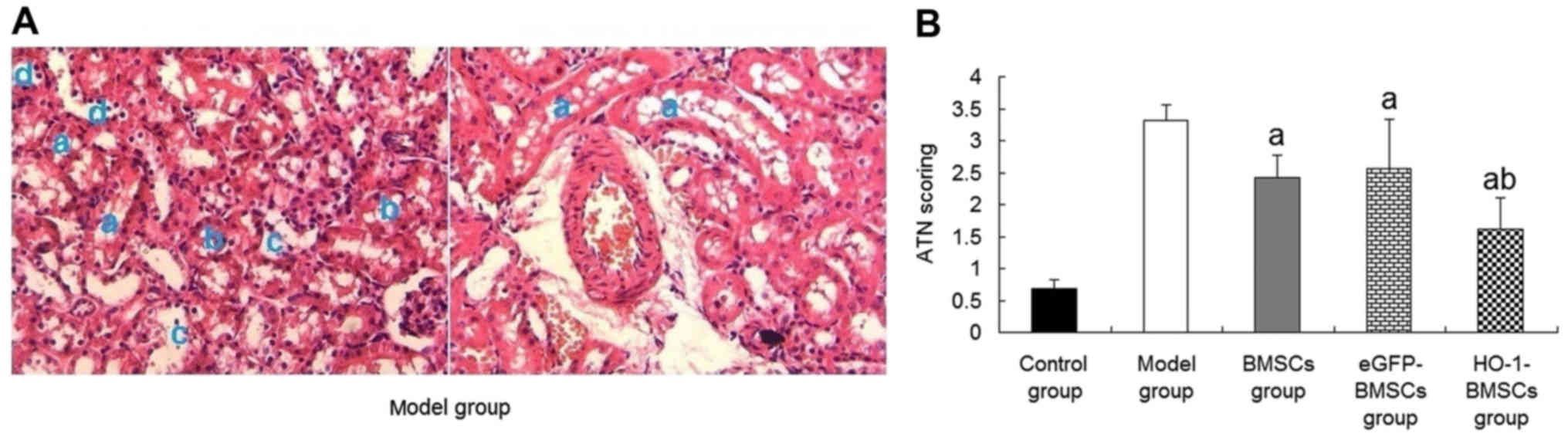
HO-1 overexpression improves the
treatment effect of BMSCs on I/R-AKI
A previous study by the present research team
demonstrated that the administration of BMSCs was able to improve
the renal function of rats with I/R-AKI. Furthermore, rats treated
with HO-1-BMSCs exhibited significantly lower levels of blood urea
nitrogen (BUN) and serum creatinine (Scr) than rats treated with
unmodified BMSCs (24). To
further substantiate these results, histological examinations of
the tubular tissues were conducted and ATN scores were evaluated.
In the model group, PAS staining (Fig. 7A) revealed that some tubules
displayed brush border loss, vacuolar degeneration and the
sloughing off of epithelial cells into the tubular lumen. The ATN
score was high. In comparison with the model group, the rats
treated with BMSCs or eGFP-BMSCs exhibited significantly reduced
ATN scores compared with the model group (P<0.05; Fig. 7B). The reduction of the ATN score
in the HO-1-BMSCs-treated rats was significantly greater than those
of the rats in the BMSCs group (P<0.05; Fig. 7B).
Discussion
BMSCs transplantation has been proposed to repair
AKI via differentiation into renal cells or by the paracrine effect
(33-36). However, the therapeutic efficacy
has been hindered by the death and low nephrogenic-differentiation
of those cells when they are implanted in vivo. Previous
studies have revealed the adverse effect of ROS on the survival and
differentiation of the implanted BMSCs trapped in the I/R-AKI
kidney (24). These toxic ROS are
generated in the ischemia tissue following reperfusion (37). Thus, reducing the level of ROS may
be an appealing approach to improve the outcome of the implanted
BMSCs and subsequently obtain a better therapeutic effect.
HO-1, which was initially identified as the
rate-limiting enzyme in the degradative pathway of heme, is now
recognized to have cytoprotective effects (38). HO-1 and its byproducts (iron,
carbon monoxide and biliverdin), possess the potential ability to
eliminate the elevated ROS levels and thereby prevent cells from
programmed cell death in the ischemic environment (18,39,40).
As mentioned above, HO-1 expression in BMSCs is
induced by many stress mediators, but in limited amounts that are
not able to counteract the injury caused by the stress, or exert a
positive effect. Therefore, in the present study, the
overexpression of HO-1 in BMSCs was achieved via gene transfection.
In a previous study conducted using these cells, it was
demonstrated that the levels of superoxide dismutase and
glutathione peroxidase were significantly increased in
HO-1-BMSCs/AKI-KHS compared with BMSCs/AKI-KHS (24). This suggests that the ROS level in
HO-1-BMSCs may be substantially decreased, and that the enhanced
HO-1 expression may have a potentially positive effect of on the
survival and differentiation of BMSCs in the AKI
microenvironment.
The results of experiments performed in the present
study confirmed this hypothesis. Following treatment with AKI-KHS,
the proportion of HO-1-BMSCs at the G0/G1 phase was decreased and
the proportion of PCNA+ HO-1-BMSCs was increased
significantly compared with those in the control BMSCs. In
agreement with observations from the in vitro experiment,
the in vivo experiment also revealed a significantly
elevated PCNA+DAPI+/DAPI+ cell
ratio in the HO-1-BMSCs group.
The effect of implanted BMSCs on AKI repair is
partly due to their direct nephrogenic differentiation process
(33,34). The effect of HO-1 overexpression
on the nephrogenic differentiation of BMSCs was also tested in the
present study. BMSCs indirectly cultured with AKI-KHS were used to
imitate implanted BMSCs trapped in the injured kidney in
vivo. Higher numbers of organelles were observed in the
cytoplasm of the BMSCs in the BMSCs/AKI-KHS group. In addition,
microvilli and intercellular junctions that are specific to
epithelial cells were also visible on the surface of the BMSCs.
These phenomena suggest the renalepithe lial differentiation of
BMSCs. These ultrastructural changes were also observed in the
HO-1-BMSCs/AKI-KHS group. CK18, a specific marker of renal tubular
epithelial cells, was also tested in the present study.
CK18+ BMSCs were observed in the BMSCs/AKI-KHS group,
the eGFP-BMSCs/AKI-KHS group and the HO-1-BMSCs/AKI-KHS group, but
the highest proportion of CK18+ BMSCs was detected in
the HO-1-BMSCs/AKI-KHS group. Consistent with this, the highest
proportion of CK18+ BMSCs was observed in the HO-1-BMSCs
group in the I/R-AKI kidney in vivo.
The improved BMSC survival and increased
renal-epithelial differentiation observed in the in vivo
experiment induced a great improvement of the renal function in the
AKI rats, including low BUN and Scr levels (24). In addition, a significantly
reduced ATN score was observed in the HO-1-BMSCs group compared
with the BMSCs group.
In the present study, the downstream signaling
pathways involved in the cytoprotective effect of HO-1
overexpression were explored. The PI3K/Akt and MEK/ERK pathways are
the most prominent survival signaling cascades that are stimulated
in different cell types (41-44). Previous studies suggest that
signaling through the PI3K pathway leads to the phosphorylation of
Akt, which in turn serves a pivotal role in the regulation of
survival in BMSCs (32,45). In addition, it has been documented
that the activation of MEK/ERK mediates the survival of BMSCs
(45). Other studies have
demonstrated that the Akt and ERK signaling pathways are involved
in the differentiation ability of BMSCs (31,32). In the in vitro experiment
in the present study, western blot analysis revealed that the
levels of pAkt and pERK were the highest in the HO-1-BMSCs.
Proliferation and differentiation assays using a PI3K-specific
inhibitor (LY294002) and MeK inhibitor (PD98059) demonstrated that
each of these inhibitors significantly decreased the proportions of
PCNA+ cells and CK18+ cells in the
HO-1-BMSCs. Therefore, it is likely that the PI3K/Akt and MEK/ERK
signaling pathways are involved in the improved survival and
renal-epithelial differentiation of BMSCs induced by the HO-1
overexpression in the AKI microenvironment.
In conclusion, the present study indicates that
overexpression of HO-1 in BMSCs enhances the survival and the
renal-epithelial differentiation of BMSCs in the AKI
microenvironment. Stimulation of PI3K/Akt and MEK/ERK signaling
pathways may be the mechanism underlying the effects of HO01. When
BMSC transplantation was used as a treatment for AKI in the rat
model, the use of HO-1-BMSCs was associated with an improved renal
function and reduced ATN score.
Acknowledgments
Not applicable.
Funding
The authors acknowledge financial support from
Shanghai Key Projects of Basic Research (grant no. 12DJ1400203),
National Natural Science Foundation of China (grant no. 81300568),
Medical Youth Training Project of PLA (grant no. 13QNP050),
Training Programme Foundation for Young Talents in Shanghai City
Health System (grant no. XYQ2013088) and Specific Project of
Nanjing Military Command (grant no. ZX07).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
NL conceived the study, drafted and revised the
manuscript. NL, HW and GH conceived the study. HW and GH
participated in the design of the study. JC, WH and JZ performed
the statistical analysis and the experiments. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was provided by the
Animal Experimentation Institution of the Second Military
University (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Lameire N, Van Biesen W and Vanholder R:
The changing epidemiology of acute renal failure. Nat Clin Pract
Nephrol. 2:364–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oeyen S, Vandijck D, Benoit D,
Decruyenaere J, Annemans L and Hoste E: Long-term outcome after
acute kidney injury in critically-ill patients. Acta Clin Belg.
62(Suppl 2): 337–340. 2007. View Article : Google Scholar
|
|
3
|
Ulusoy S, Arı D, Ozkan G, Cansız M and
Kaynar K: The frequency and outcome of acute kidney injury in a
tertiary hospital: which factors affect mortality. Artif Organs.
39:597–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morigi M, Introna M, Imberti B, Corna D,
Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, et al:
Human bone marrow mesenchymal stem cells accelerate recovery of
acute renal injury and prolong survival in mice. Stem Cells.
26:2075–2082. 2008. View Article : Google Scholar
|
|
5
|
Herrera MB, Bussolati B, Bruno S, Fonsato
V, Romanazzi GM and Camussi G: Mesenchymal stem cells contribute to
the renal repair of acute tubular epithelial injury. Int J Mol Med.
14:1035–1041. 2004.
|
|
6
|
Tögel F, Hu Z, Weiss K, Isaac J, Lange C
and Westenfelder C: Administered mesenchymal stem cells protect
against ischemic acute renal failure through
differentiation-independent mechanisms. Am J Physiol Renal Physiol.
289:F31–F42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lange C, Tögel F, Ittrich H, Clayton F,
Nolte-Ernsting C, Zander AR and Westenfelder C: Administered
mesenchymal stem cells enhance recovery from
ischemia/reperfusion-induced acute renal failure in rats. Kidney
Int. 68:1613–1617. 2005. View Article : Google Scholar
|
|
8
|
Sadek EM, Afifi NM, Elfattah LI and Mohsen
MA: Histological study on effect of mesenchymal stem cell therapy
on experimental renal injury induced by ischemia/reperfusion in
male albino rat. Int J Stem Cells. 6:55–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu N, Tian J, Wang W, Cheng J, Hu D and
Zhang J: effect and mechanism of erythropoietin on mesenchymal stem
cell proliferation in vitro under the acute kidney injury
microenvironment. Exp Biol Med (Maywood). 236:1093–1099. 2011.
View Article : Google Scholar
|
|
10
|
Frippiat C, Dewelle J, Remacle J and
Toussaint O: Signal transduction in
H2O2-induced senescence-like phenotype in
human diploid fibroblasts. Free Radic Biol Med. 33:1334–1346. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei H, Li Z, Hu S, Chen X and Cong X:
Apoptosis of mesenchymal stem cells induced by hydrogen peroxide
concerns both endoplasmic reticulum stress and mitochondrial death
pathway through regulation of caspases, p38 and JNK. J Cell
Biochem. 111:967–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mimeault M and Batra SK: Recent insights
into the molecular mechanisms involved in aging and the malignant
transformation of adult stem/progenitor cells and their therapeutic
implications. Ageing Res Rev. 8:94–112. 2009. View Article : Google Scholar
|
|
13
|
Yang Y, Yang D, Yang D, Jia R and Ding G:
Role of reactive oxygen species-mediated endoplasmic reticulum
stress in contrast-induced renal tubular cell apoptosis. Nephron
Exp Nephrol. 128:30–36. 2014. View Article : Google Scholar
|
|
14
|
Hoffmann J, Glassford AJ, Doyle TC,
Robbins RC, Schrepfer S and Pelletier MP: Angiogenic effects
despite limited cell survival of bone marrow-derived mesenchymal
stem cells under ischemia. Thorac Cardiovasc Surg. 58:136–142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YG, Yang Z, Zhang H, Wang C, Liu M,
Guo X and Xu P: Effect of negative pressure on human bone marrow
mesenchymal stem cells in vitro. Connect Tissue Res. 51:14–21.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barbagallo I, Tibullo D, Di Rosa M,
Giallongo C, Palumbo GA, Raciti G, Campisi A, Vanella A, Green CJ
and Motterlini R: A cytoprotective role for the heme oxygenase-1/CO
pathway during neural differentiation of human mesenchymal stem
cells. J Neurosci Res. 86:1927–1935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otterbein Le, Bach FH, Alam J, Soares M,
Tao Lu H, Wysk M, Davis RJ, Flavell RA and Choi AM: Carbon monoxide
has anti-inflammatory effects involving the mitogen-activated
protein kinase pathway. Nat Med. 6:422–428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Melo LG, Agrawal R, Zhang L, Rezvani M,
Mangi AA, ehsan A, Griese DP, Dell'Acqua G, Mann MJ, Oyama J, et
al: Gene therapy strategy for long-term myocardial protection using
adeno-associated virus-mediated delivery of heme oxygenase gene.
Circulation. 105:602–607. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yet SF, Perrella MA, Layne MD, Hsieh CM,
Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S and Lee
Me: Hypoxia induces severe right ventricular dilatation and
infarction in heme oxygenase-1 null mice. J Clin Invest.
103:R23–R29. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang YL, Zhao Q, Qin X, Shen L, Cheng L,
Ge J and Phillips MI: Paracrine action enhances the effects of
autologous mesenchymal stem cell transplantation on vascular
regeneration in rat model of myocardial infarction. Ann Thorac
Surg. 80:229–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng B, Ren X, Lin G, Zhu C, Chen H, Yin
J, Jiang H, Yang B and Ding D: Paracrine action of HO-1-modified
mesenchymal stem cells mediates cardiac protection and functional
improvement. Cell Biol Int. 32:1256–1264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill-Kapturczak N, Chang SH and Agarwal A:
Heme oxygenase and the kidney. DNA Cell Biol. 21:307–321. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haugen EN, Croatt AJ and Nath KA:
Angiotensin II induces renal oxidant stress in vivo and heme
oxygenase-1 in vivo and in vitro. Kidney Int. 58:144–152. 2000.
View Article : Google Scholar
|
|
24
|
Liu N, Wang H, Han G, Tian J, Hu W and
Zhang J: Alleviation of apoptosis of bone marrow-derived
mesenchymal stem cells in the acute injured kidney by heme
oxygenase-1 gene modification. Int J Biochem Cell Biol. 69:85–94.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nanmei L, Jun T, Jin C and Jinyuan Z:
Migration of CXCR4 gene-modified bone marrow-derived mesenchymal
stem cells to the acute injured kidney. J Cell Biochem.
114:2677–2689. 2013. View Article : Google Scholar
|
|
26
|
Oyama T, Watanabe H, Iwafuchi M, Maejima T
and Ajioka Y: Diagnostic value of proliferating cell nuclear
antigen for myogenic tumors of the stomach. Gartroenterol Jpn.
28:193–200. 1993. View Article : Google Scholar
|
|
27
|
Nakas-Ićindić E, Avdagić N, Mijanović M,
Prasović S, Zaciragić A, Hadzović A and Tahirović G: Nitric oxide
in gentamicin -induced acute tubular necrosis in rats. Bosn J Basic
Med Sci. 5:70–74. 2005. View Article : Google Scholar
|
|
28
|
Baer PC, Bereiter-Hahn J, Schubert R and
Geiger H: Differentiation status of human renal proximal and distal
tubular epithelial cells in vitro: differential expression of
characteristic markers. Cells Tissues Organs. 184:16–22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colić M, Pejnović N, Kataranovski M,
Stojanović N, Terzić T and Dujić A: Rat thymic epithelial cells in
culture constitutively secrete IL-1 and IL-6. Int Immunol.
3:1165–1174. 1991. View Article : Google Scholar
|
|
30
|
Xu R, Chen J, Cong X, Hu S and Chen X:
Lovastatin protects mesenchymal stem cells against hypoxia- and
serum deprivation-induced apoptosis by activation of PI3K/Akt and
ERK1/2. J Cell Biochem. 103:256–269. 2008. View Article : Google Scholar
|
|
31
|
Platt MO, Wilder CL, Wells A, Griffith LG
and Lauffenburger DA: Multipathway kinase signatures of multipotent
stromal cells are predictive for osteogenic differentiation:
tissue-specific stem cells. Stem Cells. 27:2804–2814. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai Z, Li Y, Quarles LD, Song T, Pan W,
Zhou H and Xiao Z: Resveratrol enhances proliferation and
osteoblastic differentiation in human mesenchymal stem cells via
ER-dependent eRK1/2 activation. Phytomedicine. 14:806–814. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li K, Han Q, Yan X, Liao L and Zhao RC:
Not a process of simple vicariousness, the differentiation of human
adipose-derived mesenchymal stem cells to renal tubular epithelial
cells plays an important role in acute kidney injury repairing.
Stem Cells Dev. 19:1267–1275. 2010. View Article : Google Scholar
|
|
34
|
Morigi M, Imberti B, Zoja C, Corna D,
Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N,
et al: Mesenchymal stem cells are renotropic, helping to repair the
kidney and improve function in acute renal failure. J Am Soc
Nephrol. 15:1794–1804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tögel F, Weiss K, Yang Y, Hu Z, Zhang P
and Westenfelder C: Vasculotropic, paracrine actions of infused
mesenchymal stem cells are important to the recovery from acute
kidney injury. Am J Physiol Renal Physiol. 292:F1626–F1635. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Semedo P, Wang PM, Andreucci TH, Cenedeze
MA, Teixeira VP, Reis MA, Pacheco-Silva A and Câmara NO:
Mesenchymal stem cells ameliorate tissue damages triggered by renal
ischemia and reperfusion injury. Transplant Proc. 39:421–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haugen E and Nath KA: The involvement of
oxidative stress in the progression of renal injury. Blood Purif.
17:58–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Courtney Ae and Maxwell AP: Heme oxygenase
1: does it have a role in renal cytoprotection. Am J Kidney Dis.
51:678–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vulapalli SR, Chen Z, Chua BHL, Wang T and
Liang CS: Cardio-selective overexpression of HO-1 prevents
I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart
Circ Physiol. 283:H688–H694. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ozaki KS, Yoshida J, Ueki S, Pettigrew GL,
Ghonem N, Sico RM, Lee LY, Shapiro R, Lakkis FG, Pacheco-Silva A,
et al: Carbon monoxide inhibits apoptosis during cold storage and
protects kidney grafts donated after cardiac death. Transpl Int.
25:107–117. 2012. View Article : Google Scholar
|
|
41
|
Gonzalez-Zulueta M, Feldman AB, Klesse LJ,
Kalb RG, Dillman JF, Parada LF, Dawson TM and Dawson VL:
Requirement for nitric oxide activation of 21(ras)/extracellular
regulated kinase in neuronal ischemic preconditioning. Proc Natl
Acad Sci USA. 97:436–441. 2000. View Article : Google Scholar
|
|
42
|
Brunet A, Datta SR and Greenberg Me:
Transcription-dependent and -independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Downward J: PI 3-kinase, Akt and cell
survival. Semin Cell Dev Biol. 15:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Di Santo S, Seiler S, Fuchs AL, Staudigl J
and Widmer HR: The secretome of endothelial progenitor cells
promotes brain endothelial cell activity through PI3-kinase and
MAP-kinase. PLoS One. 9:e957312014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Isele NB, Lee HS, Landshamer S, Straube A,
Padovan CS, Plesnila N and Culmsee C: Bone marrow stromal cells
mediate protection through stimulation of PI3-K/Akt and MAPK
signaling in neurons. Neurochem Int. 50:243–250. 2007. View Article : Google Scholar
|