Introduction
Neovascular age-related macular degeneration (nAMD)
may lead to central vision loss and is the leading cause of reduced
visual acuity in the elderly worldwide (1). At present, the pathogenesis of nAMD
is not completely understood; however, an increasing body of
evidence suggests that oxidative stress and iron-induced oxidative
stress are major causes of macular degeneration (2,3).
Bone morphogenetic protein 6 (BMP-6) belongs to the
transforming growth factor superfamily and may have a critical role
in nAMD pathogenesis. BMPs are expressed in various ocular tissues
and serve key roles in eye development and differentiation.
Specifically, autopsy results have indicated that BMP-6 levels are
significantly decreased in the retinal pigment epithelial (RPE)
cells of patients with early AMD (4). Furthermore, BMP-6 knockout mice
experience intrahepatic iron accumulation (5), and exhibit age-related iron
accumulation and retinal degeneration (6). BMP-6 signaling in hepatocytes is a
central transcriptional regulator of the iron-associated hormone
hepcidin, which controls the systemic iron balance (7).
At present, the role of BMP-6 in nAMD is poorly
understood. To address this issue, the present study measured the
aqueous levels of BMP-6 in patients with nAMD. Furthermore, to the
best of our knowledge, the protective effects of BMP-6 on RPE cells
have rarely been reported. Therefore, the main objective of the
present study was to investigate the role of BMP-6 in protecting
RPE cells from oxidative stress injury, and to determine the
potential signaling pathways involved in this protection. To this
end, hydrogen peroxide (H2O2) was used to
induce RPE cell damage and to establish a cell model of oxidative
stress injury. Using this model, the present study examined the
ability of BMP-6 pretreatment to protect cells from
H2O2-induced injury. In addition, the BMP-6
inhibitor noggin and inhibitors of the relevant signaling pathways
were used to identify potential signaling pathways involved in this
protection. The present study indicated that BMP-6 protects RPE
cells from oxidative damage by inhibiting the c-Jun N-terminal
protein kinase (JNK) and p38 mitogen-activated protein kinase
(MAPK) signaling pathways, thereby down-regulating pro-caspase-3
and dysregulating the balance between B-cell lymphoma 2 (Bcl-2) and
Bcl-2-associated X protein (Bax) expression.
Materials and methods
Patients and controls
This prospective cross-sectional study was performed
at the Department of Ophthalmology, Xi'an Jiao-tong University
(Xi'an, China). A total of 30 patients with untreated nAMD and 20
age- and sex-matched controls undergoing cataract surgery were
enrolled in the present study between July 1 and December 31, 2016.
The present study was approved by the Ethics Committee of the First
Affiliated Hospital of Xi'an Jiao-tong University (Xi'an, China),
and patients provided written informed consent. Patients with nAMD
were included in the study group if they met the following criteria
at their initial visit to the department: Active choroidal
neovascularization secondary to AMD, confirmed by spectral domain
optical coherence tomography and fluorescein angiography.
Patients were excluded from the study if they met
any of the following criteria: Previous treatment for nAMD,
including antiangiogenic medications such as bevacizumab,
ranibizumab or pegaptanib, laser photocoagulation or vitrectomy;
they had diabetes mellitus, diabetic retinopathy or iris rubeosis;
they had a history of ocular inflammation and vitreoretinal
diseases; and/or they had a recent myocardial infarction, cerebral
vascular accident or malignant hypertension. Control subjects were
included if they were scheduled to have cataract surgery, did not
have a history of hypertension or diabetes mellitus, and had no
retinal vascular diseases, as determined by comprehensive
ophthalmic examinations by retinal specialists.
Collection of aqueous humor samples
Aqueous humor samples were obtained at the beginning
of cataract surgery from the control patients and immediately prior
to intravitreal bevacizumab injections from patients with nAMD. All
sample collections and intravitreal injections were performed using
standard sterile methods. Prior to intravitreal bevacizumab
injection or cataract surgery, undiluted aqueous humor samples
(0.1-0.2 ml) were obtained by anterior-chamber paracentesis using a
30-gauge needle, with no complications. The samples were placed
immediately into safe-lock microcentrifuge tubes (1.5 ml) and were
stored at −80°C prior to analysis.
Measurement of BMP-6 levels using
ELISA
Aqueous BMP-6 levels were measured by ELISA using a
commercially available BMP-6 ELISA kit (#DY507; R&D Systems,
Inc., Minneapolis, MN, USA), according to the manufacturer's
protocol.
In vitro culture of RPE cells
ARPE-19 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA), and were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in an
atmosphere containing 5% CO2 and saturated humidity
(Sanyo, Osaka, Japan). The cells were subcultured by trypsinization
and were mechanically separated by pipetting, in order to prepare
single-cell suspensions.
Experimental interventions
An oxidative stress model was generated via exposure
of RPE cells to H2O2. Briefly, RPE cells were
adherently cultured in poly-D-lysine (PDL)-coated 96-well cell
culture plates. Subsequently, the RPE cells (5,000 cells/well) were
incubated with a series of H2O2 solutions of
various concentrations (10, 50, 100, 200 and 500 µM) for 3,
6 and 12 h at 37°C. Cell viability was examined using a Cell
Counting Kit-8 (CCK-8) assay, in order to determine the appropriate
H2O2 concentration and treatment duration,
which were 200 µM H2O2 and 6 h. To
evaluate the ability of BMP-6 to protect cells from
H2O2-induced cell damage, RPE cells were
pretreated with various BMP-6 concentrations (0, 1, 10, 50, 100 and
200 ng/ml; R&D Systems, Inc.) for 1 h. Subsequently, the cells
were treated with 200 µM H2O2 for 6 h.
Cell viability and apoptosis were examined using the CCK-8 method
and terminal deoxynucleotidyl transferase dUTP nick-end labeling
(TUNEL) staining, respectively. The expression levels of the
apoptotic proteins Bcl-2, Bax and pro-caspase-3 were analyzed by
western blotting. In addition, the BMP-6 antagonist noggin (Abcam,
Cambridge, UK) was used to assess the ability of BMP-6 to protect
RPE cells. Specifically, 3 µg/ml noggin was added to the
culture medium, and BMP-6 (100 ng/ml) was added to the culture
medium after 1 h. After BMP-6 treatment for 1 h, 200 µM
H2O2 was added. Following 6 h of
H2O2 treatment, TUNEL staining was performed
to analyze the apoptotic status of the cell groups. To examine the
role of JNK and p38 MAPK signaling in BMP-6-mediated protection of
oxidative stress-injured RPE cells, the cells were pretreated with
the p38-specific antagonist SB203580 (10 µM; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), the JNK-specific antagonist
SP600125 (10 µM; Sigma-Aldrich; Merck KGaA), BMP-6 (100
ng/ml) or an equal volume of normal saline for 1 h. Subsequently,
200 µM H2O2 was added to all groups,
with the exception of the control group. After 6 h of
H2O2 treatment, p38 and JNK expression were
measured by western blotting. All experiments were repeated three
times.
Measurement of cell viability
The viability of ARPE-19 cells was measured using
the CCK-8 assay kit (Sigma-Aldrich; Merck KGaA). Briefly, RPE cells
were seeded into 96-well plates at a density of 1×104
cells/well. After overnight adherent growth, the cells were divided
into the various experimental groups, and were treated and
incubated accordingly. Subsequently, CCK-8 reagent was added to all
wells containing cells, and the cells were incubated at 37°C for 2
h. The absorbance was measured at 450 nm using a microplate reader
(Model EL800; Bio-Tek Instruments, Inc., Winooski, VT, USA), and
the results were statistically analyzed.
TUNEL staining
The apoptotic rate of RPE cells was measured by
TUNEL staining. Briefly, adherent cultured RPE cells were treated
according to the experimental requirements, and were then fixed in
4% paraformaldehyde at room temperature for 30 min. Subsequently,
cells were washed three times with 0.01 M PBS (5 min/wash) and were
stained with TUNEL reagent, according to the manufacturer's
protocol. Briefly, at the end of each treatment, the cells were
fixed in 4% paraformaldehyde in PBS for 20 min at room temperature.
Following permeabilization using 0.1% Triton X-100 in 0.1% sodium
citrate for 2 min on ice, the cells were incubated with 50 ml TUNEL
reaction mixture for 1 h at 37°C. Cells were further counterstained
with DAPI (1 mg/ml) prior to mounting. After TUNEL staining, the
cells were counterstained with DAPI for 5 min and washed three
times with 0.01 M PBS (5 min/wash). The cells were then mounted
with the anti-quenching mounting medium Fluoroshield, after which
cells were examined and images were captured under a fluorescence
microscope. The TUNEL staining results were statistically
analyzed.
Western blot analysis
ARPE-19 cells were seeded into 6-well plates at a
density of 1×106 cells/well. Following the relevant
treatments, cells were washed with serum-free DMEM and harvested
with 10% FBS-containing DMEM. The protein expression levels of
Bcl-2, Bax, pro-caspase-3, p38 MAPK and JNK were examined by
western blotting. β-actin was used as the internal reference. The
relative Bcl-2, Bax, pro-caspase-3, p38 MAPK and JNK protein
expression levels were calculated and normalized to β-actin, which
was used as an internal control. To extract total proteins, the
cells were lysed using tissue lysis buffer [50 mM Tris-HCl (pH
7.6), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.5%
sodium deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, 50 mM
sodium fluoride, 20 mM Na4P2O7 and
10% glycerol], and the concentrations of the protein samples were
determined using the bicinchoninic acid protein quantification
assay, based on established standard curves. An equal amount of
protein from each sample (30 µg) was mixed with loading
buffer and denatured by heating at 97°C for 6 min. After cooling,
the proteins were separated by 12% SDS-PAGE and were then
transferred from the separating gels onto polyvinylidene fluoride
(PVDF) membranes using transfer apparatus. The PVDF membranes were
blocked with blocking solution [Tris-buffered saline-Tween 20
(TBST) + 50 g/l skim milk powder] at room temperature for 30 min,
and were then incubated with following primary antibodies:
Anti-caspase-3 (1:1,000; #9662), anti-Bcl-2 (1:1,000; #3498),
anti-Bax (1:1,000; #2774), anti-phosphorylated (p)-JNK2 (1:2,000;
#4668), anti-JNK2 (1:1,000; #4672), anti-p-p38 (1:1,000; #4511),
anti-p38 (1:2,000; #9212) and anti-β-actin (1:25,00; #3700) (all
Cell Signaling Technology, Inc., Danvers, MA, USA). Subsequently,
the membranes were washed three times with 1X TBST for 15, 5 and 5
min successively, and were incubated with appropriately diluted
anti-rabbit or anti-mouse immunoglobulin G secondary antibodies
(both 1:100,000; #A0545 and #A9044; Sigma-Aldrich; Merck KGaA) at
room temperature for 1 h. The membranes were then washed again with
1X TBST (three times for 15, 5 and 5 min) and developed using an
enhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific,
Inc.). Western blotting results were semi-quantitatively analyzed
using the gray-scale feature in ImageJ 3.5 software (National
Institutes of Health, Bethesda, MD, USA). Western blotting was
repeated three times, and each replicate yielded similar
results.
Statistical analysis
Quantitative data are presented as the means ±
standard deviation. Data were analyzed using SPSS 13.0 software
(SPSS Inc., Chicago, IL, USA). Differences between the study group
and the control group were estimated using a nonparametric
Mann-Whitney rank sum test and independent t-test when appropriate.
Fisher's exact test was used to compare noncontinuous variables.
Cell viability, apoptosis and western blotting data were compared
between different groups with one-way analysis of variance followed
by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between nAMD and aqueous
BMP-6 levels
The study population consisted of 30 patients and 20
control subjects. The clinical characteristics of the two groups
are summarized in Table I. The
nAMD and control groups had a mean age of 68.63±8.63 (range, 45-74)
and 71.05±10.73 (range, 46-75) years, respectively. In addition, 60
(18/30) and 45% (9/20) of patients were female in the nAMD and
control groups, respectively. Age and intraocular pressure were
normally distributed in both groups. The two groups did not differ
significantly in terms of age or sex distribution. The mean aqueous
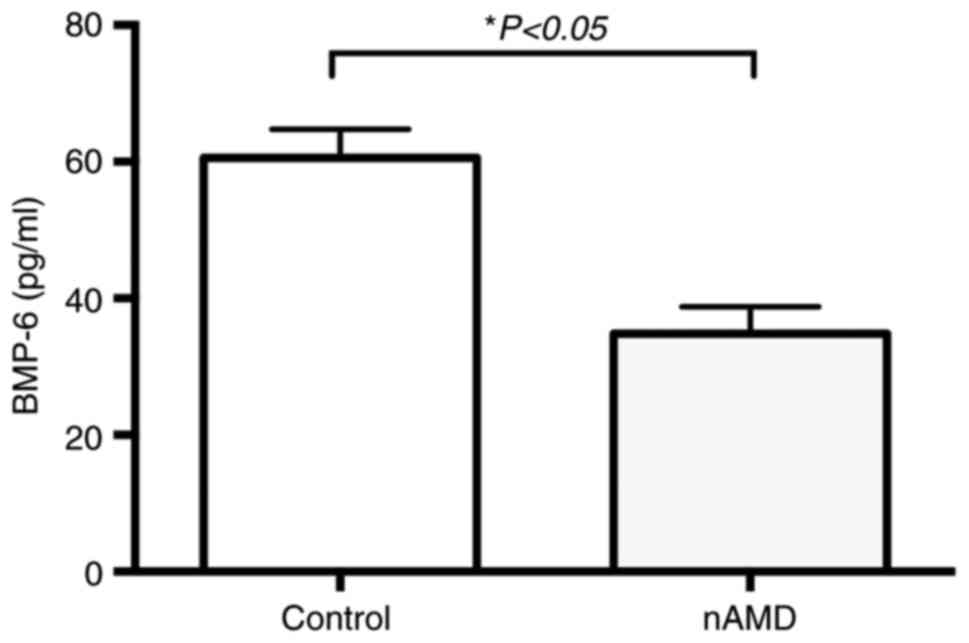
BMP-6 levels were 34.82±3.91 and 60.58±4.21 pg/ml in the nAMD and
control groups, respectively (P<0.01; Fig. 1).
 | Table IComparison between the nAMD and
control groups. |
Table I
Comparison between the nAMD and
control groups.
| Variable | nAMD (n=30) | Control (n=20) | P-value |
|---|
| Age, years | 68.63±8.63 | 71.05±10.73 | 0.281a |
| Sex (female %) | 18 (60%) | 9 (45%) | 0.388b |
| Baseline visual
acuity, LogMAR | 1.15±0.80 | 0.75±0.43 | 0.035a |
| IOP, mmHg | 13.70±2.63 | 15.05±3.34 | 0.182a |
BMP-6 attenuates the
H2O2-induced decrease in RPE cell
viability
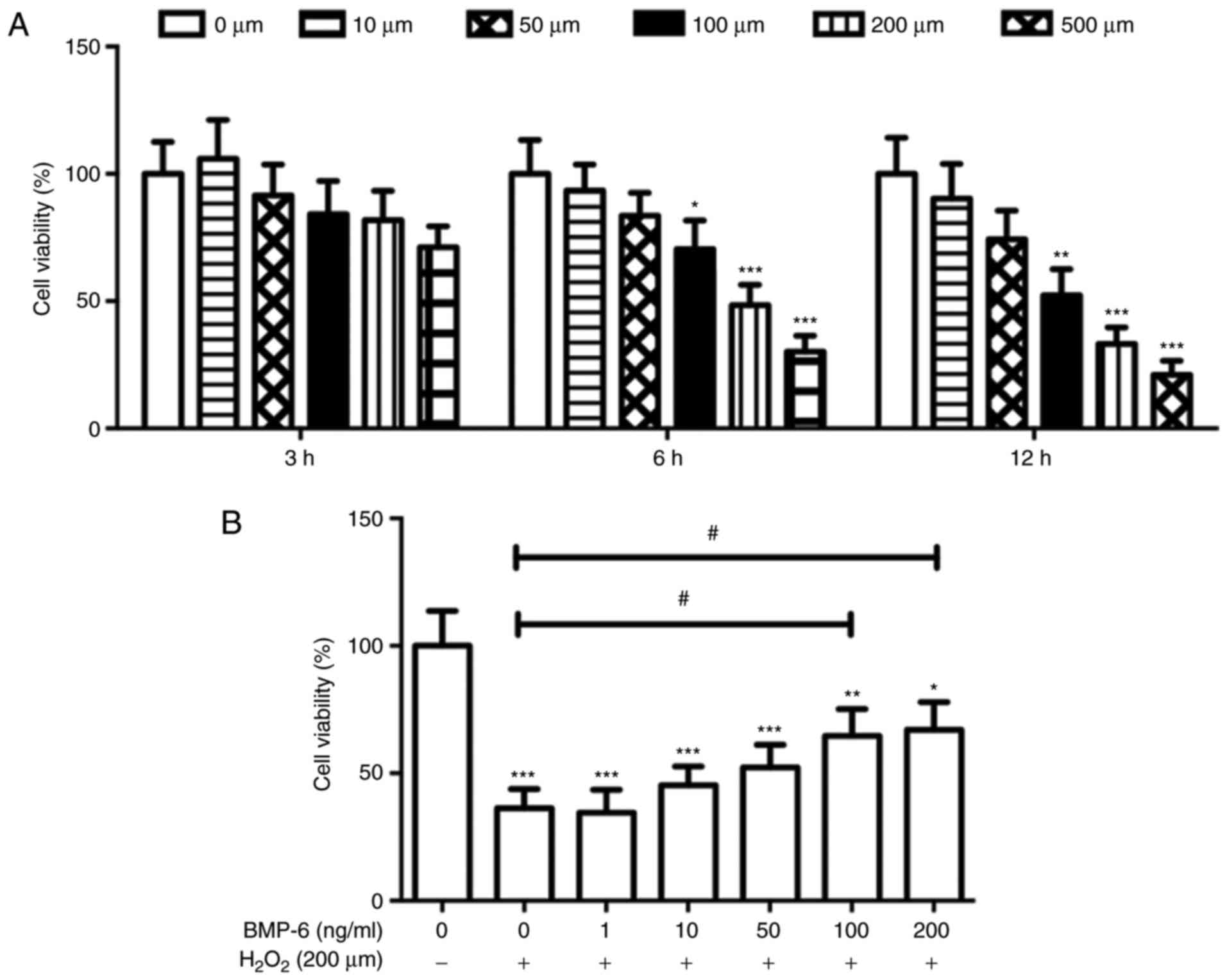
In the present study, cultured RPE cells were
treated with a specific H2O2 concentration to
establish an RPE cell model of oxidative stress injury. RPE cell
viability was inversely correlated with H2O2
concentration and treatment duration. In particular, treatment with
200 and 500 µM H2O2 for 6 and 12 h
decreased the viability of RPE cells by >50% compared with in
the control group (Fig. 2A).
Based on these results and the requirements of the subsequent
experimental treatments, a concentration of 200 µM
H2O2 and treatment duration of 6 h were
selected for subsequent experiments. To evaluate the ability of
BMP-6 to protect cells from H2O2-induced
damage, RPE cells were pretreated with various concentrations of
BMP-6 (0, 1, 10, 50, 100 and 200 ng/ml) for 1 h. Following BMP-6
treatment, the cells were treated with 200 µM
H2O2 for 6 h. Pretreatment with BMP-6
attenuated H2O2-induced oxidative damage in
RPE cells in a concentration-dependent manner (Fig. 2B). Therefore, 100 ng/ml BMP-6 was
selected for subsequent experiments.
Effects of BMP-6 on the expression of
apoptotic proteins in RPE cells following
H2O2-mediated oxidative stress injury
Alterations in the expression of apoptosis-related
proteins following BMP-6 pretreatment in RPE cells exposed to
oxidative stress injury were examined by western blotting. Briefly,
the cells were treated with various BMP-6 concentrations (0, 5, 10,
50, 100 and 200 ng/ml), and were then subjected to western blotting
to analyze the expression levels of pro-caspase-3, Bcl-2 and Bax.
Compared with in the blank control group, the protein expression
levels of pro-caspase-3 and Bcl-2 were markedly decreased, whereas
the protein levels of Bax were significantly increased following
treatment with H2O2. Compared with in cells
exposed to H2O2 only, cells pretreated with
various BMP-6 concentrations and exposed to 200 µM
H2O2 exhibited a concentration-dependent
increase in pro-caspase-3 and Bcl-2 protein levels, and a
concentration-dependent decrease in Bax protein levels (Fig. 3). These results suggested that
pretreatment with BMP-6 may inhibit the activation of
apoptosis-associated proteins; thereby protecting RPE cells from
oxidative stress injury.
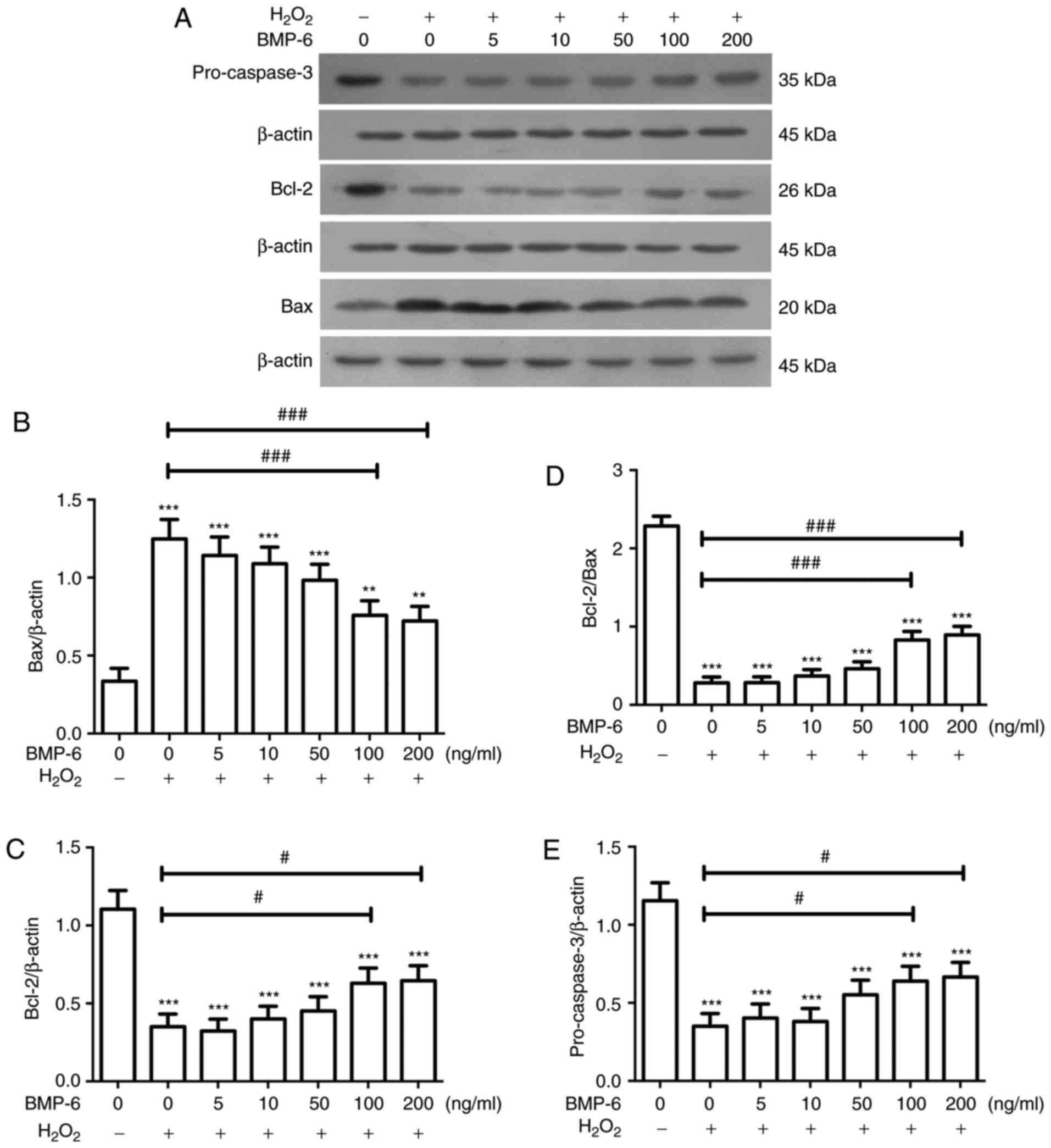 | Figure 3Effects of BMP-6 treatment on the
expression levels of apoptotic proteins in RPE cells following
H2O2-mediated oxidative stress injury. (A)
Western blot images showing the protein levels of pro-caspase-3,
Bcl-2 and Bax; β-actin was used as an internal reference. (B-E)
Expression levels of pro-caspase-3, Bcl-2 and Bax were normalized
to β-actin, and alterations in the ratio of Bcl-2 to Bax expression
were determined. Data are presented as the means ± standard
deviation. *P<0.05, **P<0.01 and
***P<0.001 vs. the blank control group.
#P<0.01, ##P<0.01 and
###P<0.001 vs. cells exposed to 200 µM
H2O2 only. Bax, Bcl-2-associated X protein;
Bcl-2, B-cell lymphoma 2; BMP-6, bone morphogenetic protein 6;
H2O2, hydrogen peroxide; RPE, retinal pigment
epithelial. |
H2O2-induced
apoptosis and the ability of BMP-6 to attenuate
H2O2-induced apoptosis based on TUNEL
staining
To confirm that BMP-6 protected RPE cells from
H2O2-induced injury, cells were also treated
with the BMP-6 antagonist noggin. Noggin (3 µg/ml) was added
to the culture medium and BMP-6 (100 ng/l) was added to the culture
medium after 1 h. After 1 h of BMP-6 treatment, cells were exposed
to 200 µM H2O2 for 6 h. TUNEL staining
was performed to examine apoptosis in each cell group (Fig. 4). The percentage of TUNEL-positive
cells exceeded 50% in the 200 µM H2O2
group, and was significantly increased compared with the percentage
in the control group. The number of TUNEL-positive cells was
markedly decreased in the BMP-6 group. Conversely, blocking the
effects of BMP-6 using the BMP-6 antagonist noggin increased the
number of TUNEL-positive cells, compared with in the
H2O2 + BMP-6 group (Fig. 4). These findings suggested that
BMP-6 may protect RPE cells from H2O2-induced
damage.
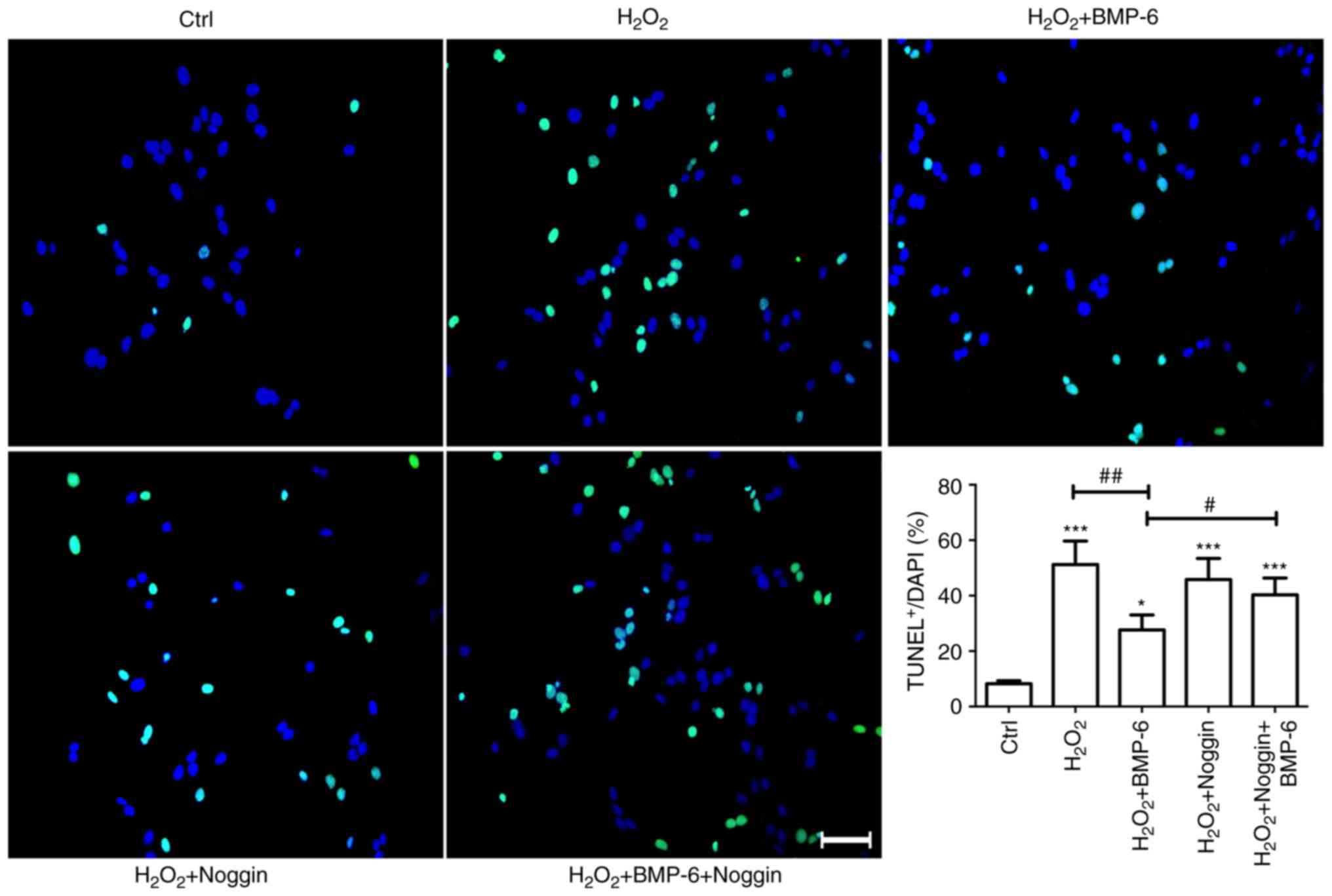 | Figure 4Effects of BMP-6 pretreatment on
apoptosis of RPE cells induced by
H2O2-mediated oxidative stress injury.
Adherently cultured RPE cells were treated with normal saline
(Ctrl), H2O2 (30 µM), BMP-6 (100
ng/ml) or noggin (3 µg/ml) + BMP-6 (100 ng/ml).
Subsequently, the cells were treated with
H2O2 (200 µM) for 6 h. Subsequently,
TUNEL staining was performed to examine apoptosis. Green staining
indicates TUNEL-positive cells, whereas nuclei labeled with DAPI
display bright blue fluorescence (magnification, ×40). Scale bar,
50 µm. The number of TUNEL-stained cells was statistically
analyzed. *P<0.05 and ***P<0.001 vs.
the Ctrl group. #P<0.01 vs. the
H2O2 + BMP-6 group. ##P<0.01
vs. cells exposed to 200 µM H2O2 only.
BMP-6, bone morphogenetic protein 6; Ctrl, control;
H2O2, hydrogen peroxide; RPE, retinal pigment
epithelial; TUNEL, terminal deoxynucleotidyl transferase dUTP
nick-end labeling. |
BMP-6 protects RPE cells by inhibiting
p38 and JNK signaling
To identify the intracellular signaling pathways
involved in regulation of the protective effects of BMP-6, the
present study examined the JNK and p38 MAPK signaling pathways.
Previous studies demonstrated that the JNK and p38 signaling
pathways may have key roles in neuroprotection, inflammatory
regulation and the oxidative stress response (8-10).
RPE cells were treated with various BMP-6 concentrations (0, 1, 5,
10, 50, 100 and 200 ng/ml) for 1 h. All groups were then exposed to
H2O2 (200 µM) for 6 h, and proteins
were and subjected to western blotting to determine the expression
levels of p-p38 and p-JNK2. Compared with in the
H2O2-treated group, BMP-6 reduced the
activity of p-p38 and p-JNK2 in a concentration-dependent manner
(Fig. 5A–C). To confirm that
BMP-6 inhibited p38 and JNK2 signaling, cells were treated with
noggin. Briefly, noggin was added to the culture medium at a
concentration of 3 µg/ml. After 1 h, BMP-6 (100 ng/ml) was
added to the culture medium for 1 h. Subsequently, 200 µM
H2O2 was added to the culture medium. After 6
h of H2O2 treatment, western blotting was
performed to examine p38 and JNK2 phosphorylation levels. The
results demonstrated that noggin suppressed BMP-6-mediated
inhibition of p38 and JNK2 activity (Fig. 5D–F).
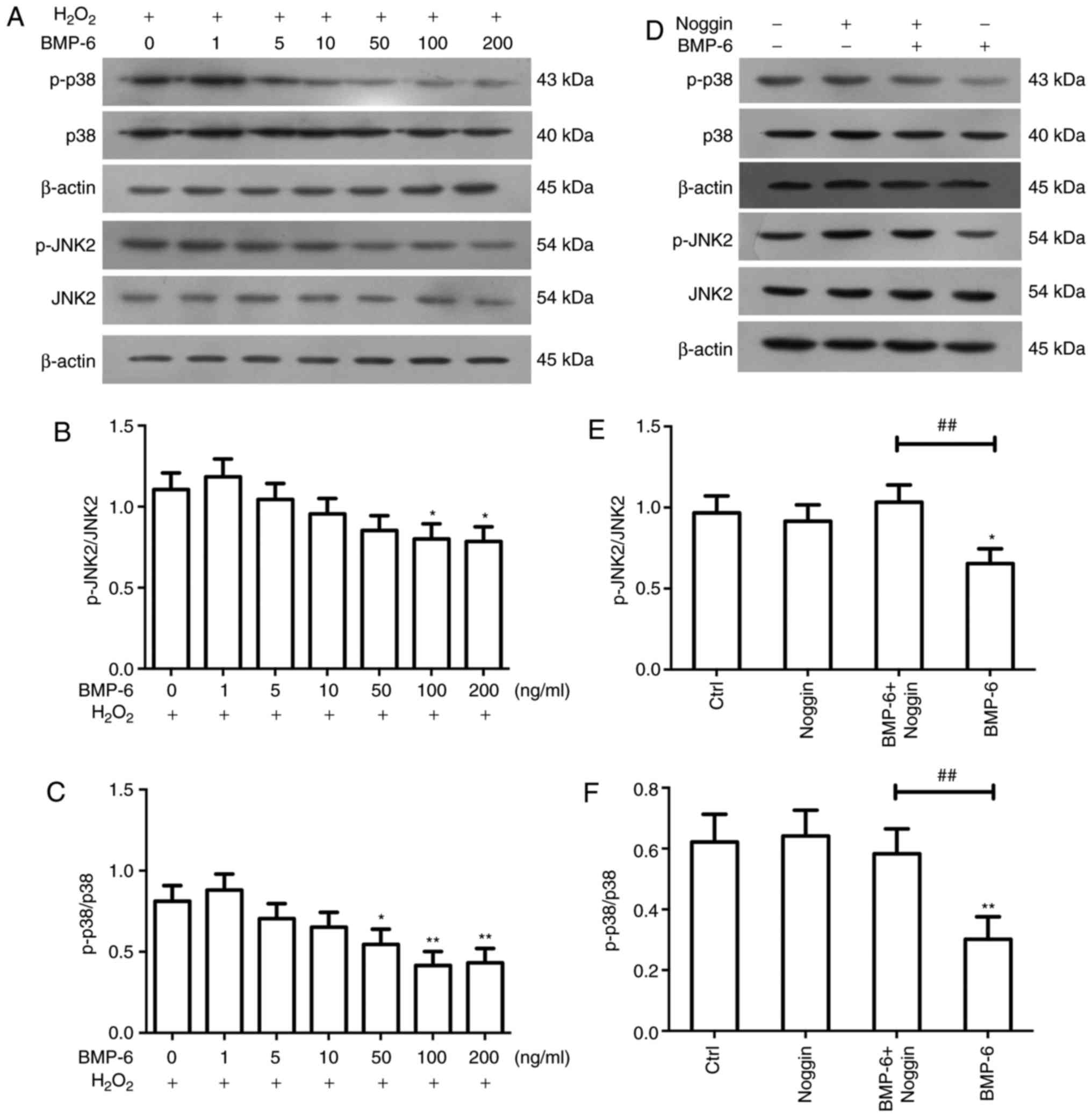 | Figure 5Effects of BMP-6 on JNK and p38
signaling in RPE cells following
H2O2-mediated oxidative stress injury. RPE
cells were pretreated with various BMP-6 concentrations and were
then treated with 200 µM H2O2 for 6 h.
(A) Subsequently, western blotting was used to determine the
expression levels of p-p38, p38, p-JNK and JNK2. β-actin was used
as an internal reference. (B and C) Alterations in the ratios of
p-p38/p38 and p-JNK2/JNK2 were determined. Data are presented as
the means ± standard deviation. *P<0.05 and
**P<0.01 vs. cells exposed to 200 µM
H2O2 only. (D) RPE cells were pretreated with
the BMP-6 inhibitor noggin and were then treated with BMP-6.
Western blotting was used to detect the expression levels of p-p38,
p38, p-JNK and JNK2. β-actin was used as an internal reference. (E
and F) Alterations in the ratios of p-p38/p38 and p-JNK2/JNK2 were
determined. Data are presented as the means ± standard deviation.
*P<0.05 and **P<0.01 vs. the Ctrl
group. ##P<0.01, vs. the BMP-6-treated group. BMP-6,
bone morphogenetic protein 6; Ctrl, control;
H2O2, hydrogen peroxide; JNK2, c-Jun
N-terminal protein kinase 2; p-, phosphorylated; RPE, retinal
pigment epithelial. |
Confirmation that BMP-6 affects the p38
and JNK pathways
The present study indicated that
H2O2 may activate the p38 and JNK pathways,
whereas BMP-6 inhibited these pathways. These findings suggested
that BMP-6 may exert its protective effect by inhibiting the
activities of the p38 and JNK pathways. Subsequently, the present
study examined whether p38 and JNK inhibition could protect RPE
cells against H2O2-mediated oxidative stress
injury. Compared with in the group treated with
H2O2 only, SB203580 and SP600125 reduced
pro-caspase-3 expression (Fig. 6A
and B), and the number of TUNEL-positive cells (Fig. 6C). Based on these results, BMP-6
may protect RPE cells from H2O2-induced
oxidative stress injury by inhibiting p38 and JNK signaling.
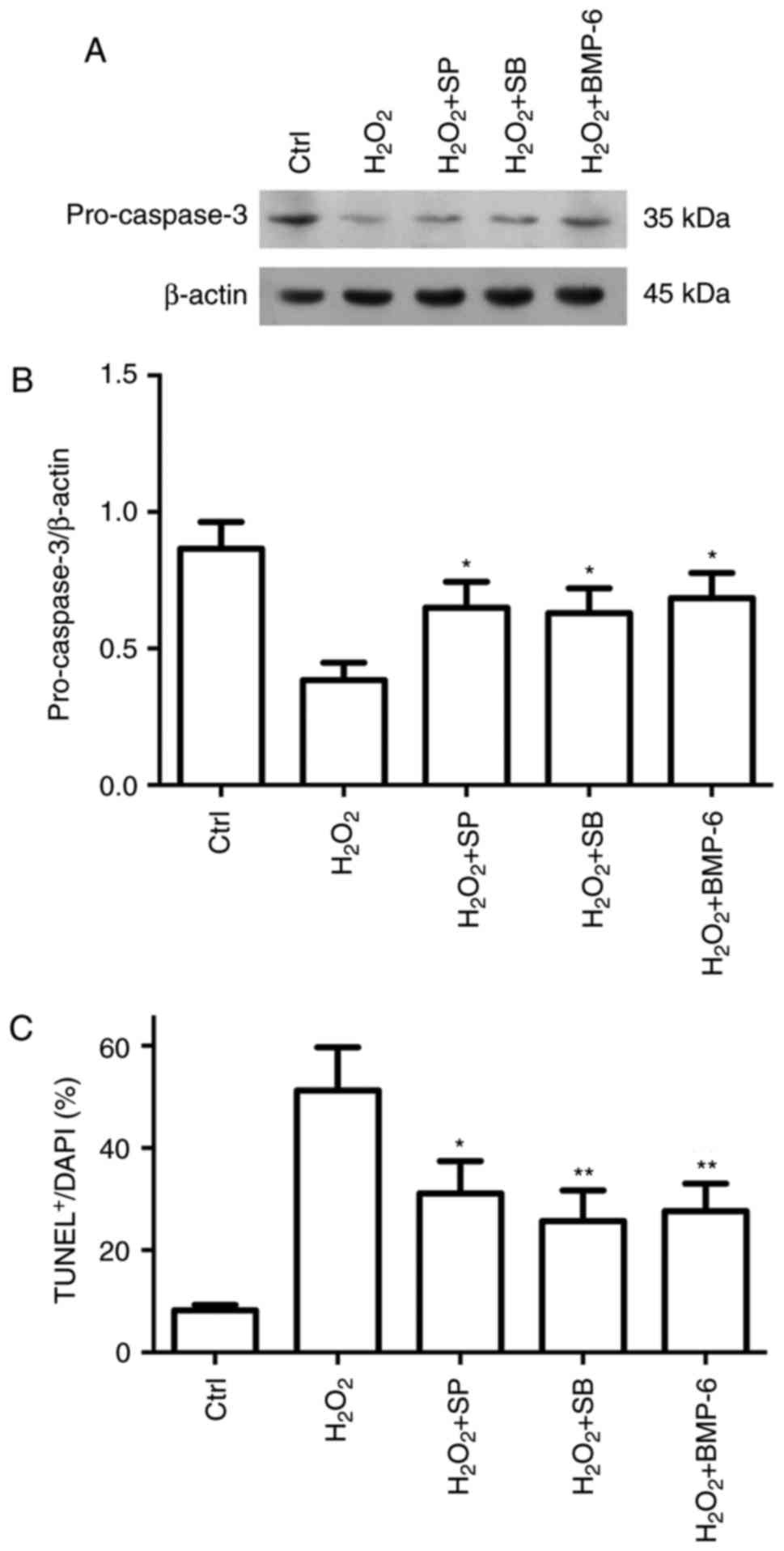 | Figure 6Protective effect of BMP-6 on
H2O2-induced RPE cell apoptosis is involved
in the inactivation of JNK and p38 MAPK pathways. Adherently
cultured RPE cells were treated with normal saline (Ctrl),
H2O2 (200 µM), SB +
H2O2, SP + H2O2 or
BMP-6 + H2O2. After 6 h of treatment, western
blotting was performed. (A and B) Pro-caspase-3 protein expression
was determined by western blotting and was normalized to β-actin.
(C) Apoptosis was examined by TUNEL staining, and the results were
statistically analyzed. *P<0.05 and
**P<0.01 vs. cells exposed to 200 µM
H2O2 only. BMP-6, bone morphogenetic protein
6; Ctrl, control; H2O2, hydrogen peroxide;
RPE, retinal pigment epithelial; SB, SB203580; SP, SP600125; TUNEL,
terminal deoxynucleotidyl transferase dUTP nick-end labeling. |
Discussion
To the best of our knowledge, the present study is
the first to report that BMP-6 expression was markedly decreased in
the aqueous humors of patients with nAMD. BMPs are a group of
highly conserved functional proteins with similar structures that
were originally identified to induce bone and cartilage formation
in vivo. In particular, BMPs are widely distributed in
various human tissues and cells, where they regulate the growth,
differentiation and apoptosis of target cells, and consequently
serve important roles in numerous processes, including embryonic
growth and development, wound healing, and tumor development and
progression (11). BMP-6 has
unique structural and functional characteristics, and its function
is fairly complex. It participates in the regulation of various
metabolic processes in vivo. Notably, BMP-6 not only has an
important role in the maintenance of physiological functions, but
also mediates the occurrence of many diseases (12). RPE cells express BMP-6, and
oxidative stress downregulates BMP-6 (13). In addition, BMP-6 expression is
markedly decreased in RPE cells isolated from the eyes of cadavers
with early macular degeneration (14). These results indicated that BMP-6
may have a key role in the development of AMD.
To the best of our knowledge, the present study was
also the first to demonstrate that BMP-6 protected human RPE cells
from oxidative stress injury, as reflected by a decrease in the
numbers of TUNEL-positive cells, a reduction in the expression of
the apoptotic signaling protein pro-caspase-3, and an increase in
the Bcl-2/Bax ratio. Further investigations revealed that BMP-6
inhibited the H2O2-induced increases in p-JNK
and p-p38 expression, and that inhibition of JNK and p38 activation
protected RPE cells from oxidative stress injury. These findings
suggested that BMP-6 may protect RPE cells by inhibiting activation
of JNK and p38 signaling.
At present, the pathogenesis of AMD remains unclear;
however, it has been hypothesized to be associated with oxidative
stress and inflammation. Due to high oxygen consumption, richness
in polyunsaturated fatty acids and exposure to light, retinal
tissues are highly susceptible to oxidative stress (15). H2O2 is an
important reactive oxygen species (ROS), and exogenous
H2O2 easily penetrates the cell membrane and
enters the cell. If transition metals are present in the cell,
H2O2 is converted to highly reactive free
radicals via the Fenton reaction, and the resultant free radicals,
including singlet oxygen and hydroxyl free radicals, cause
additional cellular damage. Since H2O2 can
rapidly penetrate the cell membrane, it is considered a good
experimental model to explore oxidative stress-induced cell injury
(16). Among all of the cell
types in the retina, RPE cells are the most prone to oxidative
stress injury due to their anatomical location proximal to the
choroid (17). In the present
study, H2O2 was used to produce ROS, which
damage RPE cells. H2O2 not only reduced cell
viability, but also induced apoptosis. These findings are
consistent with the results of a previous study (18).
H2O2-mediated apoptosis is a
mixed response involving numerous apoptotic pathways, which
includes activation of the caspase pathways and dysregulation of
the Bcl-2/Bax balance (19,20). Caspase-3 is a key regulatory
protein in the caspase-dependent apoptotic pathway (21,22), which can be activated by
caspase-8/9. Caspase family proteases are downstream targets of Bax
and Bcl-2 in the mitochondrial apoptosis signaling pathway
(23,24). Mitochondrial membrane destruction
not only downregulates the expression of the apoptosis inhibitor
Bcl-2, but also results in the accumulation of Bax and the
formation of Bax oligomers. Notably, Bax mediates apoptosis
(25,26). In the present study,
H2O2 stimulation decreased procaspase-3 and
Bcl-2 levels, and increased Bax expression levels. These results
indicated that these apoptotic signaling proteins may mediate
oxidative stress injury-induced apoptosis of RPE cells, which is
consistent with the findings of previous studies (27,28). Furthermore, the present study
revealed that BMP-6 reversed the H2O2-induced
reduction of pro-caspase-3, and maintained the balance between
Bcl-2 and Bax in vitro. Therefore, BMP-6 may protect RPE
cells from H2O2-mediated oxidative stress
injury.
As key regulatory factors in intracellular
signaling, JNK and p38 MAPK participate in the regulation of
numerous stress responses, including ionizing radiation, the
inflammatory response, ischemia and hemorrhage (29,30). Previous studies have reported that
JNK and p38 activation are associated with oxidative stress-induced
cell death in various cell types, including RPE cells (9,10).
The present study revealed that oxidative stress injury induced
apoptosis. Notably, H2O2 activated JNK and
p38, which triggered the caspase cascade and eventually led to cell
death. Previous studies have reported that inhibiting JNK and p38
activation protects cells against oxidative stress injury (31,32). In the present study, BMP-6
inhibited activation of the JNK and p38 pathways. Noggin is a
specific inhibitor of BMP-6 (33,34); treatment with noggin inhibited the
effects of BMP-6 on JNK and p38 in the present study. Although
possible synergy between these pathways upon oxidative
stress-induced injury was not examined in this study, a large
number of studies demonstrated that injuries may simultaneously
activate the JNK and p38 pathways (35,36). The present study revealed that
BMP-6 not only inhibited the H2O2-induced
activation of JNK and p38 MAPK signaling pathways in vitro,
but also inhibited caspase-3 activation and reduced apoptosis.
These results indicated that BMP-6 may protect RPE cells by
inhibiting JNK and p38 activity.
Overall, the present study demonstrated that BMP-6
attenuated H2O2-induced oxidative damage in
human RPE cells, as evidenced by changes in cell viability,
apoptosis, caspase-3 activity and MAPK signaling. In addition, the
present study suggested that the protective effects of BMP-6 were
mediated by inhibition of the JNK/p38 signaling pathways, and
subsequent suppression of caspase-3 activity. Furthermore, BMP-6
dysregulated the Bcl-2/Bax balance, thereby inhibiting apoptosis of
RPE cells. Although these findings suggested that BMP-6 may protect
RPE cells from oxidative stress injury by regulating JNK/p38
phosphorylation, this conclusion is based on in vitro
experiments.
The present study was also subject to other
limitations. Ki et al reported that pathways involving JNK
and p38 MAPK, but not extracellular signal-regulated kinase
(ERK)1/2, mediated apoptosis and were involved in the inflammatory
response (9). The present study
did not observe alterations in ERK signaling. ERK1/2 and p38 MAPK
are generally phosphorylated together in response to numerous types
of stress, including transforming growth factor-β, oxidative and
inflammatory stress. We aim to further analyze this in the future.
Zhang et al reported that numerous types of BMP receptor
(BMPR), including BMPR1A, BMPR1B and BMPR2, were expressed in the
retina and choroid (37).
Hemojuvelin (HJV) is an iron-regulatory protein and a BMP
coreceptor; HJV−/− mice have been reported to accumulate
excess iron in the retina, and exhibit aberrant vascularization and
retinal hemangiomas (38). BMP-6
binds to BMP receptor type I (BMPRI) and BMPRII cell surface
receptors, and to the coreceptor HJV (39). Arjunan et al revealed that
G-protein-coupled receptor 91 is a target of BMP-6 and HJV deletion
enhances BMP signaling in the retina (40). Further studies should investigate
the BMP-6 receptor and the BMP-6-mediated signaling pathways,
including HJV receptor and protein kinase B pathways. In addition,
the protective effects of BMP-6 on RPE cells require further
exploration in vivo.
In conclusion, the present study demonstrated that
BMP-6 may protect RPE cells from oxidative stress injury to a
certain extent, which may be associated with alterations in the
MAPK signaling pathway. This study may prove useful for the
development of more effective therapies to prevent the development
of nAMD.
Funding
The present study was supported by grants from the
Science and Technology Development Project of Shaanxi Province
(grant nos. 2012K16-11-01 and 2016SF-257) and the China
postdoctoral science foundation (grant no. 2017M623152).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC and YoL conceived and designed the study. LC,
YaL, YiL, ZZ and BM performed the experiments. ML and XL collected
the patient data and conducted the statistical analysis. LC and ML
wrote the study. LC, ZZ and YoL reviewed and edited the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol for this research project was approved
by the Ethics Committee of the First Affiliated Hospital of Xi'an
Jiao-tong University, Xi'an, Shaanxi, China, and patients provided
written informed consent.
Consent for publication
Patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
nAMD
|
neovascular age-related macular
degeneration
|
|
BMP-6
|
bone morphogenetic protein 6
|
|
RPE
|
retinal pigment epithelial
|
|
H2O2
|
hydrogen peroxide
|
|
JNK
|
c-Jun N-terminal protein kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bax
|
Bcl-2-associated X protein
|
Acknowledgments
Not applicable.
References
|
1
|
Klettner A: Age-related macular
degeneration-biology and treatment. Med Monatsschr Pharm.
38:258–66. 2015.In German.
|
|
2
|
Liu C, Cao L, Yang S, Xu L, Liu P, Wang F
and Xu D: Subretinal injection of amyloid-β peptide accelerates RPE
cell senescence and retinal degeneration. Int J Mol Med. 35:169–76.
2015. View Article : Google Scholar
|
|
3
|
Zhu Y, Zhang L, Lu Q, Gao Y, Cai Y, Sui A,
Su T, Shen X and Xie B: Identification of different macrophage
subpopulations with distinct activities in a mouse model of
oxygen-induced retinopathy. Int J Mol Med. 40:281–292. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hadziahmetovic M, Song Y, Wolkow N,
Iacovelli J, Kautz J, Roth MP and Dunaief JL: Bmp6 regulates
retinal iron homeostasis and has altered expression in age-related
macular degeneration. Am J Pathol. 179:335–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andriopoulos B Jr, Corradini E, Xia Y,
Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A,
Vukicevic S, Lin HY and Babitt JL: BMP6 is a key endogenous
regulator of hepcidin expression and iron metabolism. Nat Genet.
41:482–487. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haynes T, Gutierrez C, Aycinena JC, Tsonis
PA and Del Rio-Tsonis K: BMP signaling mediates stem/progenitor
cell-induced retina regeneration. Proc Natl Acad Sci USA.
104:20380–20385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Canali S, Zumbrennen-Bullough KB, Core AB,
Wang CY, Nairz M, Boulery R, Swirski FK and Babbit JL: Endothelial
cells produce bone morphogenetic protein 6 required for iron
homeostasis in mice. Blood. 129:405–414. 2017. View Article : Google Scholar :
|
|
8
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ki YW, Park JH, Lee JE, Shin IC and Koh
HC: JNK and p38 MAPK regulate oxidative stress and the inflammatory
response in chlorpyrifos-induced apoptosis. Toxicol Lett.
218:235–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feligioni M, Brambilla E, Camassa A, Sclip
A, Arnaboldi A, Morelli F, Antoniou X and Borsello T: Crosstalk
between JNK and SUMO signaling pathways: deSUMOylation is
protective against H2O2-induced cell injury.
PLoS One. 6:e281852011. View Article : Google Scholar
|
|
11
|
Shepherd TG, Heriault BL and Naehtigal MW:
Autocrine BMP4 signalling regulates ID3 Proto-oncogene expression
in human ovarian cancer cells. Gene. 414:95–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piubelli C, Castagna A, Marchi G, Rizzi M,
Busti F, Badar S, Marchetti M, De Gobbi M, Roetto A, Xumerle L, et
al: Identification of new BMP6 pro-peptide mutations in patients
with iron overload. Am J Hematol. 92:562–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan J, Yang S, Zhang J, Zhai C and Zhu T:
BMP6 attenuates oxidant injury in HK-2 cells via Smad-dependent
HO-1 induction. Free Radic Biol Med. 46:1275–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meynard D, Kautz L, Darnaud V,
Canonne-Hergaux F, Coppin H and Roth MP: Lack of the bone
morphogenetic protein BMP6 induces massive iron overload. Nat
Genet. 41:478–481. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhuto I and Luty G: Understanding
age-related macular degeneration (AMD): Relationships between the
photoreceptor/retinal pigment epithelium/Bruch's
membrane/choriocapillaris complex. Mol Aspects Med. 33:295–317.
2012. View Article : Google Scholar
|
|
16
|
Haudek VJ, Gundacker NC, Slany A,
Wimmersion H, Bayer E, Pablé K and Gerner C: Consequences of acute
and chronic oxidative stress upon the expression pattern of
proteins in peripheral blood mononuclear cells. J Proteome Res.
7:5138–5147. 2008. View Article : Google Scholar
|
|
17
|
Zhao L, Li Y, Song D, Song Y, Theurl M,
Wang C, Cwanger A, Su G and Dunaief JL: A high serum iron level
causes mouse retinal iron accumulation despite an intact
blood-retinal barrier. Am J Pathol. 184:2862–2867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tu G, Zhang YF, Wei W, Li L, Zhang Y, Yang
J and Xing Y: Allicin attenuates H2O2-induced
cytotoxicity in retinal pigmented epithelial cells by regulating
the levels of reactive oxygen species. Mol Med Rep. 13:2320–2326.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cia D, Vergaud-Gauduchon J, Jacquemot N
and Doly M: Epigallocatechin gallate (EGCG) prevents
H2O2-induced oxidative stress in primary rat
retinal pigment epithelial cells. Curr Eye Res. 39:944–952. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamm C, Zhivotovsky B and Ceccatelli S:
Caspase-2 activation in neural stem cells undergoing oxidative
stress-induced apoptosis. Apoptosis. 13:354–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Savory J, Rao JK, Huang Y, Letada PR and
Herman MM: Age-related hippocampal changes in Bcl-2: Bax ratio,
oxidative stress, redox-active iron and apoptosis associated with
aluminum-induced neurodegenerati: Increased susceptibility with
aging. Neurotoxicology. 20:805–817. 1999.PubMed/NCBI
|
|
22
|
Naderi J, Hung M and Pandey S: Oxidative
stress-induced apoptosis in dividing fibroblasts involves
activation of p38 MAP kinase and over-expression of Bax: Resistance
of quiescent cells to oxidative stress. Apoptosis. 8:91–100. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoshyar R, Bathaie SZ and Sadeghizadeh M:
Crocin triggers the apoptosis through increasing the Bax/Bcl-2
ratio and caspase activation in human gastric adenocarcinoma, AGS,
cells. DNA Cell Biol. 32:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosen RB, Hu DN, Chen M, McCormick SA,
Walsh J and Roberts JE: Effects of melatonin and its receptor
antagonist on retinal pigment epithelial cells against hydrogen
peroxide damage. Mol Vis. 18:1640–1648. 2012.PubMed/NCBI
|
|
26
|
Wang ZY, Shen LJ, Tu L, Hu DN, Liu GY,
Zhou ZL, Lin Y, Chen LH and Qu J: Erythropoietin protects retinal
pigment epithelial cells from oxidative damage. Free Radical Biol
Med. 46:1032–1041. 2009. View Article : Google Scholar
|
|
27
|
Paeng SH, Jung WK, Park WS, Lee DS, Kim
GY, Choi YH, Seo SK, Jang WH, Choi JS, Lee YM, et al: Caffeic acid
phenethyl ester reduces the secretion of vascular endothelial
growth factor through the inhibition of the ROS, PI3K and HIF-1α
signaling pathways in human retinal pigment epithelial cells under
hypoxic conditions. Int J Mol Med. 35:1419–1426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bokara KK, Kwon KH, Nho Y, Lee WT, Park KA
and Lee JE: Retroviral expression of arginine decarboxylase
attenuates oxidative burden in mouse cortical neural stem cells.
Stem Cells Dev. 20:527–537. 2011. View Article : Google Scholar
|
|
29
|
Sekine Y, Takeda K and Ichijo H: The
ASK1-MAP kinase signaling in ER stress and neurodegenerative
diseases. Curr Mol Med. 6:87–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamata H, Honda S, Maeda S, Chang L,
Hirata H and Karin M: Reactive oxygen species promote
TNFalpha-induced death and sustained JNK activation by inhibiting
MAP kinase phosphatases. Cell. 120:649–661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JH, Choi W, Lee JH, Jeon SJ, Choi YH,
Kim BW, Chang HI and Nam SW: Astaxanthin inhibits
H2O2-mediated apoptotic cell death in mouse
neural progenitor cells via modulation of P38 and MEK signaling
pathways. J Microbiol Biotechnol. 19:1355–1363. 2009.PubMed/NCBI
|
|
32
|
Wang C, Hao Z, Zhou J, Zhang J, Sun Y and
Liang C: Rutaecarpine alleviates renal ischemia reperfusion injury
in rats by suppressing the JNK/p38 MAPK signaling pathway and
interfering with the oxidative stress response. Mol Med Rep.
16:922–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuen HF, McCrudden CM, Grills C, Zhang SD,
Huang YH, Chan KK, Chan YP, Wong ML, Law S, Srivastava G, et al:
Combinatorial use of bone morphogenetic protein 6, noggin and SOST
significantly predicts cancer progression. Cancer Sci.
103:1145–1154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song K, Krause C, Shi S, Patterson M, Suto
R, Grgurevic L, Vukicevic S, van Dinther M, Falb D, Ten Dijke P and
Alaoui-Ismaili MH: Identification of a key residue mediating bone
morphogenetic protein (BMP)-6 resistance to noggin inhibition
allows for engineered BMPs with superior agonist activity. J Biol
Chem. 285:12169–12180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abell AN, Granger DA and Johnson GL: MEKK4
stimulation of p38 and JNK activity is negatively regulated by
GSK3beta. J Biol Chem. 282:30476–30484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun G, Li Z, Wang X, Tang W and Wei Y:
Modulation of MAPK and Akt signaling pathways in proximal segment
of injured sciatic nerves. Neurosci Lett. 534:205–210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Liu Y, Hang A, Phan E and
Wildsoet CF: Differential gene expression of BMP2 and BMP receptors
in chick retina & choroid induced by imposed optical defocus.
Vis Neurosci. 33:E0152016. View Article : Google Scholar
|
|
38
|
Tawfik A, Gnana-Prakasam JP, Smith SB and
Ganapathy V: Deletion of hemojuvelin, an iron-regulatory protein,
in mice results in abnormal angiogenesis and vasculogenesis in
retina along with reactive gliosis. Invest Ophthalmol Vis Sci.
55:3616–3625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bandyopadhyay A, Yadav PS and Prashar P:
BMP signaling in development and diseases: A pharmacological
perspective. Biochem Pharmacol. 85:857–864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arjunan P, Gnanaprakasam JP, Ananth S,
Romej MA, Rajalakshmi VK, Prasad PD, Martin PM, Gurusamy M,
Thangaraju M, Bhutia YD and Ganapathy V: Increased retinal
expression of the pro-angiogenic receptor GPR91 via BMP6 in a mouse
model of juvenile hemochromatosis. Invest Ophthalmol Vis Sci.
57:1612–1619. 2016. View Article : Google Scholar : PubMed/NCBI
|