Introduction
Myelodysplastic syndromes (MDS) are a group of
related clonal stem cell disorders characterized by ineffective
hema-topoiesis, peripheral blood cytopenia and an increased risk of
progression to acute myeloid leukemia (1). Despite aggressive treatments,
patients >60 years of age, or with secondary or therapy-related
acute myeloid leukemia have the poorest prognosis, and those with
high-risk MDS ineligible for allogeneic stem cell transplantation
have similar outcomes, predominantly due to high rates of relapse
(2). As previous studies have
shown that only 5-15% of patients with therapy-related
myelodysplasia or myeloid leukemia show long-term survival
(3), the identification of novel
effective agents with more benign toxicity profiles is required to
improve the survival rate of patients with MDS.
Natural dietary compounds obtained from fruits,
vegetables or herbs have been considered as potential
chemopreventive and chemotherapeutic agents due to their non-toxic
nature and usage for a long period of time without side effects
(4). In preclinical studies,
luteolin (3′,4′,5′,7′-tetrahydroxyflavone), a type of flavonoid
compound, has been reported to possess various pharmacological
properties, including anti-inflammatory, antioxidant and anticancer
activities (5-8), with anticancer activity having
attracted increased attention in research (9). There is accumulating evidence that
luteolin exerts its anticancer actions by affecting numerous
biochemical pathways, which are critical for the regulation of cell
proliferation, cell cycle arrest, apoptosis, angiogenesis, matrix
metalloproteinase and metastasis (10-13). Additionally, luteolin has been
demonstrated to inhibit the proliferation of leukemia cells and
other cells through a mitochondrial-dependent pathway mediated by
reactive oxygen species (ROS) (9,14–17). Currently, the effect of luteolin
on patients with MDS has not been addressed.
It is recognized that the induction of apoptosis of
cancer cells is one of the most important and direct approaches to
attenuate the development of cancer and eliminate tumors (18). Increasing evidence from multiple
studies has shown that cell apoptosis is closely associated with
the loss of mitochondrial inner membrane structure and integrity
(9,15). Mitochondria are central to the
intrinsic apoptotic pathway, which involves a cascade of molecular
events that occur entirely within the cell, and the increased ROS
generation that occurs during apoptosis. It is known that ROS are a
group of reactive, short-lived, oxygen-containing species,
including superoxide, hydrogen peroxide, hydroxyl radicals, singlet
oxygen, and peroxyl radicals (19). The overgeneration of ROS disrupts
the balance between oxidation and antioxidant defense systems and,
subsequently, it not only causes oxidative damage to proteins,
lipids and nucleic acids, but also leads to the mitochondrial
damage including collapse of the mitochondrial membrane potential
(ΔΨm) and complex IV inactivation, resulting in mitochondrial
dysfunction and consequent induction of cell apoptosis (20). Kittiratphatthana et al
(17) reported that luteolin
exerts its pro-apoptotic effect partly through generating
intracellular ROS, which then contributes to the induction of
mitochondria-mediated cell apoptosis. Others have indicated that
the overexpression of ROS may contribute to the apoptosis through
regulating B-cell lymphoma 2 (Bcl-2) family proteins, releasing
cytochrome c from the mitochondria, and activating effector
caspases, including caspase-3 and caspase-9 (21). Therefore, a promising strategy for
the prevention and treatment of MDS has emerged via inducing
apoptosis by mitochondria-mediated ROS generation.
In the present study, SKM-1 cells and primary bone
marrow (PBM) mononuclear cells from patients with intermediate- or
high- risk MDS were used as models to evaluate the
anti-proliferative effect of luteolin on MDS cells. Furthermore,
the possible molecular mechanism of luteolin-induced apoptosis in
SKM-1 cells was examined, including the p53-dependent mitochondrial
signaling pathway mediated by ROS and the expression of
apoptosis-related proteins.
Materials and methods
Reagents and culture medium
Luteolin, obtained from Sigma; EMD Millipore
(Billerica, MA, USA) was dissolved in dimethylsulfoxide (DMSO) and
its concentration adjusted to 100 mM, as stock solutions, for
storage at −20°C. This was diluted with medium prior to each
experiment, in which the final concentration of DMSO was <0.1%.
Fetal bovine serum (FBS) was obtained from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Cell Counting Kit-8 (CCK8) was
obtained from Beyotime Institute of Biotechnology (Haimen, China).
Annexin V-FITC/PI double staining was obtained from BD Biosciences
(Franklin Lakes, NJ, USA). The Fluorometric Protease Assay kit was
obtained from Beyotime Institute of Biotechnology.
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra-ethylbenzimidazolylcarbocyanine
iodide (JC-1) Mitochondrial Membrane Potential Assay Kit was
obtained from Thermo Fisher Scientific, Inc.
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) and
N-acetyl-L-cysteine (NAC) were obtained from Sigma-Aldrich; EMD
Millipore. The BCA protein assay kit was obtained from Beijing
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). The anti-p53
(cat. no. 2527T), Bcl-2-associated X protein (Bax; cat. no. 5023T),
Bcl-2 (cat. no. 2872T), apoptotic protease activating factor 1
(Apaf1; cat. no. 8969T), cytochrome c (cat. no. 11940T), and
β-actin (cat. no. 4967S) antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). A secondary
HRP-labeled goat anti-rabbit IgG (cat. no. ZB-2301) was obtained
from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing,
China).
Cell culture
The SKM-1 cell line, originally established from a
76-year-old Japanese male patient with overt monoblastic leukemia
following MDS (22), was obtained
from the Institute of Biochemistry and Cell Biology, Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences
(Shanghai, China). The SKM-1 cells were grown in RPMI-1640 medium
containing 10% heat-inactivated FBS, 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified atmosphere of 5%
CO2 at 37°C, and the cultivating media was replaced
every 2 days. SKM-1 cells in logarithmic growth phase were used in
all experiments.
Primary cell culture
Between October 2017 and February 2018, PBM cells
(>1×106/ml) were obtained from the bone marrow of a
patient with intermediate MDS (female, 71 years) and two patients
with high-risk MDS (male, 75 years; and female, 52 years) in RPMI
1640 medium containing 100 U/ml heparin, following which the PBM
mononuclear cells were isolated by gradient sedimentation using
Ficoll-Hypaque (1.077 g/ml). Subsequently, the cells were washed at
least twice with PBS and cultured as described for the culture of
SKM-1 cells above. All patients involved in the present study
provided written informed consent and the Institutional Review
Board of The Third Affiliated Hospital of Soochow University
(Changzhou, China) approved the study.
Cytotoxicity assay
The cells were seeded in triplicate at a density of
5×103 cells/well (for SKM-1 cells) or 5×104
cells/well (for fresh MDS cells) in 96-well plates, respectively.
Following incubation for 24 h, serial dilutions of luteolin (0, 10,
20, 40, 80 and 120 µM) were added and incubated for various
durations (12, 24 and 48 and 72 h), following which 10 µl of
CCK8 solution (5 g/l) was added to each well and the cells were
incubated for another 3 h at 37°C. Subsequently, the optical
density for each well was measured using a Model 550 microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a
wavelength of 450 nm according to the manufacturer's protocol. Cell
viability was calculated as the percentage of viable cells in the
luteolin-treated group versus the control group. Finally, the
obtained cell proliferation plots were used to calculate the half
maximal inhibitory concentration (IC50) value of the
luteolin.
Analysis of apoptosis
To demonstrate the apoptotic features of the cells,
the cells treated with or without luteolin were collected,
sedimented onto microscopic slides and fixed with methanol.
Wright-Giemsa staining (Yeasen Biotech, Shanghai, China) was then
performed and images of the apoptotic morphological features were
captured using an Olympus IX71 light microscope (Olympus
Corporation, Tokyo, Japan). The extent of cell apoptosis was
evaluated using Annexin V-FITC/PI double staining as previously
reported (23).
DNA fragmentation assay
Following treatment with serial dilutions of
luteolin for 24 h, the SKM-1 cells were harvested and washed twice
with sterile PBS. DNA was collected from the cells using TRIzol
reagent according to manufacturer's protocol, and the quality and
purity of the isolated DNA were then determined using a UV 2500
spectrophotometer (Shimadzu Corporation, Tokyo, Japan). The DNA
samples (15 µg) were then electrophoresed on a 1.5% agarose
gel and visualized with ethidium bromide staining under ultraviolet
light.
Measurement of ROS
Intracellular ROS levels were examined by flow
cytometry (FCM) as described previously (24). Briefly, following incubation with
serial dilutions of luteolin for 12 h, DCFH-DA working solution was
added directly to the culture medium at a final concentration of 10
µM. The cells were then incubated at 37°C for an additional
30 min, following which the cells were collected and the intensity
of fluorescence was analyzed by FACSCalibur FCM (BD
Biosciences).
Determination of ΔΨm
To demonstrate whether luteolin-induced apoptosis is
mediated through mitochondrial dysfunction, alterations in ΔΨm were
detected by JC-1 staining. Briefly, the SKM-1 cells were pretreated
with or without 5 mM NAC for 2 h and then treated with serial
dilutions of luteolin for 12 h. The cells (1×106
cells/ml) were suspended with PBS and incubated with JC-1 at a
final concentration of 2 µM for 20 min at 37°C. The cells
were then washed with 1X JC-1 buffer (2 µM) and resuspended
in PBS, and illuminated at 488 nm. Images of ΔΨm change from
monomer and aggregate were captured using an FV-1000 fluorescence
microscope (Olympus Corporation). Fluorescence-activated cell
sorting analysis was performed by FCM, and the ratio of JC-1
monomers (green fluorescence) to JC-1 aggregates (orange
fluorescence) was calculated for each concentration of luteolin and
for the medium control.
Caspase activity assay
A Fluorometric Protease Assay kit was used to detect
caspase activities according to the manufacturer's protocol.
Briefly, the SKM-1 cells were preincubated with or without 5 mM NAC
for 2 h and then incubated with serial dilutions of luteolin for 12
h. The cells (2×106 cells/ml) were collected and lysed
by incubating them with lysis buffer provided in the kit on ice for
10 min. Subsequently, 50 µg of cell lysate proteins in 200
µl of supernatant was incubated with an equal volume of
reaction buffer containing fluorogenic peptide substrate at 37°C
for 1 h. The fluorescence intensities of cleaved substrate were
then determined using a UV 2500 spectrophotometer (Shimadzu
Corporation).
Western blot analysis
Cell protein extraction and western blot analysis
were performed as described previously (25). Briefly, following treatment with
luteolin, as described in the DNA fragmentation experiments, the
total protein content of the cytosolic fraction in the supernatant
was isolated from the harvested cells and measured using a BCA
protein assay. The samples (25 µg/lane) were subjected to
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto a nitrocellulose membrane. Nonspecific binding
sites were blocked with 5% non-fat milk for 1 h. The membranes were
then incubated with primary antibodies against p53 (1:1,000), Bax
(1:1,000), Bcl-2 (1:1,000), Apaf1 (1:1,000), cytochrome c
(1:1,000), and β-actin (1:1,000) for 2 h at 4°C, and then with a
secondary HRP-labeled goat anti-mouse IgG (1:2,000) for 1 h at room
temperature. The blots were then visualized by enhanced
chemiluminescence (ECL system, GE Healthcare Life Sciences,
Chalfont, UK) and the density of β-actin served as an internal
loading control.
Statistical analysis
Data are expressed as the mean ± standard deviation
and all analyses were performed with Statistical Package for Social
Science (version 18.0; SPSS, Inc., Chicago, IL, USA). Comparisons
between two groups were made using Student's t-test and those
between three or more groups were made using one-way analysis of
variance followed by the SNK test. P<0.05 was considered to
indicate a statistically significant difference.
Results
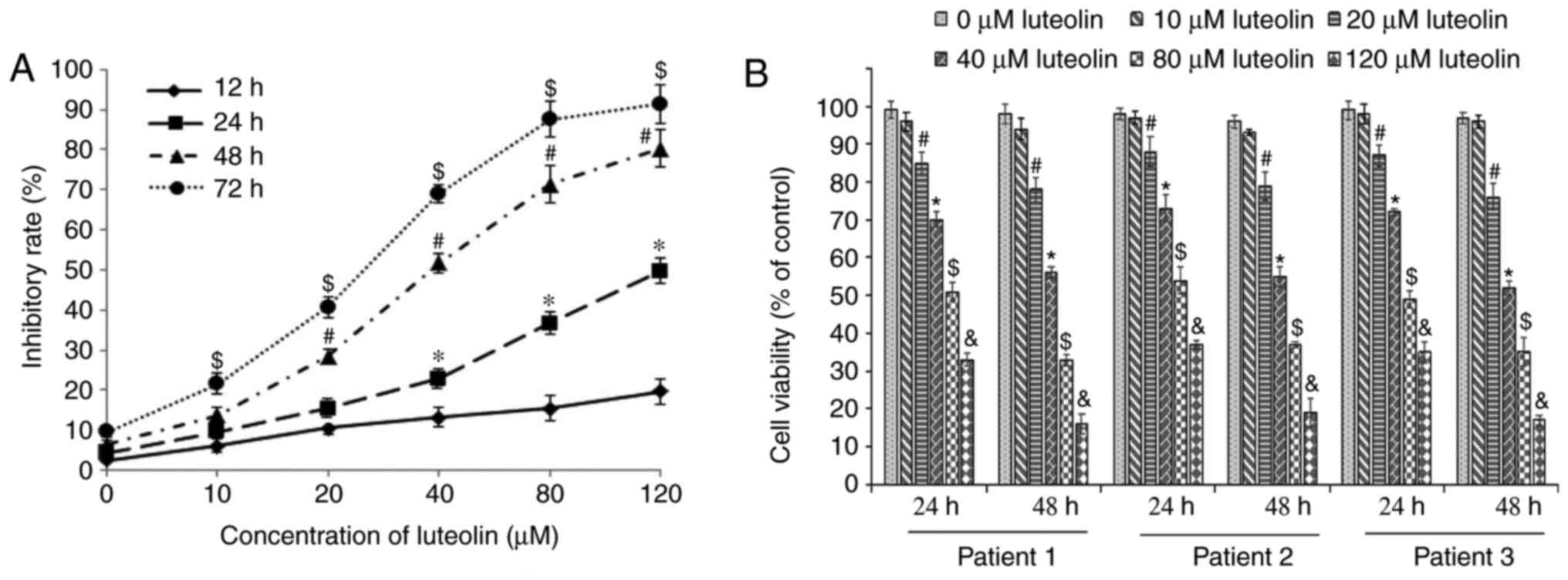
Inhibition of cell proliferation
following treatment with luteolin
Following incubation with serial dilutions of
luteolin for various durations, the CCK8 assay showed that the
proliferation of SKM-1 cells was markedly inhibited in a time- and
dose-dependent manner (Fig. 1A)
and the dose of luteolin required to yield IC50 was
139.41 µM at 24 h, 39.97 µM at 48 h, and 23.95
µM at 72 h. Notably, the cell viability of PBM mononuclear
cells from one patient with intermediate-risk MDS was significantly
decreased, compared with that in the medium control group
(P<0.05) following treatment with 10, 20, 40, 80 and 120
µM luteolin for 24 and 48 h. Similarly, the cell growth of
primary cell cultures from another two patients with high-risk MDS
was markedly inhibited, compared with that in the medium control
group following treatment with the same concentration of luteolin
for 24 and 48 h (all P<0.05; Fig.
1B), although there were no significant differences in cell
viability between patients. As luteolin exerted similar anticancer
effects on the SKM-1 cells and PBM mononuclear cells from patients
with MDS, SKM-1 cells were used as a model to examine the
underlying mechanisms of luteolin in the subsequent
experiments.
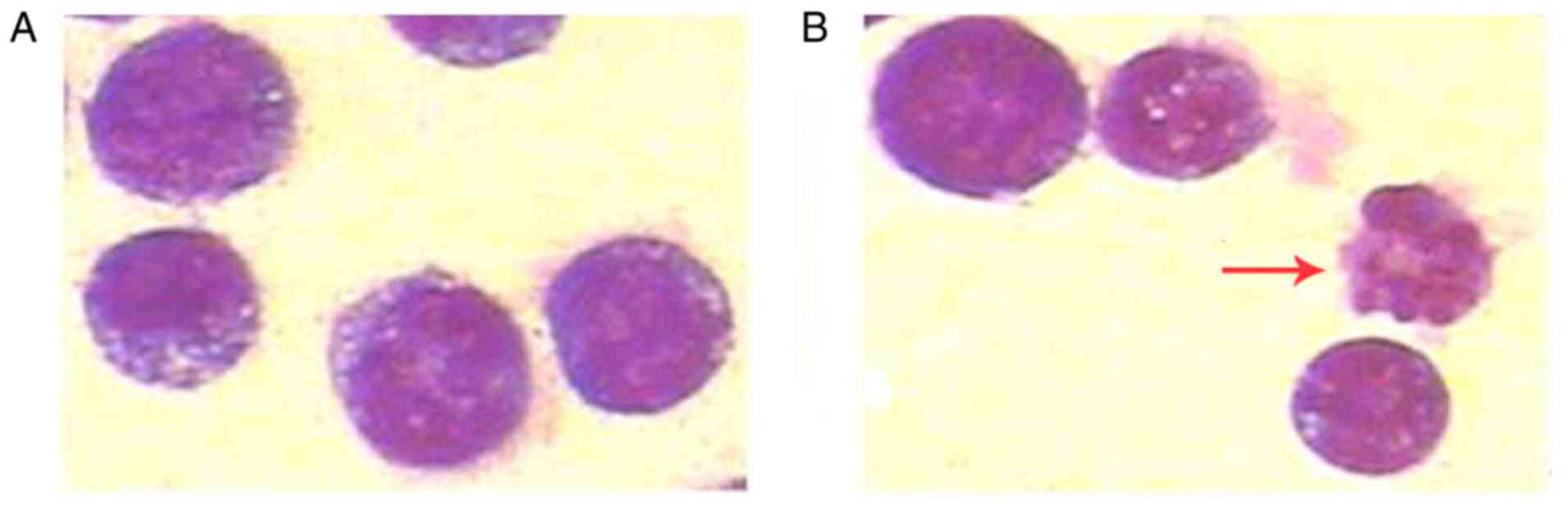
Cell morphological assessment following
treatment with luteolin
To determine whether the inhibition of cell growth
by luteolin was in fact due to cell apoptosis, Wright-Giemsa
staining was performed and the results demonstrated that the
targeted cells underwent morphological changes characteristic of
apoptosis. As expected, the typical morphological features of cell
apoptosis, including cell shrinkage, chromatin condensation and
loss of normal nuclear architecture, were observed in the
luteolin-treated group under the light microscope, which were not
observed in the control group (Fig.
2A and B).
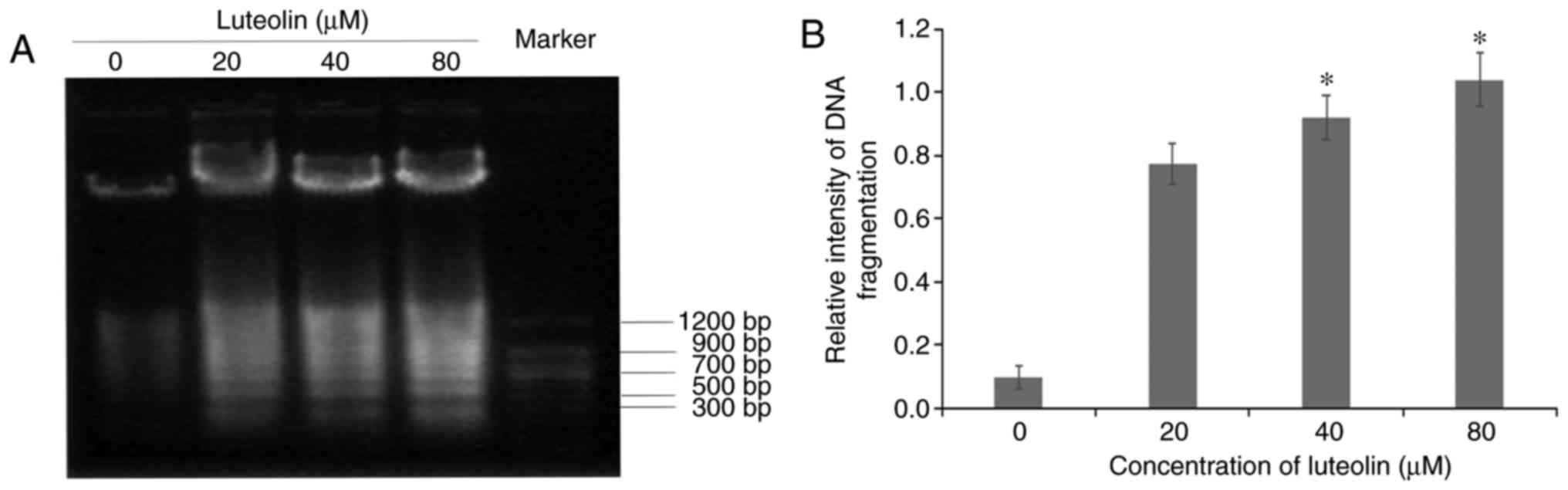
DNA fragmentation analysis following
treatment with luteolin
To confirm the induction of apoptosis by luteolin in
SKM-1 cells, DNA was isolated and analyzed by agarose gel
electrophoresis. As shown in Fig.
3A, a ladder-like pattern of DNA fragmentation became apparent
in the SKM-1 cells following treatment with serial dilutions of
luteolin for 24 h, whereas no fragments were observed in the medium
control group. In particular, a typical ladder pattern of genomic
DNA fragmentation was observed in SKM-1 cells exposed to higher
concentrations of luteolin (40 and 80 µM), in which the
bands were thicker than those of 20 µM luteolin, however, no
significant difference was observed between the two groups treated
with the two higher concentrations of luteolin (Fig. 3B).
Apoptotic induction of SKM-1 cells
following treatment with luteolin
The effect of luteolin on the extent of cell
apoptosis was further quantified by FCM. The apoptotic rates,
including the sum of cells in early and late apoptosis, for the
SKM-1 cells were increased in the luteolin-treated group, compared
with those in the control group, and luteolin induced SKM-1 cell
apoptosis in a dose-dependent manner (Fig. 4A–E), suggesting that 80 µM
luteolin had the largest pro-apoptotic effect on SKM-1 cells. In
particular, the apoptotic rates of the SKM-1 cells were increased
significantly with the prolonged duration of incubation with 40
µM luteolin (P<0.05; Fig.
4F).
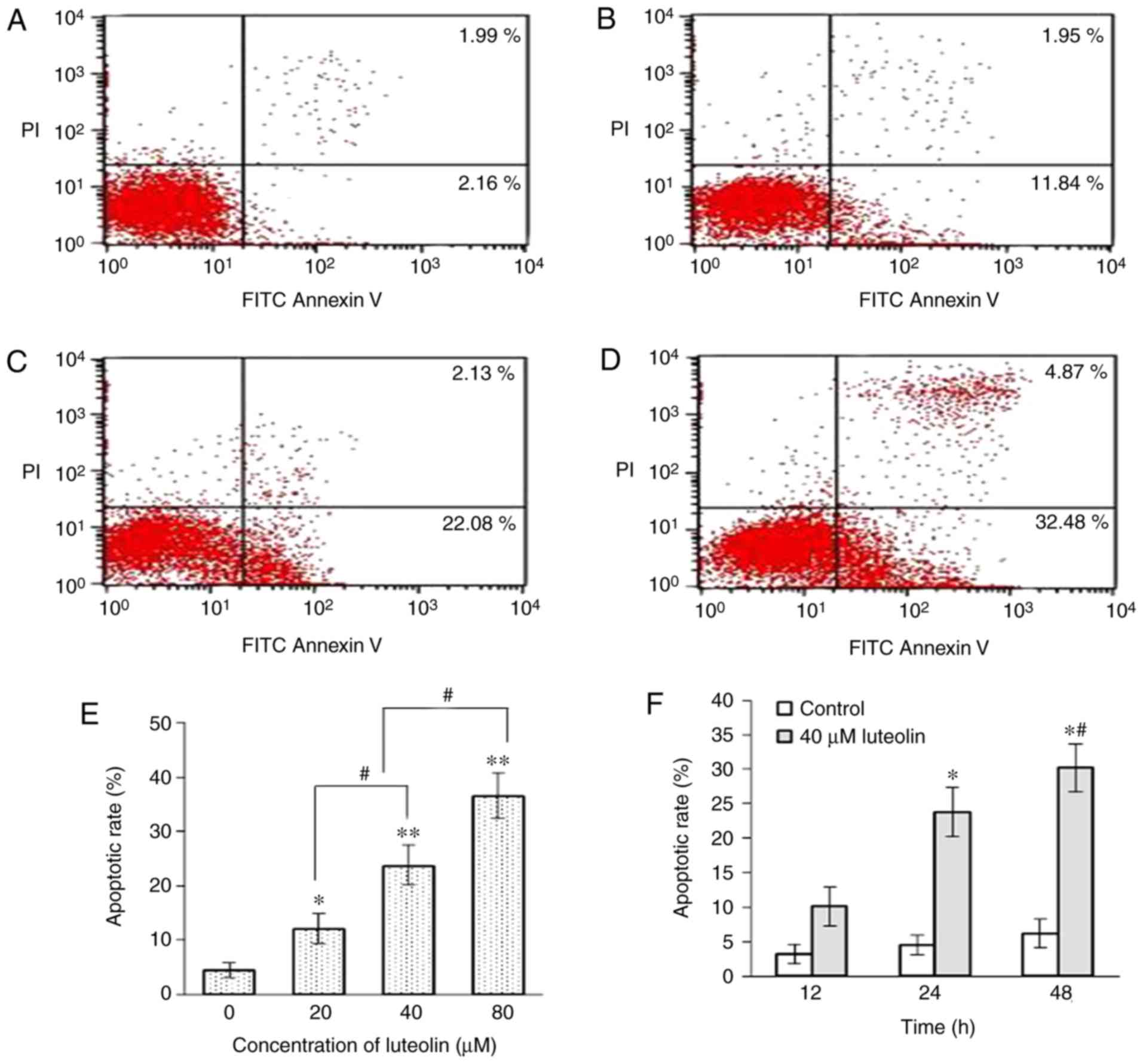 | Figure 4Effect of luteolin on the cell
apoptosis for SKM-1 cells. SKM-1 cells were treated with (A) 0, (B)
20, (C) 40 and (D) 80 µM luteolin for 24 h, and evaluated
using Annexin V-FITC/PI double staining. Results are representative
of three independent experiments. Graph quadrants: Lower left,
living cells (AV-negative/PI-negative); lower right, early
apoptotic cells (AV-positive/PI-negative); upper right, late
apoptotic cells (AV-positive/PI-positive); upper left, necrotic
cells (AV-negative/PI-positive). The numbers of cells undergoing
apoptosis represent the percentage of cells in the sum of the lower
right and upper right quadrants. (E) Apoptotic rates of SKM-1 cells
treated with serial dilutions of luteolin for 24 h were detected by
FCM. Data obtained from three independent experiments are presented
as the mean ± standard deviation. *P<0.05 and
**P<0.01, vs. control group; #P<0.05,
vs. different concentration of luteolin. (F) Apoptotic rates of
SKM-1 cells treated with 40 µM luteolin for 12, 24 and 48 h
were detected by FCM. Data obtained from three independent
experiments are presented as the mean ± standard deviation.
*P<0.01, vs. control group; #P<0.05,
vs. different time points with 40 µM luteolin. FCM, flow
cytometry. |
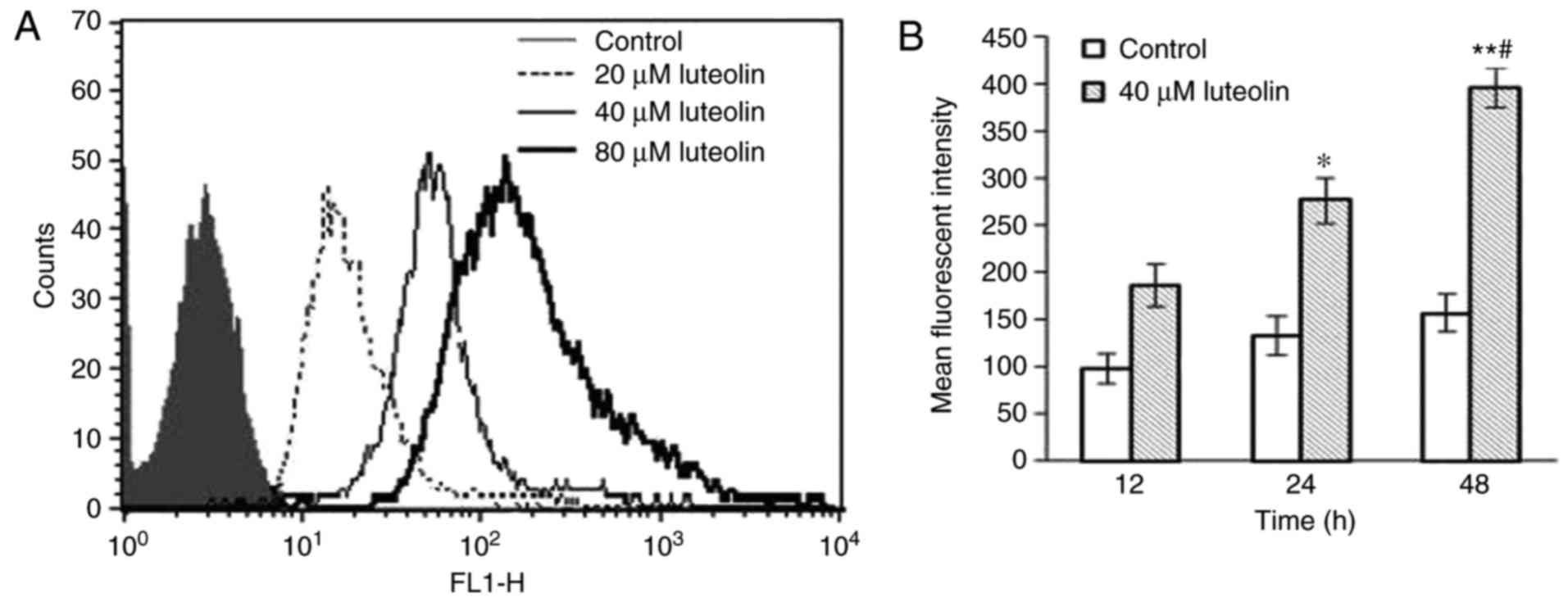
Formation of intracellular ROS following
treatment with luteolin
Following treatment with 0, 20, 40 and 80 µM
luteolin for 12 h, the mean fluorescence intensity (MFI) for the
SKM-1 cells was increased, compared with that in the control group
(P<0.05; Fig. 5A). Compared
with the corresponding time points for the serial dilutions of
luteolin, a significant increase in MFI was observed in the 80
µM luteolin group. In addition, following incubation with 40
µM luteolin for different time intervals, the MFI of the
SKM-1 cells was increased significantly with the prolonged duration
of incubation (P<0.05; Fig.
5B).
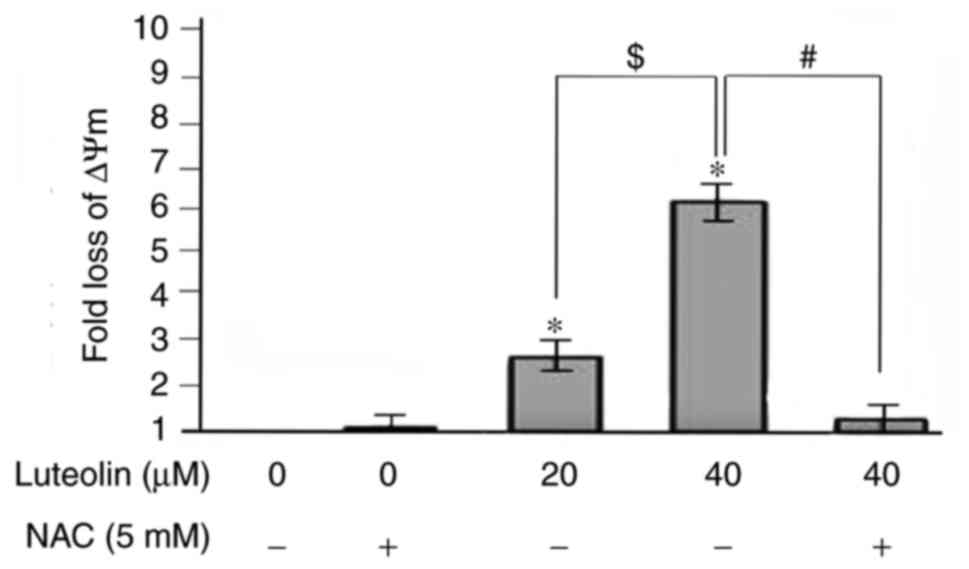
Depolarization of ΔΨm following treatment
with luteolin
The cationic dye, JC-1, has the ability to enter
into mitochondria and changes in color from green to orange
reversibly. In normal cells with high ΔΨm, JC-1 spontaneously
aggregates in mitochondria and emits red or orange fluorescence,
and in those cells with low ΔΨm, JC-1 remains monomeric and emits
green fluorescence. As shown in Fig.
6, the number of SKM-1 cells with orange fluorescence in the
control group was higher (Fig.
6A) and the percentage of cells emitting only green
fluorescence was increased following exposure of SKM-1 cells to 40
µM luteolin (Fig. 6B). A
shift from orange to green emission was observed under the
fluorescence microscope, and there were 2.7- and 6.2-fold increases
in loss of ΔΨm in response to 20 and 40 µM luteolin,
respectively, compared with those in the medium controls (Fig. 7). In particular, preincubation
with the antioxidant NAC for 2 h effectively attenuated the loss of
ΔΨm caused by luteolin, compared with that in the control group at
the same time (P<0.05).
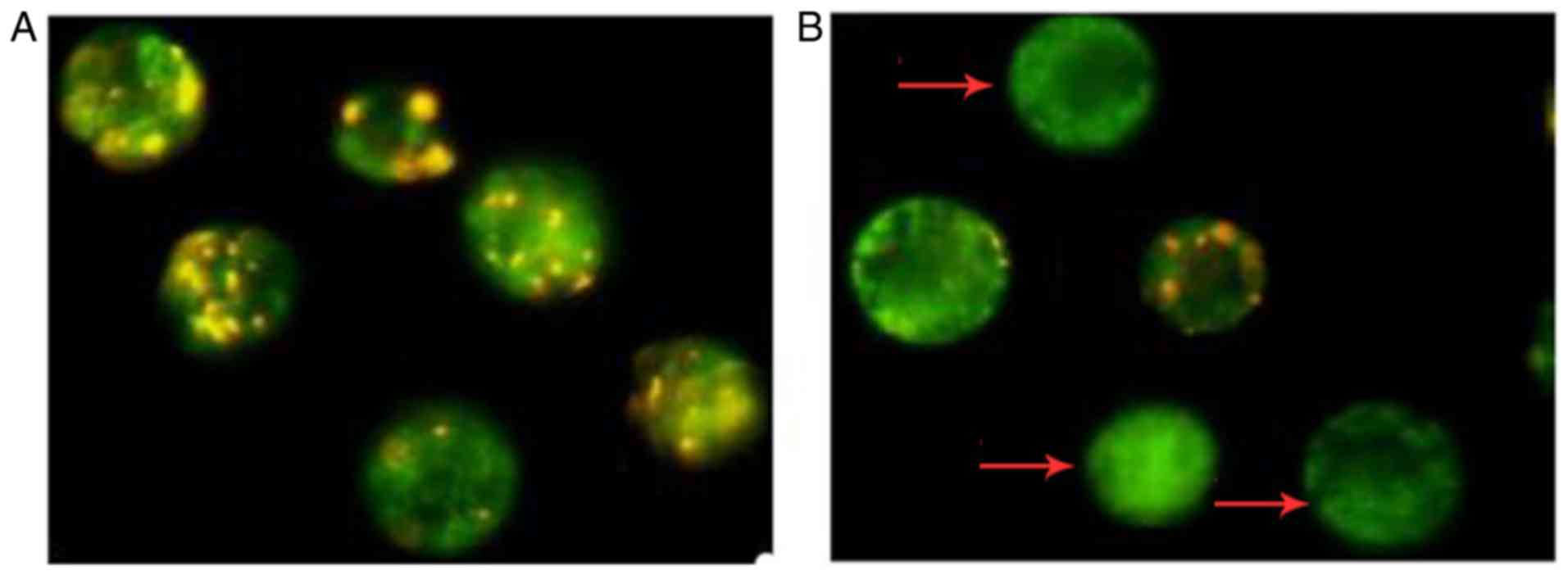 | Figure 6Typical fluorescence photomicrographs
under a fluorescence microscope (JC-1 staining, magnification,
×1,000). (A) SKM-1 cells were treated with medium for 12 h as a
control group. (B) SKM-1 cells were treated with 40 µM
luteolin for 12 h. Results shown are representative of two similar
experiments. The orange fluorescence represents JC-1 aggregates,
the green fluorescence represents JC-1 monomers, and the arrows
indicate SKM-1 cells with a lower ΔΨm in the luteolin-treated group
compared with the control group. JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocya-nine
iodide; ΔΨm, mitochondrial membrane potential. |
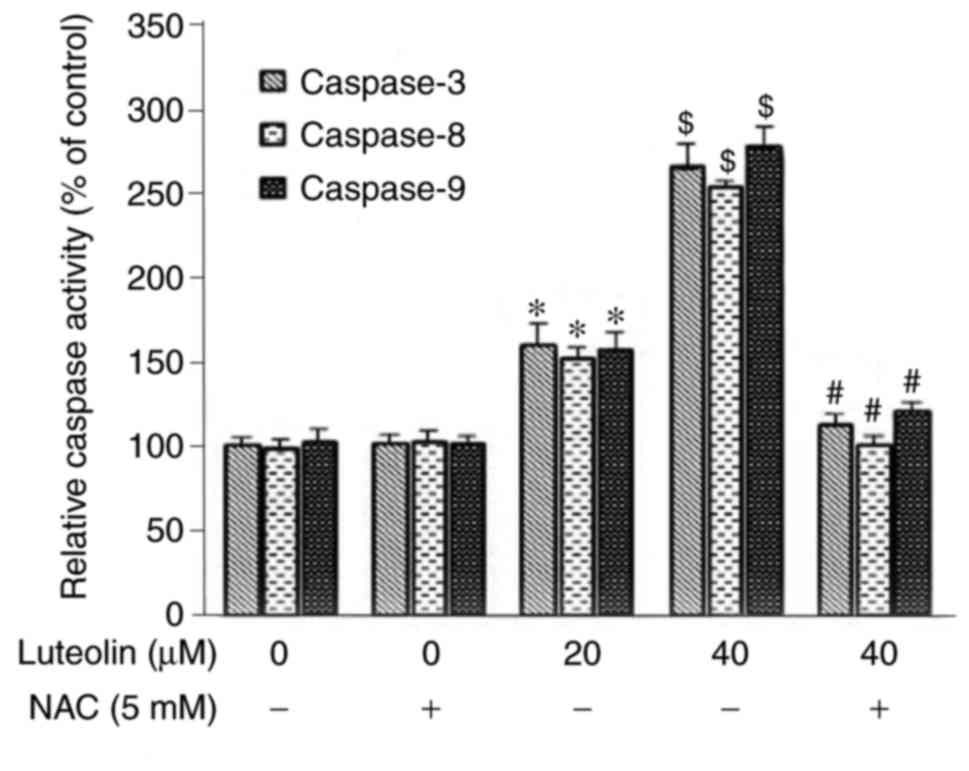
Modulation of caspase activity following
treatment with luteolin
Following incubation for 24 h, luteolin markedly
induced the activities of caspase-3, -8 and -9 in the SKM-1 cells,
and there were significant differences compared with those in
either the medium control group or NAC control group (P<0.05).
The activities of caspases were higher in the NAC (−)/40 µM
luteolin group, compared with those in the NAC(−)/20 µM
luteolin group, suggesting that the induction of caspase activity
occurred in a dose-dependent manner. Preincubation with the
antioxidant NAC for 2 h effectively suppressed the activities of
caspase-3, -8 and -9 induced by 40 µM luteolin in the SKM-1
cells (Fig. 8).
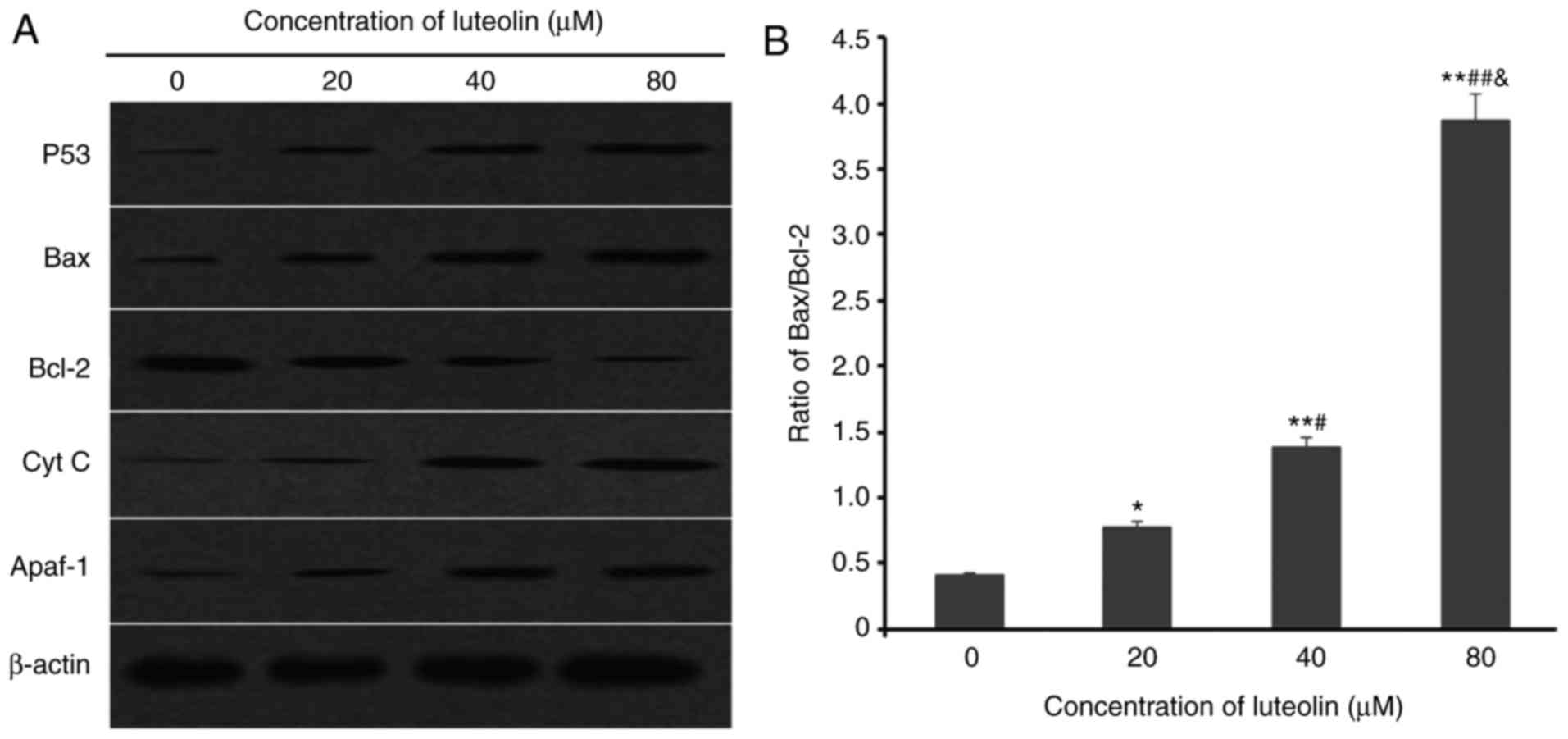
Alteration of mitochondrial apoptosis
related-proteins following treatment with luteolin
Following incubation with luteolin for 24 h, the
increased tumor suppressor protein p53, downregulation of
anti-apoptotic protein Bcl-2 and upregulation of proapoptotic
proteins Bax, Apaf-1, and cytochrome c in the SKM-1 cells
were positively associated with the dose of luteolin (Fig. 9A) and there was a significant
dose-dependent shift in the ratio of Bax to Bcl-2 (Fig. 9B).
Discussion
Cytotoxic chemotherapy is used for patients with MDS
with increasing myeloblasts and those who have progressed to acute
leukemia. The incidence of MDS has been increasing rapidly with an
aging population and an increasing number of individuals exposed to
benzene (26). However, the
unavailability of a curable approach and the blind strategy choice
present challenges for the treatment of MDS. There has been
increasing interest in the utilization of natural product-derived
compounds as safe and effective chemotherapeutic agents for the
treatment of various human diseases due to their diversity of
biological activities. As a molecular targeted drug, the anticancer
properties of luteolin have been identified to be associated with
antiproliferative effects and pro-apoptotic effects, in addition to
the inhibition of angiogenesis and metastasis (9,14,27). However, the molecular mechanisms
underlying its anticancer activities remain to be fully
elucidated.
In the present study, the antiproliferative effect
of luteolin on MDS cells was confirmed. The results of a CCK8 assay
demonstrated that the proliferation of SKM-1 cells was markedly
inhibited, and the dose of luteolin required to yield
IC50 was 39.97 µM at 48 h, and 23.95 µM at
72 h. Luteolin also markedly inhibited the proliferation of PBM
mononuclear cells from patients with intermediate- or high-risk
MDS. Using differentiated enterocytes and the Caco-2 human colon
carcinoma cell line, Abdel Hadi et al (28) demonstrated that luteolin exhibited
cytotoxic activity towards human colon cancer cells with minimal or
no effect on normal cells. These results suggested that luteolin
may be a potential chemopreventive agent with an improved safety
profile. In addition, the present study suggested that luteolin
suppressed MDS cell proliferation, mainly as a result of the
induction of apoptosis, as demonstrated by typical morphological
features of cell apoptosis, including cell shrinkage, chromatin
condensation and loss of normal nuclear architecture, under the
microscope using Wright-Giemsa staining. The formation of DNA
fragments of oligonucleosomal size (180-200 bp) is considered to be
a hallmark of apoptotic cell death in several cell types (29). In the present study, the agarose
gel electrophoresis showed a typical ladder pattern of genomic DNA
fragmentation in the nuclei isolated from SKM-1 cells (Fig. 3). This observation led to the
hypothesis that nucleosomal DNA fragmentation may be involved in
luteolin-induced apoptosis of MDS cells. In addition, to evaluate
the extent of cell apoptosis, Annexin V-FITC/PI double staining was
performed; the results of FCM showed additional evidence for the
occurrence of apoptosis induced by luteolin in a dose- and
time-dependent manner, compared with that in the control group
(Fig. 4). Consistent with these
results, it has been reported that treatment with luteolin inhibits
cell proliferation and induces cell apoptosis in other
hematopoietic diseases (14,30–32). Collectively, the results presented
in the present study and those of others provide clear evidence
that luteolin exhibits cytotoxic effects on cancer cells through
the induction of apoptosis, and a luteolin concentration of 80
µM in the present study had the most marked pro-apoptotic
effect on the SKM-1 cells.
Apoptosis, the key factor stimulating programmed
cell death in cancer cells, has become one of the major mechanisms
for the elimination of several types of cancer cell through
different pathways, including the mitochondrial pathway (intrinsic
pathway), endoplasmic reticulum pathway, or death
receptor-medicated apoptotic pathway (extrinsic pathway) (33). These three signaling pathways
communicate and interact with one another in physiological
conditions, and are important in regulating cell dissolution
(34). Accumulating evidence
suggests that mitochondria are not only the major source of
intracellular ROS, but they are also the major targets of their
detrimental effects in mammalian cells (15,35). Luteolin exerts its function as an
antioxidant and ROS-generating agent (36), and various contrasting reports
have been published regarding the effects of luteolin in the
generation of ROS. In previous studies, Zhang et al
(6) and Shelton et al
(37) reported that luteolin
possesses direct and indirect antioxidant effects by scavenging
ROS. In agreement, Liu et al demonstrated that luteolin
scavenges ROS generation to protect cells at concentrations of 1.0
and 10 µM (38), whereas
others (16,19) reported that luteolin induces ROS
accumulation in an early phase via the suppression of cellular
superoxide dismutase activity in cancer cells. This discrepancy may
be influenced by cell type and the dose of the ROS-inducing agents
examined. In the present study, a significant increase in the MFI
of SKM-1 cells was detected with the increase in luteolin
concentration. Additionally, the MFI of the SKM-1 cells increased
significantly with the prolonging of luteolin treatment duration,
suggesting that luteolin leads to an intracellular accumulation of
ROS in a dose-and time-dependent manner in MDS cells. It is well
known that the excessive generation and accumulation of
intracellular ROS can cause disruption of ΔΨm, which results in
mitochondrial dysfunction and triggers cell apoptosis through
multiple downstream signaling pathways (39). Similarly, Choi et al
reported that a decrease in ΔΨm following intense ROS generation
induced mitochondrial disruption, inhibition of mitochondrial
respiratory chain, reduction of ATP synthesis, and cell death
(40). In the present study, the
Annexin V-FITC/PI double staining and JC-1 staining showed that the
dose of luteolin used in the experiments resulted in SKM-1 cell
apoptosis, and this event was preceded by sustained
hyperpolarization of the mitochondrial membrane, as demonstrated by
the results of FCM and fluorescent microscope. A shift from orange
to green emission was observed under the fluorescence microscope,
and there was a higher fold increase in loss of ΔΨm in response to
the increase of luteolin concentration, compared with that in the
control group. Preincubation with the antioxidant NAC effectively
attenuated the loss of ΔΨm caused by luteolin, compared with that
in the control group at the same time. The results of these
experiments indicated that luteolin inhibited cell proliferation
and induced cell apoptosis through the mitochondrial pathway,
mediated by ROS, in MDS cells.
P53, a tumor suppressor protein, is involved in
inducing cell apoptosis (41).
There is increasing evidence that luteolin activates the intrinsic
apoptosis pathway through inducing DNA damage and activating the
marked p53-dependent inhibition of cell proliferation and cell
apoptosis in several cancer cell lines, including EC1 and KYSE450
esophageal carcinoma cells (9),
HepG2 cells (42), cervical
cancer cells (43), HT-29 colon
cancer cells (44) and human RPE
cells (45). Several lines of
evidence have demonstrated that overexpression of the p53 gene
suppresses the growth of human tumor cell lines (46,47). The results of the present study
confirmed that, following incubation with luteolin for 24 h, the
protein expression of p53 was augmented with the increasing
concentration of luteolin. Therefore, these findings and those of
others indicate that the p53 protein may act as a major activator
for the induction of apoptosis in luteolin-treated SKM-1 cells.
Until now, the mechanisms by which p53 regulates cell apoptosis
have not been well illustrated, however, Bcl-2 family proteins,
which comprise pro-apoptotic and anti-apoptotic members, are
important in p53-dependent apoptosis (41). It has been shown that p53 directly
activates the transcription of several genes encoding members of
the Bcl-2 family, which can positively or negatively regulate
mitochondrial outer membrane permeabilization to promote the
release of cytochrome c and other apoptotic molecules
(48). To further examine the
underlying mechanisms of p53-mediated apoptosis in luteolin-treated
SKM-1 cells, the protein expression levels of Bax and Bcl-2 were
determined by western blot analysis in the present study. The
results confirmed that the mechanisms of luteolin-induced cell
apoptosis may involve the downregulation of Bcl-2 and upregulation
of Bax proteins in a dose-dependent manner, leading to a
significant dose-dependent shift in the ratio of Bax to Bcl-2
family proteins in favor of the activation of caspase-3 in MDS
cells. These findings are consistent with the findings of other
studies that luteolin can activate the protein levels of Bax and
Bcl-2 in human osteosarcoma, lung adenocarcinoma and gliomablastoma
cells (39,49,50). Collectively, these findings
suggested that luteolin induces cell apoptosis via the
p53-dependent mitochondrial signaling pathway by modulating a
number of target proteins, particularly Bcl-2 and Bax.
It is known that the induction of apoptosis is
accompanied by the release of cytochrome c from
mitochondria, which is considered one of the most important
pro-apoptotic proteins and key in the assembly of a multimolecular
complex known as the apoptosome. Its levels are markedly reduced in
the normal cell cytoplasm and markedly increased in apoptotic cells
(51). The increased generation
of ROS induces the release of cytochrome c from mitochondria
to the cytoplasm (17), where it
binds Apaf-1. The subsequent exchange of ADP/dADP for ATP/dATP in
Apaf-1 triggers it to form an apoptosome (52). Therefore, the expression of
cytochrome c was analyzed in the present study to examine
whether cytochrome c had been released from the
mitochondrial membrane in MDS cells, and to determine how
cytochrome c activates the downstream effector caspases to
induce apoptosis in SKM-1 cells following incubation with luteolin.
The results indicated that the levels of cytochrome c
increased significantly with the processing of caspase in a
dose-dependent manner, compared with those in the control group. In
addition, following incubation with luteolin for 24 h, the
accumulation of ROS appeared to upregulate the proapoptotic protein
Apaf-1 in SKM-1 cells. Luteolin has been found to increase the
expression of Bax and caspase-3 and decrease the expression of
Bcl-2 in liver carcinoma cells, but exerted almost no effects on
normal liver HL-7702 cells (53).
Therefore, it is reasonable to suggest that luteolin-induced ROS
generation may be an early event in MDS cells, which promotes the
induction of cell apoptosis by the release of cytochrome c
from mitochondria.
Caspases are another important family of proteins
involved in the downstream events of p53-mediated apoptosis
(41). Caspase-3, an aspartic
acid-specific cysteine protease, is the most widely studied
mammalian caspase, which activates as cleaved caspase-3 by specific
shearing off the inactive subunit in the location of aspartic acid.
Additionally, it is important in both death pathways, and cleaves a
wide range of cellular substrates (54). To elucidate whether caspase
activation was involved in luteolin-induced apoptosis, a
fluorometric assay was performed to detect the activities of
caspase-3, -8, and -9. Following incubation for 24 h, luteolin
significantly induced the activation of caspase-3, -8 and -9 in
SKM-1 cells in a dose-dependent manner. Preincubation with the
antioxidant NAC for 2 h effectively suppressed the activity of
caspase-3, -8 and -9 induced by 40 µM luteolin (Fig. 8). These results supported the
hypothesis that luteolin induces ROS-mediated apoptosis by
activating caspase-dependent pathways.
In conclusion, luteolin was shown to inhibit the
proliferation of SKM-1 cells and exerted its pro-apoptotic action
partly through the p53-dependent mitochondrial signaling pathway
mediated by intracellular ROS. This provides a promising
therapeutic candidate for patients with MDS. However, further
investigation of luteolin in clinical trials is required to confirm
these results.
Funding
This study was supported by the Fund for Young
Scholars in Jiangsu Province (grant no. BK 20150253), the Fund of
Science and Technology Bureau of Changzhou, Jiangsu, China (grant
no. CJ20150418) and the Changzhou High-Level Medical Talents
Training Project (grant no. 2016ZCLJ024).
Availability of data and materials
All datasets generated and analysed in the present
study are included in this published article.
Authors' contributions
WD and WG conceived and designed the study. YaL, YC
and YuL performed all the experiments. XX performed all the
clinical diagnoses and treatments. YaL and YC analyzed the data. WD
and YaL wrote the manuscript. WG revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All patients involved in the present study provided
the written informed consent and the Institutional Review Board of
The Third Affiliated Hospital of Soochow University approved the
study.
Consent for publication
All the study participants provided consent for the
data to be published.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations
Abbreviations:
|
MDS
|
myelodysplastic syndrome
|
|
ROS
|
reactive oxygen species
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
DMSO
|
dimethylsulfoxide
|
|
CCK8
|
Cell Counting Kit-8
|
|
FCM
|
flow cytometry
|
|
DCFH-DA
|
2,7-dichlorodihydrofluorescein
diacetate
|
|
JC-1
|
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide
|
|
NAC
|
N-acetyl-L-cysteine
|
|
MFI
|
mean fluorescence intensity
|
Acknowledgments
The authors would like to thank Dr Feng Gao for
technical assistance.
References
|
1
|
Sekeres MA and Cutler C: How we treat
higher-risk myelodysplastic syndromes. Blood. 123:829–836. 2014.
View Article : Google Scholar
|
|
2
|
Ferrero D, Crisà E, Marmont F, Audisio E,
Frairia C, Giai V, Gatti T, Festuccia M, Bruno B, Riera L, et al:
Survival improvement of poor-prognosis AML/MDS patients by
maintenance treatment with low-dose chemotherapy and
differentiating agents. Ann Hematol. 93:1391–1400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith SM, Le Beau MM, Huo D, Karrison T,
Sobecks RM, Anastasi J, Vardiman JW, Rowley JD and Larson RA:
Clinical-cytogenetic associations in 306 patients with
therapy-related myelodysplasia and myeloid leukemia: The University
of Chicago series. Blood. 102:43–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan N, Afaq F, Syed DN and Mukhtar H:
Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle
arrest in human prostate cancer LNCaP cells. Carcinogenesis.
29:1049–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SX, Cao M, Xu SH, Zhang JM, Wang ZG,
Mao XD, Yao XM and Liu C: Effect of luteolin on inflammatory
responses in RAW264.7 macrophages activated with LPS and IFN-γ. J
Funct Foods. 32:123–130. 2017. View Article : Google Scholar
|
|
6
|
Zhang YC, Gan FF, Shelar SB, Ng KY and
Chew EH: Antioxidant and Nrf2 inducing activities of luteolin, a
flavonoid constituent in Ixeris sonchifolia Hance, provide
neuroprotective effects against ischemia-induced cellular injury.
Food Chem Toxicol. 59:272–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Li G, He K and Jiang W: Luteolin
exerts a marked antitumor effect in cMet-overexpressing
patient-derived tumor xenograft models of gastric cancer. J Transl
Med. 13:422015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suh KS, Chon S and Choi EM: Luteolin
alleviates methylglyoxal-induced cytotoxicity in osteoblastic
MC3T3-E1 cells. Cytotechnology. 68:2539–2552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen P, Zhang JY, Sha BB, Ma YE, Hu T, Ma
YC, Sun H, Shi JX, Dong ZM and Li P: Luteolin inhibits cell
proliferation and induces cell apoptosis via down-regulation of
mitochondrial membrane potential in esophageal carcinoma cells EC1
and KYSE450. Oncotarget. 8:27471–27480. 2017.PubMed/NCBI
|
|
10
|
Lim W, Yang C, Bazer FW and Song G:
Luteolin inhibits proliferation and induces apoptosis of human
placental choriocarcinoma cells by blocking the PI3K/AKT pathway
and regulating sterol regulatory element binding protein activity.
Biol Reprod. 95:822016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SH, Ham S, Kwon TH, Kim MS, Lee DH,
Kang JW, Oh SR and Yoon DY: Luteolin induces cell cycle arrest and
apoptosis through extrinsic and intrinsic signaling pathways in
MCF-7 breast cancer cells. J Environ Pathol Toxicol Oncol.
33:219–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pandurangan AK, Dharmalingam P, Sadagopan
SK and Ganapasam S: Luteolin inhibits matrix metalloproteinase 9
and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp
Toxicol. 33:1176–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai PH, Cheng CH, Lin CY, Huang YT, Lee
LT, Kandaswami CC, Lin YC, Lee KP, Hung CC, Hwang JJ, et al:
Dietary flavonoids luteolin and quercetin suppressed cancer stem
cell properties and metastatic potential of isolated prostate
cancer cells. Anticancer Res. 36:6367–6380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng L, Jiang L, Lin X, Tseng KF, Lu Z and
Wang X: Luteolin, a novel p90 ribosomal S6 kinase inhibitor,
suppresses proliferation and migration in leukemia cells. Oncol
Lett. 13:1370–1378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen HM, Tang XX, Zhou B, Zhou Z, Xu N and
Wang Y: A ROS-mediated mitochondrial pathway and Nrf2 pathway
activation are involved in BDE-47 induced apoptosis in Neuro-2a
cells. Chemosphere. 184:679–686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang W, Zhao FI, Zhang J and Gao D:
Luteolin induces apoptosis in mouse liver cancer cells through ROS
mediated pathway: A mechanistic investigation. Biomed Res.
28:839–845. 2017.
|
|
17
|
Kittiratphatthana N, Kukongviriyapan V,
Prawan A and Senggunprai L: Luteolin induces cholangiocarcinoma
cell apoptosis through the mitochondrial-dependent pathway mediated
by reactive oxygen species. J Pharm Pharmacol. 68:1184–1192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerl R and Vaux DL: Apoptosis in the
development and treatment of cancer. Carcinogenesis. 26:263–270.
2005. View Article : Google Scholar
|
|
19
|
Ju W, Wang X, Shi H, Chen W, Belinsky SA
and Lin Y: A critical role of luteolin-induced reactive oxygen
species in blockage of tumor necrosis factor-activated nuclear
factor-kappa B pathway and sensitization of apoptosis in lung
cancer cells. Mol Pharmacol. 71:1381–1388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding GL, Zhao JQ and Jiang DM: Allicin
inhibits oxidative stress-induced mitochondrial dysfunction and
apoptosis by promoting PI3K/AKT and CREB/ERK signaling in
osteoblast cells. Exp Ther Med. 11:2553–2560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu BX, Zhou JY, Li Y, Zou X, Wu J, Gu JF,
Yuan JR, Zhao BJ, Feng L, Jia XB and Wang RP: Hederagenin from the
leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo
colon cells through the mitochondrial pathway. BMC Complement
Altern Med. 14:4122014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakagawa T, Matozaki S, Murayama T,
Nishimura R, Tsutsumi M, Kawaguchi R, Yokoyama Y, Hikiji K, Isobe T
and Chihara K: Establishment of a leukaemic cell line from a
patient with acquisition of chromosomal abnormalities during
disease progression in myelodysplastic syndrome. Br J Haematol.
85:469–761. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia G, Chen B, Ding J, Gao C, Lu H, Shao
Z, Gao F and Wang X: Effect of magnetic Fe3O4
nanoparticles with 2-methoxyestradiol on the cell-cycle progression
and apoptosis of myelodysplastic syndrome cells. Int J
Nanomedicine. 6:1921–1927. 2011.
|
|
24
|
Kim SY, Lee YM and Cho JS: Korean red
ginseng extract exhibits neuroprotective effects through inhibition
of apoptotic cell death. Biol Pharm Bull. 37:938–946. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu H, Gao F, Shu G, Xia G, Shao Z, Lu H
and Cheng K: Wogonin inhibits the proliferation of myelodysplastic
syndrome cells through the induction of cell cycle arrest and
apoptosis. Mol Med Rep. 12:7285–7292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jing Y, Shen X, Mei Q and Han W: Spotlight
on decitabine for myelodysplastic syndromes in Chinese patients.
Onco Targets Ther. 8:2783–2790. 2015.PubMed/NCBI
|
|
27
|
Kim YS, Kim SH, Shin J, Harikishore A, Lim
JK, Jung Y, Lyu HN, Baek NI, Choi KY, Yoon HS and Kim KT: Luteolin
suppresses cancer cell proliferation by targeting vaccinia-related
kinase 1. PLoS One. 9:e1096552014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdel Hadi L, Di Vito C, Marfia G,
Ferraretto A, Tringali C, Viani P and Riboni L: Sphingosine kinase
2 and ceramide transport as key targets of the natural flavonoid
luteolin to induce apoptosis in colon cancer cells. PloS One.
10:e01433842015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devi PS, Kumar MS and Das SM: Evaluation
of antiproliferative activity of red sorghum bran anthocyanin on a
human breast cancer cell line (mcf-7). Int J Breast Cancer.
2011:8914812011. View Article : Google Scholar
|
|
30
|
Cheng AC, Huang TC, Lai CS and Pan MH:
Induction of apoptosis by luteolin through cleavage of Bcl-2 family
in human leukemia HL-60 cells. Eur J Pharmacol. 509:1–10. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ko WG, Kang TH, Lee SJ, Kim YC and Lee BH:
Effects of luteolin on the inhibition of proliferation and
induction of apoptosis in human myeloid leukaemia cells. Phytother
Res. 16:295–298. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sak K, Kasemaa K and Everaus H:
Potentiation of luteolin cytotoxicity by flavonols fisetin and
quercetin in human chronic lymphocytic leukemia cell lines. Food
Funct. 7:3815–3824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
34
|
Schwartz JT, Barker JH, Kaufman J, Fayram
DC, McCracken JM and Allen LA: Francisella tularensis inhibits the
intrinsic and extrinsic pathways to delay constitutive apoptosis
and prolong human neutrophil lifespan. J Immunol. 188:3351–3363.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marchi S, Giorgi C, Suski JM, Agnoletto C,
Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti
F, et al: Mitochondriaros crosstalk in the control of cell death
and aging. J Signal Transduct. 2012:3296352012. View Article : Google Scholar
|
|
36
|
Rao PS, Satelli A, Moridani M, Jenkins M
and Rao US: Luteolin induces apoptosis in multidrug resistant
cancer cells without affecting the drug transporter function:
Involvement of cell line-specific apoptotic mechanisms. Int J
Cancer. 130:2703–2714. 2012. View Article : Google Scholar :
|
|
37
|
Shelton LM, Park BK and Copple IM: Role of
Nrf2 in protection against acute kidney injury. Kidney Int.
84:1090–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu R, Meng F, Zhang L, Liu A, Qin H, Lan
X, Li L and Du G: Luteolin isolated from the medicinal plant
Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in
β-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y
cells. Molecules. 16:2084–2096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Q, Wang H, Jia Y, Pan H and Ding H:
Luteolin induces apoptosis by ROS/ER stress and mitochondrial
dysfunction in gliomablastoma. Cancer Chemother Pharmacol.
79:1031–1041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi IY, Lee SJ, Ju C, Nam W, Kim HC, Ko
KH and Kim WK: Protection by a manganese porphyrin of endogenous
peroxynitrite-induced death of glial cells via inhibition of
mitochondrial transmembrane potential decrease. Glia. 31:155–164.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin Y, Xu JP, Liao HH, Li L and Pan L:
Piperine induces apoptosis of lung cancer A549 cells via
p53-dependent mitochondrial signaling pathway. Tumor Biol.
35:3305–3310. 2014. View Article : Google Scholar
|
|
42
|
Yee SB, Choi HJ, Chung SW, Park DH, Sung
B, Chung HY and Kim ND: Growth inhibition of luteolin on HepG2
cells is induced via p53 and Fas/Fas-ligand besides the TGF-β
pathway. Int J Oncol. 47:747–754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ham S, Kim KH, Kwon TH, Bak Y, Lee DH,
Song YS, Park SH, Park YS, Kim MS, Kang JW, et al: Luteolin induces
intrinsic apoptosis via inhibition of E6/E7 oncogenes and
activation of extrinsic and intrinsic signaling pathways in
HPV-18-associated cells. Oncol Rep. 31:2683–2691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lim DY, Jeong Y, Tyner AL and Park JH:
Induction of cell cycle arrest and apoptosis in HT-29 human colon
cancer cells by the dietary compound luteolin. Am J Physiol
Gastrointest Liver Physiol. 292:G66–G75. 2007. View Article : Google Scholar
|
|
45
|
Hytti M, Szabó D, Piippo N, Korhonen E,
Honkakoski P, Kaarniranta K, Petrovski G and Kauppinen A: Two
dietary poly-phenols, fisetin and luteolin, reduce inflammation but
augment DNA damage-induced toxicity in human RPE cells. J Nutr
Biochem. 42:37–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ge Q, Wang C, Ruan Y, Chen Z, Liu J and Ye
Z: Overexpression of p53 activated by small activating RNA
suppresses the growth of human prostate cancer cells. Onco Targets
Ther. 9:231–241. 2016.PubMed/NCBI
|
|
47
|
Wang C, Ge Q, Zhang Q, Chen Z, Hu J, Li F
and Ye Z: Targeted p53 activation by saRNA suppresses human bladder
cancer cells growth and metastasis. J Exp Clin Cancer Res.
35:532016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Macip S, Igarashi M, Berggren P, Yu J, Lee
SW and Aaronson SA: Influence of induced reactive oxygen species in
p53-mediated cell fate decisions. Mol Cell Biol. 23:8576–8585.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang YH, Kong DL, Wang XW, Dong X, Tao Y
and Gong H: Molecular mechanisms of luteolin induced growth
inhibition and apoptosis of human osteosarcoma cells. Iran J Pharm
Res. 14:531–538. 2015.PubMed/NCBI
|
|
50
|
Chen Q, Liu S, Chen J, Zhang Q, Lin S,
Chen Z and Jiang J: Luteolin induces mitochondria-dependent
apoptosis in human lung adenocarcinoma cell. Nat Prod Commun.
7:29–32. 2012.PubMed/NCBI
|
|
51
|
Kilbride SM and Prehn JH: Central roles of
apoptotic proteins in mitochondrial function. Oncogene.
32:2703–2711. 2013. View Article : Google Scholar
|
|
52
|
Bao Q, Lu W, Rabinowitz JD and Shi Y:
Calcium blocks formation of apoptosome by preventing nucleotide
exchange in Apaf-1. Mol Cell. 25:181–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ding S, Hu A, Hu Y, Ma J, Weng P and Dai
J: Anti-hepatoma cells function of luteolin through inducing
apoptosis and cell cycle arrest. Tumour Biol. 35:3053–3060. 2014.
View Article : Google Scholar
|
|
54
|
Kobayashi T, Masumoto J, Tada T, Nomiyama
T, Hongo K and Nakayama J: Prognostic significance of the
immunohistochemical staining of cleaved caspase-3, an activated
form of caspase-3, in gliomas. Clin Cancer Res. 13:3868–3874. 2007.
View Article : Google Scholar : PubMed/NCBI
|