Introduction
Epilepsy is a syndrome caused by the paradoxical
discharge of the high synchronization of brain cells, which is
caused by either a known or unknown pathogenesis. The clinical
manifestation has certain features of a malfunction in the central
nervous system, with repeatability, paroxysms, transience and
inflexibility, showing paroxysmal feelings, and automatic nervous
dysneuria or a combination of these (1). An epidemiological survey showed that
the morbidity of epilepsy in general is 50–70 cases/100,000
individuals (2). Following
establishment of treatment, ~80% of epilepsy attacks can be
controlled, but ~20% of patients have poor outcomes (3). Epilepsy is a type of chronic or
repeatedly paroxysmal disease. Long-term repeated seizures may
result in hypophrenia, insanity, a poor memory and reductions in
the ability for social adaptation for numerous patients. This may
seriously impact the life and work of a patient and their ability
to learn (4). If children or
adolescents suffer from epilepsy, intelligence and physical
development may be more easily impacted. Epilepsy drugs
administered for a long time may make certain patients drowsy,
tired and inattentive (5).
Furthermore, it is hard for epilepsy patients, particularly
infants, to achieve a normal education and to be capable of
mastering normal work (6).
Therefore, epilepsy attacks not only bring pain to the bodies and
spirits of patients and their families, but also increases the
economic burden of health care. This directly impacts quality of
life, particularly in the case of damage to the central nervous
system caused by status epilepticus, which is serious with a longer
duration. Damage caused to hippocampal neurons is even more serious
and impacts the quality of life of patients (7).
MicroRNAs (miRNAs) are small non-coding RNA
molecules with a length of 20–24 nt. miRNA are cut by pre-miRNA
with a length of 60–70 nt and a hairpin structure (8). In plant and animal cells, miRNA can
restrain gene activity or degrade target genes to regulate
expressive activity following transcription through interaction
with the target gene's specific sequences (9). Every type of miRNA can participate
in growth, development, aging and death, as miRNAs have high
stability, conservatism, time sequence and tissue specificity in
terms of system evolution between species (10).
Among the existing miRNAs, 70% are expressed in the
brain tissues of mammals, including certain miRNAs (miRNA-124a,
miRNA-128 and miRNA-101) specific to the brain tissues. Brain
tissues are rich in miRNAs (such as miRNA-125b) (11), which express and regulate
different physiological processes and conduction pathways in the
nervous system, and serve an important role in the initiation and
development of the nervous system, neural stem cell differentiation
and cell apoptosis (12). It has
been shown that the brain miRNA-133 level in patients with
Parkinson's disease is markedly reduced compared with that in
normal control patients. miRNA-25 downregulation in Alzheimer's
disease has synergistic effects with ROS (12).
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt) signaling pathway serves an important function in the
proliferation, differentiation, survival and migration of
regulating cells, and is an extremely important conduction pathway
of 'survival signals' (it is also known as the 'apoptosis
resistance' pathway) (13). PI3K
can activate phosphorylated (p-)Akt level to develop its biological
functions, and it is an important indicator of standing for the
access activity (14). p-Akt can
activate multiple downstream proteins, including Bcl-2-associated
agonist of cell death (Bad), caspase-3, nuclear factor-κB, Kp85,
mechanistic target of rapamycin (mTOR), apoptosis regulator Bcl-2
(Bcl-2) and apoptosis regulator BAX (Bax), so as to mediate the
growth of induced cells. In the signal transduction anti-apoptosis
pathway, activation of the PI3K/Akt signal transduction pathway can
be regarded as a signal of cell growth and anti-apoptosis (15).
mTORs are highly conservative serine/threonine
protein kinases that have multiple activation methods.
PI3K/Akt/mTOR signaling pathway is the most common method to be
induced by growth factors and superfamily (16). The activated Akt can suspend its
inhibitory effects on mTOR, leading to mTOR activation by the
inhibition of tuberous sclerosis (TSC)1/TSC2 compound activity
relief. In addition to hypoxia, amino acid level and DNA damage,
multiple physiological and pathological factors may activate or
restrain mTORs (17). mTORs
generate a complicated biological effect by forming the functional
compounds mTOR complex 1 (mTORC1) and mTORC2 (16). The present study aimed to detect
the contribution of miRNA-155 to the occurrence of epilepsy, and to
investigate its neuroprotective effect and mechanism.
Materials and methods
Ethical considerations and patient
recruitment
Patients with temporal lobe epilepsy (age, 72±6.5
years) and volunteers (age, 68±5.5 years) were enrolled from the
Department of Neurology, Ya'an Hospital (Ya'an, Sichuan, China).
The protocol was approved by the Ethics Committee of Ya'an
Hospital. The study was conducted in accordance with the guidelines
and the principles expressed in Ya'an Hospital.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Blood from patients with temporal lobe epilepsy and
volunteers was collected and centrifuged at 3,000 × g for 10 min at
4°C. Total RNA was isolated from the serum using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. Total RNA (1 µg) was
reverse-transcribed using a RevertAid First Strand cDNA synthesis
kit (Thermo Fisher Scientific, Inc.). RT-qPCR was performed using
an ABI Mx3000P qPCR system (Stratagene; Agilent Technologies, Inc.,
Santa Clara, CA, USA) with the Platinum SYBR-Green qPCR Super Mix
UDG (Invitrogen; Thermo Fisher Scientific, Inc.) and according to
the manufacturer's instructions. The primer sequences for
quantitative RT-PCR were as follows: miR-155-5p forward,
5′-GGTCCTTAATGCTAATCGTGATAGGGG-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′; U6 forward, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′
and reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′. miRNA-155 expression level
was calculated by the comparative Cq method (18).
Hippocampal HT22 cell culture and
transfection
Mouse hippocampal HT22 cells were purchased from the
Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,
China) and grown in Dulbecco's modified Eagle's medium (Promega
Corporation, Madison, WI, USA) containing 10% (v/v) fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and incubated in a
humidified atmosphere (95% O2, 5% CO2) at
37°C. The cells were treated with 5 mM glutamate for 24 h.
miRNA-155 (5′-UUAAUGCUAAUCGUGAUAGGGGU-3′) and negative control
plasmid (5′-UUCUCCGAACGUGUCACGUTT-3′) were structured and purchased
by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). The HT22
cells were then seeded in a 6-well plate and transfected with 100
ng of miRNA-155 or 100 ng negative control plasmid using
Lipofectamine® 2000. Following transfection for 48 h,
the cells were used for experimentation. Following transfection for
24 h, 50 nM LY294002 was added to the HT22 cells for 24 h, which
were used for other experiments.
Determination of brain-derived
neurotrophic factor (BDNF) and caspase-3 activity by enzyme-linked
immunosorbent assay
HT22 cells were lysed in a cold RIPA lysis buffer
(Beyotime, Shanghai, China) for 30 min at 4°C and then the
supernatant was collected following centrifugation at 2,000 × g for
20 min at 4°C. Total protein concentration was determined using the
commercial bicinchoninic acid (BCA) assay kit (Beyotime Institute
of Biotechnology, Haimen, China). Total proteins (10 µg) were
incubated with BDNF (H069; Nanjing Jiancheng Biology Engineering
Institute, Nanjing, China) and caspase-3 (G015; Nanjing Jiancheng
Biology Engineering Institute) activity kits. Optical density was
obtained by a microplate reader at a wavelength of 450 or 405
nm.
Western blotting
To evaluate the function of miRNA-155 in epilepsy,
western blotting was used to measure the protein expression of BDNF
and TrkB protein expression. HT22 cells were lysed in a cold RIPA
lysis buffer (Beyotime Institute of Biotechnology) for 30 min at
4°C and then the supernatant was collected following centrifugation
at 2,000 × g for 20 min at 4°C. Total protein concentration was
determined by the commercial BCA assay kit (Beyotime Institute of
Biotechnology). Total proteins (50 µg) were separated using 6–10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then
transferred electrophoretically onto nitrocellulose membranes.
Membranes were blocked using 5% skimmed milk for 1 h at room
temperature and then incubated with anti-BDNF (1:1,000; sc-20981;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-TrkB (1:2,000;
cat. no. 4607; Cell Signaling Technology, Inc., Danvers, MA, USA)
anti-tumor protein p53 (p53; 1:1,000; sc-47698; Santa Cruz
Biotechnology), anti-Bax (1:1,000; sc-6236; Santa Cruz
Biotechnology), anti-PI3K (1:1,000; sc-1331; Santa Cruz
Biotechnology), anti-p-Akt (1:1,000; sc-7985-R; Santa Cruz
Biotechnology), anti-p-mTOR (1:1,000; sc-293133; Santa Cruz
Biotechnology) and anti-glyceraldehyde 3-phosphate dehydrogenase
(GaPDH; 1:5,000; sc-32233; Santa Cruz Biotechnology) overnight at
4°C. The membranes were washed with TBST and then incubated with
the secondary antibodies (1:5,000; sc-2004 or sc-2005; Santa Cruz
Biotechnology) for 2 h at room temperature. Protein visualization
was performed using an enhanced chemiluminescence kit and
quantified using Quantity One software (both Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard error.
Statistical significance was evaluated using Student's t-test for
two groups or one-way analysis of variance with Tukey's post hoc
test for multiple comparisons, and was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
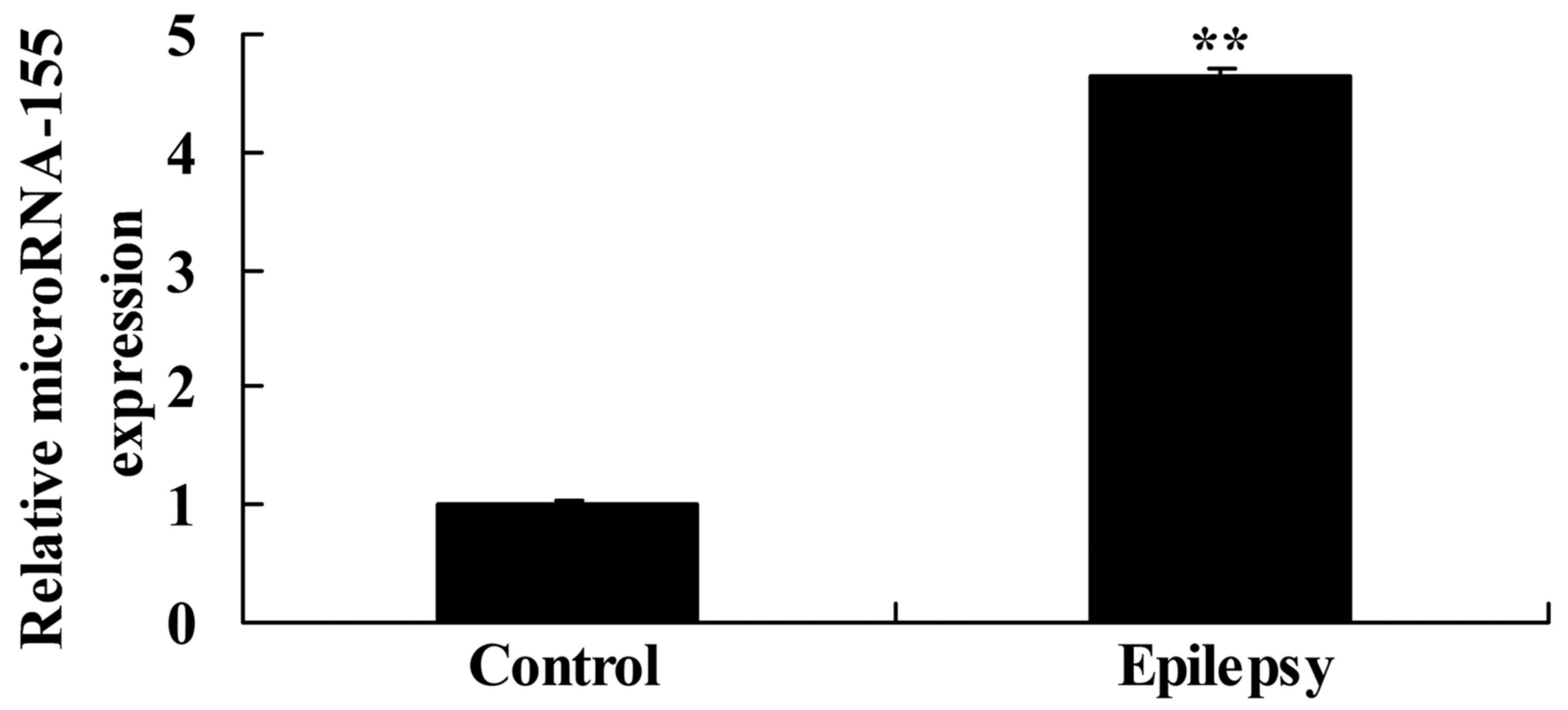
miRNA-155 expression in patients with
temporal lobe epilepsy
miRNA-155 expression was selected for assessment in
patients with temporal lobe epilepsy and volunteers in the present
study. The miRNA-155 expression in patients with temporal lobe
epilepsy was significantly higher than that of the control
volunteer group (Fig. 1).
Overexpression of miRNA-155 decreases
BDNF and tropomyosin receptor kinase B (TrkB) protein
expression
To evaluate the overexpression of miRNA-155 in
epilepsy, BDNF and TrkB protein expression in HT22 cells under
glutamate stimulation was measured (Fig. 2). As displayed in Fig. 2A, B and E, overexpression of
miRNA-155 decreased BDNF and TrkB protein expression in HT22 cells
under glutamate stimulation compared with that in the control or
negative groups.
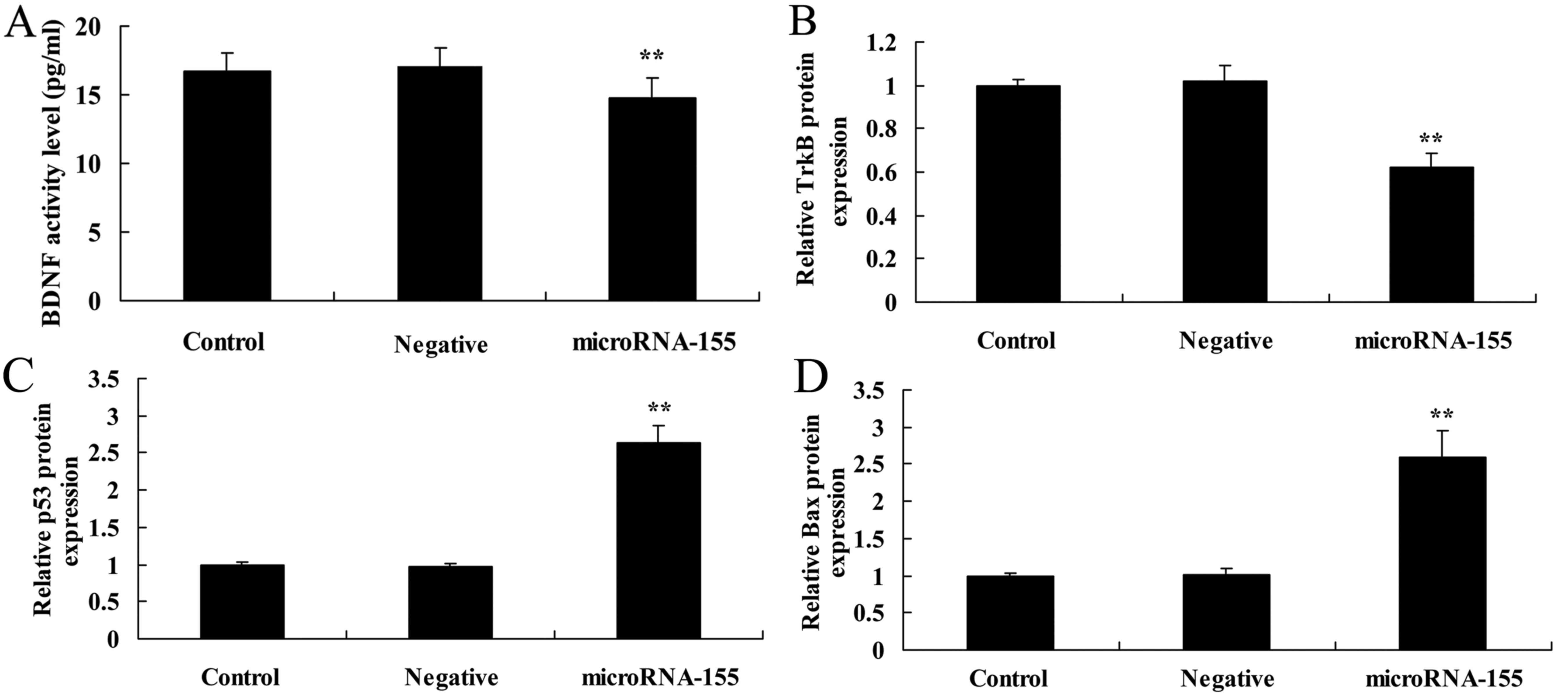 | Figure 2Overexpression of miRNA-155 decreases
BDNF and TrkB protein expression and increases p53 and Bax protein
expression. Overexpression of miRNA-155 decreased (A) BDNF and TrkB
(B) protein expression, and increased (C) p53 and (D) Bax protein
expression, as determined by statistical analysis of (E) western
blot assays. **P<0.01 compared with control. Control,
control group; negative, negative control group; miRNA-155,
overexpression of miRNA-155 group; BDNF, brain-derived neurotrophic
factor; TrkB, tropomyosin receptor kinase B; p53, tumor protein
p53; Bax, apoptosis regulator BAX; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; miRNA, microRNA. |
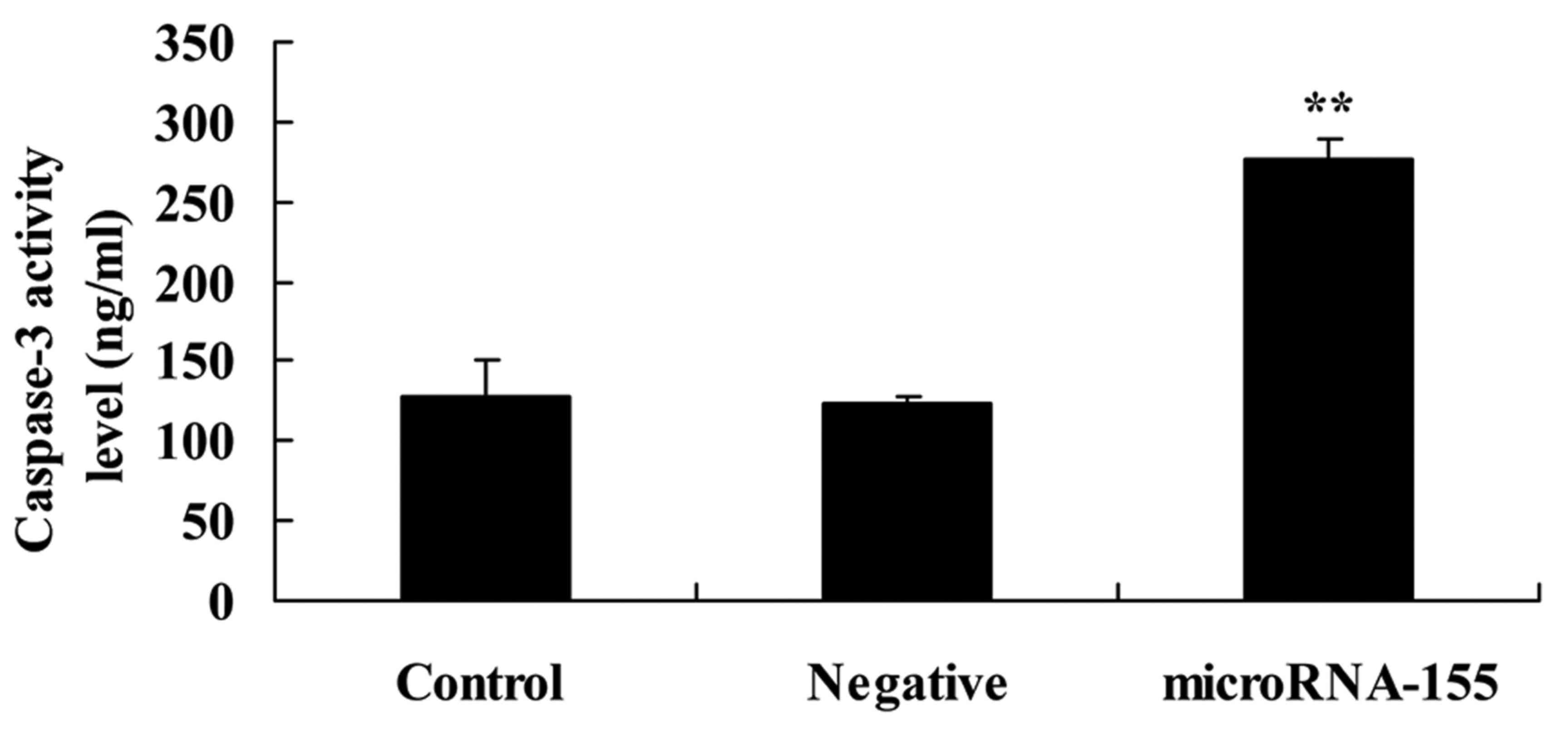
Overexpression of miRNA-155 increases
caspase-3 activity
To investigate whether the overexpression of
miRNA-155 induced apoptosis of epilepsy, caspase-3 activity in HT22
cells under glutamate stimulation was measured in the present
study. As shown in Fig. 3,
overexpression of miRNA-155 increased caspase-3 activity in the
HT22 cells under glutamate stimulation compared with that in the
negative control group.
Overexpression of miRNA-155 increases p53
protein expression
The present study further investigated whether the
overexpression of miRNA-155 induced apoptosis of epilepsy by
measuring p53 protein expression. Fig. 2C and E shows that the
overexpression of miRNA-155 could increase the p53 protein
expression in HT22 cells under glutamate stimulation compared with
that in the negative control group.
Overexpression of miRNA-155 induces Bax
protein expression
Next, the present study investigated whether the
overexpression of miRNA-155 induced Bax protein expression in
epilepsy. As shown in Fig. 2D and
E, overexpression of miRNA-155 induced Bax protein expression
in HT22 cells under glutamate stimulation compared with that in the
negative control group.
Overexpression of miRNA-155 inhibits the
PI3K/Akt signaling pathway
miRNA-155 in the PI3K/Akt signaling pathway was also
investigated in this study (Fig.
4). Fig. 4A, B and D shows
that the overexpression of miRNA-155 suppressed the PI3K/Akt
signaling pathway, and inhibited PI3K and p-Akt protein expression
in HT22 cells under glutamate stimulation compared with that in the
negative control group.
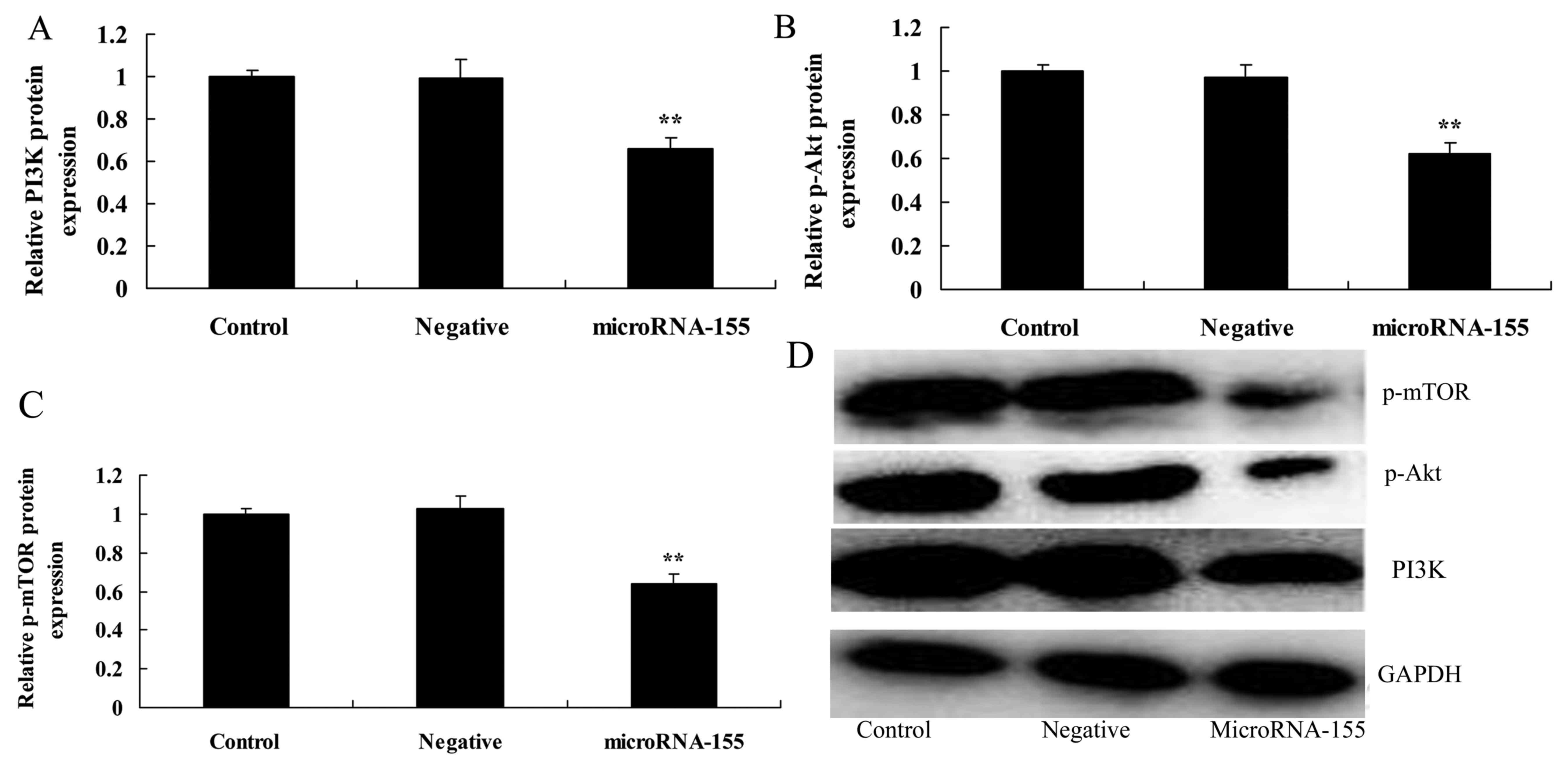 | Figure 4Overexpression of miRNA-155 inhibits
the PI3K/Akt/mTOR signaling pathway. Overexpression of miRNA-155
inhibited (A) PI3K, (B) p-Akt and (C) p-mTOR protein expression, as
determined by statistical analysis and (D) western blot assays.
**P<0.01 compared with control. Control, control
group; negative, negative control group; miRNA-155, overexpression
of miRNA-155 group; PI3K, phosphoinositide 3-kinase; Akt, protein
kinase B; mTOR, mechanistic target of rapamycin; p-,
phosphorylated; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
miRNA, microRNA. |
Overexpression of miRNA-155 inhibits mTOR
protein expression
Downstream of PI3K/Akt signaling is mTOR, which was
investigated to enrich our analysis of miRNA-155 in epilepsy. The
overexpression of miRNA-155 also inhibited the mTOR signaling
pathway, and suppressed p-mTOR protein expression in HT22 cells
under glutamate stimulation compared with that in the negative
control group (Fig. 4C and
D).
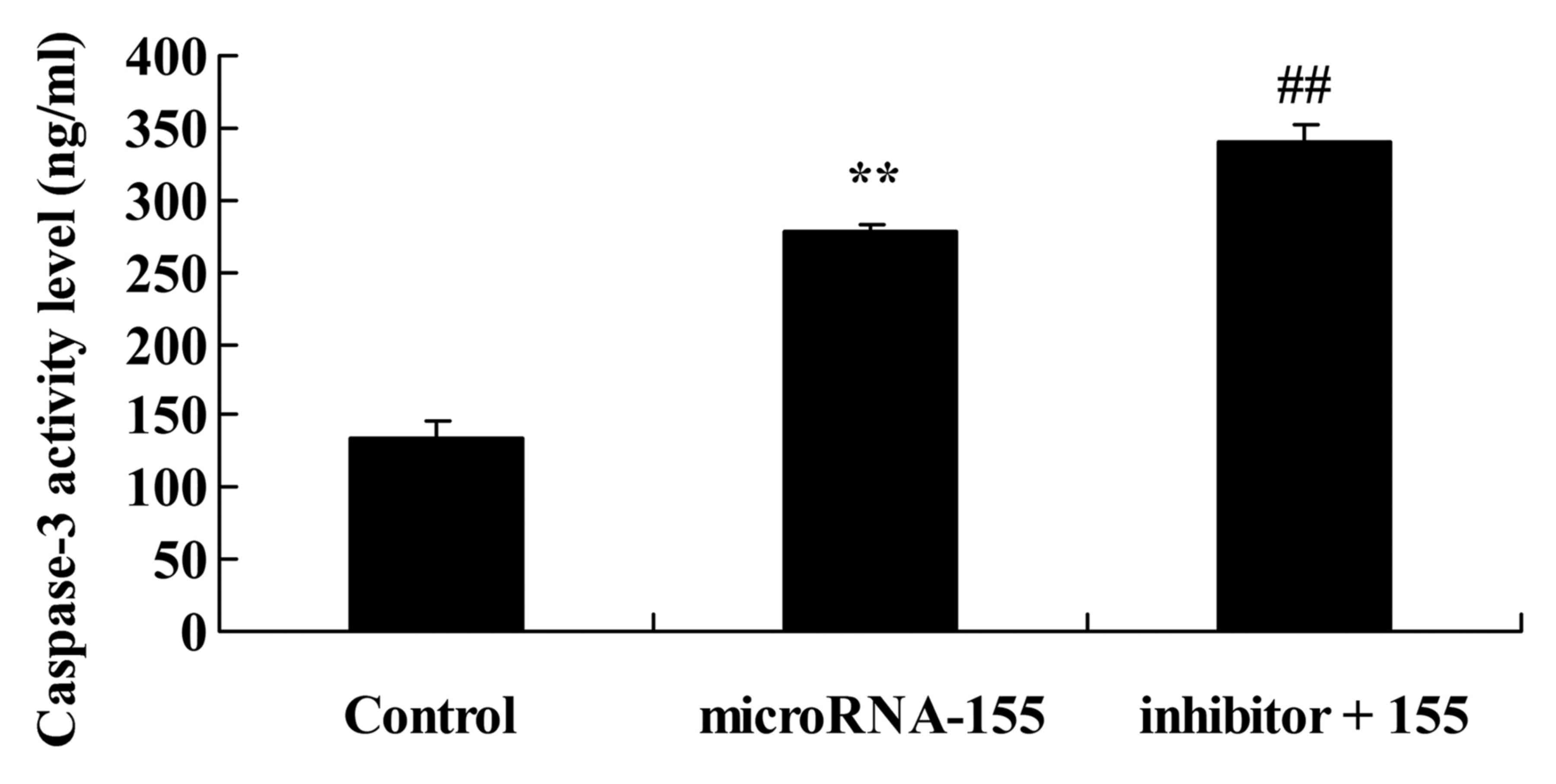
Inhibition of PI3K accelerates the effect
of miRNA-155 on the suppression of the PI3K/Akt/mTOR signaling
pathway
According to the aforementioned results, the
PI3K/Akt signaling pathway may participate in the effect of
miRNA-155 on epilepsy. PI3K inhibitor suppressed the PI3K/Akt
signaling pathway and inhibited PI3K, p-Akt and p-mTOR protein
expression in HT22 cells under glutamate stimulation following
miRNA-155 overexpression (Fig.
5).
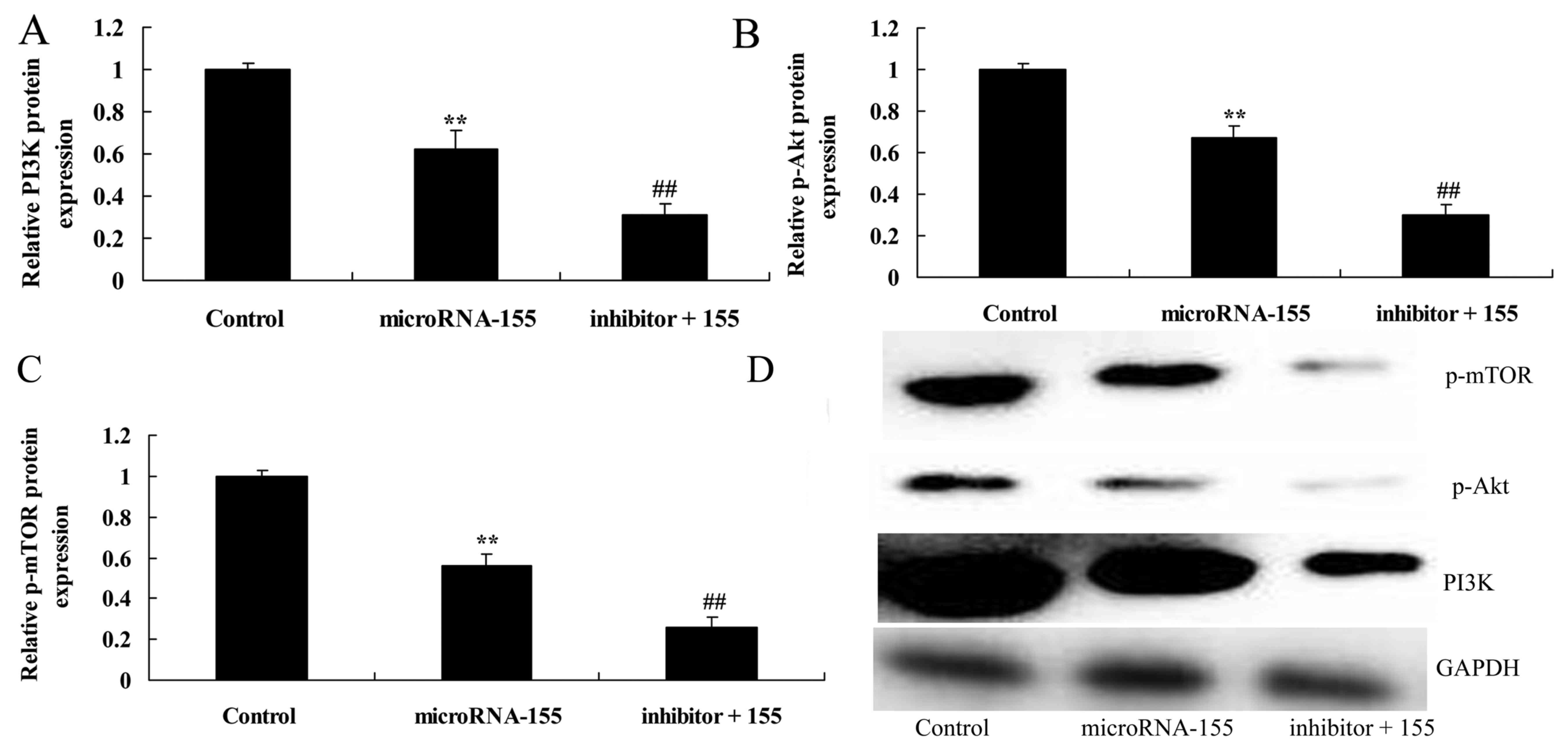 | Figure 5Inhibition of PI3K accelerates the
effect of miRNA-155 on the suppression of the PI3K/Akt/mTOR
signaling pathway. Inhibition of PI3K accelerated the effect of
miRNA-155 on the inhibition of (A) PI3K, (B) p-Akt and (C) p-mTOR
protein expression, as determined by statistical analysis and (D)
western blot assays. **P<0.01 compared with control
group; ##P<0.01 compared with miRNA-155 group.
Control, control group; miRNA-155, overexpression of miRNA-155
group; inhibitor + 115, PI3K inhibitor + overexpression of
miRNA-155 group; PI3K, phosphoinositide 3-kinase; Akt, protein
kinase B; mTOR, mechanistic target of rapamycin; p-,
phosphorylated; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
miRNA, microRNA. |
Inhibition of PI3K accelerates the effect
of miRNA-155 on the suppresion BDNF and TrkB protein
expression
The change in BDNF and TrkB protein expression was
investigated whilst assessing the effect of miRNA-155 on epilepsy
(Fig. 6). Inhibition of PI3K
decreased the BDNF and TrkB protein expression in HT22 cells under
glutamate stimulation following miRNA-155 overexpression (Fig. 6A, B and E).
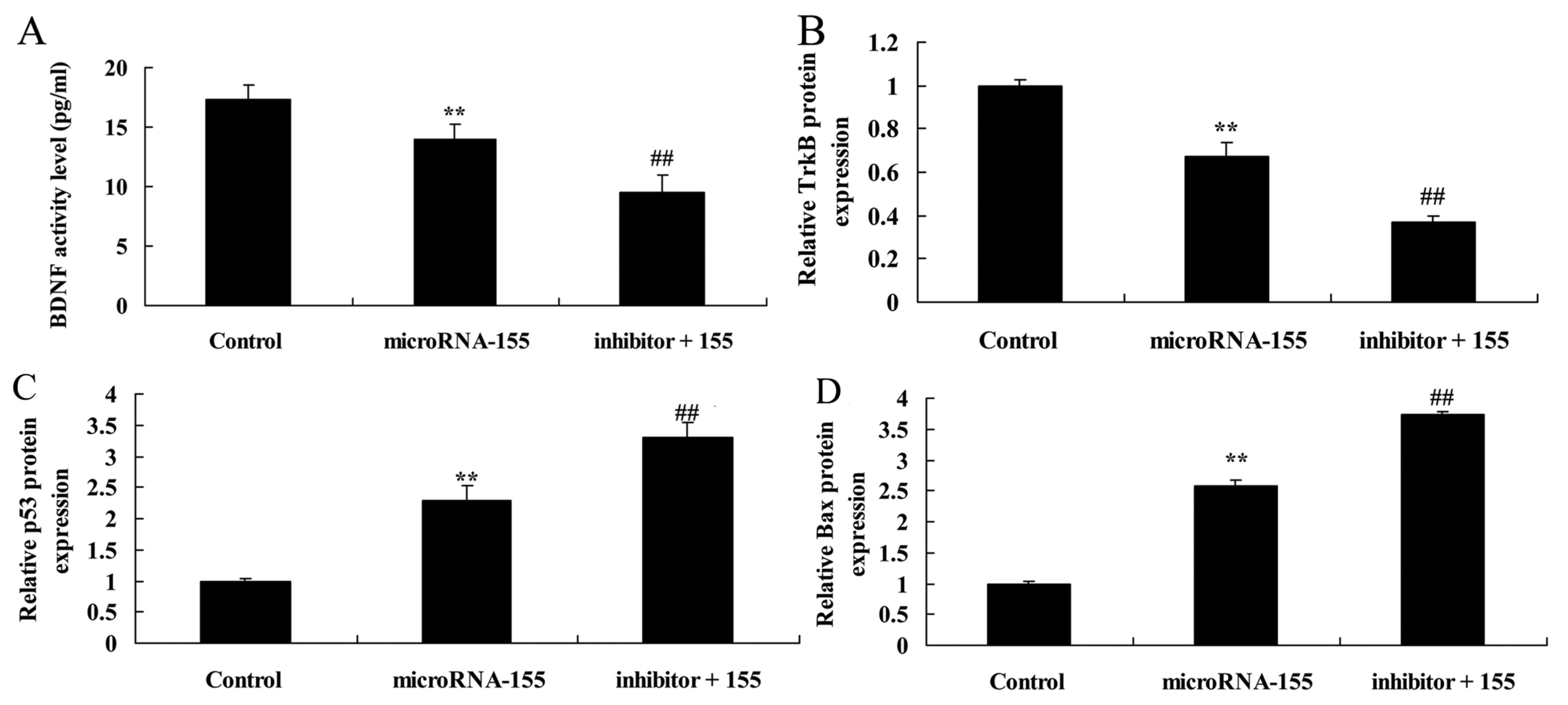 | Figure 6Inhibition of PI3K accelerates the
effect of miRNA-155 inhibition of BDNF and TrkB protein expression,
and induction of p53 and Bax protein expression. Inhibition of PI3K
accelerated the effect of miRNA-155 inhibition of (A) BDNF, (B)
TrkB, (C) p53 and (D) Bax protein expression, as determined by
statistical analysis and (E) western blot assays.
**P<0.01 compared with control;
##P<0.01 compared with miRNA-155. Control, control
group; miRNA-155, overexpression of miRNA-155 group; inhibitor +
115, PI3K inhibitor + overexpression of miRNA-155 group; PI3K,
phosphoinositide 3-kinase; BDNF, brain-derived neurotrophic factor;
TrkB, tropomyosin receptor kinase B; miRNA, microRNA; p53, tumor
protein p53; Bax, apoptosis regulator BAX; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Inhibition of PI3K accelerates the effect
of miRNA-155 on the induction of caspase-3 activity
The PI3K/Akt signaling pathway is concerned with the
apoptosis mechanism of miRNA-155 in epilepsy. As shown in Fig. 7, inhibition of PI3K increased
caspase-3 activity in HT22 cells under glutamate stimulation
following miRNA-155 overexpression.
Inhibition of PI3K accelerates the effect
of miRNA-155 on the induction of p53 and Bax protein
expression
The PI3K/Akt signaling pathway serves a critical
role in miRNA-155 in epilepsy. As shown in Fig. 6C–E, inhibition of PI3K promoted
p53 and Bax protein expression in HT22 cells under glutamate
stimulation following miRNA-155 overexpression.
Discussion
Epilepsy is a brain disease with features that
generate a susceptibility to continuous epileptic attack and that
also present with corresponding neurobiological, cognitive,
psychological and societal consequences (19). Epilepsy is a common disease of the
nervous system that seriously threatens human health, not only
doing harm to the patients, but also conferring a heavy burden on
the family and on society. A domestic epidemiological survey showed
that the prevalence rate of epilepsy is 0.7% (2). Thus, it is predicted that there are
~9 million patients globally, with 1/3 patients experiencing
refractory epilepsy. The definition of refractory epilepsy has not
been reached by consensus thus far (3). The majority of scholars suggest that
sequential or combined applications of 3 or more anti-epilepsy
drugs can provide a sufficient or tolerated dosage, and have
observed a sufficiently long course of treatment (20). In refractory epilepsy, the attack
times do not reduce or even increase slightly as a result (21). The findings of the present study
indicated that the miRNA-155 expression in patients with temporal
lobe epilepsy was significantly higher than that of the control
volunteer group.
As it is hard to extract materials from the human
brain of epilepsy patients, it is also difficult to directly detect
the miRNA expression in human brain tissues in vitro
(10). Cerebrospinal fluid is
mainly generated in the choroid plexus tissues, and is created and
absorbed into the veins constantly (22). The fluid serves a lymphatic role
in the central nervous system; it provides a certain degree of
nutrition to the brain cells, carries away metabolites of brain
tissues, regulates the acid-base balance of the central nervous
system, reduces the pressure in the brain and spinal cord, and
protects and supports the brain and spinal cord (23). Meanwhile, cerebrospinal fluid
envelops the brain parenchyma, can contact the external cell gap
directly, and can reflect the pathological and physiological
changes of brain tissues dynamically (22). Soluble molecular biomarkers in
cerebrospinal fluid are of value in studying brain diseases
(24). miRNAs in the
cerebrospinal fluid can be regarded as potential biomarkers in the
central nervous system, particularly for Alzheimer's disease,
Huntington's chorea, disseminated sclerosis, schizophrenia and
bipolar affective disorder (25).
Furthermore, the present data also evidently showed that
overexpression of miRNA-155 decreased BDNF level and TrkB protein
expression in HT22 cells under glutamate stimulation.
In the process of an epileptic attack, repeated
seizures may cause ischemia and anoxia of brain tissues, release
excitatory amino acids and cause an inward sodium current, so as to
launch a caspase chain reaction (26). Epilepsy can generate free
radicals, nerve-nitric oxide synthase, mediate apoptosis core and
execute protein kinase caspase-3, make important protein degraded
and inactivated, such as cytoplasm, cell nucleus and cytoskeleton
and result in the apoptosis and deficiency of numerous nerve cells
(27). Nerve cell damage is one
of the reasons for chronic spontaneous epilepsy (28). Finally it may form refractory TLE.
The morbidity of TLE accounts for 25% of epilepsy cases. In
refractory epilepsy, even once patients have been treated using
multiple anti-epilepsy drugs, the attacks still cannot be
controlled (28). Therefore,
controlling the deficiency of nerve cells after an epileptic attack
has important significance for refractory epilepsy. Epilepsy nerve
cell apoptosis is regulated by a series of genes, including miRNAs,
Bcl-2, Bax and p53 (29). The
abnormal expression of these genes serves an important role in the
apoptosis of nerve cells. The apoptosis of the nerve cells occurs
by launching the internal death mechanism of the cells. Studies on
relevant genes associated with epilepsy nerve apoptosis are
increasing in number. In the present study, overexpression of
miRNA-155 increased caspase-3 activity, and p53 and Bax expression,
and reduced PI3K, p-Akt and p-mTOR protein expression in epilepsy
cells.
The PI3K/Akt signal transduction pathway is widely
applied in cells. PI3K is the dimer protein with a P110 catalytic
subunit and a p85 regulating subunit. PI3K has protein kinases and
lipoid kinase activity (15). Akt
is the direct downstream substrate of PI3K with a relative
molecular mass is 60 kDa. Akt serves an important role in the
signal transduction pathway. PI3K and JAK2 activation induced
erythropoeitin (EPO), which caused p-Akt protein expression
(30). p-JAK2 makes PI3K
regulated subunits to combine. When the regulated subunit is
combined with EPO-R, the catalytic subunit is activated and
phosphorylates Akt. p-Akt induced Bcl-2 protein expression and
suppressed caspase-9 protein expression. In summary, activated Akt
can phosphorylate apoptosis proteins or change the expression level
of apoptosis genes indirectly, so as to regulate the apoptotic
process (31). The specific
mechanism refers to restraining the activation of caspase family
members and restraining the apoptosis caused by caspase; releasing
apoptosis by reducing release of CytC; regulating activity of Bcl-2
family members, making Bad and Bax residues phosphorylated and
making them inactivated and restraining apoptosis (27). The present study showed that PI3K
inhibitor accelerated the effect of miRNA-155 on the inhibition of
BDNF and TrkB protein expression, and on the promotion of caspase-3
activity and p53 and Bax protein expression in epilepsy cells.
According to existing research results, the mTOR
signaling pathway participates in multiple of pathological changes
at the modular and cellular level, including apoptosis, gliosis,
and changes to synaptic plasticity, neurotransmitter receptor, ion
channel and axon budding, associated with epilepsy (32). In genetic diseases, including TSC,
PMSE, sporadic diseases PCD and GG, and acquired epilepsy, the mTOR
signal pathway is activated abnormally (32). Sirolimus affects the mTOR
signaling pathway; it can reduce an epilepsy attack to a certain
extent or reverse pathological changes (33). Taken together, the results of the
present study indicated that the inhibition of PI3K enhanced the
effect of miRNA-155 on p-mTOR protein expression in epilepsy
cells.
In conclusion, the present study demonstrated that
miRNA-155 contributes to the occurrence of epilepsy, and also
provided evidence that miRNA-155 exhibits a neuroprotective effect
on epilepsy-induced neuronal apoptosis through the PI3K/Akt/mTOR
signaling pathway. These findings suggest that miRNA-155 induced
neuronal apoptosis in epilepsy, which may open up new avenues in
the treatment of refractory epilepsy.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XRW designed the experiment. WD and YC performed the
experiment and analyzed the data. XRW wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics Committee of
Ya'an Hospital. The study was conducted in accordance with the
guidelines and the principles expressed in Ya'an Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lazzari AA, Dussault PM, Thakore-James M,
Gagnon D, Baker E, Davis SA and Houranieh AM: Prevention of bone
loss and vertebral fractures in patients with chronic epilepsy -
anti-epileptic drug and osteoporosis prevention trial. Epilepsia.
54:1997–2004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delger AB, Avakyan GN, Oleinikova OM,
Bogomazova MA, Chromych EA and Lagutin IuV: Effects of tenoten on
anxiety and depression disorders in patients with epilepsy. Bull
Exp Biol Med. 153:704–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacoby A, Sudell M, Tudur Smith C,
Crossley J, Marson AG and Baker GA; SANAD Study Group:
Quality-of-life outcomes of initiating treatment with standard and
newer antiepileptic drugs in adults with new-onset epilepsy:
Findings from the SANAD trial. Epilepsia. 56:460–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jóźwiak S, Kotulska K, Domańska-Pakieła D,
Lojszczyk B, Syczewska M, Chmielewski D, Dunin-Wąsowicz D, Kmieć T,
Szymkiewicz-Dangel J, Kornacka M, et al: Antiepileptic treatment
before the onset of seizures reduces epilepsy severity and risk of
mental retardation in infants with tuberous sclerosis complex. Eur
J Paediatr Neurol. 15:424–431. 2011. View Article : Google Scholar
|
|
5
|
French JA, Abou-Khalil BW, Leroy RF,
Yacubian EM, Shin P, Hall S, Mansbach H and Nohria V; RESTORE
1/Study 301 Investigators: Randomized, double-blind,
placebo-controlled trial of ezogabine (retigabine) in partial
epilepsy. Neurology. 76:1555–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma S, Sankhyan N, Gulati S and
Agarwala A: Use of the modified Atkins diet for treatment of
refractory childhood epilepsy: A randomized controlled trial.
Epilepsia. 54:481–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kerr EN and Blackwell MC: Near-transfer
effects following working memory intervention (Cogmed) in children
with symptomatic epilepsy: An open randomized clinical trial.
Epilepsia. 56:1784–1792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li MM, Li XM, Zheng XP, Yu JT and Tan L:
MicroRNAs dysregulation in epilepsy. Brain Res. 1584:94–104. 2014.
View Article : Google Scholar
|
|
9
|
Ashhab MU, Omran A, Kong H, Gan N, He F,
Peng J and Yin F: Expressions of tumor necrosis factor alpha and
microRNA-155 in immature rat model of status epilepticus and
children with mesial temporal lobe epilepsy. J Mol Neurosci.
51:950–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Wang J, Jiang C, Zheng G, Lu X and
Guo H: Association of the genetic polymorphisms in pre-microRNAs
with risk of childhood epilepsy in a Chinese population. Seizure.
40:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li MM, Jiang T, Sun Z, Zhang Q, Tan CC, Yu
JT and Tan L: Genome-wide microRNA expression profiles in
hippocampus of rats with chronic temporal lobe epilepsy. Sci Rep.
4:47342014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henshall DC: MicroRNA and epilepsy:
Profiling, functions and potential clinical applications. Curr Opin
Neurol. 27:199–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue Y, Xie N, Cao L, Zhao X, Jiang H and
Chi Z: Diazoxide preconditioning against seizure-induced oxidative
injury is via the PI3K/Akt pathway in epileptic rat. Neurosci Lett.
495:130–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao Z, Peng J, Yang L, Kong H and Yin F:
Interleukin-1β plays a role in the pathogenesis of mesial temporal
lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in
hippocampal neurons. J Neuroimmunol. 282:110–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng H, Wang X, Tang Z, Zheng W and Li Z:
The PI3K/Akt and ERK1/2 signaling pathways mediate the
erythropoietin-modulated calcium influx in kainic acid-induced
epilepsy. Neuroreport. 24:335–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong M: mTOR strikes again: mTORC1
activation causes epilepsy independent of overt pathological
changes. Epilepsy Curr. 14:41–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bockaert J and Marin P: mTOR in brain
physiology and pathologies. Physiol Rev. 95:1157–1187. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Aalbers MW, Klinkenberg S, Rijkers K,
Verschuure P, Kessels A, Aldenkamp A, Vles J and Majoie M: The
effects of vagus nerve stimulation on pro- and anti-inflammatory
cytokines in children with refractory epilepsy: An exploratory
study. Neuroimmunomodulation. 19:352–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meurer WJ, Silbergleit R, Nicholas KS,
Burke JF and Durkalski V: Accounting for repeat enrollments during
an emergency clinical trial: The Rapid Anticonvulsant Medications
Prior to Arrival Trial (RAMPART). Acad Emerg Med. 22:373–377. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryvlin P, Gilliam FG, Nguyen DK, Colicchio
G, Iudice A, Tinuper P, Zamponi N, Aguglia U, Wagner L, Minotti L,
et al: The long-term effect of vagus nerve stimulation on quality
of life in patients with pharmacoresistant focal epilepsy: The
PuLsE (Open Prospective Randomized Long-term Effectiveness) trial.
Epilepsia. 55:893–900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu K, Xie YY, Zhang C, Ouyang DS, Long HY,
Sun DN, Long LL, Feng L, Li Y and Xiao B: MicroRNA expression
profile of the hippocampus in a rat model of temporal lobe epilepsy
and miR-34a-targeted neuroprotection against hippocampal neurone
cell apoptosis post-status epilepticus. BMC Neurosci. 13:1152012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zucchini S, Marucci G, Paradiso B, Lanza
G, Roncon P, Cifelli P, Ferracin M, Giulioni M, Michelucci R,
Rubboli G, et al: Identification of miRNAs differentially expressed
in human epilepsy with or without granule cell pathology. PLoS One.
9:e1055212014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reschke CR and Henshall DC: microRNA and
Epilepsy. Adv Exp Med Biol. 888:41–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manna I, Labate A, Borzì G, Mumoli L,
Cavalli SM, Sturniolo M, Quattrone A and Gambardella A: An SNP site
in primiR-124, a brain expressed miRNA gene, no contribution to
mesial temporal lobe epilepsy in an Italian sample. Neurol Sci.
37:1335–1339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tzeng TT, Tsay HJ, Chang L, Hsu CL, Lai
TH, Huang FL and Shiao YJ: Caspase 3 involves in neuroplasticity,
microglial activation and neurogenesis in the mice hippocampus
after intracerebral injection of kainic acid. J Biomed Sci.
20:902013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aanandhi MV, Bhattacherjee D, Ray A and
George PS: New avenue in the treatment of temporal lobe epilepsy by
classical anti-epileptics: A hypothetical establishment of
executioner Caspase 3 inactivation by molecular modeling. J Adv
Pharm Technol Res. 6:65–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng XJ, Wang F and Li CK: Resveratrol is
neuroprotective and improves cognition in
pentylenetetrazole-kindling model of epilepsy in rats. Indian J
Pharm Sci. 76:125–131. 2014.PubMed/NCBI
|
|
29
|
Chen X, Bao G, Hua Y, Li Y, Wang Z and
Zhang X: The effects of topiramate on caspase-3 expression in
hippocampus of baso-lateral amygdala (BLA) electrical kindled
epilepsy rat. J Mol Neurosci. 38:201–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang B and Wong M:
Pentylenetetrazole-induced seizures cause acute, but not chronic,
mTOR pathway activation in rat. Epilepsia. 53:506–511. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jansen LA, Mirzaa GM, Ishak GE, O'Roak BJ,
Hiatt JB, Roden WH, Gunter SA, Christian SL, Collins S, Adams C, et
al: PI3K/AKT pathway mutations cause a spectrum of brain
malformations from megalencephaly to focal cortical dysplasia.
Brain. 138:1613–1628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Avet-Rochex A, Carvajal N, Christoforou
CP, Yeung K, Maierbrugger KT, Hobbs C, Lalli G, Cagin U, Plachot C,
McNeill H, et al: Unkempt is negatively regulated by mTOR and
uncouples neuronal differentiation from growth control. PLoS Genet.
10:e10046242014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye M, Bi YF, Ding L, Zhu WW and Gao W:
Saikosaponin a functions as anti-epileptic effect in
pentylenetetrazol induced rats through inhibiting mTOR signaling
pathway. Biomed Pharmacother. 81:281–287. 2016. View Article : Google Scholar : PubMed/NCBI
|