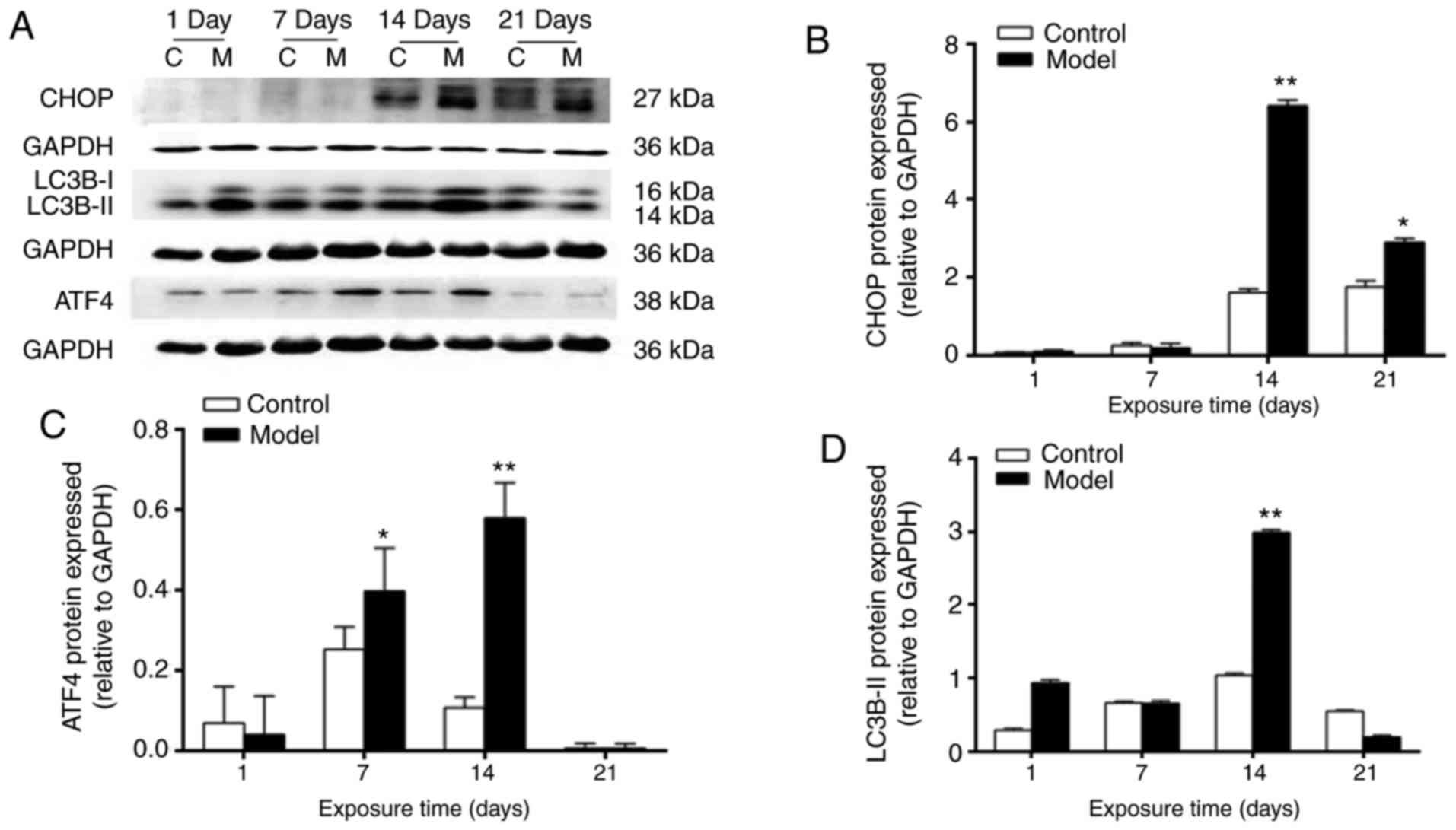
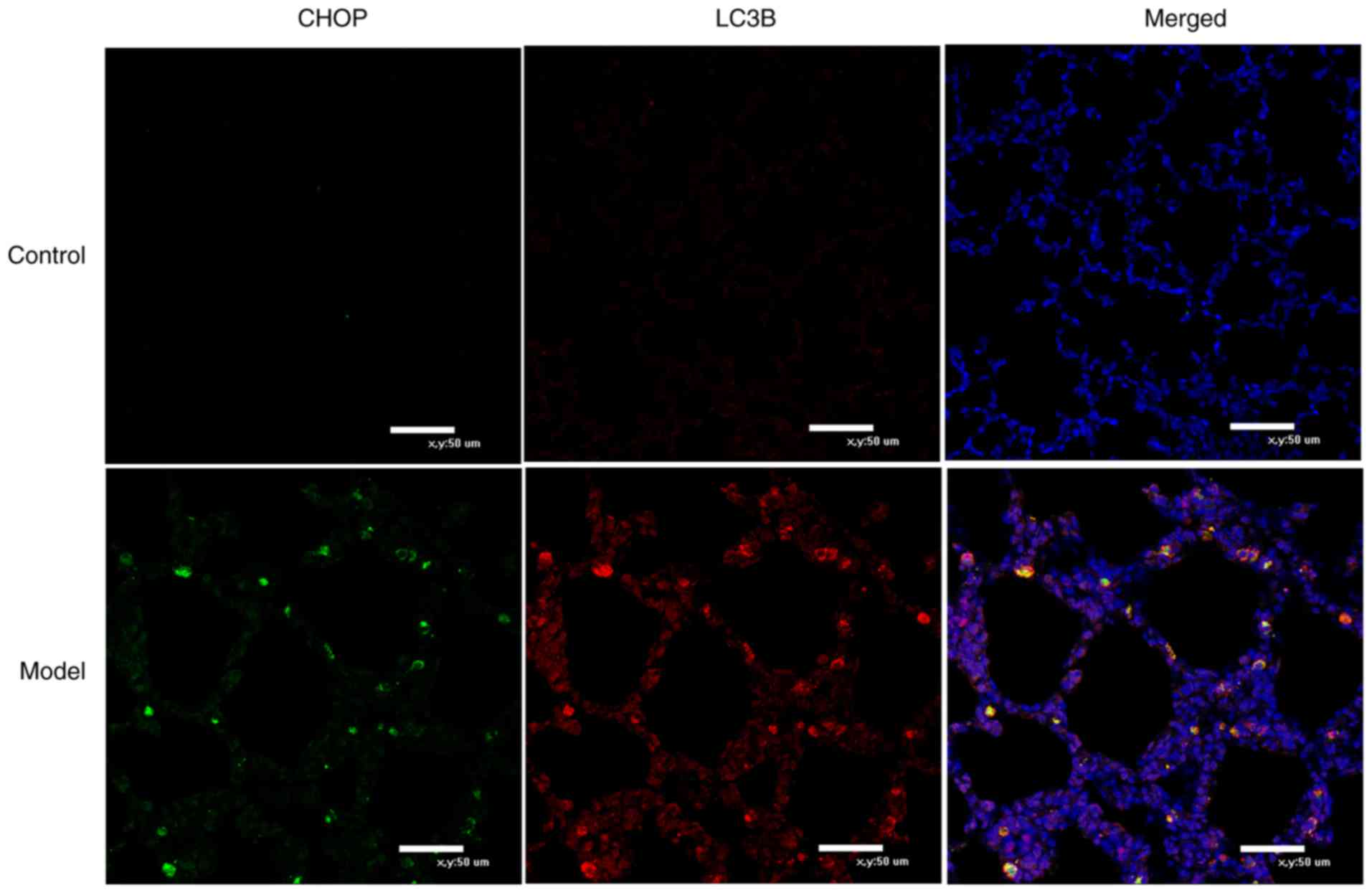
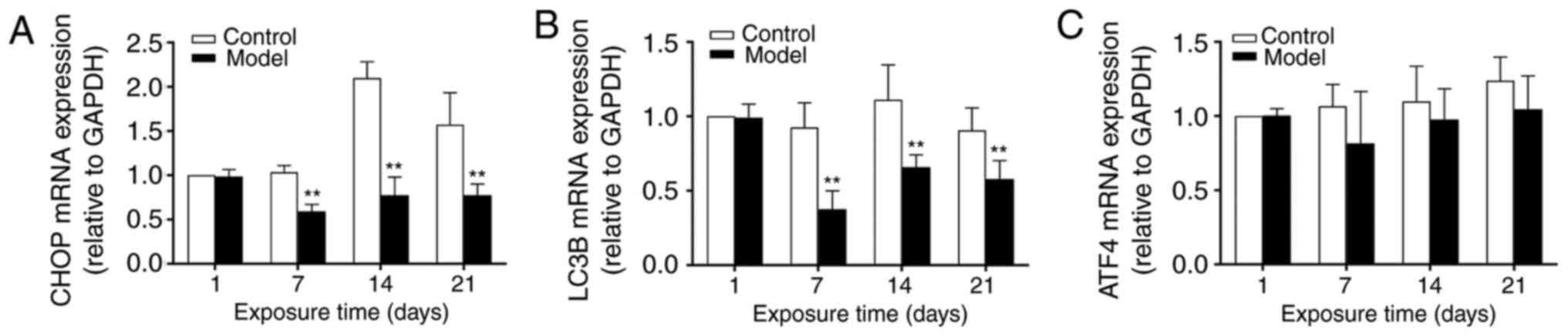
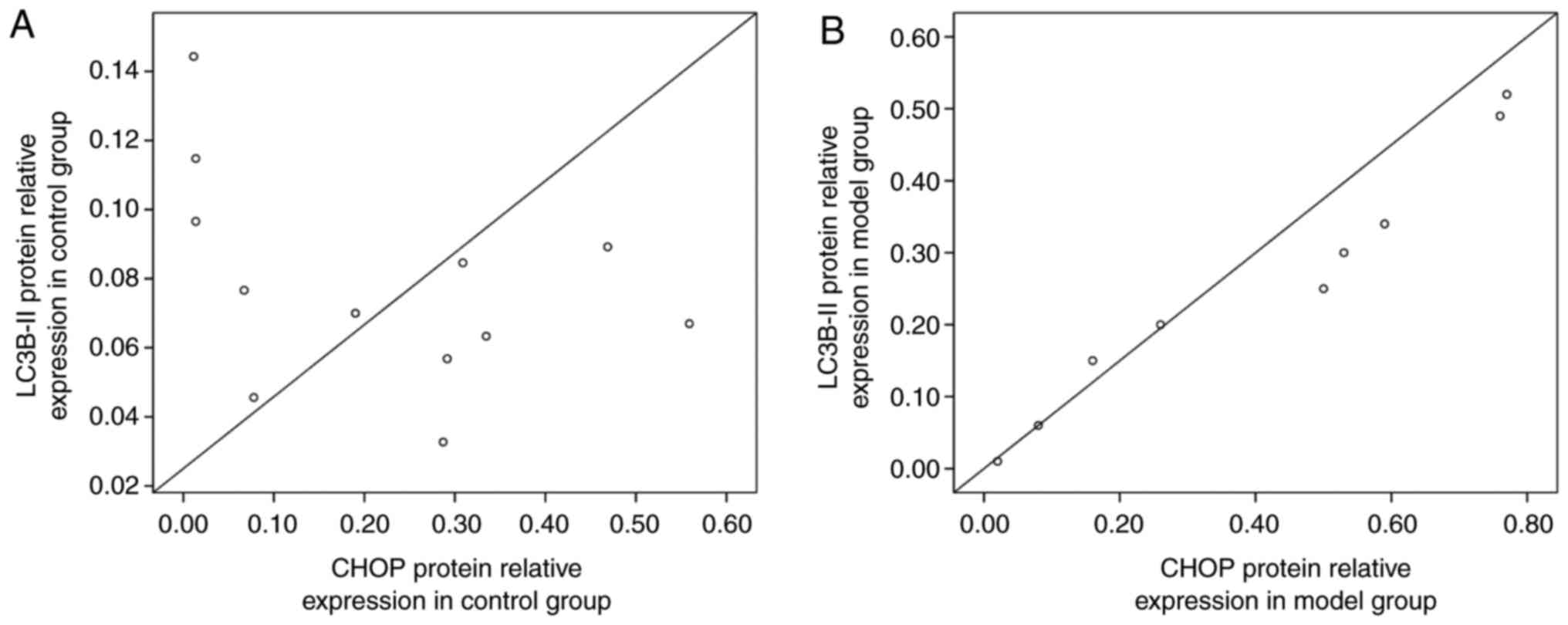
|
1
|
Isayama T, Lee SK, Mori R, Kusuda S,
Fujimura M, Ye XY and Shah PS; Canadian Neonatal Network, Neonatal
Research Network of Japan: Comparison of mortality and morbidity of
very low birth weight infants between Canada and Japan. Pediatrics.
130:e957–e965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
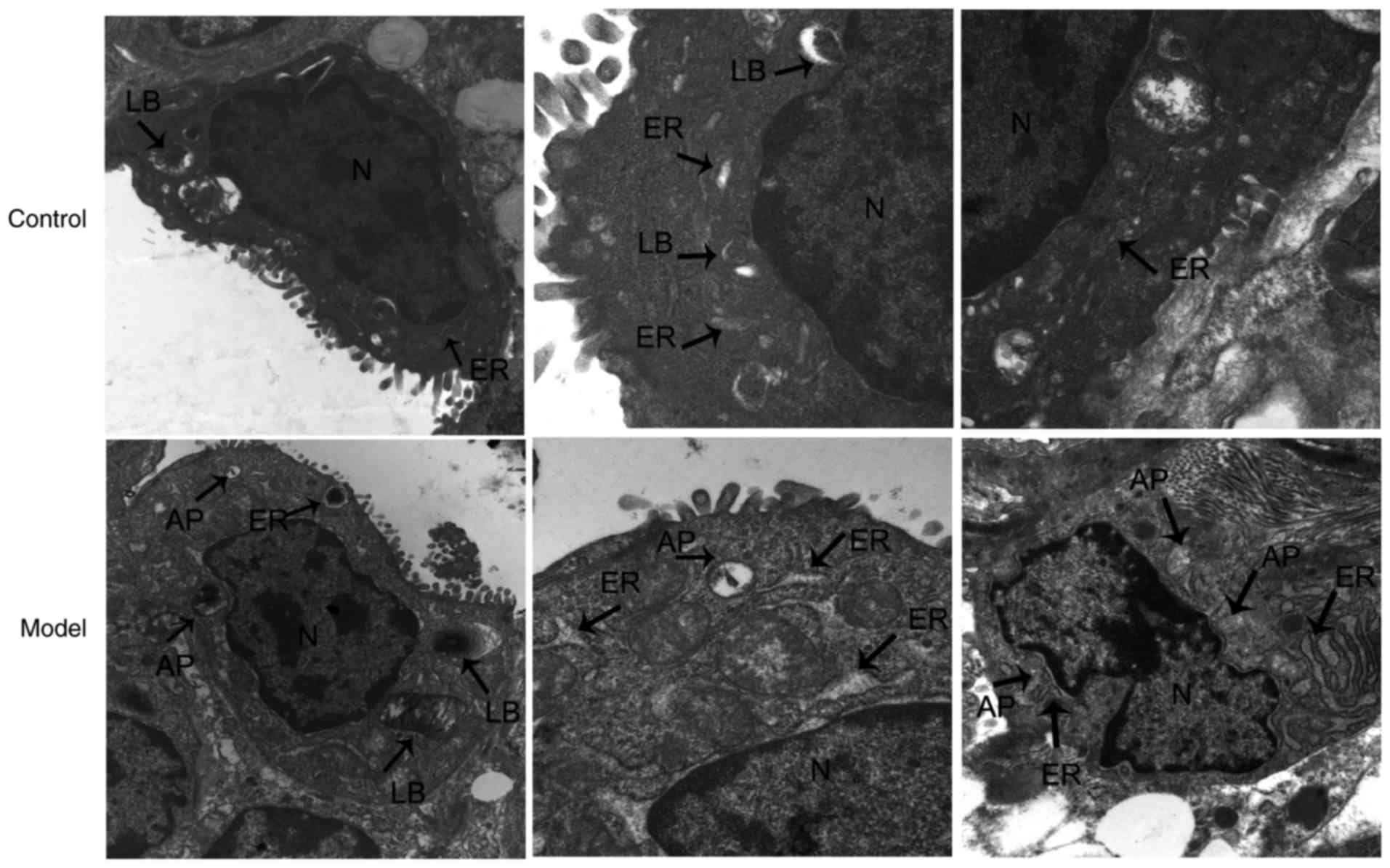
|
Silva DM, Nardiello C, Pozarska A and
Morty RE: Recent advances in the mechanisms of lung alveolarization
and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol
Lung Cell Mol Physiol. 309:L1239–L1272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dani C and Poggi C: The role of genetic
polymorphisms in antioxidant enzymes and potential antioxidant
therapies in neonatal lung disease. Antioxid Redox Signal.
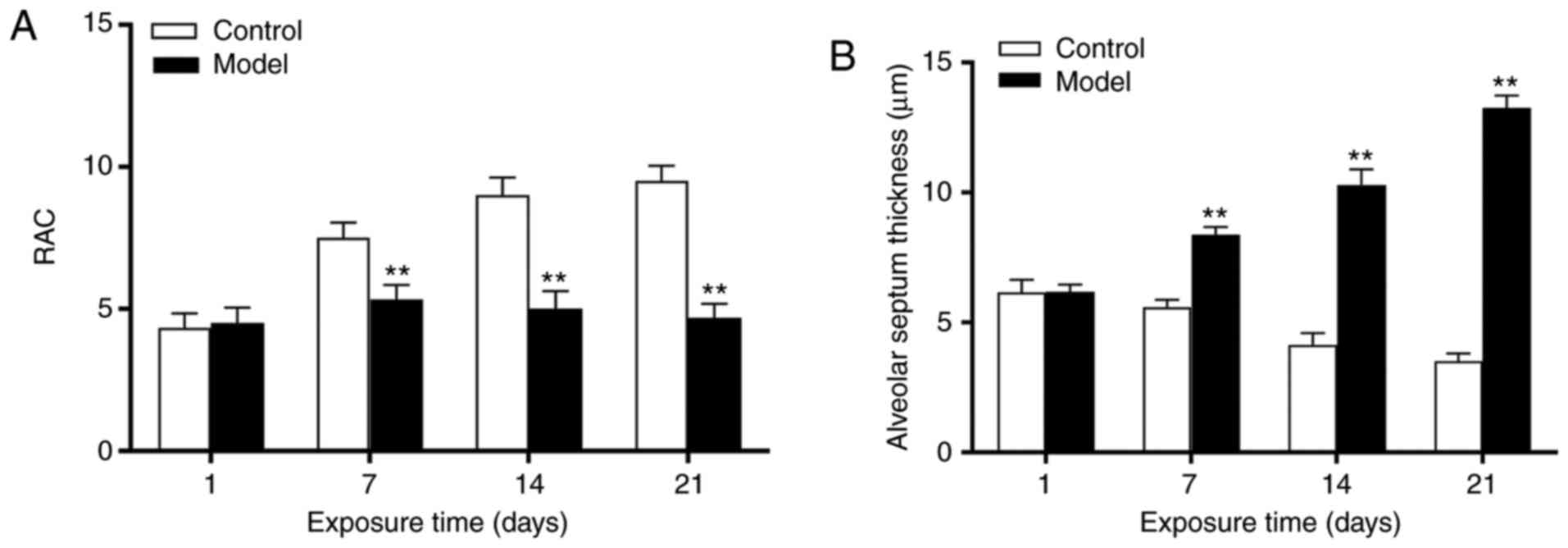
21:1863–1880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gitto E, Pellegrino S, D'Arrigo S, Barberi
I and Reiter RJ: Oxidative stress in resuscitation and ventilation
of newborns. Eur Respir J. 34:1461–1469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perrone S, Negro S, Tataranno ML and
Buonocore G: Oxidative stress and antioxidant strategies in
newborns. J Matern Fetal Neonatal Med Suppl. 3:63–65. 2010.
View Article : Google Scholar
|
|
6
|
Perrone S, Tataranno ML and Buonocore G:
Oxidative stress and bronchopulmonary dysplasia. J Clin Neonatol.
1:109–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Li W, Liu W, Cai B, Cheng T, Gao
C, Mo L, Yang H and Chang L: GSTM1 and GSTT1 gene polymorphisms as
major risk factors for bronchopulmonary dysplasia in a Chinese Han
population. Gene. 533:48–51. 2014. View Article : Google Scholar
|
|
8
|
Hsiao CC, Chang JC, Tsao LY, Yang RC, Chen
HN, Lee CH, Lin CY and Tsai YG: Correlates of elevated
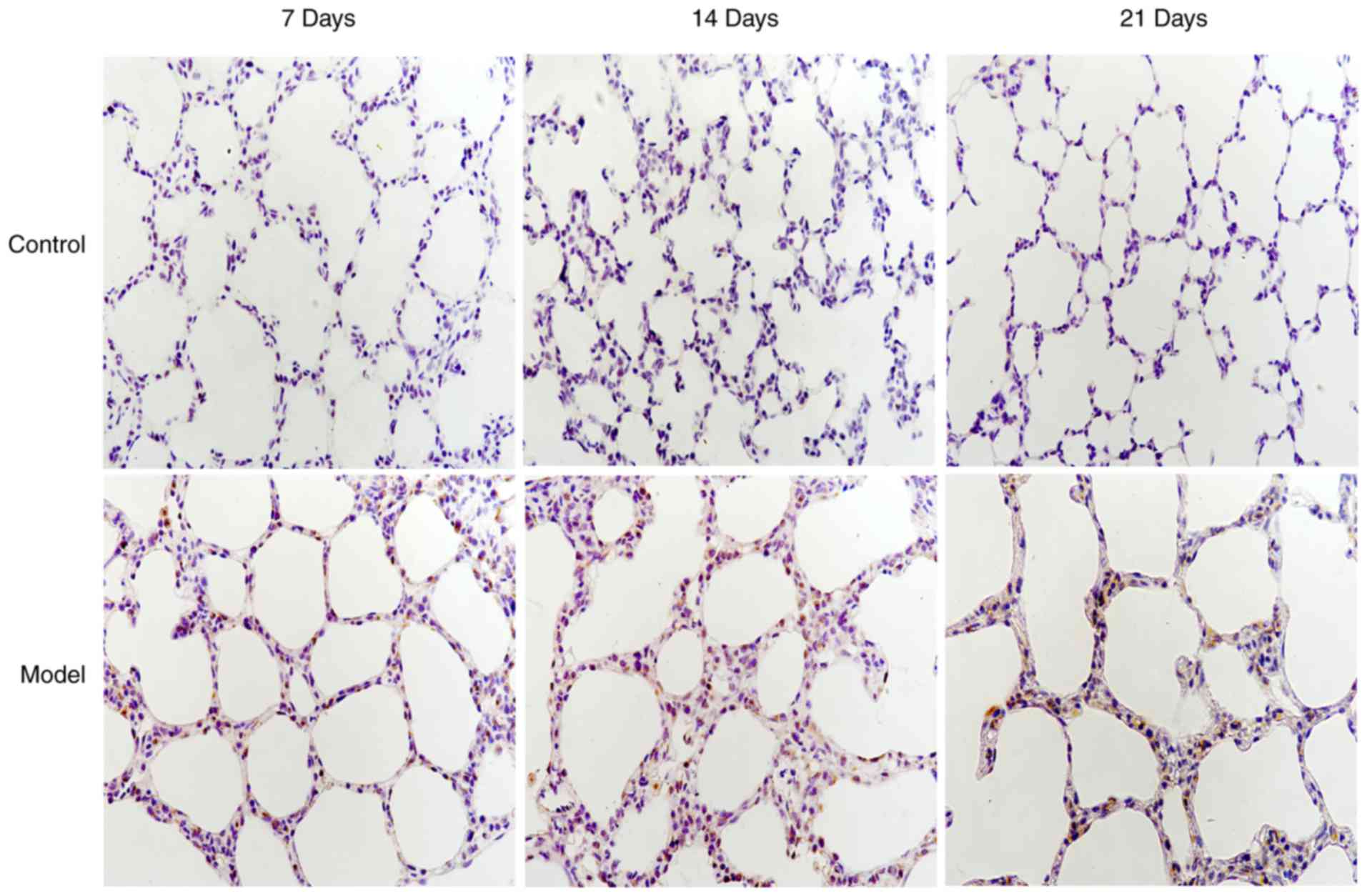
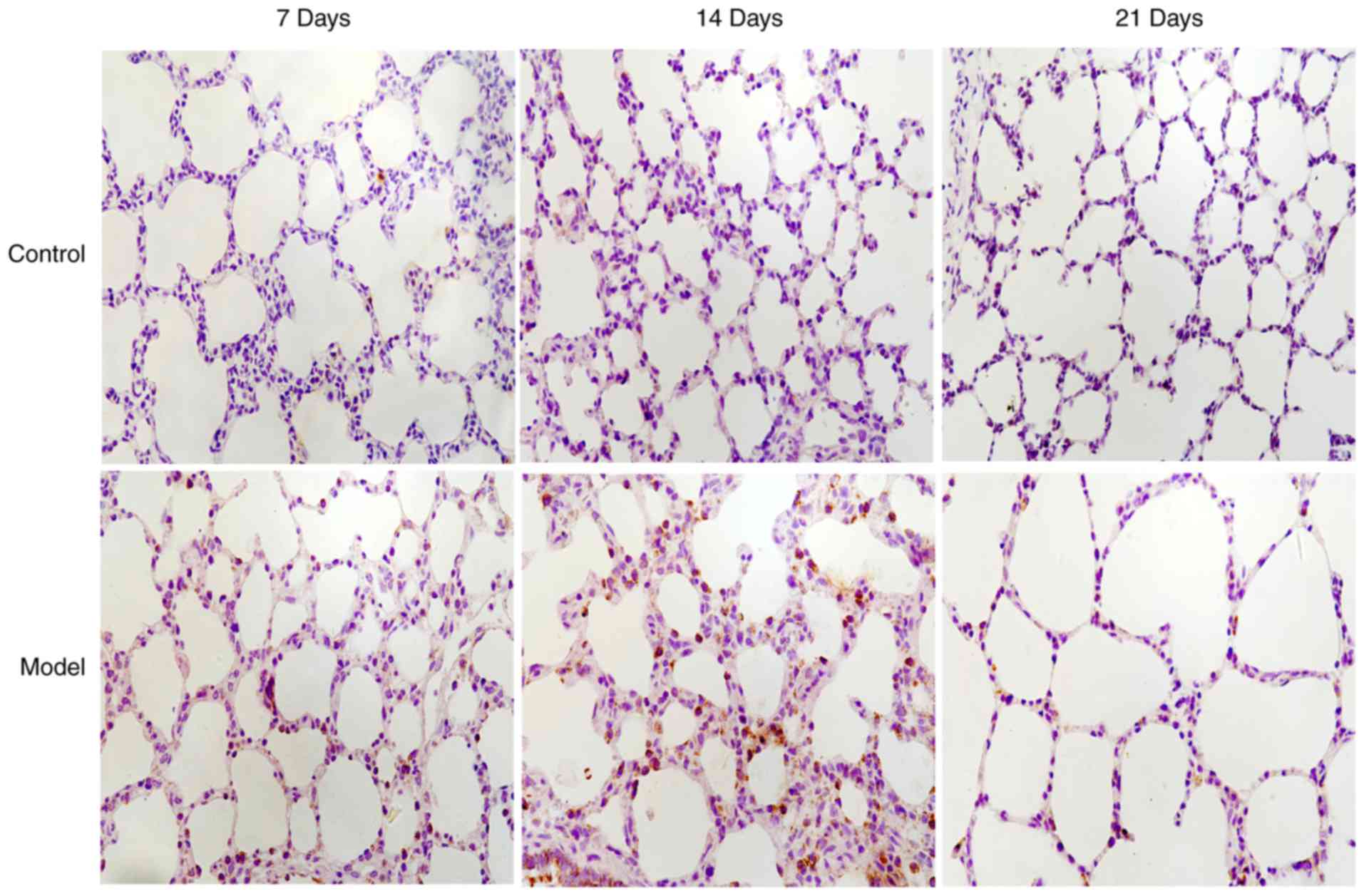
interleukin-6 and 8-Hydroxy-2′-Deoxyguanosine levels in tracheal
aspirates from very low birth weight infants who develop
bronchopulmonary dysplasia. Pediatr Neonatol. 58:63–69. 2017.
View Article : Google Scholar
|
|
9
|
Fabiano A, Gavilanes AW, Zimmermann LJ,
Kramer BW, Paolillo P, Livolti G, Picone S, Bressan K and Gazzolo
D: The development of lung biochemical monitoring can play a key
role in the early prediction of bronchopulmonary dysplasia. Acta
Paediatr. 105:535–541. 2016. View Article : Google Scholar
|
|
10
|
Kaya G, Saldir M, Polat A, Fidanci MK,
Erdem A, Erdem G, Kurt YG, Cetinkaya M, Cekmez F, Onguru O and Tunc
T: Evaluation of etanercept treatment in newborn rat model with
hyperoxic lung injury. Fetal Pediatr Pathol. 35:327–338. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Görlach A, Klappa P and Kietzmann T: The
endoplasmic reticulum: Folding, calcium homeostasis, signaling, and
redox control. Antioxid Redox Signal. 8:1391–1418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu BP and Weissman JS: Oxidative protein
folding in eukaryotes: Mechanisms and consequences. J Cell Biol.
164:341–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sevier CS and Kaiser CA: Ero1 and redox
homeostasis in the endoplasmic reticulum. Biochim Biophys Acta.
1783:549–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Younce CW, Wang K and Kolattukudy PE:
Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1
production and induction of a novel zinc-finger protein MCPIP.
Cardiovasc Res. 87:665–674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rothermel BA and Hill JA: Myocyte
autophagy in heart disease: Friend or foe? Autophagy. 3:632–634.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takemura G, Miyata S, Kawase Y, Okada H,
Maruyama R and Fujiwara H: Autophagic degeneration and death of
cardiomyocytes in heart failure. Autophagy. 2:212–214. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jian X, Xiao-yan Z, Bin H, Yu-feng Z, Bo
K, Zhi-nong W and Xin N: MiR-204 regulate cardiomyocyte autophagy
induced by hypoxia-reoxygenation through LC3-II. Int J Cardiol.
148:110–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Li YZ, Wang XR, Lu ZR, Shi DZ and
Liu XH: Panax quinquefolium saponins reduce myocardial
hypoxia-reoxygenation injury by inhibiting excessive endoplasic
reticulum stress. Shock. 37:228–233. 2012. View Article : Google Scholar
|
|
19
|
Fan T, Huang Z, Chen L, Wang W, Zhang B,
Xu Y, Pan S, Mao Z, Hu H and Geng Q: Associations between
autophagy, the ubiquitin-proteasome system and endoplasmic
reticulum stress in hypoxia-deoxygenation or ischemia-reperfusion.
Eur J Pharmacol. 791:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Zhao S, Yuan LJ, Wu HM, Jiang H,
Zhao SM, Luo G and Xue XD: Autophagy regulates hyperoxia-induced
intracellular accumulation of surfactant protein C in alveolar type
II cells. Mol Cell Biochem. 408:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Zhao S, Yuan L, Wu H, Jiang H and
Luo G: Hyperoxia-mediated LC3B activation contributes to the
impaired transdifferentiation of type II alveolar epithelial cells
(AECIIs) to type I cells (AECIs). Clin Exp Pharmacol Physiol.
43:834–843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
You K, Xu X, Fu J, Xu S, Yue X, Yu Z and
Xue X: Hyperoxia disrupts pulmonaryepithelial barrier in newborn
rats via the deterioration of occludin and ZO-1. Respir Res.
13:362012. View Article : Google Scholar
|
|
23
|
Cooney TP and Thurlbeck WM: The radial
alveolar count method of Emery and Mithal: A reappraisal
1-postnatal lung growth. Thorax. 37:572–579. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou A, Fu J, Yang H, Zhu Y, Pan Y, Xu S
and Xue X: Hyperoxia stimulates the transdifferentiation of type II
alveolar epithelial cells in newborn rats. Am J Physiol Lung Cell
Mol Physiol. 308:L861–L872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Abman SH and Groothius JR: Pathophysiology
and treatment of bronchopulmonary dysplasia. Current issues.
Pediatr Clin North Am. 41:277–315. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walsh MC, Yao Q, Gettner P, Hale E,
Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, et
al: Impact of a physiologic definition on bronchopulmonary
dysplasia rates. Pediatrics. 114:1305–1311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin JA, Hamilton BE, Sutton PD, Ventura
SJ, Mathews TJ and Osterman MJ: Births: Final data for 2008. Natl
Vital Stat Rep. 59(1): 3–71. 2010.
|
|
29
|
Chien YH, Tsao PN, Chou HC, Tang JR and
Tsou KI: Rehospitalization of extremely-low-birth-weight infants in
first 2 years of life. Early Hum Dev. 66:33–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doyle LW, Ford G and Davis N: Health and
hospitalisations after discharge in extremely low birth weight
infants. Semin Neonatol. 8:137–145. 2003. View Article : Google Scholar
|
|
31
|
Lamarche-Vadel A, Blondel B, Truffer P,
Burguet A, Cambonie G, Selton D, Arnaud C, Lardennois C, du
Mazaubrun C, N'Guyen S, et al: Rehospitalization in infants younger
than 29 weeks' gestation in the EPIPAGE cohort. Acta Paediatr.
93:1340–1345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luu TM, Lefebvre F, Riley P and
Infante-Rivard C: Continuing utilisation of specialised health
services in extremely preterm infants. Arch Dis Child Fetal
Neonatal Ed. 95:F320–F325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Smith VC, Zupancic JA, McCormick MC, Croen
LA, Greene J, Escobar GJ and Richardson DK: Rehospitalization in
the first year of life among infants with bronchopulmonary
dysplasia. J Pediatr. 144:799–803. 2004.PubMed/NCBI
|
|
34
|
Broström EB, Thunqvist P, Adenfelt G,
Borling E and Katz-Salamon M: Obstructive lung disease in children
with mild to severe BPD. Respir Med. 104:362–370. 2010. View Article : Google Scholar
|
|
35
|
Hintz SR, Kendrick DE, Vohr BR, Poole WK
and Higgins RD; National institute of child health and human
development (NICHD) neonatal research network: Community supports
after surviving extremely low-birthweight, extremely preterm birth:
Special outpatient services in early childhood. Arch Pediatr
Adolesc Med. 162:748–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Groothuis JR and Makari D: Definition and
outpatient management of the very low-birth-weight infant with
bronchopulmonary dysplasia. Adv Ther. 29:297–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang H, Fu J, Xue X, Yao L, Qiao L, Hou A,
Jin L and Xing Y: Epithelial-mesenchymal transitions in
bronchopulmonary dysplasia of newborn rats. Pediatr Pulmonol.
49:1112–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou A, Fu J, Shi Y, Qiao L, Li J, Xing Y
and Xue X: Decreased ZONAB expression promotes excessive
transdifferentiation of alveolar epithelial cells in
hyperoxia-induced bronchopulmonary dysplasia. Int J Mol Med.
41:2339–2349. 2018.PubMed/NCBI
|
|
39
|
Abdel Ghany EA, Alsharany W, Ali AA,
Younass ER and Hussein JS: Anti-oxidant profiles and markers of
oxidative stress in preterm neonates. Paediatr Int Child Health.
36:134–140. 2016. View Article : Google Scholar
|
|
40
|
Jobe AH and Bancalari E: Bronchopulmonary
dysplasia. Am J Respir Crit Care Med. 163:1723–1729. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saugstad OD: Oxygen and oxidative stress
in bronchopulmonary dysplasia. J Perinat Med. 38:571–577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Biesalski HK and Nohr D: Importance of
vitamin-A for lung function and development. Mol Aspects Med.
24:431–440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Metzler MD and Snyder JM: Retinoic acid
differentially regulates expression of surfactant associated
proteins in human fetal lung. Endocrinology. 133:1990–1998. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vento M, Moro M, Escrig R, Arruza L,
Villar G, Izquierdo I, Roberts LJ II, Arduini A, Escobar JJ, Sastre
J and Asensi MA: Preterm resuscitation with low oxygen causes less
oxidative stress, inflammation and chronic lung disease.
Pediatrics. 124:e439–e449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Perrone S, Tataranno ML, Negro S, Longini
M, Marzocchi B, Proietti F, Iacoponi F, Capitani S and Buonocore G:
Early identification of the risk for free radical-related diseases
in preterm newborns. Early Hum Dev. 86:241–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang CL, Liu C, Niu LL, Wang LR, Hou LH
and Cao XH: Surfactin-induced apoptosis through
ROS-ERS-Ca2+-ERK pathways in HepG2 cells. Cell Biochem
Biophys. 67:1433–1439. 2013. View Article : Google Scholar :
|
|
47
|
Toma L, Sanda GM, Deleanu M, Stancu CS and
Sima AV: Glycated LDL increase VCAM-1 expression and secretion in
endothelial cells and promote monocyte adhesion through mechanisms
involving endoplasmic reticulum stress. Mol Cell Biochem.
417:169–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: A vicious cycle or a
double-edged sword? Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cioffi DL: Redox regulation of endothelial
canonical transient receptor potential channels. Antioxid Redox
Signal. 15:1567–1582. 2011. View Article : Google Scholar :
|
|
50
|
Flodby P, Li C, Liu Y, Wang H, Marconett
CN, Laird-Offringa IA, Minoo P, Lee AS and Zhou B: GRP78 regulates
ER homeostasis and distal epithelial cell survival during lung
development. Am J Respir Cel Mol Biol. 55:135–149. 2016. View Article : Google Scholar
|
|
51
|
Gao Y, Gui Q, Jin L, Yu P, Wu L, Cao L,
Wang Q and Duan M: Hydrogen-rich saline attenuates hippocampus
endoplasmic reticulum stress after cardiac arrest in rats. Neurosci
Lett. 640:29–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Marciniak SJ, Yun CY, Oyadomari S, Novoa
I, Zhang Y, Jungreis R, Nagata K, Harding HP and Ron D: CHOP
induces death by promoting protein synthesis and oxidation in the
stressed endoplasmic reticulum. Genes Dev. 18:3066–3077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Song B, Scheuner D, Ron D, Pennathur S and
Kaufman RJ: Chop deletion reduces oxidative stress, improves beta
cell function, and promotes cell survival in multiple mouse models
of diabetes. J Clin Invest. 118:3378–3389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Han J, Back SH, Hur J, Lin YH,
Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M,
et al: ER-stress-induced transcriptional regulation increases
protein synthesis leading to cell death. Nat Cell Biol. 15:481–490.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang X, Shan P, Sasidhar M, Chupp GL,
Flavell RA, Choi AM and Lee PJ: Reactive oxygen species and
extracellular signal-regulated kinase 1/2 mitogen-activated protein
kinase mediate hyperoxia-induced cell death in lung epithelium. Am
J Respir Cell Mol Biol. 28:305–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cao SS and Kaufman RJ: Targeting
endoplasmic reticulum stress in metabolic disease. Expert Opin Ther
Targets. 17:437–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Roussel BD, Kruppa AJ, Miranda E, Crowther
DC, Lomas DA and Marciniak SJ: Endoplasmic reticulum dysfunction in
neurological disease. Lancet Neurol. 12:105–118. 2013. View Article : Google Scholar
|
|
59
|
Kroemer G and Blomgren K: Mitochondrial
cell death control in familial Parkinson disease. PLoS Biol.
5:e2062007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Endo M, Oyadomari S, Suga M, Mori M and
Gotoh T: The ER stress pathway involving CHOP is activated in the
lungs of LPS-treated mice. J Biochem. 138:501–507. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bek MF, Bayer M, Müller B, Greiber S, Lang
D, Schwab A, August C, Springer E, Rohrbach R, Huber TB, et al:
Expression and function of C/EBP homology protein (GADD153) in
podocytes. Am J Pathol. 168:20–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lozon TI, Eastman AJ, Matute-Bello G, Chen
P, Hallstrand TS and Altemeier WA: PKR-dependent CHOP induction
limits hyperoxiainduced lung injury. Am J Physiol Lung Cell Mol
Physiol. 300:L422–L429. 2011. View Article : Google Scholar
|
|
63
|
Choo-Wing R, Syed MA, Harijith A, Bowen B,
Pryhuber G, Janér C, Andersson S, Homer RJ and Bhandari V:
Hyperoxia and interferon-γ-induced injury in developing lungs occur
via cyclo-oxygenase-2 and theendoplasmic reticulum stress-dependent
pathway. Am J Respir Cell Mol Biol. 48:749–757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lu HY, Zhang J, Wang QX, Tang W and Zhang
LJ: Activation of the endoplasmic reticulum stress pathway
involving CHOP in the lungs of rats with hyperoxia-induced
bronchopulmonary dysplasia. Mol Med Rep. 12:4494–4500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bozi LH, Jannig PR, Rolim N, Voltarelli
VA, Dourado PM, Wisløff U and Brum PC: Aerobic exercise training
rescues cardiac protein quality control and bluntsendoplasmic
reticulum stress in heart failure rats. J Cell Mol Med.
20:2208–2212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
B'Chir W, Chaveroux C, Carraro V, Averous
J, Maurin AC, Jousse C, Muranishi Y, Parry L, Fafournoux P and
Bruhat A: Dual role for CHOP in the crosstalk between autophagy and
apoptosis to determine cell fate in response to amino acid
deprivation. Cell Signal. 26:1385–1391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kouno T, Mizuguchi M, Tanida I, Ueno T,
Kanematsu T, Mori Y, Shinoda H, Hirata M, Kominami E and Kawano K:
Solution structure of microtubule-associated protein light chain 3
and identification of its functional subdomains. J Biol Chem.
280:24610–24617. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Schönthal AH: Endoplasmic reticulum stress
and autophagy as targets for cancer therapy. Cancer Lett.
275:163–169. 2009. View Article : Google Scholar
|
|
69
|
Høyer-Hansen M and Jäättelä M: Connecting
endoplasmic reticulum stress to autophagy by unfolded protein
response and calcium. Cell Death Differ. 14:1576–1582. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bernales S, McDonald KL and Walter P:
Autophagy counterbalances endoplasmic reticulum expansion during
the unfolded protein response. PLoS Biol. 4:e4232006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cheng Y and Yang JM: Survival and death of
endoplasmic-reticulum-stressed cells: Role of autophagy. World J
Biol Chem. 2:226–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Schleicher SM, Moretti L, Varki V and Lu
B: Progress in the unraveling of the endoplasmic reticulum
stress/autophagy pathway and cancer: Implications for future
therapeutic approaches. Drug Resist Updat. 13:79–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dunn WA Jr: Studies on the mechanisms of
autophagy: Formation of the autophagic vacuole. J Cell Biol.
110:1923–1933. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ueno T, Muno D and Kominami E: Membrane
markers of endoplasmic reticulum preserved in autophagic vacuolar
membranes isolated from leupeptin-administered rat liver. J Biol
Chem. 266:18995–18999. 1991.PubMed/NCBI
|
|
76
|
Axe EL, Walker SA, Manifava M, Chandra P,
Roderick HL, Habermann A, Griffiths G and Ktistakis NT:
Autophagosome formation from membrane compartments enriched in
phosphatidylinositol 3-phosphate and dynamically connected to the
endoplasmic reticulum. J Cell Biol. 182:685–701. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hart LS, Cunningham JT, Datta T, Dey S,
Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, et al: ER
stress-mediated autophagy promotes Myc-dependent transformation and
tumor growth. J Clin Invest. 122:4621–4634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
B'chir W, Maurin AC, Carraro V, Averous J,
Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P and Bruhat
A: The eIF2α/ATF4 pathway is essential for stress-induced autophagy
gene expression. Nucleic Acids Res. 41:7683–7699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rouschop KM, van den Beucken T, Dubois L,
Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W
and Voncken JW: The unfolded protein response protects human tumor
cells during hypoxia through regulation of the autophagy genes
MAP1LC3B and ATG5. J Clin Invest. 120:127–141. 2010. View Article : Google Scholar :
|
|
80
|
Avivar-Valderas A, Salas E,
Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J and
Aguirre-Ghiso JA: PERK integrates autophagy and oxidative stress
responses to promote survival during extracellular matrix
detachment. Mol Cell Biol. 31:3616–3629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J,
Su J, Li H and Sun L: Inhibition of autophagy enhances cisplatin
cytotoxicity through endoplasmic reticulum stress in human cervical
cancer cells. Cancer Lett. 314:232–243. 2012. View Article : Google Scholar
|
|
82
|
Kouroku Y, Fujita E, Tanida I, Ueno T,
Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E and Momoi T: ER
stress (PERK/eIF2alpha phosphorylation) mediates the
polyglutamine-induced LC3 conversion, an essential step for
autophagy formation. Cell Death Differ. 14:230–239. 2007.
View Article : Google Scholar
|
|
83
|
Nijholt DA, de Graaf TR, van Haastert ES,
Oliveira AO, Berkers CR, Zwart R, Ovaa H, Baas F, Hoozemans JJ and
Scheper W: Endoplasmic reticulum stress activates autophagy but not
the proteasome in neuronal cells: Implications for Alzheimer's
disease. Cell Death Differ. 18:1071–1081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Adolph TE, Tomczak MF, Niederreiter L, Ko
HJ, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M,
Hartwig J, Hosomi S, et al: Paneth cells as a site of origin for
intestinal inflammation. Nature. 503:272–276. 2013. View Article : Google Scholar : PubMed/NCBI
|