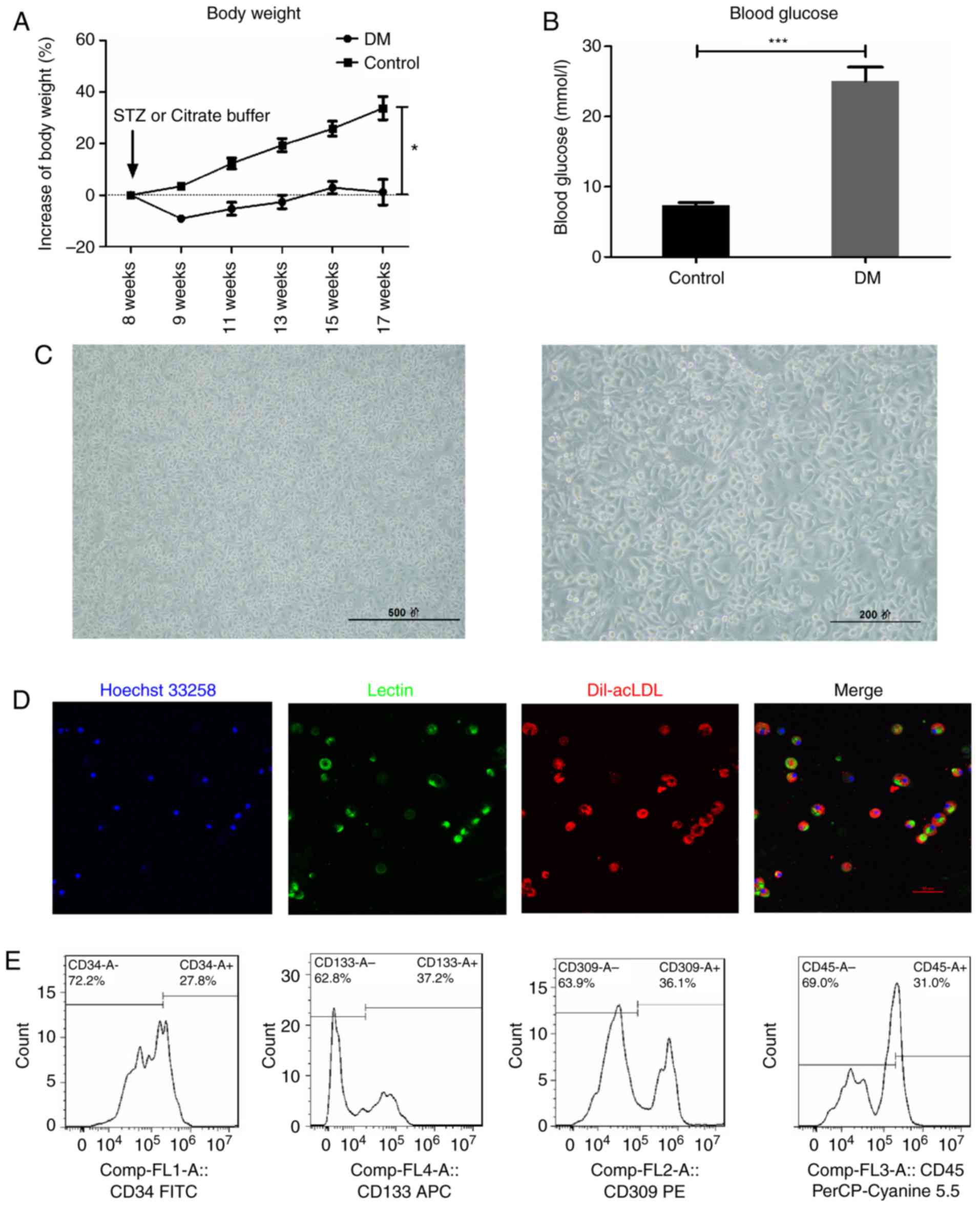
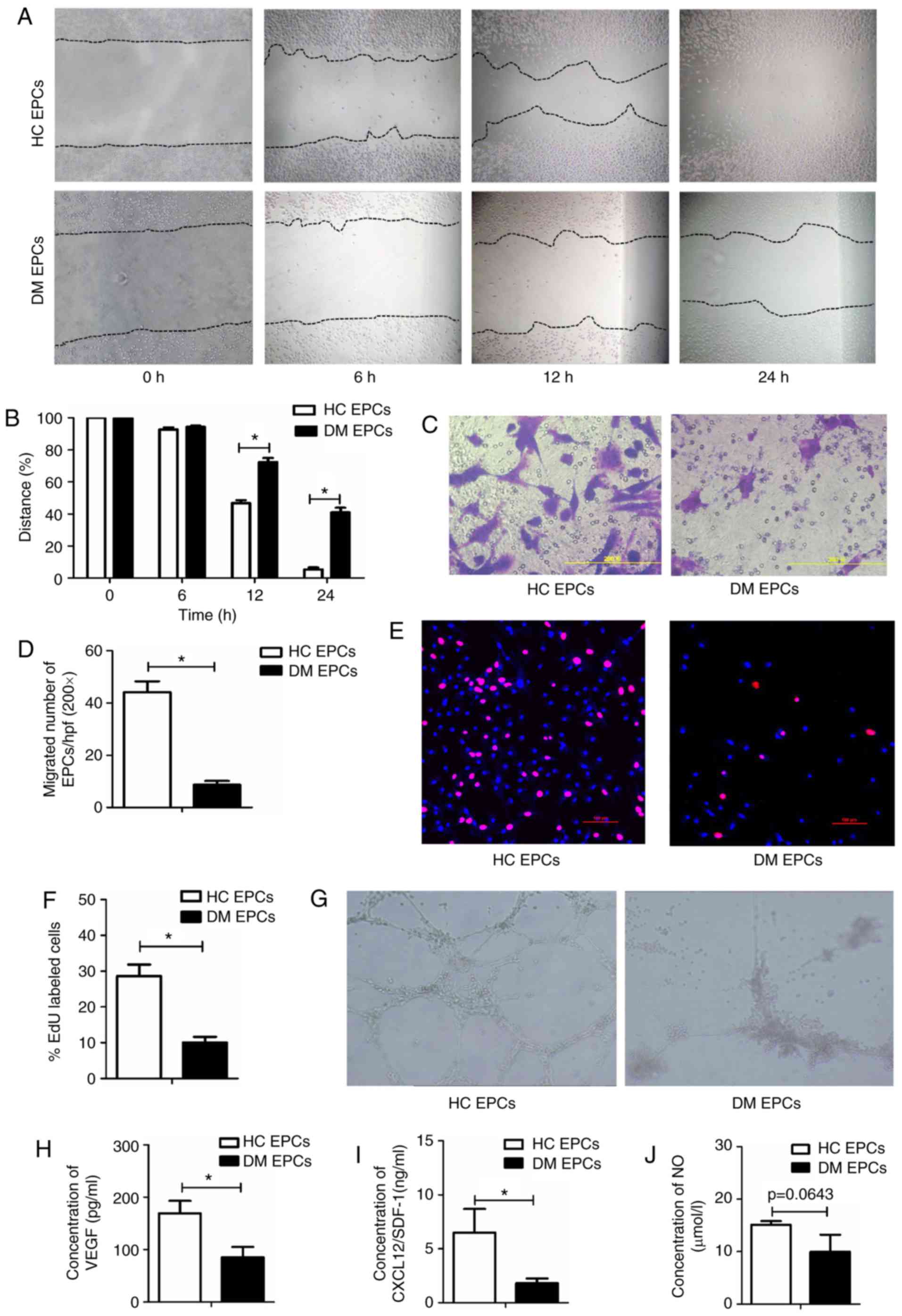
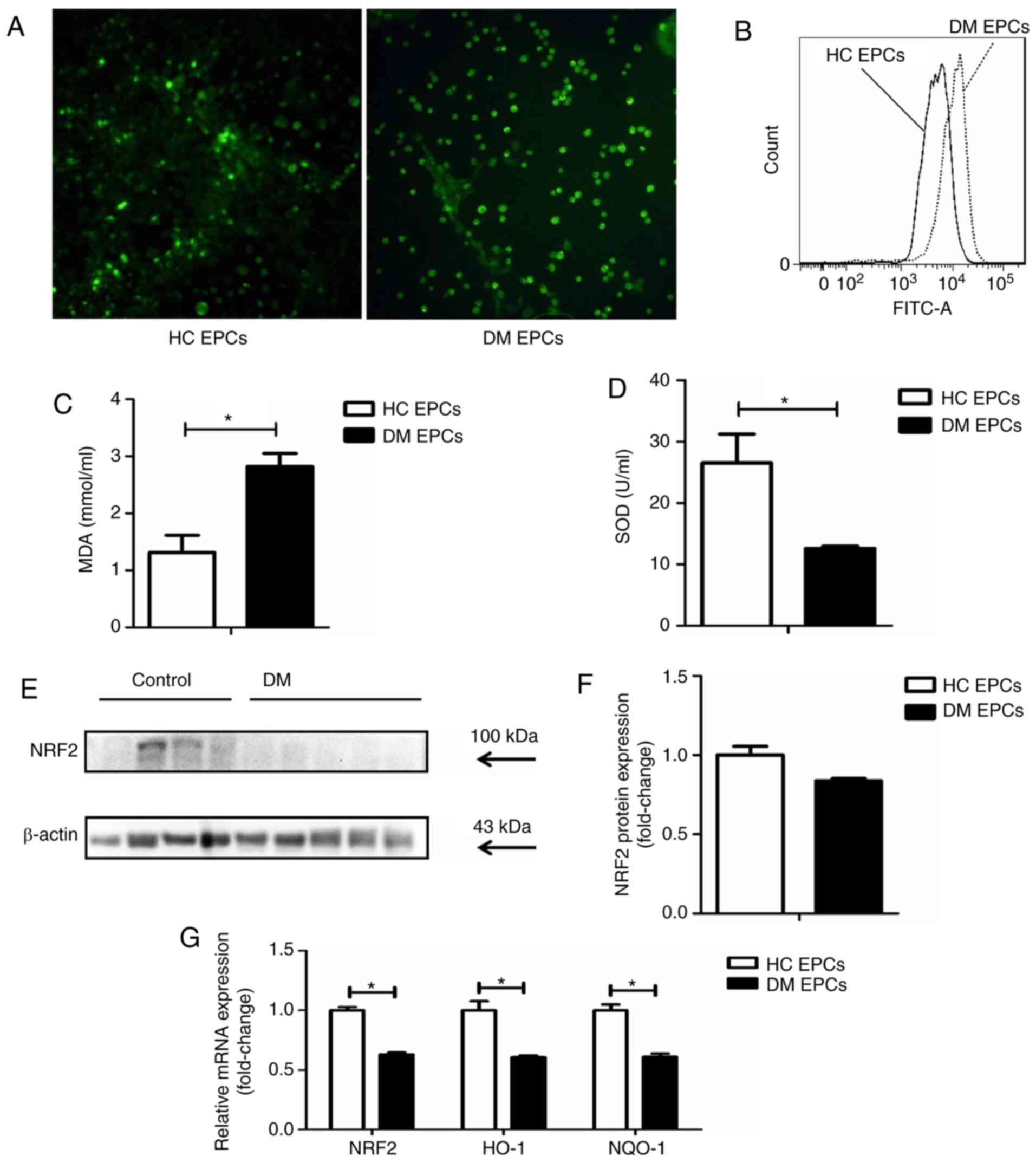
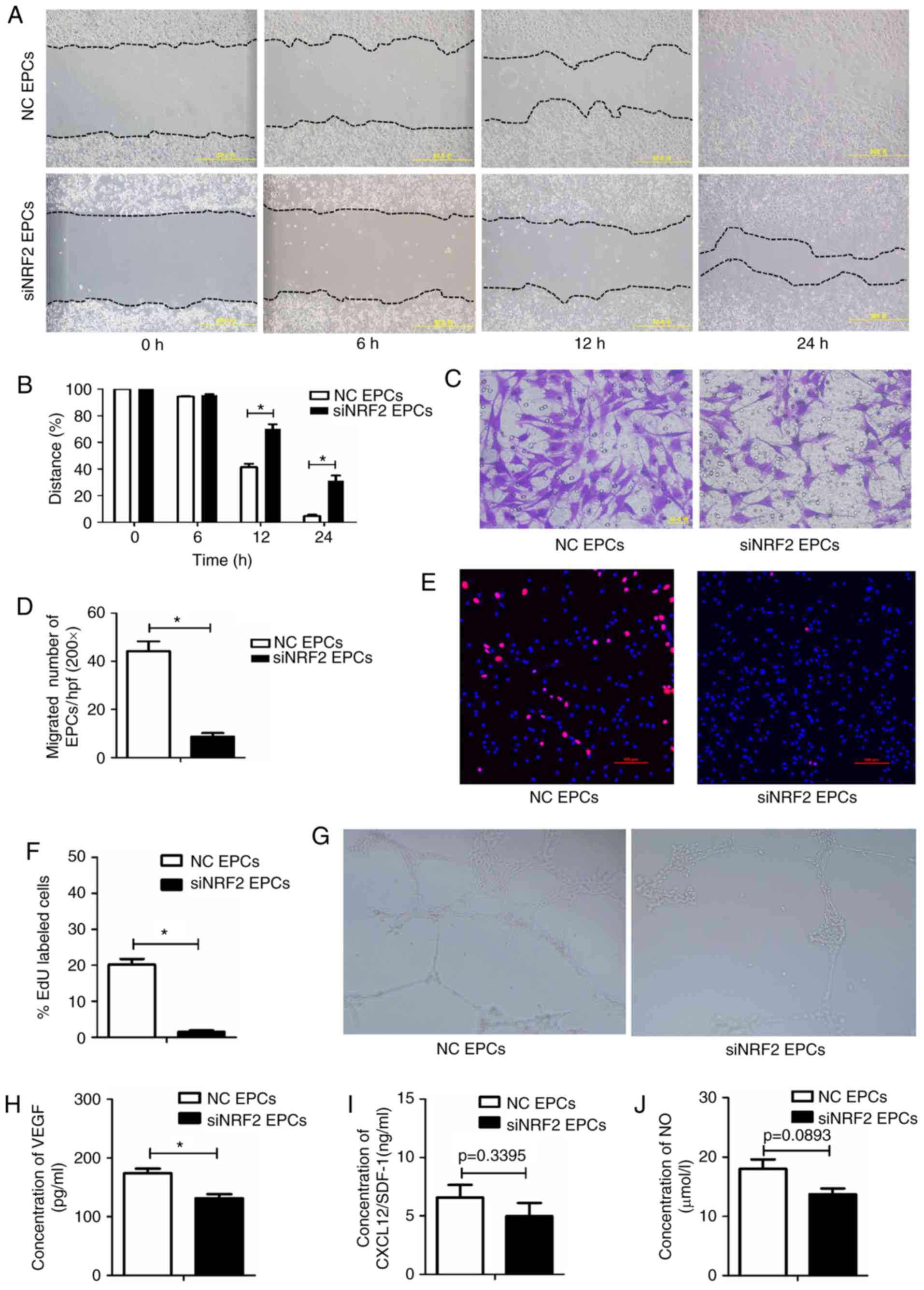
|
1
|
Abplanalp WT, Conklin DJ, Cantor JM,
Ginsberg MH, Wysoczynski M, Bhatnagar A and O’Toole TE: Enhanced
integrinα4β1-mediated adhesion contributes to a mobilization defect
of endothelial progenitor cells in diabetes. Diabetes.
65:3505–3515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soedamah-Muthu SS, Fuller JH, Mulnier HE,
Raleigh VS, Lawrenson RA and Colhoun HM: High risk of
cardiovascular disease in patients with type 1 diabetes in the
U.K.: A cohort study using the general practice research database.
Diabetes Care. 29:798–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Georgescu A: Vascular dysfunction in
diabetes: The endothelial progenitor cells as new therapeutic
strategy. World J Diabetes. 2:92–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fadini GP, Sartore S, Albiero M, Baesso I,
Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A,
Agostini C and Avogaro A: Number and function of endothelial
progenitor cells as a marker of severity for diabetic vasculopathy.
Arterioscler Thromb Vasc Biol. 26:2140–2146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zampetaki A, Kirton JP and Xu Q: Vascular
repair by endothelial progenitor cells. Cardiovasc Res. 78:413–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Williamson K, Stringer SE and Alexander
MY: Endothelial progenitor cells enter the aging arena. Front
Physiol. 3:302012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung HS, Shyu WC, Tsai CH, Hsu SH and Lin
SZ: Transplantation of endothelial progenitor cells as therapeutics
for cardiovascular diseases. Cell Transplant. 18:1003–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatwadekar AD, Shaw LC and Grant MB:
Promise of endothelial progenitor cell for treatment of diabetic
retinopathy. Expert Rev Endocrinol Metab. 5:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cantrell D: T cell antigen receptor signal
transduction pathways. Annu Rev Immunol. 14:259–274. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dinkova-Kostova AT, Holtzclaw WD, Cole RN,
Itoh K, Wakabayashi N, Katoh Y, Yamamoto M and Talalay P: Direct
evidence that sulfhydryl groups of Keap1 are the sensors regulating
induction of phase 2 enzymes that protect against carcinogens and
oxidants. Proc Natl Acad Sci USA. 99:11908–11913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McMahon M, Itoh K, Yamamoto M and Hayes
JD: Keap1-dependent proteasomal degradation of transcription factor
Nrf2 contributes to the negative regulation of antioxidant response
element-driven gene expression. J Biol Chem. 278:21592–21600. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar
|
|
16
|
Venugopal R and Jaiswal AK: Nrf1 and Nrf2
positively and c-Fos and Fra1 negatively regulate the human
antioxidant response element-mediated expression of NAD(P)H:
Quinone oxidoreductase1 gene. Proc Natl Acad Sci USA.
93:14960–14965. 1996. View Article : Google Scholar
|
|
17
|
Lee JM, Li J, Johnson DA, Stein TD, Kraft
AD, Calkins MJ, Jakel RJ and Johnson JA: Nrf2, a multi-organ
protector. FASEB J. 19:1061–1066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W and Kong AN: Molecular mechanisms of
Nrf2-mediated antioxidant response. Mol Carcinog. 48:91–104. 2009.
View Article : Google Scholar :
|
|
19
|
Maher JM, Dieter MZ, Aleksunes LM, Slitt
AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et
al: Oxidative and electrophilic stress induces multidrug
resistance-associated protein transporters via the nuclear
factor-E2-related factor-2 transcriptional pathway. Hepatology.
46:1597–1610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ and Nel AE: The role of phase II
antioxidant enzymes in protecting memory T cells from spontaneous
apoptosis in young and old mice. J Immunol. 175:2948–2959. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thimmulappa RK, Scollick C, Traore K,
Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW and
Biswal S: Nrf2-dependent protection from LPS induced inflammatory
response and mortality by CDDO-Imidazolide. Biochem Biophys Res
Commun. 351:883–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai X, Yan X, Zeng J, Chen J, Wang Y, Chen
J, Li Y, Barati MT, Wintergerst KA, Pan K, et al: Elevating CXCR7
improves angiogenic function of EPCs via Akt/GSK-3β/Fyn-Mediated
Nrf2 activation in diabetic limb ischemia. Circ Res.
120:e7-e232017. View Article : Google Scholar
|
|
23
|
You J, Sun J, Ma T, Yang Z, Wang X, Zhang
Z, Li J, Wang L, Ii M, Yang J and Shen Z: Curcumin induces
therapeutic angiogenesis in a diabetic mouse hindlimb ischemia
model via modulating the function of endothelial progenitor cells.
Stem Cell Res Ther. 8:1822017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Zhang X, Guan X, Cui X, Wang Y, Chu
H and Cheng M: Advanced glycation end products impair the
migration, adhesion and secretion potentials of late endothelial
progenitor cells. Cardiovasc Diabetol. 11:462012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sorrentino SA, Bahlmann FH, Besler C,
Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A,
Limbourg F, et al: Oxidant stress impairs in vivo
reendothelialization capacity of endothelial progenitor cells from
patients with type 2 diabetes mellitus: Restoration by the
peroxisome proliferator-activated receptor-gamma agonist
rosiglitazone. Circulation. 116:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landmesser U, Engberding N, Bahlmann FH,
Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner
D, Templin C, Kotlarz D, et al: Statin-induced improvement of
endothelial progenitor cell mobilization, myocardial
neovascularization, left ventricular function, and survival after
experimental myocardial infarction requires endothelial nitric
oxide synthase. Circulation. 110:1933–1939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bahlmann FH, De Groot K, Spandau JM,
Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H and
Fliser D: Erythropoietin regulates endothelial progenitor cells.
Blood. 103:921–926. 2004. View Article : Google Scholar
|
|
28
|
Wu Q, Qi B, Liu Y, Cheng B, Liu L, Li Y
and Wang Q: Mechanisms underlying protective effects of
trimetazidine on endothelial progenitor cells biological functions
against H2O2-induced injury: Involvement of antioxidation and
Akt/eNOS signaling pathways. Eur J Pharmacol. 707:87–94. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuliszewski MA, Ward MR, Kowalewski JW,
Smith AH, Stewart DJ, Kutryk MJ and Leong-Poi H: A direct
comparison of endothelial progenitor cell dysfunction in rat
metabolic syndrome and diabetes. Atherosclerosis. 226:58–66. 2013.
View Article : Google Scholar
|
|
30
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li WD, Hu N, Lei FR, Wei S, Rong JJ,
Zhuang H and Li XQ: Autophagy inhibits endothelial progenitor cells
migration via the regulation of MMP2, MMP9 and uPA under normoxia
condition. Biochem Biophys Res Commun. 466:376–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Takaya K, Suzuki T, Motohashi H, Onodera
K, Satomi S, Kensler TW and Yamamoto M: Validation of the multiple
sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med.
53:817–827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Yu Z, Sunchu B, Shoaf J, Dang I,
Zhao S, Caples K, Bradley L, Beaver LM, Ho E, et al: Rapamycin
inhibits the secretory phenotype of senescent cells by a
Nrf2-independent mechanism. Aging Cell. 16:564–574. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
D’Apolito M, Colia AL, Lasalvia M, Capozzi
V, Falcone MP, Pettoello-Mantovani M, Brownlee M, Maffione AB and
Giardino I: Urea-induced ROS accelerate senescence in endothelial
progenitor cells. Atherosclerosis. 263:127–136. 2017. View Article : Google Scholar
|
|
36
|
Lois N, McCarter RV, O’Neill C, Medina RJ
and Stitt AW: Endothelial progenitor cells in diabetic retinopathy.
Front Endocrinol (Lausanne). 5:442014.
|
|
37
|
Foresti R, Bucolo C, Platania CM, Drago F,
Dubois-Randé JL and Motterlini R: Nrf2 activators modulate
oxidative stress responses and bioenergetic profiles of human
retinal epithelial cells cultured in normal or high glucose
conditions. Pharmacol Res. 99:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boyko EJ, Seelig AD and Ahroni JH: Limband
person-level risk factors for lower-limb amputation in the
prospective seattle diabetic foot study. Diabetes Care. 41:891–898.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hörtenhuber T, Rami-Mehar B, Satler M,
Nagl K, Höbaus C, Höllerl F, Koppensteiner R, Schernthaner G,
Schober E and Schernthaner GH: Endothelial progenitor cells are
related to glycemic control in children with type 1 diabetes over
time. Diabetes Care. 36:1647–1653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
António N, Fernandes R, Soares A, Soares
F, Lopes A, Carvalheiro T, Paiva A, Pêgo GM, Providência LA,
Gonçalves L and Ribeiro CF: Reduced levels of circulating
endothelial progenitor cells in acute myocardial infarction
patients with diabetes or pre-diabetes: Accompanying the glycemic
continuum. Cardiovasc Diabetol. 13:1012014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ingram DA, Lien IZ, Mead LE, Estes M,
Prater DN, Derr-Yellin E, DiMeglio LA and Haneline LS: In vitro
hyperglycemia or a diabetic intrauterine environment reduces
neonatal endothelial colony-forming cell numbers and function.
Diabetes. 57:724–731. 2008. View Article : Google Scholar
|
|
42
|
Tsukada S, Masuda H, Jung SY, Yun J, Kang
S, Kim DY, Park JH, Ji ST, Kwon SM and Asahara T: Impaired
development and dysfunction of endothelial progenitor cells in type
2 diabetic mice. Diabetes Metab. 43:154–162. 2017. View Article : Google Scholar
|
|
43
|
Fadini GP, Sartore S, Schiavon M, Albiero
M, Baesso I, Cabrelle A, Agostini C and Avogaro A: Diabetes impairs
progenitor cell mobilisation after hindlimb ischaemia-reperfusion
injury in rats. Diabetologia. 49:3075–3084. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kryczek I, Lange A, Mottram P, Alvarez X,
Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, et al: CXCL12
and vascular endothelial growth factor synergistically induce
neoangiogenesis in human ovarian cancers. Cancer Res. 65:465–472.
2005.PubMed/NCBI
|
|
45
|
Uruno A, Yagishita Y and Yamamoto M: The
Keap1-Nrf2 system and diabetes mellitus. Arch Biochem Biophys.
566:76–84. 2015. View Article : Google Scholar
|
|
46
|
Florczyk U, Jazwa A, Maleszewska M, Mendel
M, Szade K, Kozakowska M, Grochot-Przeczek A, Viscardi M, Czauderna
S, Bukowska-Strakova K, et al: Nrf2 regulates angiogenesis: Effect
on endothelial cells, bone marrow-derived proangiogenic cells and
hind limb ischemia. Antioxid Redox Signal. 20:1693–1708. 2014.
View Article : Google Scholar :
|
|
47
|
Gremmels H, de Jong OG, Hazenbrink DH,
Fledderus JO and Verhaar MC: The transcription factor Nrf2 protects
angiogenic capacity of endothelial colony-forming cells in
high-oxygen radical stress conditions. Stem Cells Int.
2017:46806122017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chiu SC, Chao CY, Chiang EI, Syu JN,
Rodriguez RL and Tang FY: N-3 polyunsaturated fatty acids alleviate
high glucose-mediated dysfunction of endothelial progenitor cells
and prevent ischemic injuries both in vitro and in vivo. J Nutr
Biochem. 42:172–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vaamonde-Garcia C, Courties A, Pigenet A,
Laiguillon MC, Sautet A, Houard X, Kerdine-Römer S, Meijide R,
Berenbaum F and Sellam J: The nuclear factor-erythroid 2-related
factor/heme oxygenase-1 axis is critical for the inflammatory
features of type 2 diabetes-associated osteoarthritis. J Biol Chem.
292:14505–14515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tullet JMA, Green JW, Au C, Benedetto A,
Thompson MA, Clark E, Gilliat AF, Young A, Schmeisser K and Gems D:
The SKN-1/Nrf2 transcription factor can protect against oxidative
stress and increase lifespan in C. Elegans by distinct mechanisms.
Aging Cell. 16:1191–1194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Corenblum MJ, Ray S, Remley QW, Long M,
Harder B, Zhang DD, Barnes CA and Madhavan L: Reduced Nrf2
expression mediates the decline in neural stem cell function during
a critical middle-age period. Aging Cell. 15:725–736. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gao S, Duan X, Wang X, Dong D, Liu D, Li
X, Sun G and Li B: Curcumin attenuates arsenic-induced hepatic
injuries and oxidative stress in experimental mice through
activation of Nrf2 pathway, promotion of arsenic methylation and
urinary excretion. Food Chem Toxicol. 59:739–747. 2013. View Article : Google Scholar : PubMed/NCBI
|