Introduction
Cardiovascular disease (CVD) causes high mortality
world-wide (1). Diabetes mellitus
(DM) is a common, chronic, metabolic disease that causes a great
socioeconomic burden, and the incidence of DM is increasing.
Compared with non-diabetic individuals, patients with DM have an
increased risk of CVD (2). DM is
often accompanied by vascular complications, including vascular
remodeling and vascular growth disorders, decreased response to
hypoxia-ischemia sites, abnormal neovascularization, and impaired
endothelium regeneration (3).
Therefore, therapeutic interventions that ameliorate endothelial
dysfunction may be a promising strategy for DM.
Endothelial progenitor cell (EPC) dysregulation has
been reported in the pathogenesis of diabetic vasculopathy
(4). A number of studies have
suggested that bone-marrow derived EPCs contribute to postnatal
neovascularization and vascular endothelial repair (5,6).
When vascular injury occurs, EPCs are mobilized from the bone
marrow, enter the circulation and translocate to sites of
endothelial damage to induce neovascularization and repair vascular
damage (7). In the past decade,
endothelial progenitor cell (EPC) transplantation has been used
experimentally to treat CVD (8).
Several studies have reported that EPC transplantation is
beneficial in CVD, and transplanted EPCs may compensate for the
insufficiency of endogenous EPCs in diabetic retinopathy (9). However, in order to improve
therapeutic efficacy of EPC transplantation, it is necessary to
improve EPC survival and proliferation.
The transcription factor, nuclear factor erythroid
2-related factor 2 (Nrf2), is a receptor for exogenous toxic
substance oxidative stress. Nrf2 serves an important role in the
major defense mechanisms associated with cellular antioxidative
stress and the induction of exogenous toxic substances (10,11). Under normal conditions, Nrf2
exists in a complex with Kelch-like ECH-associating protein 1
(Keap1), its repressor protein, and is tethered to the actin
cytoskeleton in the cytosol. The interaction between Nrf2 and Keap1
causes its continual ubiquitination and degradation (12,13). Under oxidative stress, activated
Nrf2 translocates and accumulates in the nucleus, binding to the
promoters of genes that contain the antioxidant response element,
inducing transcription (14-16). Nrf2 and its target genes serve
roles in the antioxidant response, detoxification, glutathione
homeostasis and other cytoprotective functions (17-19). Previous studies have reported that
Nrf2 is inducible and upregulates Heme oxygenase-1 (HO-1), as well
as NADPH quinone oxidoreductase 1 (NQO1) in leukocytes (20,21). Therefore, Nrf2 activity can be
assessed by measuring the induction of NQO1 and HO-1 mRNA
expression. Tert-butylhydroquinone (tBHQ), one of the most studied
Nrf2 inductors, is present in the human body and is used as a food
preservative widely. Nrf2 may be the key factor in cell
homeostasis. It has been reported that CXC chemokine ligand 7
(CXCL7) upregulation improves the angiogenesis of EPCs via Nrf2
activation in diabetic ischemia (22), and that hyperglycemia induces EPC
senescence (23). However,
whether Nrf2 serves a direct role in regulating the functions and
senescence in DM remains unclear.
The aim of the present study was to investigate the
potential role of Nrf2 in the biological functions of EPCs in DM,
including migration, proliferation, secretion and angiogenesis, as
well as its relevance in oxidative stress and cell senescence.
Materials and methods
Animals and diabetic models
Male C57BL/6 male mice weighing 18-20 g (7-8 weeks)
were purchased from the Beijing Vital River Laboratory Animal
Technology Co. Ltd. (Beijing, China). The animals were housed in
cages at 22±2°C with 40±5% humidity under a 12-h light/dark cycle,
and received standard diet and water ad libitum. Mice were
handled according to the institutional animal care guidelines and
the Guide for Care and Use of Laboratory Animals published by
Tongji Medical College (Huazhong University of Science and
Technology, Wuhan, China). Mice were randomly divided into two
groups: the control group and the diabetic group (n=40). Mice in
the diabetic group were intraperitoneally administered with
streptozotocin (STZ; 60 mg/kg/day for 5 days) dissolved in 0.1 mM
sodium citrate buffer, while mice in the control group were
administered with isometric 0.1 mM sodium citrate buffer (pH 4.5).
Blood glucose levels and body weight were measured every 2 weeks
for the following 8 weeks, and blood glucose concentrations ≥16.7
mmol/l (300 mg/dl) was considered modeling success. All experiments
were approved by the Ethics Committee of Tongji Medical College,
Huazhong University of Science and Technology.
Isolation and culture of EPCs
EPCs were isolated and cultured as previously
described (24). In brief, bone
marrow mononuclear cells were extracted from the tibia and femurs
of mice and cultured on fibronectin-coated 6-well plates in
endothelial cell basal medium-2 (Lonza Group, Ltd., Basel,
Switzerland) supplemented with endothelial cell growth medium-2
(EGM-2) SingleQuot kit supplement (Lonza Group, Ltd.). Following 4
days of culture, nonadherent cells were removed by washing the
plates with PBS. The remaining cells were cultured for another 3
days (total 7 days) and used for further analysis (25). The cultured EPCs from each mouse
were used for 1-2 cell function experiments.
Characterization of EPCs
According to previous publications (5,25–28), adherent EPCs were positive for
CD45, the endothelial and hematopoietic cell marker (29), and were subjected to dual staining
for acetylated low-density lipoprotein (acLDL; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and l fluorescein
isothiocyanate (FITC)-conjugated BS-1 lectin, followed by flow
cytometry analysis. Flow cytometry was also used to assess the
expression of cell surface antigens with the following antibodies
(eBioscience; Thermo Fisher Scientific, Inc.): FITC-conjugated
anti-mouse CD34 antibody; allophycocyanin (APC)-conjugated
anti-mouse CD133 (also known as Prominin-1) antibody; phycoerythrin
(PE)-conjugated anti-mouse CD309 (also known as FLK1/VEGF-R2/KDR)
antibody and PerCP-Cyanine5.5-conjugated anti-mouse CD45 antibody.
More than 6 mice were included in this experiment.
EPC migration evaluation (Transwell and
wound healing assays)
EPC migration was evaluated using a modified
Boyden’s chamber assay as previously described (30). Briefly, cell suspensions
(5×104 cells/well) were seeded in the upper chamber, and
EGM-2 medium containing human recombinant vascular endothelial
growth factor (VEGF; 50 ng/ml; R&D Systems Inc., Minneapolis,
MN, USA) was used to fill the lower chamber. The chamber was
incubated for 24 h under 5% CO2 at 37°C, then washed
with PBS, fixed with 4% paraformaldehyde and stained with crystal
violet. Migration activity was evaluated by counting the mean
number of migrated cells under a microscope (Olympus Corp., Tokyo,
Japan) in three random high-power fields. Cell migration was also
assessed using an in vitro scratch wound healing assay as
previously described (31). EPCs
were cultured in 6-well plates with EGM-2 medium supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc.) until they
reached 100% confluence. A sterile 200 μl pipette tip was
used to make a straight scratch in the surface. Cells were washed
three times with PBS and cultured with serum free EGM-2 medium for
24 h. The shortest vertical distance of the scratch was observed
using an inverted microscope (magnification, ×100) to determine
cell migration at 0, 6, 12 and 24 h. More than 6 mice were included
in each group.
EPC proliferation evaluation
[5-ethynyl-2′-deoxyuridine (EdU) incorporation assay]
An EdU labeling/detection kit (Guangzhou Ribobio
Co., Ltd., Guangzhou, China) was used to evaluate the proliferation
of EPCs, according to the manufacturer’s protocol. Briefly, EPCs
were cultured in 24-well plates at a density of 5×104
cells/well. Following exposure to the indicated experimental
conditions, EPCs were labeled with 50 μM EdU labeling media
and incubated for 4 h at 37°C under 5% CO2, then fixed
with 4% paraformaldehyde, lysed using 0.5% Triton X-100, and
stained with anti-EdU working solution. The nuclei were labeled
with Hoechst 33258 (Guge, Wuhan, China), and the percentage of
EdU-positive EPCs was calculated following fluorescent microscopy
(Olympus Corp.). Five random fields of view were assessed for each
group. More than 4 mice were included in each group.
Tube formation assay
Matrigel matrix (BD Biosciences, Franklin Lakes, NJ,
USA) was placed in the well of a 48-well cell culture plate, after
which 2×103 Dil-labeled EPCs and 2×104 human
umbilical vein endothelial cells (HUVECs, ATCC®
CRL-1730™) which were purchased from the American Type Culture
Collection (Manassas, VA, USA), were added in each well with EGM-2.
After 18 h of incubation, images of tube morphology were captured.
More than 4 mice were included in each group.
Measurement of VEGF, stromal-derived
growth factor (SDF) and nitric oxide (NO) secretion
To measure the secretion functions of EPCs, SDF-1
and VEGF levels in the cell culture supernatants were determined
using ELISA kits (anti-mouse VEGF ELISA kit, cat. no. EMC103, Neo
Bioscience Technology, China; CXCL12/SDF-1α Quantikine ELISA kit,
cat. no. MCX120, R&D Systems, Inc.), according to the
manufacturer’s protocols. NO released from EPCs was assessed by
measuring the stable breakdown product of NO in the culture
supernatant with a commercial NO colorimetric assay kit (Enzo Life
Sciences, Inc., Farmingdale, NY, USA) according to the
manufacturer’s protocol. More than 6 mice were included in each
group.
Measurement of intracellular reactive
oxygen species (ROS)
Intracellular ROS levels were determined using
imaging and flow cytometry analysis. Following exposure to the
indicated experimental conditions, EPCs were stained with 5
μmol/l CellROX Green reagent (Thermo Fisher Scientific,
Inc.) and incubated for 40 min at 37°C, following which the medium
was removed and cells were washed three times with PBS. Cells were
fixed with 4% formaldehyde for 15 min, after which the nuclei were
stained with Hoechst 33258 and examined using a fluorescent
microscope (Olympus Corp.). For flow cytometry analysis, EPCs were
incubated with CellROX Green reagent, washed 3 times with PBS,
trypsinized and detected using a flow cytometer (FACSCalibur; BD
Biosciences). Data were analyzed using FlowJo software (FlowJo LLC,
Ashland, OR, USA). More than 6 mice were included in each
group.
Superoxide dismutase (SOD) and
malondialdehyde (MDA) assay
SOD activity and MDA content in the media were
measured using commercially available kits and colorimetric assays
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
according to the manufacturer’s protocols (31). More than 6 mice were included in
each group.
Small-interfering RNA (siRNA)
transfection
Nrf2 expression was silenced using siRNA (siRNA
mouse Silencer select Nrf2, gene ID: 18024; Guangzhou RiboBio Co.,
Ltd.), according to the manufacturer’s protocol with a riboFECT CP
Transfection kit (Guangzhou RiboBio Co., Ltd.) after determining
optimal transfection conditions (data not shown). The siRNA
sequence (sense strand) used for targeting Nrf2 was
CGACAGAAACCTCCATCTA. A Stealth RNAi Negative Control Duplex
(Guangzhou RiboBio Co., Ltd.) was used as a negative control. To
measure biological function, and to examine protein and mRNA
levels, EPCs were collected at 48 and 72 h post-transfection,
respectively. More than 6 mice were included in each group.
Western blotting
Western blotting for Nrf2 was performed in EPCs in
each group. Total proteins were prepared with RIPA lysis buffer at
4°C for 0.5 h (Beyotime Institute of Biotechnology, Haimen, China)
and quantified using a BCA kit (Beyotime Institute of
Biotechnology). Aliquots of cell lysates (50 μg) were
separated by 10% SDS-PAGE, electrotransferred to a polyvinylidene
difluoride (PVDF) membrane (Bio-Rad Laboratories, Inc.) and blocked
with TBS/0.1% Tween-20 (TBS-T) buffer with 5% nonfat milk for 1 h
at room temperature. Membranes were incubated with the appropriate
primary antibody (anti-Nrf2, 1:1,000; cat. no. 12721 or
anti-β-actin, 1:2,000, cat. no. 4970; Cell Signaling Technology,
Inc., Danvers, MA, USA; anti-p16, 1:1,000, cat. no. ARG57377, Arigo
Biolaboratories Corp., Taiwan, ROC) at 4°C overnight. The PVDF
membranes were washed with TBS-T buffer followed by incubation with
horse-radish peroxidase (HRP)-conjugated secondary antibody anti
rabbit immunoglobulin G (H+L; cat. no. ANT020; Antgene
Biotechnology Co., Ltd., Wuhan, China) at room temperature for 1 h.
Following extensive washing, the bands were detected using a
Chemiluminescence Detection System (ECL; Thermo Fisher Scientific,
Inc.). More than 4 mice were included in each group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of cultured EPCs was isolated with TRIzol
Reagent according to the manufacturer’s protocol (Thermo Fisher
Scientific, Inc.). Complementary DNA was synthesized using the
PrimeScript RT Master Mix kit (Takara Biotechnology Co., Ltd.,
Dalian, China) and then used for qPCR with a QuantiTect SYBR-Green
PCR kit (Qiagen GmbH, Hilden, Germany) on a ROCHE
LightCycler® 480 System (Roche Diagnostics GmbH, Basel,
Switzerland). The primer sequences were as follows: β-actin
forward, 5′-GGCTGTATTCCCCTCCATCG-3′ and reverse,
5′-CCAGTTGGTAACAATGCCATGT-3′; Nrf2 forward,
5′-TCCATTTCCGAGTCACTGAACCCA-3′ and reverse,
5′-TGACTCTGACTCCGGCATTTCACT-3′; HO-1 forward,
5′-AGGTACACATCCAAGCCGAGA-3′ and reverse,
5′-CATCACCAGCTTAAAGCCTTCT-3′; NQO-1 forward,
5′-AGGATGGGAGGTACTCGAATC-3′ and reverse,
5′-TGCTAGAGATGACTCGGAAGG-3′; p16 forward,
5′-CGCAGGTTCTTGGTCACTGT-3′ and reverse, 5′-TGTTCACGAAAGCCAGAGCG-3′;
interleukin 6 (IL-6) forward, 5′-TAGTCCTTCCTACCCCAATTTCC-3′ and
reverse, 5′-TTGGTCCTTAGCCACTCCTTC-3′; monocyte chemotactic
protein-2 (MCP-2) forward, 5′-TCTACGCAGTGCTTCTTTGCC-3′ and reverse,
5'-AAGGGGGATCTTCAGCTTTAGTA-3′; tumor necrosis factor α (TNF-α)
forward, 5′-CCCTCACACTCAGATCATCTTCT-3′ and reverse,
5′-GCTACGACGTGGGCTACAG-3′; vascular cell adhesion molecule 1
(VCAM-1) forward, 5′-AGTTGGGGATTCGGTTGTTCT-3′ and reverse,
5′-CCCCTCATTCCTTACCACCC-3′. The thermocycling conditions were as
follows: 95°C for 10 min, and 45 cycles of 95°C for 15 sec and 60°C
for 15 sec. The relative expression was analyzed according to the
2−ΔΔCq method (32).
qPCR was performed with 2 replicates of each sample, and more than
6 mice were included in each group.
Senescence-associated-β-galactosidase
(SA-β-gal) staining
EPCs were stained using a Senescence Cells
Histochemical Staining kit (Beyotime Institute of Biotechnology),
according to the manufacturer’s protocols, to assess senescence.
Senescence was quantitated by visual inspection of blue/green
stained cells with an inverted microscope (magnification, ×10).
More than 4 mice were included in each group.
Statistical analysis
Data are expressed as the mean ± standard error of
mean. Differences between groups were evaluated using either
two-tailed Student’s t-test or one-way analysis of variance
followed by Dunnett’s T3 post hoc test. All statistical analyses
were performed using the SPSS software version 19.0 (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of early EPCs
In the STZ-induced DM mice, body weight gain was
significantly slowed (Fig. 1A),
while blood glucose levels were significantly increased (Fig. 1B), compared with the control mice.
Following isolation and 7-day culture in EGM-2 medium, mononuclear
cells became spindle shaped (Fig.
1C), and double positive for acetylated low-density
lipoprotein-lectin (Dil-acLDL) uptake and lectin binding affinity
(Fig. 1D), which are hallmarks of
EPCs. In addition, flow cytometry analysis revealed that the
expression of CD34 (27.8 %), CD133 (37.2%), VEGF receptor 2 (36.1%)
and CD45dim (69.0%), the most widely used EPC markers in
the literature (5,22,24-28), further confirmed the
characteristics of early EPCs (Fig.
1E), consistent with previous reports (5,22,28). Therefore, the cells isolated and
cultured in the present study were considered suitable to further
examine the properties of EPCs.
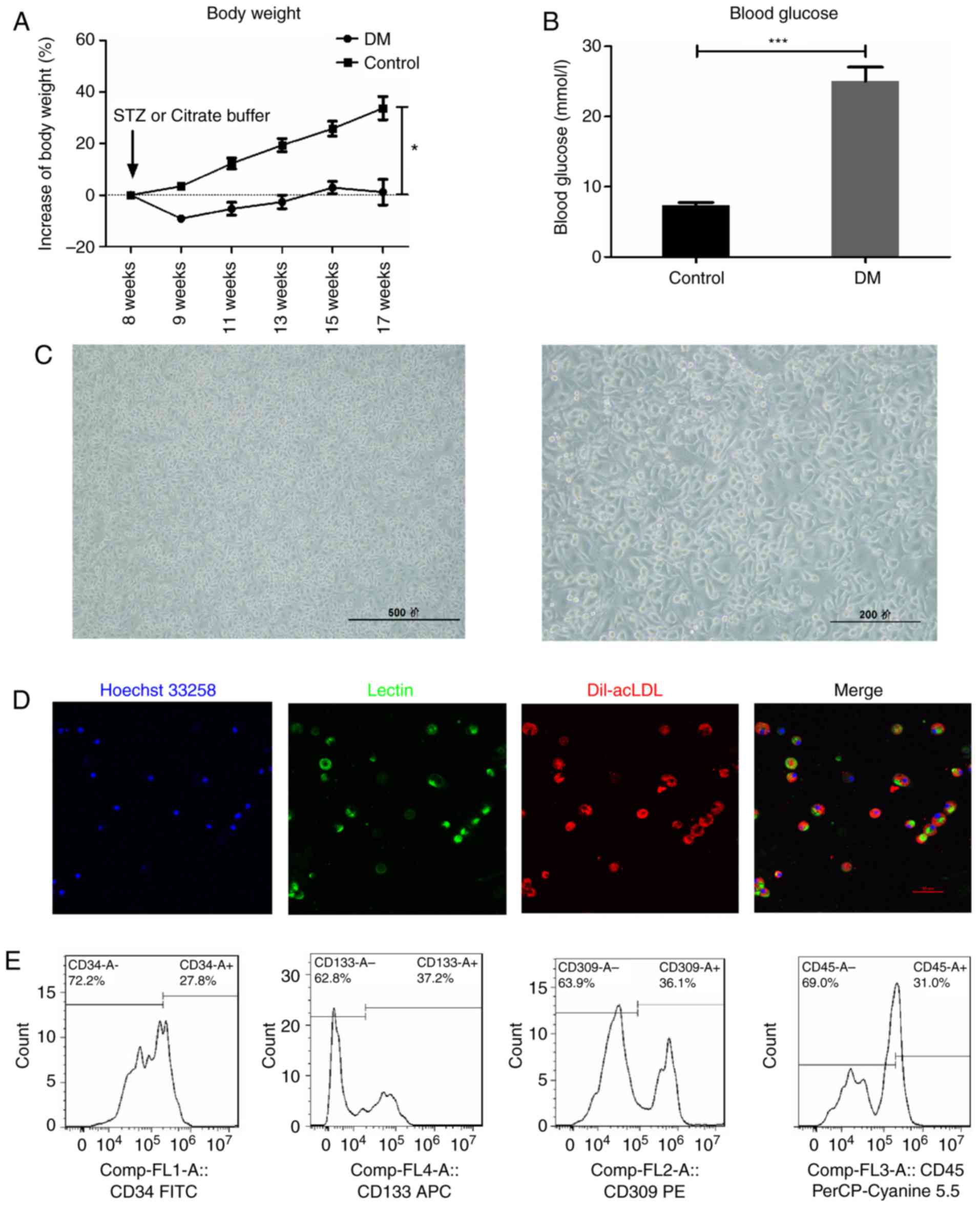 | Figure 1Characterization of early EPCs
derived from the STZ-induced DM mice. (A) Blood glucose and (B)
body weight measurements in STZ-induced DM mice. (C) The isolated
mononuclear cells became spindle-shaped following 7 days of
culture. Scale bar, 500 (left) and 200 (right) μm. (D) The
EPCs were identified as double positive for Dil-acLDL (red) and
lectin (green) following 7 days of culture. Scale bar, 50
μm. (E) Early EPCs were further confirmed by expression of
well-established cell surface markers CD34, CD133, VEGFR2 and
CD45dim. *P<0.05 and
***P<0.001 compared with control (n=10). EPCs,
endothelial progenitor cells; STZ, streptozotocin; DM, diabetes
mellitus; Dil-acLDL,
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine-labeled
acetylated low density lipoprotein; VEGFR2, vascular endothelial
growth factor receptor 2; FITC, fluorescein isothiocyanate. |
The biological functions of EPCs are
impaired in DM mice
Wound healing and Transwell assays were used to
examine the migratory capacity of EPCs isolated from mice in the DM
and control groups. Scratch wound healing assay results indicated
that the migration of EPCs was significantly decreased in DM mice
compared with the control group at 12 and 24 h (P<0.05; Fig. 2A and B). The Transwell assay
results also suggested that migration was significantly impaired in
DM mice, with 8.67±1.50 vs. 44.2±4.18 migrated cells per field in
the DM and control groups, respectively (P<0.05; Fig. 2C and D). An EdU assay was used to
detect the proliferative ability of EPCs and the results
demonstrated that proliferation was significantly reduced in DM
mice compared with the control group (10.08±1.57% vs. 28.6±3.3% in
DM and control groups respectively; P<0.05; Fig. 2E and F). Furthermore, tube-forming
activity was decreased in DM EPCs compared with the control group
(Fig. 2G), as was the secretion
of VEGF, SDF and NO (Fig. 2H–J).
These data indicate that DM decreased the migration, proliferation
and secretion abilities of EPCs.
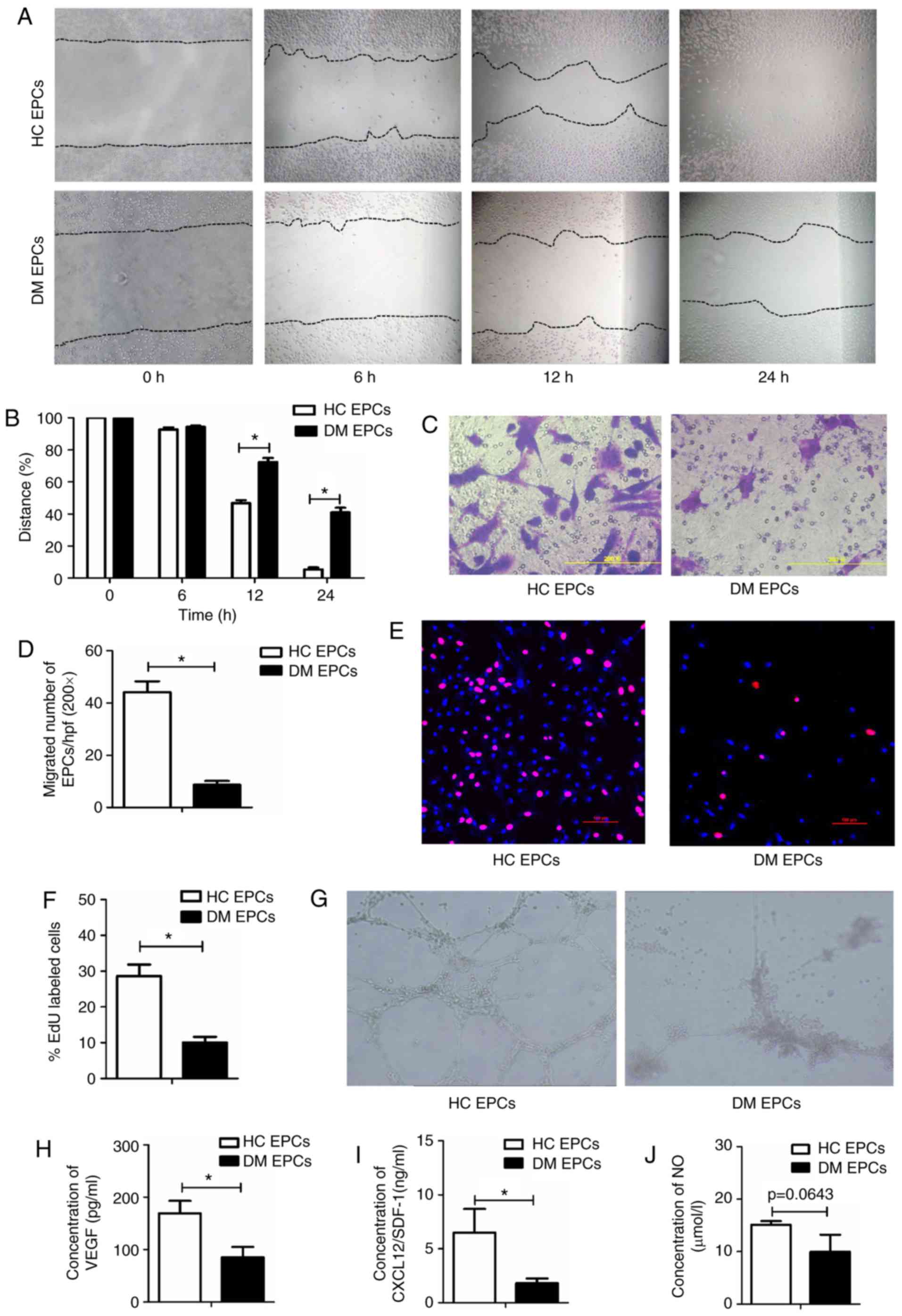 | Figure 2Impaired migration, proliferation,
secretion and angiogenesis of EPCs in DM mice. (A) Representative
images and (B) quantification of wound healing assays with EPCs
isolated from DM mice and control mice at time 0, 6, 12 and 24 h.
Magnification, ×100. The black dotted line shows the edge of the
cells. (C) Representative images and (D) quantification of crystal
violet-stained migrated cells, as assessed by Transwell assay.
Magnification, ×400. (E) Representative images and (F)
quantification of EdU staining (red), to measure the proliferative
ability of EPCs. Scale bar, 100 μm. (G) Representative
images showing tube-forming activity. Magnification, ×100. (H)
Levels of secreted VEGF, (I) SDF-1 and (J) NO in the supernatant of
cultured EPCs. *P<0.05 compared with control (n=4-8).
EPCs, endothelial progenitor cells; DM, diabetes mellitus; VEGF,
vascular endothelial growth factor; SDF-1, stromal-derived
factor-1; NO, nitric oxide; HC, healthy control. |
Nrf2 expression is decreased and
oxidative stress levels are increased in DM EPCs
ROS levels were markedly increased in EPCs derived
from DM mice compared with control EPCs, as assessed by
fluorescence detection (Fig. 3A)
and flow cytometry analysis (Fig.
3B). The levels of MDA were increased and SOD activity was
decreased in the supernatant of DM EPCs (Fig. 3C and D). Nrf2 expression was
decreased significantly in DM EPCs at the protein (Fig. 3E and F) and mRNA levels (Fig. 3G), compared with healthy EPCs. In
addition, the mRNA expression levels of HO-1 and NQO-1 were also
significantly decreased in DM EPCs (Fig. 3G). These results indicate that DM
increased oxidative stress and decreased Nrf2 expression in
EPCs.
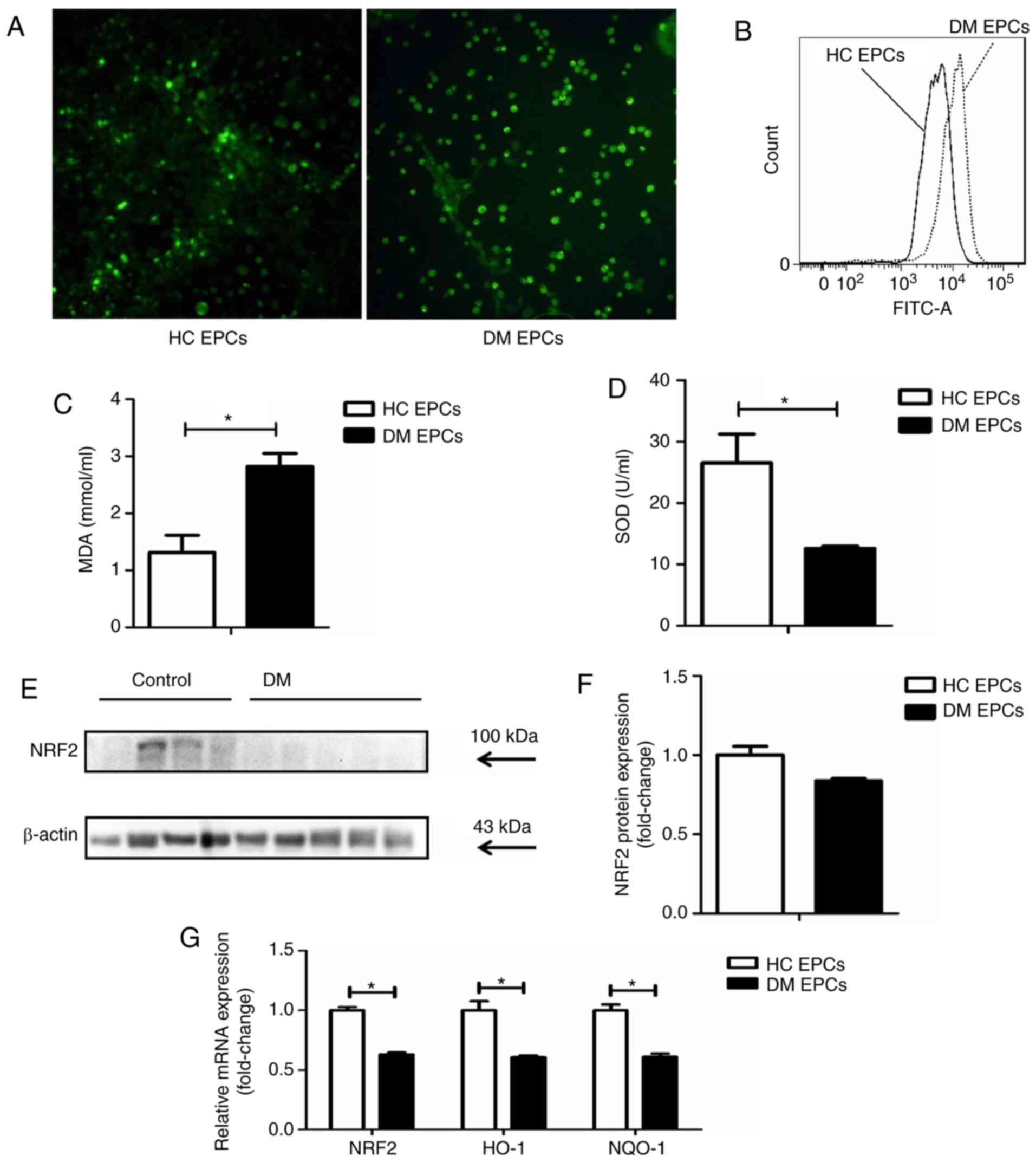 | Figure 3Decreased Nrf2 expression and
increased oxidative stress levels in diabetic EPCs. (A) ROS levels
in EPCs were detected utilizing CellROX Green reagent.
Representative fluorescent images are shown. Magnification, ×100.
(B) ROS levels in EPCs as measured by flow cytometry. (C) MDA
levels were increased and (D) SOD activity was decreased in the
supernatant of diabetic EPCs. (E) Representative blots and (F)
quantification of Nrf2 protein expression levels in control and
diabetic EPCs. (G) mRNA expression levels of Nrf2, HO-1 and NQO-1
were decreased in diabetic EPCs. *P<0.05 compared
with control (n=8). Nrf2, nuclear factor erythroid 2-related factor
2; EPCs, endothelial progenitor cells; DM, diabetes mellitus; ROS,
reactive oxygen species; MDA, malondialdehyde; SOD, superoxide
dismutase; HO-1, heme oxygenase-1; NQO-1, quinone oxidoreductase-1;
HC, healthy control. |
Nrf2 silencing impairs the migration,
proliferation, secretion and angiogenesis functions of EPCs
To investigate the role of Nrf2 in regulating the
biological function of EPCs, Nrf2 expression was silenced in normal
bone marrow-derived EPCs via siRNA transfection. Nrf2-silenced EPCs
had decreased migratory abilities (Fig. 4A–D), proliferative capacity
(Fig. 4E and F), and tube-forming
activity (Fig. 4G), as well as
lower VEGF secretion (Fig. 4H),
compared with the negative controls. SDF-1 and NO levels were lower
in the Nrf2-silenced EPCs, however these data were not
statistically significant (Fig. 4I
and J). Collectively, the functionality of EPCs was reduced
following Nrf2 silencing.
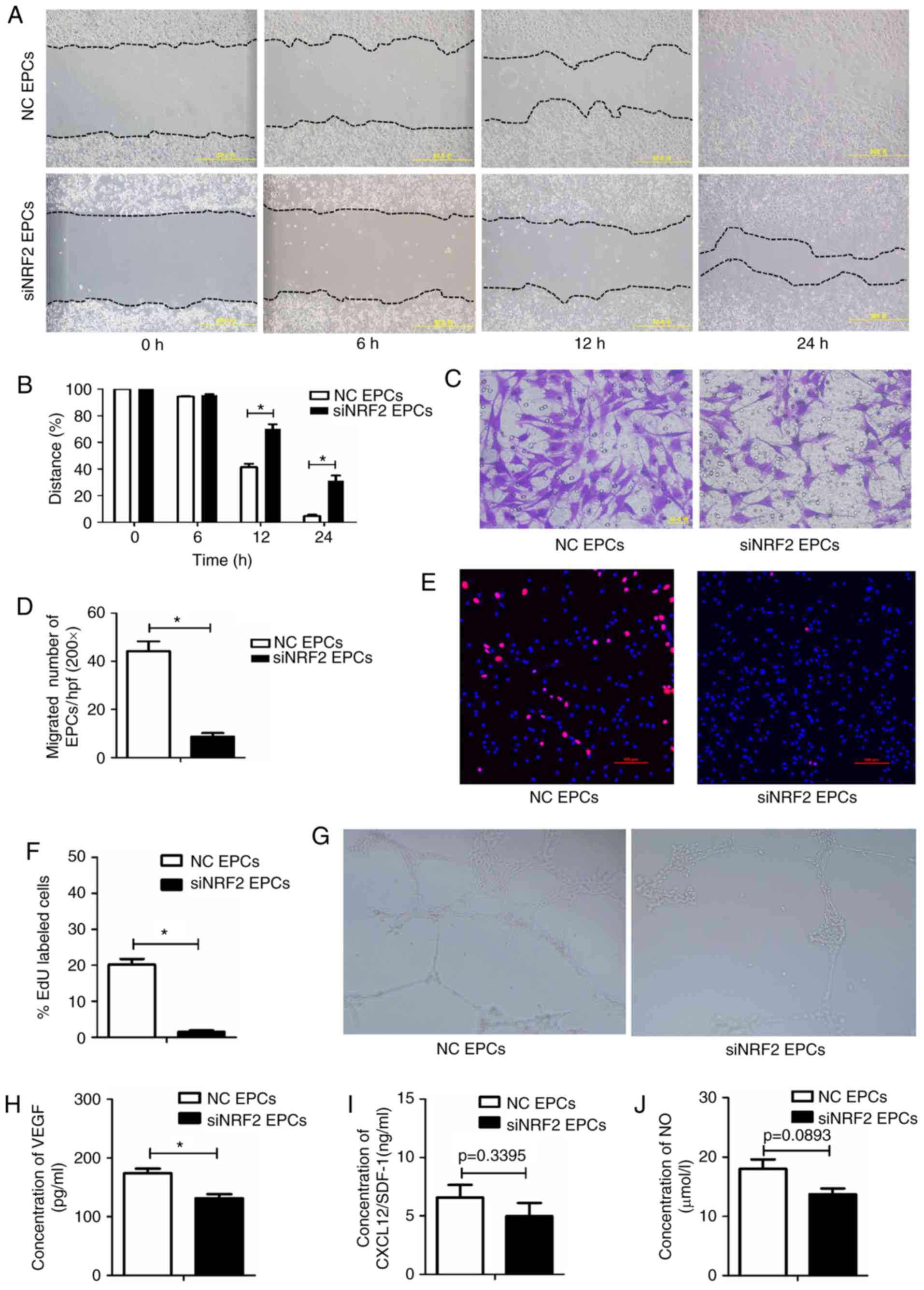 | Figure 4Nrf2 silencing reduces migration,
proliferation, secretion and angiogenesis in EPCs. (A)
Representative images and (B) quantification of wound healing
assays with Nrf2-silenced EPCs and negative controls at 0, 6, 12
and 24 h. Magnification, ×100. The black dotted line shows the edge
of the cell population. (C) Representative images and (D)
quantification of crystal violet-stained migrated cells, as
assessed by Transwell assay. Magnification, ×400. (E)
Representative images and (F) quantification of EdU staining (red),
to measure the proliferative ability of Nrf2-silenced and control
EPCs. Scale bar, 100 μm. (G) Representative images showing
tube-forming activity. Magnification, ×100. (H) Levels of secreted
VEGF, (I) SDF-1 and (J) NO in the supernatant of cultured
Nrf2-silenced and control EPCs. *P<0.05 compared with
control (n=4-6). Nrf2, nuclear factor erythroid 2-related factor 2;
EPCs, endothelial progenitor cells; VEGF, vascular endothelial
growth factor; SDF-1, stromal-derived factor-1; NO, nitric oxide;
NC, negative control; si, small interfering. |
Nrf2 silencing upregulates ROS and MDA,
and decreases the activity of SOD in EPCs
Nrf2 silencing was achieved by transfection with
siRNA (Fig. 5A and B). Following
Nrf2 silencing in the EPCs, the mRNA expression levels of HO-1 and
NQO-1 were reduced (Fig. 5C),
while ROS production was increased (Fig. 5D and E), compared with the
negative controls. In addition, Nrf2 silencing significantly
increased the expression of MDA and decreased SOD activity in the
supernatant of EPCs (Fig. 5F and
G).
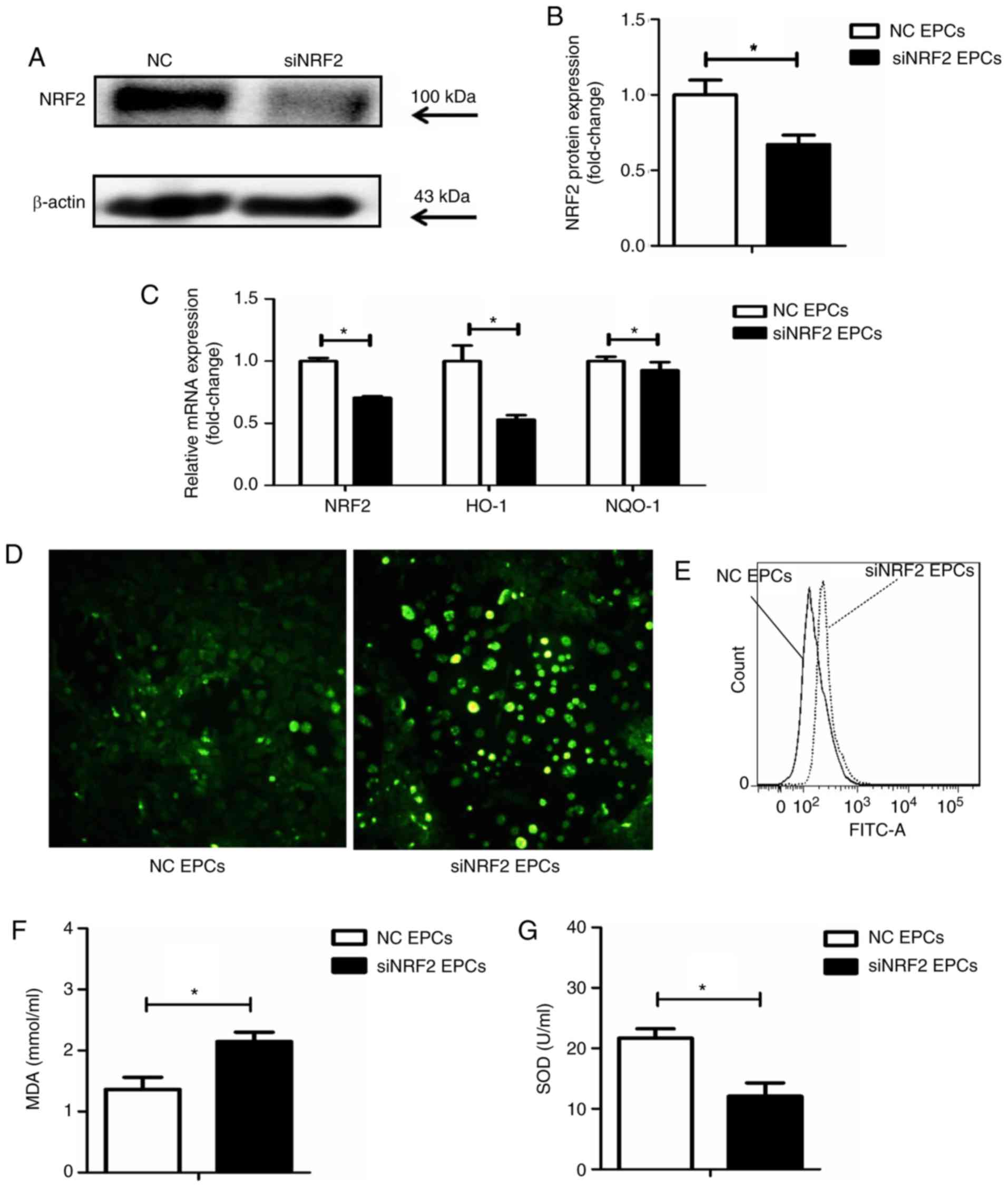 | Figure 5Nrf2 silencing increases the
expression of ROS and MDA in EPCs, while SOD activity is
downregulated. (A) Representative blots and (B) quantification of
Nrf2 protein expression levels in Nrf2-silenced and control EPCs.
(C) mRNA expression levels of Nrf2, HO-1 and NQO-1 in Nrf2-silenced
and control EPCs. (D) Representative images from fluorescence
microscopy analysis of ROS levels (green; magnification, ×100). (E)
Quantification of ROS levels as detected by flow cytometry. (F)
Nrf2 silencing upregulated MDA levels and (G) decreased SOD
activity in the supernatant of Nrf2-silenced EPCs.
*P<0.05 compared with control (n=8). Nrf2, nuclear
factor erythroid 2-related factor 2; ROS, reactive oxygen species;
MDA, malondialdehyde; EPCs, endothelial progenitor cells; SOD,
superoxide dismutase; HO-1, heme oxygenase-1; NQO-1, quinone
oxidoreductase-1; NC, negative control; si, small interfering. |
Nrf2 activation by tBHQ improves the
migration, proliferation and secretion of DM EPCs
To determine whether Nrf2 serves an important role
in EPCs, the effect of Nrf2 activation by tBHQ, a validated Nrf2
inducer (33), was assessed on
the biological function of EPCs from DM mice. The migratory ability
(Fig. 6A–D), proliferative
capacity (Fig. 6E and F) and
tube-forming activity (Fig. 6G)
of DM EPCs were increased following Nrf2 activation (with 20
μM tBHQ), compared with untreated DM EPCs. In addition,
tBHQ-treated DM-EPCs displayed a trend to increased VEGF, SDF-1 and
NO secretion, although these results were not statistically
significant (Fig. 6H–J).
Collectively, these results suggest that tBHQ-mediated Nrf2
activation improved the migration, proliferation and secretion
capacity of DM EPCs.
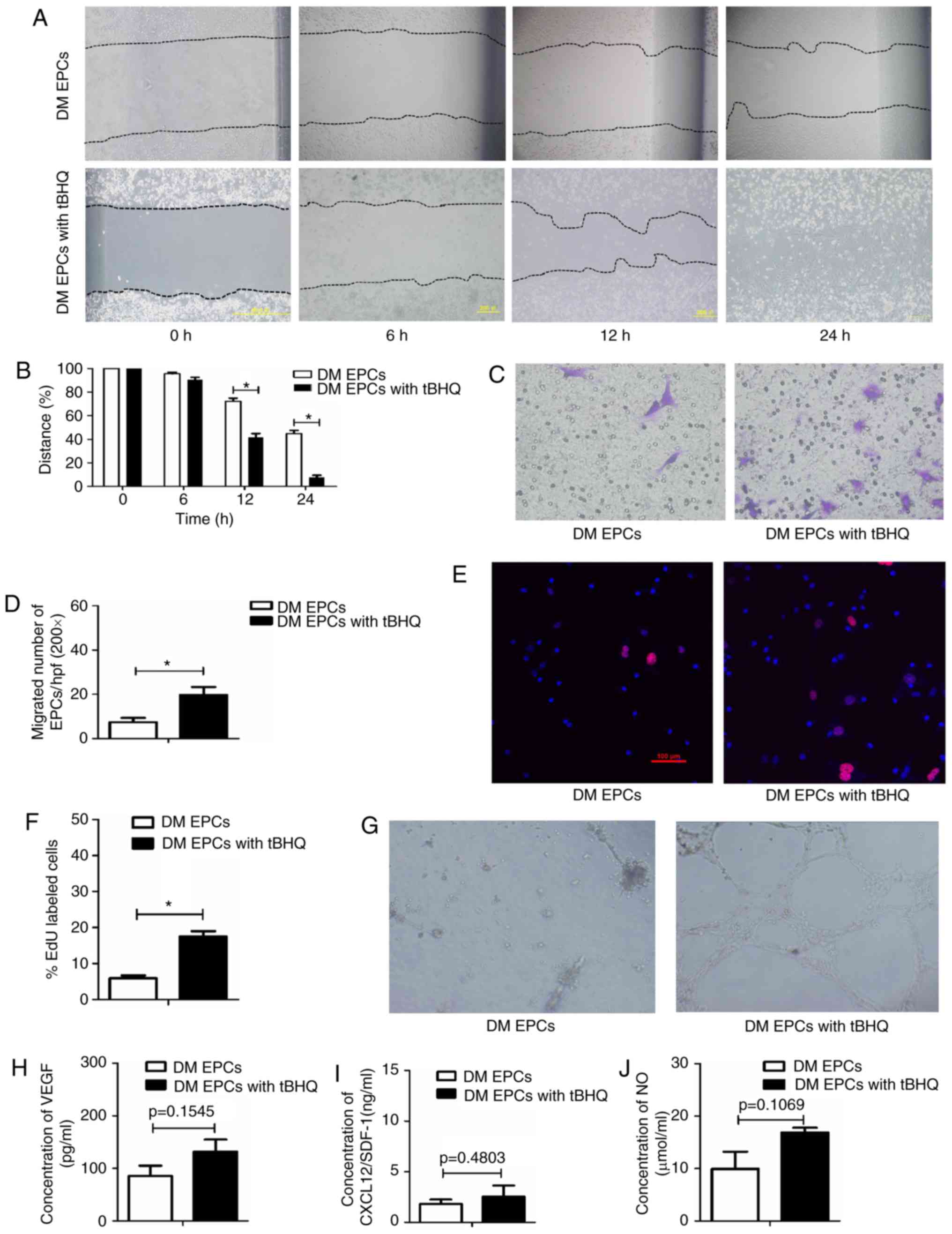 | Figure 6Nrf2 activation by tBHQ improves
migration, proliferation, secretion and angiogenesis in diabetic
EPCs. (A) Representative images and (B) quantification of wound
healing assays with DM EPCs treated with Nrf2 activator tBHQ for 0,
6, 12 and 24 h. Magnification, ×100. The black dotted line shows
the edge of the cell population. (C) Representative images and (D)
quantification of crystal violet-stained migrated cells, as
assessed by Transwell assay. Magnification, ×400. (E)
Representative images and (F) quantification of EdU staining (red),
to measure the proliferative ability of Nrf2-activated DM EPCs.
Scale bar, 100 μm. (G) Representative images showing
tube-forming activity. Magnification, ×100. (H) Levels of secreted
VEGF, (I) SDF-1 and (J) NO in the supernatant of cultured DM EPCs
treated with tBHQ. *P<0.05 compared with control
(n=4-6). Nrf2, nuclear factor erythroid 2-related factor 2; tBHQ,
tert-Butylhydroquinone; EPCs, endothelial progenitor cells; DM,
diabetes mellitus; VEGF, vascular endothelial growth factor; SDF-1,
stromal-derived factor-1; NO, nitric oxide. |
tBHQ protects against oxidative stress in
DM-EPCs
Following treatment with tBHQ, EPCs isolated from DM
mice had higher Nrf2 expression at the protein (Fig. 7A and B) and mRNA level (Fig. 7C). In addition, HO-1 and NQO-1
mRNA expression levels were increased in tBHQ-treated DM EPCs
compared with untreated DM EPCs (Fig.
7C), while ROS production was decreased (Fig. 7D and E). MDA expression was
decreased and SOD activity was increased in the supernatant of DM
EPCs treated with tBHQ compared with untreated cells (Fig. 7F and G). These results indicate
that tBHQ has the potential to ameliorate oxidative stress in DM
EPCs.
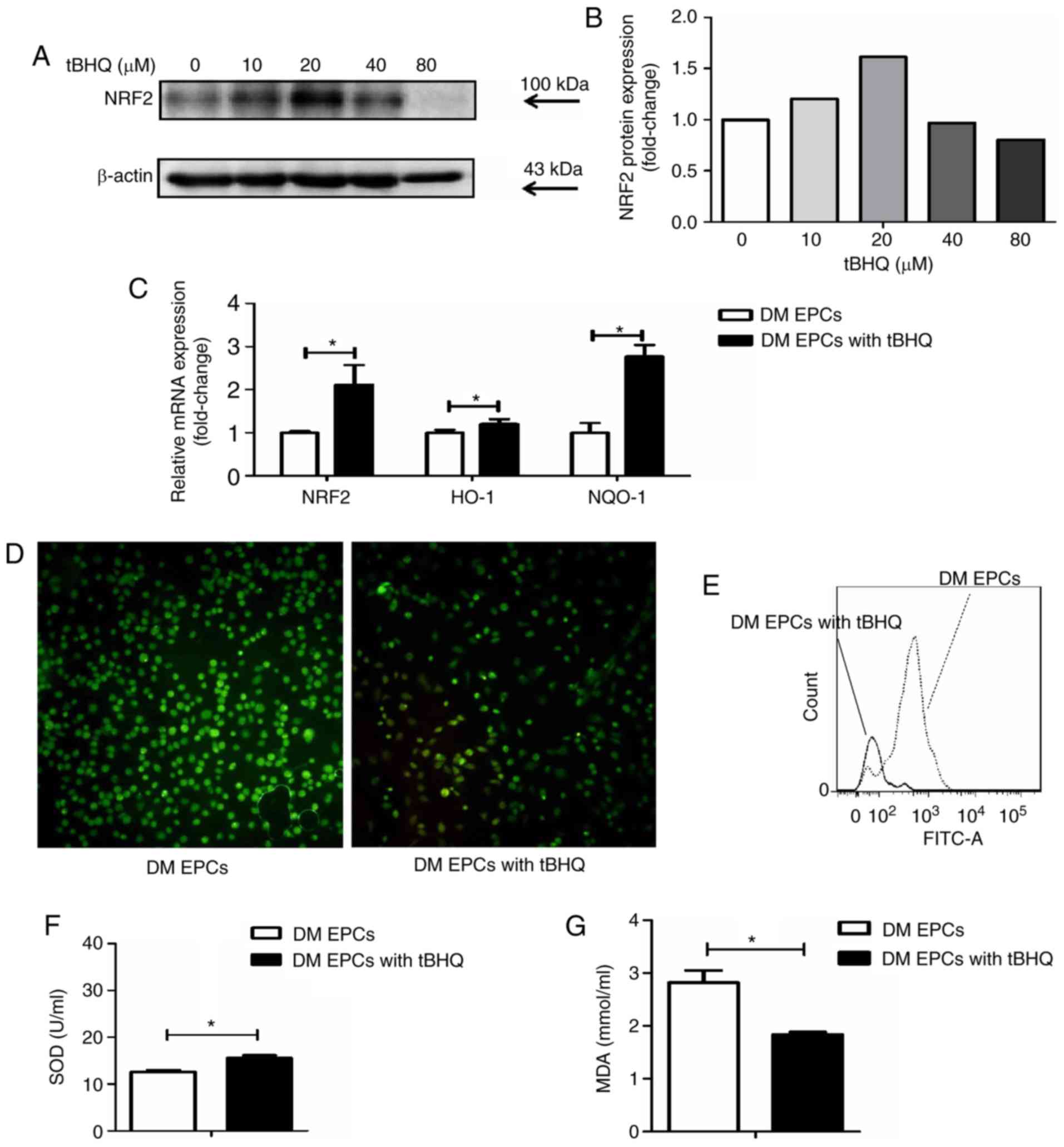 | Figure 7tBHQ protects against oxidative
stress in EPCs derived from DM mice. (A) Representative images and
(B) quantification of Nrf2 protein expression levels in
tBHQ-treated DM EPCs. (C) mRNA expression levels of Nrf2, HO-1 and
NQO-1 in Nrf2-activated DM EPCs. (D) Representative images from
fluorescence microscopy analysis of ROS levels (green;
magnification, ×100). (E) Quantification of ROS levels as detected
by flow cytometry. (F) MDA expression was decreased and (G) SOD
activity was increased in the supernatant of DM EPCs treated with
tBHQ compared with untreated controls. *P<0.05
compared with control (n=8). tBHQ, tert-Butylhydroquinone; EPCs,
endothelial progenitor cells; DM, diabetes mellitus; Nrf2, nuclear
factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; NQO-1,
quinone oxidoreductase-1; MDA, malondialdehyde; SOD, superoxide
dismutase. |
Nrf2 regulates the senescence of EPCs in
DM mice
To explore the underlying mechanism by which tBHQ
protects against damage in DM-EPCs, the senescence of EPCs was
assessed using an SA-β-gal assay, and by evaluating p16 expression
(34) and senescence-associated
secretory phenotype (SASP) (34,35). SASP was evaluated by measuring the
mRNA expression levels of IL-6, MCP-2, TNF-α and VCAM1. As
illustrated in Fig. 8A and B, DM
EPCs exhibited increased β-gal-positive cell staining compared with
the control group, while tBHQ treatment reversed this effect. In
addition, p16 and SASP expression was increased in DM EPCs compared
with the control group, and this effect was significantly reversed
by tBHQ treatment (Fig. 8C–F). In
normal healthy EPCs, an increased number of β-gal-positive cells
were observed following Nrf2 silencing (Fig. 8G and H), and p16 and SASP were
increased (Fig. 8I–K). The
results suggest that Nrf2 negatively regulated the senescence of
EPCs.
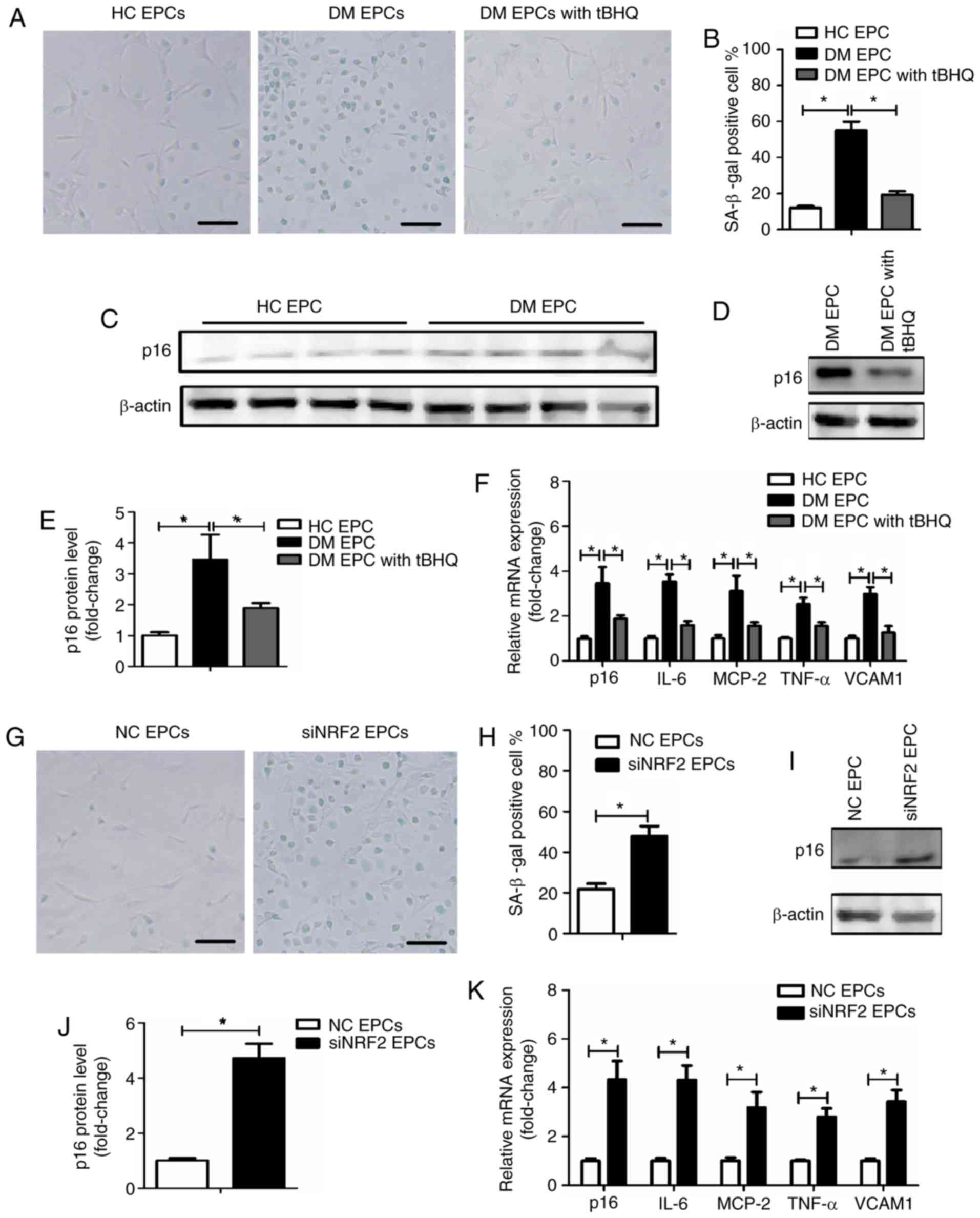 | Figure 8Nrf2 regulates EPC senescence in DM
mice. (A) Representative images and (B) quantification of β-gal
staining of HC EPCs, DM EPCs and DM EPCs treated with tBHQ. Scale
bar, 100 μm. (C-E) Expression levels of p16 protein in HC EPCs, DM
EPCs and DM EPCs treated with tBHQ. (F) mRNA expression levels of
p16, IL-6, MCP-2, TNF-α and VCAM1 in HC EPCs, DM EPCs and DM EPCs
treated with tBHQ. (G) Representative images and (H) quantification
of β-gal staining of NC EPCs and siNRF2 EPCs. Scale bar, 100 μm.
(I) Representative blots and (J) quantification of p16 protein
expression levels in NC EPCs and siNRF2 EPCs. (K) mRNA expression
levels of p16, IL-6, MCP-2, TNF-α, and VCAM1 in NC EPCs and siNRF2
EPCs. *P<0.05, with comparisons indicated by lines
(n=4-6). Nrf2, nuclear factor erythroid 2-related factor 2; EPCs,
endothelial progenitor cells; DM, diabetes mellitus; HC, healthy
control; tBHQ, tert-Butylhydroquinone; IL, interleukin; MCP,
monocyte chemotactic protein; TNF, tumor necrosis factor; VCAM1,
vascular cell adhesion molecule 1; NC, negative control; si, small
interfering. |
Discussion
The results of the present study revealed that DM
reduced the expression of Nrf2, which was accompanied by enhanced
oxidative stress, senescence and dysfunction in EPCs. Nrf2
activation protected DM EPCs against oxidative stress and
ameliorated the biological dysfunction and senescence of DM EPCs.
These results suggest that Nrf2 may serve a vital role in
regulating EPC survival and maintaining functionality under
oxidative stress via modifying cell senescence.
Patients with DM often experience serious
complications, including CVD, diabetic retinopathy and diabetic
nephropathy, which can cause death or blindness. Some EPC subtypes
have been considered as potential therapeutic modalities for DM
complications (36,37). Boyko et al (38) reported that the emergence of
arterial disease is the only limb-specific risk factors for
amputation in DM, and EPCs contribute to postnatal
neovascularization and endothelial repair. Thus, therapeutic
interventions using EPCs may be a promising strategy for the
management of DM. Previous studies have indicated that DM is able
to downregulate the number of circulating EPCs in humans (4,39,40) and in animals (28,41,42). In addition, proliferation, colony
formation, tube formation, self-renewal and mobilization in DM EPCs
were reduced (41,43). In the present study, EPCs isolated
from STZ-induced DM mice displayed decreased functionality,
including inhibited migration, proliferation and angiogenesis
abilities, as well as reduced secretion of NO, VEGF and SDF-1α, all
of which are important for the vascular recruitment repair in DM.
These results were consistent with the previous report (39-43). NO release is essential for the
survival, migration, and other biological functions of EPCs. It had
been proposed that VEGF and SDF-1α act together to stimulate
angiogenic processes (44), both
of which are also implicated in EPC mobilization.
To further explore the potential mechanisms by which
DM inhibits EPC functionality, the levels of oxidative stress in DM
EPCs were assessed. The results revealed that EPC impairment in DM
may be associated with oxidative stress, with increased ROS and MDA
content and decreased SOD activity. Nrf2 regulates the response of
cells to oxidative stress; activated Nrf2 translocates into the
nucleus, binds to antioxidant response elements and activates the
transcription of target antioxidant genes, including HO-1, to
counteract ROS (45). It has been
reported that Nrf2 knockdown reduces the biofunction of endothelial
cells, while angiogenic factors can promote tube formation in
endothelial cells via activating Nrf2 and increasing expression of
its target gene, HO-1 (46).
Increasing Nrf2 activity and its downstream target genes protects
against EPC damage in DM, and the protective role of SDF-1 is
reduced by silencing Nrf2 (22).
Nrf2 serves an important role in the angiogenesis of EPCs,
especially when cells are under oxidative stress (47). Previous reports have demonstrated
that Nrf2 is downregulated in the nuclei of EPCs under high glucose
treatment, including in DM (48,49). In the present study, total Nrf2
expression was decreased in DM EPCs compared with the control
group. Furthermore, prototype Nrf2 target genes, NQO1 and HO-1,
were downregulated, which is consistent with previous studies.
Overall, Nrf2 may modify the oxidative stress and participate in
the diabetes-induced damages of EPCs.
Previous research has suggested that Nrf2 increases
the lifespan in Caenorhabditis elegans (50) and regulates neural stem cells
during aging (51). Based on
this, the present study next explored whether senescence serves a
role in the pathogenesis of DM EPCs. The results revealed that DM
accelerated EPC senescence and reduced the expression and activity
of Nrf2. Silencing of Nrf2 resulted in an increase in normal EPC
senescence, while Nrf2 overexpression downregulated senescence in
DM EPCs and ameliorated functional impairments. Based on these
results, it can be concluded that Nrf2 serves a protective role in
ameliorating cell senescence in DM. You et al (23) reported that curcumin modulates the
function of endothelial progenitor cells and can activate the Nrf2
signaling pathway (52). Nrf2 may
be an effective target for the prevention or treatment of DM
complications, reducing diabetic amputation risk by regulating EPC
dysfunction.
In summary, the present study demonstrated that Nrf2
protected against DM-induced EPC dysfunction due to oxidative
stress, possibly via ameliorating cell senescence. These results
suggest that targeting Nrf2 may be a promising therapeutic method
for the treatment and prevention of diabetes-induced endothelial
dysfunction and microangiopathy, potentially reducing the risk of
complications associated with DM.
Acknowledgments
Not applicable.
Abbreviations:
|
EPCs
|
endothelial progenitor cells
|
|
DM
|
diabetes mellitus
|
|
STZ
|
streptozotocin
|
|
SDF-1
|
stromal-derived factor-1
|
|
NO
|
nitric oxide
|
|
SOD
|
superoxide dismutase
|
|
Dil-acLDL
|
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine-labeled
acetylated low density lipoprotein
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
|
tBHQ
|
tert-Butylhydroquinone
|
|
KEAP1
|
kelch-like ECH-associated
protein-1
|
|
HO-1
|
heme oxygenase-1
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
NQO-1
|
quinone oxidoreductase-1
|
|
SASP
|
senescence-associated secretory
phenotype
|
Funding
This work was supported by the Natural Science
Foundation of China (grant no. 81571373) and the Natural Science
Foundation of Hubei Province (grant no. 2017CFB627).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
RYW designed and performed the study, analyzed the
data and wrote the manuscript. LHL and LZ contributed to writing
the manuscript. HL, KFW and JA were involved in performing the
study. QW, YL, LJB and BMQ contributed to data analysis and
interpretation. BLQ conceived the study, participated in its design
and helped to draft the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of Tongji Medical College, Huazhong University of Science
and Technology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abplanalp WT, Conklin DJ, Cantor JM,
Ginsberg MH, Wysoczynski M, Bhatnagar A and O’Toole TE: Enhanced
integrinα4β1-mediated adhesion contributes to a mobilization defect
of endothelial progenitor cells in diabetes. Diabetes.
65:3505–3515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soedamah-Muthu SS, Fuller JH, Mulnier HE,
Raleigh VS, Lawrenson RA and Colhoun HM: High risk of
cardiovascular disease in patients with type 1 diabetes in the
U.K.: A cohort study using the general practice research database.
Diabetes Care. 29:798–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Georgescu A: Vascular dysfunction in
diabetes: The endothelial progenitor cells as new therapeutic
strategy. World J Diabetes. 2:92–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fadini GP, Sartore S, Albiero M, Baesso I,
Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A,
Agostini C and Avogaro A: Number and function of endothelial
progenitor cells as a marker of severity for diabetic vasculopathy.
Arterioscler Thromb Vasc Biol. 26:2140–2146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zampetaki A, Kirton JP and Xu Q: Vascular
repair by endothelial progenitor cells. Cardiovasc Res. 78:413–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Williamson K, Stringer SE and Alexander
MY: Endothelial progenitor cells enter the aging arena. Front
Physiol. 3:302012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung HS, Shyu WC, Tsai CH, Hsu SH and Lin
SZ: Transplantation of endothelial progenitor cells as therapeutics
for cardiovascular diseases. Cell Transplant. 18:1003–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatwadekar AD, Shaw LC and Grant MB:
Promise of endothelial progenitor cell for treatment of diabetic
retinopathy. Expert Rev Endocrinol Metab. 5:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cantrell D: T cell antigen receptor signal
transduction pathways. Annu Rev Immunol. 14:259–274. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dinkova-Kostova AT, Holtzclaw WD, Cole RN,
Itoh K, Wakabayashi N, Katoh Y, Yamamoto M and Talalay P: Direct
evidence that sulfhydryl groups of Keap1 are the sensors regulating
induction of phase 2 enzymes that protect against carcinogens and
oxidants. Proc Natl Acad Sci USA. 99:11908–11913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McMahon M, Itoh K, Yamamoto M and Hayes
JD: Keap1-dependent proteasomal degradation of transcription factor
Nrf2 contributes to the negative regulation of antioxidant response
element-driven gene expression. J Biol Chem. 278:21592–21600. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar
|
|
16
|
Venugopal R and Jaiswal AK: Nrf1 and Nrf2
positively and c-Fos and Fra1 negatively regulate the human
antioxidant response element-mediated expression of NAD(P)H:
Quinone oxidoreductase1 gene. Proc Natl Acad Sci USA.
93:14960–14965. 1996. View Article : Google Scholar
|
|
17
|
Lee JM, Li J, Johnson DA, Stein TD, Kraft
AD, Calkins MJ, Jakel RJ and Johnson JA: Nrf2, a multi-organ
protector. FASEB J. 19:1061–1066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W and Kong AN: Molecular mechanisms of
Nrf2-mediated antioxidant response. Mol Carcinog. 48:91–104. 2009.
View Article : Google Scholar :
|
|
19
|
Maher JM, Dieter MZ, Aleksunes LM, Slitt
AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et
al: Oxidative and electrophilic stress induces multidrug
resistance-associated protein transporters via the nuclear
factor-E2-related factor-2 transcriptional pathway. Hepatology.
46:1597–1610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ and Nel AE: The role of phase II
antioxidant enzymes in protecting memory T cells from spontaneous
apoptosis in young and old mice. J Immunol. 175:2948–2959. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thimmulappa RK, Scollick C, Traore K,
Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW and
Biswal S: Nrf2-dependent protection from LPS induced inflammatory
response and mortality by CDDO-Imidazolide. Biochem Biophys Res
Commun. 351:883–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai X, Yan X, Zeng J, Chen J, Wang Y, Chen
J, Li Y, Barati MT, Wintergerst KA, Pan K, et al: Elevating CXCR7
improves angiogenic function of EPCs via Akt/GSK-3β/Fyn-Mediated
Nrf2 activation in diabetic limb ischemia. Circ Res.
120:e7-e232017. View Article : Google Scholar
|
|
23
|
You J, Sun J, Ma T, Yang Z, Wang X, Zhang
Z, Li J, Wang L, Ii M, Yang J and Shen Z: Curcumin induces
therapeutic angiogenesis in a diabetic mouse hindlimb ischemia
model via modulating the function of endothelial progenitor cells.
Stem Cell Res Ther. 8:1822017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Zhang X, Guan X, Cui X, Wang Y, Chu
H and Cheng M: Advanced glycation end products impair the
migration, adhesion and secretion potentials of late endothelial
progenitor cells. Cardiovasc Diabetol. 11:462012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sorrentino SA, Bahlmann FH, Besler C,
Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A,
Limbourg F, et al: Oxidant stress impairs in vivo
reendothelialization capacity of endothelial progenitor cells from
patients with type 2 diabetes mellitus: Restoration by the
peroxisome proliferator-activated receptor-gamma agonist
rosiglitazone. Circulation. 116:163–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landmesser U, Engberding N, Bahlmann FH,
Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner
D, Templin C, Kotlarz D, et al: Statin-induced improvement of
endothelial progenitor cell mobilization, myocardial
neovascularization, left ventricular function, and survival after
experimental myocardial infarction requires endothelial nitric
oxide synthase. Circulation. 110:1933–1939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bahlmann FH, De Groot K, Spandau JM,
Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H and
Fliser D: Erythropoietin regulates endothelial progenitor cells.
Blood. 103:921–926. 2004. View Article : Google Scholar
|
|
28
|
Wu Q, Qi B, Liu Y, Cheng B, Liu L, Li Y
and Wang Q: Mechanisms underlying protective effects of
trimetazidine on endothelial progenitor cells biological functions
against H2O2-induced injury: Involvement of antioxidation and
Akt/eNOS signaling pathways. Eur J Pharmacol. 707:87–94. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuliszewski MA, Ward MR, Kowalewski JW,
Smith AH, Stewart DJ, Kutryk MJ and Leong-Poi H: A direct
comparison of endothelial progenitor cell dysfunction in rat
metabolic syndrome and diabetes. Atherosclerosis. 226:58–66. 2013.
View Article : Google Scholar
|
|
30
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li WD, Hu N, Lei FR, Wei S, Rong JJ,
Zhuang H and Li XQ: Autophagy inhibits endothelial progenitor cells
migration via the regulation of MMP2, MMP9 and uPA under normoxia
condition. Biochem Biophys Res Commun. 466:376–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Takaya K, Suzuki T, Motohashi H, Onodera
K, Satomi S, Kensler TW and Yamamoto M: Validation of the multiple
sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med.
53:817–827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang R, Yu Z, Sunchu B, Shoaf J, Dang I,
Zhao S, Caples K, Bradley L, Beaver LM, Ho E, et al: Rapamycin
inhibits the secretory phenotype of senescent cells by a
Nrf2-independent mechanism. Aging Cell. 16:564–574. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
D’Apolito M, Colia AL, Lasalvia M, Capozzi
V, Falcone MP, Pettoello-Mantovani M, Brownlee M, Maffione AB and
Giardino I: Urea-induced ROS accelerate senescence in endothelial
progenitor cells. Atherosclerosis. 263:127–136. 2017. View Article : Google Scholar
|
|
36
|
Lois N, McCarter RV, O’Neill C, Medina RJ
and Stitt AW: Endothelial progenitor cells in diabetic retinopathy.
Front Endocrinol (Lausanne). 5:442014.
|
|
37
|
Foresti R, Bucolo C, Platania CM, Drago F,
Dubois-Randé JL and Motterlini R: Nrf2 activators modulate
oxidative stress responses and bioenergetic profiles of human
retinal epithelial cells cultured in normal or high glucose
conditions. Pharmacol Res. 99:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boyko EJ, Seelig AD and Ahroni JH: Limband
person-level risk factors for lower-limb amputation in the
prospective seattle diabetic foot study. Diabetes Care. 41:891–898.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hörtenhuber T, Rami-Mehar B, Satler M,
Nagl K, Höbaus C, Höllerl F, Koppensteiner R, Schernthaner G,
Schober E and Schernthaner GH: Endothelial progenitor cells are
related to glycemic control in children with type 1 diabetes over
time. Diabetes Care. 36:1647–1653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
António N, Fernandes R, Soares A, Soares
F, Lopes A, Carvalheiro T, Paiva A, Pêgo GM, Providência LA,
Gonçalves L and Ribeiro CF: Reduced levels of circulating
endothelial progenitor cells in acute myocardial infarction
patients with diabetes or pre-diabetes: Accompanying the glycemic
continuum. Cardiovasc Diabetol. 13:1012014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ingram DA, Lien IZ, Mead LE, Estes M,
Prater DN, Derr-Yellin E, DiMeglio LA and Haneline LS: In vitro
hyperglycemia or a diabetic intrauterine environment reduces
neonatal endothelial colony-forming cell numbers and function.
Diabetes. 57:724–731. 2008. View Article : Google Scholar
|
|
42
|
Tsukada S, Masuda H, Jung SY, Yun J, Kang
S, Kim DY, Park JH, Ji ST, Kwon SM and Asahara T: Impaired
development and dysfunction of endothelial progenitor cells in type
2 diabetic mice. Diabetes Metab. 43:154–162. 2017. View Article : Google Scholar
|
|
43
|
Fadini GP, Sartore S, Schiavon M, Albiero
M, Baesso I, Cabrelle A, Agostini C and Avogaro A: Diabetes impairs
progenitor cell mobilisation after hindlimb ischaemia-reperfusion
injury in rats. Diabetologia. 49:3075–3084. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kryczek I, Lange A, Mottram P, Alvarez X,
Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, et al: CXCL12
and vascular endothelial growth factor synergistically induce
neoangiogenesis in human ovarian cancers. Cancer Res. 65:465–472.
2005.PubMed/NCBI
|
|
45
|
Uruno A, Yagishita Y and Yamamoto M: The
Keap1-Nrf2 system and diabetes mellitus. Arch Biochem Biophys.
566:76–84. 2015. View Article : Google Scholar
|
|
46
|
Florczyk U, Jazwa A, Maleszewska M, Mendel
M, Szade K, Kozakowska M, Grochot-Przeczek A, Viscardi M, Czauderna
S, Bukowska-Strakova K, et al: Nrf2 regulates angiogenesis: Effect
on endothelial cells, bone marrow-derived proangiogenic cells and
hind limb ischemia. Antioxid Redox Signal. 20:1693–1708. 2014.
View Article : Google Scholar :
|
|
47
|
Gremmels H, de Jong OG, Hazenbrink DH,
Fledderus JO and Verhaar MC: The transcription factor Nrf2 protects
angiogenic capacity of endothelial colony-forming cells in
high-oxygen radical stress conditions. Stem Cells Int.
2017:46806122017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chiu SC, Chao CY, Chiang EI, Syu JN,
Rodriguez RL and Tang FY: N-3 polyunsaturated fatty acids alleviate
high glucose-mediated dysfunction of endothelial progenitor cells
and prevent ischemic injuries both in vitro and in vivo. J Nutr
Biochem. 42:172–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vaamonde-Garcia C, Courties A, Pigenet A,
Laiguillon MC, Sautet A, Houard X, Kerdine-Römer S, Meijide R,
Berenbaum F and Sellam J: The nuclear factor-erythroid 2-related
factor/heme oxygenase-1 axis is critical for the inflammatory
features of type 2 diabetes-associated osteoarthritis. J Biol Chem.
292:14505–14515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tullet JMA, Green JW, Au C, Benedetto A,
Thompson MA, Clark E, Gilliat AF, Young A, Schmeisser K and Gems D:
The SKN-1/Nrf2 transcription factor can protect against oxidative
stress and increase lifespan in C. Elegans by distinct mechanisms.
Aging Cell. 16:1191–1194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Corenblum MJ, Ray S, Remley QW, Long M,
Harder B, Zhang DD, Barnes CA and Madhavan L: Reduced Nrf2
expression mediates the decline in neural stem cell function during
a critical middle-age period. Aging Cell. 15:725–736. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gao S, Duan X, Wang X, Dong D, Liu D, Li
X, Sun G and Li B: Curcumin attenuates arsenic-induced hepatic
injuries and oxidative stress in experimental mice through
activation of Nrf2 pathway, promotion of arsenic methylation and
urinary excretion. Food Chem Toxicol. 59:739–747. 2013. View Article : Google Scholar : PubMed/NCBI
|






















