Introduction
Ultraviolet rays can be classified into three types
according to wavelength: Ultraviolet A (UVA; 320-380 nm),
ultraviolet B (UVB; 280-320 nm), and ultraviolet C (UVC; 100-280
nm) (1). UVC is mostly absorbed
by the ozone layer, and UVA and UVB reach the surface of the Earth.
Although a small amount of UVB reaches the surface of the Earth,
UVB is 500-800 times more harmful than UVA (2). Nowadays, UVB rays reaching the
earth’s surface are the predominant risk factor causing skin
photoaging and disease, such as immune suppression and
cancerization (3).
UVB destroys keratinocyte cells on the outer layer
of the skin (epidermis) without penetrating the skin (4). Damaged keratinocyte cells secrete
proinflammatory cytokines, including interleukin (IL)-1α, IL-1β,
IL-6, IL-8, and tumor necrosis factor (TNF) α (5-9).
There is evidence that UVB-irradiated keratinocytes induce TNFα and
TNFα-dependent pathway. In specific, IL-1α induces a synergistic
induction of TNFα in keratinocytes and fibroblasts (10,11). These types of proinflammatory
cytokines activate the mitogen-activated protein kinase (MAPK) and
nuclear factor-κB (NF-κB) pathways in fibroblasts on the dermis
(12), a deeper layer of skin
(13). The activation of MAPK
cascades, such as extracellular signal-regulated kinase (ERK),
c-Jun N-terminal kinase (JNK) and p38 kinase phosphorylation, which
in turn regulate activator protein-1 (AP-1), increases matrix
metallopeptidase (MMP)-1 production (14,15). The activation of p38 MAPK leads to
the induction of multiple proteins that are key to the inflammatory
process, including a further induction of cytokine secretion. p38
MAPK signaling has a pivotal role in regulating the production of
proinflammatory cytokines, such as TNFα (16). Inhibitor κB kinase (IKK) is
activated by proinflammatory cytokines and then phosphorylates IκB
and leads to its degradation. In addition, NF-κB enhances MMP-1
production and increases the gene expression levels of
proinflammatory cytokines by translocating into the nucleus
(17). Consequently, these
pathways result in skin wrinkles by promoting the synthesis of
MMP-1 in fibroblasts, which degrade collagen (18). For these reasons, herbal products
have been investigated as candidates for anti-aging agents, as a
means to regulate the production of MMP-1 without toxicity.
Previous studies have reported the effects of
Artemisia capillaris regarding hepatitis, obesity,
inflammation, antimicrobial activity, antioxidant effects,
hemostasis, pyrexia, hypertension, cytoprotection, and choleretic
action (19-22). Several compounds have been
isolated from A. capillaris, including coumarin derivatives
such as esculetin, scoparone, and scopoletin (23), and flavonoid derivatives such as
quercetin (24), hyperoside
(25), isorhamnetin (26), and isoquercitrin (27). Scopoletin
(7-hydroxy-6-methoxychromen-2-one) is naturally derived from
coumarin and phytoalexin (28).
Scopoletin has been reported to inhibit acetylcholinesterase
(29), to have antioxidant
properties (30) and
anti-inflammatory effects (31),
and to reduce insulin resistance (32). However, no study has investigated
the effects and related mechanisms for A. capillaris ethanol
extract (ACE) with the active compound, scopoletin, in fibroblasts.
The present study evaluated the inhibition of MMP-1 protein
expression and the underlying mechanisms for scopoletin in
fibroblasts treated with conditioned medium from UVB-exposed-HaCaT
cells.
Materials and methods
Chemicals and antibodies
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Anti-MMP-1 (cat. no. ab52631, 1:1,000)
antibody was purchased from Abcam (Cambridge, UK). ERK1/2 (cat. no.
4377; 1:1,000), phosphorylated (p-) ERK1/2 (cat. no. 9101;
1:1,000), stress-activated protein kinase (SAPK)/JNK (cat. no.
9252; 1:1,000), p-SAPK/JNK (cat. no. 9251; 1:1,000), p38 MAPK (cat.
no. 8690; 1:1,000), p-p38 MAPK (cat. no. 9215; 1:1,000), IκBα (cat.
no. 2859; 1:1,000), p-IκBα (cat. no. 2078; 1:1,000), NF-κB p65
(cat. no. 9609; 1:1,000), p-NF-κB p65 (cat. no. 4887; 1:1,000), and
β-actin (cat. no. 4967; 1:1,000) antibodies were obtained from Cell
Signaling Technology, Inc., (Danvers, MA, USA). p38 inhibitor (cat.
no. SB203580, 1:1,000) was purchased from Calbiochem (Merck
KGaA).
Cell culture
HaCaT human keratinocytes were provided by Professor
Moon Je Cho (Department of Biochemistry, National University,
Cheju, Korea). Fibroblasts as human primary dermal cells were
purchased from American Type Culture Collection (Manassas, VA, USA;
cat. no. PCS-201-012TM). HaCaT cells and fibroblasts were cultured
in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone; GE Healthcare
Lifesciences, Logan, UT, USA) with 10% fetal bovine serum (FBS;
PEAK, Colorado, USA) and 1% penicillin/streptomycin (10,000 U/100
µg/ml; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in a 5% CO2 humidified atmosphere incubator at
37°C. HaCaT cells were maintained until 80% confluence and then
cultured for 24 h in medium without FBS. The cell medium was then
replaced with 5 ml PBS and the cells were exposed to UVB light. The
UVB doses were determined by irradiating the HaCaT cells with
various doses of UVB (0, 20, 40, 60, 80 and 100 mJ/cm2)
and optimizing the UVB light as 40 mJ/cm2. Cells were
cultured in DMEM medium containing 10% FBS. At 24 h
post-irradiation, HaCaT-conditioned medium was collected and added
on the fibroblasts. After 24 h incubation, the culture medium was
collected. Fibroblasts were treated with various concentrations of
scopoletin for 24 h in HaCaT-conditioned medium. Vehicle control
was serum-free medium-treated fibroblasts.
To determine the effects of scopoletin on the NF-κB
signaling pathway, fibroblasts were pretreated with scopoletin for
6 h, and then treated with HaCaT-conditioned medium (40
mJ/cm2) containing scopoletin (0, 30, 100 and 300
µM) for 15 min prior to western blot analyses. When
examining the signaling pathway activation in fibroblasts,
generally the phosphorylation reaction time is short, therefore
pretreatment with scopoletin was performed in order to provide the
required time to act on the fibroblasts.
Isolation of active compound and
structure determination
A. capillaris was purchased from
Hwasun-bul-minari Company (Hwasun, Korea). Dried A.
capillaris (1,475 g) was extracted with 100% ethanol for 3 days
at room temperature. The filtered extract was concentrated with a
vacuum evaporator (EYELA Rotary evaporator, Tokyo, Japan) and was
freeze-dried. The ethanol (EtOH) extract of A. capillaris
(71.343 g) was dissolved in H2O, and extracted with
ethyl acetate (EtOAc). The EtOAc layer (38.56 g) was evaporated to
dryness under vacuum and partitioned with 90% methanol (MeOH) and
n-Hexane. The 90% MeOH layer (24.19 g) was fractionated by Waters
MPLC system (Waters, Milford, MA, USA) using a YMC-DispoPackAT
(SIL-25; 40 g) eluted with EtOAc and n-Hexane mixture in a gradient
mode (EtOAc:n-Hexane, 2:8 to 10:0 in 60 min). The flow rate was 30
ml/min and the elution was monitored at UV 254 nm. The MMP-1
expression levels were evaluated in order to determine the effects
of the fraction layers on HaCaT-conditioned medium-treated
fibroblasts. Among the nine fractions eluted, the fraction with
inhibitory activity on MMP-1 protein expression was further
purified using a Waters prep-HPLC system using a YMC ODS A column
(YMC-pack ODS-A; 5 µm, 20×250 mm). The column was eluted
with 40% MeOH containing 0.2 mM ammonium acetate at a flow rate of
10 ml/min. The compounds were monitored by ultraviolet absorbance
at 254 nm. The scopoletin structure was confirmed via nuclear
magnetic resonance (NMR, 1D, 2D). 1H- and
13C-NMR spectra were obtained on an Advance DPX 500 MHz
NMR spectrometer (Bruker Corporation, Billerica, MA, USA), recorded
in a deuterated chloroform (CDC13) solution (33). Scopoletin was dissolved in
dimethyl sulfoxide (DMSO) and then diluted in serum-free medium for
in vitro studies.
Cell viability
Cell viability was determined by MTT assay.
Fibroblasts were seeded at 1.5×104/well in a 24-well
plate. After 24 h of incubation, the cell medium was replaced with
serum-free medium and incubated for an additional 24 h. The cells
were treated with scopoletin for 24 h in serum-free medium. MTT
solution (5 mg/ml) was added to each of the wells, and the cells
were incubated for 3 h at 37°C. The supernatants were removed, and
DMSO was added to dissolve the formazan crystals. Absorbance at 570
nm was measured using an ELISA plate reader (Tecan group Ltd.,
Mannedorf, Switzerland).
Western blot analysis
Fibroblasts were lysed in RIPA buffer
(Sigma-Aldrich, Merck KGaA) containing protease inhibitors and
phosphatase inhibitors. The lysates were centrifuged at 3,000 × g
for 15 min at 4°C, and the protein concentrations were determined
using a BCA assay. The proteins (40 µg) were separated by
10% SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were
initially blocked with 5% skimmed milk in Tris-buffered saline
containing 0.1% Tween-20 (TBS-T) for 30 min at room temperature and
then incubated with primary antibodies at 4°C overnight. The
membranes were washed with TBS-T and incubated with goat
anti-rabbit antibody conjugated to horseradish peroxidase (cat. no.
1662408edu, 1:2,500, Bio-Rad Laboratories, Inc.) at room
temperature for 1 h. The immunoreactive bands were visualized using
Clarity Western ECL Substrate (cat no. 1705060, Bio-Rad
Laboratories, Inc.) and quantified with Image J software (version
1.49v; National Institutes of Health, Bethesda, MD, USA) (34).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA samples from treated cells were isolated
using an RNeasy Mini kit (Qiagen GmbH, Hilden, Germany). The cDNA
samples were then synthesized using a Primescript 1st Strand cDNA
Synthesis kit (Takara Bio, Inc., Otsu, Japan) following the
manufacturer’s protocol. The reaction conditions were 42°C for 60
min and 95°C for 5 min. qPCR was performed with a SYBR Green Master
Mix (Bio-Rad Laboratories, Inc.) and the following primers: IL-1α,
forward 5′-CGC CAA TGA CTC AGA GGA AGA-3′ and reverse 5′-AGG GCG
TCA TTC AGG ATG AA-3′ (120 bp); TNFα. Forward 5′-TCT TCT CGA ACC
CCG AGT GA-3′ and reverse 5′-CCT CTG ATG GCA CCA CCA G-3′ (151 bp);
GAPDH, forward 5′-TGC CAC CAG AAG ACT GTG G-3′ and reverse 5′-AGC
TTC CCG TTC AGC TCA GG-3′. The thermocycling conditions were as
follows: 10 min at 94°C, followed by a total of 45 cycles of 15 sec
at 94°C and 1 min at 60°C. The expression levels of the genes
presented as the quantification cycle (Cq) value was measured using
the 2−∆∆Cq relative quantitative analysis method
(35), as automatically
determined using the LightCycler 96 Software 1.1 (Roche
Diagnostics, Basel, Switzerland).
Statistical analysis
All experiments were repeated at least three times,
and results were presented as the mean ± standard deviation from
three individual experiments. Statistical significance between two
groups was examined with two-tailed Student’s t-test using the SPSS
19.0 software package (IBM Corps, Armonk, NY, USA). Multiple-group
comparisons were performed using one-way analysis of variance
followed by Dunnett’s post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Isolation of the active substance from
the ACE and cell viability of scopoletin-treated fibroblasts
To investigate the effects of the ACE fractions on
MMP-1 protein expression inhibition, fibroblasts were treated with
the 9 fractions. The highest inhibition activity of MMP-1 protein
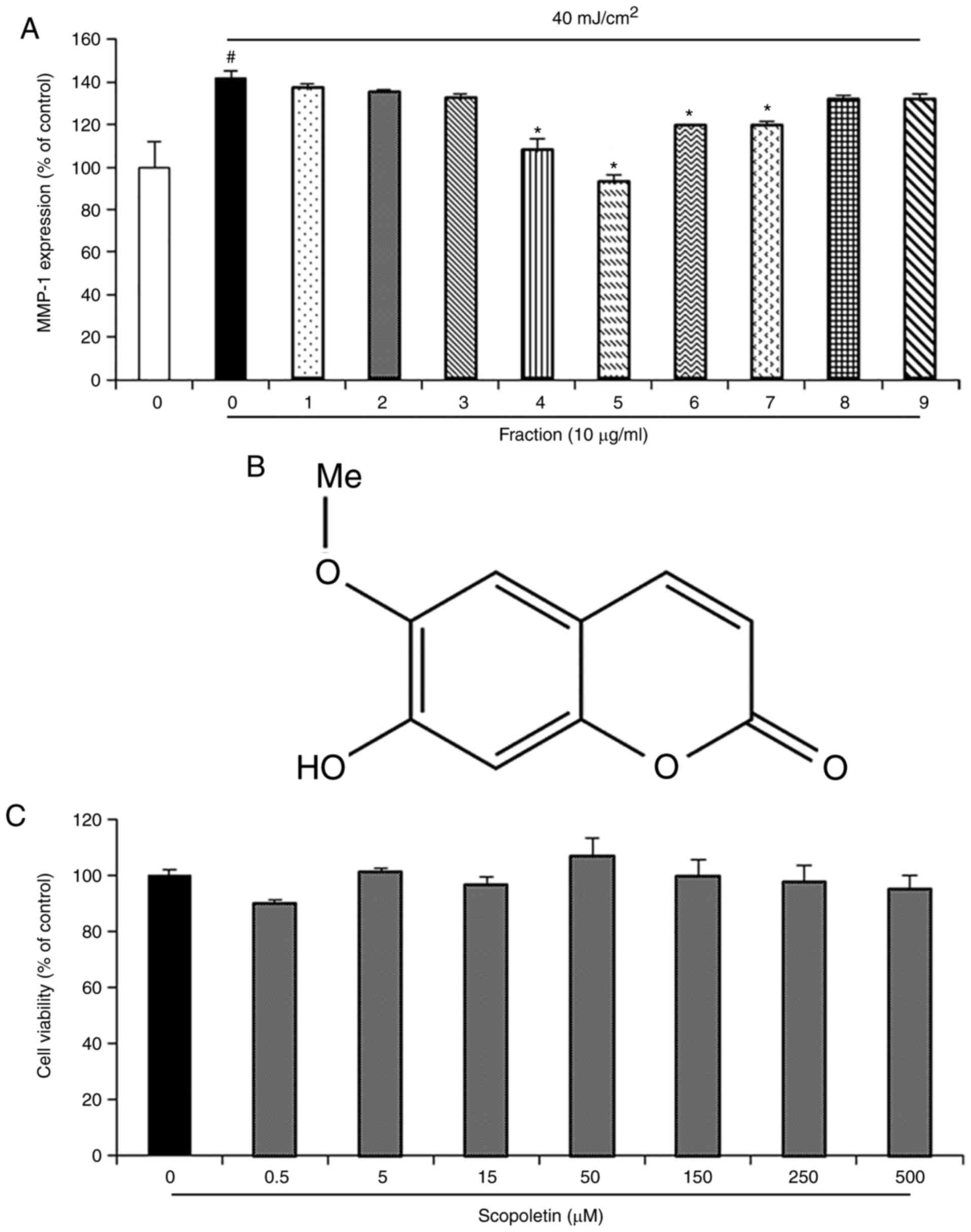
expression was observed in the Fraction 5 (Fig. 1A). A bioassay-guided fraction
aided in the isolation of a single compound from Fraction 5 that
exhibited inhibition of MMP-1 protein expression. The NMR spectrum
of this compound was identical to scopoletin, which has been
reported to be isolated from ACE in a previous study (23). The structure of this compound is
shown in Fig. 1B. To examine
whether scopoletin may exhibit cytotoxicity, the cell viability of
fibroblasts treated with scopoletin was evaluated using an MTT
assay. The results indicated that scopoletin had no cytotoxicity,
as tested at the doses of 0.5, 5, 15, 50, 150, 250 and 500
µM for 24 h (Fig. 1C).
Effects of scopoletin on MMP-1 protein
expression in fibroblasts treated with conditioned medium from UVB
exposed HaCaT cells
To determine the optimal irradiation conditions,
HaCaT cells were irradiated with various doses of UVB (0, 20, 40,
60, 80 and 100 mJ/cm2). After 24 h, HaCaT-conditioned
medium was collected and then added to the fibroblasts.
HaCaT-conditioned medium from 40 mJ/cm2 irradiation
produced the highest level of MMP-1 overexpression in fibroblasts
(Fig. 2A), therefore 40
mJ/cm2 was used in subsequent experiments. To
investigate the effects of scopoletin on MMP-1 protein expression,
fibroblasts were treated with various concentrations of scopoletin
in conditioned medium from UVB-irradiated HaCaT cells. The protein
expression levels for MMP-1 were determined by western blot
analysis. MMP-1 protein expression was not altered when the
fibroblasts were treated with serum-free medium containing
scopoletin (300 µM; Fig.
2B). However, MMP-1 expression was significantly increased in
fibroblasts treated with HaCaT conditioned medium. Scopoletin
treatment decreased the MMP-1 protein expression levels with an
inhibition rate of 44.84% at a concentration of 300 µM in
the fibroblasts (Fig. 2B).
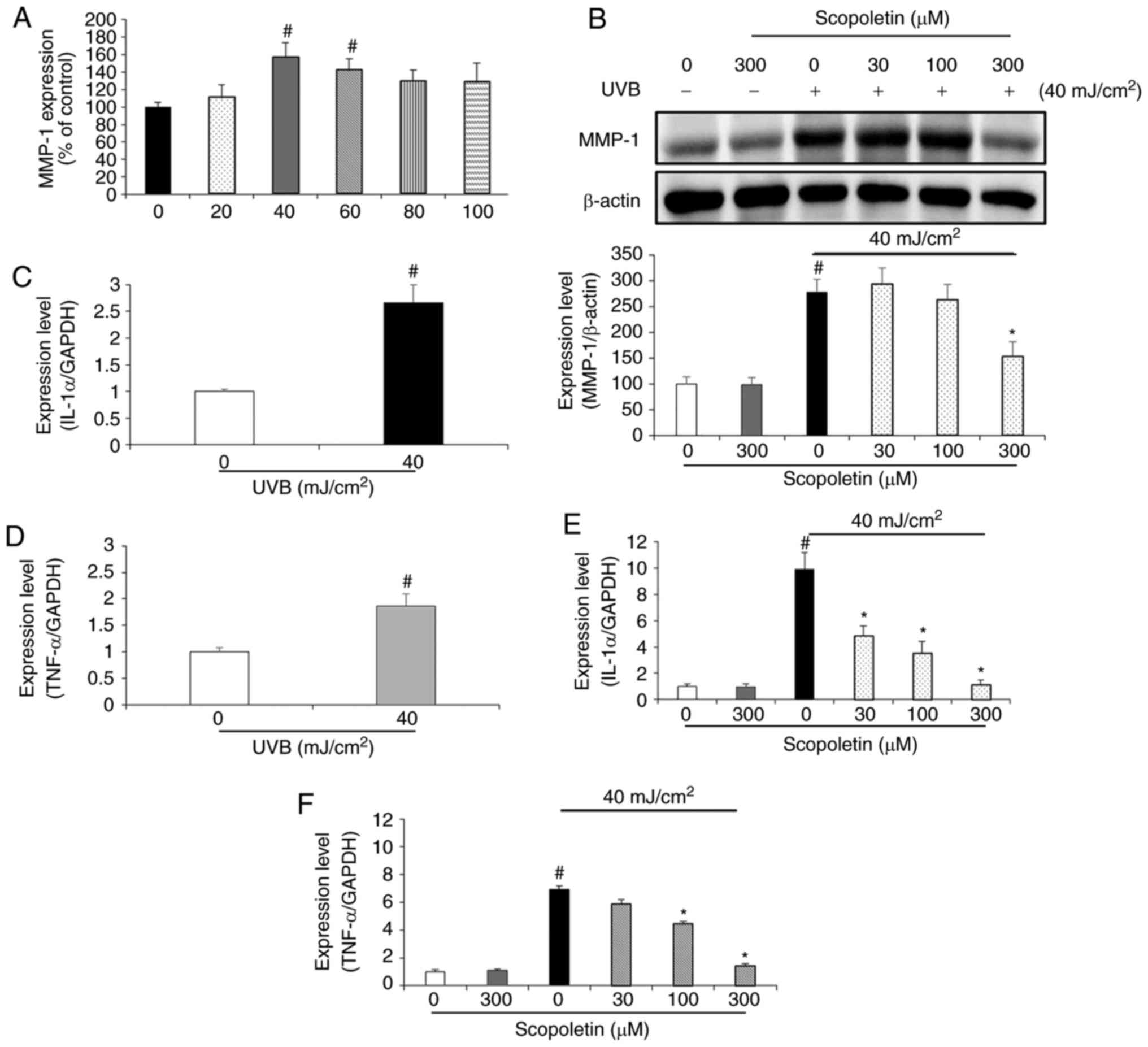 | Figure 2Effects of scopoletin on MMP-1 and
proinflammatory cytokine expression in fibroblasts treated with
conditioned medium from UVB-exposed HaCaT cells. (A) HaCaT cells
were exposed to different doses of UVB irradiation (0, 20, 40, 60,
80 and 100 mJ/cm2), and the conditioned media was
collected. The effect of the different conditioned media on the
fibroblast MMP-1 expression was assessed. (B) Effects of scopoletin
on MMP-1 protein expression in fibroblasts. Fibroblasts were
treated with HaCaT-conditioned medium containing scopoletin (0, 30
100 and 300 µM) for 24 h. MMP-1 expression levels were
assessed by western blot analysis. (C) IL-1α and (D) TNFα mRNA
expression levels were assessed in UVB-exposed HaCaT cells. (E)
Effect of scopoletin on IL-1α and (F) TNFα mRNA expression levels
in fibroblasts. Results are presented as the mean ± standard
deviation of triplicate independent experiments.
#P<0.05 compared with the vehicle control;
*P<0.05, compared with the HaCaT-conditioned medium
(40 mJ/cm2) alone-treated control. MMP-1,
metallopeptidase-1; UVB, ultraviolet B; IL-1α, interleukin-1α;
TNFα, tumor necrosis factor α. |
Effects of scopoletin on proinflammatory
cytokine mRNA expression in fibroblasts treated with conditioned
medium from UVB-exposed HaCaT cells
To examine whether UVB causes an increase in the
mRNA expression of proinflammatory cytokines, the mRNA expression
levels of IL-1α and TNFα were analyzed by RT-qPCR in HaCaT cells
exposed to UVB for 24 h. The results demonstrated that 40
mJ/cm2 UVB enhanced the mRNA levels of IL-1α and TNFα,
by 2.66- and 1.85-fold, respectively (Fig. 2C and D). After collecting this
conditioned medium, it was added on fibroblasts together with
various concentrations of scopoletin (0, 30, 100 and 300 µM)
for 24 h. In the fibroblasts treated with conditioned medium from
the UVB-exposed HaCaT cells, the mRNA levels of IL-1α and TNFα were
significantly increased compared with the control-exposed
fibroblasts (Fig. 2E and F).
Scopoletin inhibited this effect in a dose-dependent manner, in the
range of 50-88.5% for IL-1α, and 15-80% for TNFα, compared with
untreated, conditioned media-exposed fibroblasts (Fig. 2E and F).
Effects of scopoletin on NF-κB activation
in fibroblasts treated with conditioned medium from UVB-exposed
HaCaT cells
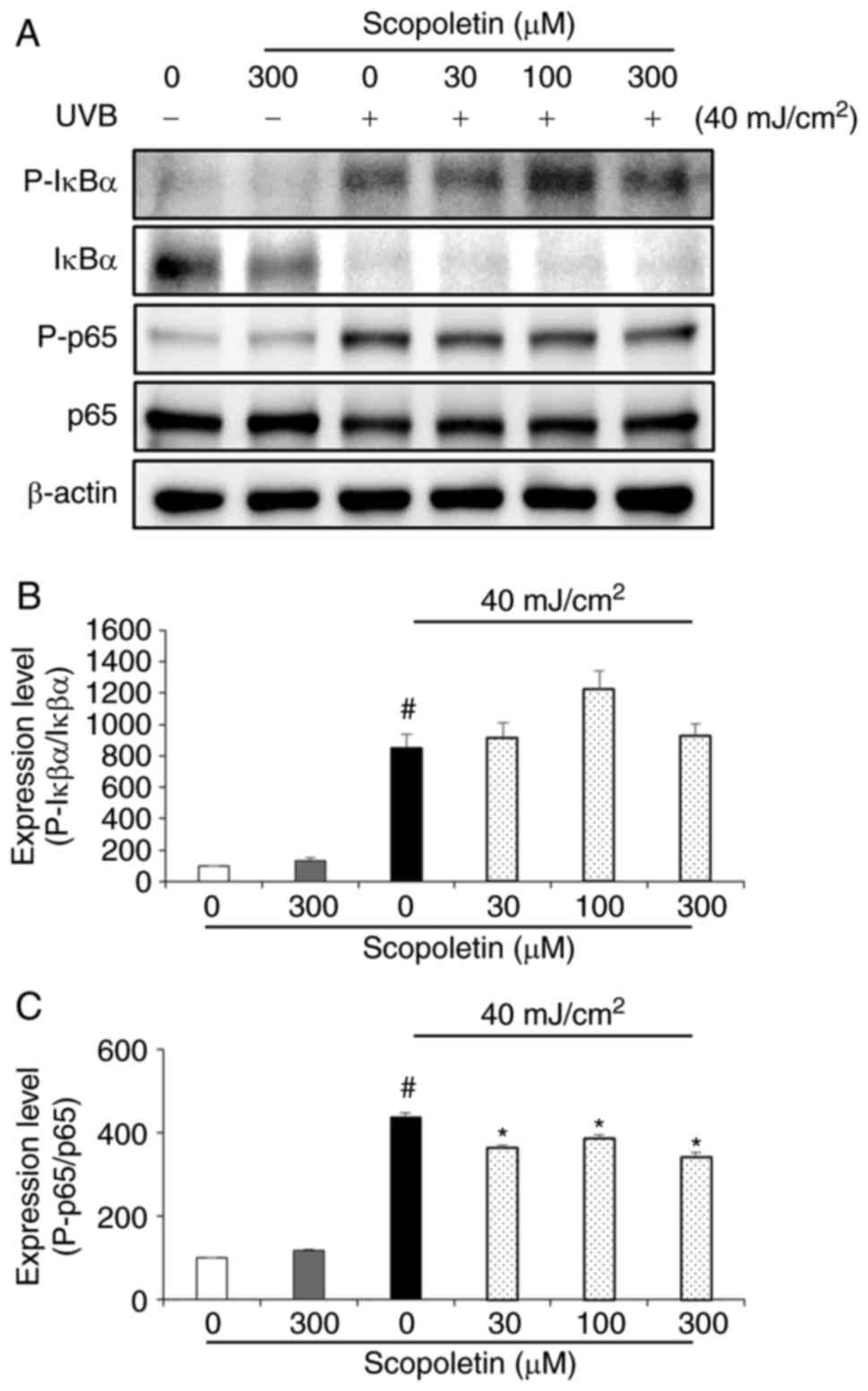
To determine the effect of scopoletin on NF-κB
activation, the phosphorylation levels of IκBα and p65 were
examined by western blot analysis (Fig. 3). No phosphorylation of IκBα and
p65 was observed when fibroblasts were treated with serum-free
medium containing scopoletin (300 µM; Fig. 3A). However, IκBα and p65
phosphorylation levels were increased when the fibroblasts were
treated with conditioned medium from UVB-exposed HaCaT cells
(Fig. 3). While IκBα
phosphorylation was unaffected (Fig.
3B), p65 phosphorylation was significantly inhibited by
scopoletin treatment (Fig. 3C).
The reduction of p65, in the rate of 11.54-21.92%, was observed at
scopoletin concentration of 300 µM (Fig. 3C).
Effects of scopoletin on MAPK activation
in fibroblasts treated with conditioned medium from UVB-exposed
HaCaT cells
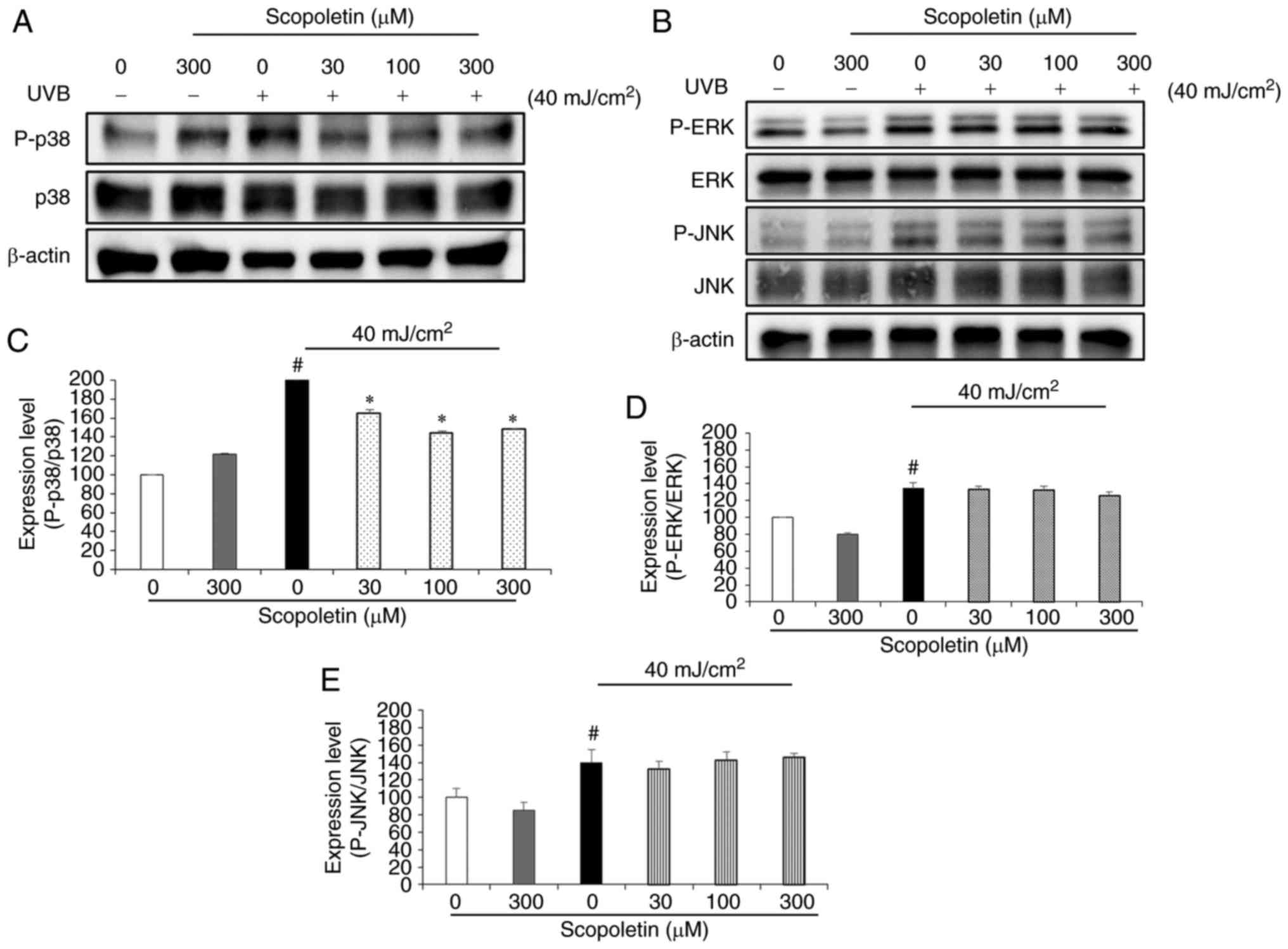
To determine whether scopoletin inhibited MMP-1
expression by blocking MAPK signaling, the phosphorylation levels
of ERK, JNK, and p38 were examined by western blot analysis. No
phosphorylation of ERK, JNK, and p38 was observed in fibroblasts
with serum-free medium containing scopoletin (300 µM;
Fig. 4A and B). By contrast, the
phosphorylation of ERK, JNK, and p38 was markedly increased in the
fibroblasts treated with conditioned medium from UVB-exposed HaCaT
cells (Fig. 4). Scopoletin
treatment significantly inhibited p38 phosphorylation, with a rate
of 17.67-28.33% observed at the scopoletin concentration of 300
µM (Fig. 4A and C). No
effect on reducing phosphorylation of ERK and JNK was observed
following scopoletin treatment in fibroblasts treated with
conditioned medium from UVB-exposed HaCaT cells (Fig. 4B, D and E).
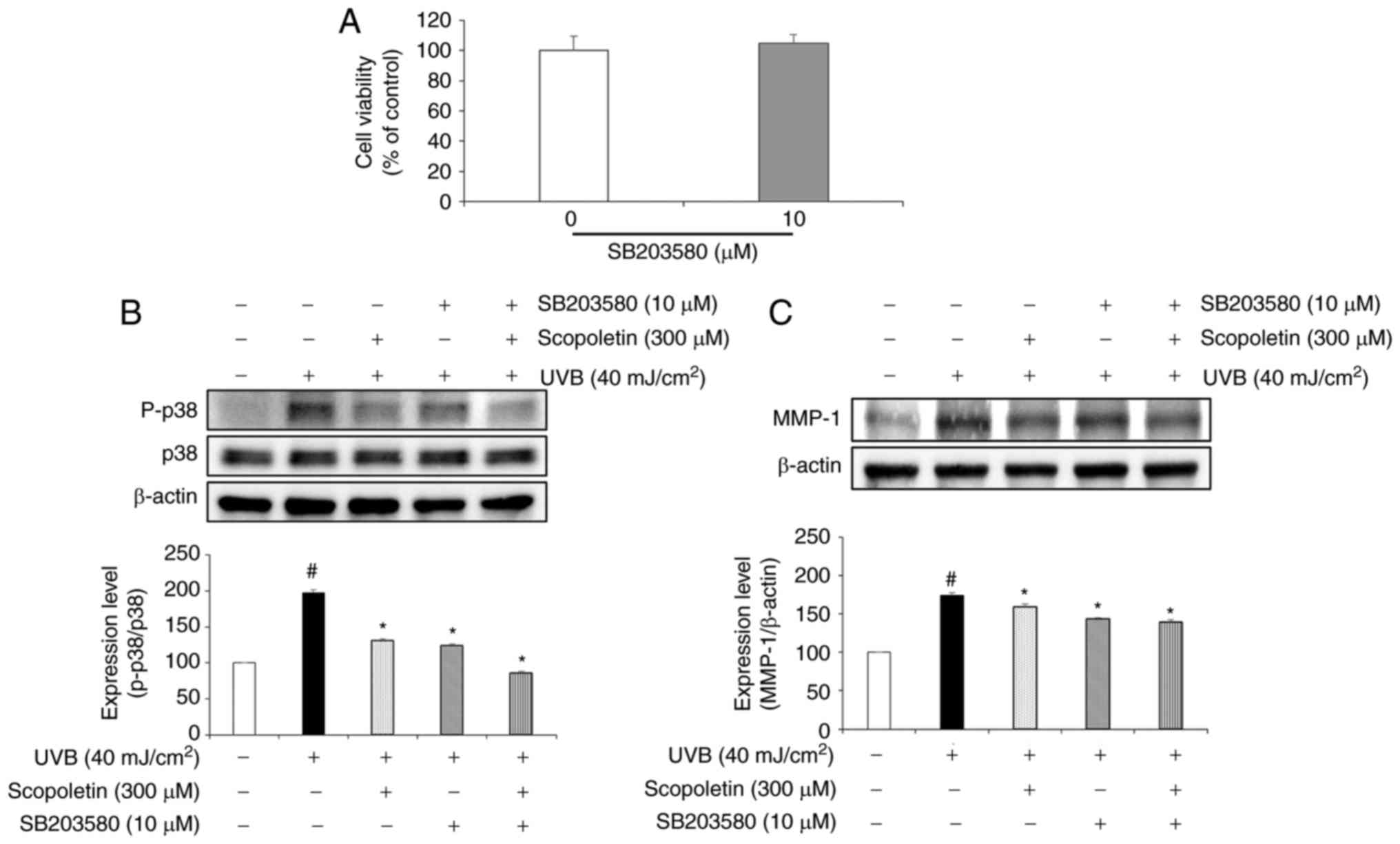
Effects of SB203580 and scopoletin on
MMP-1 protein expression in fibroblasts treated with conditioned
medium from UVB-exposed HaCaT cells
First, the potential cytotoxicity of SB203580 was
tested on the fibroblasts by MTT assay. The results indicated that
SB203580 had no cytotoxicity at the concentration of 10 µM
(Fig. 5A). Then, the inhibition
effect of scopoletin on p38 phosphorylation and MMP-1 expression
was confirmed by western blotting (Fig. 5B and C). To further explore this
pathway, the effect of SB203580, a well-known p38 inhibitor
(36-40), was assessed. The results
demonstrated that treatment with SB203580 and scopoletin
significantly inhibited the phosphorylation of p38 by 37.31 and
33.45% (Fig. 5B) and decreased
the MMP-1 protein expression by 17.39 and 8.94%, respectively
(Fig. 5C).
Discussion
Several studies have demonstrated that UVB is the
most dangerous light, causing skin cancer (4). Furthermore, UVB irradiation is
responsible for epidermal thickness and degradation of
extracellular matrix (ECM), leading to damage in skin tissue
integrity, formation of wrinkles, and inflammation (41). Therefore, protecting the skin from
UVB irradiation may prevent the processes of wrinkle formation,
photoaging, and inflammatory reactions of the skin (42).
In many studies, herbal products have been
investigated and extensively used as candidates for traditional
medicine without toxicity. Among these, A. capillaris has
been reported to possess several biological effects, including
hepatoprotective, antibacterial, antioxidant, antiobesity, and
health properties. A. capillaris contains several compounds,
including coumarin derivatives and flavonoid derivatives (23). However, no study has investigated
the effects and related mechanisms of A. capillaris ethanol
extract (ACE) with the active compound, scopoletin, in
fibroblasts.
When UVB irradiation reaches the skin, it does not
penetrate deeply into the dermis and damages keratinocyte cells in
the epidermis (43). Damaged
keratinocyte cells secrete proinflammatory cytokines, including
IL-1α, IL-1β, IL-6, IL-8, and TNFα (5-9).
Based on this knowledge, the present study used an in vitro
model where HaCaT cells were irradiated with UVB to produce an
environment similar to that of human skin, and then the conditioned
medium containing proinflammatory cytokines released from the HaCaT
cells was collected and added on fibroblasts (12). Proinflammatory cytokines, such as
IL-1α and TNFα, that were secreted from UVB-exposed keratinocyte
cells stimulate fibroblasts to express MMP-1 protein, a member of
the collagenase subfamily of MMPs. MMP-1 has a major role in skin
photoaging, by degrading the ECM to maintain the dermal skin layers
(44,45). In the present study, MMP-1 protein
expression was demonstrated to be significantly increased in
fibroblasts that were treated with HaCaT-conditioned medium (40
mJ/cm2). Scopoletin inhibited the MMP-1 protein
overexpression in fibroblasts treated with HaCaT-conditioned
medium. In addition, the mRNA levels of IL-1α and TNFα were
increased in fibroblasts treated with HaCaT-conditioned medium, and
this effect was reversed by scopoletin treatment. IL1α and TNFα are
known to induce phosphorylation of MAPKs and NF-κB in fibroblasts.
NF-κB, a regulator of gene expression associated with inflammatory
responses, is activated by IL-1α and TNFα. NF-κB activation occurs
by phosphorylation and degradation of IκBα and translocation of
NF-κB p65 (46). In addition,
MAPK signaling pathways serve a central role in regulating cell
proliferation, cell motility, MMP gene expression, cell survival
and death. Three major MAPK subfamilies in mammalian cells include
ERK, JNK and p38. The activation of p38 MAPK leads to the induction
of many proteins that are key to the inflammatory process,
including a further induction of cytokine secretion. The p38 MAPK
signaling pathway has a pivotal role in regulating the production
of proinflammatory cytokines, such as TNFα (16). When these two pathways, NF-κB and
MAPKs, are activated, MMP-1 protein and proinflammatory cytokine
mRNA are expressed in fibroblasts (47). Proinflammatory cytokines, such as
IL-1α and TNFα, are then secreted from fibroblasts to further
activate the MAPK and NF-κB pathways in an autocrine action
(48). Phosphorylation of p65
decreased slightly following scopoletin treatment of fibroblasts
stimulated with HaCaT conditioned medium. In addition, HaCaT
conditioned medium induced phosphorylation of ERK, JNK, and p38
MAPKs in fibroblasts. The phosphorylation of p38 MAPK decreased
significantly in fibroblasts treated with scopoletin, but no effect
was observed on the phosphorylation of ERK and JNK. In summary, the
present study demonstrated that scopoletin inhibited the
phosphorylation of p38 MAPK and decreased MMP-1 protein expression
in fibroblasts. To evaluate whether the inhibition of MMP-1 protein
expression in the fibroblasts is due to a reduction in the
phosphorylation of p38 MAPK, we treated fibroblasts with a p38
inhibitor (SB203580). The results demonstrated that phosphorylation
of p38 was inhibited following treatment with SB203580. Notably,
treatment with SB203580 also reduced the levels of MMP-1. These
findings indicated that scopoletin inhibited the expression of
IL-1α and TNFα mRNA by reducing the phosphorylation of p38 MAPK,
thereby decreasing the expression of MMP-1 protein in fibroblasts
treated HaCaT-conditioned medium.
In conclusion, although there is no antiwrinkle
effect of scopoletin in mice, it has been reported that A.
capillaris extract alleviates atopic dermatitis in mice
(49,50). Based on these results, it is
expected that the inhibitory effect on MMP-1 protein expression may
be tested by treating with scopoletin in UVB-irradiated mice.
Future studies will also investigate the effect of scopoletin
treatment on the expression and cellular distribution of cytokines
by immunofluorescence analysis, as well as its effects on cellular
morphology. In addition, future studies will investigate whether
the observed changes in MMP-1 levels are due to alterations at the
transcriptional, translational, or post-translational levels.
Although further studies are necessary to fully explore the use of
scopoletin in humans, the present results suggest a possible role
of scopoletin as a potential preventing factor against skin
photoaging.
Acknowledgments
Not applicable.
Funding
This work was supported by the Cooperative Research
Program for Agriculture Science & Technology Development (grant
no. PJ011299022017), funded by the Rural Development
Administration, Republic of Korea.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors’ contributions
HLK was analyzed the experimental data and was a
major contributor in writing the manuscript. SMW optimized the
HaCaT cell cultures. WRC and HSK performed the cell viability
assays. CY and JC isolated the active compounds and determined the
chemical structure. KHK and SHY performed the western blot
analysis. JWS performed the RT-PCR analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Diffey BL: What is light? Photodermatol
Photoimmunol Photomed. 18:68–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pillai S, Oresajo C and Hayward J:
Ultraviolet radiation and skin aging: roles of reactive oxygen
species, inflammation and protease activation, and strategies for
prevention of inflammation-induced matrix degradation - A review.
Int J Cosmet Sci. 27:17–34. 2005. View Article : Google Scholar
|
|
3
|
Bosch R, Philips N, Suárez-Pérez JA,
Juarranz A, Devmurari A, Chalensouk-Khaosaat J and González S:
Mechanisms of photo-aging and cutaneous photocarcinogenesis, and
photoprotective strategies with phytochemicals. Antioxidants
(Basel). 4:248–268. 2015. View Article : Google Scholar
|
|
4
|
Sanches Silveira JE and Myaki Pedroso DM:
UV light and skin aging. Rev Environ Health. 29:243–254. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta N, Chakrobarty A, Raman G and
Banerjee G: Cloning and identification of EDD gene from
ultraviolet-irradiated HaCaT cells. Photodermatol Photoimmunol
Photomed. 22:278–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Im A-R, Yeon SH, Lee JS, Um KA, Ahn Y-J
and Chae S: Protective effect of fermented Cyclopia intermedia
against UVB-induced damage in HaCaT human keratinocytes. BMC
Complement Altern Med. 16:2612016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishida T and Sakaguchi I: Protection of
human keratinocytes from UVB-induced inflammation using root
extract of Lithospermum erythrorhizon. Biol Pharm Bull. 30:928–934.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MS, Oh GH, Kim MJ and Hwang JK:
Fucosterol inhibits matrix metalloproteinase expression and
promotes type-1 procollagen production in UVB-induced HaCaT cells.
Photochem Photobiol. 89:911–918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mutou Y, Tsukimoto M, Homma T and Kojima
S: Immune response pathways in human keratinocyte (HaCaT) cells are
induced by ultraviolet B via p38 mitogen-activated protein kinase
activation. J Health Sci. 56:675–683. 2010. View Article : Google Scholar
|
|
10
|
Bashir M, Sharma M and Werth V: TNF-alpha
production in the skin. Arch Dermatol Res. 301:87–91. 2009.
View Article : Google Scholar
|
|
11
|
Werth VP and Zhang W: Wavelength-specific
synergy between ultraviolet radiation and interleukin-1alpha in the
regulation of matrix-related genes: Mechanistic role for tumor
necrosis factor-alpha. J Invest Dermatol. 113:196–201. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imokawa G, Nakajima H and Ishida K:
Biological mechanisms underlying the ultraviolet radiation-induced
formation of skin wrinkling and sagging II: Over-expression of
neprilysin plays an essential role. Int J Mol Sci. 16:7776–7795.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malinin NL, Boldin MP, Kovalenko AV and
Wallach D: MAP3K-related kinase involved in NF-kappaB induction by
TNF, CD95 and IL-1. Nature. 385:540–544. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ray A, Shakya A and Ray BK:
Inflammation-responsive transcription factors SAF-1 and c-Jun/c-Fos
promote canine MMP-1 gene expression. Biochim Biophys Acta.
1732:53–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Wenger L, Brinckerhoff CE, Misra RR
and Cheung HS: Basic calcium phosphate crystals induce matrix
metalloproteinase-1 through the Ras/Mitogen-activated protein
Kinase/c-Fos/AP-1/Metalloproteinase 1 pathway. Involvement of
transcription factor binding sites AP-1 and PEA-3. J Biol Chem.
277:1544–1552. 2002. View Article : Google Scholar
|
|
16
|
Ono K and Han J: The p38 signal
transduction pathway: Activation and function. Cell Signal.
12:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fagot D, Asselineau D and Bernerd F:
Matrix metalloproteinase-1 production observed after
solar-simulated radiation exposure is assumed by dermal fibroblasts
but involves a paracrine activation yhrough epidermal
keratinocytes. Photochem Photobiol. 79:499–505. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bora KS and Sharma A: The genus Artemisia:
A comprehensive review. Pharm Biol. 49:101–109. 2011. View Article : Google Scholar
|
|
20
|
Jang G: Jung Gyeong Jeon Seo. Publishers,
Seoul, Hollym Corp; 1975
|
|
21
|
Lee HI, Seo KO, Yun KW, Kim MJ and Lee MK:
Comparative study of the hepatoprotective efficacy of Artemisia
iwayomogi and Artemisia capillaris on ethanol-administered mice. J
Food Sci. 76:T207–T211. 2011. View Article : Google Scholar
|
|
22
|
Okuno I, Uchida K, Kadowaki M and Akahori
A: Choleretic effect of Artemisia capillaris extract in rats. Jpn J
Pharmacol. 31:835–838. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheu SJ, Chieh CL and Weng WC: Capillary
electrophoretic determination of the constituents of Artemisiae
capillaris Herba. J Chromatogr A. 911:285–293. 2001. View Article : Google Scholar
|
|
24
|
Ternai B and Markham K: Carbon-13 NMR
studies of flavonoids-I: Flavones and flavonols. Tetrahcdron.
32:565–569. 1976. View Article : Google Scholar
|
|
25
|
Markham K, Ternai B, Stanley R, Geiger H
and Mabry T: Carbon-13 NMR studies of flavonoids-III: Naturally
occurring flavonoid glycosides and their acylated derivatives.
Tetrahcdron. 34:1389–1397. 1978. View Article : Google Scholar
|
|
26
|
Sakakibara M, Difeo D Jr, Nakatani N,
Timmermann B and Mabry TJ: Flavonoid methyl ethers on the external
leaf surface of Larrea tridentata and L. divaricata.
Phytochemistry. 15:727–731. 1976. View Article : Google Scholar
|
|
27
|
Wang ZW, Tan XJ, Ma TT, Chen XH and Bi KS:
Isolation and identification of chemical constituents from
Artemisia capillaries Thunb. J Shenyang Pharm Univ. 10:781–784.
2008.
|
|
28
|
Gnonlonfin BG, Gbaguidi F, Gbenou JD,
Sanni A and Brimer L: Changes in scopoletin concentration in
cassava chips from four varieties during storage. J Sci Food Agric.
91:2344–2347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rollinger JM, Hornick A, Langer T,
Stuppner H and Prast H: Acetylcholinesterase inhibitory activity of
scopolin and scopoletin discovered by virtual screening of natural
products. J Med Chem. 47:6248–6254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SH, Ding Y, Tan XT, Kim YH and Jang
HD: Scopoletin and scopolin isolated from Artemisia iwayomogi
suppress differentiation of osteoclastic macrophage RAW264.7 cells
via scavenging reactive oxygen species. J Nat Prod. 76:615–620.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao X, Ding Z, Xia Y, Wei Z, Luo Y,
Feleder C and Dai Y: Inhibition of monosodium urate crystal-induced
inflammation by scopoletin and underlying mechanisms. Int
Immunopharmacol. 14:454–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang WY, Lee JJ, Kim Y, Kim IS, Park JS
and Myung CS: Amelioration of insulin resistance by scopoletin in
high-glucose-induced, insulin-resistant HepG2 cells. Horm Metab
Res. 42:930–935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim BG, Lee Y, Hur HG, Lim Y and Ahn JH:
Production of three O-methhylated esculetins with Escherichia coli
expressing O-methyltransferase from poplar. Biosci Biotechnol
Biochem. 70:1269–1272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:6712012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Bae JY, Choi JS, Choi YJ, Shin SY, Kang
SW, Han SJ and Kang YH: (−) Epigallocatechin gallate hampers
collagen destruction and collagenase activation in
ultraviolet-B-irradiated human dermal fibroblasts: Involvement of
mitogen-activated protein kinase. Food Chem Toxicol. 46:1298–1307.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ham SA, Kang ES, Lee H, Hwang JS, Yoo T,
Paek KS, Park C, Kim JH, Lim DS and Seo HG: PPARδ inhibits
UVB-induced secretion of MMP-1 through MKP-7-mediated suppression
of JNK signaling. J Invest Dermatol. 133:2593–2600. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu Q, Hou W, Zheng Y, Liu C, Gong Z, Lu C,
Lai W and Maibach HI: Ultraviolet A-induced cathepsin K expression
is mediated via MAPK/AP-1 pathway in human dermal fibroblasts. PLoS
One. 9:e1027322014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang B, Ji C, Chen X, Cui L, Bi Z, Wan Y
and Xu J: Protective effect of astragaloside IV against matrix
metalloproteinase-1 expression in ultraviolet-irradiated human
dermal fibroblasts. Arch Pharm Res. 34:1553–1560. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang B, Ji C, Kang J, Chen W, Bi Z and Wan
Y: Trans-Zeatin inhibits UVB-induced matrix metalloproteinase-1
expression via MAP kinase signaling in human skin fibroblasts. Int
J Mol Med. 23:555–560. 2009.PubMed/NCBI
|
|
41
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee H, Bae SK, Pyo M, Heo Y, Kim CG, Kang
C and Seyedian R: Anti-wrinkle effect of PLA2-free bee venom
against UVB-irradiated human skin cells. J Agric Life Sci.
49:125–135. 2015. View Article : Google Scholar
|
|
43
|
Rijken F, Kiekens RC and Bruijnzeel PL:
Skin-infiltrating neutrophils following exposure to solar-simulated
radiation could play an important role in photoageing of human
skin. B. r J Dermatol. 152:321–328. 2005.
|
|
44
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vincenti MP, White LA, Schroen DJ, Benbow
U and Brinckerhoff CE: Regulating expression of the gene for matrix
metalloproteinase-1 (collagenase): Mechanisms that control enzyme
activity, transcription, and mRNA stability. Criti Rev Eukaryot
Gene Expr. 6:391–411. 1996. View Article : Google Scholar
|
|
46
|
Karin M and Greten FR: NF-κB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bond M, Baker AH and Newby AC: Nuclear
factor kappaB activity is essential for matrix metalloproteinase-1
and-3 upregulation in rabbit dermal fibroblasts. Biochem Biophys
Res Commun. 264:561–567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fini ME, Strissel KJ, Girard MT, Mays JW
and Rinehart WB: Interleukin 1 alpha mediates collagenase synthesis
stimulated by phorbol 12-myristate 13-acetate. J Biol Chem.
269:11291–11298. 1994.PubMed/NCBI
|
|
49
|
Ha H, Lee H, Seo CS, Lim HS, Lee JK, Lee
MY and Shin H: Artemisia capillaris inhibits atopic dermatitis-like
skin lesions in Dermatophagoides farinae-sensitized Nc/Nga mice.
BMC Complement Altern Med. 14:1002014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Son HU, Lee S, Heo JC and Lee SH: The
solid-state fermentation of Artemisia capillaris leaves with
Ganoderma lucidum enhances the anti-inflammatory effects in a model
of atopic dermatitis. Int J Mol Med. 39:1233–1241. 2017. View Article : Google Scholar : PubMed/NCBI
|