Introduction
Colon carcinoma is one of the most common types of
malignant disease with high mortality and one of the leading causes
of cancer-associated death worldwide (1,2).
Half of colon carcinoma patients develop recurrences and
metastasis, as treatment tends to commence late due to the lack of
symptoms at the early stage (3).
Given the rapid increases in the incidence and mortality observed
in numerous counties, particularly in Eastern Europe, Asia and
South America (4), early
detection and primary prevention, which may significantly reduce
the mortality of colon carcinoma patients, are therefore imperative
(5). Unfortunately, the symptoms
are frequently not obvious at the earliest stage and there has been
little success in the screening for effective molecular markers for
colon cancer (6).
Carcinoembryonic antigen (CEA), the most utilized colon carcinoma
marker, is limited by its low specificity (7). Thus, novel molecular markers with
high sensitivity and specificity, which significantly optimize the
use of therapies and provide a benefit for patients, are
desired.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs,
which regulate post-transcription gene expression. Certain miRNAs
are aberrantly expressed during colorectal cancer (CRC) development
and progression and exert regulatory roles in cancer-associated
processes (8). Several studies
have reported that miRNAs may function as potential diagnostic and
prognostic biomarkers for colon carcinoma (9–11).
In the present study, the differential miRNA
expression profiles of 457 colon carcinoma tissues vs. 8 adjacent
non-tumorous tissues from The Cancer Genome Atlas (TCGA) database
we compared by a bioinformatics analysis. Furthermore, a novel
six-miRNA signature was proposed by using the univariate and
multivariate Cox's proportional hazard regression model, which has
the capacity to effectively predict the prognosis and survival of
colon carcinoma patients [P<0.001, area under the receiver
operating characteristic (ROC) curve (AUC)=0.763]. In addition, a
prognostic prediction model was constructed based on the six miRNAs
that were highly correlated with the tumor-nodes-metastasis (TNM)
stage. By combining the six-miRNA model and the TNM stage, an
improved prediction of the patient's survival status (AUC=0.797)
was possible. Taken together, the present results indicated that
the six miRNAs may be reliable biomarkers for monitoring the
progression of colon carcinoma and predicting the prognosis of
affected patients.
Materials and methods
miRNA expression dataset
The miRNA sequence data and clinical characteristics
were downloaded from the publicly available TCGA database
(https://cancergenome.nih.gov/).
Consequently, a total of 465 samples were enrolled in our study,
including 457 colon carcinoma tissues and 8 matched normal tissues.
Patients without complete information of clinical characteristics
or survival time were excluded from the further analysis. Finally,
we got 453 colon carcinoma patients and the clinical features
recorded included age, sex, TNM stage, tumor (T) stage, extent of
spread to the lymph nodes as indicated by the nodal (N) stage,
presence of metastasis as indicated by the metastasis (M) stage and
residual tumor. The TNM staging system of the American Joint
Committee on Cancer (AJCC) is one of the most commonly used tumor
staging systems for colon carcinoma. The data processing was in
accordance with the publication guidelines provided by TCGA
(http://cancerge-nome.nih.gov/publications/publicationguidelines).
Differentially expressed miRNAs
(DEmiRNAs)
The package 'edgeR' version 3.22.3 (http://www.bioconductor.org/packages/release/bioc/html/edgeR.html)
in R version 3.4.2 was used to identify DEmiRNAs between colon
carcinoma and normal tissues. The miRNAsb that could not be
determined in all samples were excluded. A |log2 fold change
(log2FC)| of >2 and an adjusted P-value of <0.01 were
considered as the cut-off criteria. The expression levels of the
miRNAs were log2 transformed for further analysis.
Construction of the miRNA signature
associated with overall survival (OS)
The association between DEmiRNAs and the patients'
OS was evaluated by using the univariate Cox's proportional hazard
ratio (HR) model using the 'survival' package in R. The miRNAs with
P<0.01 were selected for further multivariate Cox's proportional
hazard regression to identify the independent prognostic miRNAs.
Using the logistic regression method, a prognostic model based on
the independent prognostic miRNAs was constructed to evaluate the
survival risk of each patient. The risk score was calculated as
follows: Risk score = expmiRNA1 × βmiRNA1 +
expmiRNA2 × βmiRNA2 + …expmiRNAn ×
βmiRNAn, where exp is the expression level and β is the
regression coefficient derived from the multivariate Cox regression
model (12,13).
Utilizing the median risk score as the cut-off
point, patients with colon carcinoma were categorized into
high-risk and low-risk groups. It was investigated whether the
median survival time was significantly different between these two
groups using a Kaplan-Meier estimation and log-rank test. The
predictability of the model was evaluated by the AUC using the
'survival ROC' package in R (14).
Association between risk score and other
clinical characteristics
Next, the association between the risk score of the
six miRNAs and the clinical characteristics of patients with colon
carcinoma, including age, sex, TNM stage, T stage, N stage, M stage
and residual tumor, was assessed. Furthermore, univariate Cox
proportional hazard regression analyses were performed to
investigate the effects of various clinical features and risk score
on the OS of patients with colon adenocarcinoma. The HR and 95%
confidence interval were assessed. Multivariate Cox regression
analysis was used to verify the independent predictive capacity of
the risk score when compared with that of other clinical
factors.
Prognostic prediction for colon carcinoma
based on risk score
To assess whether the risk score may be used to
assess tumor progression, the risk score in different stages,
including TNM stage, T stage, N stage and M stage, were compared.
Furthermore, the association of integrated risk score with clinical
characteristics was assessed to evaluate its predictive value
regarding the prognosis in colon carcinoma patients. We
investigated if the median survival time was significantly
different between patients separated by both risk score and staging
(low/high-risk score + high/low stage) using a Kaplan-Meier
estimator and log-rank test. The risk score was compared via
Multivariate Cox regression analysis as aforementioned and
separated by its median. To investigate the performance of the
prognostic risk score of the six miRNAs and TNM stage in predicting
the outcome for colon carcinoma patients, the AUC of the ROC was
calculated and compared.
Functional enrichment analysis
To reveal the possible mechanism of action of the
six miRNAs involved in colon carcinoma, the target genes of
prognostic miRNAs were predicted using TargetScan (http://www.targetscan.org/), miRDB (http://www.mirdb.org/miRDB/), mirPath (https://mpd.bioinf.uni-sb.de/overview.html) and
miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php). Only
the overlapping genes were identified to further enhance the
reliability of the analysis. A Venn diagram was drawn using the
'VennDiagram' package.
To further elucidate the biological function of the
targeted genes of the six miRNAs, a Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway biological
enrichment analysis was preformed through the Database for
Annotation, Visualization and Integrated Discovery bioinformatics
tool (15). P<0.05 was set as
the cut-off criterion.
Statistical analysis
Kaplan-Meier survival analysis and
univariate/multivariate Cox proportional hazard regression analysis
were conducted using the 'survival' packageversion 2.41 (https://github.com/therneau/survival) in
R to compare each miRNA (low vs. high level) and prognostic miRNA
signatures (low vs. high-risk). Pearson correlation analysis was
used to estimate the correlation between the miRNAs and clinical
features. An independent-samples t-test was performed to examine
the difference in risk score distribution between clinical
characteristics (TNM stage), as the risk score was abnormally
distributed in those categories. P<0.05 was considered to
indicate a statistically significant difference. The statistical
analysis was performed using the SPSS 20.0 software package (IBM
Corp., Armonk, NY, USA).
Results
Identification of prognostic miRNAs in
colon carcinoma
According to the cut-off criteria (P<0.01 and
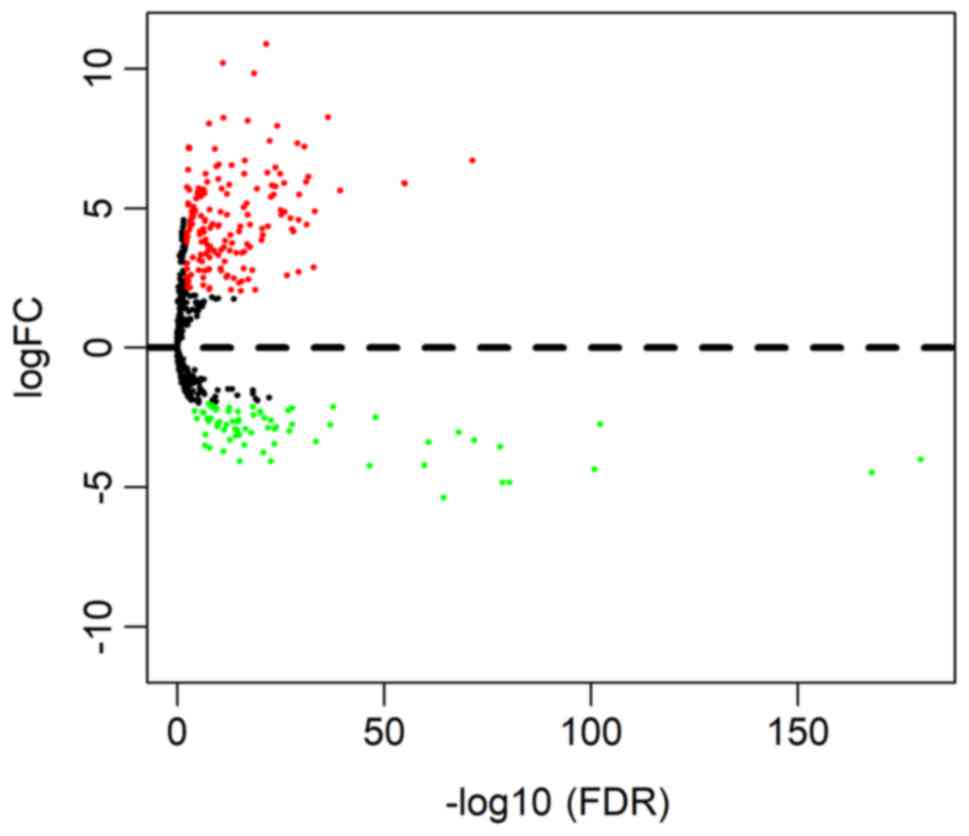
|log2FC|>2.0), a total of 245 DEmiRNAs were obtained, including
174 upregulated and 71 downregulated miRNAs. The result is
presented as volcano plot in Fig.
1.
The association between the expression of the 245
DEmiRNAs and patient survival was evaluated by using univariate Cox
proportional hazard regression. Based on the criteria (P<0.01),
eight miRNAs that were significantly associated with OS were
obtained. Furthermore, six of the eight miRNAs were screened out by
multivariate Cox regression and a diagnostic prediction model was
constructed based on the expression levels of the six miRNAs
weighed by their relative coefficient. The miRNA risk score was
calculated as follows: miRNA risk score = 0.204 ×
exphsa-mir-149 − 0.244 × exphsa-mir-3189 −
0.164 × exphsa-mir-3677 − 0.269 ×
exphsa-mir-3917 + 0.224 × exphsa-mir-4999 −
0.380 × exphsa-mir-6854. The detailed information of
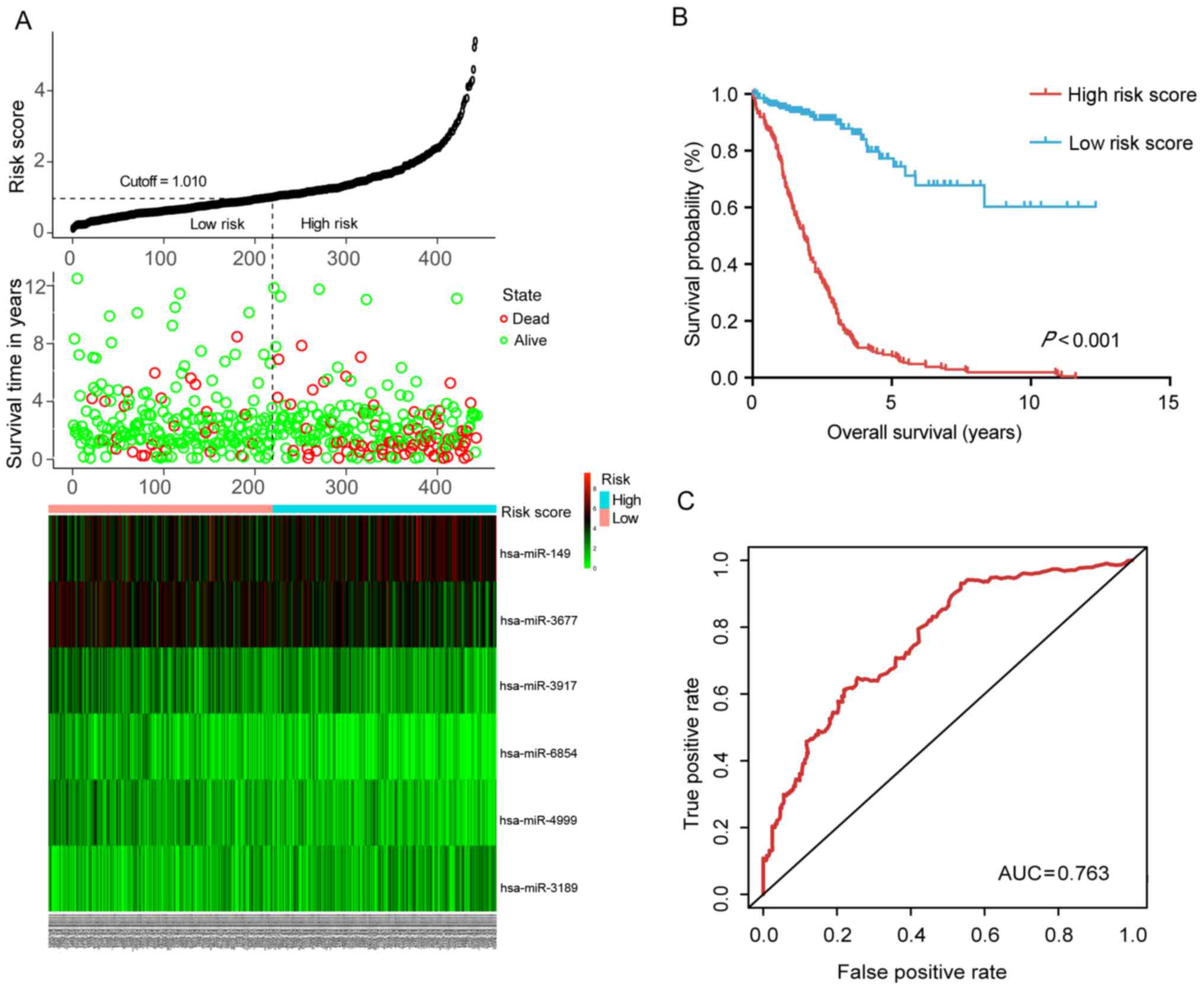
these miRNAs is presented in Fig.
2A and Table I. By dividing
the risk score according to its median (median=1.010), 453 patients
were stratified into the high-risk and low-risk groups. The
survival analysis performed using the Kaplan-Meier method revealed
that the low-risk group had a significantly better OS (P<0.001)
than the high-risk group (Fig.
2B). The ROC curve further demonstrated that the risk score
model was able to effectively predict the prognosis of colon cancer
patients (AUC=0.763; Fig.
2C).
 | Table ICharacteristics of the six deregulated
miRNAs and their regression coefficient derived from the
multivariate Cox regression model in colon carcinoma prognosis
prediction. |
Table I
Characteristics of the six deregulated
miRNAs and their regression coefficient derived from the
multivariate Cox regression model in colon carcinoma prognosis
prediction.
| miRNA | Ensembl ID | Coefficient | SE | P-value |
|---|
| hsa-miR-149 | ENSG00000207611 |
0.204 | 0.093 | 0.029 |
| hsa-miR-3917 |
ENSG00000283938 | −0.269 | 0.102 | 0.008 |
| hsa-miR-3677 |
ENSG00000266643 | −0.164 | 0.102 | 0.108 |
| hsa-miR-6854 |
ENSG00000278412 | −0.380 | 0.111 |
<0.001 |
| hsa-miR-4999 |
ENSG00000265390 |
0.224 | 0.112 | 0.045 |
| hsa-miR-3189 |
ENSG00000264175 | −0.244 | 0.130 | 0.060 |
Correlation between risk score and other
clinical characteristics
The correlation between the risk score based on the
signature of DEmiRNAs and various clinical features was then
assessed. The results indicated that the risk score was
significantly correlated with the TNM stage (P=0.007), N stage
(P<0.001), M stage (P<0.001) and residual tumor (P=0.021;
Table II).
 | Table IICorrelation between the risk score
and clinical features. |
Table II
Correlation between the risk score
and clinical features.
| Variable | Total (n) (%) | Risk score of
six-miRNA signature
| χ2
test
P-value |
|---|
| Low (n) (%) | High (n) (%) |
|---|
| Age (years) | | | | |
| <60 | 125 (27.6) | 65 (52.0) | 60 (48.0) | 0.596 |
| ≥60 | 328 (72.4) | 162 (49.4) | 166 (50.6) | |
| Sex | | | | |
| Male | 239 (52.8) | 121 (50.6) | 118 (49.4) | 0.294 |
| Female | 214 (47.2) | 106 (49.5) | 108 (50.5) | |
| TNM stage | | | | |
| I+III | 250 (56.6) | 134 (53.6) | 116 (46.4) | 0.007 |
| III+IV | 192 (43.4) | 89 (46.4) | 103 (53.6) | |
| T-stage | | | | |
| T1+T2 | 89 (19.6) | 55 (61.8) | 34 (38.2) | 0.070 |
| T3+T4 | 364 (80.4) | 172 (47.3) | 192 (52.7) | |
| N-stage | | | | |
| N0 | 266 (58.7) | 140 (52.6) | 126 (47.4) | 0.007 |
| N1+N2 | 187 (41.3) | 87 (46.5) | 100 (53.4) | |
| M-stage | | | | |
| M0 <0.001 | 332 (83.4) | 173 (52.2) | 159 (47.8) | |
| M1 | 64 (16.6) | 27 (42.2) | 37 (57.8) | |
| Residual tumor | | | | |
| R0 | 327 (92.1) | 175 (53.5) | 152 (46.5) | 0.021 |
| R1+R2 | 28 (7.9) | 13 (46.4) | 15 (53.6) | |
In addition, to further verify the independent
prognostic value of the six miRNAs, univariate and multivariate Cox
regression analysis was used to evaluate the effect of the six
miRNA-based risk score (high- vs. low-risk) as well as that of
other clinical parameters on OS. As presented in Table III, the univariate analysis
revealed that the TNM stage (HR=2.884, P<0.001), T stage
(HR=2.895, P=0.007), N stage (HR=2.538, P<0.001), M stage
(HR=4.253, P<0.001), residual tumor (HR=4.094, P<0.001) and
risk score of the six-miRNA signature (HR=3.504, P<0.001) were
significantly associated with the OS of colon carcinoma patients.
In the multivariate analysis, TNM stage (HR=5.461, P=0.007), N
stage (HR=0.331, P=0.034) and the six-miRNA signature (HR=3.991,
P<0.001) were independent prognostic factor.
 | Table IIIUni- and multivariate Cox regression
analysis for the prognostic value of various parameters in colon
carcinoma patients. |
Table III
Uni- and multivariate Cox regression
analysis for the prognostic value of various parameters in colon
carcinoma patients.
| Variable | Univariate analysis
| Multivariate
analysis
|
|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Age (≥60 vs.
<60) | 1.346
(0.845–2.144) | 0.211 | | |
| Sex (male vs.
female) | 1.124
(0.759–1.665) | 0.559 | | |
| TNM stage (III+IV
vs. I+II) | 2.884
(1.890–4.402) | <0.001 | 5.461
(1.605–18.585) | 0.007 |
| T stage (T3+T4 vs.
T1+T2) | 2.895
(1.340–6.252) | 0.007 | 6.549
(0.877–48.892) | 0.067 |
| N stage (N1+N2 vs.
N0) | 2.538
(1.699–3.792) | <0.001 | 0.331
(0.119–0.918) | 0.034 |
| M stage (M1 vs.
M0) | 4.253
(2.710–6.673) | <0.001 | 1.687
(0.788–3.612) | 0.178 |
| Residual tumor
(R1+R2 vs. R0) | 4.094
(2.249–7.450) | <0.001 | 1.809
(0.867–3.777) | 0.114 |
| Risk score (high
vs. low) | 3.504
(2.237–5.489) | <0.001 | 3.991
(2.040–7.809) | <0.001 |
Prognostication of colon carcinoma
patients based on risk score
To further assess the capacity of the risk score to
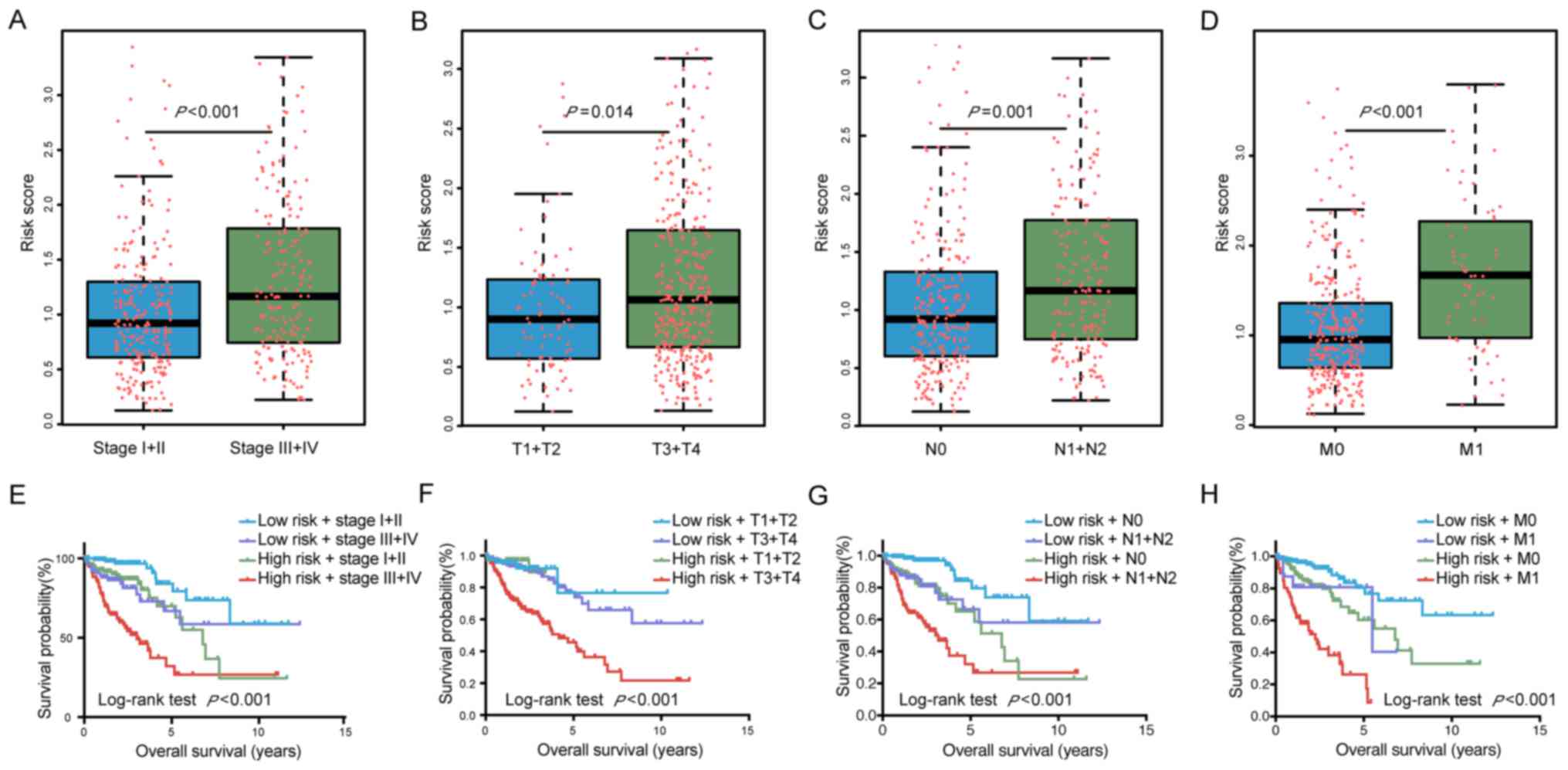
predict tumor progression, the risk score was compared different
tumor stages. Patients with a high TNM stage (stage III+IV) had
significantly higher risk scores than those with a low TNM stage
(stage I+II; P<0.001). In addition, the risk score was
significantly higher in tissues from patients with high vs. low T
(P=0.014), N (P=0.001) and M stages (P<0.001; Fig. 3A–D). Collectively, these results
suggest that the risk score of the six miRNAs may have a utility in
predicting tumor progression and surveillance for recurrence.
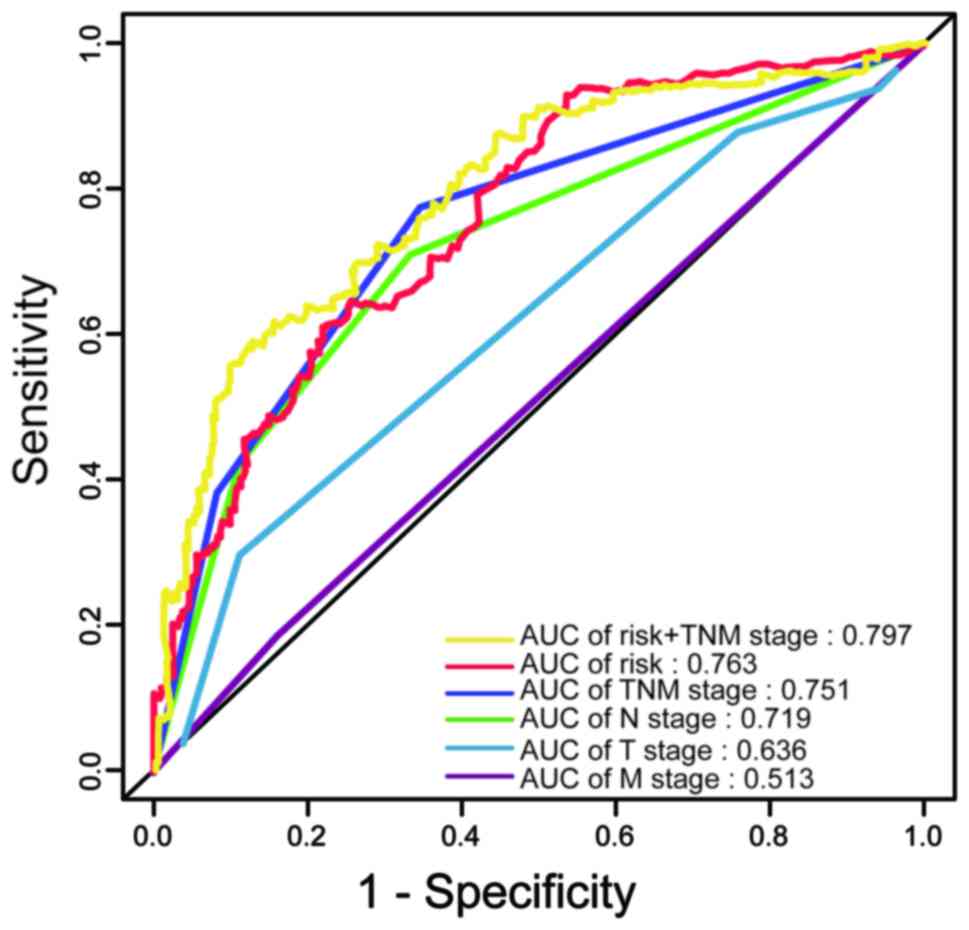
The AUC of the ROC indicated that the prediction
model had higher prognostic accuracy than any other factors. The
AJCC staging system is widely applied in various tumor types,
including colon carcinoma (16).
The data of the present study indicated that the TNM stage was able
to predict patient survival (AUC=0.751). However, combination of
the TNM stage and risk score by binary logistic regression analysis
significantly improved the prognostic value (AUC=0.797; Fig. 4).
The Kaplan-Meier curves also indicated that patient
prognosis for patients stratified by their risk score combined with
staging was significantly different (P<0.0001; Fig. 3E–H). All of these results
demonstrated that the six miRNAs may be utilized for the prediction
of the progression of colon carcinoma and the prognosis of affected
patients. Furthermore, a more accurate prognosis was obtained by
using a combination of the TNM stage and risk score.
Target prediction and functional
analysis
To elucidate the possible mechanisms that the six
miRNAs are involved in, their target genes were predicted using the
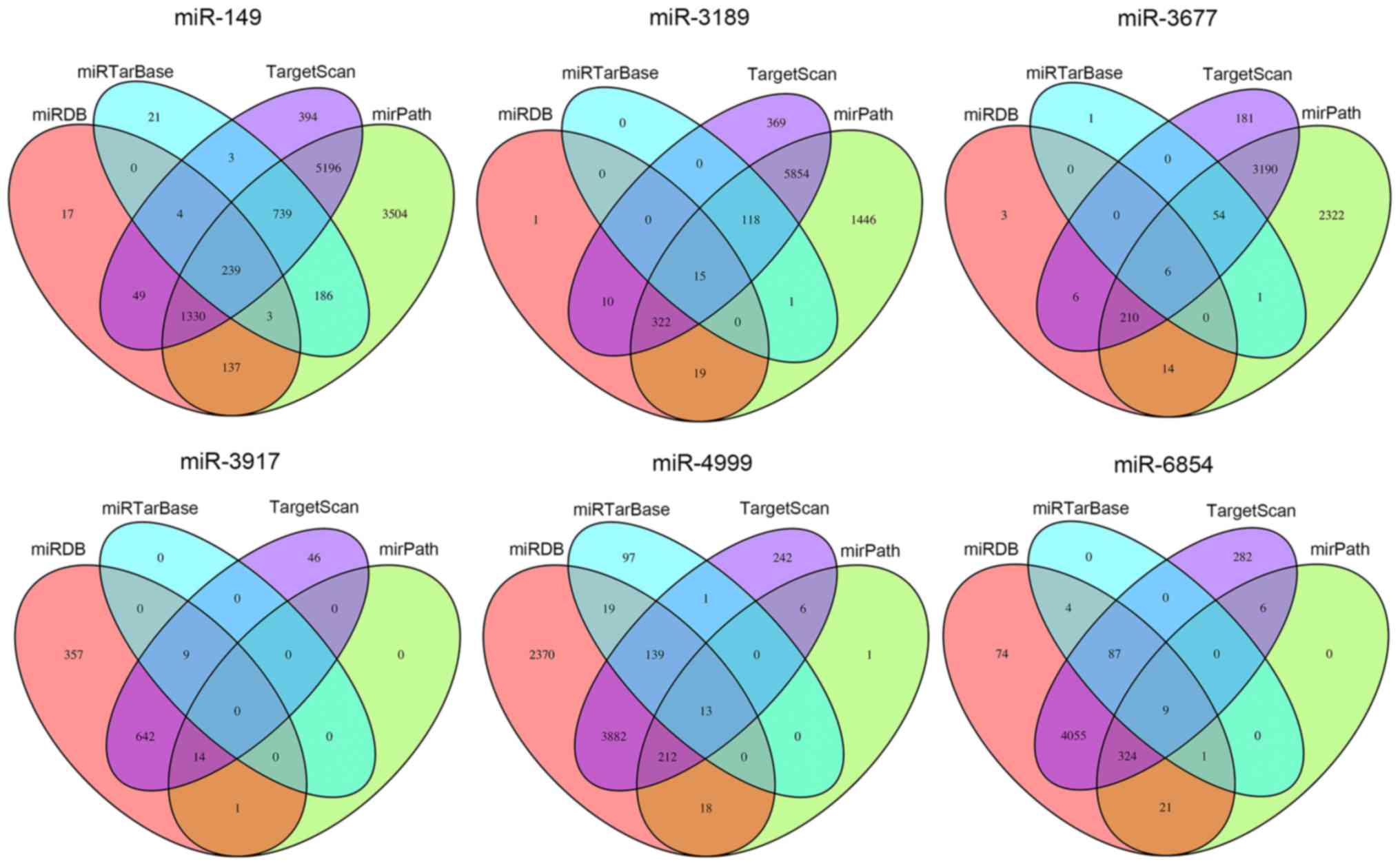
TargetScan, miRDB, mirPath and miRTarBase online analysis tools. A
total of 239 overlapping genes for miR-149, 15 overlapping genes
for miR-3189, 6 overlapping genes for miR-3677, 0 overlapping genes
for miR-3917, 13 overlapping genes for miR-4999 and 9 overlapping
genes for miR-6854 were obtained (Fig. 5). Subsequently, a bioinformatics
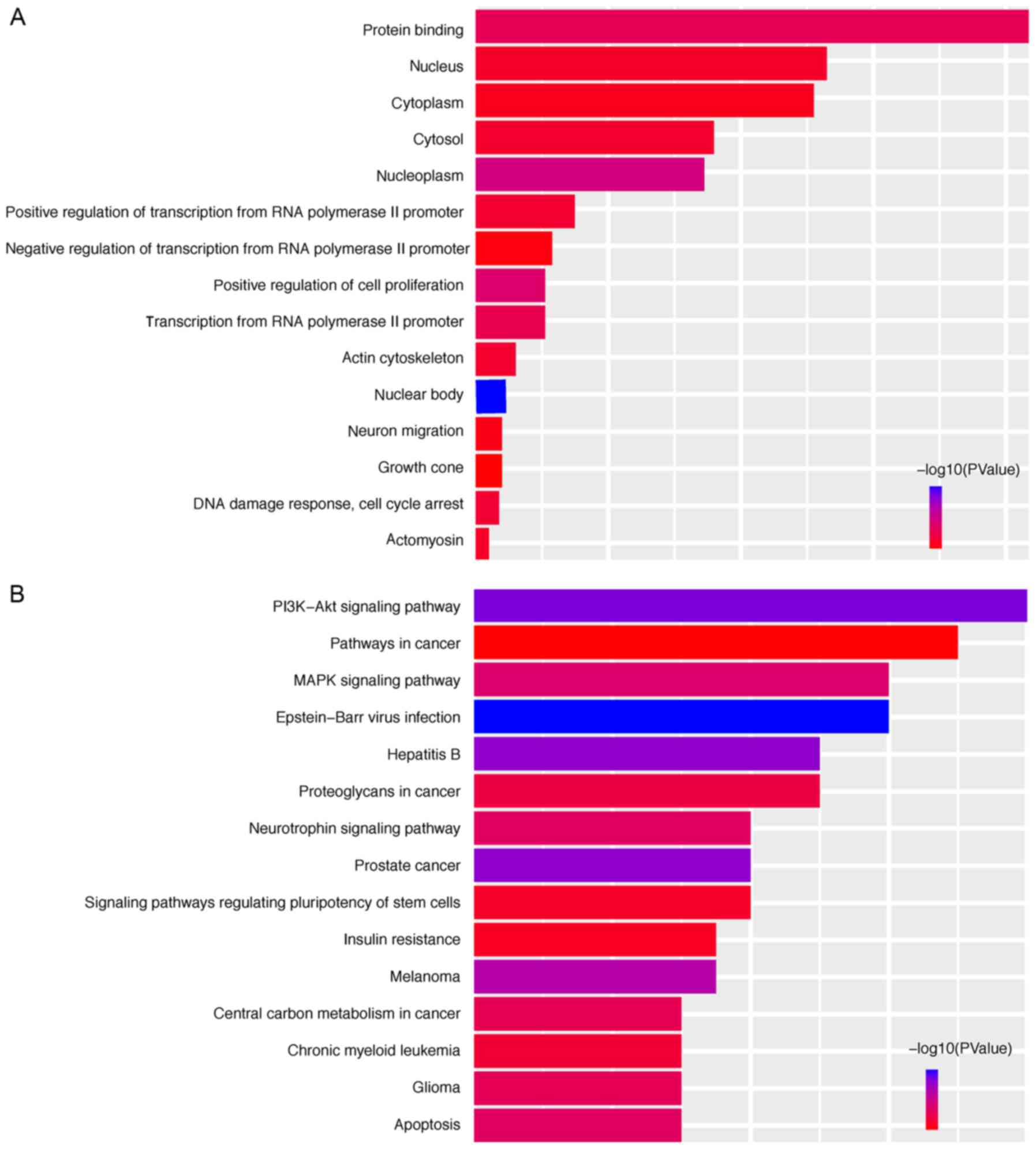
enrichment analysis was performed. The overlapping genes of the
DEmiRNAs were significantly enriched in KEGG pathways
'phosphoinositide-3 kinase (PI3K)-Akt signaling pathway', 'prostate
cancer', 'mitogen-activated protein kinase (MAPK) signaling
pathway', 'apoptosis' and 'neurotrophin signaling pathway'. The top
five GO terms were 'nuclear body', 'nucleoplasm', 'positive
regulation of cell proliferation', 'protein binding' and
'transcription from RNA polymerase II promoter' (Fig. 6). This functional enrichment
analysis revealed the potential mechanisms of the prognostic miRNAs
in the genesis/progression of colon cancer.
Discussion
Colon carcinoma is one of the leading causes of
cancer-associated mortality worldwide (2). Early diagnosis and radical resection
may improve patient prognosis (17,18). However, the symptoms are
frequently not obvious at the earliest stage, and at present, the
detection of colon carcinoma relies on screening and endoscopy
(8,19). Although the detection of serum CEA
is currently available as a non-invasive method for the diagnosis
and surveillance of colon cancer, its clinical utility is limited
by its low sensitivity and specificity (20). Therefore, it is necessary to
further elucidate the molecular mechanisms of colon cancer
development, which may lead to the identification of novel accurate
markers.
miRNAs are small non-coding RNAs, whose aberrant
expression may be involved in the initiation and progression of
colon carcinoma (21,22). Monitoring of changes in the
expression of miRNAs may aid in the diagnosis and prediction of the
prognosis of colon carcinoma and management of its recurrence
(23–25).
In the present study, DEmiRNAs between colon
carcinoma tissues and normal tissues were identified. A prognostic
model using six miRNAs was then constructed based on univariate
(data not shown) and multivariate Cox regression analysis.
Furthermore, multivariate Cox regression and stratification
analyses suggested that the six-miRNA signature was an independent
prognostic factor for survival prediction of colon carcinoma
patients. The risk score, based on which it was possible to
effectively distinguish colon cancer patients with significantly
different prognoses, was validated as an independent prognostic
risk factor.
Furthermore, previous studies indicated that miR-149
is downregulated in breast cancer and colonic carcinoma cells and
functions as a tumor suppressor by controlling breast epithelial
cell migration and invasion, which is in agreement with the present
results (26,27). miR-3189 has been previously
reported to exert antitumor effects in glioblastoma and gastric
cancer (28,29), miR-3677 may be used as a potential
molecular marker to predict the prognosis of hepatic carcinoma
patients (30,31), and miR-3917 may be useful for the
early screening of high-risk populations and early diagnosis of
lung cancer (32). Furthermore,
miR-4999 and miR-6854, which have not been previously reported in
tumors, to the best of our knowledge, may improve the accuracy of
colon carcinoma prognostication.
The mRNAs regulated by the six-miRNA signature were
enriched in pathways that were involved in cancer progression and
prognosis. The six miRNAs were able to regulate several key
signaling pathways, including the PI3K-Akt signaling pathway,
apoptosis, the MAPK signaling pathway and the neurotrophin
signaling pathway, which is in accordance with the results of the
present bioinformatics analysis (33,34). The results indicated that the
six-miRNA signature was highly correlated with cancer, which
suggested the possibility of using it as a prognostic factor for
colon carcinoma.
The current management of colon cancer relies on
clinical and histopathologic factors, including TNM stage, tumor
margin involvement, differentiation and lymphovascular invasion
(35). The TNM staging system is
the preferred staging system for colon cancer, and it may be
beneficial to identify reliable biomarkers that are able to
distinguish between different stages, to further assess tumor
progression and aid the development of therapeutic strategies. The
risk score postulated in the present study exhibited high
sensitivity and specificity, and was correlated with the TNM stage
and prognosis of colon cancer patients, which further suggests that
it may be able to predict tumor occurrence and development. In
addition, combining the risk score and TNM stage provided an
improved prediction of the patients' survival status.
Of note, the present study was is entirely based on
the TCGA dataset and experiments should be performed to further
verify the mechanisms of the miRNAs involved in the tumorigenesis
of colon cancer. In addition, further clinical investigations
should be performed to validate the utility of this model for early
diagnosis and evaluation of therapeutic efficacy.
In conclusion, the present study postulated a
six-miRNA model that provides effective mortality risk
stratification of colon carcinoma patients and may be a potential
prognostic indicator. Combination with the TNM stage further
improved the capacity of the risk score to predict patient
prognosis. It may also be possible to utilize the six miRNAs for
early diagnosis, allowing for a timely intervention.
Acknowledgments
Not applicable.
Funding
This study was supported in part by grants from the
Jiangsu Provincial Key research development program (grant nos.
BE2016794 to JF and BE2016795 to JW).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding authors on reasonable request.
Authors' contributions
JF and JW contributed to the design of the study. HZ
performed the bioinformatic analysis and wrote the manuscript. ZW
and RM were responsible for article revision. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Villar-Vázquez R, Padilla G,
Fernández-Aceñero MJ, Suárez A, Fuente E, Pastor C, Calero M,
Barderas R and Casal JI: Development of a novel multiplex
beads-based assay for autoantibody detection for colorectal cancer
diagnosis. Proteomics. 16:1280–1290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar
|
|
6
|
Pucci S, Bonanno E, Sesti F, Mazzarelli P,
Mauriello A, Ricci F, Zoccai GB, Rulli F, Galatà G and Spagnoli LG:
Clusterin in stool: A new biomarker for colon cancer screening? Am
J Gastroenterol. 104:2807–2815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Jiang X, Dong S, Shen J, Yu H,
Zhou J, Li J, Ma H, He E and Skog S: Serum TK1 is a more reliable
marker than CEA and AFP for cancer screening survey in a study of
56,286 people. Cancer Biomark. 16:529–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hollis M, Nair K, Vyas A, Chaturvedi LS,
Gambhir S and Vyas D: MicroRNAs potential utility in colon cancer:
Early detection, prognosis, and chemosensitivity. World J
Gastroenterol. 21:8284–8292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortez MA, Bueso-Ramos C, Ferdin J,
Lopez-Berestein G, Sood AK and Calin GA: MicroRNAs in body
fluids-the mix of hormones and biomarkers. Nat Rev Clin Oncol.
8:467–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng JH, Liang L, He RQ, Tang RX, Cai XY,
Chen JQ, Luo DZ and Chen G: Comprehensive investigation of a novel
differentially expressed lncRNA expression profile signature to
assess the survival of patients with colorectal adenocarcinoma.
Oncotarget. 8:16811–16828. 2017.PubMed/NCBI
|
|
13
|
Sui J, Xu SY, Han J, Yang SR, Li CY, Yin
LH, Pu YP and Liang GY: Integrated analysis of competing endogenous
RNA network revealing lncRNAs as potential prognostic biomarkers in
human lung squamous cell carcinoma. Oncotarget. 8:65997–66018.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heagerty PJ, Lumley T and Pepe MS:
Time-dependent ROC curves for censored survival data and a
diagnostic marker. Biometrics. 56:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hari DM and Bilchik AJ: Clinical
decision-making and implementation challenges with the AJCC VII
staging system for colorectal carcinoma. J Surg Oncol. 105:221–222.
2011. View Article : Google Scholar
|
|
17
|
van Rossum LG, van Rijn AF, Laheij RJ, Van
Oijen MG, Fockens P, Van Krieken HH, Verbeek AL, Jansen JB and
Dekker E: Random comparison of guaiac and immunochemical fecal
occult blood tests for colorectal cancer in a screening population.
Gastroenterology. 135:82–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar
|
|
19
|
Kanaan Z, Rai SN, Eichenberger MR, Roberts
H, Keskey B, Pan J and Galandiuk S: Plasma miR-21: A potential
diagnostic marker of colorectal cancer. Ann Surg. 256:544–551.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spratlin JL, Hui D, Hanson J, Butts C and
Au HJ: Community compliance with carcinoembryonic antigen:
Follow-up of patients with colorectal cancer. Clin Colorectal
Cancer. 7:118–125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ito M, Mitsuhashi K, Igarashi H, Nosho K,
Naito T, Yoshii S, Takahashi H, Fujita M, Sukawa Y, Yamamoto E, et
al: MicroRNA-31 expression in relation to BRAF mutation, CpG island
methylation and colorectal continuum in serrated lesions. Int J
Cancer. 135:2507–2515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colussi D, Brandi G, Bazzoli F and
Ricciardiello L: Molecular pathways involved in colorectal cancer:
Implications for disease behavior and prevention. Int J Mol Sci.
14:16365–16385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weng M, Wu D, Yang C, Peng H, Wang G, Wang
T and Li X: Noncoding RNAs in the development, diagnosis, and
prognosis of colorectal cancer. Transl Res. 181:108–120. 2017.
View Article : Google Scholar
|
|
24
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pereira DM, Rodrigues PM, Borralho PM and
Rodrigues CM: Delivering the promise of miRNA cancer therapeutics.
Drug Discov Today. 18:282–289. 2013. View Article : Google Scholar
|
|
26
|
Bischoff A, Huck B, Keller B, Strotbek M,
Schmid S, Boerries M, Busch H, Müller D and Olayioye MA: miR149
functions as a tumor suppressor by controlling breast epithelial
cell migration and invasion. Cancer Res. 74:5256–5265. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang G, Liu X, Li Y, Wang Y, Liang H, Li
K, Li L, Chen C, Sun W, Ren S, et al: EphB3-targeted regulation of
miR-149 in the migration and invasion of human colonic carcinoma
HCT116 and SW620 cells. Cancer Sci. 108:408–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeansonne D, DeLuca M, Marrero L, Lassak
A, Pacifici M, Wyczechowska D, Wilk A, Reiss K and Peruzzi F:
Anti-tumoral effects of miR-3189-3p in glioblastoma. J Biol Chem.
290:8067–8080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bian Y, Guo J, Qiao L and Sun X:
miR-3189-3p mimics enhance the effects of S100A4 siRNA on the
inhibition of proliferation and migration of gastric cancer cells
by targeting CFL2. Int J Mol Sci. 19:pii: E236. 2018. View Article : Google Scholar
|
|
30
|
Zhang J, Chong CC, Chen GG and Lai PB: A
Seven-microRNA expression signature predicts survival in
hepatocellular carcinoma. PLoS One. 10:e01286282015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu M, Kong X, Wang H, Huang G, Ye C and He
Z: A novel microRNAs expression signature for hepatocellular
carcinoma diagnosis and prognosis. Oncotarget. 8:8775–8784. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Sui J, Shen X, Li C, Yao W, Hong
W, Peng H, Pu Y, Yin L and Liang G: Differential expression
profiles of microRNAs as potential biomarkers for the early
diagnosis of lung cancer. Oncol Rep. 37:3543–3553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kandimalla R, Gao F, Matsuyama T, Ishikawa
T, Uetake H, Takahashi N, Yamada Y, Becerra CR, Kopetz S, Wang X
and Goel A: Genome-wide discovery and identification of a novel
miRNA signature for recurrence prediction in stage II and III
colorectal cancer. Clin Cancer Res. Mar 7–2018.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|