Introduction
Tongue squamous cell carcinoma (TSCC) is one of the
most common cancer types of the head and neck, often resulting in
short survival times and poor prognosis (1). At present, treatment of TSCC
involves a comprehensive therapeutic model combining surgery,
radiation and chemotherapy (2-5).
However, chemical agents kill not only tumor cells but also normal
cells, thereby exhibiting cellular toxicity, along with the
possibility of inducing resistance in tumor cells (6). Therefore, the identification of
highly efficient, low toxicity targeting drugs from natural sources
has gradually become a topic of contemporary research. In recent
years, the primary extract from green tea
epigallocatechin-3-gallate (EGCG) has demonstrated anti-tumor
bioactivities with mild side effects in normal cells (7,8).
In addition, attention has been focused on targeted therapy against
molecules, including epidermal growth factor receptor (EGFR),
cyclooxygenase-2 (COX-2), peroxisome proliferator-activated
receptor-γ (PPARγ) and progesterone receptor, to treat oral cancer
(9). Numerous in vitro
studies have reported the abilities of EGCG to reduce growth,
induce apoptosis, and inhibit the migration and invasion of TSCC
cell lines through several molecular signaling pathways (10-16). The Hippo signaling pathway is a
highly conserved signaling pathway. When this pathway is activated,
its downstream transcription coactivator with a PDZ-binding motif
tafazzin (TAZ) is translocated into the nucleus to bind the TEA
domain transcription factor family, and induce changes in the
expression of a range of genes associated with proliferation,
survival and migration (17,18). According to previous studies, the
activation of Hippo-TAZ signaling promotes proliferation, migration
and invasion, and inhibits apoptosis in TSCC cells (19,20). However, the effect EGCG on TAZ
expression has not been well evaluated in human TSCC cells. Thus,
the present study aimed to explore the possible associations
between EGCG stimulation and activation of the Hippo-TAZ signaling
pathway in TSCC cells.
Therefore, the current study was performed to
investigate how EGCG exerts its biological effects on processes,
including cell proliferation, apoptosis, migration and invasion
through the Hippo-TAZ signaling pathway in TSCC cells.
Materials and methods
Reagents and antibodies
EGCG (E8120) was purchased from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China). Simvastatin
(S6196) was purchased from Merck KGaA (Sigma-Aldrich; Darmstadt,
Germany) and dissolved in DMSO to make stock solutions. Primary
rabbit monoclonal anti-human antibodies against TAZ (cat. no.,
70148), phosphorylated (p)-TAZ (Ser89) (cat. no., 59971), large
tumor suppressor 1 (LATS1; cat. no., 3477), MOB kinase activator 1
(MOB1; cat. no., 13730), mammalian sterile 20-like 1 (MST1; cat.
no., 14946), salvador 1 (SAV1; cat. no., 13301), c-Jun N-terminal
kinase (JNK; cat. no., 9252), p-JNK (Thr183/Tyr185) (cat. no.,
4668), extracellular regulated protein kinases (Erk; cat. no.,
4695), p-Erk (Thr202/Tyr204) (cat. no., 4370), protein kinase B
(Akt) (cat. no., 4691), p-Akt (Ser473) (cat. no., 4060), B cell
lymphoma-2 (Bcl-2; cat. no., 2872), Bcl-2 associated X protein
(Bax; cat. no., 5023), poly ADP-ribose polymerase (PARP; cat. no.,
9532), cleaved PARP (cat. no., 5625), vimentin (cat. no., 5741),
and E-cadherin (cat. no., 3195), GAPDH (cat. no., 5174) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibodies were obtained from Affinity Biosciences (cat. no.,
S0001; Cincinnati, OH, USA).
Cell lines and culture
The TSCC cell lines, CAL27 and SCC15, were obtained
from the American Type Culture Collection (Manassas, VA, USA). They
were identified using short tandem repeats. CAL27 cells were
cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), while SCC15 cells were
incubated in minimum essential medium (HyClone; GE Healthcare Life
Sciences) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin-streptomycin at 37°C in a humidified atmosphere with 5%
CO2.
Proliferation assay
Cell proliferation was measured by a Cell-Counting
Kit-8 assay (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Cells were cultured in 96-well tissue culture plates
(4.0×103 cells/well) with 10% FBS for 24 h. Then, the
cells were exposed to different concentrations of EGCG (0, 40, 80,
120, 160 and 200 µM) for different durations (0, 24, 48, 72,
96 and 120 h). Following incubation, 10 µl CCK-8 solution
was added to each well, and the plates were incubated for an
additional 2 h. The absorbance was measured using a spectrometer at
a wavelength of 450 nm.
Cell proliferation was also assessed using an EdU
Apollo DNA in vitro kit (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China) following the manufacturer's protocol. CAL27
cells were seeded at a density of 1.0×105
cells/cm2 in 24-well plates and incubated for 24 h in
normal growth medium. Cells were treated with 200 µl EdU for
2 h and then fixed with 4% paraformaldehyde for 15-20 min at room
temperature. The cells were then incubated with 2 mg/ml glycine at
room temperature for 10 min, followed by washing with PBS. Then,
the cells were permeabilized with 100 µl/well
permeabilization buffer (0.5% Triton X-100 in PBS) at room
temperature for 30 min and incubated with 200 µl 1X Apollo
solution for 30 min at room temperature in the dark. Subsequently,
the cells were incubated with 200 µl 1X Hoechst 33342
solution for 30 min at room temperature in the dark. The percentage
of EdU-positive cells was determined by fluorescence microscopy
(magnification, ×100) and was calculated using Image-Pro Plus 6.0
software (Media Cybernetics Inc., Rockville, MD, USA).
Apoptosis assay
Cell apoptosis was analyzed by flow cytometry. Cells
with green fluorescent probes and empty vector were stained using
the Annexin V-APC staining kit (Sungene Biotech Co., Ltd., Tianjin,
China). Briefly, following washing with cold PBS, the cells were
suspended in 500 µl 1X binding buffer, and then incubated
with 5 µl Annexin V-fluorescein APC at room temperature in
the dark for 10 min. Finally, 5 µl 7-AAD solution was added
and cells were incubated for 5 min at room temperature in the dark.
Untreated control cells without transfection were measured using
the Annexin V-FITC/PI kit [Hangzhou Multi Sciences (Lianke) Biotech
Co., Ltd., Hangzhou, China] following the manufacturer's protocol.
The percentage of apoptotic cells was measured using a FACSCalibur
(BD Biosciences, San Jose, CA, USA), and analyzed using CellQuest
software version 5.1 (BD Biosciences).
Wound healing assay
Cells (8.0×105/well) were seeded in
6-well plates, and allowed to attach and reach 80% confluence. Cell
monolayers were wounded by scratching with 200-µl pipette
tips and then washed twice with PBS to remove floating cells. Cells
in each well were subsequently exposed to FBS-free medium with or
without EGCG for up to 48 h. Cells were imaged at ×100
magnification with a phase-contrast microscope at each time point.
The wound healing areas at different time points were measured
using ImageJ software 14.8 for Windows (National Institutes of
Health, Bethesda, MD, USA).
Transwell invasion assay
Cells (1.0×104 cells/well) were seeded in
the upper chamber of Transwell inserts (8-µm pore size)
pre-coated with Matrigel (both from Corning Incorporated, Corning,
NY, USA) and exposed to FBS-free medium with or without different
concentrations of EGCG. Medium containing 10% FBS was placed in the
lower chamber, and cells for each treatment were incubated for 24 h
at 37°C in a humidified environment with 5% CO2. Then,
non-invaded cells in the upper chamber were removed with a cotton
swab, and invaded cells were washed with PBS, fixed with 4%
para-formaldehyde for 30 min and stained with 0.1% crystal violet
(Solarbio, Shanghai, China) for 10 min. Images were captured with a
light microscope at ×200 magnification, and the number of cells
that had penetrated the membrane was counted using Image-Pro Plus
6.0 software.
Cell transfection
A total of 1×106 CAL27 cells were seeded
and transfected at ~80% confluence with culture medium with 8
µg/ml Polybrene and 100 µl overexpression TAZ
lentivirus particle LV5-homo-TAZ (NM_000116.4) (TAZ-overexpression
group). Similarly, CAL27 cells at ~80% confluence were transfected
with the culture medium with 8 µg/ml Polybrene and 100
µl empty vector lentivirus LV5-NC (NM_000116.4) (negative
control group). After 6-8 h, the medium was changed to basal medium
(HyClone; GE Healthcare Life Sciences) supplemented with 10% FBS,
and the cells were cultured for further analyses. The efficiency of
TAZ overexpression was determined using western blotting and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) assays.
Total protein isolation and western
blotting
Cells were collected and lysed in ice-cold RIPA
lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China), and the concentration of each protein sample was quantified
using a bicinchoninic acid assay. Subsequently, 20 µg of
protein from each sample was separated using 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Following blocking in 5% fat-free
milk for 1 h at room temperature, the membranes were probed with
primary antibodies (dilution 1:1,000) at 4°C overnight. Next, they
were washed with Tris-based saline-Tween-20 (20 mmol/l Tris-HCl,
150 mmol/l NaCl and 0.05% Tween-20) at room temperature three times
for 10 min each. Then, the membranes were incubated with
HRP-conjugated secondary antibodies (dilution 1:20,000) at room
temperature for 1 h. Finally, protein bands were detected using the
Immobilon Western chemiluminescent HRP substrate kit (EMD
Millipore). Quantitative analysis of protein bands was calculated
using ImageJ with 64-bit Java 1.6.0-24 program for Windows.
RNA isolation and RT-qPCR
Total RNA was extracted from cells at 80% confluence
using TRIzol® reagent (Gibco; Thermo Fisher Scientific,
Inc.) and then reverse transcribed into cDNA using the PrimeScript™
RT II reagent kit (Takara Bio, Inc., Otsu, Japan) according to
manufacturer's protocol. The generated cDNA was used as template
for Quantitative real-time PCR (RT-qPCR) using SYBR Premix Ex Taq™
kit (Takara, Tokyo, Japan) following the manufacturer's protocol.
Amplification conditions were as follows: Initial step of 95°C for
30 sec; 45 cycles of 95°C for 5 sec, 60°C for 35 sec and 72°C for
60 sec; and a final step at 40°C for 30 sec. RT-qPCR was performed
using a LightCycler® 480 II and changes in gene
expression were calculated using the 2−ΔΔCq method
(21). The expression levels of
GAPDH and TAZ were analyzed and the primers used in the present
study were as follows: TAZ forward, 5′-GCT GCT TCT GGA CCA AGT
ACA-3′ and reverse, 5′-AGA TGT GGC GGA GTT TCA GG-3′; GAPDH
forward, 5′-GCT TGT CAT CAA CGG GAA G-3′ and reverse, 5′-GAT GTT
AGT GGG GTC TCG-3′.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). Differences between multiple groups were examined using
one-way analysis of variance followed by Tukey's post hoc test and
a two-tailed t-test was used for the comparison between two groups.
All results are expressed as the mean ± standard deviation from at
least three independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
EGCG stimulation inhibits proliferation,
migration and invasion, and promotes apoptosis in CAL27 and SCC15
cells
To investigate the effect of EGCG on proliferation
of CAL27 cells, cells were treated with different concentrations of
EGCG and the changes in proliferation were measured using a CCK-8
assay. Cell proliferation was significantly decreased in response
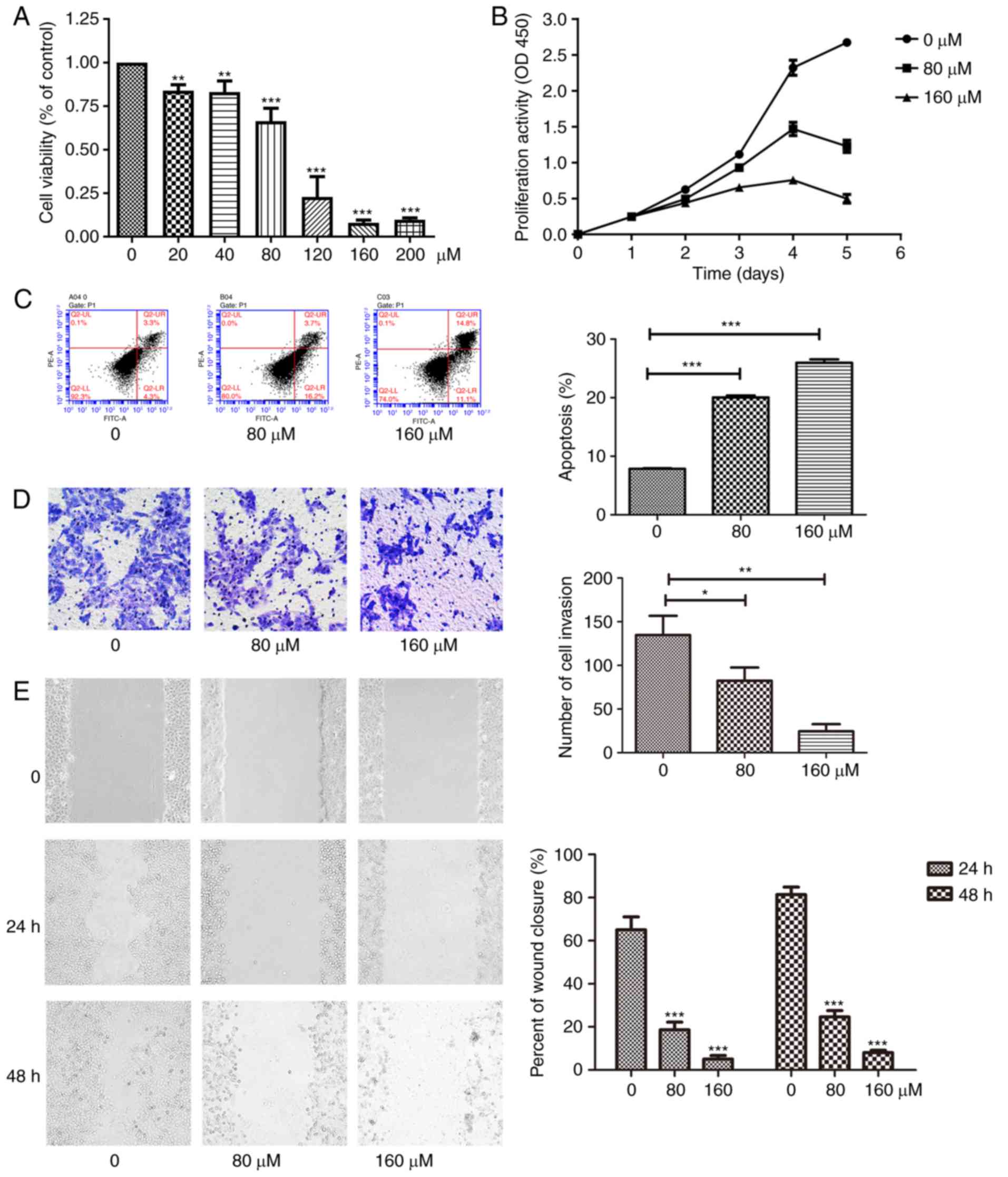
to EGCG treatment in time- and dose-dependent manners (Fig. 1A and B). Following incubation for
120 h, the cell numbers in the 160 µM EGCG group were
significantly lower compared with those in the control groups.
Eventually, 80 µM was established as the optional drug
concentration. Additionally, cell apoptosis was measured by flow
cytometry (Fig. 1C). The
apoptosis rates, total and early apoptosis, of the 80 µM
EGCG group were significantly higher compared with those of the
group without EGCG. In addition, the 160 µM EGCG group had a
higher proportion of cells in late apoptosis compared with the 80
µM EGCG group. EGCG also significantly inhibited cell
invasion in a concentration-dependent manner as evaluated using a
Transwell assay (Fig. 1D).
Similarly, as revealed using the scratch assay, EGCG significantly
reduced the time of wound healing (Fig. 1E). Therefore, the results of the
present study indicated that EGCG inhibited proliferation,
migration and invasion, and promoted apoptosis in CAL27 cells. In
order to confirm these findings, the aforementioned experiments
were repeated in SCC15 cells, whereby similar changes were observed
(Fig. 2)
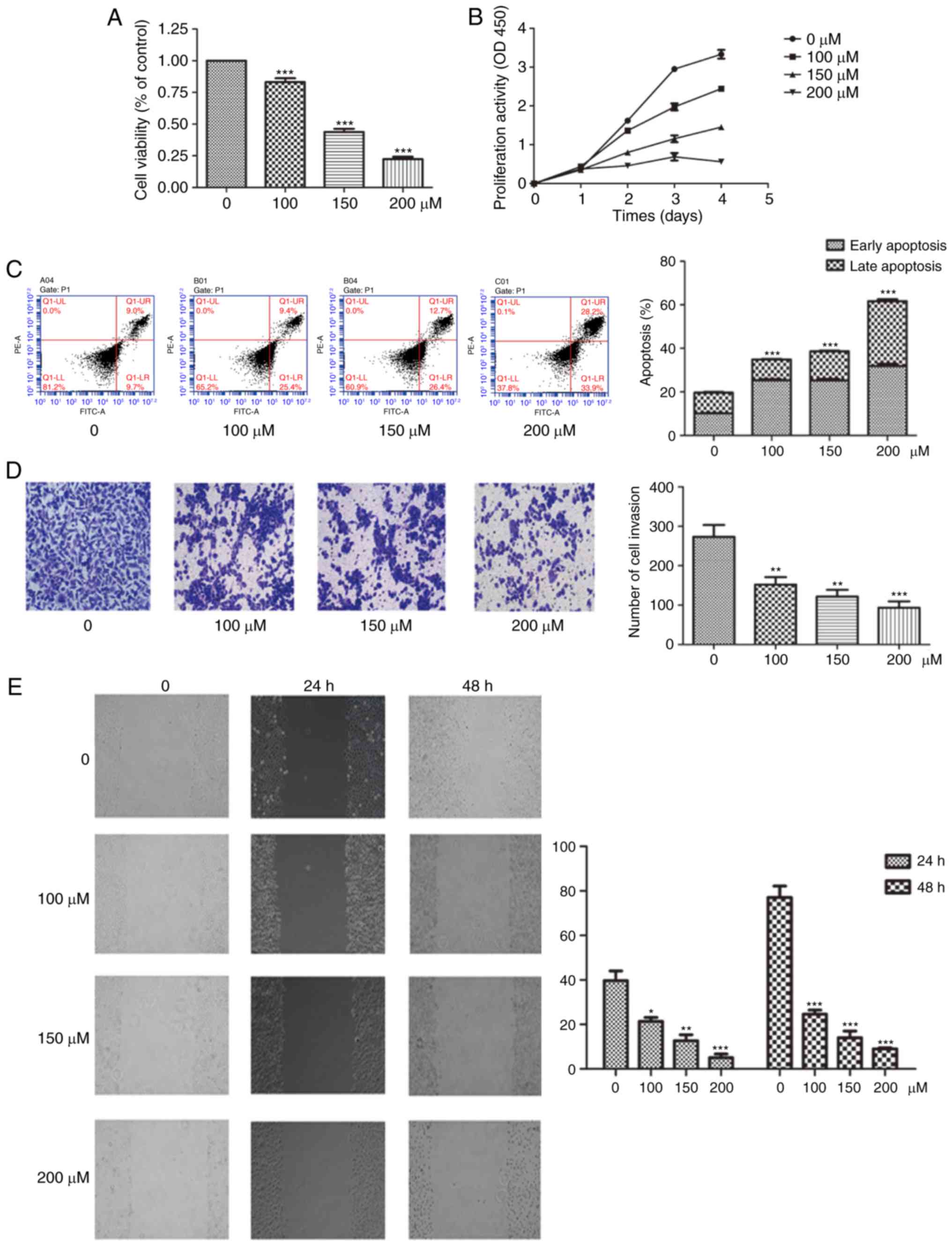 | Figure 2EGCG inhibits the proliferation,
migration and invasion of SCC15 cells in a dose-dependent manner.
(A) Cells were exposed to increasing concentrations of EGCG (0-200
µM) for 24 h. Following treatment, cell viability was
measured using a CCK-8 assay. (B) Cell growth was measured by CCK-8
assay at the indicated time points and EGCG concentrations. (C)
Cell apoptosis was determined by flow cytometry after 24 h of
treatment, and the rates of apoptosis at different EGCG
concentrations were statistically analyzed. (D) Cell invasion was
measured using a Transwell assay in response to treatment with
different concentrations of EGCG for 24 h (magnification, ×200).
(E) Cell migration was analyzed using scratch assay following
treatment with the indicated concentration of EGCG treatment for 24
and 48 h (magnification, ×100), and the percentage of wound closure
was statistically analyzed. *P<0.05,
**P<0.01 and ***P<0.001, vs. the
control (0 µM) group or as indicated. EGCG,
epigallocatechin-3-gallate; CCK-8, Cell Counting Kit-8. |
EGCG causes changes in the expression of
genes associated with proliferation, apoptosis and epithelial
mesenchymal transition (EMT) in CAL27 cells
Western blot assays were performed to confirm
whether EGCG treatment induces changes in the expression of
proteins associated with proliferation, apoptosis, migration and
invasion (Fig. 3). Akt is known
as a key molecule of the PI3K/Akt/MTOR signaling pathway (22), and Erk-1/2 is a component of the
mitogen-activated protein kinase (MAPK) signaling pathway (23), which affects the expression of
proliferation-associated proteins. According to the results of the
present study, EGCG significantly reduced the expression of
proliferation-associated proteins compared with normal (0
µM) cells, including p-Akt, while no differences were
observed in total Akt expression. Although the level of total Erk
was unaffected, changes in p-Erk levels indicate a reduction in
proliferative abilities.
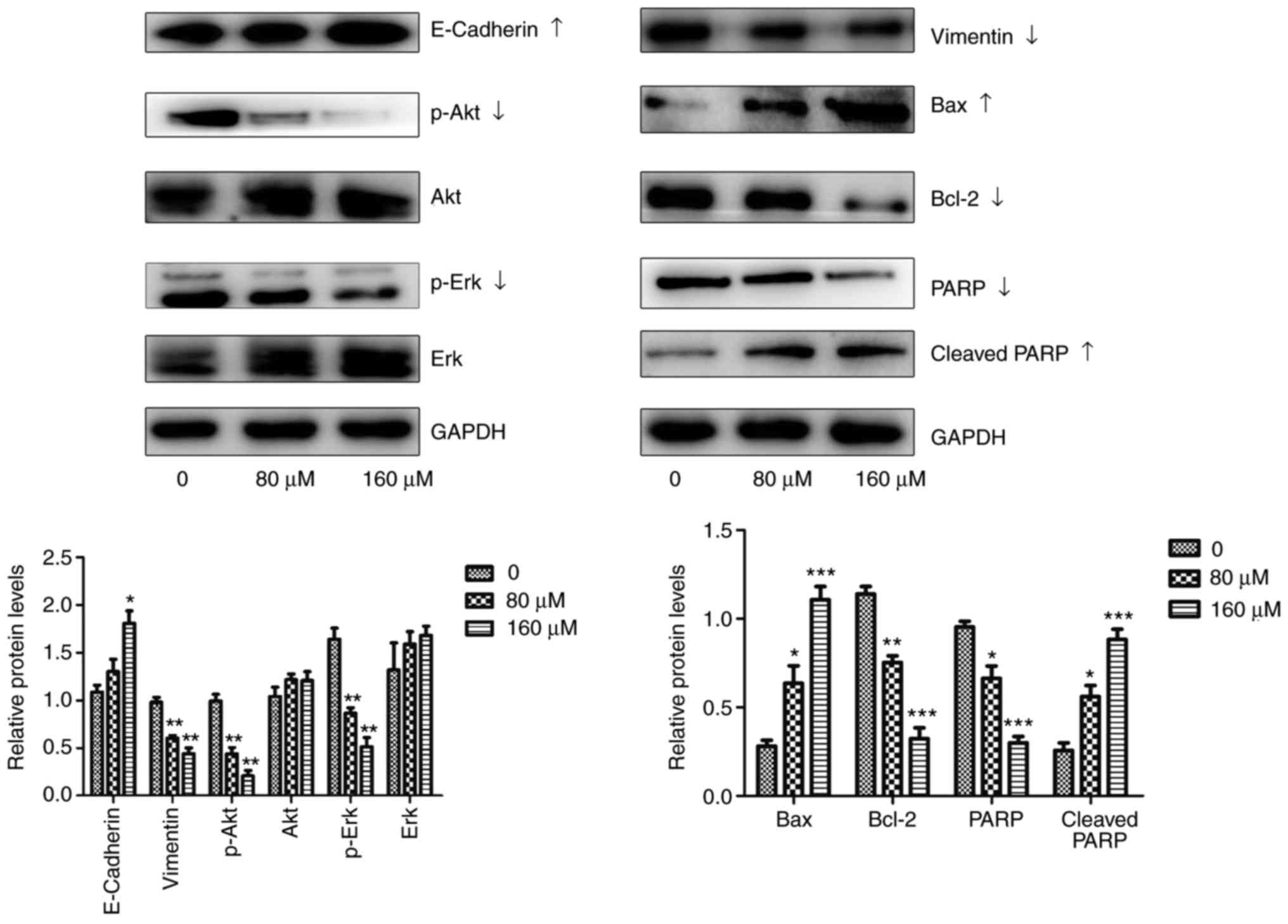 | Figure 3EGCG affects the expression of genes
associated with proliferation, apoptosis and EMT in CAL27 cells.
Changes in Erk, p-Erk, Akt, p-Akt, Bcl-2, Bax, PARP, cleaved PARP,
vimentin, E-cadherin and GAPDH expression were determined using a
western blot assay after 24 h of treatment with different
concentrations of EGCG. All data are presented as the mean ±
standard deviation from three experiments. *P<0.05,
**P<0.01 and ***P<0.001, vs. the
control (0 µM) group. EMT, epithelial-mesenchymal
transition; Erk, extracellular regulated protein kinases; p-Erk,
phosphorylated Erk; Akt, protein kinase B; p-Akt, phosphorylated
Akt; Bcl-2, B cell lymphoma/lewkmia-2; Bax, Bcl-2 associated X
protein; PARP, poly ADP-ribose polymerase; EGCG,
epigallocatechin-3-gallate. |
The apoptotic pathway is tightly regulated by pro-
and anti-apoptotic members of the Bcl-2 protein family (24). Bcl-2 is an anti-apoptotic protein
of the Bcl-2 family, whereas Bcl-2 associated X apoptosis regulator
is a pro-apoptotic protein of the same family. PARP is a
zinc-finger DNA-binding enzyme that is activated by binding to DNA
breaks. When it is activated, PARP is cleaved (25). It has been reported that EMT
affects tumor metastasis (26),
and the expression of proteins associated with EMT in the present
study changed in response to EGCG treatment, indicating that EGCG
serve a role in tumor metastasis. The expression of E-cadherin, an
epithelial protein, significantly increased with EGCG treatment
compared with untreated control cells. In contrast, the expression
of vimentin, a mesenchymal protein, significantly decreased in
response to EGCG treatment.
EGCG downregulates Hippo-TAZ signaling,
and the expression of associated upstream genes in CAL27 and SCC15
cells
In previous studies, researchers have demonstrated
that TAZ is associated with tumorigenesis-associated processes in
TSCC, including cell proliferation, survival and migration in
vitro (19,20). Therefore, whether the expression
of TAZ and its upstream signaling molecules were affected by EGCG
treatment were investigated in the present study. As expected, the
protein levels of total TAZ and p-TAZ were significantly decreased
in response to different doses of EGCG for 24 h of CAL27 cells
(Fig. 4A). To investigate whether
EGCG altered the nuclear localization of TAZ, proteins were
extracted from the nucleus and cytoplasm of cells, and the relevant
protein levels of TAZ were decreased in parallel with total TAZ
levels (Fig. 4B). Next, the
effect of EGCG on the upstream signaling pathway was determined. As
determined by the western blot assay, EGCG treatment significantly
decreased LATS1 and MOB1 protein levels compared with untreated
control cells. However, EGCG had minimal or no effect on MST1 and
SAV1 expression (Fig. 4A). Next,
total JNK and p-JNK protein levels were examined. p-JNK levels were
significantly decreased while total JNK level remained constant
(Fig. 4A) compared with untreated
control cells, consistent with the results of a previous study
(10). In order to confirm these
findings, all the aforementioned experiments were performed on
SCC15 cells, whereby Hippo-associated proteins and p-JNK protein
exhibited the same trend as CAL27 cells, indicating that EGCG
inhibited the biological behaviors of TSCC through the Hippo-TAZ
signaling pathway (Fig. 5).
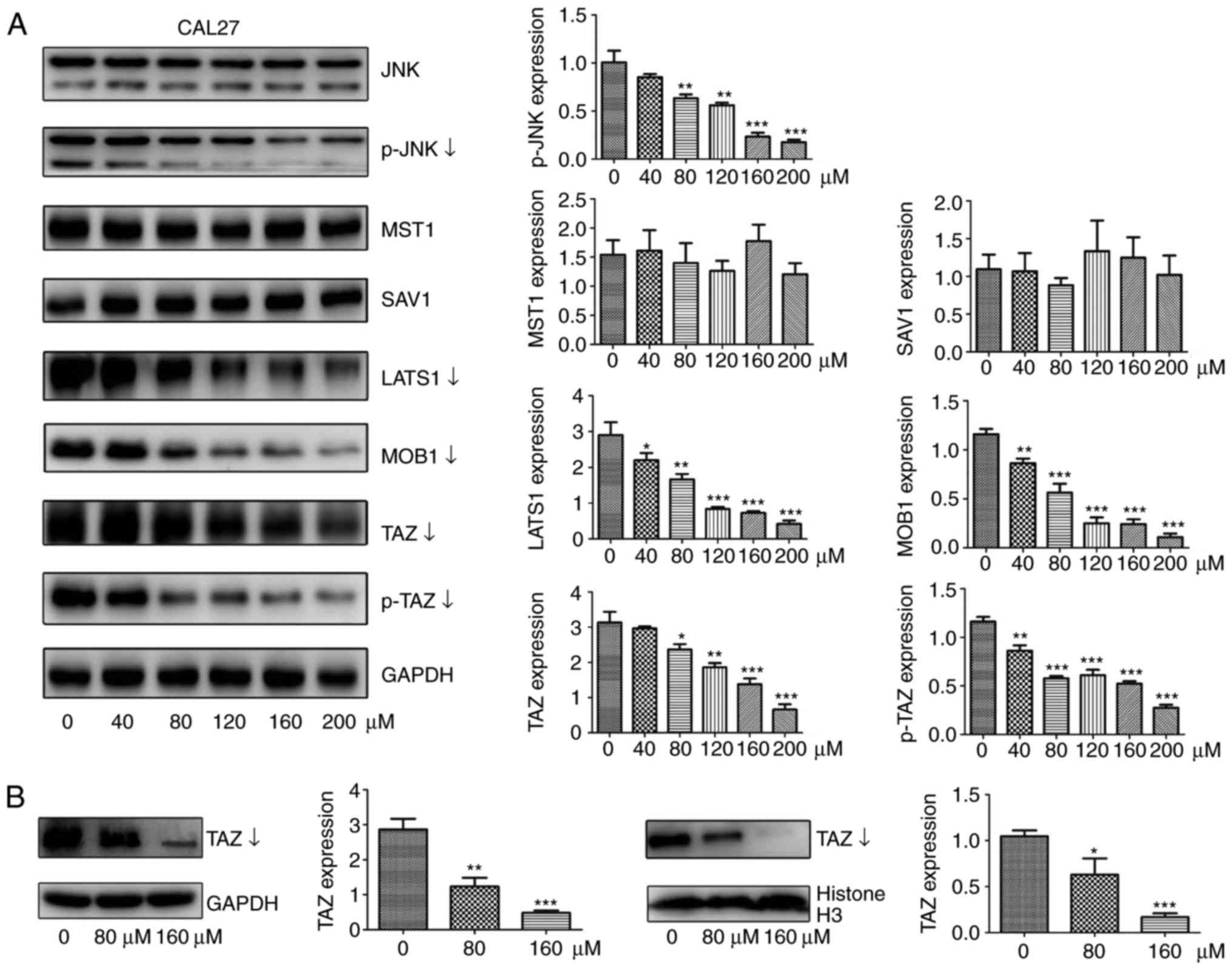 | Figure 4EGCG downregulates the protein
expression of TAZ, LATS1, MOB1 and JNK in CAL27 cells. (A) Western
blot analysis was used to measure the protein abundance of JNK,
p-JNK, p-TAZ, TAZ, LATS1, MOB1, SAV1, and MST1 in response to
treatment with different concentrations of EGCG for 24 h. (B)
Protein levels of TAZ in nuclear or cytoplasmic lysates determined
by western blotting following treatment with different doses of
EGCG for 24 h. Histone H3 and GAPDH served as internal controls for
nuclear and cytoplasmic proteins, respectively. All data are
presented as the mean ± standard deviation from three experiments.
*P<0.05, **P<0.01 and
***P<0.001, vs. the control (0 µM) group. TAZ,
tafazzin; p-TAZ, phosphorylated TAZ; LATS1, large tumor suppressor
1; MOB1, MOB kinase activator 1; SAV1, salvador 1; MST1, mammalian
sterile 20-like 1; JNK, c-Jun N-terminal kinase; p-JNK,
phosphorylated JNK; EGCG, epigallocatechin-3-gallate. |
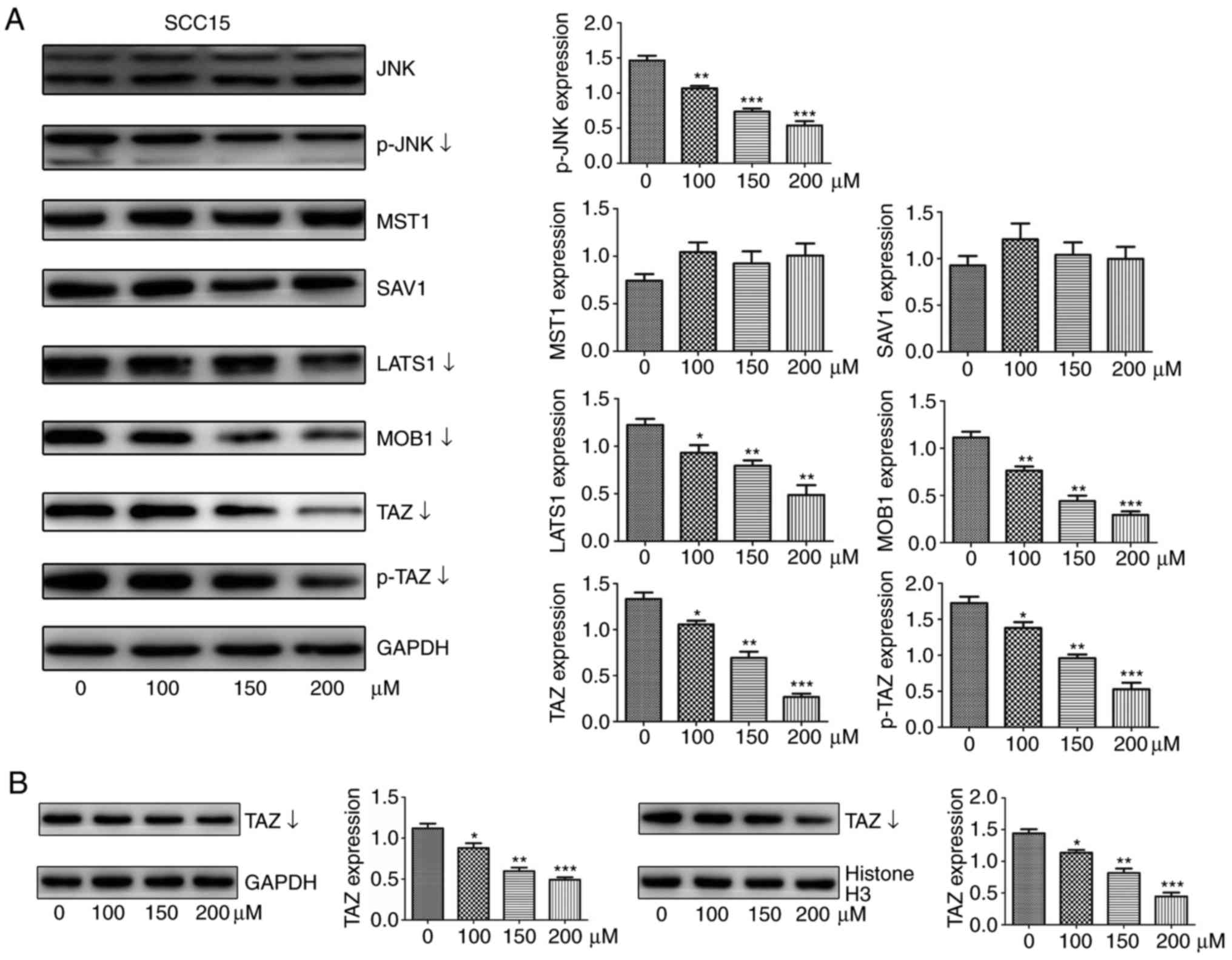 | Figure 5EGCG downregulates the protein
expression of TAZ, LATS1, MOB1 and JNK in SCC15 cells. (A) Western
blot analysis was used to measure the protein abundance of JNK,
p-JNK, p-TAZ, TAZ, LATS1, MOB1, SAV1, and MST1 in response to
treatment with different concentrations of EGCG for 24 h. (B)
Protein levels of TAZ in nuclear or cytoplasmic lysates determined
by western blotting following treatment with different doses of
EGCG for 24 h. Histone H3 and GAPDH served as internal controls for
nuclear and cytoplasmic proteins, respectively. All data are
presented as the mean ± standard deviation from three experiments.
*P<0.05, **P<0.01 and
***P<0.001, vs. the control (0 µM) group. TAZ,
tafazzin; p-TAZ, phosphorylated TAZ; LATS1, large tumor suppressor
1; MOB1, MOB kinase activator 1; SAV1, salvador 1; MST1, mammalian
sterile 20-like 1; JNK, c-Jun N-terminal kinase; p-JNK,
phosphorylated JNK; EGCG, epigallocatechin-3-gallate. |
TAZ overexpression attenuates the effects
of EGCG on CAL27 cells
To investigate whether the expression level of TAZ
influenced EGCG-treated cells, CAL27 cells were treated with TAZ
overexpression lentiviral particles or control lentiviral particles
for another 24-48 h. Western blot analysis and RT-qPCR assays
indicated the successful overexpression of TAZ (Fig. 6A and B). Treatment with EGCG
significantly inhibited proliferation, migration and invasion and
promoted apoptosis in control cells. However, TAZ overexpression
alleviated the effects of EGCG compared with that in EGCG-treated
control cells (Fig. 6C-G). No
significant differences were observed in the protein expression
levels between the empty vector group and non-transfected cell
group, which were all stimulated with EGCG, indicating that the
transfection of control lentiviral particles exhibited little
influence on CAL27 cells. The protein levels of TAZ, Bcl-2, p-Akt
and vimentin significantly increased in TAZ-overexpression cells,
and E-cadherin protein significantly decreased compared with the
non-transfected cells and the empty vector groups, which was in
parallel with the morphological features observed (Fig. 7). Taken together, these results
revealed that TAZ overexpression, at least in part, abolished the
effects of EGCG, further demonstrating that the Hippo-TAZ pathway
serves an important role in regulating CAL27 cell proliferation and
migration in response to EGCG stimulation.
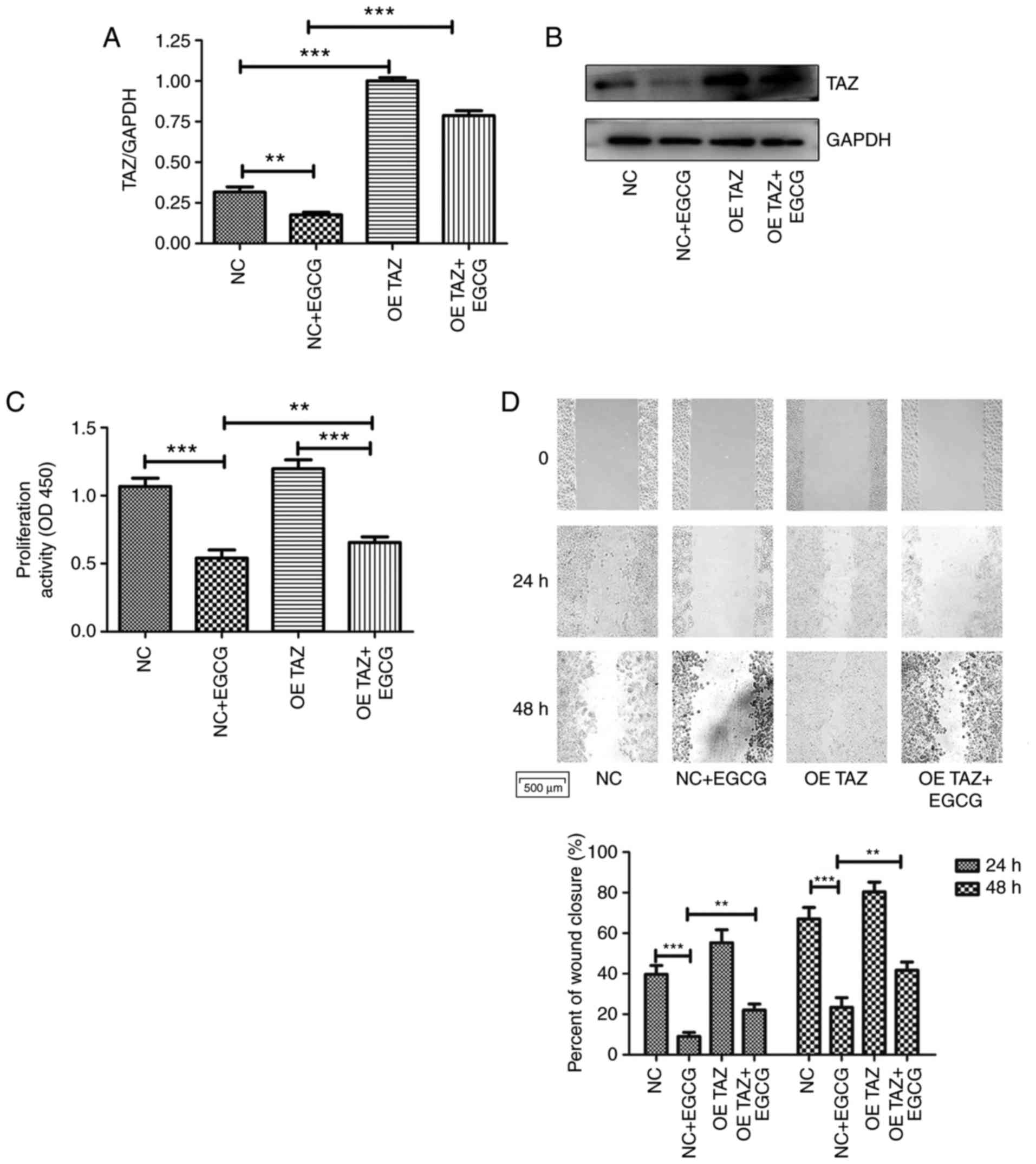 | Figure 6TAZ overexpression attenuates the
effects of EGCG on CAL27 cells on processes, including
proliferation, apoptosis, migration and invasion. (A) RT-qPCR and
(B) western blot analysis demonstrated successful TAZ
overexpression. (C) Proliferation was determined using Cell
Counting Kit-8 assay. (D) Scratch assay was performed to determine
cell migratory ability (top panel) and images of the migrating
cells were analyzed (bottom image) (magnification, ×100). (E) Cell
proliferation was also determined using an EdU assay. Images of
proliferating CAL27 cells following various treatments were
statistically analyzed (magnification, ×200). (F) Apoptosis was
analyzed by flow cytometry. (G) Invasion was evaluated using a
Transwell assay (magnification, ×200). *P<0.05,
**P<0.01 and ***P<0.001. NC, negative
control group; OE TAZ, TAZ overexpression group; TAZ, tafazzin;
EGCG, epigallocatechin-3-gallate. |
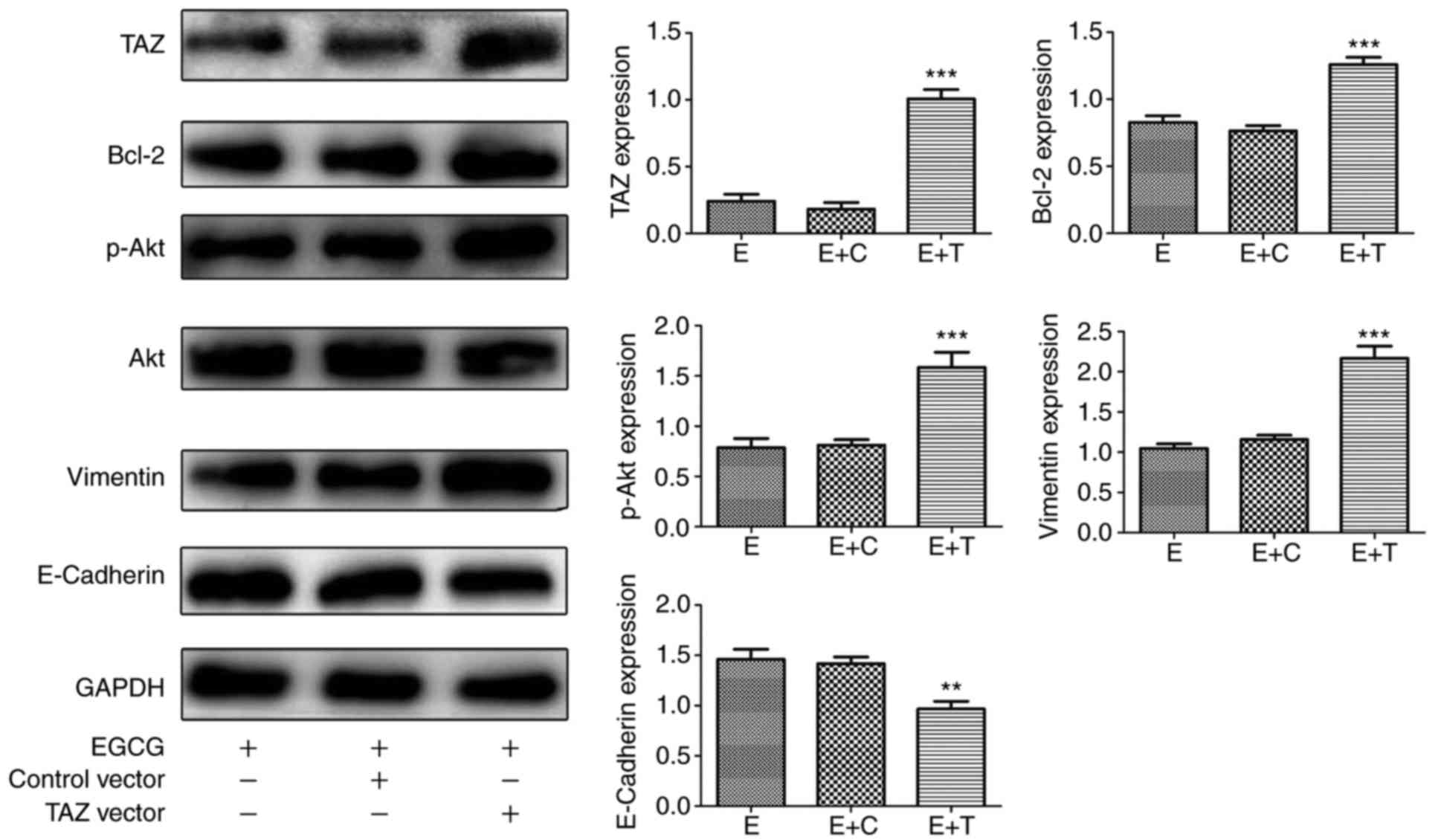 | Figure 7TAZ-overexpressing cells are more
resistant to EGCG challenge compared with negative control or
normal cells in CAL27 cells. Protein levels of TAZ, Bcl-2, Akt,
p-Akt, E-cadherin, Vimentin, and GAPDH were determined using
western blot assay. All data are presented as the mean ± standard
deviation from three experiments. **P<0.01 and
***P<0.001, vs. the EGCG group. E, EGCG; E+T,
EGCG+TAZ vector; E+C, EGCG+control vector; TAZ, tafazzin; Bcl-2, B
cell lymphoma/lewkmia-2; Akt, protein kinase B; p-Akt,
phosphorylated Akt; EGCG, epigallocatechin-3-gallate. |
EGCG and simvastatin additively inhibit
proliferation, migration and invasion, and promote apoptosis in
CAL27 cells
Simvastatin, which has been identified as the
inhibitor of TAZ, was used to treat CAL27 cells stimulated with
EGCG (20). To further determine
the additive effects of EGCG and simvastatin, CCK-8 assays, flow
cytometry, scratch and Transwell assays were used to examine the
corresponding changes in the proliferative, apoptotic, migrative
and invasive abilities of CAL27 cells (Fig. 8). Simvastatin decreased the TAZ
protein level in a dose manner, particularly at 20 µM, thus
20 µM was selected as the optional drug concentration
(Fig. 8A). It was demonstrated
that 80 µM EGCG and 20 µM simvastatin treatment
separately inhibited the proliferation, migration and invasion of
CAL27 cells, but more significant suppressive changes were detected
when cells were treated with a combination of EGCG and simvastatin
(Fig. 8B, C and E). In addition,
80 µM EGCG and 20 µM simvastatin promoted cell
apoptosis to varying degrees, the simulative effect was more
significant when cells were exposed to both drugs compared with
single treatment (Fig. 8D).
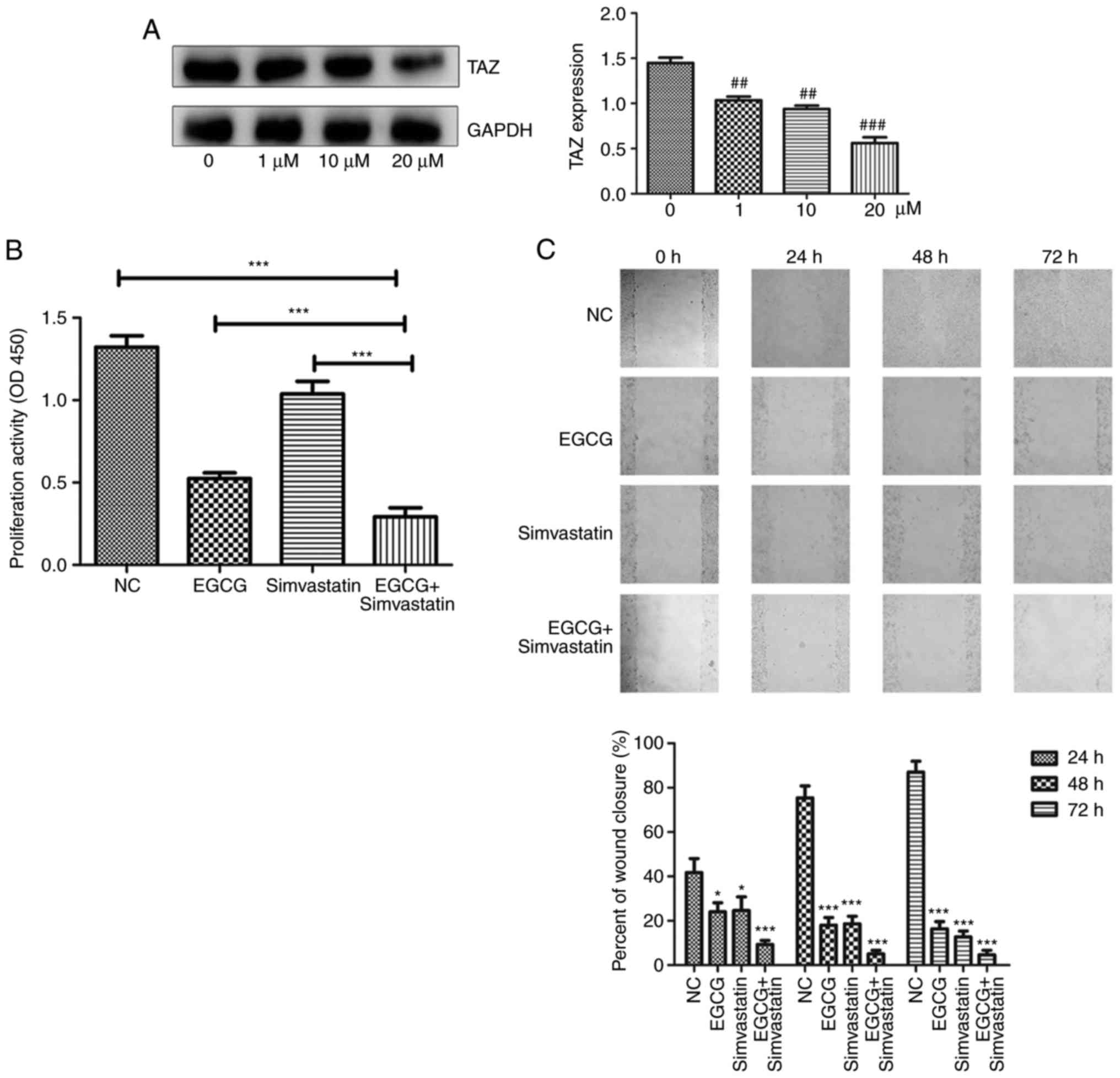 | Figure 8EGCG and simvastatin additively
inhibit proliferation, migration and invasion, and increase
apoptosis in CAL27 cells under different conditions. (A)
Simvastatin inhibited the protein expression of TAZ in a
dose-dependent manner. ##P<0.01 and
###P<0.001, vs. the control (0 µM) group. (B)
Cell proliferation was determined using a Cell Counting Kit-8
assay. (C) Scratch assay was used to detect cell migration (top
panel), and the percent of wound closure was statistically analyzed
(bottom image) (magnification, ×100). *P<0.05,
***P<0.001, vs. NC group. (D) Cells were incubated
under different conditions for 24 h and then analyzed by flow
cytometry to investigate apoptosis. (E) Transwell assay was
performed to analyze cell invasion (magnification, ×200). NC,
negative control group; TAZ, tafazzin; EGCG,
epigallocatechin-3-gallate. *P<0.05,
***P<0.001, vs. NC group. |
Discussion
Green tea is one of the most frequently and heavily
consumed beverages worldwide (8).
EGCG, as the primary active compound in green tea, accounting for
50-80% or 200-300 mg/brewed cup of green tea, has been extensively
studied for its health benefits and anti-tumor properties (7,27,28). Notably, EGCG serves an important
role in the prevention and treatment of oral cancer, likely via the
mitochondrial pathway, and exerts little toxic effects on normal
cells (29,30).
TSCC is one of the most common and malignant types
of oral cancer, and it is significantly more malignant compared
with other types of oral squamous cell carcinoma (OSCC) because the
tongue is an active organ with an abundant blood supply (31). Therefore, patient with TSCC
typically have a poor prognosis following systematic therapy
(32). At present, targeted
therapy may be a novel therapeutic approach to prevent the
occurrence and development of TSCC (9). Previous studies have demonstrated
that EGCG exerts an anti-tumor effect by suppressing the turnover
and enhancing the degradation of β-catenin (14,15). In addition, other studies have
demonstrated that EGCG treatment induces apoptosis in human OSCC
cells by suppressing hepatocyte growth factor c-Met signaling and
downstream MAPK signaling pathways, and inhibits the proliferation
of OSCC cells by upregulating Bcl-2 via p38 and Erk signaling
(10,11,13,33).
TAZ, a key effector of the Hippo signaling pathway,
is a novel oncogene with pleiotropic roles in TSCC tumorigenesis
(34,35). Notably, TAZ promotes cell
proliferation, migration and invasion, as well as EMT, and prevents
apoptosis in oral cancer (19,20,36). However, there is little research
on the association between EGCG and TAZ in TSCC cells.
In the present study, the human TSCC cell lines
CAL27 and SCC15 were used as research models, and whether EGCG
stimulation was able to inhibit the proliferation, migration and
invasion, and increase apoptosis was determined in accordance with
the results of previous studies (11,37-39). The changes in the expression of
markers of proliferation, apoptosis, invasion and migration further
confirmed the phenotypic changes upon EGCG exposure.
Mechanistically, EGCG significantly decreased the
protein levels of TAZ in a concentration-dependent manner in the
cytoplasm and nucleus of CAL27 as well as SCC15 cells.
Additionally, the protein level of p-TAZ decreased with increasing
concentrations of EGCG. The protein levels of MOB1, LATS1, SAV1 and
MST1 were also evaluated, which are relevant upstream genes of
Hippo signaling, and similar changes were observed as TAZ in MOB1
and LATS1 levels. However, EGCG had minimal or no effect on MST1
and SAV1 expression. Following hippo signal activation, MST1/2 and
SAV1 are phosphorylated, and subsequently phosphorylate LATS1/2 and
MOB1 (40). Similar to MST1/2 and
SAV1, two groups of mitogen-activated protein kinase kinase kinase
kinase (MAP4Ks), MAP4K1/2/3/5 and MAP4K4/6/7, also directly
phosphorylate LATS1/2 at their hydrophobic motifs, and promote
LATS1/2 activation (40,41). MAPK cascades classically involve
mitogen-activated protein kinase kinase kinase, mitogen-activated
protein kinase kinase, MAPK and occasionally an upstream MAP4K
(42). The MAPK signaling pathway
is known to be tightly associated with cell proliferation and
differentiation, among others, and this signaling cascade consists
of p38, JNK, ERK and ERK5. Among these effectors, researchers
reported that there may be associations between Hippo signaling and
JNK through the Ajuba family (43). When JNK is activated, it promotes
the phosphorylation of Ajuba proteins, which then bind strongly to
LATS and occupy the phosphorylation site of LATS (43). Therefore, the protein levels of
JNK and p-JNK were examined, and p-JNK was demonstrated to be
decreased in a concentration-dependent manner, while JNK level
remained invariable. These data suggest that EGCG serves an
effective role through the phosphorylation of JNK, which then
activates LATS and TAZ, but not through MST1/2 or SAV1.
Next, CAL27 cells were transfected with lentiviruses
LV5-homo-TAZ and LV5-NC, and the cells were divided into a TAZ
overexpression (OE TAZ) and a negative control (NC) groups. Indeed,
EGCG inhibited the proliferation, migration and invasion, and
increased apoptosis of the control group. However, TAZ
overexpression alleviated the effects of EGCG on CAL27 cells
compared with the EGCG-treated control group. Then, the changes in
the expression of associated proteins were examined. As expected,
the protein levels of TAZ, Bcl-2, p-Akt, E-cadherin and vimentin
also paralleled the changes in morphological features.
To further verify that EGCG affects the biological
characteristics of TSCC cells through the Hippo-TAZ pathway, a drug
that has been shown to have similar bioactivities in CAL27 cells in
a previous study was used (44).
Simvastatin is a chemical inhibitor of the enzyme HMG-CoA reductase
that catalyzes mevalonic acid production, and this effect may be
attributed to other targets beyond Hippo-TAZ (45). A previous study has reported that
simvastatin reduces TAZ protein level in a concentration-dependent
manner and that TAZ-overexpressing cells are more resistant to this
compound compared with control cells (20). Thus, simvastatin was combined with
EGCG. The combined treatment enhanced the corresponding phenotypic
changes produced by single agent treatment. These data further
demonstrate that EGCG affects the proliferation, apoptosis,
migration and invasion of TSCC cells through the Hippo-TAZ
signaling pathway.
In the present study, EGCG was demonstrated to the
affect proliferation, apoptosis, migration and invasion of TSCC
cells through the Hippo-TAZ signaling pathway. These results
provide a molecular basis for the use of green tea as a potential
chemotherapeutic agent in TSCC and offer more opportunities for
molecular targeted therapy of TSCC.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81300885),
the Shandong Provincial key research and development program (grant
nos. 2016GSF201115 and 2017GSF18117), the Shandong Provincial
National Science Foundation (grant no. ZR2018MH018), the China
Postdoctoral Science Foundation (grant no. 2017M610432), the Young
Scholars Program of Shandong University (grant no. 2015WLJH53), and
the Construction Engineering Special Fund of Taishan Scholars
(grant no. ts201511106).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KG and XC designed the experiments. AL performed the
experiments. AL, KG, XC, QW, XF and YW analyzed the data, and AL
wrote the manuscript. All authors were responsible for giving final
approval of the version to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
Acknowledgments
Not applicable.
Reference
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guidi A, Codecà C and Ferrari D:
Chemotherapy and immunotherapy for recurrent and metastatic head
and neck cancer: A systematic review. Med Oncol. 35:372018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haddad RI and Shin DM: Recent advances in
head and neck cancer. N Engl J Med. 359:1143–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferris RL, Blumenschein G Jr, Fayette J,
Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE,
Even C, et al: Nivolumab for recurrent squamous-cell carcinoma of
the head and neck. N Engl J Med. 375:1856–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colevas AD: Chemotherapy options for
patients with metastatic or recurrent squamous cell carcinoma of
the head and neck. J Clin Oncol. 24:2644–2652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Afzal M, Safer AM and Menon M: Green tea
polyphenols and their potential role in health and disease.
Inflammopharmacology. 23:151–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen ZM and Lin Z: Tea and human health:
Biomedical functions of tea active components and current issues. J
Zhejiang Univ Sci B. 16:87–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamakawa H, Nakashiro K, Sumida T,
Shintani S, Myers JN, Takes RP, Rinaldo A and Ferlito A: Basic
evidence of molecular targeted therapy for oral cancer and salivary
gland cancer. Head Neck. 30:800–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang CM, Chang PY, Tu MG, Lu CC, Kuo SC,
Amagaya S, Lee CY, Jao HY, Chen MY and Yang JS: Epigallocatechin
gallate sensitizes CAL-27 human oral squamous cell carcinoma cells
to the anti-metastatic effects of gefitinib (Iressa) via
synergistic suppression of epidermal growth factor receptor and
matrix metalloproteinase-2. Oncol Rep. 28:1799–1807. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irimie AI, Braicu C, Zanoaga O, Pileczki
V, Gherman C, Berindan-Neagoe I and Campian RS:
Epigallocatechin-3-gallate suppresses cell proliferation and
promotes apoptosis and autophagy in oral cancer SSC-4 cells. Onco
Targets Ther. 8:461–470. 2015.PubMed/NCBI
|
|
12
|
Kanlaya R, Khamchun S, Kapincharanon C and
Thongboonkerd V: Protective effect of epigallocatechin-3-gallate
(EGCG) via Nrf2 pathway against oxalate-induced epithelial
mesenchymal transition (EMT) of renal tubular cells. Sci Rep.
6:302332016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JC, Chung LC, Chen YJ, Feng TH, Chen
WT and Juang HH: Upregulation of B-cell translocation gene 2 by
epigallocatechin-3-gallate via p38 and ERK signaling blocks cell
proliferation in human oral squamous cell carcinoma cells. Cancer
Lett. 360:310–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oh S, Gwak J, Park S and Yang CS: Green
tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting
GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation.
Biofactors. 40:586–595. 2014. View Article : Google Scholar
|
|
15
|
Shin YS, Kang SU, Park JK, Kim YE, Kim YS,
Baek SJ, Lee SH and Kim CH: Anti-cancer effect of
(-)-epigallocatechin-3-gallate (EGCG) in head and neck cancer
through repression of transactivation and enhanced degradation of
β-catenin. Phytomedicine. 23:1344–1355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masuda M, Wakasaki T, Toh S, Shimizu M and
Adachi S: Chemoprevention of head and neck cancer by green tea
extract: EGCG-The role of EGFR signaling and 'lipid raft'. J Oncol.
2011:5401482011. View Article : Google Scholar
|
|
17
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hiemer SE, Zhang L, Kartha VK, Packer TS,
Almershed M, Noonan V, Kukuruzinska M, Bais MV, Monti S and Varelas
X: A YAP/TAZ-regulated molecular signature is associated with oral
squamous cell carcinoma. Mol Cancer Res. 13:957–968. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang
W, Qi B, Qiu J, Song X, pagesYe J, et al: The Hippo transducer TAZ
promotes epithelial to mesenchymal transition and cancer stem cell
maintenance in oral cancer. Mol Oncol. 9:1091–1105. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PcR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
22
|
Lim HJ, Crowe P and Yang JL: Current
clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment
of human cancer. J Cancer Res Clin Oncol. 141:671–689. 2015.
View Article : Google Scholar
|
|
23
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Curtin NJ: PARP inhibitors for cancer
therapy In: Poly(ADP-Ribosyl)ation Molecular Biology Intelligence
Unit. Springer; Boston, MA: pp. 218–233. 2006
|
|
26
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Oncol. 4:62015.
|
|
27
|
Fujiki H, Sueoka E, Watanabe T and
Suganuma M: Synergistic enhancement of anticancer effects on
numerous human cancer cell lines treated with the combination of
EGCG, other green tea catechins, and anticancer compounds. J Cancer
Res Clin Oncol. 141:1511–1522. 2015. View Article : Google Scholar
|
|
28
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramshankar V and Krishnamurthy A:
Chemoprevention of oral cancer: Green tea experience. J Nat Sci
Biol Med. 5:3–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tao L, Park JY and Lambert JD: The
differential pro-oxidative effects of the green tea polyphenol,
(−)-epigallocatechin-3-gallate, in normal and oral cancer cells are
related to differences in sirtuin 3 signaling. Mol Nutr Food Res.
59:203–211. 2015. View Article : Google Scholar
|
|
31
|
Martinez VD, Garnis C and Wan LL: Oral
cancer In: Encyclopedia of Cancer. Schwab M: Springer; Berlin: pp.
3243–3245. 2017
|
|
32
|
Schwam ZG and Judson BL: Improved
prognosis for patients with oral cavity squamous cell carcinoma:
Analysis of the National Cancer Database 1998–2006. Oral Oncol.
52:45–51. 2016. View Article : Google Scholar
|
|
33
|
Chen LL, Han WF, Geng Y and Su JS: A
genome-wide study of DNA methylation modified by
epigallocatechin-3-gallate in the CAL-27 cell line. Mol Med Rep.
12:5886–5890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong JH, Hwang ES, Mcmanus MT, Amsterdam
A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp
PA, et al: TAZ, a transcriptional modulator of mesenchymal stem
cell differentiation. Science. 309:1074–1078. 2005. View Article : Google Scholar
|
|
35
|
Wei Z, Wang Y, Li Z, Yuan C, Zhang W, Wang
D, Ye J, Jiang H, Wu Y and Cheng J: Overexpression of Hippo pathway
effector TAZ in tongue squamous cell carcinoma: Correlation with
clini-copathological features and patients' prognosis. J Oral
Pathol Med. 42:747–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu C, Huang W and Lei Q: Regulation and
function of the TAZ transcription co-activator. Int J Biochem Mol
Biol. 2:247–256. 2011.PubMed/NCBI
|
|
37
|
Ren QP, Fang YM, Zhu XH and Wang JX:
Effects of EGCG on the proliferation and apoptosis of human oral
squamous cell carcinoma Tca8113 cell line. J Oral Sci. 28:405–408.
2012.In Chinese.
|
|
38
|
Sakagami H: Apoptosis-inducing activity
and tumor-specificity of antitumor agents against oral squamous
cell carcinoma. Jpn Dent Sci Rev. 46:173–187. 2010. View Article : Google Scholar
|
|
39
|
Hwang YS, Park KK and Chung WY:
Epigallocatechin-3 gallate inhibits cancer invasion by repressing
functional invadopodia formation in oral squamous cell carcinoma.
Eur J Pharmacol. 715:286–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng Z, Moroishi T and Guan KL: Mechanisms
of Hippo pathway regulation. Genes Dev. 30:1–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meng Z, Moroishi T, Mottier-Pavie V,
Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al:
MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2
in the Hippo pathway. Nat Commun. 6:83572015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Champion A, Picaud A and Henry Y:
Reassessing the MAP3K and MAP4K relationships. Trends Plant Sci.
9:123–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun G and Irvine KD: Ajuba family proteins
link JNK to Hippo signaling. Sci Signal. 6:ra812013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang SS, Chen YH, Chen N, Wang LJ, Chen
DX, Weng HL, Dooley S and Ding HG: Hydrogen sulfide promotes
autophagy of hepatocellular carcinoma cells through the
PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 8:e26882017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stine JE, Hui G, Sheng X, Han X,
Schointuch MN, Gilliam TP, Gehrig PA, Zhou C and Bae-Jump VL: The
HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic
and anti-tumorigenic effects in ovarian cancer. Oncotarget.
7:946–960. 2016. View Article : Google Scholar :
|