Introduction
Keratoconus (KC) is the leading cause of eye
blindness. Typically, KC begins in adolescence and progresses until
the patient is aged 30-40 years, when it usually arrests (1). KC is characterized by the conical
protrusion of the cornea and is accompanied by corneal stromal
thinning (2). Patients with KC
have anomalous corneal astigmatism and myopia, which can result in
progressive visual impairment and may necessitate corneal
transplantation in order to maintain or improve vision (1). Corneal transplantation is expensive
and donated corneas are scarce. To date, there have been no
investigations of drugs for KC. Therefore, there is a pressing need
to identify effective drugs to maintain normal function of the
cornea and to stop or slow down the development of KC.
There is currently no therapeutic target associated
with the disease mechanism of KC, as the pathology remains to be
fully elucidated. Certain factors are associated with the
pathogenesis of KC, including environmental and genetic factors
(3,4), oxidative stress and cytokine
signaling. Numerous studies have demonstrated the effect of
oxidative stress on the pathogenesis of KC disease, and oxidative
damage occurs in this corneal disorder (5-8).
Oxidative stress is a sign of reactive oxygen species (ROS)
accumulation, and cultured KC fibroblasts exhibit increased ROS
generation compared with normal cultures (7,8).
Therefore, there has been considerable interest in the potential of
developing antioxidative therapies for KC-associated damage.
Nuclear factor E2-related factor 2 (Nrf-2) is key
role in antioxidant/electrophile response element
(ARE/EpRE)-regulated gene expression (9), via binding to ARE/EpRE and
trans-activating downstream target genes. ARE/EpRE-regulated phase
II detoxifying antioxidants and enzymes, including heme oxygenase-1
(HO-1), are important antioxidants involved in regulating elevated
oxidative stress and maintaining the redox status in numerous
biological settings (10,11). Nrf-2 is sequestered in the cytosol
by Kelch-like ECH-associated protein 1 (Keap1) and is targeted for
proteasomal degradation under physiological conditions (9,12).
Under oxidative stress conditions, Nrf-2 is dissociated from Keap1,
constitutively accumulates in the nucleus and stimulates the
transcription of cytoprotective genes encoding antioxidant enzymes,
including the stress response protein HO-1 (9,12).
The Nrf-2 activator sulforaphane (SF), as a potent
inducer of phase II enzymes, is a natural compound derived from
cruciferous vegetables, particularly broccoli (13). It has been demonstrated that SF
may have a neuroprotective function in several experimental animal
models (14-16). Numerous pathological features can
be manifestations of ROS-associated functional defects. Antioxidant
exposure has a favorable effect on the Nrf-2/ARE signaling pathway
in several types of tissues and cells. It is well established that
SF has protective effects that lead to an increase in the
expression of numerous antioxidant proteins (17). SF promotes the translation of
cytoprotective proteins by activating the Keap1/Nrf-2/ARE pathway
and has a catalytic effect. It has also been reported that other
antioxidative compounds increase antioxidant gene expression and
downregulate ROS-generating oxidase gene expression in KC stromal
cells (18). The present study
aimed to investigate whether the treatment of KC keratocytes with
the antioxidant SF advantageously alters the protein expression of
molecules involved in ROS-associated pathways (endogenous
antioxidant proteins and ROS-synthesizing oxidases). As the
Nrf-2/ARE axis is being considered for therapeutic drug
identification (19), the data
presented in the present highlight the importance of monitoring the
pharmacological actions of Nrf-2 activators (SF) in conditions that
simulate the pathological states associated with KC.
Materials and methods
Animals
A total of 56 female New Zealand white rabbits, 6-7
months old, weighing 3.0-3.5 kg, were used in the present study.
The animals were obtained from Beijing FYY Laboratory Animal Co.,
Ltd. (Beijing, China; SCXK 2014-0012). The rabbits were housed in a
controlled environment with a 12-h light/dark cycle. Food and water
were available ad libitum. Continuous clinical care (24 h
per day/7 days per week) was provided throughout the investigation
to ensure timely intervention when required. The experimental
protocol was approved by the Ethical Committee of Peking University
First Hospital (Beijing, China). All animals used in the present
study were treated in accordance with the Association for Research
in Vision and Ophthalmology Statement for the Use of Animals in
Ophthalmic and Vision Research (https://www.arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/).
Animal model of KC
Briefly, collagenase type II (Worthington
Biochemical Corporation, Lakewood, NJ, USA) was obtained in powder
form and dissolved in balanced salt solution with 15% dextran
(Adamas Reagent Co., Ltd., Shanghai, China) to a final
concentration of 5 mg/ml. The rabbits were anesthetized
intravenously with 0.6 ml/kg of 5% sodium pentobarbital (30 mg/kg).
Topical anesthesia using 0.4% oxybuprocaine hydrochloride eye drops
was applied to the eyes. Following epithelial debridement, corneal
trephines were placed on the cornea. In the right eye, 200
µl of 5 mg/ml collagenase type II solution was transferred
into the corneal trephines, and the cornea was immersed in
collagenase type II solution at room temperature (24°C) for 30 min
(20). The solution was then
removed with cotton swabs, and the cornea was rinsed with 0.9%
sodium chloride solution. The right eyes were treated as the
experimental eyes throughout the experiment. The left eyes did not
undergo any treatment. Prior to surgery, the rabbit eyes underwent
slit-lamp examinations, which were repeated every day during the
14-day study.
Experimental design
At 24 h following induction of the KC model, SF
(D,L-sulforaphane, 5 mg/ml, Sigma-Aldrich; Merck KGaA, Darmstadt
Germany; dissolved in maize oil) (21) or maize oil (placebo) was
administered via a subconjunctival (s.c.) injection daily for a
total of 2 weeks until the animals were sacrificed. As an HO-1
inhibitor, zinc (II) protoporphyrin IX (ZnPP IX, 5 mg/ml;
Sigma-Aldrich; Merck KGaA), was administered 24 h following
application of collagenase type II solution via s.c. injection in
combination with SF. The ZnPP IX was dissolved in 0.1 mol/l NaOH (1
ml). The pH of the solution was adjusted to 7.4 using 1 mol/l HCl,
and then diluted to the final concentration required using 0.9%
NaCl (22,23). All drug treatments were
administered at a fixed time each day. A total of 56 rabbits were
divided randomly into four experimental groups (n=14 per group):
Sham-operated group (eyes subjected to the same protocol as model
rabbits, but the applied solution lacked collagenase type II;
Control); placebo group (rabbits were injected s.c. with maize oil
24 h following corneal KC model establishment; KC); SF-treated
group (rabbits were injected s.c. with SF 24 h following corneal KC
model establishment; KC + SF); the ZnPP-treated group (SF and ZnPP
IX were injected s.c. 24 h following corneal KC model
establishment; KC + SF + ZnPP). The rabbits were sacrificed by
administering an overdose of pentobarbital sodium 14 days following
establishment of the corneal KC model.
Corneal keratometry (Km)
Km was performed on the day prior to surgery and 14
days following surgery using a handheld keratometer (Suowei;
Tianjin Suowei, Tianjin, China). Eight measurements were recorded
at each time point, and the mean Km was recorded in diopters (D) in
all experimental groups.
Corneal pachymetry
Central cornea thickness (CCT) was recorded on the
day prior to surgery and 14 days following surgery using a handheld
pachymeter (PachPen; Acctome Ultrasound, Malvern, PA, USA) under
topical anesthesia. Six measurements were recorded at each time
point and the mean CCT (µm) was recorded in all experimental
groups.
Detection of ROS production
ROS production was evaluated based on
dihydroethidium (DHE; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) measurements, as previously described (24). Following removal of the eyeball,
the fresh cornea was harvested and immediately flash-frozen in
liquid nitrogen (n=4 per group). The frozen sections (10-µm
thick) were washed three times with 0.01 M PBS (pH 7.2-7.4) and
then incubated with 5 mM DHE dissolved in PBS solution for 30 min
at room temperature. DHE specifically reacted with superoxide anion
free radicals and was transformed into red fluorescent compounds.
The sections were observed and images were captured with a
fluorescence microscope (Eclipse Ci-E; Nikon Corporation, Tokyo,
Japan) under the same exposure conditions, and the average optical
density of the fluorescent dye in the corneal stroma layer was
measured using randomly selected images. Three fields of view were
observed per animal. The fluorescence intensities of the DHE-tagged
cells were quantified with Adobe Photoshop CS5 (Adobe Systems,
Inc., Beijing, China).
Immunohistochemical staining
The rabbits were sacrificed by intravenous injection
with an overdose of pentobarbital sodium on day 14 (n=4 per group).
The eyeballs were then removed and post-fixed with 4%
paraformaldehyde overnight, processed into paraffin wax, cut into
5-µm thick sections and placed on glass slides. The sections
were dehydrated at 37°C overnight, dewaxed and then rehydrated.
Hematoxylin and eosin (H&E) staining was performed to detect
changes in corneal tissue structure in the four experimental
groups. Hydrogen peroxide (3%) was used to block endogenous
peroxidase for 20 min, and the sections were then heated to promote
antigen repair in pH 6.0 citrate buffer. The sections were then
placed into cold water prior to immunostaining. Following 30 min of
incubation with normal serum (I5506-100MG; Sigma-Aldrich; Merck
KGaA), the sections were incubated with primary antibodies at 4°C
for 24 h. The primary antibodies were as follows: Rabbit polyclonal
anti-NADPH oxidase (Nox)-2 (1:200; cat. no. ab80508; Abcam,
Cambridge, MA, USA), rabbit polyclonal anti-Nox-4 (1:200; cat. no.
NB110-58849; Novus Biologicals, LLC, Littleton, CO, USA), rabbit
polyclonal anti-Nrf-2 (1:500; cat. no. bs-1074R; Bioss, Beijing,
China) or rabbit polyclonal anti-HO-1 (1:100; cat. no.
ADI-SPA-895-F, Enzo Life Sciences, Inc., Farmingdale, NY, USA). The
immunoreaction was localized using a 2-step Plus Poly-HRP
Anti-Mouse/Rabbit IgG Detection system (PV-9000, OriGene
Technologies, Inc., Beijing, China). HRP activity was shown via
dipping the sections in a compound containing 3,3′-diaminobenzidine
(DAB) chromogen and DAB substrate (ZLI-9018, OriGene Technologies,
Inc.) at room temperature for 5 min. The sections were mounted,
air-dried, dehydrated and cover-slipped. No positive signals were
detected in any negative control samples. Images of the stained
sections were captured using an Olympus optical microscope (Olympus
Corporation, Tokyo, Japan).
Western blot analysis
The rabbits were sacrificed by intravenous injection
of an overdose of sodium pentobarbital on day 14 (n=3 per group).
Briefly, the cornea was separated and frozen at −80°C within 2 min
of enucleation. The cornea was then sonicated on ice with lysis
buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
protein concentration of the extracts was detected (n=3 per group)
using a bicinchoninic acid protein quantification kit (cat. no.
P1511, Applygen Technologies, Inc., Beijing, China). Equal
quantities of protein (20 µg/channel) were resolved by 10%
or 12% SDS-PAGE. The proteins were then electro-phoretically
transferred onto Immun-Blot PVDF membranes (cat. no. 162-0177,
Bio-Rad Laboratories, Inc., Hercules, CA, USA), which were blocked
with 5% non-fat milk and then incubated with the following
antibodies overnight at 4°C: Rabbit polyclonal anti-Nox-2 (1:1,000;
cat. no. ab80508), rabbit polyclonal anti-Nox-4 (1:500; cat. no.
NB110-58849); rabbit polyclonal anti-Nrf-2 (1:500; cat. no.
bs-1074R), rabbit polyclonal anti-HO-1 (1:500; cat. no.
ADI-SPA-895-F) and mouse monoclonal antibody against β-actin
(1:2,000; cat. no. A1978, Sigma-Aldrich; Merck KGaA). Then, the
membranes were washed and incubated with a Peroxidase-AffiniPure
Goat Anti-Mouse IgG (H+L) (1:2,000; cat. no. 115-035-003; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) and a
peroxidase-conjugated donkey anti-rabbit IgG (H+L) (1:2,000; cat.
no. 711-035-152; Jackson ImmunoResearch Laboratories, Inc.) for 1 h
at room temperature. The protein bands were visualized and analyzed
using Super ECL hypersensitive luminescent solution (cat. no.
P1020; Applygen Technologies, Inc.) and a gel image analysis system
(GBOX-CHEMI-XT4; Synoptics Ltd., Cambridge, UK) according to the
manufacturer's protocol. The immunoblot experiments were repeated
at least three times independently for quantification (n=3 per
group) and ImageJ software (version 1.4.3; National Institutes of
Health, Bethesda, MD, USA) was used for analysis.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The mRNA expression of all samples was evaluated by
RT-qPCR analysis as previously described (25,26). Briefly, total RNA was extracted
using the RNA Mini extraction kit (cat. no. 12183555; Thermo Fisher
Scientific, Inc.). The purity and concentration of RNA were
quantified using a Nanodrop Spectrophotometer ND-1000 (n=3 per
group). cDNA synthesis was performed using a PrimeScript RT reagent
kit (cat. no. RR047A; Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's protocol, with an ARKTIK
Thermal Cycler (Thermo Fisher Scientific, Inc.). qPCR was performed
using SYBR Premix Ex Taq II (cat. no. RR820A; Takara Biotechnology
Co., Ltd.). PCR was performed with 10 ng cDNA in a 20-µl
reaction system (2 µl cDNA, 0.8 µl 10 µM
forward primer, 0.8 µl 10 µM reverse primer, 10
µl PCR mix buffer, and 6.4 µl sterilized distilled
water) using a PikoReal 96 Real-Time PCR system (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The PCR
conditions were as follows: 2 min of 50°C, 30 sec at 94°C and 40
cycles of 5 sec at 94°C and 34 sec at 60°C. The primers used are
listed in Table I (AuGCT DNA-SYN
Biotechnology Co., Ltd., Beijing, China). The relative mRNA
expression was normalized against the expression of β-actin and
calculated using the 2−ΔΔCq method (26). By analyzing the melting curve, the
property and purity of the amplified products were determined. All
experiments were repeated at least three times.
 | Table IGene markers and corresponding
primers. |
Table I
Gene markers and corresponding
primers.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| Nox-2 |
TGTGAATGCCCGAGTCAACA |
AACCGCGTTACAGCCACTAA |
| Nox-4 C |
TAGAGGGCGGTGCTTTACC |
GCCACCAGTGCTGGACATAG |
| Nrf-2 |
ACACAGGTGAATTCGGAAGACAGAG |
GCATAGCAGAGAGCTGGATCAGAAG |
| HO-1 |
TGAACTCCCTGGAGATGACC |
GGTGGAGTCTTGGGTCCTG |
| β-actin C |
ATCCACGAGACCACCTTCAACT |
GATGATCTTGATCTTCATGGTGCTG |
Statistical analysis
All values are expressed as the mean ± standard
deviation. Data analysis was performed using GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). The results of
multiple groups were compared using one-way analysis of variance
followed by Tukey's post hoc test with SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in corneal tissue structure among
the four experimental groups
No obvious inflammatory reaction was observed in any
of the rabbits following the procedure in the four experimental
groups (Control, KC, KC + SF, and KC + SF + ZnPP groups). The
results of the H&E staining indicated that the corneal tissue
structures of the four experimental groups were intact at day 14
post-surgery, and the cell morphology was normal (Fig. 1). The corneal endothelial cells
were lost in certain tissues due to section preparation and other
reasons. The corneal epithelium in the four groups consisted of 5-6
layers of stratified squamous epithelium, and was closely connected
with the stroma, suggesting that the epithelium had returned to
normal (Fig. 1A-D). Compared with
the Control group, the corneal stromal fibers were loosely arranged
and the gap between the collagen fibers was increased in the KC
group. Compared with the Control group, there was no marked change
in the corneal tissue structures of the KC + SF and KC + SF + ZnPP
groups (Fig. 1E-L). SF treatment
did not visibly change the corneal structure.
 | Figure 1Changes in corneal tissue structure
among the four experimental groups. H&E staining was performed
to detect changes in corneal tissue structure among the four
experimental groups. Representative micrographs of corneal sections
stained with H&E from (A) Control, (B) KC, (C) KC + SF, and (D)
KC + SF + Znpp groups with a 10X objective lens (×100
magnification; scale bar=100 µm); (E) Control, (F) KC, (G)
KC + SF, and (H) KC + SF + Znpp groups with a 20X objective lens
(×200 magnification; scale bar=50 µm); and (I) Control, (J)
KC, (K) KC + SF, and (L) KC + SF + Znpp groups with a 40X objective
lens (×400 magnification, scale bar=25 µm). Control,
sham-operated group (eyes subjected to the same protocol but the
applied solution lacked collagenase type II); KC, vehicle group
(injected s.c. with maize oil 24 h following corneal KC model
establishment); KC + SF, SF-treated group (animals were injected
s.c. with SF 24 h following corneal KC model establishment); KC +
SF + ZnPP, SF and ZnPP-treated group (SF and ZnPP IX injected s.c.
24 h following corneal KC model establishment). H&E,
hematoxylin and eosin; s.c., subconjunctivally; KC, keratoconus;
SF, sulforaphane; ZnPP IX, zinc (II) protoporphyrin IX. |
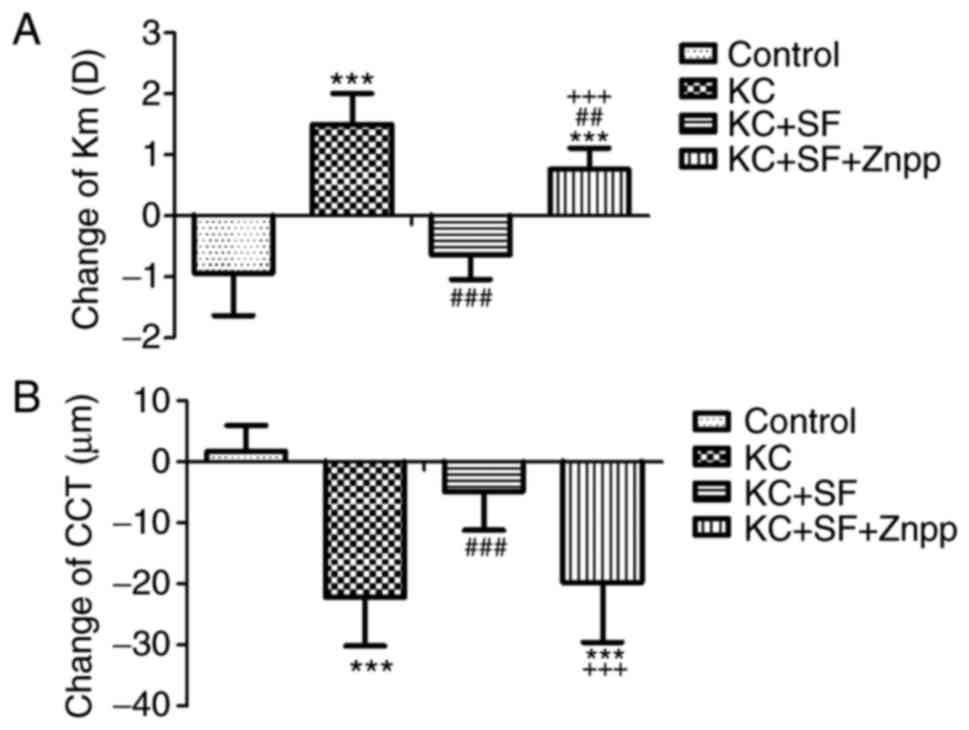
Changes in Km and CCT among the four
experimental groups
Prior to surgery, there was no marked difference in
Km among the four experimental groups (n=14 per group; Table II; 47.56±1.71, 47.55±1.60,
46.90±1.68 and 47.43±1.78 D, respectively, P=0.700) or CCT
(356.36±21.31, 353.93±24.11, 356.57±18.17 and 354.86±13.55
µm, respectively, P=0.982). Following surgery, the changes
in Km values in the four experimental groups were −0.94±0.70,
1.51±0.50, −0.64±0.40 and 0.77±0.35 D, respectively (Table II). The changes in CCT in the
four experimental groups were 1.77±4.17, −22.14±8.00, −4.86±6.34
and −19.71±9.81 µm, respectively (Table II). All values represent a change
from the pre-surgery baselines (day 14-day 0). Graphic
quantification of the changes in Km and CCT in all experimental
groups is presented in Fig. 2. Km
in the KC group was significantly increased compared with that in
the Control group (Fig. 2A;
P<0.001). Km in the KC + SF group was significantly reduced
compared with that in the KC group (Fig. 2A; P<0.001). The change in Km in
the KC + SF + ZnPP group was more marked compared with that in the
KC + SF group (Fig. 2A;
P<0.001). CCT in the KC group was significantly reduced compared
with that in the Control group (Fig.
2B; P<0.001). CCT in the KC + SF group was significantly
enhanced compared with that in the KC group (Fig. 2B; P<0.001). The change in CCT
in the KC + SF + ZnPP group was significantly = decreased compared
with that in the KC + SF group (Fig.
2B; P<0.001). The KC model exhibited a significant increase
in Km and a significant decrease in CCT, and these effects were
weakened or reversed by SF. ZnPP IX, the HO-1 inhibitor,
neutralized the protective effect of SF on the KC cornea.
 | Table IIComparison of the Km and CCT in the
four experimental groups (n=14 per group). |
Table II
Comparison of the Km and CCT in the
four experimental groups (n=14 per group).
| Factor | Group | Day 0 | Day 14 | Day 14-Day 0 |
|---|
| Km (D) | Control | 47.56±1.71 | 46.62±2.22 | −0.94±0.70 |
| KC | 47.55±1.60 | 49.06±1.59 | 1.51±0.50 |
| KC + SF | 46.90±1.68 | 47.54±1.41 | −0.64±0.40 |
| KC + SF + Znpp | 47.43±1.78 | 48.19±1.74 | 0.77±0.35 |
| P-value | 0.700 | 0.005 | <0.001 |
| CCT
(µm) | Control | 356.36±21.31 | 358.14±18.93 | 1.77±4.17 |
| KC | 353.93±24.11 | 331.79±18.10 | −22.14±8.00 |
| KC + SF | 356.57±18.17 | 351.71±16.12 | −4.86±6.34 |
| KC + SF + Znpp | 354.86±13.55 | 335.14±18.64 | −19.71±9.81 |
| P-value | 0.982 | <0.001 | <0.001 |
SF downregulates ROS generation in KC
corneas
The production of ROS in fresh corneas was measured
with DHE staining in all groups. As shown in Fig. 3, the basal level of ROS was low in
the Control group corneas (Fig.
3A). However, ROS generation was increased markedly in the KC
corneas (Fig. 3B), and this
effect was decreased with SF treatment (Fig. 3C). The production of ROS was
enhanced in the KC + SF + ZnPP group (Fig. 3D). Representative images of
corneal sections stained with DAPI were obtained from each group
(Fig. 3E-H). The merged images
with double-label immunofluorescent staining using antibodies
against DHE and DAPI are shown in Fig. 3I-L. ROS level analysis in the
stroma of the cornea was consistent with the immunofluorescent
results (Fig. 3M, n=4 per
group).
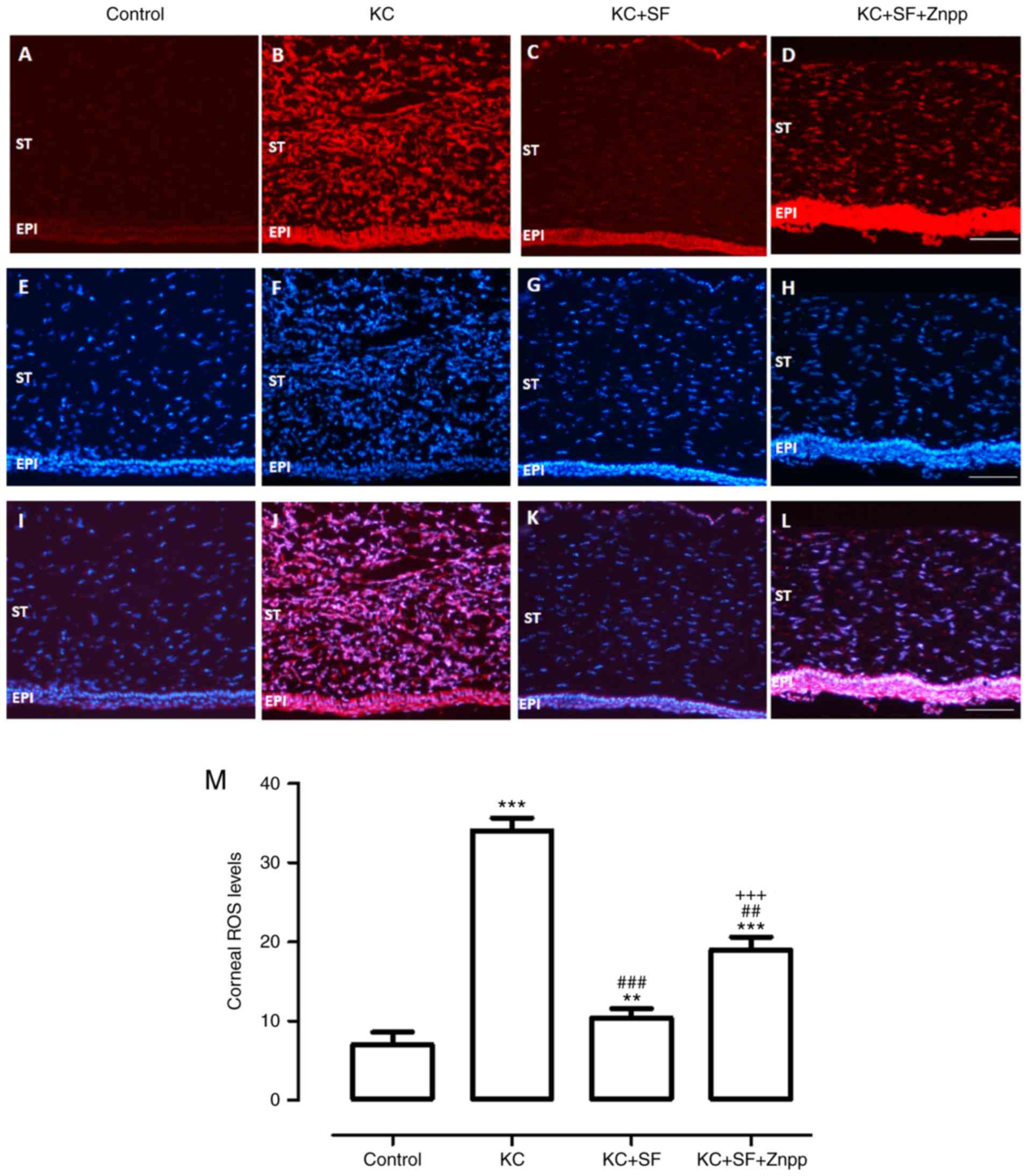 | Figure 3SF downregulates ROS generation in KC
corneas. ROS generation in fresh corneas was examined by DHE
staining. Representative micrographs of corneal sections stained
with DHE (red) in the (A) Control, (B) KC, (C) KC + SF, and (D) KC
+ SF + Znpp groups. Representative micrographs of corneal sections
stained with DAPI (blue) in the (E) Control, (F) KC, (G) KC + SF,
and (H) KC + SF + Znpp groups. Merged images of staining in the (I)
Control, (J) KC, (K) KC + SF, and (L) KC + SF + Znpp groups.
Magnification, ×200 scale bar=50 µm. (M) Quantitative
analysis of ROS levels in the entire cornea. The fluorescent
intensities of DHE-labeled cornea were quantified using ImageJ.
Data are presented as the mean ± SD; n=4 per group.
**P<0.01 and ***P<0.001, vs. Control;
###P<0.001, vs. KC group; +++P<0.001,
vs. KC+SF group. KC, keratoconus; SF, sulforaphane; ZnPP IX, zinc
(II) protoporphyrin IX; DHE, dihydro-ethidium; ROS, reactive oxygen
species; ST, corneal stroma; EPI, corneal epithelial layer. |
SF downregulates the expression levels of
Nox-2 and Nox-4 in KC corneas
The Nox family of proteins is considered to be one
of the most important ROS sources in the cornea, and Nox-2 and
Nox-4 proteins are expressed at high levels in the corneal stromal
cells of patients with KC (8,27).
Therefore, changes in the expression of Nox-2 and Nox-4 were
evaluated in the corneas of the four experimental groups (n=4 per
group). Images of the results are shown in Fig. 4A-L. The Control group had
relatively low Nox-2 immunoreactivity throughout the cornea
(Fig. 4A, E and I). In the KC
group, there was more marked Nox-2 immunoreactivity throughout the
entire cornea (Fig. 4B, F and J).
The expression of Nox-2 in the KC + SF group was reduced compared
with that in the KC group (Fig. 4C, G
and K). The expression of Nox-2 in the KC + SF + ZnPP group was
markedly enhanced compared with that in the KC + SF group (Fig. 4D, H and L). Western blot analysis
revealed that the basal level of Nox-2 in the Control group corneas
was lower than that in the KC group corneas. KC induced a
significant increase in the expression of Nox-2 in the cornea (n=3,
Fig. 4M; P<0.001). The level
of Nox-2 in the KC + SF group was significantly decreased compared
with that in the KC group (n=3, Fig.
4M; P<0.001). The level of Nox-2 in the KC + SF + ZnPP group
was significantly increased compared with that in the KC + SF group
(n=3, Fig. 4M; P<0.05). As
shown in Fig. 5, the Control
group exhibited relatively low Nox-4 immunoreactivity throughout
the cornea (n=4, Fig. 5A, E and
I). In the KC group, there was more marked Nox-4
immunoreactivity throughout the entire cornea (n=4, Fig. 5B, F and J). The expression of
Nox-4 in the KC + SF group was more markedly decreased compared
with that in the KC group (n=4, Fig.
5C, G and K). The expression of Nox-4 in the KC + SF + ZnPP
group was enhanced compared with that in the KC + SF group (n=4,
Fig. 5D, H and L). Western blot
analysis revealed that the basal level of Nox-4 in the control
cornea was lower than that in the KC cornea. KC induced a
significant increase in the expression of Nox-4 in the cornea (n=3,
Fig. 5M; P<0.001). The level
of Nox-4 in the KC + SF group was significantly reduced compared
with that in the KC group (n=3, Fig.
5M; P<0.001). The level of Nox-4 in the KC + SF + ZnPP group
was enhanced significantly compared with that in the KC + SF group
(n=3, Fig. 5M; P<0.05).
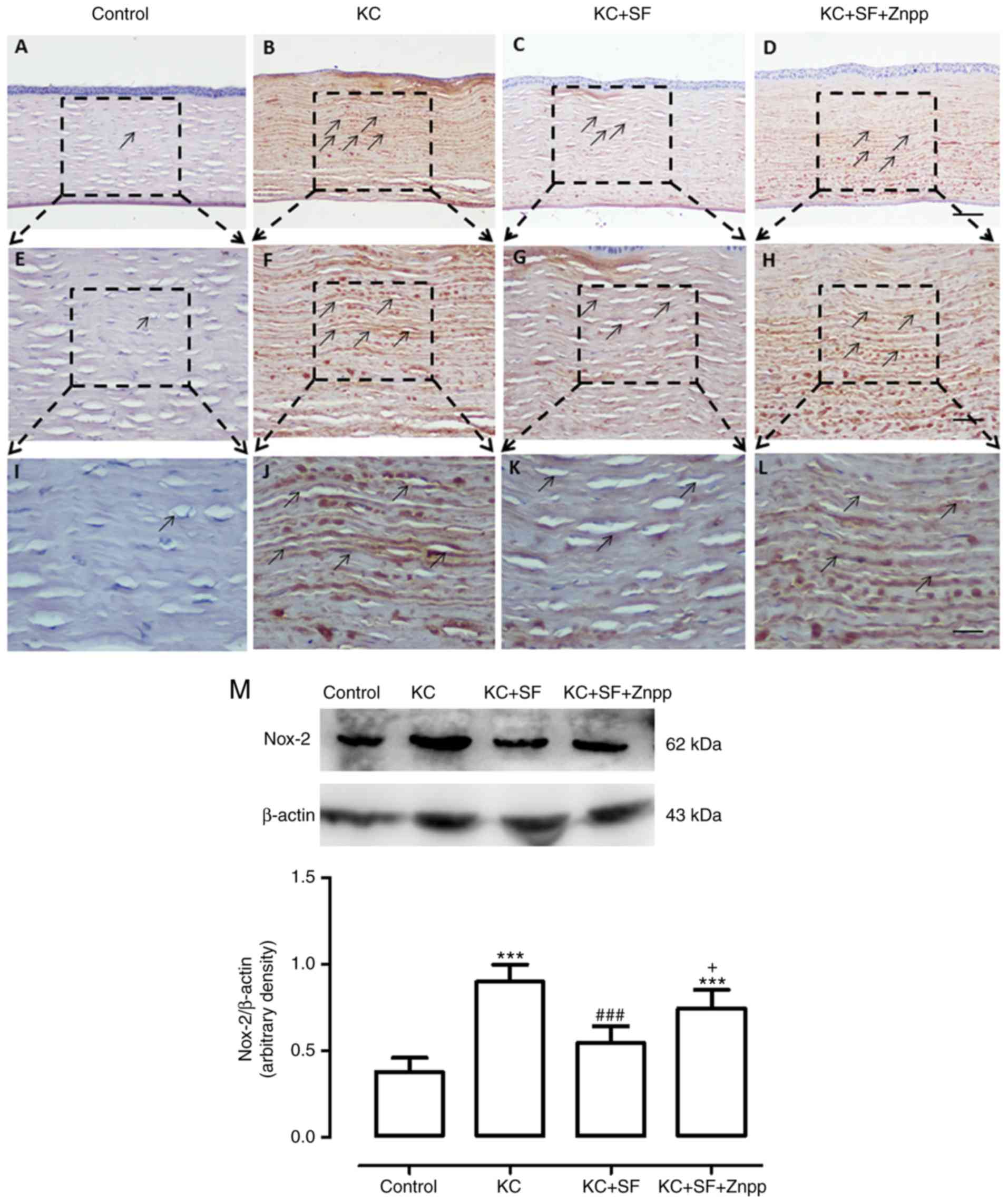 | Figure 4SF downregulates the expression level
of Nox-2 in KC corneas. Representative micrographs of corneal
sections obtained from each group stained with anti-Nox-2 antibody.
Black arrows indicate Nox-2-positive cells. (A) Control, (B) KC,
(C) KC + SF, and (D) KC + SF + Znpp groups with 10X objective lens
(×100 magnification; scale bar=100 µm. (E) Control, (F) KC,
(G) KC + SF, and (H) HKC + SF + Znpp groups with 20X objective lens
(×200 magnification; scale bar=50 µm). (I) Control, (J) KC,
(K) KC + SF, and (L) KC + SF + Znpp groups with a 40X objective
lens (×400 magnification scale bar=25 µm). (M)
Representative immunoblotting indicating Nox-2 protein levels in
the entire cornea (upper panel) and densitometric analysis of the
expression of Nox-2 relative to the loading control (lower panel).
Data are presented as the mean ± standard deviation; n=3 per group.
***P<0.001, vs. Control; ###P<0.001,
vs. KC group; +P<0.05, vs. KC + SF group. KC,
keratoconus; SF, sulforaphane; ZnPP IX, zinc (II) protoporphyrin
IX; Nox, NAPDH oxidase. |
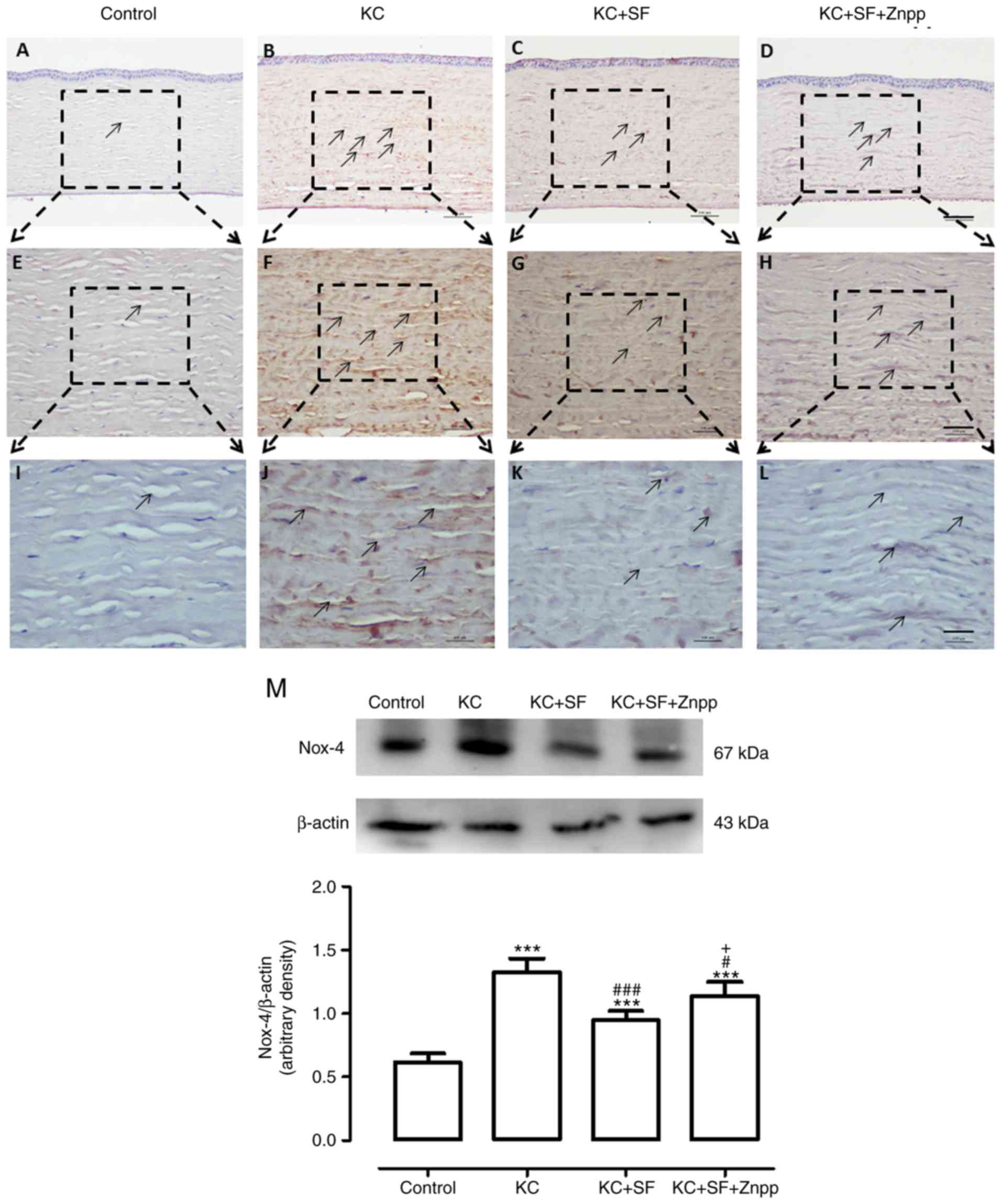 | Figure 5SF downregulates the expression level
of Nox-4 in KC corneas. (A-L) Representative micrographs of corneal
sections obtained from each group stained with anti-Nox-4 antibody.
Black arrows indicate Nox-4-positive cells. (A) Control, (B) KC,
(C) KC + SF, and (D) KC + SF + Znpp groups with 10X objective lens
(×100 magnification; scale bar=100 µm). (E) Control, (F) KC,
(G) KC + SF, and (H) KC + SF + Znpp groups with a 20X objective
lens (×200 magnification; scale bar=50 µm). (I) Control, (J)
KC, (K) KC + SF, and (L) KC + SF + Znpp groups with a 40X objective
lens (×400 magnification; scale bar=25 µm). (M)
Representative immunoblotting indicating protein levels of Nox-4 in
the entire cornea (upper panel) and densitometric analysis of the
expression of Nox-4 relative to the loading control (lower panel).
Data are presented as the mean ± standard deviation; n=3 per group.
***P<0.001, vs. Control; #P<0.05 and
###P<0.001, vs. KC group; +P<0.05, vs.
KC+SF group. KC, keratoconus; SF, sulforaphane; ZnPP IX, zinc (II)
protoporphyrin IX; Nox, NAPDH oxidase. |
SF upregulates the nuclear translocation
of Nrf-2 in keratocytes of KC corneas
Under oxidative stress, Nrf-2 translocates into the
cell nucleus and binds with ARE regions of the promoters of genes
that encode antioxidant and phase II detoxifying enzymes to weaken
cellular oxidative stress (28).
Therefore, the present study examined whether the excessive free
radicals in KC corneas were caused by the upregulation of Nrf-2.
Images of staining are shown in Fig.
6A-L, The Control group exhibited relatively low Nrf-2
immunoreactivity throughout the cornea (n=4, Fig. 6A, E and I). In the KC group, there
was higher Nrf-2 immunoreactivity throughout the entire cornea
(n=4, Fig. 6B, F and J). The
expression of Nrf-2 in the KC + SF group was increased more
markedly compared with that in the KC group (n=4, Fig. 6C, G and K). The expression of
Nrf-2 in the KC + SF + ZnPP group was lower, compared with that in
the KC + SF group (n=4, Fig. 6D, H
and L). Western blot analysis revealed that the basal level of
Nrf-2 in the Control cornea was lower than that in the KC cornea.
KC induced a significant increase in the expression of Nrf-2 in the
corneas (n=3, Fig. 6M;
P<0.01). The level of Nrf-2 in the KC + SF group was
significantly higher compared with that in the KC group (n=3,
Fig. 6M; P<0.001). The level
of Nrf-2 in the KC + SF + ZnPP group was decreased significantly
compared with that in the KC + SF group (n=3, Fig. 6M; P<0.001). SF-induced Nrf-2
pathway activation provided protection against corneal oxidative
damage. ZnPP IX, the HO-1 inhibitor, reduced the activity of Nrf-2
on the KC + SF cornea.
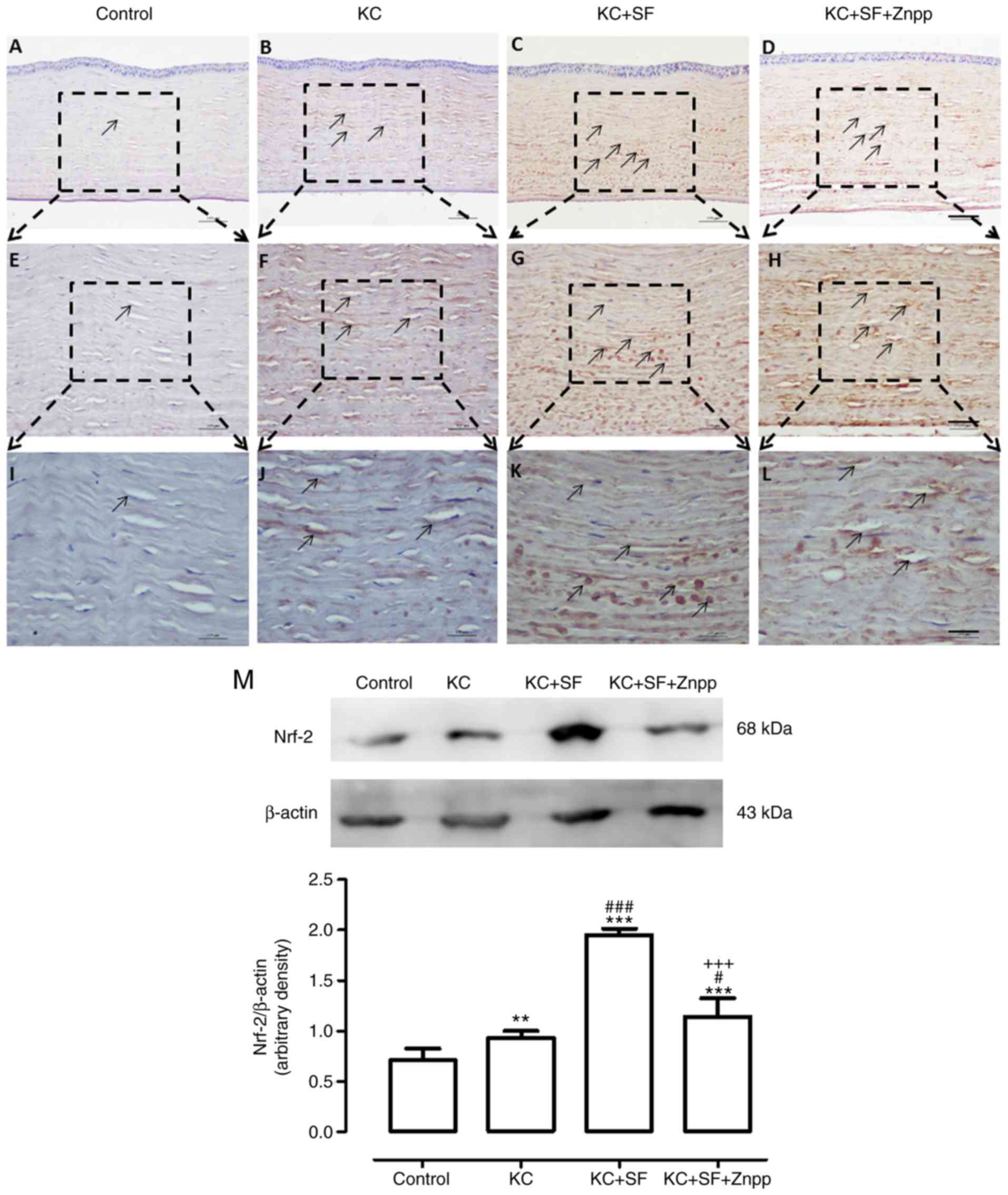 | Figure 6SF upregulates the expression level
of Nrf-2 in KC corneas. Representative micrographs of corneal
sections obtained from each group stained with anti-Nrf-2 antibody.
Black arrows indicate Nrf-2-positive cells. (A) Control, (B) KC,
(C) KC + SF, and (D) KC + SF + Znpp groups with a 10X objective
lens (×100 magnification, scale bar=100 µm). (E) Control,
(F) KC, (G) KC + SF, and (H) KC + SF + Znpp groups with a 20X
objective lens (×200 magnification; scale bar=50 µm). (I)
Control, (J) KC, (K) KC + SF, and (L) KC + SF + Znpp groups with a
40X objective lens (×400 magnification; scale bar=25 µm).
(M) Representative immunoblotting indicating protein levels of
Nrf-2 in the entire cornea (upper panel) and densitometric analysis
of the expression of Nrf-2 relative to the loading control (lower
panel). Data are presented as the mean ± standard deviation; n=3
per group. **P<0.01 and ***P<0.001 vs.
Control; #P<0.05 and ###P<0.001 vs. KC
group; +++P<0.001, vs. KC+SF group. KC, keratoconus;
SF, sulforaphane; ZnPP IX, zinc (II) protoporphyrin IX; Nrf-2,
nuclear factor E2-related factor 2. |
SF upregulates the expression of HO-1 in
KC corneas
Nrf-2 is a nuclear transcription factor that
regulates the expression of HO-1 (29). Therefore, the expression of HO-1,
a downstream signaling pathway gene of Nrf-2, was investigated by
immunohistochemical and western blot analyses in the present study.
As shown in the images in Fig.
7A-L, the Control group had weak HO-1 immunoreactivity in the
entire cornea (n=4, Fig. 7A, E and
I). In the KC group, intense HO-1 immunoreactivity was observed
in the entire cornea (n=4, Fig. 7B, F
and J). HO-1 immunoreactivity in the KC + SF group was
increased more markedly compared with that in the KC group (n=4,
Fig. 7C, G and K). HO-1
immunoreactivity in the KC + SF + ZnPP group was decreased compared
with that in the KC+SF group (n=4, Fig. 7D, H and L). Western blot analysis
revealed that the basal expression of HO-1 in the control cornea
was lower than that in the KC cornea. The expression level of HO-1
was significantly enhanced in KC corneas (n=3, Fig. 7M; P<0.01). The level of HO-1 in
the KC + SF group was significantly higher compared with that in
the KC group (n=3, Fig. 7M;
P<0.01). The level of HO-1 in the KC + SF + ZnPP group was
reduced significantly compared with that in the KC+SF group (n=3,
Fig. 7M; P<0.001). SF
stimulated and induced the protein expression of HO-1 in KC
corneas.
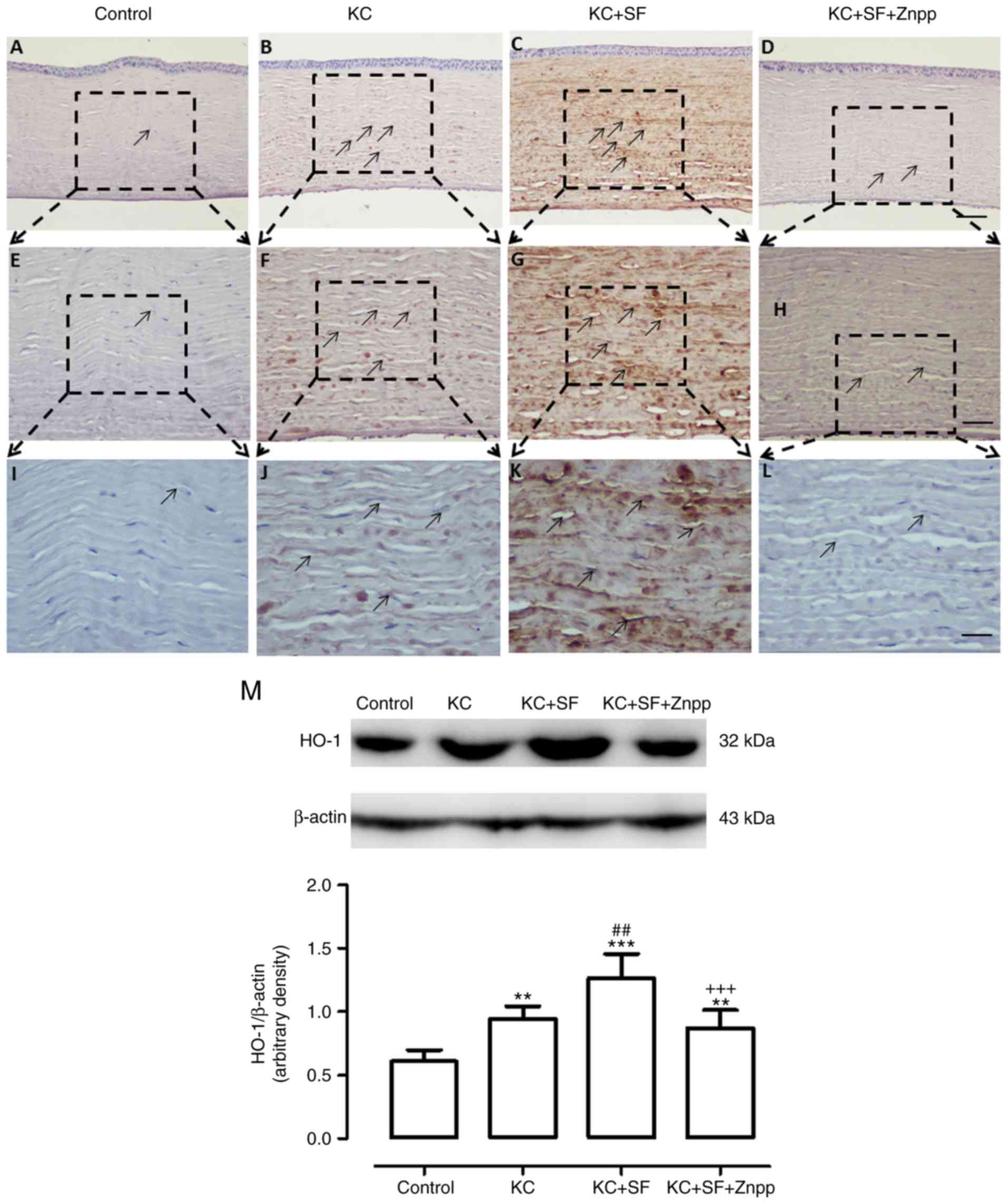 | Figure 7SF upregulates the expression level
of HO-1 in KC corneas. Representative micrographs of corneal
sections obtained from each group stained with anti-HO-1 antibody.
Black arrows indicate HO-1-positive cells. (A) Control, (B) KC, (C)
KC + SF, and (D) KC + SF + Znpp groups with a 10X objective lens
(×100 magnification; scale bar=100 µm; (E) Control, (F) KC,
(G) KC + SF, and (H) KC + SF + Znpp groups with a 20X objective
lens (×200 magnification); scale bar=50 µm). (I) Control,
(J) KC, (K) KC + SF, and (L) KC + SF + Znpp groups with a 40X
objective lens (×400 magnification; scale bar=25 µm. (M)
Representative immunoblotting indicating protein levels of HO-1 in
the entire cornea (upper panel) and densitometric analysis of the
expression of HO-1 relative to the loading control (lower panel).
Data are presented as the mean ± standard deviation; n=3 per group.
**P<0.01 and ***P<0.001, vs. Control;
##P<0.01, vs. KC group; +++P<0.001, vs.
KC+SF group. KC, keratoconus; SF, sulforaphane; ZnPP IX, zinc (II)
protoporphyrin IX; HO-1, heme oxygenase-1. |
SF decreases the mRNA levels of Nox-2 and
Nox-4, and increases the mRNA Nrf-2 and HO-1 in KC corneas
The expression levels of relevant antioxidant and
oxidation genes, including Nox-2, Nox-4, Nrf-2 and HO-1, were
evaluated in corneal stromal cells by RT-qPCR analysis in all
experimental groups. As shown in Fig.
8, the RT-qPCR results indicated that, compared with the
Control group, the mRNA expression levels of Nox-2, Nox-4, Nrf-2
and HO-1 in the KC group were significantly increased (n=3, all
P<0.05), which was consistent with the results of the western
blot analysis. The mRNA levels of Nox-2 and Nox-4 in the KC + SF
group were decreased significantly compared with those in the KC
group (n=3, Fig. 8; P<0.01).
The mRNA levels of Nrf-2 and HO-1 in the KC + SF group were
increased significantly compared with those in the KC group (n=3,
Fig. 8; P<0.01). The mRNA
levels of Nox-2 and Nox-4 in the KC + SF + ZnPP group were
increased significantly compared with those in the KC+SF group (n=3
per group, Fig. 8; P<0.05).
The mRNA levels of Nrf-2 and HO-1 in the KC + SF + ZnPP group were
decreased significantly compared with those in the KC + SF group
(n=3 per group, Fig. 8;
P<0.05). SF decreased the mRNA levels of Nox-2 and Nox-4, and
increased the mRNA levels of Nrf-2 and HO-1 in the KC corneas. ZnPP
IX lessened the protective effect of SF on the KC corneas.
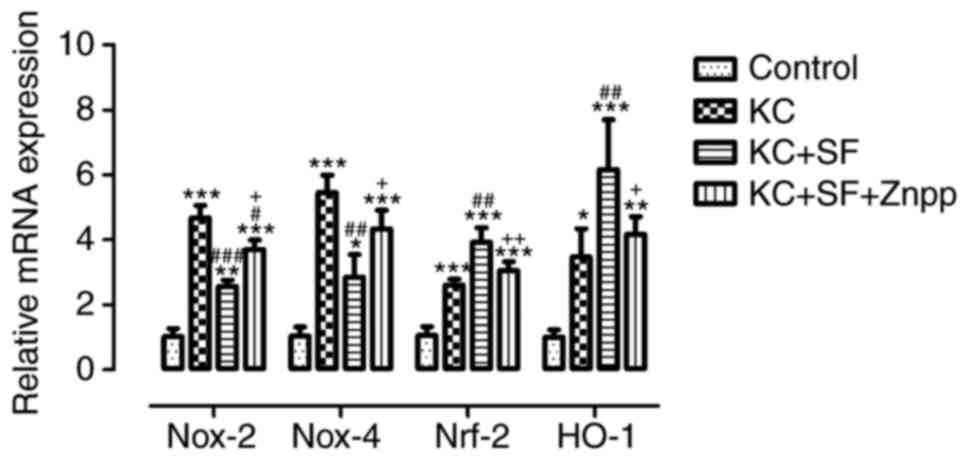 | Figure 8SF decreases the mNRA expression of
Nox-2 and Nox-4, and increases the mRNA expression of HO-1 and
Nrf-2 in KC corneas. The expression levels of the relevant genes in
corneal stromal cells, including HO-1, Nox-2, Nox-4 and Nrf-2, were
assessed in the four groups (Control, KC, KC + SF, and KC + SF +
ZnPP). Fold change in gene expression relative to β-actin was
calculated as 2−ΔΔCq. Data are presented as the mean ±
standard deviation; n=3 per group. *P<0.05,
**P<0.01 and ***P<0.001, vs. Control;
#P<0.05, ##P<0.01 and
###P<0.001, vs. KC group; +P<0.05 and
++P<0.01 vs. KC + SF group. KC, keratoconus; SF,
sulforaphane; ZnPP IX, zinc (II) protoporphyrin IX; Nox, NAPDH
oxidase; Nrf-2, nuclear factor E2-related factor 2; HO-1, heme
oxygenase-1. |
Discussion
The present study was designed to clarify the
possible protective mechanism of SF in a KC rabbit model. SF exerts
a protective effect against oxidative stress in numerous biological
settings. This protective effect may be induced through the
Nrf-2-mediated induction of HO-1. The present study analyzed the
expression of an ARE/EpRE-mediated antioxidant protein, HO-1, in
the corneas of KC rabbits and examined the possible effect of Nox
on the activation of HO-1. Following surgery, KC caused a
significant enhancement of ROS production, leading to a significant
increase in Km and a significant decrease in CCT. These changes
caused by KC were weakened or neutralized by SF treatment.
Furthermore, SF treatment significantly lowered the protein
expression of Nox-2 and Nox-4 and increased the levels of Nrf-2 and
HO-1 in the KC cornea. The HO-1 inhibitor, ZnPP IX, reduced the
protective effect of SF on the KC cornea. Therefore, the present
study may be the first to demonstrate that the protective effect of
SF on the KC cornea is, at least be partially, accomplished via the
Nrf-2/HO-1 antioxidant pathway.
The corneal KC model mimicked clinical features,
including a significant increase in Km and a significant decrease
in CCT compared with the Control group. Previous studies have
indicated that corneal ectasia may be generated in vitro and
in vivo by treatment with collagenase type II, with
increased corneal Km and decreased CCT (20,30,31). Collagenase type II preferentially
degrades collagen I, which is the main collagen component of
cornea; therefore, it is possible that exposure to collagenase
generates a KC model (32). When
considering the natural flattening of the cornea and the reduction
in keratometry in both eyes, the total keratometry of the
experimental eyes was increased by >2 D. Furthermore, the
increase in keratometry and decrease in CCT following collagenase
exposure lasted for 2 weeks, suggesting that the rabbit model of
corneal ectasia generated by collagenase treatment is suitable for
use in basic investigations of KC.
In the past two decades, the main focuses of KC
biomechanical investigations have been on alterations in the
composition of collagen fibers in the corneal stroma, the
connection between fibrin layers, and the role of proteases,
however, this has ignored the role of corneal stromal cells.
Although corneal stromal cells account for <5% of the total
corneal matrix, corneal stromal cells are able produce collagen
fibers (33-35). Therefore, corneal stromal cells
may be the root cause of the role of corneal collagen fibers in KC.
Furthermore, corneal stromal cells possess the function of
synthesizing glycoproteins and mucins, and may be regulated by
various chemical substances (34,36). The present study focused on the
role of corneal stromal cells in the pathogenesis of KC, the
specific mechanism of oxidative stress in KC stromal cells, and the
drug treatment response to this pathogenesis based on the KC model
used.
The overproduction of ROS has been regarded as a
pivotal event in KC corneas in certain studies (37,38), and this was also observed in the
present study. Accumulating evidence suggests that oxidative stress
is critical in the pathogenesis of KC (5,7,38,39). This hypothesis was supported by
excessive ROS and interference at the transcriptional level and/or
antioxidant enzyme activity in KC corneas compared with controls.
However, the application and investigation of antioxidants in the
prevention and treatment of KC have been limited. It has been
reported that the antioxidant riboflavin upregulates the expression
of antioxidant compounds in KC stromal cells, downregulates the
expression of oxidase genes, and reduces ROS levels in KC (18). Quercetin may be used to reduce
oxidative stress regulatory signals involved in the pathogenesis of
KC (40,41). These antioxidative stress drugs
may have potential in the clinical prevention and treatment of
KC.
SF is a member of the isothiocyanate family and is
obtained from cruciferous vegetables, including Brussels sprouts,
cabbage and broccoli (42). As an
Nrf-2 activator, SF has been identified to have multiple protective
effects, and its role primarily depends on the activation and
induction of the phase II expression of antioxidant enzymes
(13,17). Numerous studies have reported that
SF protects the heart, kidneys, liver and brain from ischemic
injury via activation of the Nrf-2/ARE signaling pathway (17,43-45). The protective effects of SF in
response to ocular diseases have also been demonstrated. SF has
been shown to upregulate primary ARE/EpRE-mediated antioxidants and
alleviate oxidative stress-induced corneal endothelial cell
apoptosis in Fuchs endothelial corneal dystrophy through enhancing
the nuclear translocation of Nrf-2 (46). SF treatment has also been reported
to protect retinal pigment epithelial and photoreceptor cells from
optical damage and postpone retinal photoreceptor cell degeneration
in mice through upregulating Nrf-2 (47,48). However, to the best of our
knowledge, there has been no report on the effect of SF on corneal
stromal cells in KC and its clinical features. In the present
study, it was demonstrated that SF significantly inhibited ROS
generation in KC stromal cells, enhanced corneal Km and reduced CCT
in KC, suggesting that SF exhibits an antioxidant function in KC
corneas and delays the progress of KC disease. This is the first
evidence, to the best of our knowledge, of the antioxidative effect
of SF on corneal stromal cells in a KC model.
The mechanism underlying the effect of SF in
reducing oxidative stress response in KC was evaluated further by
analyzing the expression of genes associated with the Nrf-2/ARE
signaling pathway. Unlike other oxidoreductases, the Nox family is
considered to be a distinct enzymatic source of cellular ROS
generation as these enzymes are among the most effective ROS
producers (49). Cornea stromal
cells have been demonstrated to be capable of producing ROS
(8,27). In the present study, KC induced
the generation of ROS, Nox-2 and Nox-4 in corneal stromal cells.
The production of ROS in KC corneas may be through the activation
of Nox-2 and Nox-4. In the present study, it was demonstrated that
SF significantly downregulated the expression levels of Nox-2 and
Nox-4 in the KC corneas. It was suggested that the downregulation
in the expression of Nox-2 and Nox-4 following treatment with SF
may act as an underlying contributor to the Nrf-2/ARE signaling
pathway and regulated gene expression, and may be involved in the
antioxidative stress protective mechanism in KC corneas. This may
provide valuable information on the mechanisms involved in the
pathogenesis of KC. The results suggested that SF has an
anti-Nox-dependent ROS generating role in the KC cornea, possibly
by inactivating the expression of Nox-2 and Nox-4.
The downregulation of Nox-2 and Nox-4 following SF
treatment may promote activation of the Nrf-2/ARE signaling pathway
and regulate the expression of associated genes, and may be
involved in the protective mechanism of SF on oxidative stress in
KC. Nrf-2 is critical to maintain the level of intracellular
glutathione and redox balance, and it is also important in
resisting oxidative stress via activating the expression of several
ROS-detoxifying enzymes and stimulating the production of
antioxidants (50). Under normal
physiological conditions, Nrf-2 is sequestered in the cell cytosol
via an interaction with Keap1. Under oxidative stress conditions,
Nrf-2 dissociates from Keap1, accumulates in the nucleus, and then
binds to ARE regions in the promoters of antioxidants, including
Nrf-2 itself and HO-1 combined with small Maf proteins (51,52). Several types of cellular stressors
induce the protein expression of HO-1 through redox-sensitive
factors, including Nrf-2, which has been identified as a pivotal
transcription factor controlling antioxidant gene expression
(53,54). HO-1 catalyzes the rate-limiting
process of heme oxidative degradation to biliverdin, releasing
carbon monoxide and iron. HO-1 is important in sustaining
oxidative/antioxidant equilibrium (55). In the present study, it was
observed that SF treatment increased the accumulation of nuclear
Nrf-2 and upregulated HO-1 in the corneas of the KC rabbit model.
The HO-1 inhibitor, ZnPP IX, reduced the activity of Nrf-2 in the
KC + SF cornea. The upregulation of Nrf-2 by SF treatment was
associated with an increased level of HO-1 in the KC corneas. The
induction of HO-1 and Nrf-2 by SF may decrease damage in corneal
stromal cells via recovering the balance of antioxidants and
pro-oxidants in the cornea. This suggested that the activation of
HO-1 and Nrf-2 may represent a key signaling pathway for mitigating
the degree of cell damage attributed to oxidative stress in KC
corneas.
The findings of the present study suggested that the
activation of Nrf-2, and the protective effect of SF, were likely
to be due to control of the oxidative stress response via the
induction of HO-1. SF induced a significant decrease of ROS
production and increase of HO-1 protein in the present study. The
ability of SF-mediated free radical production and its reaction
with cysteine residues of Keap1 (56) lead to Nrf-2 defense signaling
pathway activation. ZnPP IX, an HO-1 inhibitor, attenuated the
protective effects of SF against oxidative stress injury in the KC
cornea. These observations suggested that the Nrf-2/HO-1
antioxidant signaling pathway was associated with the protective
effect of SF on KC-induced injury in the rabbit cornea. SF may
reduce the damage from oxidative stress to corneal stromal cells by
activating the Nrf-2/HO-1 axis, thereby reducing the corneal Km and
increasing CCT, and delaying the progression of KC. SF may provide
a novel and important theoretical basis for KC drug treatment; it
may function as an underlying prophylactic drug resisting oxidative
stress in corneal KC injury.
N-acetylcysteine (NAC) is a compound containing
active sulfhydryl groups, which enhances antioxidation and
anti-free radical damage by direct antioxidation effects (57). NAC, an antioxidant and ROS
scavenger, is able to inhibit or prevent damage and cell death in
corneas (58). The majority of
reports consider NAC to be a direct, fast-acting, short-acting
antioxidant. In addition, various ophthalmic formulations of NAC
have been widely utilized for other corneal disorders (59,60). Compared with NAC, SF is a notable
indirect antioxidant. It functions by stimulating the free radical
scavenging system of the body and promoting the body to produce
increased free radical scavenging enzymes. SF, a long-lasting
antioxidant, has a long half-life and is not consumed in the
antioxidation process. Therefore, the antioxidant response of SF
was examined in the present study, with a focus on the protective
effect of SF on KC, including reducing the curvature of the KC and
increasing the thickness of the KC. The specific mechanisms of this
protective effect were examined. However, further investigations
can be performed to detect the protective effect of NAC on KC
corneas, or combined NAC and SF drugs, and provide further ideas
for the treatment of KC.
Previous studies have indicated that the
pathogenesis of KC consists of corneal thinning together with a
reduced number of keratocytes, attributed to excessive oxidative
stress and increased catalase activity (61). No significant reduction in the
number of corneal stromal cells was observed in the present study.
This may be due to the fact that the level of ROS production was
not high enough, the duration was short, and the endogenous
compensatory mechanism was involved, thus reducing damage to
corneal stromal cells by oxidative stress in the KC model. The
antioxidant SF may have a discernible beneficial effect on the
inferred pathogenesis of KC by moderating the presence of ROS.
These results support the potential effectiveness of antioxidants
as a potential therapy, which is directed at the pathogenesis of
the disease by promoting normal synthesis and reducing ROS levels.
However, further investigations are warranted to solve important
problems, including the assessment of HO-1 activities in various
settings by measuring bilirubin spectrophotometrically, changes in
the Nrf-2/HO-1 signaling pathway in the cell culture of KC stroma
cells, increasing the time window of targeting oxidative stress
with SF, and assessing the functional state of rescued corneal
stroma cells.
In conclusion, KC induced an increase in ROS
generation, and caused a marked increase in Km and a marked
decrease in CCT. These effects were neutralized or reversed by SF
treatment. Treatment with SF significantly decreased the expression
levels of Nox-2 and Nox-4 and increased the expression levels of
Nrf-2 and HO-1 in the KC corneas. It was found that oxidative
stress was present in the rabbit KC model, and ROS production
primarily originated from Nox-2 and Nox-4 in the KC corneas. SF
activated the Nrf-2/HO-1 pathway as a target to resist the
oxidative stress response of KC, thereby reducing corneal Km,
increasing corneal CCT, and preventing the progression of KC. These
results suggested that SF may be an appropriate drug for the
treatment of KC. Overall, these experimental results suggested that
the protective effect of SF on KC corneas was mediated at least in
part by activating the Nrf-2/HO-1 antioxidant pathway. These novel
findings may improve current understanding of KC disease signaling
pathways and thus reinforce the clinical treatment and management
of KC.
Funding
This study was supported by funding from the
National Natural Science Foundation of China (grant no.
11372011).
Availability of data and materials
All data generated or analyzed during this study are
included in this article.
Authors' contributions
All authors conceived and designed the experiments.
RL performed the experiments and analyzed the data. RL wrote the
manuscript. XY modified the manuscript. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving
animals were approved by the Ethical Committee of Peking University
First Hospital. All animals used in the present study were treated
in accordance with the Association for Research in Vision and
Ophthalmology Statement for the Use of Animals in Ophthalmic and
Vision Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Rabinowitz YS: Keratoconus. Surv
Ophthalmol. 42:297–319. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katsoulos C, Karageorgiadis L, Vasileiou
N, Mousafeiropoulos T and Asimellis G: Customized hydrogel contact
lenses for kera-toconus incorporating correction for vertical coma
aberration. Ophthalmic Physiol Opt. 29:321–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abu-Amero KK, Al-Muammar AM and Kondkar
AA: Genetics of keratoconus: Where do we stand? J Ophthalmol.
2014:6417082014.PubMed/NCBI
|
|
4
|
Nielsen K, Hjortdal J, Pihlmann M and
Corydon TJ: Update on the keratoconus genetics. Acta Ophthalmol.
91:106–113. 2013. View Article : Google Scholar
|
|
5
|
Kenney MC, Chwa M, Atilano SR, Tran A,
Carballo M, Saghizadeh M, Vasiliou V, Adachi W and Brown DJ:
Increased levels of catalase and cathepsin V/l2 but decreased
TIMP-1 in keratoconus corneas: Evidence that oxidative stress plays
a role in this disorder. Investig Ophthalmol Vis Sci. 46:823–832.
2005. View Article : Google Scholar
|
|
6
|
Chwa M, Atilano SR, Hertzog D, Zheng H,
Langberg J, Kim DW and Kenney MC: Hypersensitive response to
oxidative stress in keratoconus corneal fibroblasts. Investig
Ophthalmol Vis Sci. 49:4361–4369. 2008. View Article : Google Scholar
|
|
7
|
Buddi R, Lin B, Atilano SR, Zorapapel NC,
Kenney MC and Brown DJ: Evidence of oxidative stress in human
corneal diseases. J Histochem Cytochem. 50:341–351. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chwa M, Atilano SR, Reddy V, Jordan N, Kim
DW and Kenney MC: Increased stress-induced generation of reactive
oxygen species and apoptosis in human keratoconus fibroblasts.
Investig Ophthalmol Vis Sci. 47:1902–1910. 2006. View Article : Google Scholar
|
|
9
|
Jeong WS, Jun M and Kong AN: Nrf2: A
potential molecular target for cancer chemoprevention by natural
compounds. Antioxid Redox Signal. 8:99–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siow RC, Ishii T and Mann GE: Modulation
of antioxidant gene expression by 4-hydroxynonenal:
Atheroprotective role of the Nrf2/ARE transcription pathway. Redox
Rep. 12:11–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng X, Siow RCM and Mann GE: Impaired
redox signaling and antioxidant gene expression in endothelial
cells in diabetes: A role for mitochondria and the nuclear
factor-E2-related factor 2-Kelch-like ECH-associated protein 1
defense pathway. Antioxid Redox Signal. 14:469–487. 2011.
View Article : Google Scholar
|
|
13
|
Zhang Y, Talalay P, Cho CG and Posner GH:
A major inducer of anticarcinogenic protective enzymes from
broccoli: Isolation and elucidation of structure. Proc Natl Acad
Sci USA. 89:2399–2403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Kobori N, Aronowski J and Dash PK:
Sulforaphane reduces infarct volume following focal cerebral
ischemia in rodents. Neurosci Lett. 393:108–112. 2006. View Article : Google Scholar
|
|
15
|
Danilov CA, Chandrasekaran K, Racz J,
Soane L, Zielke C and Fiskum G: Sulforaphane protects astrocytes
against oxidative stress and delayed death caused by oxygen and
glucose deprivation. Glia. 57:645–656. 2009. View Article : Google Scholar :
|
|
16
|
Tarozzi A, Morroni F, Merlicco A, Hrelia
S, Angeloni C, Cantelli-Forti G and Hrelia P: Sulforaphane as an
inducer of glutathione prevents oxidative stress-induced cell death
in a dopaminergic-like neuroblastoma cell line. J Neurochem.
111:1161–1171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piao CS, Gao S, Lee GH, Kim DS, Park BH,
Chae SW, Chae HJ and Kim SH: Sulforaphane protects ischemic injury
of hearts through antioxidant pathway and mitochondrial K(ATP)
channels. Pharmacol Res. 61:342–348. 2010. View Article : Google Scholar
|
|
18
|
Cheung IMY, Mcghee CN and Sherwin T:
Beneficial effect of the antioxidant riboflavin on gene expression
of extracellular matrix elements, antioxidants and oxidases in
keratoconic stromal cells. Clin Exp Optom. 97:349–355.
2014.PubMed/NCBI
|
|
19
|
Clark JE, Foresti R, Green CJ and
Motterlini R: Dynamics of haem oxygenase-1 expression and bilirubin
production in cellular protection against oxidative stress. Biochem
J. 348(Pt 3): 615–619. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiao J, Li H, Tang Y, Song W, Rong B, Yang
S, Wu Y and Yan X: A rabbit model of corneal Ectasia generated by
treatment with collagenase type II. BMC Ophthalmol. 18:942018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng H, Whitman SA, Wu W, Wondrak GT,
Wong PK, Fang D and Zhang DD: Therapeutic potential of Nrf2
activators in streptozotocin-induced diabetic nephropathy.
Diabetes. 60:3055–3066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park J, Kang JW and Lee SM: Activation of
the cholinergic anti-inflammatory pathway by nicotine attenuates
hepatic ischemia/reperfusion injury via heme oxygenase-1 induction.
Eur J Pharmacol. 707:61–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Wang F, Shi L, Jia X and Lin L:
Role of heme oxygenase-1/carbon monoxide system in pulmonary
ischemia-reperfusion injury. Interact Cardiovasc Thorac Surg.
9:159–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He M, Pan H, Xiao C and Pu M: Roles for
redox signaling by NADPH oxidase in hyperglycemia-induced heme
oxygenase-1 expression in the diabetic retina. Investig
Opthalmology Vis Sci. 54:4092–4101. 2013. View Article : Google Scholar
|
|
25
|
Fleige S, Walf V, Huch S, Prgomet C, Sehm
J and Pfaffl MW: Comparison of relative mRNA quantification models
and the impact of RNA integrity in quantitative real-time RT-PCR.
Biotechnol Lett. 28:1601–1613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
O'Brien WJ, Heimann T and Rizvi F: NADPH
oxidase expression and production of superoxide by human corneal
stromal cells. Mol Vis. 15:2535–2543. 2009.PubMed/NCBI
|
|
28
|
Kermer P, Ankerhold R, Klöcker N,
Krajewski S, Reed JC and Bähr M: Caspase-9: Involvement in
secondary death of axotomized rat retinal ganglion cells in vivo.
Brain Res Mol Brain Res. 85:144–150. 2000. View Article : Google Scholar
|
|
29
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the aminoterminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hong CW, Sinha-Roy A, Schoenfield L,
Mcmahon JT and Dupps WJ Jr: Collagenase-mediated tissue modeling of
corneal ectasia and collagen cross-linking treatments. Investig
Ophthalmol Vis Sci. 53:2321–2327. 2012. View Article : Google Scholar
|
|
31
|
Wang X, Huang Y, Jastaneiah S, Majumdar S,
Kang JU, Yiu SC, Stark W and Elisseeff JH: Protective effects of
soluble collagen during ultraviolet-A crosslinking on
enzyme-mediated corneal ectatic models. PLoS One. 10:e01369992015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Davidson AE, Hayes S, Hardcastle AJ and
Tuft SJ: The pathogenesis of keratoconus. Eye (Lond). 28:189–195.
2014. View Article : Google Scholar
|
|
34
|
Meek KM and Knupp C: Corneal structure and
transparency. Prog Retin Eye Res. 49:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elsheikh A, Ross S, Alhasso D and Rama P:
Numerical study of the effect of corneal layered structure on
ocular biomechanics. Curr Eye Res. 34:26–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jester JV, Moller-Pedersen T, Huang J, Sax
CM, Kays WT, Cavangh HD, Petroll WM and Piatigorsky J: The cellular
basis of corneal transparency: Evidence for 'corneal crystallins'.
J Cell Sci. 112:613–622. 1999.PubMed/NCBI
|
|
37
|
Karamichos D, Hutcheon a EK, Rich CB,
Trinkaus-Randall V, Asara JM and Zieske JD: In vitro model suggests
oxidative stress involved in keratoconus disease. Sci Rep.
4:46082014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arnal E, Peris-Martínez C, Menezo JL,
Johnsen-Soriano S and Romero FJ: Oxidative stress in keratoconus?
Invest Ophthalmol Vis Sci. 52:8592–8597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gondhowiardjo TD and van Haeringen NJ:
Corneal aldehyde dehydrogenase, glutathione reductase, and
glutathione S-transferase in pathologic corneas. Cornea.
12:310–314. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McKay TB, Sarker-Nag A, Lyon D, Asara JM
and Karamichos D: Quercetin modulates keratoconus metabolism in
vitro. Cell Biochem Funct. 33:341–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McKay TB, Lyon D, Sarker-Nag A,
Priyadarsini S, Asara JM and Karamichos D: Quercetin attenuates
lactate production and extracellular matrix secretion in
keratoconus. Sci Rep. 5:90032015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fahey JW, Holtzclaw WD, Wehage SL, Wade
KL, Stephenson KK and Talalay P: Sulforaphane bioavailability from
glucoraphanin-rich broccoli: Control by active endogenous
myrosinase. PLoS One. 10:e01409632015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao HD, Zhang F, Shen G, Li YB, Li YH,
Jing HR, Ma LF, Yao JH and Tian XF: Sulforaphane protects liver
injury induced by intestinal ischemia reperfusion through Nrf2-ARE
pathway. World J Gastroenterol. 16:3002–3010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ping Z, Liu W, Kang Z, Cai J, Wang Q,
Cheng N, Wang S, Wang S, Zhang JH and Sun X: Sulforaphane protects
brains against hypoxic-ischemic injury through induction of
Nrf2-dependent phase 2 enzyme. Brain Res. 1343:178–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoon HY, Kang NI, Lee HK, Jang KY, Park JW
and Park BH: Sulforaphane protects kidneys against
ischemia-reperfusion injury through induction of the Nrf2-dependent
phase 2 enzyme. Biochem Pharmacol. 75:2214–2223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ziaei A, Schmedt T, Chen Y and Jurkunas
UV: Sulforaphane decreases endothelial cell apoptosis in Fuchs
endothelial corneal dystrophy: A novel treatment. Investig
Ophthalmol Vis Sci. 54:6724–6734. 2013. View Article : Google Scholar
|
|
47
|
Tanito M, Masutani H, Kim YC, Nishikawa M,
Ohira A and Yodoi J: Sulforaphane induces thioredoxin through the
antioxidant-responsive element and attenuates retinal light damage
in mice. Investig Ophthalmol Vis Sci. 46:979–987. 2005. View Article : Google Scholar
|
|
48
|
Kong L, Tanito M, Huang Z, Li F, Zhou X,
Zaharia A, Yodoi J, McGinnis JF and Cao W: Delay of photoreceptor
degeneration in tubby mouse by sulforaphane. J Neurochem.
101:1041–1052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar
|
|
51
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang KW, Lee SJ and Kim SG: Molecular
mechanism of nrf2 activation by oxidative stress. Antioxid Redox
Signal. 7:1664–1673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ishii T, Itoh K, Ruiz E, Leake DS, Unoki
H, Yamamoto M and Mann GE: Role of Nrf2 in the regulation of CD36
and stress protein expression in murine macrophages: Activation by
oxidatively modified LDL and 4-hydroxynonenal. Circ Res.
94:609–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wakabayashi N, Slocum SL, Skoko JJ, Shin S
and Kensler TW: When NRF2 talks, who's listening? Antioxid Redox
Signal. 13:1649–1663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ryter SW, Alam J and Choi AMK: Heme
oxygenase-1/carbon monoxide : From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hu C, Eggler AL, Mesecar AD and Van
Breemen RB: Modification of Keap1 cysteine residues by
sulforaphane. Chem Res Toxicol. 24:515–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kizilgun M, Poyrazoglu Y, Oztas Y, Yaman
H, Cakir E, Cayci T, Akgul OE, Kurt YG, Yaren H, Kunak ZI, et al:
Beneficial effects of N-acetylcysteine and ebselen on renal
ischemia/reperfusion injury. Ren Fail. 33:512–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rosani U, Tarricone E, Venier P and Brun
P, Deligianni V, Zuin M, Martines E, Leonardi A and Brun P:
Atmospheric-pressure cold plasma induces transcriptional changes in
ex vivo human corneas. PLoS One. 10:e01331732015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yalçin E, Altin F, Cinhüseyinoglue F and
Arslan MO: N-acetylcysteine in chronic blepharitis. Cornea.
21:164–168. 2002. View Article : Google Scholar
|
|
60
|
Schmidl D, Werkmeister R, Kaya S,
Unterhuber A, Witkowska KJ, Baumgartner R, Höller S, O'Rourke M,
Peterson W, Wolter A, et al: A controlled, randomized double-blind
study to evaluate the safety and efficacy of
chitosan-N-acetylcysteine for the treatment of dry eye syndrome. J
Ocul Pharmacol Ther. 33:375–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ishii T, Miyazawa M, Onouchi H, Yasuda K,
Hartman PS and Ishii N: Model animals for the study of oxidative
stress from complex II. Biochim Biophys Acta. 1827:588–597. 2013.
View Article : Google Scholar
|