Introduction
The dental follicle (DF) is a loose connective
tissue sac surrounding the enamel organ and dental papilla of a
developing tooth germ prior to eruption. This tissue is believed to
contain DF stem/progenitor cells (DFSCs) that are able to give rise
to cementoblasts, periodontal ligament cells and osteoblasts
(1). In vitro, DFSCs have
the potential to differentiate into osteoblasts (2). The Wnt/β-catenin signaling pathway
is critical for normal bone and tooth formation and development
(3). Multiple pathways, including
bone morphogenetic protein (BMP), Notch, insulin-like growth factor
and Wnt signaling, are involved in the differentiation of DFSCs
into osteoblasts (3). Previous
studies have revealed that the expression of Wnts has spatial and
temporal specificity during tooth development (4-6)
and that the Wnt/β-catenin pathway participates in the
differentiation of DFSCs into osteoblasts (7-11).
The enhanced and reduced activity of the Wnt/β-catenin pathway may
result in abnormalities in tooth development (12-14).
Drosophila naked cuticle (Nkd), a
Drosophila segment polarity gene, encodes an inducible
antagonist for the Wnt signaling molecule Wingless (Wg) (15) and Nkd functions as a
signal-inducible feedback antagonist of Wg in fly embryos (16). Nkd homolog 2 (Nkd2) is one of the
mammalian homologs of Nkd, and has been demonstrated to negatively
regulate the canonical Wnt signaling pathway by interacting with
Dishevelled (Dvl) (17,18). In 293T cells, myristoylated Nkd2
interacts with Dvl-1 at the plasma membrane, which results in the
ubiquitin-mediated proteasomal degradation of the two proteins,
thus attenuating Wnt signaling (19). Nkd2 also suppresses canonical
Wnt/β-catenin signaling during multiple stages of early development
in zebrafish (20). An additional
role for Nkd2 is to escort transforming growth factor-α
(TGF-α)-containing vesicles to the basolateral surface of polarized
epithelial cells (21). Nkd2 may
regulate Wnt and epidermal growth factor receptor signaling via its
function in TGF-α delivery and Dvl stabilization (22). Nevertheless, the functions and
mechanisms of Nkd2 during the differentiation of rat DFSCs (rDFSCs)
into osteoblasts remain largely unknown.
In the present study, it was hypothesized that Nkd2
regulates the Wnt/β-catenin pathway during the differentiation of
rDFSCs into osteoblasts. Therefore, the function of Nkd2 during
rDFSC differentiation into osteoblasts was examined, and its
function in Wnt/β-catenin signaling was assessed. It was
demonstrated that Nkd2 is able to promote the differentiation of
rDFSCs into osteoblasts through the Wnt/β-catenin pathway, results
that may provide novel insight into the molecular mechanism of
regeneration of tooth root and alveolar bone.
Materials and methods
Immunohistochemistry
All animal procedures were ethically approved by the
Ethics Committee of the Guanghua College of Stomatology, Sun
Yat-sen University [Guangzhou, China; approval no. ERC-(2014)-21].
Sprague-Dawley rats (Laboratory Animal Center, Sun Yat-sen
University) were housed in a specific pathogen-free laboratory
animal center at a temperature of 20-26°C, 35 pa higher than the
standard atmosphere, light-dark cycle 12/12 h, and fed using
standard rodent chow (ad libitum access to food and water).
A total of 70 rats (1, 3, 5, 7, 9, 11 and 13 days old; 10 rats for
each group, nonsexual selection with 38 female mice and 32 male
mice, weight 5-20 g) were obtained from the Laboratory Animal
Center and euthanized by cervical dislocation immediately.
Mandibles containing the first molars were
dissected, fixed in 4% paraformaldehyde at 4°C for 24 h,
decalcified in methanoic acid for 3-7 days and embedded in
paraffin. Furthermore, serial 5-mm sections were then cut,
collected on 3-aminopropyltriethoxysilane-coated slices and air
dried. Mesiodistal sections of mandibular tissues were
deparaffinized with two washes in dimethyl benzene for 10 min
succeeded by rehydration with serial dilutions (95, 90, 80 and 70%)
of ethanol for 5 min respectively. Sections were heated in a water
bath (10 min at 95°C) in citrate buffer for antigen retrieval.
Following incubation with blocking buffer consisting of 1% bovine
serum albumin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at room temperature for 1 h, the slides were incubated
overnight at 4°C with the primary antibody Nkd2 (1:100; cat. no.
sc163145; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or with
PBS as a negative control. Subsequent to washing using PBS, the
slides were incubated with the horseradish peroxidase
(HRP)-conjugated secondary antibody rabbit anti-goat IgG (1:200;
cat. no. A21030; Abbkine Scientific Co., Ltd., Wuhan, China) at
room temperature for 1 h and images were obtained using an electron
optical microscope (magnification, x200; Zeiss AG, Oberkochen,
Germany) as previously described (23). Subsequent to image acquisition,
image analysis was performed using ImageJ 1.48 version (National
Institutes of Health, Bethesda, MD, USA), as previously described
(24). Quantification of the
immunoscore of all images was blinded for histopathological
diagnosis. A specific brown color was selected as the standard for
all positive image scores as previously described (24). The mean gray value of the negative
group was used to set the threshold adjustment, and the mean gray
value of each image was obtained. The results were evaluated using
SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA) with a
paired Student's t-test (each group was compared with the negative
group), and the data are expressed as the mean ± standard
deviation, with P<0.05 considered to indicate a statistically
significant difference.
Cell culture and induction of osteoblast
differentiation
DFs were gently isolated from the first mandibular
molars of Sprague-Dawley rats (5 to 7 days old) and digested with
0.1% collagenase type I (Collagen-I) and 10 U/ml dispase
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30 min at 37°C
to obtain rDFSCs, as previously described (7). To ensure a uniform cell population,
the rDFSCs were cultured in α-modified Eagle's medium (α-MEM;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin for 3 to 4 passages in a 37°C incubator
with 5% CO2. rDFSCs at passages 3-5 were used for all
experiments.
rDFSCs were cultured until sub-confluent (>80%)
in standard cell culture medium prior to the induction of
cementoblast/osteoblast differentiation, and then stimulated with
osteoblast induction medium containing 10 mmol β-glycerophosphate
(Sigma-Aldrich; Merck KGaA), 0.2 mmol ascorbic acid (Sigma-Aldrich;
Merck KGaA) and 100 nmol dexamethasone (Sigma-Aldrich; Merck KGaA).
Differentiation of rDFSCs was determined by Alizarin Red staining
and the expression of osteoblast markers.
Immunofluorescence
rDFSCs were fixed with 4% paraformaldehyde for 15
min at room temperature and permeabilized with 0.1% Triton X-100 in
PBS for 20 min. The cells were blocked with 1% bovine serum albumin
at 4°C for 1 h and then incubated overnight at 4°C with an anti-rat
Nkd2 antibody (1:100; cat. no. sc163145; Santa Cruz Biotechnology,
Inc.). Fluorescein isothiocyanate-conjugated immunoglobulin G
secondary antibody (1:1,000; cat. no. E031230; EarthOx, LLC,
Millbrae, CA, USA) was added to the cells at 37°C for 2 h as the
secondary antibody prior to nuclear staining with 10 µg/ml DAPI at
37°C for 2 min. The cells were finally washed with PBS 3 times and
examined under a confocal laser-scanning microscope (magnification,
×400; Zeiss AG).
Small interfering RNA (siRNA)
transfection
rDFSCs (passages 3-5) were seeded in 6-well culture
plates with a density of 105/ml and 200 ml cell
suspension per well, and cultured until sub-confluent (>80%)
prior to transfection with 50 nmol/l siRNA targeting the rat Nkd2
gene (si-Nkd2) using Lipofectamine RNAiMax (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 8 h according to the
manufacturer's protocol. Cells transfected with non-targeting siRNA
(si-NC) or cells incubated without siRNA (mock transfection) were
used as negative controls. The oligonucleotides si-Nkd2 (forward,
5′ GCACUCCAGUGUGAUGUCUdTdT 3' and reverse, 3′
dTdTCGUGAGGUCACACUACAGA 5′) and si-NC were synthesized by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China).
Cell Counting Kit-8 (CCK-8) assay for
proliferation
DFSCs were seeded in 96-well culture plates at a
density of 4×103/ml, 200 µl cell suspension per well and
transfected with si-Nkd2 or si-NC for 24, 48 or 72 h.
Mock-transfected cells were used as negative controls. CCK-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) reagent was
added to each well at 10% of the volume, and the samples were
incubated at 37°C in the dark for 2 h. Optical density was measured
at 450 nm using a microplate reader (Tecan Group, Ltd., Mannedorf,
Switzerland).
Cell cycle distribution and apoptosis
analyses
DFSCs transfected with si-Nkd2 or si-NC for 72 h
were used for cell cycle distribution and apoptosis analyses. For
the cell cycle distribution assay, 1×106 treated cells
were fixed in 70% pre-chilled ethanol overnight at -20°C.
Subsequently, the cells were labeled with Forevergen Cell Cycle
kits (Forevergen; Guangzhou Yongnuo Biotechnology Co., Ltd.,
Guangzhou, China) according to the manufacturer's protocol. The
Annexin V/propidium iodide apoptosis assay was performed using the
PE Annexin V Apoptosis Detection kit I (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer's protocols. All
samples were analyzed using an Accuri C6 Flow Cytometer and CFlow
Plus 1.0.264.15 Software (BD Biosciences).
Cell migration assay
si-Nkd2- or si-NC-transfected rDFSCs were
trypsinized with 0.25% Trypsin-EDTA solution (cat. no. 25200-056;
Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 1 min and
resuspended in serum-free DMEM (Gibco; Thermo Fisher Scientific,
Inc.) at a density of 3×105/ml, and 200 ml of the cell
suspension was seeded in the upper chamber of a Costar Transwell
System (Corning Incorporated, Corning, NY, USA). The lower chamber
was filled with 600 ml α-MEM containing 10% FBS. The cells were
allowed to migrate through 8-mm pores for 16 h at 37°C and the
cells in the upper chamber were removed with a cotton swab. The
cells present beneath the membrane were stained with 10 µg/ml DAPI
at 37°C for 2 min. Fluorescence microscopy was performed with a
electron fluorescence microscope (magnification, x100; Axiovert;
Zeiss AG, Oberkochen, Germany). Ten fields per sample were randomly
selected for the quantitative analysis of cell migration by
counting cell nuclei.
Alkaline phosphatase activity (ALP)
assay
si-Nkd2- or si-NC-transfected rDFSCs seeded at a
density of 2×104/ml and 100 µl cell suspension per well
in 96-well culture plates were grown in conditioned medium for 7
days. The plates were washed twice, and 55 µl 1% Triton X-100 was
added to each well. Subsequent to incubation overnight at 4°C, 30
µl cell lysate per well was subjected to an ALP activity assay
using an ALP kit (Nanjing Jiancheng Bioengingeering Institute,
Nanjing, China). To normalize enzyme activity, the protein
concentration was measured with a BCA protein assay kit (Boster
Biological Technology, Pleasanton, CA, USA).
Alizarin Red staining
si-Nkd2- or si-NC-transfected rDFSCs were seeded at
a density of 2×104/ml and 100 µl cell suspension per
well in 96-well culture plates. Subsequent to osteogenic induction
for 7 days, si-Nkd2- or si-NC-transfected rDFSCs were fixed with 4%
paraformaldehyde at 4°C for 24 h and stained with 1% Alizarin Red S
(Sigma-Aldrich; Merck KGaA) at 37°C for 15 min. Following the
addition of 10% cetylpyri-dine, the cells were incubated at 37°C
for 60 min. The extent of mineralization was detected at 562 nm
using a microplate reader (Tecan Group, Ltd.).
Western blotting
Control and induced (0, 1, 2, 3 and 4 weeks), and
si-Nkd2- or si-NC-transfected cells (≥106 cells per
group) were harvested with radioimmunoprecipitation assay (RIPA)
lysis buffer at 0°C for 30 min (10 mmol/l Tris-HCl, 1 mmol/l EDTA,
1% sodium dodecyl sulfate, 1% Nonidet P-40, 1:1,000 proteinase
inhibitor cocktail, 50 mmol/l β-glycerophosphate and 50 mmol/l
sodium fluoride) to obtain total proteins. Nuclear proteins were
extracted using a CelLytic™ NuCLEAR™ Extraction kit (Sigma-Aldrich;
Merck KGaA) according to the manufacturer's protocol. Proteins were
quantified using a BCA Protein Assay kit (cat. no. P0012S; Beyotime
Institute of Biotechnology, Shanghai, China), and 30 µg protein per
lane was separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto 0.22-mm nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA). The nitrocellulose
membranes were blocked using 1% bovine serum albumin for 20 min at
37°C. Subsequent to washing with tris-buffered saline (TBS; 10 mM
Tris, 154 mM NaCl, pH=7.5 with 0.1% Tween-20) for 5 min at room
temperature 3 times, the membranes were incubated overnight at 4°C
with goat anti-rat Nkd2 (1:100; cat. no. sc163145; Santa Cruz
Biotechnology, Inc.), rabbit anti-rat β-catenin (1:1,000; cat. no.
ab6302; Abcam, Cambridge, MA, USA), rabbit anti-rat runt-related
transcription factor 2 (Runx2; 1:1,000; cat. no. ab23981; Abcam),
rabbit anti-rat osteocalcin (OCN; 1:200; cat. no. AB10911; EMD
Millipore), rabbit anti-rat β-actin (1:1,000; cat. no. G3202; Wuhan
Saiwei Biotechnology Co., Ltd., Wuhan, China), rabbit anti-rat
Cyclin D1 (1:1,000; cat. no. #2978; Cell Signaling Technology,
Inc., Danvers, MA, USA), rabbit anti-rat c-Myc (1:1,000; cat. no.
#5605; Cell Signaling Technology, Inc.), mouse anti-rat GAPDH
(1:1,000; cat. no. #5174; Cell Signaling Technology, Inc.) and
rabbit anti-rat histone H3 (1:500; cat. no. ab8580; Abcam)
antibodies. The membranes were washed with TBS for 5 min at room
temperature 3 times. Proteins were detected by incubated with
HRP-conjugated secondary antibody rabbit anti-goat immunoglobulin G
(IgG; 1:3,000; cat. no. A21030; Abbkine Scientific Co., Ltd.), goat
anti-rabbit IgG (1:3,000; cat. no. A0208; Beyotime Institute of
Biotechnology) and goat anti-mouse IgG (1:3,000; cat. no. A0216;
Beyotime Institute of Biotechnology) at 37°C for 2 h and according
to the enhanced chemiluminescence method (EMD Millipore).
Densitometric analysis of the protein bands was performed using
ImageJ software 1.48 version (National Institutes of Health,
Bethesda, MD, USA).
Simple western size assay
Cells (si-Nkd2- or si-NC-transfected, and control or
treated by Wnt3a) at a density of ≥106 per group were
harvested with RIPA lysis buffer (10 mmol/l Tris-HCl, 1 mmol/l
EDTA, 1% sodium dodecyl sulfate, 1% Nonidet P-40 and 1:1,000
proteinase inhibitor cocktail) for 30 min at 0°C for protein
extraction and western blot analysis. Cellular extracts were
prepared with Lysis kit-RIPA buffer (ProteinSimple, San Jose, CA,
USA). Wes Antibody Diluent2 included in the respective Wes Master
kit was used as the blocking buffer and used undiluted. Cellular
extracts were blocked with the Wes Antibody Diluent2 for 30 min at
room temperature. Immunoreactive proteins were detected using
Wes-Rabbit (12-230 kDa) Master kit (cat. no. PS-MK01;
ProteinSimple) and Wes-Mouse (12-230 kDa) Master kit (cat. no.
PS-MK02; ProteinSimple) by the Wes™ Simple Western System
(ProteinSimple) according to the manufacturer's protocol. Rabbit
anti-rat Runx2 (1:20; cat. no. ab23981; Abcam), Collagen-I (1:10;
cat. no. ab90395; Abcam), ALP (1:10; cat. no. ab83259; Abcam) and
β-catenin (1:50; cat. no. ab23981; Abcam) antibodies were used as
primary antibodies for detecting Runx2, Collagen-I and ALP,
respectively. Cellular extracts were incubated with the primary
antibodies for 30 min at room temperature. As the loading control,
β-tubulin was detected using a mouse anti-rat β-tubulin monoclonal
antibody (1:50; cat. no. AF7011; Affinity Biosciences, Cincinnati,
OH, USA). The secondary antibodies were included in the respective
Wes Master kit and used undiluted. Cellular extracts were incubated
with the secondary antibodies for 30 min at room temperature.
Quantitation of the detected proteins and image preparation were
performed with Compass Software 2.5.8.0 (ProteinSimple).
Parameters: Sample separation time, 25 min; separation voltage, 375
V.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of control and induced (0, 1, 2, 3 and
4 weeks), and si-Nkd2- or si-NC-transfected cells was isolated with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. mRNA quantification was
performed using an ABI 7300 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Complementary DNA
(cDNA) was synthesized with a RevertAid First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Inc.) in a total volume of 20 µl. RT
was performed at 37°C for 1 h followed by incubation for 10 min at
70°C to inactivate the reverse transcriptase, following which 5 µl
of the reaction mixture was incubated with FastStart Universal SYBR
Green Master (Roche Diagnostics, Basel, Switzerland) in a total
volume of 10 µl. The primers used for qPCR are listed in Table I. All reactions were performed
with a hot-start preincubation step of 10 min at 95°C, followed by
40 cycles of 15 sec at 95°C, 60 sec at 60°C and a final 5 min step
at 60°C. The amount of template was quantified using the
comparative cycle quantification cycle method according to the ABI
7300 Real-Time PCR System manufacturer's protocol. Relative gene
expression was calculated with GAPDH as an internal control using
the formula 2−ΔΔCq (25).
 | Table IReverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Gene | Primer
sequences |
|---|
| Runt related
transcription factor 2 | Forward:
5′-TAGAGGGGATGCCTTAGTG |
| Reverse:
5′-GAGGATGGAGGGAAACAA |
| Osteocalcin | Forward:
5′-AGCAGGAGGGCAGTAAGG |
| Reverse:
5′-TCCAGGGGATCTGGGTAG |
| β-catenin | Forward:
5′-TGGTGAAAATGCTTGGGTCG |
| Reverse:
5′-TCTGAAGGCAGTCTGTCGTAATAG |
| Naked cuticle
homolog 2 | Forward:
5′-ATCTGCCGCTGTGTGAGTGT |
| Reverse:
5′-CGTTTGTTTCCCATCTCTTTAGGT |
| β-actin | Forward:
5′-TGCTATGTTGCCCTAGACTTCG |
| Reverse:
5′-GTTGGCATAGAGGTCTTTACGG |
Luciferase assay
Activation of the Wnt/β-catenin signaling pathway
was measured using the activity of the transcription factor T-cell
factor (TCF)/lymphoid enhancer factor (LEF), a direct downstream
modulator of Wnt signaling, as previously described (26). The luciferase assay was performed
following the RT protocol described by the manufacturer of TCF/LEF
Reporter Assay kit (Qiagen GmbH, Hilden, Germany), which contains a
TCF/LEF luciferase reporter construct in addition to a negative
control (Cignal negative control; non-inducible firefly luciferase
reporter construct) and a positive control (Cignal positive
control; constitutively expressed firefly lucif-erase construct).
The pGL4 Luciferase Reporter Vectors were used as the plasmid
vectors as described by the manufacturer of TCF/LEF Reporter Assay
kit. The reporter was a mixture of inducible transcription factor
responsive construct and constitutively expressing Renilla
luciferase construct (40:1). The inducible transcription
factor-responsive construct encodes the firefly luciferase reporter
gene under the control of a basal promoter element (TATA box)
joined to tandem repeats of a specific Transcriptional Response
Element. The negative control was a mixture of non-inducible
reporter construct and constitutively expressing Renilla
luciferase construct (40:1). The noninducible reporter construct
encodes firefly luciferase under the control of a basal promoter
element (TATA box), without any additional transcriptional response
elements. The positive control was a constitutively expressing GFP
construct, pre-mixed with a constitutively expressing firefly
luciferase construct, and a constitutively expressing
Renilla luciferase construct (40:1:1).
For co-transfection with si-Nkd2, rDFSCs were seeded
at a density of 4×105 cells/ml and cultured in α-MEM
containing 5% FBS without antibiotics. The constructs were diluted
in 25 ml Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.),
mixed with Lipofectamine RNAiMax reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated at room temperature for 20 min to
allow formation of the transfection complex. Subsequently, the
mixture was added to cells in 96-well culture plates. After 24 h of
transfection at RT, signals were assessed using
Dual-Luciferase® Reporter Assay System (Promega
Corporation, Madison, WI, USA). Luciferase activity in control
(si-NC-transfected) cells was used for calibration.
Rescue experiment
si-Nkd2-transfected rDFSCs were seeded in 6-well
culture plates at a density of 105/ml and 200 ml cell
suspension per well. si-Nkd2-transfected rDFSCs were treated with
100 ng/ml Wnt3a or mock-treated and osteoblast induction medium at
37°C for 7 days. rDFSCs cultured in osteoblast induction medium at
37°C for 7 days served as the control. A simple western size assay
was performed to assess the levels of ALP, Runx2 and β-catenin
proteins.
Statistical analysis
All experiments were performed at least 3 times.
Unpaired Student's t-test was used to compare the means of two
groups. One-way analysis of variance was applied to compare more
than two means and Bonferroni's test was performed to compare the
means between the groups. The Kruskal-Wallis test was used to
assess the variance of heterogeneity. All results were evaluated
using SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA).
The data were expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significance
difference.
Results
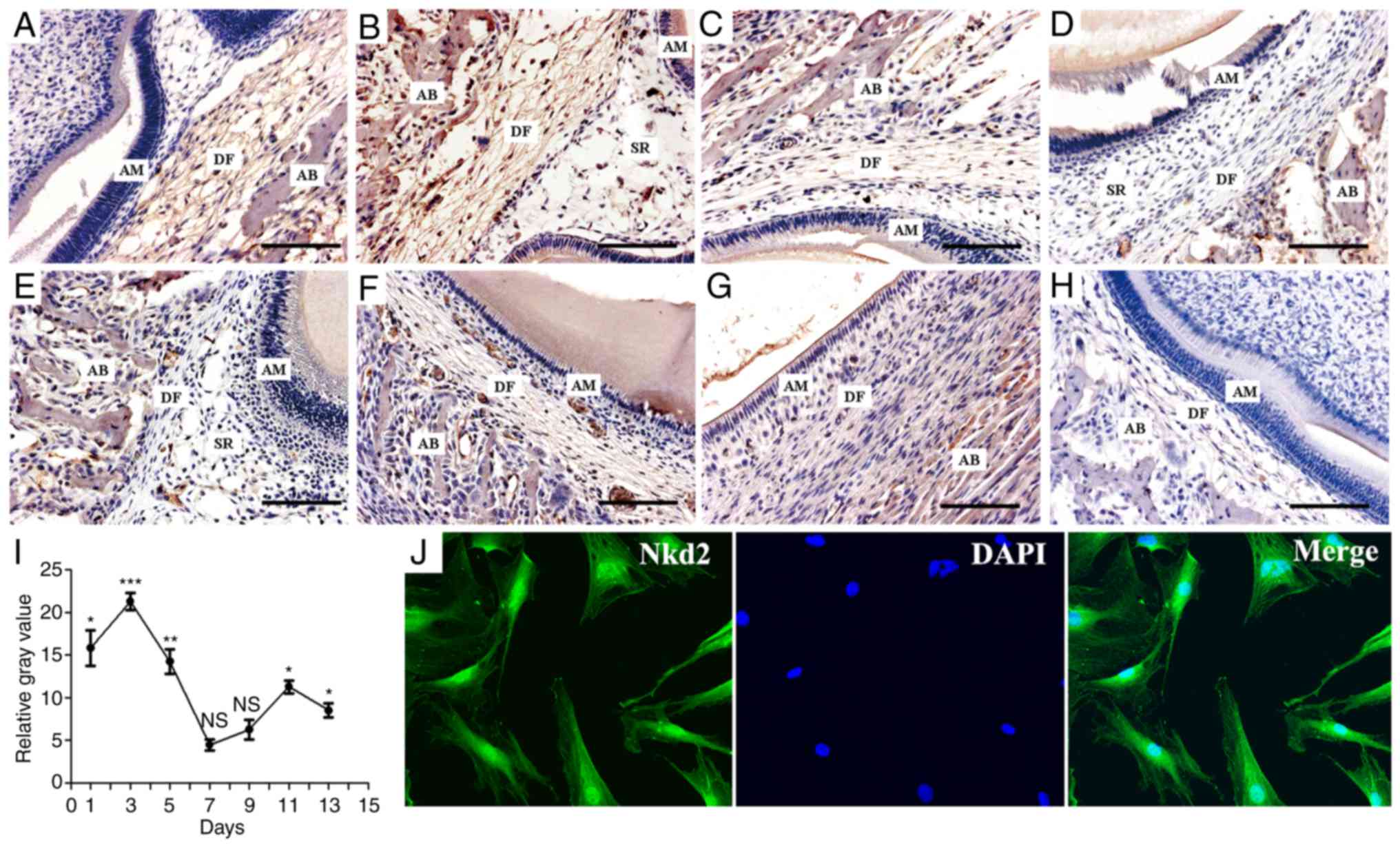
Expression of Nkd2 in rDFs
Nkd2 staining was positive on postpartum day 1 in
the DF of newborn rats (Fig. 1A).
Subsequently, Nkd2 expression peaked on day 3 (Fig. 1B) and became weaker on day 5
(Fig. 1C). Nkd2 staining was not
detected on days 7 and 9 (Fig. 1D and
E) but was detected again on days 11 (Fig. 1F) and 13 (Fig. 1G). The expression of Nkd2 in the
DF at postpartum days 1, 3, 5, 11 and 13 were significantly higher
compared with the respective days in the negative control group
(Fig. 1H) (P<0.05, P<0.001,
P<0.01, P<0.05 and P<0.05, respectively. Fig. 1I). However, at postpartum days 7
and 9, the difference was not significant (P>0.05). In addition,
primary rDFSCs were isolated from the first mandibular molars of
Sprague-Dawley rats, and the localization of Nkd2 in the
cytomembrane, cytoplasm and nucleus of rDFSCs was observed by
immunofluorescence and confocal laser-scanning microscopy (Fig. 1J).
Nkd2 expression is upregulated in rDFSCs
following osteogenic induction
Subsequent to culturing for 4 weeks in osteoblast
induction medium, RT-qPCR (Fig.
2A) and western blotting (Fig.
2B) revealed that Nkd2 expression was upregulated in rDFSCs
following osteogenic induction. This result was consistent with the
observed expression of β-catenin, Runx2 and OCN. The expression of
Nkd2 mRNA was significantly increased at week 1, 2, 3 and 4
compared with the control group (P<0.01, P<0.001, P<0.001
and P<0.001, respectively), which was the similar to the mRNA
expression of β-catenin (P<0.001, P<0.001, P<0.001 and
P<0.001, respectively), Runx2 (P<0.001, P<0.001,
P<0.001 and P<0.001, respectively) and OCN (P>0.05,
P>0.05, P>0.05 and P<0.001, respectively) compared with
the control group. The expression of Nkd2 protein was also
significantly upregulated on week 3 and 4 compared with the control
group (P<0.05 and P<0.01) similar to the protein levels of
β-catenin (P<0.001 and P<0.001), Runx2 (P<0.001 and
P<0.001) and OCN (P<0.05 and P<0.01).
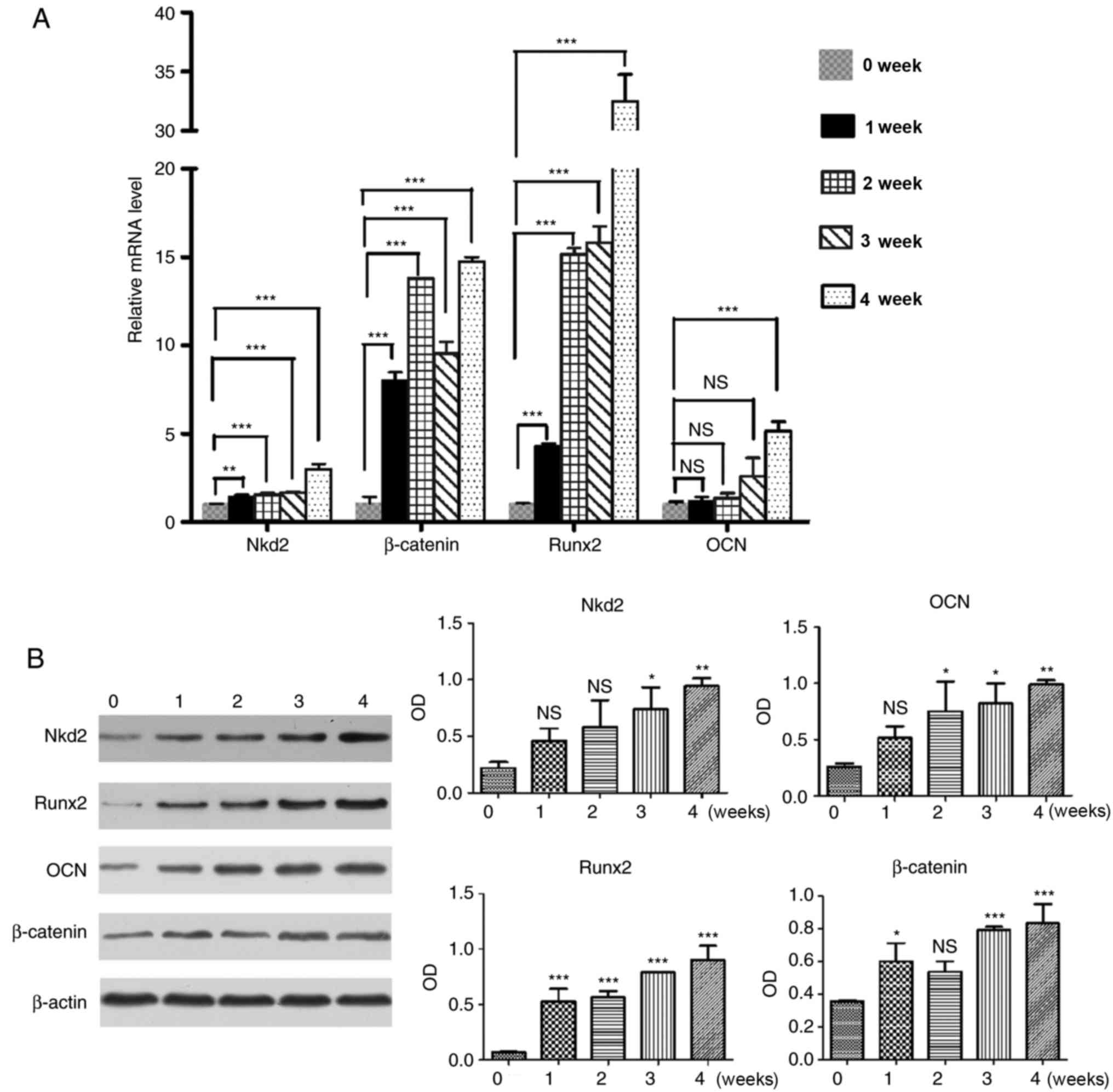 | Figure 2Reverse transcription-quantitative
polymerase chain reaction and western blotting were performed in
rDFSCs following 1, 2, 3 and 4 weeks of culturing in osteogenic
induction medium to detect the expression of Nkd2, β-catenin, Runx2
and OCN. rDFSCs harvested prior to the addition of osteogenic
induction medium (at 80% confluence) served as controls. (A)
Expression of Nkd2 mRNA was upregulated in rDFSCs following
culturing in osteogenic induction medium, consistent with the
expression of β-catenin, Runx2 and OCN. (B) Expression of Nkd2
protein was upregulated in rDFSCs following culturing in osteogenic
induction medium, consistent with expression of β-catenin, Runx2
and OCN. *P<0.05, **P<0.01 and
***P<0.001 vs. values at week 0. rDFSC, rat dental
follicle stem/progenitor cell; Nkd2, naked cuticle homolog 2;
Runx2, runt-related transcription factor 2; OCN, osteocalcin; OD,
optical density; NS, not significant. |
Involvement of Nkd2 in rDFSC
proliferation and migration abilities
RT-qPCR and western blotting were employed to
investigate the efficiency of si-Nkd2 transfection in rDFSCs. The
mRNA and protein levels of Nkd2 were significantly reduced compared
with the si-NC transfected group (P<0.05 and P<0.01,
respectively; Fig. 3A and B).
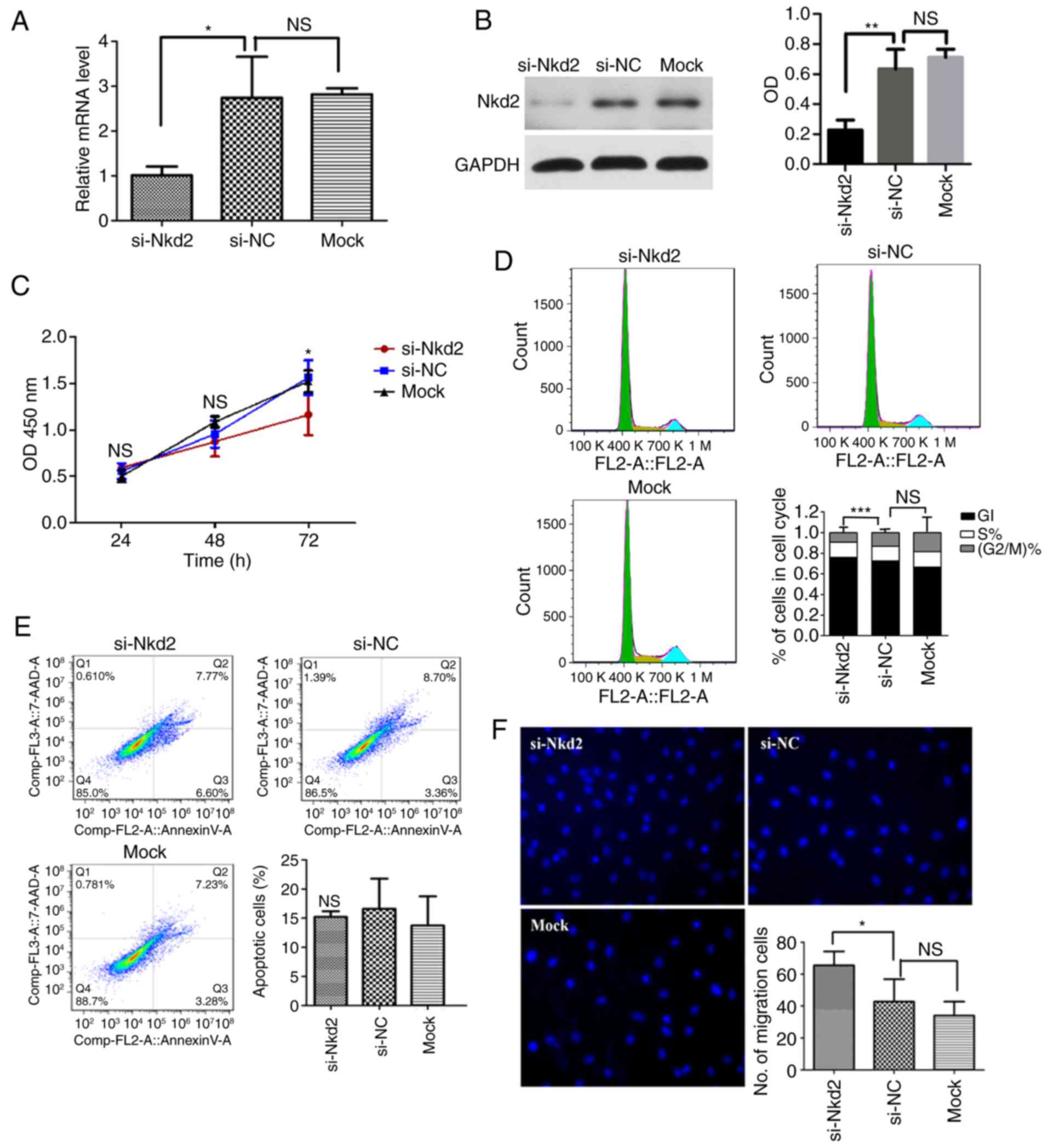 | Figure 3Effect of si-Nkd2 transfection on
rDFSCs. (A) Efficiency of si-Nkd2 transfection in rDFSCs determined
by reverse transcription-quantitative polymerase chain reaction. A
total of 48 h after si-Nkd2 transfection, the levels of Nkd2 mRNA
in the si-Nkd2 group were significantly lower compared with that in
the si-NC group, but there was no difference between the si-NC and
Mock groups. (B) Efficiency of si-Nkd2 transfection in rDFSCs
determined by western blotting. A total of 72 h after si-Nkd2
transfection, the Nkd2 protein levels in the si-Nkd2 group were
significantly lower compared with that in the si-NC group, but
there was no difference between the si-NC and Mock groups. (C) Cell
Counting Kit-8 assay results revealed that the proliferation of
si-Nkd2-transfected rDFSCs was significantly lower compared with
that of si-NC-transfected rDFSCs at 72 h after transient
transfection. (D) A total of 72 h after transient transfection,
cells were arrested in the G1 phase, and their rate of entry into
G2 phase was diminished. (E) Silencing of Nkd2 had no significant
effect on apoptosis rates in rDFSCs. (F) Transwell assay results
demonstrated significantly increased migration in Nkd2-silenced
rDFSCs. *P<0.05 and **P<0.01 with
comparisons shown by lines. si-, small interfering RNA; Nkd2, naked
cuticle homolog 2; NC, negative control; NS, not significant; OD,
optical density; rDFSC, rat dental follicle stem/progenitor
cell. |
Silencing of Nkd2 expression significantly decreased
the proliferation of rDFSCs after 72 h of transfection compared
with the si-NC group (P<0.05; Fig.
3C). To assess whether si-Nkd2-transfected rDFSCs exhibited
decreased proliferation or whether these cells underwent apoptosis,
the cell cycle distribution and apoptosis rate were investigated.
It was revealed that Nkd2 silencing arrested cells in the G1 phase
and significantly reduced their rate of entry into the G2 phase
compared with the si-NC group (P<0.001; Fig. 3D). Apoptosis analysis revealed
that fewer cells underwent apoptosis due to si-Nkd2 transfection
compared with the si-NC group, however this difference was not
significant (P>0.05; Fig. 3E).
Therefore, si-Nkd2 inhibited cell cycle progression instead of
affecting survival. Furthermore, a Transwell assay revealed the
significantly increased migration of rDFSCs silenced for Nkd2
compared with the si-NC group (P<0.05; Fig. 3F).
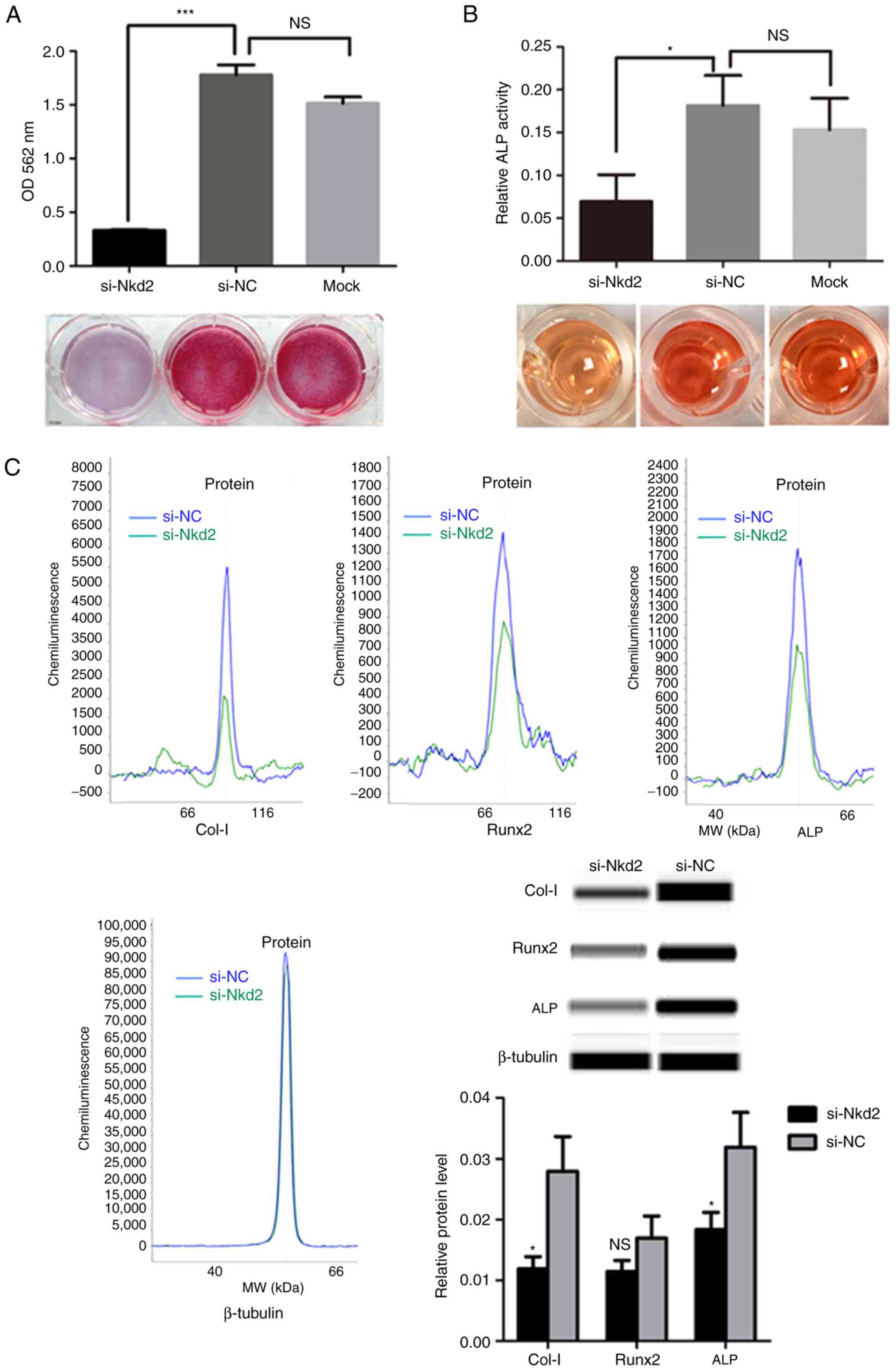
siRNA-mediated depletion of nkd2 reduces
differentiation of rDFSCs to osteoblasts
Subsequent to culturing in osteoblast induction
medium for 7 days, Alizarin Red staining demonstrated a significant
reduction in the formation of mineralized nodules for Nkd2-silenced
rDFSCs compared with the si-NC group (P<0.001; Fig. 4A). Furthermore, ALP activity was
significantly lower in si-Nkd2-transfected cells compared with in
si-NC- and Mock-transfected cells (P<0.05; Fig. 4B). As an indication of osteoblast
differentiation, the expression of osteoblast-associated markers
were examined using a simple western size assay. Collagen-I and ALP
protein levels were significantly lower in si-Nkd2-transfected
rDFSCs compared with in si-NC-transfected rDFSCs subsequent to
culturing in osteoblast induction medium for 7 days (P<0.05).
Runx2 expression was also reduced in Nkd2-silenced rDFSCs, albeit
in a non-significant manner (P>0.05; Fig. 4C).
Effect of Nkd2 on Wnt/β-catenin signaling
in rDFSCs
A luciferase activity assay at 72 h after si-Nkd2
transfection revealed that TCF/LEF activity was significantly
reduced in Nkd2-silenced rDFSCs with Cignal reporter compared with
the si-NC transfected group with the Cignal reporter (P<0.05) or
compared with the Cignal positive control (P<0.001; Fig. 5A). Furthermore, western blotting
of the nuclear proteins revealed that the expression of β-catenin,
cyclin D1 and c-Myc was significantly lower in si-Nkd2-transfected
rDFSCs compared with in si-NC-transfected rDFSCs (P<0.05,
P<0.01 and P<0.05, respectively; Fig. 5B).
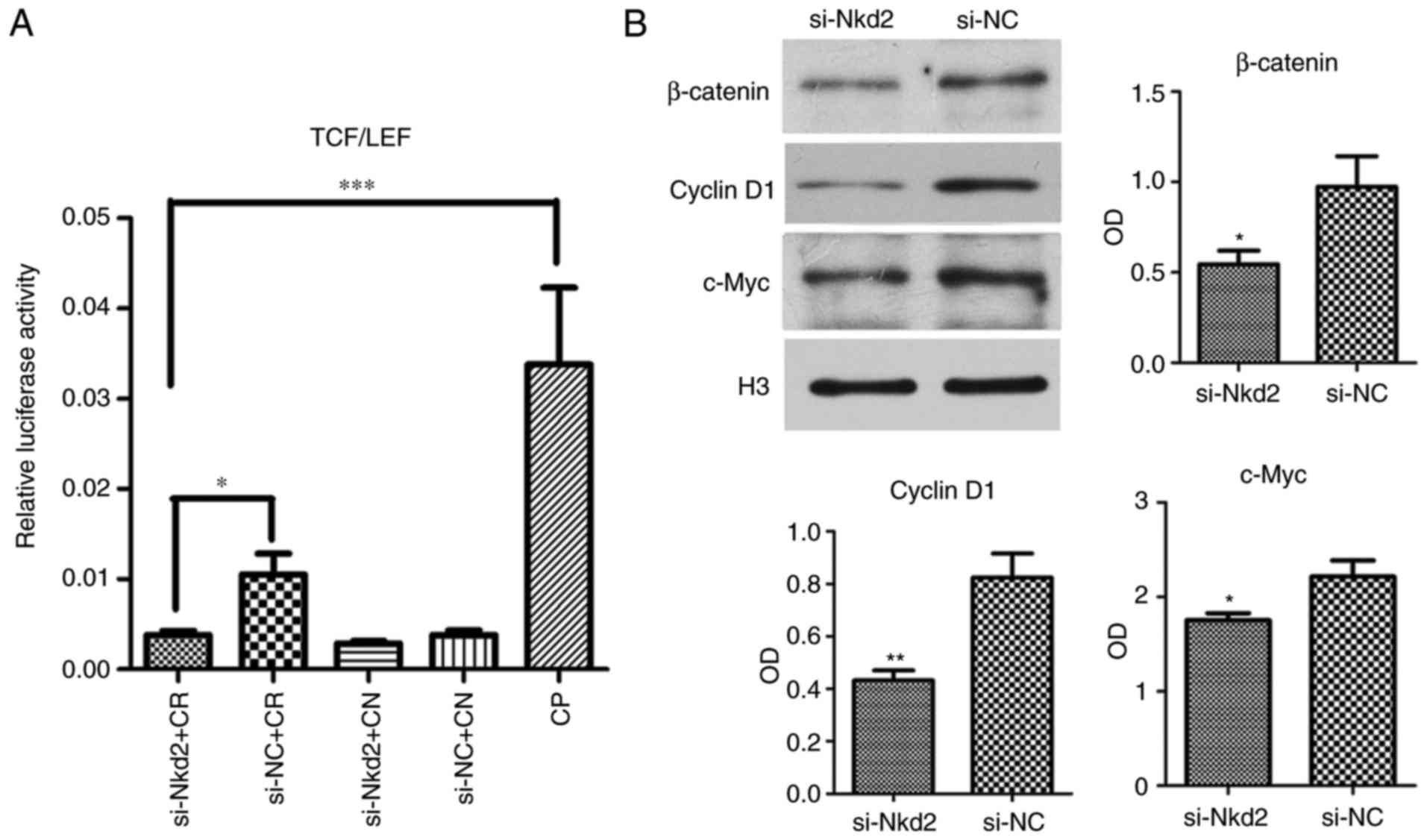 | Figure 5si-Nkd2 transfection reduced activity
of the Wnt/β-catenin pathway. (A) Luciferase activity at 72 h after
transfection was significantly lower in si-Nkd2-transfected rDFSCs
compared with in si-NC-transfected rDFSC. (B) Western blotting
revealed that the expression of β-catenin, cyclinD1 and c-Myc was
signficantly lower in si-Nkd2-transfected rDFSCs compared with in
si-NC-transfected rDFCs. *P<0.05,
**P<0.01 or ***P<0.001 with comparisons
shown by lines or vs. the si-NC group. TCF, LEF, si-, Nkd2, CR,
Cignal reporter; CN, Cignal negative control; NC, negative control;
CP, Cignal positive control; rDFSC, rat dental follicle
stem/progenitor cell. |
Next, si-Nkd2-transfected rDFSCs were treated with
Wnt3a or mock-treated and cultured in osteoblast induction medium
for 7 days. According to the results of the simple western size
assay, the protein levels of ALP, Runx2 and β-catenin were
significantly increased in Wnt3a-treated si-Nkd2-transfected rDFSCs
compared with si-Nkd2-transfected rDFSCs (P<0.001, P<0.05 and
P<0.05, respectively; Fig.
6).
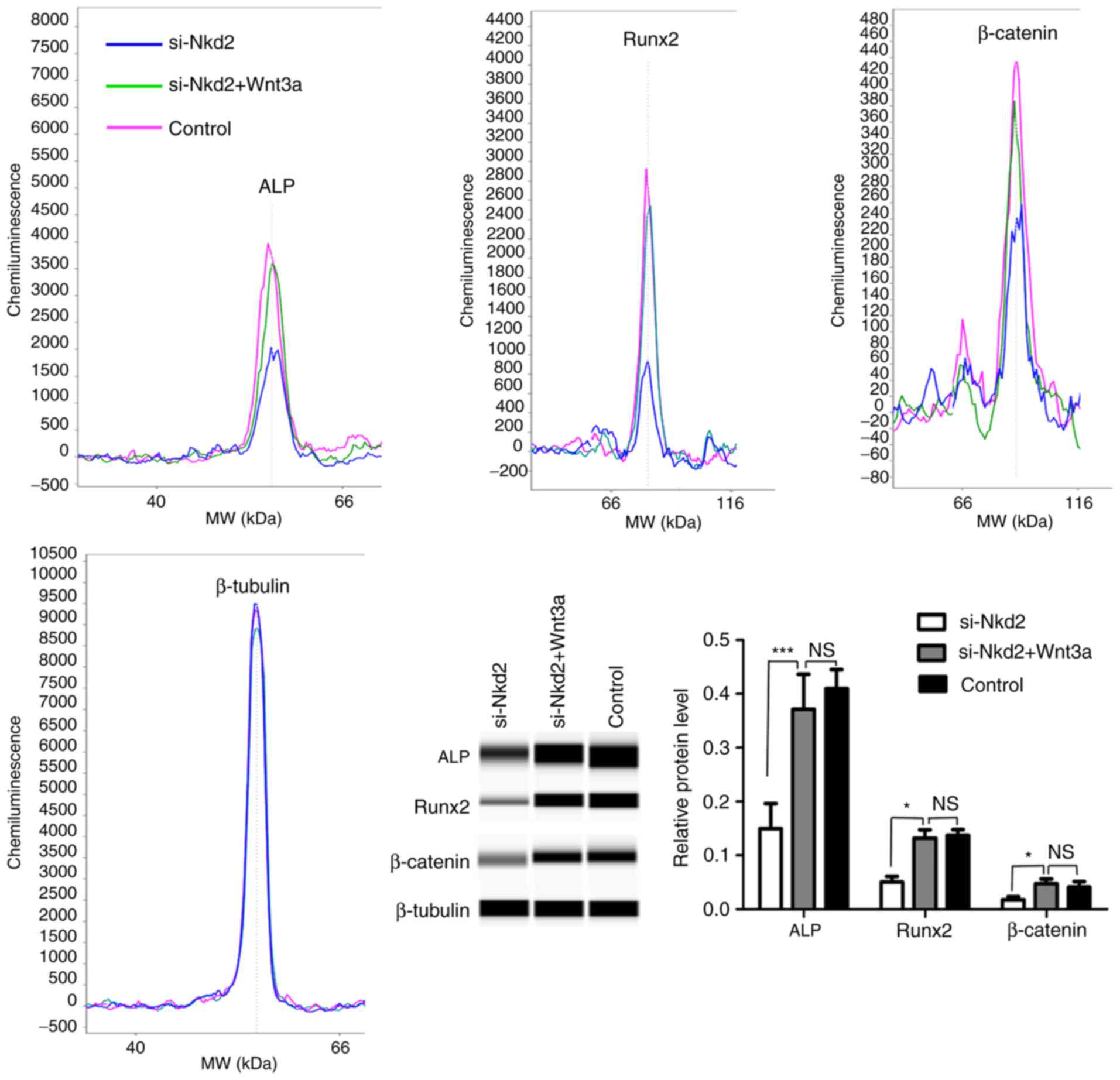 | Figure 6A simple western size assay
demonstrating the levels of ALP, Runx2 and β-catenin proteins in
Wnt3a- or mock-treated si-Nkd2-transfected rDFSCs.
si-Nkd2-transfected rDFSCs were treated with Wnt3a or mock-treated
and cultured in osteoblast induction medium for 7 days. Cells were
harvested, and the protein levels of ALP, Runx2 and β-catenin
proteins were analyzed by a simple western size assay.
Significantly lower levels of ALP, Runx2 and β-catenin proteins
were observed in si-Nkd2-transfected rDFSCs, and higher levels of
proteins were observed in Wnt3a-treated cells compared with the
control. rDFSCs cultured in osteoblast induction medium served as
the control. *P<0.05 and ***P<0.001
with comparisons shown by lines. NS, not significant; si-, small
interfering RNA; Nkd2, naked cuticle homolog 2; ALP, alkaline
phosphatase; Runx2, runt-related transcription factor 2; rDFSC, rat
dental follicle stem/progenitor cell. |
Discussion
During tooth development, DFSCs give rise to the
cementoblasts of the tooth and osteoblasts of the alveolar bone
(27). Previously, rDFSCs with
multipotential differentiation abilities were produced using tissue
explants combined with enzymatic digestion (7). Nkd2 is an inhibitor of the
Wnt/β-catenin pathway and is transcriptionally regulated by
β-catenin. This signal-inducible feedback antagonist serves an
important role during embryogenesis; in addition, Nkd2 is
associated with the suppressive effects of Wnt proteins on
development (16) and is
differentially expressed in different tissues (28).
Using in situ hybridization, Wang et
al (5) identified similar
expression patterns for Wnt/β-catenin signaling components,
including Wnt ligands (Wnt3 and Wnt5A), receptors [Frizzled class
receptor (FZD)4, FZD6 and LDL receptor related protein (LRP)5],
transducers (β-catenin), transcription factors (transcription
factor 4 and lymphoid enhancer binding factor 1) and antagonists
(Dickkopf WNT signaling pathway inhibitor 1 and sclerostin domain
containing 1) in the development of human and mouse tooth germ.
These results suggest an essential role of Wnt/β-catenin signaling
in the regulation of mammal tooth development. β-catenin is a key
component of the canonical Wnt pathway, and in a previous study,
the expression of this protein was absent in dental follicles on
days 1 and 3 in vivo, but progressively increased from days
5 to 13 (7). In the present
study, the expression of Nkd2 was high at an early stage, unlike
β-catenin, but was similar at later stages, suggesting its
involvement in the development of teeth.
Studies have revealed that Wnt/β-catenin signaling
may be involved in the osteogenic differentiation of DFSCs. In
mice, the Wnt/β-catenin pathway regulates the bone morphogenetic
protein 2-mediated differentiation of DFSCs (9), and the protein and mRNA levels of
ALP, Runx2 and osterix are increased by Wnt3a treatment (11). Hertwig's epithelial root sheath
cells regulate osteogenic differentiation of DFSCs through the Wnt
pathway (10). Additionally, a
previous study revealed that LiCl treatment upregulated the levels
of total β-catenin, nuclear β-catenin, OCN, Runx2 and Collagen-I
and suggested that Wnt/β-catenin signaling pathway positively
regulates cement-oblast/osteoblast differentiation of DFCs
(7). Runx2 and OCN are important
markers of osteoblast differentiation (29-31). Runx2 is an important transcription
factor that mediates osteogenesis. As the expression of Runx2 in
the mesenchyme occurs prior to formation of bone, it is known as an
early regulator of osteoblast differentiation. Gaur et al
(32) revealed that the
Wnt/β-catenin pathway promotes osteogenesis by directly stimulating
Runx2 gene expression. In the present study, Nkd2 expression was
upregulated in rDFSCs following osteogenic induction in
vitro, which was consistent with the expression of β-catenin,
Runx2 and OCN. Further analyses demonstrated that fewer mineralized
nodules formed in Nkd2-silenced rDFSCs compared with si-NC
transfected cells. Overall, the lower ALP activity (P<0.05) of
si-NKd2-transfected cells combined with the significantly lower
protein expression of osteoblast-associated markers Collagen-I and
ALP (P<0.05 and P<0.05, respectively) in si-Nkd2-transfected
rDFSCs indicated that the silencing of Nkd2 inhibited the
differentiation of rDFSCs into osteoblasts. Runx2 expression was
also reduced in Nkd2-silenced rDFSCs, albeit in a non-significant
manner. Thus, it was hypothesised that Nkd2 is involved in the
differentiation of rDFSCs into osteoblasts through Wnt/β-catenin
signaling.
In the Wnt/β-catenin pathway, following the
canonical Wnt ligand binding to the LRP5/LRP6 complex, the Dvl
protein is recruited to the membrane, which results in the
disassembly of the 'Wnt destruction complex', which includes Axin,
APC, WNT signaling pathway regulator, casein kinase 1 α 1 and
glycogen synthase kinase 3 β (GSK-3β). Inactivation of GSK-3β is
the central step of canonical Wnt signaling, allowing β-catenin
stabilization and translocation to the nucleus (33). Nkd2 has been reported to be
expressed in the cytomembrane of epithelial cells; myristoylated
Nkd2 was demonstrated to interact with Dvl-1, resulting in their
mutual ubiquitin-mediated proteasomal degradation, which
antagonizes Wnt signaling (17,19). However, in the present study, Nkd2
was observed in the cytomembrane, cytoplasm and nucleus of rDFSCs,
suggesting that the function of Nkd2 in rDFSCs may be different
from that in epithelial cells. Further investigation demonstrated
that the activity of TCF/LEF and the expression of β-catenin,
cyclin D1 and c-Myc were decreased in Nkd2-silenced rDFSCs, which
validated the hypothesis that Nkd2 sustains the Wnt/β-catenin
pathway in rDFSCs, despite being reported as a Wnt inhibitor
(17,19). These characteristics of Nkd2 are
similar to those of APC downregulated 1 (APCDD1), which is a
membrane-bound glycoprotein. In hair follicle cells, APCDD1
inhibits the canonical Wnt/β-catenin pathway, and its inactivation
is associated with an autosomal dominant form of hair loss
(26). However, during the
osteogenic differentiation of DFSCs, APCDD1 sustains the expression
and activation of β-catenin (26).
The expression levels of Cyclin D1, a protein
required for progression through the G1 phase of the cell cycle
(34), were decreased in the
nuclei of Nkd2-silenced rDFSCs in the present study. Cyclin D1
dimerizes with cyclin-dependent kinase 4/6 to regulate
G1/S-phase transition and entry into S phase (35). In the present study, Nkd2-silenced
rDFSCs were arrested in the G1 phase, and few cells underwent
apoptosis. Therefore, Nkd2 silencing inhibited cell cycle
progression as opposed to affecting cell survival. The effect of
Nkd2 on cell migration remains controversial. Adamowicz et
al (36) utilized a DNA
microarray analysis to reveal the high expression of Nkd2 in
subregions of malignant peripheral nerve sheath tumor types,
suggesting that Nkd2 is able to reduce the adhesion of tumor cells,
resulting in tumor metastasis. Other studies have reported that
Nkd2 suppresses tumor growth and metastasis in gastric (37) and breast cancer (38), and osteosarcoma (39). In the present study, the increased
migratory ability of si-Nkd2 rDFSCs was observed, which may have
been caused by a reduction in β-catenin levels and cell
adhesion.
The Wnt3a ligand has been recognized as a canonical
Wnt activator. ALP is another marker of osteoblast differentiation
(40). The present study detected
the protein expression of ALP, Runx2 and β-catenin in Nkd2-silenced
rDFSCs treated with Wnt3a or mock-treated and revealed the higher
levels of these proteins in Nkd2-silenced rDFSCs treated with
Wnt3a, which is able to activate the Wnt/β-catenin pathway. This
result suggested that the repression of rDFSC differentiation into
osteoblasts by si-Nkd2 transfection may be rescued by Wnt3a; thus,
Nkd2 regulates the differentiation of DFSCs into osteoblasts,
potentially via Wnt/β-catenin signaling.
It was concluded that Nkd2 serves a role in DFSCs
during osteoblast differentiation; it promotes the differentiation
of DFSCs into osteoblasts, potentially through the activation of
Wnt/β-catenin signaling. However, further studies are required in
order to reveal the mechanism of Wnt/β-catenin signaling activation
by Nkd2 in DFSCs.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170932 and
81700967), the Natural Science Foundation of Guangdong Province
(grant nos. 2017A030310595 and 2015A030313048) and the Science and
Technology Research Foundation of Shenzhen (grant no.
JCYJ20170303155435885).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CC and JL conceived and designed the study. CC, JZ,
YD and YH performed the experiments. CC and JZ wrote the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Ethics
Committee of the Guanghua College of Stomatology, Sun Yat-sen
University [Guangzhou, China; approval no. ERC-(2014)-21].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Felthaus O, Gosau M, Ettl T, Prantl L and
Morsczeck C: Migration of human dental follicle cells in vitro. J
Periodontal Res. 49:205–212. 2014. View Article : Google Scholar
|
|
2
|
Pan K, Sun Q, Zhang J, Ge S, Li S, Zhao Y
and Yang P: Multilineage differentiation of dental follicle cells
and the roles of Runx2 over-expression in enhancing
osteoblast/cement-oblast-related gene expression in dental follicle
cells. Cell Prolif. 43:219–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morsczeck C and Schmalz G: Transcriptomes
and proteomes of dental follicle cells. J Dent Res. 89:445–456.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu F and Millar SE: Wnt/β-catenin
signaling in oral tissue development and disease. J Dent Res.
89:318–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang B, Li H, Liu Y, Lin X, Lin Y, Wang Y,
Hu X and Zhang Y: Expression patterns of WNT/β-CATENIN signaling
molecules during human tooth development. J Mol Histol. 45:487–496.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiang L, Chen M, He L, Cai B, Du Y, Zhang
X, Zhou C, Wang C, Mao JJ and Ling J: Wnt5a regulates postnatal
dental follicle stem/progenitor cells. Stem Cell Res Ther.
5:1352014. View
Article : Google Scholar
|
|
7
|
Du Y, Ling J, Wei X, Ning Y, Xie N, Gu H
and Yang F: Wnt/β-catenin signaling participates in
cementoblast/osteoblast differentiation of dental follicle cells.
Connect Tissue Res. 53:390–397. 2012. View Article : Google Scholar
|
|
8
|
Nemoto E, Koshikawa Y, Kanaya S, Tsuchiya
M, Tamura M, Somerman MJ and Shimauchi H: Wnt signaling inhibits
cement-oblast differentiation and promotes proliferation. Bone.
44:805–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silvério KG, Davidson KC, James RG, Adams
AM, Foster BL, Nociti FH Jr, Somerman MJ and Moon RT: Wnt/β-catenin
pathway regulates bone morphogenetic protein (BMP2)-mediated
differentiation of dental follicle cells. J Periodontal Res.
47:309–319. 2012. View Article : Google Scholar
|
|
10
|
Yang Y, Ge Y, Chen G, Yan Z, Yu M, Feng L,
Jiang Z, Guo W and Tian W: Hertwig's epithelial root sheath cells
regulate osteogenic differentiation of dental follicle cells
through the Wnt pathway. Bone. 63:158–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nemoto E, Sakisaka Y, Tsuchiya M, Tamura
M, Nakamura T, Kanaya S, Shimonishi M and Shimauchi H: Wnt3a
signaling induces murine dental follicle cells to differentiate
into cementoblastic/osteoblastic cells via an osterix-dependent
pathway. J Periodontal Res. 51:164–174. 2016. View Article : Google Scholar
|
|
12
|
Bae CH, Lee JY, Kim TH, Baek JA, Lee JC,
Yang X, Taketo MM, Jiang R and Cho ES: Excessive Wnt/β-catenin
signaling disturbs tooth-root formation. J Periodontal Res.
48:405–410. 2013. View Article : Google Scholar
|
|
13
|
Kim TH, Lee JY, Baek JA, Lee JC, Yang X,
Taketo MM, Jiang R and Cho ES: Constitutive stabilization of
β-catenin in the dental mesenchyme leads to excessive dentin and
cementum formation. Biochem Biophys Res Commun. 412:549–555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang R, Yang G, Wu X, Xie J, Yang X and
Li T: Disruption of Wnt/β-catenin signaling in odontoblasts and
cementoblasts arrests tooth root development in postnatal mouse
teeth. Int J Biol Sci. 9:228–236. 2013. View Article : Google Scholar :
|
|
15
|
Martinez Arias A, Baker NE and Ingham PW:
Role of segment polarity genes in the definition and maintenance of
cell states in the Drosophila embryo. Development. 103:157–170.
1988.PubMed/NCBI
|
|
16
|
Zeng W, Wharton KA Jr, Mack JA, Wang K,
Gadbaw M, Suyama K, Klein PS and Scott MP: Naked cuticle encodes an
inducible antagonist of Wnt signalling. Nature. 403:789–795. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rousset R, Mack JA, Wharton KA Jr, Axelrod
JD, Cadigan KM, Fish MP, Nusse R and Scott MP: Naked cuticle
targets dishevelled to antagonize Wnt signal. Genes Dev.
15:658–671. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan D, Wallingford JB, Sun TQ, Nelson AM,
Sakanaka C, Reinhard C, Harland RM, Fantl WJ and Williams LT: Cell
autonomous regulation of multiple Dishevelled-dependent pathways by
mammalian Nkd. Proc Natl Acad Sci USA. 98:3802–3807. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu T, Li C, Cao Z, Van Raay TJ, Smith JG,
Willert K, Solnica-Krezel L and Coffey RJ: Myristoylated Naked2
antago-nizes Wnt-beta-catenin activity by degrading Dishevelled-1
at the plasma membrane. J Biol Chem. 285:13561–13568. 2010.
View Article : Google Scholar :
|
|
20
|
Van Raay TJ, Coffey RJ and Solnica-Krezel
L: Zebrafish Naked1 and Naked2 antagonize both canonical and
non-canonical Wnt signaling. Dev Biol. 309:151–168. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Franklin JL, Graves-Deal R, Jerome
WG, Cao Z and Coffey RJ: Myristoylated Naked2 escorts transforming
growth factor alpha to the basolateral plasma membrane of polarized
epithelial cells. Proc Natl Acad Sci USA. 101:5571–5576. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu T and Li C: Convergence between
Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 9:2362010.
View Article : Google Scholar
|
|
23
|
Chen C, Wei X, Ling J and Xie N:
Expression of matrilin-2 and -4 in human dental pulps during
dentin-pulp complex wound healing. J Endod. 37:642–649. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mane DR, Kale AD and Belaldavar C:
Validation of immunoexpression of tenascin-C in oral precancerous
and cancerous tissues using ImageJ analysis with novel
immunohistochemistry profiler plugin: An immunohistochemical
quantitative analysis. J Oral Maxillofac Pathol. 21:211–217. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
26
|
Viale-Bouroncle S, Klingelhöffer C, Ettl T
and Morsczeck C: The WNT inhibitor APCDD1 sustains the expression
of β-Catenin during the osteogenic differentiation of human dental
follicle cells. Biochem Biophys Res Commun. 457:314–317. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morsczeck C, Götz W, Schierholz J,
Zeilhofer F, Kühn U, Möhl C, Sippel C and Hoffmann KH: Isolation of
precursor cells (PCs) from human dental follicle of wisdom teeth.
Matrix Biol. 24:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Cagatay T, Amanai M, Zhang M,
Kline J, Castrillon DH, Ashfaq R, Oz OK and Wharton KA Jr: Viable
mice with compound mutations in the Wnt/Dvl pathway antagonists
nkd1 and nkd2. Mol Cell Biol. 27:4454–4464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kitagawa M, Tahara H, Kitagawa S, Oka H,
Kudo Y, Sato S, Ogawa I, Miyaichi M and Takata T: Characterization
of established cementoblast-like cell lines from human
cementumlining cells in vitro and in vivo. Bone. 39:1035–1042.
2006. View Article : Google Scholar
|
|
30
|
Lengner CJ, Drissi H, Choi JY, van Wijnen
AJ, Stein JL, Stein GS and Lian JB: Activation of the bone-related
Runx2/Cbfa1 promoter in mesenchymal condensations and developing
chondrocytes of the axial skeleton. Mech Dev. 114:167–170. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gaur T, Lengner CJ, Hovhannisyan H, Bhat
RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS,
et al: Canonical WNT signaling promotes osteogenesis by directly
stimulating Runx2 gene expression. J Biol Chem. 280:33132–33140.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Libro R, Bramanti P and Mazzon E: The role
of the Wnt canonical signaling in neurodegenerative diseases. Life
Sci. 158:78–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coqueret O: Linking cyclins to
transcriptional control. Gene. 299:35–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adamowicz M, Radlwimmer B, Rieker RJ,
Mertens D, Schwarzbach M, Schraml P, Benner A, Lichter P,
Mechtersheimer G and Joos S: Frequent amplifications and abundant
expression of TRIO, NKD2, and IRX2 in soft tissue sarcomas. Genes
Chromosomes Cancer. 45:829–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jia Y, Cao B, Yang Y, Linghu E, Zhan Q, Lu
Y, Yu Y, Herman JG and Guo M: Silencing NKD2 by promoter region
hypermethylation promotes gastric cancer invasion and metastasis by
up-regulating SOX18 in human gastric cancer. Oncotarget.
6:33470–33485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong Y, Cao B, Zhang M, Han W, Herman JG,
Fuks F, Zhao Y and Guo M: Epigenetic silencing of NKD2, a major
component of Wnt signaling, promotes breast cancer growth.
Oncotarget. 6:22126–22138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao S, Kurenbekova L, Gao Y, Roos A,
Creighton CJ, Rao P, Hicks J, Man TK, Lau C, Brown AM, et al: NKD2,
a negative regulator of Wnt signaling, suppresses tumor growth and
metastasis in osteosarcoma. Oncogene. 34:5069–5079. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manzano-Moreno FJ, Ramos-Torrecillas J,
Melguizo-Rodríguez L, Illescas-Montes R, Ruiz C and García-Martínez
O: Bisphosphonate modulation of the gene expression of different
markers involved in osteoblast physiology: Possible implications in
bisphosphonate-related osteonecrosis of the Jaw. Int J Med Sci.
15:359–367. 2018. View Article : Google Scholar : PubMed/NCBI
|