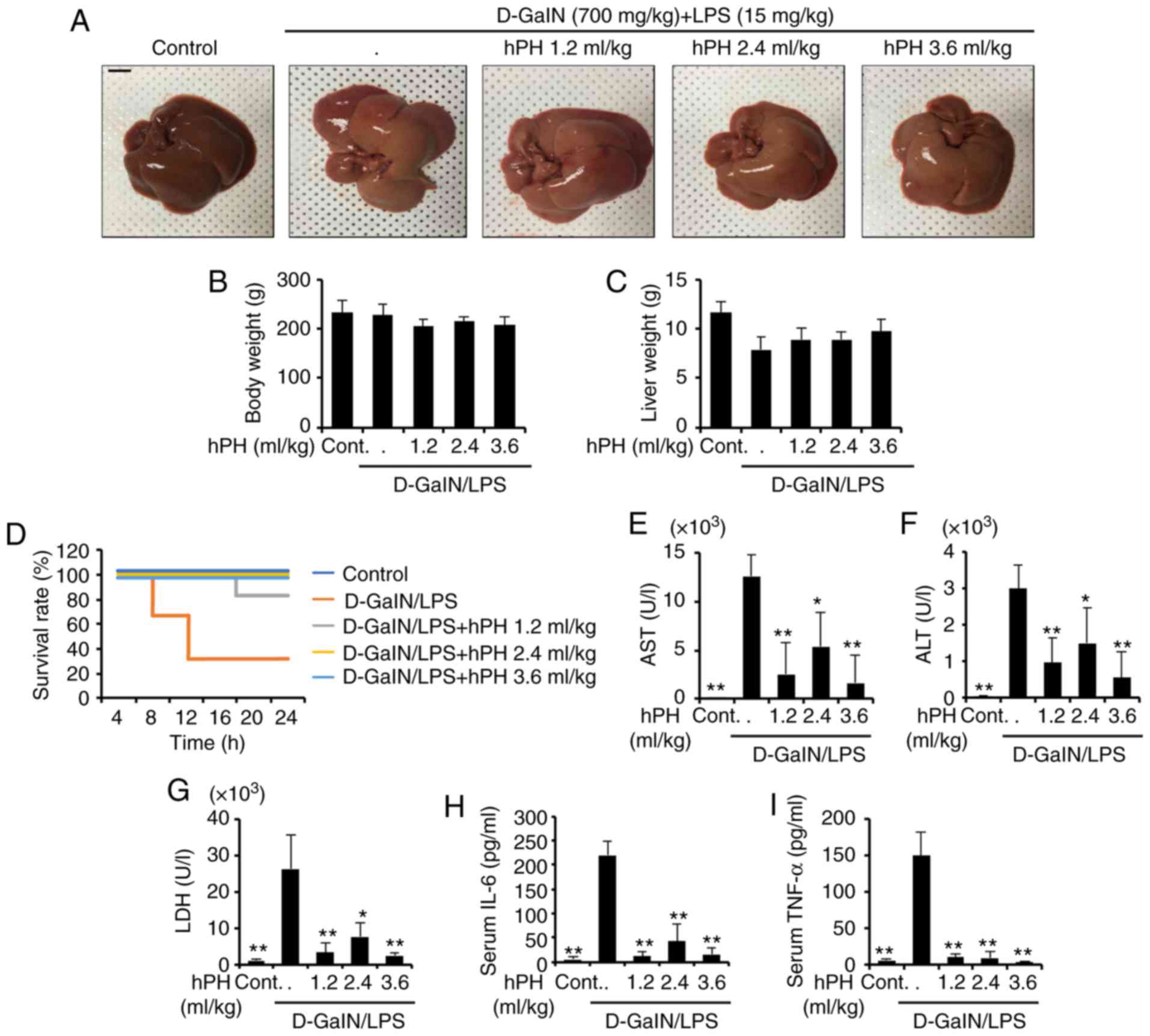
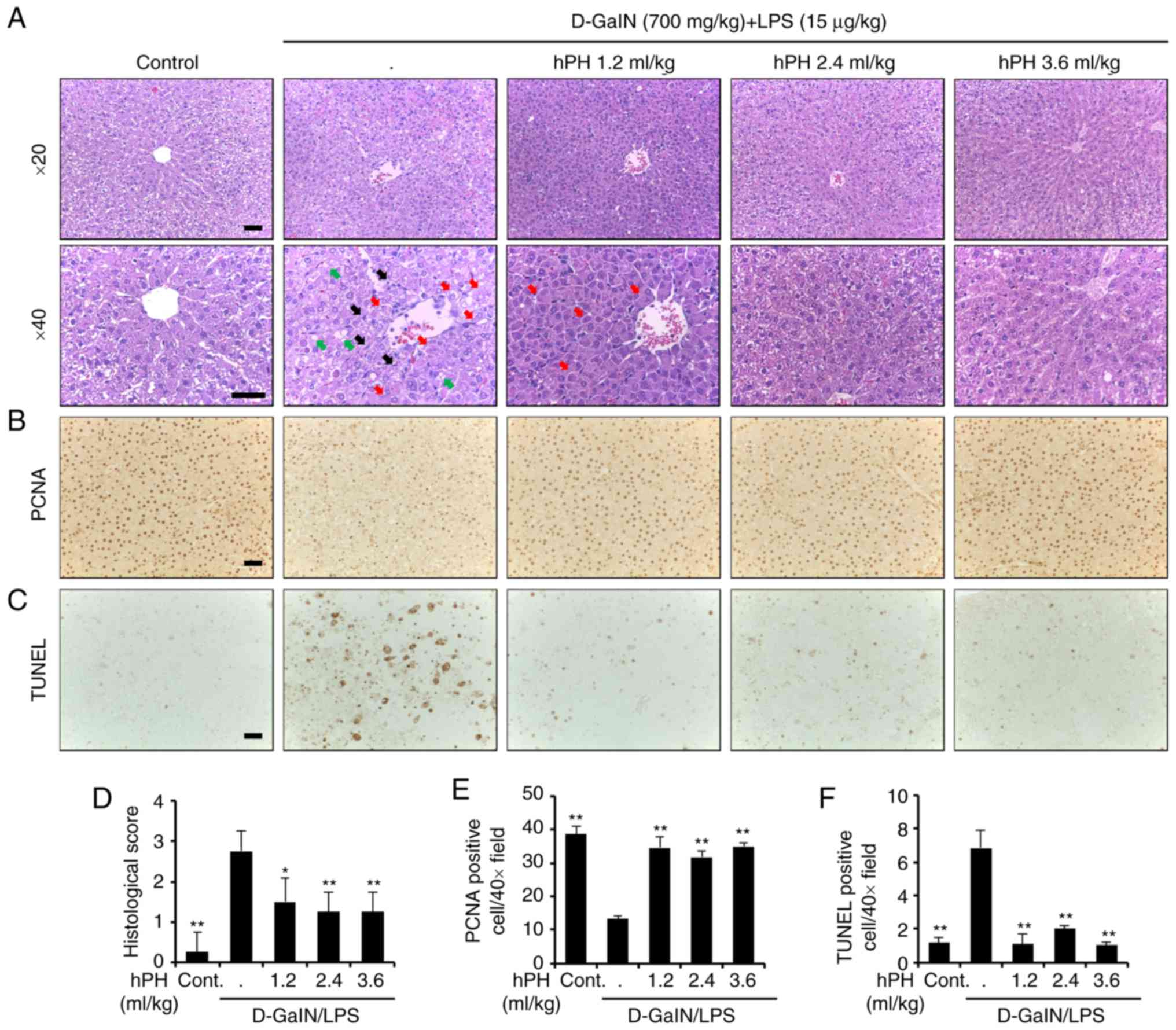
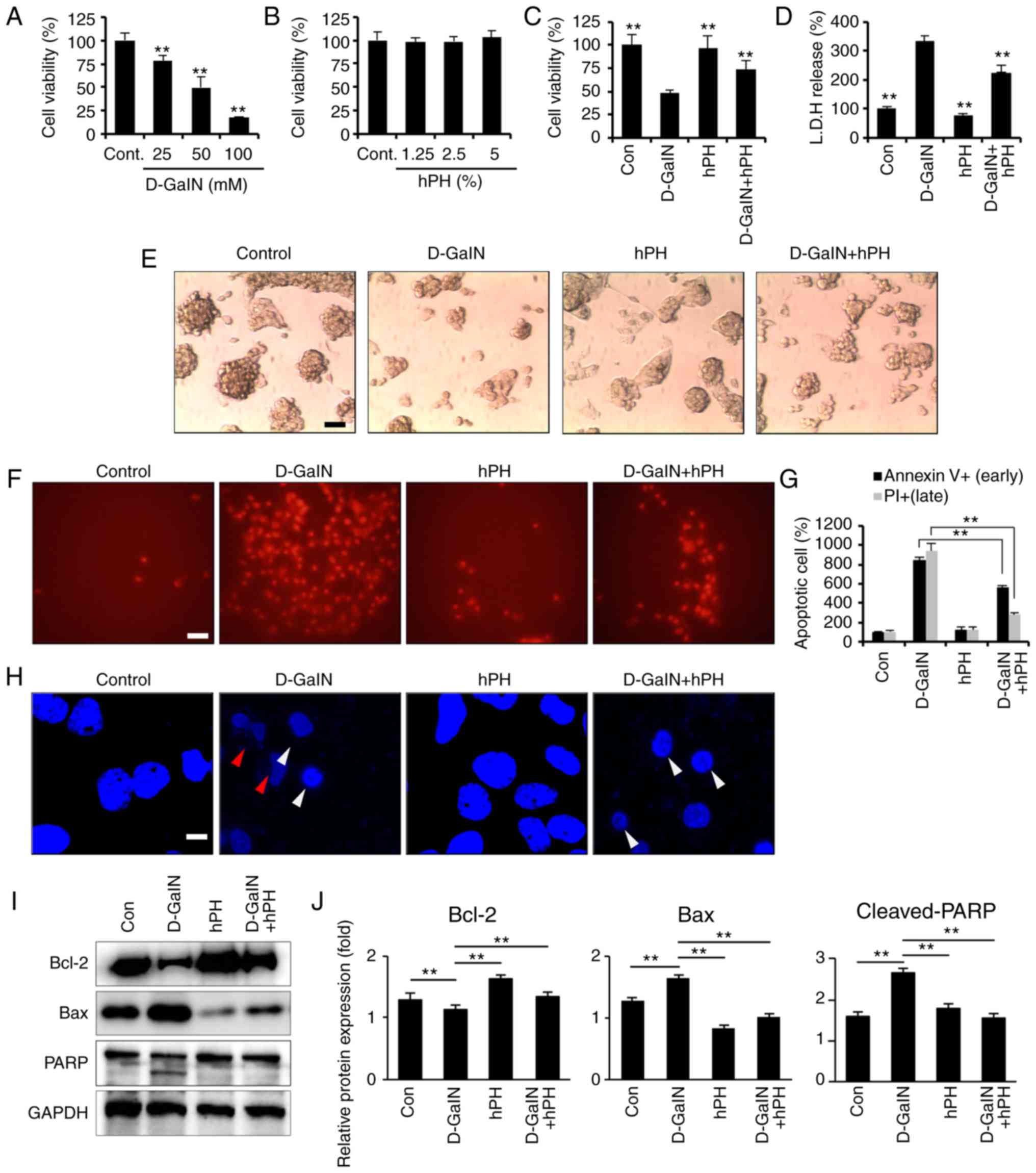
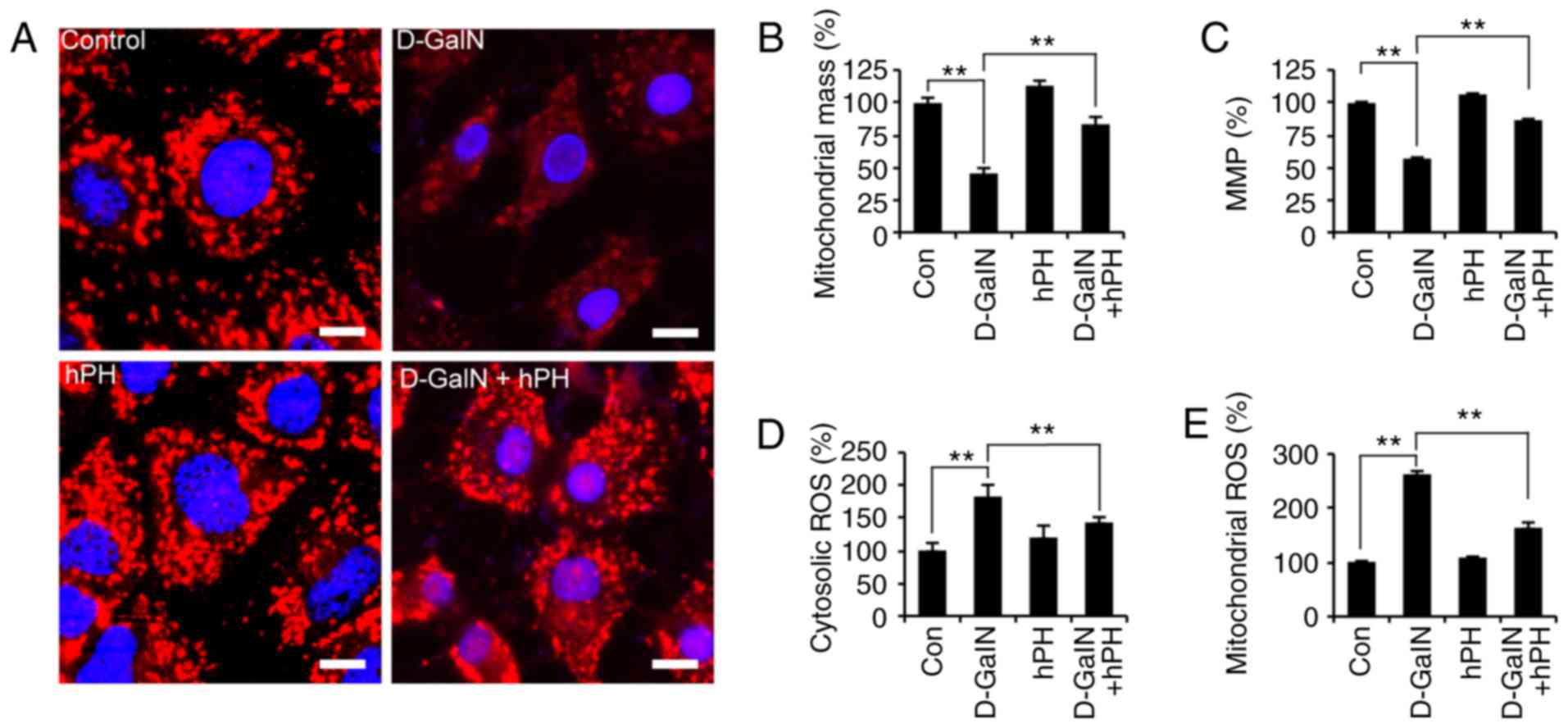
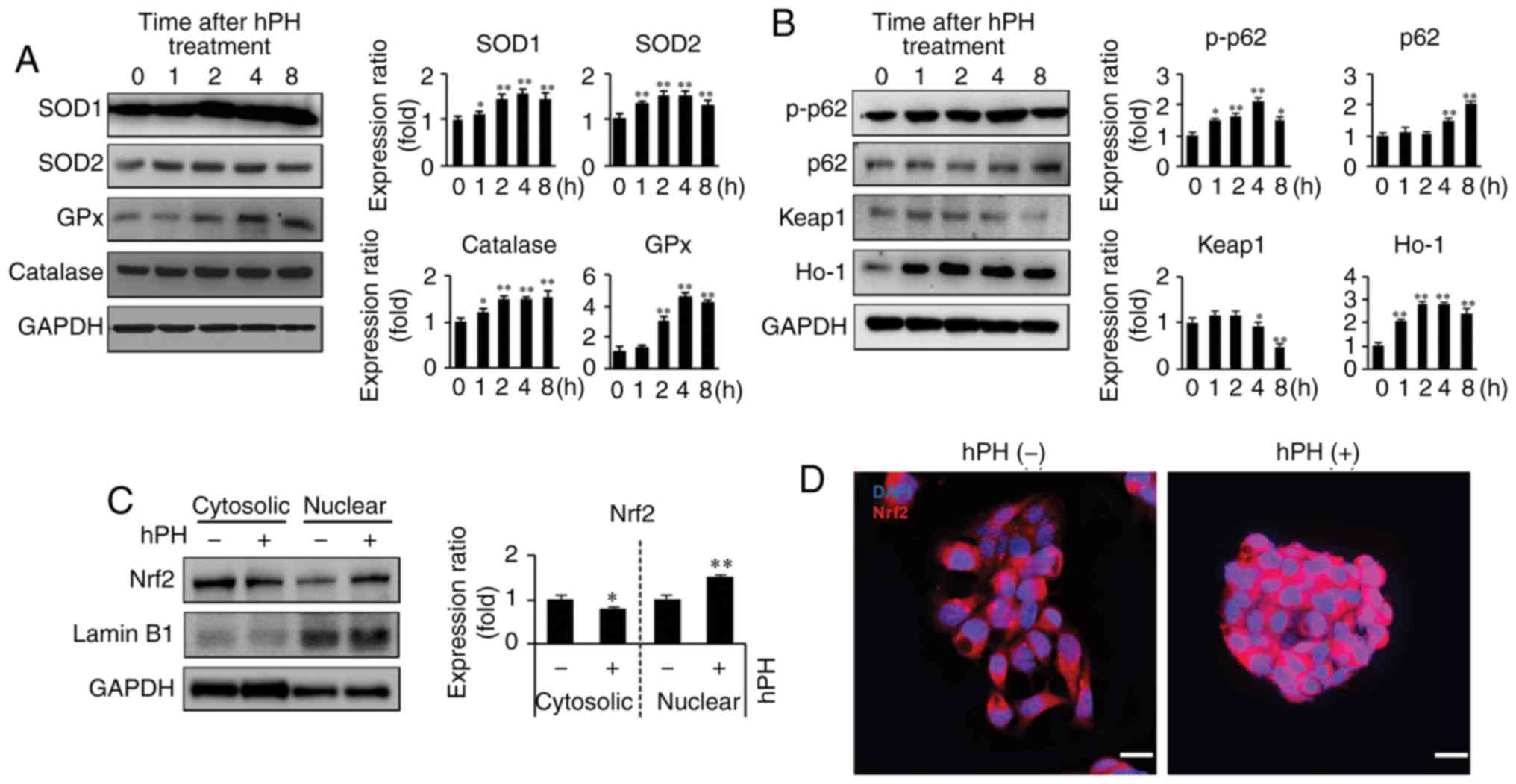
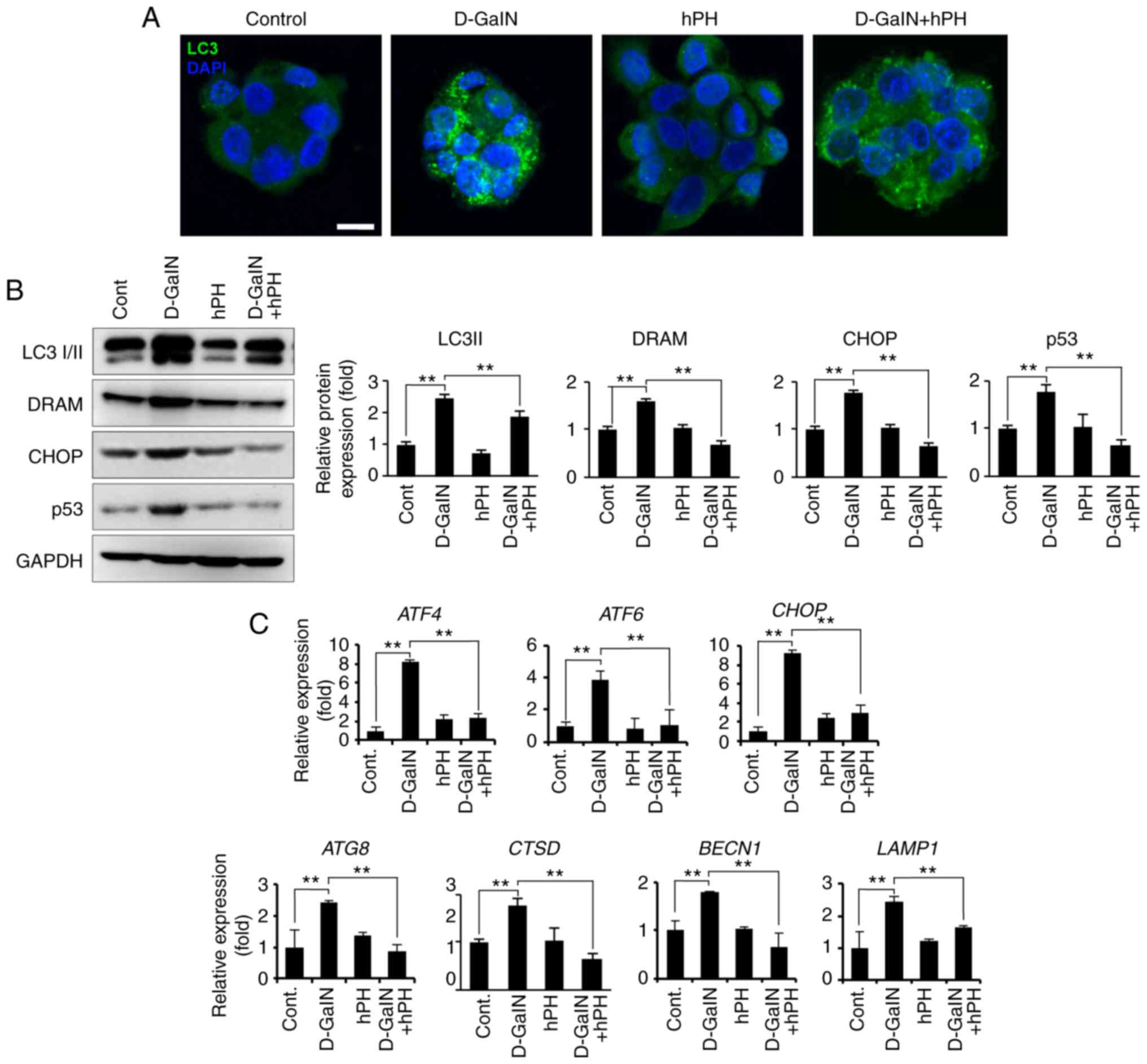
|
1
|
Bernal W, Auzinger G, Dhawan A and Wendon
J: Acute liver failure. Lancet. 376:190–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han DW: Intestinal endotoxemia as a
pathogenetic mechanism in liver failure. World J Gastroenterol.
8:961–965. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sathivel A, Balavinayagamani, Hanumantha
Rao BR and Devaki T: Sulfated polysaccharide isolated from Ulva
lactuca attenuates d-galactosamine induced DNA fragmentation and
necrosis during liver damage in rats. Pharm Biol. 52:498–505. 2014.
View Article : Google Scholar
|
|
4
|
Masaki T, Chiba S, Tatsukawa H, Yasuda T,
Noguchi H, Seike M and Yoshimatsu H: Adiponectin protects
LPS-induced liver injury through modulation of TNF-α in KK-Ay obese
mice. Hepatology. 40:177–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Z, Han M, Chen T, Yan W and Ning Q:
Acute liver failure: Mechanisms of immune-mediated liver injury.
Liver Int. 30:782–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheriff SA, Shaik Ibrahim S, Devaki T,
Chakraborty S, Agarwal S and Pérez-Sánchez H: Lycopene prevents
mitochondrial dysfunction during
d-galactosamine/lipopolysaccharide-induced fulminant hepatic
failure in albino rats. J Proteome Res. 16:3190–3199. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong L, Yin L, Quan H, Chu Y and Lu J:
Hepatoprotective effects of
kaempferol-3-O-α-l-arabinopyranosyl-7-O-α-l-rhamn opyranoside on
d-Galactosamine and lipopolysaccharide caused hepatic failure in
mice. Molecules. 22:E17552017. View Article : Google Scholar
|
|
8
|
Lee SB, Kang JW, Kim SJ, Ahn J, Kim J and
Lee SM: Afzelin ameliorates D-galactosamine and
lipopolysaccharide-induced fulminant hepatic failure by modulating
mitochondrial quality control and dynamics. Br J Pharmacol.
174:195–209. 2017. View Article : Google Scholar
|
|
9
|
Decker CW, Casian JG, Nguyen KT, Horton
LA, Rao MP, Silkwood KH and Han D: The critical role of
mitochondria in drug-induced liver injury. Molecules, Systems and
Signaling in Liver Injury. Springer; pp. 159–181. 2017, View Article : Google Scholar
|
|
10
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bryan HK, Olayanju A, Goldring CE and Park
BK: The Nrf2 cell defence pathway: Keap1-dependent and-independent
mechanisms of regulation. Biochem Pharmacol. 85:705–717. 2013.
View Article : Google Scholar
|
|
12
|
Zhou R, Lin J and Wu D: Sulforaphane
induces Nrf2 and protects against CYP2E1-dependent binge
alcohol-induced liver steatosis. Biochim Biophys Acta.
1840:209–218. 2014. View Article : Google Scholar
|
|
13
|
Jiang T, Huang Z, Lin Y, Zhang Z, Fang D
and Zhang DD: The protective role of Nrf2 in streptozotocin-induced
diabetic nephropathy. Diabetes. 59:850–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi A, Kang MI, Okawa H, Ohtsuji M,
Zenke Y, Chiba T, Igarashi K and Yamamoto M: Oxidative stress
sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to
regulate proteasomal degradation of Nrf2. Mol Cell Biol.
24:7130–7139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bataille A and Manautou J: Nrf2: A
potential target for new therapeutics in liver disease. Clin
Pharmacol Ther. 92:340–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jian Z, Li K, Song P, Zhu G, Zhu L, Cui T,
Liu B, Tang L, Wang X, Wang G, et al: Impaired activation of the
Nrf2-ARE signaling pathway undermines H response: A possible
mechanism for melanocyte degeneration in 2O2-induced oxidative
stress vitiligo. J Invest Dermatol. 134:2221–2230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gozuacik D and Kimchi A: Autophagy and
cell death. Curr Top Dev Biol. 78:217–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tonello G, Daglio M, Zaccarelli N,
Sottofattori E, Mazzei M and Balbi A: Characterization and
quantitation of the active poly-nucleotide fraction (PDRN) from
human placenta, a tissue repair stimulating agent. J Pharm Biomed
Anal. 14:1555–1560. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sur TK, Biswas TK, Ali L and Mukherjee B:
Anti-inflammatory and anti-platelet aggregation activity of human
placental extract. Acta Pharmacol Sin. 24:187–192. 2003.PubMed/NCBI
|
|
23
|
Chakraborty PD and Bhattacharyya D:
Isolation of fibronectin type III like peptide from human placental
extract used as wound healer. J Chromatogr B Analyt Technol Biomed
Life Sci. 818:67–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi JY, Lee K, Lee SM, Yoo SH, Hwang SG,
Choi JY, Lee SW, Hwang JS, Kim KK, Kang HC, et al: Efficacy and
safety of human placental extract for alcoholic and nonalcoholic
steato-hepatitis: An open-label, randomized, comparative study.
Biol Pharm Bull. 37:1853–1859. 2014. View Article : Google Scholar
|
|
25
|
Shimokobe H, Sumida Y, Tanaka S, Mori K,
Kitamura Y, Fukumoto K, Kakutani A, Ohno T, Kanemasa K, Imai S, et
al: Human placental extract treatment for non-alcoholic
steato-hepatitis non-responsive to lifestyle intervention: A pilot
study. Hepatol Res. 45:1034–1040. 2015. View Article : Google Scholar
|
|
26
|
Park S, Phark S, Lee M, Lim J and Sul D:
Anti-oxidative and anti-inflammatory activities of placental
extracts in benzo[a] pyrene-exposed rats. Placenta. 31:873–879.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Camargo CA Jr, Madden JF, Gao W, Selvan RS
and Clavien P: Interleukin-6 protects liver against warm
ischemia/reperfusion injury and promotes hepatocyte proliferation
in the rodent. Hepatology. 26:1513–1520. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okoh VO, Felty Q, Parkash J, Poppiti R and
Roy D: Reactive oxygen species via redox signaling to PI3K/AKT
pathway contribute to the malignant growth of 4-hydroxy
estradiol-transformed mammary epithelial cells. PLoS One.
8:e542062013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jasek E, Lis GJ, Jasińska M, Jurkowska H
and Litwin JA: Effect of histone deacetylase inhibitors
trichostatin A and valproic acid on etoposide-induced apoptosis in
leukemia cells. Anticancer Res. 32:2791–2799. 2012.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Cassidy W and Reynolds T: Serum lactic
dehydrogenase in the differential diagnosis of acute hepatocellular
injury. J Clin Gastroenterol. 19:118–121. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rolando N, Wade J, Davalos M, Wendon J,
Philpott-Howard J and Williams R: The systemic inflammatory
response syndrome in acute liver failure. Hepatology. 32:734–739.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guicciardi M and Gores GJ: Apoptosis: A
mechanism of acute and chronic liver injury. Gut. 54:1024–1033.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stachlewitz RF, Seabra V, Bradford B,
Bradham CA, Rusyn I, Germolec D and Thurman RG: Glycine and uridine
prevent d-galactosamine hepatotoxicity in the rat: Role of kupffer
cells. Hepatology. 29:737–745. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thabrew MI, Hughes RD and McFarlane IG:
Screening of hepa-toprotective plant components using a HepG2 cell
cytotoxicity assay. J Pharm Pharmacol. 49:1132–1135. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
González R, Ferrín G, Hidalgo AB, Ranchal
I, López-Cillero P, Santos-Gónzalez M, López-Lluch G, Briceño J,
Gómez MA, Poyato A, et al: N-acetylcysteine, coenzyme Q10 and
superoxide dismutase mimetic prevent mitochondrial cell dysfunction
and cell death induced by d-galactosamine in primary culture of
human hepatocytes. Chem Biol Interact. 181:95–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao Y, Li S, Childs EE, Kuharsky DK and
Yin XM: Activation of pro-death Bcl-2 family proteins and
mitochondria apoptosis pathway in tumor necrosis factor-α-induced
liver injury. J Biol Chem. 276:27432–27440. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuznetsov AV, Kehrer I, Kozlov AV, Haller
M, Redl H, Hermann M, Grimm M and Troppmair J: Mitochondrial ROS
production under cellular stress: Comparison of different detection
methods. Anal Bioanal Chem. 400:2383–2390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee J, Giordano S and Zhang J: Autophagy,
mitochondria and oxidative stress: Cross-talk and redox signalling.
Biochem J. 441:523–540. 2012. View Article : Google Scholar :
|
|
40
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar :
|
|
41
|
Liu GY, Jiang XX, Zhu X, He WY, Kuang YL,
Ren K, Lin Y and Gou X: ROS activates JNK-mediated autophagy to
counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta
Pharmacol Sin. 36:1473–1479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cichoż-Lach H and Michalak A: Oxidative
stress as a crucial factor in liver diseases. World J
Gastroenterol. 20:8082–8091. 2014. View Article : Google Scholar
|
|
44
|
Jenner P: Oxidative stress in Parkinson's
disease. Ann Neurol. 53(Suppl 3): S26–S38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kujoth G, Hiona A, Pugh T, Someya S,
Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA,
et al: Mitochondrial DNA mutations, oxidative stress, and apoptosis
in mammalian aging. Science. 309:481–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Radi E, Formichi P, Battisti C and
Federico A: Apoptosis and oxidative stress in neurodegenerative
diseases. J Alzheimers Dis. 42(Suppl 3): S125–S152. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Osakabe N, Yasuda A, Natsume M, Sanbongi
C, Kato Y, Osawa T and Yoshikawa T: Rosmarinic acid, a major
polyphenolic component of Perilla frutescens, reduces
lipopolysaccharide (LPS)-induced liver injury in D-galactosamine
(D-GalN)-sensitized mice. Free Radic Biol Med. 33:798–806. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nowak M, Gaines GC, Rosenberg J, Minter R,
Bahjat FR, Rectenwald J, MacKay SL, Edwards CK III and Moldawer LL:
LPS-induced liver injury in D-galactosamine-sensitized mice
requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol
Regul Integr Comp Physiol. 278:R1202–R1209. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu YL, Lian LH, Wan Y and Nan JX:
Baicalein inhibits nuclear factor-κB and apoptosis via c-FLIP and
MAPK in D-GalN/LPS induced acute liver failure in murine models.
Chem Biol Interact. 188:526–534. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen L, Ren F, Zhang H, Wen T, Piao Z,
Zhou L, Zheng S, Zhang J, Chen Y, Han Y, et al: Inhibition of
glycogen synthase kinase 3β ameliorates D-GalN/LPS-induced liver
injury by reducing endoplasmic reticulum stress-triggered
apoptosis. PloS One. 7:e452022012. View Article : Google Scholar
|
|
51
|
Wang H, Xu DX, Lv JW, Ning H and Wei W:
Melatonin attenuates lipopolysaccharide (LPS)-induced apoptotic
liver damage in D-galactosamine-sensitized mice. Toxicology.
237:49–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kawakatsu M, Urata Y, Goto S, Ono Y and Li
TS: Placental extract protects bone marrow-derived stem/progenitor
cells against radiation injury through anti-inflammatory activity.
J Radiat Res. 54:268–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Park JY, Lee J, Jeong M, Min S, Kim SY,
Lee H, Lim Y and Park HJ: Effect of Hominis Placenta on cutaneous
wound healing in normal and diabetic mice. Nutri Res Pract.
8:404–409. 2014. View Article : Google Scholar
|
|
54
|
Lee KH, Kim TH, Lee WC, Kim SH, Lee SY and
Lee SM: Anti-inflammatory and analgesic effects of human placenta
extract. Nat Prod Res. 25:1090–1100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee KW, Ji HM, Kim DW, Choi SM, Kim S and
Yang EJ: Effects of Hominis placenta on LPS-induced cell toxicity
in BV2 microglial cells. J Ethnopharmacol. 147:286–292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Akagi H, Imamura Y, Makita Y, Nakamura H,
Hasegawa N, Fujiwara SI and Wang PL: Evaluation of collagen type-1
production and anti-inflammatory activities of human placental
extracts in human gingival fibroblasts. J Hard Tissue Biol.
25:277–281. 2016. View Article : Google Scholar
|
|
57
|
Watanabe S, Togashi S, Takahashi N and
Fukui T: L-tryptophan as an antioxidant in human placenta extract.
J Nutri Sci Vitaminol (Tokyo). 48:36–39. 2002. View Article : Google Scholar
|
|
58
|
Togashi SI, Takahashi N, Iwama M, Watanabe
S, Tamagawa K and Fukui T: Antioxidative collagen-derived peptides
in human-placenta extract. Placenta. 23:497–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Rozanova S, Cherkashina Y, Repina S,
Rozanova K and Nardid O: Protective effect of placenta extracts
against nitrite-induced oxidative stress in human erythrocytes.
Cell Mol Biol Lett. 17:240–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Halliwell B and Gutteridge JM: Free
Radicals in Biology and Medicine. Oxford University Press; USA:
2015, View Article : Google Scholar
|
|
61
|
Wells PG and Winn LM: Biochemical
toxicology of chemical teratogenesis. Crit Rev Biochem Mol Biol.
31:1–40. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Avissar N, Whitin JC, Allen PZ, Wagner DD,
Liegey P and Cohen HJ: Plasma selenium-dependent glutathione
peroxidase. Cell of origin and secretion. J Biol Chem.
264:15850–15855. 1989.PubMed/NCBI
|
|
63
|
Thomas EL, Learn DB, Jefferson MM and
Weatherred W: Superoxide-dependent oxidation of extracellular
reducing agents by isolated neutrophils. J Biol Chem.
263:2178–2186. 1988.PubMed/NCBI
|
|
64
|
Kankofer M: Antioxidative defence
mechanisms against reactive oxygen species in bovine retained and
not-retained placenta: Activity of glutathione peroxidase,
glutathione transferase, catalase and superoxide dismutase.
Placenta. 22:466–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mochizuki H and Kada T: Restorative
effects of human placenta extract in X-ray-irradiated mice. J
Radiat Res. 23:403–410. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
González R, Collado JA, Nell S, Briceño J,
Tamayo MJ, Fraga E, Bernardos A, López-Cillero P, Pascussi JM,
Rufián S, et al: Cytoprotective properties of α-tocopherol are
related to gene regulation in cultured D-galactosamine-treated
human hepato-cytes. Free Radic Biol Med. 43:1439–1452. 2007.
View Article : Google Scholar
|
|
67
|
Siendones E, Fouad D, Abou-Elella AMKE,
Quintero A, Barrera P and Muntané J: Role of nitric oxide in
d-galactos-amine-induced cell death and its protection by PGE 1 in
cultured hepatocytes. Nitric Oxide. 8:133–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mahmoud MF, Hamdan DI, Wink M and
El-Shazly AM: Hepatoprotective effect of limonin, a natural
limonoid from the seed of Citrus aurantium var. bigaradia, on
D-galactosamine- induced liver injury in rats. Naunyn-Schmiedebergs
Arch Pharmacol. 387:251–261. 2014. View Article : Google Scholar
|
|
69
|
Wang Y, Li Y, Xie J, Zhang Y, Wang J, Sun
X and Zhang H: Protective effects of probiotic Lactobacillus casei
Zhang against endotoxin-and d-galactosamine-induced liver injury in
rats via anti-oxidative and anti-inflammatory capacities. Int
Immunopharmacol. 15:30–37. 2013. View Article : Google Scholar
|
|
70
|
Xia X, Su C, Fu J, Zhang P, Jiang X, Xu D,
Hu L, Song E and Song Y: Role of α-lipoic acid in LPS/d-GalN
induced fulminant hepatic failure in mice: Studies on oxidative
stress, inflammation and apoptosis. Int Immunopharmacol.
22:293–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shin JW, Wang JH, Park HJ, Choi MK, Kim HG
and Son CG: Herbal formula CGX ameliorates
LPS/D-galactosamine-induced hepatitis. Food Chem Toxicol.
49:1329–1334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee HJ, Oh YK, Rhee M, Lim JY, Hwang JY,
Park YS, Kwon Y, Choi KH, Jo I, Park SI, et al: The role of
STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial
transmembrane potential during hepatic cell death induced by
LPS/d-GalN. J Mol Biol. 369:967–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu LM, Zhang JX, Luo J, Guo HX, Deng H,
Chen JY and Sun SL: A role of cell apoptosis in lipopolysaccharide
(LPS)-induced nonlethal liver injury in D-galactosamine
(D-GalN)-sensitized rats. Dig Dis Sci. 53:1316–1324. 2008.
View Article : Google Scholar
|
|
74
|
Soriano ME, Nicolosi L and Bernardi P:
Desensitization of the permeability transition pore by cyclosporin
A prevents activation of the mitochondrial apoptotic pathway and
liver damage by tumor necrosis factor-alpha. J Biol Chem.
279:36803–36808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Indo HP, Davidson M, Yen HC, Suenaga S,
Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T and Majima HJ:
Evidence of ROS generation by mitochondria in cells with impaired
electron transport chain and mitochondrial DNA damage.
Mitochondrion. 7:106–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Farombi EO, Shrotriya S, Na HK, Kim SH and
Surh YJ: Curcumin attenuates dimethylnitrosamine-induced liver
injury in rats through Nrf2-mediated induction of heme oxygenase-1.
Food Chem Toxicol. 46:1279–1287. 2008. View Article : Google Scholar
|
|
77
|
Klaassen CD and Reisman SA: Nrf2 the
rescue: Effects of the antioxidative/electrophilic response on the
liver. Toxicol Appl Pharmacol. 244:57–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xu W, Shao L, Zhou C, Wang H and Guo J:
Upregulation of Nrf2 expression in non-alcoholic fatty liver and
steatohepatitis. Hepatogastroenterology. 58:2077–2080. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhao HD, Zhang F, Shen G, Li YB, Li YH,
Jing HR, Ma LF, Yao JH and Tian XF: Sulforaphane protects liver
injury induced by intestinal ischemia reperfusion through Nrf2-ARE
pathway. World J Gastroenterol. 16:3002–3010. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pan CW, Pan ZZ, Hu JJ, Chen WL, Zhou GY,
Lin W, Jin LX and Xu CL: Mangiferin alleviates lipopolysaccharide
and D-galactosamine-induced acute liver injury by activating the
Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J
Pharmacol. 770:85–91. 2016. View Article : Google Scholar
|
|
81
|
Beyer TA, Xu W, Teupser D, auf dem Keller
U, Bugnon P, Hildt E, Thiery J, Kan YW and Werner S: Impaired liver
regeneration in Nrf2 knockout mice: Role of ROS-mediated
insulin/IGF-1 resistance. EMBO J. 27:212–223. 2008. View Article : Google Scholar
|
|
82
|
Duarte TL, Caldas C, Santos AG,
Silva-Gomes S, Santos-Gonçalves A, Martins MJ, Porto G and Lopes
JM: Genetic disruption of NRF2 promotes the development of
necroinflam-mation and liver fibrosis in a mouse model of
HFE-hereditary hemochromatosis. Redox Biol. 11:157–169. 2017.
View Article : Google Scholar
|
|
83
|
Sahin K, Orhan C, Akdemir F, Tuzcu M,
Sahin N, Yılmaz I and Juturu V: β-Cryptoxanthin ameliorates
metabolic risk factors by regulating NF-κB and Nrf2 pathways in
insulin resistance induced by high-fat diet in rodents. Food Chem
Toxicol. 107:270–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Uchiyama Y, Shibata M, Koike M, Yoshimura
K and Sasaki M: Autophagy-physiology and pathophysiology. Histochem
Cell Biol. 129:407–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Amir M, Zhao E, Fontana L, Rosenberg H,
Tanaka K, Gao G and Czaja MJ: Inhibition of hepatocyte autophagy
increases tumor necrosis factor-dependent liver injury by promoting
caspase-8 activation. Cell Death Differ. 20:878–887. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Donohue TM Jr: Autophagy and
ethanol-induced liver injury. World J Gastroenterol. 15:1178–1185.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lin Z, Wu F, Lin S, Pan X, Jin L, Lu T,
Shi L, Wang Y, Xu A and Li X: Adiponectin protects against
acetaminophen-induced mitochondrial dysfunction and acute liver
injury by promoting autophagy in mice. J Hepatol. 61:825–831. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chang CP and Lei HY: Autophagy induction
in T cell-independent acute hepatitis induced by concanavalin A in
SCID/NOD mice. Int J Immunopathol Pharmacol. 21:817–826. 2008.
View Article : Google Scholar
|
|
91
|
Gotoh K, Lu Z, Morita M, Shibata M, Koike
M, Waguri S, Dono K, Doki Y, Kominami E, Sugioka A, et al:
Participation of autophagy in the initiation of graft dysfunction
after rat liver transplantation. Autophagy. 5:351–360. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liu K, Lou J, Wen T, Yin J, Xu B, Ding W,
Wang A, Liu D, Zhang C, Chen D and Li N: Depending on the stage of
hepa-tosteatosis, p53 causes apoptosis primarily through either
DRAM-induced autophagy or BAX. Liver Int. 33:1566–1574.
2013.PubMed/NCBI
|
|
93
|
Maiuri MC, Malik SA, Morselli E, Kepp O,
Criollo A, Mouchel PL, Carnuccio R and Kroemer G: Stimulation of
autophagy by the p53 target gene Sestrin2. Cell Cycle. 8:1571–1576.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Salazar M, Carracedo A, Salanueva ÍJ,
Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C,
Torres S, García S, et al: Cannabinoid action induces
autophagy-mediated cell death through stimulation of ER stress in
human glioma cells. J Clin Invest. 119:1359–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xi H, Kurtoglu M, Liu H, Wangpaichitr M,
You M, Liu X, Savaraj N and Lampidis TJ: 2-Deoxy-D-glucose
activates autophagy via endoplasmic reticulum stress rather than
ATP depletion. Cancer Chemother Pharmacol. 67:899–910. 2011.
View Article : Google Scholar :
|
|
96
|
Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao
M, Liu D, Qiao L, Li N, Zheng J and Chen D: CHOP mediates
ASPP2-induced autophagic apoptosis in hepatoma cells by releasing
Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2.
Cell Death Dis. 5:e13232014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tuñón MJ, San-Miguel B, Crespo I, Laliena
A, Vallejo D, Álvarez M, Prieto J and González-Gallego J: Melatonin
treatment reduces endoplasmic reticulum stress and modulates the
unfolded protein response in rabbits with lethal fulminant
hepatitis of viral origin. J Pineal Res. 55:221–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|