Introduction
Curcumin (diferuloylmethane) is a natural product
derived from the rhizome of Curcuma longa. Curcumin is one
of the most common ingredients in Asian cuisine. Curcumin also has
been found to have many potent effects, including anti-oxidative
stress, anti-inflammatory, anti-proliferative, anticancer and
neuroprotective properties (1–3).
It was previously observed that curcumin enhanced the neurogenesis
of neural progenitor cells by decreasing histone H3 and H4
acetylation (4) as well as
stimulating developmental and adult hippocampal neurogenesis
through the activation of ERK and p38 kinase (4,5).
However, whether the effect of curcumin on NSCs is associated with
autophagy has yet to be determined.
Autophagy is a highly regulated sequential process
that delivers cytoplasmic macromolecules and damaged organelles to
lysosomes for degradation, including mitochondria, endoplasmic
reticulum, peroxisomes and misfolded proteins (6,7).
Autophagy contributes to cell growth, cell development and cell
homeostasis in normal conditions (7). Autophagic flux may be stimulated by
multiple forms of cellular stress, including nutrient deprivation,
energy limitations, hypoxia, toxins, radiation, DNA damage and
intracellular pathogens (8,9).
In recent years, increasing evidence has supported
that autophagy has a beneficial role in neurodegenerative
disorders, including Huntington's, Alzheimer's, Parkinson's and
amyotrophic lateral sclerosis (7,10,11). However, other evidence has
indicated that excessive autophagy could contribute to neuronal
death in cerebral ischemia (7,12–14). Furthermore, autophagy is also
associated with cell aging, survival and proliferation (15–17).
In the present study, it was identified that
curcumin actively suppressed the differentiation of NSCs into
astrocytes and immature neurons while they were in adherent
culture, and suppressed cell cycle progression and apoptosis in
NSCs in suspension. Furthermore, transmission electron microscopy
(TEM) revealed that the cytoplasm of the NSCs displayed
autophagosomes following treatment with curcumin. Finally, it was
identified that curcumin affected autophagy by inducing a decrease
in autophagy-related gene (Atg)7 and p62 protein expression in NSCs
in different culture states.
Materials and methods
Preparation of NSCs
NSCs were prepared from pregnant female
Sprague-Dawley rats supplied by the Animal Breeding Center of
Chinese Academy of Sciences (Shanghai, China) according to the
method previously established by our group (18,19). All animal-related procedures were
approved by the Institutional Animal Care and Use Committee of
Wenzhou Medical University (Wenzhou, China), and were conducted in
accordance with the university's guidelines.
Briefly, embryonic cerebral cortices were collected
and dissected from embryonic day 14.5 (n=8; E14.5) rats. The cells
were isolated by mechanical pipetting with a fire-polished Pasteur
pipette. The suspension was filtered through a 70-µm nylon
mesh, seeded into a T25 Corning tissue culture flask (Corning
Incorporated, Corning, NY, USA) at a density of 105
cells/ml and incubated at 37°C in a humidified, 5% CO2
atmosphere. The culture medium was composed of DMEM/F12, B27, N2
(all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100×
Penicillin-Streptomycin Solution (Beyotime Institute of
Biotechnology, Haimen China), heparin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), HEPES and glutamine, with 20 ng/ml epidermal
growth factor (EGF) and 20 ng/ml basic fibroblast growth factor
(bFGF; Gibco; Thermo Fisher Scientific, Inc.) added, which is
henceforth described as proliferation medium. At day 5,
neurospheres were collected and passaged. Passage 2 NSCs were used
for the subsequent assays.
To induce the differentiation of NSCs, dissociated
cells in a single-cell suspension were seeded onto poly-L-lysine
(cat. no. P-2636; Sigma-Aldrich; Merck KGaA) coated coverslips in a
24-well plate at a density of 5×104/coverslip.
Differentiation medium contained 1% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) without EGF or bFGF. The cultures
were allowed to differentiate for 6 days prior to being fixed for
immunostaining.
The adherent and suspension cultures of NSCs were
incubated in differentiation medium or proliferation medium,
respectively, for 72 h prior to the preparation of protein extracts
for western blotting. The medium was changed every 48 h.
Antibodies and reagents
Glial fibrillary acidic protein (GFAP) was detected
by chicken polyclonal anti-GFAP (cat. no. AB5541; Merck KGaA).
Other antibodies were as follows: Rabbit polyclonal anti-light
chain (LC)3B (cat. no. 2775), rabbit monoclonal anti-Atg7 (D12B11;
cat. no. 8558), anti-cyclin dependent kinase (CDK)2 (78B2; cat. no.
2546; Cell Signaling Technology, Inc.) and anti-p62 (cat. no.
P0067), mouse monoclonal anti-nestin (cat. no. N5413) (both
Sigma-Aldrich; Merck KGaA), anti-βIII-tubulin (cat. no. AB15708;
Merck KGaA), anti-O4 (cat.no. O7139; Sigma-Aldrich; Merck KGaA),
anti-β-actin (cat. no. sc-47778; Santa Cruz Biotechnology, Inc.),
anti-tubulin βIII (cat. no. MAB1637; Merck KGaA), anti-doublecortin
(DCX; cat. no. ab2253) and anti-caspase-3 antibody (cat. no. 9662;
Cell Signaling Technology, Inc.). Curcumin (cat. no. C1386-5G) and
monodansylcadaverine (MDC) were purchased from Sigma-Aldrich; Merck
KGaA (cat. no. D4008-100MG). 3-Methyladenine (3MA, an inhibitor of
phosphatidylinositol 3-kinases and autophagosome formation; cat.
no. s2767) was purchased from Selleck Chemicals (Shanghai,
China).
Drug treatment
Curcumin, MDC and 3MA stocks of 1 mg/ml were
prepared in dimethylsulfoxide (DMSO) and stored at −20°C, in the
dark. Subsequent to passaging, NSCs were treated with 10 µM
curcumin or 10 µM 3MA for 72 h by adding the stock solutions
to the medium. Then, they were fixed in 4% paraformaldehyde for 15
min, and processed for immunostaining as subsequently described.
The 3MA group was used as a positive control.
Determination of cell viability
Cell viability was determined by a water-soluble
tetrazolium salts (WST-1 Cell Proliferation and Cytotoxicity Assay
kit; Beyotime Institute of Biotechnology) assay (20). Neural stem cells in suspension
were seeded at a density of 2×104 cells/well into a
96-well plate. Following treatment with curcumin for 72 h, WST-1
was added to each well and incubated for 2 h at 37°C. The optical
density was measured at 450 nm using a microplate reader. The data
were presented as the mean from four independent experiments in
quadruplicate.
Immunocytochemistry assay
Immunofluorescence was used to characterize the
differentiation of NSCs in vitro, as described previously
(18,19). Briefly, cells on poly-L-lysine
coated cover-slips were fixed with 4% paraformaldehyde for 10 min
at room temperature (RT), washed and stored in 0.01 mol/l PBS (pH
7.4). The sections of neurospheres or cell culture were blocked in
10% goat serum (cat. no. S26-100 ML; Merck KGaA) in PBS (for O4) or
0.3% Triton X-100-containing 10% goat serum in PBS (for GFAP,
βIII-tubulin and nestin) for 1 h at RT, and incubated with the
following primary antibodies overnight at 4°C: Monoclonal mouse
antibodies against nestin (dilution, 1:800) for NSCs, βIII-tubulin
(dilution, 1:400) for neurons, GFAP (dilution, 1:500) for
astrocytes and O4 (dilution, 1:100) for oligodendrocytes. After
washing three times with PBS, the cultures were incubated with
rhodamine-conjugated goat anti-mouse or DyLight 488 (cat. no.
611-545-215) or 594-conjugated goat anti-rabbit antibodies (cat.
no. 111-005-047) (dilution, 1:150; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for 1 h at 37°C and washed
three times with PBS. Subsequently, the cells were incubated with
Hoechst 33258 (Beyotime Institute of Biotechnology) for 3 min at RT
to stain the nuclei. Finally, the coverslips were mounted onto
slides in 70% glycerol. Stained sections were observed and scanned
under a fluorescence microscope (Olympus BX53; Olympus Corporation,
Tokyo, Japan).
MDC staining
Neural stem cells were seeded on slides. Cells were
incubated and treated with curcumin or 3MA for 72 h in
proliferation medium, finally incubated with 0.05 mM MDC for 1 h at
37°C, and then washed four times with PBS (pH 7.4) (21). Cells were immediately visualized
with a fluorescence microscope (Olympus BX53; Olympus Corporation).
The fluorescence intensity values of all groups were digitally
quantified using ImageJ image analyzer software (version 1.45;
National Institutes of Health, Bethesda, MD, USA).
Transmission electron microscopy
(TEM)
NSCs were seeded in 100-mm dishes. Cells were
incubated and treated with curcumin or 3MA for 72 h in the
proliferation medium. At the end of incubation, cell monolayers
were washed with PBS and scraped gently with a plastic cell
scraper. The harvested cells were pelleted by centrifugation at
13,000 × g for 10 min, and fixed in 2.5% glutaraldehyde and 2%
paraformaldehyde in cacodylate buffer. After rinsing with
cacodylate buffer, the samples were post-fixed in 2% osmium
tetroxide for 1 h. The samples were rinsed with water and
dehydrated in a graded alcohol series (50, 75, 80 and 100%). The
samples were then embedded in epoxy resin. Representative areas
were chosen for ultra-thin sectioning, and viewed with a Hitachi
7000 STEM transmission electron microscope (Hitachi, Ltd., Tokyo,
Japan) (6).
Western blot analysis
Cells were plated at a density of 1×106
cells per 100-mm culture dish and treated with curcumin or 3MA as
previously described. Cells were washed twice with PBS and lysed in
RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1.0 mM
Na3VO4, 1 mM EDTA, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS, 100 µg/ml phenylmethylsulfonyl
fluoride, 30 µl/ml aprotinin and 4 µg/ml leupeptin,
pH 7.5). Lysates were centrifuged and the supernatants diluted in
sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50
mM DTT and 0.1% bromophenol blue), then boiled for 5 min. Equal
amounts of protein were resolved on 12% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. The membranes were blocked at RT
for 1 h in 5% (w/v) dry skim milk in TBS plus 0.1% Tween-20 (TBST),
rinsed in TBST and incubated with primary antibodies at 4°C
overnight. The primary antibodies used were mouse antibodies
specific for β-actin, βIII-tubulin, GFAP, caspase-3 DCX, p62, Atg7,
LC3B and CDK2 (all dilution, 1:1,000). After rinsing, blots were
incubated in TBST with peroxidase-conjugated secondary antibodies
at RT for 1 h. The secondary antibodies used included goat
anti-mouse IgG and goat anti-rabbit IgG (both dilution, 1:1,000).
The peroxidase reaction was visual-ized with an enhanced
chemiluminescence reagent. Films were digitized and densitometry
was performed using ImageJ software.
Statistical analysis
Data were analyzed by one-way analysis of variance
followed by Dunnett's post hoc test to determine whether there were
significant differences between individual groups. All test
assumptions regarding distribution and variance were met for each
data set. All analyses were based on biological replicates (n=3)
from the same independent experiment, not technical replicates or
combined experiments. P<0.05 was considered to represent a
statistically significant difference.
Results
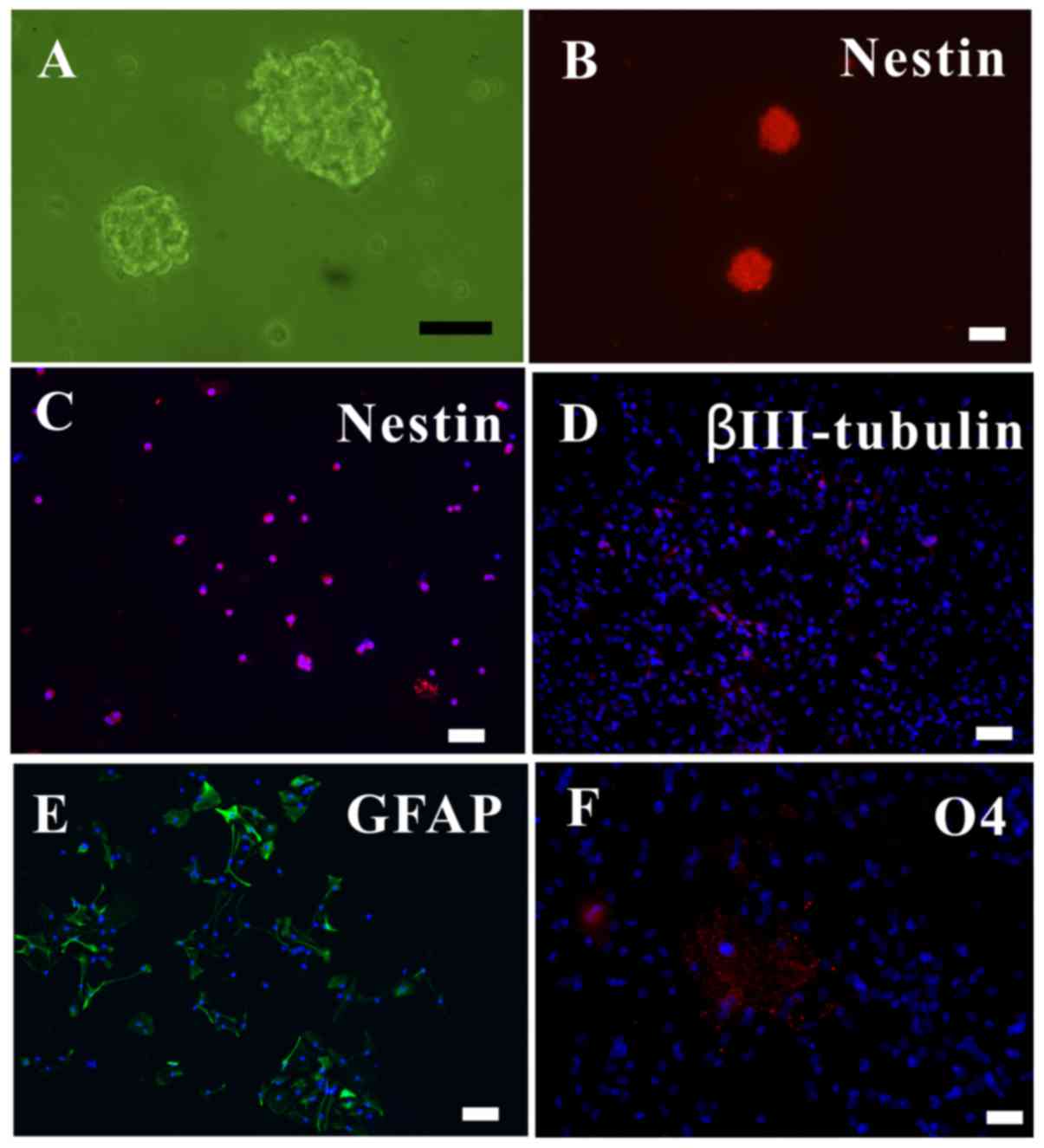
NSCs can be successfully isolated while
retaining their differentiation ability
The in vitro cells proliferated to form
neurospheres, which were observed under an inverted microscope
(Fig. 1A). As nestin is a marker
of NSCs (19), the
nestin+ neurospheres and dissociated single cells were
observed with immunofluorescence (Fig. 1B and C).
To ascertain the differentiation ability of the
NSCs, the NSCS were subjected to differentiating media for 5 days,
after which they were immunolabeled for neurons
(βIII-tubulin+; Fig.
1D), astrocytes (GFAP+; Fig. 1E) and oligodendrocytes
(O4+; Fig. 1F). Thus,
it was verified that the extracted NSCs could proliferate,
self-renew and differentiate into the three major neural
lineages.
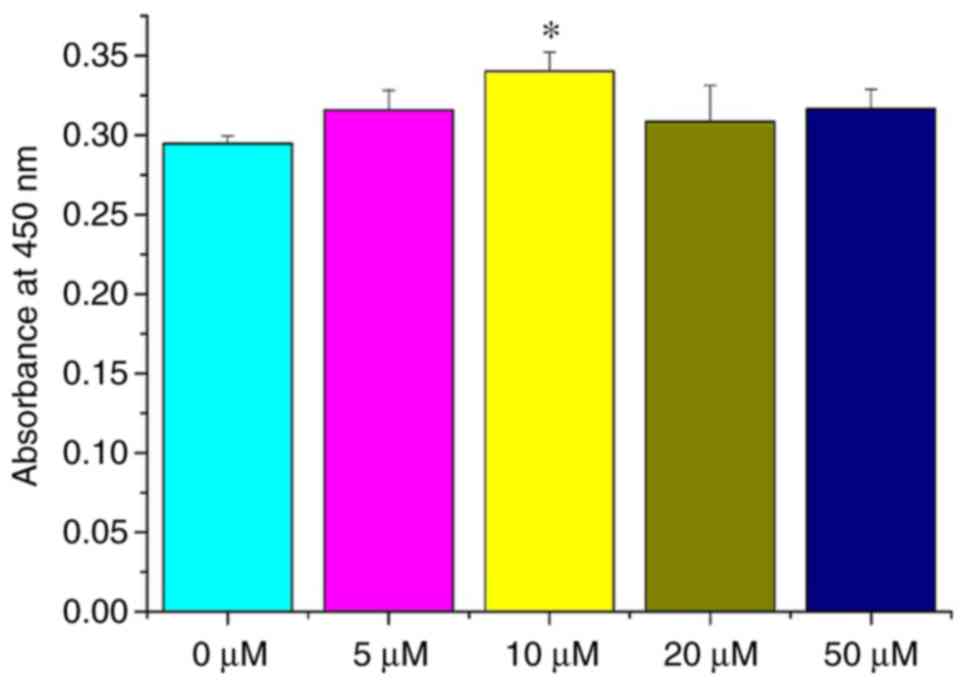
Curcumin (10 µM) enhances NSC
viability
In order to investigate the effect of different
curcumin concentrations on NSCs in vitro, cell viability was
determined by a WST-1 assay. NSCs were treated with 0, 5, 10, 20
and 50 µM curcumin. At 72 h, the viability of NSCs treated
with 10 µM curcumin was significantly different compared
with NSCs treated with 0 µM (P<0.05; Fig. 2), while differences among the 0,
5, 20 and 50 µM groups were all non-significant (P>0.05;
Fig. 2). These results
demonstrate that a 10-µM dose of curcumin can promote the
proliferation of NSCs in vitro. Thus, a dose of 10 µM
curcumin was selected for the following studies.
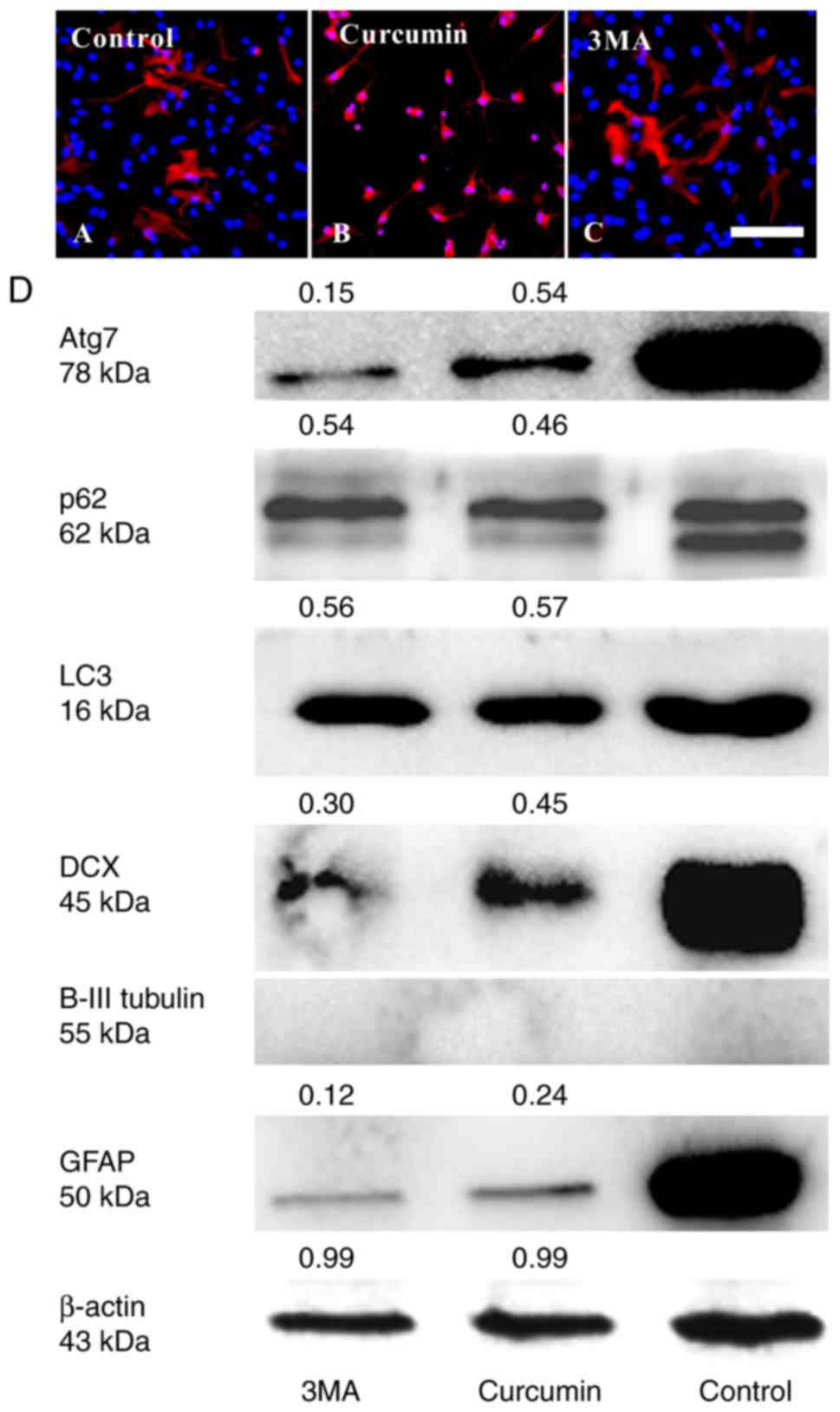
Curcumin inhibits the differentiation of
adherent NSCs by decreasing Atg7 and p62 expression
It was investigated whether treating with curcumin
would result in changes in cell differentiation using
immunocytochemistry and western blot analyses. After 3 days culture
in differentiation medium, treatment with curcumin evidently
decreased the number of NSCs differentiating into GFAP+
astrocytes (Fig. 3A–D) and
DCX+ immature neurons compared with in the control
group. However, βIII-tubulin+ expression could not be
detected in all groups (Fig. 3D).
The result of curcumin treatment was similar to that of 3MA
treatment.
To further validate the role of autophagy in the
effect of curcumin on NSC differentiation, changes in the
expression of autophagy markers, including LC3, Atg7 and p62, were
determined (Fig. 3D). LC3, is an
essential component of autophagosomes widely used as autophagy
marker. The degree of conversion of cytosolic LC3I to membrane
bound LC3II indicates the level of autophagic activity (6). It was identified that the total LC3
(16 kDa) in the curcumin and 3MA groups was lower than in the
control group. In addition, the LC3II isoform was not detected in
any of the groups. As total LC3 is not a good marker for autophagy
(22), it was not possible to
ascertain whether NSC differentiation was associated with LC3.
Another approach is to detect the Atg7 and p62 expression levels.
Atg7 is essential for the early elongation and closure of the
autophagosomal membrane (9,23).
The level of p62 degradation is used to detect autophagic flux
(24); p62 accumulates when
autophagy is inhibited, and decreased levels are observed when
autophagy is induced (25).
Therefore, the reduction in the number of GFAP+
astrocytes and DCX+ immature neurons may be due to
differences in the protein levels of Atg7 and p62.
Following exposure to 3MA or curcumin, Atg7 and p62
levels were much lower than in the untreated control group
(Fig. 3D). 3MA is a known
inhibitor of type III phosphatidylinositol 3-kinase (PI3K) and
autophagy induction. Thus, the results preliminarily indicated that
curcumin may have inhibited the differentiation of NSCs through
PI3K inhibition (26,27) or decreasing the protein levels of
Atg7 and p62.
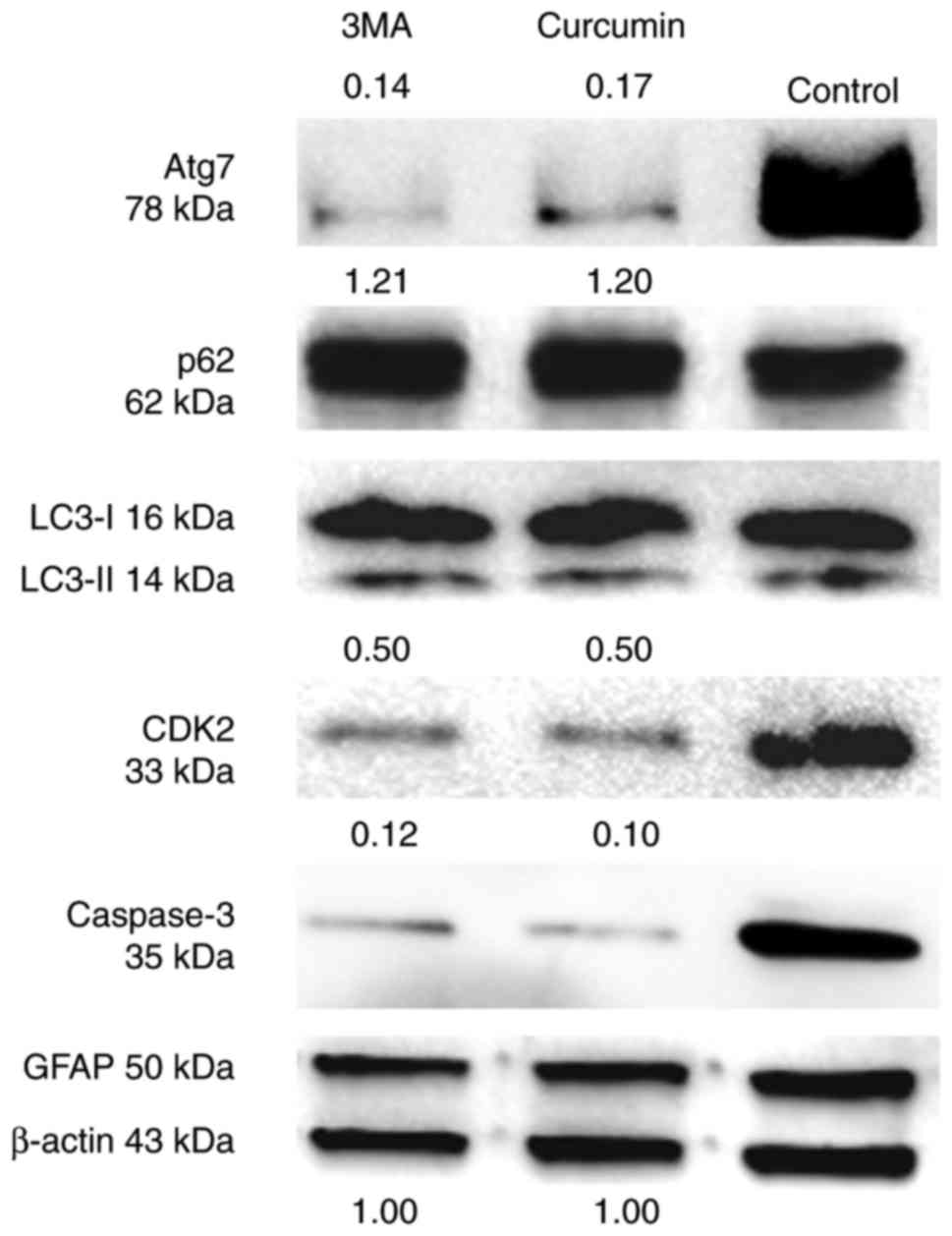
Curcumin inhibits the cell cycle
progression and apoptosis of suspended NSCs by decreasing Atg7 and
increasing p62
The effect of treatment with curcumin on the cell
cycle and apoptosis of NSCs was then considered. As NSCs also
express GFAP (28), there was no
change in the expression of GFAP protein between the three groups
(Fig. 4).
CDK2 is a marker of the cell cycle required during S
phase (29), and caspase-3 is a
critical executioner of apoptosis. The results suggested that
curcumin or 3MA could markedly decrease the protein level of these
two markers compared with untreated cells (Fig. 4). Additionally, the changes in the
expression of autophagy markers in suspended NSCs was assessed. As
identified through western blot analysis, LC3I and II protein
levels were unchanged between the three groups. However, Atg7
protein levels were much lower following treatment with curcumin or
3MA compared with the control group. The level of p62 protein was
slightly increased in the curcumin and 3MA treatment groups
compared with the control group (Fig.
4). Taken together, these findings indicate that curcumin can
affect the progression of NSCs from G1 to S phase, and
prevent their apoptosis. Furthermore, they suggest that increased
autophagic flux and decreased Atg7 expression are involved in the
process of NSC S-phase arrest and reduced apoptosis.
Autophagic vacuoles (AVOs) decreased in
curcumin-treated NSCs
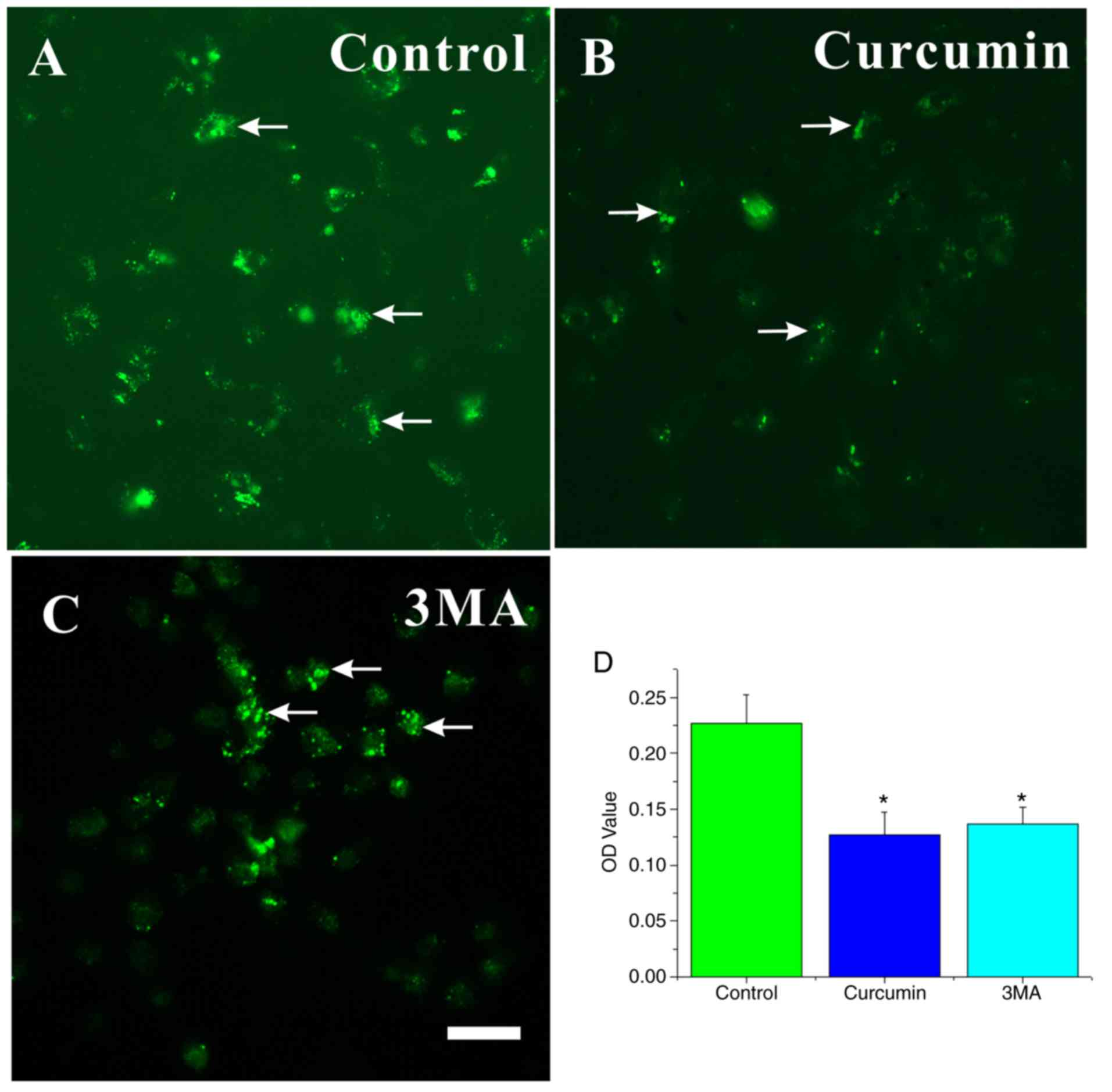
MDC is a specific marker for autolysosomes and AVOs,
which appear as spherical structures in the cytoplasm and the
perinuclear region (30). Thus,
the MDC staining of AVOs was used to assess the extent of autophagy
in the cells. Large dots indicative of AVOs appeared in the
cytoplasm of NSCs in the control group (Fig. 5A). The fluorescence intensity of
MDC in NSCs significantly decreased in the curcumin- or 3MA-treated
groups compared with the control group (P<0.05; Fig. 5B–D).
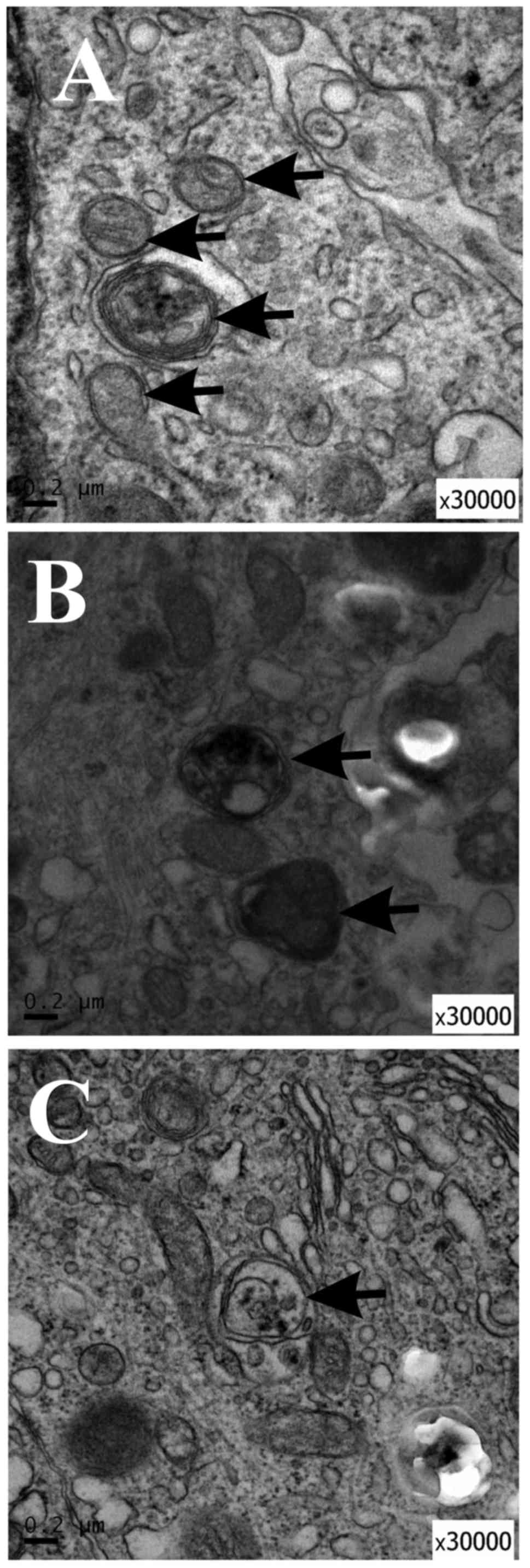
TEM demonstrated autophagosome formation
in curcumin-treated NSCs
In order to study the ultrastructural changes of
NSCs treated with curcumin or 3MA, TEM was performed to identify
AVOs, as previously described (31). AVOs containing extensively
degraded organelles, including mitochondria and endoplasmic
reticulum, were detected in the cytoplasm of NSCs (Fig. 6A). In contrast, NSCs treated with
curcumin or 3MA had relatively few autophagosomes in the cytoplasm
(Fig. 6B and C). These results
suggest that curcumin treatment affected autophagy initiation in
NSCs.
Discussion
Curcumin has been used for many centuries as a
traditional agent in treating inflammatory diseases and other
ailments. Curcumin is reported to contribute to the proliferation
and neurogenesis of NSCs (5).
Additionally, curcumin can promote the differentiation of
glioma-initiating cells by inducing autophagy (32) and induce glioma stem-like cell
formation (33). However, the
role and mechanism of curcumin in NSCs remains to be characterized.
In the present study, curcumin treatment prevented the
differentiation of NSCs in the adherent state via a reduction in
p62 expression. Curcumin treatment impeded cell cycle progression
and reduced the rate of apoptosis by decreasing the Atg7 protein
level and increasing the autophagic flux of p62. Taken together,
these data strongly suggest that curcumin inhibits the
differentiation and cell cycle of NSCs by adjusting Atg7 and p62
protein levels. It thus appears that the outcomes of curcumin
treatment may differ depending on the conditions of culture.
In the present study, the effect of curcumin on the
survival of NSCs in vitro was assessed using the WST method.
The viability of NSCs was higher following treatment with 10
µM curcumin compared with the control group. No cytotoxicity
was observed following treatment with 10 or 20 µM curcumin.
Additionally, the caspase-3 protein level decreased in suspended
NSCs treated with curcumin as detected by western blotting.
Caspase-3 is activated in apoptotic cells. Therefore, the data
showed that curcumin may prevent apoptosis and promote NSC
survival.
However, CDK2, a regulator of cell cycle progression
from G1 to S phase, was also reduced in suspended NSCs.
This is not contrary to the WST results, as cellular proliferation
may be not associated with CDK2 (34–36). Autophagy exerts a major influence
on the G1 and S phases of the cell cycle (37). For example, a previous study has
indicated that Atg7 is required for the p53-dependent expression of
p21CDKN1A and the cell cycle arrest of mouse embryonic fibroblasts
starved of serum and amino acids (38). However, another study reported
that the knockdown of Atg7 specifically increased p27 protein
abundance; p27 is a CDK inhibitor that prevents cell proliferation
(39). These two studies suggest
opposing roles for Atg7 in the cell cycle. Accordingly, we
hypothesize that curcumin promotes NSC survival through reducing
Atg7 to decrease the expression of CDK inhibitors.
Five CDKs active in the cell cycle have been
identified so far, including during G1 (CDK4, 6 and 2),
S (CDK2), G2 and M (CDK1). The present study has
demonstrated that curcumin treatment may inhibit G1-to-S
progression by downregulating CDK2. However, whether other factors
affecting the cell cycle, including CDK1, 4 and 6, and CDK
inhibitors, are also involved in the effect of curcumin on the NSC
cell cycle is unclear. Further research in this area is required to
fully understand the mechanisms of the effect.
Accumulating data have confirmed that the
association between autophagy and apoptosis is complex. Caspases
can cleave various autophagy-related proteins, and the cleaved
fragments generated have different functional activities and
cellular localization (40).
Caspase-8 contributes to the cleavage of Atg3, preventing its
pro-autophagic activity (41),
whereas caspase-9 can interact with Atg7 to facilitate autophagy
(42). A recent study has also
indicated that caspase-3 has both anti- and pro-autophagic effects
(43). The knockdown of Atg12
leads to a marked inhibition in caspase activity, including that of
caspase-3 (44). The present
study revealed that curcumin could decrease Atg7 protein level and
downregulate caspase-3 expression, indicating that a decrease in
Atg7 may have led to the inhibition of caspase-3, potentially
impairing Atg7-mediated autophagosome formation. These data are in
line with a previous study (42).
It was hypothesized that an increase in p62 level may decrease the
sensitivity of NSCs to caspase-3.
A previous study identified that the inhibition of
autophagy through the deletion of Atg5, Atg16L1 or Atg7 did not
impair the maintenance and differentiation of postnatal NSCs,
whereas p62 accumulation promoted the apoptosis of
autophagy-deficient NSCs by increasing the superoxide concentration
(45). In the present study, it
was shown that curcumin treatment prevented the differentiation of
NSCs into astrocytes or immature neurons accompanied by a reduction
in the Atg7 and p62 protein levels. Therefore, we hypothesize that
Atg7 or p62 may be involved in the effect on NSC differentiation
mediated by curcumin. p62 expression can prevent oxidative stress
(46,47) and be used to detect the state of
reactive oxygen species buffering systems (48). On the other hand,
H2O2 exposure can increase the neurogenesis
and oligodendrogenesis of NSCs (49), and curcumin has anti-oxidative
effects (50); thus, it was
hypothesized that the oxidative stress and p62 protein expression
decrease induced by curcumin treatment may result in the
dysfunction of NSC differentiation.
Consistent with previous research (9), for NSCs in a suspended culture
state, the levels of p62 increased, whereas the Atg7 levels
decreased, compared with untreated control cells. An increase in
LC3-II is not a measure of autophagic flux per se, since it can
also indicate the inhibition of autophagosome clearance (51). However, there were no differences
in LC3-II expression among the control and the curcumin- and
3MA-treated groups. Thus, the results demonstrated that curcumin
could enhance the autophagic flux of p62 and suppress the Atg7
protein level in suspended NSCs.
The MDC-labeled vesicles were also assessed in the
groups. A previous study has indicated that MDC-labeled vesicles
are not exclusively autolysosomes, and that MDC labels any acidic
compartment (21). In addition,
MDC dots can still be detected in Atg5−/− mouse
embryonic stem cells (52).
Therefore, TEM was also applied; the TEM results were consistent
with the MDC-labeling.
It was previously identified that NSC dynamics can
be modulated by different ion channels, such as K+,
Na+, Cl+ and TRP channels (53). Moreover, curcumin also affects the
functions of these channels (54,55). Thus, curcumin may also affect NSC
differentiation, proliferation and apoptosis through an effect on
these channels.
In summary, to the best of our knowledge, it was
demonstrated for the first time that curcumin inhibited the
differentiation, cell cycle progression and apoptosis of NSCs
through modulating the expression of Atg7 and p62 in vitro.
However, the results also suggest that effect of curcumin may be
dependent on the cell culture state. p62, a marker of autophagic
flux, was evidently decreased in adherent NSCs and increased in
suspended NSCs. 3MA, used as a positive control as an inhibitor of
autophagy, induced similar effects to curcumin. Thus, we
hypothesize that curcumin may also affect PI3K in NSCs. However,
the results of the present study were preliminary; it is not yet
possible to be certain whether Atg7 modulation mediated the effects
of curcumin intervention. Employing gene overexpression and
knockdown would further demonstrate the roles of Atg7 and p62 in
the effects of curcumin treatment. Furthermore, the connections
between Atg7 and p53 in the effect of curcumin on NSCs will need to
be considered in the future.
Funding
The study was supported by the Zhejiang Provincial
Natural Science Foundation of China (grant nos. LY18H090013 and
LY14F020035) and Wenzhou Municipal Science and Technology Bureau
Fund (grant no. Y20170165).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JJW and CJX analyzed the data; JLW, ZNC, CJX
performed the experiments and data analysis; CJX wrote the
manuscript; JJW and CJX designed the experiments. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal-related procedures were approved by the
Institutional Animal Care and Use Committee of Wenzhou Medical
University (Wenzhou, China), and were conducted in accordance with
the university's guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: Role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang R, Li Y, Li Y, Xu Y, Wu H and Li X:
Curcumin protects against glutamate excitotoxicity in rat cerebral
cortical neurons by increasing brain-derived neurotrophic factor
level and activating. TrkB Brain Res. 1210:84–91. 2008. View Article : Google Scholar
|
|
3
|
Ramadan G, Al-Kahtani MA and El-Sayed WM:
Anti-inflammatory and anti-oxidant properties of curcuma longa
(turmeric) versus zingiber officinale (ginger) rhizomes in rat
adjuvant-induced arthritis. Inflammation. 34:291–301. 2011.
View Article : Google Scholar
|
|
4
|
Kang SK, Cha SH and Jeon HG:
Curcumin-induced histone hypoacetylation enhances
caspase-3-dependent glioma cell death and neurogenesis of neural
progenitor cells. Stem Cells Dev. 15:165–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SJ, Son TG, Park HR, Park M, Kim MS,
Kim HS, Chung HY, Mattson MP and Lee J: Curcumin stimulates
proliferation of embryonic neural progenitor cells and neurogenesis
in the adult hippocampus. J Biol Chem. 283:14497–14505. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long DX, Hu D, Wang P and Wu YJ: Induction
of autophagy in human neuroblastoma SH-SY5Y cells by
tri-ortho-cresyl phosphate. Mol Cell Biochem. 396:33–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi R, Weng J, Zhao L, Li XM, Gao TM and
Kong J: Excessive autophagy contributes to neuron death in cerebral
ischemia. CNS Neurosci Ther. 18:250–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomez-Puerto MC, Folkerts H, Wierenga ATJ,
Schepers K, Schuringa JJ, Coffer PJ and Vellenga E: Autophagy
proteins ATG5 and ATG7 are essential for the maintenance of human
CD34+ hematopoietic stem-progenitor cells. Stem Cells.
34:1651–1663. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ouyang L, Zhang L, Zhang S, Yao D, Zhao Y,
Wang G, Fu L, Lei P and Liu B: Small-molecule activator of
UNC-51-like kinase 1 (ULK1) that induces cytoprotective autophagy
for Parkinson's disease treatment. J Med Chem. 61:2776–2792. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karabiyik C, Lee MJ and Rubinsztein DC:
Autophagy impairment in Parkinson's disease. Essays Biochem.
61:711–720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kabuta T, Suzuki Y and Wada K: Degradation
of amyotrophic lateral sclerosis-linked mutant Cu, Zn-superoxide
dismutase proteins by macroautophagy and the proteasome. J Biol
Chem. 281:30524–30533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fornai F, Longone P, Ferrucci M, Lenzi P,
Isidoro C, Ruggieri S and Paparelli A: Autophagy and amyotrophic
lateral sclerosis: The multiple roles of lithium. Autophagy.
4:527–530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nixon RA, Wegiel J, Kumar A, Yu WH,
Peterhoff C, Cataldo A and Cuervo AM: Extensive involvement of
autophagy in Alzheimer disease: An immunoelectron microscopy study.
J Neuropathol Exp Neurol. 64:113–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cianfanelli V and Cecconi F: AMBRA1: When
autophagy meets cell proliferation. Autophagy. 11:1705–1707. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Revuelta M and Matheu A: Autophagy in stem
cell aging. Aging Cell. 16:912–915. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Codogno P and Meijer AJ: Autophagy and
signaling: Their role in cell survival and cell death. Cell Death
Differ. 2(Suppl 12): S1509–S1518. 2005. View Article : Google Scholar
|
|
18
|
Zhang C, Wu JM, Liao M, Wang JL and Xu CJ:
The ROCK/GGtase pathway are essential to the proliferation and
differentiation of neural stem cells mediated by simvastatin. J Mol
Neurosci. 60:474–485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu CJ, Xu L, Huang LD, Li Y, Yu PP, Hang
Q, Xu XM and Lu PH: Combined NgR vaccination and neural stem cell
transplantation promote functional recovery after spinal cord
injury in adult rats. Neuropathol Appl Neurobiol. 37:135–155. 2011.
View Article : Google Scholar
|
|
20
|
Peng ZW, Xue F, Wang HN, Zhang RG, Chen
YC, Wang Y, Zhang LY, Fan J and Tan QR: Paroxetine up-regulates
neurogenesis in hippocampus-derived neural stem cell from fetal
rats. Mol Cell Biochem. 375:105–113. 2013.
|
|
21
|
Vázquez CL and Colombo MI: Chapter 6
assays to assess autophagy induction and fusion of autophagic
vacuoles with a degradative compartment, using monodansylcadaverine
(MDC) and DQ-BSA. Methods Enzymol. 452:85–95. 2009. View Article : Google Scholar
|
|
22
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komatsu M, Waguri S, Ueno T, Iwata J,
Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et
al: Impairment of starvation-induced and constitutive autophagy in
Atg7-deficient mice. J Cell Biol. 169:425–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bjørkøy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: p62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bjørkøy G, Lamark T, Pankiv S, Øvervatn A,
Brech A and Johansen T: Monitoring autophagic degradation of
p62/SQSTM1. Methods Enzymol. 452:181–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamzehzadeh L, Atkin SL, Majeed M, Butler
AE and Sahebkar A: The versatile role of curcumin in cancer
prevention and treatment: A focus on PI3K/AKT pathway. J Cell
Physiol. 233:6530–6537. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X and Zhu R: Curcumin suppresses the
progression of laryngeal squamous cell carcinoma through the
upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR
pathway. Onco Targets Ther. 11:3521–3531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahmed AI, Shtaya AB, Zaben MJ, Owens EV,
Kiecker C and Gray WP: Endogenous GFAP-positive neural
stem/progenitor cells in the postnatal mouse cortex are activated
following traumatic brain injury. J Neurotrauma. 29:828–842. 2012.
View Article : Google Scholar :
|
|
29
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2010.
View Article : Google Scholar
|
|
30
|
Munafó DB and Colombo MI: A novel assay to
study autophagy: Regulation of autophagosome vacuole size by amino
acid deprivation. J Cell Sci. 114:3619–3629. 2001.PubMed/NCBI
|
|
31
|
Gukovskaya AS and Gukovsky I: Autophagy
and pancreatitis. Am J Physiol Gastrointest Liver Physiol.
303:G993–G1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhuang W, Long L, Zheng B, Ji W, Yang N,
Zhang Q and Liang Z: Curcumin promotes differentiation of
glioma-initiating cells by inducing autophagy. Cancer Sci.
103:684–690. 2012. View Article : Google Scholar
|
|
33
|
Shi L, Wang Z and Sun G: Curcumin induces
glioma stem-like cell formation. Neuroreport. 26:167–172. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Méndez J: Cell proliferation without
cyclin E-CDK2. Cell. 114:398–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tetsu O and Mccormick F: Proliferation of
cancer cells despite CDK2 inhibition. Cancer Cell. 3:233–245. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kelly TJ and Brown GW: Regulation of
chromosome replication. Annu Rev Biochem. 69:829–880. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tasdemir E, Maiuri MC, Tajeddine N, Vitale
I, Criollo A, Vicencio JM, Hickman JA, Geneste O and Kroemer G:
Cell cycle-dependent induction of autophagy, mitophagy and
reticulophagy. Cell Cycle. 6:2263–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee IH, Kawai Y, Fergusson MM, Rovira II,
Bishop AJ, Motoyama N, Cao L and Finkel T: Atg7 modulates p53
activity to regulate cell cycle and survival during metabolic
stress. Science. 336:225–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu J, Yang L, Tian Z, Hua X, Gu J, Li J,
Liu C, Jin H, Wang Y, Jiang G, et al: ATG7 overexpression is
crucial for tumorigenic growth of bladder cancer in vitro and in
vivo by targeting the ETS2/miRNA196b/FOXO1/p27 axis. Mol Ther
Nucleic Acids. 7:299–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ojha R, Ishaq M and Singh SK:
Caspase-mediated crosstalk between autophagy and apoptosis: Mutual
adjustment or matter of dominance. J Cancer Res Ther. 11:514–524.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oral O, Oz-Arslan D, Itah Z, Naghavi A,
Deveci R, Karacali S and Gozuacik D: Cleavage of Atg3 protein by
caspase-8 regulates autophagy during receptor-activated cell death.
Apoptosis. 17:810–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han J, Hou W, Goldstein LA, Stolz DB,
Watkins SC and Rabinowich H: A complex between atg7 and caspase-9:
A novel mechanism of cross-regulation between autophagy and
apoptosis. J Biol Chem. 289:6485–6497. 2014. View Article : Google Scholar :
|
|
43
|
Chang JL, Chow JM, Chang JH, Wen YC, Lin
YW, Yang SF, Lee WJ and Chien MH: Quercetin simultaneously induces
G0/G1-phase arrest and caspase-mediated crosstalk between apoptosis
and autophagy in human leukemia HL-60 cells. Environ Toxicol.
32:1857–1868. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rubinstein AD, Eisenstein M, Ber Y, Bialik
S and Kimchi A: The autophagy protein atg12 associates with
antiapoptotic BCL-2 family members to promote mitochondrial
apoptosis. Mol Cell. 44:698–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang C, Chen S, Yeo S, Karsli-Uzunbas G,
White E, Mizushima N, Virgin HW and Guan JL: Elevated p62/SQSTM1
determines the fate of autophagy-deficient neural stem cells by
increasing superoxide. J Cell Biol. 212:545–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taniguchi K, Yamachika S, He F and Karin
M: p62/SQSTM1-Dr. Jekyll and Mr. Hyde that prevents oxidative
stress but promotes liver cancer. FEBS Lett. 590:2375–2397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Cano M and Handa JT: p62 provides
dual cytoprotection against oxidative stress in the retinal pigment
epithelium. Biochim Biophys Acta. 1843:1248–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carroll B, Otten EG, Manni D, Stefanatos
R, Menzies FM, Smith GR, Jurk D, Kenneth N, Wilkinson S, Passos JF,
et al: Oxidation of SQSTM1/p62 mediates the link between redox
state and protein homeostasis. Nat Commun. 9:2562018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Perez Estrada C, Covacu R, Sankavaram SR,
Svensson M and Brundin L: Oxidative stress increases neurogenesis
and oligodendrogenesis in adult neural progenitor cells. Stem Cells
Dev. 23:2311–2327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Panchal HD, Vranizan K, Lee CY, Ho J, Ngai
J and Timiras PS: Early anti-oxidative and anti-proliferative
curcumin effects on neuroglioma cells suggest therapeutic targets.
Neurochem Res. 33:1701–1710. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mizushima N: Methods for monitoring
autophagy. Int J Biochem Cell Biol. 36:2491–2502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yasuda T and Adams DJ: Physiological roles
of ion channels in adult neural stem cells and their progeny. J
Neurochem. 114:946–959. 2010.PubMed/NCBI
|
|
54
|
Zhang X, Chen Q, Wang Y, Peng W and Cai H:
Effects of curcumin on ion channels and transporters. Front
Physiol. 5:942014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nalli M, Ortar G, Schiano Moriello A, Di
Marzo V and De Petrocellis L: Effects of curcumin and curcumin
analogues on TRP channels. Fitoterapia. 122:126–131. 2017.
View Article : Google Scholar : PubMed/NCBI
|