Introduction
Aquaporins (AQPs), a large family of integral
membrane proteins termed the major intrinsic proteins (MIPs), have
been identified in nearly all organisms. Indeed, these water
channel proteins are conserved in unicellular (archaea, bacteria,
yeasts and protozoa) and multicellular (plants, animals and humans)
organisms. However, the numbers of AQP isoforms vary significantly
between species. For example, two isoforms were detected in
Escherichia coli, one being an AQP (AqpZ) and the other an
AQP-like sequence (glycerol facilitator GlpF) (1). The yeast Saccharomyces
cerevisiae possesses two AQPs (ScAqy1 and ScAqy2) and two
aquaglyceroporins (YFL054Cp and ScFps1) (2). Since the completion of the human
genome project, 13 mammalian AQPs (AQP0-12) with varying degrees of
homology have been defined; these are expressed in a number of
epithelial and endothelial cells involved in fluid transport, as
well as in skin, fat, brain, liver and urinary bladder cells that
may not be involved in fluid transport (3). Based on the primary sequences and a
phylogenetic framework (rather than function), human AQPs are
classified into three major paralogous groups: i) Orthodox or
classical AQPs (AQP0, 1, 2, 4, 5, 6 and 8), considered to be
specific water channels; ii) aquaglyc-eroporins (AQP3, 7, 9 and
10), permeable to glycerol and perhaps urea and other small solutes
in addition to water; and iii) superAQPs (S-AQPs) (AQP11 and 12),
also known as unorthodox S-AQPs or subcellular AQPs, a third
subfamily present in animals, but not in plants, fungi or bacteria,
and with uncertain permeability (4). AQPs are implicated in numerous
physiological processes and in the pathophysiology of a wide range
of clinical disorders, including mediating allergic responses
(5), encouraging the developing
of novel approaches for the treatment of human diseases based on
AQP function or dysfunction (6,7).
Two conserved regions of amino acids (NPA motifs)
are considered markers for a common origin of water channel
proteins (8-10). These highly conserved regions can
be used to design degenerate polymerase chain reaction (PCR)
primers to enable amplification of an MIP gene fragment. Indeed,
this was performed in the insect Rhodnius prolixus, in which
the full-length cDNA encoding a protein termed R.
prolixus-MIP was isolated from malpighian tubules and amplified
using reverse-transcription PCR (RT-PCR) with degenerate primers to
highly conserved regions of the members of the AQP family (11). Similarly, an open reading frame
was obtained from a λ lgt-11 cDNA library constructed from an adult
buffalo fly by screening with degenerate PCR primers designed from
highly conserved regions of amino acids found in all members of the
AQP gene family (12). Thus,
certain information on AQPs in insects has been gained by cloning.
Studies of AQPs allow precise biophysical measurements of their
functions and exploration of high affinity inhibitors for control
of these insects.
Molecular cloning with degenerate PCR primers,
however, cannot fully resolve all AQP-coding genes within a given
organism due to sequence diversity. By contrast, transcriptomic
approaches such as RNA-sequencing (RNA-seq) can reveal the full
complement of messenger RNA molecules, and de novo assembly
of RNA-seq data enables the study of genes without the requirement
for a genome sequence (13). The
use of transcriptomics approaches opens novel possibilities for
advancing our understanding of insect genomes (14). In our recent study, RNA-seq,
combined with tandem mass spectroscopy, was used to identify and
study potential allergens from Tyrogphagus putrescentiae
(15), a mite species with
importance for human allergic disease.
Another domestic mite species, Blomia
tropicalis, is an important source of indoor allergens
associated worldwide with asthma, as well as rhinoconjunctivitis
and atopic eczema. B. tropicalis mites coexist with
Dermatophagoides pteronyssinus mites in tropical and
subtropical regions; although D. pteronyssinus contributes
to more allergic disease worldwide, B. tropicalis allergens
are probably more important clinically than D. pteronyssinus
allergens in these regions (16,17). To the best of our knowledge, to
date, no AQP-related proteins have been identified in mite species.
Thus, in the present study, RNA-seq was conducted in B.
tropicalis, its AQPs were annotated by BLASTX and five AQPs
were identified by molecular cloning techniques. To the best of our
knowledge, this is the first report of AQPs in any house dust or
storage mite species.
Materials and methods
Mite culture
Mites were collected from dust samples from Haikou,
Hainan, located in the South China Sea. Each mite isolated under a
stereomicroscope was cultivated in a small flask (5 ml) with
culture medium in the bottom and filter paper covering the top.
After 2 months, the mites were removed, mounted on slides and
viewed under a stereomicroscope. Any mites identified as B.
tropicalis were placed in a large flask (200 ml) for continuous
culture. Flasks were placed in an artificial climate incubator
(catalog no. RXZ-280D; Jiangnan Instrument Factory, Ningbo, China)
at 25±1°C and 70±5% relative humidity. The culture medium was
comprised of fish meal and yeast powder.
Isolation of total RNA
Total RNA was isolated from mites using an RNAiso
Plus kit (catalog no. 9108Q; Takara Biotechnology Co., Ltd.,
Dalian, China) according to the manufacturer's protocols. Briefly,
isolated cultured mites were rapidly frozen with liquid nitrogen,
and then 2 ml RNAiso Plus was added. Mites were then homogenized in
a PowerGen 125 Tissue Homogenizer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) starting at 5,000 rpm and gradually increasing to
~20,000 rpm for 30-60 sec at room temperature. Samples were
transferred to an Eppendorf tube for RNA isolation. To evaluate the
quality of the total RNA, the concentrations, 28S/18S and RNA
Integrity Number (RIN) were detected on an Agilent 2100 bioanalyzer
with Agilent RNA 6000 nano Reagents Port 1 (Agilent Technologies
GmbH, Waldbronn, Germany). Finally, total RNA was dissolved in
RNase-free water and stored at -80°C.
Transcriptome library construction,
sequencing, de novo assembly and annotation
Previously described methods were used to sequence
and assemble the transcriptome of B. tropicalis (15). Briefly, to create a pooled cDNA
library for large-scale sequencing, a SMART™ cDNA Library
Construction kit (catalog no. 634901; Clontech Laboratories, Inc.,
Mountainview, CA, USA) was used. mRNA was isolated using magnetic
beads with Oligo(dT). Fragmented mRNA was used as a template for
cDNA production. Elution buffer (10 mM Tris ·Cl, pH 8.5) was used
for end repair and single nucleotide adenine addition, then
fragments were connected with adapters. Suitable fragments were
selected as templates for PCR amplification.
The library was sequenced using Illumina HiSeq™ 2000
(Illumina, Inc., San Diego, CA, USA). Image data were transformed
by base calling into sequence data and stored in Fastq format. Raw
data were cleaned by removing reads with adaptors, unknown
nucleotides >5% and low-quality reads (base quality ≤10)
>20%.
Trinity program v2.2.0 (13) was used to align contigs and
generate unigenes. Unigenes were aligned by BLASTX (E-value
≤1.0x10-5; https://blast.ncbi.nlm.nih.gov/Blast.cgi) to databases
in the priority order non-redundant (NR), Nucleotide (NT),
Swiss-Prot, Kyoto Encyclopedia of Genes and Genomes (KEGG),
Clusters of Orthologous Groups (COG) and Gene Ontology (GO)
database. Unigenes aligned to a high priority database were not
aligned to databases of lower priority. The process ended when all
alignments were finished. Proteins with the highest ranks in BLAST
results were selected to decide the coding region sequences of
unigenes, and then the coding region sequences (CDS) were
translated into amino sequences with the standard codon table.
Unigenes that could not be aligned to any database were scanned by
the program ESTScan (18)
producing nucleotide sequence (5′-3′) direction and amino sequences
of the predicted coding region.
Simple sequence repeats (SSRs) were identified with
the program MicroSAtellite using unigene as a reference.
Single-nucleotide polymorphisms (SNPs) were identified using the
Short Oligonucleotide Analysis Package (http://soap.genomics.org.cn).
Identification and cDNA cloning of AQPs
from Blomia tropicalis
During functional annotation by BLASTX in
transcriptomic analysis, AQP sequences were selected and clustered
to create an Excel table. Using total RNA of B. tropicalis
as a template, these sequences were then tested by RT-PCR with the
Takara PrimeScript™ RT-PCR kit (catalog no. bRR014A) and the Takara
Tks Gflex DNA Polymerase kit (catalog no. R060A) (both Takara
Biotechnology Co., Ltd.). The RT-PCR products were analyzed by
agarose electrophoresis (1.0%), visualized with
ImageMaster® VDS (Pharmacia Biotech Inc., San Francisco,
CA, USA), recovered with a Takara MiniBEST Agarose Gel DNA
Extraction kit Ver.4.0 (catalog no. 9762; Takara Biotechnology Co.,
Ltd.), and submitted to direct sequencing. Following alignment by
BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi), candidate
sequences were obtained. Finally, the full cDNA encoding these AQPs
were directly synthesized by RT-PCR from the total RNA of B.
tropicalis with the primers listed in Table I and using the temperature
protocols mentioned in the RT-PCR section. Subsequent to agarose
gel electrophoresis, the recovered PCR products were inserted into
expression plasmid pET-28a(+) vector (kit catalog no. N72770;
Novagen; Merck KGaA, Darmstadt, Germany) with In-Fusion®
HD Cloning kit (catalog no. 639633; Clontech Laboratories, Inc.)
for sequence determination.
 | Table IIdentification of AQPs from Blomia
tropicalis. |
Table I
Identification of AQPs from Blomia
tropicalis.
| AQPs | GenBank accession
number | cDNA length,
bp | Deduced no. of
amino acids | Deduced molecular
weight, Da | Homolog
similaritya (%) | Forward primers in
PCR | Reverse primers in
PCR |
|---|
| BlotAQP1 | KX655540 | 843 | 282 | 30518.8 | ANC28171 (48) | AATGGGTCGCGGATCCATG
GTCAATTGGTCAATTAAAA ATGC |
TCAGTGGTGGTGGTGGTGGTGC
TCGAGTGCGGCCGCCTAGTTCT GGGTGACTCGTACA |
| BlotAQP2 | KX655541 | 918 | 305 | 33164.6 | KRX48204 (41) | AATGGGTCGCGGATCCATG
AATAATATTTGGAAAGAAT C |
T:CAGTGGTGGTGGTGGTGGTG
TCGAGTGCGGCCGCTCAATAA CGTGAATGTGGTG |
| BlotAQP3 | KX655542 | 771 | 256 | 28598.7 | KFM59126 (51) | AATGGGTCGCGGATCCATG
TTACCGCACGATATCATCC GTACA |
TCAGTGGTGGTGGTGGTGGTGC
TCGAGTGCGGCCGCTTATTCCA ATTTTTCCCTGGGC |
| BlotAQP4 | KX655543 | 1,377 | 458 | 49839.3 | XP_001866597
(43) | AATGGGTCGCGGATCCATG
ATGGCAACACCAAAATTG GGTC |
TCAGTGGTGGTGGTGGTGGTGC
TCGAGTGCGGCCGCTTAGAATT TGGGATTCACACCA |
| BlotAQP5 | KX655544 | 825 | 274 | 30087.6 | KRX92759 (35) | AATGGGTCGCGGATCCATG
AAACGTGCTGCAAAAGAG |
TCAGTGGTGGTGGTGGTGGTGC
TCGAGTGCGGCCGCTCATTGTA CTAATATATTTT |
RT-PCR
RT was performed using the total RNA isolated from
mites with Takara PrimeScript™ RT-PCR kit (catalog no. RR014A) in
the PCR Thermal Cycler Dice (catalog no. TP600) (both Takara
Biotechnology Co., Ltd.). The reaction mix contained 1 µl
total RNA, 1 µl Random 6 (20 mM), 1 µl dNTPs (10
mmol/l each) and 5.5 µl RNase-free dH2O. The
reaction mix was placed at 65°C for 5 min, and then on an ice-bath
for 2 min. Additionally, 2 µl 5X PrimeScript RT Buffer, 0.25
µl RNase inhibitor (40 U/µl), 0.5 µl
PrimeScript RTase and 10 µl of the final reaction mixture
were incubated at 30°C for 10 min, 45°C for 30 min and 95°C for 5
min. Next, the RT product was used as the template for PCR in the
same thermal cycler with PrimeSTAR HS DNA Polymerase (catalog no.
DR010A; Takara Biotechnology Co., Ltd.). The total reaction mixture
contained 2 µl RT products, 25 µl 2X Gflex PCR Buffer
(Mg2+, dNTP plus), 1 µl Tks Gflex DNA Polymerase
(1.25 U/µl), 1 µl forward primer (10 µM), 1
µl reverse primer (10 µM) and 20 µl
dH2O. PCR conditions comprised an initial incubation for
1 min at 94°C, followed by 30 cycles of 10 sec at 98°C, 15 sec at
55°C and 1 min at 68°C. Subsequent to a final incubation for 5 min
at 68°C, 5 µl of the PCR product was analyzed by agarose
electrophoresis (1.0%) and visualized with ImageMaster®
VDS (Pharmacia Biotech Inc.). The PCR products were recovered by
Takara MiniBEST Agarose Gel DNA Extraction kit Ver.4.0 (catalog no.
9762; Takara Biotechnology Co., Ltd.).
Preparation of vector DNA
The vector pET28a(+) (Novagen; Merck KGaA) was
digested with BamHI and NotI. Digestion mixture (50
µl) contained 10 µl pET28a(+) (100 ng/µl), 2.5
µl 10X K Buffer, 1.5 µl BamHI (15
U/µl), 1.5 µl NotI (15 U/µl), 5
µl 0.1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) and
29.5 µl dH2O. Digestion was performed at 37°C for
3 h, and then samples were separated by 1% agarose gel
electrophoresis. The PCR products were recovered by Takara MiniBEST
Agarose Gel DNA Extraction kit Ver.4.0.
Cloning and DNA sequencing
The cDNA fragments coding for AQPs were sub-cloned
into the expression vector pET28a(+) to create recombinant plasmids
using the DNA Ligation kit (catalog no. D6023; Takara Biotechnology
Co., Ltd.). E. coli JM109 competent cells (catalog no.
D9052; Takara Biotechnology Co., Ltd.) were transformed with
recombinant plasmids, and positive clones were selected by
blue/white screening. Clones were sequenced on an ABI PRISM™ 377XL
DNA Sequencer (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Bioinformatics
Amino acid sequences were translated from the
nucleotide sequencing results by the Expasy Translate tool
(http://www.expasy.org/) and aligned with Clustal
Omega (http://www.ebi.ac.uk/). A tree was
constructed using amino acid sequences with the neighbor-joining
method using MEGA 6.0 (https://www.megasoftware.net/mega6/). Homology
modeling was used to construct a tertiary structure of these AQPs
from B. tropicalis one by one. BLASTp search with default
parameters was performed against the Protein Data Bank (https://www.rcsb.org/) to find suitable templates
(high score, low E-value and maximum sequence identity). MODELLER
v9.16 (19) was used to predict
the tertiary structure of the aforementioned AQPs. Estimating the
quality of structural models is a vital step in protein structure
construction. PROCHECK (20)
checked the stereochemical quality of the constructed structure.
ERRAT (21) analyzed statistics
of non-bonded interactions between different atom types. VERIFY_3D
(22) determined the
compatibility of an atomic model (3-dimensional) with its the amino
acid sequence (1-dimensional) and compared the results with
favorable structures. Superimposition of query and template
structure, and visualization of generated models was performed
using UCSF Chimera 1.10.2 (23).
Results
Sequencing, assembly and annotation of B.
tropicalis transcriptome
Total RNA was obtained at a concentration of 508
ng/µl and a RIN value of 7.2, which suggested that the total
RNA was of high quality. A cDNA library was constructed from the
RNA template, and sequencing runs were performed. Trinity v2.2.0
was used for the de novo assembly of 57,492,140 raw reads.
Using default assembly parameters, raw reads were screened and
trimmed of 5′ and 3′ adapters, and low quality reads were removed.
A total of 55,113,524 total clean reads were obtained with
4,960,217,160 bp. The Q20 value, describing the proportion of
nucleotides with a quality value of >20 in reads, was 97.96%,
and the GC percentage, describing the proportion of guanidine and
cytosine nucleotide among total nucleotides, was 40.55%. Overall,
this assembly obtained 68,658 unigenes (transcripts) with a total
length of 62,406,675 bp and a mean length of 909 bp. A total of
36,645 unigenes were annotated with 33,545 in the non-redundant
(NR) database, 12,475 in the nucleotide (NT) database, 30,057 in
Swiss-Prot, 26,686 in KEGG, 15,995 in COG and 18,426 in the Gene
Ontology (GO) database. In total, 45,632 CDS were generated,
including 34,195 CDS by BLASTX protein database searches and 11,437
predicted as aforementioned. The NR database queries revealed B.
tropicalis sequences to closely match sequences of Ixodes
dammini (11.7%), Galendromus occidentalis (7.5%),
Daphnia pulex (3.6%), Crassostrea gigas (2.6%),
Pediculus humanus corporis (2.7%) and Capitella spp.
(2.5%).
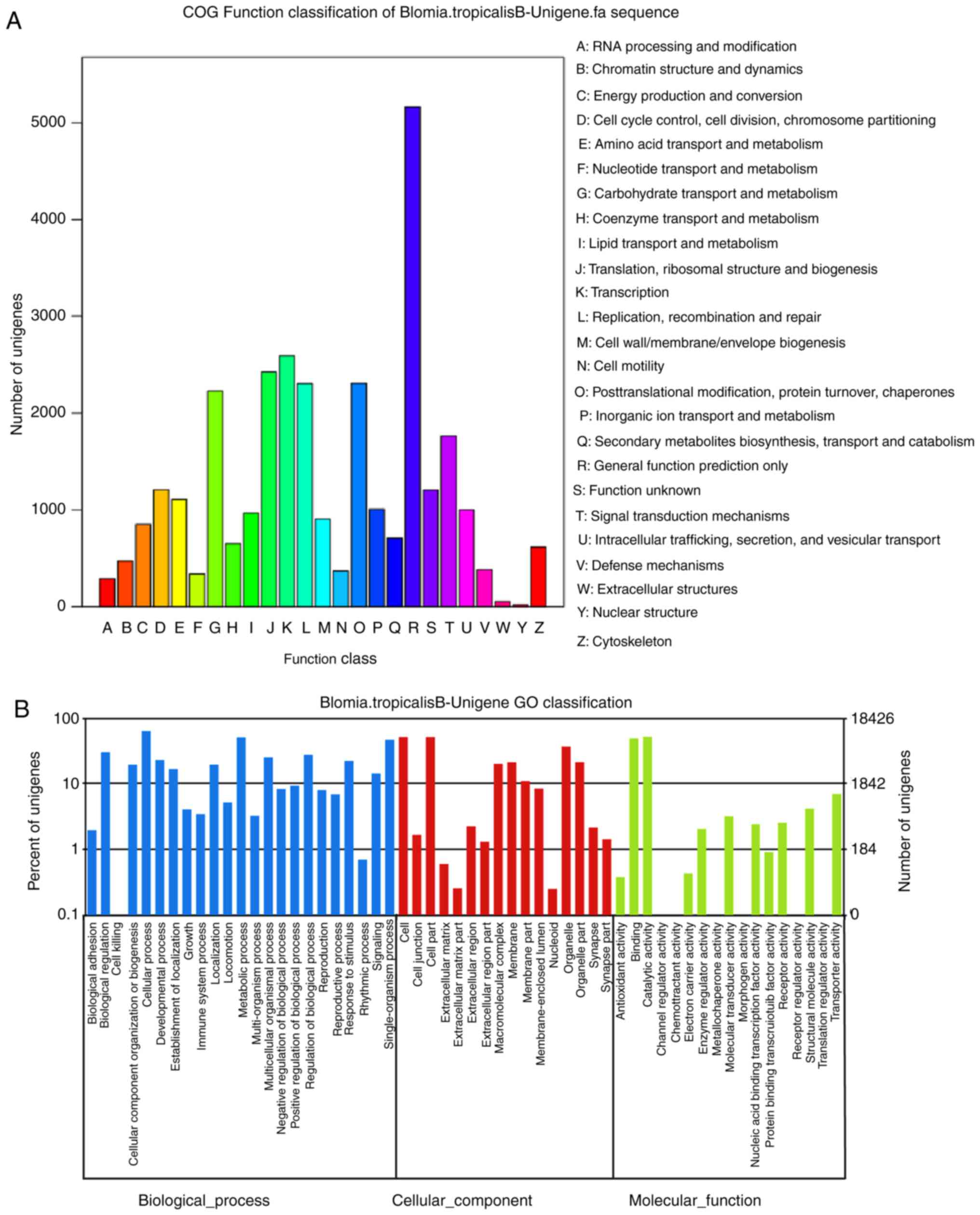
Functional annotation performed by searching against
the COG database resulted in a classification of 25 categories. The
cluster for ‘cytoskeleton’ constituted the major group (n=5,161;
16.68%), followed by ‘nuclear structure’ (n=2,593; 8.38%),
‘extracellular structures’ (n=2,424; 7.83%) and ‘defense
mechanisms’ (n=2,306; 7.45%); only 5 unigenes were allocated to
‘RNA processing and modification’ (Fig. 1A).
In the GO annotation, the 18,426 unigenes were
allocated to one or more GO terms based on sequence similarity. The
three main categories of GO annotations were biological process
(n=6,016; 38.1%), cellular component (n=4,793; 30.36%) and
molecular function (n=4,979; 31.54%) (Fig. 1B). In the category of biological
process, the term ‘cellular process’ was in the highest proportion
of annotations, followed by ‘metabolic process’, ‘single-organism
process’, ‘biological regulation’ and ‘regulation of biological
process’. For cellular component, genes involved in ‘cell’ and
‘cell part’ were most represented. In the category of molecular
function, the most frequent GO term was ‘catalytic activity’,
followed by ‘binding’.
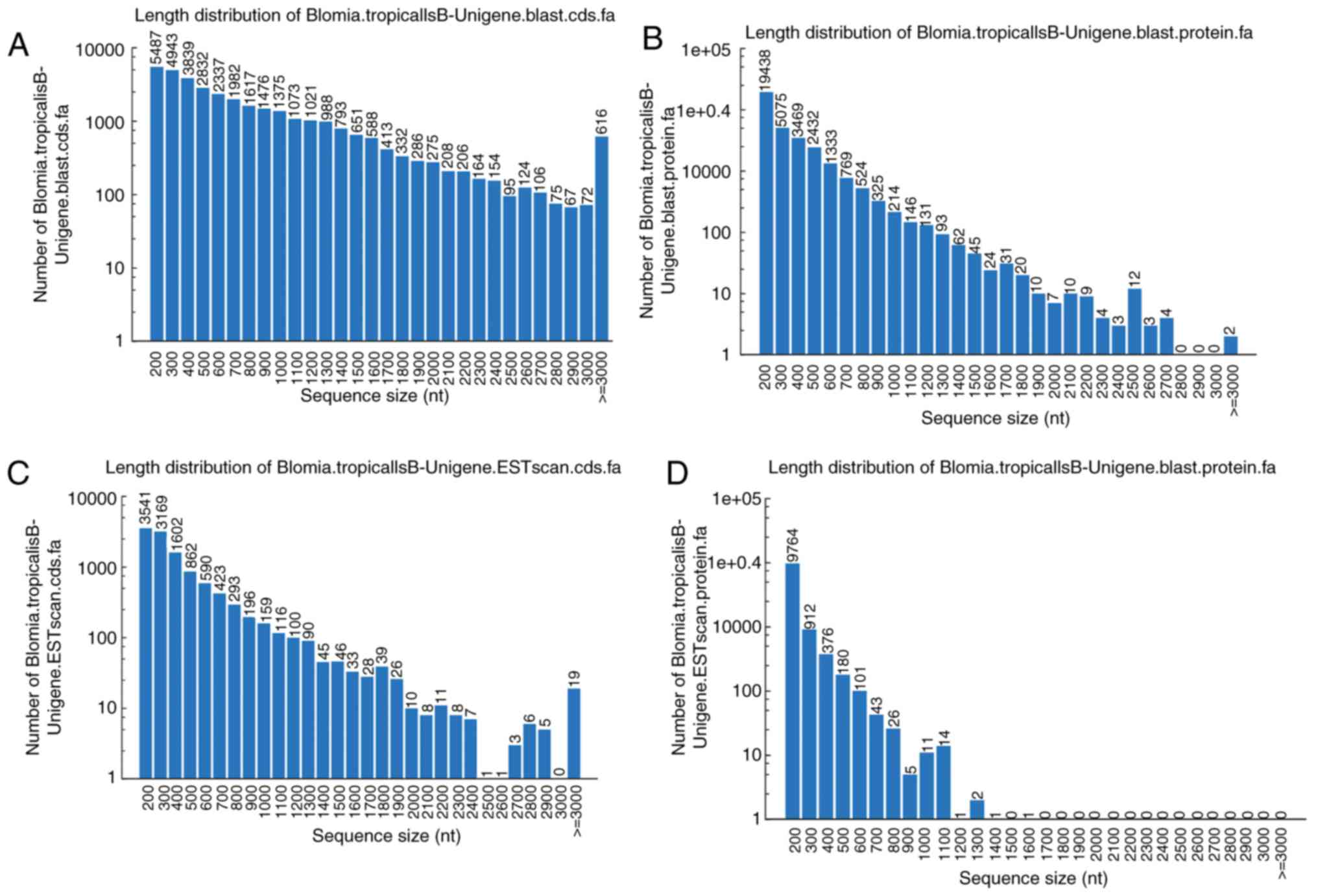
In total, CDS of 34,195 unigenes were generated by
BLASTX protein database searches. The ratio of gap lengths to the
sizes of unigene CDS were analyzed. The majority of the unigene CDS
(n=24,513; 71.69%) had <1,000 bp, and 8,307 had ≥1,000 bp. The
size frequency distributions of these unigene CDS and proteins are
depicted in Fig. 2A and B,
respectively. A total of 11,437 CDS of unigenes were scanned that
could not be aligned to any database by ESTScan. Of these, the
majority of the unigene CDS assigned by ESTScan (n=10,676; 93.34%)
were shorter than 1,000 bp (Fig.
2C); this was also the case for protein sequences obtained from
ESTScan (Fig. 2D).
Discovery of microsatellites
SSRs, or DNA microsatellites, can be used for
genomic mapping, DNA fingerprinting and marker-assisted selection.
To investigate the SSR profile in unigenes of B. tropicalis,
in the 68,658 investigated unigene sequences, 13,298 SSRs were
detected, with 3,896 (29.29%) sequences containing more than one
SSR. Six types of SSRs were found, among which the tri-nucleotide
repeats motif represented the largest group (n=10,394), followed by
the di-nucleotide repeats (n=7,653), quad-nucleotide repeats
(n=541), mono-nucleotide repeats (n=336), penta-nucleotide repeats
(n=182) and hexa-nucleotide repeats (n=87).
Finally, based on the alignment of short reads to
unigenes with the corresponding sequencing quality scores, a total
of 78,391 SNPs, both transition and transversion, were identified
for B. tropicalis. In the total 53,761 transition variants,
there were 27,032 ‘A-G’ and 26,729 ‘C-T’. In the total 24,630
trans-version variants, there were 5,981 ‘A-C’, 9,954 ‘A-T’, 3,113
‘C-G’ and 5,582 ‘G-T’.
Gene cloning and nucleotide sequencing of
AQPs from B. tropicalis
According to functional annotation in transcriptome
analysis, the unigenes coding for AQPs were selected and clustered
to create an Excel table. Based on these unigenes sequences, the
primers were designed, synthesized and used in RT-PCR for
amplification of the sequences from the total RNA of B.
tropicalis. By agarose electrophoresis analysis, five genes
were obtained, which were then recovered and inserted into the
vector pET28a(+) to create recombinant plasmids. The recombinant
plasmids were transformed into competent cells for subsequent
selection of positive clones. Sequencing of the positive clones
produced five full-length genes of 843, 918, 771, 1,377 and 825 bp.
The five sequences were registered in GenBank under accession
numbers KX655540, KX655541, KX655542, KX655543 and KX655544, and
termed BlotAQP1, BlotAQP2, BlotAQP3, BlotAQP4 and BlotAQP5,
respectively.
Phylogenic tree, amino acid sequences
alignment and homolog modeling
Fig. 3 shows the
molecular evolution tree constructed with the aforementioned five
amino acid sequences and human AQPs (AQP0-12), from which it can be
observed that BlotAQP3 was clustered with hAQP11 and hAQP12,
BlotAQP4 was clustered with hAQP1, hAQP2, hAQP4, hAQP5, hAQP6 and
hAQP8, and BlotAQP1, BlotAQP2 and BlotAQP5 were clustered with
hAQP3, hAQP7 hAQP9 and hAQP10. By Clustal Omega, the five amino
acid sequences from Blomia tropicalis were aligned with the
human orthodox AQPs (AQP0, 1, 2, 4, 5, 6 and 8), aquaglyceroporins
and S-AQPs (AQP11 and 12). Alignments are shown in Fig. 4A-D.
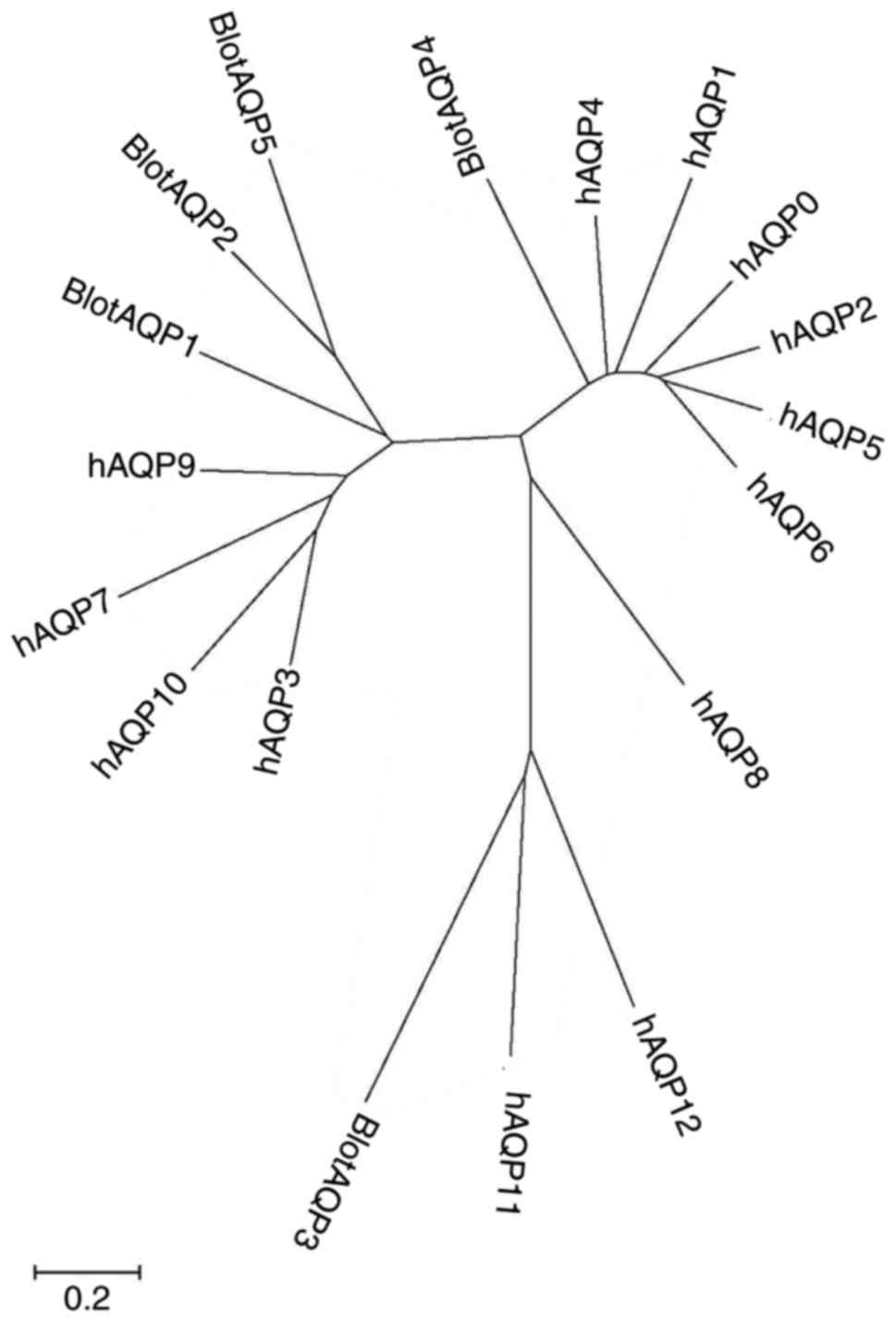 | Figure 3Phylogenetic relationship of AQPs
from Blomia tropicalis and Homo sapiens. Tree
constructed using amino acid sequences with neighbor-joining method
using MEGA (6.0). Scale bar represents an estimate of the number of
amino acid substitutions per site. Accession and database sequences
identifiers are as follows: hAQP1 (no. AB451275), hAQP2 (no.
AH007817), hAQP3 (no. BT007199), hAQP4 (no. BC022286), hAQP5 (no.
AH006636), hAQP6 (no. NM_001652), hAQP7 (no. BC119672), hAQP8 (no.
AF067797), hAQP9 (no. AB008775), AQP10 (no. BC069607), AQP11 (no.
BC040443), AQP12 (no. AB040748), BlotAQP1 (no. KX655540), BlotAQP2
(no. KX655541), BlotAQP3 (no. KX655542), BlotAQP4 (no. KX655543)
and BlotAQP5 (no. KX655544). AQP, aquaporin. |
 | Figure 4AQP amino acid sequence alignments.
Amino acid sequence alignments (A) among AQPs of Blomia
tropicalis; (B) among BlotAQP4 and hAQP1, hAQP2, hAQP4, hAQP5,
hAQP6 and hAQP8. Alignments performed using Clustal omega
(http://www.ebi.ac.uk/Tools/msa/clustalo/). ‘*’
indicates that residues or nucleotides in that column are identical
in all sequences in the alignment; ‘:’ indicates conserved
substitutions; and ‘.’ indicates semi-conserved substitutions. AQP,
aquaporin. AQP amino acid sequence alignments. (C) among BlotAQP1,
BlotAQP2, BlotAQP5 and hAQP3, hAQP7, hAQP9 and hAQP10; (D) among
BlotAQP3, hAQP11 and hAQP12. Alignments performed using Clustal
omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). ‘*’
indicates that residues or nucleotides in that column are identical
in all sequences in the alignment; ‘:’ indicates conserved
substitutions; and ‘.’ indicates semi-conserved substitutions. AQP,
aquaporin. |
By BLASTp, the sequence similarity between BlotAQP4
and Rattus norvegicus AQP is 42.3%, whereas the sequence
similarities between BlotAQP1, BlotAQP3, BlotAQP5, BlotAQP2 and
Plasmodium falciparum AQP (PDB ID: 3C02) are 68.5, 56.7 and
63.8%, respectively (Table II).
Homolog models of the AQPs identified from B. tropicalis
were built based on the crystal structures of Rattus
norvegicus AQP4 (PDB ID: 2D57) for BlotAQP4, and Plasmodium
falciparum AQP (PDB ID: 3C02) for BlotAQP1, BlotAQP3, BlotAQP5
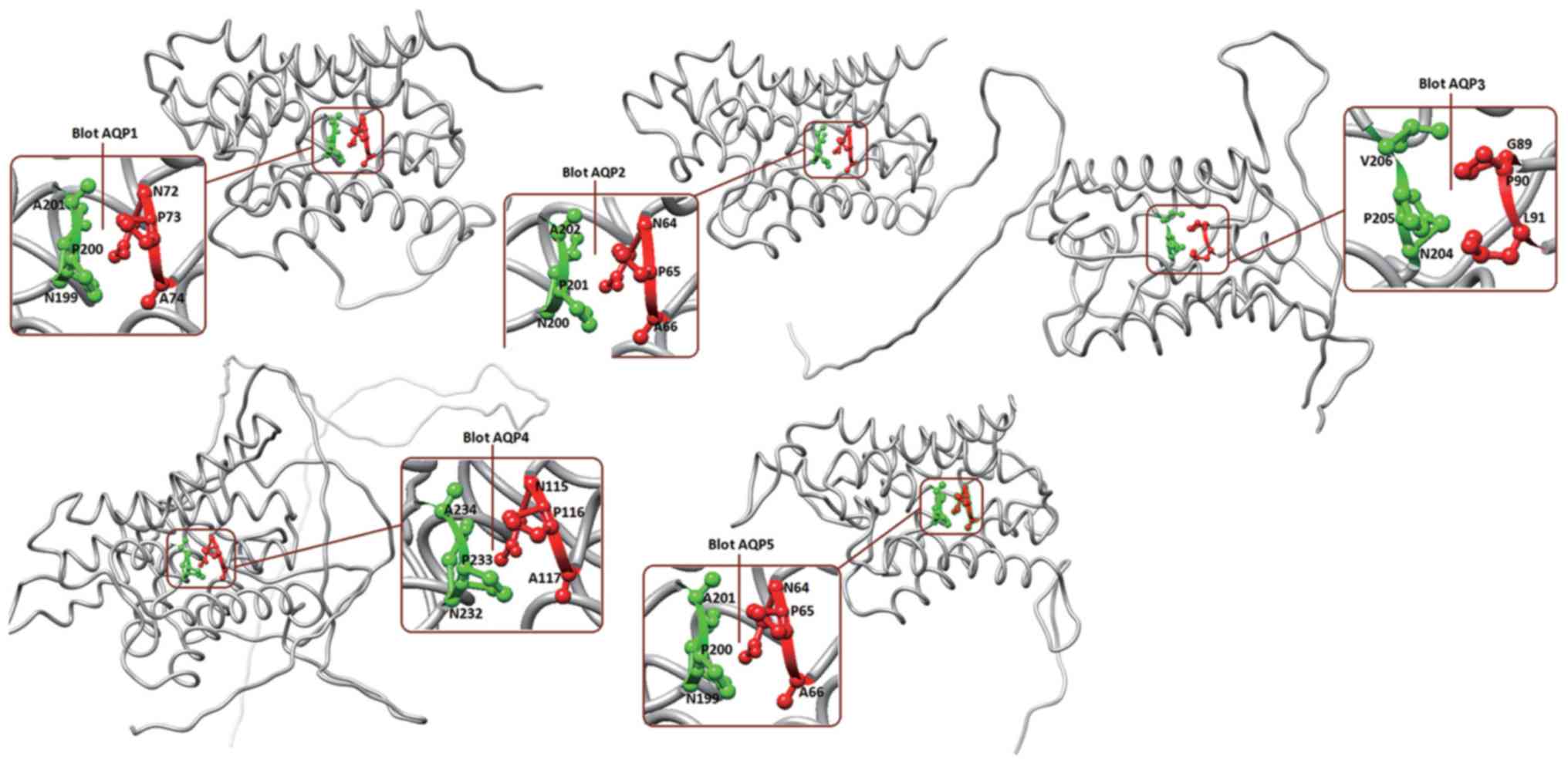
and BlotAQP2, respectively, by MODELLER v9.16. The predicted
crystal structures were then evaluated by PROCHECK in Table II. For each AQP from B.
tropicalis, 20 homology models were built and the one with the
best discrete optimized protein energy score was chosen. The
conserved motifs are marked on Fig.
5: these are N72-P73-A74 and N199-P200-A201 for BlotAQP1,
N64-P65-A66 and N200-P201-A202 for BlotAQP2, G89-P90-L91 and
N204-P205-V206 for BlotAQP3, N115-P116-A117 and N232-P233-A234 for
BlotAQP4, and N64-P65-A66 and N199-P200-A201 for BlotAQP5. From the
sequences alignment and homolog modeling, these mite AQPs can be
divided into three clades: BlotAQP4 clustered with hAQP1, hAQP2,
hAQP4, hAQP5, hAQP6 and hAQP8 (AQPs); BlotAQP1, BlotAQP2 and
BlotAQP5 clustered with hAQP3, hAQP7, hAQP9 and hAQP10
(aquaglyceroporins); and BlotAQP3 clustered with hAQP11 and hAQP12
(S-AQPs).
 | Table IIEvaluation parameters for tertiary
structures of AQPs. |
Table II
Evaluation parameters for tertiary
structures of AQPs.
| AQPs | Template
(.pdb) | Identity, % | PROCHECK | ERRAT | VERIFY 3D, % |
|---|
| BlotAQP1 | 3C02 | 68.5 | 88.9% core 8.9%
allowed 0.4% generously allowed 1.7% disallowed | 83.209 | 69.29 |
| BlotAQP2 | 3C02 | 63.8 | 91.5% core 7.7%
allowed 0.8% generously allowed 0.0% disallowed | 68.889 | 70.33 |
| BlotAQP3 | 3C02 | 56.7 | 85.5% core 11.0%
allowed 3.1% generously allowed 0.4% disallowed | 61.134 | 65.55 |
| BlotAQP4 | 2D57 | 42.3 | 91.4% core 7.6%
allowed 0.8% generously allowed 0.3% disallowed | 55.016 | 67.26 |
| BlotAQP5 | 3C02 | 65.0 | 88.3% core 9.6%
allowed 1.7% generously allowed 0.4% disallowed | 63.910 | 66.35 |
Discussion
Mites belong to the taxon Acari, which also includes
other acarines such as ticks. Ball et al (24) constructed a phylogenetic tree of
tick AQPs that branched into two clear groups: One is tick AQP1,
which was found in R. sanguineus, R. appendiculatus,
A. variegatum and I. scapularis; the other is tick
AQP2, found only in I. scapularis in addition to the
original D. variabilis source, and considerably different
from the tick AQP1s. The present results identified five AQP-like
proteins by direct PCR, which is in accordance with the
identification of at least four AQPs in the genome of the yellow
fever mosquito, Aedes aegypti (25). To the best of our knowledge, the
present study is the first to suggest the existence of at least
five AQP family members in mites. Further, the predicted proteins
were divided into three clades (i.e., paralogs), as has been
demonstrated for human AQPs. Therefore, it may be concluded that
all three subgroups, i.e., AQPs, aquaglyceroporins and S-AQPs, are
present in mites.
All AQPs share a relatively conserved overall
molecular structure, containing six transmembrane domains with five
connecting loops (A-E), and the amino and carboxyl termini are
located in the cytoplasm. Two ‘NPA’ motifs are embedded into the
loop connecting the second and third transmembrane domains (loop
B), and in the loop connecting the fifth and sixth transmembrane
domains (loop E), respectively (9,16,26). In the present study, following
alignment of the five sequences from Blomia tropicalis, two
conserved NPA motifs were identified, except in BlotAQP3, which had
GPL and NPV motifs. Furthermore, five other fully conserved
residues were found in these mite AQPs, including a conserved ‘GA’
~26 residues downstream from the first NPA motif, an ‘L’ ~23
residues and a ‘G’ ~4 residues upstream from the second NPA motif,
and a ‘G’ ~38 residues downstream from the second NPA motif.
There are several residues that characterize each of
the three clades (i.e., paralogs) and are likely responsible for
their specific functional properties. In the first clade of AQPs,
BlotAQP4 clustered with hAQP1, hAQP2, hAQP4, hAQP5, hAQP6 and
hAQP8. AQP0, AQP1, AQP2, AQP4 and AQP5 have been demonstrated to be
permeated by water (27); AQP6 is
also permeated by anions such as nitrate (28), and AQP8 perhaps by water and urea
(29). By sequence alignment of
BlotAQP4 and hAQP1, hAQP2, hAQP4, hAQP5, hAQP6 and hAQP8, three
fairly conserved motifs are observed. An AEF box that is located
~60 residues upstream from the first NPA motif is replaced by AEC
in BlotAQP4. The highly conserved ‘E’, ‘T’, ‘IG’, ‘L’ and ‘G’
residues located ~50, ~46, ~20, ~11 and ~4 residues, respectively,
upstream from the second NPA motif were present in all of these
AQPs from mites and humans. A ‘HW-W-GPL’ motif was also found ~16
residues downstream of the second NPA motif. This sequence
alignment supports the hypothesis that the NPA box could be
included within a larger, less-stringent consensus ‘SG-H-NPAVT’
motif, whereas a second NPA box could be part of a ‘G-NPAR-GP’
motif (30).
In the second clade of AQPs, according to the
molecular evolution tree constructed by amino acid sequences coding
for AQPs from B. tropicalis and humans, BlotAQP1, BlotAQP2
and BlotAQP5 clustered with hAQP3, hAQP7 hAQP9 and hAQP10. Human
AQP3, 7, 9 and 10 are the aquaglyceroporins, which transport small
neutral solutes such as glycerol and urea, as well as water.
Aquaglyceroporins also facilitate the diffusion of charged and
non-charged molecules of the metalloids arsenic and antimony, and
serve a crucial role in metalloid homeostasis (31). The sequence alignment in the
present study satisfied the two highly conserved NPA motifs in
BlotAQP1, BlotAQP2 and BlotAQP5. Furthermore, the second NPA box
could be included within a larger common consensus ‘N-G-NPSRD-PRL’
motif. A highly conserved ‘AQYLGAF’ motif (~22 residues downstream
from the first NPA motif) was observed in these three proteins.
There are certain other residues that characterize these paralogs
and are likely to be responsible for their specific functional
properties. Although the AEF box only appears in BlotAQP2 and
hAQP10, the ‘E’ is highly conserved.
In the last clade of AQPs, BlotAQP3 was clustered
with hAQP11 and hAQP12, ‘S-AQPs’, with two deviated NPA motifs. The
first NPA motif was ‘CPC’ in BlotAQP3, ‘NPC’ in hAQP11 and ‘NTP’ in
hAQP12. The second NPA motif could be included within a larger
common consensus ‘FNPALA’ motif. Unexpectedly, a conserved motif
‘VYWL, ~20 residues downstream from the second NPA motif, was found
in all three of these sequences. The substrate specificities for
hAQP11 and hAQP12 remain to be investigated, but water transport
activity has been shown for hAQP11 (32), while another study failed to
detect any water or glycerol transport activities (33).
Notably, from the B. tropicalis
transcriptome, eight sequences were predicted that appeared to be
water channel proteins, but only five gene fragments were obtained
by RT-PCR using the total RNA of B. tropicalis. These five
full-length cDNA were not expressed in E. coli BL21 (DE3)
T1R cells. The current study presents these five genes in full
length, and use bioinformatics to classify them into three
paralogous groups, i.e. AQPs, aquaglyceroporins and S-AQPs. The
signature sequences summarized in the present study will be useful
for the investigation of mite AQPs in the future. AQP proteins
identified in other insects are implicated in excretion (34), and excretions are a major source
of allergens from mites (35).
Thus, these proteins may represent key mediators of allergic
disease in humans. Furthermore, AQPs have been noted as potential
drug targets (36); in the case
of mite species, the identification of AQPs presents the potential
to design or identify drugs that, by targeting AQPs, may enable
control of the population.
Acknowledgements
The authors would like to thank Ms. Xiaowei Zhang,
Ms. Ying Han and Ms. Liwei Lin (Takara Biotechnology Co., Ltd.) for
assisting with the molecular techniques, and Mr. Zhiyuan Huang
(LC-Bio Co. Ltd., Hangzhou, China) for assistance with the
transcriptomic analyses.
Funding
This study was supported by the National Natural
Sciences Foundation of China (grant no. NSFC31272369), the 333
project of Jiangsu Province in 2017, Medical Innovation Team of
Jiangsu Province (grant no. CXTDB 2017016) and the Major Program of
Wuxi health and Family Planning Commission (grant nos. z201606 and
Z201701)
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC conceived and designed the experiments. YZ, LL
and HJ performed the experiments. JQ and YC performed the
bioinformatics analysis, prepared the figures and wrote the paper.
All authors reviewed the results and approved the submitted version
of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Calamita G, Bishai WR, Preston GM, Guggino
WB and Agre P: Molecular cloning and characterization of AqpZ, a
water channel from Escherichia coli. J Biol Chem. 270:29063–29066.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soveral G, Prista C, Moura TF and
Loureiro-Dias MC: Yeast water channels: An overview of orthodox
aquaporins. Biol Cell. 103:35–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verkman AS: More than just water channels:
Unexpected cellular roles of aquaporins. J Cell Sci. 118:32252005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishibashi K, Tanaka Y and Morishita Y: The
role of mammalian superaquaporins inside the cell. Biochim Biophys
Acta. 1840:1507–1512. 2014. View Article : Google Scholar
|
|
5
|
Ikezoe K, Oga T, Honda T, Hara-Chikuma M,
Ma X, Tsuruyama T, Uno K, Fuchikami J, Tanizawa K, Handa T, et al:
Aquaporin-3 potentiates allergic airway inflammation in
ovalbumin-induced murine asthma. Sci Rep. 6:257812016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castle NA: Aquaporins as targets for drug
discovery. Drug Discov Today. 10:485–493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishibashi K, Hara S and Kondo S: Aquaporin
water channels in mammals. Clin Exp Nephrol. 13:107–117. 2009.
View Article : Google Scholar
|
|
8
|
Gonen T and Walz T: The structure of
aquaporins. Q Rev Biophys. 39:361–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao CX, Shao HB and Chu LY: Aquaporin
structure-function relationships: water flow through plant living
cells. Colloids Surf B Biointerfaces. 62:163–172. 2008. View Article : Google Scholar
|
|
10
|
Benga G: Foreword to the special issue on
water channel proteins (aquaporins and relatives) in health and
disease: 25 years after the discovery of the first water channel
protein, later called aqua-porin 1. Mol Aspects Med. 33:511–513.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Echevarría M, Ramírez-Lorca R, Hernández
CS, Gutiérrez A, Méndez-Ferrer S, González E, Toledo-Aral JJ,
Ilundáin AA and Whittembury G: Identification of a new water
channel (Rp-MIP) in the Malpighian tubules of the insect Rhodnius
prolixus. Pflugers Arch. 442:27–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elvin CM, Bunch R, Liyou NE, Pearson RD,
Gough J and Drinkwater RD: Molecular cloning and expression in
Escherichia coli of an aquaporin-like gene from adult buffalo fly
(Haematobia irritans exigua). Insect Mol Biol. 8:369–380. 2010.
View Article : Google Scholar
|
|
13
|
Haas BJ, Papanicolaou A, Yassour M,
Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber
M, et al: De novo transcript sequence reconstruction from RNA-seq
using the Trinity platform for reference generation and analysis.
Nat Protoc. 8:1494–1512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang QL and Yuan ML: Progress in insect
transcriptomics based on the next-generation sequencing technique.
Acta Entomologica Sinica. 56:1489–1508. 2013.
|
|
15
|
Cui Y, Yu L, Teng F, Zhang C, Wang N, Yang
L and Zhou Y: Transcriptomic/proteomic identification of allergens
in the mite Tyrophagus putrescentiae. Allergy. 71:1635–1639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arlian LG, Vyszenski-Moher DL and
Fernandez-Caldas E: Allergenicity of the mite, Blomia tropicalis. J
Allergy Clin Immunol. 91:1042–1050. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernández-Caldas E: Allergenicity of
Blomia tropicalis. J Investig Allergol Clin Immunol. 7:4021997.
|
|
18
|
Iseli C, Jongeneel CV and Bucher P:
ESTScan: A program for detecting, evaluating, and reconstructing
potential coding regions in EST sequences. Proc Int Conf Intell
Syst Mol Biol. 99:138–148. 1998.
|
|
19
|
Eswar N, Eramian D, Webb B, Shen MY and
Sali A: Protein structure modeling with MODELLER. Methods Mol Biol.
426:145–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laskowski RA, Rullmannn JA, MacArthur MW,
Kaptein R and Thornton JM: AQUA and PROCHECK-NMR: Programs for
checking the quality of protein structures solved by NMR. J Biomol
NMR. 8:477–486. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colovos C and Yeates TO: Verification of
protein structures: Patterns of nonbonded atomic interactions.
Protein Sci. 2:1511–1519. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bowie JU, Luthy R and Eisenberg D: A
method to identify protein sequences that fold into a known
three-dimensional structure. Science. 253:164–170. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pettersen EF, Goddard TD, Huang CC, Couch
GS, Greenblatt DM, Meng EC and Ferrin TE: UCSF Chimera-A
visualization system for exploratory research and analysis. J
Comput Chem. 25:2010.
|
|
24
|
Ball A, Campbell EM, Jacob J, Hoppler S
and Bowman AS: Identification, functional characterization and
expression patterns of a water-specific aquaporin in the brown dog
tick, Rhipicephalus sanguineus. Insect Biochem Mol Biol.
39:105–112. 2009. View Article : Google Scholar
|
|
25
|
Duchesne L, Hubert JF, Verbavatz JM,
Thomas D and Pietrantonio PV: Mosquito (Aedes aegypti) aquaporin,
present in tracheolar cells, transports water, not glycerol, and
forms orthogonal arrays in Xenopus oocyte membranes. Febs J.
270:422–429. 2003.
|
|
26
|
Benga G: On the definition, nomenclature
and classification of water channel proteins (aquaporins and
relatives). Mol Aspects Med. 33:514–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Agre P, Sasaki S and Chrispeels MJ:
Aquaporins: A family of water channel proteins. Am J Physiol.
265:F4611993.PubMed/NCBI
|
|
28
|
Yasui M, Kwon TH, Knepper MA, Nielsen S
and Agre P: Aquaporin-6: An intracellular vesicle water channel
protein in renal epithelia. Proc Natl Acad Sci USA. 96:5808–5813.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishibashi K, Kuwahara M, Gu Y, Kageyama Y,
Tohsaka A, Suzuki F, Marumo F and Sasaki S: Cloning and functional
expression of a new water channel abundantly expressed in the
testis permeable to water, glycerol, and urea. J Biol Chem.
272:20782–20786. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zardoya R and Villalba S: A phylogenetic
framework for the aquaporin family in eukaryotes. J Mol Evol.
52:391–404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bienert GP, Schussler MD and Jahn TP:
Metalloids: Essential, beneficial or toxic? Major intrinsic
proteins sort it out. Trends Biochem Sci. 33:20–26. 2008.
View Article : Google Scholar
|
|
32
|
Yakata K, Hiroaki Y, Ishibashi K, Sohara
E, Sasaki S, Mitsuoka K and Fujiyoshi Y: Aquaporin-11 containing a
divergent NPA motif has normal water channel activity. Biochim
Biophys Acta. 1768:688–693. 2007. View Article : Google Scholar
|
|
33
|
Gorelick DA, Praetorius J, Tsunenari T,
Nielsen S and Agre P: Aquaporin-11: A channel protein lacking
apparent transport function expressed in brain. BMC Biochem.
7:142006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spring JH, Robichaux SR and Hamlin JA: The
role of aquaporins in excretion in insects. J Exp Biol.
212:358–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Platts-Mills TA, Vervloet D, Thomas WR,
Aalberse RC and Chapman MD: Indoor allergens and asthma: Report of
the third international workshop. J Allergy Clin Immunol.
100:S2–S24. 1997. View Article : Google Scholar
|
|
36
|
Frigeri A, Nicchia GP and Svelto M:
Aquaporins as targets for drug discovery. Curr Pharm Des.
13:2421–2427. 2007. View Article : Google Scholar : PubMed/NCBI
|