Introduction
Cynanchum auriculatum (C. auriculatum)
Royle ex Wight, a member of the Asclepiadaceae family, is
widely distributed in China. The root tuber of C.
auriculatum Royle ex Wight, a well-known traditional Chinese
herbal medicine known as ‘Baishouwu’ has been used as a local tonic
and medicine for >1,000 years since the Tang Dynasty in China
(1). Modern phytochemical and
pharmacological studies have demonstrated that C-21 steroidal
glycosides are the major active components of Baishouwu (2,3).
The total C-21 steroidal glycosides (TCSGs), isolated from
Baishouwu, possess various pharmacological activities, including
antitumor (4–11), aging-attenuating (12), free radical-scavenging (13), immunity-enhancing (14), depression-reducing (15) and fungus-suppressing (16) activities. Recently, it has been
reported that the C-21 steroidal glycosides isolated from Baishouwu
exhibit notable hepatoprotective effects in vivo (17). However, the underlying mechanisms
remain largely unknown.
Pathological and experimental evidence has suggested
that multiple mechanisms of hepatic injury are implicated in
oxidative damage, inflammation, the dysfunction of intracellular
targets and the innate immune system (18–20). It has been well established that
cell oxidative stress damage induced by reactive oxygen species
(ROS) is a principal mechanism of hepatic injury. When there is an
imbalance in the levels of intracellular oxidative factors and
antioxidants, oxidative stress can result in a disruption in redox
signaling and cellular injury (21,22). An increasing body of evidence has
indicated that superoxide anion and hydrogen peroxide
(H2O2) are associated with various
pathological diseases, such as viral hepatitis (23), alcoholic hepatitis (24) and non-alcoholic fatty liver
diseases (NAFLD) (25).
H2O2-induced hepatic injury is a common cell
model for investigating the potential hepatoprotective activity
(26). Lipid peroxidation is one
of the significant causes of H2O2-induced
hepatic injury and can be monitored by detecting the content of
intracellular malondialdehyde (MDA). The disruption of the hepatic
antioxidant defense system is characterized by increased MDA and/or
altered enzymatic antioxidants, including superoxide dismutase
(SOD), catalase (CAT) and glutathione peroxidase (GSH-Px). The
superoxide radical (O2−) is an oxygen free
radical that damages the body, which is then converted to
O2 and H2O2 by the action of SOD
and detoxified to water by CAT or GSH-Px. The activities of these
antioxidants have been used to evaluate oxidative stress levels in
cells (27). Excessive ROS levels
induced by H2O2 disrupt the balance between
ROS production and the antioxidant defense system.
It has been reported that excessive ROS levels
induced by H2O2 can induce nuclear factor
(NF)-κB activation, and subsequently increase NF-κB p65 subunit
nuclear translocation (28). The
activation of NF-κB is enhanced by increasing the degradation and
phosphorylation of inhibitor of nuclear factor-κB (IκB), which
further modulates the hepatic injury by regulating proinflammatory
cytokine production, such as tumor necrosis factor (TNF)-α and
interleukin (IL)-6, and the expression of inflammatory mediators
including inducible nitric oxide synthase (iNOS) and cyclooxygenase
(COX)-2. Nitric oxide (NO) plays a critical role in hepatic injury
caused by H2O2. H2O2
exposure generates excessive levels of NO via the activation of
iNOS, thereby leading to hepatic tissue damage (29).
In addition, a previous study revealed that the
nuclear factor erythroid 2-related factor 2 (Nrf2) signaling
pathway played an important role in the intracellular defense
against oxidative stress (30).
The nuclear transcription factor Nrf2 binds to antioxidant response
elements (AREs) in order to activate antioxidant genes that are
involved in the elimination of free radicals by promoting the
expression of antioxidant enzymes, such as SOD and CAT (31). The role of the Nrf2/ARE signaling
pathway in liver disease pathogenesis and its possible application
as a underlying therapeutic target to block and treat chronic
hepatitis, and alcoholic as well as non-alcoholic hepatic injury,
have been extensively investigated (32).
The critical roles of oxidative stress and
inflammation in the initiation and progression of liver injury have
attracted increasing levels of attention (33). Previous studies have reported that
H2O2-derived ROS production induced oxidative
stress and promoted the generation of proinflammatory cytokines via
the Nrf2 and NF-κB signaling pathway (34–37). Thus, redox-sensitive transcription
factors, such as Nrf2 and NF-κB, are essential transcription
factors that modulate an array of antioxidant responses and
proinflammatory gene expression in the liver (38).
Natural products derived from herbal medicines are
attracting increasing attention as alternative treatment options
for the prevention and treatment of liver diseases. TCSGs, the most
important active components of Baishouwu, are commonly considered
to be strong antioxidants due to their high free radical scavenging
properties. Recent studies have revealed that TCSGs affect cellular
inflammatory stress and inhibit tumors by regulating
carcinogenesis-associated processes (3,9,10,39). In addition, it has been
demonstrated that TCSGs exert hepatoprotective effects against
liver injury induced by carbon tetrachloride in vivo
(17). To date, few studies have
provided conclusive results, particularly with regard to the
molecular mechanism of TCSGs in protecting hepatocytes against
oxidative damage and inflammatory response.
The aim of the present study was to explore the
possible protective effects of TCSGs on human liver cells against
H2O2-induced oxidative damage by detecting
oxidative stress indicators and inflammatory markers. More
specifically, the potential molecular mechanisms of action of TCSGs
were investigated by examining antioxidant and inflammatory
signaling pathways. These results may elucidate the underlying
protective mechanisms of TCSGs in liver injury.
Materials and methods
Plant material and preparation of
TCSGs
The peeled root tubers of C. auriculatum were
collected from Binhai County (Jiangsu, China) in December, 2015.
The materials were identified and authenticated by Professor
Shi-Hui Qian (Jiangsu Province Academy of Traditional Chinese
Medicine, Jiangsu, China). A voucher specimen (no. WFC-20151225)
was deposited at the Department of Natural Product Chemistry,
Jiangsu Province Academy of Traditional Chinese Medicine (Nanjing,
China). Chemical reference substances, namely
caudatin-2,6-dideoxy-3-O-methy-β-D-cymaropyrano side (no. 1),
wilfoside C1N (no. 2), caudatin (no. 3), wilfoside
K1N (no. 4), wilfoside C1G (no. 5),
cynauricuoside A (no. 6), cynauricuoside C (no. 7) and auriculoside
IV (no. 8) (Fig. 1), were
isolated from C. auriculatum (40) and identified based on infrared,
ultraviolet, mass spectrometry (MS) and nuclear magnetic resonance
spectroscopic analyses (data not shown). The purity of these
compounds was determined to be >98% by high-performance liquid
chromatography (HPLC) analysis. The root tubers were cut into small
sectoins and boiled in 95% ethanol (1:10) 2 times, 2 h each time,
filtered through gauze and concentrated under reduced pressure to
produce the ethanol extract. The ethanolic extract was extracted
with ethyl acetate 3 times and then merged and evaporated to obtain
the TCSG extract, as previously described (41). The main chemical components of the
TCSGs were identified and determined by the ultra high-performance
liquid chromatography triple quadrupole tandem mass spectrometry
method according to previously reported protocols (41). An Acquity UHPLC BEH C18
column (2.1×100 mm, 1.7 µm) was used for separations. The
mobile phase was composed of (A) water [0.1% (v/v) formic acid] and
(B) acetonitrile, and a linear gradient elution was used. MS was
operated using an electrospray ionization (ESI) source in negative
mode and the ESI-MS spectra were acquired by multiple reaction
monitoring (MRM). Chlorzoxazone was used as the internal standard.
A series of concentrations of standard solution were prepared for
the establishment of the calibration curves. It was revealed that
the TCSGs mainly contained 8 C-21 steroidal glycoside components,
including caudatin-2,6-dideoxy-3-O-methy-β-D-cymaropyranoside
(20.11 mg/g), wilfoside C1N (5.13 mg/g), caudatin (3.96
mg/g), wilfoside K1N (7.90 mg/g), wilfoside
C1G (73.25 mg/g), cynauricuoside A (80.16 mg/g),
cynauricuoside C (7.20 mg/g) and auriculoside IV (3.07 mg/g). The
chemical structures and the total ion chromatograms of the 8
compounds are presented in Fig.
1. The total glycosides content of the TCSGs was 73.5%, as
detected according to the vanillin-vitriol colorimetric method
(42). The standard curve was
constructed using the cynauricuoside A standard.
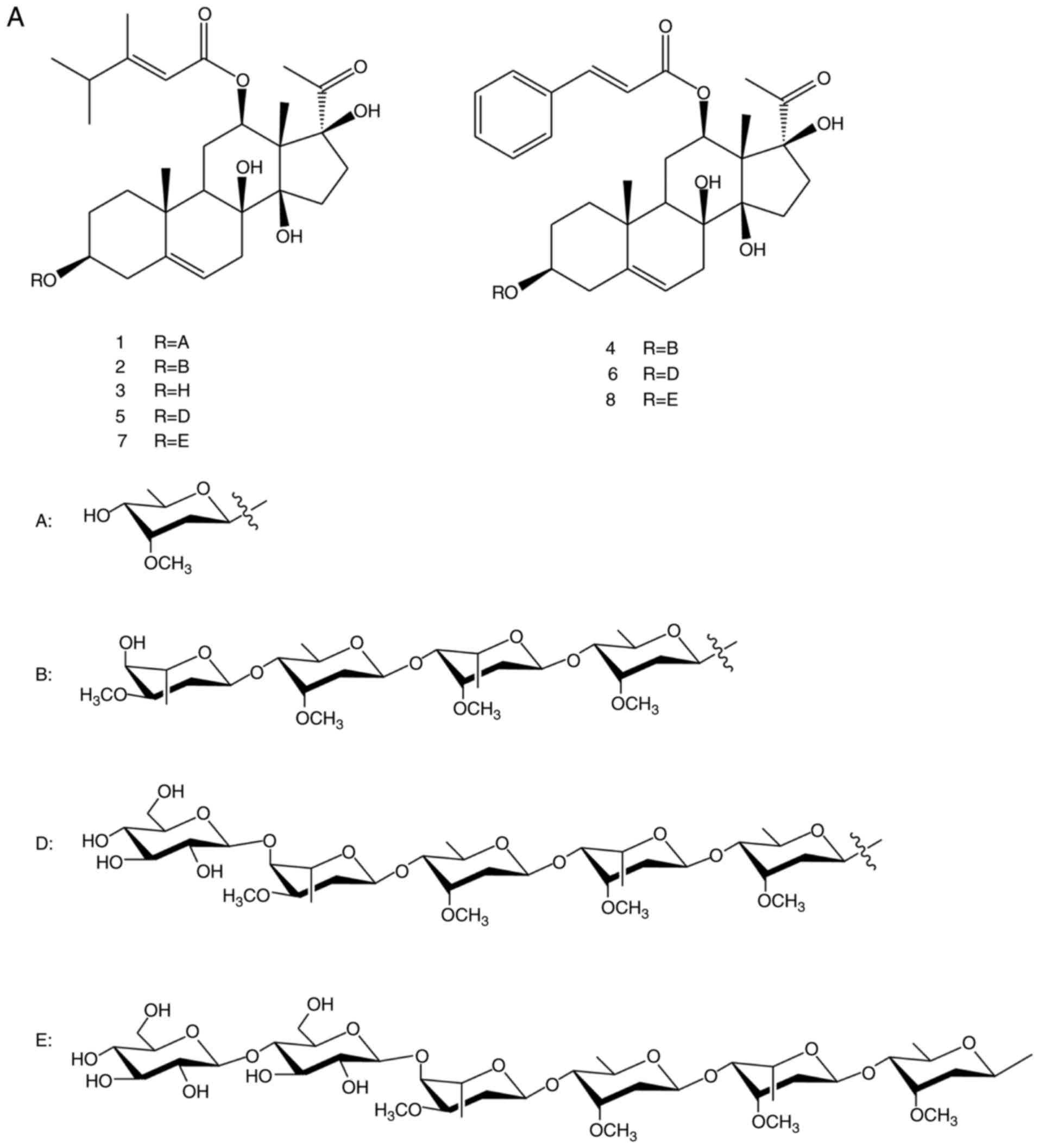 | Figure 1(A) Chemical structures of the 8
compounds. (B) The total ion chromatograms of 8 standard compounds
and TCSGs by multiple reaction monitoring. No. 1,
caudatin-2,6-dideoxy-3-O-methy-β-D-cymaropyranoside; no. 2,
wilfoside C1N; no. 3, caudatin; no. 4, wilfoside K1N; no. 5,
wilfoside C1G; no. 6, cynauricuoside A; no. 7, cynauricuoside C;
no. 8, auriculoside IV. TCSGs, total C-21 steroidal glycosides. |
Reagents
H2O2 was purchased from
Nanchang Baiyun Pharmaceutical Co., Ltd. (Nanchang, China). Fetal
bovine serum (FBS) was purchased from Gibco/Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Alanine aminotransferase (ALT;
cat. no. C009-2), aspartate aminotransferase (AST; cat. no.
C010-2), lactate dehydrogenase (LDH; cat. no. C020-2) and NO (cat.
no. C013-2) assay kits were obtained from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). SOD (cat. no. S0101),
CAT (cat. no. S0051), GSH-Px (cat. no. S0058), MDA (cat. no. S0131)
and ROS (cat. no. S0033) assay kits were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). Dulbecco’s modified
Eagle’s medium (DMEM), the BCA protein quantification kit and the
cytoplasmic-nuclear protein extraction kit were obtained from
KeyGEN Biotech Co., Ltd. (Nanjing, China). Anti-iNOS (cat. no.
13120), anti-NF-κB (cat. no. 8242), anti-phospho-NF-κB p65 (cat.
no. 3033) and anti-GAPDH (cat. no. 5174) antibodies were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-IκBα
(cat. no. abp57568) antibody was obtained from AmyJet Scientific
(Wuhan, China). Anti-phospho-IκBα (cat. no. orb13487) antibody was
obtained from Biorbyt (Wuhan, China). Anti-COX-2 (cat. no. ab15191)
and anti-Lamin B1 (cat. no. ab133741) antibodies were purchased
from Abcam (Cambridge, UK). Anti-heme oxygenase-1 (HO-1; cat. no.
Pb0212) antibody was purchased from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). Anti-Nrf2 (cat. no. Abs120634)
antibody was obtained from Absin (Shanghai, China). Goat
anti-rabbit (cat. no. AP307P) and mouse IgG (cat. no. AP124P)
antibodies were purchased from EMD Millipore (Billerica, MA,
USA).
Cell culture
L02 cells, a normal human hepatic cell line, were
obtained from KeyGEN Biotech Co., Ltd. (cat. no. KG063) and used in
the subsequent experiments, as previously described (43). The L02 cells were incubated at
37°C and 5% CO2 in DMEM supplemented with 10% FBS and
100 U/ml penicillin-streptomycin. Once cell confluence reached
~80%, the cells were passaged.
Cell viability assay
The viability of the L02 cells was determined using
the 3-(4,5-dimethyl-2-thiazolyl)-2,5-di-phenyl-2-H-tetrazolium
bromide (MTT) method. Firstly, the effective concentrations and
treatment durations for TCSGs and H2O2 were
determined using the cell viability data. The L02 cells at the
exponential growth phase were suspended in DMEM (5×103
cells/well in 100 µl) and seeded into Corning disposable
96-well plates. Cell treatments were performed as follows: i) The
cells were incubated with the TCSGs (0, 5, 10, 15, 20, 25, 30, 35,
40 and 45 µg/ml) for 24, 48 and 72 h; ii) The cells were
cultured for 72 h and then treated with H2O2
(0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.5 and 2 mM) for 24 h. The cells
were then washed with PBS, and 10 µl of MTT solution (5
mg/ml) were added to each well and the plate was incubated for 4 h.
Then cell supernatants were discarded and 150 µl dimethyl
sulfoxide were added to each well. The formazan crystals in the
wells were dissolved and the absorbance was read at 570 nm using
the Universal Microplate Reader (Thermo Fisher Scientific, Inc.).
During these experiments, the TCSG group (0 µg/ml) or the
H2O2 group (0 mM) was considered to be the
control group (negative control) in each test, respectively. Based
on the results obtained from the above-mentioned experiments, the
effect of TCSGs on the viability of the L02 cells damaged by
H2O2 was investigated as the selected
protocol. There were 5 groups during this test: The control group
(negative control), the H2O2 group (positive
control), and the TCSGs I (10 µg/ml), TCSGs II (20
µg/ml) and TCSGs III (40 µg/ml) groups. Following
exposure to the TCSGs (0, 10, 20 and 40 µg/ml) for 48 h, the
cells were treated with or without H2O2 (1.5
mM) for 24 h. MTT assay was then performed as described above.
Cell morphological changes
assessment
Briefly, the L02 cells (2×105 cells/well)
were incubated in corning disposable 6-well plates at 37°C and 5%
CO2 for 24 h. The cells were pretreated with the TCSGs
(0, 10, 20 and 40 µg/ml) for 48 h, and then stimulated with
or without H2O2 (1.5 mM) for 24 h. Cell
morphological changes were imaged under a contrast microscope
(Zeiss, Axiovert 200; Zeiss GmbH, Jena, Germany).
Assay of AST, ALT and LDH activities and
NO levels
The levels of NO and the activities of
hepatocellular leakage enzymes were assayed using commercial assay
kits according to the manufacturer’s instructions, respectively.
Exponential growth phase cells were cultured in corning disposable
96-well plates (5×103 cells/well in 100 µl) for
24 h and pretreated with the TCSGs (0, 10, 20 and 40 µg/ml)
for 48 h, followed by exposure to, or the absence of, 1.5 mM
H2O2 for 24 h. The cell culture media were
then collected for the detection of AST, ALT and LDH activities,
and NO levels. The data were expressed as units per liter (U/l) and
µmol/l, respectively.
Determination of SOD, CAT and GSH-Px
activities, and MDA levels
Following treatment with TCSGs and/or
H2O2 as described above, the cells were
washed and lysed with radioimmunoprecipitation assay (RIPA) buffer
containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and maintained
on ice for 30 min. Cell lysates were collected and centrifuged at
10,000 x g for 5 min. The supernatants were used to detect the
activities of SOD, CAT and GSH-Px, and the levels of MDA following
the manufacturer’s instructions. The protein contents of the
supernatants were assayed using a BCA protein quantification
kit.
Detection of ROS levels
To evaluate the direct effects of TCSGs on
H2O2-induced oxidative stress in L02 cells,
the intracellular ROS levels were observed and analyzed via a
fluorescence microplate assay and fluorescence microscopy.
According to the manufacturer’s instructions and as previously
described (44), the
intracellular ROS levels were measured using the ROS assay kit.
Briefly, exponential growth phase L02 cells were pretreated with or
without TCSGs for 48 h, followed by exposure to, or the absence of,
1.5 mM H2O2 for 24 h. Following 3 washes with
PBS, the L02 cells were treated with 10 µM of the
fluorescent probe, 2′,7′-dichlorodihy-drofluorescein diacetate
(DCFH-DA; 1:1,000), and incubated for 20 min at 37°C in the dark.
Finally, the cells preloaded with DCFH-DA were imaged with a
fluorescence microscope (Olympus Corporation, Tokyo, Japan), and
the DCF fluorescence intensity was detected with an EnSpire
Multifunctional Microplate Reader (PerkinElmer Inc., Waltham, MA,
USA) under a 488 nm excitation wavelength and 525 nm emission
wavelength.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
detection
Total RNA was extracted from the L02 cells using
TRIzol reagent (Invitrogen/Thermo Fisher Scientific, Inc.) and
first-stand cDNA was obtained using the Thermo Fisher K1622
first-stand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
Gene-specific primer sequences were designed and synthesized by
GenScript Inc., Nanjing, China, as previously described (45). The primer sequences utilized are
presented in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) | Accession
number |
|---|
| TNF-α | F:
GTCAACCTCCTCTCTGCCATCAAG | NM_000594 |
| TNF-α | R:
CTGAGTCGGTCACCCTTCTCCA | |
| IL-6 | F:
CTCAATATTAGAGTCTCAACCCCCA | NM_000600 |
| IL-6 | R:
GAAGGCGCTTGTGGAGAAGG | |
| iNOS | F:
GGAGCCAGCTCTGCATTATC | NM_000620 |
| iNOS | R:
TTTTGTCTCCAAGGGACCAG | |
| COX-2 | F:
TGCACTACATACTTACCCACTTCAA | NM_000963 |
| COX-2 | R:
CAAATGTGATCTGGATGTCAACACA | |
| GAPDH | F:
GAAGGTCGGAGTCAACGGAT | NM_002046 |
| GAPDH | R:
CCTGGAAGATGGTGATGGG | |
The qPCR reactions were carried out in 20 µl
reaction mixtures containing 10 µl 2X Real Time PCR Master
Mix (SYBR-Green), 2 µl primer mix (including forward and
reverse primers), and 1 µl cDNA diluted in RNase-free water.
The housekeeping gene, GAPDH, was used as an internal standard. The
levels of relative mRNA were analyzed using the 2−ΔΔCq
comparative approach (46).
Western blot analysis
The L02 cells were lysed in RIPA buffer (containing
1 mM PMSF) on ice for 30 min and total proteins were collected. The
cytoplasmic and nuclear protein samples were extracted using the
cytoplasmic-nuclear protein extraction kits. The protein
concentration was detected using the BCA protein quantification
kit. Equal amounts of proteins (50 µg/lane) were separated
on 10% SDS-PAGE and elec-troblotted onto polyvinylidene difluoride
membranes (EMD Millipore). Following blocking with 5% skim milk for
1 h, the membranes were incubated overnight with primary antibodies
(anti-iNOS, 1:1,000; anti-COX-2, 1:800; anti-NF-κB p65, 1:1,000;
anti-phospho-NF-κB p65, 1:1,000; anti-IκBα, 1:1,000;
anti-phospho-IκBα, 1:1,000; anti-Nrf2, 1:500; anti-HO-1, 1:800;
anti-Lamin B1, 1:1,000; and anti-GAPDH, 1:1,000) at 4°C. Following
3 washes, the membranes were incubated for 1 h with goat
anti-rabbit IgG (1:2,000) and goat anti-mouse IgG (1:2,000)
peroxidase-conjugated secondary antibodies at room temperature. The
transferred proteins were visualized using an enhanced
chemiluminescence detection kit (Beyotime Institute of
Biotechnology, Shanghai, China) and the grayscale values of each
band were quantified using Tanon-5200 Analyzer software. Target
protein expression levels were normalized based on the internal
controls (GAPDH and Lamin B1).
Statistical analysis
All results are presented as the means ± standard
deviation, and analyzed using one-way analysis of variance followed
by Tukey’s post hoc test for multiple comparisons with SPSS
software (version 22.0; IBM Corp., Armonk, NY, USA). All histograms
were plotted using GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA). A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of TCSGs on
H2O2-induced cytotoxicity in and morphology
of L02 cells
The cell viability data indicated that the TCSGs
exerted no significant toxicity towards the L02 cells, even at high
concentrations (40 µg/ml) following treatment for 24 and 48
h. However, TCSGs at the concentrations exceeding 40 µg/ml
and treatment for longer than 48 h markedly decreased cell
viability (P<0.05; Fig. 2A).
As shown in Fig. 2B, with
increasing concentrations of H2O2, cell
viability decreased in a dose-dependent manner, and the 50%
inhibitory concentration of H2O2 treatment
was 1.49 mM, as determined by SPSS software. Based on these
results, treatment with 10, 20 and 40 µg/ml TCSGs and 1.5 mM
H2O2, as well as the time points of 48 h for
the TCSGs and 24 h for H2O2, were employed in
the subsequent experiments. With this selected protocol, the
effects of TCSGs on H2O2-induced cytotoxicity
in and morphology of the L02 cells were investigated. The results
revealed that pretreatment with the TCSGs significantly increased
the cell survival rate in the cells exposed to
H2O2 (Fig.
2C). As shown in Fig. 2D, the
control cells exhibited an irregular polygon morphology, with only
a few cells being spindle-shaped. Following exposure to
H2O2 (1.5 mM), the cells became smaller.
However, pretreatment with the TCSGs (10, 20 and 40 µg/ml)
for 48 h, attenuated the morphological changes of the L02 cells
observed with exposure to H2O2.
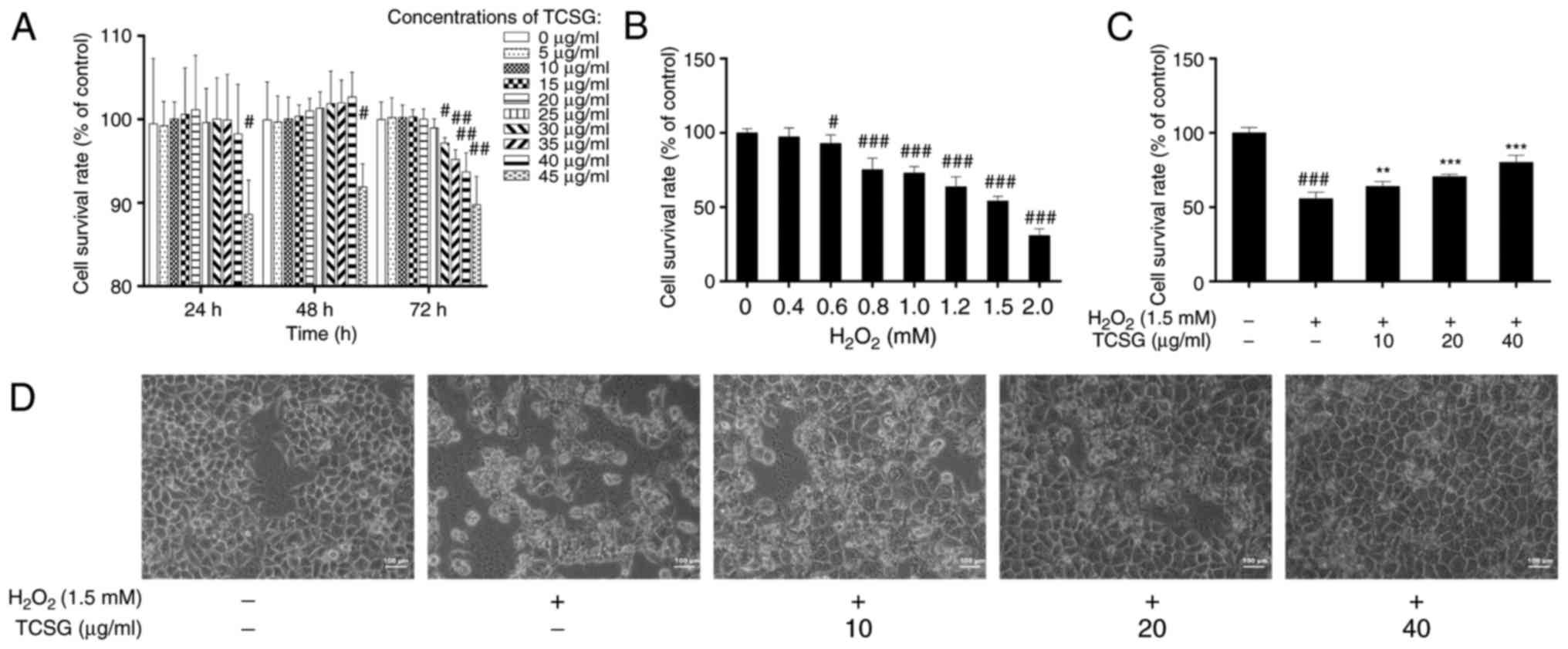 | Figure 2Effect of TCSGs and
H2O2 on L02 cell morphological changes and
cell viability. (A) Cells were treated with the TCSGs (0, 5, 10,
15, 20, 25, 30, 35, 40 and 45 µg/ml) for 24, 48 and 72 h.
(B) Cells were cultured for 72 h and then exposed to
H2O2 (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.5
and 2 mM) for 24 h. (C) Pretreatment with TCSGs (10, 20 and 40
µg/ml) for 48 h, followed by treatment with
H2O2 (1.5 mM) for 24 h. (D) L02 cells were
cultured with TCSGs (10, 20 and 40 µg/ml) for 48 h, followed
by the incubation with 1.5 mM H2O2 for 24 h.
Cell morphology was imaged using an inverted phase contrast
microscope (magnification, ×200; scale bar, 100 µm). All
data are presented as the means ± standard deviation (n=6).
#P<0.05, ##P<0.01 and
###P<0.001 vs. the control group (0; no treatment);
**P<0.01 and ***P<0.001 vs.
H2O2 group. TCSGs, total C-21 steroidal
glycosides; H2O2, hydrogen peroxide. |
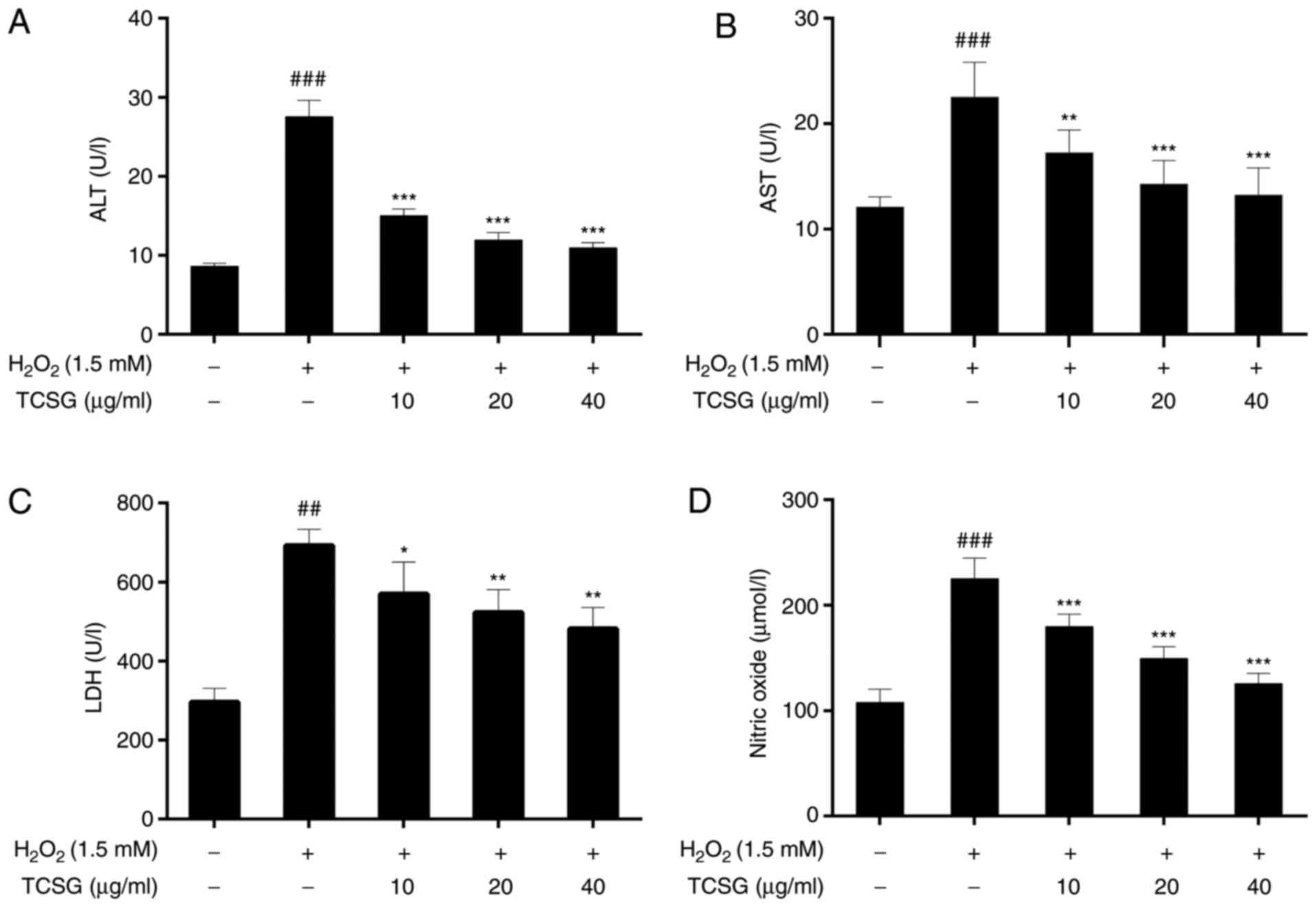
Effect of TCSGs on the activities of ALT,
AST, LDH and NO levels in L02 cells
As shown in Fig.
3, exposure to H2O2 led to a significant
elevation in the levels of hepatocellular leakage enzymes,
including ALT, AST and LDH, which are indicative of hepatic injury.
When compared with the control group, the NO levels in the group
exposed to H2O2 were markedly increased
(P<0.01). Following pretreatment with the TCSGs, there was a
significant attenuation in the activities of ALT, AST and LDH, and
the NO levels when compared with the H2O2
group (P<0.05, P<0.01 or P<0.001). The results thus
suggested that TCSGs attenuated H2O2-induced
injury in L02 cells.
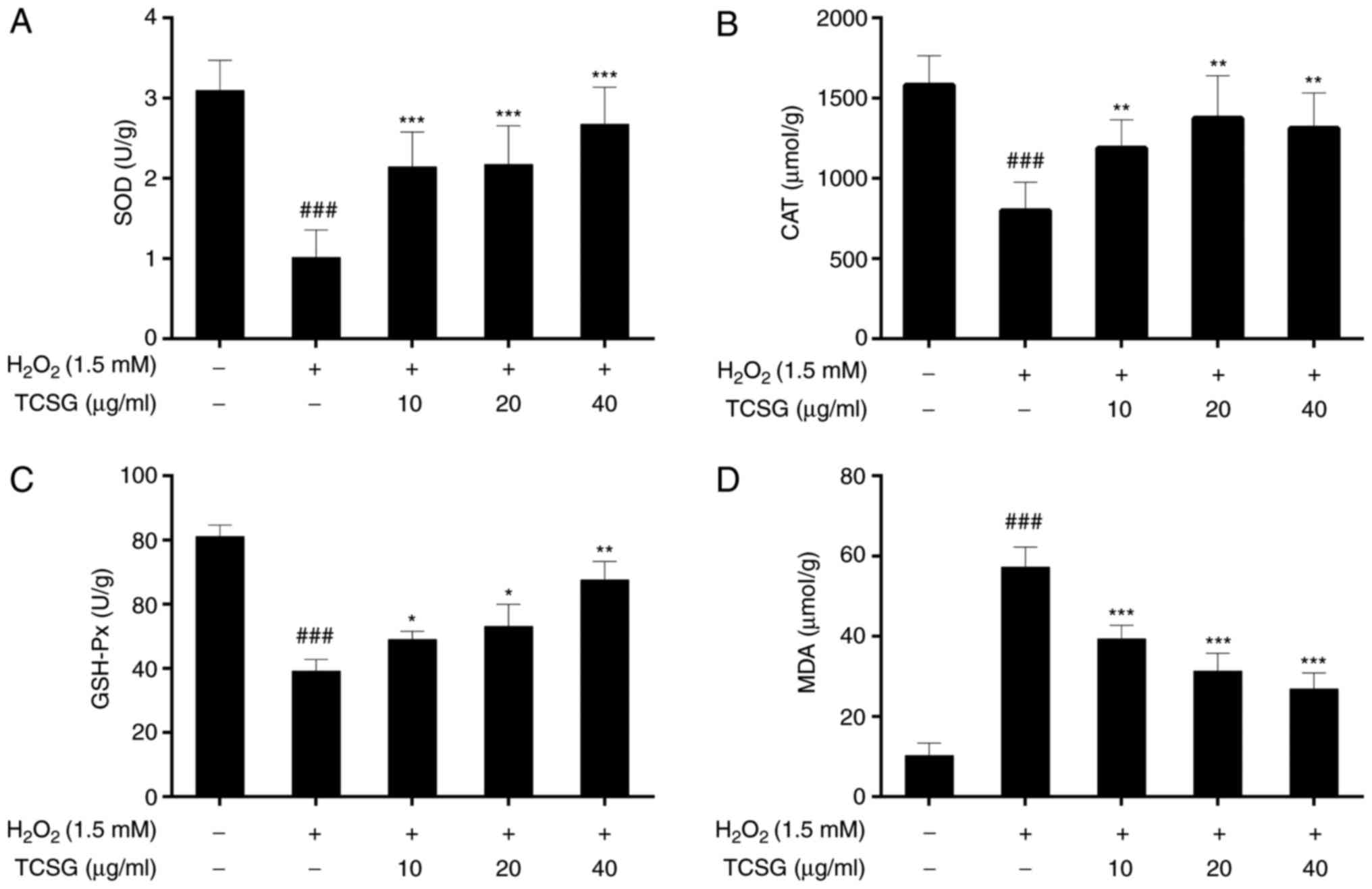
Effect of TCSGs on the activities of SOD,
CAT and GSH-Px, and MDA levels in L02 cells
The effects of TCSGs on the activities of SOD, CAT
and GSH-Px in the L02 cells are presented in Fig. 4A–C. H2O2
significantly (P<0.001) decreased the activities of SOD, CAT and
GSH-Px in the L02 cells. However, pretreatment with the TCSGs
alleviated the inhibition of antioxidant enzyme activities in a
dose-dependent manner under oxidative stress.
The effects of TCSGs on the levels of MDA in the
H2O2-exposed L02 cells are presented in
Fig. 4D. Exposure to
H2O2 significantly increased the levels of
MDA in the L02 cells when compared with the control group
(P<0.001). However, pretreatment with the TCSGs dose-dependently
inhibited the increase in the MDA levels when compared with the
H2O2 group (P<0.001).
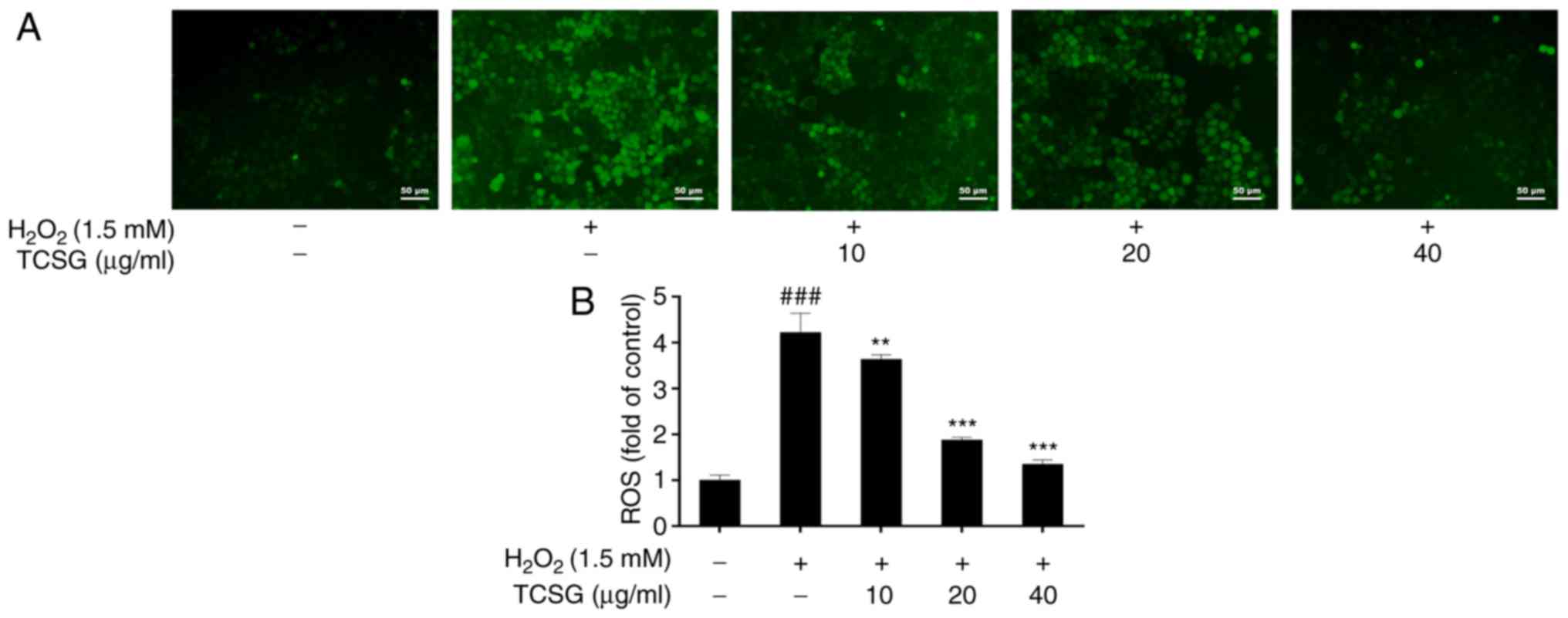
Effect of TCSGs on ROS levels in L02
cells
The effects of TCSGs on the production of ROS in the
H2O2-exposed L02 cells are presented in
Fig. 5B. Exposure to
H2O2 significantly increased intracellular
ROS levels in the L02 cells (P<0.001), and this effect was
markedly suppressed by the TCSGs. The results revealed that the
TCSGs dose-dependently attenuated
H2O2-induced ROS production in the L02 cells.
In addition, each group of images was recorded using a digital
camera attached to an inverted fluorescence microscope. As shown in
Fig. 5A, the results were in
agreement with the fluorescence intensity assays.
Effect of TCSGs on
H2O2-induced production of inflammatory
mediators and cytokines in L02 cells
iNOS and COX-2 are used as inflammatory markers and
play critical roles in the pathogenesis of chronic inflammation.
The effects of TCSGs on the expression levels of iNOS and COX-2 in
the L02 cells are presented in Fig.
6. The protein and mRNA expression levels of iNOS (Fig. 6A, B and D) and COX-2 (Fig. 6A, C and D) increased significantly
in the H2O2-exposed group; however,
pretreatment with the TCSGs dose-dependently inhibited the increase
in the iNOS and COX-2 levels. Furthermore, the results of RT-qPCR
demonstrated that the TCSGs markedly attenuated the upregulation in
the mRNA expression levels of TNF-α and IL-6 (Fig. 6D) induced by
H2O2 in the L02 cells, which was consistent
with the iNOS and COX-2 expression levels mentioned above.
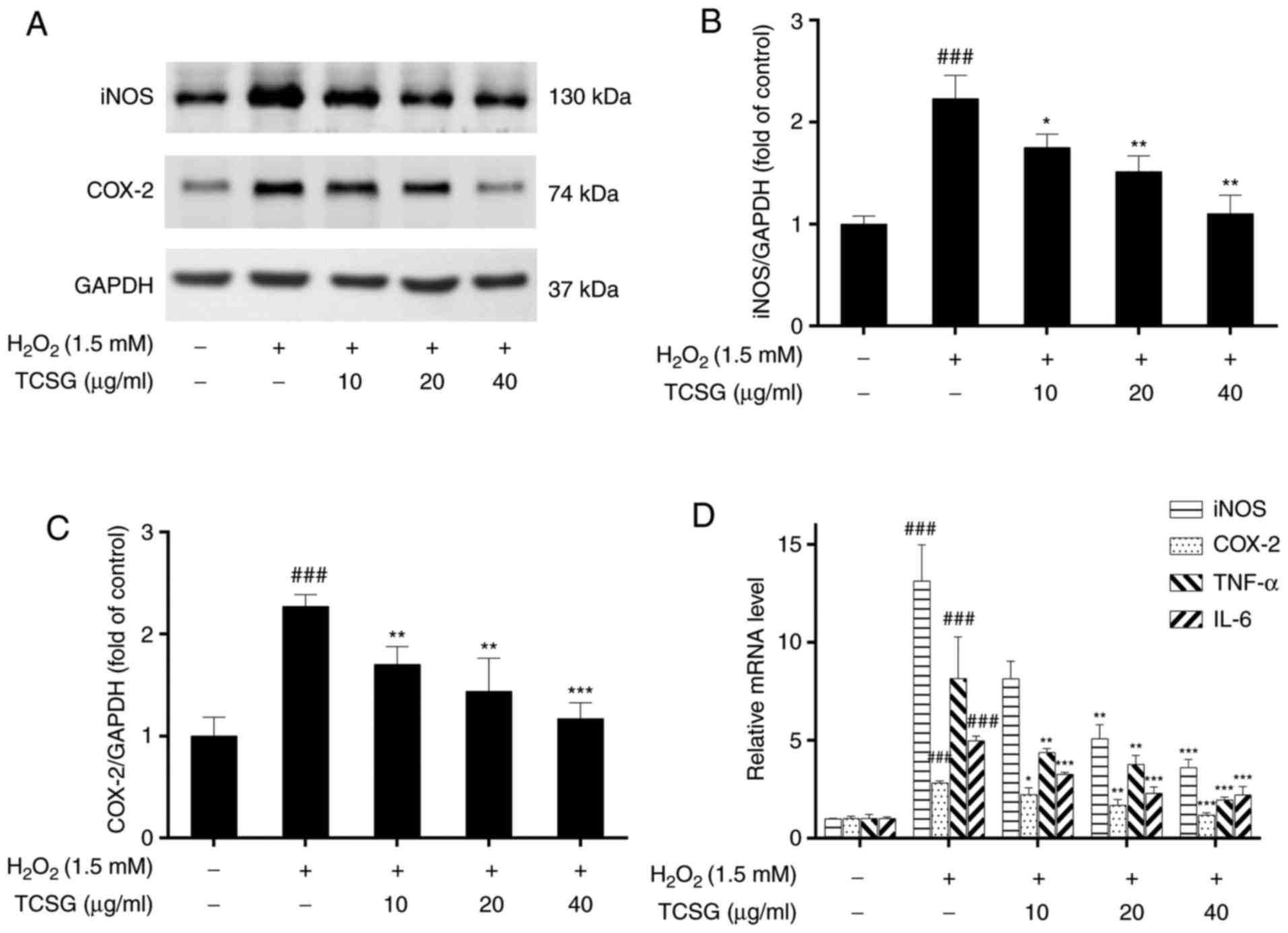 | Figure 6Effect of TCSGs on the
H2O2-induced expression of inflammatory
mediators and cytokines in L02 cells. Equal quantities of proteins
(50 µg/lane) were subjected to 10% SDS-PAGE, and the protein
expression levels of (A) iNOS, COX-2 and GAPDH were detected by
western blot analysis in H2O2-exposed L02
cells. The results of (B) iNOS and (C) COX-2 protein expressions
were represented. The mRNA expression levels of (D) iNOS and COX-2
were detected by RT-qPCR in the H2O2-exposed
L02 cells. The mRNA expression levels of (D) TNF-α and IL-6 were
detected by RT-qPCR in the H2O2-exposed L02
cells. All data are presented as the means ± standard deviation
(n=3). ###P<0.001 vs. the control group (no
treatment); *P<0.05, **P<0.01 and
***P<0.001 vs. H2O2 group.
TCSGs, total C-21 steroidal glycosides; H2O2,
hydrogen peroxide; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; iNOS, inducible nitric oxide synthase;
COX-2, cyclooxygenase-2; TNF, tumor necrosis factor; IL,
interleukin. |
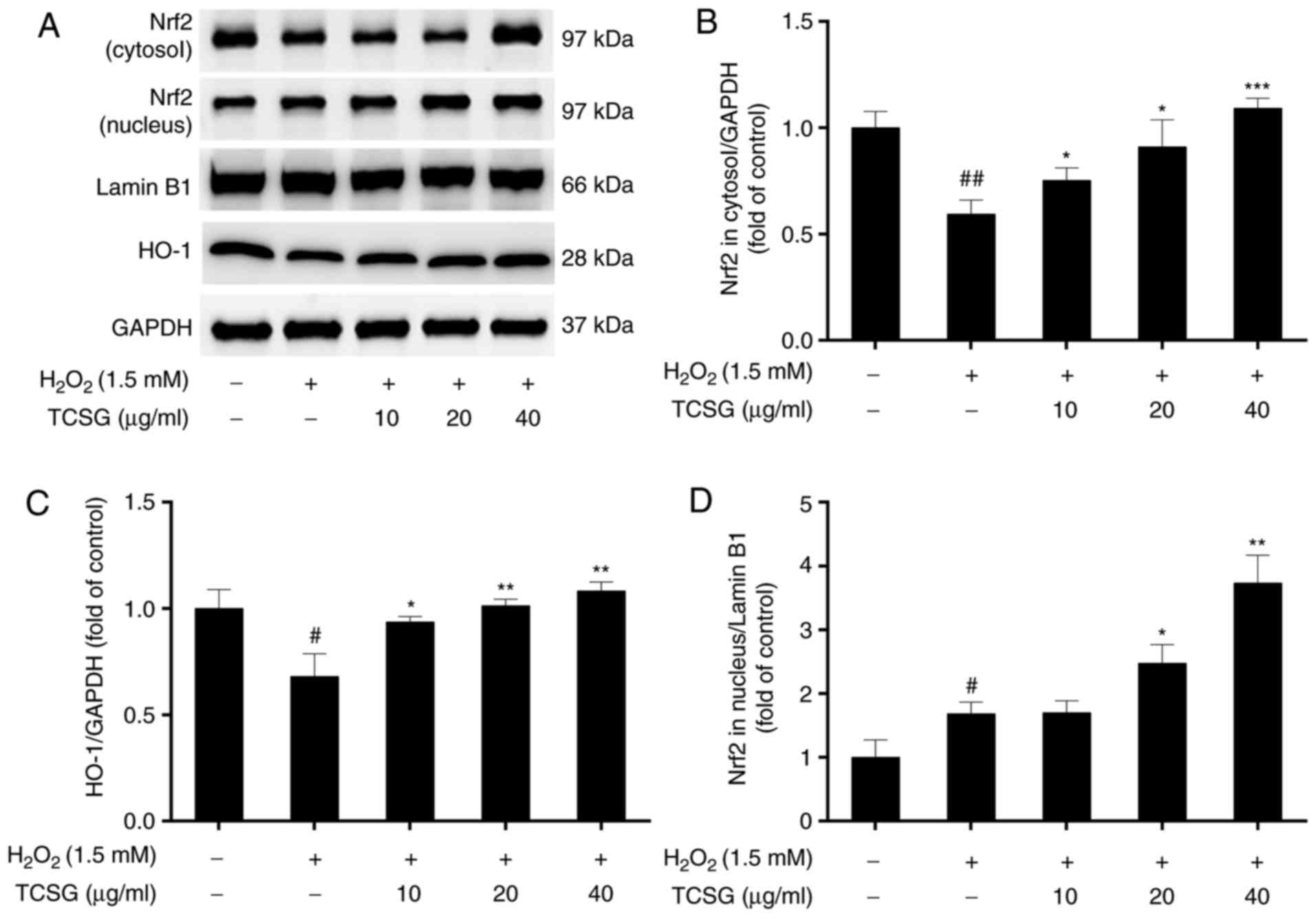
Effect of TCSGs on the Nrf2 signaling
pathway in L02 cells
In order to determine the antioxidative mechanisms
of action of the TCSGs, the protein expression levels of Nrf2 and
HO-1, as well as the translocation of Nrf2 into the nucleus were
measured by western blot analysis (Fig. 7). The results revealed that
H2O2 significantly decreased Nrf2 expression
in the cytosol and increased Nrf2 expression in the nucleus,
indicating that nuclear Nrf2 translocation had occurred (Fig. 7A, B and D).
H2O2 also downregulated the protein
expression of HO-1 in the L02 cells (Fig. 7C). Pretreatment with the TCSGs
resulted in the enhanced expression of Nrf2 and HO-1, suggesting
that treatment with the TCSGs activated the Nrf2 signaling
pathway.
Effect of TCSGs on the activation of the
NF-κB signaling pathway in L02 cells
To further elucidate the mechanisms responsible for
the suppression of the inflammatory mediators by the TCSGs in the
H2O2-exposed L02 cells, the NF-κB signaling
pathway was investigated (Fig.
8). It was revealed that pretreatment with the TCSGs
dose-dependently downregulated NF-κB p65 and phospho-NF-κB p65
levels in the nucleus, and enhanced NF-κB p65 expression in the
cytosol in the H2O2-injured L02 cells
(Fig. 8A–C and G). As shown in
Fig. 8A and E, the TCSGs
significantly inhibited the H2O2-induced
phosphorylation of IκBα in the L02 cells. These results indicated
that the TCSGs prevented the translocation of NF-κB by inhibiting
the phosphorylation of NF-κB, as well as the degradation and
phosphorylation of IκBα in the H2O2-exposed
L02 cells.
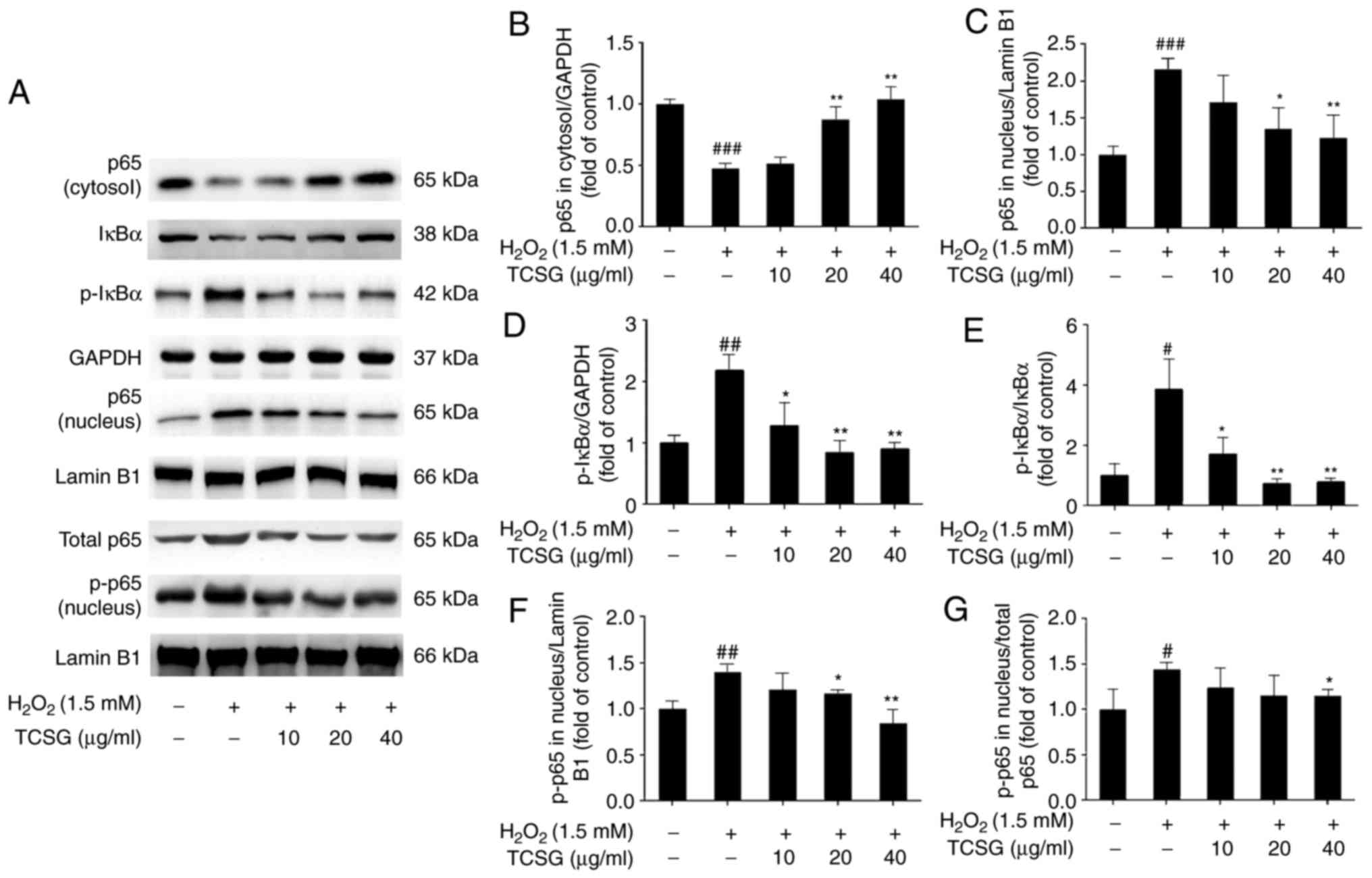 | Figure 8Effect of TCSGs on the activation of
the NF-κB signaling pathway in H2O2-induced
L02 cells. Cells were pretreated with the TCSGs (10, 20 and 40
µg/ml) for 48 h, and stimulated with
H2O2 (1.5 mM) for 24 h. The samples of
nuclear and cytosolic proteins were prepared to detect the
expression of (A–C) NF-κB p65, (A, D and E) IκBα and p-IκBα, and
(A, G and F) p-NF-κB p65 using specific antibodies by western blot
analysis, respectively. GAPDH and Lamin B1 were used as the
internal controls. All results are presented as the means ±
standard deviation (n=3). #P<0.05,
##P<0.01 and ###P<0.001 vs. the control
group (no treatment); *P<0.05 and
**P<0.01 vs. H2O2 group. TCSGs,
total C-21 steroidal glycosides; H2O2,
hydrogen peroxide; NF-κB, nuclear factor-κB; IκBα, inhibitor of
nuclear factor-κBα; p-, phosphorylated. |
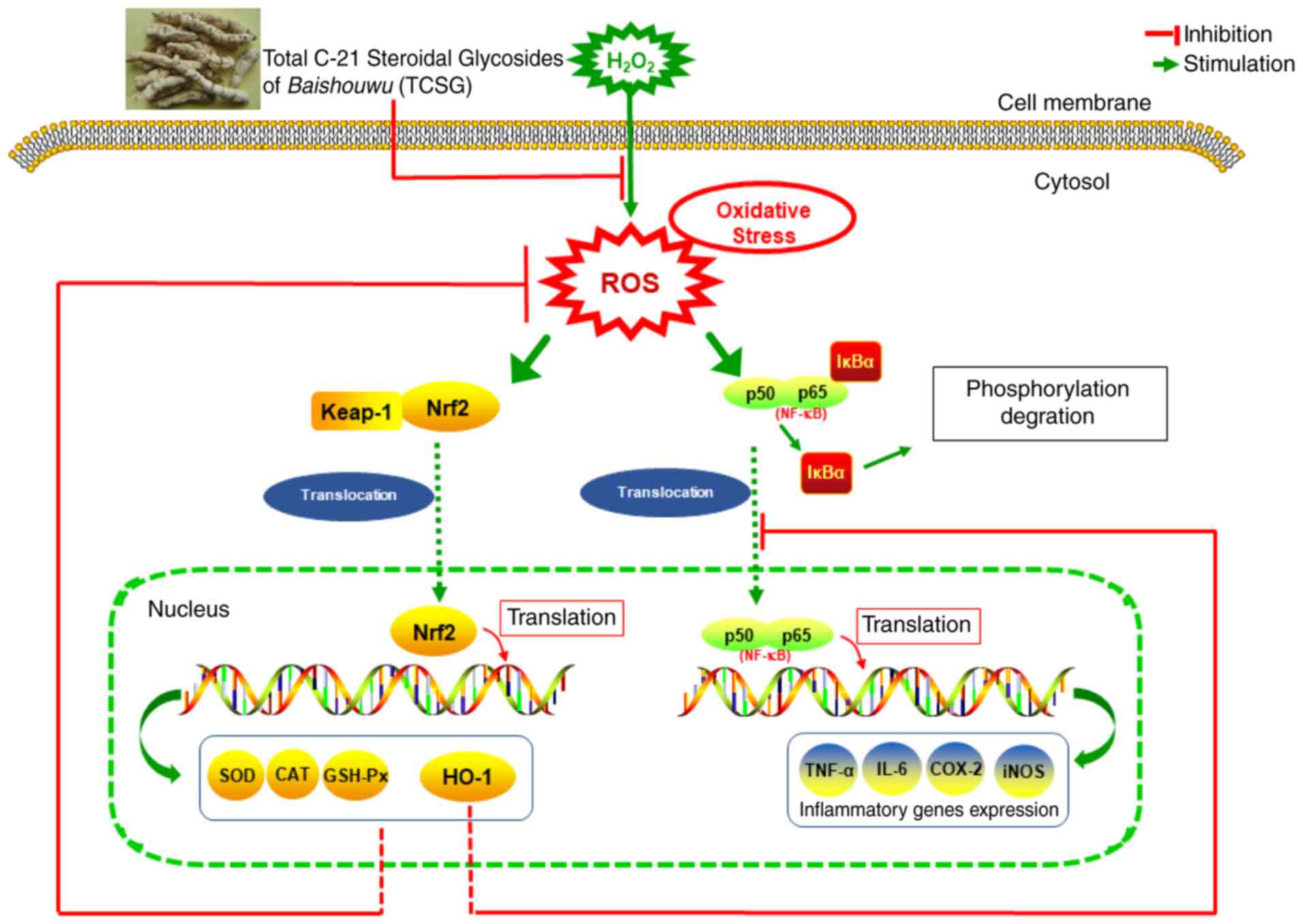
Taken together, the potential molecular mechanisms
of action of TCSGs in human liver cells and their protective
effects against H2O2-induced oxidative injury
and inflammation are presented in Fig. 9.
Discussion
Hepatic injury is a leading cause of hepatic
fibrosis and cancer development. Several factors, including
oxidative stress, inflammation and immune responses, have been
considered to be involved in the pathogenesis of hepatic injury. An
accumulating body of evidence has indicated that oxidative stress
and inflammation play important roles in the pathogenesis of liver
disease (47). High levels of
oxidative stress cause cellular damage, such as lipid peroxidation
(48). Inflammatory cytokines
also induce oxidant formation and cause liver injury.
Anti-oxidative stress-based treatments are promising therapeutic
strategies for chronic liver diseases.
TCSGs, the main bioactive components of Baishouwu,
have been reported to have potent free radical scavenging
properties and hepatoprotective effects (13,17). In the present study, it was
confirmed that TCSGs was able to ameliorate
H2O2-induced toxicity in the L02 human
hepatic cell line. The results of cell viability assay revealed
that treatment with the TCSGs markedly protected the cells from
damage caused by H2O2, and improved the cell
survival rate. As shown in Fig.
2B, treatment with TCSGs at a concentration exceeding 40
µg/ml or given or for a prolonged period of time (72 h)
seemed to be toxic to the cells. To the best of our knowledge, C-21
steroidal glycosides at higher concentrations may exert cytotoxic
effects in vitro (49–52). We speculate the reasons are as
follows: i) Chemical structures of C-21 steroidal glycosides
usually contain hydrophilic and lipophilic groups, which probably
affect the cell membrane and produce a surfactant-like action
(53); ii) DNA damage may be
involved in the cytotoxic mechanisms of C-21 steroidal glycosides.
It has been reported that C-21 steroidal glycosides exert growth
inhibitory effects on human glioma cells by triggering DNA damage
(54). To date, the majority of
reports on the cytotoxicity of C-21 steroidal glycosides have
mainly focused on tumor cells lines, and few on normal cell lines.
From this, it can be deduced that the cytotoxic effects of C-21
steroidal glycosides on normal cells may differ from those on
cancer cell lines. Thus, in the future, we aim to carry out further
investigations on the cytotoxic effects and mechanisms of action of
C-21 steroidal glycosides.
Oxidative stress leads to cellular damage and cell
death via the generation of ROS. Excess ROS primarily induces lipid
peroxidation, as indicated by the production of MDA. MDA is a
critical marker of lipid peroxidation and is one of the final
products of the peroxidation of unsaturated fatty acids. The levels
of MDA reflect the extent of cell oxidative damage (55). The antioxidant defense system,
containing SOD, CAT, and GSH-Px, scavenges ROS to prevent cell
damage in response to oxidative stress (56,57). The present study demonstrated that
the enzyme activities of SOD, CAT and GSH-Px, decreased following
exposure to H2O2. In addition, the ROS levels
and MDA levels increased in the L02 cells exposed to
H2O2. However, treatment with the TCSGs
attenuated these effects. The results indicated that TCSGs
inhibited H2O2-induced oxidative damage by
suppressing ROS production and lipid peroxidation, and enhancing
the antioxidant defense systems.
The pro-inflammatory enzymes, iNOS and COX-2, play
important roles in liver damage. The activation of iNOS induces the
production of high levels of NO and further damages hepatocytes
(58,59). The present study demonstrated that
the TCSGs significantly inhibited COX-2 and iNOS expression, which
was consistent with the reduced NO levels in the L02 cells exposed
to H2O2 but pretreated with the TCSGs. It has
been reported that the upregulation in the mRNA levels of iNOS and
COX-2 in hepatic tissues and cells is a result of the increased
expression of pro-inflammatory cytokines, such as TNF-α and IL-6,
and the activation of NF-κB (60,61). Cytokines, including TNF-α and IL-6
have been the focus of previous investigations evaluating the
inflammatory injury of tissues or cells, as they activate the
inflammatory and immune responses, exacerbating hepatic injury
(62). The results of the present
study revealed that the increased levels of TNF-α and IL-6 induced
by H2O2 were markedly suppressed by
pretreatment with the TCSGs. Notably, treatment with the TCSGs also
markedly downregulated the expression of NF-κB p65 and p-NF-κB p65
in the nucleus, upregulated NF-κB p65 expression in the cytosol,
and reduced the degradation and phosphorylation of IκBα induced by
H2O2 in the L-02 cells. Oxidative stress is
known to activate NF-κB, leading to the induction of inflammatory
genes, which in turn increases neutrophil recruitment to the liver
and exacerbates oxidative stress and inflammation. Taken together,
the results of the present study indicated that TCSGs exerted
hepatoprotective effects against H2O2-induced
oxidative stress via the inhibition of the NF-κB signaling pathway
and the reduction in COX-2 and iNOS levels, resulting in the
reduction of inflammatory factors.
Nrf2 is a pivotal transcription factor, which can
encode detoxification enzymes and antioxidant proteins to against
oxidative stress (63). Nrf2
regulates the expression of various antioxidant enzymes, including
CAT, GSH-Px, and SOD, by using GSH as their substrate (64). The activation of Nrf2 in cells
provides an indirect strategy to enhance antioxidative capacity
(65,66). Under oxidative stress conditions,
Nrf2 is released from Kelch-like ECH-associated protein 1 and
translocates to the nucleus; it then recognizes the ARE and
regulates the expression of antioxidant enzymes including HO-1. In
the present study, the antioxidant ability of TCSGs in the
regulation of redox mechanisms by augmenting the expression of
antioxidant genes via the Nrf2 signaling pathway, was evaluated.
Following exposure to H2O2, Nrf2 expression
in the L02 cells decreased in the cytoplasm, but increased in the
nucleus when compared with the control group, which indicated that
the nuclear Nrf2 translocation and Nrf2 activation had occurred. To
the best of our knowledge, the effects of
H2O2 on Nrf2 activation are strongly
dependent on H2O2 concentrations and the
method of H2O2 delivery (67). Different results however, have
been obtained under different experimental conditions. Ma et
al reported that treatment with 200 µM
H2O2 for 30 min exerted no significant effect
on the cytosolic Nrf2 expression in HepG2 cells (68). In addition, in another study, no
significant influence on the expression of nuclear Nrf2 was
observed in melanocyte cells exposed to 1 mM
H2O2 for 24 h (69). Some researchers have proven that
the nuclear Nrf2 translocation occurs in melanocytes or human
dental pulp stem cells following exposure to
H2O2 (36,70). Other studies have found that
exposure to 300 µM H2O2 for 4 h
significantly decreased the expression of Nrf2 at both the mRNA and
protein level in PC12 cells (71). In this study,
H2O2 decreased Nrf2 expression in the
cytoplasm and increased it in the nucleus, which indicated that
nuclear Nrf2 translocation and Nrf2 activation occurred. It was
speculated that Nrf2 may have been slightly activated as an
adaptive defense against oxidative stress under these conditions.
In the groups treated with the TCSGs, Nrf2 expression and the
translocation of Nrf2 into the nucleus were markedly increased when
compared with the H2O2 group, indicating that
there was an enhanced activation of the Nrf2 signaling pathway.
Furthermore, the TCSGs markedly upregulated the expression of
downstream target proteins of the Nrf2 signaling pathway, such as
HO-1. HO-1 is a key rate-limiting enzyme in the degradation of
heme, preventing free heme from participating in the oxidative
reaction. The overexpression of HO-1 inhibited ROS generation,
NF-κB activation and nuclear translocation, and protected against
oxidative stress and inflammatory injury (72). Combined with the findings of the
present study, these results demonstrated that TCSGs protected L02
cells against H2O2-mediated oxidative injury
by activating the Nrf2 signaling pathway and inducing HO-1
expression.
In conclusion, in this study, it was demonstrated
that pretreatment with the TCSGs protected the L02 cells against
H2O2-induced oxidative damage by increasing
the expression of Nrf2 and HO-1, medicated by the NF-κB signaling
pathway. The present study also indicated that TCSGs isolated from
Baishouwu possessed marked anti-oxidative stress and
anti-inflammatory effects against
H2O2-induced hepatic injury via Nrf2
activation and inhibition of the NF-κB signaling pathway (Fig. 9). Further studies on the potential
effects of TCSGs on any other cell signaling pathways involved in
the pathogenesis of H2O2-induced
hepatotoxicity are still ongoing. Based on these results, TCSGs may
be proven to be a promising strategy for preventing inflammation
and reducing the risk of hepatic disease.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81774178, 81373888
and 81102884) and Jiangsu Province Natural Science Foundation of
China (grant no. BK20151606).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors’ contributions
PW and YP designed the experiments. ZW, YWa and XM
performed the experiments. XW, ZL and SQ equally contributed in
extraction and analysis of chemical constituents. YWe, LS and YD
analyzed the results. ZW drafted the manuscript. All the authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Shan L, Liu RH, Shen YH, Zhang WD, Zhang
C, Wu DZ, Min L, Su J and Xu XK: Gastroprotective effect of a
traditional Chinese herbal drug ‘Baishouwu’ on experimental gastric
lesions in rats. J Ethnopharmacol. 107:389–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Zhang J, Gu X, Peng Y, Huang W and
Qian S: Two new cytotoxic pregnane glycosides from Cynanchum
auriculatum. Planta Med. 74:551–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang DY, Hua X, Ye JL, Li JJ, Li Q and Yan
HS: Antitumor effect of C21 steroidal glycosides in Radix Cynanchi
bungei on Heps rats and its influence on hematopoiesis. J Clin Med
Pract. 18:6–8. 2014.
|
|
4
|
Peng YR, Li YB, Liu XD, Zhang JF and Duan
JA: Antitumor activity of C-21 steroidal glycosides fro. Cynanchum
auriculatum Royle ex Wight Phytomedicine. 15:1016–1020. 2008.
|
|
5
|
Zhang RS, Ye YP and Liu XL: Studies on in
vitro antitumor activity of total steroidal glycoside from the root
of Cynanchum auriculatum. Chin Tradit Herbal Drugs. 31:599–601.
2000.
|
|
6
|
Zhang RS, Ye YP, Shen YM and Liang HL: Two
new cytotoxic C-21 steroidal glycosides from the root of Cynanchum
auriculatum. Tetrahedron. 56:3875–3879. 2000. View Article : Google Scholar
|
|
7
|
Wang YQ, Zhang SJ, Lu H, Yang B, Ye LF and
Zhang RS: A C21-steroidal glycoside isolated from the roots of
Cynanchum auriculatum induces cell cycle arrest and apoptosis in
human gastric cancer SGC-7901 cells. Evid Based Complement Alternat
Med. 2013.180839:2013.
|
|
8
|
Wang DY, Zhang HQ and Li X: Apoptosis
induced by the C21 sterols in Baishouwu and its mechanism of action
in hepatoma. Yao Xue Xue Bao. 42:366–370. 2007.In Chinese.
PubMed/NCBI
|
|
9
|
Shan L, Zhang WD, Zhang C, Liu RH, Su J
and Zhou Y: Antitumor activity of crude extract and fractions from
root tuber of Cynanchum auriculatum Royle ex Wight. Phytother Res.
19:259–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng YR, Li YB, Liu XD, Zhang JF and Duan
JA: Apoptosis induced by caudatin in human hepatoma cell line
SMMC7721. Chin J Nat Med. 6:210–213. 2008. View Article : Google Scholar
|
|
11
|
Peng YR, Ding YF, Wei YJ, Shu B, Li YB and
Liu XD: Caudatin-2,6-dideoxy-3-O-methy-β-D-cymaropyranoside 1
induced apoptosis through caspase 3-dependent pathway in human
hepatoma cell line SMMC7721. Phytother Res. 25:631–637. 2011.
View Article : Google Scholar
|
|
12
|
Zhang SX, Li X, Yin JL, Chen LL and Zhang
HQ: Effect of C21 steroidal glycoside from root of Cynanchum
auriculatum on D-galactose induced aging model mice. Zhongguo Zhong
Yao Za Zhi. 32:2511–2514. 2007.In Chinese.
|
|
13
|
Song JM and Ding XL: Study on the
scavenging effect of Baishouwu on superoxide free radicals. Chin
Wild Plant Resources. 17:1–4. 1997.
|
|
14
|
Gu LG, Gong SS, Tao JD, Liu CH and Zhou Y:
Studies on the regulation effect of Baishouwu on the immunity of
mouse. Chin J Int Med. 7:37–41. 1987.
|
|
15
|
Ji CX, Li XY, Jia SB, Liu LL, Ge YC, Yang
QX and Zhang JJ: The antidepressant effect of Cynanchum auriculatum
in mice. Pharm Biol. 50:1067–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon MY, Choi NH, Min BS, Choi GJ, Choi
YH, Jang KS, Han SS, Cha B and Kim JC: Potent in vivo antifungal
activity against powdery mildews of pregnane glycosides from the
roots of Cynanchum wilfordii. J Agric Food Chem. 59:12210–12216.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv W, Zhang A, Xu S and Zhang H: Effects
of general glycosides in Cynanchum auriculatum of Jiangsu province
on liver fibrosis of rats. Zhongguo Zhong Yao Za Zhi. 34:2508–2511.
2009.In Chinese.
|
|
18
|
Jaeschke H: Reactive oxygen and mechanisms
of inflammatory liver injury: Present concepts. J Gastroenterol
Hepatol. 26(Suppl 1): S173–S179. 2011. View Article : Google Scholar
|
|
19
|
Miller AM, Wang H, Park O, Horiguchi N,
Lafdil F, Mukhopadhyay P, Moh A, Fu XY, Kunos G, Pacher P and Gao
B: Anti-inflammatory and anti-apoptotic roles of endothelial cell
STAT3 in alcoholic liver injury. Alcohol Clin Exp Res. 34:719–725.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dey A and Cederbaum AI: Alcohol and
oxidative liver injury. Hepatology. 43(Suppl 1): S63–S74. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sies H, Berndt C and Jones DP: Oxidative
stress. Annu Rev Biochem. 86:715–748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weng D, Lu Y, Wei Y, Liu Y and Shen P: The
role of ROS in microcystin-LR-induced hepatocyte apoptosis and
liver injury in mice. Toxicology. 232:15–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukiyama-Kohara K: Role of oxidative
stress in hepatocarcinogenesis induced by hepatitis C virus. Int J
Mol Sci. 13:15271–15278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das S, Maras JS, Hussain MS, Sharma S,
David P, Sukriti S, Shasthry SM, Maiwall R, Trehanpati N, Singh TP
and Sarin SK: Hyperoxidized albumin modulates neutrophils to induce
oxidative stress and inflammation in severe alcoholic hepatitis.
Hepatology. 65:631–646. 2017. View Article : Google Scholar
|
|
25
|
Chen G, Ni Y, Nagata N, Xu L and Ota T:
Micronutrient antioxidants and nonalcoholic fatty liver disease.
Int J Mol Sci. 17:pii: E13792016. View Article : Google Scholar
|
|
26
|
Wang M, Ma HL, Liu B, Wang HB, Xie H, Li
RD and Wang JF: Pinus massoniana bark extract protects against
oxidative damage in L-02 hepatic cells and mice. Am J Chin Med.
38:909–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sies H: Hydrogen peroxide as a central
redox signaling molecule in physiological oxidative stress:
Oxidative eustress. Redox Biol. 11:613–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hseu YC, Wu FY, Wu JJ, Chen JY, Chang WH,
Lu FJ, Lai YC and Yang HL: Anti-inflammatory potential of Antrodia
Camphorata through inhibition of iNOS, COX-2 and cytokines via the
NF-kappaB pathway. Int Immunopharmacol. 5:1914–1925. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee D, Park S, Bae S, Jeong D, Park M,
Kang C, Yoo W, Samad MA, Ke Q, Khang G and Kang PM: Hydrogen
peroxide-activatable antioxidant prodrug as a targeted therapeutic
agent for ischemia-reperfusion injury. Sci Rep. 5:165922015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W and Kong AN: Molecular mechanisms of
Nrf2-mediated antioxidant response. Mol Carcinog. 48:91–104. 2009.
View Article : Google Scholar :
|
|
31
|
Yu J, Zhu X, Qi X, Che J and Cao B:
Paeoniflorin protects human EA.hy926 endothelial cells against
gamma-radiation induced oxidative injury by activating the
NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol Lett.
218:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin SM, Yang JH and Ki SH: Role of the
Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev.
2013.763257:2013.
|
|
33
|
Huo X, Liu C, Gao L, Xu X, Zhu N and Cao
L: Hepatoprotective effect of aqueous extract from the seeds of
orychophragmus violaceus against liver injury in mice and HepG2
cells. Int J Mol Sci. 18:pii: E11972017. View Article : Google Scholar
|
|
34
|
Conde de la Rosa L, Schoemaker MH, Vrenken
TE, Buist-Homan M, Havinga R, Jansen PL and Moshage H: Superoxide
anions and hydrogen peroxide induce hepatocyte death by different
mechanisms: Involvement of JNK and ERK MAP kinases. J Hepatol.
44:918–929. 2006. View Article : Google Scholar
|
|
35
|
Vaziri ND: Oxidative stress in uremia:
Nature, mechanisms, and potential consequences. Semin Nephrol.
24:469–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim D, Kim H, Kim K and Roh S: The
protective effect of indole-3-acetic acid (IAA) on H2O2-damaged
human dental pulp stem cells is mediated by the AKT pathway and
involves increased expression of the transcription factor nuclear
factor-erythroid 2-related factor 2 (Nrf2) and its downstream
target heme oxygenase 1 (HO-1). Oxid Med Cell Longev.
2017.8639485:2017.
|
|
37
|
Cao YJ, Zhang YM, Qi JP, Liu R, Zhang H
and He LC: Ferulic acid inhibits H2O2-induced oxidative stress and
inflammation in rat vascular smooth muscle cells via inhibition of
the NADPH oxidase and NF-κB pathway. Int Immunopharmacol.
28:1018–1025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tian Y, Li Z, Shen B, Zhang Q and Feng H:
Protective effects of morin on
lipopolysaccharide/d-galactosamine-induced acute liver injury by
inhibiting TLR4/NF-κB and activating Nrf2/HO-1 signaling pathways.
Int Immunopharmacol. 45:148–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tan ZW, Xie S, Hu SY, Liao T, Liu P, Peng
KH, Yang XZ, He ZL, Tang HY, Cui Y, et al: Caudatin targets
TNFAIP1/NF-κB and cytochrome c/caspase signaling to suppress tumor
progression in human uterine cancer. Int J Oncol. 49:1638–1650.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu XJ, Yao N, Qian SH, Li YB and Li P:
Four new C21 steroidal glycosides from the root of Cynanchum
auriculatum. Helvetica Chimica Acat. 92:88–97. 2009. View Article : Google Scholar
|
|
41
|
Wang XJ, Li ZL, Lv XH, Zuo QY, Zhao YM,
Ding YF, Pu SB, Qian SH and Peng YR: Antitumor evaluation and
multiple analysis on different extracted fractions of the root of
Cynanchum auriculatum Royle ex Wight. J Sep Sci. 40:3054–3063.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao YN, Wang ZL, Dai JG, Chen L and Huang
YF: Preparation and quality assessment of high-purity ginseng total
saponins by ion exchange resin combined with macroporous adsorption
resin separation. Chin J Nat Med. 12:382–392. 2014.PubMed/NCBI
|
|
43
|
Hu X, Yang T, Li C, Zhang L, Li M, Huang W
and Zhou P: Human fetal hepatocyte line, L-02, exhibits good liver
function in vitro and in an acute liver failure model. Transplant
Proc. 45:695–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Myhre O, Andersen JM, Aarnes H and Fonnum
F: Evaluation of the probes 2′,7′-dichlorofluorescin diacetate,
luminol, and lucigenin as indicators of reactive species formation.
Biochem Pharmacol. 65:1575–1582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ding YF, Wu ZH, Wei YJ, Shu L and Peng YR:
Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular
carcinoma induced by diethylnitrosamine. J Cancer Res Clin Oncol.
143:821–834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
47
|
Wei Y, Chen K, Whaley-Connell AT, Stump
CS, Ibdah JA and Sowers JR: Skeletal muscle insulin resistance:
Role of inflammatory cytokines and reactive oxygen species. Am J
Physiol Regul Integr Comp Physiol. 294:R673–R680. 2008. View Article : Google Scholar
|
|
48
|
Sid B, Verrax J and Calderon PB: Role of
oxidative stress in the pathogenesis of alcohol-induced liver
disease. Free Radic Res. 47:894–904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dong J, Peng X, Li L, Lu S, Zhou L and Qiu
M: C21 steroidal glycosides with cytotoxic activities from
Cynanchum otophyllum. Bioorg Med Chem Lett. 28:1520–1524. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang YQ, Yang B, Zhang RS and Wei EQ:
Inhibitive effect of C-21 steroidal glycosides of Cynanchum
auriculatum on rat glioma cells in vitro. Zhejiang Da Xue Xue Bao
Yi Xue Ban. 40:402–407. 2011.In Chinese. PubMed/NCBI
|
|
51
|
Wang YB, Su SS, Chen SF, Tang MX, Chen G,
Zhao D, Sang XN, Si YY, Wang HF and Pei YH: C 21 steroidal
glycosides with cytotoxic activity from Cynanchum taihangense.
Phytochem Lett. 20:218–223. 2017. View Article : Google Scholar
|
|
52
|
Kim CS, Ju YO, Sang UC and Lee KR:
Chemical constituents from the roots of Cynanchum paniculatum, and
their cytotoxic activity. Carbohydr Res. 381:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang DY and Zhang HQ: The current
situation and progress of Baishouwu planted in Jiangsu. Chin Wild
Plant Resources. 24:13–15. 2005.
|
|
54
|
Fu XY, Zhang S, Wang K, Yang MF, Fan CD
and Sun BL: Caudatin inhibits human glioma cells growth through
triggering DNA damage-mediated cell cycle arrest. Cell Mol
Neurobiol. 35:953–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Su M, Yu T, Zhang H, Wu Y, Wang X and Li
G: The antiapoptosis effect of glycyrrhizate on HepG2 cells induced
by hydrogen peroxide. Oxid Med Cell Longev. 2016.6849758:2016.
|
|
56
|
Zhao L, Chen J, Su J, Li L, Hu S, Li B,
Zhang X, Xu Z and Chen T: In vitro antioxidant and
antiproliferative activities of 5-hydroxymethylfurfural. J Agric
Food Chem. 61:10604–10611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Herken H, Uz E, Ozyurt H, Söğüt S, Virit O
and Akyol O: Evidence that the activities of erythrocyte free
radical scavenging enzymes and the products of lipid peroxidation
are increased in different forms of schizophrenia. Mol Psychiatry.
6:66–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pan MH, Yang JR, Tsai ML, Sang S and Ho
CT: Anti-inflammatory effect of Momordica grosvenori, Swingle
extract through suppressed LPS-induced upregulation of iNOS and
COX-2 in murine macrophages. J Funct Foods. 1:145–152. 2009.
View Article : Google Scholar
|
|
59
|
Wang WW, Smith DL and Zucker SD: Bilirubin
inhibits iNOS expression and NO production in response to endotoxin
in rats. Hepatology. 40:424–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li J, Zhang X and Huang H: Protective
effect of linalool against
lipopolysaccharide/D-galactosamine-induced liver injury in mice.
Int Immunopharmacol. 23:523–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dai C, Li B, Zhou Y, Li D, Zhang S, Li H,
Xiao X and Tang S: Curcumin attenuates quinocetone induced
apoptosis and inflammation via the opposite modulation of Nrf2/HO-1
and NF-κB pathway in human hepatocyte L02 cells. Food Chem Toxicol.
95:52–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Arranz J, Soriano A, Garcia I, García I,
Concepción MT, Navarro J, Arteaga A, Filella X, Bravo P, Barrera M,
et al: Effect of proinflammatory cytokines (IL-6, TNF-alpha,
IL-1beta) on hemodynamic performance during orthotopic liver
transplantation. Transplant Proc. 35:1884–1887. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim HJ and Vaziri ND: Contribution of
impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in
chronic renal failure. Am J Physiol Renal Physiol. 298:F662–F671.
2010. View Article : Google Scholar
|
|
65
|
Fahey JW, Haristoy X, Dolan PM, Kensler
TW, Scholtus I, Stephenson KK, Talalay P and Lozniewski A:
Sulforaphane inhibits extracellular, intracellular, and
antibiotic-resistant strains of Helicobacter pylori and prevents
benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA.
99:7610–7615. 2002. View Article : Google Scholar
|
|
66
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar
|
|
67
|
Covas G, Marinho HS, Cyrne L and Antunes
F: Activation of Nrf2 by H2O2: De novo
synthesis versus nuclear translocation. Methods Enzymol.
528:157–171. 2013. View Article : Google Scholar
|
|
68
|
Ma Z, Li C, Qiao Y, Lu C, Li J, Song W,
Sun J, Zhai X, Niu J, Ren Q and Wen A: Safflower yellow B
suppresses HepG2 cell injury induced by oxidative stress through
the AKT/Nrf2 pathway. Int J Mol Med. 37:603–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chang Y, Li S, Guo W, Yang Y, Zhang W,
Zhang Q, He Y, Yi X, Cui T, An Y, et al: Simvastatin protects human
melanocytes from H2O2-induced oxidative
stress by activating Nrf2. J Invest Dermatol. 137:1286–1296. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jian Z, Li K, Song P, Zhu G, Zhu L, Cui T,
Liu B, Tang L, Wang X, Wang G, et al: Impaired activation of the
Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress
response: A possible mechanism for melanocyte degeneration in
vitiligo. J Invest Dermatol. 134:2221–2230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mao J, Li Z, Lin R, Zhu X, Lin J, Peng J
and Chen L: Preconditioning with Gua Lou Gui Zhi decoction enhances
H2O2-induced Nrf2/HO-1 activation in PC12
cells. Exp Ther Med. 10:877–884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee IT, Luo SF, Lee CW, Wang SW, Lin CC,
Chang CC, Chen YL, Chau LY and Yang CM: Overexpression of HO-1
protects against TNF-alpha-mediated airway inflammation by
down-regulation of TNFR1-dependent oxidative stress. Am J Pathol.
175:519–532. 2009. View Article : Google Scholar : PubMed/NCBI
|