Introduction
While developing countries are fighting against the
spread of infectious diseases, developed countries are currently
focusing on combating non-communicable diseases, including obesity,
which has exhibited a progressive rise in prevalence over the past
years. However, although obesity is a disease of developed
countries, its prevalence in developing countries is also on a rise
(1). Thus, it can be seen as one
of the most serious public health challenges of the 21st century.
Obesity can actually be defined as the accumulation of excess body
fat. The accumulation of fat results from a high-fat diet (HFD),
caloric-dense diet, sedentary lifestyle, increased urbanization and
psychosocial stress (2). Obesity
is a risk factor for atherosclerosis, cancer, type 2 diabetes,
dyslipidemia and metabolic syndrome. A study has even linked
obesity with excess adipocyte accumulation, lung problems, and
respiratory symptoms. In that study, accumulation of fat tissue in
the abdominal wall and around the abdominal organs hampered the
movement of diaphragm, reduced lung expansion during inspiration
and also decreased lung capacity (3). In addition, the increase in adipose
tissue in obesity has been linked to the production of systemic
oxidative stress, through the peroxisomal and mitochondrial
oxidation of fatty acids, and the overconsumption of oxygen,
producing reactive oxygen species (4). Furthermore, liver damage in obesity
has been reported, as diet-induced obesity is associated with liver
inflammation (5).
Several drugs, including phentermine, fluoxetine,
orlistat, sibutramine and rimonabant, as well as lifestyle
modifications and even surgery are used for the treatment of
obesity. However, the associated side effects of nausea, dizziness,
insomnia, diarrhea, dyspepsia and constipation cannot be
overlooked. A healthy lifestyle can be difficult to maintain, while
surgery is reserved for terminal obesity cases. Thus, researchers
are focusing on the need for new natural therapies and different
methods of combating obesity. Plant extracts with phytochemicals
including polyphenols, curcumin, resveratrol and proanthocyanidins
have become increasingly popular in the past decades due to their
anti-adipogenic properties (6-8).
Herbal medicines have been used to control weight and for the
treatment of obesity. For instance, the Garcinia cambogia
fruit extract, containing hydroxycitric acid, has frequently been
used for weight control, and has demonstrated no toxic effects
(9).
Diospyros (D.) lotus, which
belongs to the Ebenaceae family, is a deciduous tree native to
China, Korea, Japan, Brazil, Turkey and Italy, and its fruit is
widely consumed for its sedative, antidiabetic, antiseptic and
antitumor properties (10).
Notably, compounds isolated from D. lotus fruit and three
new dimeric naphthoquinones isolated from D. lotusroots have
been demonstrated to have antiproliferative and cytotoxic effects
on a series of cancer cell lines, including COR-L23, C32, A375,
CaCo-2 and mouse T-cell lymphoma cell lines (11-13). Furthermore, D. lotus leaves
have been widely used for the treatment of muscle and joint pain of
the lower back (14).
A study on the leaf extract of another species,
D. kaki, revealed that this extract improved
hyperglycemia, dyslipidemia and liver fat accumulation in type 2
diabetic db/db mice (15).
Another recently published study indicated that D.
kaki fruit in synergy with Citrus unshiu peel
demonstrated an anti-obesity effect by inhibiting pancreatic lipase
(16). However, to the best of
our knowledge, no studies have examined the anti-obesity effect of
D. lotus leaves. The present study was, therefore,
conducted to investigate the anti-obesity effect of D. lotus
leaf water extract (DLE) on adipocyte differentiation in both in
vitro and in vivo studies, using a cell model and an
HFD-induced obesity model in mice.
Materials and methods
Chemicals
Glucose, triglyceride (TG), total cholesterol (TC)
and high-density lipoprotein cholesterol (HDL-C) assay kits were
purchased from Asan Pharmaceutical Co., Ltd. (Seoul, Korea).
Apigenin, 3-isobutyl-1-methylxanthine (IBMX) and Oil red O were
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Aspartate
transaminase (AST), alanine transaminase (ALT) and alkaline
phosphatase (ALP) activity colorimetric assay kits were purchased
from BioVision, Inc. (Milpitas, CA, USA). Catalase (CAT),
glutathione (GSH), and glutathione peroxidase (GPx) assay kits were
obtained from Cayman Chemical Company (Ann Arbor, MI, USA).
Superoxide dismutase (SOD) and malondialdehyde (MDA) quantification
kits were purchased from Calbiochem (EMD Millipore, Billerica, MA,
USA) and Cell Biolabs, Inc. (San Diego, CA, USA), respectively. A
Mouse Leptin Quantikine enzyme-linked immunosorbent assay (ELISA)
kit and ultra-sensitive mouse insulin ELISA kit were from R&D
Systems, Inc. (Minneapolis, MN, USA) and Crystal Chem, Inc. (Elk
Grove Village, IL, USA), respectively. Ethanol, xylene, and
paraffin wax came from Daejung Co., Ltd., (Busan, Korea), Junsei
Chemical Co., Ltd., (Tokyo, Japan) and Leica Biosystems (Wetzlar,
Germany), respectively. All other chemicals used were of reagent
grade and purchased from Sigma-Aldrich (Merck KGaA), unless
otherwise stated.
Plant material and extract
preparation
D. lotus leaves used in the present study
were harvested from Bugwi-Myeon, Jinan-Gun, Jeonbuk, Korea on June
30th, 2015. The plant was identified and authenticated by Professor
Hong-Jun Kim from the College of Oriental Medicine, Woosuk
University (Jeonju, Korea) and a voucher specimen (no.
2015-06-30-DLE) was deposited in our laboratory. The leaves were
washed four times with distilled water, steamed for 5 min, dried at
40°C for 12 h and then extracted (100 g) in distilled water (2,000
ml) at 100°C for 30 min. The extract was passed through a
0.45-μm pore size filter paper (ADVANTEC, Togo, Japan). The
filtered extract was concentrated by vacuum evaporation and
lyophilized to obtain the dry extract (15.2 g), which was stored at
−20°C for use in further experiments.
Cell culture and Oil Red O staining
3T3-L1 pre-adipocytes obtained from American Type
Culture Collection (Manassas, VA, USA) were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 units/ml penicillin and 0.1 mg/ml streptomycin in
an incubator with temperature of 37°C and 5% CO2. To
induce adipocyte differentiation, the 3T3-L1 pre-adipocytes were
seeded in 6-mm cell culture dishes at a density of 5×104
cells/ml. After 2 days, when the cells had reached 100% confluence,
differentiation was induced by replacing the media with DMEM
containing 10% FBS and 0.5 mM IBMX, 1 μM dexamethasone and 1
μg/ml insulin (designated hereafter as MDI), along with 200
or 400 μg/ml DLE, or with 10 μg/ml apigenin as the
positive control. After 3 days of culture, the medium was replaced
with DMEM containing serum, insulin, and DLE or apigenin, followed
by replacement with fresh DMEM with the same constituents after a
further 2 days of incubation (temperature 37°C and 5%
CO2).
For Oil red O staining, cells (5×105
cells/ml) in 60 mm culture dishes were fixed with 10% formalin for
1 h and then washed with 60% isopropanol, followed by incubation in
5 ml Oil red O working solution for 5 min at room temperature.
Subsequent to incubation, the cells were immediately washed five
times with distilled water, and images were captured using a Leica
light microscope (Leica Microsystems GmbH, Wetzlar, Germany). Next,
Oil red O was dissolved in 100% isopropanol, and the absorbance
were measured by a micro-plate reader (Molecular Devices LLC,
Sunnyvale, CA, USA) at 540 nm.
TG content in 3T3-L1 adipocytes
Total TG content in 3T3-L1 adipocytes was determined
using a commercial TG assay kit according to the manufacturer’s
protocol.
Animals and diet
The in vivo study involved 4-week-old male
C57BL/6J mice, obtained from Orient Bio, Inc. (Iksan, Korea). The
mice were fed with commercial standard laboratory diet and water
ad libitum, and maintained in an air-conditioned room with a
temperature of 22±2°C, humidity of 50-60%, and a 12-h light-dark
cycle. The animal experiment was approved by Jeonju University
Institutional Animal Care and Used Committee (no.
JJU-IACUC-2015-04) and the protocols were performed following the
guidelines set by the institution.
Following 1 week of acclimation, the mice were
randomly divided into five groups of 6 mice each, including the
normal control, HFD control, HFD + 200 mg/kg DLE, HFD + 400 mg/kg
DLE, and HFD + 20 mg/kg apigenin groups. The normal control group
received a commercial standard diet (AIN-76A; Research Diet, Inc.,
New Brunswick, NJ, USA), throughout the experimental period, while
the HFD model and experimental groups were fed with a diet high in
fat content (Rodent Diet with 45 kcal% fat; Research Diet Inc.) for
8 weeks to induce obesity. After obesity was induced, the mice in
the HFD control and experimental groups were maintained on the
high-fat diet while being administered orally with DLE and Apigenin
for a further 10 weeks. Apigenin was used as a positive
control.
Following completion of all treatments, the mice
were fasted for 12 h and then anesthetized with ether. Blood was
collected by a cardiac puncture and then the mice were euthanized.
The blood samples were allowed to clot for 30 min at room
temperature and centrifuged at 2,000 x g for 15 min at 4°C. The
liver and abdominal fat tissues were also completely excised and
cleansed with saline. Moisture was completely removed with a filter
paper, and the weights were measured using an electronic balance.
The tissue samples were quickly frozen with liquid nitrogen and
stored at -80°C until used for further studies.
Weight gain, food intake and food
efficiency ratio (FER)
The body weight and food intake of mice were
measured once per week. The FER was calculated according to the
following formula: FER (%)=[body weight gain (g/day)/food intake
(g/day)] ×100.
Biochemical analysis
Serum glucose, TG, TC and HDL-C levels were measured
using the corresponding assay kits, according to the manufacturer’s
protocol. Low-density lipoprotein cholesterol (LDL-C) was
calculated according to previously described methods (17), using the following formula:
LDL-C=[TC-HDL-C-(TG/5)]. Insulin and leptin levels in the serum
samples were evaluated via commercial ELISA kits, according to the
manufacturer’s instructions. The atherogenic index was calculated
using the following formula: (TC-HDL-C)/HDL-C. Furthermore, the
activities of hepatic enzymes AST and ALT in the serum were
measured using commercial assay kits, according to the
manufacturer’s protocol.
Histological analysis
For histological analysis, the liver tissues were
fixed in 10% neutral formalin for 3 days at room temperature,
washed in 4 changes of phosphate-buffered saline (1 h each),
dehydrated in a series of graded ethanol (from 60-100%, 30 min
each), cleared twice in xylene (30 min each) and embedded in of
paraffin wax three separate times at 70°C (30 min each). The
tissues were then fixed with paraffin wax overnight at 4°C and
sectioned (5-μm) using a microtome. Next, the tissues
sections were stained with hematoxylin for 3 min and eosin for 0.5
min (H&E) at room temperature.
Determination of oxidative stress and
antioxidant markers
After the mice were sacrificed, liver tissues (0.3
g) were homogenized in 0.5 ml phosphate-buffered saline and then
centrifuged at 2,000 x g for 15 min at 4°C to obtain the
supernatant which was stored at -70°C for subsequent use. Hepatic
MDA and GSH levels were measured using commercially available assay
kits, according to the manufacturer’s protocol. Liver SOD, CAT, and
GPx activities were also measured as described in the corresponding
kit manuals.
High-performance liquid chromatography
(HPLC) analysis
HPLC was performed to determine the active compounds
in DLE using an Agilent 1100 series system (Agilent Technologies,
Inc., Santa Clara, CA, USA), equipped with a binary pump delivery
system, a degasser (G1379A), an autosampler (G1313A), a diode array
detector (G1315B) and Agilent Eclipse XDB-C18 column (4.6×250 mm,
5-μm particles). The mobile phase was composed of 0.5%
formic acid in water (solvent A) and acetonitrile (solvent B), and
the gradient elution were as follows: 0 min, 5% solvent B; 10 min,
10% solvent B; 50 min, 40% solvent B; 54 min, 100% solvent B; and
then held for 10 min prior to returning to the initial conditions.
Other HPLC conditions were as follows: Flow rate, 1 ml/min; UV
detection wavelength, 280 nm; sample injection volume, 20
μl; and column temperature, 30°C. All standards were
identified based on retention time. The integration of each
component on the chromatogram was processed using the Agilent
Chemstation software (Agilent Technologies, Inc.).
Statistical analysis
The data were analyzed using the IBM SPSS statistics
program (version 22; IBM Corp., Armonk, NY, USA) with one-way
analysis of variance, followed by Duncan’s multiple range test. A
P<0.05 was considered to indicate a statistically significant
difference. All data are presented as the mean ± standard
deviation.
Results
Inhibitory effect of DLE on lipid
accumulation in 3T3-L1 cells
To evaluate the effect of DLE on adipocyte
differentiation, lipid accumulation was evaluated using the Oil red
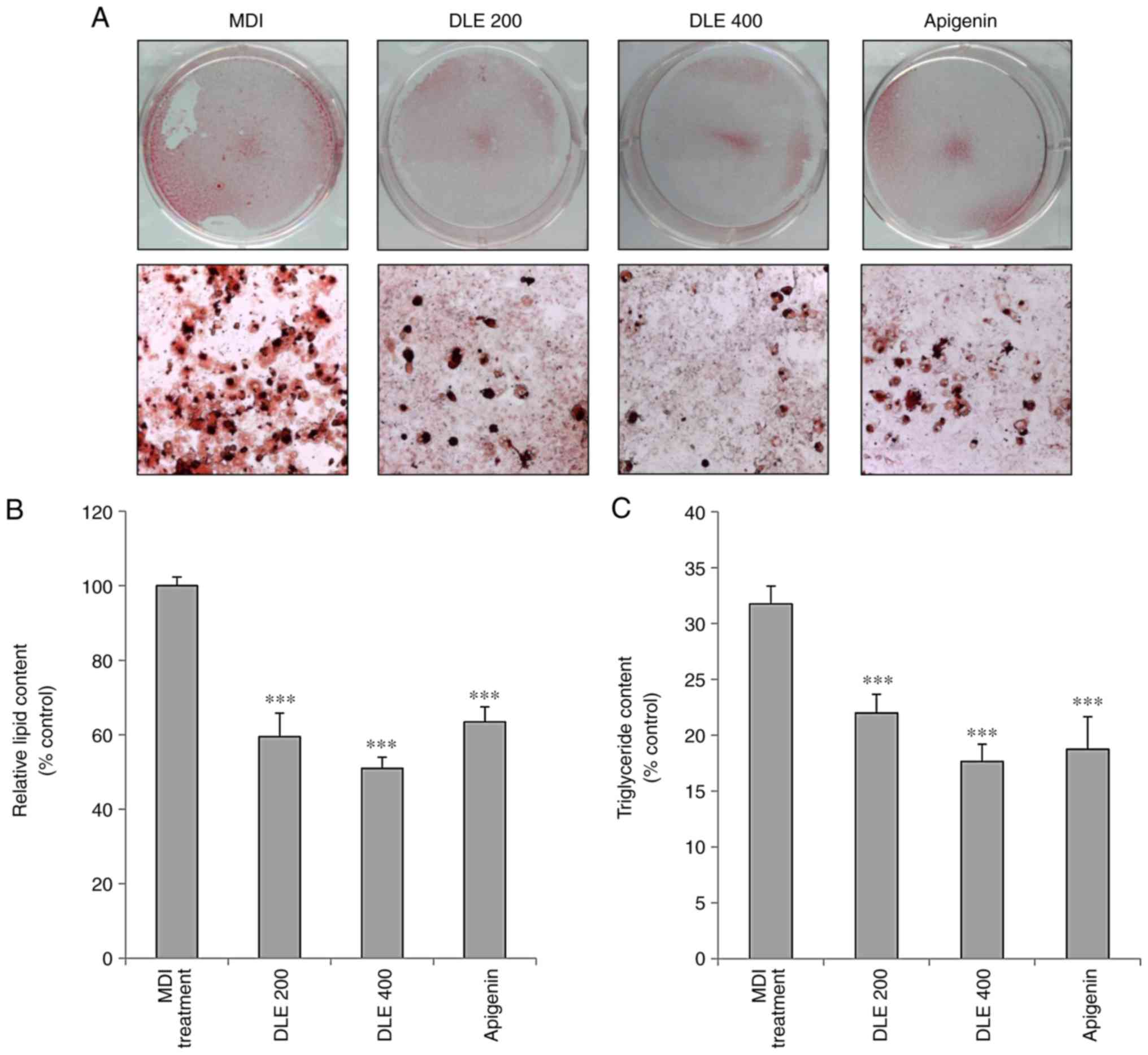
O staining method. As presented in Fig. 1A and B, DLE treatment
significantly inhibited lipid accumulation at the doses of 200 and
400 μg/ml in 3T3-L1 adipocytes. TG accumulation was also
examined in differentiated 3T3-L1 adipocytes. DLE treatment
significantly diminished the TG content in 3T3-L1 adipocytes
(Fig. 1C). Apigenin used as a
positive control demonstrated similar effects.
DLE reduces body weight, food intake,
visceral fat and liver weight in mice with HFD-induced obesity
To investigate the anti-obesity effect of DLE, mice
were fed with HFD in the presence or absence of DLE (200 and 400
mg/kg), and the body, liver and visceral fat weights of the mice
were measured. As presented in Table
I, the final body weight gain in the HFD model group was
significantly higher compared with that in the normal control
group. However, body weight gain was markedly decreased by DLE
administration at doses of 200 and 400 mg/kg, without significantly
altering the food intake. The FER (%) was significantly higher in
the HFD group as compared with that in the normal control group,
whereas this ratio was significantly reduced by oral administration
of DLE at doses of 200 and 400 mg/kg. Furthermore, the liver and
visceral fat weights were significantly increased in the HFD group,
while DLE administration at 200 and 400 mg/kg markedly decreased
these weights. Apigenin administration also suppressed the body
weight gain, FER, and liver and visceral fat weights in HFD-fed
mice.
 | Table IEffect of DLE on body weight, weight
gain, food intake, FER, liver weight and visceral fat weight in
HFD-induced obese mice. |
Table I
Effect of DLE on body weight, weight
gain, food intake, FER, liver weight and visceral fat weight in
HFD-induced obese mice.
| Group | Normal control | HFD | HFD + 200 mg/kg
DLE | HFD + 400 mg/kg
DLE | HFD + apigenin |
|---|
| Initial body weight
(g) | 29.83±0.99 | 39.53±2.49b | 39.63±1.88 | 39.33±5.77 | 38.54±2.08 |
| Final body weight
(g) | 31.13±1.00 | 47.13±1.43b | 44.50±2.25 | 42.95±5.37 | 43.42±1.69c |
| Weight gain
(g) | 1.30±0.34 | 7.60±1.17b | 4.88±0.48d | 3.63±1.90c | 4.88±1.31c |
| Food intake
(g/day) | 3.12±0.33 | 2.35±0.33b | 2.14±0.21 | 2.42±0.36 | 2.43±0.32 |
| FER (%) | 3.21±1.26 | 29.58±5.06b | 19.81±6.91c | 17.27±8.52c | 20.04±5.37c |
| Liver weight
(g) | 1.21±0.35 | 1.84±0.24a | 1.55±0.15 | 1.39±0.26 | 1.48±0.21 |
| Visceral fat weight
(g) | 0.38±0.09 | 2.11±0.15b | 1.46±0.39c | 1.34±0.26c | 1.84±0.37 |
Effect of DLE on serum lipid and glucose
levels
The effects of DLE on the serum lipid levels of
HFD-fed mice were investigated at the end of the experimental
period (Fig. 2A–E). Serum TG, TC,
LDL-C and LDL-C/HDL-C ratio levels in the HFD group were
significantly higher compared with those in the normal control
group (P<0.05). However, oral administration of DLE at doses of
200 and 400 mg/kg led to a decrease in the level of TG, and TC,
with a significant difference obtained only with the group treated
with 400 mg/kg of DLE (P<0.01). Also, the levels of LDL-C and
LDL-C/HDL-C ratio were significantly decreased with 200 and 400
mg/kg DLE treatment (P<0.05). Apigenin significantly reduced
these levels, with the exception of TC levels (Fig. 2A, B, D, and E). HDL-C levels were
markedly increased in the HFD group, while DLE and apigenin
administration slightly increased HDL-C levels (Fig. 2C). However, there was no
significant difference in the HDL-C levels between the HFD group
and the DLE or apigenin-treated groups. Furthermore, the serum
glucose levels were significantly increased in the HFD group
compared with the control. However, the glucose levels were
decreased in HFD-fed mice following treatment with 200 and 400
mg/kg DLE, with significant difference obtained only with 400 mg/kg
treatment group. Apigenin treatment demonstrated a similar effect
with DLE 400 mg/kg treatment (Fig.
2F).
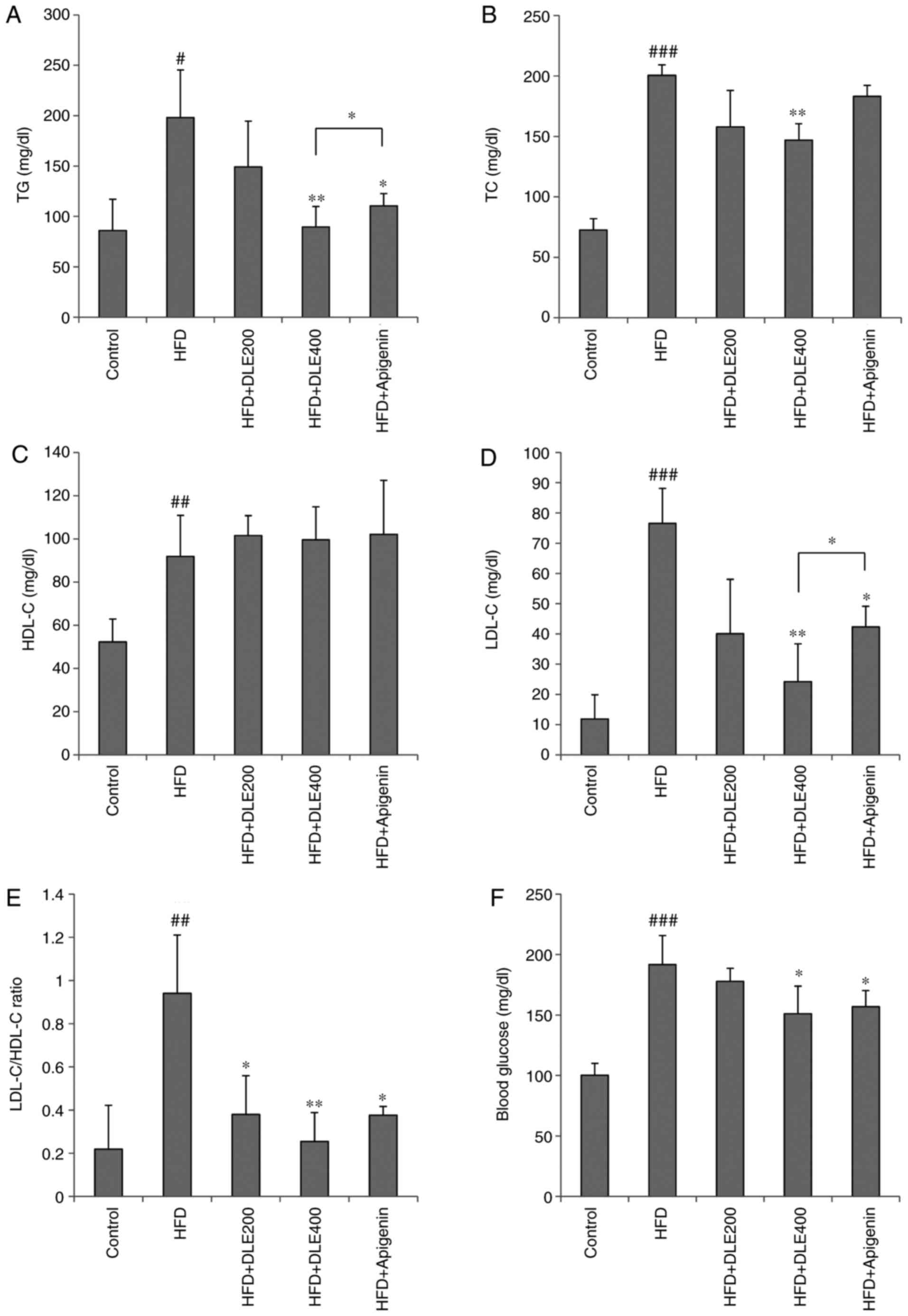 | Figure 2Effect of DLE on lipid profile and
glucose levels in the serum of mice with HFD-induced obesity. At
the end of the experimental period, serum lipid profiles and
glucose levels were measured. (A) TG, (B) TC, (C) HDL-C, (D) LDL-C,
(E) LDL-C/HDL-C ratio, and (F) glucose levels. Results are
presented as the mean ± standard deviation. #P<0.05,
##P<0.01, and ###P<0.001 vs. the
control group; *P<0.05 and **P<0.01 vs.
the HFD group. DLE, Diospyros lotus leaf water extract; HFD,
high-fat diet; TG, triglyceride; TC, total cholesterol; HDL-C,
high-density lipoprotein cholesterol; LDL-C, low-density
lipoprotein cholesterol. |
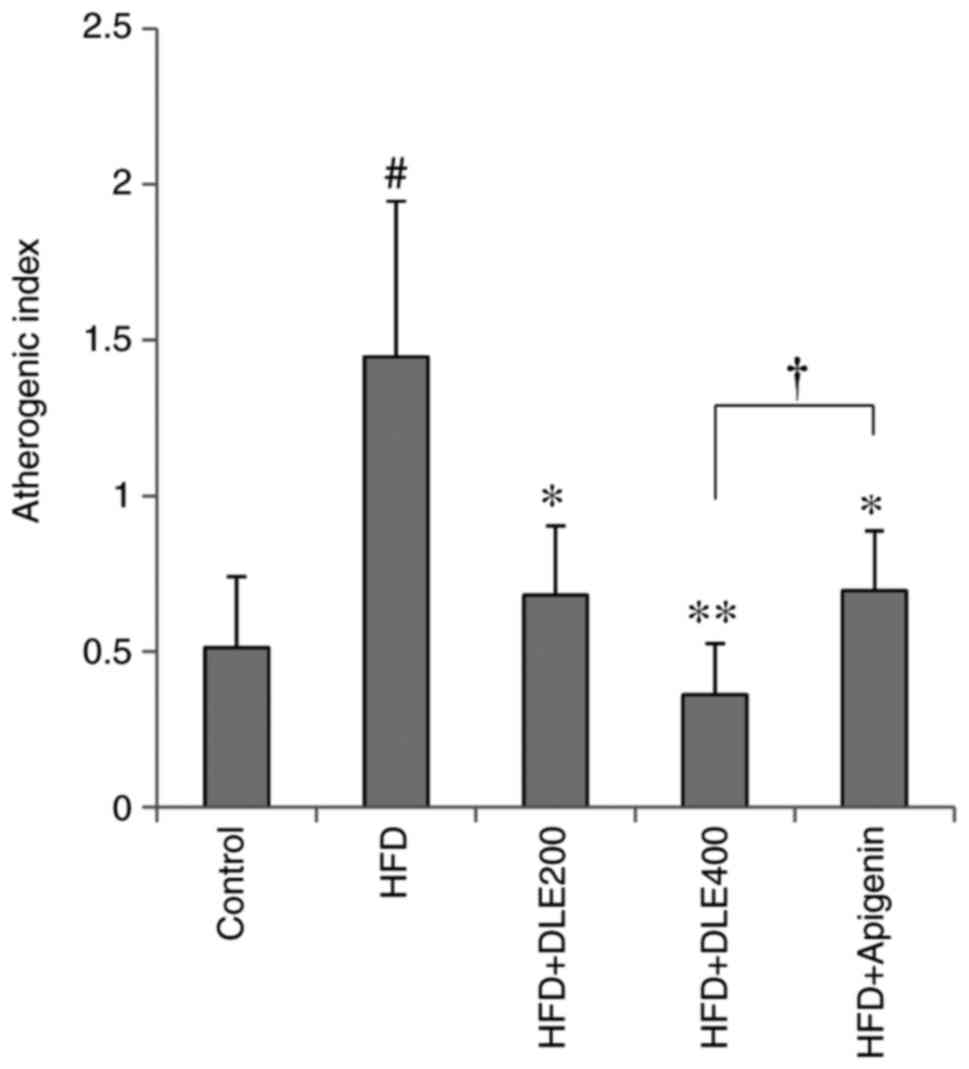
Effect of DLE on the atherogenic
index
The atherogenic index was determined using the
values of TC and HDL-C. As presented in Fig. 3, the atherogenic index was
signifi-cantly higher in the HFD group as compared with that in the
normal control group. However, this increase in the atherogenic
index was significantly reduced by oral administration of DLE at
doses of 200 and 400 mg/kg. Apigenin had a similar effect, although
DLE at the dose of 400 mg/kg significantly decreased the
atherogenic index in comparison with apigenin (P<0.05).
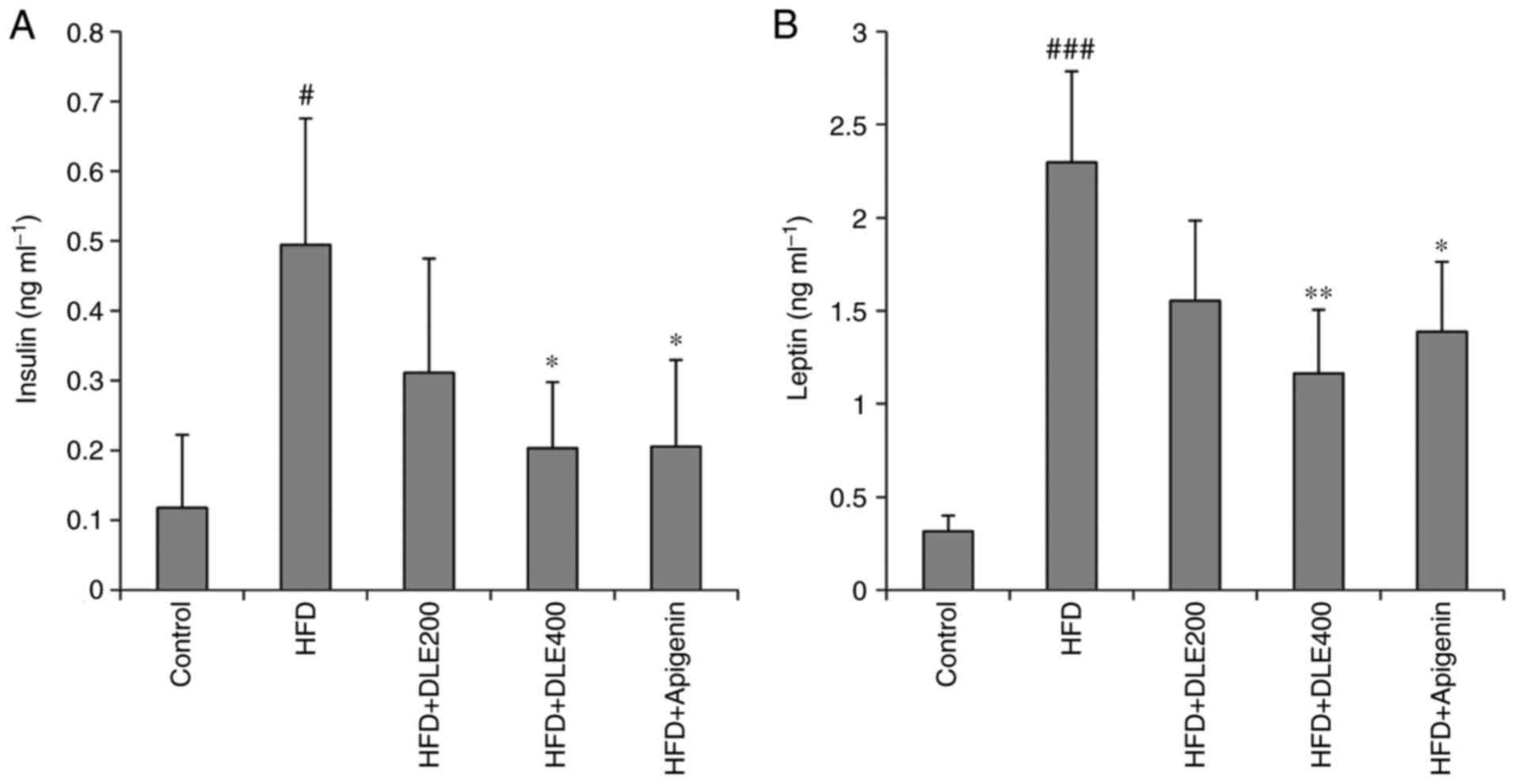
Effect of DLE on serum insulin and leptin
levels
The serum insulin and leptin levels were also
determined. As presented in Fig.
4A, serum insulin was increased significantly in the HFD group,
while oral administration of DLE at the doses of 200 and 400 mg/kg
decreased the serum insulin levels with a significant difference
obtained with the 400 mg/kg DLE-treated group (P<0.05). The
serum leptin levels were also significantly increased in the HFD
group (P>0.001), whereas they were decreased in the DLE-treated
group at doses of 200 and 400 mg/kg (Fig. 4B). However, only the 400 mg/kg
treated group exhibited a significant difference. Apigenin
administration also reduced the elevation of serum insulin and
leptin levels similar to the effect of DLE at both doses, in mice
fed with the high-fat diet.
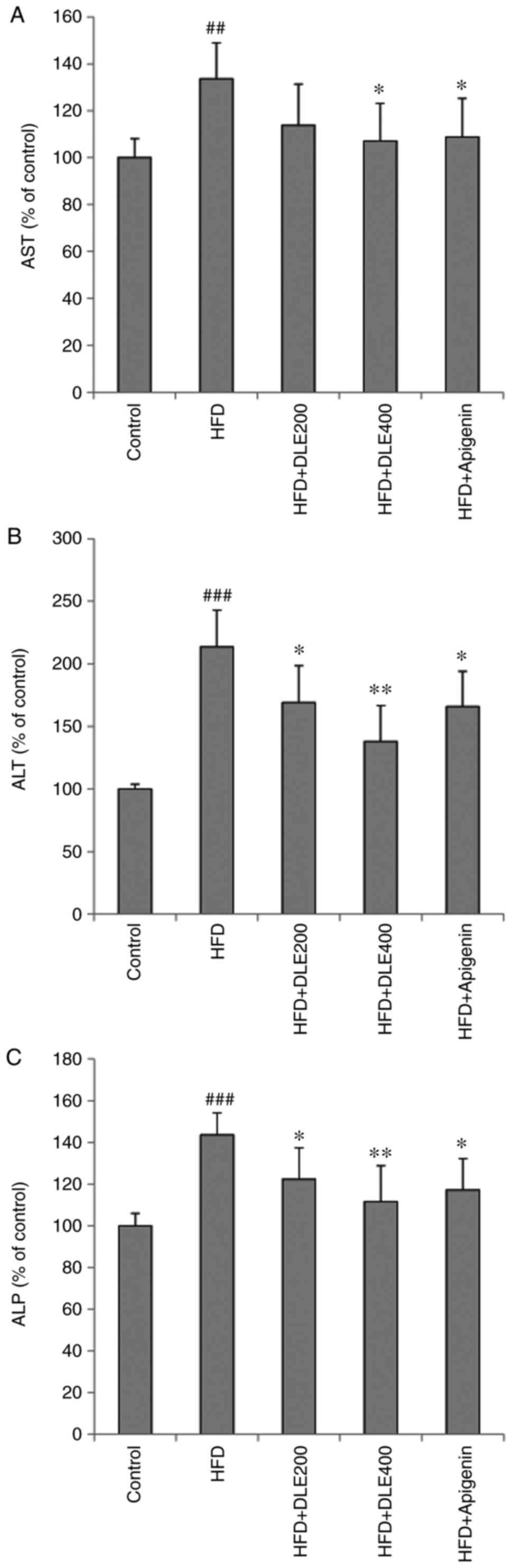
Effect of DLE on liver function
parameters
Changes in serum liver function parameters,
including AST, ALT and ALP levels, are indicated in Fig. 5. The levels of the liver function
parameters were significantly increased in the HFD group when
compared with those in the normal control group. However, DLE
administration at both doses, significantly decreased the ALT and
ALP levels in HFD-fed mice, except for AST levels which
demonstrated a significant difference only in the 400 mg/kg-treated
group. Similar to the DLE 200 and 400 mg/kg treated, apigenin
treatment also resulted in a reduction of these parameters in the
mice with HFD-induced obesity, with no significant differences
among the group.
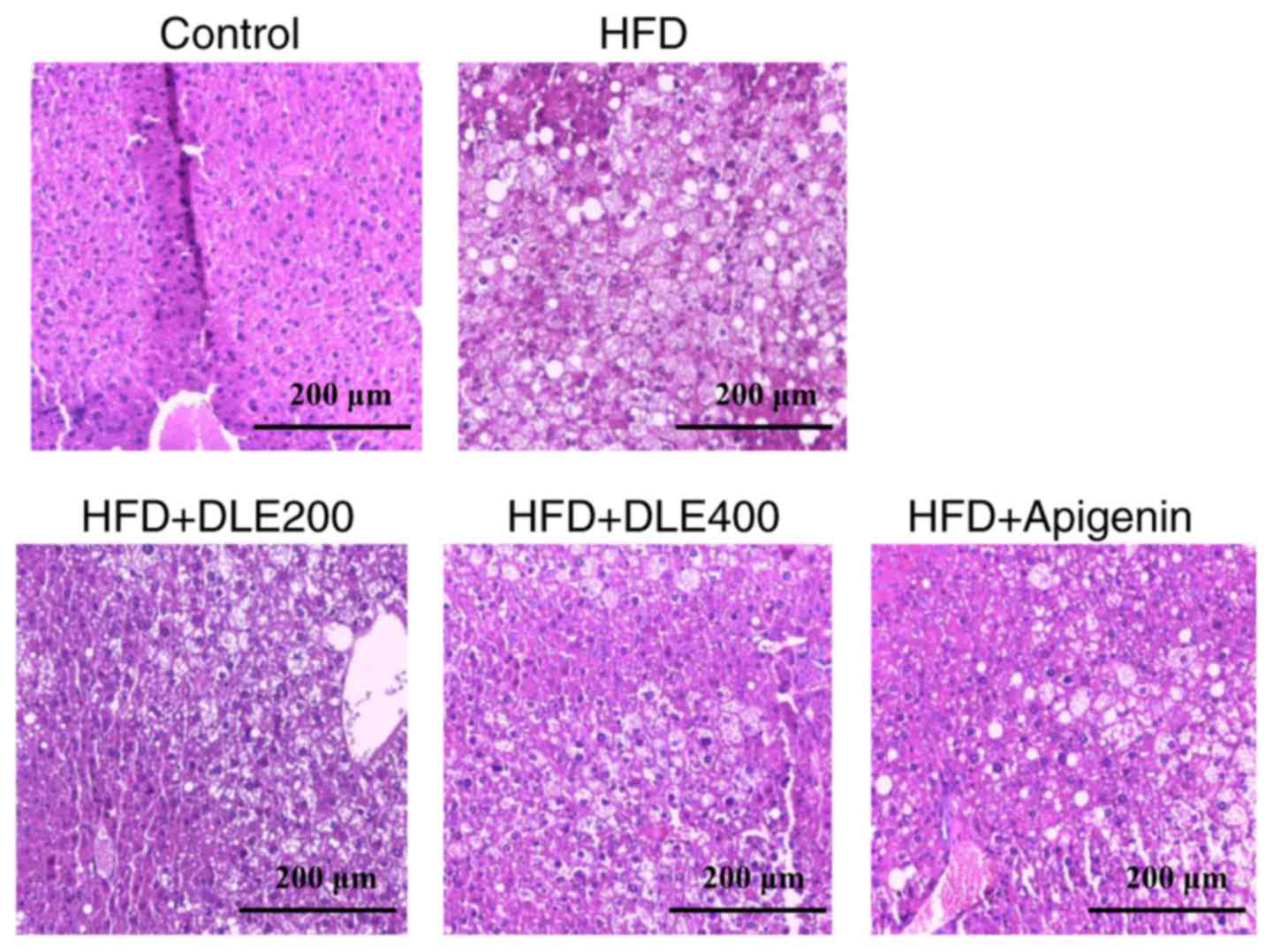
Effect of DLE on liver histology
Next, the study evaluated the effect of DLE on liver
morphology by H&E staining. As presented in Fig. 6, the lipid droplets were increased
significantly in the HFD group in comparison with those observed in
the normal control group. However, DLE at 400 mg/kg and apigenin
administration significantly decreased the lipid droplets in the
mice with HFD-induced obesity.
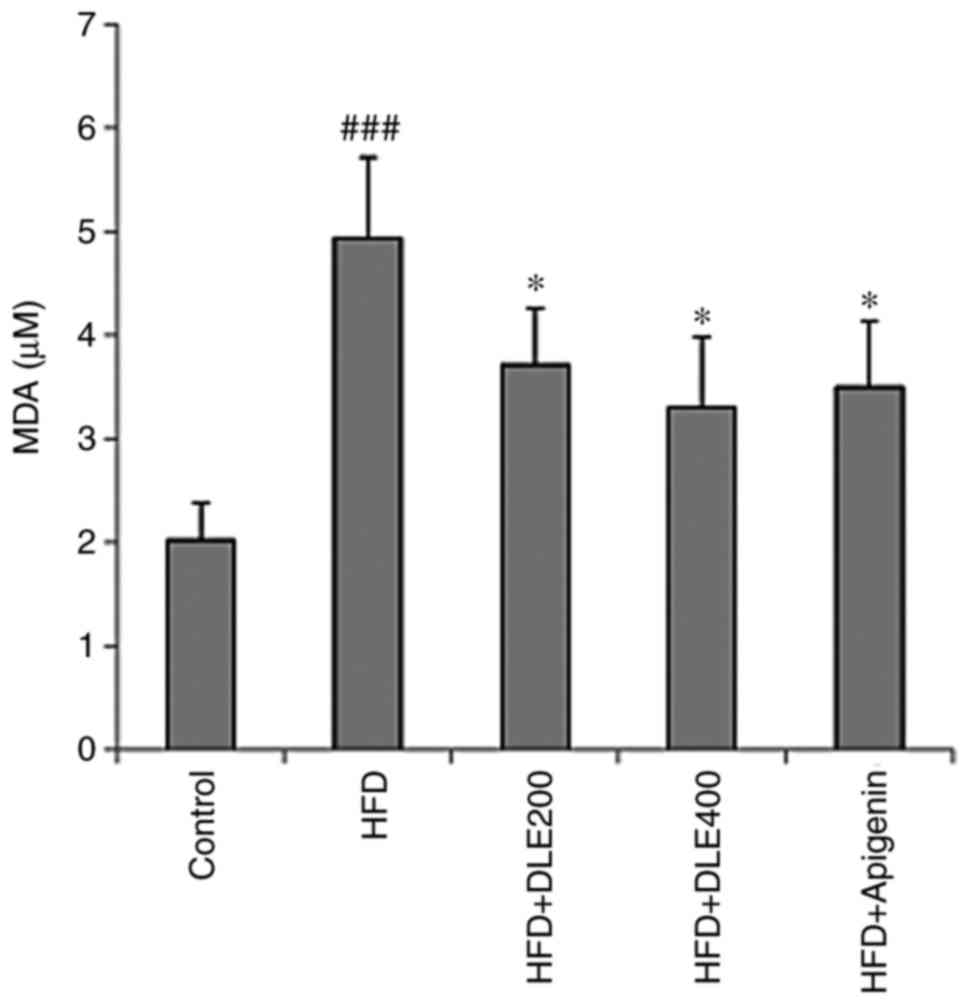
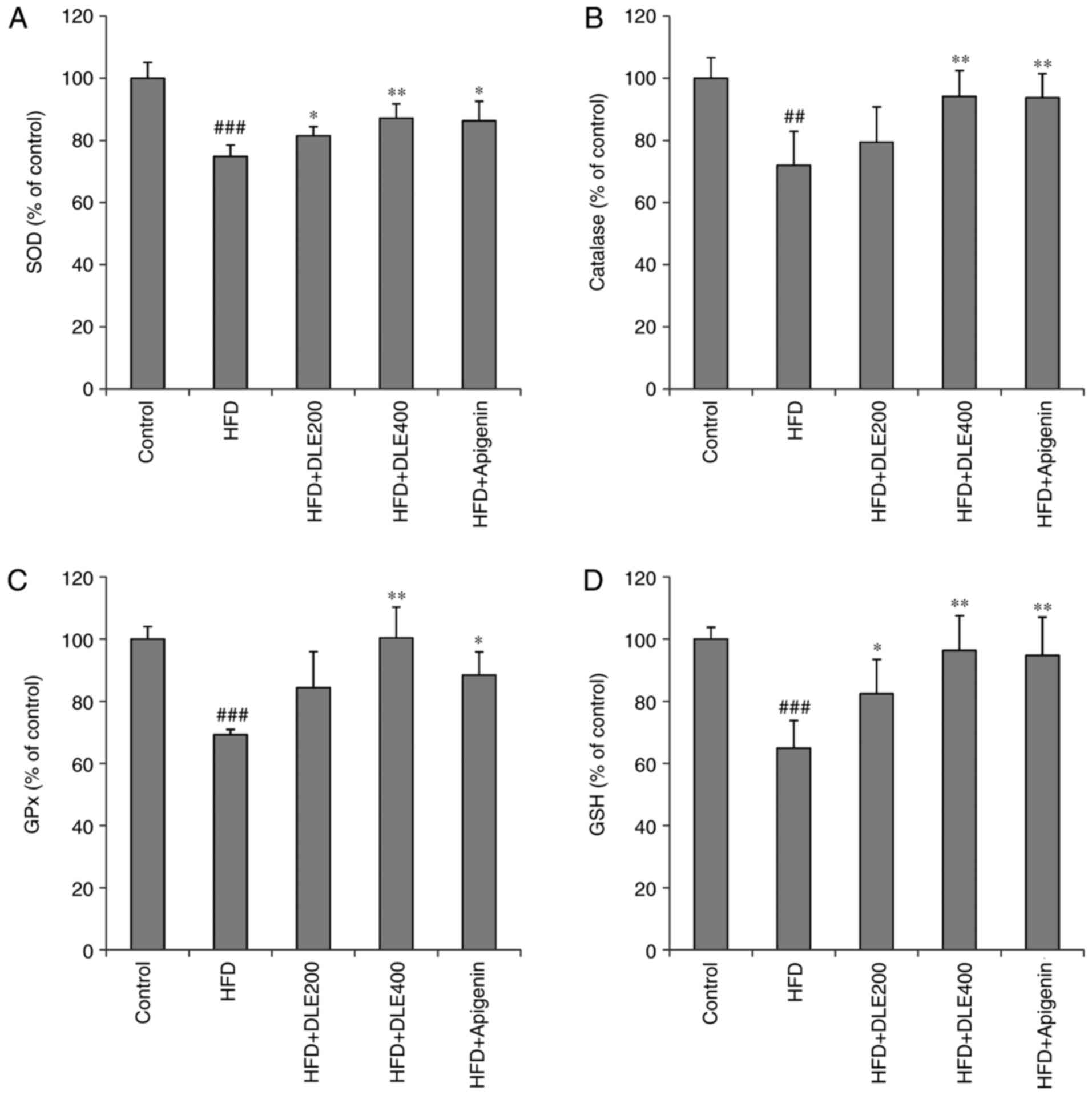
DLE treatment reduces oxidative stress in
HFD-induced obese mice
MDA is a known marker of lipid peroxidation involved
in oxidative stress. In the present study, hepatic MDA levels were
significantly increased in the HFD group. However, DLE at both
doses and apigenin administration significantly reduced the MDA
levels (Fig. 7). There was no
statistically significant difference between the DLE and apigenin
groups. In addition, the activities of antioxidant enzymes SOD, CAT
and GPx were markedly diminished, while the hepatic GSH antioxidant
was significantly reduced in the HFD group, compared with the
normal control group. However, DLE administration at doses of 200
and 400 mg/kg resulted in a significant increase in SOD and CAT
activities, while GPx activities and GSH levels were significantly
increased only with 400 mg/kg treatment, compared with the HFD
group levels. Apigenin administration demonstrated similar effects
as those observed by treatment with 400 mg/kg DLE (Fig. 8).
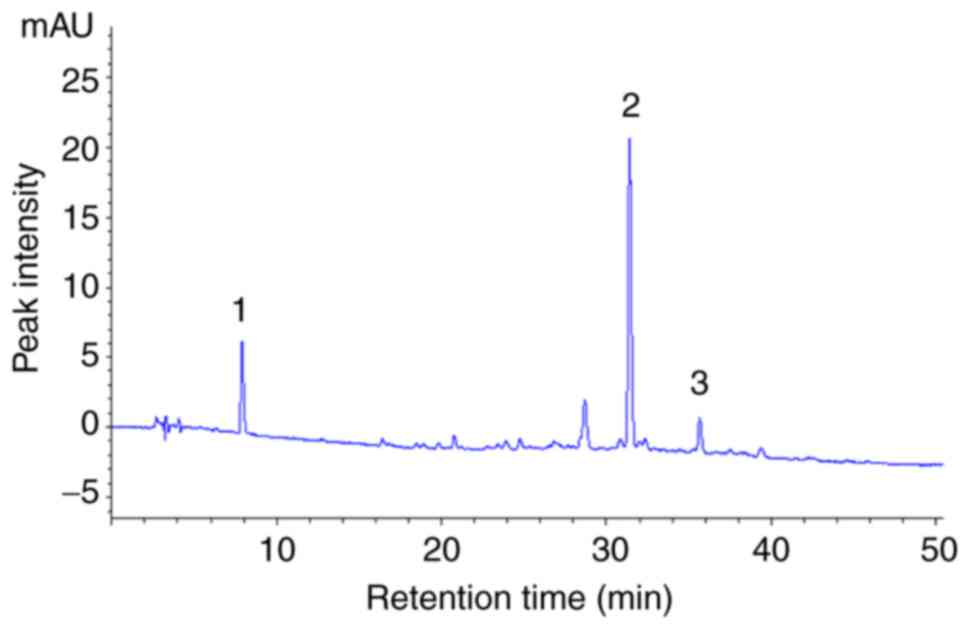
Chemical composition of DLE
HPLC analysis was performed to characterize the
active compounds in DLE. The results of the HPLC analysis revealed
that gallic acid (peak 1), myricitrin (peak 2) and astragalin (peak
3) were the active compounds in DLE (Fig. 9).
Discussion
Previous studies by Uddin et al (10), Rauf et al (11,12,14), and Loizzo et al (13) have verified various functions of
D. lotus, including its antioxidant, anti-inflammatory,
antiseptic, antidi-abetic, antitumor and sedative properties. In
the current study, in order to elucidate the potential of DLE as a
food ingredient for preventing obesity, we focused on the
regulation of adipo-genesis and lipogenesis in vitro and
in vivo. The in vitro results revealed that DLE
treatment ameliorated lipid and TG accumulation in 3T3-L1
adipocytes, indicating that DLE was able to inhibit adipocyte
differentiation. Next, the present study demonstrated the
anti-obesity effects of DLE in HFD-induced obesity in mice. The
results indicated that DLE inhibited HFD-induced obesity by
preventing the increase of body weight and visceral fat weight, the
alteration of lipid profile and liver function parameters, and the
increase in insulin and leptin levels, as well as by improving the
antioxidant defense system. According to the findings of a previous
study (18), apigenin was used as
a positive control, and it was also able to reverse these
conditions in mice with HFD-induced obesity.
Obesity is associated with numerous diseases,
including atherosclerosis, cancer, type 2 diabetes, dyslipidemia
and metabolic syndrome (3).
Several studies have demonstrated that HFD causes elevation of body
weight, liver weight and fat mass, as well as increased TG, TC,
HDL-C, LDL-C and glucose levels in the serum (19-21). In the present study, DLE clearly
decreased body weight gain, liver weight, visceral fat accumulation
and FER levels, while it also reduced serum TG, TC, LDL-C, and
glucose levels, with the exception of HDL-C levels. TG, TC, and
LDL-C are associated with fat accumulation and are major risk
factors for dyslipidemia (22).
The ratio of LDL-C to HDL-C is associated with cardiovascular
disease and dyslipidemia (19,23). The TC/HDL-C and TG/HDL-C molar
ratios are associated with coronary heart disease (24). Furthermore, the atherogenic index
is used as a predictor of atherosclerosis and coronary artery
disease (25,26). The increase in cholesterol is
associated with the risk of fatty liver and atherosclerosis
(5). The results of the current
study indicated that these levels were lower in the DLE-treated
mice in comparison with the untreated HFD-fed group. Therefore, the
presented results imply that DLE was able to decrease the levels of
the lipid profile, and ameliorate cardiovascular disease,
atherosclerosis and dyslipidemia in HFD-induced obesity.
Obesity is also known to cause insulin resistance,
which serves a key role in type 2 diabetes. Previous studies have
indicated that in mice fed with HFD, an increase in fasting blood
glucose and insulin levels was induced, while plant extracts were
demonstrated to suppress the HFD-induced increase in these levels
in obese animals (27-29). In the present study, the HFD also
led to an increase in serum glucose and insulin levels and this
increase in glucose and insulin levels were markedly decreased when
mice fed with the HFD were administered DLE.
Leptin is secreted by adipocytes and is a hormone
involved in energy balance and in the regulation of glucose
metabolism. It serves a role in reducing appetite and increasing
energy consumption (30,31). It has been reported that leptin
levels in the serum are increased in HFD-induced obese mice
(32,33), causing leptin resistance (34). In the present study, it was proven
that DLE reduced serum leptin levels in HFD-induced obese mice.
Therefore, the results suggested that DLE may ameliorate insulin
and leptin resistance induced by HFD in obese mice.
Serum AST, ALT and ALP levels have been widely used
as major markers of liver damage. It has been reported that HFD
administration increased serum AST, ALT and ALP levels in obese
animals (35-37). To assess liver function in the
current study, these markers were measured in the serum of mice
with HFD-induced obesity. The results revealed that DLE
administration reduced the levels of AST, ALT and ALP, suggesting
that DLE attenuated the liver damage caused by HFD. The author’s
previous study has also demonstrated similar activities in
acetaminophen-induced acute liver damage in mice (38).
It has been reported that obesity also induces
hepatic steatosis, characterized by excessive fat accumulation in
the liver, and exhibits liver cell injury caused by oxidative
stress (27,37). The data of the current study
indicated that DLE diminished hepatic steatosis caused by HFD,
corresponding with the reduction of liver parameters. Furthermore,
HFD-induced obesity is known to cause oxidative damage through
lipid peroxidation, the depletion of endogenous antioxidants and
the reduction of the activities of antioxidant enzymes, such as
SOD, CAT and GPx (19,39,40). In the present study, HFD feeding
of mice resulted in oxidative damage as seen by the reduction of
the major endogenous antioxidant GSH, the decrease in SOD, CAT and
GPx activities, and the induction of lipid peroxidation products.
However, DLE administration not only significantly prevented lipid
peroxidation, but also recovered GSH levels, and SOD, CAT and GPx
activities in the liver. These results suggest that DLE may
ameliorate HFD-induced hepatic steatosis through inhibition of
oxidative stress. In previous studies, plant extracts have been
reported to protect against obesity-induced oxidative damage in
mice (41,42). Our previous study also
demonstrated that DLE protects against UVB-induced oxidative damage
and acetaminophen-induced oxidative liver damage in mice (38,43). Therefore, it can be suggested that
DLE may be a potent antioxidant used for preventing the oxidative
stress associated with HFD-induced obesity.
HPLC analysis revealed that DLE contains myricitrin,
gallic acid and astragalin. In a previous study, we reported that
ethanol extracts of D. lotus leaf contained myricitrin as
the main flavonoid compound (43). It has been demonstrated that plant
extracts containing myricitrin exhibit anti-obesity effects in
HFD-induced obese mice (4). A
recent study has demonstrated that myricitrin protects against
diabetic cardiomyopathy in mice (44). In addition, oral administration of
gallic acid in HFD-induced obese mice ameliorated impaired glucose
and lipid homeostasis (45). A
previous study has also reported that astragalin stimulate
lipolysis in the visceral adipose tissue of mice (46). Therefore, the anti-obesity effect
of DLE may be attributed to myricitrin, gallic acid and astragalin
flavonoids. However, this needs to be confirmed by further
studies.
In conclusion, the present study demonstrated that
DLE administration restored the body, liver and visceral fat
weights, as well as the TG, TC, HDL-C, LDL-C, glucose, insulin,
leptin, AST, ALP and ALP levels in mice with HFD-induced obesity.
In addition, it was also revealed that DLE was able to reverse the
obesity-induced the hepatic steatosis, lipid peroxidation, and GSH
depletion, as well as activities of antioxidant enzymes, including
SOD, CAT, and GPx in the liver of obese mice. These data suggest
that DLE may be an effective ingredient for the treatment and
prevention of HFD-induced obesity.
Acknowledgments
The authors would like to thank Mr. Denis Nchang Che
(Department of Food Science and Technology, Chonbuk National
University, Jeonju, Republic of Korea) for grammar editing and Mr.
Jae Young Shin (Department of Health Management, Jeonju University,
Jeonju, Republic of Korea) for animal care.
Funding
The present study was financially supported by the
Ministry of SMEs and Startups, Korea, under the ‘Regional
Specialized Industry Development Program (R&D, Project number
R0006168)’ supervised by the Korea Institute for Advancement of
Technology.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
BMK, BOC and SIJ designed the research. BMK and BOC
performed the study. BMK, BOC and SIJ analyzed the data and wrote
the manuscript. SIJ supervised the research project. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocols were performed following
approval from Jeonju University Institutional Animal Care and Use
Committee (no. JJU-IACUC-2015-04).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Popkin BM and Doak CM: The obesity
epidemic is a worldwide phenomenon. Nutr Rev. 56:106–114. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen XM, Lane J, Smith BR and Nguyen NT:
Changes in inflammatory biomarkers across weight classes in a
representative US population: A link between obesity and
inflammation. J Gastrointest Surg. 13:1205–1212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shore SA: Obesity and asthma: Implications
for treatment. Curr Opin Pulm Med. 13:56–62. 2007. View Article : Google Scholar
|
|
4
|
White PA, Cercato LM, Batista VS, Camargo
EA, De Lucca W Jr, Oliveira AS, Silva FT, Goes TC, Oliveira ER,
Moraes VR, et al: Aqueous extract of Chrysobalanus icaco leaves, in
lower doses, prevent fat gain in obese high-fat fed mice. J
Ethnopharmacol. 179:92–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang TW, Chang CL, Kao ES and Lin JH:
Effect of Hibiscus sabdariffa extract on high fat diet-induced
obesity and liver damage in hamsters. Food Nutr Res. 59:290182015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li KK, Liu CL, Shiu HT, Wong HL, Siu WS,
Zhang C, Han XQ, Ye CX, Leung PC and Ko CH: Cocoa tea (Camellia
ptilophylla) water extract inhibits adipocyte differentiation in
mouse 3T3-L1 preadipocytes. Sci Rep. 6:201722016. View Article : Google Scholar :
|
|
7
|
Noh JR, Kim YH, Hwang JH, Gang GT, Yeo SH,
Kim KS, Oh WK, Ly SY, Lee IK and Lee CH: Scoparone inhibits
adipo-cyte differentiation through down-regulation of peroxisome
proliferators-activated receptor γ in 3T3-L1 preadipocytes. Food
Chem. 141:723–730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ku HC, Liu HS, Hung PF, Chen CL, Liu HC,
Chang HH, Tsuei YW, Shih LJ, Lin CL, Lin CM and Kao YH: Green tea
(-)-epigallocatechin gallate inhibits IGF-I and IGF-II stimulation
of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor,
but not AMP-activated protein kinase pathway. Mol Nutr Food Res.
56:580–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sethi A: A review on Garcinia cambogia-a
weight controlling agent. Int J Pharm Res Dev. 3:13–24. 2011.
|
|
10
|
Uddin G, Rauf A, Siddiqui BS and Shah SQ:
Preliminary comparative phytochemical screening of Diospyros lotus
Stewart. Middle-East J Sci Res. 10:78–81. 2011.
|
|
11
|
Rauf A, Uddin G, Siddiqui BS, Molnár J,
Csonka Á, Ahmad B, Szabó D, Farooq U and Khan A: A rare class of
new dimeric naphthoquinones from Diospyros lotus have multidrug
reversal and antiproliferative effects. Front Pharmacol. 6:2932015.
View Article : Google Scholar
|
|
12
|
Rauf A, Shaheen U, Raza M, Uddin G, Hadda
TB, Mabkhot YN, Jehan N, Ahmad B, Raza S, Molnar J, et al:
Multidrug resistance reversal activity of extract and a rare
dimeric naphthoquinone from Diospyros lotus. Pak J Pharm Sci.
31:821–825. 2018.PubMed/NCBI
|
|
13
|
Loizzo MR, Said A, Tundis R, Hawas UW,
Rashed K and Menichini F, Frega NG and Menichini F: Antioxidant and
anti-proliferative activity of Diospyros lotus L. extract and
isolated compounds. Plant Foods Hum Nutr. 64:264–270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rauf A, Uddin G, Siddiqui BS, Muhammad N
and Khan H: Antipyretic and antinociceptive activity of Diospyros
lotus L. in animals. Asian Pac J Trop Biomed. 4(Suppl 1):
S382–S386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung UJ, Park YB, Kim SR and Choi MS:
Supplementation of persimmon leaf ameliorates hyperglycemia,
dyslipidemia and hepatic fat accumulation in type 2 diabetic mice.
PLoS One. 7:e490302012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim GN, Shin MR, Shin SH, Lee AR, Lee JY,
Seo BI, Kim MY, Kim TH, Noh JS, Rhee MH and Roh SS: Study of
antiobesity effect through inhibition of pancreatic lipase activity
of Diospyros kaki fruit and Citrus unshiu peel. Biomed Res Int.
2016:17230422016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
18
|
Jung UJ, Cho YY and Choi MS: Apigenin
ameliorates dyslipidemia, hepatic steatosis and insulin resistance
by modulating metabolic and transcriptional profiles in the liver
of high-fat diet-induced obese mice. Nutrients. 8:E3052016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El Ayed M, Kadri S, Smine S, Elkahoui S,
Limam F and Aouani E: Protective effects of grape seed and skin
extract against high-fat-diet-induced lipotoxicity in rat lung.
Lipids Health Dis. 16:1742017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sunil V, Shree N, Venkataranganna MV,
Bhonde RR and Majumdar M: The anti diabetic and anti obesity effect
of Memecylon umbellatum extract in high fat diet induced obese
mice. Biomed Pharmacother. 89:880–886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rahman HA, Sahib NG, Saari N, Abas F,
Ismail A, Mumtaz MW and Hamid AA: Anti-obesity effect of ethanolic
extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat
diet. BMC Complement Altern Med. 17:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hokanson JE and Austin MA: Plasma
triglyceride level is a risk factor for cardiovascular disease
independent of high-density lipoprotein cholesterol level: A
meta-analysis of population-based prospective studies. J Cardiovasc
Risk. 3:213–219. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jukema JW, Liem AH, Dunselman PH, van der
Sloot JA, Lok DJ and Zwinderman AH: LDL-C/HDL-C ratio in subjects
with cardiovascular disease and a low HDL-C: Results of the RADAR
(Rosuvastatin and Atorvastatin in different Dosages and Reverse
cholesterol transport) study. Curr Med Res Opin. 21:1865–1874.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan MH, Johns D and Glazer NB:
Pioglitazone reduces athero-genic index of plasma in patients with
type 2 diabetes. Clin Chem. 50:1184–1188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai G, Shi G, Xue S and Lu W: The
atherogenic index of plasma is a strong and independent predictor
for coronary artery disease in the Chinese Han population. Medicine
(Baltimore). 96:e80582017. View Article : Google Scholar
|
|
26
|
Nikniaz Z, Mahdavi R, Nikniaz L, Ebrahimi
A and Ostadrahimi A: Effects of Elaeagnus angustifolia L. on lipid
profile and atherogenic indices in obese females: A randomized
controlled clinical trial. J Diet Suppl. 13:595–606. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim YJ, Choi JY, Ryu R, Lee J, Cho SJ,
Kwon EY, Lee MK, Liu KH, Rina Y, Sung MK and Choi MS: Platycodon
grandi-florus root extract attenuates body fat mass, hepatic
steatosis and insulin resistance through the interplay between the
liver and adipose tissue. Nutrients. 8:E5322016. View Article : Google Scholar
|
|
28
|
Sabater AG, Ribot J, Priego T, Vazquez I,
Frank S, Palou A and Buchwald-Werner S: Consumption of a mango
fruit powder protects mice from high-fat induced insulin resistance
and hepatic fat accumulation. Cell Physiol Biochem. 42:564–578.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leng J, Chen MH, Zhou ZH, Lu YW, Wen XD
and Yang J: Triterpenoids-enriched extract from the aerial parts of
Salvia miltiorrhiza regulates macrophage polarization and
ameliorates insulin resistance in high-fat fed mice. Phytother Res.
31:100–107. 2017. View Article : Google Scholar
|
|
30
|
Myers MG, Cowley MA and Münzberg H:
Mechanisms of leptin action and leptin resistance. Annu Rev
Physiol. 70:537–556. 2008. View Article : Google Scholar
|
|
31
|
Gilbert ER, Fu Z and Liu D: Development of
a nongenetic mouse model of type 2 diabetes. Exp Diabetes Res.
2011:4162542011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung YY, Kim DS, Kim SH and Kim HK:
Anti-obesity activity, acute toxicity, and chemical constituents of
aqueous and ethanol Viola mandshurica extracts. BMC Complement
Altern Med. 17:2972017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim Y, Kwon MJ, Choi JW, Lee MK, Kim C,
Jung J, Aprianita H, Nam H and Nam TJ: Anti-obesity effects of
boiled tuna extract in mice with obesity induced by a high-fat
diet. Int J Mol Med. 38:1281–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Myers MG Jr, Leibel RL, Seeley RJ and
Schwartz MW: Obesity and leptin resistance: Distinguishing cause
from effect. Trends Endocrinol Metab. 21:643–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parasuraman S, Zhen KM, Banik U and
Christapher PV: Ameliorative effect of curcumin on
olanzapine-induced obesity in sprague-dawley rats. Pharmacognosy
Res. 9:247–252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou X, Chen S and Ye X: The anti-obesity
properties of the proanthocyanidin extract from the leaves of
Chinese bayberry (Myrica rubra Sieb. et Zucc). Food Funct.
8:3259–3270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Seo M, Goo TW, Chung MY, Baek M, Hwang JS,
Kim MA and Yun EY: Tenebrio molitor larvae inhibit adipogenesis
through AMPK and MAPKs signaling in 3T3-L1 adipocytes and obesity
in high-fat diet-induced obese mice. Int J Mol Sci. 18:E5182017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cho BO, Yin HH, Fang CZ, Kim SJ, Jeong SI
and Jang SI: Hepatoprotective effect of Diospyros lotus leaf
extract against acetaminophen-induced acute liver injury in mice.
Food Sci Biotechnol. 24:2205–2212. 2015. View Article : Google Scholar
|
|
39
|
Annamalai S, Mohanam L, Raja V, Dev A and
Prabhu V: Antiobesity, antioxidant and hepatoprotective effects of
Diallyl trisulphide (DATS) alone or in combination with Orlistat on
HFD induced obese rats. Biomed Pharmacother. 93:81–87. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agil R, Patterson ZR, Mackay H, Abizaid A
and Hosseinian F: Triticale bran alkylresorcinols enhance
resistance to oxidative stress in mice fed a high-fat diet. Foods.
5:E52016. View Article : Google Scholar
|
|
41
|
Perez Gutierrez RM, Madrigales Ahuatzi D,
Horcacitas Mdel C, Garcia Baez E, Cruz Victoria T and Mota-Flores
JM: Ameliorative effect of hexane extract of Phalaris canariensis
on high fat diet-induced obese and streptozotocin-induced diabetic
mice. Evid Based Complement Alternat Med. 2014:1459012014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Veeramani C, Alsaif MA and Al-Numair KS:
Lavatera critica, a green leafy vegetable, controls high fat diet
induced hepatic lipid accumulation and oxidative stress through the
regulation of lipogenesis and lipolysis genes. Biomed Pharmacother.
96:1349–1357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cho BO, Che DN, Shin JY, Kang HJ, Kim JH,
Kim HY, Cho WG and Jang SI: Ameliorative effects of Diospyros lotus
leaf extract against UVB-induced skin damage in BALB/c mice. Biomed
Pharmacother. 95:264–274. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang B, Shen Q, Chen Y, Pan R, Kuang S,
Liu G, Sun G and Sun X: Myricitrin alleviates oxidative
stress-induced inflammation and apoptosis and protects mice against
diabetic cardiomyopathy. Sci Rep. 7:442392017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chao J, Huo TI, Cheng HY, Tsai JC, Liao
JW, Lee MS, Qin XM, Hsieh MT, Pao LH and Peng WH: Gallic acid
ameliorated impaired glucose and lipid homeostasis in high fat
diet-induced NAFLD mice. PLoS One. 9:e969692014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ohkoshi E, Miyazaki H, Shindo K, Watanabe
H, Yoshida A and Yajima H: Constituents from the leaves of Nelumbo
nucifera stimulate lipolysis in the white adipose tissue of mice.
Planta Med. 73:1255–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|