Introduction
The allo-transplantation of vascularized composite
tissue (VCA) has been increasingly used in recent years to
reconstruct tissues following amputation or severe injury (1,2).
However, the immunosuppressive agents that are administered
post-transplantation may cause life-threatening complications,
including nephrotoxicity, opportunistic infections and
tumorigenesis (3). The cumulative
side effects of chronic immunosuppression outweigh the benefits of
VCA. In the last decade, cellular therapy has attracted increased
attention in the field of allo-transplantation. Mesenchymal stem
cells (MSCs), particularly adipose-derived stem cells (ADSCs), can
regulate the host immune response to allografts and, in addition to
immunosuppressants, improve graft survival (4,5).
The combination therapy of MSCs and
immunosuppres-sants induces immune tolerance to VCA, indicating
that a cellular component can reduce the dependency on
immuno-suppressants in maintaining allografts. However, cell-based
therapy also has safety concerns, including the increased risk of
infection during ex vivo expansion and cell aggregation
during systematic infusion (6,7).
Therefore, it is essential to understand the interactions between
ADSCs and host immune cells in order to improve the outcomes of
cellular therapy in allo-transplantation.
ADSCs secrete immunomodulatory cytokines, including
prostaglandin E2 (PGE-2), which inhibit the proliferation of
peripheral blood mononuclear cells (PBMCs) in a mixed lymphocyte
reaction (8), and express higher
levels of cyclooxygenase-2 (COX-2) and indoleamine-2,3- dioxygenase
when co-cultured with lymphocytes or pro-inflammatory cytokines
(9). In addition, ADSCs and other
MSCs regulate the function of T cells, the major driver of
allo-rejection, and dendritic cells and macrophages during allo-
transplantation (10,11).
The studies performed so far on the mechanisms of
ADSC-mediated immunosuppression have not analyzed the molecular
changes induced by ADSCs in lymphocytes. The aim of the present
study was to determine the effect of ADSCs on T cells; to this end,
ADSCs were isolated from adipose tissues and their interaction with
the human Jurkat T cell line was investigated.
Materials and methods
Isolation and expansion of ADSCs, and
co-culture with Jurkat cells
The human ADSCs were cultured as described
previously (12). Briefly,
adipose tissue was obtained by liposuction of the abdominal wall
from three different donors (samples 1, 2 and 3; females aged 36,
54 and 56 years; Shanghai 9th People's Hospital, Shanghai, China),
who had provided informed consent. The tissues were digested in
0.01% collagenase IV (Roche Diagnostics GmbH, Mannheim, Germany)
for 1 h, washed twice with PBS, and seeded in 10-cm culture dishes
at the density of 1x105 cells/ml with low-glucose
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; ScienCell Research Laboratories, Inc., San
Diego, CA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
cells were cultured at 37˚C under 5% CO2 until they
reached 80-90% confluence, following which they were dissociated
with 0.05% Trypsin-EDTA and passaged. The cells of passages 2-5
were combined, and used for further characterization and in
vitro differentiation. The ADSCs were identified by immune-
detection of surface CD29 (1:100, cat. no. B195249), CD44 (1:100,
cat. no. B162932), CD90 (1:100, cat. no. B205317), CD34 (1:100,
cat. no. B203565) and CD45 (1:100, cat. no. B215193) (all
BioLegend, Inc., San Diego, CA, USA). The cells were stained with
the labeled antibodies for 15 min in the dark at 4˚C and analyzed
using the BD FACSCalibur flow cytom-eter (BD Biosciences, San Jose,
CA, USA). Adipogenesis, osteogenesis and chondrogenesis were
induced by suitable differentiation media (human adipose-derived
stem cell adipogenic differentiation medium, HUXMD-90031; human
adipose-derived stem cell osteogenic differentiation medium,
HUXMD-90021; human adipose-derived stem cell chondro-genic
differentiation medium, HUXMD-9004; all Cyagen Bioscience, Inc.,
Guangzhou, China) at 37˚C under 5% CO2 for >28 days,
and the ensuing differentiated cells were identified by staining
with oil red, alizarin red and alcian blue, respectively. Images
were captured using an inverted microscope (Leica Microsystems
GmbH, Wetzlar, Germany).
The Jurkat cells (purchased from GENE, Inc.,
Shanghai, China) were suspended in RPMI 1640 medium (HyClone; GE
Healthcare, Logan, UT, USA) with 10% FBS, 100 U/ml penicillin and
100 mg/ml streptomycin, and seeded in 100-mm dishes at the density
of 1x106 cells each. The culture medium was replaced
every second day. The ADSCs and Jurkat cells were co-cultured for
subsequent experiments in the same media in a 0.4-µm Transwell
system (Corning Incorporated, Corning, NY, USA), wherein the ADSCs
were seeded in the upper chamber and Jurkat cells in the lower
chamber at the ratio of 1:5. The Jurkat cells were treated with 40
µM of the JNK inhibitor SP600125 (Selleck Chemicals, Houston, TX,
USA) or DMSO (1 µl/ml cell suspension) for 30 min at 37°C per the
requirements of the experiment.
Proliferation, cell cycle and
apoptosis assays
The effect of the ADSCs on Jurkat cell proliferation
was measured using a CCK-8 (Doijndo Molecular Technologies, Inc.,
Kumamoto, Japan) assay according to the manufacturer's protocol.
The Jurkat cells were seeded into the lower chamber of a 24-well
Transwell plate at a density of 1×105 cells/ml per well
in 600 µl medium. The upper chambers were filled with either ADSC
suspension or sterile culture medium (control). The cells were
cultured for 1, 3, or 5 days, and then incubated with 60 µl CCK-8
per well at 37°C for 3 h. The supernatants were collected and the
absorbance was measured at 450 nm with a microplate reader
(Molecular Devices LLC, Sunnyvale, CA, USA). Each test sample was
assayed in triplicate and the experiment was repeated three
times.
For cell cycle profiling, the Jurkat cells were
harvested following 24 h of mono- or co-culture, and fixed
overnight with 70% ethanol. The cells were stained with the
reagents provided in a Cell Cycle kit (Qihai Biotechnology,
Shanghai, China) as per the manufacturer's protocol. To detect
apoptosis, the mono- and co-cultured Jurkat cells were harvested
and washed twice with phosphate-buffered saline (PBS), and stained
using the FITC-Annexin V/PI Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA) as per the manufacturer's
protocol. All stained samples were analyzed by flow cytometry (BD
Biosciences, San Jose, CA, USA), and the ModFit LT v2.0 program (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the Jurkat cells
harvested following 48 h of mono- or co-culture using an RNA
isolation kit (Takara Bio, Inc., Otsu, Japan). The purity of the
RNA was evaluated by calculating the A260/A280 ratio, and samples
with ratios between 1.8 and 2.0 were used for further analysis. The
mRNA was reverse transcribed into cDNA using the PrimeScript™ II
First Strand cDNA Synthesis kit (Takara Bio, Inc.). The RT-qPCR
analysis was accomplished with a SYBR-Green PCR Master mix kit
(Applied Biosystems; Thermo Fisher Scientific, Inc., MA, USA)
containing 2 µl cDNA and 0.5 µl of each primer (10 µM), according
to the manufacturer's protocol. The following primer pairs were
used for gene amplification: TGF-β1, forward 5'-ACA CCA ACT ATT GCT
TCA G-3' and reverse 5'-TGT CCA GGC TCC AAA TG-3'; TNF-a, forward
5'-CTC GAA CCC CGA GTG ACA AG-3' and reverse 5'-TGA GGT ACA GGC CCT
CT G AT-3'; β-actin, forward 5'-AAG CAG GAG TAT GAC GAG TCC G-3'
and reverse 5'-GCC TTC ATA CAT CTC AAG TTG G-3', with the following
thermal cycling conditions for 40 cycles in total: 10 min at 95°C;
15 sec at 95°C; and 30 sec at 60°C. The relative gene expression
levels were calculated using the 2-ΔΔCq method (13), and normalized against those of
β-actin. Three independent experiments were performed.
ELISA
After 72 h, the supernatants were collected from the
mono- and co-cultured Jurkat cells and stored at -80°C until use.
The levels of TGF-β1 were measured using an ELISA kit (R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
Western blotting
Total protein was extracted from the Jurkat cells
harvested following 24, 48 or 72 h of co-culture with RIPA lysis
buffer as described previously (14). The nuclear proteins were extracted
with NE-PER nuclear and cytoplasmic extraction reagents (Pierce;
Thermo Fisher Scientific, Inc.) from the 48 and 72 h co-cultured
cells. Equal quantities of protein (40 µg) per sample were loaded
on an 12% SDS-polyacrylamide gel, electrophoresed and transferred
onto a PVDF membrane and blocked with TBS with Tween-20 containing
5% non-fat dry milk for 1 h at room temperature. The membranes were
probed with primary antibodies against phosphorylated (p)-P65
(1:2,000, cat. no. AF2006, Affinity Biosciences, Cambridge, UK),
P65 (1:2,000, cat. no. 10745-1-AP, ProteinTech Group, Inc., Wuhan,
China), inhibitor of NF-κB (IκB) kinase (IKK)β (1:1,000, cat. no.
Ab124957, Abcam, Cambridge, MA, USA), IκBα (1:1,000, cat. no. 4812,
Cell Signaling Technology, Inc., Danvers, MA, USA), B-cell lymphoma
2 (Bcl-2; 1:2,000, cat. no. 12789-1-AP, ProteinTech Group, Inc.),
Bcl-2-associated X protein (Bax; 1:5,000, cat. no. 50599-2-IG,
Protein Tech Group, Inc), JNK1/2 (1:1,000, cat. no. 9252, Cell
Signaling Technology, Inc.), p-JNK1/2 (1:1,000, cat. no. 4668, Cell
Signaling Technology, Inc.), extracellular signal-regulated kinase
(ERK)1/2 (1:1,000, cat. no. 9102, Cell Signaling Technology, Inc.),
p-ERK1/2 (1:2,000, cat. no. 4370, Cell Signaling Technology, Inc.),
P38 (1:1,000, cat. no. 9212, Cell Signaling, Technology, Inc.),
p-P38 (1:1,000, cat. no. 9211, Cell Signaling Technology, Inc.),
mothers against decapentaplegic (Smad)2/3 (1:1,000, cat. no.
AF6367, Affinity Biosciences), p-Smad2/3 (1:200, cat. no. MAB8935,
R&D Systems, Inc.) and the loading control β-actin (1:200, cat.
no. BM0627, Boster Biological Technology, Wuhan, China). This step
was followed by incubation with horseradish peroxidase-conjugated
secondary antibodies (1:50,000, goat anti-mouse, cat no. BA1051,
Boster Biological Technology) for 2 h at room temperature. The
specific protein bands were visualized using an enhanced
chemiluminescence detection kit (GE Healthcare Life Sciences,
Chalfont, UK), and the ratio of the grayscale values of the target
protein were calculated.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical significance was calculated with Student's
t-test and one-way analysis of variance; Tukey's multiple
comparison test was used for post hoc analysis. Statistical
analyses were performed using Prism 6.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of ADSCs on Jurkat cell
proliferation, cell cycle and apoptosis
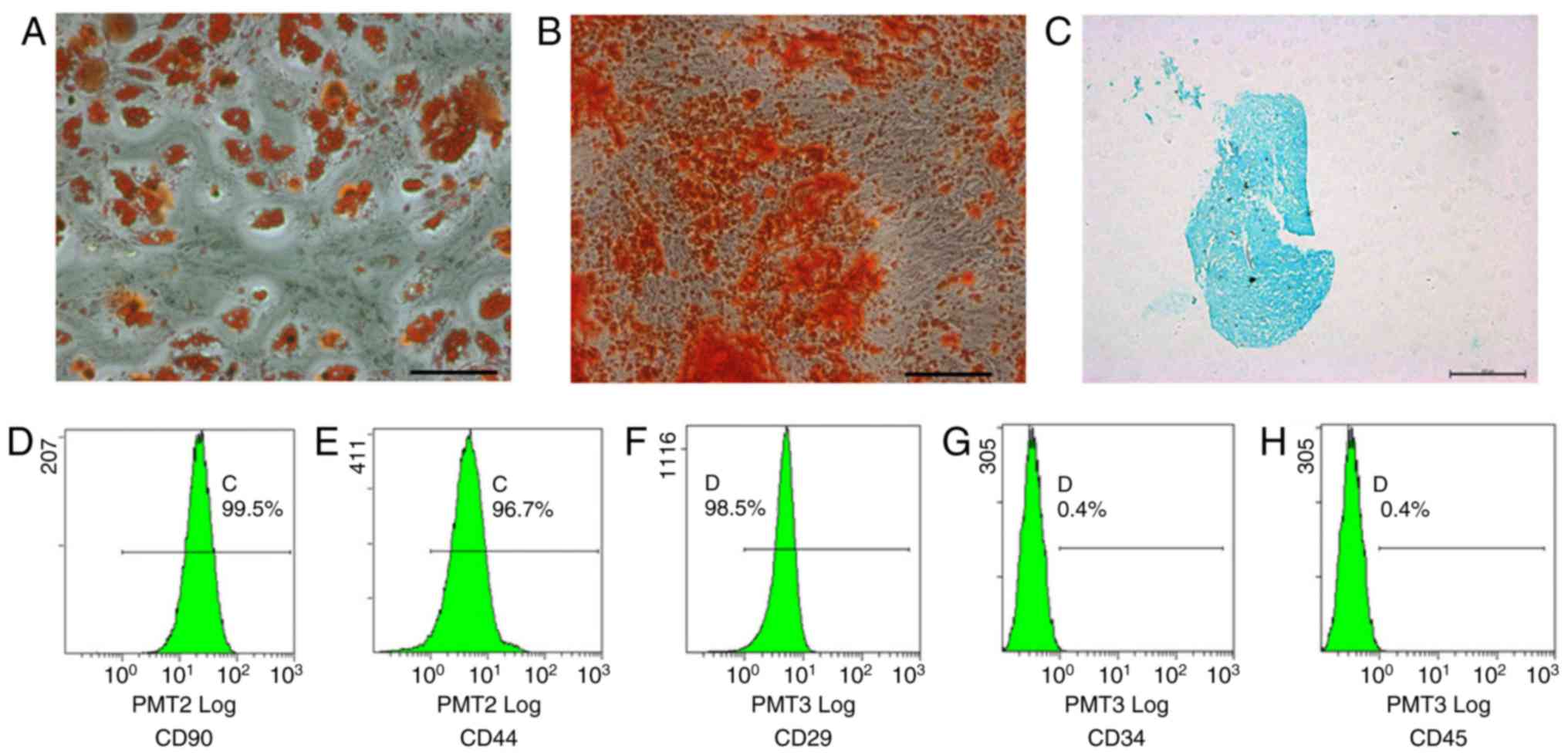
The ADSCs isolated from human adipose tissue were
characterized using MSC surface markers CD90, CD44, CD29, CD34 and
CD45 (12). The multipotency of
the ADSCs was determined by the adipogenic, osteogenic and
chondrogenic differentiation assays (Fig. 1A-H).
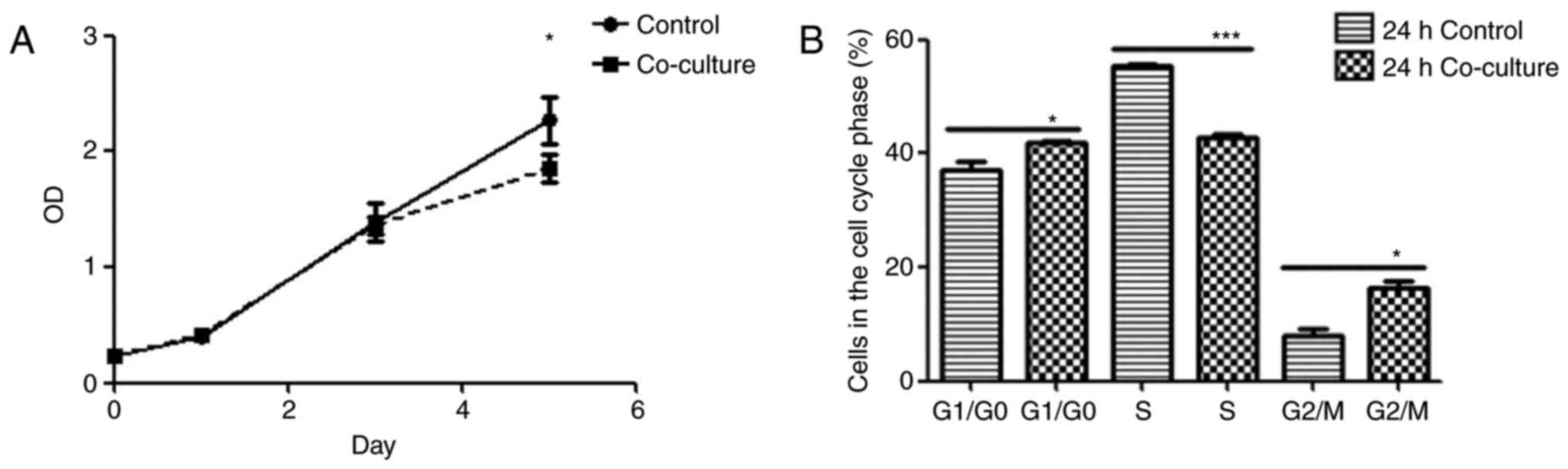
The effect of ADSCs on Jurkat cell behavior was
assessed using established proliferation, cell cycle and apoptosis
assays. Although no significant differences were observed in the
proliferation rates of the mono- and co-cultured Jurkat cells on
days 1 and 3, the ADSC-co-cultured cells exhibited a significantly
lower proliferation rate on day 5 compared with the control cells
(P<0.05; Fig. 2A). Following
24 h of co-culture with ADSCs, the proportion of Jurkat cells in
the G0/G1 and G2/M phases
increased significantly and there was a concomitant decrease in the
proportion of cells in the S phase compared with the control cells
(Fig. 2B). Finally, the Jurkat
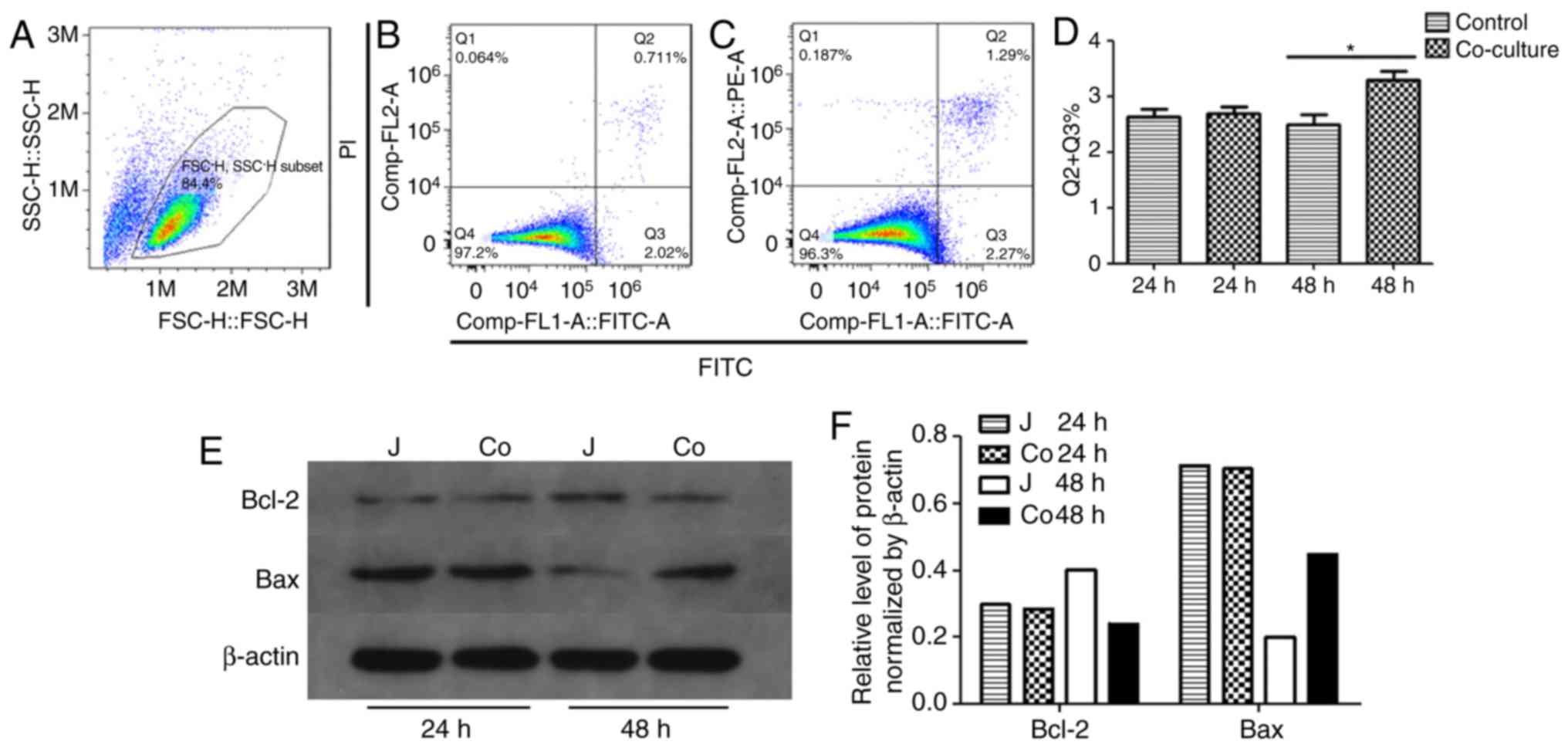
cells co-cultured with ADSCs for 48 h exhibited significantly
higher apoptotic rates than the control cells (Fig. 3A-D). The Jurkat cells co-cultured
with ADSCs for 48 h also showed significantly higher levels of
pro-apoptotic Bax and lower levels of anti-apoptotic Bcl-2 compared
with the control cells (Fig. 3E).
Taken together, the ADSCs suppressed Jurkat cell proliferation by
arresting them at the G0/G1 and
G2/M phases, and inducing apoptosis.
Effect of ADSCs on Jurkat cell cytokine
secretion
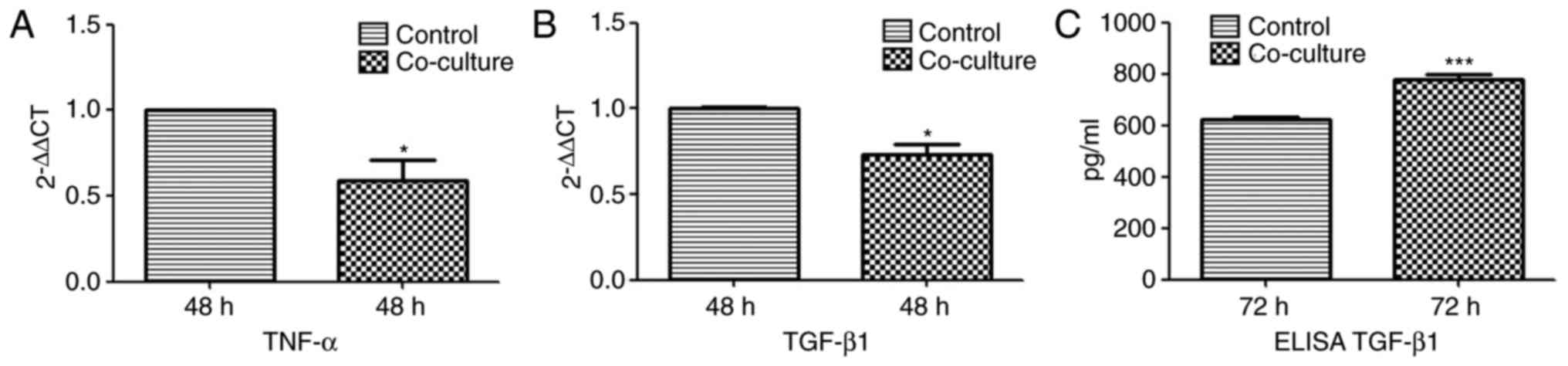
The mRNA and protein levels of TNF-α and TGF-β1 were
measured in the Jurkat cells co-cultured with ADSCs by RT-qPCR and
ELISA analyses, respectively. Compared with the control cells, the
mRNA levels of TNF-α and TGF-β1 were signifi-cantly decreased in
the co-cultured cells at 48 h, whereas the secreted levels of
TGF-β1 were significantly increased in the supernatants of the
co-cultured cells at 72 h. (Fig.
4).
Regulation of Jurkat cell signaling
pathways by ADSCs
In addition to the cells co-cultured with ADSCs for
48 h showing higher levels of pro-apoptotic Bax and lower levels of
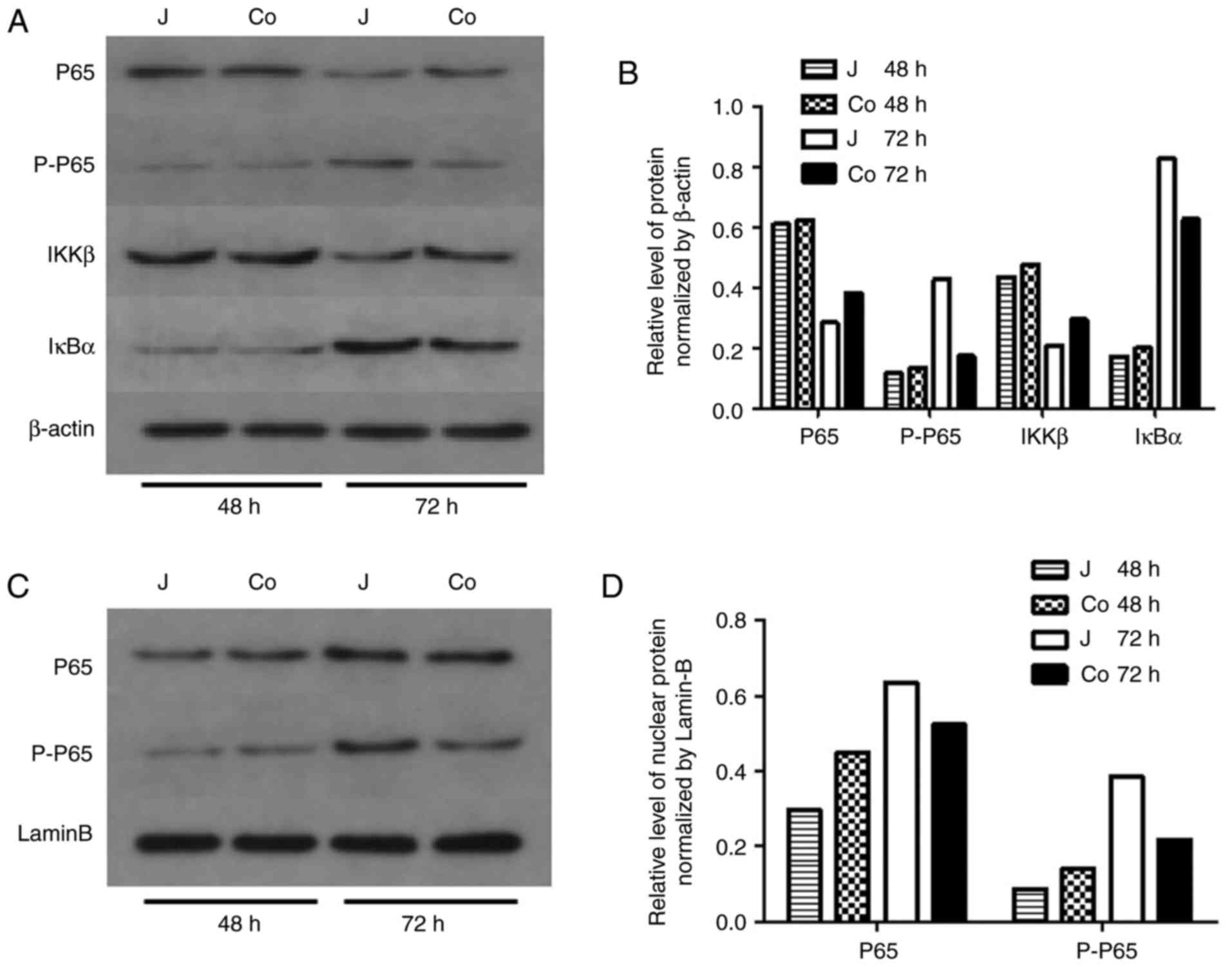
anti-apoptotic Bcl-2, the NF-κB p-P65 subunit levels in the
cellular and nuclear fractions were marginally lower in the
co-cultured cells compared with those in the control cells after 72
h. No apparent differences were observed in the levels of P65, IκBα
or IKKβ between the two groups, as well as the level of Erk, P38
and Smad2/3 (Figs. 5A-D and
6). By contrast, the level of
p-JNK1/2 was higher in the co-cultured cells after 48 h (Fig. 6A-C). These findings indicated that
the ADSCs induced apoptosis of the Jurkat cells by suppressing
NF-κB P65 subunit phosphorylation and activating the JNK signaling
pathway.
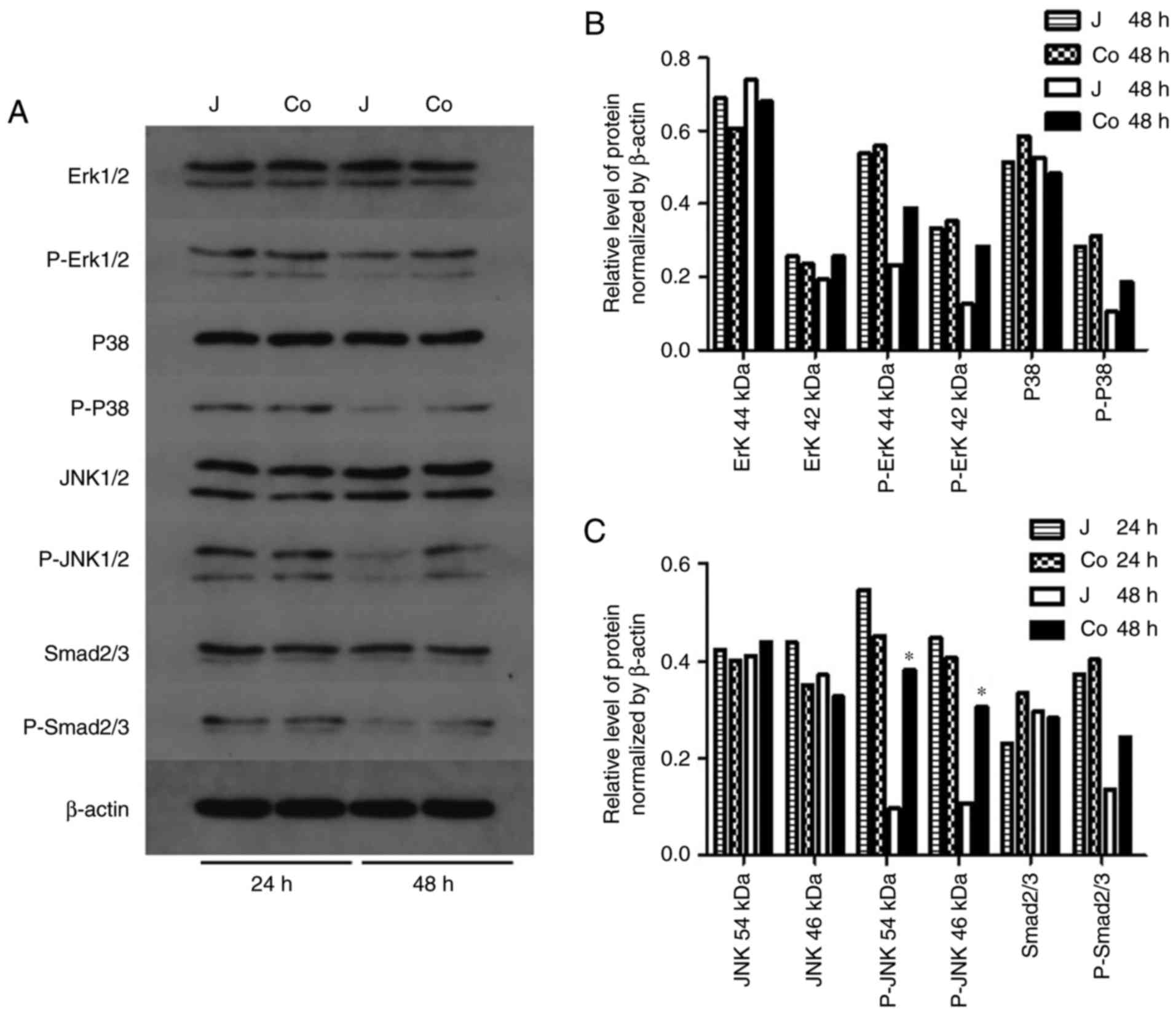 | Figure 6Signaling pathways. (A) Protein
levels of Erk, p-Erk, P38, p-P38, JNK, p-JNK, Smad2/3 and P-Smad2/3
in the control and co-cultured Jurkat cells. (B) Densitometric
analysis of the expression of Erk, p-Erk, P38 and p-P38. (C)
Densitometric analysis of the expression of JNK, p-JNK, Smad2/3 and
p-Smad2/3, showing upregulation of p-JNK following 48 h of
co-culture compared with the control group at 48 h.
(*P<0.05 vs. J 48 h). J, control; co, co-cultured;
Erk, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; Smad2/3, mothers against decapentaplegic; p-,
phosphorylated. |
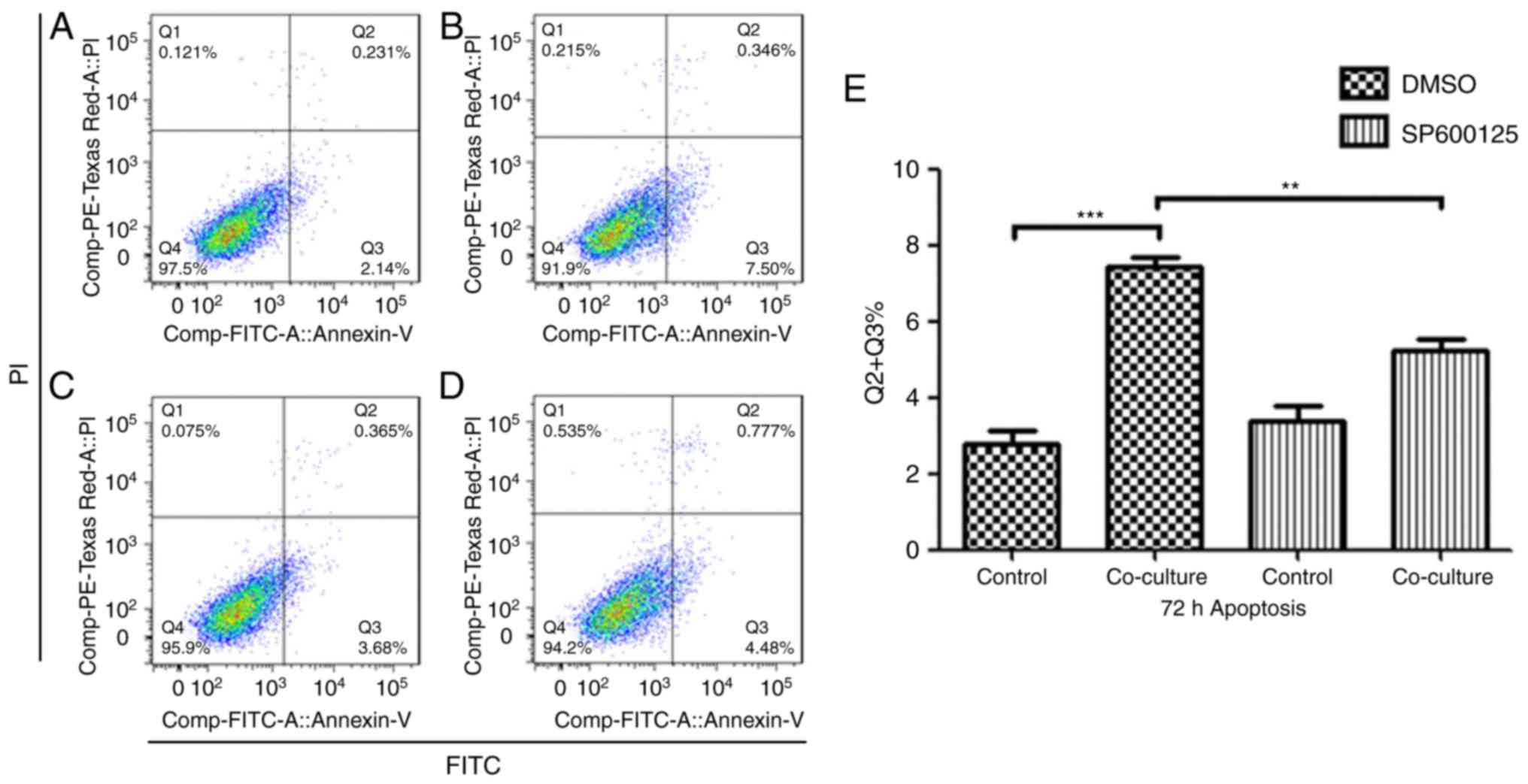
ADSC induces apoptosis in Jurkat cells
via the JNK pathway
In order to evaluate the role of the activation of
JNK in ADSC-induced Jurkat cell apoptosis, the latter were treated
with 40 µM of the specific JNK inhibitor SP600125 prior to the
co-culture, and the percentage of apoptotic cells was assessed. As
shown in Fig. 7A-E, the
inhibition of JNK in Jurkat cells significantly inhibited
ADSC-induced apoptosis.
Discussion
As the prognosis of allo-transplantation is
dependent on the host immune response to the allografts, reducing T
cell-mediated acute rejection is vital in order to protect the
allograft. The immunomodulatory effects of ADSCs observed in
pre-clinical studies make it a suitable addition to routine
immunosuppressants for preventing allograft rejection (4,5).
Therefore, it is important to determine the specific effects of
ADSCs on immune cells, particularly CD4+ and
CD8+ T cells, which are the major elicitors of acute and
chronic rejection (15,16).
Gonzalez-Rey et al (17) reported suppressive effects of
human ADSCs on the proliferation of activated PBMCs and production
of TNF-α. A study on the miniature swine hind-limb model showed
that the infusion of ADSCs prolonged allograft survival, increased
regulatory T cells in allografts and blood, and decreased the
levels of circulating pro-inflammatory cytokine TNF-α (4). In addition, Matula et al
(18) reported a similar
inhibitory effect of ADSCs on Jurkat T cells following 3 and 4 days
of co-culture. However, Mousavi et al (19) did not report any differences in
the proliferation rates of Jurkat cells cultured with or without
ADSC for 2 and 3 days. In the present study, ADSC co-culture
reduced Jurkat cell viability on day 5, with no significant effects
observed until day 3, indicating a time-dependent effect of the
ADSCs on Jurkat cells.
The NFAT and NF-κB signaling pathways
synergistically activate T cells (20). NF-κB is a transcription factor
that regulates the expression of inflammatory cytokines and genes
essential for the proliferation and survival of T cells (21). MSCs have been shown to inhibit
NF-κB signaling in responder cells (22-24). Consistent with this, the present
study observed a reduction in the levels of p-p65 and its nuclear
translocation in Jurkat cells co-cultured with ADSCs, thereby
confirming the suppressive effect of ADSCs on T cells.
Consistent with the pro-apoptotic effects of MSCs on
T cells reported in previous studies (25,26), the present study observed
increased apoptosis in the Jurkat cells co-cultured with ADSCs.
Previous studies have also reported an inhibitory effect of MSCs on
T cell apoptosis (27-29). This ambiguity may be the result of
differences in responder cells (28), different tissue sources of MSCs
(e.g. bone marrow, placenta or adipose tissue) (27,29), and different T-cell stimulators
(e.g., PHA or anti-Fas antibody) (27,29) used in these studies. Therefore,
MSCs from different origins may affect T cell function via
different mechanisms.
Apoptosis can be induced by either inhibiting
survival signals, for example, BCL-2 as an inner mitochondrial
membrane anti-apoptotic protein, or by increasing death signals,
for example BAX as a pro-apoptotic effector of mitochondrial outer
membrane permeabilization (30).
Furthermore, Bcl-2-associated apoptosis in Jurkat cells is
associated with the activation of JNK (31). The present study observed
increased levels of p-JNK1/2 in the co-cultured Jurkat cells,
indicating the ADSC-mediated activation of JNK. The involvement of
the JNK pathway in ADSC-induced apoptosis was further verified by
the JNK-specific inhibitor SP600125 inhibiting apoptosis in the
co-cultured Jurkat cells. However, the inhibitory effect was not
complete, indicating the involvement of other pathways in
ADSC-induced apoptosis. Higher levels of secreted TGF-β1, an
activator of JNK, were present in the superna-tants of the
co-cultured Jurkat cells. However, the mRNA levels of TGF-β1 were
significantly lower in the co-cultured Jurkat cells; it is likely
that TGF-β1 was secreted by the ADSCs and not Jurkat cells. TGF-β1
is an important component of the ADSC secretome, and has
immunosuppressive and apoptotic effects on T cells (32,33). Taken together, the ADSCs induced
apoptosis in Jurkat cells by activating the JNK pathway.
MSCs have shown potential in improving
transplantation outcomes (34-36). A previous study showed that the
inclusion of bone marrow-derived MSCs (BMSCs) during renal
transplantation improved renal function at the one-year follow-up
(34). In addition, the BMSCs
also reduced tubulitis, interstitial fibrosis and tubule atrophy
post-transplantation (35). The
immunomodulatory effects ADSCs, although evident in pre-clinical
and experimental studies, have not been verified in clinical
trials. In the present study, the mechanism underlying the effect
of ADSC on T cells was elucidated, to further underscore the
clinical potential of ADSCs in immunological diseases and
transplantation, and to improve current understanding of stem
cell-based therapy.
In conclusion, the present study showed that ADSCs
suppressed Jurkat T cell viability by inducing apoptosis and cell
cycle arrest by activating the JNK signaling and mitochondrial
apoptotic pathways. These findings provide novel insights into the
molecular mechanisms underlying the immunomodulatory effects of
ADSCs, and provide an experimental basis for the clinical
application of ADSCs.
Funding
The present study was supported by the National
Natural Science Foundation for Young Scholars (grant no.
81401613).
Availability of data and materials
The data used during the present study are available
from the corresponding authors on reasonable request.
Authors' contributions
FL conceived and designed the experiments, and JY
guided the design of the experiments. YMW and XXW performed the
experiments under the direction of JY. ZZ and XYZ were involved in
the completion of the experiments. YMW and XXW analyzed the data.
YMW wrote the manuscript. FL and JY revised the manuscript. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of Shanghai Ninth People's Hospital, Shanghai Jiao Tong
University School of Medicine (no. 2017-452-T348). The donors
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ADSCs
|
adipose-derived stem cells
|
|
VCA
|
vascularized composite
allotransplantation
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MSCs
|
mesenchymal stem cells
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
FBS
|
fetal bovine serum
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
DMSO
|
dimethyl sulfoxide
|
Acknowledgments
The authors would like to thank the Laboratory of
Tissue Engineering at Shanghai Ninth People's Hospital, Shanghai
Jiao Tong University (Shanghai, China) for the provision of
experimental equipment and technical guidance necessary to complete
the study.
References
|
1
|
Khalifian S, Brazio PS, Mohan R, Shaffer
C, Brandacher G, Barth RN and Rodriguez ED: Facial transplantation:
The first 9 years. Lancet. 384:2153–2163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuo KJ and Olson JL: The evolution of
functional hand replacement: From iron prostheses to hand
transplantation. Plast Surg (Oakv). 22:44–51. 2014. View Article : Google Scholar :
|
|
3
|
Bamoulid J, Staeck O, Halleck F,
Khadzhynov D, Brakemeier S, Durr M and Budde K: The need for
minimization strategies: Current problems of immunosuppression.
Transpl Int. 28:891–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo YR, Chen CC, Chen YC and Chien CM:
Recipient Adipose-derived stem cells enhance recipient cell
engraftment and prolong allotransplant survival in a miniature
swine hind-limb model. Cell Transplant. 26:1418–1427. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plock JA, Schnider JT, Zhang W, Schweizer
R, Tsuji W, Kostereva N, Fanzio PM, Ravuri S, Solari MG, Cheng HY,
et al: Adipose- and bone marrow-derived mesenchymal stem cells
prolong graft survival in vascularized composite
allotransplantation. Transplantation. 99:1765–1773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Munir H and McGettrick HM: Mesenchymal
stem cell therapy for autoimmune disease: Risks and rewards. Stem
Cells Dev. 24:2091–2100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boltze J, Arnold A, Walczak P, Jolkkonen
J, Cui L and Wagner DC: The dark side of the force-constraints and
complications of cell therapies for stroke. Front Neurol.
6:1552015. View Article : Google Scholar
|
|
8
|
Cui L, Yin S, Liu W, Li N, Zhang W and Cao
Y: Expanded adipose-derived stem cells suppress mixed lymphocyte
reaction by secretion of prostaglandin E2. Tissue Eng.
13:1185–1195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crop MJ, Baan CC, Korevaar SS, Ijzermans
JN, Pescatori M, Stubbs AP, van Ijcken WF, Dahlke MH, Eggenhofer E,
Weimar W and Hoogduijn MJ: Inflammatory conditions affect gene
expression and function of human adipose tissue-derived mesenchymal
stem cells. Clin Exp Immunol. 162:474–486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuo YR, Chen CC, Goto S, Lin PY, Wei FC
and Chen CL: Mesenchymal stem cells as immunomodulators in a
vascularized composite allotransplantation. Clin Dev Immunol.
2012.854846:2012.
|
|
11
|
Zhao Q, Ren H and Han Z: Mesenchymal stem
cells: Immunomodulatory capability and clinical potential in immune
diseases. J Cell Immunotherapy. 2:3–20. 2016. View Article : Google Scholar
|
|
12
|
Bunnell BA, Flaat M, Gagliardi C, Patel B
and Ripoll C: Adipose-derived stem cells: Isolation, expansion and
differentiation. Methods. 45:115–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Hwang GS, Hu S, Lin YH, Chen ST, Tang TK,
Wang PS and Wang SW: Arecoline inhibits interleukin-2 secretion in
Jurkat cells by decreasing the expression of alpha7-nicotinic
acetylcho-line receptors and prostaglandin E2. J Physiol Pharmacol.
64:535–543. 2013.PubMed/NCBI
|
|
15
|
Liu Z, Fan H and Jiang S: CD4(+) T-cell
subsets in transplantation. Immunol Rev. 252:183–191. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yap M, Brouard S, Pecqueur C and Degauque
N: Targeting CD8 T-cell metabolism in transplantation. Front
Immunol. 6:5472015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonzalez-Rey E, Gonzalez MA, Varela N,
O'Valle F, Hernandez-Cortes P, Rico L, Büscher D and Delgado M:
Human adipose-derived mesenchymal stem cells reduce inflammatory
and T cell responses and induce regulatory T cells in vitro in
rheumatoid arthritis. Ann Rheum Dis. 69:241–248. 2010. View Article : Google Scholar
|
|
18
|
Matula Z, Németh A, Lőrincz P, Szepesi Á,
Brózik A, Buzás EI, Lőw P, Német K, Uher F and Urbán VS: The role
of extracellular vesicle and tunneling Nanotube-mediated
intercellular cross-talk between mesenchymal stem cells and human
peripheral T cells. Stem Cells Dev. 25:1818–1832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mousavi Niri N, Jaberipour M, Razmkhah M,
Ghaderi A and Habibagahi M: Mesenchymal stem cells do not suppress
lymphoblastic leukemic cell line proliferation. Iran J Immunol.
6:186–194. 2009.
|
|
20
|
Bronk CC, Yoder S, Hopewell EL, Yang S,
Celis E, Yu XZ and Beg AA: NF-κB is crucial in proximal T-cell
signaling for calcium influx and NFAT activation. Eur J Immunol.
44:3741–3746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reale C, Zotti T, Scudiero I, Vito P and
Stilo R: Chapter nine-the NF-κB family of transcription factors and
its role in thyroid physiology. Vitamins and Hormones. Litwack G:
Academic Press; pp. 195–210. 2018, View Article : Google Scholar
|
|
22
|
Letourneau PA, Menge TD, Wataha KA, Wade
CE, S Cox C Jr, Holcomb JB and Pati S: Human bone marrow derived
mesenchymal stem cells regulate Leukocyte-endothelial interactions
and activation of transcription factor NF-Kappa B. J Tissue Sci
Eng. 3(Suppl): S0012011.
|
|
23
|
Onishi R, Ohnishi S, Higashi R, Watari M,
Yamahara K, Okubo N, Nakagawa K, Katsurada T, Suda G, Natsuizaka M,
et al: Human amnion-derived mesenchymal stem cell transplantation
ameliorates dextran sulfate sodium-induced severe colitis in rats.
Cell Transplant. 24:2601–2614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang PP, Xie DY, Liang XJ, Peng L, Zhang
GL, Ye YN, Xie C and Gao ZL: HGF and direct mesenchymal stem cells
contact synergize to inhibit hepatic stellate cells activation
through TLR4/NF-kB pathway. PLoS One. 7:e434082012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Plumas J, Chaperot L, Richard MJ, Molens
JP, Bensa JC and Favrot MC: Mesenchymal stem cells induce apoptosis
of activated T cells. Leukemia. 19:1597–1604. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akiyama K, Chen C, Wang D, Xu X, Qu C,
Yamaza T, Cai T, Chen W, Sun L and Shi S:
Mesenchymal-stem-cell-induced immunoregulation involves
FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell.
10:544–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benvenuto F, Ferrari S, Gerdoni E,
Gualandi F, Frassoni F, Pistoia V, Mancardi G and Uccelli A: Human
mesenchymal stem cells promote survival of T cells in a quiescent
state. Stem Cells. 25:1753–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramasamy R, Lam EW, Soeiro I, Tisato V,
Bonnet D and Dazzi F: Mesenchymal stem cells inhibit proliferation
and apoptosis of tumor cells: Impact on in vivo tumor growth.
Leukemia. 21:304–310. 2007. View Article : Google Scholar
|
|
29
|
Gu YZ, Xue Q, Chen YJ, Yu GH, Qing MD,
Shen Y, Wang MY, Shi Q and Zhang XG: Different roles of PD-L1 and
FasL in immunomodulation mediated by human placenta-derived
mesenchymal stem cells. Hum Immunol. 74:267–276. 2013. View Article : Google Scholar
|
|
30
|
Zheng JH, Viacava Follis A, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–2700. 2016. View Article : Google Scholar
|
|
31
|
Lee SH, Park SW, Pyo CW, Yoo NK, Kim J and
Choi SY: Requirement of the JNK-associated Bcl-2 pathway for human
lactoferrin-induced apoptosis in the Jurkat leukemia T cell line.
Biochimie. 91:102–108. 2009. View Article : Google Scholar
|
|
32
|
Kapur SK and Katz AJ: Review of the
adipose derived stem cell secretome. Biochimie. 95:2222–2228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Guan E, Roderiquez G and Norcross
MA: Synergistic induction of apoptosis in primary CD4(+) T cells by
macrophage-tropic HIV-1 and TGF-beta1. J Immunol. 167:3360–3366.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan J, Wu W, Xu X, Liao L, Zheng F,
Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, et al: Induction
therapy with autologous mesenchymal stem cells in living-related
kidney transplants: A randomized controlled trial. JAMA.
307:1169–1177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reinders ME, de Fijter JW, Roelofs H,
Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP,
Roelen DL, van Kooten C, et al: Autologous bone marrow-derived
mesen-chymal stromal cells for the treatment of allograft rejection
after renal transplantation: Results of a phase I study. Stem Cells
Transl Med. 2:107–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W,
Li X, Chen Z, Ma J, Liao D, et al: Donor-derived mesenchymal stem
cells combined with low-dose tacrolimus prevent acute rejection
after renal transplantation: A clinical pilot study.
Transplantation. 95:161–168. 2013. View Article : Google Scholar
|