Introduction
Intervertebral discs are the primary component of
the human spine, which provides mechanical support and spinal
motion for daily activities (1).
Intervertebral disc degeneration (IDD) is an important contributor
to neck, back and radicular pain, and a leading cause of disability
worldwide (2). Commonly, IDD is
characterized by disc space narrowing, water content reduction in
the nucleus pulposus (NP) tissues, the appearance of fibrosis that
is reflected in an increase in thickness and spacing of collagen
fibers, and in the secretion of proinflammatory cytokines (3,4).
Specifically, NP tissues destruction and deceleration of the
synthesis of disc components are the two primary causes of IDD
pathogenesis (5). Several factors
have been demonstrated to contribute to IDD, including hip
osteoarthritis (6), obesity
(7) and genetic factors, but the
exact mechanisms of IDD remain unclear. With the development of
medical technology in previous years, several methods have been
explored to treat IDD, including anti-inflammatory medication,
analgesics, physical therapy and surgery, but the outcome is
unsatisfactory (8,9). Therefore, it is necessary to explore
novel therapeutic methods to improve the treatment of IDD.
The extracellular matrix (ECM) primarily consists of
proteoglycans, aggrecan, collagens and matrix metalloproteinases
(MMPs) in NP. Degradation of the ECM, particularly collagen II and
aggrecan, is considered to be an important cause of IDD (10). Collagen II is an important
component of ECM, and its expression is significantly downregulated
during the IDD process (11,12). Mutation of collagen type II alpha
1 chain, the encoding gene of collagen II, is commonly identified
in spondyloepiphyseal dysplasia congenita, knee osteoarthritis,
kniest dysplasia and type II collagenopathies (13-15). C-telopeptide of type II collagen
(CTX-II) is a degradation product of collagen II, and is usually
upregulated in the sera of patients with osteoarthritis (16). Yang et al (17) suggested that E2 inhibited aberrant
apoptosis of NP cells via upregulating the expression of collagen
II and aggrecan, and downregulating the expression of MMP-13.
MMP-13 has been identified to degrade collagen II in IDD (18). Considering these variations in
ECM, therapeutics for degenerative diseases may be developed by
increasing the synthesis of collagen II and aggrecan, while
inhibiting the expression of MMPs.
Bone morphogenetic proteins (BMPs) belong to the
transforming growth factor β (TGF-β) family, and are commonly known
to function as underlying regulators of bone and cartilage
formation (19). Specifically,
BMP2 has been widely demonstrated to be involved in the regulation
of diseases, including non-union fractures, osteoporosis and spinal
fusion, as reviewed in previous literatures (20,21). BMP2 was also suggested to protect
the growth and decrease the apoptosis of NP cells (22), but the exact mechanism remains
unclear. An animal model demonstrated that combination therapy of
BMP2 and a receptor activator of nuclear factor κB ligand inhibitor
promoted bone healing in a critical-sized femoral defect mouse
model (23). Additionally, BMP2
inhibited the growth and metastasis of hepatocellular carcinoma by
inhibiting the phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt) pathway, which serves an important role in osteogenesis
(24,25). Concurrently, activation of the
PI3K/Akt signaling pathway may protect against IDD via enhancing
the production of aggrecans and collagen II (26). However, whether BMP2 alleviates
IDD via the PI3K/Akt pathway remains unclear.
In light of the aforementioned data, the present
study aimed to explore the underlying mechanisms of BMP2 in
alleviating IDD in a rat model and NP cells in vitro. The
present study indicated that BMP2 alleviated IDD via activating the
PI3K/Akt signaling pathway to inhibit NP cells apoptosis and the
decrease in matrix protein degradation, particularly aggrecans and
collagen II. These data may provide novel insights into the
understanding and treatment of IDD in clinical settings.
Materials and methods
Preparation of adeno-associated virus
serotype 2 (AAV)-BMP2 adenovirus
The 293T cells were purchased from the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China), and maintained
in Dulbecco’s modified Eagle’s medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a humidity
incubator at 37°C with 5% CO2. Subsequently, 1 μg
recombinant plasmid pDC315-BMP2 (Shanghai GenePharma Co., Ltd.,
Shanghai, China) was co-transfected for 4 h with 10×106
pfu pBGHE3 (Shanghai GenePharma Co., Ltd.) into 293T cells using
Lipofectamine 2000® (Invitrogen; Thermo Fisher
Scientific, Inc.) to obtain the recombinant BMP2 adenovirus.
Following this, the titer of viral particles was estimated by dot
blot assay as described previously (27) and purified stocks were maintained
at ~1012 particles/ml.
Construction of IDD model and
grouping
The experimental procedures of the present study
were approved by the Second Xiangya Hospital, Central South
University (Changsha, China). All the procedures involving animals
and their care were performed in accordance with the Guidelines for
the Care and Use of Laboratory Animals of the Second Xiangya
Hospital, Central South University. A total of 48 male Lewis rats
aged 13-14 weeks were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China), and housed in a 12-h light/dark
cycle sterile room at ~23°C with access to food and water ad
libitum. Rats were randomly divided into six groups (n=8 per
group): Control; punctured (model); puncture + PBS injection (5
μl, containing 1010 AAV); punctured +
106 AAV-BMP2 (5 μl); punctured + 108
AAV-BMP2 (5 μl); and punctured + 1010 AAV-BMP2 (5
μl). The IDD model was generated by using the annulus
fibrosus needle puncture method (28). Briefly, rats were anesthetized
intraperitoneally with sodium pentobarbital (30 mg/kg). A 18G
syringe needle was inserted into the C6-C7 and C8-C9 discs in the
vertical direction with 5 mm depth. Following full penetration, the
needle was rotated 360° and held for 20 sec. For the control group,
the discs were exposed, but not punctured. For the later groups,
PBS (containing 1010 AAV) or AAV-BMP2 was injected into
punctured location on week 2 subsequent to puncture. All rats were
sacrificed following 8 weeks by inhalation of 7.5% isoflurane, and
the NP tissues and blood samples were collected.
Magnetic resonance imaging (MRI)
acquisition
A total of 2 months after surgery, rats were
anesthetized as aforementioned to limit movement during the MRI
examination. Images were obtained using a 3.0 T MRI machine
(Philips Healthcare, Amsterdam, The Netherlands) with a dedicated
coil for small animals. Following this, the tails of the rats were
immersed in 0.1 M CuSO4 solution in a tube, to increase
the contrast of image. Subsequently, a 2-D spin echo-dual echo
sequence was performed with the following parameters: Repetition
time=9,000 ms, flip angle=90°, echo times=16 and 80 ms, slice
thickness=0.6, number of averages=2, field of view=40×40
mm2, and in-plane resolution=0.1 mm with 30 sagittal
slices. The disc signal intensity was calculated by using the
T2-weighted image (echo time=80 ms) to monitor the disc hydration.
Then, the mean signal intensity (brightness) in the control disc
was used as reference for the signal intensity of the injured discs
in each group. Therefore, the value of normalized intensity for the
injured discs ranged from 0-1, as described previously (28).
Histological analysis
Following MRI examination, rats were sacrificed and
the whole discs with the vertebrae adjacent to the punctured C6-C7
and C8-C9, and non-punctured C7-C8 were isolated, fixed with 4%
paraformaldehyde at 4°C for 24 h, decalcified in 10% EDTA for 30
days, embedded in paraffin and cut into 5-μm thick slices.
Then, the slices were dehydrated with an ethanol gradient (70, 90%)
and stained for 30 min at room temperature with hematoxylin and
eosin (H&E; Nanjing Jiancheng Bioengineering Institute,
Nanjing, Jiangsu, China) according to manufacturer’s protocols.
Concurrently, parts of the slices were stained for 30 min at room
temperature with 1% Alcian Blue (pH 2.5; Newcomer Supplu Inc.,
Middleton, WI, USA) following dehydration. Following this, sections
were permeabilized with 100% xylene and sealed with neutral resins.
Subsequently, the stained slides were analyzed under a light
microscope at ×400 magnification (Nikon E800 microscope, Nikon
Instruments Inc., Melville, NY, USA).
NP cells isolation and treatment
NP cells were isolated as described previously
(29,30). Following isolation, NP cells were
resuspended in DMEM medium supplied with 10% FBS, 100 μg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.), and 100
U/ml penicillin (Invitrogen; Thermo Fisher Scientific, Inc.) at
37°C in a humidified atmosphere with 5% CO2. The medium
was changed every two days, and the second passage was used for
subsequent experiments.
For treatment, NP cells were first treated with
different concentrations of recombinant human BMP2 (rhBMP2; 0, 25,
50, 100, 200, 400 and 800 ng/ml) to assess the effects of rhBMP2 on
cell proliferation, and for the selection of appropriate
concentrations for subsequent experimentation. NP cells were
treated with 10 ng/ml interleukin 1β (IL-1β; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) to imitate the IDD model in vitro.
For subsequent experiments, cells were pretreated with 10 μM
LY294002 (PI3K signal pathway inhibitor) for 1 h at 37°C to verify
the mechanism of the signal pathway.
MTT assay
NP cells were seeded in 96-well plates at a density
of 1.0×103/well and cultured with complete DMEM medium
containing different concentrations of rhBMP2 (0, 25, 50, 10 0,
200, 400 and 800 ng/ml). Then, cells were cultured for 24 and 48 h.
Subsequently, 20 μl MTT solution (5 mg/ml) was added into
each well and plates were incubated at 37°C for 4 h. Then, the
supernatant was discarded and 150 μl dimethyl sulfoxide was
added into each well, and plates were agitated at room temperature
for 10 min. Following this, the optical density (OD) values were
determined at 570 nm.
Apoptosis assay
The apoptosis of cells was determined by flow
cytometry with Kaluza 2017 Analysis Software 1.3 (Beckman Coulter,
Inc., Brea, CA, USA) following staining with fluorescein
isothiocyanate (FITC)-conjugated Annexin V and propidium iodide
(PI; Beyotime Institute of Biotechnology, Haimen, China). Briefly,
1×106 cells were seeded in 6-well plates with DMEM
containing 10% FBS overnight at 37°C. Then, cells were treated with
rhBMP2 and IL-1β as aforementioned for 48 h. Following this, the
cells were stained using the FITC Annexin V/PI apoptosis Detection
kit I (BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) for
20 min at room temperature according to the manufacturer’s
protocol. Subsequently, the levels of apoptosis of the cells were
detected using flow cytometry. Annexin V+ cells were
considered early apoptotic cells, Annexin
V+/PI+ cells were considered end-stage
apoptotic cells and PI+ cells were considered necrotic
cells.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from rat NP tissues and
cells using TRIzol® reagent (Life Technologies; Thermo
Fisher Scientific, Inc.) according to manufacturer’s protocol.
Following this, the mRNA was reverse transcribed into cDNA using a
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China) according to manufacturer’s protocol. Then, the cDNA was
used as the template to perform qPCR (BeyoFast™ SYBR
Green qPCR Mix; Beyotime Institute of Biotechnology) with the
following thermocycler conditions: 95°C for 5 sec, then 45 cycles
comprising denaturing at 95°C for 30 sec, annealing at 60°C for 20
sec and extending at 72°C for 30 sec on an ABI 7500 Real-time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
primers used are listed in Table
I. Gene expression levels were normalized to GAPDH, and were
analyzed using the 2−ΔΔCq method (31).
 | Table IPrimers for reverse transcription
quantitative polymerase chain reaction. |
Table I
Primers for reverse transcription
quantitative polymerase chain reaction.
| Gene | Primers | Sequences
(5′-3′) |
|---|
| Collagen-II-h | Forward |
GGCAATAGCAGGTTCACGTACA |
| Reverse C |
GATAACAGTCTTGCCCCACTT |
| SOX9-h | Forward |
AGCGAACGCACATCAAGAC |
| Reverse C |
TGTAGGCGATCTGTTGGGG |
| Aggrecan-h | Forward |
TCCACAAGGGAGAGAGGGTA |
| Reverse |
GTAGGTGGTGGCTAGGACGA |
| MMP-13-h | Forward |
GGCTCCGAGAAATGCAGTCTTTCTT |
| Reverse |
ATCAAATGGGTAGAAGTCGCCATGC |
| IL-6-h | Forward |
ATGAACTCCTTCTCCACAAGC |
| Reverse C |
TACATTTGCCGAAGAGCCCTCAGGCTGGACTG |
| IL-10-h | Forward |
AGGGCACCCAGTCTGAGAACA |
| Reverse C |
GGCCTTGCTCTTGTTTTCAC |
| TNF-α-h | Forward |
ATGAGCACTGAAAGCATGATC |
| Reverse |
TCACAGGGCAATGATCCCAAAGTAGACCTGCCC |
| GAPDH-h | Forward |
AAGGTCGGAGTCAACGGATTT |
| Reverse |
AGATGATGACCCTTTTGGCTC |
| Collagen-II-r | Forward |
ACGCTCAAGTCGCTGAACAA |
| Reverse |
TCAATCCAGTAGTCTCCGCTCT |
| SOX9-r | Forward |
TCCAGCAAGAACAAGCCACA |
| Reverse C |
GAAGGGTCTCTTCTCGCTC |
| Aggrecan-r | Forward |
TCCAAACCAACCCGACAAT |
| Reverse |
TCTCATAGCGATCTTTCTTCTGC |
| MMP-13-r | Forward |
ATGCAGTCTTTCTTCGGCTTAG |
| Reverse |
ATGCCATCGTGAAGTCTGGT |
| IL-6-r | Forward CC |
TCTGGTCTTCTGGAGTACC |
| Reverse |
ACTCCTTCTGTGACTCCAGC |
| IL-10-r | Forward |
ATAACTGCACCCACTTCCCA |
| Reverse |
GGGCATCACTTCTACCAGGT |
| TNF-α-r | Forward |
ATGAGCACAGAAAGCATGA |
| Reverse |
AGTAGACAGAAGAGCGTGGT |
| GAPDH-r | Forward |
GGAAAGCTGTGGCGTGAT |
| Reverse |
AAGGTGGAAGAATGGGAGTT |
ELISA assay
The level of CTX-II in rat serum and culture
supernatant following 1,000 × g centrifugation at room temperature
for 5 min was detected using CTX ELISA kit (cat. no. E-EL-M0368c;
Ela bscience, Wuhan, China) according to the manufacturer’s
protocols.
Western blot analysis
Total proteins in NP tissues and cells were isolated
by using the radioimmunoprecipitation assay lysis buffer (Beyotime,
Nanjing, China) containing 1X protease inhibitor cocktail and 1X
PhosStop (both, Roche Diagnostics, Indianapolis, IN, USA). The
concentration of protein was determined by using a BCA protein
assay kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer’s protocol. Then, the protein extracts were boiled
with equal volumes of loading buffer (Beyotime Institute of
Biotechnology) for 10 min. Following this, 15 μg total
protein was loaded onto 10% SDS-PAGE gels for electrophoresis, and
then electro-transferred onto polyvinylidene fluoride membranes.
Following this, the membranes were blocked with 5% non-fat milk
solution in TBS-Tween 20 at room temperature for 1 h. Subsequently,
membranes were incubated with the following anti-rat primary
antibodies (1:1,000) at 4°C overnight: Cleaved caspase-3 (cat. no.
9662; Cell Signaling Technology, Inc., Danvers, MA, USA), PARP
(cat. no. 9542; Cell Signaling Technology, Inc.), collagen II (cat.
no. ab34712; Abcam, Cambridge, United Kingdom), aggrecan (cat. no.
ab3778; Abcam), transcription factor SOX9 (SOX9; cat. no. ab185966;
Abcam), MMP-13 (cat. no. 94808; Cell Signaling Technology, Inc.),
phosphorylated (p)-PI3K (cat. no. 4228; Cell Signaling Technology,
Inc.), PI3K (cat. no. 4249; Cell Signaling Technology, Inc.), p-AKT
(cat. no. 4685; Cell Signaling Technology, Inc.), AKT (cat. no.
4060; Cell Signaling Technology, Inc.) and GAPDH (cat. no. ab8245;
Abcam). Following this, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies
(anti-rabbit, cat. no. ab150077; anti-mouse, cat. no. ab6785; both,
1:5,000; both, Abcam) at room temperature for 1 h. The protein
bands in the membranes were then visualized using the enhanced
chemiluminescent method (EMD Millipore, Billerica, MA, USA), and
quantified using Quantity One software 4.2.1 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). GAPDH was used as the internal control to
normalize the expression of the proteins.
Statistical analysis
In the present study, all data are presented as mean
± standard deviation. Statistical analyses were performed using
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical evaluation was performed using Student’s t-test
(two-tailed) between two groups, or one-way analysis of variance
followed by Tukey’s post hoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
BMP2 treatment alleviates IDD in
rats
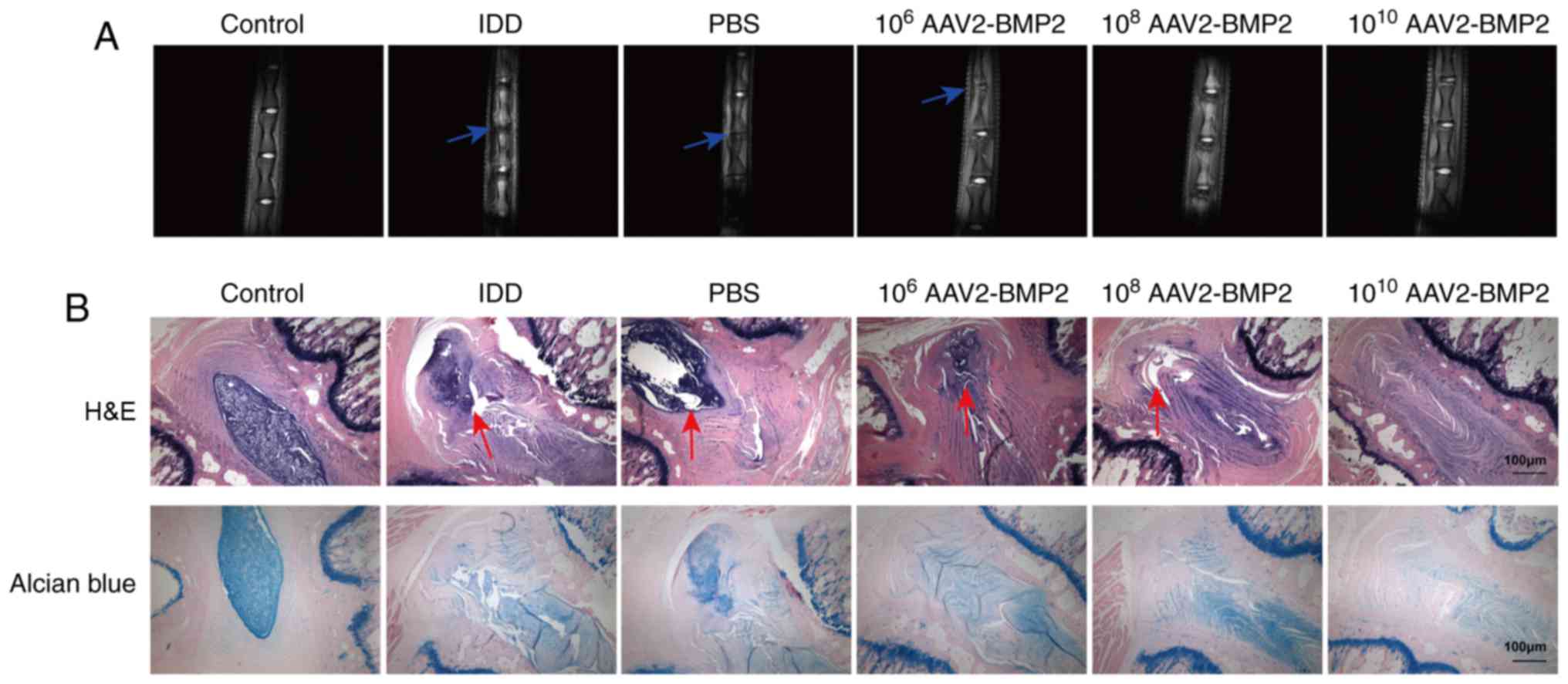
The pathogenic symptoms of IDD in each group were
determined using MRI. Compared with the control group, the interval
between punctured discs was significantly decreased in the IDD rat
model, as indicated by the blue arrows, while AAV2-BMP2 treatment
markedly inhibited the decrease in intervals between punctured
discs in the IDD model in a dose-dependent manner (Fig. 1A). Additional H&E assays
revealed that there were clearly intact discs with well-opposed
lamellae and unbroken annulus fibrosus with clearly-defined borders
in the control group, but the IDD model group presented severe
lamellar disorganization and broken annulus fibrosus, as indicated
by the red arrows. AAV2-BMP2 treatment markedly alleviated the
disorganization of lamellae and protected the annulus fibrosus
structure of the discs (Fig. 1B).
Alcian Blue staining indicated that the secretion of glycoproteins
was significantly downregulated in the IDD model, and that
AAV2-BMP2 injection attenuated this downregulation (Fig. 1B). All these data indicate that
BMP2 may alleviate the symptoms of IDD in vivo.
BMP2 treatment regulates the levels of
IDD-associated biomarkers and inflammatory cytokines
Following sacrifice, the levels of collagen II,
aggrecan, SOX9, MMP-13 and CTX-II in NP tissues of each group were
determined by RT-qPCR. As a result, the levels of collagen II,
aggrecan and SOX9 were significantly downregulated in the IDD group
compared with the control group, while BMP2 overexpression
significantly attenuated these changes in IDD in a dose-dependent
manner (Fig. 2A). However, the
serum levels of MMP-13 in NP tissues and CTX-II were significantly
increased in IDD group compared with the control group, and
overexpression of BMP2 significantly inhibited the upregulation of
MMP-13 and CTX-II in a dose-dependent manner (Fig. 2A and B). IL-6, TNF-α and IL-10
levels in NP tissues were additionally examined using RT-qPCR. It
was identified that BMP2 significantly inhibited the mRNA levels of
proinflammatory cytokines IL-6 and TNF-α, but significantly
increased the levels of anti-inflammatory cytokine IL-10 mRNA, in a
dose-dependent manner (Fig. 2C).
Taken together, these results suggest that BMP2 may suppress the
degradation of the extracellular matrix and inflammatory response
during IDD.
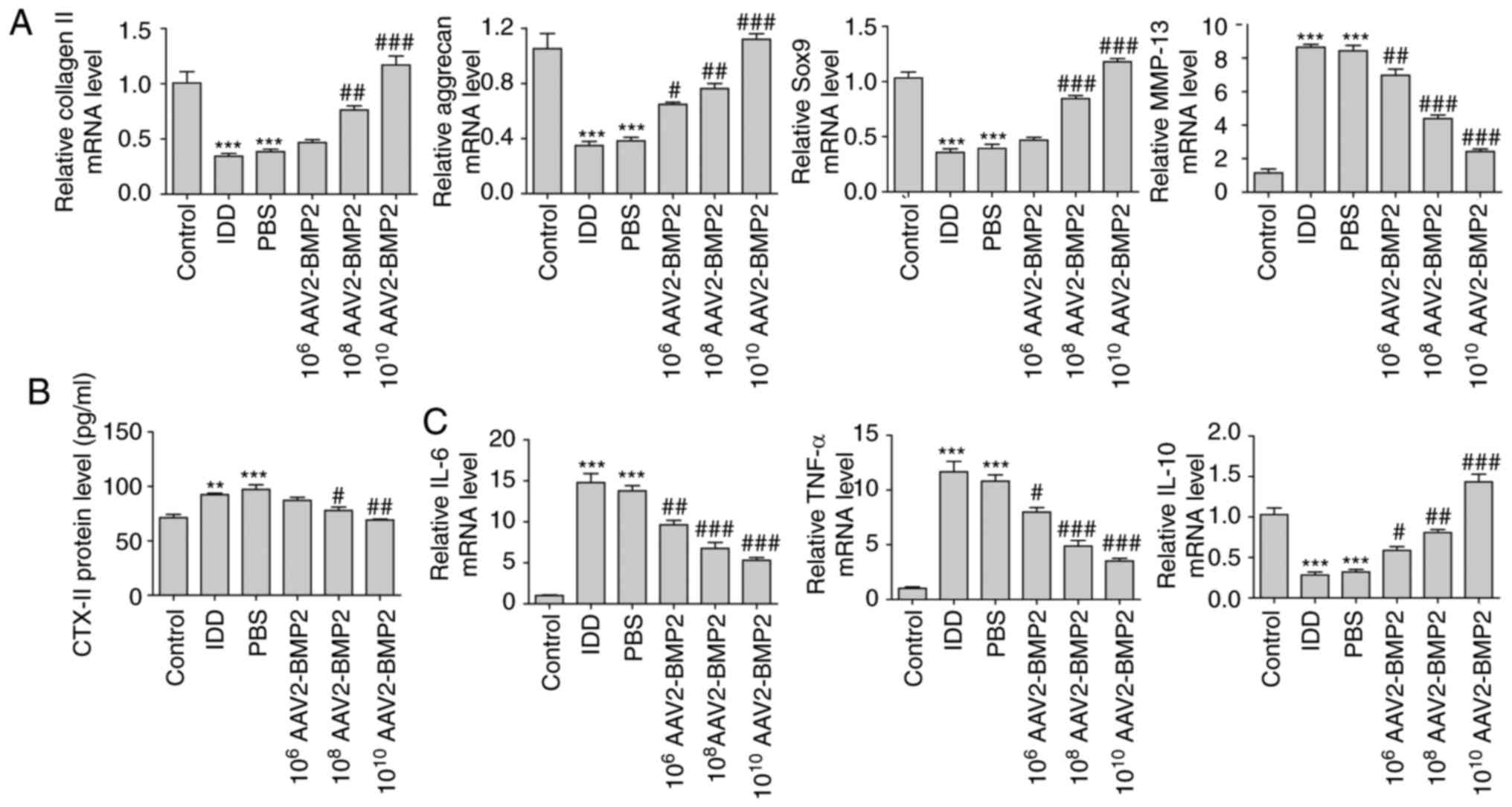 | Figure 2BMP2 treatment regulates the levels
of IDD-associated biomarkers and inflammatory cytokines in NP
tissues. (A) Relative mRNA levels of collagen II, aggrecan, SOX9
and MMP-13 in NP tissues determined using RT-qPCR. (B) Level of
CTX-II in the serum of rats determined using ELISA. (C) Relative
mRNA levels of IL-6, TNF-α and IL-10 determined using RT-qPCR.
**P<0.01 and ***P<0.001 vs. control
group. #P<0.05, ##P<0.01 and
###P<0.001 vs. IDD group. IDD, intervertebral disc
degeneration; AAV2-BMP2, adeno-associated virus serotype 2-bone
morphogenic protein 2; SOX9, transcription factor SOX9; MMP-13,
matrix metalloproteinase 13; CTX-II, C-telopeptide of type II
collagen; IL, interleukin; TNF-α, tumor necrosis factor α; RT-qPCR,
reverse transcription quantitative polymerase chain reaction; NP,
nucleus pulposus. |
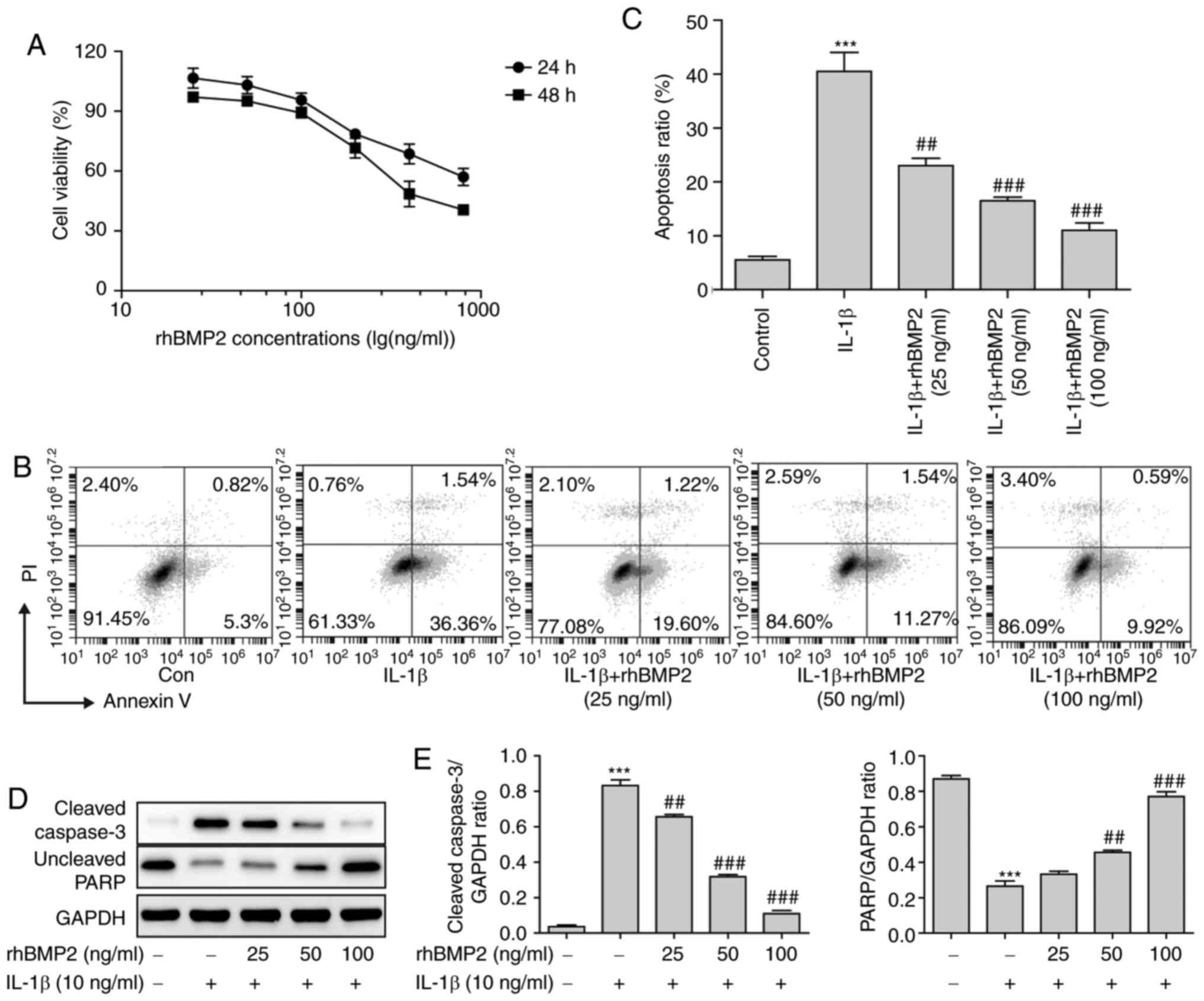
rhBMP2 treatment inhibits the apoptosis
of NP cells during IDD
To explore the underlying effects of BMP2 on the
alleviation of IDD in vitro, an IDD cell model was
constructed using IL-1β, and the viability of NP cells following
treatment with rhBMP2 was determined. As a result, rhBMP2 markedly
inhibited the survival ability of NP cells when being treated with
high concentrations at 24 and 48 h (Fig. 3A). Therefore, the lower
concentrations (25, 50 and 100 ng/ml) were selected for subsequent
experiments. Compared with the control group, IL-1β treatment
significantly increased the levels of apoptosis of NP cells, and
rhBMP2 pretreatment markedly attenuated the apoptosis ratio induced
by IL-1β in the NP (Fig. 3B and
C). Concurrently, the levels of apoptosis-associated proteins
were also determined by western blot analysis. IL-1β treatment
significantly increased the level of cleaved caspase-3 but
decreased the level of uncleaved (full length) poly [adenosine
5′-diphosphate (ADP)-ribose] polymerase (PARP) compared with the
control group, while rhBMP2 pretreatment significantly inhibited
these changes in a dose-dependent manner (Fig. 3D and E). These results indicated
that BMP2 inhibited apoptosis and promoted the survival ability of
NP cells in a dose-dependent manner during the pathogenesis of
IDD.
rhBMP2 attenuates the levels of
IDD-associated proteins and inflammatory response induced by IL-1β
in NP cells
The levels of collagen II, aggrecan, SOX9 and MMP-13
in cells, and levels of CTX-II in the supernatant were also
determined in vitro. It was identified that the mRNA levels
of collagen II, aggrecan and SOX9 were significantly downregulated,
while the level of MMP-13 was significantly increased in the cells
following treatment with IL-1β. Additionally, rhBMP2 pretreatment
significantly attenuated these changes in a dose-dependent manner
(Fig. 4A). The western blot
analysis results also indicated that IL-1β treatment markedly
decreased the protein levels of collagen II, aggrecan and SOX9, but
increased the level of MMP-13 in NP cells, while rhBMP2 treatment
inhibited these changes in a dose-dependent manner (Fig. 4B and C). In addition, the ELISA
assay results demonstrated that IL-1β significantly increased the
level of CTX-II, and rhBMP2 pre-treatment suppressed this increase
in a dose-dependent manner (Fig.
4D). IL-6, TNF-α and IL-10 levels in the NP cells were also
examined using RT-qPCR, as demonstrated in Fig. 4E. It was identified that rhBMP2
exhibited similar effects on the levels of IL-6, TNF-α and IL-10 in
NP tissues when its concentration was at 25 and 50 ng/ml. When
using high doses (100 ng/ml) of BMP2, no significant difference in
the effect was observed. All of these data indicated that rhBMP2
may alleviate the decrease in matrix protein secretion and inhibit
the inflammatory response in NP cells.
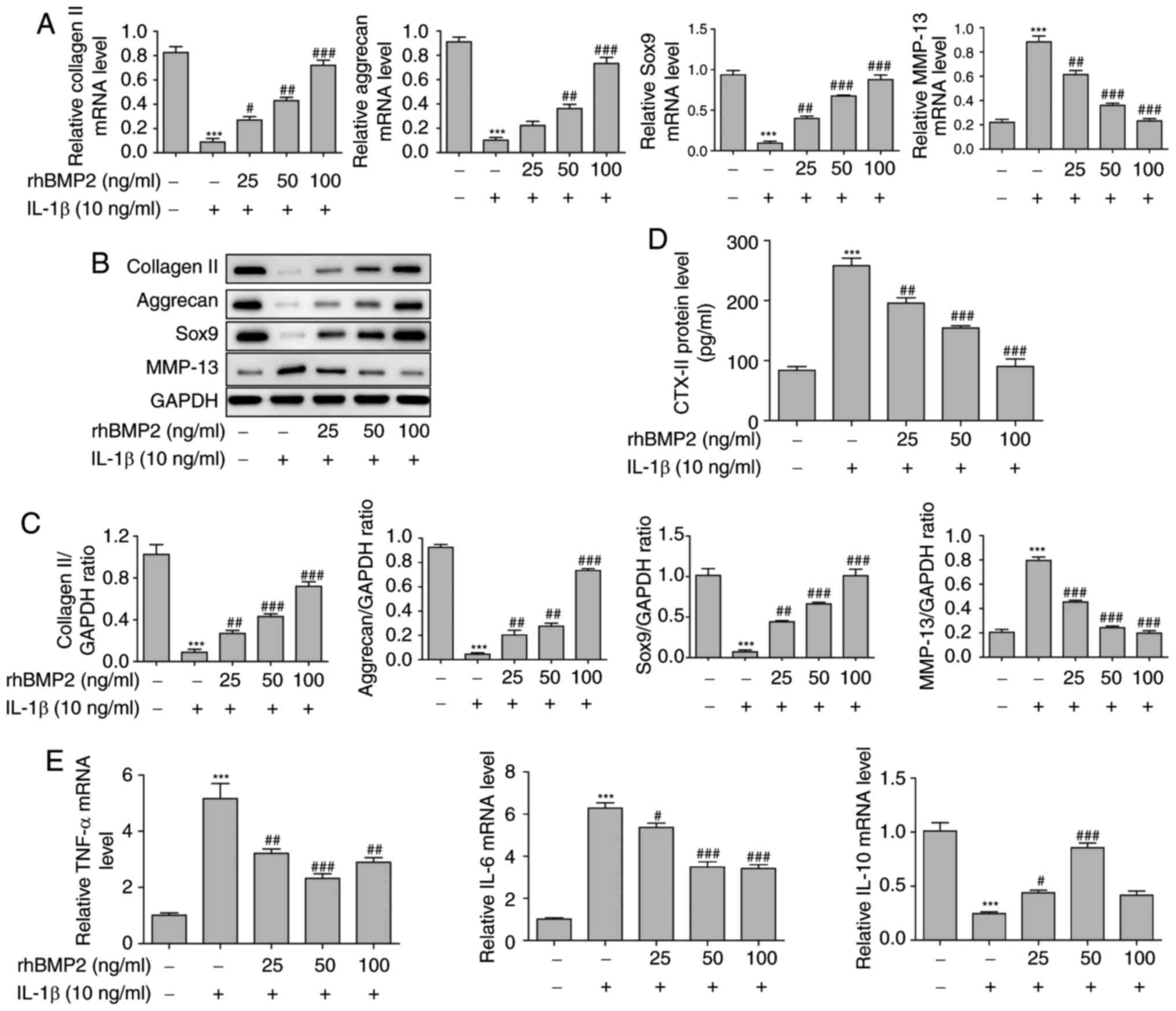 | Figure 4rhBMP2 attenuates the IDD-associated
proteins and inflammatory response induced by IL-1β in NP cells.
(A) Relative mRNA levels of collagen II, aggrecan, SOX9 and MMP-13
in NP cells determined by using RT-qPCR. (B) Levels of collagen II,
aggrecan, SOX9 and MMP-13 in NP cells determined by western blot
analysis. (C) Quantification of western blot analysis results of
collagen II, aggrecan, SOX9 and MMP-13 in NP cells. (D) Level of
CTX-II in the supernatant of NP cells determined by ELISA. (E)
Relative mRNA levels of IL-6, TNF-α and IL-10 in NP cells
determined by RT-qPCR. ***P<0.001 vs. control group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. the IL-1β treated group. SOX9,
transcription factor SOX9; MMP-13, matrix metalloproteinase 13;
CTX-II, C-telopeptide of type II collagen; IL, interleukin; TNF-α,
tumor necrosis factor α; RT-qPCR, reverse transcription
quantitative polymerase chain reaction; rhBMP2, recombinant human
bone morphogenic protein 2; NP, nucleus pulposus. |
rhBMP2 inhibits the levels of
extracellular matrix degradation and apoptosis of NP cells via the
PI3K/Akt signaling pathway
To additionally reveal the mechanisms of
extracellular matrix degradation and apoptosis of NP cells during
IDD, the proteins involved in the PI3K/Akt signaling pathway were
examined. It was identified that the levels of total PI3K and Akt
protein were not changed following treatment of NP cells with IL-1β
and rhBMP2. However, the levels of phosphorylation of PI3K and Akt
in NP cells were significantly decreased following IL-1β treatment.
Additionally, rhBMP2 pretreatment significantly upregulated the
levels of phosphorylation of PI3K and Akt in a dose-dependent
manner (Fig. 5A and B). To
additionally confirm whether the PI3K/Akt pathway was involved in
IDD, LY294002, an inhibitor for PI3K, was utilized. Firstly, levels
of phosphorylated Akt and total Akt in response to IL-1β, rhBMP2
and LY294002 treatment we examined by western blot analysis to
verify the function of LY294002 and the PI3K/Akt pathway, and the
results indicated that LY294002 markedly inhibited the level of
phosphorylation of Akt and reversed the effects induced by rhBMP2
(Fig. 5C and D). Additional
results indicated that LY294002 treatment significantly attenuated
the increase in collagen II, aggrecan and SOX9 levels, and the
decrease in MMP-13 and CTX-II levels caused by rhBMP2 in
1L-1β-treated NP cells (Fig. 5E and
F). Pretreatment with LY294002 also markedly inhibited the
decrease of cleaved caspase-3 and the increase of uncleaved PARP
induced by rhBMP2 in 1L-1β-treated NP cells (Fig. 5G). Flow cytometry was also used to
examine cell apoptosis following treatment with combinations of
IL-1β, rhBMP2 and LY294002. It was identified that IL-1β
significantly increased the apoptosis rate of NP cells, but rhBMP2
significantly inhibited the IL-1β-induced apoptosis rate of NP
cells. Additionally, LY294002 reversed the effects of rhBMP2, and
caused a significant increase in the apoptosis rate of
rhBMP2-treated NP cells (Fig. 5H
and 5I). All of these results
suggest that rhBMP2 attenuated the levels of apoptosis and
extracellular matrix degradation of NP cells, potentially via the
PI3K/Akt signaling pathway.
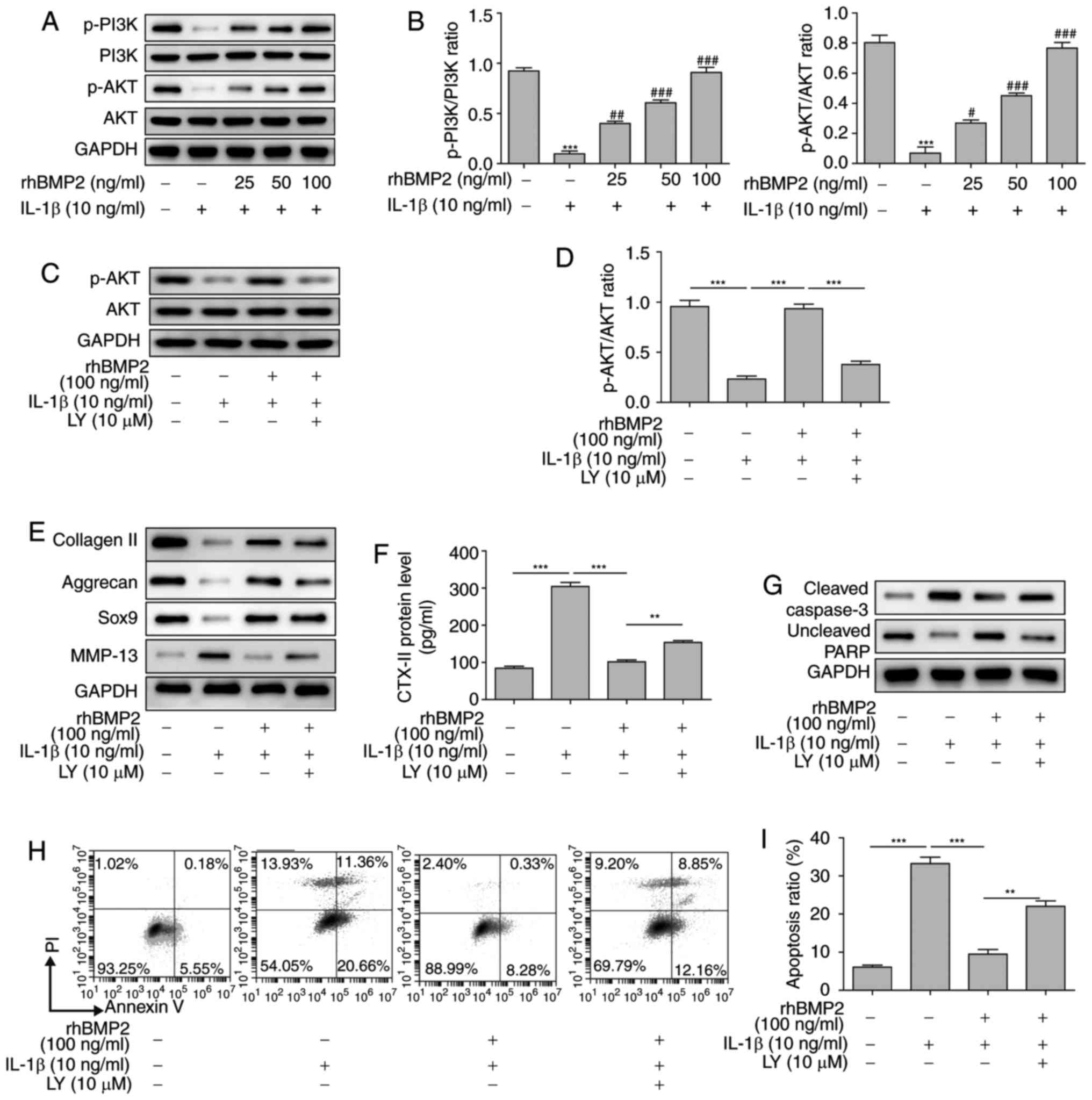 | Figure 5rhBMP2 inhibits the extracellular
matrix degradation and apoptosis of NP cells via the PI3K/Akt
signaling pathway. (A) Levels of phosphorylation of PI3K and Akt
determined by western blot analysis. (B) Quantification of western
blot analysis results from part A. (C) Levels of phosphorylated Akt
and total Akt in response to IL-1β, rhBMP2 and LY294002 treatment
as determined by western blot analysis. (D) Quantification of
western blot analysis results from part C. (E) Effects of LY294002
treatment on the protein levels involved in the extracellular
matrix as determined by western blot analysis. (F) Effects of
LY29004 treatment on the level of CTX-II in the supernatant of NP
cells determined by ELISA. (G) Levels of apoptosis-associated
proteins as determined by western blot analysis. (H) Flow cytometry
analysis of cell apoptosis following treatment with combinations of
rhBMP2, IL-1β and LY294002. (I) Quantification of flow cytometry
results from part H. **P<0.01;
***P<0.001 vs. control group. #P<0.05,
##P<0.01 and ###P<0.001 vs.
IL-1β-treated group. rhBMP2, recombinant human bone morphogenic
protein 2; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B;
IL, interleukin; LY, LY29004; SOX9, transcription factor SOX9;
MMP-13, matrix metalloproteinase 13; CTX-II, C-telopeptide of type
II collagen; p-phosphorylated; PI, propidium iodide; NP, nucleus
pulposus. |
Discussion
IDD is a common chronic disease contributing to
lower back pain, and is considered to be one of the most important
public health problems worldwide (32). Although a number of trials have
been performed to identify treatments for IDD, the situation
remains unresolved, owing to the lack of clear understanding of the
initiation and pathogenesis of IDD. Considering this, BMP2 was
utilized in the present study as a novel promising treatment for
IDD via targeting ECM degradation, and proliferation and apoptosis
levels of NP cells. Furthermore, the underlying mechanism of BMP2
in treating IDD was examined to provide novel insights on the
pathogenesis and treatment of IDD.
To explore the pathogenesis of IDD, a needle
puncture model in rats was constructed. The results demonstrated
that AAV2-BMP2 injection significantly alleviated the fibrous ring
rupture and glycoproteins degradation of IDD in a dose-dependent
manner, as determined by MRI and histological analyses. Leckie
et al (33) also
identified that BMP2 pretreatment delayed the onset of degenerative
changes, as measured by MRI, histological examination and serum
biomarkers. BMP2 is one of a group of BMPs belonging to the TGF-β
family, and serves critical roles in skeletal development and
repair (34). Concurrently, BMP2
may also directly promote the synthesis of collagen via stimulating
chondrocytes (35). Clinical data
suggested that rhBMP2 may also promote spinal fusion and bone
healing (23). Due to this
function, a significant inhibition in the degradation of collagen
and significant decrease in the production of CTX-II was observed
following AAV2-BMP2 injection in the present study. In addition,
the MMP-13 level was also significantly downregulated following
treatment with AAV2-BMP2 in IDD. These results suggest that BMP2
may promote the synthesis of collagen and inhibit the degradation
of collagen in ECM against IDD. Concomitantly, aggrecan, an
additional component of ECM, and its transcription factor SOX9
(36), were significantly
upregulated following AAV2-BMP2 injection in IDD. These data
indicate that BMP2 upregulated the level of aggrecan via promotion
of the transcription of SOX9 to attenuate the pathology of IDD.
Previous studies have indicated that certain pro-inflammatory
cytokines, including TNF-α, may stimulate the degradation of the
ECM of intervertebral discs (37,38). In the present study, it was
demonstrated that BMP2 inhibited TNF-α and IL-6 expression levels
and increased IL-10 expression, suggesting that BMP2 may alleviate
intervertebral disc ECM degradation by regulating inflammatory
factors. Considering these results, we hypothesize that BMP2 may
promote the synthesis of collagen and aggrecan, and inhibit the
degradation of ECM, to inhibit the pathogenesis of IDD.
To additionally reveal the underlying mechanism of
BMP2 in alleviating IDD (5), the
molecular mechanism of BMP2 in NP cells was explored in
vitro. As the secretion of inflammatory cytokines is a common
characteristic of IDD, IL-1β was used to induce an IDD cell model
in NP cells in the present study. Subsequently, it was identified
that pretreatment with rhBMP2 significantly inhibited the apoptosis
levels of NP cells. In addition, rhBMP2 pretreatment also
significantly upregulated the levels of collagen-II, aggrecan and
SOX9, but downregulated the levels of MMP-13 and CTX-II in NP
cells. The results were consistent with the results identified in
the IDD model of the present study. Previous studies have
demonstrated that the synthesis of aggrecan was closely correlated
with the phosphorylation of the PI3K/Akt signaling pathway, which
also performs crucial roles in cell growth, proliferation,
migration and invasion (26,39). Inhibiting the PI3K signaling
pathway leads to a decrease in the expression levels of aggrecan,
collagen II and SOX9 (26,40,41).
In the present study, no significantly differences in the levels of
total Akt and PI3K following treatment with IL-1β alone or in
combination with rhBMP2 were observed, but the levels of
phosphorylation of PI3K and Akt were significantly decreased
following treatment with IL-1β, and rhBMP treatment markedly
upregulated the levels of phosphorylation of PI3K and Akt in a
dose-dependent manner. The PI3K inhibitor LY29400 significantly
attenuated the effects of rhBMP2 on the phosphorylation of PI3K and
Akt, and reversed the effects on the levels of apoptosis and
IDD-associated proteins induced by rhBMP2 in IL-1β-treated NP
cells. Cheng et al (42)
also documented that PI3K/Akt regulated the expression levels of
aggrecan and SOX9 in NP cells. Taken together, all of these data
indicate that BMP2 may alleviate the levels of apoptosis and ECM
degradation in IDD via the PI3K/Akt signaling pathway.
In conclusion, inhibition of ECM degradation and NP
cells apoptosis, which are two primary characteristics of IDD, may
delay the process of IDD. Therefore, BMP2 may provide a potential
strategy for IDD treatment by increasing the production of collagen
II, aggrecan and SOX9, decreasing the levels of CTX-II and MMP-13
in the PI3K/Akt signaling pathway and increasing the survival of NP
cells. The data of the present study indicate that BMP2 may serve
as a promising therapeutic method in treating IDD. However, the
safety of BMP2 in clinical applications requires additional
examination.
Acknowledgments
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81401842).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
YT is the guarantor of integrity of the entire
study, and was responsible for the study concept, design and
manuscript preparation. XY was responsible for study conception and
literature research. ZD performed experiments and data acquisition.
YW conducted data analysis and statistical analysis. GL performed
manuscript drafting and conducted analysis and interpretation of
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental procedures were approved by the
Second Xiangya Hospital, Central South University (Changsha,
China). All the procedures in the present study involving animals
and their care were performed in accordance with the Guidelines for
the Care and Use of Laboratory Animals of the Second Xiangya
Hospital, Central South University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah AM, Kwon SYJ, Chan WC and Chan D:
Intervertebral disc degeneration. Cartilage: Springer; pp. 229–261.
2017
|
|
2
|
Lim KZ, Daly CD, Ghosh P, Jenkin G, Oehme
D, Cooper-White J, Naidoo T and Goldschlager T: Ovine lumbar
intervertebral disc degeneration model utilizing a lateral
retroperitoneal drill bit injury. J Vis Exp. May 23–2017.
View Article : Google Scholar
|
|
3
|
Muriuki MG, Havey RM, Voronov LI,
Carandang G, Zindrick MR, Lorenz MA, Lomasney L and Patwardhan AG:
Effects of motion segment level, Pfirrmann intervertebral disc
degeneration grade and gender on lumbar spine kinematics. J Orthop
Res. 34:1389–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Phillips KL, Cullen K, Chiverton N,
Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK
and Le Maitre CL: Potential roles of cytokines and chemokines in
human intervertebral disc degeneration: Interleukin-1 is a master
regulator of catabolic processes. Osteoarthritis Cartilage.
23:1165–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang S, Liu C, Sun Z, Yan P, Liang H,
Huang K, Li C and Tian J: IL-1β increases asporin expression via
the NF-κB p65 pathway in nucleus pulposus cells during
intervertebral disc degeneration. Sci Rep. 7:41122017. View Article : Google Scholar
|
|
6
|
Nelson AE and Jordan JM: Osteoarthritis:
Epidemiology and classification. Rheumatology. Hochberg M, Silman
AJ, Smolen J, Weinblatt M and Weisman M: 6th edition. Elsevier;
Philadelphia PA: pp. 1433–1440. 2014
|
|
7
|
Segar A, Urban J and Fairbank JCT:
Adipokines and the intervertebral disc: A biochemical link exists
between obesity, intervertebral disc degeneration and low back
pain. Spine J. 16(Suppl): S2252016. View Article : Google Scholar
|
|
8
|
Dong S, Peng L, Jie C, Ma Z, Liu J and Qin
T: Correlation between intervertebral disc degeneration, paraspinal
muscle atrophy, and lumbar facet joints degeneration in patients
with lumbar disc herniation. BMC Musculoskeletal Disorders.
18:1672017. View Article : Google Scholar
|
|
9
|
Sakai D and Grad S: Advancing the cellular
and molecular therapy for intervertebral disc disease. Adv Drug
Deliv Rev. 84:159–171. 2015. View Article : Google Scholar
|
|
10
|
Wang AM, Cao P, Yee A, Chan D and Wu EX:
Detection of extracellular matrix degradation in intervertebral
disc degeneration by diffusion magnetic resonance spectroscopy.
Magn Reson Med. 73:1703–1712. 2015. View Article : Google Scholar
|
|
11
|
Gou S, Oxentenko SC, Eldrige JS, Xiao L,
Pingree MJ, Wang Z, Perez-Terzic C and Qu W: Stem cell therapy for
intervertebral disk regeneration. Am J Phys Med Rehabil. 93(11
Suppl 3): S122–S131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Deng X, Xu H, Wu S, Yuan L, Yang
Z, Yang Y and Deng H: Identification of a novel mutation in the
COL2A1 gene in a Chinese Family with Spondyloepiphyseal dysplasia
Congenita. PLoS One. 10:e01275292015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barathouari M, Sarrabay G, Gatinois V,
Fabre A, Dumont B, Genevieve D and Touitou I: Mutation update for
COL2A1 gene variants associated with type II collagenopathies. Hum
Mutat. 37:7–15. 2016. View Article : Google Scholar
|
|
15
|
Xu YQ, Zhang ZH, Zheng YF and Feng SQ:
Dysregulated miR-133a mediates loss of type II collagen by directly
targeting matrix metalloproteinase 9 (MMP9) in human intervertebral
disc degeneration. Spine (Phila Pa 1976). 41:E717–E724. 2016.
View Article : Google Scholar
|
|
16
|
Xin L, Wu Z, Qu Q, Wang R, Tang J and Chen
L: Comparative study of CTX-II, Zn2+, and Ca2+ from the urine for
knee osteoarthritis patients and healthy individuals. Medicine
(Baltimore). 96:e75932017. View Article : Google Scholar
|
|
17
|
Yang SD, Yang DL, Sun YP, Wang BL, Ma L,
Feng SQ and Ding WY: 17β-estradiol protects against apoptosis
induced by interleukin-1β in rat nucleus pulposus cells by
down-regulating MMP-3 and MMP-13. Apoptosis. 20:348–357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller SL, Coughlin DG, Waldorff EI, Ryaby
JT and Lotz JC: Pulsed electromagnetic field (PEMF) treatment
reduces expression of genes associated with disc degeneration in
human intervertebral disc cells. Spine J. 16:770–776. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye S, Ju B, Wang H and Lee KB: Bone
morphogenetic protein-2 provokes interleukin-18-induced human
intervertebral disc degeneration. Bone Joint Res. 5:412–418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khosla S, Westendorf JJ and Oursler MJ:
Building bone to reverse osteoporosis and repair fractures. J Clin
Invest. 118:421–428. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
McKay WF, Peckham SM and Badura JM: A
comprehensive clinical review of recombinant human bone
morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop.
31:729–734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sobajima S, Shimer AL, Chadderdon RC,
Kompel JF, Kim JS, Gilbertson LG and Kang JD: Quantitative analysis
of gene expression in a rabbit model of intervertebral disc
degeneration by real-time polymerase chain reaction. Spine J.
5:14–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bougioukli S, Jain A, Sugiyama O, Tinsley
BA, Tang AH, Tan MH, Adams DJ, Kostenuik PJ and Lieberman JR:
Combination therapy with BMP-2 and a systemic RANKL inhibitor
enhances bone healing in a mouse critical-sized femoral defect.
Bone. 84:93–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng Y, Wang X, Wang H, Yan W, Zhang Q
and Chang X: Bone morphogenetic protein 2 inhibits hepatocellular
carcinoma growth and migration through downregulation of the
PI3K/AKT pathway. Tumor Biol. 35:5189–5198. 2014. View Article : Google Scholar
|
|
25
|
Qu Z, Guo S, Fang G, Cui Z and Ying L: AKT
pathway affects bone regeneration in nonunion treated with
umbilical cord-derived mesenchymal stem cells. Cell Biochem
Biophys. 71:1543–1551. 2015. View Article : Google Scholar :
|
|
26
|
Ouyang ZH, Wang WJ, Yan YG, Wang B and Lv
GH: The PI3K/Akt pathway: A critical player in intervertebral disc
degeneration. Oncotarget. 8:57870–57881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonino F, Heermann KH, Rizzetto M and
Gerlich WH: Hepatitis delta virus: Protein composition of delta
antigen and its hepatitis B virus-derived envelope. J Virol.
58:945–950. 1986.PubMed/NCBI
|
|
28
|
Issy AC, Castania V, Castania M, Salmon
CE, Nogueira-Barbosa MH, Bel ED and Defino HL: Experimental model
of intervertebral disc degenerationby needle puncture in Wistar
rats. Braz J Med Biol Res. 46:235–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G
and Liu J: Leptin induces cyclin D1 expression and proliferation of
human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK
pathways. PLoS One. 7:e531762012. View Article : Google Scholar
|
|
30
|
Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G
and Liu J: The role of leptin on the organization and expression of
cytoskeleton elements in nucleus pulposus cells. J Orthop Res.
31:847–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Wang XF, Zhang AP, Sun ZY, Liu C, Kuang LH
and Tian JW: Expression of NF-κB in a degenerative human
intervertebral disc model. Zhonghua Yi Xue Za Zhi. 97:1324–1329.
2017.In Chinese. PubMed/NCBI
|
|
33
|
Leckie SK, Bechara BP, Hartman RA, Sowa
GA, Woods BI, Coelho JP, Witt WT, Dong QD, Bowman BW, Bell KM, et
al: Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus
slows the course of intervertebral disc degeneration in an in vivo
rabbit model. Spine J. 12:7–20. 2012. View Article : Google Scholar
|
|
34
|
Leung VY, Zhou L, Tam WK, Sun Y, Lv F,
Zhou G and Cheung KMC: Bone morphogenetic protein -2 and -7 mediate
the anabolic function of nucleus pulposus cells with discrete
mechanisms. Connect Tissue Res. 58:573–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lafont JE, Poujade FA, Pasdeloup M, Neyret
P and Mallein-Gerin F: Hypoxia potentiates the BMP-2 driven COL2A1
stimulation of human articular chondrocytes via p38 MAPK.
Osteoarthritis Cartilage. 24:856–867. 2016. View Article : Google Scholar
|
|
36
|
Kumar A, Oz MB, Elayyan J, Reich E,
Binyamin M, Kandel L, Liebergall M, Steinmeyer J and Lefebvre V:
SOX9 acetylation reduces aggrecan expression in adult human
chondrocytes. Osteoarthritis and Cartilage. 24:S1542016. View Article : Google Scholar
|
|
37
|
Xu K, Wang X, Zhang Q, Liang A, Zhu H,
Huang D, Li C and Ye W: Sp1 downregulates proinflammatory
cytokine-induced catabolic gene expression in nucleus pulposus
cells. Mol Med Rep. 14:3961–3968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walter BA, Purmessur D, Moon A,
Occhiogrosso J, Laudier DM, Hecht AC and Iatridis JC: Reduced
tissue osmolarity increases TRPV4 expression and pro-inflammatory
cytokines in intervertebral disc cells. Eur Cell Mater. 32:123–136.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kita K, Kimura T, Nakamura N, Yoshikawa H
and Nakano T: PI3K/Akt signaling as a key regulatory pathway for
chondrocyte terminal differentiation. Genes Cells. 13:839–850.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li N, Cui J, Duan X, Chen H and Fan F:
Suppression of type I collagen expression by miR-29b via PI3K, Akt,
and Sp1 pathway in human Tenon’s fibroblasts. Invest Ophthalmol Vis
Sci. 53:1670–1678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng CC, Uchiyama Y, Hiyama A, Gajghate
S, Shapiro IM and Risbud MV: PI3K/AKT regulates aggrecan gene
expression by modulating Sox9 expression and activity in nucleus
pulposus cells of the intervertebral disc. J Cell Physiol.
221:668–676. 2009. View Article : Google Scholar : PubMed/NCBI
|