Introduction
Congenital heart disease (CHD) is the most common
congenital defect globally (1-3).
The incidence of neonatal morbidity is 0.8-1.0% (4), and 30% of newborns with CHD succumb
without radical treatment. Therefore, further investigation into
CHD prevention and treatment is of vital importance. The heart is
the first organ to become functional during embryological
development. Its developmental process is complex and disturbed
easily, involving the precise regulation of a number of gene
signals. The minor errors during development can lead to the lesion
of the atrium, ventricle, valve and other heart structures, which
results in the occurrence of CHD.
microRNAs (miRNAs/miRs) are a type of non-coding
single-stranded RNA of ~22 nucleotides in length, which have
important regulatory functions within cells (5). In recent years, miRNAs have received
increasing attention in the field of heart development (6-8).
We previously identified that miR-29c-3p in serum from patients
with CHD, and that its expression was significantly increased in
the serum of pregnant women carrying fetuses with CHD compared with
in that of normal pregnant women (9). Although miR-29c-3p may be associated
with the development of CHD, the majority of current miR-29c-3p
research focuses on cancer, with few studies describing its
function in heart development. Shu et al (10) identified that the expression of
miR-29c-5p was significantly downregulated in gallbladder carcinoma
and was markedly associated with lymph node metastasis. Han et
al (11) identified that loss
of miR-29c-3p expression was an early event in the initiation of
gastric carcinogenesis. Other studies have revealed that miR-29c-3p
was associated with lung cancer (12), nasopharyngeal carcinoma (13), acute myeloid leukemia (14) and breast cancer (15).
The phosphoinositide 3-kinase (PI3K)/protein kinase
Bγ (Akt3) signaling pathway serves an important function in cardiac
development and disease occurrence. The PI3K/Akt3 signaling pathway
is involved in heart ischemia/reperfusion injury (16), myocardial hypertrophy (17) and myocardial cell reshaping
(18), but it can also affect
normal heart development (19) by
regulating cardiac progenitor cell proliferation. As an important
factor in the PI3K/Akt3 signaling pathway, Akt3 was selected for
investigation in the present study.
The P19 cell line is derived from the CH3/He mouse
teratocarcinoma and can differentiate into three germ layers:
Endodermal phenotype, mesodermal phenotype and neuronal phenotype
(20). At low concentrations of
dimethylsulfoxide (DMSO), P19 cells aggregate and are induced to
differentiate into cardiomyocytes (21-24). Therefore, P19 cells are used
widely as a myocardial cell model of cardiomyocyte differentiation
in vitro (25,26).
The aim of the present study was to investigate the
function of miR-29c-3p and Akt3 in the occurrence of CHD.
Transfection technology was used to research the effect of
miR-29c-3p overexpression on the function of P19 cells, which are a
classic model of embryonic heart development.
Materials and methods
Cell culture and differentiation
Murine P19 embryonal carcinoma cells were obtained
from Nanjing Maternity and Child Health Care Hospital Affiliated to
Nanjing Medical University (Jiangsu Province, China). Cells were
cultured in α-minimal essential medium with 10% fetal bovine serum
(both from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in
an incubator containing 5% CO2. To induce P19 cell
differentiation, DMSO (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to the culture medium at a final concentration
of 1.0% (27-29). P19 cells aggregated over the
following 4 days to form embryoid bodies (EBs), during which time
the medium was replenished every 24 h. On day 4, the EBs were
transferred into a 6-well plate and cultured in DMSO-free medium to
allow adherent proliferation of P19 cells. The culture medium was
refreshed every 48 h during the ensuing differentiation. The P19
cells were confirmed to be successfully induced into cardiomyocytes
when the beating cardiomyocytes were observed under a microscope.
Images of the EBs and differentiated cell patches were captured
microscopically using a Primovert ZM300FL fluorescence microscope
(Carl Zeiss AG, Oberkochen, Germany).
Transfection of miR-29c-3p mimic and
small interfering RNA (siRNA)
At 1 day before transient transfection, P19 cells
were plated in 6-well plates at a density of 2×104
cells/well and incubated at 37°C. A miR-29c-3p mimic (forward,
5′-UAG CAC CAU UUG AAA UCG GUU A-3′; reverse, 5′-UAA CCG AUU UCA
AAU GGU GCU A-3′; 100 nM) synthesized by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China) was used to produce
miR-29c-3p-overexpressing cells, 100 nM si-Akt3 (5′-GCT CAT TCA TAG
GCT ATA A-3′; Guangzhou RiboBio Co., Ltd.) was transfected for Akt3
knockdown, whereas scrambled oligo-nucleotides served as negative
controls. Transfection of cells at 30-40% confluence was achieved
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer’s protocol.
Briefly, two mixtures were prepared separately: Mixture A [125
µl Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) and 5
µl Lipofectamine 3000] and mixture B (125 µl Opti-MEM
and 10 µl mimic). si-Akt3 and negative control mixtures were
prepared in the same manner. The two mixtures were left to stand at
room temperature for 5 min, then mixed and left for a further 30
min. The mixture was then added to each well of the 6-well plate.
At 48 h after transfection, the transfection efficiency was
determined. miR-29c-3p fluorescence was determined using
fluorescence microscopy, whereas miR-29c-3p and Akt3 mRNA
expression were determined using the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), and
Akt3 protein levels were determined by western blotting.
Cell proliferation and cell cycle
assays
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay was used to determine
the proliferation rate of P19 cells. P19 cells at a density of
1×103 cells/well were plated in 96-well plates.
Following incubation for 24 h, transfection of the miR-29c-3p
mimic/si-Akt3/negative control was performed as aforementioned.
Following a further 24 h, 10 µl CCK-8 reagent was added to
each well, according to the manufacturer’s protocol, and the cells
were maintained at 37°C for 2 h under 5% CO2. The cell
proliferation was determined by measuring the optical density (OD)
at 450 nm using a Multiskan GO microplate reader (Thermo Fisher
Scientific, Inc.). Cells were monitored every 24 h over the
following 4 days.
For cell cycle analysis, P19 cells were plated in
6-well plates at a density of 2×104 cells/well and were
transfected as aforementioned after 24 h. When the cells had
adhered, trypsin digestion was used to harvest them at 24-h
intervals. Pelleted cells were washed twice with PBS at 4°C, and
resuspended in 70% ethanol at −20°C overnight. The cells were then
washed once with PBS and resuspended in 300 µl DNA staining
solution and 3 µl permeabilization solution [Cell Cycle
Staining kit; Multisciences (Lianke) Biotechnology LLC, Hangzhou,
China] for 20 min at room temperature. Subsequently, cells were
analyzed by flow cytometry using a FACSCalibur (BD Biosciences,
Franklin Lakes, NJ, USA) instrument, and the proportion of cells in
each phase of the cell cycle was determined according to the DNA
content using FlowJo software (version 7.6.1; Tree Star, Inc.,
Ashland, OR, USA).
Cell apoptosis
P19 cells were plated in 6-well plates at a density
of 2×104 cells/well and were transfected as
aforementioned after 24 h. After 48 h, the medium was removed and
0.5 ml paraformaldehyde was added to each well for 10 min, then
removed. Following washing with PBS, cells were stained with 0.5 ml
Hoechst 33258 (Beyotime Institute of Biotechnology, Haimen, China)
per well for 5 min. Fluorescence was analyzed using a fluorescence
microscope at a magnification of ×100.
Flow cytometry was used to evaluate the apoptotic
status of P19 cells. Cells were transfected as aforementioned,
harvested using trypsin and washed twice in ice-cold PBS. Cells
were centrifuged at 100 × g for 5 min at 4°C, resuspended in 200
µl 1X binding buffer [10 mM
4-(2-hydroxyethyl)-1-pipera-zineethanesulfonic acid, 140 mM NaCl, 1
mM MgCl2, 5 mM KCl and 2.5 mM CaCl2], and 5
µl Annexin V-fluorescein isothiocyanate and 5 µl
propidium iodide (Cell Cycle Staining kit) were added immediately.
Samples were analyzed using flow cytometry.
RNA isolation and extraction
When P19 cells achieved 80-90% confluence, total RNA
was isolated using an RNAiso Plus kit (Takara Bio, Inc., Otsu,
Japan). RNA concentrations were determined using a NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA).
Determination of miR-29c-3p expression by
RT-qPCR
miR-29c-3p was converted into cDNA using a
PrimeScript™ RT Reagent kit (Takara Bio, Inc.), according to the
manufacturer’s protocol, from 1 µg total RNA isolated as
aforementioned. RT-qPCR was performed using an ABI 7500 Fast
real-time PCR cycler (Applied Biosystems; Thermo Fisher Scientific,
Inc.). U6 was used as an internal control. The primers for
miR-29c-3p and U6 were purchased from Realgene (Nanjing, China).
Amplification was performed with the SYBR® Premix Ex
Taq™ (Takara Bio, Inc.), according to the manufacturer’s protocol.
Primer sequences were as follows: miR-29c-3p forward, 5′-GCG CGC
GTA GCA CCA TTT GAA AT-3′; miR-29c-3p reverse, 5′-ATC CAG TGC AGG
GTC CGA GG-3′; U6 forward, 5′-CTC GCT TCG GCA GCA CATA TAC T-3′; U6
reverse, 5′-ACG CTT CAC GAA TTT GCG TGT C-3′; and RT primer, 5′-GTC
GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACT AAC
CG-3′.
RT-qPCR analysis of Akt3, B-cell
lymphoma-2 (Bcl-2), Bcl-2- associated X protein (Bax), α-myosin
heavy chain (aMHC), cardiac troponin T (cTnT), myocyte enhancer
factor 2c (Mef2c) and GATA-binding protein 4 (GATA4)
transcripts
RT of 1 µg RNA isolated from P19 cells into
cDNA was performed using the PrimeScript™ RT Reagent kit, according
to the manufacturer’s protocol. qPCR was performed using a
SYBR-Green kit (Takara Bio, Inc.)-based detection system. The
thermocycling conditions (30)
were as follows: 95°C for 180 sec, then 40 cycles of 95°C for 10
sec, 60°C for 30 sec, 70°C for 10 sec; melting curve analysis: 95°C
for 15 sec, 60°C for 60 sec and 95°C for 15 sec. Results were
analyzed using the 2−ΔΔCq method (31). GAPDH primers were used for
normalization. All primers were purchased from Realgene (Table I).
 | Table IPrimer sequences for the quantitative
polymerase chain reaction. |
Table I
Primer sequences for the quantitative
polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| GAPDH |
AACTTTGGCATTGTGGAAGG |
CACATTGGGGGTAGGAACAC |
| Akt3 |
TGGCGGAGAGCTGTTTTTCC |
GGCCATCCTTATCTAGCATCAAA |
| Bax |
AGACAGGGGCCTTTTTGCTAC |
AATTCGCCGGAGACACTCG |
| Bcl-2 |
ATGCCTTTGTGGAACTATATGGC |
GGTATGCACCCAGAGTGATGC |
| cTnT |
GGAGTACGAGGAGGAACAGG |
GTCCACTCTCTCTCCATCGG |
| αMHC |
ACCAACCTGTCCAAGTTCCG |
GTCGTGCATCTTCTTGGCAC |
| Mef2c |
TGTCCAGCCATAACAGTTTGG |
CCTTGTGAACATGAAGTCCTCTT |
| GATA4 |
CCAACTGCCAGACTACCAC |
GGACCAGGCTGTTCCAAGA |
Western blot analysis
Protein was extracted from P19 cell samples using
the Whole Cell Lysis assay (Nanjing KeyGen BioTech Co., Ltd.,
Nanjing, China), and protein concentrations were determined using
the Bicinchoninic Acid Protein assay kit (Beyotime Institute of
Biotechnology). Subsequently, 50 µg denatured proteins were
separated by SDS-PAGE (10% gels) and transferred onto
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were then blocked at room
temperature with 5% bovine serum albumin suspended in Tris-buffered
saline containing 0.1% Tween-20 (TBST) for 2 h, washed in TBST, and
incubated at 4°C overnight with monoclonal rabbit anti-mouse Bax
antibody (1:1,000 dilution; cat. no. WL01637; Wanleibio Co., Ltd.,
Shanghai, China), monoclonal rabbit anti-mouse Bcl-2 antibody
(1:1,000 dilution; cat. no. WL01556; Wanleibio Co. Ltd.),
monoclonal rabbit anti-mouse Akt3 antibody (1:500 dilution; cat.
no. 4059; Biogot Technology, Co., Ltd., Nanjing, China) or
monoclonal mouse anti-mouse β-actin antibody (1:2,000 dilution;
cat. no. ab8226; Abcam, Cambridge, UK). The membranes were washed
with TBST and incubated with horseradish peroxidase-conjugated
polyclonal goat anti-rabbit immunoglobulin G (IgG) secondary
antibody [1:3,000 dilution; cat. no. 85-11-4839-81; Multisciences
(Lianke) Biotech Co., Ltd.] or horseradish peroxidase-conjugated
polyclonal goat anti-mouse IgG secondary antibody (1:3,000
dilution; cat. no. 70-GAM001; Multisciences (Lianke) Biotech Co.,
Ltd.) for 2 h at room temperature. Following a further three washes
with TBST, the blots were visualized using Enhanced
Chemiluminescence Plus reagents [Multisciences (Lianke) Biotech
Co., Ltd.]. For western blot analysis, β-actin was used as an
internal control. Protein expression levels were quantified using
ImageJ2× software (version 2.1.4.5; Rawak Software, Inc.,
Stuttgart, Germany).
Luciferase reporter assays
To further investigate the miR-29c-3p regulatory
mechanisms in cardiac development, miRTarBase (www.mirbase.org), TargetScan (www.targetscan.org) and miRNApath
(snf-515788.vm.okeanos.grnet.gr) were used to predict potential
target genes. Akt3 was identified as a likely target of miR-29c-3p,
as the same result was obtained across all platforms. To validate
this prediction, functional luciferase assays were performed. To
determine whether miR-29c-3p was able to bind to the
3′-untranslated region (UTR) of Akt3, wild-type (wt), Akt3 3′-UTR
sequence (Pgl3-Akt3 3′-UTR-wt) and pGL3-Akt3 3′-UTR-mutant (mut)
sequence (comprising the Akt3 3′-UTR sequence with a number of
mutations) luciferase reporter plasmids were used (Promega
Corporation, Madison, WI, USA). A total of 1×105 cells
was seeded in triplicate in 48-well plates for 24 h, then
transfected with 100 ng luciferase reporter plasmid and 100 nM
miRNA using Lipofectamine 3000. At 48 h after transfection, the
cells were lysed and luciferase activity was assayed using a
Dual-Luciferase Reporter assay system (Promega Corporation). The
following primers were used: mmu-miR-29c-3p, 5′-UAG CAC CAU UUG AAA
UCG GUUA-3′; Akt3 wt, 5′-TTC AGA TTA ACC CTT TGG TGC TA-3′; and
Akt3 mut, 5′-TTC AGA TTA ACC CTT ACC ACG AT-3′.
Statistical analysis
Results are presented as the mean ± standard
deviation. The statistical significance of the results was analyzed
using one-way analysis of variance, and the least significant
difference post-hoc test was used for further multiple comparisons.
P-values were two-sided, and P<0.05 was considered to indicate a
statistically significant difference. Analyses were performed using
SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA).
Results
Overexpression of miR-29c-3p suppresses
P19 cell proliferation and promotes apoptosis
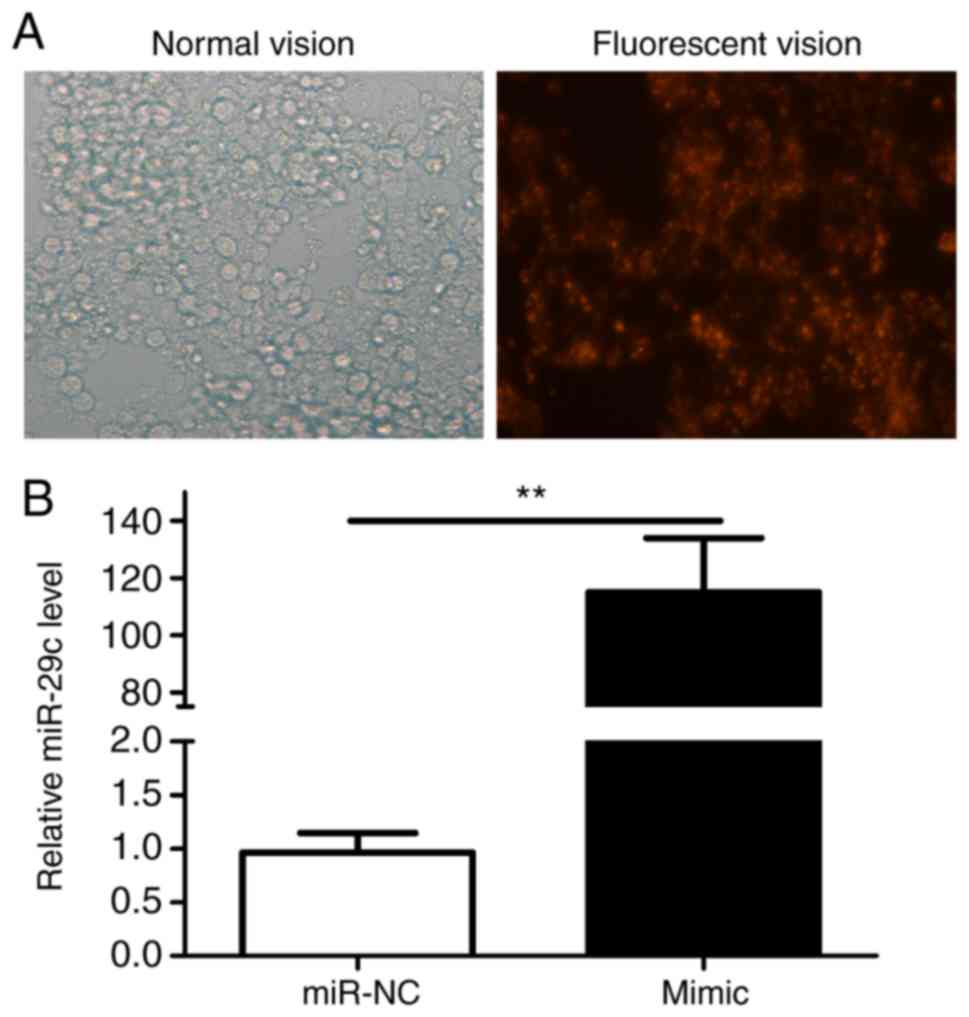
The expression of miR-29c-3p following the
transfection of P19 cells with the miR-29c-3p mimic was analyzed by
fluorescence microscopy (Fig. 1A)
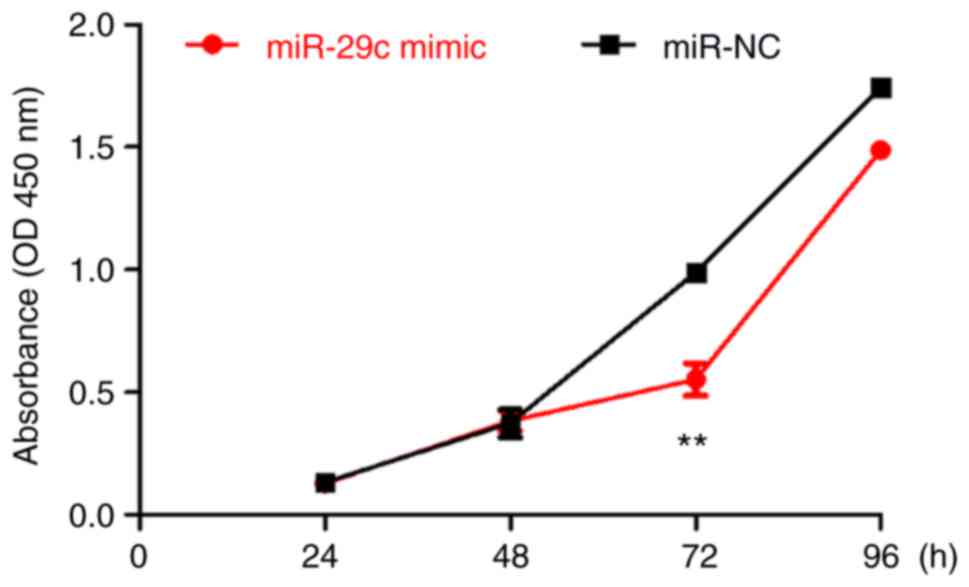
and was revealed to be significantly increased (Fig. 1B). The CCK-8 assay indicated that
the standard deviation of the OD value at each point was small, and
that the cells grew exponentially. Differences in proliferation
rates emerged at 48 h post-transfection, and became obvious at 72
h. At 72 h, the OD value of the miR-29c-3p-overexpression group was
significantly decreased compared with that of control cells
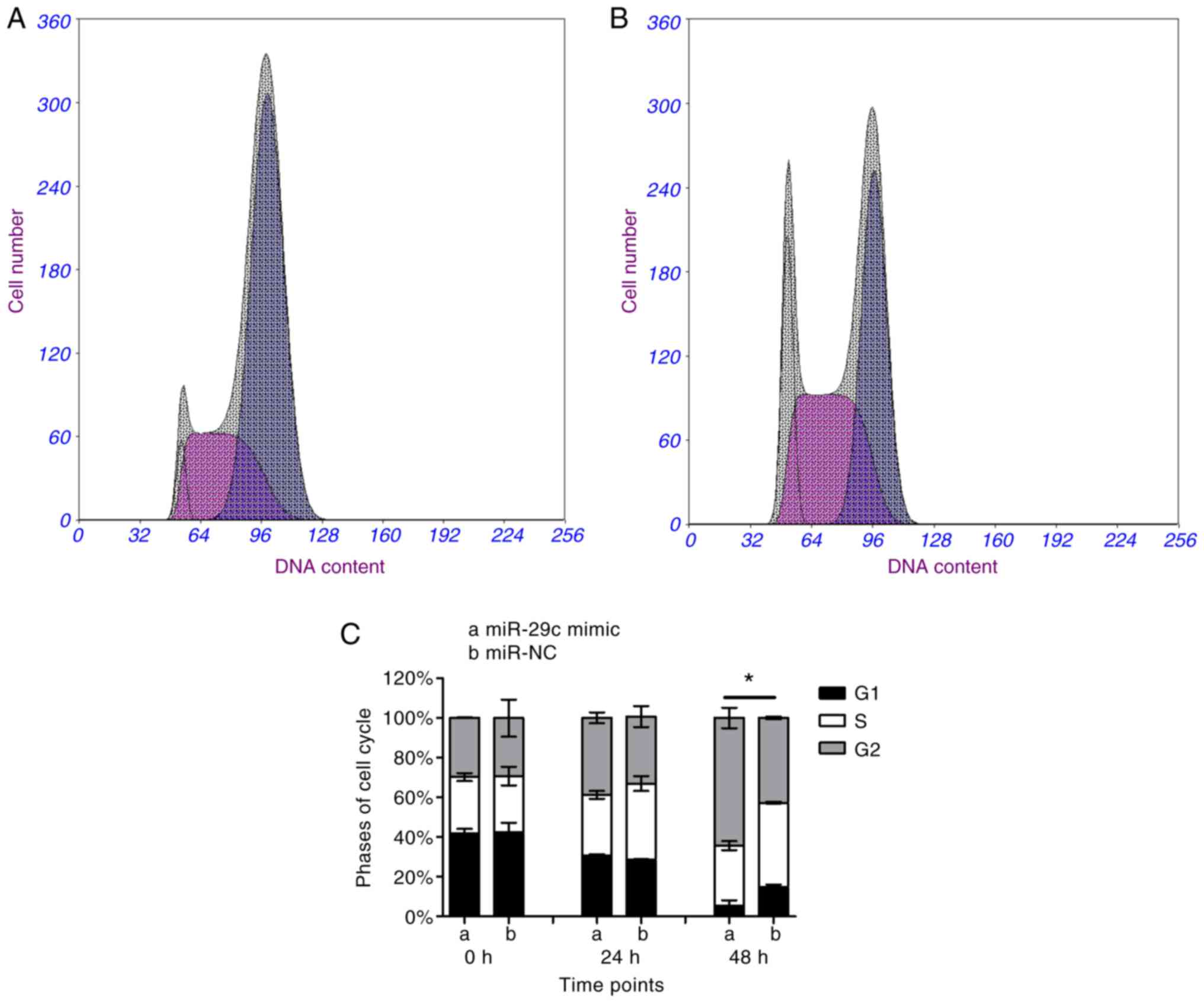
(Fig. 2). Flow cytometric results
(Fig. 3A and B) indicated that
the proportion of cells in S phase was significantly decreased in
miR-29c-3p-overexpressing cells compared with in control cells
(Fig. 3C).
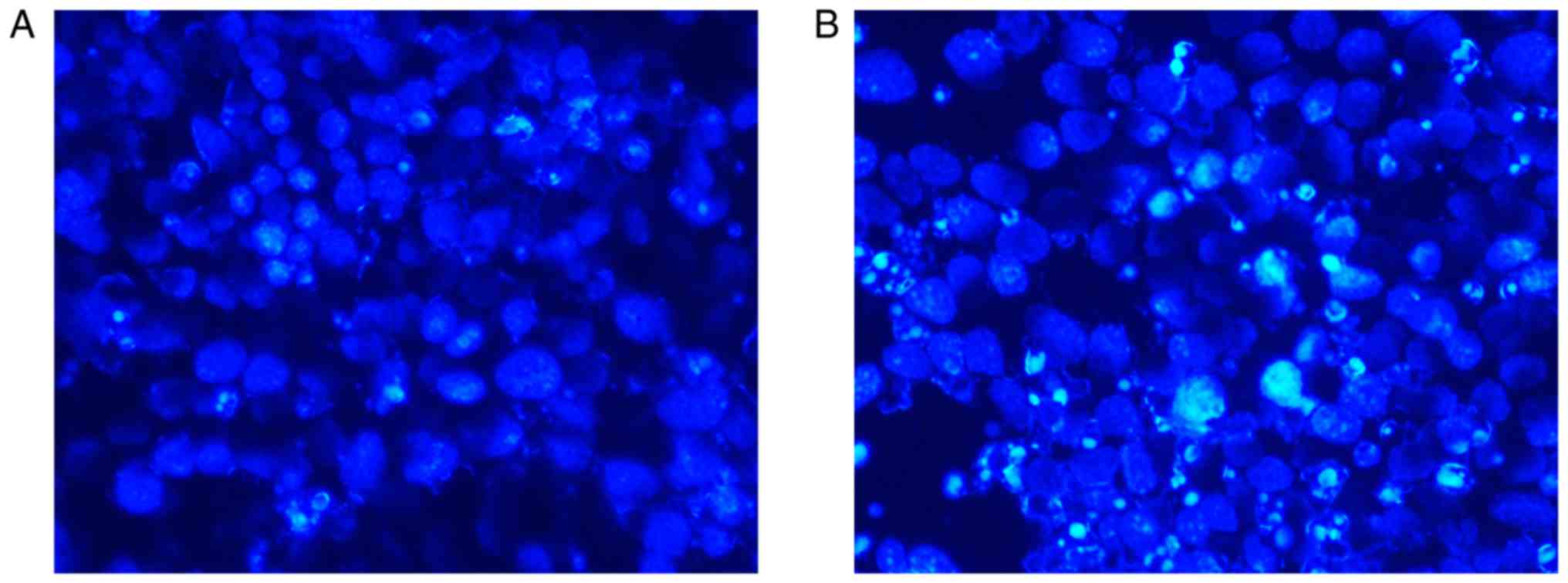
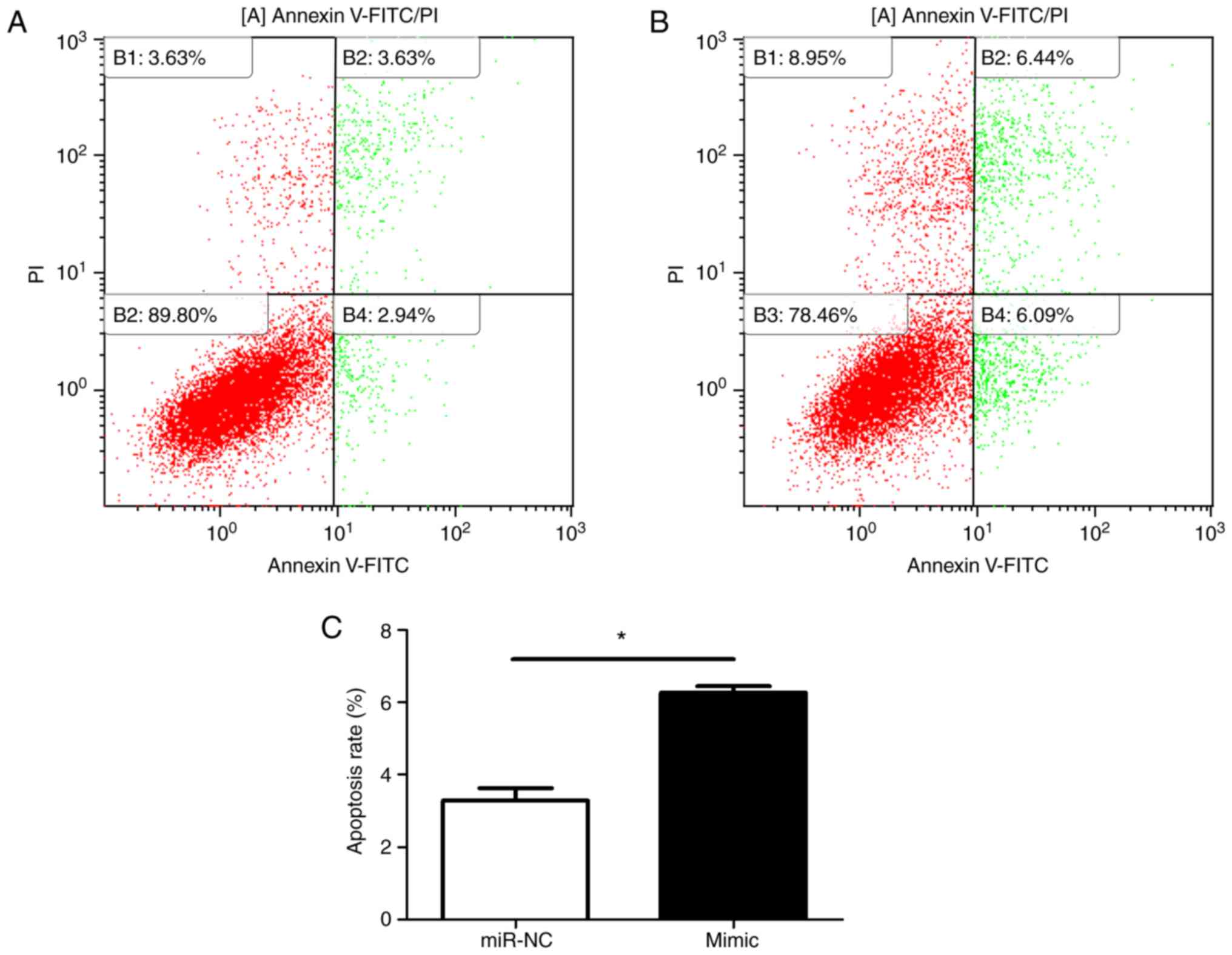
Hoechst staining revealed an increased number of
apoptotic cells in the miR-29c-3p-overexpression group compared
with in the control group. Furthermore, the nuclei of these cells
appeared as fragmented or dense spots, respectively, under a
fluorescence microscope (Fig. 4A and
B). To verify the Hoechst staining results, flow cytometry was
performed (Fig. 5A and B). At 48
h, the apoptotic rate of miR-29c-3p-overexpressing cells was
significantly increased compared with that of the negative control
cells (Fig. 5C). Furthermore,
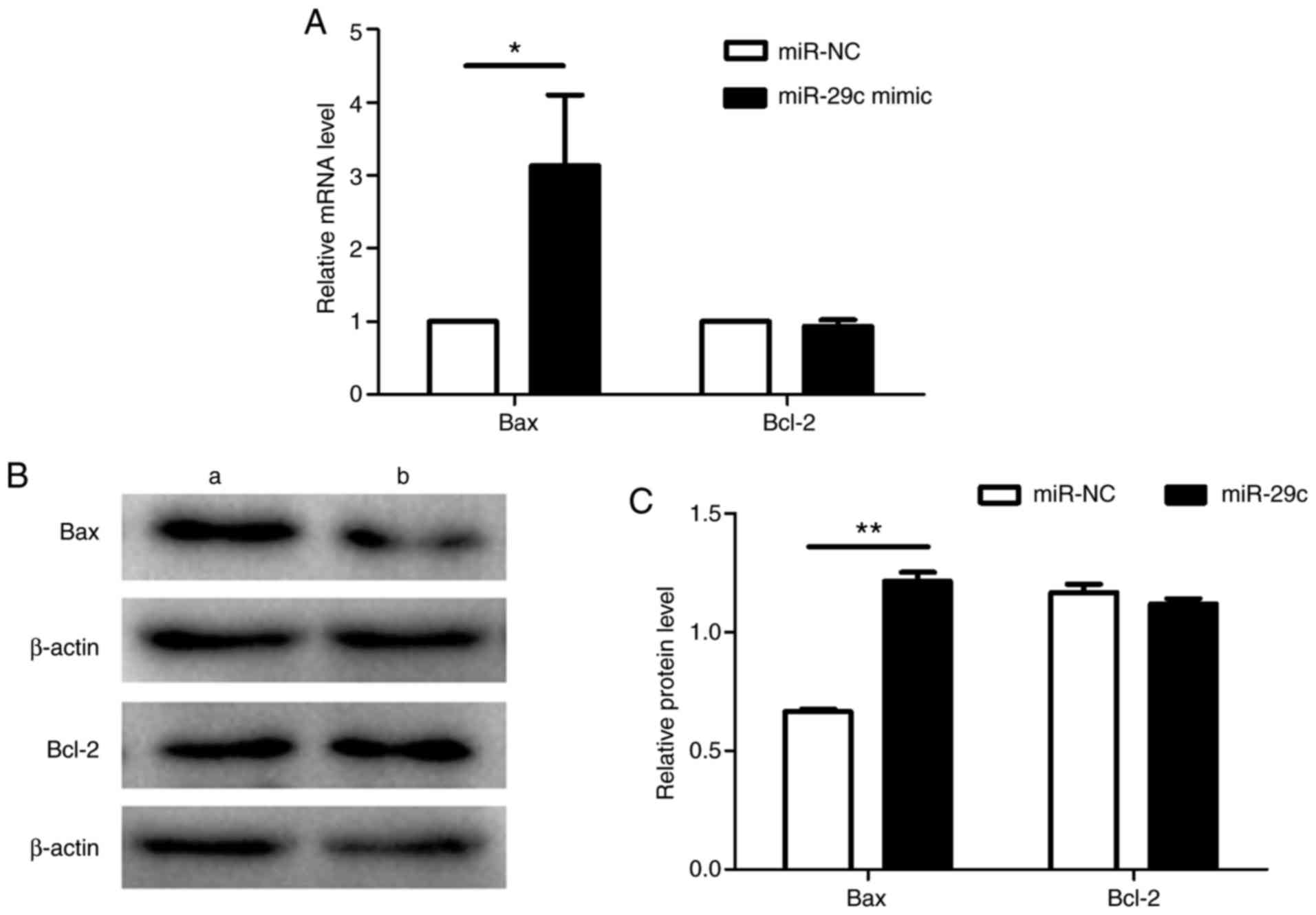
RT-qPCR (Fig. 6A) and western
blotting (Fig. 6B and C)
indicated that expression of the anti-apoptotic Bcl-2 was similar
between the two groups, but that of the pro-apoptotic Bax was
increased in the miR-29c-3p-overexpressing cells compared with in
the negative control cells, a difference that was determined to be
significant.
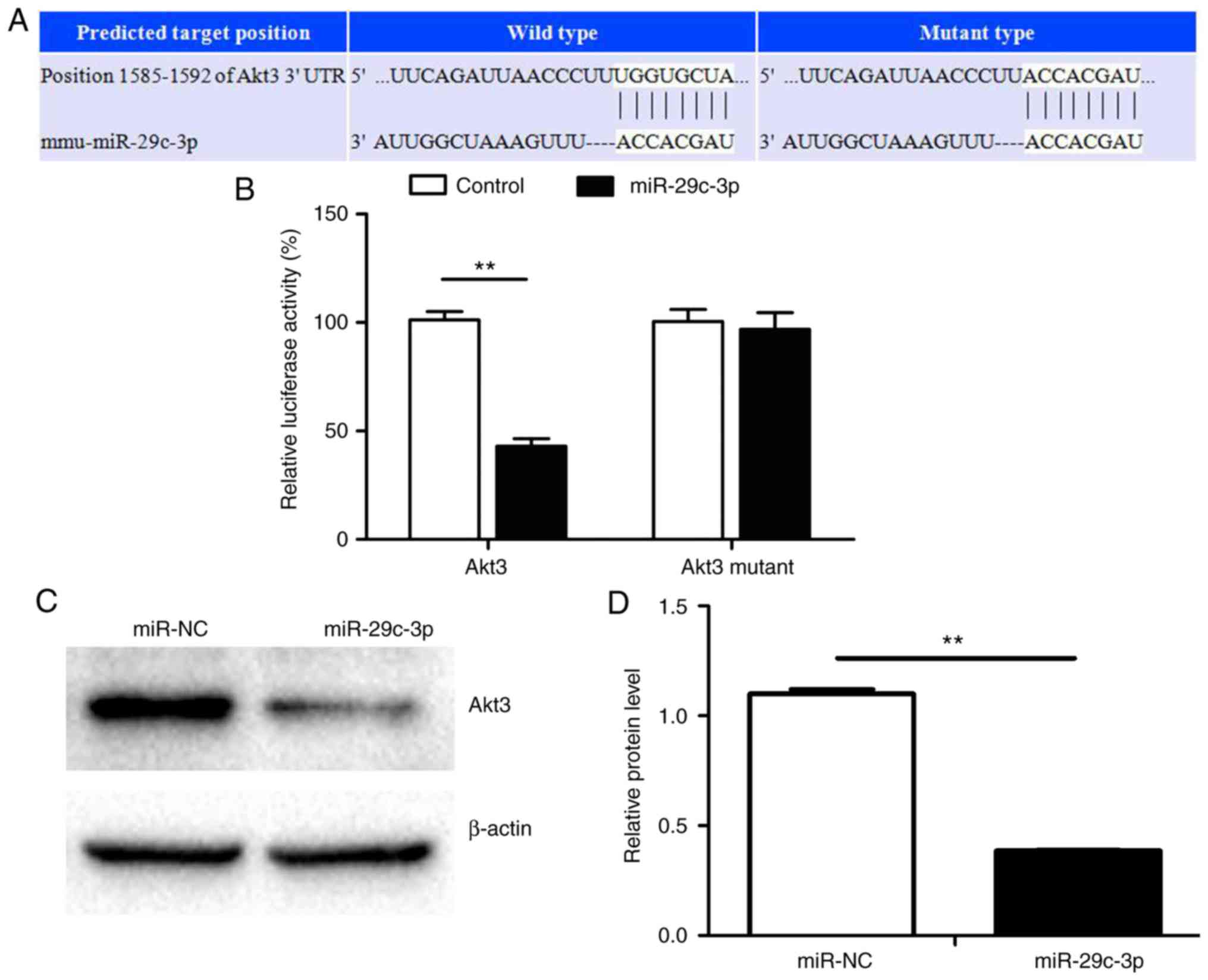
miR-29c-3p decreases Akt3 expression by
targeting the Akt3 3′-UTR in P19 cells
Bioinformatics analysis was used to identify
additional novel targets of miR-29c-3p, and it was identified that
miR-29c-3p was able to bind to the 3′-UTR of Akt3 mRNA. Therefore,
it is possible that miR-29c-3p inhibits Akt3 by binding directly to
its 3′-UTR (Fig. 7A). Luciferase
reporter assays indicated that P19 cells co-transfected with
plasmids carrying wild-type Akt3 3′-UTR and miR-29c-3p mimic
exhibited significantly decreased activity levels compared with
controls, but that there was no difference in cells carrying the
mutant construct (Fig. 7B).
Western blotting indicated that miR-29c-3p overexpression in P19
cells suppressed Akt3 protein expression (Fig. 7C and D). These results support the
post-transcriptional regulation of Akt3 by miR-29c-3p.
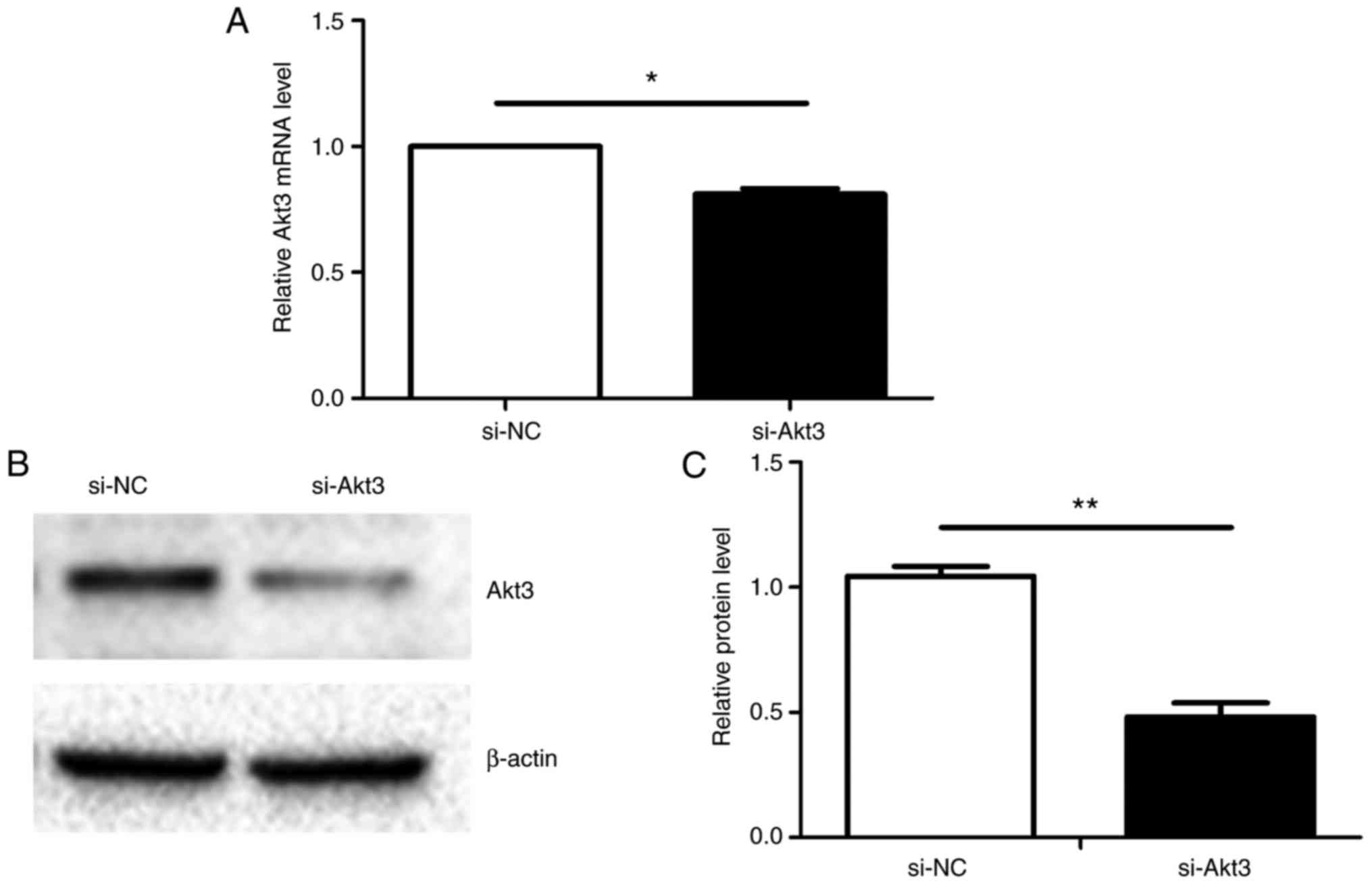
Knockdown of Akt3 inhibits proliferation
and cell cycle progression of P19 cells
Akt3 mRNA expression was identified to be
significantly decreased 48 h after transient transfection (Fig. 8A) and the protein level of Akt3
decreased similarly (Fig. 8B and
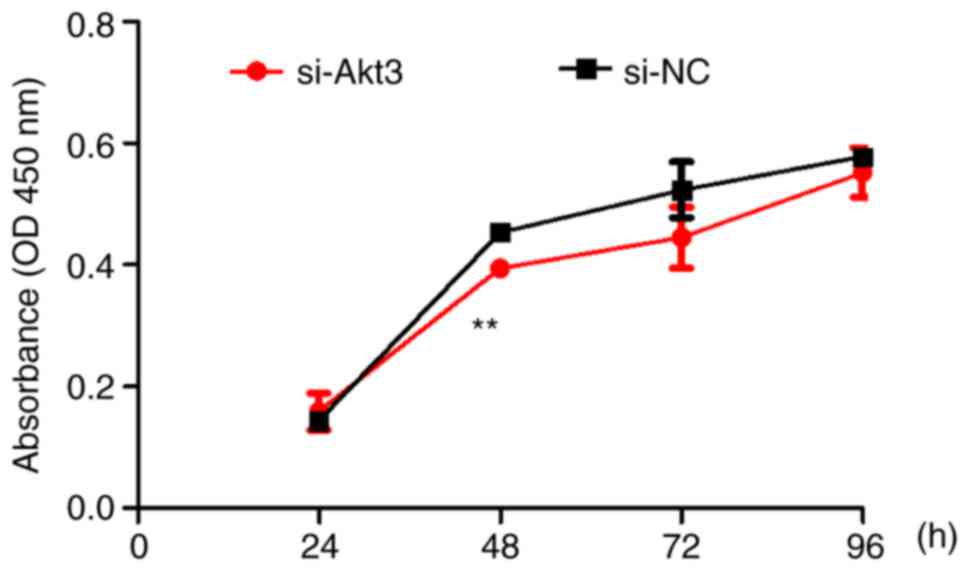
C). Continuous 72-h monitoring using the CCK-8 assay determined
that proliferation was decreased in the Akt3-knockdown group
compared with that of the control group (Fig. 9). At 48 h, the proliferation rate
of P19 cells reached its maximum. In the next phase, the
proliferation rate of si-Akt3 and si-NC decreased possibly because
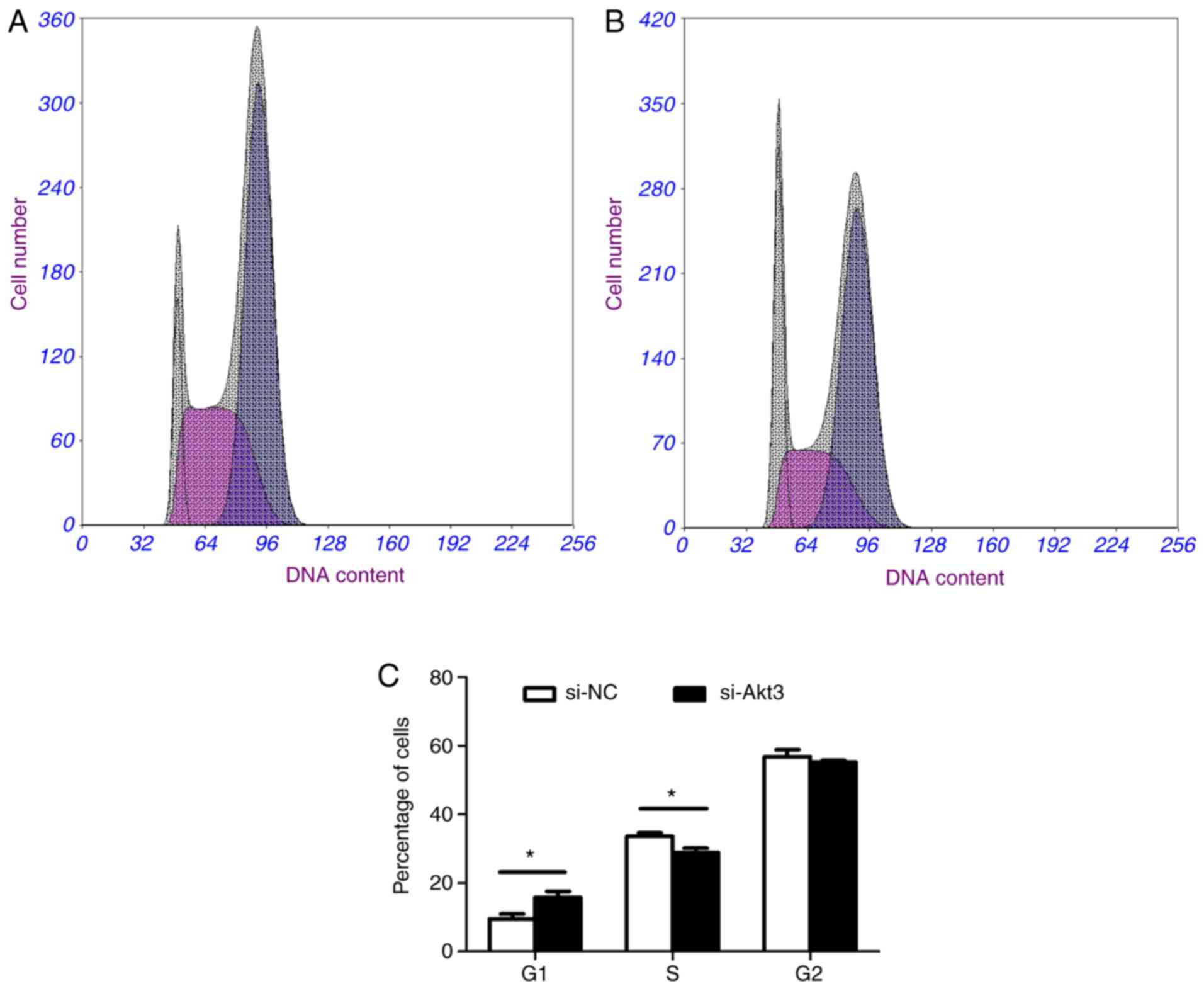
of the contact inhibition mechanism. Flow cytometry revealed an
increased proportion of G1 phase cells and a decreased
proportion of S phase cells in the Akt3-knockdown group in contrast
with the control group (Fig. 10A, B
and C).
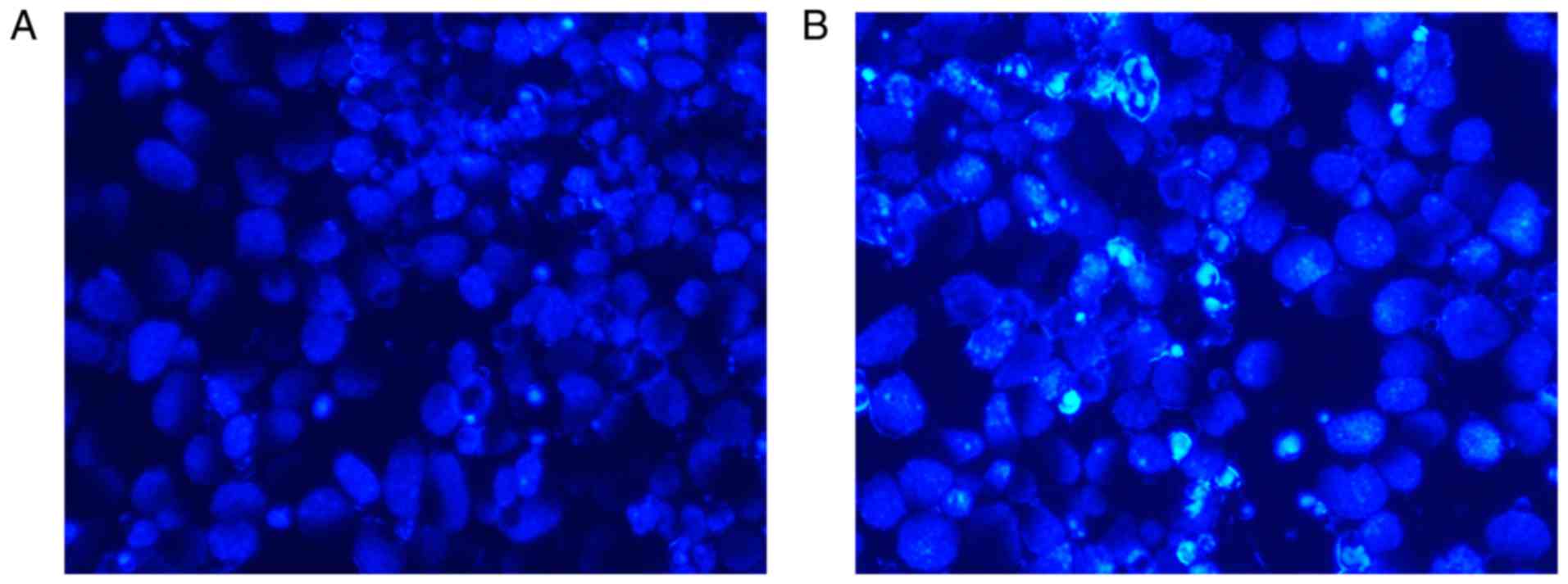
The impact of Akt3 knockdown on the P19 cell
apoptotic response was assessed by Hoechst staining, flow cytometry
and the detection of apoptosis-associated genes. As presented in
Fig. 11A and B, Akt3 knockdown
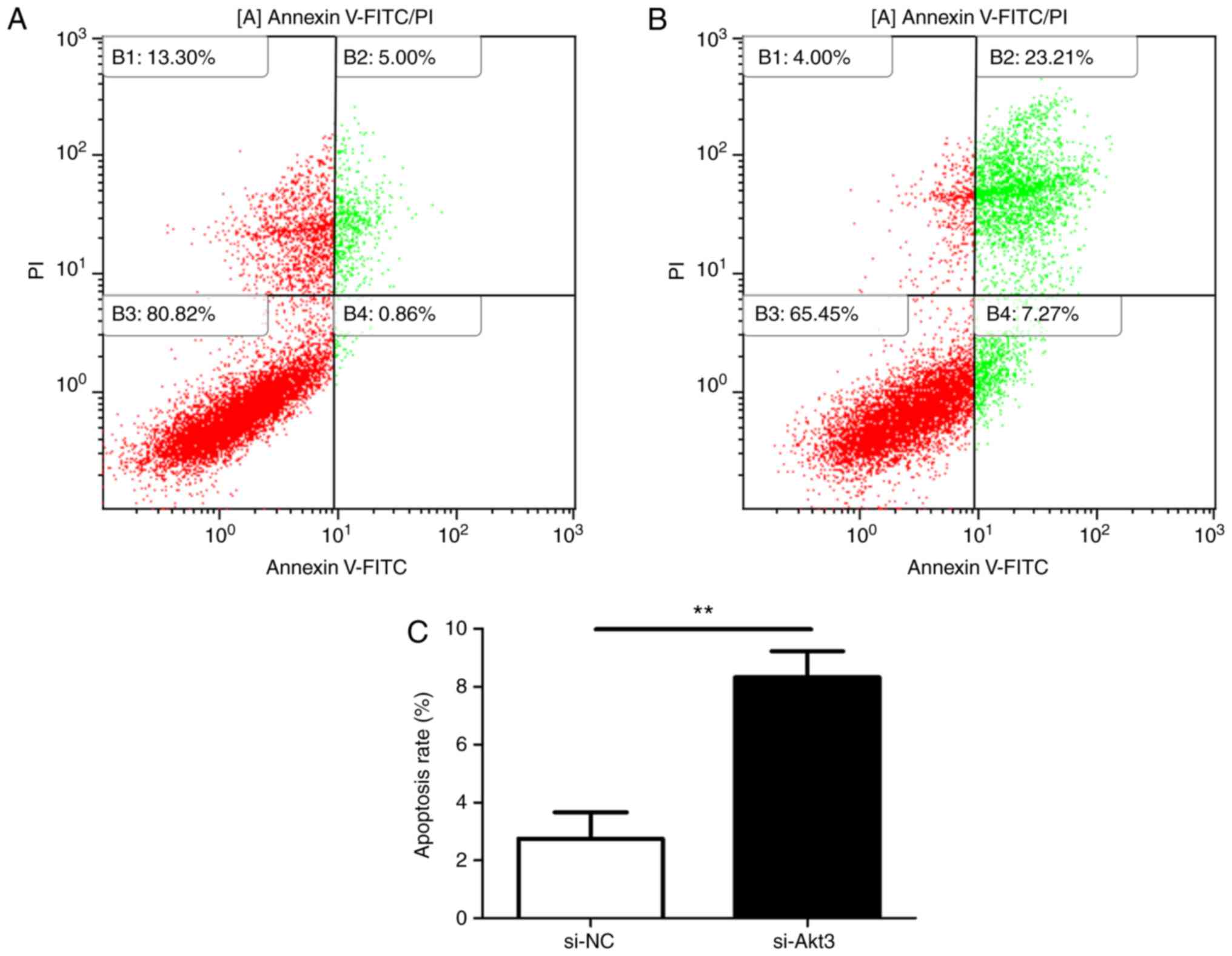
increased cell apoptosis. Flow cytometry (Fig. 12A and B) also revealed that the
knockdown of Akt3 significantly increased the apoptotic rate of P19
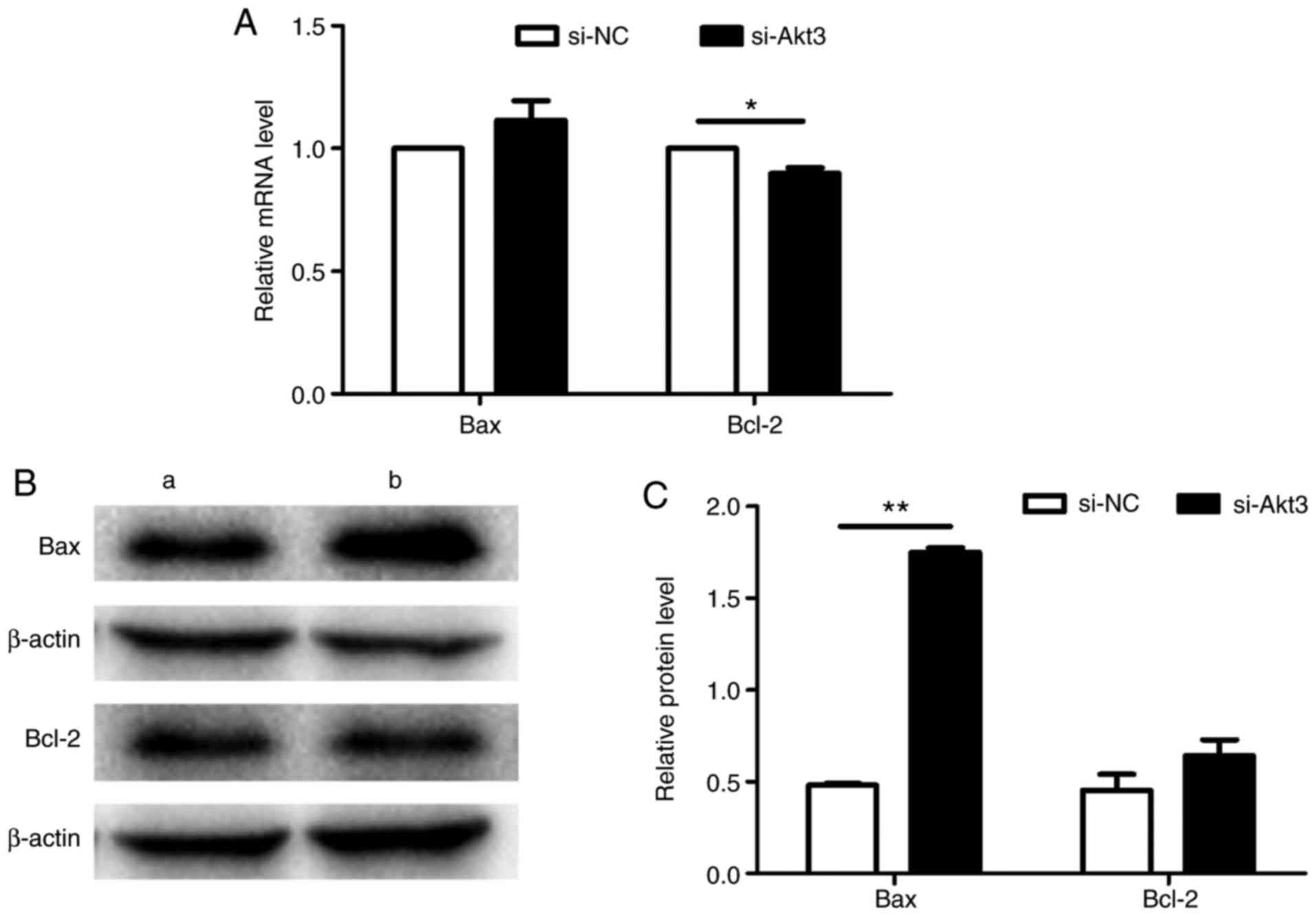
cells (Fig. 12C). Additionally,
Akt3 knockdown decreased Bcl-2 mRNA expression and increased Bax
protein expression in P19 cells compared with in control cells,
indicating that Akt3 promotes P19 cell apoptosis; however, there
was no difference in the expression of Bax mRNA and Bcl-2 protein
between the two groups (Fig.
13A-C).
miR-29c-3p promotes the differentiation
of cardiomyocytes by decreasing Akt3 in P19 cells
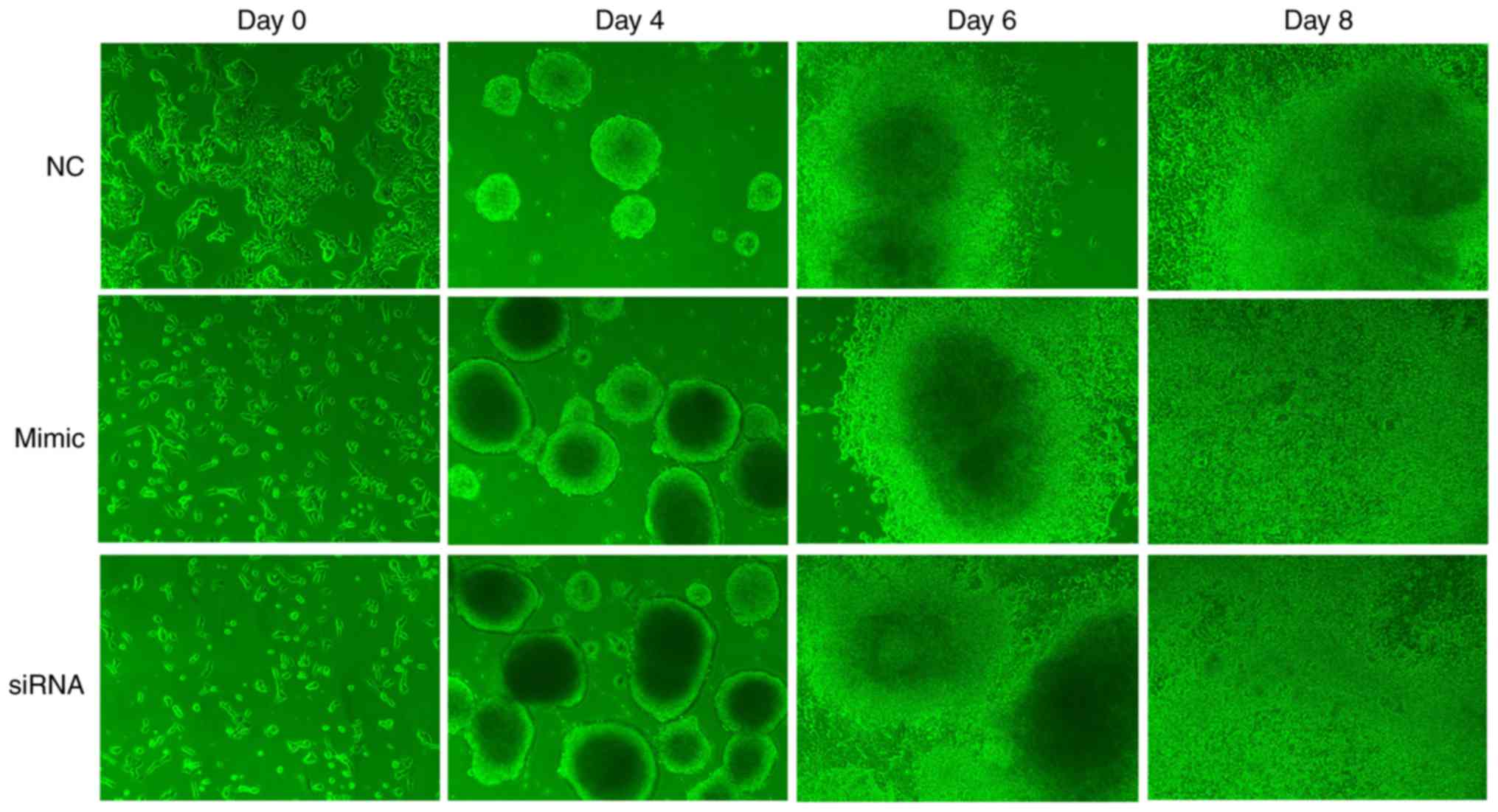
The process by which P19 cells were induced into
cardiomyocytes was observed using an inverted microscope. P19 cells
underwent a series of morphological changes (Fig. 14). On day 4, cells in the
miR-29c-3p-overexpression group and si-Akt3 group formed more
regular and larger EBs when cultured under the same conditions as
the control group. Following transfer to 6-well culture plates on
day 6, cells in the miR-29c-3p-overexpression group and si-Akt3
group exhibited more uniform outward proliferation, centered on the
EBs, whereas control group cells appeared more disordered. On day
8, beating cardiomyocytes were observed, but the
miR-29c-3p-overexpression group and si-Akt3 group cells were more
active compared with those of the control group. On days 0, 4, 6, 8
and 10 of differentiation, relative mRNA expression of the
myocardial-specific marker genes αMHC, Mef2c, GATA4 and cTnT was
determined using RT-qPCR (Fig. 15A
and B). Gene expression levels were significantly increased
during the course of differentiation. In the
miR-29c-3p-overexpression group and si-Akt3 group, the expression
levels of these marker genes were increased compared with in
controls.
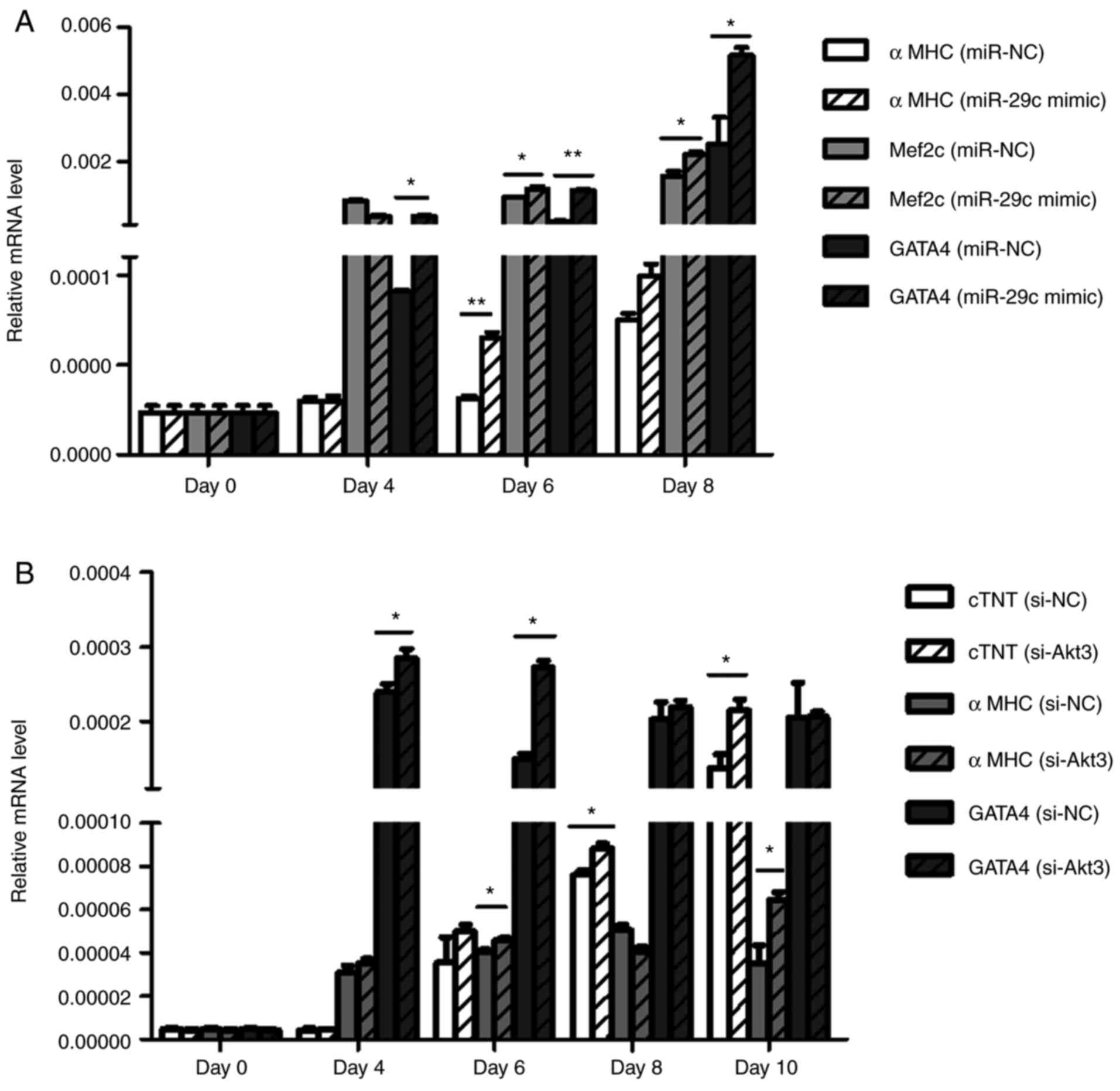 | Figure 15miR-29c-3p promotes the
differentiation of cardiomyocytes via the decreased in Akt3 in P19
cells. (A) RT-qPCR analysis of relative αMHC, Mef2c and GATA4 mRNA
expression during differentiation between the
miR-29c-3p-overexpression and control groups (n=3).
*P<0.05, **P<0.01. (B) RT-qPCR analysis
of relative cardiac αMHC, GATA4 and cTnT mRNA expression during
differentiation between the si-Akt3 and control groups (n=3).
*P<0.05, **P<0.01. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; miR,
microRNA; Akt3, protein kinase Bγ; αMHC, α-myosin heavy chain;
Mef2c, myocyte enhancer factor 2c; GATA4, GATA-binding protein 4;
cTNT, cardiac troponin T; si, small interfering RNA; NC; negative
control. |
Discussion
miRNAs have recently become a focus of research.
Their main function is post-transcriptional gene regulation in
plants and animals (32-34), and studies have increasingly
focused on their function in heart development (35-37). We identified previously that
miR-29c-3p is upregulated in the serum of pregnant women carrying
fetuses with CHD (9). In the
present study, the function of miR-29c-3p in cardiac development
was investigated using transfection technology to simulate the
internal environment of miR-29c-3p overexpression. miRNA mimics are
a means of simulating and enhancing the function of endogenous
miRNA (38), whereas miRNA
inhibitors suppress the function of endogenous miRNAs (39).
In the present study, murine P19 cells were used to
investigate cardiac cell differentiation and development. Following
DMSO induction, P19 cells differentiate into beating
cardio-myocytes. It was identified that miR-29c-3p overexpression
suppressed proliferation, and promoted apoptosis and
differentiation of P19 cells, and that Akt3 is a potential binding
target of miR-29c-3p.
The proliferation curve indicated that the OD value
of the miR-29c-3p-overexpression group was significantly lower
compared with that of the control group at 72 h post-transfection,
and similar results were obtained for the si-Akt3 group at 48 h.
These results indicate that miR-29c-3p upregulation and Akt3
knockdown inhibit the proliferation of P19 cells. The differences
between the two groups diminished following these time points,
which may be due to cellular contact inhibition.
The formation of EBs is a key step in the process of
P19 cell differentiation. Following DMSO induction, EBs were more
regular and stable in the miR-29c-3p-overexpression group and
si-Akt3 group compared with the control group. From day 4 of
differentiation, the expression level of myocardium-specific marker
genes was significantly increased compared with that in the control
group, suggesting that miR-29c-3p upregulation and Akt3 knockdown
promote the differentiation of P19 cells into cardiomyocytes.
Infinite proliferation and universal differentiation
are typical characteristics of embryonal carcinoma cells. During
proliferation, cells proliferate at a certain density, whereas
pluripotent stem cells transform into different tissues in the
differentiation process (40).
These features enable P19 cells to transform into cardiomyocytes
and develop into beating hearts. The results of the present study
revealed that miR-29c-3p overexpression and Akt3 knockdown inhibit
proliferation and promote the differentiation of P19 cells. This
contributes to inefficiency and imbalance in embryonic heart
development, which is why miR-29c-3p overexpression appears to be
associated with CHD.
Cardiac morphogenesis is a complex process, during
which the heart develops from a simple tube into a structure with
four cavities and a separate outflow tract (41-43). During this process, a number of
specific structures emerge and disappear, and apoptosis serves a
crucial function. Apoptotic cells stained with Hoechst dye emit
fluorescence which may be observed under a fluorescent microscope.
Furthermore, the apoptotic cell nucleus is lobulated, fragmented
and light blue, compared with the round blue normal nucleus with
dark blue grains. In the present study, significantly more
apoptotic cells were observed in the miR-29c-3p-overexpression
group and si-Akt3 group compared with in the control group after 48
h. This observation was confirmed by flow cytometry. The apop-totic
rate of the miR-29c-3p-overexpression group and si-Akt3 group was
also significantly increased compared with that in the control
group.
The Bcl family is one of a number of gene families
regulating apoptosis (44,45).
Bcl-2 and Bax are members of this family, but have opposing
functions, with Bcl-2 inhibiting apoptosis and Bax promoting it.
Together, they regulate the internal balance of apoptosis with
other family members (46).
Increased gene expression of Bax was observed in the
miR-29c-3p-overexpression group compared with in the control group,
and decreased gene expression of Bcl-2 was observed in the si-Akt3
group compared with in the control group. Therefore, the
pro-apoptotic effect of miR-29c-3p on P19 cells may be due to the
regulation of apoptosis-associated genes.
Previous studies have identified that Akt3
transgenic mice have notable cardiac hypertrophy, suggesting that a
potential function of Akt3 is to promote cardiomyocyte
proliferation. Cardiac growth at 4 weeks of age was also identified
to resemble compensated hypertrophy, whereas hypertrophy at 20
weeks of age was clearly pathological on the basis of measurements
of interstitial fibrosis and cardiac performance (17). In the early embryo, the Akt3
signal is beneficial for cardiac growth; however, the sustained
activation of Akt3 signaling may have adverse effects and result in
maladaptive hypertrophy. The results of the present study indicate
that Akt3 is a target of miR-29c-3p, and that the overexpression of
miR-29c-3p decreases Akt3 expression levels. Taken together, these
results suggested that regulating Akt3 expression may be one of the
ways in which miR-29c-3p affects cardiac development.
The results of the present study revealed that
miR-29c-3p overexpression decreases proliferation, and promotes
apoptosis and differentiation in P19 cells. The underlying
molecular mechanism may involve regulation of the miR-29c-3p target
gene Akt3. These results may contribute to an improved
understanding of heart development, and may assist in preventing
the development of CHD and other serious cardiac disorders.
Funding
The present study was supported by the Maternal and
Child Health Research Project of Jiangsu Province (grant no.
F201509) and National Natural Science Foundation of China (grant
no. 81573234).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
TC and SJL performed the major examination, TC was a
major contributor in writing the manuscript. BC, QH, XYK and CS
analyzed the data of this study. HTG made substantial contributions
to the conception and design of the study, acquisition of data,
analysis and interpretation of data. XWW revised the paper
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We thank Dr Sarah Williams for editing the English
text of a draft of this manuscript.
References
|
1
|
Wang J and Liu B: Exercise and congenital
heart disease. Adv Exp Med Biol. 1000:95–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Junghare SW and Desurkar V: Congenital
heart diseases and anaesthesia. Indian J Anaesth. 61:744–752. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Itani M, Matesan M, Ahuja J, Bermo M,
Habib AS, Goiney C, Krieger EV and Vesselle H: The role of
pulmonary scintigraphy in the evaluation of adults with congenital
heart disease. Semin Nucl Med. 47:660–670. 2017. View Article : Google Scholar
|
|
4
|
Suradi HS and Hijazi ZM: Adult congenital
interventions in heart failure. Interv Cardiol Clin. 6:427–443.
2017.PubMed/NCBI
|
|
5
|
Chien PS, Chiang CB, Wang Z and Chiou TJ:
MicroRNA-mediated signaling and regulation of nutrient transport
and utilization. Curr Opin Plant Biol. 39:73–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Lucia C, Komici K, Borghetti G,
Femminella GD, Bencivenga L, Cannavo A, Corbi G, Ferrara N, Houser
SR, Koch WJ and Rengo G: microRNA in cardiovascular aging and
age-related cardiovascular diseases. Front Med (Lausanne).
4:742017. View Article : Google Scholar
|
|
7
|
Stanley-Hasnain S, Hauck L, Grothe D and
Billia F: Control of cardiomyocyte proliferation through
p53/Mdm2-regulated MicroRNAs. J Heart Lung Transpl. 35(Suppl 1):
S183–S184. 2016. View Article : Google Scholar
|
|
8
|
Zhou Y, Jia WK, Jian Z, Zhao L, Liu CC,
Wang Y and Xiao YB: Downregulation of microRNA-199a-5p protects
cardiomyocytes in cyanotic congenital heart disease by attenuating
endoplasmic reticulum stress. Mol Med Rep. 16:2992–3000. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu S, Cao L, Zhu J, Kong L, Jin J, Qian
L, Zhu C, Hu X, Li M, Guo X, et al: Identification of maternal
serum MicroRNAs as novel noninvasive biomarkers for prenatal
detection of fetal congenital heart defects. Clin Chim Acta.
424:66–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shu YJ, Bao RF, Jiang L, Wang Z, Wang XA,
Zhang F, Liang HB, Li HF, Ye YY, Xiang SS, et al: MicroRNA-29c-5p
suppresses gallbladder carcinoma progression by directly targeting
CPEB4 and inhibiting the MAPK pathway. Cell Death Differ.
24:445–457. 2017. View Article : Google Scholar :
|
|
11
|
Han TS, Hur K, Xu G, Choi B, Okugawa Y,
Toiyama Y, Oshima H, Oshima M, Lee HJ, Kim VN, et al: MicroRNA-29c
mediates initiation of gastric carcinogenesis by directly targeting
ITGB1. Gut. 64:203–214. 2015. View Article : Google Scholar
|
|
12
|
Zhang HW, Wang EW, Li LX, Yi SH, Li LC, Xu
FL, Wang DL, Wu YZ and Nian WQ: A regulatory loop involving miR-29c
and Sp1 elevates the TGF-β1 mediated epithelial-to-mesenchymal
transition in lung cancer. Oncotarget. 7:85905–85916.
2016.PubMed/NCBI
|
|
13
|
Niu M, Gao D, Wen Q, Wei P, Pan S, Shuai
C, Ma H, Xiang J, Li Z, Fan S, et al: MiR-29c regulates the
expression of miR-34c and miR-449a by targeting DNA
methyltransferase 3a and 3b in nasopharyngeal carcinoma. BMC
Cancer. 16:2182016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Butrym A, Rybka J, Baczyńska D, Poręba R,
Kuliczkowski K and Mazur G: Clinical response to azacitidine
therapy depends on microRNA-29c (miR-29c) expression in older acute
myeloid leukemia (AML) patients. Oncotarget. 7:30250–30257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nygren MK, Tekle C, Ingebrigtsen VA,
Mäkelä R, Krohn M, Aure MR, Nunes-Xavier CE, Perälä M, Tramm T,
Alsner J, et al: Identifying microRNAs regulating B7-H3 in breast
cancer: The clinical impact of microRNA-29c. Br J Cancer.
110:2072–2080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Zhu Y and Qiu R: Shikonin protects
H9C2 cardiomyocytes against hypoxia/reoxygenation injury through
activation of PI3K/Akt signaling pathway. Biomed Pharmacother.
104:712–717. 2018. View Article : Google Scholar
|
|
17
|
Taniyama Y, Ito M, Sato K, Kuester C, Veit
K, Tremp G, Liao R, Colucci WS, Ivashchenko Y, Walsh K and Shiojima
I: Akt3 over-expression in the heart results in progression from
adaptive to maladaptive hypertrophy. J Mol Cell Cardiol.
38:375–385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiojima I, Sato K, Izumiya Y, Schiekofer
S, Ito M, Liao R, Colucci WS and Walsh K: Disruption of coordinated
cardiac hypertrophy and angiogenesis contributes to the transition
to heart failure. J Clin Invest. 115:2108–2118. 2005. View Article : Google Scholar :
|
|
19
|
Yoshioka K, Yoshida K, Cui H, Wakayama T,
Takuwa N, Okamoto Y, Du W, Qi X, Asanuma K, Sugihara K, et al:
Endothelial PI3K-C2α, a class II PI3K, has an essential role in
angiogenesis and vascular barrier function. Net Med. 18:1560–1569.
2012. View
Article : Google Scholar
|
|
20
|
Kanungo J: Retinoic acid signaling in P19
stem cell differentiation. Anticancer Agents Med Chem.
17:1184–1198. 2017. View Article : Google Scholar
|
|
21
|
Wang L, Song G, Liu M, Chen B, Chen Y,
Shen Y, Zhu J and Zhou X: MicroRNA-375 overexpression influences
P19 cell proliferation, apoptosis and differentiation through the
notch signaling pathway. Int J Mol Med. 37:47–55. 2016. View Article : Google Scholar
|
|
22
|
McBurney MW, Jones-Villeneuve EM, Edwards
MK and Anderson PJ: Control of muscle and neuronal differentiation
in a cultured embryonal carcinoma cell line. Nature. 299:165–167.
1982. View
Article : Google Scholar
|
|
23
|
Mueller I, Kobayashi R, Nakajima T, Ishii
M and Ogawa K: Effective and steady differentiation of a clonal
derivative of P19CL6 embryonal carcinoma cell line into beating
cardiomyocytes. J Biomed Biotechnol. 2010:3805612010. View Article : Google Scholar
|
|
24
|
Song G, Shen Y, Ruan Z, Li X, Chen Y, Yuan
W, Ding X, Zhu L and Qian L: LncRNA-uc 167 influences cell
proliferation, apoptosis and differentiation of P19 cells by
regulating Mef2c. Gene. 590:97–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Heyden MA and Defize LH: Twenty
one years of P19 cells: What an embryonal carcinoma cell line
taught us about cardiomyocyte differentiation. Cardiovasc Res.
58:292–302. 2003. View Article : Google Scholar
|
|
26
|
Mcburney MW: P19 embryonal carcinoma
cells. Int J Dev Biol. 37:135–140. 1993.
|
|
27
|
Ai F, Zhang Y and Peng B: miR-20a
regulates proliferation, differentiation and apoptosis in P19 cell
model of cardiac differentiation by targeting smoothened. Biol
Open. 5:1260–1265. 2016. View Article : Google Scholar
|
|
28
|
Wang D, Liu C, Wang Y, Wang W, Wang K, Wu
X, Li Z, Zhao C, Li L and Peng L: Impact of miR-26b on
cardiomyocyte differentiation in P19 cells through regulating
canonical/non-canonical wnt signaling. Cell Proliferation.
50:e123712017. View Article : Google Scholar
|
|
29
|
Li H, Jiang L, Yu Z, Han S, Liu X, Li M,
Zhu C, Qiao L and Huang L: The role of a novel long noncoding RNA
TUC40- in cardiomyocyte induction and maturation in P19 cells. Am J
Med Sci. 354:608–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Poucke M, Van Zeveren A and Peelman
LJ: [Letter to the editor] Combined FAM-labeled TaqMan probe
detection and SYBR green I melting curve analysis in multiprobe
qPCR genotyping assays. Biotechniques. 52:81–86. 2012. View Article : Google Scholar
|
|
31
|
Wang J, Meng X, Dobrovolskaya OB, Orlov YL
and Chen M: Non-coding RNAs and their roles in stress response in
plants. Genomics Proteomics Bioinformatics. 15:301–312. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Grossi I, Salvi A, Abeni E, Marchina E and
De Petro G: Biological function of MicroRNA193a-3p in health and
disease. Int J Genomics. 2017:59131952017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar V, Khare T, Shriram V and Wani SH:
Plant small RNAs: The essential epigenetic regulators of gene
expression for salt-stress responses and tolerance. Plant Cell Rep.
37:61–75. 2018. View Article : Google Scholar
|
|
35
|
Chen C, Ponnusamy M, Liu C, Gao J, Wang K
and Li P: MicroRNA as a therapeutic target in cardiac remodeling.
Biomed Res Int. 2017:12784362017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wojciechowska A, Braniewska A and
Kozar-Kamińska K: MicroRNA in cardiovascular biology and disease.
Adv Clin Exp Med. 26:865–874. 2017. View Article : Google Scholar
|
|
37
|
Sun W, Zhao L, Song X, Zhang J, Xing Y,
Liu N, Yan Y, Li Z, Lu Y, Wu J, et al: MicroRNA-210 modulates the
cellular energy metabolism shift during H2O2-induced oxidative
stress by repressing ISCU in H9c2 cardiomyocytes. Cell Physiol
Biochem. 43:383–394. 2017. View Article : Google Scholar
|
|
38
|
Chu D, Zhao Z, Li Y, Li J, Zheng J, Wang
W, Zhao Q and Ji G: Increased microRNA-630 expression in gastric
cancer is associated with poor overall survival. PLoS One.
9:e905262014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qiao P, Li G, Bi W, Yang L, Yao L and Wu
D: microRNA-34a inhibits epithelial mesenchymal transition in human
cholangio-carcinoma by targeting Smad4 through transforming growth
factor-beta/Smad pathway. BMC Cancer. 15:4692015. View Article : Google Scholar
|
|
40
|
Rijlaarsdam MA and Looijenga LH: An
oncofetal and developmental perspective on testicular germ cell
cancer. Semin Cancer Biol. 29:59–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Paraskevas G, Koutsouflianiotis K and
Iliou K: The first descriptions of various anatomical structures
and embryological remnants of the heart: A systematic overview. Int
J Cardiol. 227:674–690. 2017. View Article : Google Scholar
|
|
42
|
Gittenbergerde Groot AC, Bartelings MM,
Poelmann RE, Haak MC and Jongbloed MR: Embryology of the heart and
its impact on understanding fetal and neonatal heart disease. Semin
Fetal Neonatal Med. 18:237–244. 2013. View Article : Google Scholar
|
|
43
|
Abu-Elmagd M and Wheeler G: Frizzled-7
expression during early cardiogenesis of Xenopus laevis embryo. BMC
Genomics. 15:1–2. 2014. View Article : Google Scholar
|
|
44
|
Vitagliano O, Addeo R, D’Angelo V, Indolfi
C, Indolfi P and Casale F: The Bcl-2/Bax and Ras/Raf/MEK/ERK
signaling pathways: Implications in pediatric leukemia pathogenesis
and new prospects for therapeutic approaches. Expert Rev Hematol.
6:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|