Introduction
Osteosarcoma (OS) is a rare malignant bone tumor
that commonly occurs in children and adolescents. OS usually causes
pain and swelling of the long bones of the legs and arms (1-3).
The 5-year survival rate of young-onset OS is 61.6% globally due to
advances in therapeutic strategies, including combinatorial
chemotherapy and radiotherapy, over the last decade (4). Only a few oncogenes and tumor
suppressors have been identified in the disease, limiting the
development of potential novel therapeutic targets for OS treatment
(5,6). Therefore, investigations on the
mechanisms underlying the initialization, progression, and
metastasis of OS are required to improve the therapeutic efficiency
of the treatments. MicroRNAs (miRNAs) act as an important type of
regulatory molecules in eukaryotic cells and consist of ~22
nucleotides (7,8). miRNAs have been widely reported to
exhibit ectopic expression in various types of cancer, including
stomach, colorectal, lung, squamous, and pancreatic cancer, and OS
(9-15). In a previous study, a total of 268
miRNAs were identified to be either upregulated or downregulated in
human OS cell lines. For example, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis has demonstrated that miRNA-9, miRNA-99, miRNA-195,
miRNA-148a and miRNA-181a are upregulated, whereas miRNA-143,
miRNA-145, miRNA-335 and miRNA-539 are downregulated in the MG-63
OS cell line (16). Different
miRNAs can have different effects on cancer. Certain miRNAs promote
the progression of tumors, whereas others inhibit the development
of tumors (17,18).
miRNA-101-3p has been shown to be downregulated in
several human malignancies (19-21), including OS (22). This miRNA acts as a tumor
suppressor. Chang et al (23) suggested that miRNA-101 inhibits
the autophagy of OS cells, thereby promoting chemotherapeutic
efficiency. Furthermore, miRNA-101 was shown to inhibit OS
metastasis by regulating enhancer of zeste 2 polycomb repressive
complex 2 subunit (24). In
another report, mammalian target of rapamycin (mTOR) was shown to
be a target of miRNA-101 in OS cells; mTOR induced cell apoptosis
and inhibited cell proliferation (25). Overall, miRNA-101 is considered to
be a tumor suppressor in OS. However, the mechanism underlying the
regulation of OS cell proliferation, migration, invasion and
apoptosis by miRNA-101 remains to be fully elucidated.
In addition, the expression of miRNA-101-3p is
regulated by several other factors in cancer cells (26). Long non-coding RNA (lncRNA), which
consists of >200 nucleotides, is a class of non-coding RNA that
is important in the development of human cancer (27,28). The dysregulation of lncRNA has
been identified in several types of cancer, including renal cell
carcinoma, melanoma, glioma cells, non-small cell lung cancer, and
OS cells (29,30). In non-small cell lung cancer, Cui
et al (19) found that the
lncRNA SNHG1 was upregulated and inhibited the expression of
miRNA-101-3p, which significantly promoted the progression of
cancer. However, the mechanism underlying the regulation of the
expression of miRNA-101by lncRNAs and the effect of lncRNAs on
cancer development in OS remain to be elucidated.
Rho-associated coiled-coil-containing protein kinase
1 (ROCK1) is considered to be a potential target unit of
miRNA-101-3p in different types of cancer, including OS (31-34). ROCK1 is one of the four kinases
whose inhibition induces cell apoptosis and poor cell viability in
OS cells (22). The knockdown of
ROCK1 in OS cells has been reported to decrease cell proliferation
and promote cell death in OS cell lines. By contrast, the
overexpression of ROCK1 in patients with OS is usually associated
with poor prognosis, suggesting that it may be used as a prognosis
marker and therapeutic target.
miRNA-101 has been shown to inhibit the progression
of several types of cancer by targeting ROCK1 (22). However, the regulation of
miRNA-101-3p by the lncRNA SNHG1; the subsequent association
between the regulated miRNA-101-3p and its potential target, ROCK1;
and the resulting OS development have not been fully investigated.
Therefore, in the present study, the associations among the lncRNA
SNHG1, miRNA-101-3p, and ROCK1 were investigated in OS cell lines.
Furthermore, the molecular mechanism of this lncRNA
SNHG1-miRNA-101-3p-ROCK1 pathway in regulating the proliferation,
migration, invasion, and apoptosis of OS cells was examined, which
may provide a potential prognostic marker and therapeutic target
for OS treatment.
Materials and methods
Patients and specimens
The present study was approved by the Research
Ethics Committee of Nanfang Hospital, Southern Medical University
(Guangzhou, China). Written informed consent was obtained from all
participants. A total of 43 OS samples were obtained from patients
who had surgical resection between 2015 and 2016 at Nanfang
Hospital, Southern Medical University. Normal osteoblast samples
were obtained from 12 individuals who succumbed to mortality in
traffic accidents between 2015 and 2016 at Nanfang Hospital,
Southern Medical University.
Cell culture and reagents
The MG63, U2OS, and Saos-2 human OS cell lines and
the hFOB1.19 human osteoblast cell line were purchased from the
Shanghai Institutes for Biological Sciences Cell Resource Center
(Shanghai, China). Dulbecco's modified Eagle's medium (DMEM;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) with high
glucose which supplemented with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used for the cell
culture. The humidified incubator for cell culture was set to 37°C
with 5% CO2.
RT-qPCR analysis
Total RNAs were collected from the patient specimens
and cultured cells using TRIzol reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RNA (2 µg)
was reversed transcribed into cDNA using TaqMan™ Reverse
Transcription reagents (Thermo Fisher Scientific, Inc.). The
expression levels of miRNA-101-3p and the lncRNA SNHG1 were
analyzed using the DyNAmo ColorFlash SYBR-Green qPCR kit (Thermo
Fisher Scientific, Inc.) with the ABI Prism 7700 Sequence Detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). U6 and
GAPDH were used as the internal controls. The sequences of the
primers for miRNA-101-3p were as follows: Forward,
5′-GCGCGCATACAGTACTGTGATA-3′ and reverse,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACT GGATACGACTTCAGT-3′. The
sequences of the primer for the lncRNA SNHG1 were as follows:
Forward, 5′-ACGTT GGAACCGAAGAGAGC-3′ and reverse, 5′-GCAGCTGAA
TTCCCCAGGAT-3′ The sequences of the primers for U6 were as follows:
Forward, CTCGCTTCGGCAGCACA and reverse, AACGCTTCACGAATTTGCGT. The
sequences of the primers for GAPDH were as follows: Forward, CCAGG
TGGTCTCCTCTGACTT and reverse, GTTGCTGTAGCCA AATTCGTTGT. The
reaction conditions were: 95°C for 30 sec, followed by 40 cycles of
95°C for 3 sec and 60°C for 34 sec. The relative expression of
target genes was calculated using the 2−ΔΔCq method
(35).
miRNA transfection
miRNA-101-3p mimic (5′-UACAG
UACUGUGAUAACUGAACAGUUAUCACAGUACUGU AUU-3′), miRNA-101-3p
mimic-inhibitor (5′-UUCAGUUAU CACAGUACUGUA-3′), small interfering
RNA (si)-SNHG1, si-ROCK1 and the negative control (5′-UUCUCCGAACGU
GUCACGUTT-3′) were obtained from GenePharma Co., Ltd. (Shanghai,
China). Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.) was used for miRNA and siRNA transfection. The sequences for
si-SNHG1 were sense, 5′-CUUAAAGUG UUAGCAGACATT-3′ and antisense,
5′-AAUGUCUGCUAA CACUUUAAG-3′; the siROCK1 sequences were sense,
5′-GCACCAGTTGTACCCGATTTA-3′ and antisense, 5′-TAA
ATCGGGTACAACTGGTGC-3′.
3-(4,5-Dimethylthiazol-2-yl)-2, 5
diphenyltetrazolium bromide (MTT) assay
In 96-well plates, following the transfection in
different groups, the MG63 (2,500 cells/well) and U2OS (3,000
cells/well) were cultured in DMEM/F12 supplemented with 10% FBS for
24, 48, 72 and 96 h. Subsequently, 10 µl MTT was added into
the culture medium for 60 min. The culture media was removed, and
100 µl DMSO was added into each well to dissolve the
resulting crystals with agitation for 10 min. The optical density
(OD) at 490 nm was measured using a microplate reader.
Analysis of cell cycle and apoptosis via
flow cytometry
For the cell cycle analysis, the transfected cells
were harvested and stained with propidium iodide using the Cell
Cycle Analysis kit (Biyuntian; Jiangsu, China), followed by
assessment using flow cytometry. Using FlowJo software 7.6 (Tree
Star, Inc., Ashland OR, USA), the percentage of cells in different
phases was counted. An FITC Annexin V Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA) was used to analyze
apoptosis. The transfected cells were harvested and re-suspended in
binding buffer, and Annexin V-FITC and propidium iodide were used
to stain the cells. Flow cytometry was performed according to the
manufacturer's protocol.
Dual luciferase activity assay
The dual luciferase activity assay was used for
miRNA target validation. The SNHG1 cDNA containing the putative
miRNA-101-3p-binding site was amplified by PCR and was cloned into
the luciferase reporter psiCHECK2 vector (Promega Corporation,
Madison, WI, USA), termed SNHG1 wild-type (WT). The mutant (Mut) of
SNHG1 was 5′-CTGTCATGCTGTCATGACTA-3′. Subsequently, the MG63
(1.5×104) and U2OS (1.8×104) cells were
seeded in 24-well plates and were transfected with the SNHG1 WT or
SNHG1 Mut and miRNA-101-3p or a scramble using Lipofectamine 3000
(Thermo Fisher Scientific, Inc.). The luciferase activity in the
transfected cells was analyzed using the dual-luciferase assay
system (Promega Corporation), following the manufacturer's
protocol.
Invasion and migration assay
A cell invasion assay was performed with Transwells
coated with Matrigel (BD Biosciences). Briefly, MG63
(1×105) and U2OS (1.2×105) cells were
re-suspended in a serum-free medium. Subsequently, 200 µl of
the cell suspension was added to the top chamber. To prepare the
chemoattractant in the lower chamber, 600 µl of medium
containing 10% FBS was added into the lower chamber. The Transwell
was incubated for 48 h at 37°C under 5% CO2.
Subsequently, the cells in the top chamber were wiped off using a
cotton swab, and the cells in the lower chamber were fixed in 4%
formaldehyde and were stained with 0.1% crystal violet. Under a
microscope (1×71; Olympus Corporation, Tokyo, Japan), the cell
numbers were counted. The average data were collected from three
independent repeats.
A migration assay was performed using a two-chamber
migration assay with a pore size of 8 mm. The top chamber was
seeded with 200 µl of cells in serum-free medium, and 600
µl of complete medium was added to the lower chamber.
Following incubation at 37°C for 24 h, the traversed cells on the
lower chamber were stained with crystal violet and were counted.
The data were collected from three independent experiments.
Western blot analysis
The proteins were extracted using
immunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) with protease inhibitors (Roche,
Diagnostics GmbH, Mannheim, Germany). Protein concentration was
determined with a bicinchoninic acid protein assay. Equal
quantities of protein (10 µg/lane) from cell lysates were
separated via 8% SDS-polyacrylamide gel electrophoresis and were
transferred onto polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The western blot analysis
was performed using a Bio-Rad Bis-Tris Gel system according to the
manufacturer's protocol. Briefly, primary antibodies (All purchased
from Abcam, Cambridge, MA, USA) against ROCK1 (cat. no. ab45171;
1:2,000), anti-phosphoinositide 3-kinase (PI3K; cat. no. ab151549;
1:1,000), phosphorylated (p)-PI3K (cat. no. ab182651; 1:1,000),
anti-protein kinase B (AKT; cat. no. ab8805; 1:1,000), anti-p-AKT
(cat. no. ab38449; 1:1,000), anti-E-cadherin (cat. no. ab1416;
1:1,000), anti-N-cadherin (cat. no. ab76057; 1:1,000) were
incubated with the membrane at 4°C overnight with 5% blocking
buffer. Horseradish peroxidase-conjugated secondary antibodies,
including goat anti-rabbit (cat. no. ab7090; 1:5,000; Abcam), goat
anti-mouse (cat. no. ab97040; 1:5,000; Abcam) were subsequently
added for 1 h at room temperature, following washing the membrane
three times with Tris buffered saline with 0.05% Tween-20.
Following rinsing, the chemiluminescence signal was visualized
following the addition of 200 µl Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA),
and the bands were quantified using Image Lab™ 3.0 software
(Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated at least three times,
and data are expressed as the mean ± standard deviation. All the
data were analyzed with SPSS 20.0 (IBM Corp., NY, Armonk, USA). The
difference between two groups was compared using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miRNA-101 is downregulated and lncRNA
SNHG1 is upregulated in OS tissues and OS cell lines
To determine the role of miRNA-101-3p and the lncRNA
SNHG1 in OS, the present study examined the expression levels of
miRNA-101-3p and the lncRNA SNHG1 via RT-qPCR analysis in OS
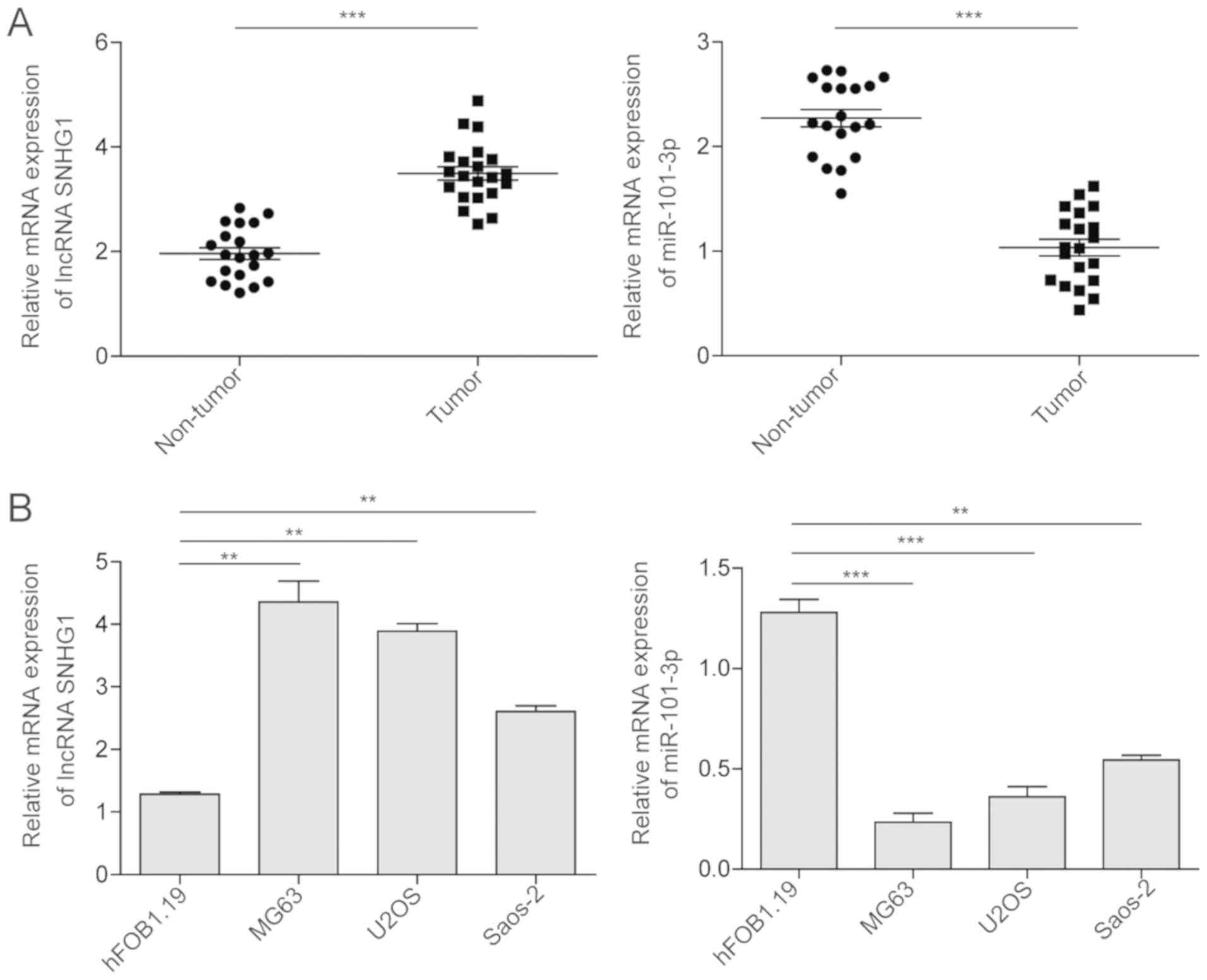
tissues samples and OS cell lines. As shown in Fig. 1A, miRNA-101-3p was downregulated
in the OS tissues compared with the expression in the adjacent
non-tumor tissues. However, the lncRNA SNHG1 was upregulated in the
OS tumor tissues compared with its expression in the adjacent
non-tumor tissues. The sample distribution and tumor stage in these
patients were also analyzed (Table
I). The results indicated that the expression of the lncRNA
SNHG1 did not differ significantly with patient age or gender.
However, patients with a large tumor size (>8 cm), later
Enneking stage or distant metastasis exhibited a significantly
higher expression level of lncRNA SNHG1. Furthermore, similar
trends were observed in the OS tumor cell lines (Fig. 1B). All three OS cancer cell lines
(MG63, U2OS, and Saos-2 cells), exhibited a significantly lower
expression of miRNA-101-3p and higher expression of lncRNA SNHG1
compared with the expression levels in the normal hFOB1.19 cell
line.
 | Table IExpression of lncRNA SNHG1 in
relation to clinicopathologic features. |
Table I
Expression of lncRNA SNHG1 in
relation to clinicopathologic features.
|
Characteristics | n | lncRNA SNHG1
| P-value |
|---|
| Negative | Positive |
|---|
| Patients | 43 | 13 | 30 | |
| Age (years) | | | | 0.864 |
| ≤20 | 19 | 6 | 13 | |
| >20 | 24 | 7 | 17 | |
| Sex | | | | 0.370 |
| Male | 22 | 8 | 14 | |
| Female | 21 | 5 | 16 | |
| Tissue size | | | | 0.002a |
| D <8 cm | 15 | 9 | 6 | |
| D ≥8 cm | 28 | 4 | 24 | |
| Enneking stage | | | | 0.004a |
| I | 6 | 5 | 1 | |
| II | 24 | 7 | 17 | |
| III | 13 | 1 | 12 | |
| Distant
metastasis | | | | 0.034a |
| Present | 13 | 1 | 12 | |
| Absent | 30 | 12 | 18 | |
lncRNA SNHG1 promotes cell
proliferation
To investigate the function of the lncRNA SNHG1 in
OS tumorigenesis, si-lncRNA SNHG1 was transfected into two OS tumor
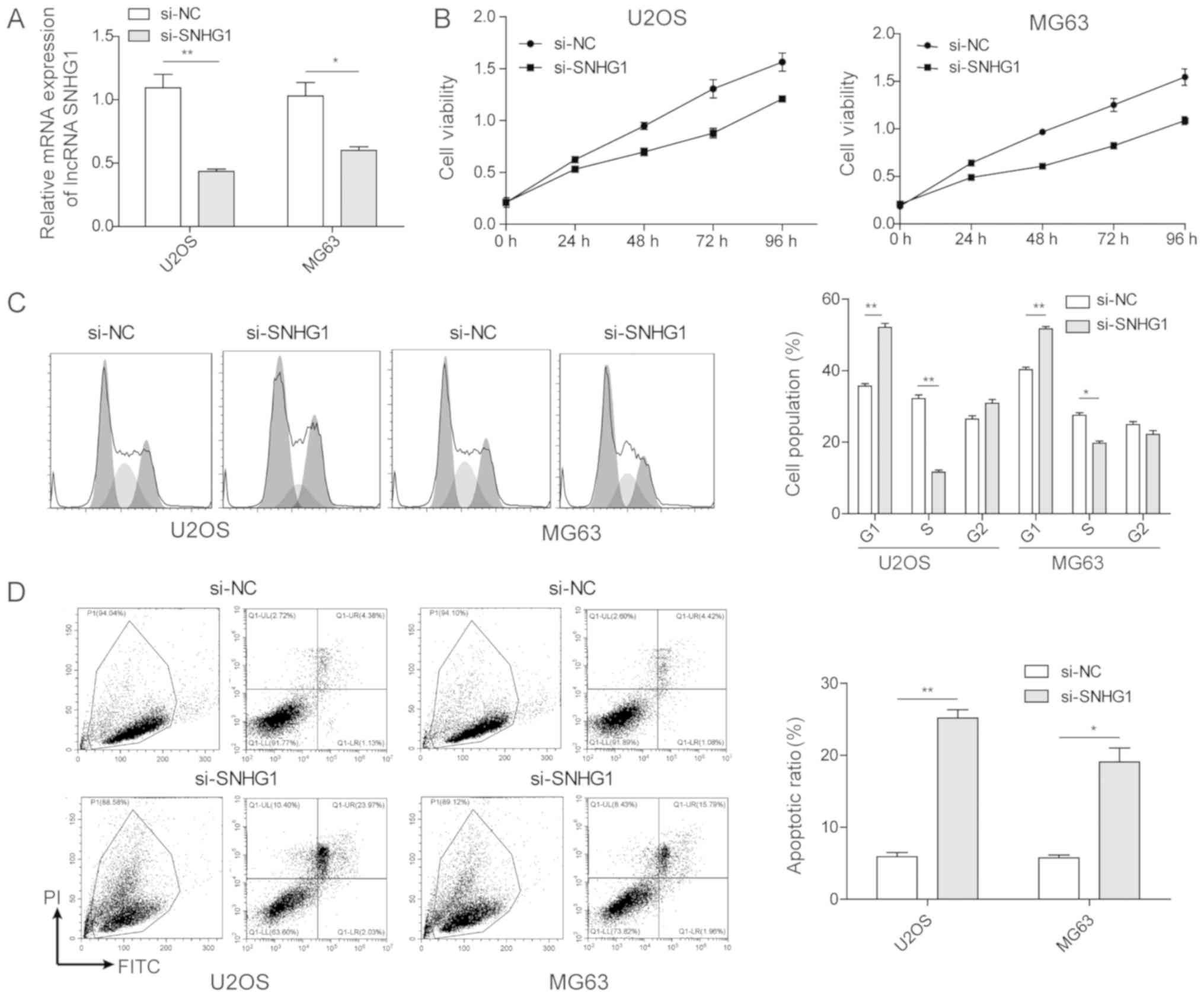
cell lines. As shown in Fig. 2A
successful transfection was achieved, which induced a lower
expression of lncRNA SNHG1, as determined via RT-qPCR analysis.
With these transfected cell lines, an MTT assay was performed to
investigate the effect of the lncRNA SNHG1 on cell proliferation.
As shown in Fig. 2B, the MTT OD
values in the OS cell lines transfected with si-lncRNA SNHG1 at 48,
72 and 96 h were significantly lower than those of the control
groups, demonstrating that inhibiting the lncRNA SNHG1
significantly decreased cell proliferation rates. These results
indicated that the overexpression of lncRNA SNHG1 in OS tumor cell
lines promoted OS cell proliferation in vitro.
Subsequently, flow cytometric analysis was used to
evaluate the effect of the lncRNA SNHG1 on OS cell proliferation.
As shown in Fig. 2C, the
inhibition of lncRNA SNHG1 in OS cancer cell lines decreased the
percentages of cells at the G0/G1 and S phases, demonstrating that
the lncRNA SNHG1 promoted cell cycle progression of the OS cancer
cells. Furthermore, flow cytometric analysis was used to analyse
cell apoptosis through Annexin V-FITC/propidium-iodide dual
staining. The results showed that the proportion of apoptotic OS
cells with si-lncRNA SNHG1 transfection was significantly enhanced
compared with that in the control groups. Therefore, in the OS
tumor cells, the high expression of lncRNA SNHG1 inhibited OS cell
apoptosis.
lncRNA SNHG1 inhibits the expression of
miRNA-101-3p
To determine the interaction between the lncRNA
SNHG1 and miRNA-101-3p, the miRDB database was used to examine the
potential complementary sequences between the lncRNA SNHG1 and
miRNA-101-3p (Fig. 3A).
Subsequently, dual-luciferase reporters containing either SNHG1 WT
or SNHG1 Mut were constructed. First, the U2OS cells were
co-transfected with miRNA-101-3p and SNHG1-WT or SNHG1-Mut and the
luciferase intensity was measured. As shown in Fig. 3B, the SNHG1-WT and
miRNA-101-3p-transfection groups demonstrated a significant
decrease in luminescence intensity, which suggested that there may
be a direct interaction between miRNA-101-3p and the lncRNA SNHG1.
Subsequently, the expression of miRNA-101-3p was examined in OS
cancer cell lines following knockdown of the lncRNA SNHG1 via siRNA
(Fig. 3C). The results showed a
significant enhancement of the expression of miRNA-101-3p in OS
cancer cell lines with SNHG1 knockdown compared with that in the
control groups. A similar investigation was performed following
manipulation of the expression of miRNA-101-3p. However,
miRNA-101-3p transfection in OS cell lines did not affect the
expression level of the lncRNA SNHG1. These results indicated that
the lncRNA SNHG1 inhibited the expression of miRNA-101-3p in OS
cancer cell lines.
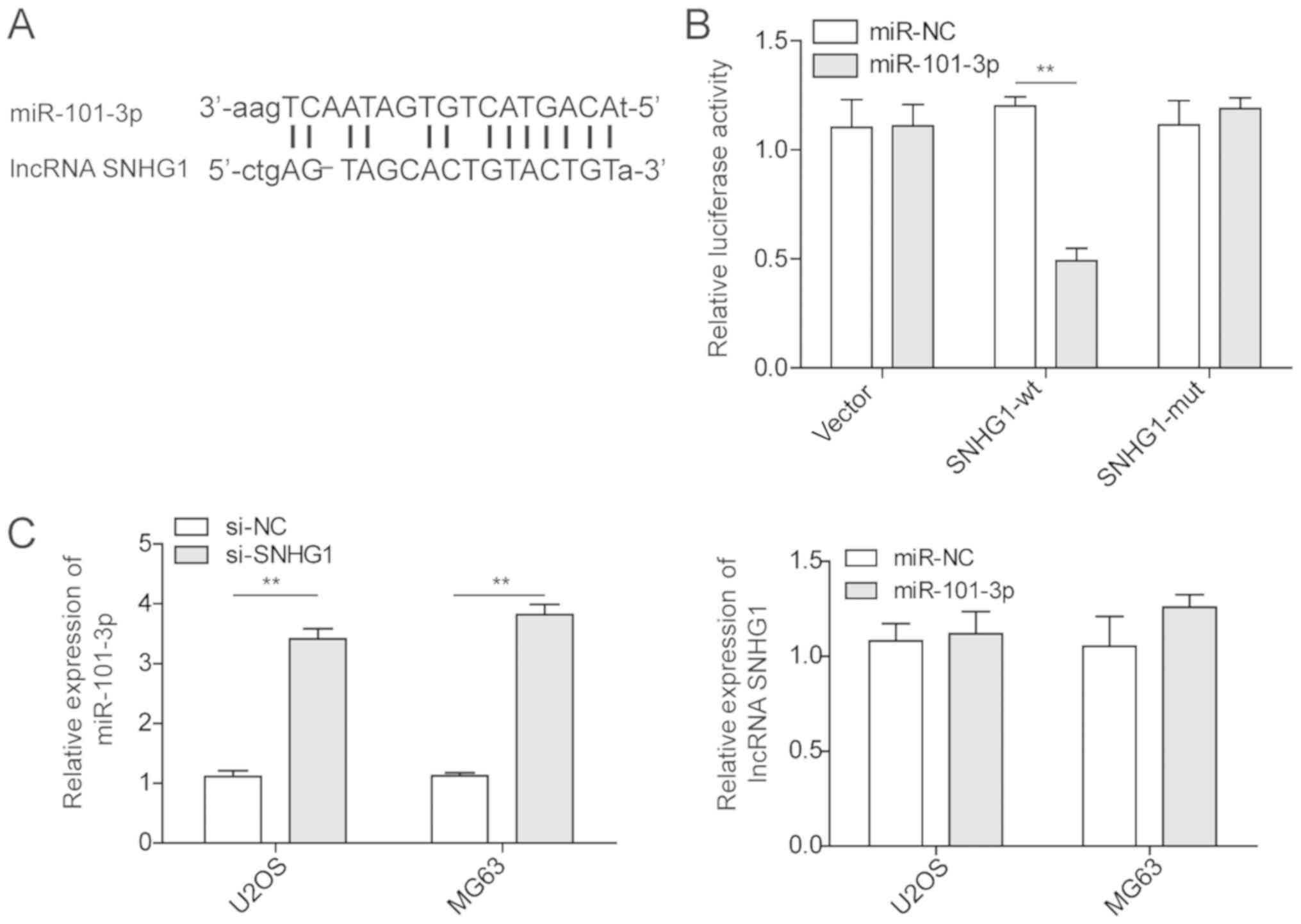 | Figure 3Regulation of miR-101-3p by the
lncRNA SNHG1 in osteosarcoma cancer cells. (A) Prediction of
miR-101-3p binding sites on the lncRNA SNHG1 transcript. (B)
Luciferase activities in U2OS cells co-transfected with miR-101-3p
or miR-NC and luciferase reporters containing empty vector, lncRNA
SNHG1 WT, or lncRNA SNHG1 Mut. Data are presented as a relative
ratio. (C) Relative expression levels of miR-101-3p in U2OS and
MG63 cells transfected with si-SNHG1 or si-NC, as detected by
RT-qPCR analysis. The relative expression levels of the lncRNA
SNHG1 in U2OS and MG63 cells transfected with miR-101-3p or miR-NC
were detected via RT-qPCR analysis. The mean ± standard deviation
in the graph represents the relative levels from three replicant
experiments. **P<0.01. lncRNA, long non-coding RNA;
si-, small interfering RNA; miR, microRNA; NC, negative control;
WT, wild-type; Mut, mutant; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Identification of ROCK1 as a target of
miRNA-101-3p
The present study investigated the association
between ROCK1 and miRNA-101-3p via a dual-luciferase assay. From
the prediction of the miRDB database and the reported literature,
ROCK1 was identified as a potential target of miRNA-101-3p
(Fig. 4A). A ROCK1-WT and a
ROCK1-Mut vector were constructed and were used to transfect U2OS
cells with either miRNA-101-3p or miRNA-NC. The luciferase activity
in the group transfected with ROCK1-WT and miRNA-101-3p was lower
than the activity in the other control groups, indicating that
ROCK1 was a target of miRNA-101-3p (Fig. 4B).
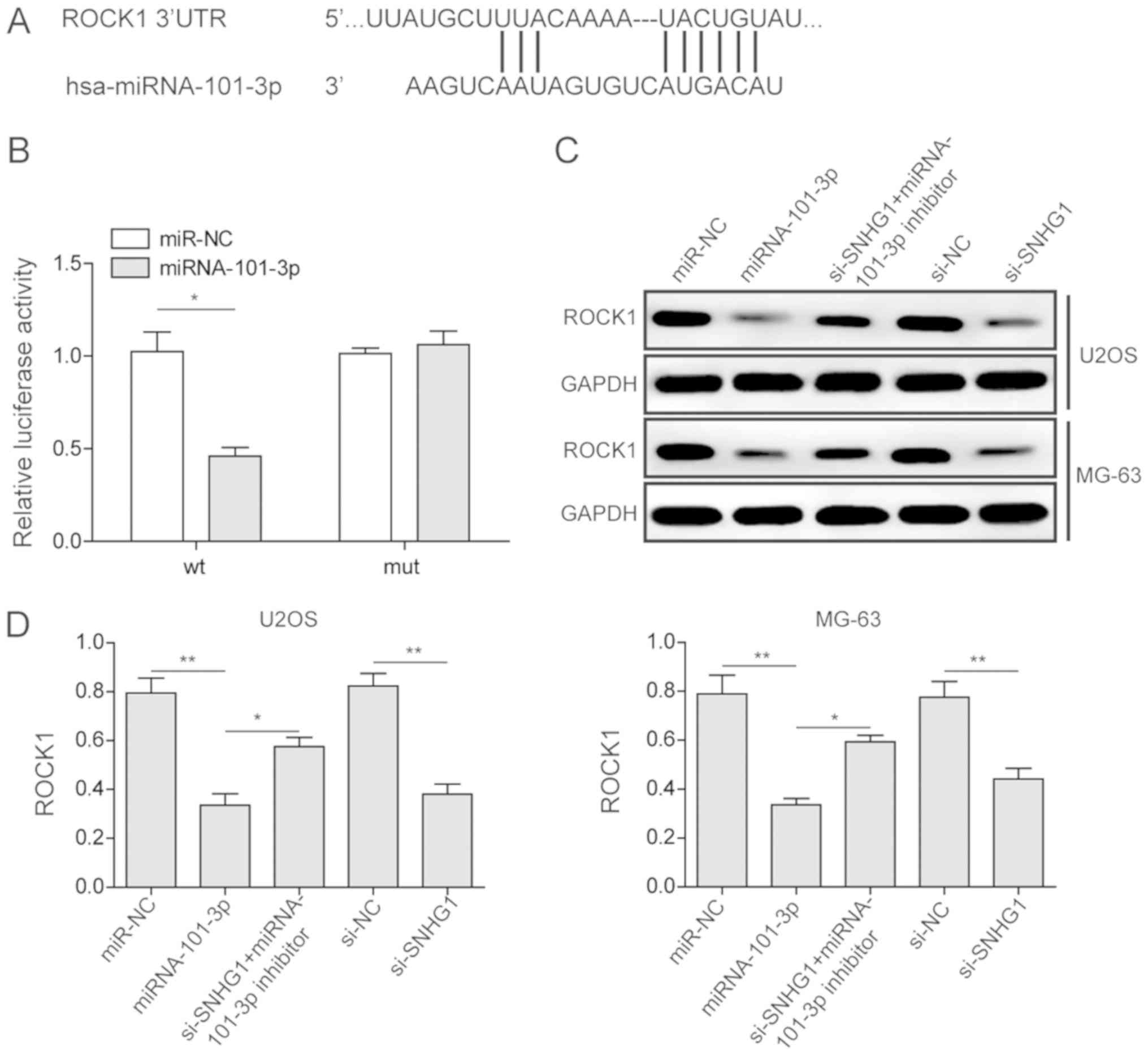 | Figure 4Regulation of ROCK1 via miR-101-3p in
osteosarcoma cancer cells. (A) Prediction of miR-101-3p-binding
sites on the ROCK1 transcript. (B) Luciferase activities in U2OS
cells co-transfected with miR-101-3p or miR-NC and luciferase
reporters containing ROCK1 WT or ROCK1 Mut. (C) Relative expression
levels of ROCK1 in U2OS and MG63 cells transfected with miR-101-3p,
miR-NC, si-SNHG1, si-NC, and si-SNHG1 + miR-101-3p inhibitor, as
detected via western blot analysis. (D) Quantitative analysis of
ROCK1 expression in U2OS and MG63 cells. The mean ± standard
deviation in the graph represents the relative levels from three
replicate experiments. *P<0.05;
**P<0.01. miR, microRNA; NC, negative control; WT,
wild-type; Mut, mutant; si-, small interfering RNA; ROCK1,
Rho-associated coiled-coil-containing protein kinase 1. |
miRNA-101-3p controlled by lncRNA SNHG1
inhibits cell proliferation, migration and invasion by inhibiting
ROCK1
From the previous results, it was shown that the
lncRNA SNHG1 significantly inhibited the expression of miRNA-101-3p
and that ROCK1 was a target of miRNA-101-3p. Therefore, the
association among the lncRNA SNHG1, miRNA-101-3p and ROCK1 was
investigated using western blot analysis. As shown in Fig. 4C, U2OS and MG63 cells were
transfected with miRNA-NC, miRNA-101-3p, si-SNHG1 with miRNA-101-3p
inhibitor, si-NC and si-SNHG1. Compared with its expression
following miRNA-NC transfection, ROCK1 was significantly decreased
in the group treated with miRNA-101-3p, which was consistent with
the results of the dual-luciferase assay. Furthermore, by
inhibiting the lncRNA SNHG1 and miRNA-101-3p or inhibiting the
lncRNA SNHG1 only, ROCK1 was significantly inhibited. Combed with
the previous results, it was concluded that the lncRNA SNHG1
inhibited the expression of miRNA-101-3p, which further enhanced
the expression of ROCK1 in OS cancer cell lines.
The present study also investigated the associations
among the lncRNA SNHG1, miRNA-101-3p and ROCK1, and their effect on
cell viability, migration and invasion. The U2OS and MG-63 cells
were transfected with miRNA-NC, miRNA-101-3p, miRNA-101-3p
inhibitor, si-SNHG1 with miRNA-101-3p inhibitor, and si-ROCK1. As
shown in Fig. 5A, the MTT assay
results indicated that miRNA-101-3p and si-ROCK1 treatments
decreased the cell viability of OS cancer cells, which suggested
that any pathways that inhibited ROCK1 inhibit the cell viability
of OS cancer cells. Additionally, a Transwell assay was used to
investigate their effect on OS cell migration and invasion. As
shown in Fig. 5B and 5C, transfection with miRNA-101-3p mimics
and si-ROCK1 inhibited cell migration and invasion; however, the
miRNA-101-3p inhibitor and si-SNHG1 with miRNA-101-3p inhibitor
enhanced cell migration. All of the above results suggested that
the lncRNA SNHG1 inhibited the expression of miRNA-101-3p, leading
to the activation of ROCK1 in OS cancer cells, which significantly
enhanced cell proliferation, migration and invasion ability.
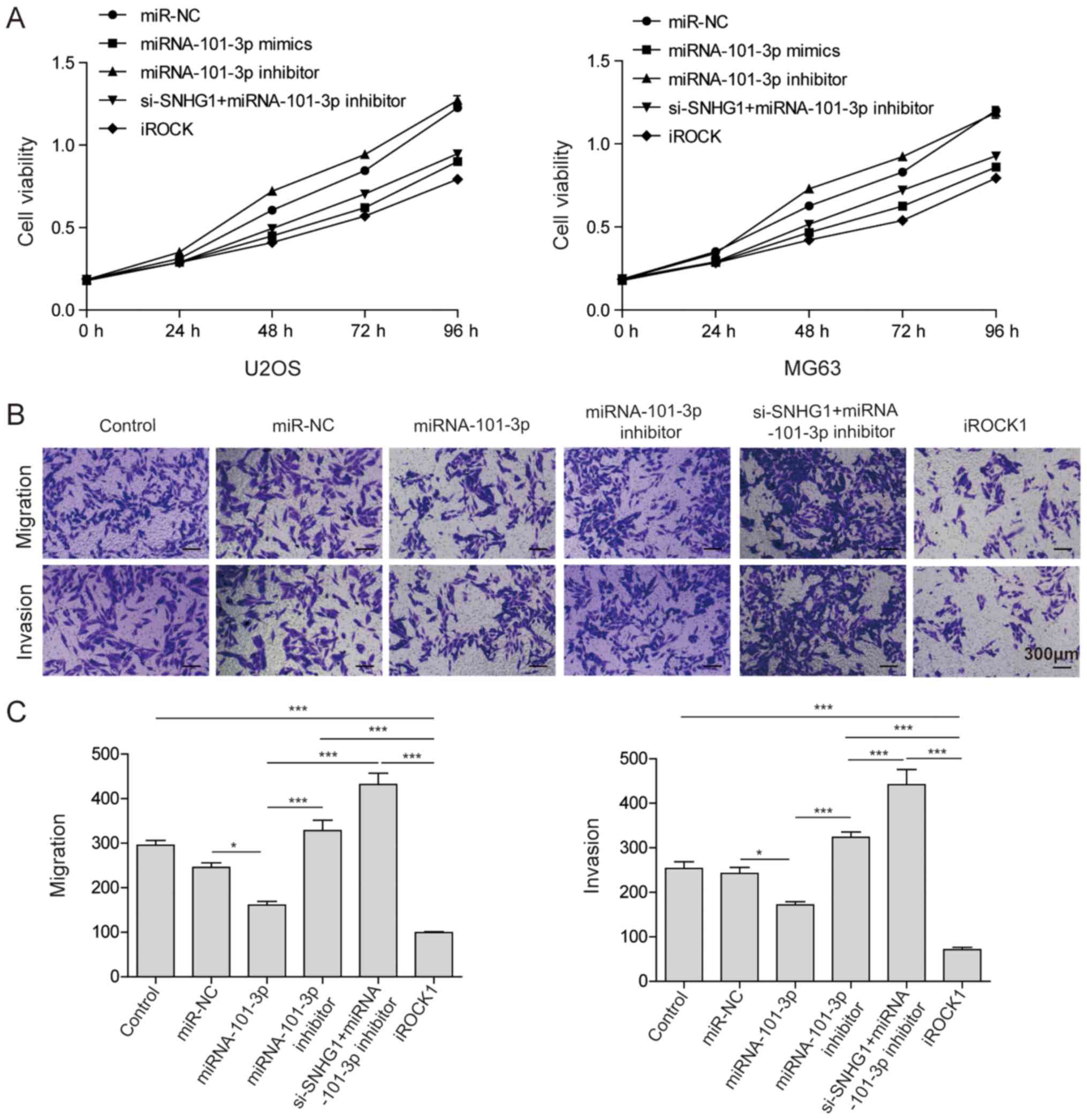 | Figure 5lncRNA SNHG1 promotes osteosarcoma
cell proliferation, invasion and migration. (A) U2OS and MG63 cells
were transfected with miR-NC, miR-101-3p, miR-101-3p inhibitor,
si-lncRNA SNHG1 + miR-101-3p inhibitor, and iROCK1. An MTT assay
was performed to determine the cell viability. (B) Migration and
invasion abilities of U2OS cells following no transfection or
transfection with miR-NC, miR-101-3p, miR-101-3p inhibitor,
si-lncRNA SNHG1 + miR-101-3p inhibitor, and iROCK1 were
investigated via a Transwell assay. Magnification, ×100. (C) Cell
numbers in the bottom chambers for migration and invasion assays
were determine in the groups. The mean ± standard deviation in the
graphs represent the relative levels from three replicate
experiments. *P<0.05; ***P<0.001.
lncRNA, long non-coding RNA; miR, microRNA; NC, negative control;
WT, wild-type; Mut, mutant; si-, small interfering RNA; ROCK1,
Rho-associated coiled-coil-containing protein kinase 1; iROCK1,
si-ROCK1. |
Phosphoinositide 3-kinase (PI3K)/AKT
pathway is activated and EMT is induced
To clarify the signaling pathways involved in the
function of the lncRNA SNHG1, miRNA-101-3p, and ROCK1 in OS cancer
cells, the expression of the components of the PI3K/AKT and EMT
pathways were measured via western blot analysis. As shown in
Fig. 6A and B, the overexpression
of miRNA-101-3p in the group transfected with miRNA-101-3p mimics
resulted in a downregulation in the expression of ROCK1, p-PI3K,
and p-AKT. However, the knockdown of miRNA-101-3p by the
miRNA-101-3p inhibitor alone significantly enhanced the expression
of ROCK1, p-PI3K, and p-AKT. In addition, the co-transfection of
si-SNHG1 and the miRNA-101-3p inhibitor enhanced the expression of
these three proteins compared with miRNA-101-3p-mimics
transfection. These results indicated that the PI3K/AKT pathway was
activated in OS cancer cells.
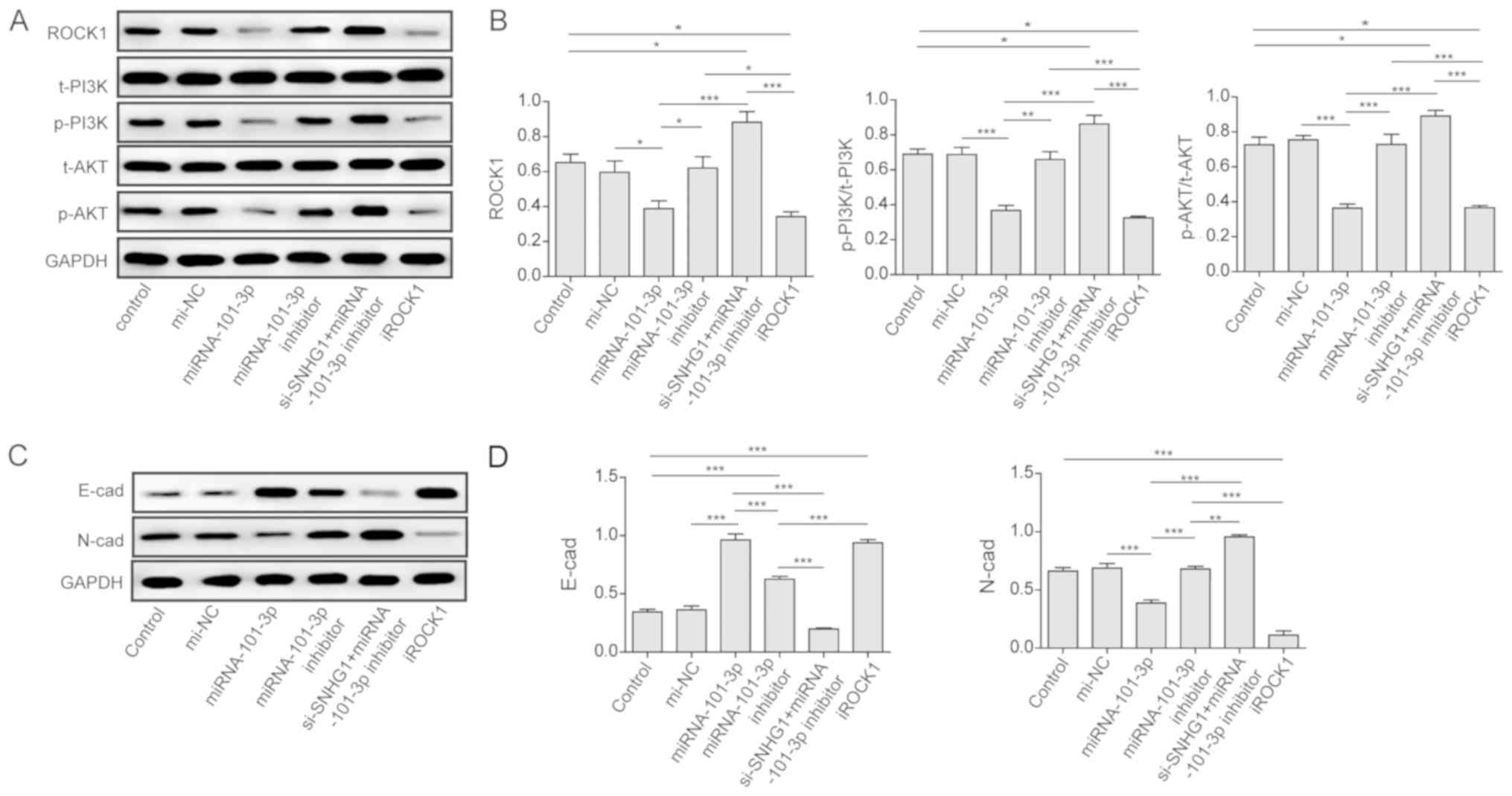 | Figure 6PI3K/AKT pathway and EMT activation.
(A) Overexpression of miR-101-3p inhibited the PI3K/AKT pathway via
the downregulation of ROCK1. Western blot analysis was used to
analyse the expressions of related components in U2OS cells
transfected with nothing, miR-NC, miR-101-3p, miR-101-3p inhibitor,
si-lncRNA SNHG1 + miR-101-3p inhibitor, and iROCK1. (B) Expression
levels of ROCK1, p-PI3K/t-PI3K, and p-AKT/t-AKT in the different
groups. (C) Overexpression of miR-101-3p inhibited the activation
of EMT by promoting the expression of E-cad and inhibiting N-cad.
Western blot analysis was used to analyse the expressions of N-cad
and E-cad in U2OS cells transfected with nothing, miR-NC,
miR-101-3p, miR-101-3p inhibitor, si-lncRNA SNHG1 + miR-101-3p
inhibitor, and iROCK1. (D) Expression levels of E-cad in the
different groups. The mean ± standard deviation in the graph
represents the relative levels from three replicate experiments.
*P<0.05; **P<0.01;
***P<0.001. lncRNA, long non-coding RNA; miR,
microRNA; NC, negative control; WT, wild-type; Mut, mutant; si-,
small interfering RNA; ROCK1, Rho-associated coiled-coil-containing
protein kinase 1; iROCK1, si-ROCK1; PI3K, phosphoinositide
3-kinase; p-, phosphorylated; t-, total; EMT,
epithelial-mesenchymal transition; E-cad, E-cadherin; N-cad,
N-cadherin. |
Usually, activation of the PI3K/AKT pathway induces
EMT in cancer cells. Therefore, the present study also investigated
the EMT pathway. As shown in Fig. 6C
and D, miRNA-101-3p mimics and si-ROCK1 promoted the expression
of E-cadherin and inhibited the expression of N-cadherin. By
contrast, treatment with the miRNA-101-3p inhibitor alone or
si-SNHG1 with the miRNA-101-3p inhibitor inhibited the expression
of E-cadherin and promoted the expression of N-cadherin. These
results demonstrated that EMT was also activated in OS cancer
cells, and that the inhibition of lncRNA SNHG1 or overexpression of
miRNA-101-3p were able to inhibit activation of the EMT process in
OS cells.
Discussion
Osteosarcoma (OS) is a common aggressive
mesenchyme-derived bone tumor that primarily affects children and
adolescents (3). The main
strategies for OS treatment include surgical resection,
chemotherapy, and radiotherapy (8). Despite the efforts of clinicians and
researchers, the overall survival rate of patients with OS remains
poor, particularly for those who are at advanced clinical stages
(6). Therefore, the
identification a novel diagnostic biomarker and the mechanism
involved in OS tumorigenesis and metastasis, which may offer a
potential therapeutic target, is crucial. Previously, the
dysregulation of lncRNAs has been reported during tumorigenesis and
progression in several types of malignant tumors. For example, Wang
et al (36) reported that
the lncRNA AK093407 promoted OS cell proliferation and inhibited
its apoptosis by activating signal transducer and activator of
transcription 3. Therefore, the present study focused on the
abnormal expression of the lncRNA SNHG1 in OS and its underlying
molecular mechanism in regulating OS tumorigenesis.
In the present study, the lncRNA SNHG1 was found to
be overexpressed in OS tumor tissues compared with its expression
in adjacent non-tumor tissues. In addition, it was found that the
expression of lncRNA SNHG1 in the OS tumor cell lines was
significantly upregulated compared with the expression in the
non-tumor cell line, which was consistent with the results reported
by Jiang et al (37)
previously. Wang et al (38) also found that the lncRNA SNHG1
promoted tumorigenesis in OS. In all OS tumor tissues and cell
lines, the expression of miRNA-101-3p was significantly decreased
compared with that in the control groups, which indicated a
potential association between the lncRNA SNHG1 and miRNA-101-3p.
Furthermore, using an MTT assay, RT-qPCR analysis, and flow
cytometric analysis, the present study determined the effect of the
lncRNA SNHG1 on OS cell proliferation, cell cycle phase, and
apoptosis. First, by silencing the expression of lncRNA SNHG1, all
three OS tumor cell lines exhibited lower cell viability, and the
flow cytometric analysis indicated that tumor cell apoptosis
increased following transfection with the si-lncRNA SNHG1. In
addition, the cell cycle phase was analyzed following lncRNA SNHG1
inhibition. The result revealed that the low OS cell proliferation
was caused by G1/G0 phase arrest and cell apoptosis. These data
suggested that the lncRNA SNHG1 may be considered as a tumor
oncogene in OS progression.
Previous reports have indicated that the expression
and activity of miRNA can also be regulated by lncRNAs. For
example, Li et al (39)
found that the lncRNA UCA1 promoted glutamine metabolism by
targeting miRNA-16 in human bladder cancer. Cui et al
(19) reported that the lncRNA
SNHG1 contributed to the progression of non-small cell lung cancer
by inhibiting miRNA-101-3p. Wang et al (38) reported that the lncRNA SNHG1
inhibited miRNA-326 and promoted tumorigenesis in OS. However, the
association between the lncRNA SNHG1 and miRNA-101-3, and their
effect on tumorigenesis and progression in OS remain to be
elucidated and require documentation to provide an efficient
therapeutic strategy. In the present study, it was found that
miRNA-101-3p was an inhibitory target of the lncRNA SNHG1 in OS
using sequence complementarity analysis and a dual-luciferase
assay. In OS tissues and cell lines, the lncRNA SNHG1 and
miRNA-101-3p showed a significant negative correlation. By
inhibiting the expression of the lncRNA SNHG1 in OS cancer cells,
the expression of miRNA-101-3p was significantly enhanced. However,
miRNA-101-3p transfection did not affect the expression of the
lncRNA SNHG1. These data indicated that miRNA-101-3p was a direct
target of the lncRNA SNHG1. Furthermore, miRNA has been reported to
inhibit the expression of ROCK1 in OS cancer cells to prevent cell
proliferation, migration and invasion. Therefore, the present study
also examined the associations among lncRNA SNHG1, miRNA-101-3p and
ROCK1 in OS cancer cell lines using a dual-luciferase assay and
western blot analysis. The results showed that ROCK1 was a direct
target of miRNA-101-3p in OS cancer cells. In addition, by knocking
down the expression of lncRNA SNHG1 using si-SNHG1 in U2OS and
MG-63 cells, the expression of ROCK1 was significantly decreased,
which showed a similar effect as for the overexpression of
miRNA-101-3p. However, co-transfection with si-SNHG1 and
miRNA-101-3p inhibitor promoted the expression of ROCK1 in the two
cell lines. These results suggested that, in OS cancer cell lines,
ROCK1 was a direct target of miRNA-101-3p and that ROCK1 can be
directly controlled by the expression of lncRNA SNHG1.
Furthermore, the present study examined how the
associations among the lncRNA SNHG1, miRNA-101-3p, and ROCK1 affect
cell proliferation, migration and invasion. Using an MTT assay and
Transwell assay, it was found that the overexpression of
miRNA-101-3p in OS cancer cell lines inhibited cell proliferation,
cell migration and invasion; this result was similar to that
obtained when the cells were transfected with si-ROCK1. By
contrast, treatment with the miRNA-101-3p inhibitor or
co-transfection with si-SNHG1 and miRNA-101-3p inhibitor promoted
cell proliferation, migration and invasion.
A previous report indicated that miRNA-101-3p
inhibited OS tumor growth and migration by regulating the PI3K/AKT
pathway (22). In OS tumors, the
PI3K/AKT pathway is the most frequently activated signal
transduction pathway and contributes to cancer initiation,
development and metastasis. Therefore, the present study also
investigated the mechanism underlying lncRNA SNHG1 regulation in OS
cancer cell lines using western blot analysis. Through the
transfection of miRNA-101-3p mimics or si-SNHG1 in U2OS cells, the
expression levels of PI3K and AKT were significantly inhibited. By
contrast, co-transfection with si-SNHG1 and miRNA-101-3p inhibitor,
or transfection with miRNA-101-3p inhibitor alone, promoted the
activation of the PI3K/AKT pathway in OS cells. Furthermore, it was
found that EMT was inactivated by treatment with miRNA-101-3p or
si-SNHG1. EMT can be divided into three general subtypes based on
the phenotype of the output cells (40). Type 1 EMT involves primitive
epithelial cell transition into mobile mesenchymal cells. Type 2
EMT transitions cells to resident tissue fibroblasts through
involvement of the secondary epithelial or endothelial cells. Type
3 EMT occurs in epithelial carcinoma cells, generating secondary
tumor nodules by transforming these cells to metastatic tumor cells
that migrate to metastatic sites (40). Therefore, it was hypothesized that
miRNA-101-3p or si-SNHG1 can be used to inhibit type 3 EMT during
cancer development.
In conclusion, the present study indicated that the
lncRNA SNHG1 acted as an oncogene in OS cancer; it downregulated
the expression of miRNA-101-3p and promoted cancer cell
proliferation, migration and invasion through activating the
expression of ROCK1, the PI3K/AKT pathway and EMT. The results
provided a possible molecular mechanism of tumorigenesis and
development in OS cancer involving the lncRNA SNHG1 and
miRNA-101-3p, which may be used for the development of a novel
therapeutic strategy for OS.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RD, JC and JZ conceived and designed the study. RD
and JZ performed the experiments; acquired, analyzed and
interpreted the data; and edited the manuscript. RD was involved in
the clinical study and preparation of the manuscript. JC reviewed
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Nanfang Hospital, Southern Medical University
(Guangzhou, China). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare the that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lamoureux F, Trichet V, Chipoy C,
Blanchard F, Gouin F and Redini F: Recent advances in the
management of osteosarcoma and forthcoming therapeutic strategies.
Expert Rev Anticancer Ther. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Z, He R, Xia H, Wei YU and Wu S:
MicroRNA-101 has a suppressive role in osteosarcoma cells through
the targeting of c-FOS. Exp Ther Med. 11:1293–1299. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo J, Reddick WE, Glass JO, Ji Q, Billups
CA, Wu J, Hoffer FA, Kaste SC, Jenkins JJ, Ortega Flores XC, et al:
Dynamic contrast-enhanced magnetic resonance imaging as a
prognostic factor in predicting event-free and overall survival in
pediatric patients with osteosarcoma. Cancer. 118:3776–3785. 2012.
View Article : Google Scholar
|
|
5
|
El-Naggar AM, Veinotte CJ, Cheng H,
Grunewald TG, Negri GL, Somasekharan SP, Corkery DP, Tirode F,
Mathers J, Khan D, et al: Translational Activation of HIF1α by YB-1
Promotes Sarcoma Metastasis. Cancer Cell. 27:682–697. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, Function and Role in Cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar
|
|
8
|
Yang J and Zhang W: New molecular insights
into osteosarcoma targeted therapy. Curr Opin Oncol. 25:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT,
Xia YJ, Ye ZY and Tao HQ: MicroRNA-101 is down-regulated in gastric
cancer and involved in cell migration and invasion. Eur J Cancer.
46:2295–2303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JG, Guo J-F, Liu D-L, Liu Q and Wang
J-J: MicroRNA-101 exerts tumor-suppressive functions in non-small
cell lung cancer through directly targeting enhancer of zeste
homolog 2. J Thorac Oncol. 6:671–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K,
Ren G, Su T, Pan Y, Feng B, et al: MiR-150 promotes gastric cancer
proliferation by negatively regulating the pro-apoptotic gene EGR2.
Biochem Biophys Res Commun. 392:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma Y, Zhang P, Wang F, Zhang H, Yang J,
Peng J, Liu W and Qin H: miR-150 as a potential biomarker
associated with prognosis and therapeutic outcome in colorectal
cancer. Gut. 61:1447–1453. 2012. View Article : Google Scholar
|
|
15
|
Farhana L, Dawson MI, Murshed F, Das JK,
Rishi AK and Fontana JA: Upregulation of miR-150* and miR-630
induces apoptosis in pancreatic cancer cells by targeting IGF-1R.
PLoS One. 8:e610152013. View Article : Google Scholar
|
|
16
|
Hu H, Zhang Y, Cai XH, Huang JF and Cai L:
Changes in microRNA expression in the MG-63 osteosarcoma cell line
compared with osteoblasts. Oncol Lett. 4:1037–1042. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aguda BD, Kim Y, Piper-Hunter MG, Friedman
A and Marsh CB: MicroRNA regulation of a cancer network:
Consequences of the feedback loops involving miR-17-92, E2F, and
Myc. Proc Natl Acad Sci USA. 105:19678–19683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui Y, Zhang F, Zhu C, Geng L, Tian T and
Liu H: Upregulated lncRNA SNHG1 contributes to progression of
non-small cell lung cancer through inhibition of miR-101-3p and
activation of Wnt/β-catenin signaling pathway. Oncotarget.
8:17785–17794. 2017.PubMed/NCBI
|
|
20
|
Jin J, Chu Z, Ma P, Meng Y and Yang Y:
Long non-coding RNA SPRY4-IT1 promotes proliferation and invasion
by acting as a ceRNA of miR-101-3p in colorectal cancer cells.
Tumour Biol. 39:10104283177162502017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li CY, Pang YY, Yang H, Li J, Lu HX, Wang
HL, Mo WJ, Huang LS, Feng ZB and Chen G: Identification of
miR-101-3p targets and functional features based on bioinformatics,
meta-analysis and experimental verification in hepatocellular
carcinoma. Am J Transl Res. 9:2088–2105. 2017.PubMed/NCBI
|
|
22
|
Jiang R, Zhang C, Liu G, Gu R and Wu H:
MicroRNA-101 inhibits proliferation, migration and invasion in
osteosarcoma cells by targeting ROCK1. Am J Cancer Res. 7:88–97.
2017.PubMed/NCBI
|
|
23
|
Chang Z, Huo L, Li K, Wu Y and Hu Z:
Blocked autophagy by miR-101 enhances osteosarcoma cell
chemosensitivity in vitro. ScientificWorldJournal. 2014:7947562014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang K, Zhang Y, Ren K, Zhao G, Yan K and
Ma B: MicroRNA-101 inhibits the metastasis of osteosarcoma cells by
downregulation of EZH2 expression. Oncol Rep. 32:2143–2149. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin S, Shao NN, Fan L, Ma XC, Pu FF and
Shao ZW: Effect of microRNA-101 on proliferation and apoptosis of
human osteosarcoma cells by targeting mTOR. J Huazhong Univ Sci
Technolog Med Sci. 34:889–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar
|
|
28
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang Y and Fullwood MJ: Roles, Functions,
and Mechanisms of Long Non-coding RNAs in Cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mouraviev V, Lee B, Patel V, Albala D,
Johansen TE, Partin A, Ross A and Perera RJ: Clinical prospects of
long noncoding RNAs as novel biomarkers and therapeutic targets in
prostate cancer. Prostate Cancer Prostatic Dis. 19:14–20. 2016.
View Article : Google Scholar
|
|
31
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Villalonga P, Fernández de Mattos S and
Ridley AJ: RhoE inhibits 4E-BP1 phosphorylation and eIF4E function
impairing cap-dependent translation. J Biol Chem. 284:35287–35296.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Whatcott CJ, Ng S, Barrett MT, Hostetter
G, Von Hoff DD and Han H: Inhibition of ROCK1 kinase modulates both
tumor cells and stromal fibroblasts in pancreatic cancer. PLoS One.
12:e01838712017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maskey N, Li D, Xu H, Song H, Wu C, Hua K,
Song J and Fang L: MicroRNA-340 inhibits invasion and metastasis by
downregulating ROCK1 in breast cancer cells. Oncol Lett.
14:2261–2267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Wang Y, Liang T, Wang Y, Huang Y and Li Y:
Long non-coding RNA AK093407 promotes proliferation and inhibits
apoptosis of human osteosarcoma cells via STAT3 activation. Am J
Cancer Res. 7:892–902. 2017.PubMed/NCBI
|
|
37
|
Jiang Z, Jiang C and Fang J: Up-regulated
lnc-SNHG1 contributes to osteosarcoma progression through
sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin
pathway. Biochem Biophys Res Commun. 495:238–245. 2018. View Article : Google Scholar
|
|
38
|
Wang J, Cao L, Wu J and Wang Q: Long
non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326
and promotes tumorigenesis in osteosarcoma. Int J Oncol. 52:77–88.
2018.
|
|
39
|
Li HJ, Li X, Pang H, Pan JJ, Xie XJ and
Chen W: Long non-coding RNA UCA1 promotes glutamine metabolism by
targeting miR-16 in human bladder cancer. Jpn J Clin Oncol.
45:1055–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|