Introduction
Bone homeostasis is dependent on the balance between
bone formation and bone resorption, which are mediated by
osteoblasts and osteoclasts (1).
In patients with osteoporosis, principally postmenopausal women,
the rate of osteoclastogenesis surpasses that of osteogenesis,
resulting in decreased bone density and strength (2-4).
Osteoporosis is associated with an increased incidence of bone
fractures, which are particularly dangerous in the elderly, and
with a reduced quality of life (5,6).
The prevalence of osteoporosis is increasing worldwide (7). In the United States, ~10 million
individuals aged >50 years suffer from osteoporosis and 34
million suffer from osteopenia. In China, the prevalence of
osteoporosis was 14.94% prior to 2008 and 27.96% between 2012 and
2015 (8).
Osteoclasts differentiate from the mononuclear
phagocyte system and are involved in bone re sorption. Inflammation
leads to overactivation of osteoclasts, and thus suppression of
inflammation inhibits osteoclastogenesis (3). Osteoclastogenesis has been reported
to involve several signaling pathways (6,9,10).
Receptor activator for nuclear factor-κB ligand (RANKL) is vital
for the formation and function of osteoclasts (11,12), and binding of RANKL to its
receptor RANK results in activation of the nuclear factor (NF)-κB,
mitogen-activated protein kinase (MAPK) and protein kinase B (AKT)
signaling pathways. The activation of these pathways induces the
expression of several osteoclast-specific genes, including
cathepsin K, calcitonin receptor (CTR), matrix metalloproteinase 9
(MMP9), TNF receptor-associated factor 6 (TRAF6), and
tartrate-resistant acid phosphatase (TRAP) (13-15).
Magnolol is the active component of the Chinese
medicine Magnolia officinalis and is used clinically for its
anti-inflammatory and antioxidant effects (16-19). Magnolol reportedly inhibits
RANKL-induced osteoclastic differentiation of RAW264.7 macrophages
(20). However, further research
is required to assess the effect of magnolol on osteoporosis and
the underlying mechanism(s). Therefore, the present study aimed to
investigate the effect of magnolol on osteoclastogenesis in
vivo and in vitro, as well as to examine the underlying
mechanism at the molecular level.
Materials and methods
Reagents and cells
Magnolol was provided by Shanghai Nodel Biotech Co.,
Ltd. (Shanghai, China) and reconstituted in phosphate-buffered
saline (PBS), serving as the vehicle. RAW264.7 cells were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). Fetal bovine serum (FBS), penicillin, streptomycin and
antibodies were purchased from BioTNT (Shanghai, China). The
primary antibodies (all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) used included anti-cathepsin K (1:500; cat. no. sc-6506),
anti-CTR (1:200; cat. no. sc-8858), anti-MMP9 (1:400; cat. no.
sc-21733), anti-TRAF6 (1:250; cat. no. sc-8409), anti-TRAP (1:350;
cat. no. sc-100314), anti-p65 (1:350; cat. no. sc-398442),
anti-phosphorylated (p)-p65 (1:500; cat. no. sc-136548), anti-p50
(1:250; cat. no. sc-166580), anti-p-p50 (1:400; cat. no.
sc-271908), anti-inhibitor of κB (IκB; 1:350; cat. no. sc-74451),
anti-p-IκB (1:500; cat. no. sc-8404), anti-extracellular
signal-regulated kinase (ERK; 1:200; cat. no. sc-135900),
anti-p-ERK (1:400; cat. no. sc-81492), anti-c-Jun N-terminal kinase
(JNK; 1:500; cat. no. sc-7345), anti-p-JNK (1:400; cat. no.
sc-135642), anti-p38 (1:300; cat. no. sc-136210), anti-p-p38
(1:300; cat. no. sc-7973) and anti-β actin (1:1,000; cat. no.
sc-8432). Horseradish peroxidase-conjugated anti-mouse (cat. no.
ZB2301) and anti-rabbit (cat. no. ZB2305) antibodies (1:5,000;
OriGene Technologies, Inc., Beijing, China) were also used.
Dulbecco’s modified Eagle’s medium was purchased from Corning Inc.
(Corning, NY, USA).
Animals and experimental design
Experiments involving mice were approved by the
Ethics Committee on Animal Experiments of the Central Hospital of
Zibo (Shandong University, Zibo, China). A total of 36 C57BL/6
female mice (8-week-old; weight, 20-25 g) were obtained from
Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The
mice were housed under standard laboratory conditions (20-25°C;
humidity, 40-70%; 16/8-h light/dark cycle), and provided with food
and water ad libitum. The mice were randomly divided into
the sham-operated, saline-treated ovariectomy (OVX), and
magnolol-treated OVX groups (n=6 per group). OVX was performed
using a standard protocol (21)
Sham operation was performed following the same procedure as OVX
without the resection of ovaries. At 24 h postoperatively, the OVX
mice were treated with an intraperitoneal injection of 10 ml/kg
magnolol or saline daily for 6 weeks. The mice were then euthanized
by cervical dislocation, and femur and blood samples were
obtained.
Bone histomorphometric analysis
Femur samples were decalcified for 2 weeks in an
aqueous solution of 10% tetrasodium-EDTA at 4°C. Next, bone
sections (4 µm) were stained with hematoxylin and eosin (H&E)
at room temperature for 5-10 min, followed by TRAP staining (cat.
no. BB-4421-1; BestBio, Shanghai, China) to identify osteoclasts
according to the manufacturer’s protocol. Bone trabecula was
observed in H&E-stained sections, and the osteoclasts were
observed in TRAP-stained sections. Histomorphometric measurements
were then performed under a microscope, and the trabecular bone
area was calculated using Image-Pro Plus 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA).
Microcomputed tomography analysis
Bone samples were analyzed by microcomputed
tomography (Skyscan; Bruker Corporation, Billerica, MA, USA) using
the following parameters: 80 kV, 124 µA and 8-µm
resolution. The built-in software was used to analyze various
indices, including bone mineral density (BMD), bone surface
area/bone volume (BS/BV), BS/total volume (BS/TV), BV/TV and
trabecular number (Tb.N).
Serum biochemistry
Following anesthesia, blood samples were collected
from the fundus venous plexus. Subsequent to centrifugation (1,811
× g; 15 min; 4°C), the serum levels of C-terminal telopeptide of
type 1 collagen (CTX-1; cat. no. MBS726456; MyBiosource, Inc., San
Diego, USA), interleukin (IL)-6 (cat. no. MEC1008; Anogen-Yes
Biotech Laboratories, Ltd., Mississauga, Canada), tumor necrosis
factor α (TNF-α; cat. no. MEC1003; Anogen-Yes Biotech Laboratories,
Ltd.) and TRAP 5b (TRAcp5B; cat. no. MBS776993; MyBiosource, Inc.)
were determined using enzyme-linked immunosorbent assay (ELISA)
kits according to the manufacturer’s protocol.
In vitro osteoclastogenesis assay
Bone marrow monocytes (BMMCs) were isolated from the
bone marrow of healthy C57BL/6 mice by flushing femurs and tibias
with α-MEM and culturing in α-MEM with 10% FBS, 100 U/ml
penicillin, 100 µg/ml streptomycin and macrophage
colony-stimulating factor (M-CSF; 30 ng/ml) overnight. Non-adherent
cells were collected and further cultured in the presence of M-CSF
(30 ng/ml) for 3 days. Floating cells were discarded and adherent
cells were used as bone marrow-derived macrophages. BMMCs and
RAW264.7 cells were cultured in Dulbecco’s modified Eagle’s medium
supplemented with glucose, 10% heat-inactivated FBS, penicillin
(100 U/ml) and streptomycin (100 mg/ml) at 37°C in an atmosphere
containing 5% CO2. The culture medium was changed once
per day during the first 3 days. After removing nonadherent cells,
colonies were cultured for 14 days, then passaged and subcultured.
BMMCs treated with 0, 5, 10, 20, 40, 80 or 160 µg/ml
magnolol were subjected to an MTT assay.
Based on the MTT assay results, third-generation
BMMCs (5×103/well) and RAW264.7 cells
(3×103/well) were cultivated in 96-well plates and
divided into a control group and four magnolol-treated (0, 5, 10
and 20 µg/ml) groups. To induce osteoclast formation, 20
ng/ml M-CSF and 50 ng/ml RANKL were applied. After 5 days, staining
for TRAP was performed using the TRAP Staining kit (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). TRAP-positive cells with three or
more nuclei were regarded as osteoclasts. In addition, in order to
visualize F-actin rings by fluorescent microscopy, cells treated
with M-CSF/RANKL were cultured for 48 h with 4% paraformaldehyde in
PBS, permeabilized with 0.1% Triton X-100 and incubated with
rhodamine-conjugated phalloidin (cat. no. ab235138; Abcam,
Cambridge, MA, USA) for 60 min at room temperature. Fluorescein
isothiocyanate-conjugated F-actin antibodies (1:600; cat. no.
ab205; Abcam) were used to detect F-actin. Assays were performed in
at least triplicate, and mean values were calculated.
Bone-pit formation assay
RAW264.7 cells were divided into five groups,
including the untreated control and four groups treated with
magnolol of various concentration. Next, cells
(3×103/well) were seeded in 48-well plates containing a
collagen gel matrix, and then treated with 20 ng/ml M-CSF and 50
ng/ml RANKL for 6 days. The resulting mature osteoclasts were
digested with collagenase and seeded onto a biosynthetic bone
surface (Osteo Assay Surface 24-well Multiple-Well Plates; Corning,
Inc., Corning, NY, USA) and incubated for 7 days at room
temperature. The medium was changed on day 3, and the plates were
washed with PBS and air-dried for 3 h on day 7. The bone resorption
area was assessed by light microscopy (BX53; Olympus Corporation,
Tokyo, Japan) and Image-Pro Plus software.
Western blotting
In order to assess the effects of magnolol on the
NF-κB and MAPK signaling pathways, RAW264.7 cells
(3×103/well) were seeded into 6-well plates and treated
with RANKL without or with magnolol (0 or 20 µg/ml).
Following incubation for 0, 15, 30, 45 or 60 min, the
phosphorylation levels of p38, p50, p65, IκB, ERK and JNK were
evaluated by western blotting. In addition, in order to assess the
protein expression levels of osteoclastogenesis markers, RAW264.7
cells (3×103/well) were cultured for 7 days in the
presence of RANKL without or with 0, 5, 10, or 20 µg/ml
magnolol. Subsequently, the expression levels of cathepsin K, CTR,
MMP9, TRAF6, and TRAP were assessed by western blotting using
antibodies obtained from Abcam (Cambridge, MA, USA).
The total protein was extracted from the cells using
M-PER mammalian protein extraction reagent (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Equal amounts of protein (10
µg/lane), estimated by a bicinchoninic acid protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.) were loaded onto 11%
SDS-PAGE gels and transferred onto nitrocellulose membranes.
Blocking was performed with blocking solution (cat. no. P0023B;
Beyotime Institute of Biotechnology, Haimen, China) at room
temperature for 1 h. Membranes were then incubated with the primary
(4°C; overnight) and secondary antibodies (room temperature; 1 h)
detailed above (p38, p50, p65, IκB, ERK, JNK, cathepsin K, CTR,
MMP9, TRAF6 and TRAP). Bands were detected by chemiluminescence
(cat. no. E003-1007; Sea Biotech, Shanghai, China) and imaged with
X-ray films. β-actin was used as an endogenous reference for
normalization. ImageJ software (v1.46; National Institutes of
Health, Bethesda, MD, USA) was used to analyze densitometry.
Immunofluorescence staining
The effect of magnolol on the nuclear translocation
of p65 was evaluated by immunofluorescence staining. Briefly, BMMCs
treated with RANKL in the absence or presence of 20 µg/ml
magnolol were fixed with 4% paraformaldehyde for 10 min and
permeabilized with 0.1% Triton X-100 in PBS for 5 min. The cells
were washed with 0.1% BSA-PBS and incubated with an anti-p65
monoclonal antibody overnight at 4°C, followed by a biotinylated
goat anti-mouse IgG and fluorescein-conjugated streptavidin (Vector
Laboratories, Burlingame, CA, USA) for 1 h at room temperature and
Hoechst (Vector Laboratories) was used for counterstaining for 2
days. The localization of p65 was visual-ized by immunofluorescence
analysis (×400 magnification) and the percentage of nuclei
fluorescence intensity of p65 in 3 random fields were measured in
each group with ImageJ software.
Statistical analysis
Data are presented as the mean ± standard deviation.
Student’s t-test was used for comparisons of two groups, while
one-way analysis of variance was performed to compare three or more
groups. A value of P<0.05 was considered to denote a difference
that was statistically significant.
Results
Magnolol prevents OVX-induced bone
loss
According to H&E staining, magnolol prevented
the loss of trabecular bone in OVX mice (Fig. 1A). This was further confirmed by
microcomputed tomography, and the three-dimensional structure of
the bone is shown in Fig. 1B.
When comparing Sham and OVX groups, it was observed that the BMD
(P<0.001), BS/BV (P<0.05), BS/TV (P<0.001), BV/TV
(P<0.001) and Tb.N (P<0.001) values were decreased in OVX
mice. When comparing OVX and OVX+Magnolol groups, the BMD
(P<0.05), BS/BV (P<0.01), BS/TV (P<0.001), BV/TV
(P<0.01) and Tb.N (P<0.001) values were increased in
OVX+Magnolol mice, indicating that magnolol treatment reversed
OVX-induced bone loss (Fig.
1C).
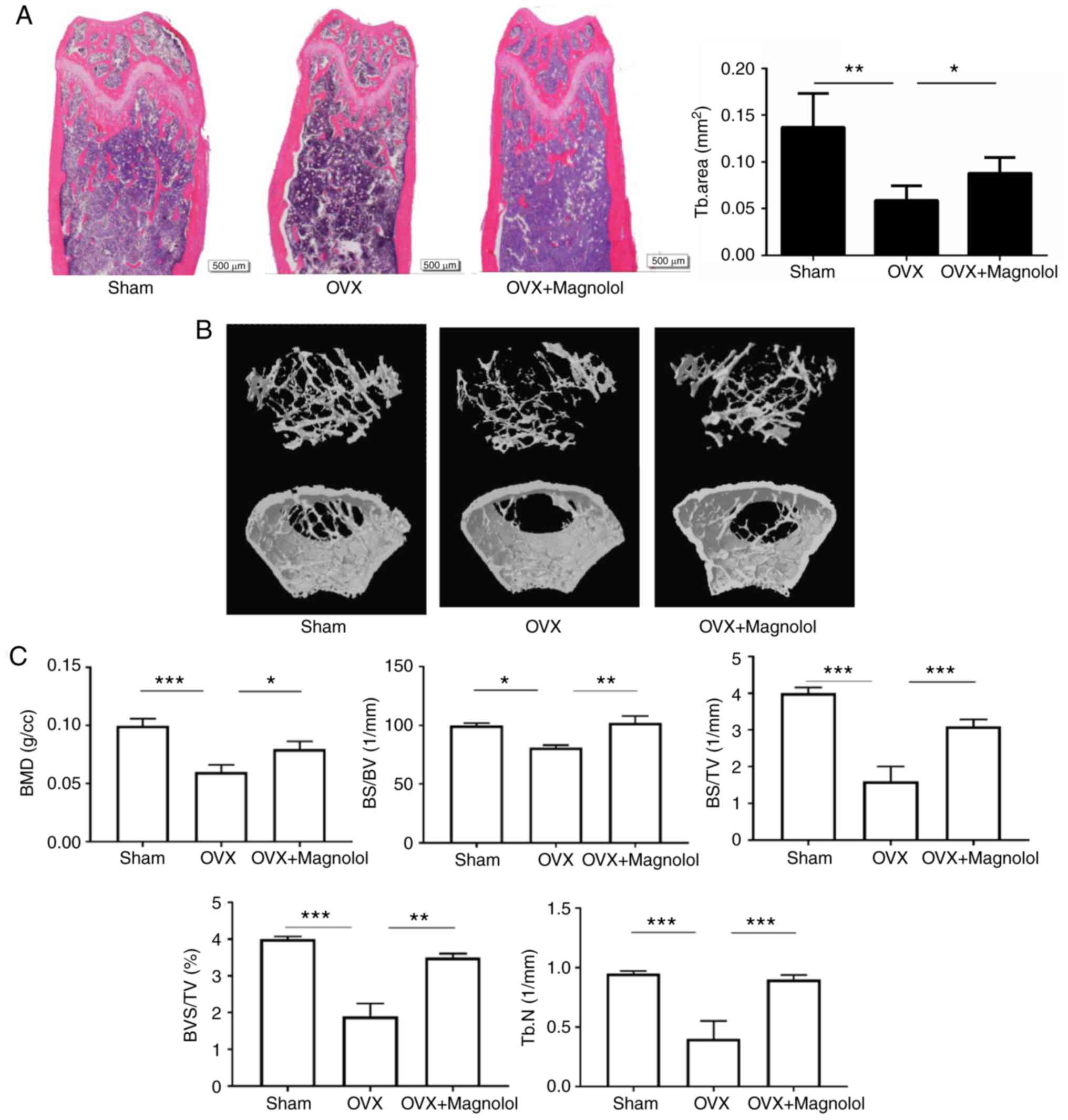 | Figure 1Magnolol prevents OVX-induced bone
loss. (A) Representative femurs and trabecular areas stained with
hematoxylin and eosin at 6 weeks postoperatively (magnification,
×40; bar=500 µm; n=6). (B) Representative three-dimensional
structures of femurs examined by microcomputed tomography. (C) BMD,
BS/BV, BS/TV, BV/TV and Tb.N values. *P<0.05,
**P<0.01 and ***P<0.001. OVX,
ovariectomy; BMD, bone mineral density; BS, bone surface area; BV,
bone volume; TV, total volume; Tb.N, trabecular number. |
Magnolol inhibits osteoclastogenesis in
vivo
To determine whether magnolol suppressed OVX-induced
bone loss by inhibiting the formation of osteoclasts, TRAP staining
was performed, and the serum levels of markers of
osteoclastogenesis and inflammation were assessed. It was observed
that there were more TRAP-positive cells in OVX mice when compared
with the sham group. However, magnolol reduced the number
TRAP-positive cells in OVX+Magnolol mice compared with the OVX
group (Fig. 2A). As determined by
ELISA, the serum CTX-1 (P<0.001), IL-6 (P<0.001), TNF-α
(P<0.01) and TRAcp5B (P<0.001) levels were significantly
increased in OVX mice compared with those in the sham group.
However, magnolol treatment markedly decreased the serum levels of
CTX-1 (P<0.001), IL-6 (P<0.001), TNF-α (P<0.01) and
TRAcp5B (P<0.001) in the OVX+Magnolol group compared with the
OVX group (Fig. 2B), suggesting
inhibition of osteoclastogenesis and inflammation.
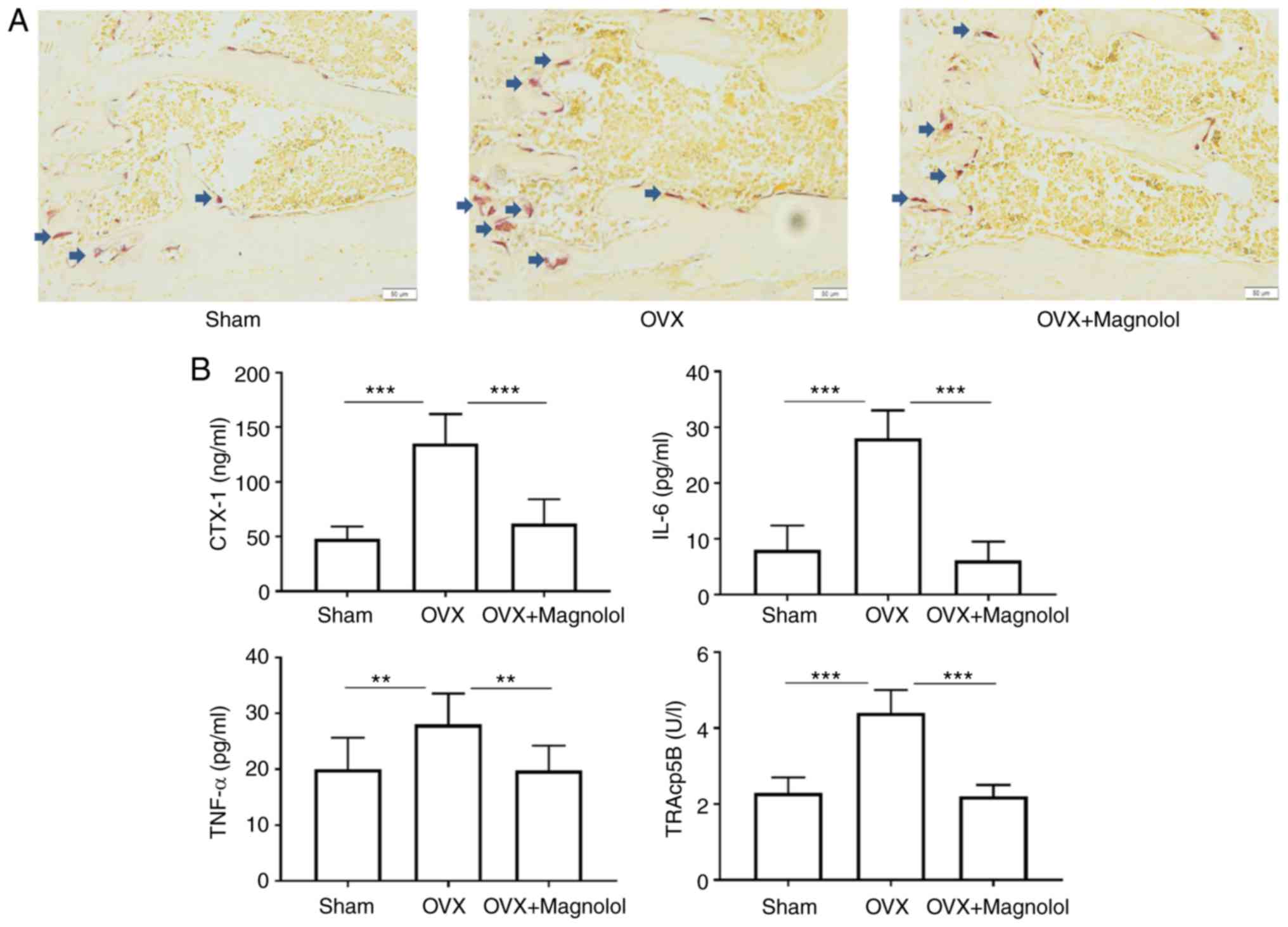 | Figure 2Magnolol inhibits osteoclastogenesis
in vivo. (A) Representative TRAP-stained femur tissues from
the sham, OVX model and magnolol-treated OVX groups (magnification,
×40; bar=50 µm; n=6). Arrows indicate the TRAP+ cells. (B)
Serum CTX-1, IL-6, TNF-α and TRAcp5B levels, detected by
enzyme-linked immunosorbent assay. **P<0.01 and
***P<0.001. OVX, ovariectomy; TRAP,
tartrate-resistant acid phosphatase; CTX-1, C-terminal telopeptide
of type 1 collagen; IL-6, interleukin-6; TNF-α, tumor necrosis
factor α; TRAcp5B, tartrate-resistant acid phosphatase 5b. |
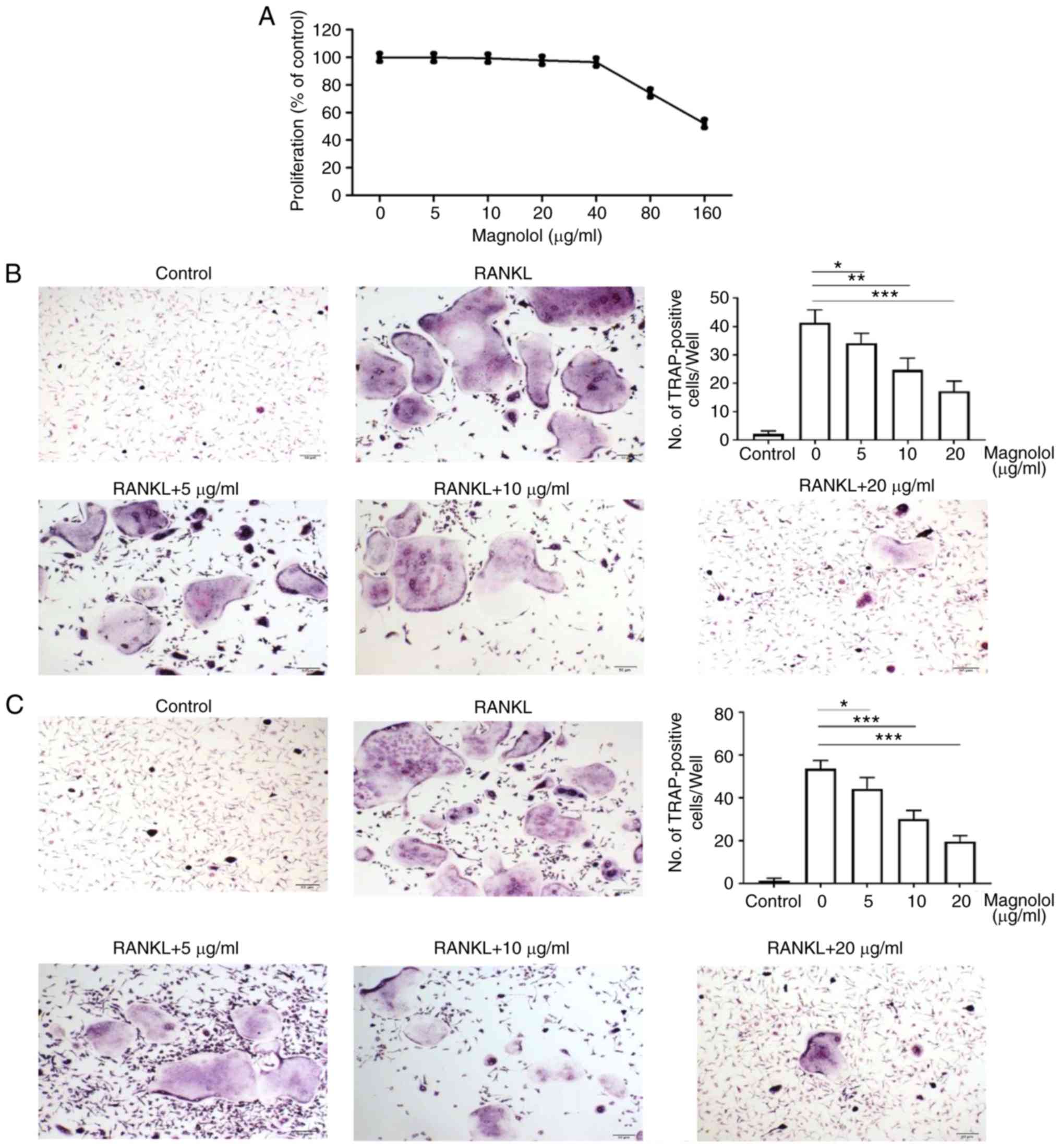
Magnolol inhibits osteoclastogenesis in
vitro
Based on the results of the MTT assays, magnolol
concentrations of 0, 5, 10 and 20 µg/ml were selected for
use in subsequent experiments, since the cell proliferation was not
markedly reduced following treatment at these doses (Fig. 3A). Compared with the positive
control (RANKL alone), magnolol decreased the number of
TRAP-positive BMMCs (Fig. 3B) and
RAW264.7 cells (Fig. 3C) in a
concentration-dependent manner. Therefore, magnolol appeared to
suppress RANKL-induced osteoclastogenesis in BMMCs and RAW264.7
cells.
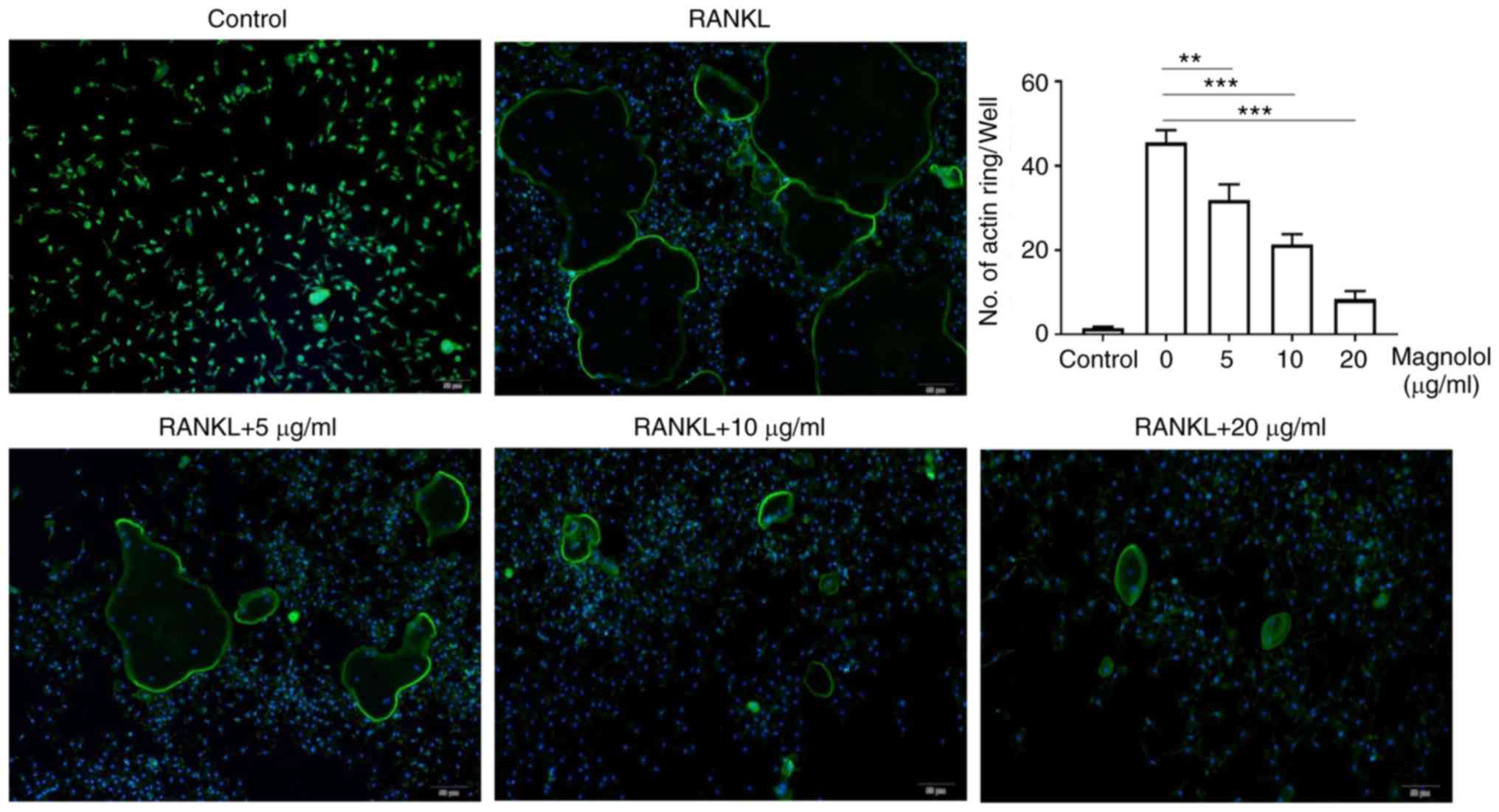
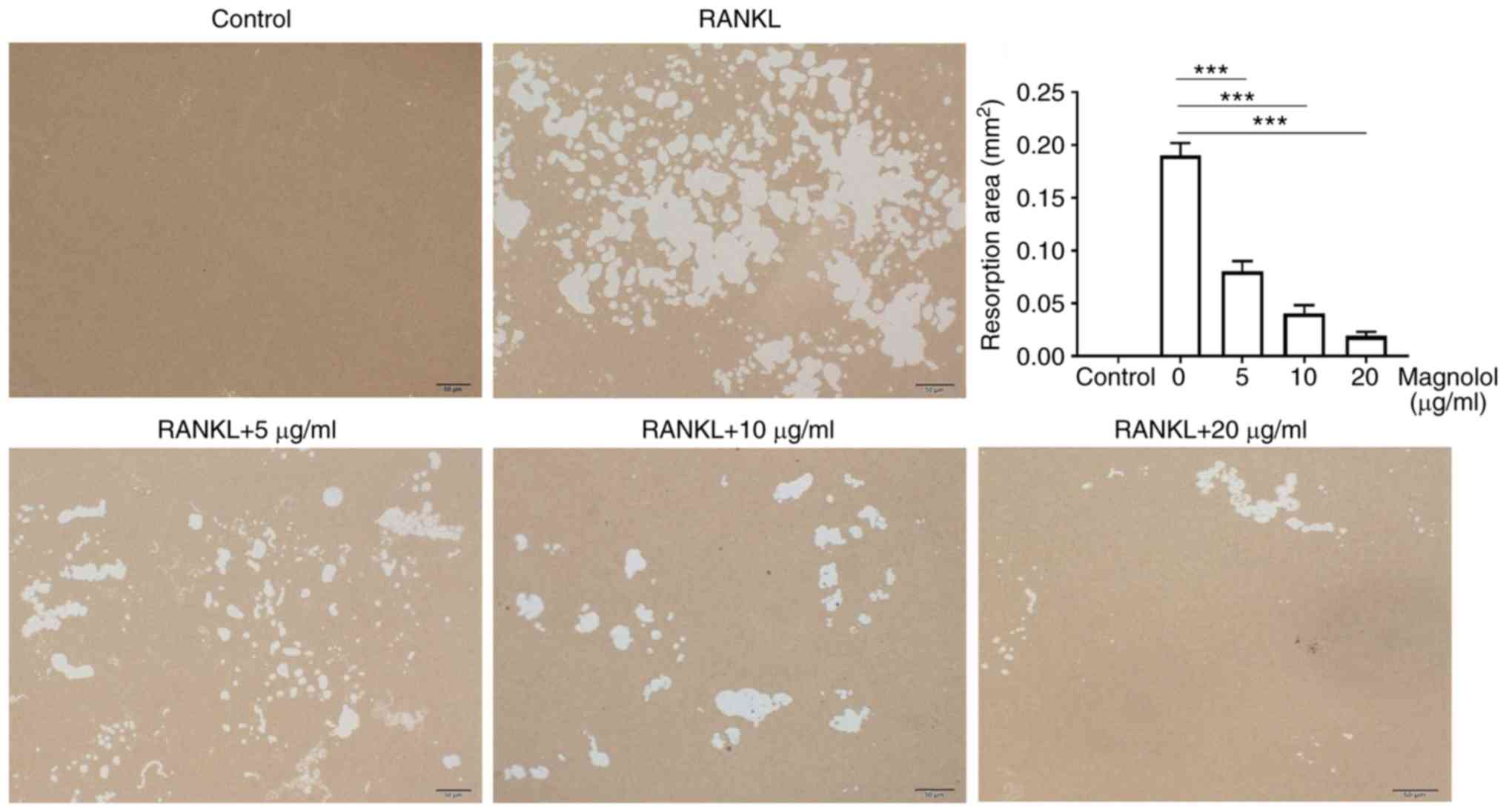
Magnolol inhibits osteoclast function in
vitro
To evaluate the effect of magnolol on osteoclast
function in vitro, F-actin ring formation and bone-pit
formation were assayed. Magnolol inhibited F-actin ring formation
in M-CSF-stimulated and RANKL-stimulated RAW264.7 cells in a
concentration-dependent manner (Fig.
4). In addition, magnolol significantly decreased the area of
bone resorption pits (Fig. 5).
Taken together, these results suggested that magnolol inhibited
RANKL-induced osteoclast activity.
Magnolol suppresses the expression of
osteoclastogenesis markers
To evaluate the mechanism underlying the effect of
magnolol, the protein expression levels of cathepsin K, CTR, MMP9,
TRAF6 and TRAP were assessed. It was observed that magnolol
significantly suppressed the RANKL-induced expression of cathepsin
K, CTR, MMP9, TRAF6 and TRAP (Fig.
6). Therefore, magnolol suppressed the expression levels of
osteoclastogenesis markers.
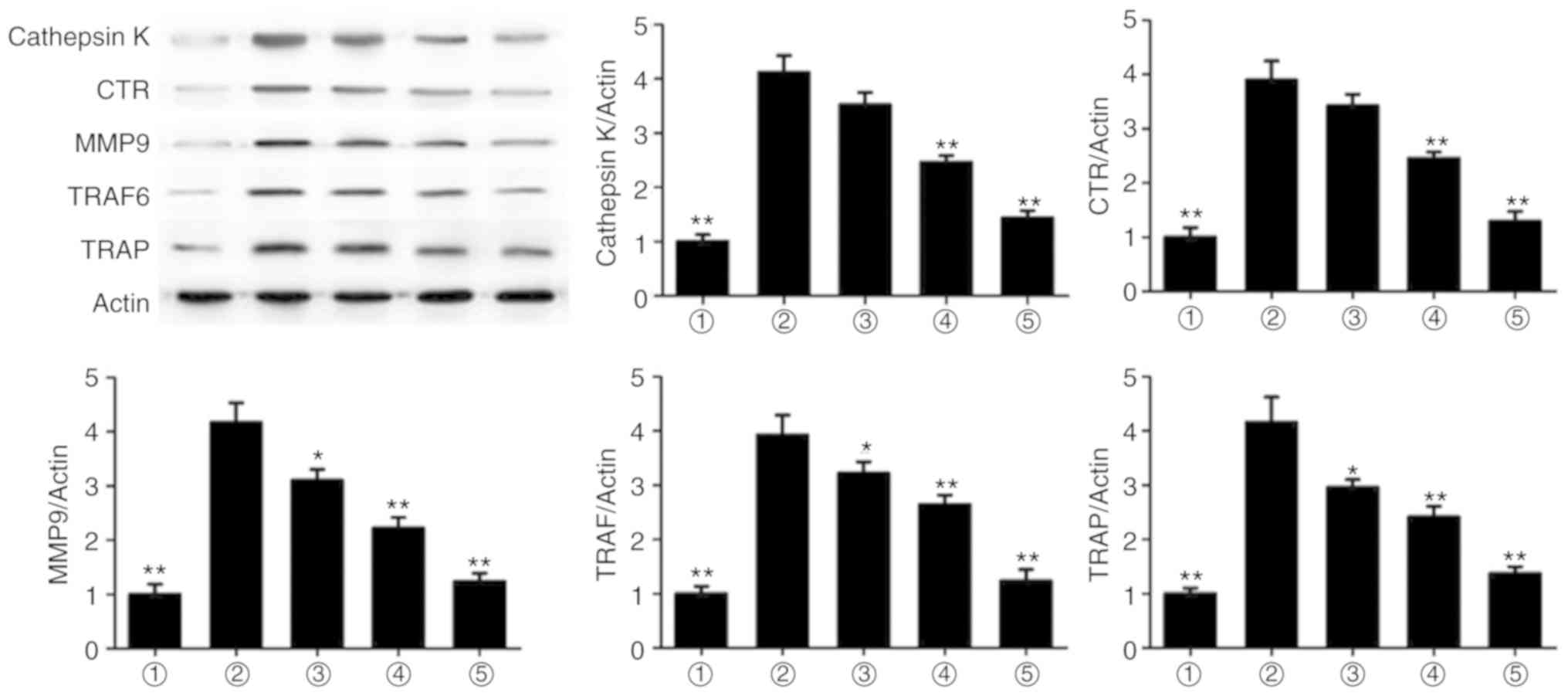 | Figure 6Magnolol suppresses the expression
levels of the osteoclastogenesis markers cathepsin K, CTR, MMP9,
TRAF6 and TRAP in RAW264.7 cells. *P<0.05 and
**P<0.01, vs. RANKL-only group. 1, Control; 2, RANKL;
3, RANKL+5 µg/ml; 4, RANKL+10 µg/ml; 5, RANKL+20
µg/ml. RANKL, receptor activator for nuclear factor-κB
ligand; CTR, calcitonin receptor; MMP9, matrix metalloproteinase 9;
TRAF6, TNF receptor-associated factor 6; TRAP, tartrate-resistant
acid phosphatase. |
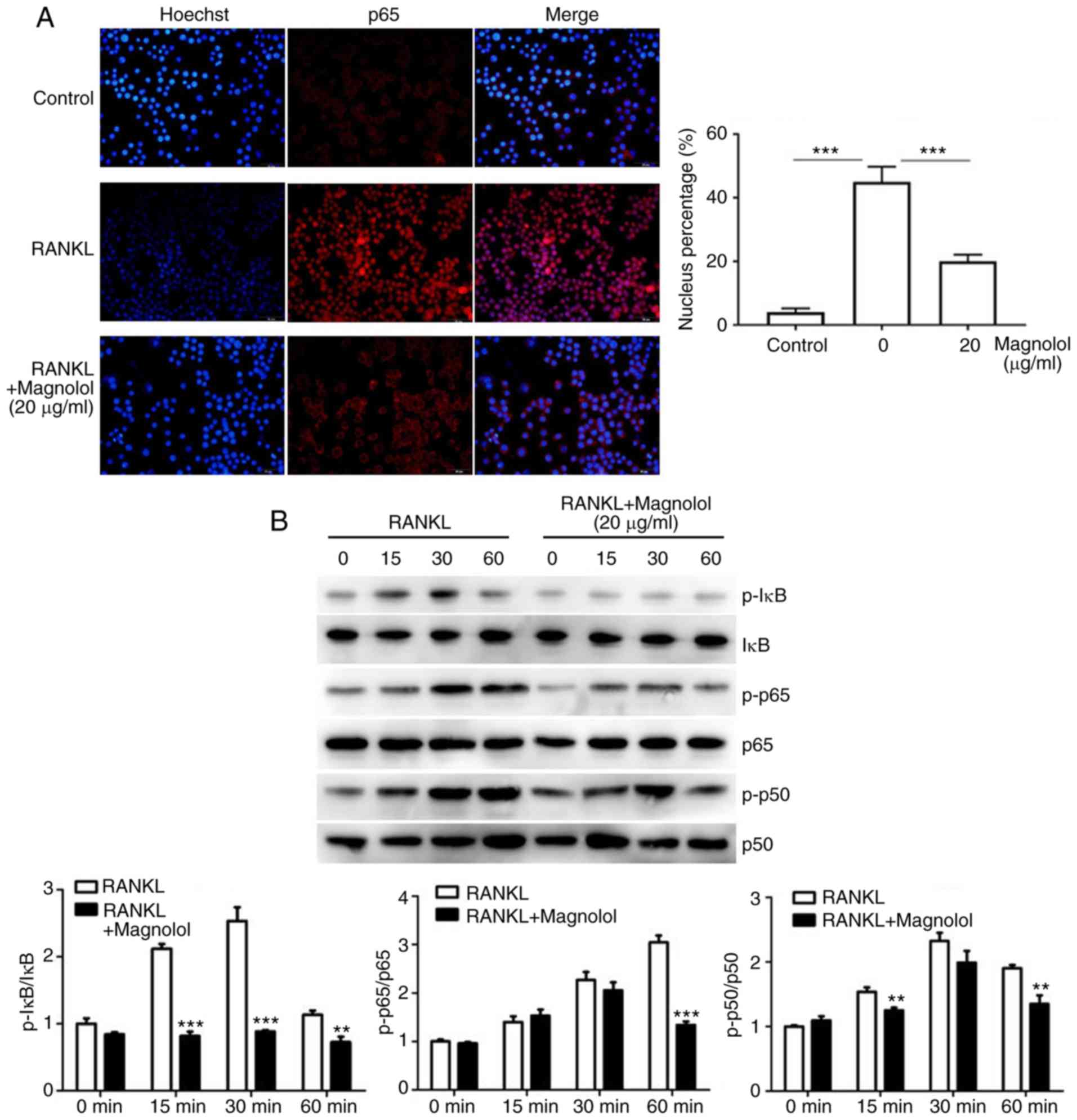
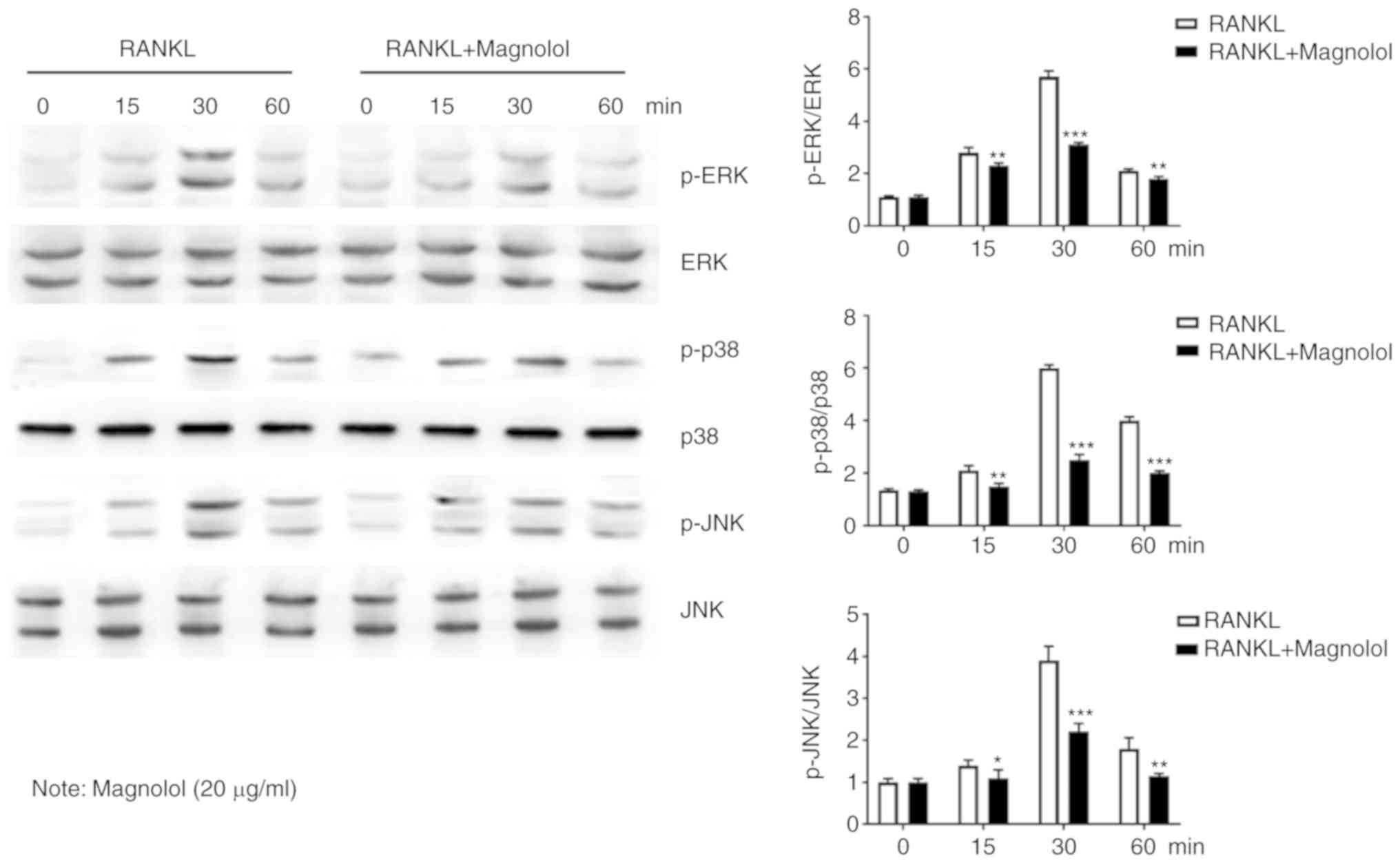
Magnolol suppresses the NF-κB and MAPK
pathways
To assess the effect of magnolol on the NF-κB
pathway, immunofluorescence staining of p65 was performed. The
results revealed that the RANKL-induced translocation of p65 to the
nucleus was inhibited by magnolol (Fig. 7A). Furthermore, the
phosphorylation of the key proteins of the NF-κB pathway, namely
IκB, p65 and p50, were assessed by western blotting. Magnolol
inhibited the phosphorylation of IκB, p65 and p50 (Fig. 7B) at different time points. These
results indicate that magnolol suppressed the NF-κB signaling
pathway.
The MAPK pathway is also important in
osteoclastogenesis (13,15); thus, the phosphorylation of the
key proteins, namely ERK, p38 and JNK were further examined by
western blotting. It was observed that magnolol suppressed the
RANKL-induced phosphorylation of ERK, p38 and JNK at different time
points (Fig. 8). Therefore,
magnolol may also suppress the activation of the MAPK signaling
pathway.
Discussion
A large number of middle-aged and elderly
individuals worldwide suffer from osteoporosis (2,22).
The pathogenesis of osteoporosis involves an imbalance between bone
formation and resorption (23),
which reflects an imbalance between osteoblasts and osteoclasts
(24). Patients with osteoporosis
are more vulnerable to fractures, which may lead to hypostatic
pneumonia, bedsores, deep-vein thrombosis, pulmonary embolism, and
urinary tract infection due to long-term immobilization (2,6,23).
Calcium supplements and vitamin D are used to reverse bone loss
(25); however, the clinical
outcomes are unsatisfactory (6,23,25). For this reason, effort has been
focused on developing drugs that are effective against osteoporosis
(13,15,26).
Estrogen suppresses inflammation and inhibits
osteoclast formation and function in bone tissue; therefore,
postmenopausal women are more likely to develop osteoporosis
(27,28). In the present study, the femurs of
OVX mice were subjected to H&E staining and microcomputed
tomography, as bone loss typically begins in regions with abundant
cancellous bone (24). The data
indicated that OVX-induced bone loss was significantly attenuated
by magnolol treatment. To identify the underlying mechanism,
TRAP-positive cells were counted and the concentration of TRAcp5B
was determined, which reflects the degree of osteoclastogenesis
(15); the results suggested that
magnolol inhibits osteoclastogenesis. Therefore, magnolol assists
the restoration of the osteoclast-osteoblast balance by suppressing
the formation of osteoclasts. Magnolol alleviated OVX-induced
osteoporosis by restraining osteoclastogenesis in a
concentration-dependent manner. However, magnolol at greater than
threshold concentrations suppressed cell proliferation.
Furthermore, inflammation is an important factor in the
pathogenesis of osteoporosis (3).
In the current study, the elevated serum IL-6 and TNF-α levels in
OVX mice were reversed by magnolol treatment. Thus, inflammation
serves an important role in osteoclastogenesis and bone resorption,
and consequently anti-inflammatory agents may be useful against
osteoporosis.
Traditional Chinese medicine provides an abundant
source for drug development. A number of active components of
Chinese herbs, such as matrine and epoxyeicosatrienoic acids, can
prevent bone loss (26,29,30). Magnolol is extracted from
Magnolia officinalis, and has been reported to exhibit
antioxidant, anti-inflammatory and anticancer effects (16-21). In addition, magnolol promotes the
growth of osteoblasts and inhibits the differentiation of
osteoclasts in vitro (27). Previous research has demonstrated
that magnolol was able to ameliorate ligature-induced periodontitis
in rats, which is an osteoclast-associated disease (31).
In the present study, magnolol suppressed
osteoclastogenesis in vivo and in vitro by blocking
the NF-κB and MAPK signaling pathways. In vitro, TRAP
staining revealed that magnolol inhibited RANKL-induced
osteoclastogenesis by BMMCs and RAW264.7 cells. Osteoclast
function, in terms of F-actin ring formation and bone-pit
formation, was also inhibited by magnolol. Furthermore, the
expression levels of the osteoclastogenesis markers cathepsin K,
CTR, MMP9, TRAF6 and TRAP were suppressed by magnolol.
RANKL serves a vital role in osteoclast maturation
(32). RANKL binds to RANK on
immature osteoclasts, resulting in the activation of the NF-κB and
MAPK pathways (11,12,20). The NF-κB pathway is closely
associated with osteoclastogen-esis and inflammation, and agents
targeting this pathway are used to treat osteoporosis and
inflammatory conditions (13,28). Following the induction of RANKL,
p65 translocates to the nucleus to activate gene transcription, and
this translocation was suppressed by magnolol in the present study.
Furthermore, magnolol decreased the phosphorylation of IκB, p65 and
p50 in a time-dependent manner, which subsequently suppressed gene
transcription. It was also observed that magnolol suppressed the
MAPK pathway, as indicated by inhibition of the phosphorylation of
ERK, p38 and JNK.
As mentioned earlier, magnolol was demonstrated to
inhibit osteoclastogenesis in vivo and in vitro;
however, it remains unclear whether magnolol affects osteoblast
formation. A previous study reported that magnolol promoted the
function of MC3T3-E1 osteoblasts (27), but further research is required on
this subject. Furthermore, the present study demonstrated that
magnolol inhibited osteoclast maturation by suppressing the NF-κB
and MAPK pathways. However, whether magnolol affects the AKT
pathway or other signaling transduction pathways remains unclear.
Identification of the genes targeted by magnolol that mediate its
inhibition of osteoclast formation would provide further insight on
the pathways involved. Finally, in the current study, magnolol
inhibited bone loss in a model involving estrogen; however, the
effects of magnolol on other osteoclastogenesis-associated
diseases, including arthritis and bone tumors, warrant further
investigation.
In conclusion, the current data indicated that
magnolol suppressed osteoclastogenesis in vivo and in
vitro by blocking the NF-κB and MAPK pathways, suggesting its
potential use in the treatment of osteoporosis and associated
disorders.
Funding
The present study was supported by funds from the
Young Medical Key Talent Project of Jiangsu province (grant no.
QNRC2016458), Jiangsu Provincial Medical Innovation Team (grant no.
CXTDB2017004) and Clinical Study of Jiangsu University (grant no.
JLY20160005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
The study was conceived and designed by WYF and TL.
WYF and LJQ conducted most of the experiments with assistance from
QH and PQZ. The paper was written by WYF, with contributions from
TL. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments on mice were approved by the Ethics
Committee on Animal Experiments of the Central Hospital of Zibo
(Zibo, China).
Patient consent for publication
No patients were involved in this study.
Competing interests
The authors declare that they have no competing
interests and no financial associations to disclose.
Acknowledgments
The authors would like to thank the Orthopedic Basic
and Translational Research Center in Wuxi, China, and Shanghai
Geekbiotech Company for technical support.
References
|
1
|
Bateman ME, Strong AL, McLachlan JA, Burow
ME and Bunnell BA: The effects of endocrine disruptors on
adipogenesis and osteogenesis in mesenchymal stem cells: A review.
Front Endocrinol (Lausanne). 7:1712016.
|
|
2
|
Noel SE, Mangano KM, Griffith JL, Wright
NC, Dawson-Hughes B and Tucker KL: Prevalence of osteoporosis and
low bone mass among Puerto Rican older adults. J Bone Miner Res.
33:396–403. 2018. View Article : Google Scholar :
|
|
3
|
Aldaghri NM, Aziz I, Yakout S, Aljohani
NJ, Al-Saleh Y, Amer OE, Sheshah E, Younis GZ and Al-Badr FB:
Inflammation as a contributing factor among postmenopausal Saudi
women with osteoporosis. Medicine (Baltimore). 96:e57802017.
View Article : Google Scholar
|
|
4
|
Paschalis EP, Gamsjaeger S, Hassler N,
Fahrleitner-Pammer A, Dobnig H, Stepan JJ, Pavo I, Eriksen EF and
Klaushofer K: Vitamin D and calcium supplementation for three years
in postmenopausal osteoporosis significantly alters bone mineral
and organic matrix quality. Bone. 95:41–46. 2017. View Article : Google Scholar
|
|
5
|
Gielen E, Bergmann P, Bruyère O, Cavalier
E, Delanaye P, Goemaere S, Kaufman JM, Locquet M, Reginster JY,
Rozenberg S, et al: Osteoporosis in frail patients: A consensus
paper of the belgian bone club. Calcif Tissue Int. 101:111–131.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ensrud KE and Crandall CJ: Osteoporosis
Ann Intern Med. 167:ITC17–ITC32. 2017. View Article : Google Scholar
|
|
7
|
Office of the Surgeon General (US): Bone
Health and Osteoporosis: A Report of the Surgeon General. Office of
the Surgeon General; Rockville, MD: 2004
|
|
8
|
Peng C, Li Z and Hu Y: Prevalence of
osteoporosis in China: A meta-analysis and systematic review. Bmc
Public Health. 16:10392016. View Article : Google Scholar
|
|
9
|
Zhou Y, Guan X, Chen X, Yu M, Wang C, Chen
X, Shi J, Liu T and Wang H: Angiotensin II/Angiotensin II receptor
blockade affects osteoporosis via the AT1/AT2-mediated
cAMP-dependent PKA pathway. Cells Tissues Organs. 204:25–37. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu L, Zhang L, Zhang H, Yang Z, Qi L, Wang
Y and Ren S: The participation of fibroblast growth factor 23
(FGF23) in the progression of osteoporosis via JAK/STAT pathway. J
Cell Biochem. 119:3819–3828. 2018. View Article : Google Scholar
|
|
11
|
Wang Y, van Assen AHG, Reis CR,
Setroikromo R, van Merkerk R, Boersma YL, Cool RH and Quax WJ:
Novel RANKL DE-loop mutants antagonize RANK-mediated
osteoclastogenesis. FEBS J. 284:2501–2512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okabe I, Kikuchi T, Mogi M, Takeda H, Aino
M, Kamiya Y, Fujimura T, Goto H, Okada K, Hasegawa Y, et al: IL-15
and RANKL play a synergistically important role in
osteoclastogenesis. J Cell Biochem. 118:739–747. 2017. View Article : Google Scholar
|
|
13
|
Yang X, Gao W, Wang B, Wang X, Guo H, Xiao
Y, Kong L and Hao D: Picroside II Inhibits RANKL-mediated
Osteoclastogenesis by attenuating the NF-κB and MAPKs signaling
pathway in vitro and prevents bone loss in lipopolysaccharide
treatment mice. J Cell Biochem. 118:4479–4486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueno M, Cho K, Isaka S, Nishiguchi T,
Yamaguchi K, Kim D and Oda T: Inhibitory effect of sulphated
polysaccharide porphyran (isolated from Porphyra yezoensis) on
RANKL-induced differentiation of RAW264.7 cells into osteoclasts.
Phytother Res. 32:452–458. 2018. View
Article : Google Scholar
|
|
15
|
Chen X, Zhi X, Pan P, Cui J, Cao L, Weng
W, Zhou Q, Wang L, Zhai X, Zhao Q, et al: Matrine prevents bone
loss in ovariectomized mice by inhibiting RANKL-induced
osteoclastogenesis. FASEB J. 31:4855–4865. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong J, Ding H, Liu Y, Yang Q, Xu N, Yang
Y and Ai X: Magnolol protects channel catfish from Aeromonas
hydrophila infection via inhibiting the expression of aerolysin.
Vet Microbiol. 211:119–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Li M, Wang F, Wu X, Zhang X, Zhang
B, Wu N, Wang W, Weng Z, Liu HS, et al: Magnolol inhibits growth of
gallbladder cancer cells through the p53 pathway. Cancer Sci.
106:1341–1350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei W, Dejie L, Xiaojing S, Tiancheng W,
Yongguo C, Zhengtao Y and Naisheng Z: Magnolol inhibits the
inflammatory response in mouse mammary epithelial cells and a mouse
mastitis model. FEBS J. 38:16–26. 2015.
|
|
19
|
Yang B, Xu Y, Yu S, Huang Y, Lin L and
Liang X: Anti-angiogenic and anti-inflammatory effect of Magnolol
in the oxygen-induced retinopathy model. Inflamm Res. 65:81–93.
2016. View Article : Google Scholar
|
|
20
|
Park KR, Kim JY, Kim EC, Yun HM and Hong
JT: RANKL-induced osteoclastogenesis is suppressed by
4-O-methylhonokiol in bone marrow-derived macrophages. Arch Pharm
Res. 40:933–942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong JH, Jin ES, Kim JY, Lee B, Min J,
Jeon SR, Lee M and Choi KH: The effect of biocomposite screws on
bone regeneration in a rat osteoporosis model. World Neurosurg.
106:964–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen P, Li Z and Hu Y: Prevalence of
osteoporosis in China: A meta-analysis and systematic review. Bmc
Public Health. 16:10392016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McClung M, Baron R and Bouxsein M: An
update on osteoporosis pathogenesis, diagnosis, and treatment.
Bone. 98:372017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM
and Xie C: Osteoblast-osteoclast interactions. Connect Tissue Res.
59:99–107. 2018. View Article : Google Scholar
|
|
25
|
Stoecker WV, Carson A, Nguyen VH, Willis
AB, Cole JG and Rader RK: Addressing the crisis in the treatment of
osteoporosis: Better paths forward. J Bone Miner Res. 32:1386–1387.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Q, Wang T, Zhou L, Song F, Qin A, Feng
HT, Lin XX, Lin Z, Yuan JB, Tickner J, et al: Nitidine chloride
prevents OVX-induced bone loss via suppressing NFATc1-mediated
osteoclast differentiation. Sci Rep. 6:366622016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwak EJ, Lee YS and Choi EM: Effect of
magnolol on the function of osteoblastic MC3T3-E1 cells. Mediators
Inflamm. 2012.829650:2012.
|
|
28
|
Park JW, Yoon HJ, Kang WY, Cho S, Seong
SJ, Lee HW, Yoon YR and Kim HJ: G protein-coupled receptor 84
controls osteoclastogenesis through inhibition of NF-κB and MAPK
signaling pathways. J Cell Physiol. 233:1481–1489. 2018. View Article : Google Scholar
|
|
29
|
Chen X, Zhi X, Cao L, Weng W, Pan P, Hu H,
Liu C, Zhao Q, Zhou Q, Cui J and Su J: Matrine derivate MASM
uncovers a novel function for ribosomal protein S5 in
osteoclastogenesis and postmenopausal osteoporosis. Cell Death Dis.
8:e30372017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan H, Zhao L, Cao H, Chen A and Xiao J:
Epoxyeicosanoids suppress osteoclastogenesis and prevent
ovariectomy-induced bone loss. FASEB J. 29:1092–1101. 2015.
View Article : Google Scholar
|
|
31
|
Lu SH, Huang RY and Chou TC: Magnolol
ameliorates ligature-induced periodontitis in rats and
osteoclastogenesis: In vivo and in vitro study. Evid Based
Complement Alternat Med. 2013.634095:2013.
|
|
32
|
Kanzaki H, Shinohara F, Itohiya K,
Yamaguchi Y, Katsumata Y, Matsuzawa M, Fukaya S, Miyamoto Y, Wada S
and Nakamura Y: RANKL induces Bach1 nuclear import and attenuates
Nrf2-mediated antioxidant enzymes, thereby augmenting intracellular
reactive oxygen species signaling and osteoclastogenesis in mice.
FASEB J. 31:781–792. 2017. View Article : Google Scholar
|