Introduction
Ameloblastoma (AM) is a benign but locally invasive
odontogenic tumor, accounting for ~1% of oral tumors and 11-18% of
odontogenic tumors (1). In the
4th edition of the World Health Organization Classification of Head
and Neck Tumors published in 2017, AM was classified into three
main types: AM, unicystic, and extraosseous/peripheral types, among
which classic AM is the most common accounting for 91% of all cases
(2). AM initially presents as a
concealed central bone lesion, which gradually leads to bone and
tooth root resorption. If ignored further, the bone can become
perforated and the tumor will eventually invade the soft tissue
(3).
Previous studies of the invasiveness of AM in the
bone have mainly evaluated the following aspects: Cell cycle
proliferation (4,5), apoptosis (6,7),
invasiveness (8) and matrix
metalloproteinases (9-11). However, bone represents a
particularly mineralized harsh environment for tumor progression
characterized by a high rigidity and modulus. Although the invasive
capacities of tumor cells are important for tumor progression, the
stimulation of bone resorption by tumor cells is required to invade
the skeleton (12). It is widely
accepted that osteolytic lesions predominantly result from
increased osteoclast recruitment, activation, differentiation and
function (13,14). In the current study, numerous
osteoclasts were detected in osteolytic lesions of AM, which were
located not only adjacent to tumor cells but also at more distant
sites, indicating the potential role of osteoclast formation in AM
development in the bone. However, coculture of osteoclast
precursors with AM cells resulted in only a slight increase in
osteoclastogenic activity. Therefore, whether other possible
mechanisms underlie osteoclast activation in AM was evaluated.
Previously, interactions between tumor cells and
cells in the bone microenvironment have been proposed as potential
drivers of tumor progression in the bone (15). The understanding of these
interactions has mostly been derived from studies of bone
metastatic diseases, including breast cancer (16,17), prostate cancer (18) and bone malignancies including
multiple myeloma (19-24). During these interactions, the bone
marrow microenvironment is enriched by a variety of inflammatory
factors including parathyroid hormone-associated protein, tumor
necrosis factor-α (TNF-α), interleukin 1 (IL)-1, IL-6, IL-8, IL-11,
activin A and receptor activator of NF-κB ligand (RANKL) (16-26), which further stimulate tumor
growth and osteoclastogenesis or inhibit osteoblast formation,
supporting tumor progression in the bone.
RANKL is the final extracellular mediator that
promotes osteoclast precursors to differentiate into mature
osteoclasts in the presence of macrophage-colony stimulating factor
(M-CSF), an indispensable factor for early development of
osteoclasts and survival of mature osteoclasts (27,28). Bone resorbing factors, including
IL-1, IL-6, IL-8, IL-11 and IL-17, have been reported to trigger
RANKL expression in bone marrow stromal cells (14,15,29). Osteoprotegerin (OPG) produced by
stromal cells acts as a soluble decoy receptor for RANKL and
prevents osteoclast formation by interfering with the interactions
between RANKL and its receptor RANK (27,28).
In AM, communication between tumor cells and host
cells and the underlying cellular and molecular mechanisms remain
poorly understood. A previously study by Fuchigami et al
(30) suggested that direct
interactions between tumor cells and stromal fibroblasts support
proliferation of tumor cells in AM.
The aim of the present study was to clarify the role
of the interactions between AM cells and bone marrow stromal cells
in osteoclastogenesis. The present study provides experimental
evidence demonstrating that IL-8 and activin A were induced in
stromal cells following interacting with AM cells. These two
factors, in combination with RANKL, served critical roles in
osteoclastogenesis in AM.
Materials and methods
Reagents
Anti-TNF-α, activin A and IL-8 antibodies, as well
as recombinant RANKL, OPG, activin A and nonspecific mouse
immunoglobulin (Ig)G were purchased from R&D Systems, Inc.,
(Minneapolis, MN, USA). Anti-RANKL, cathepsin K, and acid
phosphatase 5, tartrate resistant (TRAP) antibodies were purchased
from Abcam (Cambridge, UK). Anti-JUN N-terminal kinase (JNK),
anti-phosphorylated (p)-JNK and anti-nuclear factors of activated
T-cells (NFATc-1) antibodies were purchased from Cell Signaling
Technology, Inc., (CST; Danvers, MA, USA). Recombinant human IL-8
and recombinant murine M-CSF were purchased from Sino Biological
(Beijing, China). The JNK pathway inhibitor SP600125 was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Tissue samples and cell culture
The AM tissues were obtained from 2 male patients
(27 and 29 years old) and 2 female patients (23 and 24 years old)
treated at the Department of Oral and Maxillofacial Surgery
(Hospital of Stomatology, Sun Yat-sen University, Guangzhou, China)
from June 2017 to February 2018. Informed consent was obtained
according to a protocol approved by the Ethical Committee of the
Guanghua School of Stomatology, Hospital of Stomatology and Sun
Yat-sen University [Guangzhou, China; ERC-(2017)-5]. Principles
outlined in the Declaration of Helsinki were followed. All AM
tissues were resected from the mandible, two were from plexiform
and two were from follicular AM (Table SI). Normal bone tissue was
obtained from a 24-year-old female patient with dento-maxillofacial
deformities during the orthognathic surgery.
Primary culture of AM cells was performed as
previously described (31).
Briefly, the specimen was diced into pieces at an approximate size
of 1 mm3 following removing the soft connective tissue,
placed into plates coated with collagen I (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and incubated at 37°C in
a 5% (v/v) CO2 atmosphere for 5 h. Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
containing 15% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) was added and used for subculture of the
proliferative cells. The epithelial cells were purified and
collected with a differential adhesion method.
Mouse bone marrow-derived monocyte/macrophage
precursor cells (BMMs) were isolated as previously described with
slight modifications (32).
Briefly, bone marrow cells were collected from the femur and tibiae
of 12 6-week-old female C57BL/6J mice (mean weight, 17.2 g). The
mice were purchased from the Laboratory Animal Center of Sun
Yat-sen University. Following washing, the cells were resuspended
in α-minimum essential medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS. Following 16 h of culture, the
nonadherent cells were collected and incubated in M-CSF (25 ng/ml)
at a density of 3×105 cells/ml in flasks. The cells were
used as BMMs following 3 days of culture. The study was performed
in accordance with the Guidelines laid down by the National
Institute of Health (Bethesda, MD, USA) in the USA regarding the
care and use of animals for experimental procedures, and in
accordance with local laws and regulations. Adequate measures were
taken to minimize the pain or discomfort of the mice. The
experiment was approved by the Institutional Animal Care and Use
Committee of Sun Yat-sen University (IACUC-DB-2017-0605).
The AM cell line, hTERT-AM, was previously
established by the authors' group (31). HS-5, a human bone marrow stromal
cell line, was purchased from the American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in DMEM containing 10%
FBS at 37°C in a 5% (v/v) CO2 atmosphere.
Cocultures of hTERT-AM cells with HS-5
cells
The hTERT-AM cells and HS-5 cells were directly
cocultured in 1:1 ratio or independently cultured at a density of
2.5×105 cells/ml for 24 h. For indirect cocultures, HS-5
cells (1.5×105 cells) were seeded in the lower chamber
of a 24-tran-swell plate (0.4-mm pore size; Corning-Costar; Conig,
Inc., Corning, NY, USA) and 100 µl medium containing
5×104 AM cells was added to the upper chamber. Cells
were cultured for 24 h. The culture media (CM) was collected and
stored at -80°C until use. Cells were used for RNA extraction.
Cytokine array
A commercial antibody-based protein microarray assay
was performed using G-Series Human Bone Metabolism Array 1
(RayBiotech Life, Norcross, GA, USA). In brief, array glass slides
were incubated in serum-free media from HS-5 cultures,
hTERT-AM/HS-5 cocultures and hTERT-AM cultures (500 µg/ml)
for 2 h. Following washing, the glass slides were incubated with a
cocktail of 31 biotinylated antibodies and the remaining
experimental procedure was carried out following the manufacturer's
protocol. The intensity of the signal was normal-ized to that of
the internal positive control.
ELISA
The serum-free medium from cell cultures were
collected and the level of TNF-α (cat. no. CSB-E04740h; Cusabio,
Wuhan, China), IL-8 (cat. no. D8000C; R&D Systems, Inc.), and
activin A (cat. no. DAC00B; R&D Systems, Inc.) were analyzed in
accordance with the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) and reverse synthesized to cDNA
using a PrimeScript RT Reagent kit (Takara Bio Inc., Otsu, Japan).
The following thermal cycling steps were used: 37°C for 15 min,
85°C for 5 sec, and store at 4°C. GAPDH was used as internal
control. The primers used in the present study are presented in
Table SII. Amplification of the
cDNA template was conducted using LightCycler 480 SYBR-Green I
Master (Roche Diagnostics, Basel, Switzerland). PCR cycles were run
as follows: 95°C for 5 min, 40 cycles of 95°C for 10 sec, 60°C for
20 sec and 72°C for 20 sec. Finally followed by 95°C for 5 sec and
65°C for 1 min. Following RT-PCR, the PCR products were separated
on a 2% agarose gel and stained with a new type of DNA dye GoldView
(cat. no. G8140; Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China), which is an ideal substitute for ethidium
bromide. Expression of each gene was normalized to that of GAPDH as
a loading control.
Western blot analysis
Total cellular proteins were extracted with
Radioimmunoprecipitation assay lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology, Shanghai, China) containing 10
mg/ml aprotinin, 10 mg/ml leupeptin and 100 mM PMSF. The
bicinchoninic acid assay was used for protein quantification. The
aliquots (containing 40 µg protein) were separated by 8%
SDS-PAGE, transferred to polyvinyli-dene difluoride membrane. The
membranes were incubated at 4°C overnight with antibodies against
TRAP (cat. no. ab191406; Abcam; 1:1,000), NFATc-1 (cat. no. 8032;
CST; 1:1,000), Cathepsin K (cat. no. ab187647; Abcam; 1:1,000),
RANKL (cat. no. ab97864; Abcam; 1:1,000), JNK (cat. no. 9252; CST;
1:1,000) and p-JNK (cat. no. 4668; CST; 1:1,000). Anti-β-actin
(cat. no. 4970; CST; 1:1,000) was used as a loading control. After
washing, the membranes were incubated with IRDye® 800CW
goat anti-Rabbit IgG (cat. no. 926-32211; LI-COR Biosciences,
Lincoln, NE, USA; 1:10,000) or Alexa Fluor® 680 goat
anti-rabbit IgG (cat. no. ab175773; Abcam; 1:10,000) for 1 h at
room temperature. The target proteins were visual-ized and
quantification was performed with the Odyssey Clx Infrared Imaging
System (LI-COR Biosciences) and Image Studio 5.0 software (LI-COR
Biosciences).
Osteoclast formation assay
Isolated BMMs (3×104 cells/well) were
cultured in a 96-well plate in medium containing M-CSF (25 ng/ml),
RANKL (50 ng/ml), IL-8 (100 ng/ml), activin A (50 ng/ml), the
culture mediums of hTERT-AM cells, HS-5 cells and the coculture
medium with or without corresponding antibodies for 5 days. The
cultures were fed every 2 days with fresh medium. To detect
osteoclast formation, the cells were subjected to TRAP staining
using an Acid Phosphatase Leukocyte kit (Sigma-Aldrich; Merck
KGaA). In addition, multinucleated TRAP-positive cells (≥3 nuclei)
were counted as osteoclasts.
Pit formation assay
BMMs (3×104 cells/well) were seeded onto
6 mm-diameter dentin slices in 96-well plates and cultured in
medium containing M-CSF (25 ng/ml), RANKL (50 ng/ml) with or
without activin A (50 ng/ml), or the CM from HS-5 cells, hTERT-AM
cells and HS-5/hTERT-AM cocultures for 15 days. CM was changed
every 2 days. The cells were lysed with 0.25% ammonia water and the
slices were stained with toluidine blue for 4 min at room
temperature. To evaluate bone resorption, the areas covering the
pit were calculated by counting the number of mesh squares (100×100
µm) with a light microscope (magnification, ×100; Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was conducted using SPSS
version 23.0 software (IBM, Corps., Armonk, NY, USA). Data are
presented as the mean ± standard deviation. Student's t-tests were
used in two-group comparisons. One-way analysis of variance with
multiple comparisons followed by Tukey's test was used for multiple
groups comparisons. All tests were two-tailed and a value of
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was performed in triplicate.
Results
Osteolysis and osteoclasts in AM
A total of two subtypes of AM were first tested for
their ability to form osteolytic lesions and induce osteoclast
formation. The histological examination results indicated a
follicular and a plexiform AM, respectively (Fig. 1A). The sections were then stained
with TRAP, a specific enzyme of osteoclasts. TRAP-positive
osteoclasts were located adjacent to tumor cells, but were more
commonly demonstrated at more distant sites from tumor cells,
forming resorption pits. However, the normal bone demonstrated no
TRAP-positive cells and a smooth cortical bone surface (Fig. 1B). Additionally, The AM tissues
and primary AM cells as well as hTERT-AM cells were screened for
the expression of osteoclast-activating factors including RANKL,
M-CSF, IL-1α, IL-1β, TNF-α, IL-6 and IL-8 by RT-PCR. Due to
different sources or conditions of the tissues and cells, these
factors demonstrated various expression levels among the specimens;
however, RANKL expression was only detected in AM tissues rather
than in AM cells (Fig. 1C).
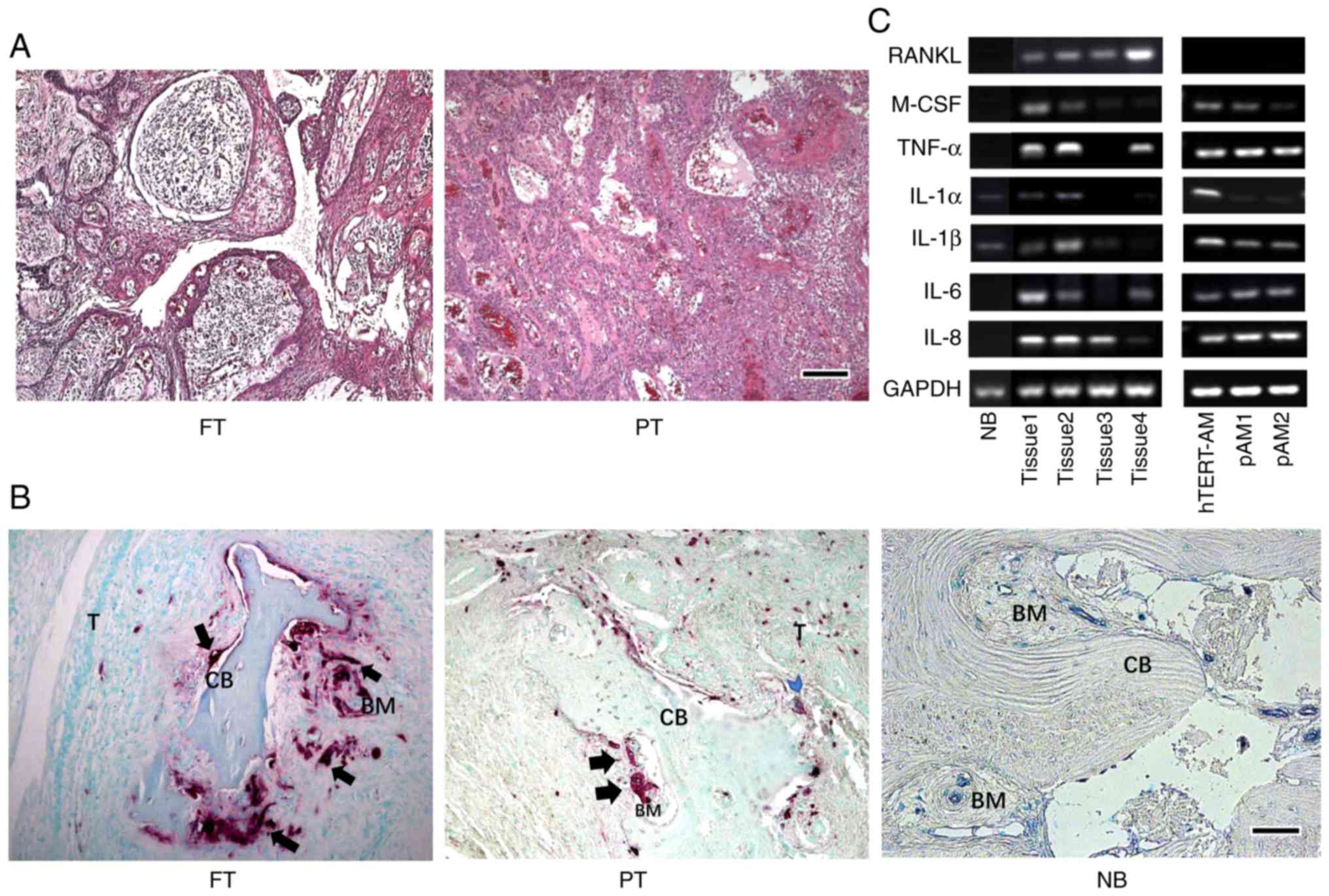 | Figure 1AMs develop osteolytic lesions and
induce osteoclastogenesis. (A) Hematoxylin and eosin staining of
histological sections of the tumors from two patients: One FT and a
PT. Scale bar, 250 µm. (B) TRAP-positive cells were
presented on the surface of the bone adjacent to tumor cells (blue
arrowhead), but more commonly located at a distant site from the
tumors (black arrow). Scale bar, 250 µm. (C) Expression of
osteoclast-activating factors in AM tissues and cells. The
polymerase chain reaction products were separated on a 2% agarose
gel and stained with gold view and expression of each gene was
normalized to GAPDH, which was used as a loading control. The
experiment was performed in triplicate. PAM, primary AM cells; FT,
follicular type; PT, plexiform type; NB, normal bone tissue; T,
tumor; CB, cortical bone; BM, bone marrow; RANKL, receptor
activator of NF-κB ligand; IL, interleukin; TNF, tumor necrosis
factor; M-CSF, macrophage-colony stimulating factor; AM,
ameloblastoma; TRAP, acid phosphatase 5, tartrate resistant. |
Coculture medium of AM cells and bone
marrow stromal cells induces osteoclast differentiation and
function
Mouse BMMs were treated with culture media from
hTERT-AM. A slightly increased number of TRAP-positive cells was
observed [Fig. 2A(c)], suggesting
that the soluble factors released from AM cells have a limited
potential to stimulate osteoclast formation. It was observed that
osteoclasts were mostly located in the bone marrow far from the
tumor cells, which prompted the investigation of whether cells in
the bone microenvironment, particularly BMSCs, serve a role in
osteoclastogenesis in AM. Therefore a cell-to-cell coculture of
hTERT-AM cells, HS-5 cells and a human BMSC line was established
and the coculture medium was added to the culture of mouse BMMs.
Notably, the average number of TRAP-positive cells was identified
to be significantly increased when mouse BMMs were treated with
culture medium from the coculture [P<0.05; Fig. 2A(d) and B]. However, BMSCs plated
in the absence of hTERT-AM cells did not induce osteoclast
formation [Fig. 2A(b)]. A pit
formation assay was further performed to investigate the potential
of the multi-nucleated cells to resorb calcified tissue. The
results demonstrated that the formation of resorption pits was seen
on the dentin slices treated with hTERT-AM CM or HS-5/ hTERT-AM CM
and the pit area of wells containing HS-5/hTERT-AM CM was ~4-fold
greater than those seeded with hTERT-AM CM (Fig. 2C). Furthermore, mRNA expression of
regulatory genes of osteoclast proliferation (PU.1),
differentiation (NFATc-1), fusion (DC-STAMP and MITF) and function
(Cathepsin K, integrin β3, and TRAP) were identified to be elevated
in BMMs following treatment with HS-5/AM CM (Fig. 2D). These, in combination with an
elevated protein level of NFATc-1, Cathepsin K and TRAP (Fig. 2E), which suggested that
interactions between AM cells and BMSCs are essential for
osteoclast differentiation and function.
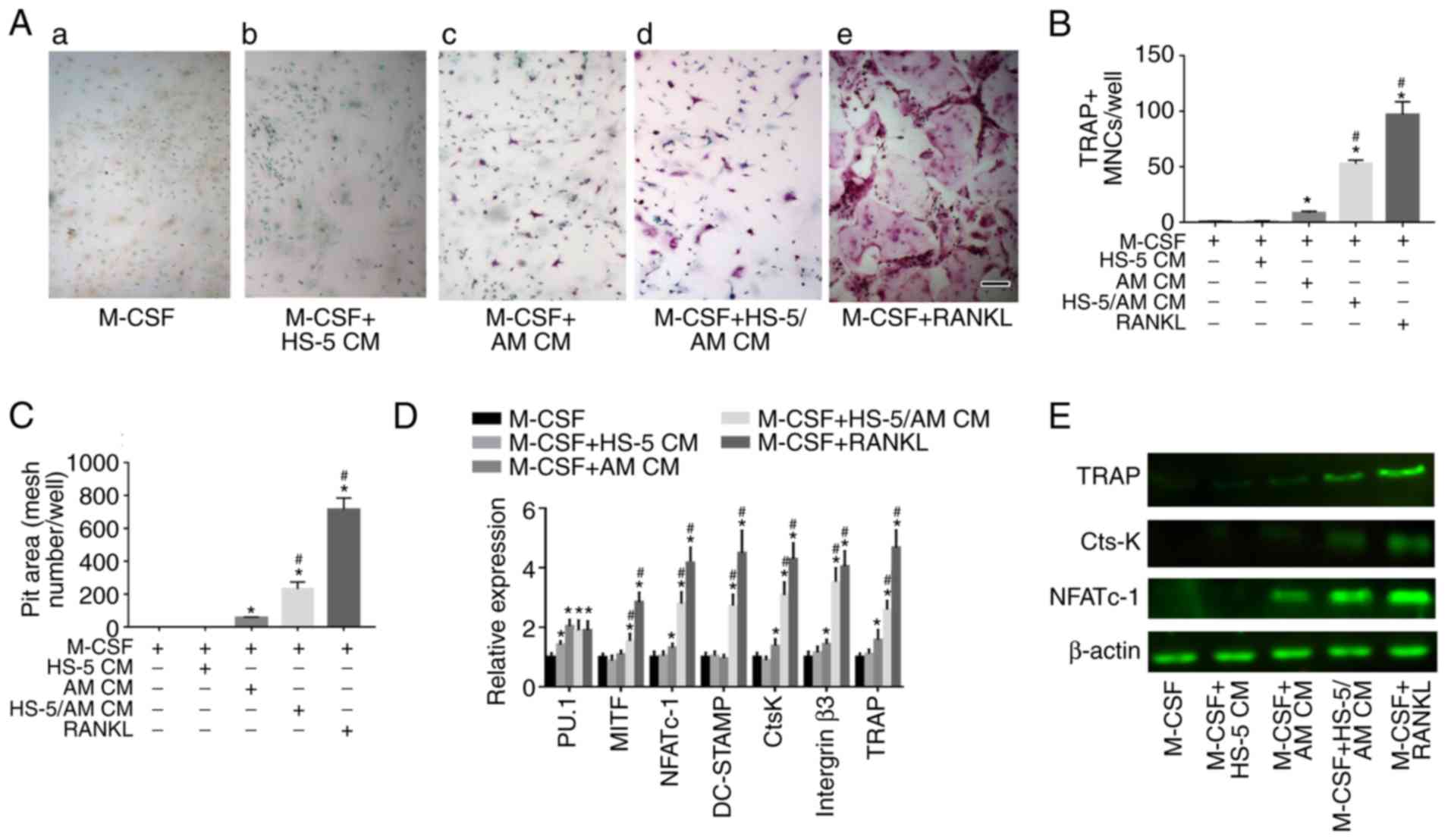 | Figure 2Coculture medium of hTERT-AM cells
and HS-5 cells increases osteoclast formation and function. (A)
Mouse BMMs (3×104 cells/well) were cultured in
osteoclastogenic medium with 25 ng/ml M-CSF, or in the same medium
containing either 50 ng/ml RANKL or CM from HS-5 cells, hTERT-AM
cells and hTERT-AM/HS-5 cultures at 50% dilution for 5 days. Scale
bar, 250 µm. (B) TRAP positive multinucleated cells (≥3
nuclei) were counted as mature osteoclasts. (C) BMMs were seeded
onto 6 mm-diameter dentin slices in 96-well plates and cultured in
medium as described above for 15 days. Culture media was altered
every 2 days. Then, pit areas were measured by counting mesh
numbers. (D) mRNA expression of PU.1, MITF, NFATc-1, DC-STAMP,
cathepsin K, integrin β3 and TRAP in BMMs were determined by
reverse transcription-quantitative polymerase chain reaction.
*P<0.05 vs. BMMs treated with M-CSF,
#P<0.05 vs. BMMs treated with M-CSF+hTERT-AM CM. (E)
NFATc-1, cathepsin K and TRAP proteins in BMMs were detected by
western blotting. β-actin was used as loading control. The
experiment was performed in triplicate. CtsK, cathepsin K; M-CSF,
macrophage colony stimulating factor; TRAP, acid phosphatase 5,
tartrate resistant; NFATc-1, nuclear factors of activated T-cells;
BMMs, bone marrow-derived monocyte/macrophage precursor cells; CM,
conditioned medium; RANKL, receptor activator of NF-κB ligand. |
Interactions between AM cells and BMSCs
triggers IL-8 and activin A expression
A total of 31 cytokines and growth factors
potentially involved in bone metabolism were screened for in the
culture media of monoculture of hTERT-AM cells, HS-5 cells and
coculture of the two cell lines using a cytokine array. The
analysis indicated that certain cytokines were relatively abundant
in the HS-5/ hTERT-AM cell coculture medium. Notably, the
expression of IL-8 and TNF-α in the CM of hTERT-AM cells was
significantly increased compared with HS-5 cells and a markedly
elevated level of activin A was observed in the coculture medium of
the two cell lines (Fig. 3A and
Table SIII). These three factors
were previously observed to be involved in the process of bone
resorption (13,33,34).
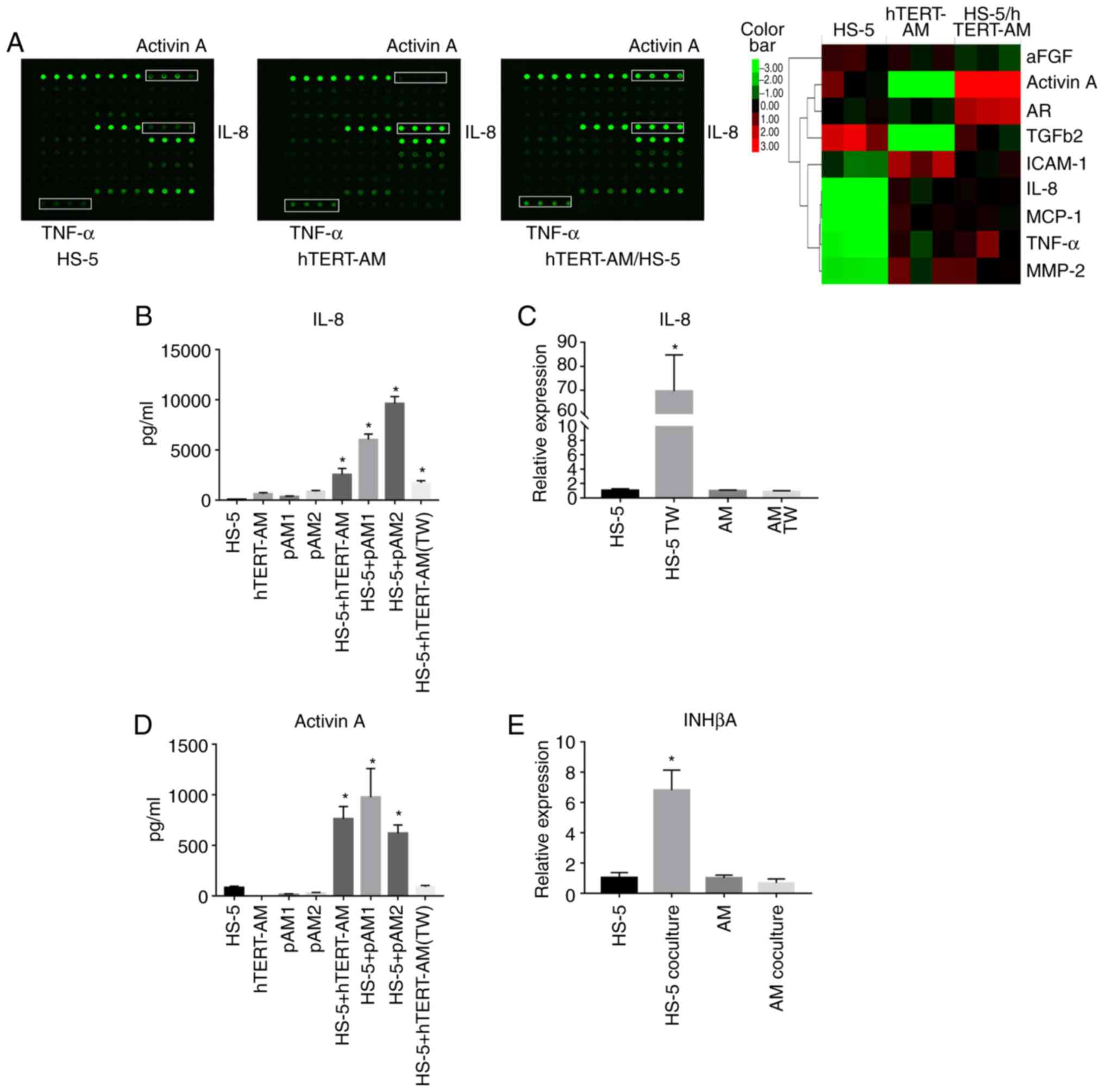 | Figure 3Interactions between AM cells and
BMSCs stimulated secretion of IL-8 and activin A. (A) Cytokine
array of the conditioned media of HS-5 cells, hTERT-AM cells and
the coculture of the two cell types. The hTERT-AM cells and HS-5
cells were directly co-cultured in 1:1 ratio or independently
cultured at a density of 2.5×105 cells/ml for 24 h, the
serum-free mediums were collected for array analysis. A1-A4: POS1;
A5-A8: POS2; A9-A12: Activin A; B1-B4: FGF-1; B5-B8: Amphiregulin;
B9-B12: bFGF; C1-C4: BMP-4; C5-C8: BMP-9; C9-C12: E-Selectin;
D1-D4: CD54; D5-D8: IGF-1; D9-D12: IL-1α; E1-E4: IL-1β; E5-E8:
IL-6; E9-E12: IL-8; F1-F4: IL-11; F5-F8: IL-17A; F9-F12: MCP-1;
G1-G4: M-CSF; G5-G8: MIP-1α; G9-G12: MMP-2; H1-H4: MMP-9; H5-H8:
MMP-13; H9-H12: Osteoactivin; I1-I4: P-Cadherin; I5-I8: RANK;
I9-I12: Stromal-cell derived factor-1α; J1-J4: Sonic hedgehog-N;
J5-J8: TGFβ1; J9-J12: TGFβ2; K1-K4: TNF-α; K5-K8: CD106; K9-K12:
CDH5. A heat map (right) demonstrating the semiquantitative results
of cytokine levels. (B) Protein levels of IL-8 in the serum-free
culture media from AM cells (hTERT-AM cells, primary AM cells),
HS-5 cells or direct or Transwell co-cultures measured by ELISA.
*P<0.05 vs. wells containing HS-5 cells or the
corresponding AM cells. (C) hTERT-AM cells and HS-5 cells were
cultured separately with a Transwell plate for 24 h, RT-qPCR was
performed to detect relative mRNA expression of IL-8 to GAPDH.
*P<0.05 vs. HS-5 monoculture. (D) Protein levels of
activin A in the serum-free culture media from AM cells (hTERT-AM
cells, primary AM cells), HS-5 cells or direct or Transwell
co-cultures measured by ELISA. *P<0.05 vs. wells
containing HS-5 cells or the corresponding AM cells. (E) hTERT-AM
cells and HS-5 cells were cocultured for 24 h. Following cell
separation, RT-qPCR was performed to detect relative mRNA
expression of INHβA to GAPDH. *P<0.05 vs. HS-5
monoculture. The experiment was performed in triplicate. pAM,
primary AM cells; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; IL, interleukin; CD, cluster of
differentiation; BMP, bone morpho-genic protein; AM, ameloblastoma;
MMP, matrix metalloproteinase; TGF, transforming growth factor;
TNF, tumor necrosis factor; bFGF, basic fibroblast growth factor;
SDF, stromal-cell derived factor. |
To validate the findings of the cytokine array, IL-8
levels were quantified by ELISA in the 24-h serum-free culture
media from the HS-5 cells, AM cells, or coculture of the two cell
types. The results revealed that AM cells secreted increased
amounts of IL-8 compared with HS-5 cells and a dramatic increase in
the production of IL-8 protein was observed following direct or
indirect co-culture of the two cell types (Fig. 3B). To define the source of IL-8 in
the coculture, RT-qPCR was performed to compare IL-8 expression in
AM cells and HS-5 cells cultured separately using a Transwell
plate. The results revealed a significant increase in IL-8
expression in HS-5 cells following indirect coculture with hTERT-AM
cells, whereas IL-8 expression in hTERT-AM cells remained nearly
unchanged (P<0.05; Fig. 3C).
These data indicate that AM cells stimulated the IL-8 secretion of
BMSCs either though cell-cell contact or soluble factors.
In addition, activin A production was observed to be
relatively high in HS-5 cells, but was nearly undetectable in AM
cells. Only a direct interaction between AM and HS-5 cells could
increase activin A production in the coculture medium (Fig. 3D). To investigate the source of
activin A, RT-qPCR was used to examine the expression levels of
inhibin-βA subunits, in which activin A is a dimer. The results
indicated that the upregulated activin A was derived from BMSCs
(Fig. 3E). These findings suggest
that cell-to-cell contact was essential for triggering activin A
secretion in BMSCs.
Furthermore, ELISA analysis verified increased
production of TNF-α in AM cells compared with in HS-5 cells and
that coculture of AM and HS-5 cells did not lead to alteration in
TNF-α protein level (Fig.
S1).
Neutralization of IL-8 or activin A
results in decreased osteoclastogenesis induced by AM/BMSCs CM
To investigate the role of AM/BMSCs CM-derived IL-8
and activin A in osteoclastogenesis, CM from hTERT-AM/HS-5
cocultures, with or without IL-8 or activin A neutralizing
antibodies, were added to the culture of mouse BMMs in the presence
of M-CSF. It was demonstrated that osteoclast formation provoked by
the coculture medium was decreased either by neutralization of IL-8
or activin A (Fig. 4), which
ascertained that the two proteins are involved in
osteoclastogenesis induced by AM/BMSCs interactions and that IL-8
seems to serve a more important role in osteoclastogenesis in
AM.
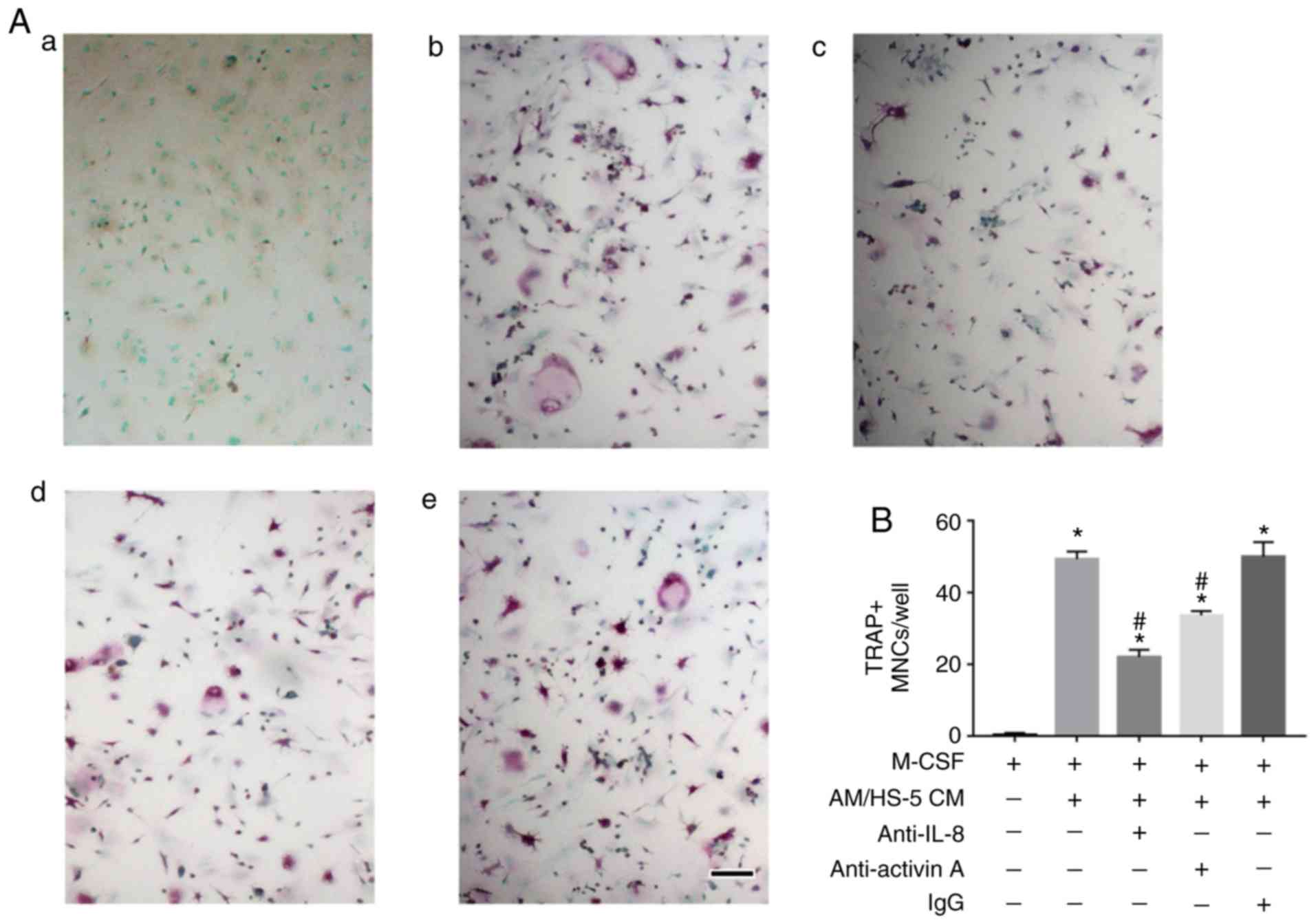 | Figure 4Neutralization of IL-8 or activin A
resulted in decreased osteoclastogenesis induced by AM/bone marrow
stromal cells CM. (A) Mouse bone marrow-derived monocyte/macrophage
precursor cells were cultured in osteoclastogenic medium with 25
ng/ml M-CSF, or in the same medium containing the CM from
HS-5/hTERT-AM cocultures at 1:1 dilution. Anti-IL-8 antibody (1
µg/ml) or anti-activin A antibody (100 ng/ml) was added to
some of the CM-containing wells, the cells were cultured for 5
days. (a) +M-CSF, (b) +M-CSF+AM/HS-5 CM, (c) +M-CSF+AM/HS-5
CM+anti-IL-8, (d) +M-CSF+AM/HS-5 CM+anti-activin A and (e)
+M-CSF+AM/HS-5 CM+ immunoglobulin G. Scale bar, 250 µm. (B)
The number of TRAP+ MN cells (≥3 nuclei) was indicated.
*P<0.05 vs. cells treated with M-CSF,
#P<0.05 vs. wells containing M-CSF+AM/HS-5 CM. The
experiment was performed in triplicate. IL, interleukin; M-CSF,
macrophage-colony stimulating factor; CM, conditioned media; AM,
ameloblatoma; TRAP, acid phosphatase 5, tartrate resistant; MN,
multinucleated. |
AM-derived TNF-α upregulates IL-8
expression in BMSCs and IL-8 directly induces osteoclast
formation
Studies have demonstrated that IL-8 production can
be induced in response to multiple signals including IL-1α, IL-1β
and TNF-α (30,35). In the present study, TNF-α was
observed to be highly expressed in hTERT-AM cells (Fig. 3A), therefore anti-TNF-α antibody
was added to the culture medium of hTERT-AM cells to examine the
effect of TNF-α on IL-8 expression in HS-5 cells. The results
demonstrated that neutralizing TNF-α significantly downregulated
IL-8 expression in HS-5 cells induced by AM cells (P<0.05;
Fig. 5A). ELISA analysis further
indicated that the protein level of IL-8 in the coculture medium of
the two cell lines was significantly inhibited by addition of the
anti-TNF-α antibody (P<0.05; Fig.
5B). Additionally, it was also identified that IL-8 directly
induced osteoclast formation in vitro (Fig. 5C and D), which was consistent with
previous studies (36,37).
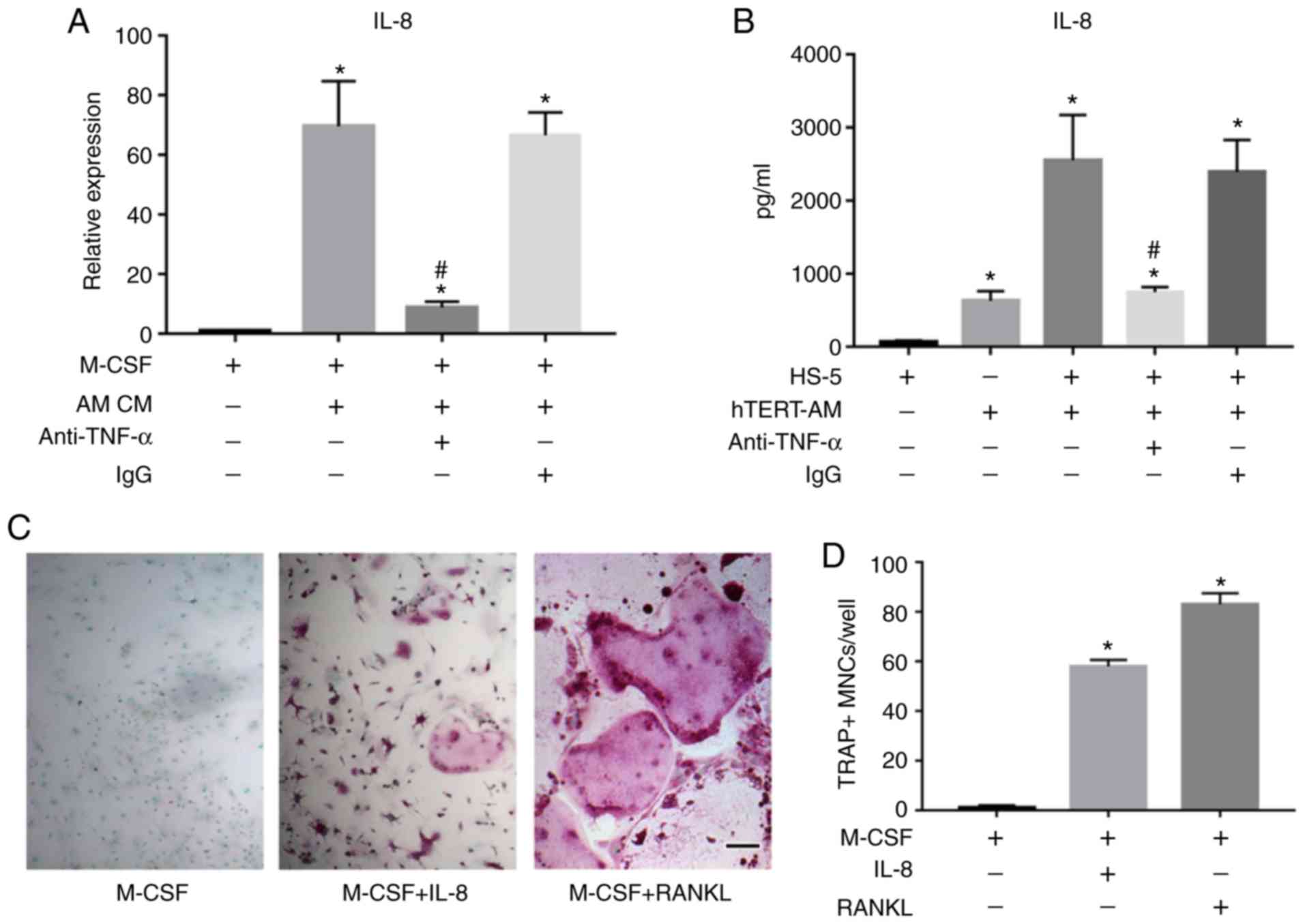 | Figure 5AM-derived TNF-α upregulated IL-8
expression in bone marrow stromal cells and IL-8 directly triggered
osteoclastogenesis. (A) HS-5 cells were treated with CM from
hTERT-AM with or without TNF-α antibody for 24 h and reverse
transcription-quantitative polymerase chain reaction was performed
to detect IL-8 expression in HS-5 cells. *P<0.05 vs.
cells treated with M-CSF, #P<0.05 vs. wells
containing M-CSF+AM/HS-5 CM. (B) HS-5 Cells and hTERT-AM were
cultured independently or directly cocultured with or without TNF-α
antibody (50 ng/ml) for 24 h, IL-8 levels in the culture media were
detected by ELISA. *P<0.05 vs. control,
#P<0.05 vs. coculture of HS-5 and hTERT-AM. (C)
Osteoclast formation stimulated by recombinant IL-8 or RANKL. Bone
marrow-derived monocyte/macrophage precursor cells were cultured in
osteoclastogenic medium with 25 ng/ml M-CSF, or in the same medium
containing RANKL (50 ng/ml) or IL-8 (100 ng/ml). Scale bar, 250
µm. (D) TRAP positive MN cells (≥3 nuclei) were counted as
mature osteoclasts. *P<0.05 vs. cells treated with
M-CSF. The experiment was performed in triplicate. IL, interleukin;
M-CSF, macrophage-colony stimulating factors; TNF, tumor necrosis
factor; RANKL, receptor activator of NF-κB ligand; AM,
ameloblastoma; CM, conditioned media; TRAP, acid phosphatase 5,
tartrate resistant; MN, multinucleated; Ig, immunoglobulin. |
Upregulated IL-8 from the coculture
system triggers RANKL expression in BMSCs
RT-qPCR was performed to examine whether AM-derived
CM could induce RANKL expression in BMSCs. The results revealed
that CM derived from hTERT-AM cells significantly increased RANKL
expression in BMSCs (P<0.05). The expression of OPG, the decoy
receptor of RANKL, was not altered (Fig. 6A). Additionally, adding OPG to the
coculture medium slightly decreased osteoclast formation in mouse
BMMs (Fig. 6B and C).
Furthermore, it was identified that recombinant IL-8 induced RANKL
level in BMSCs (Fig. 6D and E)
and addition of anti-IL-8 antibody to AM-derived CM significantly
decreased RANKL expression in BMSCs (P<0.05; Fig. 6E). These results indicate that
hTERT-AM cells upregulated the expression of RANKL in HS-5 cells in
an IL-8-dependent manner and RANKL further influenced osteoclast
formation.
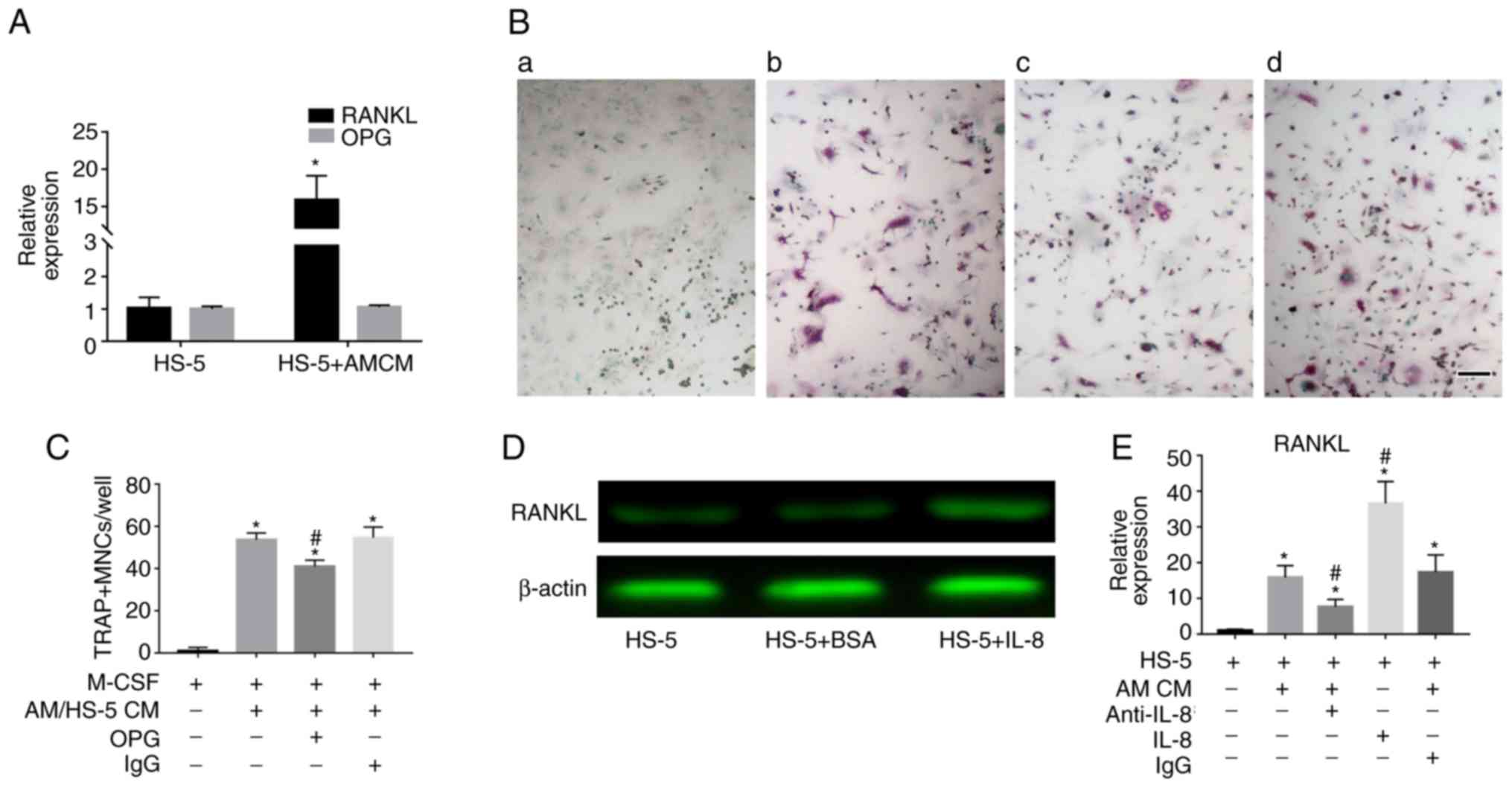 | Figure 6Upregulated IL-8 from the coculture
system supported RANKL expression on bone marrow stromal cells. (A)
HS-5 cells were cultured in the CM of hTERT-AM for 24 h. RT-qPCR
was performed to detect expressions of RANKL and OPG in HS-5 cells.
*P<0.05 vs. untreated HS-5. (B) Mouse bone
marrow-derived monocyte/macrophage precursor cells were cultured in
osteoclastogenic medium with 25 ng/ml M-CSF, or in the same medium
containing the CM from HS-5/hTERT-AM cocultures at 1:1 dilution.
OPG (100 ng/ml) was added to some of the CM-containing wells. (a)
+M-CSF, (b) +M-CSF+AM/HS-5 CM, (c) +M-CSF+AM/HS-5 CM+OPG, (d)
+M-CSF+AM/HS-5 CM+ IgG. Scale bar, 250 µm (C) The number of
TRAP+ MN cells (≥3 nuclei) was indicated. *P<0.05 vs.
cells treated with M-CSF, #P<0.05 vs. wells
containing HS-5/hTERT-AM CM. (D) HS-5 cells were cultured with or
without recombinant IL-8 (100 ng/ml) for 24 h. Cells were harvested
to analyze RANKL by western blotting. (E) HS-5 cells were treated
with recombinant IL-8 (100 ng/ml) or CM from hTERT-AM with or
without IL-8 antibody for 24 h. RT-qPCR was performed to detect
RANKL expression in HS-5 cells. *P<0.05 vs. HS-5,
#P<0.05 vs. cells treated with hTERT-AM CM. The
experiment was performed in triplicate. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; IL,
interleukin; BMSCs, bone marrow stromal cells; RANKL, receptor
activator of NF-κB ligand; OPG, Osteoprotegerin; CM, conditioned
medium; Ig, immunoglobulin; AM, ameloblastoma; TRAP, acid
phosphatase 5, tartrate resistant; MN, multinucleated. |
JNK activation is associated with
AM-induced secretion of activin A in BMSCs
Previous studies demonstrated that activin A
secretion was regulated by p38-dependent and JNK-dependent
pathways, and that a highly conserved c-Jun-binding sequence was
present in the INHβA promoter (38). Whether activation of the JNK
pathway was associated with activin A production was therefore
investigated by coculture of HS-5 cells and hTERT-AM cells. The
results indicated that direct coculture of the two cell lines
activated the JNK pathway, as phosphorylation of JNK was observed
in HS-5 cells in the presence of fixed AM cells (Fig. 7A). It was also demonstrated that
adding the specific JNK inhibitor SP600125 significantly reduced
activin A secretion in the coculture medium (P<0.05; Fig. 7B). Furthermore, although activin A
itself did not stimulate osteoclast formation, it did slightly
support RANKL to induce osteoclastogenesis (Fig. 7C and D). Furthermore, the pit
formation assay revealed that the area of resorption pits on the
dentin slices treated with RANKL and activin A was about 1.3-fold
greater than those seeded with RANKL alone (Fig. 7E). This suggests that activin A
acts as a cofactor of RANKL to induce osteoclast formation and
function and has a more effective triggering function of
osteoclasts.
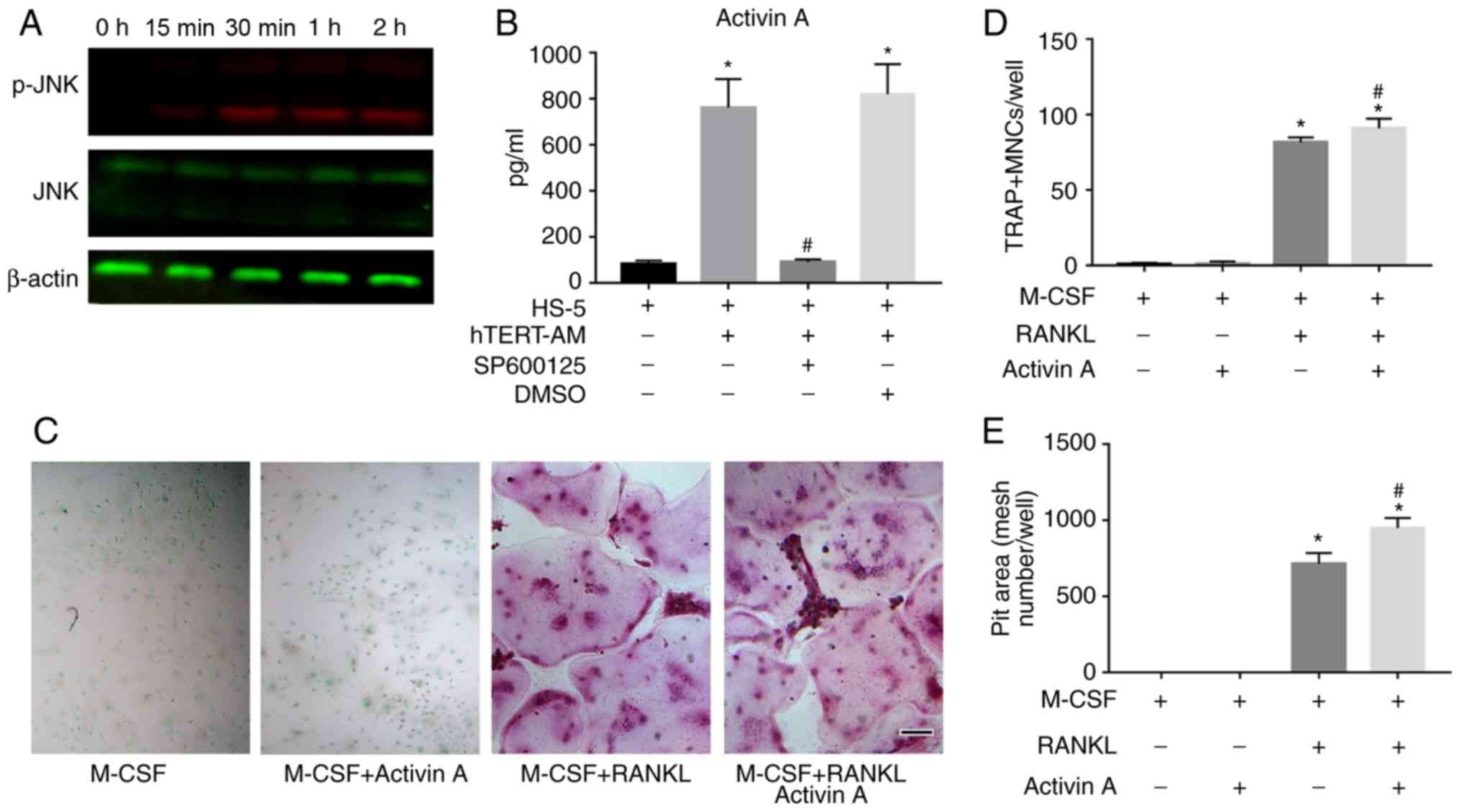 | Figure 7HS-5 cells secretion of activin A was
induced by hTERT-AM cells via JNK pathway activation. (A) HS-5
cells were cocultured with fixed hTERT-AM cells and harvested at
the indicated time points to analyze JNK phosphorylation by western
blotting. (B) hTERT-AM cells and HS-5 cells were cocultured for 24
h. In the last 15 h, JNK inhibitor (SP600125, 10 µM) or 0.1%
DMSO was added. The supernatants were analyzed for activin A levels
by ELISA. *P<0.05 vs. HS-5 CM, #P<0.05
vs. hTERT-AM/HS-5 CM. (C) Activin A stimulates RANKL-stimulated
osteoclast differentiation. Mouse BMMs were differentiated to
osteoclast with RANKL (50 ng/ml) and M-CSF (25 ng/ml) with or
without activin A (50 ng/ml), and cultured for 3 days. (D) TRAP+
multinucleated cells (≥3 nuclei) were counted. Scale bar, 250
µm. (E) BMMs were seeded onto 6 mm-diameter dentin slices in
96-well plates and cultured in medium with RANKL (50 ng/ml) and
M-CSF (25 ng/ml) with or without activin A (50 ng/ml for 15 days).
Culture media was changed every 2 days. Pit areas were measured by
counting mesh numbers. *P<0.05 vs. cells treated with
M-CSF, #P<0.05 vs. wells containing M-CSF+RANKL. The
experiment was performed in triplicate. JNK, c-Jun N-terminal
kinase 1; M-CSF, macrophage-colony stimulating factor; RANKL,
receptor activator of NF-κB ligand; BMMs, bone marrow-derived
monocyte/macrophage precursor cells; TRAP, acid phosphatase 5,
tartrate resistant; AM, ameloblastoma; CM, conditioned medium. |
Discussion
The present study revealed that interactions between
AM cells and BMSCs trigger osteoclastogenesis by upregulating IL-8
and activin A. Production of IL-8 in BMSCs was enhanced by
AM-derived TNF-α and IL-8 further induced osteoclast formation
directly or by upregulating RANKL expression in BMSCs. It was also
demonstrated that activin A secretion was stimulated in BMSCs via
activation of the JNK pathway in the presence of AM cells;
furthermore, activin A acted as a cofactor of RANKL in stimulating
osteoclast formation and function.
RANKL is known as an essential factor for osteoclast
differentiation. It can be released in soluble form from tumor
cells and can stimulate osteoclast formation directly in the
absence of stromal cells (39).
Sandra et al (3) revealed
that 10X CM of AM-1 cells, an AM cell line, stimulated
osteoclastogenesis because of RANKL secretion. However, the results
of the present study demonstrated only a slight increase in
osteoclast formation of osteoclast precursors in response to AM
cells and no evidence of RANKL expression in AM cells. Yoshimoto
et al (40) also failed to
observe detectable expression of RANKL in AM cells. Interestingly,
clear expression of IL-8 was observed in AM cells and
neutralization of IL-8 prevented osteoclastogenesis induced by the
CM of AM cells (data not shown), suggesting that tumor-derived IL-8
is a key factor that directly induces osteoclast differentiation in
AM and that the soluble RANKL in the 1X CM of AM cells is too low
to induce osteoclastogenesis.
Previous studies have emphasized the importance of
tumor-microenvironment interactions in tumor cell growth and
invasion as well as osteoclastogenesis in a variety of tumors
(15,16,41). For example, adhesion of myeloma
cells to BMSCs stimulated IL-6 expression by BMSCs, which was
further involved in modulating tumor growth and invasiveness.
Interactions between multiple myeloma cells and stromal cells
induced osteoclastogenesis by upregulating IL-8 expression in
stromal cells (13). Consistent
with these findings, Fuchigami et al (30) demonstrated that reciprocal
cell-cell interactions between tumor cells and stromal fibroblasts
resulted in increased production of IL-6 and IL-8 by fibroblasts,
and increased proliferation of AM cells. In the present study, it
was observed that the interaction between AM cells and BMSCs led to
augmented secretion of IL-8 and activin A.
IL-8, a member of the CXC chemokine family, was
originally described as a chemoattractant of neutrophils (42). It is highly expressed in a variety
of human cancer cells with high metastatic potential (36). Overexpression of IL-8 not only
correlates with tumor growth, angiogenesis and metastasis (43,44), but also serves a critical role in
osteoclast formation (36,37,45).
To investigate the role of upregulated IL-8 in the coculture system
in osteoclastogenesis, the neutral-izing anti-IL8 antibody was
tested on osteoclast formation. The findings demonstrated a
dramatically reduced osteoclast formation induced by AM/BMSCs
coculture medium. These findings again verified that IL-8 is a
vital regulator of osteoclastogenesis in AM. According to previous
studies, IL-8 production can be induced in response to multiple
signals including IL-1α, IL-1β and TNF-α (30,35). TNF-α is a proinflammatory cytokine
that has been implicated in inflammatory and immune responses
(46). It was demonstrated to
regulate tumor growth and invasiveness by upregulating IL-6 and
matrix metalloproteinase-9 in AM cells (34). The expression of TNF-α was
increased in AM cells compared with in BMSCs, prompting
investigation of the role of tumor-derived TNF-α in IL-8 secretion
in AM. The data demonstrated that neutralizing TNF-α partially
inhibited IL-8 production in hTERT-AM/HS-5 coculture, indicating
that AM-derived TNF-α is critical in modulating osteoclastogenesis
through upregulation of IL-8 secretion in BMSCs.
Previous studies demonstrated that RANKL-positive
cells were more commonly distributed throughout the stroma than in
tumor cells in AM (40,47,48). The results of the present study
indicated that RANKL mRNA was positively expressed in AM tissues
rather than in AM cells, confirming that stromal cells are the
major source of RANKL in AM. In addition, hTERT-AM/HS-5 coculture
increased RANKL expression in BMSCs and RANKL-neutralization
suppressed osteoclast formation induced by the coculture medium.
These findings verified the role of stromal-derived RANKL in
osteoclastogenesis in AM. Similarly, studies demonstrated that
multiple myeloma cells (21-24) can stimulate osteoclastogenesis by
increasing RANKL production in BMSCs and osteoblasts. Furthermore,
RANKL is triggered by multiple bone-resorbing factors including
parathyroid hormone-associated protein, IL-1, IL-6, IL-8, IL-11 and
IL-17 (14,15,29). Interestingly, neutralization of
IL-8 in the AM/BMSCs coculture medium suppressed RANKL expression
in BMSCs, suggesting that interactions between the AM cells and
BMSCs induced RANKL expression through an IL-8-dependent
pathway.
Activin A is a member of the transforming growth
factor (TGF)-β superfamily, which was identified as a regulator of
the pituitary follicular stimulating hormone (49), embryogenesis (50), cell proliferation (51), apoptosis (51,52) and differentiation (53). Interestingly, this factor was also
proposed to be involved in bone remodeling because of its dual role
in stimulating osteoclast formation (33,54) and inhibiting osteoblast
differentiation (25). The
results of the present study demonstrated that activin A secretion
was increased by direct interactions between AM cells and BMSCs. As
previous studies revealed that a highly conserved c-Jun-binding
sequence was present in the INHβA promoter (38) and that cell-to-cell contact
triggers activation of the JNK signaling pathway (55), the role of the JNK pathway on
activin A production by AM/BMSC coculture was further investigated.
The results indicated that adhesion between BMSCs and AM cells
activated the JNK pathway in BMSCs and the JNK inhibitor reduced
activin A secretion in the coculture, confirming involvement of the
JNK pathway in activin A production by BMSCs in response to AM
cells. Additionally, neutralization of activin A in coculture
resulted in decreased osteoclast formation. Furthermore, although
activin A could only slightly enhance osteoclast formation in BMMs
in the presence of RANKL, it increased the potential of osteoclasts
to resorb calcified tissues, suggesting that activin A acts as a
synergist for RANKL, which serves a greater role in stimulating
osteoclast function than osteoclast formation. However, further
research should be done in the future to elucidate the possible
mechanisms of this phenomenon.
The limitation of the present study may be that CM
from human cells is used to stimulate the BMMs from mice to
evaluate their ability to induce osteoclast formation. Indeed, it
would be more convincing if human BMMs were used in this
experiment. However, normal human BMMs are hard to obtain in the
authors' institution. Previous studies have conducted cocultures of
cells from humans and other species including mice (3,56-58), rats (59) or rabbits (60) to investigate their interactions.
This approach was therefore used in the current study. As expected,
it was identified that the coculture medium from human AM cells and
BMSCs increased osteoclast formation from mouse BMMs. Detailed
analysis of organs, tissues, cells and molecules exhibits a number
of similarities between humans and mice, ranging from embryonic
development to diseases including diabetes and cancer, despite
their striking anatomical difference. In addition, for ~99% of
mouse genes, a counterpart can be identified in the human genome,
which indicated high homologly between the mouse and human genomes
(61). Furthermore, most
cytokines, including fibroblast growth factor (62), and neurotrophin [brain derived
neurotrophic growth factor (DNF), Glial cell DNF and ciliary
neurotrophic factor) (63), TGF-β
(64)], are highly conserved
molecules, exhibiting strong cross-species bioactivity among
different species. These results further support the feasibility of
this method. However, there are a few exceptions. For example,
human IL-6 can bind to the human IL-6 receptor (IL-6R) and the
mouse IL-6R, while mouse IL-6 only binds to the mouse IL-6R
(57). Therefore, to overcome
this potential limitation, the authors' intend to choose cells from
the same species to check their communication in future
research.
In conclusion, the results of the present study
support that tumor-microenvironment interactions induce
osteoclastogenesis and the process was modulated by upregulated
IL-8, and activin A. IL-8 is a key regulator of osteoclast
formation, which not only induced osteoclast differentiation
directly but also triggered RANKL expression. In combination with
activin A, which acted as a synergist of RANKL, a favorable
microenvironment was established for AM invasion in the bone.
Supplementary Materials
Funding
The present study was supported by the Science and
Technology Planning Project of Guangdong Province (grant number
2017A020211025).
Availability of data and materials
All data of this study are included in this
article.
Authors' contributions
QT and XL conceived and designed the study. XL, ZC
and TL performed the experiments. PL analyzed the data. XL and ZC
wrote the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
the Guanghua School of Stomatology, Hospital of Stomatology and Sun
Yat-sen University [Guangzhou, China; ERC-(2017)-5] and the
Institutional Animal Care and Use Committee of Sun Yat-sen
University (IACUC-DB-2017-0605).
Patient consent for publication
Patient consent for publication was obtained
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Jhamb T and Kramer JM: Molecular concepts
in the pathogenesis of ameloblastoma: Implications for
therapeutics. Exp Mol Pathol. 97:345–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wright JM and Vered M: Update from the 4th
edition of the world health organization classification of head and
neck tumours: Odontogenic and maxillofacial bone tumors. Head Neck
Pathol. 11:68–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandra F, Hendarmin L, Kukita T, Nakao Y,
Nakamura N and Nakamura S: Ameloblastoma induces
osteoclastogenesis: A possible role of ameloblastoma in expanding
in the bone. Oral Oncol. 41:637–644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ong'uti MN, Cruchley AT, Howells GL and
Williams DM: Ki-67 antigen in ameloblastomas: Correlation with
clinical and histological parameters in 54 cases from Kenya. Int J
Oral Maxillofac Surg. 26:376–379. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandra F, Mitsuyasu T, Nakamura N,
Shiratsuchi Y and Ohishi M: Immunohistochemical evaluation of PCNA
and Ki-67 in ameloblastoma. Oral Oncol. 37:193–198. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandra F, Nakamura N, Mitsuyasu T,
Shiratsuchi Y and Ohishi M: Two relatively distinct patterns of
ameloblastoma: An anti-apoptotic proliferating site in the outer
layer (periphery) and a pro-apoptotic differentiating site in the
inner layer (centre). Histopathology. 39:93–98. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo HY, Yu SF and Li TJ: Differential
expression of apoptosis-related proteins in various cellular
components of ameloblastomas. Int J Oral Maxillofac Surg.
35:750–755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang C, Zhang Q, Shanti RM, Shi S, Chang
TH, Carrasco L, Alawi F and Le AD: Mesenchymal stromal cell-derived
interleukin-6 promotes epithelial-mesenchymal transition and
acquisition of epithelial stem-like cell properties in
ameloblastoma epithelial cells. Stem Cells. 35:2083–2094. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Zeng D, Huang H, Wang J, Tao Q,
Pan C, Xu J, Zhang B and Wang A: Tissue inhibitor of
metalloproteinase-2 inhibits ameloblastoma growth in a new mouse
xenograft disease model. J Oral Pathol Med. 39:94–102. 2010.
View Article : Google Scholar
|
|
10
|
Zhang B, Zhang J, Huang HZ, Xu ZY and Xie
HL: Expression and role of metalloproteinase-2 and endogenous
tissue regulator in ameloblastoma. J Oral Pathol Med. 39:219–222.
2010. View Article : Google Scholar
|
|
11
|
Wang A, Zhang B, Huang H, Zhang L, Zeng D,
Tao Q, Wang J and Pan C: Suppression of local invasion of
ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro.
BMC Cancer. 8:1822008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin TJ: Manipulating the environment of
cancer cells in bone: A novel therapeutic approach. J Clin Invest.
110:1399–1401. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herrero AB, García-Gómez A, Garayoa M,
Corchete LA, Hernández JM, San Miguel J and Gutierrez NC: Effects
of IL-8 Up-regulation on cell survival and osteoclastogenesis in
multiple myeloma. Am J Pathol. 186:2171–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sottnik JL and Keller ET: Understanding
and targeting osteoclastic activity in prostate cancer bone
metastases. Curr Mol Med. 13:626–639. 2013. View Article : Google Scholar :
|
|
15
|
Kovacic N, Croucher PI and McDonald MM:
Signaling between tumor cells and the host bone marrow
microenvironment. Calcif Tissue Int. 94:125–139. 2014. View Article : Google Scholar
|
|
16
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Azim H and Azim HA Jr: Targeting RANKL in
breast cancer: Bone metastasis and beyond. Expert Rev Anticancer
Ther. 13:195–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen G, Sircar K, Aprikian A, Potti A,
Goltzman D and Rabbani SA: Expression of RANKL/RANK/OPG in primary
and metastatic human prostate cancer as markers of disease stage
and functional regulation. Cancer. 107:289–298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Croucher PI, Shipman CM, Lippitt J, Perry
M, Asosingh K, Hijzen A, Brabbs AC, van Beek EJ, Holen I, Skerry
TM, et al: Osteoprotegerin inhibits the development of osteolytic
bone disease in multiple myeloma. Blood. 98:3534–3540. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sezer O, Heider U, Jakob C, Eucker J and
Possinger K: Human bone marrow myeloma cells express RANKL. J Clin
Oncol. 20:353–354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farrugia AN, Atkins GJ, To LB, Pan B,
Horvath N, Kostakis P, Findlay DM, Bardy P and Zannettino AC:
Receptor activator of nuclear factor-kappaB ligand expression by
human myeloma cells mediates osteoclast formation in vitro and
correlates with bone destruction in vivo. Cancer Res. 63:5438–5445.
2003.PubMed/NCBI
|
|
22
|
Giuliani N, Bataille R, Mancini C,
Lazzaretti M and Barillé S: Myeloma cells induce imbalance in the
osteoprotegerin/osteoprotegerin ligand system in the human bone
marrow environment. Blood. 98:3527–3533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pearse RN, Sordillo EM, Yaccoby S, Wong
BR, Liau DF, Colman N, Michaeli J, Epstein J and Choi Y: Multiple
myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to
trigger bone destruction and promote tumor progression. Proc Natl
Acad Sci USA. 98:11581–11586. 2001. View Article : Google Scholar
|
|
24
|
Shipman CM and Croucher PI:
Osteoprotegerin is a soluble decoy receptor for tumor necrosis
factor-related apoptosis-inducing ligand/Apo2 ligand and can
function as a paracrine survival factor for human myeloma cells.
Cancer Res. 63:912–916. 2003.PubMed/NCBI
|
|
25
|
Vallet S, Mukherjee S, Vaghela N,
Hideshima T, Fulciniti M, Pozzi S, Santo L, Cirstea D, Patel K,
Sohani AR, et al: Activin a promotes multiple myeloma-induced
osteolysis and is a promising target for myeloma bone disease. Proc
Natl Acad Sci USA. 107:5124–5129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renema N, Navet B, Heymann MF, Lezot F and
Heymann D: RANK-RANKL signalling in cancer. Biosci Rep. 36:pii:
e003662016. View Article : Google Scholar
|
|
27
|
Chikatsu N, Takeuchi Y, Tamura Y, Fukumoto
S, Yano K, Tsuda E, Ogata E and Fujita T: Interactions between
cancer and bone marrow cells induce osteoclast differentiation
factor expression and osteoclast-like cell formation in vitro.
Biochem Biophys Res Commun. 267:632–637. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sisay M, Mengistu G and Edessa D: The
RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer
stem cell: Potential targets for anticancer therapy. Onco Targets
Ther. 10:3801–3810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
30
|
Fuchigami T, Kibe T, Koyama H, Kishida S,
Iijima M, Nishizawa Y, Hijioka H, Fujii T, Ueda M, Nakamura N, et
al: Regulation of IL-6 and IL-8 production by reciprocal
cell-to-cell interactions between tumor cells and stromal
fibroblasts through IL-1α in ameloblastoma. Biochem Biophys Res
Commun. 451:491–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao Q, Lv B, Qiao B, Zheng CQ and Chen ZF:
Immortalization of ameloblastoma cells via reactivation of
telomerase function: Phenotypic and molecular characteristics. Oral
Oncol. 45:e239–e244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wani MR, Fuller K, Kim NS, Choi Y and
Chambers T: Prostaglandin E2 cooperates with TRANCE in osteoclast
induction from hemopoietic precursors: Synergistic activation of
differentiation, cell spreading, and fusion. Endocrinology.
140:1927–1935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fuller K, Bayley KE and Chambers TJ:
Activin A is an essential cofactor for osteoclast induction.
Biochem Biophys Res Commun. 268:2–7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohta K, Naruse T, Ishida Y, Shigeishi H,
Nakagawa T, Fukui A, Nishi H, Sasaki K, Ogawa I and Takechi M:
TNF-α-induced IL-6 and MMP-9 expression in immortalized
ameloblastoma cell line established by hTERT. Oral Dis. 23:199–209.
2017. View Article : Google Scholar
|
|
35
|
Kline M, Donovan K, Wellik L, Lust C, Jin
W, Moon-Tasson L, Xiong Y, Witzig TE, Kumar S, Rajkumar SV and Lust
JA: Cytokine and chemokine profiles in multiple myeloma;
signifi-cance of stromal interaction and correlation of IL-8
production with disease progression. Leuk Res. 31:591–598. 2007.
View Article : Google Scholar
|
|
36
|
Bendre MS, Margulies AG, Walser B, Akel
NS, Bhattacharrya S, Skinner RA, Swain F, Ramani V, Mohammad KS,
Wessner LL, et al: Tumor-derived interleukin-8 stimulates
osteolysis independent of the receptor activator of nuclear
factor-kappaB ligand pathway. Cancer Res. 65:11001–11009. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bendre MS, Montague DC, Peery T, Akel NS,
Gaddy D and Suva LJ: Interleukin-8 stimulation of
osteoclastogenesis and bone resorption is a mechanism for the
increased osteolysis of metastatic bone disease. Bone. 33:28–37.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanimoto K, Yoshida E, Mita S, Nibu Y,
Murakami K and Fukamizu A: Human activin betaA gene. Identification
of novel 5′ exon, functional promoter, and enhancers. J Biol Chem.
271:32760–32769. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshimoto S, Morita H, Matsubara R,
Mitsuyasu T, Imai Y, Kajioka S, Yoneda M, Ito Y, Hirofuji T,
Nakamura S and Hirata M: Surface vacuolar ATPase in ameloblastoma
contributes to tumor invasion of the jaw bone. Int J Oncol.
48:1258–1270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suva LJ, Washam C, Nicholas RW and Griffin
RJ: Bone metastasis: Mechanisms and therapeutic opportunities. Nat
Rev Endocrinol. 7:208–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yoshimura T, Matsushima K, Tanaka S,
Robinson EA, Appella E, Oppenheim JJ and Leonard EJ: Purification
of a human monocyte-derived neutrophil chemotactic factor that has
peptide sequence similarity to other host defense cytokines. Proc
Natl Acad Sci USA. 84:9233–9237. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim SJ, Uehara H, Karashima T, McCarty M,
Shih N and Fidler IJ: Expression of interleukin-8 correlates with
angiogenesis, tumorigenicity, and metastasis of human prostate
cancer cells implanted orthotopically in nude mice. Neoplasia.
3:33–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hwang YS, Lee SK, Park KK and Chung WY:
Secretion of IL-6 and IL-8 from lysophosphatidic acid-stimulated
oral squamous cell carcinoma promotes osteoclastogenesis and bone
resorption. Oral Oncol. 48:40–48. 2012. View Article : Google Scholar
|
|
46
|
Baud V and Karin M: Signal transduction by
tumor necrosis factor and its relatives. Trends Cell Biol.
11:372–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
da Silva TA, Batista AC, Mendonca EF,
Leles CR, Fukada S and Cunha FQ: Comparative expression of RANK,
RANKL, and OPG in keratocystic odontogenic tumors, ameloblastomas,
and dentigerous cysts. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 105:333–341. 2008. View Article : Google Scholar
|
|
48
|
Siar CH, Tsujigiwa H, Ishak I, Hussin NM,
Nagatsuka H and Ng KH: RANK, RANKL, and OPG in recurrent
solid/multicystic amelo-blastoma: Their distribution patterns and
biologic significance. Oral Surg Oral Med Oral Pathol Oral Radiol.
119:83–91. 2015. View Article : Google Scholar
|
|
49
|
Vale W, Rivier J, Vaughan J, McClintock R,
Corrigan A, Woo W, Karr D and Spiess J: Purification and
characterization of an FSH releasing protein from porcine ovarian
follicular fluid. Nature. 321:776–779. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xia Y and Schneyer AL: The biology of
activin: Recent advances in structure, regulation and function. J
Endocrinol. 202:1–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen YG, Lui HM, Lin SL, Lee JM and Ying
SY: Regulation of cell proliferation, apoptosis, and carcinogenesis
by activin. Exp Biol Med (Maywood). 227:75–87. 2002. View Article : Google Scholar
|
|
52
|
Chen YG, Wang Q, Lin SL, Chang CD, Chuang
J and Ying SY: Activin signaling and its role in regulation of cell
proliferation, apoptosis, and carcinogenesis. Exp Biol Med
(Maywood). 231:534–544. 2006. View Article : Google Scholar
|
|
53
|
Nicks KM, Perrien DS, Akel NS, Suva LJ and
Gaddy D: Regulation of osteoblastogenesis and osteoclastogenesis by
the other reproductive hormones, activin and inhibin. Mol Cell
Endocrinol. 310:11–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kajita T, Ariyoshi W, Okinaga T, Mitsugi
S, Tominaga K and Nishihara T: Mechanisms involved in enhancement
of osteoclast formation by activin-A. J Cell Biochem.
119:6974–6985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Snider JL, Allison C, Bellaire BH, Ferrero
RL and Cardelli JA: The beta1 integrin activates JNK independent of
CagA, and JNK activation is required for Helicobacter pylori
CagA+-induced motility of gastric cancer cells. J Biol
Chem. 283:13952–13963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ohshiba T, Miyaura C, Inada M and Ito A:
Role of RANKL-induced osteoclast formation and MMP-dependent matrix
degradation in bone destruction by breast cancer metastasis. Br J
Cancer. 88:1318–1326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zheng Y, Chow SO, Boernert K, Basel D,
Mikuscheva A, Kim S, Fong-Yee C, Trivedi T, Buttgereit F,
Sutherland RL, et al: Direct crosstalk between cancer and
osteoblast lineage cells fuels metastatic growth in bone via
auto-amplification of IL-6 and RANKL signaling pathways. J Bone
Miner Res. 29:1938–1949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bussard KM, Venzon DJ and Mastro AM:
Osteoblasts are a major source of inflammatory cytokines in the
tumor microenvironment of bone metastatic breast cancer. J Cell
Biochem. 111:1138–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sohara Y, Shimada H, Minkin C,
Erdreich-Epstein A, Nolta JA and DeClerck YA: Bone marrow
mesenchymal stem cells provide an alternate pathway of osteoclast
activation and bone destruction by cancer cells. Cancer Res.
65:1129–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qian Y and Huang HZ: The role of RANKL and
MMP-9 in the bone resorption caused by ameloblastoma. J Oral Pathol
Med. 39:592–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guénet JL: The mouse genome. Genome Res.
15:1729–1740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cilvik SN, Wang JI, Lavine KJ, Uchida K,
Castro A, Gierasch CM, Weinheimer CJ, House SL, Kovacs A, Nichols
CG and Ornitz DM: Fibroblast growth factor receptor 1 signaling in
adult cardiomyocytes increases contractility and results in a
hypertrophic cardiomyopathy. PLoS One. 8:e829792013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sunagar K, Fry BG, Jackson TN, Casewell
NR, Undheim EA, Vidal N, Ali SA, King GF, Vasudevan K, Vasconcelos
V and Antunes A: Molecular evolution of vertebrate neurotrophins:
Co-option of the highly conserved nerve growth factor gene into the
advanced snake venom arsenalf. PLoS One. 8:e818272013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shen J, Li S and Chen D: TGF- β signaling
and the development of osteoarthritis. Bone Res. 2:pii: 140022014.
View Article : Google Scholar
|





















